Abstract
Aplasia cutis congenita (ACC) is a congenital disorder that can be classified into nine types, with Type I ACC being the most common. Type V ACC associated with fetus papyraceus is a rare subtype of ACC. We report the case of a Type V ACC in a male newborn with extensive abdominal skin defects. The patient received conservative treatment using hydrogel foam and silicone foam dressings. Approximately five weeks later, the patient was discharged when more than 60% of the skin had completed epithelialization. After discharge from West China Second University Hospital, Chengdu , the patient continued to be followed up regularly at the Burns and Plastic Surgery Clinic at local hospital in Gansu. We followed up the child by telephone. After 4 months of follow-up, scar tissue formation was observed in the trunk area. The infant is 2 years and 5 months old now, physical examination did not reveal any organ problems.
Keywords: Type V aplasia cutis congenital, Conservative treatment, Hydrogel foam, Silicone foam dressings
Introduction
Aplasia cutis congenita (ACC) is a rare, congenital disorder characterized by localized or widespread absence of skin at birth with various etiologies and heterogeneous clinical presentation [1]. The precise incidence of ACC is unknown but thought to be around 0.5/10 000 in Europe [2]. However, the actual incidence may be higher, because cases of mild ACC are probably underreported [3]. Skin lesions can occur anywhere on the body, but 80% and even more cases of ACC have involved the scalp, others (15%) could be found on the trunk and limbs [2, 4]. ACC may be an isolated skin malformation involving one or more skin districts, and it may also merge with multiple other organ malformations [5]. The defect may be superficial, depth varies from the absence of epidermis and upper dermis only to subcutaneous tissue. In some severe cases, it may extend to deeper tissues, such as the muscle or bone (periosteum, skull, and dura).
The most widely used classification of ACC was proposed by Frieden in 1986. Frieden grouped ACC into nine subtypes based on its associated abnormalities, inheritance pattern, and body area affected [6]. Type I ACC is the most common type, and most of them are without underlying syndromes, malformations, or other abnormalities. Type V is rare, associated with fetus papyraceus or placental infarction, and characterized by a symmetrical and H-shaped distribution over the trunk and extremities [7]. Here, we successfully treated a patient with extensive abdominal skin defects with conservative treatment.
Case history
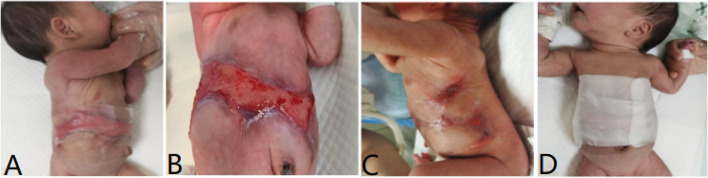
An infant with a birth weight of 2350g, was delivered through cesarean section at 37 weeks of gestation at a local hospital in Gansu (438 km far from West China Second University Hospital, Chengdu). The mother was a 35-year-old healthy primigravid woman who conceived a monocorionic diamniotic twin pregnancy naturally. The mother experienced fetal demise of one fetus at 13-14 weeks of gestation. During the pregnancy, the mother had no history of taking medicine and did not experience remarkable complications during the rest of the pregnancy. The surviving one was admitted to our hospital less than 24 hours after birth due to significant skin defects on the trunk. There were symmetrical skin defects in the abdomen and bilateral trunk, measuring approximately 17 × 4 cm. The lesions appeared to be a complete absence of epidermis, dermis, and subcutaneous fat. The defects exhibited clear boundaries and were covered only by a thin gelatinous translucent membrane, revealing visible ribs and blood vessels (Fig. 1). No skin defects were observed in other areas, and the remaining physical examination and complementary exams such as ultrasound of the chest and abdomen showed no abnormality. These indicated that the patient has no combination of deformities or defects other than abdominal skin defects.
Fig. 1.
