Abstract
Background
Low back pain (LBP) is one of the most common reasons for consultation in general practice. Currently, LBP is categorised into specific and non-specific causes. However, extravertebral causes, such as abdominal aortic aneurysm or pancreatitis, are not being considered.
Methods
A systematic literature search was performed across MEDLINE, Embase, and the Cochrane library, complemented by a handsearch. Studies conducted between 1 January 2001 and 31 December 2020, where LBP was the main symptom, were included.
Results
The literature search identified 6040 studies, from which duplicates were removed, leaving 4105 studies for title and abstract screening. Subsequently, 265 publications were selected for inclusion, with an additional 197 publications identified through the handsearch. The majority of the studies were case reports and case series, predominantly originating from specialised care settings. A clear distinction between vertebral or rare causes of LBP was not always possible. A range of diseases were identified as potential extravertebral causes of LBP, encompassing gynaecological, urological, vascular, systemic, and gastrointestinal diseases. Notably, guidelines exhibited inconsistencies in addressing extravertebral causes.
Discussion
Prior to this review, there has been no systematic investigation into extravertebral causes of LBP. Although these causes are rare, the absence of robust and reliable epidemiological data hinders a comprehensive understanding, as well as the lack of standardised protocols, which contributes to a lack of accurate description of indicative symptoms. While there are certain disease-specific characteristics, such as non-mechanical or cyclical LBP, and atypical accompanying symptoms like fever, abdominal pain, or leg swelling, that may suggest extravertebral causes, it is important to recognise that these features are not universally present in every patient.
Conclusion
The differential diagnosis of extravertebral LBP is extensive with relatively low prevalence rates dependent on the clinical setting. Clinicians should maintain a high index of suspicion for extravertebral aetiologies, especially in patients presenting with atypical accompanying symptoms.
Keywords: Extravertebral, Non-vertebral, Non-spinal, Low back pain
Background
Fundamentally, low back pain (LBP) represents a symptom rather than an aetiological diagnosis per se. Since establishing a definite pathophysiological diagnosis is often neither necessary nor possible, most clinical guidelines pragmatically distinguish between non-specific LBP, specific LBP, and sciatica/radiculopathy [1]. Furthermore, extravertebral or non-spinal medical disorders may mimic or present clinically as LBP. Consequently, some guidelines recommend considering extravertebral or non-spinal diseases in the differential diagnosis. Recognising extravertebral causes is crucial to avoid misdiagnosis and inappropriate management of potentially life-threatening diseases. In settings where patients have direct access to specialised care, such as orthopaedics and physiotherapy, the likelihood of considering non-musculoskeletal disease may be lower [2]. Deyo and Weinstein once estimated that approximately 2% of patients presenting with LBP in primary care have what they referred to as “visceral” disease. However, this percentage lacks a specific data source [3], yet it has been consistently cited in subsequent literature [4–16]. Within a specialist setting, it has been estimated that up to 10-25% of patients presenting with back pain do not have a vertebral pathology [17].
While LBP caused by conditions such as abdominal aortic aneurysm, peripheral arterial disease [18–20], or renal calculus can unambiguously be classified as extravertebral or non-spinal diseases, in many instances, categorisation remains challenging. For example, determining whether conditions involving intramedullary tumours, metabolic diseases (e.g., spinal gout), or hip pathology [21] mimicking radiculopathy should be attributed to spinal or extravertebral causes of pain remains debatable (Fig. 1). At times, these conditions are collectively referred to as unusual or rare causes for LBP [22–34]. The term “extravertebral” or “non-spinal” LBP is anatomically incorrect since it refers only to the bony structures of the back. Currently, there is no consensus on a definition or terminology to classify serious LBP not typically covered by “red flags”. Red flags are warning signs related to pathologies like tumours, fractures, inflammations or infections of the spine [35–37].
Fig. 1.
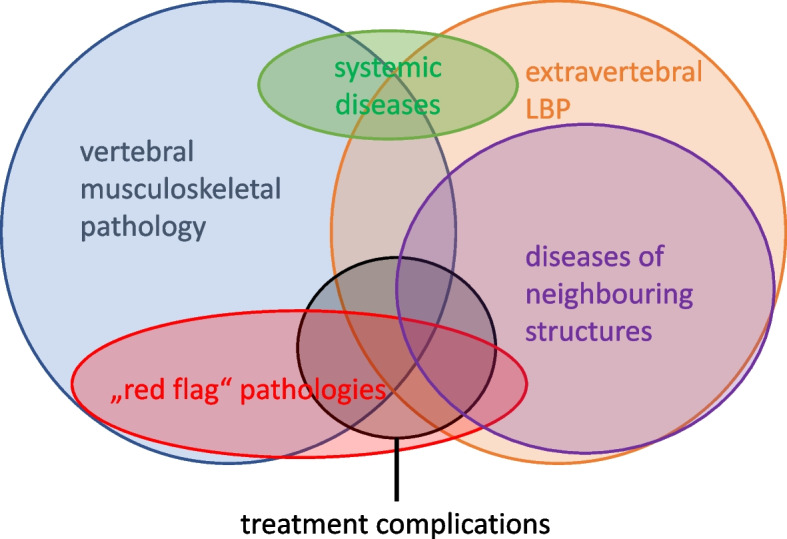
Overlap of definitions with bubble sizes approximately representing epidemiology. LBP = low back pain
The aim of this scoping review is to summarise what is known on the epidemiology and presentation of extravertebral or non-spinal LBP, in order to help clinicians assessing patients with LBP to recognise when it is appropriate to include this in the differential diagnosis.
Methods
This is a scoping review conducted according to the PRISMA extension for scoping reviews (Appendix 1) [38]. Since PROSPERO does not register scoping reviews, a protocol was not registered. A scoping review was chosen due to several factors, including the absence of a universally accepted definition, the broad spectrum of diseases, and the limited existing literature on the topic. Furthermore, this methodology was selected to facilitate a comprehensive overview of the field, identify current research gaps, and provide recommendations for future research.
Search strategy
The authors searched three electronic databases (MEDLINE, Embase, and the Cochrane Library). In 2001, Deyo and Weinstein published the first major narrative review of extravertebral causes of LBP [3]. Therefore, the search scope was limited to publications from 1 January 2001 to 31 December 2020. The detailed search strategy is outlined in Fig. 2, and the specific search terms used are available in Appendix 2. Where required, search terms were amalgamated using Boolean logic and database-specific filters. All publications available in either German or English languages were included.
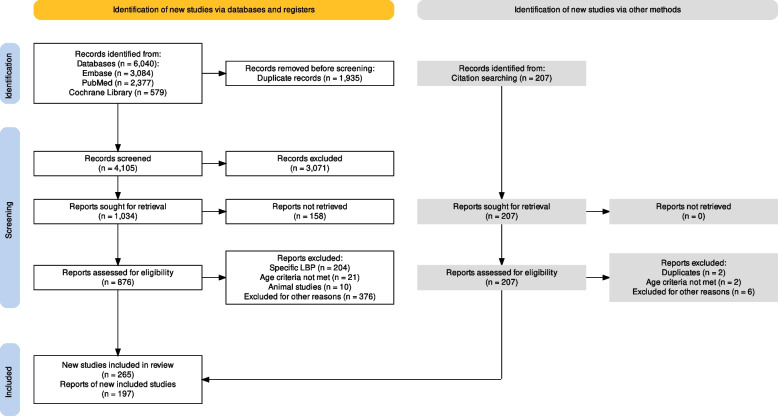
Fig. 2.
Search flow diagram of the literature review process for studies on extravertebral low back pain according to the PRISMA2020 Statement [39]. LBP = low back pain
Study selection
There is no universally accepted definition allowing to separate “extravertebral” unambiguously from “vertebral” LBP (Fig. 1). Furthermore, the terminologies are not formulated clearly and are insufficient for classifying both included and excluded diseases. Alternatively, the terms “extraspinal” or “non-spinal” back pain are also used. During the review process, the authors encountered difficulties in finding an accurate definition due to overlapping terms and classification systems. Nevertheless, an attempt was made to classify the diseases related to low back pain.
Inclusion criteria comprised publications of case reports, case series, case-control studies, cohort studies, randomised controlled trials, observational studies, and reviews reporting low back pain as a symptom of non-primary vertebral/musculoskeletal disease in adults, including systemic diseases. The MeSH-terms are available in Appendix 2.
Exclusion criteria comprised publications with patients under the age of 18 and pain primarily reported in thoracic and cervical spine. Furthermore, “red flags” indicating pathologies, such as infections, rheumatic diseases, tumours, and fractures were excluded. A complete summary of excluded pathologies can be seen in Appendix 3.
After the removal of duplicates, two authors screened the titles and abstracts independently. The included articles were discussed among the authors according to relevance, data extraction, and quality. Dissents were solved by consensus. This was followed by a handsearch.
Clinical guideline selection
All clinical guidelines listed in the most recent review of LBP guidelines [1] written in English or German language were reviewed to find recommendations regarding extravertebral LBP.
Data extraction
Descriptive characteristics were extracted from each manuscript, including, author’s name(s), year of publication, country, study design and setting. Depending on the type of publication, further data was extracted.
For case reports and case series, extracted data included participant characteristics (sample size, sex, age, and co-morbidities), pain characteristics (acute vs chronic LBP, pain description, neurological and other symptoms), diagnostic characteristics (laboratory results, imaging, biopsy/other diagnostic, diagnostic confirmation, and physical examination) and differential diagnosis.
For case-control or cohort studies, the following data were extracted: data collection (retrospective/prospective), inclusion criteria, baseline, follow-up, LBP (acute/chronic), other symptoms, pain description, differential diagnosis and other information (e.g., epidemiological information, risk factors, and physical examination findings).
For reviews encompassing LBP in general, the following data points were extracted: classification, terminology, causes, estimated prevalence, symptoms pointing toward a non-spinal pathology, diagnostic values (e.g., patient history, physical examination, laboratory results, and imaging) and whether Weinstein and Deyo 2001 was cited or not. If specific pathologies were mentioned, only unique information about LBP associated with those diseases were extracted.
Results
After elimination of duplicates and resolving disagreement between the reviewers, a total of 4105 manuscripts were screened. Additionally, a handsearch was carried out and a further 197 manuscripts were included in the review (Fig. 2), thereby bring the total number of manuscripts included in this review to 462. Various extravertebral causes of LBP were identified and are illustrated in Fig. 3.
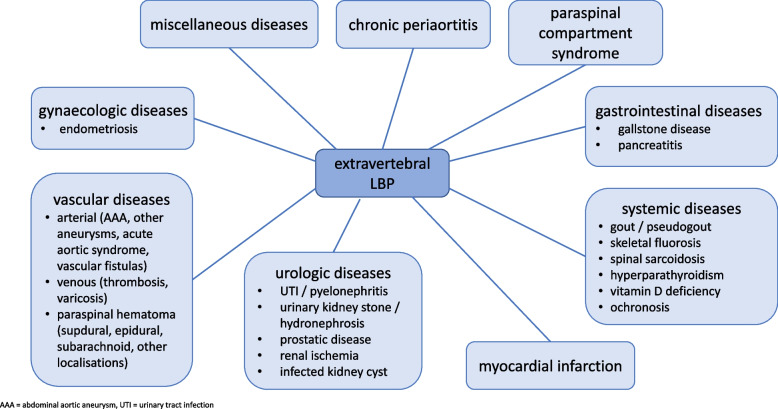
Fig. 3.
Overview: causes of extravertebral low back pain organised by pathologies. AAA = abdominal aortic aneurysm. LBP = low back pain. UTI = urinary tract infection
Description of studies
Case reports and case series
Various case reports mentioned LBP as part of the clinical presentation, but it often remained unclear, if LBP was the chief complaint. Case reports, where back pain was mentioned but no connection to the final diagnosis could be made or it seemed that back pain was a coincidence, were excluded. None of the case reports claimed adherence to the standards of reporting from CARE [40].
Case control and cohort studies
Only a few case-control or cohort studies were included. In these studies, various diseases, their therapies, and diagnostic methods were examined. If LBP was reported, comparisons were often made between pre- and post-interventional symptoms to draw conclusions regarding the association between pathology and LBP.
Narrative and systematic reviews
The content of narrative or systematic reviews often dealt with low back pain in general or in association with other pathologies, where LBP was a reported symptom. Reviews of other pathologies often omitted information regarding duration or localisation of LBP, while frequently including associated symptoms.
Guidelines
The included guidelines were examined with regards to the recommendations for dealing with extravertebral low back pain.
Results of the guideline review
Guidelines featured in the latest review of clinical practice guidelines on LBP were examined by Oliveira et al. 2018 [41]. They presented a total of 15 guidelines across various countries, of which 11 guidelines available in German or English were reviewed. Four guidelines mentioned extravertebral, non-vertebral, or systemic causes of LBP. One guideline reported that an “alternative diagnosis” should be considered [42]. The rest did not mention the possibility of an extravertebral origin of low back pain.
Results organised by pathology
Systemic diseases
Spinal gout
Gout is a systemic disease where monosodium urate crystal deposit in various joints, such as, facet, sacroiliac or interverbal joints as well as discs. Rheumatic diseases are pathologies indicated by red flags and usually refer to axial spondyloarthropathies excluding gout. Spinal or axial gout was first described in 1950 [43]. Since then, several case reports and case series have been published (Table 1). Toprover et al. published a review of 131 cases of spinal gout. We decided to disregard all case reports featured in their review in Table 1 [30, 44–52]. Furthermore, many of them were not within the time frame of this review. The majority (roughly 75%) had a history of gout. It is frequently concluded that axial gout is more common than assumed. However, no conclusions about the prevalence of axial gout can be drawn from the case reports and case series. The case series reveal a prevailing trend wherein a significant number of patients with axial gout have a confirmed diagnosis of gout, frequently accompanied by the presence of peripheral tophi. In most cases, the diagnosis was confirmed through intraoperative biopsies or fluoroscopy [43]. The case series conducted by de Mello et al. [53], stands out due to its investigation of spinal computed tomography (CT) scans in individuals who had a confirmed diagnosis of gout. Possible evidence of axial gout was found in 12/42 (29%) and peripheral tophi were associated with CT-findings suggestive for gout. The findings were not associated with current pain. Therefore, the claim that spinal gout is more frequent than assumed is weak, assuming that despite radiological findings, many patients with urate deposition in the spine are asymptomatic.
Table 1.
Case reports of gout presenting with low back pain
| Author / year / country | Setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation / differential diagnosis |
|---|---|---|---|---|---|
| Cardoso et al., 2014, Brazil [54] | ED send by GP | male, 55 y | chronic LBP, paraparesis | fever, no h/o gout, chronic kidney disease, diabetes | elevated ESR, CT-guided biopsy |
| Lu et al., 2017, China [55] | ED | male, 68 y | chronic LBP | no h/o gout | biopsy during surgery |
| Wang et al., 2017, China [56] | ED | male, 62 y | chronic LBP | h/o gout | biopsy during surgery |
| Ribeiro et al., 2018, Portugal [57] | ED | male, 77 y | acute LBP, paraparesis | h/o gout | biopsy during surgery |
| Qin et al., 2018, China [58] | ED | male, 56 y | subacute LBP | h/o gout | biopsy |
| Alqatari et al., 2018, Ireland [59] | specialist care | male, 55 y | chronic LBP | h/o gout, tophi and psoriatic arthropathy with non-response to TNF-blocker | elevated ESR, imaging (MRI, CT) |
| Zou et al., 2019, China [60] | specialist care | male, 55 y | acute LBP | intermittent claudication, h/o gout | elevated ESR, biopsy during surgery |
| Chen et al., 2020, China [61] | ED | male, 55 y | chronic LBP | no h/o gout | biopsy during surgery |
CT computer tomography, ED emergency department, ESR erythrocyte sedimentation rate, GP general practitioner, h/o history of, LBP low back pain, MRI magnetic resonance imaging, TNF-blocker tumour necrosis factor blocker, y years
Pseudogout
Pseudogout is a rare disease with calcium-pyrophosphate deposition, which can affect any joint, including facet joints, causing inflammatory arthritis. Five case reports and case series of pseudogout presenting with low back pain (Table 2) were included. Symptoms are non-specific and diagnosis is usually made incidentally due to suspicious findings leading to operative exploration with biopsies. Only one patient had a history of pseudogout.
Table 2.
Case reports or case series of pseudogout presenting with low back pain
| Author / year / country | Setting | Patient(s) | Presentation / clinical history | Diagnostic confirmation |
|---|---|---|---|---|
| Fujishiro et al., 2002, Japan [62] | ED | female, 71 y | acute LBP, h/o pseudogout, elevated ESR and CRP | joint aspiration |
| Gadgil et al., 2002, UK [63] | ED | female, 67 y | chronic LBP, sciatica, CT-Scan with calcified cyst with nerve compression | biopsy during surgery |
| Mahmud et al., 2005, UK [44] | not reported |
6 patients (4 with gout, 2 with pseudogout): female, 70 y |
subacute LBP, sciatica, MRI-Scan with cyst and nerve compression | biopsy during surgery |
| male, 81 y | chronic LBP, claudication, MRI-scan with severe spinal stenosis due to deformity and cysts | |||
| Namazie et al., 2012, New Zealand [64] | not reported | female, 69 y | subacute LBP, h/o scleroderma, CT-Scan with large, calcified mass | biopsy |
| Shen et al., 2019, China [65] | ED | male, 53 y | chronic LBP, h/o gout, elevated ESR and CRP, CT-Scan with mass and bone destruction of facet joints | biopsy |
CRP c-reactive protein, CT computer tomography, ED emergency department, ESR erythrocyte sedimentation rate, h/o history of, LBP low back pain, MRI magnetic resonance imaging, y years
Skeletal fluorosis
Two case reports were identified where the diagnosis of skeletal fluorosis, contributing to the onset of chronic metabolic bone disease, was associated with chronic LBP (Table 3). Skeletal fluorosis is a rare disease caused by increased ingestion of fluoride. It is endemic in some parts of Asia (e.g., China, India), where elevated fluoride concentrations are found in soil and water. Industrial exposure, accidental ingestion of fluoride containing medication or toothpaste and substance abuse are other possible causes. Mottling of teeth is a clinical sign of excessive exposure to fluoride as an infant. The condition is typically diagnosed incidentally based on osteosclerosis and ligamentous calcification on X-ray. There is no established treatment.
Table 3.
Case reports of skeletal fluorosis presenting with low back pain
| Author / year / country | Setting | Patient | Clinical history | Diagnostic confirmation | Comment |
|---|---|---|---|---|---|
| Peicher et al., 2017, USA [66] | not reported | male, 33 y | progressive LBP for 3 years | X-ray (generalised osteosclerosis) | fluoride inhalation (huffing of cleaner) |
| Shetty et al., 2015, India [67] | not reported | male, 35 y | compressive myelopathy with paraparesis with LBP for 3 years, mottling of teeth | X-ray (generalised osteosclerosis and calcifications of the longitudinal ligament) | most likely endemic due to increased fluoride in drinking water |
LBP low back pain, y years
Spinal sarcoidosis
Sarcoidosis is a multisystem granulomatous disease, which most commonly affects the lung. It is estimated that 1-3% of patients with sarcoidosis have some form of osseous disease, which is mostly asymptomatic. A total of 6 case reports highlighting spinal sarcoidosis associated with LBP were included (Table 4). Back pain can be caused by either spinal osseous involvement or medullary disease. Improvement following treatment, e.g., with corticosteroids, has been reported. In some patients, the diagnosis of sarcoidosis was pre-existing, while in other cases, suspicious findings on magnetic resonance imaging (MRI) prompted bone biopsies that lead to diagnosis [68, 69]. The radiological findings, however, lack specificity. Given the array of potential differential diagnoses encompassing osseous metastasis, myeloma, lymphoma, tuberculosis, and osteomyelitis, the verification of the diagnosis primarily relied on bone biopsy. No discernible clinical clue beyond a pre-existing diagnosis of sarcoidosis were evident.
Table 4.
Case reports of sarcoidosis presenting with low back pain
| Author / year / country | Setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Ludwig et al., 2003, USA [68] | not reported | female, 51 y | LBP (no duration reported) | - | MRI, PET-Scan, bone biopsy |
| Ashamalla et al., 2016, USA [69] | not reported | female, 60 y | LBP (no duration reported) | h/o Crohn’s disease | MRI, PET-Scan, bone biopsy |
| Rice et al., 2011, UK [70] | not reported | female, 62 y | LBP (no duration reported) | h/o sarcoidosis | MRI, bone biopsy, response to therapy with corticosteroids |
| Valencia et al., 2009, USA [71] | not reported | female, 48 y | LBP (no duration reported) | diffuse arthralgia, abdominal pain, dyspnoea | MRI, PET-Scan, bone biopsy |
| Packer et al., 2005, USA [72] | specialist care | male, 47 y | chronic LBP | h/o pulmonary sarcoidosis, weight loss | MRI, PET-Scan, bone biopsy |
| Barazi et al., 2008, UK [73] | not reported | female, 44 y | chronic LBP, altered sensation in the lower limbs | - | MRI, bone biopsy |
h/o history of, LBP low back pain, MRI magnetic resonance imaging, PET positron emission tomography, y years
Hyperparathyroidism
Hyperparathyroidism is another rare condition that can arise from either primary origin, such as, adenomas (and rarely carcinomas), or as a secondary manifestation of end stage kidney disease (ESKD). Presenting complaints typically include general and non-specific symptoms such as weakness, thirst, polyuria, weight loss, and musculoskeletal pain. Only five case reports describing hyperparathyroidism as a cause of LBP were found and included (Table 5). Up to 3% of individuals with hyperparathyroidism will develop brown tumours (osteitis fibrosa cystica), which are neoplastic and can cause LPB and neurological symptoms due to compression if located in the spine. The presence of the mass lesion is typically identified through imaging as a consequence of neurological symptoms [74]. Symptoms suggesting the need to consider hyperparathyroidism in the differential diagnosis include a patient’s previous history of urolithiasis and ESKD in the context of chronic LBP. The diagnosis is likely with elevated serum calcium and alkaline phosphatase, low serum level of phosphate and confirmed by measuring serum hyperparathyroid hormone.
Table 5.
Case reports and reviews of hyperparathyroidism presenting with low back pain
| Author / country / year | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Khalatbari et al., 2014, Iran [74] | CR and | 4 cases with brown tumours (50% females), but only 2 in the lumbar spine | chronic LBP and progressive weakness of the lower extremity, radicular pain | muscular weakness | laboratory work up, surgery |
| review of 15 previously reported CR / not reported | 15 cases of brown tumour, only 6 in the lumbar spine | ||||
| Hoshi et al., 2008, Japan [75] | CR / outpatient clinic | female, 23 y | chronic LBP | h/o urolithiasis | laboratory work up, biopsy |
| Yu et al., 2012, Taiwan [76] | CR / not reported | female, 28 y | chronic LBP | weakness, thirst, constipation, h/o urolithiasis | laboratory work up, surgery |
| Anastasilakis et al., 2011, Greece [77] | CR / outpatient clinic | female, 70 y | chronic LBP, kyphosis | weakness, arthralgias | laboratory work up, surgery |
| Wiederkehr, 2020, USA [78] | CR / not reported | female, 33 y | chronic LBP | end stage kidney disease | laboratory work up, biopsy |
CR case report, h/o history of, LBP low back pain, y years
Vitamin D deficiency / insufficiency
Osteomalacia arises from a deficiency in vitamin D, an essential substance for maintaining bone health, consequently leading to the manifestation of LBP [79]. However, most people with low 25-hydroxyvitamin (25-OH) D3 (calcidiol) level do not develop osteomalacia. A connection between low calcidiol and LBP was initially made by Al Faraj et al. [80], in an observational study, which subsequently resulted in numerous studies (Table 6). The documented deficiency of calcidiol in 83% of individuals experiencing lower back pain (LBP), along with the observed cessation of LBP in all patients with low levels following supplementation within an unspecified time frame, prompted the initiation of cross-sectional and case-control studies into the potential correlation between LBP and vitamin D insufficiency or deficiency. (Table 6). Most studies, except one [81], concluded that vitamin D deficiency was contributing to LBP and even recommended screening patients with chronic LBP. However, no specific symptoms, which could help to identify patients with vitamin D deficiency, were described. Additionally, the definitions of vitamin D deficiency or insufficiency were heterogenous. A Cochrane review on the effectiveness of vitamin D for chronic pain, including LBP, found no consistent evidence for the effectiveness of vitamin D substitution [82].
Table 6.
Studies reporting on vitamin D and low back pain
| Author / country / year | Design / setting | Patients | Inclusion criteria / exclusion criteria | 25-OH Vitamin D3 status | Main finding and conclusion |
|---|---|---|---|---|---|
| Al Faraj et al., 2003, Saudi Arabia [80] |
observational study / specialist clinic |
360 patients (90% female) | LBP, 15-52 years (90 % female), no red flag pathologies, no renal impairment or chronic liver disease | 299 (83%) had low 25-OH-vit D (< 22.5 nmol/L) | substitution of vitamin D: cessation of LBP (95%) (100% with low vitamin D and 69% with normal vitamin D level) |
| Rkain et al., 2013, Morocco [83] |
case control study / specialist clinic |
105 cases / 44 controls (100% female) | postmenopausal women 42-80 years with LBP, no red flag pathologies | 79 % cases vs 61.4 % controls had low vitamin D (<20 ng/ml) | association between vitamin D deficiency and chronic LBP in Moroccan post-menopausal women |
| Johansen et al., 2013, Denmark [81] | cohort study / specialist clinic | 902 patients screened; 152 patients included (48% female) | chronic LBP 19-64 years, no specific LBP, disk herniation or spinal stenosis | 99 (65.1%) had normal vitamin D levels (> 50 nmol/L), 36 (23.7%) had mild vitamin D deficiency (25–50 nmol/L) and 17 (11.2%) patients had moderate/severe deficiency (< 25 nmol/L) | vitamin D deficiency not more common than in the general population, no relation to clinical symptoms |
| Baykara et al., 2014, Turkey [84] |
case control study / specialist clinic |
60 cases (62% female) / 30 controls (63% female) | chronic LBP, 20-50 years, no red flag pathologies | 53 (88%) cases & 11 (37%) controls had low vitamin D (<20 ng/ml) | vitamin D level: significantly lower in the patient group |
| Rehman et al., 2020, Pakistan [85] | cross-sectional observational study / outpatient clinic | 182 patients (36% female) | LBP, no exclusion criteria described | 20 (11%) with vitamin D deficiency (< 50 ng/ml), 132 (74%) with vitamin D insufficiency (< 20 ng/ml) | vitamin D: contributing to LBP; conclusion not justified by study design. |
| Bahinipati et al., 2020, India [86] |
cross-sectional observational study / specialist clinic |
196 patients (61% female) | chronic LBP | 35 (18%) had normal vitamin D levels (> 30 ng/ml), 59 (30%) had insufficiency (21–29 ng/ml) and 52 (52%) had deficiency (< 20 - ng/ml) | pain intensity measured by visual analogue scale score: significantly higher with decrease in vitamin D levels. |
LBP low back pain, 25-OH-vit D 25-hydroxyvitamin D
Ochronosis / Alkaptonuria
Ochronosis, also known as alkaptonuria, is a rare autosomal recessive genetic disorder leading to accumulation of homogentisic acid in the body. Ochronotic arthritis gives rise to chronic back pain typically occurring during the fourth and fifth decade of life, mimicking ankylosing spondylitis including the marked spine stiffness. However, this condition commonly extends its involvement to other joints as well. A total of 12 case reports were found, all focussing on chronic LBP (Table 7). Diagnostic signs, such as pigmentation of the sclera and ear, and darkening of morning urine, were not always present. Intervertebral disc calcification on imaging can be considered pathognomonic and was observed in all case reports. The diagnosis was confirmed with measurement of homogentisic acid in the urine or sometimes from specimens obtained during surgery [87–89]. Only a few patients had been previously diagnosed during childhood, and one patient received a diagnosis during prior surgery.
Table 7.