Symmetrical skin defects in the abdomen and bilateral trunk
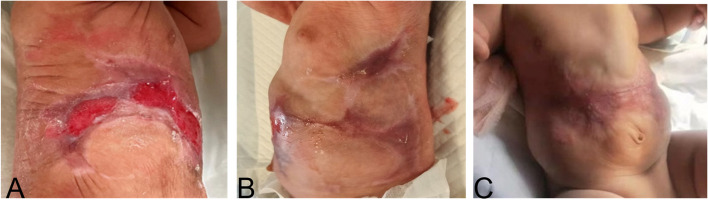
The patient was diagnosed with "Type V aplasia cutis congenita" based on the clinical manifestations and the Frieden classification. Due to the large defect skin and the presence of vital organs in the abdominal area such as the liver, and intestines. The primary focus of the patient was the local wound management. The main principles of wound treatment included maintaining adequate moisture in the wound, promoting epithelial tissue growth, and preventing infection. The patient received treatment with lipid hydrogel foam dressing (Urgotul®), silicone foam dressing (ALLEVYN GENTLE BORDER), and growth factors (Fig. 2). Additionally, prophylactic antibiotics were administered intravenously using Cefoperazone sodium tazobactam sodium. Following approximately five weeks of the treatment, the patient was discharged from the neonatal intensive care unit (NICU) when more than 60% of the lesion showed epithelialization. Throughout the hospitalization period, the patient did not experience systemic or local infections, nor did they develop severe complications such as electrolyte imbalance or bleeding. After discharge from the West China Second University Hospital, Chengdu, the patient continued to be followed up regularly at the Burns and Plastic Surgery Clinic for dressing changes and observation of wound healing at local hospital in Gansu. We followed up the child by telephone. After 4 months of follow-up, scar tissue formation was observed in the trunk area (Fig. 3). The infant is 2 years and 5 months old now, physical examination did not reveal any organ problems.
Fig. 2.
Significant improvement after 5 weeks with lipid hydrogel foam dressing, silicone foam dressing, and growth factors
Fig. 3.
More than 60% of the lesions showed epithelialization (A, B), and scar formation was observed in the trunk area after 4 months (C)
Discussion and conclusions
Type V ACC is rare, associated with fetus papyraceus or placental infarction, and characterized by a symmetrical and H-shaped distribution over the trunk and extremities. The pathogenesis of ACC is unknown, multiple factors might be contributing to the development of ACC. ACC is associated with chromosomal abnormalities, amniotic irregularities, vascular disorders, fetus papyraceus, and pregnancy infection (herpes simplex virus and varicella virus). Otherwise, the use of some teratogenic drugs during the mother’s pregnancy might increase the risk of ACC, such as misoprostol, benzodiazepines, valproic acid, cocaine, and methimazole [8]. Besides, several genes are causing Adams-Oliver Syndrome (diagnosed in the presence of ACC of the scalp and terminal transverse limb defects), such as DLL4, NOTCH1, DOCK6 and EOGT [9, 10]. Several heterozygous mutations in COL7A1 may result in Bart’s syndrome, which is characterized by ACC and epidermolysis bullosa (EB) [11, 12]. Genetic mutations in the COL7A1 gene are also associated with dystrophic EB [13]. Moreover, ACC can be associated with physical defects or syndromes. In the case of children diagnosed with trisomy 18, membrane ACC has been observed on the skull [14, 15].
Several theories have been proposed to explain the pathomechanism of Type V ACC. Although the exact cause is not yet known, most recent evidence tended to support transient hypovolemia as the primary cause [16]. Intrauterine death of one twin results in rapid blood shunting from the surviving fetus to the dying one [17]. Especially in monochorionic pregnancies, this acute transfusion between twins could induce acute hypovolemia in the living twin, resulting in ischemia of the skin and other organs [18, 19]. Monochorionic twin pregnancies have greater morbidity risk than dichorionic twin pregnancies, possibly because placental vascular anastomoses produce anomalous fetal-fetal transfusion [20]. Arteriovenous vascular anastomoses are found in 90-95% of monochorionic placentas [21]. Otherwise, studies suggested that in the presence of late first to early second-trimester fetal death in an initial twin gestation pregnancy, small abdominal circumference, detectable acetylcholinesterase, high amniotic and maternal alpha-fetoprotein could suggest the diagnosis of ACC with fetus papyraceous [22].