Case reportsa of ochronosis presenting with chronic low back pain
| Author / country / year | Setting | Patient | Spinal symptoms | Extraspinal symptoms |
|---|---|---|---|---|
| Capkin et al., 2007, Turkey [90] | outpatient clinic | male, 50 y | stiffness, restriction of movements | yellowish green ochronotic pigmentation of cartilage & ears, reduced chest expansion |
| Al-Mahfoud et al., 2008, UK [91] | outpatient clinic | female, 58 y | not reported | darkening of the urine, diagnosis established previously during surgical intervention (hip replacement) |
| Grasko et al., 2009, Australia [92] | outpatient clinic | male, 38 y | progressive severe LBP, decreased lumbar flexion | hip pain, urine discolouration changing to black after prolonged standing, renal colic, passing black calculus, angular pigmentation in sclera and bluish discolouration of auricle |
| Ahmed et al., 2010, Pakistan [93] | not reported | male, 38 y | progressively increasing stiffness, reduced ability to bend forward | degenerative changes of both radiocarpal joints and metatarso-phalangeal joints |
| Effelsberg et al., 2010, Switzerland [94] | outpatient clinic | male, 38 y | limited spine mobility, slight scoliosis | none, diagnosis of ochronosis established in childhood |
| Amiri et al., 2012, Iran [95] | hospital | female, 54 y | severe low back pain and limitation of motion | discolouration of the sclera, knee pain, renal colic and subsequently passing black urine |
| Sebastian et al., 2012, South Africa [28] | rheumatology clinic | male, 46 y | limited extension of the spine | bluish discolouration of the pinnae bilaterally, 2 mm bilateral blue nodules between the joints on the thumbs |
| Seidhamed et al., 2012, Qatar [32] | emergency department | male, 45 y | not reported | generalised joint pain |
| Mirzashahi et al., 2016, Iran [87] | outpatient clinic | male, 51 y | weakness of both lower limbs | none |
| Etzkorn et al., 2014, USA [96] | not reported | female, 55 y | decreased range of motion, morning stiffness, scoliosis | yellowish green ochronotic pigmentation of cartilage and ears, reduced chest expansion |
| Bozkurt et al., 2017, Turkey [89] | outpatient clinic | male, 47 y | tingling, and numbness and weakness in both legs, increased kyphosis | darkening of the urine |
| Alkasem et al., 2019, Irak [88] | not reported | female, 56 y | severe kyphosis, inability to stand straight | hip pain, discoloration of urine that changed to black after urination, three angular pigmentations in sclera and bluish discolouration of auricle |
LBP low back pain, y years
aall reported chronic low back pain
Vascular diseases - arterial
Abdominal aortic aneurysm
An aneurysm is an outward bulging of the vessel wall usually caused by wall weakness. Most aneurysms develop slowly and are initially asymptomatic. Symptoms of abdominal aortic aneurysm (AAA), such as LBP and abdominal pain, can vary depending on the location and type, i.e., acute versus chronic contained ruptured. Several case reports, three cohort studies and nine narrative reviews were identified documenting AAA featuring LBP within the clinical manifestation (Table 8). Mainly middle-aged to older male individuals were affected. Initially 8-18 % of non-inflammatory AAA are symptomatic, while patients with inflammatory AAA exhibit symptoms in 65 to 90% of cases [97]. The majority of symptomatic patients report chronic LBP, occasionally characterised by a progressive exacerbation. Acute or subacute back pain presentations are also possible. The presence of LBP as part of the clinical presentation of an AAA ranged from 32% [98] to 72% [99]. The co-occurrence of LBP and abdominal pain was 29.4% [100]. Furthermore, abdominal pain and a pulsatile abdominal mass in patients with LBP were indicative of the presence of an abdominal aortic aneurysm [97, 99–123]. The presence of the complete triad of LBP or abdominal pain with hypotension and a pulsatile abdominal mass is rather low at 21% [100] and usually observed during rupture [117, 118, 124]. In some cases, history of smoking was reported [97, 102–104, 106, 108, 110, 111, 115, 117, 119, 121–123, 125–132]. Other common risk factors are atherosclerotic disease, hypertension, positive family history for AAA and other aneurysms, collagen vascular disease and Marfan and Ehlers-Danlos-syndromes [106, 117, 119, 121–123, 133].
Table 8.
Abdominal aortic aneurysm presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information |
|---|---|---|---|---|---|---|
| Al-Koteesh et al., 2005, UK [134] | CR / not reported | male, 46 y | acute LBP, numbness (upper left thigh) | - | laparotomy | type: chronic contained rupture of AAA, similar attacks for past years |
| Arici et al., 2012, Italy [135] | CR+NR / not reported | male, 73 y | chronic LBP | uncontrolled hypertension | spinal MRI | type: chronic contained rupture of AAA; review: most prevalent symptom = LBP (78.6%) |
| Aydogan et al., 2008, Turkey [101] | CR / hospital | male, 51 y | chronic LBP | - | MRI | type: chronic contained rupture of AAA; physical examination: pulsatile mass |
| Caynak et al., 2008, Turkey [103] | CR / hospital | male, 75 y | chronic LBP, increased | - | CTA | type: chronic contained rupture of AAA; h/o smoking; physical examination: palpable mass |
| Copetti et al., 2017, Italy [104] | CR / ED | male, 85 y | chronic LBP | - | abdominal CT scan | type: chronic contained rupture of AAA; h/o smoking; physical examination: pulsatile abdominal mass |
| Dobbeleir et al., 2007, Belgium [105] | CR / ambulatory care | male, 79 y | acute LBP | dyspnoea | CT | type: chronic contained rupture of AAA; physical examination: palpable mass |
| Gandini et al., 2007, Italy [136] | CR / hospital | male, 69 y | LBP (no duration reported) | fever |
msC T-guided needle aspiration |
type: chronic contained rupture of AAA |
| Martos et al., 2018, Spain [137] | CR / ambulatory care | male, 73 y | chronic LBP | - | plain radiology | type: AAA without rupture |
| Henderson et al., 2003, USA [125] | CR / ED | male, 67 y | acute LBP | - | CT | type: rupture of AAA; h/o smoking (10 cigarettes per day) |
| Horowitz et al., 2005, Israel [138] | CR / not reported | male, 82 y | LBP (no duration reported) | - | MRI lumbar spine | type: AAA without rupture |
| Jimenez Viseu Pinheiro et al., 2014, Spain [126] | CR / hospital | female, 75 y | chronic LBP, difficulties walking properly | - | CTA | type: chronic contained rupture of AAA; 45-pack-year history |
| Kim et al., 2013, Korea [139] | CR / hospital | male, 73 y | chronic LBP, claudication, left leg paralysis | pitting oedema of both legs | abdominal contrast-enhanced CT | type: AAA without rupture |
| Lai et al., 2008, Taiwan [140] | CR / not reported | male, 67 y | chronic LBP | - | CT and radiography | type: chronic contained rupture of AAA |
| Lucas et al., 2018, Portugal [141] | CR / ED | male, 74 y | acute LBP, radiating | - | abdominal ultrasonography | type: AAA without rupture |
| Mechelli et al., 2008, Italy [127] | CR / ambulatory care | male, 38 y | subacute LBP, unable to run | frequent awakening | CT | type: AAA without rupture; h/o smoking (10 cigarettes per day) |
| Moos et al., 2009, USA [106] | CR / ambulatory care | female, 31 y | chronic LBP | chronic abdominal pain | CT aortogram | type: AAA without rupture; h/o smoking, physical examination: pulsatile abdominal mass |
| Nakano et al., 2013, Japan [142] | CR / not reported | male, 62 y | acute LBP, right leg pain | - | open biopsy | type: chronic contained rupture of AAA |
| Nguyen et al., 2018, Vietnam [107] | CR / not reported | male, 49 y | subacute LBP | - | biopsy, spinal surgery | type: chronic contained rupture of AAA; physical examination: pulsatile abdominal mass |
| Patel et al., 2005, USA [124] | CR / not reported | male, 69 y | acute LBP, radiating, weakness right leg | - | CTA | type: AAA without rupture |
| Seçkin et al., 2006, Turkey [143] | CR / not reported | female, 38 y | acute LBP, difficulty walking, right buttock pain | - | MR | type: AAA without rupture |
| Tan et al., 2019, Singapore [128] | CR / not reported | male, 81 y | acute LBP | hoarseness | CT | type: AAA without rupture; 4-pack / year-history (has quit 35 years ago) |
| Terai et al., 2015, Japan [144] | CR / not reported | male, 89 y | LBP (no duration reported), bilateral weakness in lower extremities | - | CT | type: AAA without rupture |
| Wyngaarden et al., 2014, USA [108] | CR / not reported | male, 58 y | acute LBP | abdominal pain, difficulty falling asleep | CT | type: AAA without rupture; h/o smoking early 1970s, abdominal pulsation |
| Walker et al., 2017, USA [129] | CR / ED | male, 57 y | acute LBP | weight loss | MRI | type: chronic contained rupture of AAA; 20 pack-year history |
| Jukovic et al., 2016, Serbia [130] | CR / hospital | male, 60 y | chronic LBP | - | CT | type: chronic contained rupture of AAA; h/o smoking |
| Alshafei et al., 2015, Bahrain [131] | CR / not reported | male, 63 y | chronic LBP, 1-year history of bilateral intermittent claudication, later: rigors | later: fever, elevated white cell count and a rise of CRP | surgery | type: chronic contained rupture of AAA; h/o smoking |
| Bogie et al., 2008, The Netherlands [109] | CR / ED | male, 55 y | acute LBP, sudden onset, loss of motor function in both legs | nausea, abdominal pain, 3 days previously: abdominal discomfort and nausea | CT | type: AAA without rupture; no h/o smoking; used antihypertensive medication |
| Chieh et al., 2003, USA [145] | CR / ambulatory care | female, 23 y | back pain (no duration or localisation reported) | dyspnoea on exertion | CT | type: multiple isolated aneurysms in Takayasu’s aortitis |
| De Boer et al., 2010, The Netherlands [110] | CR / ambulatory care | male, 74 y | chronic LBP (slowly developing, 5/10 pain scale, radiating to the knee, worsening with walking, standing, stair climbing, diminished when lying down) | later: abdominal pain while lying supine, aggravated by lying – sharp intermittent pain | ultrasound, surgery | type: AAA without rupture; previous treatments without permanent results, heavy prior cigarette use but stopped 15 years ago |
| Defraigne et al., 2001, Belgium [146] | CR / not reported | 5 patients (1 with LBP): male, 73 y | subacute LBP (sciatic pain), crural neuropathy | - | CT, ultrasound | type: chronic contained rupture of AAA; no inflammatory syndrome, no vertebral erosion |
| Dorrucci et al., 2001, Italy [147] | CR / hospital | female, 87 y | subacute back pain (no localisation reported) | mild jaundice, abdominal fullness, dyspepsia | CT, surgery | type: chronic contained rupture of AAA |
| Lombardi et al., 2016, Brazil [113] | CR / ED | male, 66 y | back pain (no duration or localisation reported), exacerbation, unsteady gait | lower left quadrant pain | CT | type: chronic contained rupture of AAA |
| Sakai et al., 2007, Japan [148] | CR / not reported | male, 72 y | chronic LBP | - | CT | type: chronic contained rupture of AAA |
| Whitwell et al., 2002, UK [149] | CR / ED | male, 70 y | acute back pain (no localisation reported), loss of power and sensation in both legs | collapse | laparotomy | type: AAA with rupture |
| Kamano et al., 2005, Japan [150] | CR / hospital | male, 40 y | LBP (no duration reported) (mild), bilateral lower limb paralysis | - | surgery | type: AAA with rupture |
| Hocaoglu et al., 2007, Turkey [115] | CR / not reported | male, 67 y | chronic LBP (radiating to the abdomen and shoulder, pressure sensation towards rectum, increasing at night), difficulty walking | flushing, sweating, abdominal distension associated with pain, weight loss (3 kg in 3 weeks) | ultrasound, CT / MRI | type: AAA without rupture; h/o smoking, LBP resistant to medication and rest |
| Metcalfe et al., 2016, USA [100] | CHS / hospital | 85 (17.6% female), median age: 76 y | LBP (54.1%) (no duration reported) | abdominal pain (61.2%), groin pain (11.8%), atypically distributed pain (8.2%), loin pain (4.7%), palpable AAA (70%), distracting symptoms (38.8%), hypotension (37.6%), LOC (36.5%), tachycardia (18.8%), gastrointestinal symptoms (17.6%) | - | combination of abdominal and LBP (29.4%), complete triad of back or abdominal pain, hypotension, and palpable mass (21%) |
| Takeyachi et al., 2006, Japan [98] | CHS / hospital | 34 (5.9% female), median age: 72 y | LBP (32%) (no duration reported) | not reported | - | - |
| Tsuchie et al., 2013, Japan [151] | CHS / hospital | 23 (34.8% female), mean age: 77 y | LBP (52.2%) (no duration reported) | not reported | - | - |
| Crawford et al., 2003, Australia [116] | NR / - | - | mostly asymptomatic at the beginning; most common symptom: LBP (no duration reported) | fullness or pulsations in the abdomen, abdominal pain | diagnostic methods: physical examination (tender, palpable, pulsatile abdominal mass and bruit) | 91% with symptoms at first presentation |
| Anderson et al., 2001, USA [117] | NR / - | age: 50-60 y, male:female = 5:1 | back pain radiating to abdomen, back pain in case of vertebral body erosion (no duration or localisation reported) | palpation of a pulsatile abdominal mass, vague abdominal pain with radiation to flank, groin; early satiety / nausea / vomiting, gastrointestinal bleeding (in case of fistula), lower extremity ischaemia, venous thrombosis, flank / groin pain | diagnostic methods: US = preferred first method, diagnostic confirmation: CT / CTA or MRI / MRA | risk factors: cigarette use, hypertension, coronary artery disease, COPD, hyperlipidaemia |
| Assar et al., 2009, USA [118] | NR / - | - | severe back pain (no duration or localisation reported), chronic contained rupture: chronic LBP may radiate to the groin, maybe lumbar vertebral erosion, lumbar spondylitis-like symptoms; unusual: transient lower limb paralysis; chronic contained rupture: left lower limb weakness or neuropathy, crural neuropathy | right hypochondrial pain, nephroureterolithiasis, groin pain, testicular pain, testicular ecchymosis (blue scrotum sign of Bryant), iliofemoral venous thrombosis, inguinoscrotal mass mimicking a hernia; chronic contained rupture: left psoas muscle haematoma and obstructive jaundice | diagnostic methods: physical examination (pulsatile epigastric mass); diagnostic confirmation: CT | - |
| Isselbacher et al., 2005, USA [119] | NR / - | - | acute LBP, typically steady and gnawing lasting hours to days | pain (hypogastrium) | diagnostic methods: physical examination (pulsatile abdominal mass), ultrasound, CT / CTA, MRI | risk factors: cigarette use, age, hypertension, hyperlipidaemia, atherosclerosis, positive family history for AAAs, male:female = 10:1, abrupt onset associated with rupture |
| Kumar et al., 2017, USA [120] | NR / - | - | back pain (no duration or localisation reported) | mostly asymptomatic and often incidentally detected; unruptured aneurysms (uncommonly abdominal pain or a pulsatile mass), ruptured aneurysms (severe abdominal pain, hypotension and shock, high mortality: 59-83%) | diagnostic methods: ultrasound, CT / CTA, MRA, DSA | - |
| Metcalfe et al., 2011, UK [133] | NR / - | - | back pain (no duration or localisation reported) | from asymptomatic to showing overt signs of rupture; pain in the abdomen or loin; distal embolisation | diagnostic methods: physical examination (pulsatile mass), ultrasound | risk factors: age, positive family history (not otherwise specified), male sex, cigarette use, hypertension, ethnicity (white > Asian), diabetes) |
| Sakalihasan et al., 2005, Belgium [122] | NR / - | male: 1.3-8.9%, female: 1.0-2.2% | LBP, recent onset, severe (no duration reported) | non-ruptured: generally asymptomatic, essentially diagnosed incidentally; chronic vague abdominal pain, ureterohydronephrosis; ruptured: sudden-onset pain in the mid-abdomen or flank, shock, pulsatile abdominal mass | diagnostic methods: physical examination (pulsatile abdominal mass), CT / CTA, MRI / MRA, aortography | risk factor: cigarette use, familial clustering; causes: trauma, acute infection, chronic infection, inflammatory diseases, connective tissue disorders (Marfan syndrome, Ehlers-Danlos-syndrome) |
| Rogers et al., 2004, USA [123] | NR / - | 4-8% older than 65 y have an AAA | back pain (no duration or localisation reported) | chest pain and neurological abnormalities, chest pain in association with abdominal pain and chest pain that radiates into the back | - | reasons for delayed diagnosis: absence classic triad; risk factors: atherosclerotic diseases, advanced age, cigarette use, male sex, hypertension, strong familial association, patients with connective tissue disorders (Ehlers-Danlos, Marfan), white race; DDx: urological diseases, gastrointestinal bleeding, neuropathy, diverticulitis |
| Winters et al., 2006, USA [9] | NR / - | male:female = 10:1, advanced age | more commonly: back pain, lower-extremity paraesthesia | triad of hypotension, abdominal pain, and a pulsatile abdominal mass (< 50% of patients); flank pain, left lower-quadrant pain, syncope | diagnostic methods: physical examination (palpable pulsatile mass, abdominal bruit, diminished lower-extremity pulses, tender left lower-quadrant mass) | risk factors: hypertension, h/o tobacco use, hyperlipidaemia, atherosclerotic vascular disease, diabetes, connective tissue disorders, positive family history for AAA |
AAA abdominal aortic aneurysm, CHS cohort study, COPD chronic obstructive pulmonary disease, CR case report, CRP c-reactive protein CT computed tomography, CTA computed tomography angiography, DSA digital subtraction angiography ED emergency department, h/o history of, LBP low back pain, LOC loss of consciousness, MR magnetic resonance, MRA magnetic resonance angiography, MRI magnetic resonance imaging, msCT multi-slice computed tomography, NR narrative review, US ultrasound, y years
In summary, in middle-aged and elderly males with chronic back pain and a pulsatile mass, abdominal pain, or other present risk factors, an AAA should be considered. The median time to diagnosis of an AAA is 7.3 years [99], with imaging studies (CT, MRI) typically used to confirm the diagnosis. In patients presenting with LBP as chief complaint and without other accompanying symptoms, an AAA is usually an incidental finding in lumbar radiographs [124]. Subsequent differential diagnoses include spinal tumours, metastasis, retroperitoneal tumours, iliopsoas muscle abscess, rheumatoid arthritis, osteoporosis and osteomalacia [103], especially when AAA leads to vertebral erosion.
Other aneurysms
A total of twelve case reports and a cohort study revealed instances of patients with aneurysms in locations other than the abdominal artery experiencing LBP as part of their clinical presentation (Table 9). Visceral artery aneurysms, for example, account for 1-2 % of non-aortic aneurysms. Of these, 60% affect the splenic artery [152]. Here, LBP was mostly described as acute pain [25, 153–159]. Extraspinal symptoms varied depending on the location of the aneurysm. For example, a splenic artery aneurysm showed gastrointestinal symptoms [152], while an aneurysm of the artery of Adamkiewicz showed neurological/vegetative symptoms [153]. The aetiology of non-aortic aneurysms is diverse and also includes infections, such as Takayasu arteritis, albeit rarely [154]. Moreover, additional underlying diseases can further contribute. For example, the majority of intercostal artery aneurysms arise in association with neurofibromatosis.
Table 9.
Other aneurysms presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Affected artery | Spinal symptoms | Extravertebral symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|---|
| Hanschke et al., 2002, Germany [152] | CR / hospital | male, 46 y | splenic artery | chronic LBP, left sided, analgesic resistant | feeling full, stool irregularities | CT |
| Iihoshi et al., 2011, Japan [153] | CR / hospital | female, 60 y | artery of Adamkiewicz | acute LBP, severe, left lower limb pain | headache, nausea | CT, DSA |
| Kellner et al., 2019, Germany [25] | CR / hospital | male, 38 y | 7. and 8. intercostal artery | acute LBP, immobilisation, increase in vigilance | vomiting, general malaise | CT |
| Matsumoto et al., 2015, Japan [154] | CR / hospital | female, 51 y | superior mesenteric artery | acute LBP | sustained fever | angiography |
| Nakamura et al., 2020, Japan [155] | CR / not reported | male, 66 y | artery of Adamkiewicz | acute LBP, progressive posterior cervical pain | fever | DSA |
| Nogueira et al., 2010, USA [156] | CR / not reported | male, age not reported | lateral sacral artery | acute LBP, lower extremity paraesthesia, weakness, numbness of the genitalia | urinary hesitance | angiography |
| Ferrero et al., 2001, Italy [160] | CR / hospital | male, 43 y | splenic artery | back pain (no duration or localisation reported) | severe state of shock, abdominal pain | CT |
| Takebayashi et al., 2020, Japan [157] | CR / hospital | female, 67 y | ruptured posterior spinal artery aneurysm | acute LBP, worsened with movement, right thigh pain | sudden nausea | MRI + CE-CT |
| Bushby et al., 2010, Australia [159] | CR / ED | male, 67 y | iliac artery aneurysm | acute LBP, left sided, radiation into the left leg, numbness, weakness | weight loss (10 kg in 3 months), no bowel movements since symptom onset | CT |
| Bell et al., 2014, USA [158] | CR / ED | female, 68 y | ruptured posterior spinal artery pseudoaneurysm | acute LBP (severe, sharp, developed suddenly during physical exertion) | - | MRI/MRA |
| Caglar et al., 2005, Turkey [161] | CR / ED | female, 74 y | ruptured posterior spinal artery of the conus medullaris | LBP (severe, no duration reported), radiation into the right leg | - | DSA, histopathological examination |
| Gonzalez et al., 2005, USA [162] | CR / hospital | 4 (all male), mean age: 56 y | spinal aneurysms | acute onset of back pain (no localisation reported), paraesthesia of lower extremities (sometimes bilateral), radicular pain | presented with ruptured aneurysms, spinal SAH concomitant headache (bilateral) | - |
| Knol et al., 2002, Belgium [163] | CHS / hospital | 51 (2% female), mean age: 69.1 y | aortic or iliac | 39% acute LBP (or abdominal pain) | 12% haemorrhagic shock, 49% haemorrhagic shock and abdominal pain or LBP | - |
CE-CT contrast enhanced computed tomography, CHS cohort study, CR case report, CT computed tomography, DSA digital subtraction angiography, ED emergency department, LBP low back pain, MRA magnetic resonance angiography, MRI magnetic resonance imaging, SAH subarachnoid haemorrhage, y years
Acute aortic syndrome
The acute aortic syndrome includes pathologies, such as aortic dissection (AD), intramural haematoma (IMH), and penetrating aortic ulcer (PAU). An aortic dissection is a tear in the inner wall of the aorta, which is potentially life-threatening and often occurs in patients with underlying diseases that weaken the aortic wall, e.g., hypertension, atherosclerosis, and AAA. A distinction is made between type A und type B dissection. Type A is a proximal aortic dissection involving the ascending aorta, while type B affects the descending aorta. The IMH is an atypical aortic dissection and characterised by bleeding into the aortic wall without an intimal tear. A PAU is an ulcerative defect of the intima of the aorta, which breaks through the internal membrane into the tunica media. Acute aortic syndromes usually present with sudden onset of symptoms, like devastating chest pain, which can radiate into the back, including the lower back, and mainly affect middle-aged men. Eleven case reports, two case series, ten cohort studies, nine register studies, a chart review study, an interventional study, thirteen narrative and one systematic review have documented acute aortic syndrome concomitant with LBP (Table 10). Wu et al. published a systematic review and meta-analysis which examines various studies on acute aortic syndrome (partly included in Table 10), where the incidence of back pain varies greatly between 10 % and 75% [164]. Most patients present with a sudden onset of acute severe LBP (pain scale: 7/10, [165]). Possible accompanying symptoms are chest discomfort, abdominal pain, nausea/vomiting, and dyspnoea [9, 166–204]. To confirm the diagnosis, imaging studies, such as computed tomography angiography (CTA), are used [167, 170, 189, 194, 205–208].
Table 10.