Most ACCs were generally treated with conservative measures and took approximately 6 weeks to fully epithelialize. Conservative treatment focuses on keeping the wound sufficiently moist, promoting epithelial tissue growth, and preventing infection [23]. Conservative treatment includes various dressings, topical antimicrobials, and systemic antibiotics [24]. Dressings used include ionic silver dressing, moist exposed burn ointment, hydrogels, adhesive or nonadherent dressings, basic fibroblast growth factor, and recombinant human epidermal growth factor [25–28]. The benefit of conservative treatment of ACC is the avoidance of the risks associated with surgery. However, when there is a large area of skin defect in important areas, such as the scalp, surgical approaches can be used to prevent morbidity and mortality from central nervous system related complications such as meningitis, sagittal sinus bleeding, and cerebral hernia. Surgical options include local or free flaps, skin grafting, acellular dermal matrix, allogenic dermis, and cultured epithelial allografts [29–35]. A child with a large total body surface area of ACC (37%) involving the entire scalp, chest, and trunk was successfully treated using acellular dermal matrix and cultured epithelial allografts [32]. Other surgical treatments of ACC on the scalp or skull include delayed cranioplasty, early composite cranioplasty, and tissue expanders [36].
The patient received conservative treatment with lipid hydrogel foam dressing (Urgotul®), silicone foam dressing (ALLEVYN GENTLE BORDER), and growth factors. Following approximately five weeks of the treatment, the patient was discharged from the NICU when more than 60% of the lesion showed epithelialization. Most children with Type V ACC can be treated conservatively with satisfactory results.
Acknowledgements
We thank all the participants in this study and all clinicians who were involved in the collection.
Abbreviations
- ACC
Aplasia cutis congenita
- NICU
Neonatal intensive care unit
- EB
Epidermolysis bullosa
Authors’ contributions
YS and RY wrote and revised the manuscript. ZYS, JY and SLH collected the data. XWL and XFZ critically reviewed the manuscript and provided supervision. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The parents of this patient consented to the publication of the case and any accompanying images with written informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan song and Ru yang contributed equally to this work.
Contributor Information
Xiaowen li, Email: 1281984307@qq.com.
Xiufang zhao, Email: xiufangzh@163.com.
References
- 1.Humphrey SR, et al. A practical approach to the evaluation and treatment of an infant with aplasia cutis congenita. J Perinatol. 2018;38(2):110–117. doi: 10.1038/jp.2017.142. [DOI] [PubMed] [Google Scholar]
- 2.Coi A, et al. Epidemiology of aplasia cutis congenita: A population-based study in Europe. J Eur Acad Dermatol Venereol. 2023;37(3):581–589. doi: 10.1111/jdv.18690. [DOI] [PubMed] [Google Scholar]
- 3.Hioki T, et al. Infant bald patch: ultrasonographic diagnosis of aplasia cutis congenita. J Eur Acad Dermatol Venereol. 2017;31(6):e276–e277. doi: 10.1111/jdv.14018. [DOI] [PubMed] [Google Scholar]
- 4.Sathishkumar D, Ogboli M, Moss C. Classification of aplasia cutis congenita: a 25-year review of cases presenting to a tertiary paediatric dermatology department. Clin Exp Dermatol. 2020;45(8):994–1002. doi: 10.1111/ced.14331. [DOI] [PubMed] [Google Scholar]