Acute aortic syndrome presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Extravertebral symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|
| Fojtik et al., 2003, USA [166] | CR / ED | male, 48 y | acute LBP (sharp, sudden onset, radiating into the groin) | diaphoresis, dyspnoea, nausea | CT | h/o smoking cigarettes |
| Hughes et al., 2016, USA [205] | CR / ED | female, 56 y | acute LBP, bilateral lower extremity paraesthesia and paralysis | - | CTA | h/o smoking cigarettes |
| Itoga et al., 2017, USA [167] | CR / not reported | male, 59 y | acute LBP, left sided pain and paraesthesia, bilateral buttock stabbing discomfort, resting pain in thigh and calf | abdominal discomfort (radiating to the back) | CTA | - |
| Johnson et al., 2008, USA [165] | CR / ED | male, 49 y | acute LBP (7/10 NRS, intensifying with prolonged periods of sitting still, moderate breathing difficulties) | - | CT | hypertension |
| Hsu et al., 2008, China [209] | CR / ED | male, 32 y | acute LBP (acute onset developed during the night) | - | CT | - |
| Sixsmith et al., 2005, USA [169] | CR / ED | male, 27 y | acute LBP (radiating from upper abdominal area) | diffuse abdominal cramping, bloody diarrhoea, frank blood per rectum | autopsy | - |
| male, 60 y | acute LBP, severe left sided flank pain | - | autopsy | hypertension | ||
| Stäubli et al., 2004, Switzerland [210] | CR / ED | male, 49 y | acute LBP (sudden, severe, later radiating to the abdomen) | pale, sweating | spiral CT, MRI | h/o smoking cigarettes |
| Takahashi et al., 2017, Japan [170] | CR / hospital | male, 73 y | acute LBP (severe) | chest pain | CTA | hypertension |
| Morris-Stiff et al., 2008, UK [211] | CR / ambulatory care | male, 47 y | acute LBP (acute onset, commenced during coitus, radiating down the right leg, increased, claudication-type pain) | - | CT, TTE | hypertension and hyperlipidaemia |
| Furui et al., 2012, Japan [199] | CR / hospital | male, 83 y | acute back pain (no localisation reported) | chest pain | CT | - |
| Ahmed et al., 2012, UK [206] | CR / ED | male, 45 y | acute LBP, weakness in lower limbs | collapse | CTA | h/o smoking cigarettes, family history of ischaemic heart disease |
| Asouhidou et al., 2009, Greece [175] | CS / ED | 49 (16.3% female), mean age: 54.8 y | back pain (no duration or localisation reported) with chest pain (8.1%), paralysis of lower extremities (4.1%), hemiparesis (2%) | only chest pain (36.7%), chest pain with neurological deficit (12.2%), chest pain with syncope (8.1%), chest pain with CHF (6.1%), CHF (10.2%), syncope (6.1%), intubated (4.1%), syncope with pulselessness of the lower extremities (2%) | - | - |
| Nathan et al., 2012, USA [202] | CS / hospital | 315 (39.8% female), mean age: 73.2 y | back pain (or chest pain) (11%,) (no duration or localisation reported) | chest pain | - | - |
| Kalko et al., 2008, Turkey [171] | CHS / hospital | 8 (12.5% female), mean age: 62.5 y | severe back pain (and abdominal pain) (57%) (no duration or localisation reported), acute ischaemia with paraplegia of the lower limb (14.3%) | abdominal pain, haemodynamic collapse, and shock (28.6%) | - | - |
| Hsu et al., 2005, Taiwan [172] | CHS / hospital | 107 (23.4% female), mean age: 58.4 y | back pain (or chest pain) (91.6%) (no duration or localisation reported) | intramural haematoma (12.1%), leg ischaemia (8.4%), hypotension / shock (0.9%) | - | - |
| Li et al., 2012, China [173] | CHS / hospital | 1812 (total: 22.5% female), mean age: 51.1 y | acute back pain (69.1%) (no localisation reported), other neurological deficits (1.5%) | pain (88.1%), abrupt onset (70.3%), anterior chest pain (69.4%), abdominal pain (12.3%), migrating pain (8.7%), leg pain (1.7%), pulse deficit (14.1%), aortic regurgitation murmur (9.2%), syncope (5.7%), shock (5.3%), stroke (5.0%), heart failure (4.1%), systolic blood pressure (143.2 +/- 24.4), diastolic blood pressure (81.8 +/- 13.8) | - | - |
| Xu et al., 2006, China [212] | CHS / hospital | 63 (6.3% female), mean age: 50.4 y | back pain (100%) (no duration or localisation reported) | shock (3.2%), hoarseness (1.6%) | - | - |
| Falconi et al., 2005, Argentina [174] | CHS / hospital | AD: 76 (34% female), mean age: 69 y, IMH: 27 (30% female), mean age: 71 y | acute back pain (72%) (no localisation reported) | abrupt onset of pain (85%), chest or back pain (25%) | - | - |
| Sen et al., 2020, USA, Switzerland [200] | CHS / hospital | 14 (29% female), median age: 73 y (range: 44-90 y) | acute back pain (7%) (no localisation reported) | abdominal pain (14%), hypotension (7%) | - | - |
| Ho et al., 2011, China (Classic AD) [201] | CHS / hospital | classic AD: 56 (32.1% female), mean age: 60.5 y | back pain (64.3%) (no duration or localisation reported) | chest pain (75%), abdominal pain (23.2%), stroke (21.4%) | - | - |
| IMH: 34 (47.1% female), mean age: 69.7 y | back pain (64.7%) (no duration or localisation reported) | chest pain (70.6%), abdominal pain (26.5%), stroke (0%) | - | - | ||
| Jansen Klomp et al., 2016, Netherlands [184] | CHS / hospital | 200 (39% female), mean age: 64 y |
comparison between part of first DDx (fDDx) and not part of first DDx (nfDDx): back pain: (fDDx: 56.1%, nfDDx: 31%) (no duration or localisation reported), focal neurological deficit (fDDx: 15.8%, nfDDx: 9.9%) |
chest pain (64.7% vs 61.3%), abdominal pain (23.7% vs 24.0%), TLOC (19.5% vs 10.7%), coma (12.1% vs 10.7%); signs: any pulse deficit (27.7% vs 9.9%), median heart rate (73 vs 78), median blood pressure (120/104 vs 120/102), median haemoglobin (7.8 vs 8.0), median creatinine (104 vs 102) | - | - |
| Collins et al., 2004, USA [185] | CHS / hospital | 617 (100 with previous surgery (w), 517 without previous surgery (wo)), female (overall: 33.1%), mean age overall: 61.1 y | back (or chest pain) (overall: 84.6%, w: 67.4%, wo: 87.9%) (no duration or localisation reported), abrupt onset of back or chest pain (overall: 91%, w: 83.9%, wo: 92%) | migrating pain (overall: 14.8%, w: 8.8%, wo: 15.9%) | - | - |
| Imamura et al., 2011, Japan [186] | CHS / hospital |
98, painless (pl): 16 (44% female, mean age: 71 y), painful (pf): 82 (46% female, mean age: 65 y) |
pf: back pain (60%) (no duration or localisation reported), focal neurological deficit (pl: 19%, pf: 2%), weakness of lower extremities (pl: 6%, pf: 12%) | chest pain (71%), abdominal pain (27%), disturbance of consciousness (transient: pl: 25%, pf: 1%; persistent: pl: 44%, pf: 6%), dyspnoea (pl: 6%, pf: 2%), nausea and vomiting (pl: 6%, pf: 7%), abdominal fullness (pl: 6%, pf: 0%), bleeding tendency (pl: 6%, pf: 0%), pyrexia (pl: 0%, pf: 2%), haematemesis (pl: 0%, pf: 1%) | - | - |
| Januzzi et al., 2004, USA [176] | register study / hospital |
1049 (Marfan (marf): 5.1%, non-Marfan (nmarf): 94.9%); marf: 26% female, mean age: 35 y; nmarf: 32% female, mean age: 64 y |
LBP (marf: 60%; nmarf: 55%) (no duration reported) | chest pain (marf: 75%, nmarf: 76%), migrating pain (marf: 16%, nmarf: 18%), syncope (marf: 10%, nmarf: 13%), congestive heart failure (marf: 10%, nmarf: 5%), coma / altered consciousness (marf: 6%, nmarf: 10%), systolic blood pressure > 140 mmHg (marf: 27%, nmarf: 44%), diastolic blood pressure > 90 mmHg (marf: 19%, nmarf: 23%), murmur of aortic regurgitation (marf: 46%, nmarf: 32%) | - | - |
| Nallamothu et al., 2002, USA [177] | register study / hospital | 728 (31% female), mean age: 63 y | back pain (or chest pain) (83%) (no duration or localisation reported) | syncope (13%) | - | - |
| Nienaber et al., 2004, Germany [178] | register study / hospital | 1078 (32.1% female), mean age: 62.4 y) | back pain (54.4 %) (no duration or localisation reported), any focal neurological deficits (14.3%), ischaemic peripheral neuropathy (2%) | abrupt onset of pain (87.1%), chest pain (75.5%), migrating pain (17.9%), hypotension / shock / tamponade (19.2%), syncope (13.1%), shock / tamponade (11.5%), coma / altered consciousness (10.3%), shock (10.0%), congestive heart failure (6.6%), cardiovascular accident (6.1%), tamponade (3.6%), mean systolic BP (142 +/- 43), mean diastolic BP (82 +/- 23), any pulse deficit (27.7%) | - | - |
| Suzuki et al., 2003, Germany [179] | register study / hospital | 384 (28.6% female), mean age: 64.4 y | back pain (and / or chest pain) (86%) of abrupt nature (89%) (no duration or localisation reported) | migrating pain (uncommon, 25%), hypertension (69%), pulse deficits (21%), spinal cord ischaemia (3%) hypotension / shock (3%) | - | - |
| Bossone et al., 2013, Italy [180] | register study / hospital | 1354 (36.4% female), mean age: 62.8 y | back pain (59.6%) (no duration or localisation reported) | any pain reported (93.8%), abrupt onset (83%), chest pain (73%), radiating pain (44.1%), abdominal pain (32.6%), migrating pain (7%), syncope (10.3%) | - | - |
| Evangelista et al., 2005, USA [181] | register study / hospital | classic AD: 952 (30.9% female, mean age: 61.7 y); IMH: 58 (39.7% female, mean age: 68.7 y) | acute back pain (no localisation reported): AD: 54.2%; IMH: 63.8%; abrupt onset described | abrupt onset (AD: 87.3% / IMH: 87.5%), chest pain (AD: 75.6% / IMH: 75.9%), pain rated as worst ever (AD: 20.0% / IMH: 39.6%), abdominal pain (AD: 28.2% / IMH: 31.0%), pain migration (AD: 18.2% / IMH: 15.5%), leg pain (AD: 10.8% / IMH: 1.7%) | - | - |
| Harris et al., 2012, USA [182] | register study / hospital | type A: AD: 1744 (32.3% female, mean age: 61.4 y), IMH: 64 (42.2% female, mean age: 69.6 y); type B: AD: 651 (33.5% female, mean age: 62.9 y), IMH (34.4% female, mean age 68.6 y) | back pain (no duration or localisation reported) (type A: AD (42.8%), IMH (41.0%); type B: AD (70.3%), IMH (78.7%)) | pain severity - severe or worst ever pain (type A: AD (91.6%), IMH (98.1%); type B: AD (93.6%), IMH (94.7%)), abrupt onset of pain (type A: AD (82.6%), IMH (86.7%); type B: AD (87.4%), IMH (82.6%)), chest pain (type A: AD (81.5%), IMH (82.5%); type B: AD (67.4%), IMH (77.3%)), radiating pain (type A: AD (36.3%), IMH (45.9%); type B: AD (44.7%), IMH (35.3%)), abdominal pain (type A: AD (26.0%), IMH (13.1%); type B: AD (43.9%), IMH (36.8%)) | - | - |
| Vagnarelli et al., 2015, Italy [183] | register study / hospital | 398 (33.2% female), mean age: 66.7 y | back pain (48.7%) (no duration or localisation reported) | chest pain (65.6%), abdominal pain (27.6%), migratory pain (12.8%), autonomic symptoms (38.9%), dyspnoea (14.6%), shock within 12 hours of admission (14.3%), pain + shock (11.1%), pain + syncope (8.5%), pain + cerebrovascular accident (3.0%), pain + paraplegia (2.5%) | - | - |
| Wang et al., 2014, China [204] | register study / hospital | Sino-RAD: 1003 (22.2% female), mean age: 51.8 y; IRAD: 464 (34.7% female), mean age: 63.1 y | back pain (no duration or localisation reported) (Sino-RAD: 77%, IRAD: 53.2%) | any pain reported (Sino-RAD: 89.6%, IRAD: 95.5%), abrupt onset (Sino-RAD: 68.5%, IRAD: 84.8%), chest pain (Sino-RAD: 17.3%, IRAD: 72.7%), abdominal pain (Sino-RAD: 12%, IRAD: 29.6%), syncope (Sino-RAD: 2.1%, IRAD: 9.4%), heart failure (Sino-RAD: 0.2%, IRAD: 6.6%) | - | - |
| Tsai et al., 2009, Taiwan [203] | chart-review study / hospital | 18 (16.7% female), mean age: 32 y | LBP (11.1%) (no duration reported) | chest pain and / or tightness (38.9%), chest-back pain (27.8%), epigastric pain (11.1%), abdominal pain (5.6%), lower limb weakness or numbness (11.1%), sudden cardiac arrest (5.6%) | - | - |
| Li et al., 2010, China [187] | interventional study / hospital | stent graft: 33 (27% female, mean age: 60 y); treated medically: 23 (35% female, mean age: 56 y) | back pain (or chest pain) (100% of all patients) (no localisation or duration reported) | - | - | - |
| Léon Ayala et al., 2011, Taiwan [188] | NR / - | male (68%) > female (32%) | acute back pain (no localisation reported), sudden onset, neurological deficits: paraplegia | most common symptom: sudden onset of severe chest pain, radiating to neck or shoulders; hypotension and / or shock (type A), hypertension (type B); other findings: fever, diaphoresis, absence of pulse, cerebrovascular manifestations, acute abdominal pain, aortic regurgitation related to cardiac failure, cardiac tamponade, syncope | diagnostic methods: laboratory testing, ECG, TEE / TTE, CT, MRI, aortography, chest x-ray | diagnosis was missed up in 38%; signs: pulse deficit (< 20%), diastolic murmur, jugular venous distention, distant heart sounds, pulsus paradoxus, ostium or coronary artery involved (7%); risk factors: cocaine, pregnancy, iatrogenic trauma |
| Golledge et al., 2008, USA [189] | NR / - | incidence: 3 / 100000 people per year, age: 61-65 y, male > female | back pain (or chest pain) (85%) (no duration or localisation reported), abrupt onset; any focal neurological deficit (12%) | severe or worst-ever pain (90%), abrupt onset of pain (90%), pain presenting within 6 hrs. of symptom onset (79%), abdominal pain (30%), migrating pain (19%), hypertension at presentation (49%), aortic regurgitation (32%), any pulse deficit (27%), hypotension / shock / tamponade (18%) | diagnostic methods: laboratory testing, ECG, chest radiograph, CT, echocardiography, CTA, MRI | risk factor: hypertension |
| Khan et al., 2002, USA [190] | NR / - | male > female, mean age: 50-55 y proximal and 60-70 y distal; patients with Marfan syndrome tend to be younger | back pain (53%) (no duration or localisation reported, depending on location of dissection); cerebral ischaemia / stroke (5-10%), spinal cord ischaemia / ischaemic peripheral neuropathies (up to 10%), various spinal cord syndromes | pain (95%) (typically catastrophic / abrupt onset (85%), sharp (64%), ripping or tearing (51%) or knife-like in nature (no percentage reported)), chest pain (73%), abdominal pain (30%), acute cardiac decompensation and shock, hypotension and shock, pericardial effusion, obstruction of the superior vena cava or from cardiac tamponade, neurological deficits (18-30%), syncope (12%), acute GI haemorrhage / acute abdomen / dysphagia; signs: aortic regurgitation (18-50%), diastolic murmur (25%), systolic BP < 100 mmHg (25%), left ventricular regional wall motion abnormalities (10-15%), low coronary perfusion, pulse differential (38%), bruits | diagnostic methods: TEE, CT, MRI, chest radiograph, TTE, aortography, serum smooth muscle myosin heavy chain | predisposing factors: hypertension, aortic disease, direct iatrogenic trauma, cocaine, pregnancy |
| Mussa et al., 2016, USA [191] | SR / - | patients with AAD: median age: 61 y, 19-50% female; incidence 15/100000 patient-years | back pain (or chest pain) (84.8%) (no duration or localisation reported); stroke (11.3%) | sharp pain (64.4%); weak carotid, brachial or femoral pulse (pulse deficit) (30%), hypotension (> 25%), syncope (13%), regurgitation murmur (31.6%), abdominal pain (type B: 42.7%, type A: 21.6%), IRAD (syncope (33.9%), congestive heart failure (19.7%), painless aortic dissection (6.4%)) | diagnostic methods: ECG, chest x-ray, CT / MRI, TEE, serologic biomarkers (e.g., d-dimer) | most common comorbidity: hypertension (45-100%), h/o smoking (20-85%), chronic renal insufficiency (3-79%), COPD (5-36%), stroke / transient ischaemic attack (0-20%); |
| patients with IMH | back pain (61.6%) (no duration or localisation reported) | abrupt chest pain (77.9%), hypertension (68-96%) | diagnostic methods: CT / MRI = gold standards | smokers: 18-67% | ||
| Nienaber et al., 2002, Germany [192] | NR / - | - | back pain (no duration or localisation reported) | chest pain | - | risk factors: hypertension (85%), coronary artery disease (61%), abdominal or thoracic aneurysm (53%, chronic renal insufficiency (31%), peripheral artery disease (17%), cerebrovascular accidents (12%) |
| Nienaber et al., 2003, Germany [193] | NR / - | IMH prevalence: 10 – 30 % | instantaneous onset of severe back pain (64%) (no localisation reported); stroke (21%), peripheral ischaemic neuropathy (encountered on occasion) | chest pain (63%), sudden abdominal pain (43%), ischaemic leg (encountered on occasion) | diagnostic methods: physical examination (pulse deficits: <20%, diastolic murmur: 40-50%), chest x-ray, ECG, TTE, TEE, CT, MRI, angiography | risk factors: smoking, dyslipidaemia, cocaine / crack, Marfan syndrome, Ehlers-Danlos-syndrome, bicuspid aortic valve, coarctation, giant cell arteritis, Takayasu arteritis, Behcet’s disease, syphilis, Ormond’s disease, trauma, iatrogenic factors, pregnancy related |
| Thrumurthy et al., 2011, UK [194] | NR / - | female: 32% | back pain (no localisation or duration reported); neurological deficit | sharp, tearing, stabbing chest pain radiating to neck (type A) or interscapular region (type B), abrupt onset of ripping, tearing, stabbing pain in chest or abdomen, shock | diagnostic methods: CTA, echocardiography, MRA | risk factors: hypertension (40-75%), race (79% white), connective tissue diseases (Marfan syndrome: 15-50% in patients under 40 years), congenital cardiovascular abnormalities, aortic vasculitis disease, cocaine misuse, pregnancy, iatrogenic (5%) |
| Tsai et al., 2005, USA [195] | NR / - | incidence: 2.6-3.5 cases per 100.000 person-years | back pain (64% type B vs. 47% type A) (no duration or localisation reported) | cataclysmic onset, chest pain (blunt, severe, sometimes radiating); chest pain (79% type A vs. 63% type B), abdominal pain (43% type B vs. 22% type A) | diagnostic methods: ECG, chest x-ray, TTE, TEE, CT, MRI, aortography, coronary angiography | risk factors: smoking, dyslipidaemia, cocaine / crack, Marfan syndrome, Ehlers-Danlos-syndrome, bicuspid aortic valve, coarctation, giant cell arteritis, Takayasu arteritis, Behcet’s disease, syphilis, Ormond’s disease, trauma, iatrogenic factors |
| Vilacosta et al., 2001, Spain [196] | NR / - | - | acute back pain (no localisation reported) | severely intense, acute, tearing or tearing, throbbing and migratory chest pain = AAS, anterior chest, neck, throat and even jaw pain = ascending aorta; back and abdominal pain = descending aorta | diagnostic methods: laboratory tests (CK, troponin), ECG, chest x-ray, CT, MRI, TEE | risk factors: severe hypertension, disorders of elastic tissue |
| Vilacosta et al., 2009, Spain [197] | NR / - | - | acute back pain (no localisation reported) | severely intense, acute, tearing or ripping, pulsating and migratory chest pain = AAS; chest pain irradiating to the neck, throat or jaw indicates = ascending aorta; back or abdominal pain: descending aorta | diagnostic methods: laboratory tests, electrocardiography, chest x-ray, CT, MRI, TEE | risk factors: moderate to severe hypertension, disorders of elastic tissue; physical examination: murmur of aortic regurgitation, pulse differentials |
| Siegal et al., 2006, USA [198] | NR / - | incidence: 5-30 cases per 1 million people per year, 34% female, age: 65 y | back pain (or abdominal or chest pain) (95%) (no localisation or duration reported), severe or worst ever, sharp (64%), tearing, ripping; neurologic deficits | pulse deficits, hypotension, hypertension, end-organ ischaemia; other clinical findings: acute myocardial ischaemia / infarction, pericardial friction rub, syncope, pleural effusion or frank haemothorax, acute renal failure, mesenteric ischaemia | - | patients history: systemic hypertension (72%), atherosclerosis, h/o prior cardiac surgery, aortic aneurysm, collagen diseases, bicuspid aortic valve, aortic coarctation, Turner syndrome, strenuous exercise, large vessel arteritis (giant cell, Takayasu's, syphilis), cocaine and methamphetamine ingestion, third-trimester pregnancy, blunt chest trauma or high-speed deceleration injury, iatrogenic injury; DDx: acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, oesophageal spasm or rupture, acute pancreatitis and acute pericarditis |
| Lech et al., 2017, USA [207] | NR / - | patients with AD: male predominance (40% female); patients with IMH typically older and more frequently in Asia than in America or Europe; patients with PAU: age >70 y | acute back pain (no localisation reported) | can be missed up to 40%; most common: sudden onset and severe pain located in the chest, abdomen, flank; painless, pain: tearing and ripping, radiating to the back; other specific symptoms: paresis, paraplegia, syncope; other signs: myocardial ischaemia, cardiac tamponade, cardiogenic shock, acute aortic regurgitation, mesenteric ischaemia; hypertension | diagnostic methods: physical examination (pulse deficit, aortic murmur; gastrointestinal bleeding, haematuria, anuria), CT / CTA, ultrasonography, MRI / MRA | causes / risk factors: hypertension (70%), h/o connective tissue disease, h/o atherosclerosis, cigarette use, illicit drug use, dyslipidaemia, h/o blunt trauma, recent aortic manipulation, family h/o aortic disease, inflammatory disorders (autoimmune and infectious), pregnancy |
| Corvera et al., 2016, USA [208] | NR / - | 1/3 female, average age of male: 63 y and female: 67 y | acute back pain (no localisation reported), tearing, ripping severe | acute onset of severe chest pain, tearing or ripping; other symptoms: syncope, neurological deficit including stroke and paraplegia, acute congestive heart failure, myocardial ischaemia, lower extremity ischaemia, abdominal pain and shock | diagnostic methods: physical examination (aortic murmur (44%), pulse deficit (20-30% in type A), hypertension (1/3 in type A); diagnostic: CTA, TTE, TEE, MRI, catheter angiography, no biomarkers | risk factors: hypertension, atherosclerosis, prior cardiac surgery, known aneurysm and Marfan syndrome |
| Winters et al., 2006, USA [9] | NR / - | male:female = 5:1, advanced age (50-70 years) | descending aorta: more commonly back pain (no duration or localisation reported) with radiation to hip and legs, neurological symptoms (quadriplegia, paraplegia, unilateral paraesthesia) | instantaneous onset of chest pain (maximal at onset, knifelike, ripping or tearing), syncope, abdominal pain, gastrointestinal bleeding, dysphagia, and hoarseness | diagnostic methods: physical examination: hypertension / hypotension, bilateral blood pressure difference; imaging: chest radiography, CT, MRI, TEE | risk factors: chronic hypertension, smoking, hyperlipidaemia, connective tissue syndromes (Marfan syndrome, Ehlers Danlos syndrome), chromosomal disorders (Turner’s syndrome, Noonan’s syndrome), bicuspid aortic valve, coarctation of the aorta, decelerating trauma, inflammatory conditions of the aorta, aortic instrumentation, pregnancy, cocaine use |
AAD acute aortic dissection, AAS acute aortic syndrome, AD aortic dissection, BP blood pressure, CHF congestive heart failure, CHS cohort study, CK creatine kinase, CR case report, CS case series, CT computed tomography, CTA computed tomography angiography, COPD chronic obstructive pulmonary disease, ECG electrocardiogram, ED emergency department, GI gastrointestinal, h/o history of, IMH intramural haematoma, IRAD International Registry of Aortic Dissection, LBP low back pain, marf marfan-group, MRA magnetic resonance angiography, MRI magnetic resonance imaging, nmarf non-marfan group, NR narrative review, NRS numeric rating scale, PAU penetrating aortic ulcer, pf painful, pl painless, Sino-RAD Registry of Aortic Dissection in China, SR systematic review, TLOC transient loss of consciousness, TEE transoesophageal echocardiogram, TTE transthoracic echocardiogram, w with previous surgery, wo without previous surgery, y years
Fistula
A fistula is an uncommon connection between two structures, such as organs or vessels. A total of twelve case reports, five case series, seven cohort studies, a chart review, and nine narrative reviews describing different fistulas (e.g., aorto-enteric, aorto-caval, aorto-venous) presenting with LBP (Table 11) were found. Middle-aged and elderly men were most commonly affected. The aetiology varies greatly depending on the localisation, for example, aorto-enteric fistulas are often (up to 80% [213]) caused by AAAs. LBP is described as a frequently accompanying symptom of fistulas, ranging from 1.7% [214] to 93% [215] of affected patients. Accompanying symptoms depend on the structures affected and can include abdominal pain or vomiting [118, 120, 213, 216–219]. Neurological symptoms like paraplegia and sensory disorders can also occur, especially when it is an aorto-venous fistula affecting the spine. The diagnosis is made incidentally during imaging studies especially when patients present with marked symptoms. CT is often used initially [118, 120, 216, 217, 219–228], followed by other possible imaging methods such as duplex sonography, MRI, and digital subtraction angiography (DSA). In some cases, surgery was necessary for diagnosis [215, 221, 229].
Table 11.
Vascular fistulas presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Diagnosis | Spinal symptoms | Extravertebral symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|---|
| Mehmood et al., 2007, Ireland [216] | CR / ED | male, 59 y | aorto-enteric fistula | acute LBP | vomiting, central abdominal pain, chronic fatigue | CT | - |
| Ogura et al., 2020, Japan [230] | CR / hospital | male, 73 y | aorto-venous fistula | chronic LBP, numbness in lower extremities, gait disturbance | - | angiography | - |
| Ozcakir et al., 2008, USA [213] | CR / ED | male, 60 y | aorto-enteric fistula | acute LBP, severe | abdominal pain, vomiting of blood | laparotomy | - |
| Patelis et al., 2018, Greece [217] | CR / ED | male, 60 y | aorto-caval fistula | acute LBP, sudden onset | abdominal pain | CTA | - |
| Verma et al., 2015, USA [231] | CR / ED | male, 54 y | aorto-venous fistula | chronic LBP motion dependent, left lower extremity radiculopathy | - | spinal angiogram | - |
| Kopp et al., 2006, Germany [220] | CR / hospital | male, 68 y | aorto-caval fistula | acute right sided back pain (no localisation reported), acutely developed, persistent | - | CT | - |
| Nakazawa et al., 2014, Japan [229] | CR / ambulatory care | male, 82 y | aorto-caval fistula | back pain (no duration or localisation reported), worsening | - | surgery | - |
| Takazawa et al., 2001, Japan [221] | CR / hospital | male, 65 y | aorto-caval fistula | acute LBP | nausea, haematuria, oedema, cyanosis, coldness in both lower extremities | CT, surgery | - |
| Koch et al., 2004, Germany [232] | CR / not reported | female, 46 y | dural arterio-venous fistula of the lumbar spine | acute LBP, radiating to both legs, neck stiffness | frontal and occipital headache, nausea | angiography | - |
| Oldfield et al., 2002, USA [233] | CR / not reported | male, 64 y | spinal dural arterio-venous fistula | LBP (no duration reported), intermittent, gait disturbance (progressively worsening) | urinary hesitance and constipation | spinal arteriogram | - |
| Pevec et al., 2010, USA [222] | CR / hospital | male, 84 y | aorto-caval fistula | acute back pain (no localisation reported) | ashen, anxious | CT | - |
| Siepe et al., 2009, Germany [223] | CR / hospital | male, 66 y | aorto-caval fistula | back pain (no duration or localisation reported), severe, incomplete paraplegia | shock developing during neurological examination | CT | - |
| Kotsikoris et al., 2012, Greece [215] | CS / hospital | 14 (sex and age not clearly reported) | aorto-venous fistula | LBP (92.8%) (no duration reported) | abdominal tenderness (78.6%), palpitation (57.1%), dyspnoea (42.8%), haemorrhagic shock (28.6%), congestive heart failure (21.4%) | - | - |
| Cinara et al., 2005, Serbia [234] | CS / hospital | 26 (7.7% female), mean age: 65.3 y | aorto-caval fistula | LBP (92.3%) with palpable abdominal aortic aneurysm | haemorrhagic shock at admission (65.3%), congestive heart failure (11.5%), dyspnoea (11.5%), palpitation (23.1%), collapse (7.0%), leg oedema (50%) (secondary to deep vein thrombosis (3.8%)), anuria (7.0%), haematuria (7%), scrotal oedema or haematoma (23.0%), haematemesis (3.8%) | - | - |
| Kiyosue et al., 2017, Japan [214] | CS / hospital | 168 (dural: 17.6% female, mean age: 64.4 y); (epidural: 27% female, mean age: 66.6 y) | spinal dural and extradural arterio-venous fistula | back pain (dural: 3.7%, epidural: 1.7%) (no duration or localisation reported); dural: myelopathy (most frequently: 97.2%), radiculopathy (4.6%), subarachnoid haemorrhage (0.9%), intramedullary haemorrhage (0.9%); epidural: myelopathy (most frequent, 91.5%), radiculopathy (11.9%) | - | - | - |
| Muralidharan et al., 2011, USA [235] | CS / hospital | 153 (22.2% female), mean age: (female: 62 y, male: 64.3 y) | spinal dural arterio-venous fistula | back pain (no duration or localisation reported), slow progression or stepwise worsening deficits, bilateral lower extremity weakness (82%), unilateral lower extremity weakness (16%), bilateral upper extremity weakness (2%), bilateral lower extremity numbness (39%), unilateral lower extremity numbness (17%), bilateral lower extremity dysesthesias / paraesthesia (29%), unilateral lower extremity dysesthesias / paraesthesia (15%), back pain +/- radiation to lower extremities (77%), pain confined to one or both extremities (23%), sphincter disturbances (neurogenic bowel or bladder, 3.9%), dyspnoea (0.6%) | - | - | - |
| Davidovic et al., 2008, Serbia [236] | CS / hospital | primary aorto-duodenal fistula: 5 (20% female, mean age; 63 y); aortocaval fistula: 25 (8% female, mean age: 65.6 y) | aorto-duodenal or aorto-caval fistula | ADF: LBP (80%) (no duration reported), ACF: LBP (100%) (no duration reported) | ADF: lower gastrointestinal bleeding, haemorrhagic shock (60%), ACF: haemorrhagic shock (72%), dyspnoea (16%), CHF (12%), severe leg swelling (56%), anuria (12%), haematuria (8%), scrotal oedema or haematoma (24%) | - | - |
| Davidovic et al., 2011, Serbia [237] | CHS / hospital | 50 (8% female) mean age: 67 y | aorto-caval fistula | LBP (or abdominal pain) (84%) (no duration reported) | pulsatile abdominal mass (100%), abdominal bruit (50%), shock (48%), lower limb oedema (44%), haematuria (32%), congestive heart failure (26%), oliguria (24%), scrotal oedema or haematoma (20%), unconsciousness (10%), anuria (8%), lower limb deep vein thrombosis (8%) | - | - |
| Davidovic et al., 2002, Yugoslavia [238] | CHS / hospital | 16 (6.2% female), mean age: 61.3 y | aorto-caval fistula | LBP (81.25%) and pulsating abdominal mass were predominant symptoms | haemorrhagic shock (68.75%), congestive heart failure (18.7%), dyspnoea and palpitation (37.5%), extensive oedema of lower extremities (31.2%), haematuria (12.5%), scrotal oedema (25%) | - | - |
| Narvid et al., 2008, USA [239] | CHS / hospital | 63 (21% female), mean age: 62 y | spinal dural arterio-venous fistula | back pain (institutional: 24%, review: 22%) (no duration or localisation reported), lower extremity weakness (institutional: 52%, review: 48%), lower extremity paraesthesia (institutional: 30%, review: 35%) | urinary symptoms (institutional: 6%, review: 7%) | - | - |
| Rangel-Castilla et al., 2011, USA [240] | CHS / not reported | 7 (29% female), mean age: not reported | spinal extradural arterio-venous fistula | back pain (28.6%) (no duration or localisation reported), unilateral or bilateral lower-extremity radiculopathy (71.5%); bowel and bladder dysfunction (57.1%), foot drop (14.3%) | - | - | - |
| Gemmete et al., 2013, USA [241] | CHS / hospital | 33 (21.2% female), mean age: 64.6 y | spinal dural arterio-venous fistula | nonspecific back pain (52%) (no duration or localisation reported), lower extremity weakness (88%), patchy dermatomal symptoms (76%) | urinary symptoms (27%) | - | - |
| Maeda et al., 2007, Japan [218] | CHS / hospital | 5 (all male), mean age: 63 y | aorto-caval or aorto-iliacal fistula | acute LBP (or abdominal pain) (80%) | chest pain associated with angina pectoris (20%); classic triad of abdominal or back pain, pulsatile mass, and abdominal bruit (40%), leg oedema (20%), hypovolaemic shock (60%), congestive heart failure (80%), renal dysfunction (80%) | - | - |
| Van Dijk et al., 2002, Canada [242] | CHS / hospital | 49 (20.4% female), mean age: 63 y) | dural arteriovenous fistulas | back pain (no duration or localisation reported) (39%); leg weakness or paraparesis (96%), sensory numbness or paraesthesia (90%) | urinary incontinence or retention (82%), bowel problems (65%) | - | - |
| Ruiz-Juretschke et al., 2011, Spain [243] | chart review study / hospital | 19 (5.3% female), mean age: 62 y | spinal dural arterio-venous fistula | lumbar or radicular pain (47.3%) (no duration reported), spastic paresis (89.5%), urinary sphincter dysfunction (78.9%), hypoesthesia (68.4%), clinical course followed: progressive myelopathy and / or radiculopathy (89.5%) | complete urinary incontinence (26%), erectile dysfunction (32%) | - | - |
| Assar et al., 2009, USA [118] | NR / - | - | aorto-caval fistula | back pain (no duration or localisation reported) | abdominal pain, pulsatile abdominal mass, continuous bruit (50-90%), high output heart failure, dyspnoea, tachycardia, wide pulse pressure, cyanosis, lower limb oedema, angina, palpitations, hypotension, fever, oliguria, haematuria, pulsatile peripheral veins, diminished lower limb pulses | diagnostic methods: CT | 3-4% with ruptured AAA, diagnosis was missed in 50% |
| Brightwell et al., 2012, UK [219] | NR / - | - | aorto-caval fistula | LBP (no duration reported) | dyspnoea, increased jugular venous pressure, pulmonary oedema, widened pulse pressure, abdominal bruit / thrill, palpable abdominal aneurysm, oliguria, leg oedema with / without cyanosis, pulsating varicose veins, haematuria and rectal bleeding, shock, abdominal pain, chest pain, scrotal oedema, tenesmus, priapism, and poor peripheral pulses | diagnostic methods: physical examination (pulsatile abdominal mass, abdominal bruit or thrill) CT, doppler ultrasound, arteriography / venography, angiography; diagnostic confirmation: CT, MRI, radioisotope studies | 80% result of a spontaneous rupture |
| Koch et al., 2006, Germany [224] | NR / - | mean age: 60 y | spinal dural arterio-venous fistula | LBP (no duration reported); early complaints (paraesthesia, sensory and gait disturbance), paraparesis, tetraparesis, flaccid paresis | impairment of micturition (anuresis or urinary incontinence, any type of bowel or bladder dysfunction (60-90%), pain (25-50% and more) | diagnostic methods: LP, MRI, DSA, aortography, CTA | 70% of all spinal arteriovenous malformation, 15% acute to subacute onset, delay 10 to 15 months |
| Marcus et al., 2013, USA [225] | NR / - | male predominance (80%), age: 50-60 y | spinal dural arterio-venous fistula | LBP (no duration reported), lower extremity weakness, gait disturbances (96%), sensory symptoms, bowel / bladder disturbances, radiculopathies, neurological deficits secondary to progressive myelopathy | sexual dysfunction, bowel and bladder incontinence and urinary retention (urinary dysfunction = 59%) | diagnostic methods: MRI, CTA / MRA, spinal angiography | 70% of all spinal arteriovenous malformation, relatively underdiagnosed; predisposing factors: thrombosis of the extradural spinal veins and trauma, usually progressive with an insidious development of disability, delay: 12-44 months |
| Krings et al., 2004, Germany [227] | NR / - | spinal dural arterio-venous fistulae = 70% of all AV shunts of the spine, male > female, age: 40-60 y | dural arterio-venous fistula | back pain (no duration or localisation reported), may irradiate to the lower legs, congestive myelopathy, hypo- and paraesthesia, paraparesis, impotence, sphincter disturbances | - | diagnostic methods: MRI, MRA, spinal angiography | slowly progressive, DDx: polyneuropathy, glioma, degenerative disc disease syringohydromyelia, inflammatory lesion or spinal ischaemia |
| Da Costa et al., 2009, Canada[244] | NR / - | 3rd decade of life, no sex predominance | spinal cord arterio-venous malformation | acute, severe back pain (no localisation reported), sudden onset of new or worsening pre-existing neurological deficits, motor and sensory symptoms, sexual, bladder and bowel dysfunction, weakness | haemorrhage (50%), bruit | diagnostic methods: MRI, angiography | - |
| Klopper et al., 2009, USA [228] | NR / - | 20% female, 80% > 40 y | spinal dural arterio-venous fistula | nonspecific, back pain (50% with radicular pain); radicular pain (50% with back pain), lower extremity weakness (33%), impaired sensation or paraesthesia (33%) | disturbance of micturition, defecation, or sexual function (10%) | diagnostic methods: laparotomy, myelography, CT, MRI, spinal angiography, MRA / CTA | mostly: lower thoracic or thoracolumbar region |
| Krings et al., 2009, Canada [226] | NR / - | male > female, mean age 35-60 y | spinal dural arterio-venous fistula | LBP (no duration reported), difficulty in climbing stairs, gait disturbances, sensory symptoms | bowel and bladder incontinence, erectile dysfunction, urinary retention | diagnostic methods: MRI / MRA, DSA, CTA, selective angiography | 70% of AVM, DDx: polyneuropathy, tumour, degenerative disc disease, prostatic hypertrophy (urinary retention), another type of spinal vascular malformation, glioma, inflammatory lesion, spinal ischaemia |
| Thron et al., 2001, Germany [245] | NR / - | 5-10 cases/ million people/ year, mean age: 60 y, male > female = 5:1 | spinal dural arterio-venous fistula | back pain (no duration or localisation reported); sensory disturbances with numbness and thermodysaesthesia | non-specific, buckling of the legs, less common pain in the muscles or legs, erectile dysfunction, sphincter disorders | diagnostic confirmation: MRI, myelography, LP, selective spinal angiography | DDx: polyneuropathy, spinal stenosis, disc protrusion, glioma, syringomyelia, spinal infarction |
AAA abdominal aortic aneurysm, ACF aorto-caval fistula, ADF aorto-duodenal fistula, AV arterio-venous, AVM arterio-venous malformation, CHF congestive heart failure, CHS cohort study, CR case report, CT computed tomography, CTA computed tomography angiography, CS case series, DSA digital subtraction angiography, ED emergency department, LBP low back pain, LP lumbar puncture, MRI magnetic resonance imaging, NR narrative review, MRA magnetic resonance angiography, y years
Miscellaneous
Six case reports, one cohort study and two narrative reviews reporting miscellaneous vascular disorders presenting with LBP, e.g., aortic thrombosis or coronary artery dissection, were found (Table 12). These disorders commonly present with acute LBP [168, 246–250] with accompanying symptoms like chest discomfort and weakness of lower extremities [227, 247, 251, 252].