- 5.Mesrati H, et al. Aplasia cutis congenita: report of 22 cases. Int J Dermatol. 2015;54(12):1370–1375. doi: 10.1111/ijd.12707. [DOI] [PubMed] [Google Scholar]
- 6.Frieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol. 1986;14(4):646–660. doi: 10.1016/S0190-9622(86)70082-0. [DOI] [PubMed] [Google Scholar]
- 7.Silva Díaz E, et al. Type V aplasia cutis congenita in a preterm newborn successfully resolved. Dermatol Ther. 2020;33(6):e13888. doi: 10.1111/dth.13888. [DOI] [PubMed] [Google Scholar]
- 8.Karg E, et al. Aplasia cutis congenita after methimazole exposure in utero. Pediatr Dermatol. 2004;21(4):491–494. doi: 10.1111/j.0736-8046.2004.21417.x. [DOI] [PubMed] [Google Scholar]
- 9.Zepeda-Romero LC, et al. Intrafamilial phenotypic variability in autosomal recessive DOCK6-related Adams-Oliver syndrome. Eur J Med Genet. 2022;65(12):104653. doi: 10.1016/j.ejmg.2022.104653. [DOI] [PubMed] [Google Scholar]
- 10.Rojnueangnit K, et al. A novel DLL4 mutation in Adams-Oliver syndrome with absence of the right pulmonary artery in newborn. Am J Med Genet A. 2022;188(2):658–664. doi: 10.1002/ajmg.a.62562. [DOI] [PubMed] [Google Scholar]
- 11.Han YM, et al. Bart’s Syndrome with Novel Frameshift Mutations in the COL7A1 Gene. Fetal Pediatr Pathol. 2019;38(1):72–79. doi: 10.1080/15513815.2018.1543370. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, et al. Bart's syndrome in a family affected three consecutive generations with mutation c.6007G>A in COL7A1. J Dermatol. 2018;45(8):1000–1002. doi: 10.1111/1346-8138.14352. [DOI] [PubMed] [Google Scholar]
- 13.Chiaverini C, et al. Aplasia cutis congenita with dystrophic epidermolysis bullosa: clinical and mutational study. Br J Dermatol. 2014;170(4):901–906. doi: 10.1111/bjd.12741. [DOI] [PubMed] [Google Scholar]
- 14.Cammarata-Scalisi F, et al. Membranous aplasia cutis congenita in trisomy 18. Ital J Pediatr. 2020;46(1):120. doi: 10.1186/s13052-020-00885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.raMeSh PotPalle D. Edward syndrome with aplasia cutis congenita: a rare case report. Indian J Neonatal Med Res. 2015;4(1):7–9. [Google Scholar]
- 16.Pieretti ML, et al. Aplasia Cutis Congenita Associated with Fetus Papyraceus. Pediatric Dermatol. 2015;32(6):858–861. doi: 10.1111/pde.12651. [DOI] [PubMed] [Google Scholar]
- 17.Lewi L, et al. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol. 2003;15(2):177–194. doi: 10.1097/00001703-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Klein RQ, et al. Symmetric aplasia cutis congenita associated with fetus papyraceus: report of two cases. Pediatr Dermatol. 2011;28(4):467–469. doi: 10.1111/j.1525-1470.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 19.Louise L, et al. Fetus papyraceus: congenital pulmonary anomalies associated with congenital aplasia cutis on the surviving twin. Pediatr Dermatol. 2013;30(6):e143–e145. doi: 10.1111/pde.12096. [DOI] [PubMed] [Google Scholar]
- 20.Lewi L. Monochorionic diamniotic twin pregnancies. Am J Obstet Gynecol MFM. 2022;4(2S):100501. doi: 10.1016/j.ajogmf.2021.100501. [DOI] [PubMed] [Google Scholar]
- 21.Society for Maternal-Fetal Medicine. Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol. 2013;208(1):3–18. doi: 10.1016/j.ajog.2012.10.880. [DOI] [PubMed] [Google Scholar]
- 22.Mazza JM, et al. Aplasia cutis congenita in a setting of fetus papyraceus associated with small fetal abdominal circumference and high alpha-fetoprotein and amniotic acetylcholinesterase. Pediatr Dermatol. 2015;32(1):138–140. doi: 10.1111/pde.12228. [DOI] [PubMed] [Google Scholar]
- 23.Başterzi Y, et al. Aplasia cutis congenita of the scalp and calvarium: conservative wound management with novel wound dressing materials. J Craniofac Surg. 2007;18(2):427–429. doi: 10.1097/01.scs.0000246500.84935.4f. [DOI] [PubMed] [Google Scholar]
- 24.Bernbeck B, et al. Aplasia cutis congenita of the scalp: how much therapy is necessary in large defects? Acta Paediatr. 2005;94(6):758–760. doi: 10.1111/j.1651-2227.2005.tb01977.x. [DOI] [PubMed] [Google Scholar]
- 25.Lei G-F, et al. Treating aplasia cutis congenita in a newborn with the combination of ionic silver dressing and moist exposed burn ointment: A case report. World J Clin Cases. 2019;7(17):2611–2616. doi: 10.12998/wjcc.v7.i17.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahiri A, Nishikawa H. A nonadherent dressing for aplasia cutis congenita. J Plast Reconstr Aesthet Surg. 2006;59(7):781–782. doi: 10.1016/j.bjps.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Orgun D, et al. Conservative Treatment of Large Aplasia Cutis Congenita of the Scalp With Bone Defect With Basic Fibroblast Growth Factor Application. J Craniofac Surg. 2017;28(2):e154–e158. doi: 10.1097/SCS.0000000000003347. [DOI] [PubMed] [Google Scholar]
- 28.Yang X-F, Shi S-W, Chen K. Case report: Recombinant human epidermal growth factor gel plus kangfuxin solution in the treatment of aplasia cutis congenita in a case with Adams-Oliver syndrome. Front Surg. 2022;9:1072021. doi: 10.3389/fsurg.2022.1072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park ES, et al. Clinical Application of Acellular Dermal Matrix in the Treatment of Aplasia Cutis Congenita on Scalp. J Craniofac Surg. 2017;28(8):e788–e789. doi: 10.1097/SCS.0000000000004050. [DOI] [PubMed] [Google Scholar]
- 30.Simman R, Priebe CJ, Simon M. Reconstruction of aplasia cutis congenita of the trunk in a newborn infant using acellular allogenic dermal graft and cultured epithelial autografts. Ann Plast Surg. 2000;44(4):451–454. doi: 10.1097/00000637-200044040-00019. [DOI] [PubMed] [Google Scholar]
- 31.Trah J, et al. Integra®-Dermal Regeneration Template and Split-Thickness Skin Grafting: A Therapy Approach to Correct Aplasia Cutis Congenita and Epidermolysis Bullosa in Carmi Syndrome. Dermatol Ther. 2018;8(2):313–321. doi: 10.1007/s13555-018-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui CLY, et al. A rare case of extensive aplasia cutis congenita: Our surgical approach. J Plast Reconstr Aesthet Surg. 2023;80:193–199. doi: 10.1016/j.bjps.2023.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Mericli AF, et al. Calvarial Regeneration With Use of Acellular Dermal Matrix in Aplasia Cutis Congenita. J Craniofac Surg. 2015;26(6):1960–1962. doi: 10.1097/SCS.0000000000002024. [DOI] [PubMed] [Google Scholar]
- 34.Scotti A, et al. A Case of Large Aplasia Cutis Congenita with Underlying Skull Defect: Effective Surgical Treatment with Integra® Dermal Regeneration Template. Pediatr Neurosurg. 2021;56(3):268–273. doi: 10.1159/000512022. [DOI] [PubMed] [Google Scholar]
- 35.Bharti G, et al. Aplasia cutis congenita: clinical management of a rare congenital anomaly. J Craniofac Surg. 2011;22(1):159–165. doi: 10.1097/SCS.0b013e3181f73937. [DOI] [PubMed] [Google Scholar]
- 36.Hadad I, Meara JG, Rogers-Vizena CR. A Novel Local Autologous Bone Graft Donor Site After Scalp Tissue Expansion in Aplasia Cutis Congenita. J Craniofac Surg. 2016;27(4):904–907. doi: 10.1097/SCS.0000000000002620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.