Table 12.
Miscellaneous vascular disorders presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Diagnosis | Spinal symptoms | Extravertebral symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|---|
| Calderon et al., 2011, Spain [246] | CR / ED | male, 45 y | floating thrombus in aorta | acute LBP, sudden onset | syncope | TEE | - |
| Triantafyllo-poulos et al., 2011, Greece [247] | CR / ED | male, 56 y | infrarenal aortic thrombus | acute LBP, sudden onset, weakness of lower extremities | - | CTA | - |
| Cataldo et al., 2019, Italy [248] | CR / ED | male, 48 y | renal artery dissection in Ehlers Danlos syndrome | acute LBP (sudden, excruciating after sexual intercourse) | - | genetic assessment and CTA | - |
| Marshman et al., 2007, UK [251] | CR / not reported | male, 62 y | lumbar extradural arteriovenous malformation | chronic LBP (exercise-related, progressive), bilateral sciatica, bilateral leg weakness, altered sensation in L4-S1 dermatomes; walking distance limited to 10-20 yards, both feet felt cold dusky and swollen; no sphincteric disturbances | - | CT | - |
| Suntharalingam et al., 2014, Germany [249] | CR / hospital | female, 57 y | arterio-venous malformation | temporary back pain (no localisation reported), hypaesthesia in digiti two to four of her left foot | tumour in her lower ankle | MRI | - |
| Korkut et al., 2020, Turkey [168] | CR / not reported | male, 25 y | renal artery dissection | acute LBP, left-sided flank pain | colic, nausea, and vomiting | CTA | h/o cigarette smoking |
| Luong et al., 2017, Canada [252] | CHS / not reported | 196 (90.8% female), mean age: 52.2 y | coronary artery dissection | LBP (13.8%) (no duration reported) | chest discomfort (96%), radiation to arm (52.0%), nausea or vomiting (23.5%), radiation to neck (22.1%), diaphoresis (21.4%), dyspnoea (19.5%), dizziness (8.7%), ventricular tachycardia (7.1%), fatigue (5.1%), headache (1.5%), cardiac arrest (1.0%), syncope (0.5%) | - | - |
| Heldner et al., 2012, Switzerland [250] | NR / - | - | acute spinal cord ischaemia syndrome | back pain (no duration or localisation reported); complete or incomplete myelopathy, para- or quadriparesis, loss of sensation (especially pain and temperature), loss of bladder function | - | - | rare condition |
| male > female | spinal cord haemorrhage | acute back pain (no localisation reported); neck pain, (intense, knife-like, often show a radicular component), meningeal irritation with headache, neck stiffness, disturbance of consciousness and epileptic seizures | - | - | rare, causes: trauma, anticoagulation, hereditary or acquired bleeding disorders, bleeding from spinal vascular malformation, spinal artery aneurysms, primary spinal cord tumours; could be intramedullary, subarachnoid, subdural or epidural | ||
| Krings et al., 2004, Germany [227] | NR / - | fistulous AVM become symptomatic in young patients | spinal cord arterio-venous malformation | back pain (no duration or localisation reported), hypo- or paraesthesia, weakness and diffuse back and muscle pain; sensorimotor symptoms (slowly or acutely) | acute haemorrhage | diagnostic methods: MRI, spinal angiography, spinal MRA | 20-30% of all spinal vascular shunts, glomerular AVMs can become symptomatic by venous congestion alone, DDx: spinal haemangioblastoma, perimedullary fistulous AVMs type I, cavernoma |
AVM arteriovenous malformation, CHS cohort study, CR case report, CT computed tomography, CTA computed tomography angiography, ED emergency department, h/o history of, LBP low back pain, MRA magnetic resonance angiography, MRI magnetic resonance imaging, NR narrative review, TEE transoesophageal echography, y years
Vascular diseases – venous
Pathologies within the venous system, such as deep venous thrombosis (DVT) involving the lower extremities or the inferior vena cava (IVC) [253], may present with LBP. Numerous case reports were found, where acute LBP formed a component of the clinical manifestation, frequently co-occurring with symptoms suggestive of DVT like leg swelling and oedema (Table 13). Some patients exhibited a history of DVT or factors that predispose them to venous thrombosis. Within many of the documented case reports, there was an additional observation of stenosis, aplasia, and hypoplasia affecting the IVC. Malformation of the IVC can contribute to LBP through two distinct mechanisms. Firstly, they directly elevate the risk of DVT. Secondly, they might induce engorgement (varicosis) of the epi- and intradural veins surrounding the spinal cord, thereby causing neural compression that results in radicular symptoms even in the absence of a DVT. Despite the identification of a compressing mass prior to surgery in patients with radicular symptoms, the definitive diagnosis of thrombosed or non-thrombosed spinal varices exerting pressure on nerve roots was established intraoperatively. It is estimated that between 1-4% of radicular symptoms are due to vascular compression [254, 255]. Most patients experienced clinical improvement after surgical decompression confirming the cause-and-effect relationship with LBP.
Table 13.
Venous diseases associated with low back pain
| Author / year / country | Design / setting | Diagnosis | Patient(s) | Spinal symptoms | Extraspinal symptoms, comorbidity | Differential diagnosis | Diagnostic confirmation |
|---|---|---|---|---|---|---|---|
| Deep venous thrombosis with and without venous malformation | |||||||
| Bozkurt et al., 2006, Turkey [256] | CR / ED | IVC stenosis associated with Budd-Chiari Syndrome | female, 27 y | chronic LBP | leg pain | - | abdominal sonography |
| Nowak et al., 2008, Germany [257] | CR / neurology department | bilateral iliac vein thrombosis and congenital IVC aplasia | male, 18 y | chronic LBP | leg pain, superficial venous engorgement | disc herniation | MRI (absence of vena cava) |
| Dudeck et al., 2007, Germany [258] | CR / ED | DVT, IVC agenesis | male, 26 y | subacute LBP | leg pain | retroperitoneal mass, lymphoma | CT |
| Vasco et al., 2009, Spain [259] | CR / ED | DVT, IVC agenesis | female, 36 y | acute LBP | leg oedema | - | vascular sonography |
| Kogias et al., 2011, Germany [31] | CR / ED | DVT, IVC hypoplasia | male, 21 y | acute LBP | leg pain | lumbar disc herniation | CT, MRI |
| Aday et al., 2016, USA [260] | CR / ED | iliofemoral DVT | female, 29 y | acute LBP | difficulties to walk, leg oedema | - | vascular sonography |
| Di Nicolo et al., 2016, Italy [24] | CR / not reported | IVC malformation / duplication | male, 33 y | acute, mechanical LBP | haematuria / h/o urolithiasis | urolithiasis | abdominal sonography |
| Williams et al., 2017, USA [261] | CR / not reported | IVC atresia complicated by extensive DVT | male, 27 y | acute exacerbation of chronic LBP | right groin numbness, oedema of both legs with tenderness to palpation over the medial aspect of the left thigh | - | MRI |
| Langer et al., 2017, Portugal [262] | CR / ED | DVT, IVC hypoplasia | male, 30 y | acute LBP | leg pain, difficulties to walk, leg oedema | - | vascular sonography, CT |
| Adachi et al., 2018, Japan [263] | CR / referred to specialist care | DVT, IVC hypoplasia | male, 32 y | acute LBP | pain radiating to both thighs, leg oedema | - | vascular sonography |
| Ikeda et al., 2019, Japan [264] | CR / ED | pulmonary embolism | male, 45 y | acute LBP | chest pain | - | CT |
| Umar et al., 2019, USA [265] | CR / ED | IVC stenosis associated with Budd-Chiari Syndrome | male, 47 y | acute LBP and unresponsive to NSAIDs | developed cyanosis of both legs, loss of sensation, and the absence of distal pulses shortly after admission | - | vascular sonography |
| Epi- or intradural varicosis leading to radicular symptoms | |||||||
| Genevay et al., 2002, France [266] | CR / not reported | epidural varicosis | female, 67 y | chronic LBP unresponsive to treatment | leg pain, no other neurologic symptoms | disc herniation | intraoperatively |
| female, 70 y | acute LBP | leg pain, bilateral paresis | |||||
| Hammer et al. 2003, UK [491] | CS / hospital | epidural varicosis | 6 patients, 4 female (age: 38, 29, 50, 30 y), 2 male (20, 35 y) | acute or chronic LBP | leg pain | disc herniation | MRI, intraoperatively |
| Moonis et al., 2003, USA [267] | CR / not reported | intradural varicosis | male, 82 y | chronic LBP | leg pain, no other neurological symptoms | intradural nerve sheath tumour | MRI, intraoperatively |
| Paksoy et al., 2004, Turkey [268] | CS / not reported | epidural venous plexus enlargement with IVC obstruction or occlusion | 13 patients, 69.2% female, age: 23-40 y | LBP (no duration reported) | leg pain, h/o Behcet’s disease | - | MRI |
| Pennekamp et al., 2007, Germany [269] | CR / ED | epidural venous varicosis | female, 40 y | acute LBP | leg pain, paresis | disc herniation | MRI |
| Aoyama et al., 2008, Japan [255] | CR / neuro-surgery de-partment | epidural venous varicosis | male, 33 y | subacute LBP | leg pain, loss of patellar tendon reflex | cystic nerve sheath tumour | MRI (lumbar mass) |
| Paldor et al., 2010, Israel [270] | CR / neuro-surgery de-partment | intradural lumbar varicosis | male, 55 y | LBP (no duration reported) | pain radiating to the buttocks and right thigh, neurological examination was normal | intradural tumour, suspected to represent a schwannoma or ependymoma | MRI with breath hold and Valsalva |
| Lee et al., 2011, Korea [271] | CR / not reported | calcified epidural varicosis | female, 72 y | chronic LBP | pain radiating into the left buttock, weakness in her left hip flexor with numbness in the left L1 and L2 dermatomes | disc herniation | CT (calcified lesion in the left epidural space) |
| Pacult et al., 2018, USA [272] | CR / ED | gluteal varicosis | female, 76 y | chronic LBP | leg numbness and weakness | disc herniation | MRI |
| Im et al., 2018, Korea [273] | CR / neuro-surgery de-partment | epidural varicosis | female, 36 y | acute LBP | leg pain, paresis | facet joint synovial cyst | MRI, CT-angiography |
| Hallan et al., 2020, USA [274] | CR / referred to ED | dilated epidural venous plexus | female, 50 y | acute LBP | bilateral lower extremity weakness, systemic Lupus | - | MRI (vascular mass) |
| female, 60 y | chronic progressive LBP | leg pain, h/o DVT | - | - | |||
CR case report, CT computed tomography, CS case series, DVT deep venous thrombosis, ED emergency department, h/o history of, IVC inferior vena cava, LBP low back pain, MRI magnetic resonance imaging, NSAIDs non-steroid anti-inflammatory drugs, y years
Ikeda et al. [264], documented a case of pulmonary embolism originating from an IVC calcification, which manifested with symptoms of back and chest pain. However, the specific anatomical site of the back pain was not provided in their report.
Ovarian vein syndrome (OVS) is a rare condition caused by varicose, dilated ovarian veins inducing chronic ureteral obstruction. In one case series, the majority (12/13) of women reported back pain; however, the clinical presentation was dominated by urological symptoms [275].
A completely different venous pathology was described by Kalender et al. [276], who reported the rupture of an iliac vein leading to a retroperitoneal haematoma. The patient presented with LBP associated with abdominal pain [276].
Paraspinal haematoma
Spinal subdural, epidural, or subarachnoid haematomas are infrequent occurrences that can lead to acute spinal cord compression, giving rise to symptoms like radicular syndromes, paraparesis, or cauda equina syndrome (characterised by urinary and faecal incontinence or constipation). A total of 17 case reports or case series depicting paraspinal haematoma-induced LBP were identified (Table 14). They are mostly caused by trauma, lumbar puncture, and spinal surgery, which are beyond the scope of this review. However, they can also manifest spontaneously in individuals with coagulating disorders, oral anticoagulation, underlying vascular malformations (e.g., aneurysms or arteriovenous fistulas), neoplasms, and other vulnerabilities of the vessel walls. It can be assumed that a diagnosis is seldom missed due to the severity of symptoms, which usually prompt the utilisation of advanced imaging techniques that ultimately facilitate accurate diagnosis.
Table 14.
Case reports or case series of paraspinal haematoma presenting as back pain
| Author / country / year | Setting | Diagnosis | Patient(s) | Spinal symptoms | Extraspinal symptoms / comorbidity | Differential diagnosis | Diagnostic confirmation |
|---|---|---|---|---|---|---|---|
| Subdural haematoma | |||||||
| Vermeulen et al., 2015, Belgium [277] | hospital | spinal subdural and epidural haematomas and ruptured aneurysm with retroperitoneal haematoma | male, 61 y | acute LBP, acute paraparesis, paraesthesia | oral anticoagulation | - | MRI |
| Castillo et al., 2015, USA [278] | hospital | spontaneous spinal subdural haematoma | male, 69 y | sudden severe LBP and paraparesis | oral anticoagulation for atrial fibrillation | initially misdiagnosed as transverse myelitis | MRI |
| McHaourab et al., 2019, UK [279] | ED and primary care | spontaneous spinal subdural haematoma | male, 68 y | acute LBP with progression of paraparesis over several days | urinary and faecal retention | initially misdiagnosed as syringomyelia | MRI |
| Joubert et al., 2019, France [280] | not reported | spontaneous spinal subdural haematoma | female, 82 y | acute LBP, paraparesis | urinary and faecal retention | - | MRI |
| Yokota et al., 2020, Japan [281] | hospital | acute spinal subdural haematoma | male, 59 y and review of 37 case reports | severe acute LBP | urinary and faecal incontinence | - | MRI |
| Epidural haematoma | |||||||
| Braun et al., 2007, Spain [282] | not reported | spinal subdural and epidural haematomas | 7 patients (4 with non-lumbar pain), 3 patients: female, 87 y, female, 22 y, male 43) | back pain and paraparesis or paraplegia | 1x on oral anticoagulation, 1x after epidural catheter | - | MRI (location of lesion thoracic possible) |
| Baek et al., 2008, Korea [283] | not reported | spontaneous spinal epidural haematoma |
2 patients: male, 64 y female, 69 y |
acute LBP and paraparesis | one on aspirin | - | MRI |
| DeSouza et al., 2014, UK [284] | ED | spontaneous spinal epidural haematoma secondary to pseudogout | male, 75 y | sudden onset back pain (no localisation reported) and paraparesis | - | - | MRI |
| Matsui et al., 2014, Japan [285] | hospital | chronic spontaneous spinal epidural haematoma | male, 74 y | chronic LBP, leg pain | hypertension | - | MRI |
| Goyal et al., 2016, India [286] | hospital | spontaneous spinal epidural haematomas | male, 54 y | sudden onset of LBP, paraparesis, paraesthesia | oral anticoagulation | - | MRI |
| Ismail et al., 2017, Libanon [287] | ED | spontaneous spinal epidural haematomas | male, 72 y | acute mechanical LBP | oral anticoagulation | - | MRI |
| Subarachnoid haematoma | |||||||
| Massand et al., 2005, US [288] | not reported | spinal artery aneurysm rupture and subarachnoid haemorrhage | 4 patients | all acute LBP | not reported | - | CT, MRI |
| male, 30 y | paraparesis | not reported | |||||
| male, 69 y | radicular symptoms | stroke | |||||
| male, 54 y | radicular symptoms | none | |||||
| male, 73 y | paraparesis | not reported | |||||
| Toi et al., 2011, Japan [289] | hospital | subarachnoid haemorrhage associated with paraspinal arteriovenous fistula | male, 60 y | sudden onset LBP and paraparesis | constipation and faecal retention | - | MRI |
| Steele et al., 2019, UK [290] | ED | vertebral artery dissection and subarachnoid haemorrhage | male, 55 y | LBP (no duration reported), paraesthesia | constipation and faecal retention | - | MRI |
| Other locations | |||||||
| Yamasaki et al., 2005, Japan [291] | hospital | haematoma in the psoas muscle | male, 53 y | sudden LBP | abnormal chest shadow | - | CT, MRI |
| Fukuda et al., 2007, Japan [292] | not reported | juxta-facet haematoma | male, 67 y | chronic LBP, radicular pain, paraesthesia | - | - | MRI |
| Taghipour et al., 2008, Iran [293] | not reported | intradural nerve root haematoma | male, 32 y | severe acute LBP radiating into both legs | - | - | MRI |
CT computed tomography, ED emergency department, LBP low back pain, MRI magnetic resonance imaging, y years
Chronic periaortitis
Retroperitoneal fibrosis
Chronic periaortitis is a term used to describe a group of rare inflammatory diseases, such as retroperitoneal fibrosis (RPF) and inflammatory abdominal aortic aneurysm (IAAA). RPF is characterised by benign proliferation of fibrotic tissue in the retroperitoneal space, which can result in compression of the aorta, sometimes called periaortitis, and the ureters. It is nowadays classified as immunoglobulin G4 (IgG4)-related autoimmune disorder [294]. Multiple case reports or case series and narrative reviews, six cohort studies, one case-control study, one register study, and one randomised controlled trial related to LBP in the context of retroperitoneal fibrosis were identified (Table 15). Due to the location of the fibrotic tissue, certain characteristic symptoms and complications manifest. The predominant initial presenting symptoms typically include LBP accompanied with abdominal pain, with flank pain occasionally reported [294–308]. RPF predominantly presents with subacute or chronic back pain, with acute LBP being a rare occurrence. The nature of the pain is typically described as dull [303, 309, 310], but can also be colicky, if complications such as unilateral or bilateral ureteral stenosis develop [303]. The occurrence of LBP in the presence of RPF varies greatly between 10% [311] and 100% [312]. Other accompanying symptoms are malaise, fever, anorexia, weight loss, unilateral or bilateral lower limb oedema, and scrotal swelling [27, 294–303, 305–311, 313–352]. The patients in the case reports and case series were mostly men aged 40 to 60 years. Confirmation of the diagnosis primarily relied upon biopsies or imaging studies with a reported diagnostic delay between 7 weeks and 16 months [300, 353].
Table 15.
Retroperitoneal fibrosis presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|
| Blanc et al., 2007, France [295] | CR / not reported | male, 55 y | subacute LBP | abdominal pain radiating into the testis, fatigue, weight loss | CT (thorax, abdomen, pelvis) | - |
|
Cavalleri et al., 2008, Monaco [354] |
CR / not reported | male, 50 y | LBP (no duration reported) | no other symptoms | biopsy | - |
| male, 47 y | chronic LBP | no other symptoms | biopsy | - | ||
| Doshi et al., 2013, UK [309] | CR / hospital | male, 45 y | subacute / chronic LBP (dull) | lethargy, anorexia, acute deterioration in renal function, left testicular swelling | biopsy | - |
| Drieskens et al., 2002, Belgium [296] | CR / not reported | female, 41 y | LBP (no duration reported) | radiating to abdomen, sleep disturbance, fatigue, anorexia | biopsy + FDG-PET | - |
| Nemec et al., 2008, Czech Republic [27] | CR / not reported | male, 59 y | chronic LBP (continuous, blunt in character), radiating to loins, buttock and thighs bilaterally, worsening during walking | weight loss, sore throat with fever, night sweat | CT-guided needle biopsy | - |
| Paetzold et al., 2013, Austria [297] | CR / ambulatory care | female, 46 y | acute LBP | fatigue, diffuse abdominal pain, skin mycosis | MRI, 18 FDG-PET | - |
| Reilly et al., 2005, USA [298] | CR / ED | male, 60 y | acute LBP | radiating to lower abdomen and suprapubic region, fatigue, nausea | biopsy | - |
| Tritschler et al., 2014, Switzerland [313] | CR / hospital | male, 47 y | subacute / chronic LBP | fatigue, loss of appetite, weight loss, subfebrile temperatures | biopsy | - |
| Yang et al., 2018, China [355] | CR / ED | male, 62 y | acute LBP (persistent, not relieved after taking NSAIDs) | not reported | CT abdomen | - |
| Zen et al., 2006, Japan [310] | CR / hospital | male, 52 y | LBP (no duration reported) (dull, paraspinal, without tenderness) | low-grade fever (37°C) | CT-guided needle biopsy | - |
| Brodman et al., 2003, Austria [314] | CR / not reported | male, 62 y | LBP (no duration reported) | lower abdominal pain, weight loss, fatigue | CT, FDG-PET | - |
| Famularo et al., 2009, Italy [356] | CR / not reported | male, 53 y | back pain (no duration and localisation reported) | arthralgias | CT | - |
| Young et al., 2008, USA [357] | CR / outpatient clinic -> hospital | male, 67 y | chronic LBP (gradually worsening) | not reported | CT | - |
| Jois et al., 2004, UK [315] | CR / not reported | male, 67 y | subacute LBP (intermittent, worse at night and disturbing sleep) | weight loss | CT | - |
| Maritati et al., 2012, Italy [316] | CR / hospital | female, 49 y | back pain (no localisation and duration reported) | malaise | laparoscopic biopsy | - |
| Vaglio et al., 2008, Italy [317] | CR / hospital | male, 76 y | LBP (no duration reported) | fatigue, comorbidities: surgery for IAAA 1 year ago | CT | - |
| female, 54 y | LBP (no duration reported) | hypogastric pain | CT | - | ||
| female, 63 y | LBP (no duration reported) | abdominal pain | MRI | - | ||
| Kawamoto et al., 2003, Japan [318] | CR / not reported | female, 40 y | back pain (no duration or localisation reported) | low-grade fever, general fatigue | CT / MRI | - |
| Oshiro et al., 2005, Japan [319] | CR / hospital | male, 58 y | left sided back pain (no duration or localisation reported), claudication | left lower extremity oedema, left inguinal mass, anaemia, left hydronephrosis | biopsy | - |
| Wu et al., 2002, USA [358] | CR / not reported | male, 39 y | chronic back pain (no localisation reported) | bilateral hydronephrosis | US | - |
| Hamano et al., 2002, Japan [359] | CR / hospital | male, 60 y | first and second presentation: back pain (no duration or localisation reported) | first presentation: hydronephrosis left sided; second presentation: epigastric pain, jaundice | first presentation: histological assessment; second presentation: exploratory biopsy | - |
| Pizzini et al., 2007, Italy [360] | CR / hospital | female, 68 y | LBP (no duration reported) | dyspnoea, palpitations | MRI + laparoscopy / biopsy | - |
| Zeina et al., 2007, Israel [352] | CR / not reported | male, 53 y | chronic LBP (moderate intensity, unaffected by motion, not relieved by bed rest) | weight loss (4 kg) | CT | - |
| Al-Hammouri et al., 2019, Jordan [361] | CS / hospital | 116 (27% female), mean age: 50.5 y | LBP (79%) (no duration reported) | bilateral ureteral obstruction (58.6%), acute renal failure and uraemic symptoms (27%), unilateral ureteral obstruction (20.6%), no obstruction (17.2%), asymptomatic (13.8%), new onset hypertension (10.3%), anejaculation (3.4%) | - | - |
| Chiba et al., 2013, Japan [311] | CS / hospital | 10 (40% female), mean age: 70.1 y | LBP (10%) (no duration reported) | joint pain (10%), swelling of lacrimal / salivary glands (30%), visual disturbance (20%), fever (10%), dry mouth (10%), oedema of the lower extremities (10%), dyspnoea (10%) | - | associated diseases: AIP, sialadenitis, dacryoadenitis, lymphadenopathy, pulmonary pseudotumor, pituitary pseudotumor |
| Hadzi-Djokic et al., 2015, Serbia [353] | CS / hospital | 15 (27% female), mean age: 56.4 y | LBP (75%) (no duration reported) | unilateral ureteral obstruction (47%), uraemia (26.7%), urinary tract infection (13.3%) | - | diagnostic delay: 15.8 months |
| Li et al., 2011, China [294] | CS / hospital | 61 (21% female), mean age: 55.7 y | LBP (38%) (no duration reported) | abdominal pain (32.8%), loin tenderness (19.7%), abdominal tenderness (11.5%), abdominal mass (8.2%), fever (4.9%), weight loss (4.9%), hypertension (3.3%), abdominal swelling (1.6%), decreased appetite (1.6%), haematuria (1.6%), lower limb oedema (1.6%) | - | mean duration of symptoms: 8 months |
| van Bommel et al., 2009, Netherlands [299] | CS / hospital | 53 (23% female), mean age: 64 y | LBP (60%) (no duration reported), upper leg claudication (11%) | abdominal pain (57%), testicular pain (46%), discomfort (92%), urinary frequency (47%), flank pain (42%), weight loss (40%), constipation (30%), hydrocele (29%), nausea / vomiting (25%), fever / rigors (17%), lower extremity oedema (8%), anejaculation (7%) | - | median duration of symptoms: 6 months |
| Yachoui et al., 2016, USA [300] | CS / hospital | 26 (23% male), median age: 58 y | LBP (29%) (no duration reported) | abdominal pain (58%), flank pain (42%), constitutional (33%), vomiting (17%), constipation (12%), asymptomatic (8%), diarrhoea (4%), arthralgia (4%), lower extremity oedema (4%) | - | diagnostic delay: 7 weeks |
| Corradi et al., 2007, Italy [322] | CS / hospital | 24 (33% female), median age: 56 y | back pain (88%) (no duration or localisation reported) | ureteral involvement (83%), constitutional symptoms (malaise, anorexia, weight loss and fever) (79%), hydrocele and / or varicocele (69%), acute renal failure (54%), constipation (13%), deep vein thrombosis of lower limbs (8%) | - | associated autoimmune disease (33%) |
| Ilie et al., 2006, UK [323] | CS / not reported | 28 (29% female), mean age: 64.1 y | back pain (29%) (no duration or localisation reported), left anterior thigh claudication (4%) | loin pain (40%), groin and testicular pain (18%), abdominal pain (18%), associated high blood pressure (36%), oedema (11%), hydrocele (7%) | - | coexisting: AAA (36%), diabetes (7%), myocardial infarction (11%), venous thrombosis (7%), primary sclerosing cholangitis & small vessel vasculitis & ureteric stone & TCC (7%) |
| Jansen et al., 2010, The Netherlands [324] | CS / hospital | 26 (35% female), mean age: 67 y | back pain (92%) (no duration or localisation reported), claudication (4%) | discomfort (96%), hydrocele / testicular pain (53%), weight loss (50%), pollakiuria (38%), nausea / vomiting (23%), constipation (19%), fever / rigors (4%), lower extremity oedema (4%) | - | decrease in visual PET score correlated with decrease in ESR over time, but not with CRP level or CT-documented mass regression over time |
| Kermani et al., 2011, USA [326] | CS / hospital | 185 (39% female), mean age at diagnosis: 57.6 y | back pain (38%) (no duration or localisation reported), lower extremity claudication (2%) | abdominal pain (40%), flank pain (21%), testicular pain (13%), weight loss (27%), nausea (20%), vomiting (13%), fatigue (13%), new lower extremity oedema (13%), constipation (12%), subjective fever (9%), anorexia (9%), arthralgias (5%), night sweats (4%) | - | - |
| Nakajo et al., 2007, Japan [327] | CS / PET centre | 6 (all male), mean age: 64 y | back pain (33%) (no duration or localisation reported) | abdominal pain (17%), ureteral involvement (50%), general fatigue (17%), anorexia (17%), asymptomatic (17%) | - | - |
| Van Bommel et al., 2007, The Netherlands [328] | CS / hospital | 34 (21% female), mean age: 63 y; 35% idiopathic, 65% secondary | back pain (88%) (no duration or localisation reported), claudication (15%) | discomfort (94%), pollakiuria (42%), weight loss (36%), constipation (24%), hydrocele / testicular pain (23%), fever / rigors (15%), anejaculation (15%) | - | mean duration of symptoms: 6 months |
| Vega et al., 2009, Chile [329] | CS / not reported | 7 (14% female), median age: 64 y | back pain (57%) (no duration or localisation reported), myalgias (14%), leg claudication (14%) | abdominal pain (57%), testicular pain (29%), ureteral colic pain (14%), constitutional symptoms (43%), constipation (29%), deep vein thrombosis of 1 leg (29%), renal insufficiency (29%), hydrocele (14%), renal colic (14%), oedema of lower limbs (14%), varicocele (14%), periumbilical mass (14%), acute renal failure (14%), prolonged fever (14%) | - | - |
| Zen et al., 2009, Japan [330] | CS / hospital | 17 (35% female), mean age: 62 y | back pain (35%), LBP (6%) (no duration reported) | lower abdominal pain (12%), asymptomatic (29%), oedema in lower extremities (29%), fever (6%) | - | 42.9% with back ache: IgG4 related |
| Salvarani et al., 2005, Italy [331] | CS / hospital | 7 (43% female), median age: 60 y | back pain (57%) (no duration or localisation reported) | abdominal pain (71%), testicular pain (14%), constitutional symptoms (fatigue, anorexia, weight loss, fever, diffuse myalgias, arthralgias) (57%), ureteral obstructive disease (57%), varicocele (29%), thrombosis (29%), hydrocele (14%), inferior vena cava syndrome (14%) | - | - |
|
Vaglio et al., 2003, Italy [332] |
CS / hospital | 16 (37.5 % female), median age: 61 y | back pain (25%) (no duration or localisation reported), claudication (12.5%) | abdominal pain (62.5%), ureteral colic pain (19%), testicular pain (19%), ureteral obstructive disease (75%), constitutional symptoms (75%), oliguria (12.5%), inferior vena cava syndrome (6%), rheumatoid arthritis (6%), dyspnoea (6%) | - | - |
|
Warnatz et al., 2005, Germany [333] |
CS / hospital | 20 (30% female), median age RPF (49 y) and IAAA (62 y) | back pain (69%) (no duration or localisation reported) | pain (94%), abdominal pain (44%), flank pain (31%), weight loss (56%), renal failure (5%) | - | leriche syndrome (10%) |
| Paravastu et al., 2009, UK [304] | CS / hospital | 38 (8% female), mean age: 69 y | back pain (or abdominal pain) (42%) (no duration or localisation reported) | hypertension (58%), ischaemic heart disease (34%) | - | 58% incidental finding, 78% smokers, male:female = 12:1 |
| Zhou et al., 2015, China [334] | CS / hospital | 30 (23% female), mean age: 56.7 y | back pain (13%) (no duration or localisation reported) | flank pain (26.7%), abdominal pain (13.3%), weight loss (30.0%), fatigue (20.0%), anorexia (16.7%), nausea and vomiting (16.7%), lower extremity oedema (13.3%), oliguria (13.3%), asymptomatic (13.3%), frequency and urgency (10.0%), anuria (6.7%), haematuria (6.7%), scrotal oedema (3.3%), fever (3.3%) | - | smoking (60%), hypertension (53.3%) |
| Ha et al., 2011, Korea [335] | CS / hospital | 27 (19% female), mean age: 55.7 y | back pain (3.7%) (no duration or localisation reported) | flank pain (37%), abdominal pain (26%), fever (26%), weight loss (26%), generalised weakness (26%), oliguria (26%), nausea / vomiting (22%), lower extremity oedema (22%), anorexia (19%), haematuria (15%), constipation (11%), urinary frequency (7%), thromboembolism (4%), anejaculation (4%) | hypertension (37%), dyslipidaemia (7%), smoking (52%) | |
| Adler et al., 2008, Switzerland [320] | CHS / outpatient department | 9 (22% female), mean age: 58.5 y | back pain (33%) (no duration or localisation reported) | abdominal pain (11%), hydronephrosis (100%), malaise (56%) | diagnostic methods: chest-x-ray to rule out malignancy | double J stenting in 78%; 67% treated for hypertension; all patients experienced regression of IRF in CT / MRI |
| Fry et al., 2008, UK [362] | CHS / hospital | 24 (8% female), mean age: 63 y | back pain (58%) (no duration or localisation reported) | not reported | - | - |
| Kardar et al., 2002, Pakistan [325] | CHS / tertiary care referral centre | 12 (25% female), mean age: 48.5 y | back pain (25%), LBP (8%) (no duration reported), migraine (8%) | loin pain (50%), abdominal pain (33%), flank pain (8%), groin pain (8%), bilateral ureteral obstruction (58%), dysuria (33%), weight loss (25%), fever (17%), leg swelling (17%), nausea / vomiting (8%), oliguria (8%), hydrocele (8%) | - | pain relief in 4 to 10 days after start of prednisolone therapy, obstruction relief in 6 to 8 weeks in (80%) |
| Simone et al., 2008, Italy [312] | CHS / not reported | 6 (all male), mean age: 47 y | back pain (100%) (no duration or localisation reported) | hydronephrosis (17%) | - | - |
| Zhang et al., 2017, China [336] | CHS / hospital | RPF: 19 (32% female, mean age: 56.7 y); Lymphoma: 23 (39% female, mean age: 57.4 y) | back pain (79%) (no duration or localisation reported) | fatigue (37%), fever (32%), proteinuria (11%) | diagnostic methods: laboratory (high ESR in 16%) | - |
| Moroni et al., 2006, Italy [305] | CHS / hospital | 17 (41% female), mean age: 56 y | back pain (or abdominal pain) (65%) (no duration or localisation reported) | ureteral obstruction (100%) plus unilateral hydronephrosis (29%) or bilateral hydronephrosis (71%), weakness / weight loss (35%), polyuria (35%), oligoanuria (18%), arterial hypertension, leg oedemas (18%), mild fever (6%) | - | - |
| Ceresini et al., 2015, Italy [306] | CCS / hospital | idiopathic RPF: 73 (37% female, mean age: 55 y); Controls: 71 (sex and age matched with idiopathic RPF) | lumbar back pain (or abdominal pain) (83%) (no duration reported) | constitutional symptoms (74%), testicular symptoms (59%), deep vein thrombosis (15%), constipation (29%), renal involvement (ureteral obstruction (71%), acute renal failure (41%)) | - | - |
| Brandt et al., 2011, Germany [321] | register study / hospital | 204 (31.9% female), mean age: 55.6 y | back pain (66%) (no duration or localisation reported) |
flank pain (66.3%), lower abdominal pain (27.3%), upper abdominal pain (25.0%), leg pain (20.4%), fatigue (52.9%), weight loss (36.6%), malaise / vomiting (32.0%), night sweats (27.9%), fever (19.8%) later: hydronephrosis (95.6%), vascular complication (27.5%), renal atrophy (22.5%), large bowel compression (2.0%) |
- | pre-existing immune disease (9.8%), coexisting fibrotic changes in other organ systems (3.4%), history of smoking (75.6%; active: 73.9%) |
| Pipitone et al., 2010, Germany [337] | NR / - | mean age: 40-60; male:female = 2-3:1 | back pain is leading symptom together with abdominal pain or flank pain (no duration or localisation reported), claudication abdominally or of the lower extremities | sometimes colic pain, oedema of the lower extremities, occasionally thrombophlebitis, scrotal swelling, varicocele, hydrocele, fever, fatigue, weight loss | diagnostic methods: physical examination (tender abdomen), laboratory: ESR / CRP elevated, anaemia, leucocytosis, imaging: US, duplex-US, CT, MRI, CTA / MRA, PET-CT | - |
| Li et al., 2011, China [294] | NR / - | - | LBP (most common symptom) (no duration reported) | insidious onset, abdominal pain, malaise, fever, anorexia, weight loss, unilateral or bilateral lower limb oedema, scrotal swelling | - | association with other autoimmune diseases, may relate to inflammatory abdominal aortic aneurysm |
| Liu et al., 2014, China [301] | NR / - | male:female = 2-3:1, mean age: 55-60 y | LBP (most common symptom) (no duration reported) | insidious onset, abdominal pain, malaise, fever, anorexia, weight loss, unilateral or bilateral lower limb oedema, scrotal swelling | - | association with autoimmune diseases, most common complication: acute and chronic renal failure secondary to ureteral obstruction |
| Pipitone et al., 2012, Italy [303] | NR / - | mean age: 55-60 y, male:female = 2-3:1 | LBP (most common symptom) (no duration reported), colicky or dull | abdominal pain and flank pain (most common presenting symptoms), varicocele, hydrocele, scrotal swelling, constipation, nausea, vomiting, fever, weight loss, fatigue, night sweats | - | - |
| Hara et al., 2014, Japan [338] | NR / - | - | back pain (no duration or localisation reported) | 50% symptom free, abdominal pain, lower limb oedema, ureteral obstruction (45-65%): hydronephrosis or renal failure | diagnostic methods: CT, MRI | - |
| Burkhardt Soares et al., 2008, Germany [339] | NR / - | male:female = 2-3:1; age: 50-60 | back pain (no duration or localisation reported), claudication | flank pain, testicular pain, upper and lower abdominal pain, pain in iliac fossa, fatigue, appetite & weight loss, fever, headache, nausea, vomiting, deep vein thrombosis, leg oedema, hydrocele, varicocele, polyuria, pollakiuria, dysuria, oliguria, UTI, erectile dysfunction, obstipation, hypertension, bladder dysfunction |
diagnostic confirmation = CT-guided biopsy |
DDx: malignancy, other inflammatory reactions |
| Caiafa et al., 2013, Spain [340] | NR / - | age: 40-65 y, male:female = 2-3:1 | LBP (no duration reported), claudication | malaise, anorexia, weight loss, low-grade fever, pain (flank, abdomen), extremity oedema, deep vein thrombosis, scrotal swelling, varicocele, hydrocele, constipation, intestinal ischaemia | - | rare condition, 56-100% obstructive uropathy (oliguria, anuria nausea, vomiting and altered consciousness) |
| Cronin et al., 2008, USA [341] | NR / - | age: 40-60 y, male:female = 2-3:1 | LBP (no duration reported) (severe, dull, increasing) |
early symptoms: abdominal pain, flank pain, lower extremity swelling and discomfort late symptoms: deep venous thrombosis, anuria, nausea, vomiting, altered consciousness relating to uraemia, hypertension, mesenteric ischaemia, bowel obstruction renal or ureteral involvement |
- | diagnosis often delayed; DDx: malignancy, drugs, therapy or chemotherapy, amyloidosis, infection, renal trauma, haemorrhage, inflammatory conditions, after irradiation |
| Fairweather et al., 2013; UK [363] | NR / - | age: 40-60, male:female = 2-3:1 | LBP (90%) (no duration reported) (dull, non-colicky pain, not affected by movement) |
abdominal pain (90%), loss of appetite, weight loss, low-grade fever, loin pain later: progressive ureteric obstruction (usually bilateral) with loin pain (colicky) |
- | 70% cause unknown, common associated finding: hydronephrosis |
| Swartz, 2009, USA [342] | NR / - | - | back pain (no duration or localisation reported) | abdominal pain, constitutional symptoms, ureteral obstruction, intestinal or biliary-pancreatic involvement, lower-extremity venous obstruction, aortic or branch arterial compression, involvement of other pelvic organs, peripheral nerve involvement | - | secondary RPF: vasculitis, granulomatosis, inflammation in patients with Crohn’s disease, primary biliary cirrhosis, granulomatosis with polyangiitis, Sjogren’s syndrome, Chester-Erdheim disease or other autoimmune syndromes; DDx: malignant process, drugs, asbestos |
| Palmisano et al., 2009, Italy [343] | NR / - | age: 50-60 y, male:female = 2-3:1 | LBP (80%) (no duration reported) (insidious, persistent, dull, unmodified by movement or rest, colicky if ureteral involvement), claudication | testicular pain, fatigue, anorexia, weight loss, low-grade fever, scrotal swelling, varicocele and hydrocele, oedema, deep vein thrombosis of the lower limbs, constipation, nausea, and vomiting | - | most frequent complication: ureteral obstruction (50-80%); DDx: inflammatory myofibroblastic tumour, sclerosing mesenteritis, Erdheim-Chester-disease, |
| Vaglio et al., 2005, 2006, 2006-2, 2007, 2011-2, 2016, Italy [344–347, 349, 350] | NR / - | age: 40-60 y, male:female = 2-3:1 | back pain / LBP (no duration reported) (radiating to abdomen, persistent, insidious, dull, constant, colicky if ureteral involvement, not exacerbated by movement or palpation, transiently responds to NSAIDs), claudication | pain (80%, usually dull, poorly localised: side pain, abdominal pain), constitutional symptoms (fatigue, anorexia, weight loss, nausea, vomiting, myalgia, low-grade fever (40-80%), sleep disturbance), anaemia, constipation, lower limb oedema and / or deep venous thrombosis, varicocele, hydrocele, testicular pain, erectile dysfunction, small bowel obstruction, intestinal ischaemia, obstructive uropathy (50% or more), haematuria, polyuria, urinary infections, urinary frequency, dysuria, oligoanuria, symptoms related to uraemia, hypertension, fluid and electrolyte disturbance | diagnostic methods: physical examination: (abdominal or lumbar tenderness, palpable, pulsatile, and tender abdominal mass, periumbilical bruit) laboratory: ESR, CRP elevated (> 80%), anaemia, autoantibodies positive, serum IL-6 elevated; diagnostic confirmation: CT, MRI, biopsy (percentage of biopsy-proven cases: 24-77%) | associated diseases: autoimmune diseases; risk factors: asbestos exposure, smoking, ergot derivates; delay between onset of symptoms and diagnosis |
| Geoghegan et al., 2007, Ireland [351] | NR / - | incidence: 1/200.000, mean age: 40-60, male:female = 2-3:1 | back pain (no duration or localisation reported), neurological manifestations | generalised malaise, loss of appetite, lethargy, nausea or vomiting; long-term complications: hydrocele formation, scrotal oedema, duodenal or small bowel obstruction, common bile duct compression, obstruction of the large bowel | diagnostic methods: physical examination (hypertension, pyrexia), imaging: IVU (past), US, CT, MRI | delay in diagnosis, associated with SLE, granulomatosis with polyangiitis, polyarteritis nodosa, ankylosing spondylitis, Hashimoto, sclerosing cholangitis, hypoplasia of the right hepatic lobe, amyloidosis, diffuse idiopathic skeletal hyperostosis, actinomycosis, asbestosis exposure |
| Gornik et al., 2008, USA [307] | NR / - | incidence: 3-10% | back pain (or abdominal pain) with fever (no duration or localisation reported) | idiopathic isolated aortitis: constitutional symptoms, abdominal pain, elevated inflammatory markers, acute renal failure | diagnostic methods: laboratory (ESR, CRP, complete blood count, kidney / liver function, blood cultures, rheumatological screening); imaging: CTA / MRA, US, PET-CT, biopsy | risk factors: tobacco use, younger age, family history of AAA; DDx: lymphoma |
| Mahajan et al., 2014, USA [308] | NR / - | - | poorly localised pain in back (no duration reported) | flank pain, abdominal pain, constitutional symptoms, acute renal failure | diagnostic methods: laboratory (elevated inflammatory markers) | diagnostic delay |
| Vaglio et al., 2011, Italy [348] | RCT / hospital | prednisone group (P) (18, 33% female, mean age: 56 y); tamoxifen group (T) (18, 39% female, mean age: 61 y) | back pain (or abdominal pain) (P: 89%, T: 94%) | fatigue, anorexia, weight loss, low-grade fever, diffuse myalgias, arthralgias (P: 83%, T: 72%); testicular symptoms (testicular pain, varicocele, hydrocele) (P: 58%, T: 55%), constipation (P: 33%, T: 22%), ureteral obstruction (P: 78%, T: 72%) | - | 6% of prednisone group and 39% of tamoxifen group relapsed while on treatment |
AAA abdominal aortic aneurysm, AIP autoimmune pancreatitis, CCS case-control study, CHS cohort study, CR case report, CRP c-reactive protein, CS case series, CT computed tomography, CTA computed tomography angiography, ED emergency department, ESR erythrocyte sedimentation rate, FDG-PET fluorodeoxyglucose-positron emission tomography, IAAA inflammatory abdominal aortic aneurysm, IgG4 immunoglobulin G4, IL-6 interleukin 6, IRF idiopathic retroperitoneal fibrosis, IVU intravenous urogram, LBP low back pain, MRA magnetic resonance angiography, MRI magnetic resonance imaging, NR narrative review, NSAIDs non-steroidal anti-inflammatory drugs, PET positron emission tomography, RCT randomised controlled trial, RPF retroperitoneal fibrosis, SLE systemic lupus erythematosus, TCC transitional cell carcinoma, US ultrasound, UTI urinary tract infection, y years
Inflammatory abdominal aortic aneurysm
Inflammatory abdominal aortic aneurysms represent a subtype of aortic aneurysm characterised by the thickening of the aortic wall and periaortic tissue, occasionally involving fibrotic remodelling. A total of two case reports, one case-control study, one cohort study, and two narrative reviews pertaining to IAAA linked with LBP were identified (Table 16). The incidence of LBP varies between 58% [364] and 80% [365]. Predominantly, it presents as chronic LBP, often accompanied by typical symptoms such as anorexia, fatigue, night sweats, nausea and vomiting [97, 111, 121, 365, 366] or lower abdominal pain [111, 121, 365, 366]. While the two case reports centred around female individuals, findings from the case control and case cohort studies indicated a higher prevalence among males. The typical age of onset ranged from 50 to 70 years. Notably, the narrative review highlighted that IAAAs constitute 2-10% of all AAAs [366]. This review further underscored the tendency for IAAA patients to exhibit a younger age profile compared to those with an AAA [121].
Table 16.
Inflammatory abdominal aortic aneurysms presenting with back pain
| Author / year/ country | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|
| Ahlawat et al., 2002, UK [97] | CR / ambulatory care | female, 71 y | chronic LBP (insidious onset, non-radiating, moderate intensity, worse at night, partially relieved with acetaminophen or aspirin; constant, dull, aching sensation) | anorexia (weight loss of 6.8 kg), decreased energy, occasional night sweats | abdominal CT | - |
| Sharif et al., 2008, UK [111] | CR / ED | female, 32 y | chronic LBP | lower abdominal pain; end of first week in hospital: increasing pain, nausea, and vomiting | CT, biopsy | - |
| Tambyraja et al., 2004, Scotland [364] | CHS / hospital | 24 (8% female), median age 69 y | back pain (58%) (no duration or localisation reported) | abdominal pain (58%), collapse (33%), haematemesis (4%), haematuria / oliguria / renal failure (4%) | - | contained retroperitoneal haematoma (67%), free intraperitoneal blood (12,5%); in-hospital mortality (42%) |
| Goldoni et al., 2014, Italy [365] | CCS / hospital | 90 (31% female, mean age: 58 y) | LBP (80%) (no duration reported) | abdominal pain (80%), ureteral obstruction (76%), systemic symptoms (fatigue, anorexia, weight loss, fever, diffuse myalgia, and arthralgia) (71%), testicular symptoms (testicular pain, varicocele, hydrocele) (63%), acute renal failure (47%), constipation (24%), deep venous thrombosis (13%) | - | - |
| Kasashima et al., 2011, Japan [366] | NR / - | patients with IAAA tend to be younger than patients with AAA | nonspecific dull back pain (no duration or localisation reported) | nonspecific and dull abdominal pain, low-grade fever, weight loss, general fatigue | diagnostic methods: laboratory (elevated: ESR, CRP, white blood counts; frequent: elevated serum IgG levels and autoantibodies (ANA)) | 2-10% of all AAAs |
| Tang et al., 2005, UK [121] | NR / - | patients are 5-10 years younger than in AAA | back pain (no duration or localisation reported) (80%) | flank or abdominal pain (80%), weight loss and anorexia (40%), very few asymptomatic | diagnostic methods: physical examination (tender abdominal pulsatile mass (30%)), laboratory (ESR elevated (40-88%), raised temperature / white cell count, elevated CRP); imaging: US, CT, MRI, IVU (past), nuclear medicine | risk factors: coincidence with smoking (77-100%), positive family history (17%), HLA-DR B1 locus in IAAA |
AAA abdominal aortic aneurysm, ANA antinuclear antibody, CHS cohort study, CCS case control study, CR case report, CRP c-reactive protein, CT computed tomography, ED emergency department, ESR erythrocyte sedimentation rate, HLA-DR human leukocyte antigen – DR isotype, IAAA inflammatory abdominal aortic aneurysm, IVU intravenous urogram, LBP low back pain, MRI magnetic resonance imaging, NR narrative review, US ultrasound, y years
Myocardial infarction
One case report, two case series, one chart review study, and two observational studies documenting instances of myocardial infarction presenting with back pain were identified (Table 17). The exact location of back pain was often not reported. In one case report, one of the patients presented with increasing back pain over a two-day period. Despite being in shock at admission, the initial presenting symptom was back pain [367]. An observational study indicated a higher prevalence of back pain as a symptom of myocardial infarction in women compared to men, whereas men typically presented more frequently with chest pain. Other accompanying symptoms were, for example, chest pain or discomfort, shoulder pain, cold sweat, or nausea [368–372].
Table 17.
Myocardial infarction presenting with back pain
| Author /year / country | Design / setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Ichinose et al., 2009, Japan [367] | CR / hospital | female, 68 y | acute back pain (no localisation reported), severe, migratory from chest | - | coronary angiogram, CE-CT |
| male, 66 y | acute back pain (no localisation reported), exacerbated over 2 days | state of pre-shock on admission | CE-CT, ultrasonic echocardiography | ||
| Kosuge et al., 2006, Japan [371] | CS / hospital | 457 (23.2% female), mean age: f: 72 y, m: 62 y | acute back pain (f: 24%, m: 12%) (no localisation reported) | anterior chest pain (f: 89%, m: 88%), throat and neck pain (f: 13%, m: 5%), left shoulder pain (f: 12%, m: 5%), left arm / forearm / hand pain (f: 11%, m: 5%), jaw pain (f: 9%, m: 3%), right shoulder pain (f: 9%, m: 4%), right arm / forearm / hand pain (f: 8%, m: 3%), epigastric region (f: 8%, m: 9%), cold sweating (f: 63%, m: 78%), shortness of breath (f: 62%, m: 52%), nausea (f: 49%, m: 36%), vomiting (f: 25%, m: 15%) | - |
| Lovlien et al., 2006, Norway [372] | CS / hospital | 533 (28% female), mean age: f: 61.2 y, m: 58.5 y | back pain (f: 26%, m: 16%) (no duration or localisation reported) | chest symptoms (pain, discomfort, pressure, tightness) (f: 86%, m: 92%), left arm pain (f: 52%, m: 49%), right arm pain (f: 34%, m: 27%), between scapulae pain (f: 34% , m: 24%), shoulder pain (f: 32%, m: 28%), jaw / throat pain (f: 32%, m: 24%), headache (f: 19%, m: 16%), abdominal pain (f: 18%, m: 15%), sweating (f: 58%, m: 56%), dyspnoea (f: 47%, m: 38%), nausea (f: 46%, m: 29%), fatigue (f: 46%, m: 38%), dizziness (f: 39%, m: 33%), palpitations (f: 38%, m: 18%), hot flashes (f: 29%, m: 22%), fainting (f: 13%, m: 7%) | - |
| Berg et al., 2009, Sweden [369] | chart review study / hospital | 225 (23.1% female), mean age: f 61.2 y, m: 59.3 y | back pain (f: 42.3%, m: 14.5%) (no duration or localisation reported) | chest pain (f: 88.5%, m: 94.8%), arm and shoulder pain (f: 61.5%, m: 59.5%), neck pain (f: 25%, m: 22%), abdominal pain (f: 15.4%, m: 7.5%), jaw pain (f: 7.7%, m: 9.2%), nausea (f: 53.8%, m: 29.5%), diaphoresis (f: 34.6%, m: 42.8%), fatigue (f: 30.8%, m: 19.1%), dizziness (f: 17.3%, m: 7.5%), vomiting (f: 13.5%, m: 6.9%), palpitations (f: 11.5%, m: 2.9%), syncope / light-headedness (f: 3.8%, m: 9.8%) | - |
| Lawesson et al., 2018, Sweden [368] | observational study / hospital | 532 STEMI patients (24% female), (mean age: f: 69.7 y vs m: 64.3 y) | back pain (m: 12%, f: 29%) (no duration or localisation reported) | chest pain / discomfort (m: 93%, f: 74%), throat / neck pain (m: 18%, f: 34%), shoulder pain (m: 15%, f: 33%), nausea (m: 29%, f: 49%), fear (m: 17%, f: 31%), cold sweat (no gender difference), fatigue and / or weakness (no gender differences) | - |
| Culic et al., 2002, Croatia [370] | observational study / hospital | 1996 (30.1 % female), mean age: f: 63 y, m: 57 y | back pain (f: 10.6%, m: 5.2%) (no duration or localisation reported) | any pain (m: 93.2%, f: 86.2%), chest pain (m: 87.6%, f: 79.7%), left arm pain (m: 65.8%, f: 71%), right arm pain (m: 40.1%, f: 47.4%), left shoulder pain (m: 45.2%, f: 43.8%), right shoulder pain (m: 35.3%, f: 30.8%), epigastric pain (m: 16.1%, f: 13.3%), neck pain (m: 10.7%, f: 17.3%), jaw pain (m: 5.5%, f: 9.2%), headache (m: 4.4%, f: 10.8%), only non-chest pain (m: 4.9%, f: 8.2%); any non-pain symptom (m: 71.2%, f: 84.2%), sweating (m: 59.7%, f: 48.1%), weakness (m: 48.1%, f: 45.8%), nausea (m: 40.9%, f: 57.4%), dyspnoea (m:34.3%, f: 48.4%), vomiting (m: 17.6%, f: 21%), belching (m: 16.9%, f: 12.8%), cough (m: 8.1%, f: 15.5%), vertigo (m: 5.7%, f: 7.8%), faintness (m: 5.1%, f: 3.5%), hiccups (m: 3.4%, f: 1.5%), tinnitus (m: 1.9%, f: 2.8%) | - |
CE-CT contrast-enhance computed tomography, CR case report, CS case series, f female, m male, STEMI ST-elevation myocardial infarction, y years
Gastrointestinal diseases
Gallstone disease / cholecystitis
Gallstone disease and cholecystitis usually present with colicky upper abdominal pain. Two case reports and one randomised controlled trial (RCT) investigating LBP as an initial complaint associated with gallstone disease or cholecystitis were identified (Table 18). The RCT compared two treatment strategies for managing gallstone disease and reported baseline symptoms encompassing pain radiating to the back. Among the case reports, one focussed on cholecystitis [373], while the other delved into symptomatic cholecystolithiasis [374], both involving female patients. LBP can present both acutely (particularly with inflammation) [373] as well as chronically (with symptomatic cholecystolithiasis without inflammation) [374]. Accompanying symptoms often comprise abdominal pain [373, 374], predominantly localised in the right upper quadrant or epigastric region [375]. Other symptoms include gastrointestinal symptoms such as fat intolerance, nausea and vomiting, diarrhoea, and difficulty in defecation [375].
Table 18.
Gallstone disease / cholecystitis presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Kinoshita et al., 2002, Japan [373] | CR / not reported | female, 75 y | acute LBP | upper abdominal pain at night | MRCP, PTGBA |
| Petersen et al., 2018, USA [374] | CR / ambulatory primary care -> ambulatory specialist care -> ED | female, 30 y | chronic LBP (intermittent, persistent and sharper), upper back pain radiating to arms and legs | abdominal pain, left posterior lateral thoracic pain, left sided torso pain, morning stiffness in all joints, increased sweating 2 to 3 times a week | pelvic ultrasound |
| van Dijk et al., 2019, The Netherlands [375] | RT / hospital | 1067 (74% female), mean age: 48.5 y | pain radiating to the back (68%) (no duration or localisation reported) | pain located in right upper quadrant or epigastric region (91%), pain radiating to the chest (25%), nausea and vomiting (60%), bloating (51%), fat intolerance (46%), burping (40%), flatulence (36%), acid reflux (31%), difficult defecation (19%), diarrhoea (18%) | - |
CR case report, ED emergency department, LBP low back pain, MRCP magnetic resonance cholangiopancreatography, PTGBA percutaneous transhepatic gallbladder aspiration, RT randomised trial, y years
Pancreatitis
Pancreatitis, an inflammatory condition of the pancreas, can either manifest acutely or chronically. Two case reports, one narrative review and one guideline were found detailing LBP as presenting symptom attributed to pancreatitis (Table 19). Accompanying symptoms were abdominal pain, loss of appetite and weight, or jaundice [376–379]. Furthermore, one narrative review [378] was identified, outlining back pain as radiating pain originating from the epigastric region.
Table 19.
Pancreatitis presenting with back pain
| Author / year / country | Design / setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|
| Matsubara et al., 2005, Japan [376] | CR / hospital | male, 63 y | LBP (no duration reported) | appetite loss, jaundice | PTGBD, wedge biopsy, intraoperative cholangiography | - |
| Nishimura et al., 2004, Japan [377] | CR / hospital | female, 47 y | sudden onset acute back pain (no localisation reported) | upper abdominal pain | ERC / biopsy | - |
| Rompianesi et al., 2017, Italy [378] | NR / - | - | pain radiating to the back (no duration or localisation reported) | epigastric pain (persistent, severe) or diffuse abdominal pain starting in the epigastric region | diagnostic methods: radiological tests | serum lipase and amylase do not reliably diagnose acute pancreatitis, especially with prolonged interval between symptom onset and testing |
| Okazaki et al., 2009, Japan [379] | guideline / - | - | back pain (15%) (no duration or localisation reported) | 1/3 to 1/2 with obstructive jaundice or mild abdominal pain, weight loss, in some cases: polydipsia / polyuria or malaise, xerostomia / xerophthalmia or hydronephrosis | diagnostic methods: laboratory (elevated biliary enzymes, pancreatic enzymes and total bilirubin) US / CT / MRI, Ga-67 and FDG accumulation, ERCP; IgG4 highest diagnostic value but not specific | association with: sclerosing cholangitis, diabetes mellitus, sclerosing sialadenitis / dacryoadenitis or retroperitoneal fibrosis; frequently: pancreatic exocrine and endocrine dysfunction |
CR case report, CT computed tomography, ERC endoscopic retrograde cholangiography, ERCP endoscopic retrograde cholangiopancreatography, FDG fluorodeoxyglucose, Ga-67 gallium-67, IgG4 immunoglobulin G4, LBP low back pain, MRI magnetic resonance imaging NR narrative review, PTGBD percutaneous transhepatic gallbladder drainage,. US ultrasound, y years
Miscellaneous
Five case reports featuring different gastrointestinal diseases as the primary presentation of LBP were identified (Table 20). These include intussusception [23], coeliac disease [380], pyeloduodenal fistula [381], liver abscess [34], and acute appendicitis [382]. While LBP predominantly presents acutely [34, 381, 382], it could also manifest chronically, as observed in coeliac disease [380]. Depending on the disease, accompanying symptoms such as occasional fever with nausea and diarrhoea (intussusception) [23] or weight loss (coeliac disease) [380, 382] indicated an extravertebral origin of LBP. However, the patient with the liver abscess presented solely with acute LBP [34]. Diagnosis was confirmed either by imaging [23, 34, 381, 382] or biopsy [380, 382].
Table 20.
Miscellaneous gastrointestinal diseases presenting with back pain
| Author / year / country | Design / setting | Patient / diagnosis | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Barbee et al., 2008, USA [23] | CR / ED | female, 20 y, intussusception | chronic LBP, radiating to the left anterior / superior hip; intermittent, aching, spasmodic, 10/10 at its worst; hip pain on most days for the past 4 months | occasional fever, occasional nausea, and diarrhoea | CT |
| Hoffman et al., 2004, USA [380] | CR / hospital | male, 42 y, coeliac disease | chronic LBP, no significant improvement with NSAR, difficulty walking | hip, ankle, and feet pain, weight loss | biopsy |
| Kobayashi et al., 2018, USA [381] | CR / ED | female, 89 y, pyeloduodenal fistula | acute LBP, radiating to the legs, progressively worsening, paroxysms of pain involving lumbar region, spasmodic cramping of bilateral buttocks and thighs | - | Tc-99m MAG3-scintigraphy |
| Tseng et al., 2015, Taiwan [34] | CR / ED | male, 46 y, liver abscess | acute right LBP | - | CT |
| Kuday Kaykisiz et al., 2018, Turkey [382] | CR / ED | male, 36 y, acute appendicitis | acute back pain (no localisation reported) | urinary difficulty, anorexia | CT + surgery / histological examination |
CR case report, CT computed tomography, ED emergency department, LBP low back pain, NSAR non-steroidal anti-rheumatic drug, Tc-99m MAG3-scintigraphy Technetium-99m mercaptoacetyltriglycine scintigraphy, y years
Paraspinal compartment syndrome
Compartment syndrome, marked by fluid accumulation in a muscle compartment leading to increased pressure and compromised blood supply, predominantly affects the lower leg but can involve other muscle groups, including paraspinal muscles. Identified were ten case reports and one narrative review reporting instances of paraspinal compartment syndrome in individuals presenting with acute LBP (Table 21). Across all case reports, only males were affected. The pain was described as abrupt, severe, sharp, throbbing, constant, or exacerbated by movement [22, 29, 383–390]. Typical accompanying symptoms were pain radiating into the leg and groin, numbness, and sensory deficits [22, 29, 256, 383–385, 389, 390]. Other extravertebral signs and symptoms were generally absent, aside from occurrences of dark urine due to myoglobinuria [22, 385] or fatigue [389]. Notably, all case reports described symptoms that started after weightlifting or heavy exercising. The review also reported aetiologies such as downhill skiing, surfboarding, or weightlifting, as well as iatrogenic causes like aortic or gastric bypass. An elevated creatine kinase (CK) in patients with LBP is a diagnostic clue for paraspinal compartment syndrome. Diagnosis was confirmed by imaging studies or measurement of intramuscular pressure. Compartment syndrome is an emergency which requires immediate referral when suspected.
Table 21.
Paraspinal compartment syndrome presenting with low back pain
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|
| Allerton et al., 2012, Australia [22] | CR* / ED | male, 25 y | LBP (no duration reported) (severe, gradual increase in severity over 4-6 hours, exacerbated by movement, radiating to right groin), altered sensation affecting his right leg | dark urine | MRI, paraspinal compartment pressure | - |
| Anaya et al., 2014, USA [383] | CR* / ED | male, 35 y | acute LBP (abrupt in onset, sharp, 10/10 on pain scale, constant, midline with no radiation, exacerbated by any flexion, extension, or rotation), hip pain, numbness, and lack of sensation in the lower portion of his back (right > left) | - | MRI | - |
| Chavez et al., 2013, USA [29] | CR* / ED | male, 25 y | acute LBP (severe, rapidly progressive stabbing and radiating down the left leg), difficulty walking | - | MRI | - |
| Minnema et al., 2008, USA [384] | CR* / ED | male, 32 y | acute LBP (excruciating, right sided, radiating down the posterior side of his leg), tenderness | - | MRI, paraspinal compartment pressure, elevated CK | - |
| Wik et al., 2010, Canada [386] | CR* / ED | male, 30 y | acute LBP (severe, bilateral, throbbing, exacerbated by lateral movement, diminished when standing perfectly straight) | - | MRI, paraspinal compartment pressure, elevated CK | - |
| Khan et al., 2005, Australia [385] | CR* / ED | male, 35 y | acute LBP (spontaneous, severe, unrelenting, right sided, exacerbated by movement, radiating across the abdomen to the groin), numbness in right lumbosacral area | dark urine | MRI, paraspinal compartment pressure, elevated CK | - |
| Paryavi et al., 2010, USA [387] | CR* / ED | male, 20 y | acute LBP (progressive, severe, debilitating back pain, bilateral) | - | MRI, paraspinal compartment pressure, elevated CK | - |
| Karam et al., 2010, USA [388] | CR* / ED | male, 23y | acute LBP (rapidly increasing, throbbing) | - | MRI, elevated CK | - |
| Kitajima et al., 2002, Japan [389] | CR* / ED | male, 25 y | acute LBP (severe, during night, decreased sensation) | fatigue | MRI, paraspinal compartment pressure, elevated CK | - |
| Xu et al., 2009, China [390] | CR* / not reported | male, 25 y | acute LBP (increasing during training, subsided afterwards, discomfort remained), sensation of tightness (during training) | - | MRI, paraspinal compartment pressure | - |
| Nathan et al., 2012, USA [391] | NR / - | 11 case reports (10% female, 24-67 y) | excruciating LBP (100%) (no duration reported), localised tenderness over the paraspinal region, localised sensory loss paraspinal region, atrophy of paraspinal muscle (9.1%) | - |
diagnostic methods: physical examination (absent abdominal sounds, deep abdominal tenderness on palpation) laboratory (CK elevated (90.1%)) |
aetiology: downhill skiing (27.3%), direct trauma (9.1%), aortic bypass (27.3%), surfboarding (9.1%), gastric bypass (9.1%), weightlifting (9.1%) |
* following weight lifting, cross-fit exercise, skiing, or surfboarding
CK creatine kinase, CR case report, ED emergency department, LBP low back pain, MRI magnetic resonance imaging, NR narrative review, y years
Gynaecological diseases
Endometriosis
Endometriosis, a gynaecological disease characterised by the presence of endometrial tissue outside the uterus, predominantly affects women in their childbearing years. Given the varying locations of the endometriosis foci, a range of non-specific symptoms, including LBP, can arise. A total of ten case reports, two case series, ten cohort studies, one case-control-study, one cross-sectional study, four narrative and one systematic review were identified (Table 22). The incidence of LBP associated with endometriosis varied greatly from 14.48% [392] to 93.4% [393]. An observational study by Darai et al. [392] suggested a causal relationship between LBP and endometriosis, noting LBP improvement in 55% of women following intervention. However, 18% reported worsening or no change of LBP, indicating that not all reported LBP among endometriosis patients can be directly attributed to endometriosis. Symptoms pointing towards endometriosis were chronic LBP, cyclical LBP, and increasing pain intensity [394–400]. Furthermore, patients with endometriosis commonly complain of dysmenorrhoea (up to 90% [401]) and dyspareunia (up to 85% [401]). Other accompanying symptoms depend on the localisation of the endometriosis foci and include urological symptoms like dysuria [395, 402–404] or gastrointestinal symptoms like rectal bleeding [396], cyclic and non-cyclic dyschezia [402–404], and alterations in bowel habits (constipation, diarrhoea) [405–409]. Due to the delay between the onset of symptoms and the diagnosis of endometriosis [399, 400, 410], endometriosis should be considered, especially in young women with chronic low back pain.
Table 22.
Endometriosis presenting with back pain
| Author / year / country | Design / setting | Patient(s) | Diagnosis | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|---|
| Agrawal et al., 2006, India [394] | CR / not reported | female, 40 y | intramedullary endometriosis of the conus medullaris | chronic LBP (cyclical), sensory loss below L1 | weakness in lower limbs, constipation, urinary hesitance | surgery, histological examination | - |
| Hsieh et al., 2010, Taiwan [395] | CR / ED | female, 42 y | ureteral endometriosis | chronic + acute LBP (cyclical, dull) | dysuria, costovertebral angle tenderness | surgery, histological examination | - |
| Kanthimathinathan et al., 2007, USA [396] | CR / ED | female, 30 y | intestinal endometriosis | chronic LBP (cyclical) | rectal bleeding | surgery, histological examination | - |
| Kondo et al., 2009, Japan [411] | CR / not reported | female, 44 y | ureteral endometriosis | chronic + acute LBP | - | biopsy, histological examination | - |
| Seyam et al., 2014, Saudi Arabia [412] | CR / not reported | female, 32 y | ureteral endometriosis | chronic + acute LBP | - | surgery | - |
| Uppal et al., 2017, USA [413] | CR / not reported | female, 39 y | endometriosis | chronic LBP (radiating) | abdominal pain | Lupron hormone antagonist therapy | - |
| Steinberg et al., 2014, USA [397] | CR / not reported | female, 29 y | intramedullary endometriosis of the conus medullaris | chronic LBP (cyclical, radicular) | difficulty walking | - | - |
| Troyer et al., 2007, USA [414] | CR / ED | female, 25 y | endometriosis | acute + chronic LBP | abdominal pain | laparoscopy and biopsy | - |
| Generao et al., 2005, USA [398] | CR / not reported | female, 49 y | ureteral endometriosis | chronic LBP | right flank pain, abdominal pain | biopsy | - |
| Kumar et al., 2012, India [415] | CR / not reported | female, 29 y | urinary tract endometriosis | back pain (no duration or localisation reported) | irritative symptoms | diagnostic laparoscopy with partial cystectomy | - |
| female, 36 y | back pain (no duration or localisation reported) | irritative symptoms | - | - | |||
| Mu et al., 2014, China [416] | CS / not reported | 23 females (age: 22-50 y) | ureteral endometriosis | LBP (73.9%) (no duration reported) | hypogastralgia (64.7%), dyspareunia (29.4%), menoxenia (29.4%), dysmenorrhoea (29.4%), haematuria (11.8%) | not reported | - |
| Carmignani et al., 2010, Italy [417] | CS / tertiary referral centre | 23 females, mean age: 35.6 y | endometriosis | LBP (26.1%) (left: 17.4%, right: 8.7%) (no duration reported) | urinary symptoms (43.5 %), renal colic (17.4%) (left: 13%, right: 4.3%), arterial hypertension (4.3%) | - | - |
| Byrne et al., 2018, UK [403] | CHS / hospital | 4721 females, median age: 35.1 y | rectovaginal endometriosis | LBP (87.9%) (no duration reported), difficulty emptying bladder | premenstrual pain, menstrual pain, non-cyclical pelvic pain, deep dyspareunia, cyclical dyschezia, non-cyclical dyschezia, bladder pain or pain passing urine, frequent bowel movements, urgent bowel movements, incomplete emptying sensation, constipation, melaena | - | - |
| Chudzinski et al., 2017, France [405] | CHS / hospital | 17 females, mean age: 34.72 y | pelvic endometriosis | LBP (47%) (no duration reported) | dysmenorrhea (64.7%), urinary functional signs (47%), dyspareunia (35.2%), primary infertility (35.2%), recurrent pyelonephritis (35.2%), digestive functional signs (29.4%), haematuria (29.4%), dyschezia (17.6%), constipation (17.6%), algomenorrhoea (17.6%), diarrhoea (11.7%), cystalgia (5.8%) | - | - |
| Darai et al., 2007, France [392] | CHS / hospital | 81 females, age: 33.2 y | colorectal endometriosis | LBP (49.4%) (no duration reported) | dysmenorrhea (74.1%), dyspareunia (70.4%), pain or cramping on bowel movement (63%), asthenia (55.6%), pain on defecation (38.3%) | - | - |
| Darai et al., 2010, France [401] | CHS / hospital | 29 females, age: 41.4 y | extensive pelvic endometriosis | LBP (14.48%) (no duration reported) | dysmenorrhea, dyspareunia, diarrhoea, constipation, tenesmus, cramping, dyschezia, asthenia | - | - |
| Dubernard et al. 2006, France [406] | CHS / hospital | 58 females, median age: 31 y | colorectal endometriosis | LBP (51.7%) (no duration reported), after surgery: LBP disappeared (57%), decreased (23%) or remained the same (20%); symptom intensity preoperative (3.5/10), postoperative (0/10) | dysmenorrhoea (84.48%), dyspareunia (74.13%), pain or cramping on bowel movement (68.97%), constipation (58.62%), asthenia (58.62%), pain on defecation (46.55%), tenesmus (37.93%), diarrhoea (25.86%), rectorrhagia (8.62%) | - | - |
| Redwine et al., 2001, UK [407] | CHS / hospital | 84 females, age: 34.98 y | endometriosis | moderate LBP (89.55%) (no duration reported), after surgery: LBP improved (65%), worsened (3%), unchanged (32%) | uterine cramps with menses (94.03%), painful bowl movements (94.03%), constipation (92.54%), diarrhoea (92.54%), fatigue (92.54%), intestinal cramping (91.04%), menstrual pain other than cramps (88.06%), non-menstrual pelvic pain (86.57%), tenderness on exam (83.58%), dyspareunia (52.24%), pelvic pain with exercise (29.85%) | - | - |
| Stepniewska et al., 2011, Italy [402] | CHS / tertiary referral centre |
20 females, mean age: 35 y |
endometriosis | LBP (20%) (no duration reported) | dysmenorrhoea (90%), dyspareunia (85%), dyschezia (65%), dysuria (30%), renal colic (5%), recurrent cystitis (5%) | - | - |
| Thomassin et al., 2004, France [408] | CHS / hospital | 27 females, median age: 32 y | colorectal endometriosis | LBP (26%) (no duration reported), postoperatively: LBP disappeared (57%) or decreased (43%), mean pain intensity preoperative: 6 (0-10) versus postoperative: 2.5 (0-8)) | tenderness (while palpation of endometriotic nodule) (100%), dysmenorrhea (89%), dyspareunia (89%), rectal symptoms (70%), pain on defecation (63%), non-menstrual pelvic pain (41%), rectorrhagia (33%), asthenia (30%), pain or cramping on bowel movement (26%), diarrhoea and / or constipation (15%) | - | - |
| Ballard et al., 2010, UK [418] | CHS / hospital |
113 females, mean age: 32 y |
chronic pelvic pain undergoing a diagnostic laparoscopy | back pain (70%) (no duration or localisation reported) | pain in: hypochondrium, right loin, umbilicus, left loin, right iliac fossa, suprapubic, left iliac fossa, left scapula, middle back, right scapular, left sacroiliac, sacrum, right sacroiliac, legs, perineal | - | - |
| Schliep et al., 2015, USA [404] | CHS / hospital | 190 females with endometriosis, mean age: 32 y; 147 females with other gynaecological conditions, mean age: 33.5 y | endometriosis | LBP (72.1% in patients with endometriosis (EM), 68% in patients with other gynaecological diseases (gynD), 67.7% in patients with normal pelvis (normPel)) (no duration reported) | pain at ovulation (EM: 67.4%; gynD: 49.0%; normPel: 52.2%), dysuria (EM: 22.6%; gynD: 19.1%; normPel: 11.0%), dyschezia (EM: 44.2%; gynD: 32.7%; normPel: 25.7%), pain in groin when lifting (EM: 26.3%; gynD: 27.2%; normPel: 19.9%), pain when bladder is full (EM: 53.2%; gynD: 51.0%; normPel: 42.7%), abdominal pain (EM: 51.1%; gynD: 49.7%; normPel: 44.1%), muscle / joint pain (EM: 53.3%; gynD: 46.0%; normPel: 47.7%), migraine (EM: 53.6%; gynD: 44.6%; normPel: 49.2%) | - | - |
| Markham et al., 2019, Australia [393] | CCS / hospital |
737 females, mean age: 34.9 y (with endometriosis: 529, mean age: 34.7 y) |
endometriosis | LBP in endometriosis (93.4%), LBP in women without a gynaecological complaint (70%) (no duration reported) | dysmenorrhoea (100%) in different pain intensities, pain at ovulation (87%), rectal pain (67%), dysuria (43%), pelvic pain other than during menses, ovulation, urination, or intercourse (77%), dyspareunia (85%) | - | - |
| Soliman et al., 2017, USA [419] | CSS / web-based survey | 1269 females, mean age: 34.3 y | endometriosis | LBP 76% (no duration reported) | pelvic pain or cramping during menstrual period (88%), anxiety or stress (75%), fatigue or weariness or anaemia (75%), depressed feelings / mood swings (65%), bloating (65%), heavy bleeding during periods (51%), non-menstrual pelvic pain (47%), dyspareunia (32%) | - | - |
| Chiantera et al., 2017, Germany [410] | NR / - | - | - | LBP, radicular pain (no duration reported) | dysmenorrhoea, dyspareunia, dyschezia, bowel and bladder complaints, vomiting / emesis, gastric disorders, headache, dizziness, painful ovulation, irregular pelvic pain, chronic fatigue | - | long delay between onset of symptoms and official diagnosis |
| Jackson et al., 2006, Canada [420] | NR / - | incidence peak: 40 years | - | LBP (no duration reported) | dysmenorrhea, dyspareunia, pelvic pain, pelvic mass, infertility, bowel and bladder symptoms | diagnostic methods: physical examination often unremarkable | often asymptomatic |
| Engemise et al., 2010, UK [421] | NR / - | - | - | LBP (29 %) (no duration reported) | dysmenorrhoea (40-87%), chronic pelvic pain (20-80%), deep dyspareunia (19-42%), bloating (42%), lethargy (40%), constipation (29%), dyschezia (13-30%), infertility (9%), cyclical rectal bleeding (9%), diarrhoea (8%), menorrhagia (7%), haematuria (3%) | - | clinical presentation varies considerably, significant proportion of those with endometriosis being asymptomatic and diagnosed incidentally |
| Al Khodairy et al., 2007, Switzerland [399] | NR / - | - | - | sciatica with or without LBP (no duration reported) | pain in the hip and the buttock radiating to leg and foot, onset few days before menstruation | - | delay until diagnosis: 4 months – 15 years; cyclic pattern of sciatic pain is highly suggestive of endometriosis |
| Young et al., 2015, Australia [400] | SR / - | - | - | back pain (no duration or localisation reported) (“crippling”, “contractions”, “horrific”, “sharp”, “stabbing”, “overwhelmed every other sense in your body”), associated with menstrual cycle, random pain, or continuous pain | pain in pelvis, bladder, bowel, gastrointestinal tracts, and joints, in association with intercourse | - | diagnostic delay more prevalent in primary care settings |
CCS case control study, CHS cohort study, CR case report, CS case series, ED emergency department, EM endometriosis, gynD other gynaecologic diseases, LBP low back pain, normPel normal pelvis, NR narrative review, SR sytematic review, y years
Miscellaneous
Six case reports and one narrative review encompassing various gynaecological diseases, such as benign cystadenoma [422], endosalpingiosis [423, 424], uterine fibroid [425], uterus-like structure of Müllerian origin [426], spinal intradural Müllerianosis [41], retroverted uterus, and tuboovarian abscess [399] associated with LBP, were identified (Table 23). The patients frequently presented with chronic radicular LBP, especially concurrent with the presence of tumorous tissue. Across all identified studies, notable findings during physical examinations included various neurological signs, e.g., decreasing muscular strength [426], tenderness on palpation [423], positive straight leg test [425], or sensory loss [426]. Therefore, when taking a patient’s history, more attention should be directed toward the presence of chronic LBP [41, 422–424, 426], occasionally exhibiting cyclical patterns [41, 426], primarily among pre-menopausal women [423, 425, 426]. Ahmad et al. [422] also reported abdominal swelling and pain, indicating that these aspects should also be included in history taking and physical examination as potential accompanying symptoms.
Table 23.
Miscellaneous gynaecological diseases presenting with low back pain
| Author / year / country | Design / setting | Diagnosis | Patient(s) | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation | Other information / comment |
|---|---|---|---|---|---|---|---|
| Ahmad et al., 2014, UK [422] | CR / ambulatory care | benign cystadenoma | female, 58 y | chronic LBP, weakness, paraesthesia, numbness | general abdominal swelling & pain | surgery and histological examination | - |
| Barresi et al., 2017, Italy [423] | CR / not reported | endosalpingiosis | female, 49 y | chronic LBP (radiating) | not reported | surgery and histological examination | - |
| Murphy et al., 2010, USA [425] | CR / ambulatory care | uterine fibroid | female, 44 y | acute LBP (radiating) | left leg pain | pelvic MRI | - |
| Sharma et al., 2007, India [426] | CR / not reported | tethered cord syndrome with a uterus-like structure of Müllerian origin | female, 24 y | chronic LBP (cyclical, radiating) | not reported | surgery and histological examination | - |
| Scheel et al., 2013, Germany [424] | CR / not reported | endosalpingiosis | female, 48 y | chronic LBP | not reported | surgery and histological examination | - |
| Barresi et al., 2006, Italy [41] | CR / not reported | spinal intradural Müllerianosis | female, 42 y | chronic LBP (in association with menstruation, worsened over time), exacerbated by sciatica mostly involving left lower limb | not reported | surgery and histological examination | - |
| Al Khodairy et al., 2007, Switzerland [399] | NR / - | retroverted uterus | female, 47 y | LBP (sciatic pain with L5 and S1 root involvement) (no duration reported) | - | - | - |
| tuboovarian abscess | female, 25 y | acute LBP (severe right sciatic pain, unilateral with LBP; after a fall 3 weeks before admission) | low grade fever | - | delay until diagnosis: 3 weeks | ||
| fibroids | 4 females, age: not reported | unilateral sciatic pain and LBP (no duration reported) | - | - | time / delay to diagnosis from immediate to 4.5 years |
CR case report, LBP low back pain, MRI magnetic resonance imaging, NR narrative review, y years
The inclusion of ovarian cancer invading the spine within the context of red flags remains uncertain. The Cochrane review addressing screening for malignancy in patients with LBP does not refer to ovarian cancer [36]. While this review omits all forms of cancers, it is important to note that LBP has been documented as a symptom in 23% of ovarian cancer cases [427].
Urological diseases
Urinary tract infection and pyelonephritis
Urinary tract infection (UTI) can cause inflammation in any part of the urogenital tract, such as in the kidney, bladder, ureters, or urethra. It commonly presents with symptoms like painful urination, urinary frequency, and sometimes fever [428]. While cystitis usually presents with exactly these symptoms, pyelonephritis is an inflammatory disease of the kidney, which can also present with LBP. It commonly occurs in middle aged and older women. Three case reports, and one case series describing acute LBP as initial clinical presentation of pyelonephritis were included. Additionally, one case report, one cross-sectional study, and two reviews have expanded upon UTI as a potential cause of LBP (Table 24). Notably, 23.3% of patients diagnosed with pyelonephritis report LBP [428], indicating it as a reliable predictor [429]. In a majority of cases, the LBP can be localised as either left- or right-sided [430]. While UTI can be a cause of LBP, it lacks a significant association [431]. On the other hand, LBP has been identified as a symptom associated with an increased probability of urinary tract infection [432]. This connection is especially pertinent in patients with neurogenic bladder and sensory deficits, where LBP can manifest as a non-specific symptom of UTI [433]. In women presenting with acute LBP alongside accompanying symptoms such as general fatigue, signs of poor health or fever, UTI should be considered as differential diagnosis. Other clinical indicators encompass prior sexual intercourse, recent utilisation of spermicidal products, asymptomatic bacteriuria, or previous history of cystitis [433].
Table 24.
Urinary tract infections and pyelonephritis presenting with low back pain
| Author / year / country | Design / setting | Patient(s) / diagnosis | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
|
Germani et al., 2002, Switzerland [434] |
CR / ED |
male, 52 y / pyelonephritis |
acute LBP, radiating | weight loss, malaise, fever | laboratory and abdominal / renal ultrasonography |
|
Nakamura et al., 2005, Japan [435] |
CR / hospital |
female, 84 y / pyelonephritis |
acute LBP | malaise, fever | laboratory and abdominal / renal ultrasonography |
| Roy et al., 2001, France [436] | CR / hospital | 1 male (72 y), 4 females (81 y, 47 y, 54 y, 65 y) / emphysematous pyelitis | LBP (no duration reported) | nausea, chills, fever | CT |
|
Derouiche et al., 2009, Tunisia [430] |
CR / hospital |
5 females, 1 male (mean age: 55 y) / pyelonephritis |
acute LBP (100%) | fever (83.3%), malaise (83.3%) | laboratory and abdominal / renal ultrasonography |
| Soler et al., 2015, Ireland [429] | CS / not reported | Malta: 9896 (age and sex not reported), the Netherlands: 15318 (age and sex not reported) / UTI | LBP (no duration reported) | flank / axilla symptom, dysuria, fever, abdominal pain / cramps, vomiting, urinary frequency or urgency, nausea | - |
|
Kinouani et al., 2017, France [428] |
CSS / hospital |
340 patients, patients with urine dipstick test: median age 44 y; patients without urine dipstick test: median age 57 y / UTI |
pyelonephritis: LBP (23.3%) (no duration reported) | cystitis: painful urination, urinary frequency; pyelonephritis: fever, painful urination, asthenia | - |
| MeReC Bulletin, 2006, UK [432] | NR / - | - | back pain (no duration or localisation reported) | dysuria, frequency, haematuria | - |
| Giesen et al., 2010, Ireland [431] | SR / - | - | back pain (no duration or localisation reported) | dysuria, frequency, fever, flank pain, haematuria, lower abdominal pain, nocturia, urgency, vaginal discharge | - |
CR case report, CS case study, CSS cross-sectional study, CT computed tomography, ED emergency department, LBP low back pain, NR narrative review, SR systematic review, y years
Urinary kidney stone and hydronephrosis
Urinary kidney stones can develop due to various factors, potentially resulting in complications like ureteral obstructions, which can lead to colic and subsequent hydronephrosis. Only one case report was identified, where acute LBP was the leading presenting complaint (Table 25). The individual had a history of urolithiasis a few years ago. Laboratory tests and pyelography were used for definitive diagnosis. The presence of urinary tract symptoms or a history of urolithiasis may serve as clinical clues in patients with acute LBP [437].
Table 25.
Urinary kidney stones and hydronephrosis causing low back pain
| Author / year / country | Design / setting | Patient / diagnosis | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Nakamura et al., 2002, Japan [437] | CR / ambulatory care |
female, 32 y / kidney stone |
acute LBP, right sided | cloudiness of urine | pyelography |
| Mantle et al., 2003, UK [26] | CR / not reported |
male, 47 y / hydronephrosis secondary to ureteral hernia |
chronic LBP with exacerbation, radiation to the left loin | - | CT |
| Tieppo Francio et al., 2018, USA [33] | CR / outpatient clinic |
male, 60 y / hydronephrosis |
chronic LBP with exacerbation, radicular, antalgic gait, paraesthesia, mild limp | - | CT |
CR case report, CT computed tomography, LBP low back pain, y years
Hydronephrosis is a disease characterised by renal pelvis expansion resulting from obstructed urinary outflow and subsequent retention. In most cases, it is caused by urinary kidney stones. Two case reports were identified, showing an association between LBP and hydronephrosis (Table 25). The reports detailed exacerbated and chronic LBP, accompanied by neurological symptoms like radicular pain, paraesthesia, and mild limping [33]. Otherwise, there were no other clinical signs or symptoms implicating hydronephrosis as an underlying cause of LBP. Diagnosis frequently occurs incidentally, prompted by imaging studies ordered for evaluation of neurological symptoms.
Prostatic diseases
Prostatic diseases (including prostatitis, prostatic calculi, and cysts) can also present with acute LBP. Five relevant case reports (Table 26) on the subject were identified. Prostatitis can manifest in young males, while prostatic calculi predominantly occur in older male individuals. Clinical clues that point to prostatic pathology as underlying cause for acute LBP are symptoms of urinary tract infections [438], fever, and occasional incontinence [439].
Table 26.
Prostatic diseases presenting with low back pain
| Author / year / country | Design / setting | Patient / diagnosis | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Bajaj et al., 2008, USA [439] | CR / hospital | male, 66 y / bilateral prostatitis | acute LBP | fever, chills, decreasing renal function, frequency, urgency, and occasional incontinence | SPECT-CT |
| Godara et al., 2003, India [438] | CR / not reported | male, 50 y / prostatic calculi | acute LBP | symptoms of urinary tract infection | surgery and histopathological examination |
| Qiu et al., 2018, China [440] | CR / hospital | male, 24 y / prostatic cyst | acute LBP, left-sided | - | abdominal ultrasonography |
| Rau et al., 2018, Switzerland [441] | CR / ambulatory care | male, 74 y / prostatitis | acute + chronic LBP, radiating in buttock, groins, and thighs; immobility | - | laboratory |
| Mateos et al., 2003, Spain [442] | CR / not reported | male, 57 y / prostatitis + orchiepididymitis | bilateral acute LBP | fever, chills, perineal pain | ultrasonography + scintigraphy |
CR case report, LBP low back pain, SPECT-CT single photon emission computed tomography – computed tomography, y years
Renal infarction
Renal infarction can result from an embolism entering the renal vein or artery. A case report documenting LBP as part of the clinical presentation was found (Table 27). Accompanying symptoms were vomiting and chest discomfort. There may be abnormalities seen in the blood test, such as elevated troponin I levels [443, 444].
Table 27.
Low back pain in association with renal infarction
| Author / year / country | Design / setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Cahill et al., 2012, UK [443] | CR / not reported | male, 44 y | acute LBP | vomiting, exertional chest discomfort | arterial phase contrast enhanced CT of abdomen |
CR case report, CT computed tomography, LBP low back pain, y years
Renal ischaemia
Renal ischaemia is a rare condition that can result from various causes, for example, exercising. A case report outlining acute LBP within the context of renal ischaemia was identified (Table 28). Additional symptoms encompassed nausea, vomiting, and abdominal pain [443, 444].
Table 28.
Low back pain in association with renal ischaemia
| Author / year / country | Design / setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Maekawa et al., 2017, Japan [444] | CR / ED | male, 26 y | acute LBP, myalgia in lower extremities | abdominal pain, severe nausea and vomiting, low grade fever | CT and renal biopsy |
CR case report, CT computed tomography, ED emergency department, LBP low back pain, y years
Infected kidney cysts
Kidney cysts, fluid-filled sacs found in kidneys, can vary in size and can be solitary or multiple. In total, three case reports documented instances of acute LBP associated with infected kidney cysts (Table 29). In patients presenting with acute LBP along with symptoms like fever and vomiting [445], kidney cysts should be considered as differential diagnosis. Furthermore, if the patient’s past medical history includes previous kidney diseases, this could serve as a valuable clinical clue.
Table 29.
Infected kidney cysts presenting with low back pain
| Author / year/ country | Design / setting | Patient | Spinal symptoms | Extraspinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|---|
| Ito et al., 2016, Japan [445] | CR / hospital | male, 58 y | acute LBP | fever, vomiting, and feeling of abdominal enlargement | blood culture and CT |
| Jensen et al., 2020, Denmark [446] | CR / hospital | female, 74 y | severe acute LBP (NRS: 9/10) | low grade fever | blood culture and PET/CT |
| Mandai et al., 2014, Japan [447] | CR / hospital | male, 48 y | acute LBP, right sided | fever, arthralgia, anorexia | MRI + laboratory |
CR case report, CT computed tomography, LBP low back pain, MRI magnetic resonance imaging, NRS numeric rating scale, PET positron emission tomography, y years
Low back pain
Multiple narrative reviews and one systematic review were identified (Table 30). Major differences emerged regarding the classification of LBP causes and terminology. The most commonly proposed classification systems are based on either mechanical/non-mechanical [3–6, 8, 10, 12, 448–450] or specific/non-specific [37, 451–454] causes of LBP. In certain instances, degenerative diseases [455, 456] or radiculopathy [12, 13, 16, 449, 453–455, 457–459] were also designated as major categories. Several classification systems further isolate extravertebral diseases as a distinct class [3–6, 8, 10, 12, 13, 16, 37, 448–450, 454–461]. Various terms have emerged to describe these extravertebral causes, such as visceral diseases, non-spinal diagnoses or aetiologies, medical causes, and referred pain. The lack of a clear definition of extravertebral LBP was also reflected in the listing of various diseases according to the classification system. For example, intestinal infections were included in non-mechanical LBP [4] despite explicit classification of them as an extravertebral cause. Many classification systems adopt the concept of red flags as indications of specific LBP, which generally do not explicitly cover extravertebral pathologies. The most frequently referenced publication regarding extravertebral pathologies was the work by Deyo and Weinstein in 2001 [3]. They estimated a prevalence of 2% for extravertebral LBP without specifying the clinical setting, such as ambulatory care versus emergency room, or providing a data source for this assumption. However, recent research studies have found disparities from this estimate with prevalences ranging from <1% [458] to 10% [8], which can be explained by the assortment of pathologies grouped within the extravertebral LBP category.
Table 30.
Publications of low back pain in general
| Author / year / country | Design | Classification | Extravertebral causes of LBP | Reported prevalence | Citation of Deyo and Weinstein 2001 |
|---|---|---|---|---|---|
| Deyo et al., 2001, USA [3] | NR | mechanical LBP and leg pain, nonmechanical spinal conditions, visceral disease | prostatitis, endometriosis, chronic pelvic inflammatory disease, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, cholecystitis, penetrating ulcer | mechanical: 97%, nonmechanical: 1%, visceral: 2% | - |
| Müller et al., 2001, Germany [4] | NR | mechanical, non-mechanical, visceral |
visceral: prostatitis, endometriosis, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, pancreas carcinoma, cholecystitis, penetrating ulcer; non-mechanical: epidural abscess, herpes zoster, intestinal infections |
2% | yes |
| Atlas et al., 2001, USA [448] | NR | mechanical LBP, nonmechanical LBP, visceral disease | prostatitis, endometriosis, chronic pelvic inflammatory disease, nephrolithiasis, pyelonephritis, perinephric abscess, AAA, aortoiliac disease, pancreatitis, cholecystitis, perforated bowel | not reported | no |
| Hicks et al., 2002, USA [5] | NR | mechanical diseases, nonmechanical spinal diseases, visceral diseases | nephrolithiasis, pyelonephritis, prostatitis, pelvic inflammatory disease, endometriosis, AAA | < 2% (caused by malignancy, infection, or visceral cause) | yes |
| Zimmermann et al., 2002, USA [462] | NR | “referred pain” | - | not reported | no |
| Jarvik et al., 2002, USA [6] | NR | mechanical LBP or leg pain, nonmechanical spinal conditions, visceral disease | pelvic organ involvement (prostatitis, endometriosis, chronic pelvic inflammatory disease), renal involvement (nephrolithiasis, pyelonephritis, perinephric abscess), aortic aneurysm, gastrointestinal involvement (pancreatitis, cholecystitis, penetrating ulcer) | 2% | yes |
| Carragee et al., 2004, USA [450] | NR | mechanical, nonmechanical, visceral disease | nephrolithiasis, prostatitis, pelvic inflammatory disease | 2% (malignancies, infection, visceral causes and other “red herrings”) | no |
| Devereaux et al., 2004, USA [7] | NR | not reported | dissecting aortic aneurysm, renal colic, tumours of multiple abdominal organs | not reported | yes |
| Stowell et al., 2005, Lebanon [463] | NR | not reported | AAA, gynaecological diseases (i.e., endometriosis, pelvic inflammatory disease, ovarian cyst), gastrointestinal infection (i.e., peritonitis, appendicitis, pancreatitis), renal disorders (i.e., nephrolithiasis, pyelonephritis, UTI), intestinal obstruction | not reported | no |
| Diamond et al., 2006, USA [8] | NR | mechanical LBP, nonmechanical LBP, visceral disease | prostatitis, endometriosis, chronic pelvic inflammatory disease, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, cholecystitis, penetrating ulcer | mechanical disorders: 90%, manifestation of systemic illness: 10% | yes |
| Winters et al., 2006, USA [9] | NR | not reported | cardiovascular, pulmonary, gastrointestinal, and urogenital system disease | not reported | yes |
| Klineberg et al., 2007, USA [464] | NR | not reported | dissecting aortic aneurysm, ectopic pregnancy, myocardial infarction, acute pancreatitis, duodenal ulcers, pyelonephritis, visceral trauma, cholecystolithiasis, endometriosis, fibroids, nephrolithiasis, pelvic inflammatory disease, pregnancy, prostatitis, UTI | not reported | no |
| Ponka et al., 2007, Canada [465] | NR | not reported | pelvic infection, nephrolithiasis, pancreatic disease and AAA, UTI, prostate cancer | not reported | no |
| Weiland et al., 2007, Germany [466] | NR | not reported | aortic bifurcation syndrome, aortic aneurysm, zoster radiculitis, nephrolithiasis, pancreatitis, ruptured aortic aneurysm | not reported | no |
| Kinkade et al., 2007, USA [10] | NR | mechanical LBP, nonmechanical spinal conditions, non-spinal / visceral disease | pelvic organs (prostatitis, pelvic inflammatory disease, endometriosis), renal organs (nephrolithiasis, pyelonephritis), aortic aneurysm, gastrointestinal system (pancreatitis, cholecystitis, peptic ulcer), shingles | 2% | yes |
| Graw et al., 2008, USA [460] | NR | spinal diagnoses, non-spinal diagnoses | neoplasm, infection, systemic medical conditions, intrapelvic, gynaecological conditions, renal disease, AAA, sacroiliac joint dysfunction, hip pathology, diabetic neuropathy | not reported | yes |
| Cohen et al., 2008, USA [449] | NR | mechanical, neurogenic, nonmechanical, referred visceral pain, other | gastrointestinal diseases (inflammatory bowel disease, pancreatitis, diverticulitis), renal disease (nephrolithiasis, pyelonephritis), abdominal aortic aneurysm | 1-2% | yes |
| Ludwig et al., 2010, Germany [461] | NR | vertebral & extravertebral causes of LBP | visceral diseases, gynaecological diseases, urologic diseases | not reported | not reported |
| Dagenais et al., 2010, USA [467] | SR | not reported, specific causes of LBP | aortic aneurysm, enteropathic disease, endocarditis, nephrolithiasis, or pancreatitis | not reported | no |
| Miura et al., 2011, Japan [11] | NR | not reported | aortic disease (dissection aortic aneurysm, true aortic aneurysm), oesophageal rupture, pancreatitis, cholecystitis and cholelithiasis, kidney and urinary tract disease | not reported | yes |
| Manusov et al., 2012, USA [12] | NR | mechanical, non-mechanical and visceral LBP; or: nonspecific LBP, radicular back pain and worrisome or medical red flags | prostatitis, endometriosis, chronic pelvic inflammatory disease, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, cholecystitis, penetrating ulcer | 2% | yes |
| Schulte-Mattler et al., 2013, Germany [456] | NR | idiopathic back pain, degenerative process of intervertebral discs or facet joints, mechanical spinal causes, nonmechanical spinal causes, visceral causes | prostatitis, endometriosis, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, cholecystitis, penetrating ulcer, pregnancy; non-mechanical spinal causes: epidural abscess, zoster | 2% | not reported |
| Amirdelfan et al., 2014, USA [459] | NR | structural aetiologies of LBP, neurogenic aetiologies of LBP, extraspinal aetiologies | rheumatological conditions (rheumatoid arthritis, ankylosing spondylitis, ossification of posterior longitudinal ligament, Paget’s disease, osteoporosis, Reiter's syndrome, psoriatic spondylitis, polymyalgia rheumatica); renal and urologic conditions (obstructive (stones, prostate), inflammatory, neoplastic, infectious (pyelonephritis)); gastrointestinal conditions (ischaemic bowel disease, motility disorders, gastrointestinal inflammation, gastrointestinal ulceration and perforation, gallbladder disease, diverticulitis, appendicitis (retrocecal), pancreatitis); pelvic and gynaecological disorders (pregnancy, menstruation, ovarian cysts, endometriosis, uterine fibroids, pelvic adhesions, ectopic pregnancy, foreign body, inflammatory pelvic disorders); cardiovascular disorders (vascular insufficiency, superior mesenteric artery syndrome, renal artery stenosis, venous obstruction, AAA, endocarditis, aortoiliac disease); infection (discitis, osteomyelitis, Pott's disease, postherpetic neuralgia); neoplasm (neurospinal aetiology, abdominal aetiology, metastatic); psychological (hypochondria, somatoform disorder, factitious disorder, secondary gain issues, malingering) | not reported | no |
| Golob et al., 2014, USA [13] | NR | nonspecific “mechanical”, back pain with lower extremity symptoms, systemic and visceral diseases | prostatitis, endometriosis, chronic pelvic inflammatory disease, nephrolithiasis, pyelonephritis, perinephric abscess, aortic aneurysm, pancreatitis, cholecystitis, penetrating ulcer | serious systemic pathologic condition: 5% | yes |
| Jones et al., 2014, UK [457] | NR | simple mechanical LBP, LBP with radiculopathy, serious pathological LBP, visceral disease masquerading as spine pathology | AAA, duodenal ulceration, cholecystolithiasis, nephrolithiasis, prostatitis, UTI, fibroids | 2% | no |
| Melcher et al., 2014, Germany [452] | continuous medical education | nonspecific (includes non-vertebral), specific | AAA, iliac artery occlusion, pancreatitis, urolithiasis, pyelonephritis, psoas abscess, psoas haematoma, pelvic mass, fracture of the femoral neck, coxitis, trochanteric bursitis, piriformis syndrome, polyneuropathy | not reported | no |
| Patrick et al., 2014, USA [453] | NR | nonspecific LBP, back pain associated with radiculopathy or spinal stenosis, back pain associated with a specific spinal cause | nephrolithiasis, pyelonephritis, prostatitis, endometriosis, ovarian cysts, esophagitis, gastric and peptic ulcer disease, cholelithiasis and cholecystitis, pancreatitis, diverticulitis, other intra-abdominal infection, abdominal or thoracic aortic aneurysm, cardiac ischaemia or myocardial infarction, intramedullary spinal cord tumours | not reported | no |
| Chou et al., 2014, USA [454] | NR | nonspecific LBP, back pain potentially associated with radiculopathy or spinal stenosis, back pain potentially associated with another specific systemic or spinal cause | intra-abdominal visceral disease: gastrointestinal (peptic ulcer or pancreatitis), genitourinary (nephrolithiasis, pyelonephritis, prostatitis, pelvic infection, or tumour), vascular (aortic dissection) | not reported | no |
| Hooten et al., 2015, USA [14] | NR | medical (non-musculoskeletal), musculoskeletal | neoplastic (metastatic carcinoma, multiple myeloma, lymphoma, leukaemia, spinal cord tumours), inflammatory (ankylosing spondylitis, psoriatic spondylitis, rheumatoid arthritis, Reiter’s syndrome, enteropathic spondylitis), visceral (endometriosis, prostatitis, nephrolithiasis, aortic aneurysm, pancreatitis), infectious (osteomyelitis, epidural abscess, discitis, herpes zoster, pyelonephritis), vascular (aortic aneurysm, aortic dissection, spinal haemangioma, inferior vena cava obstruction, sickle cell crisis), endocrine (osteoporotic fracture, Paget’s disease), traumatic (vertebral fracture, rib fracture, pelvic fracture, hip fracture) | not reported | yes |
| Selkirk et al., 2016, USA [15] | NR | mechanical back pain, nonspecific LBP, referred pain |
prostatitis, pancreatic disease, gallbladder disease, aortic aneurysm, pyelonephritis; infectious causes: epidural abscess, paraspinous abscess, herpes zoster, varicella zoster; causes of radiculopathy and radicular pain: piriformis syndrome, diabetic amyotrophy |
not reported | yes |
| Singleton et al., 2016, USA [16] | NR | benign, self-limited musculoskeletal causes, spinal pathologies that can cause severe neurologic disability, other abdominal or retroperitoneal processes that can present with back pain | aortic diseases (aneurysm, dissection, ulceration and aortitis), genitourinary disease (ureteral colic, renal infarction and tumour, prostatitis), gastrointestinal causes (pancreatitis and pancreatic cancer, penetrating peptic ulcer, cholecystitis and cholangitis), retroperitoneal haematoma, systemic infections including endocarditis, psoas abscess and other localized abscess | not reported | yes |
| Verhagen et al., 2016, The Netherlands [468] | NR | not reported | aneurysm | not reported | no |
| Maher et al., 2017, Australia [451] | NR | specific, non-specific LBP | - | 2% | yes |
| Vlaeyen et al., 2018, Belgium [458] | NR | visceral disorder, specific spinal disease, radicular syndromes or nonspecific LBP | - | < 1% (caused by visceral or spinal disease) | yes |
| Chenot, 2018, Germany [37] | NR | specific, non-specific, extravertebral | cardiac ischaemia, aortic aneurysm, pyelonephritis, nephrolithiasis, ischaemia / thrombosis of intraabdominal organs, iliosacral joint discomfort, gynaecological / urological diseases, cholelithiasis, pancreatitis, penetrating ulcer, nephrolithiasis | 2% | no |
| Reith et al., 2020, Germany [455] | NR | unspecific / degenerative, radicular, acute vertebral / extravertebral | - | unspecific degenerative: 85-90%, radicular: 1-2%, acute vertebral / extravertebral: <10% | no |
AAA abdominal aortic aneurysm, LBP low back pain, NR narrative review, SR systematic review, UTI urinary tract infection
Solely a singular case series involving 95 patients (34.7% female) from Japan presenting themselves at an emergency department (ED) with LBP [469] was identified. Within this group, a total of 66.2% were diagnosed to have a urological disease. Other reported disorders were vascular diseases like AAA or aortic dissection and pancreatitis. In most cases the diagnosis was confirmed by using a CT. However, given that primary care services in Japan are frequently attached to hospitals, the generalisability of this finding is limited.
Miscellaneous
Spinal epidural lipomatosis
Spinal epidural lipomatosis is a rare condition characterised by excessive proliferation of adipose tissue within the epidural space leading to spinal canal stenosis. In total, nine case reports, one case series, one cohort study and one narrative review reporting LBP associated with spinal epidural lipomatosis were identified (Table 31). Predominantly, this condition manifested in middle-aged and older male individuals. An association with elevated body mass index (BMI), previous treatment with corticosteroids or other endocrinological disorders, e.g., Cushing syndrome, is postulated [470]. Due to neurological symptoms, such as limb weakness or numbness [470–473], an MRI was usually performed, ultimately confirming the diagnosis.
Table 31.
Spinal epidural lipomatosis presenting with low back paina
| Author / year / country | Design / setting | Patient(s) | Spinal symptoms | Diagnostic confirmation |
|---|---|---|---|---|
| Chan et al., 2009, Taiwan [471] | CR / hospital outpatient depart-ment | male, 56 y, BMI: 24.1 kg/m2 | chronic LBP (sustained, worsening), left leg weakness and numbness, inability to walk long distances | MRI |
| male, 44 y, BMI 23.4 kg/m2 | chronic LBP (progressive worsening, inability to walk for long distances), left extremity weakness and numbness | MRI | ||
| Duran et al., 2016, Turkey [474] | CR / not reported | female, 46 y, BMI: 32 kg/m2 | chronic LBP (exacerbating over three months, deep, sharp), radicular pain in the proximal leg | MRI |
| Min et al., 2007, South Korea [475] | CR / not reported | male, 70 y, BMI: normal | chronic LBP (VAS 8/10, aggravating by standing and walking, relieved by sitting and leaning forward during walking), radiating to both lower limbs. | MRI |
| McCormick et al., 2014, USA [476] | CR / hospital | male, 79 y, BMI: 42.1 kg/m2 | chronic LBP (progressive, VAS 8/10, stabbing, relieved by sustained lumbar flexion positions and exacerbated by walking or standing), radiation to the right anterolateral thigh | MRI |
| male, 47 y, BMI: 32.5 kg/m2 | chronic LBP (VAS 9/10, burning and tingling, exacerbated by sitting, standing, and walking), radiation to anterolateral thigh and lateral posterior leg | MRI | ||
| male, 50 y, BMI: 48 kg/m2 | acute LBP (severe, VAS 10/10, achy and numb, began upon standing from a seated position, patient felt a “pop”, exacerbated by standing, walking, and ascending stairs) | MRI | ||
| Al-Khawaja et al., 2008, Australia [473] | CR / not reported | male, 68 y, BMI: not reported | chronic LBP (aggravating by standing longer than 10 minutes or walking greater than 200 m), claudication right anterior thigh and lateral lower leg | MRI |
| Botwin et al., 2004, USA [477] | CR / not reported | female, 78 y, BMI: 28.2 kg/m2 | chronic LBP (VAS 8/10, aggravated by standing and walking, relieved by sitting and leaning forward), radiation to the left lower limb | MRI |
| male, 68 y, BMI: 32.6 kg/m2 | chronic LBP (VAS 8/10, aggravated by standing and walking, relieved by sitting and leaning forward during walking), radiation to the right lower limb | MRI | ||
| Choi et al., 2012, Korea [478] | CR / not reported | male, 67 y, BMI: 25.5 kg/m2 | back pain (no duration or localisation reported), leg tightness in both legs, neurogenic claudication | MRI, MR myelogram |
| Lisai et al., 2001, Italy [479] | CR / not reported | male, 67 y, BMI: not reported | chronic LBP, bilateral pain radiating to his feet | CT / MRI |
| male, 54 y, BMI: not reported | chronic LBP, radiating pain in both legs and intermittent sacral nerve dysfunction including penile erection, incontinence, perineal pain | MRI | ||
| Maillot et al., 2006, France [480] | CR / hospital | male, 63 y, BMI: 32.6 kg/m2 | chronic LBP (diurnal on standing and walking, walking limited to 200 m), pain in lower limbs, radicular pain in the left calf | CT myelography |
| Borré et al., 2003, Argentina [470] | CS / hospital | 2528 (57% female), mean age: 47.3 y | non-specific LBP (no duration reported) (LEL grade I: 0%; LEL grade II: up to 14.5%; LEL grade III: up to 100%); radicular pain, numbness and dysaesthesias, neurogenic claudication, cauda equina syndrome; LEL grade I: no symptomatic patients; LEL grade II: 14.5% were symptomatic with weakness and / or dysaesthesia (no other substantial pathologic findings in MRIs); LEL grade III: 100% were symptomatic, 42.3% showed other pathological findings | - |
| Ishikawa et al., 2006, Japan [472] | CHS / hospital | 7 (29% female), mean age: 65.7 y, mean BMI: 27.1 kg/m2 | LBP (queried) (no duration reported), neurological deficits in the lower extremities, with intermittent claudication as seen in cauda equina syndrome | - |
| Al-Khawaja et al., 2008, Australia [473] | NR / - |
111 cases: 44% idiopathic: 10% female, 70% obese, mean age: 46 y 56% secondary: 20% female, 70% obese, mean age: 44 y |
idiopathic lumbar lipomatosis: 65% back (no duration or localisation reported) or leg pain, 30% leg weakness, 30% claudication; secondary lumbar lipomatosis: 50% back (no duration or localisation reported) or leg pain, 25% leg weakness, 10% claudication |
- |
ain all publications no extraspinal symptoms were reported
BMI body mass index, CHS cohort study, CR case report, CS case series, CT computed tomography, LBP low back pain, LEL lumbosacral epidural lipomatosis, MR magnetic resonance, MRI magnetic resonance imaging, NR narrative review, VAS visual analogue scale, y years
Episacral lipoma – Back mice
Back mice are subfascial fat herniations in the back. They are often accompanied by painful swellings, but one case report showed that they can also cause LBP (Table 32). Ultrasound examination is used to confirm the diagnosis.
Table 32.
Case reports of back mice presenting with low back pain
| Author / year / country | Setting | Patient | Symptoms | Diagnostic confirmation |
|---|---|---|---|---|
| Tiegs-Heiden et al., 2018, USA [481] | hospital | female, 47 y | chronic LBP (intermittent, lasting about a week at a time and then remitting), palpable mass in the right lower back | ultrasound |
LBP low back pain, y years
Hip pathology
Hip and lumbar spine pathologies often occur in combination and may be difficult to separate [482–484]. Pathologies of the lower back, e.g. in the iliosacral joint, or radicular symptoms can present predominantly with hip pain or pain in the thigh. Hip pain was frequently reported in the case reports reviewed, e.g. in systemic diseases affecting the hip and facet joints [88, 91, 92], but it was also part of the presentation in cases involving other organs [23, 380]. On the other hand, hip pathology can lead to LBP. An observational study of 25 patients (32-84 years) with hip and spinal pain showed improvement of back pain following total hip replacement [482]. Limited range of motion of the hip has been observed in patients with chronic LBP compared to healthy individuals, with improvement noted following hip exercise [485]. This is in line with other studies and a recent systematic review, despite low certainty evidence that hip strenghtening can improve LBP [486]. Examination of the hip (forced internal rotation) should be part of the clinical examination in patients presenting with pain radiating into the thigh.
Summary of clinical clues for the diagnosis of extravertebral LBP (Table 33)
Table 33.
Summary of clinical clues for the diagnosis of extravertebral low back pain
| Disease | Patient characteristics | Acute / chronic LBP | Clinical clue / symptoms | Diagnostic |
|---|---|---|---|---|
| Systemic diseases | ||||
| Gout | male > female, older age | acute and chronic | h/o gout, tophaceous gout | dual energy CT |
| Pseudogout | older age | mostly subacute or chronic | - | biopsy during surgery |
| Skeletal fluorosis | endemic environmental, industrial and accidental exposure to fluoride | chronic, progressive | neurological manifestation (e.g., paraparesis), mottling teeth | x-ray |
| Spinal sarcoidosis | middle age | chronic | h/o sarcoidosis | MRI, bone biopsy |
| Hyperparathyroidism | female > male | chronic | constitutional symptoms (e.g., weakness), prior history of urolithiasis and ESKD | laboratory assessment, surgery / biopsy |
| Vitamin D deficiency / insufficiency | female > male, older age (postmenopausal) | chronic | - | laboratory assessment |
| Ochronosis / alkaptonuria | male > female, middle age | chronic | pigmentation of the sclera and ear and darkening of morning urine, intervertebral disc calcification on imaging | laboratory assessment (measurement of homogentisic acid in the urine) |
| Arterial diseases | ||||
| Abdominal aortic aneurysm | middle aged and older, male, cardiovascular risk factors | chronic > acute (depending on type) | pulsating abdominal mass, abdominal pain, progressive exacerbation of symptoms | ultrasound, confirmation: CT, MRI |
| Acute aortic syndrome (including aortic dissection, intramural haematoma and penetrating aortic ulcer) | middle aged, male | acute | chest discomfort, abdominal pain, nausea / vomiting, dyspnoea | CTA |
| Fistula (including aorto-enteric, aorto-venous and, aorto-caval fistula) | middle-aged to older, male | acute and chronic | depending on affected structures: abdominal pain, vomiting, neurological symptoms | CT / MRI |
| Peripheral arterial disease | male | acute and chronic | leg pain | vascular examination, Doppler |
| Venous diseases | ||||
| Deep vein thrombosis | younger age | rather acute | leg swelling, radicular symptoms | ultrasound, CT / MRI |
| Varicosis (epidural, intradural, gluteal) | female > male, any age | acute and chronic | leg pain, neurological symptoms (e.g., paresis) | MRI |
| Paraspinal haematoma | ||||
| Subdural, epidural, subarachnoid, other localisations | male > female, middle aged to older age | acute | sudden onset paraparesis, symptoms suggestive of cauda equina syndrome (urinary and faecal incontinence), oral anticoagulation | MRI |
| Chronic periaortitis | ||||
| Retroperitoneal fibrosis | male, age: 40-60 years | acute and chronic | constitutional symptoms, symptoms of ureteral obstruction, autoimmune or IgG4-related diseases in patients’ history | CT / MRI + biopsy |
| Inflammatory abdominal aortic aneurysm | 5-10 years younger than patients with AAA | chronic | constitutional symptoms (e.g., fever), abdominal pulsating mass, abdominal pain | CT + biopsy |
| Cardiological diseases | ||||
| Myocardial infarction | middle aged to older | acute | chest pain, shock symptoms | CT |
| Gastroenterological diseases | ||||
| Gallstone disease / cholecystitis | female > male | acute and chronic | upper abdominal pain | ultrasound, MRI / MRCP |
| Pancreatitis | - | acute | abdominal pain, appetite loss, jaundice | ERC / biopsy |
| Paraspinal compartment syndrome | ||||
| - | male > female, younger or middle aged | acute | heavy exercise | MRI, pressure measurement |
| Gynaecological diseases | ||||
| Endometriosis | women of childbearing age | rather chronic | dyspareunia, dysmenorrhoea, cyclical pain | referral to gynaecologist |
| Urological diseases | ||||
| UTI / pyelonephritis | female > male, increasing with age | rather acute | malaise, fever | urinary and / or blood culture |
| Urinary kidney stone / hydronephrosis | male > female, middle aged | acute or exacerbation | haematuria | urine dipstick and ultrasound |
| Prostatic disease | male, any age | rather acute | urgency, fever | urine dipstick, referral to urologist |
| Renal infarction/renal ischaemia | male > female, younger or middle aged | rather acute | abdominal pain, nausea and vomiting | ultrasound, CT |
AAA abdominal aortic aneurysm, CT computed tomography, CTA computed tomography angiography, ERC endoscopic retrograde cholangiography, ESKD end stage kidney disease, h/o history of, IgG4 immunoglobulin G4, MRCP magnetic resonance cholangiopancreatography, MRI magnetic resonance imaging, UTI urinary tract infection
Discussion
Summary of evidence
It can be difficult to distinguish clinically between vertebral and extravertebral causes of LBP and there is limited research to date. This is further complicated by the wide range of differential diagnoses and the rare incidence of extravertebral disorders that mimic LBP. This scoping review attempts to provide a comprehensive overview of the aetiologies underlying extravertebral LBP, with a particular focus on identifying symptoms indicative of extravertebral pathology. The available body of evidence, largely derived from case reports and retrospective cohort studies, does not allow epidemiological conclusions to be drawn regarding the prevalence of extravertebral LBP. The diagnosis of extravertebral pathology is frequently made incidentally by imaging or intraoperatively. However, as summarised in Table 33, this review has identified clinical signs and symptoms that may facilitate the identification of specific aetiologies of extravertebral LBP.
Interpretation of the results
The large number of case reports highlights the clinical relevance of individuals presenting with LBP ultimately attributed to extravertebral causes. However, the clinical relevance of these reports varies widely, ranging from life-threatening conditions to those of lesser clinical consequence. This variability is not surprising, given the diverse range of extravertebral causes for LBP. Accurately estimating the prevalence of extravertebral LBP proves challenging and most likely depends on the clinical setting. It is reasonable to hypothesise that the prevalence is lower in the primary care setting. Deyo, without providing a specific source, estimated the prevalence at 2 % in primary care [3], a figure that has subsequently been cited in numerous LBP reviews [4, 7–11, 13, 14, 16, 449, 451, 458, 460, 487], albeit this has never been confirmed in an epidemiological study.
Many case reports lack details regarding the clinical setting, but it is reasonable to assume that they predominantly originate from specialist clinics or hospitals, as these comprise the majority of reports. Unfortunately, only a limited number of reports indicated whether patients were referred from primary care and the specific reasons for their referral [54, 137, 211, 229, 263, 274, 279, 297, 374]. It has been suggested that non-mechanical back pain may indicate the possibility of extravertebral LBP [3, 464]. Although this assumption seems plausible, there is currently a lack of empirical evidence to substantiate it. This is consistent with the situation of the “red flags” for specific low back pain, which similarly also lack a solid epidemiological foundation [77, 468, 488].
Retrospective case series focussing on specific pathologies often demonstrate LBP as part of the clinical presentation, as seen, for example, in endometriosis [416, 417] or retroperitoneal fibrosis [294, 300, 304, 320, 322, 323, 326, 327, 330, 333, 334, 353, 361, 362]. The practical utility of these observational studies for the clinician, who frequently encounter LBP, is limited due to the relative rarity of these pathologies in patients presenting with LBP as leading symptom. However, the definitive causal relationship with LBP remains uncertain, and the possibility of coincidence must be considered. Nonetheless, when patients present with an unusual combination of symptoms that deviate from the usual clinical picture, consideration of extravertebral pathologies becomes important. Certain extravertebral pathologies are less likely overlooked, as they present with symptoms such as paraplegia [171, 183, 188, 230, 247, 281], and always warrant advanced imaging or surgical exploration, ultimately leading to the correct diagnosis.
Case reports frequently conclude that especially primary care providers should remain vigilant for specific pathologies in patients presenting with LBP. However, given that LBP is a common reason for consultation and the majority of patients with low back pain do not exhibit serious underlying vertebral or extravertebral pathology, this approach appears impractical. The extensive examinations and test required to cover all potential differentials would exceed what is feasible and reasonable. Therefore, a primary care strategy of watchful waiting seems appropriate in the absence of serious symptoms necessitating urgent investigation.
This scoping review identified two categories of extravertebral back pain, classifying them based on symptoms that led to incidental diagnoses or cases with symptoms prompting clinical suspicion of non-vertebral pathology, such as a pulsatile swelling and AAA [101, 102, 104, 106–110, 115, 127, 131, 147, 149]. This differentiation holds forensic implications when patients litigate against health professional for misdiagnosis. Negligence can only be assumed if the clinical presentation rendered an extravertebral cause of LBP reasonably probable.
The majority of clinical guidelines [1] did not explicitly recommend considering extravertebral causes in the evaluation of low back pain. Based on the findings of this scoping review, it is recommended that the clinical assessment of LBP should incorporate a brief consideration of possible extravertebral causes as a measure to improve patient safety.
Comparison with existing literature
There is limited comprehensive literature on the topic, with the majority of reviews on red flags focussing on spinal pathologies, neglecting extraspinal pathologies [36, 468, 487, 488]. Reviews addressing extravertebral causes tend to be narrative in nature, lacking a systematic literature search dedicated to extravertebral causes [3, 7–11, 13, 14, 37, 449, 451, 458, 460]. Siddiq et al. conducted a focused systematic review on differential diagnosis of sciatica, emphasising musculoskeletal causes, which incidentally identified a few extraspinal causes of sciatica [489]. Maselli et al. provided a more targeted review of red flags in thoracolumbar pain, highlighting myocardial infarction, reflux, and pulmonary disease as important differential diagnosis [35]. Our review, focused on lumbar pain, excluded thoracic pain and treatment complications. The clinical clues for extravertebral LBP identified in our review (Table 33) exhibit limited reliability, akin to “red flags” for spinal pathologies [35, 36, 468, 487, 488].
Strength and limitations
To the best of current knowledge, this scoping review is the first attempt to comprehensively evaluate extravertebral LBP and provide an overview of associated clinical clues (Table 33). A detailed review addressing extravertebral causes that mimic radicular pain, encompassing infectious pathologies and post-injection complications, has already been published and was consequently excluded from this scoping review [489].
Limitations of the review
Clear decisions regarding the inclusion or exclusion of case reports often presented challenges, particularly in distinguishing between “red flag” pathologies and extravertebral diseases. Furthermore, the pragmatic decision to exclude cancers, metastases, and benign tumours from this review was made due to the extensive spectrum of pathologies involved.
Determining whether LBP was the primary complaint in the clinical presentation and differentiating it from related neurological diseases and leg pain was often impossible based on the publications. Despite the focus on low back pain, precise descriptions of the localisation of back pain were frequently absent. Furthermore, given low back pain’s high prevalence and chronic nature, it was not always feasible to differentiate between LBP as an independent condition or as part of the clinical manifestation of an underlying disease. As a result, some of the exclusion decisions may seem arbitrary. Moreover, not all reports provided details on the outcomes following specific interventions.
Complications from prior surgeries, pharmaceutical treatments, invasive or local procedures associated with LBP were excluded, as it was assumed that clinicians assessing the presenting complaint would consider these aspects. It is worth noting that this review’s search was limited to English and German only, therefore potentially introducing language bias. However, we assume that missing some case reports or case series published in other languages would have minimal impact on the scope of the review. Including those case reports and case series would not allow for a better inference on the epidemiology of extravertebral LBP.
Limitations of the studies
In numerous studies, LBP was mentioned as part of the clinical presentation; however, it was not consistently stated how the reported presence of LBP related to accompanying symptoms. The description of back pain was often inadequate in terms of location (lumbar versus thoracic or cervical pain), duration, and other important clinical circumstances, such as movement-related (mechanical) pain. Although a standard for reporting case reports has been issued [490], we did not attempt a formal quality assessment of the case reports or case series, since the majority of them did not meet the standard set and many of them where published before the CARE guidelines where established.
Some retrospective cohort studies examined the presence of LBP in patients with an established diagnosis, e.g., aortic dissection. From the perspective of clinicians evaluating patients with LBP, this information is of limited value given the epidemiological aspects of LBP. No prospective studies investigating the epidemiology of extra-vertebral pathologies presenting with LBP were found.
Conclusion
The differential diagnosis of extravertebral LBP is extensive. However, it is reasonable to assume that the prevalence is relatively low and varies depending on the clinical setting. It is essential that a distinction is made between two categories of extravertebral LBP: cases where clinical presentation indicates a likely extravertebral cause, and those where extravertebral LBP is diagnosed incidentally, such as through advanced imaging or intraoperatively.
Implication for practice
Extravertebral LBP is often diagnosed incidentally in the absence of symptoms indicative of extravertebral pathologies. However, this review identified symptoms suggestive of possible extravertebral LBP, yet the association is predominantly weak and lacks reliable quantification. Clinicians should therefore consider potential extravertebral causes when evaluating patients with LBP, particularly in instances where LBP appears in combination with atypical symptoms such as abdominal pain or leg swelling, or in patients with demographic characteristics (age and sex) predisposing to specific pathologies.
Implication for research
Given the diverse aetiology and rarity of extravertebral LBP, it is unlikely that more reliable data on its prevalence and presentation will emerge, particularly in primary care settings. In such settings, the prevalence of serious spinal pathologies is already low, and that of extravertebral ones is likely even lower. Therefore, specific settings within specialist care may be more conducive to systematic evaluations for extravertebral LBP. Prospective studies should prioritise reporting on non-spinal pathologies presenting with low back pain. Additionally, case reports and case series should offer a more comprehensive basis for investigating LBP. Moreover, more focused reviews targeting specific pathologies could enhance guidance for clinicians in identifying when to suspect a particular pathology.
Implication for clinical guideline makers
Guidelines on low back pain should address extravertebral causes of LBP beyond the conventional spinal pathologies typically highlighted by “red flags”. Moreover, achieving consensus on the terminology used to denote such causes is imperative. The integration of an evaluative step within the treatment algorithm of LBP guidelines, tailored to assess the potential for extravertebral LBP, could help to improve recognition and management for these conditions.
Acknowledgements
Not applicable.
Abbreviations
- 25-OH
25-Hydroxy
- AAA
Abdominal aortic aneurysm
- AAD
Acute aortic dissection
- AAS
Acute aortic syndrome
- ACF
Aorto-caval fistula
- AD
Aortic dissection
- ADF
Aorto-duodenal fistula
- AIP
Autoimmune pancreatitis
- ANA
Antinuclear antibody
- AV
Arterio-venous
- AVM
Arterio-venous malformation
- BMI
Body mass index
- BP
Blood pressure
- CADASIL
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukencephalopathy
- CARASIL
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukencephalopathy
- CCS
Case-control study
- CE-CT
Contrast enhanced computed tomography
- CHF
Congestive heart failure
- CHS
Cohort study
- CK
Creatine kinase
- COPD
Chronic obstructive pulmonary disease
- CR
Case report
- CRP
C-reactive protein
- CS
Case series
- CSS
Cross-sectional study
- CT
Computed tomography
- CTA
Computed tomography angiography
- DDx
Differential diagnosis
- DSA
Digital subtraction angiography
- DVT
Deep venous thrombosis
- e.g.
Exempli gratia
- ECG
Electrocardiogram
- ED
Emergency department
- EM
Patients with endometriosis
- ERC
Endoscopic retrograde cholangiography
- ERCP
Endoscopic retrograde cholangiopancreatography
- ESKD
End stage kidney disease
- ESR
Erythrocyte sedimentation rate
- f
Female
- fDDx
Part of first differential diagnosis
- FDG
Fluorodeoxyglucose
- FDG-PET
Fluorodeoxyglucose-positron emission tomography
- Ga-67
Gallium-67
- GI
Gastrointestinal
- GNE
Glucosamine (UDP-N-acetyl)-2-epimerase/N-acetylmannosamine kinase gene
- GP
General practitioner
- gynD
Gynaecologic diseases
- h/o
History of
- HLA-DR
Human leukocyte antigen – DR isotype
- IAAA
Inflammatory abdominal aortic aneurysm
- IgG4
Immunoglobulin G4
- IL-6
Interleukin 6
- IMH
Intramural haematoma
- IRAD
International Registry of Aortic Dissection
- IRF
Idiopathic retroperitoneal fibrosis
- IVC
Inferior vena cava
- IVU
Intravenous urogram
- LBP
Low back pain
- LEL
Lumbosacral epidural lipomatosis
- LOC
Loss of consciousness
- LP
Lumbar puncture
- m
Male
- marf
Marfan
- MeSH
Medical Subject Headings
- MR
Magnetic resonance
- MRA
Magnetic resonance angiography
- MRCP
Magnetic resonance cholangiopancreatography
- MRI
Magnetic resonance imaging
- msCT
multi-slice computed tomography
- nfDDx
Not part of first differential diagnosis
- nmarf
Non-Marfan
- NR
Narrative review
- NRS
Numeric rating scale
- NSAID
Non-steroidal anti-inflammatory drugs
- NSAR
Non-steroidal anti-rheumatic drug
- normPel
Normal pelvis
- PAU
Penetrating aortic ulcer
- PET
Positron emission tomography
- pf
Painful
- pl
Painless
- PTGBA
Percutaneous transhepatic gallbladder aspiration
- PTGBD
Percutaneous transhepatic gallbladder drainage
- RCT
Randomised controlled trial
- RPF
Retroperitoneal fibrosis, retroperitoneal fibrosis
- SAH
Subarachnoid haemorrhage
- Sino-RAD
Registry of Aortic Dissection in China
- SLE
Systemic lupus erythematosus
- SPECT-CT
Single photon emission tomography - computed tomography
- SR
Systematic review
- STEMI
ST-elevation myocardial infarction
- Tc-99m MAG3-scintigraphy
Technetium-99m mercaptoacetyltriglycine scintigraphy
- TCC
Transitional cell carcinoma
- TEE
Transoesophageal echocardiogram
- TLOC
Transient loss of consciousness
- TNF
Tumor necrosis factor
- TTE
Transthoracic echocardiogram
- UDT
Urine dipstick test
- US
Ultrasonography
- UTI
Urinary tract infection
- VAS
Visual analog scale
- vit
Vitamin
- w
With
- wo
Without
- y
Years
Appendix 1
Table 34.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist
| Section | Item | Prisma-scr checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 2-3 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 3-4 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 4 |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | Not applicable. PROSPERO does not register scoping reviews |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 5 |
| Information sources* | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 5 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 86-87 |
| Selection of sources of evidence† | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 5 |
| Data charting process‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 6 |
| Critical appraisal of individual sources of evidence§ | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | Most case reports were published before CARE was released |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | Not applicable |
| Results | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 5 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | Not applicable |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | Not applicable |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 9-40 (+ tables page 90-128) |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 40-42 |
| Discussion | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 42-44 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 45-47 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 47-48 |
| Funding | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 49 |
Appendix 2
Table 35.
MeSH-terms for MEDLINE, Embase and Cochrane Library
| MeSH-Terms MEDLINE and Embase |
|---|
| uncommon OR unusual OR rare OR medical condition AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| Differential Diagnos* AND low* AND Back Pain NOT joint NOT osteoporos* NOT fract* NOT disc* NOT spondyl* NOT spinal stenosis NOT radic* NOT musc* NOT child* NOT vertebroplast* NOT coccydynia |
| Prostatitis OR Endometriosis OR Extrauterine pregnancy OR Ovarian carcinoma OR Hematocolpos AND low* AND Back Pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| Chronic pelvic inflammatory disease OR Nephrolithiasis OR Pyelonephritis OR Perinephric Abscess OR Renal Ischemia OR Renal Vein Thrombosis OR Kidney cell Carcinoma AND low* AND Back Pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| Aortic aneurysm OR Myocardial infarction OR Pulmonary embolism OR Aortic dissection OR Aortic rupture OR Vena cava thrombosis OR Lung Cancer AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| Pancreatitis OR Cholecystitis OR Penetrating ulcer OR Intestinal ischemia OR Thrombosis of intraabdominal organs OR Hepatic vein Thrombosis OR Duodenal ulcer OR Gall stones OR Retroperitoneal fibrosis AND low* AND Back Pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| Lymphoma OR Gout OR Ochronosis OR Hypovitaminosis OR Vitamin D Deficiency OR hyperparathyroidism OR Hypercortisolism OR Neuroschistosomiasis AND low* AND Back Pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| atypical AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| medical condition* AND cause* AND acute back pain NOT child* NOT vertebroplast* NOT coccydynia |
| cancer* AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| infect* AND fever* AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| metabol* disease AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| genet* disease AND cause* AND low* AND back pain NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| unusual OR uncommon OR rare OR medical condition AND cause* AND lumbar AND radiculopathy NOT fract* NOT child* NOT vertebroplast* NOT coccydynia |
| MeSH-Terms Cochrane |
| uncommon cause* AND low* AND back pain NOT fract* |
| unusual cause* AND low* AND back pain NOT fract* |
| rare cause* AND low* AND back pain NOT fract* |
| differential diagnos* AND low* AND back pain NOT fract* |
| medical condition AND cause* AND low* AND back pain NOT fract* |
| Prostatitis AND low* AND back pain NOT fract* |
| Endometriosis AND low* AND back pain NOT fract* |
| Extrauterine pregnancy AND low* AND back pain NOT fract* |
| Ovarian carcinoma AND low* AND back pain NOT fract* |
| Hematocolpos AND low* AND back pain NOT fract* |
| Chronic pelvic inflammatory disease AND low* AND back pain NOT fract* |
| Nephrolithiasis AND low* AND back pain NOT fract* |
| Pyelonephritis AND low* AND back pain NOT fract* |
| Perinephric abscess AND low* AND back pain NOT fract* |
| Renal ischemia AND low* AND back pain NOT fract* |
| Renal vein thrombosis AND low* AND back pain NOT fract* |
| Kidney cell Carcinoma AND low* AND Back Pain NOT fract* |
| Aortic aneurysm AND low* AND Back Pain NOT frac* |
| Myocardial infarction AND low* AND Back Pain NOT fract* |
| Pulmonary embolism AND low* AND Back Pain NOT fract* |
| Aortic dissection AND low* AND Back Pain NOT fract* |
| Aortic rupture AND low* AND Back Pain NOT fract* |
| Vena cava thrombosis AND low* AND Back Pain NOT fract* |
| Lung Cancer AND low* AND Back Pain NOT fract* |
| Pancreatitis AND low* AND Back Pain NOT fract* |
| Cholecystitis AND low* AND Back Pain NOT fract* |
| Penetrating ulcer AND low* AND Back Pain NOT fract* |
| Intestinal ischemia AND low* AND Back Pain NOT fract* |
| Thrombosis of intraabdominal organs AND low* AND Back Pain NOT fract* |
| Hepatic vein Thrombosis AND low* AND Back Pain NOT fract* |
| Duodenal ulcer AND low* AND Back Pain NOT fract* |
| Gall stones AND low* AND Back Pain NOT fract* |
| Retroperitoneal fibrosis AND low* AND Back Pain NOT fract* |
| Lymphoma AND low* AND Back Pain NOT fract* |
| Gout AND low* AND Back Pain NOT fract* |
| Ochronosis AND low* AND Back Pain NOT fract* |
| Hypovitaminosis AND low* AND Back Pain NOT fract* |
| Vitamin D Deficiency AND low* AND Back Pain NOT fract* |
| Hyperparathyreoidism AND low* AND Back Pain |
| Hypercortisolism AND low* AND Back Pain |
| Neuroschistosomiasis AND low* AND Back Pain |
| atypical AND cause* AND low* AND back pain NOT fract* |
| medical condition* AND cause* AND acute back pain |
| cancer* AND cause* AND low* AND back pain NOT fract* |
| infect* AND fever AND cause* AND low* AND back pain |
| metabol* disease AND cause* AND low* AND back pain NOT fract* |
| genet* disease AND cause* AND low* AND back pain NOT fract* |
| unusual AND cause* AND lumbar AND radiculopathy NOT fract* |
| uncommon AND cause* AND lumbar AND radiculopathy NOT fract* |
| rare AND cause* AND lumbar AND radiculopathy NOT fract* |
| medical condition AND cause* AND lumbar AND radiculopathy NOT fract* |
Appendix 3
Table 36.
Excluded pathologies
| Category of excluded pathologies | Excluded pathologies |
|---|---|
| Infection of the spine and paravertebral muscles (red flag pathology) |
• inflammatory spondyloarthropathy • axial spondyloarthropathy / spondylarthritis • Bechterew’s disease / ankylosing spondylitis • discitis / septic discitis • septic arthritis (zygapophyseal) • osteomyelitis • lumbar interspinous bursitis • spondylodiscitis • juxtafacet disc infection • myelitis • helminthosis: hydatid, cysticercosis • abscess (spinal, intradural extramedullary, subdural, epidural) • psoas abscess • arachnoiditis (spinal, ossificans) • Potts disease / spinal tuberculosis • Elsberg-syndrome (radiculomyelitis) • meliodosis • neuroschistosomiasis • tuberculosis of liver and other organs • Guillain Barré syndrome • zoster and herpes infection • borreliosis with radiculopathy |
| Benign and malignant tumour (red flag pathology) |
• benign and malignant tumour of the spine and spinal structures including metastases, invasive infiltration and intramedullary tumour • plasmacytoma • Non-Hodgkin-Lymphoma • thymoma • multiple myeloma • lymphangioma • intestine mesenteric low-grade fibromyxoid sarcoma • adenocarcinoma • pheochromocytoma • tumour in other organs: renal, rectal, lung, prostate, ovaries, neuroendocrine, liver, pancreas, lipomatosis, uterus, (intra)peritoneal • pigmented villonodular synovitis |
| Selected neurological disorders |
• bowstring disease (caused by nerve compression lesion) • dorsal root ganglion compression • lumbar dorsal ramus syndrome • foot drop • meralgia paraesthetica • superior cluneal nerve entrapment |
| Rheumatic inflammatory disease |
• rheumatoid arthritis • rheumatoid nodules • axial psoriatic spondyloarthropathy • Behcet’s disease |
| Specific LBP with anatomical changes of the spine and disc, including malformation of the spine, spinal cord, and meninges |
• Modic changes • lumbar lordosis • scoliosis / pseudoscoliosis • Scheuermans disease • lumbar disc herniation / spinal cord herniation / Schmorl node / ruptured anulus fibrosus • degenerative disc disease • internal disc rupture • lumbar facet joint syndrome • Baastrup disease • cysts (discal, lumbar synovial, extradural meningeal, cystic dilatation of ventriculus terminalis, extraforaminal, epidermoid, tarlov, perineural, ependymal, sacral extradural arachnoid cyst, lateral sacral, zygapophyseal, zygapophyseal joint synovial) • malformation (pseudomeningocele, meningocele, myelomeningocele, duplicated filum terminal, tethered cord) • spondylolisthesis / retrolisthesis • spinal stenosis • spondylolysis • pseudarthrosis • Bertolotti syndrome • progressive necrotic myelopathy • ectopic ganglion in cauda equina • dural ectasia |
| Functional diagnosis |
• sacro-iliac-joint syndrome • piriformis syndrome / pyomyositis • fibromyalgia (as a functional physical discomfort) • lumbar spinal instability • lumbago • coccygodynia • zygapophyseal joint pain • quadratus femoris syndrome / muscle rupture • GNE myopathy |
| Bone disease |
• osteoporosis • osteopetrosis • melorheostosis • Morbus Paget • osteonecrosis of the spine • fibrous dysplasia |
| Complication of interventions |
• textiloma • retrievable filter • injection therapies • surgery • drug-treatment complication (e.g., epidural hematoma due to aspirin / anticoagulant) • erythema ab igne • ice-pack induced perniosis • epidural pneumorrhachis • lost intrauterine device |
| Trauma / injury / fracture (red flag pathology) |
• injury • traumatic compartment syndrome • non-traumatic fracture |
| Pregnancy-related LBP | • complication of treatment / pregnancy / birth / afterbirth |
| Other |
• depression, somatoform disorders • Stiff-Person-Syndrome • thoracic or cervical pain • Whipple’s disease • decompression sickness • histiocytosis (Rosai-Dorfman, Erdheim-Chester) • CARASIL / CADASIL • ligamentum flavum haematoma • pelvic congestion syndrome • animal model • children and adolescents |
Authors’ contributions
AK und JFC analysed and interpreted the data from the literature review and were major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin C-WC, Chenot J-F, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 2.Maselli F, Rossettini G, Viceconti A, Testa M. Importance of screening in physical therapy: vertebral fracture of thoracolumbar junction in a recreational runner. BMJ Case Rep 2019;12(8). 10.1136/bcr-2019-229987. [DOI] [PMC free article] [PubMed]
- 3.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 4.Müller G. Diagnostik des Rückenschmerzes - Wo liegen die Probleme? Schmerz. 2001;15(6):435–441. doi: 10.1007/s004820100029. [DOI] [PubMed] [Google Scholar]
- 5.Hicks GS, Duddleston DN, Russell LD, Holman HE, Shepherd JM, Brown CA. Low back pain. Am J Med Sci. 2002;324(4):207–211. doi: 10.1097/00000441-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137(7):586–597. doi: 10.7326/0003-4819-137-7-200210010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Devereaux MW. Low back pain. Prim Care. 2004;31(1):33–51. doi: 10.1016/S0095-4543(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 8.Diamond S, Borenstein D. Chronic low back pain in a working-age adult. Best Pract Res Clin Rheumatol. 2006;20(4):707–720. doi: 10.1016/j.berh.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Winters ME, Kluetz P, Zilberstein J. Back pain emergencies. Med Clin North Am. 2006;90(3):505–523. doi: 10.1016/j.mcna.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Kinkade S. Evaluation and treatment of acute low back pain. Am Fam Physician. 2007;75(8):1181–1188. [PubMed] [Google Scholar]
- 11.Miura K, Ishihara T. Examination procedures for low back pain in an emergency room. Japan Med Assoc J. 2011;54(2):117–22. [Google Scholar]
- 12.Manusov EG. Evaluation and diagnosis of low back pain. Prim Care. 2012;39(3):471–479. doi: 10.1016/j.pop.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Golob AL, Wipf JE. Low back pain. Med Clin North Am. 2014;98(3):405–428. doi: 10.1016/j.mcna.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Hooten WM, Cohen SP. Evaluation and Treatment of Low Back Pain: A Clinically Focused Review for Primary Care Specialists. Mayo Clin Proc. 2015;90(12):1699–1718. doi: 10.1016/j.mayocp.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Selkirk SM, Ruff R. Low back pain, radiculopathy. Handb Clin Neurol. 2016;136:1027–1033. doi: 10.1016/B978-0-444-53486-6.00053-3. [DOI] [PubMed] [Google Scholar]
- 16.Singleton J, Edlow JA. Acute Nontraumatic Back Pain: Risk Stratification, Emergency Department Management, and Review of Serious Pathologies. Emerg Med Clin North Am. 2016;34(4):743–757. doi: 10.1016/j.emc.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine (Phila Pa 1976) 2009;34(1):E27–32. doi: 10.1097/BRS.0b013e31818b8882. [DOI] [PubMed] [Google Scholar]
- 18.Feller D, Giudice A, Maritati G, Maselli F, Rossettini G, Meroni R et al. Physiotherapy Screening for Referral of a Patient with Peripheral Arterial Disease Masquerading as Sciatica: A Case Report. Healthcare (Basel) 2023;11(11). 10.3390/healthcare11111527. [DOI] [PMC free article] [PubMed]
- 19.Brindisino F, Pennella D, Giovannico G, Rossettini G, Heick JD, Maselli F. Low back pain and calf pain in a recreational runner masking peripheral artery disease: A case report. Physiother Theory Pract. 2021;37(10):1146–1157. doi: 10.1080/09593985.2019.1683922. [DOI] [PubMed] [Google Scholar]
- 20.Faletra A, Bellin G, Dunning J, Fernández-de-Las-Peñas C, Pellicciari L, Brindisino F, et al. Assessing cardiovascular parameters and risk factors in physical therapy practice: findings from a cross-sectional national survey and implication for clinical practice. BMC Musculoskelet Disord. 2022;23(1):749. doi: 10.1186/s12891-022-05696-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MD, Gomez-Marin O, Brookfield KFW, Li PS. Differential diagnosis of hip disease versus spine disease. Clin Orthop Relat Res. 2004;419:280–284. doi: 10.1097/00003086-200402000-00044. [DOI] [PubMed] [Google Scholar]
- 22.Allerton C, Gawthrope IC. Acute paraspinal compartment syndrome as an unusual cause of severe low back pain. Emerg Med Australas. 2012;24(4):457–459. doi: 10.1111/j.1742-6723.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbee GA. An unusual cause of low back and hip pain in a 20-year-old female. JAAPA. 2008;21(10):23–4, 26, 31. doi: 10.1097/01720610-200810000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Di Nicolò P, Zanoli L, Figuera M, Granata A. An unusual cause of lumbar pain after physical exercise: Caval vein duplicity and its detection by ultrasound. J Ultrasound. 2016;19(4):289–293. doi: 10.1007/s40477-016-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellner P, Abendroth M, Beckmann S, Paul C, Burbelko M. An unusual cause of low back pain. Notfall und Rettungsmedizin. 2019;22(7):628–34. doi: 10.1007/s10049-019-0628-7. [DOI] [Google Scholar]
- 26.Mantle M, Kingsnorth AN. An unusual cause of back pain in an achondroplastic man. Hernia. 2003;7(2):95–96. doi: 10.1007/s10029-002-0097-6. [DOI] [PubMed] [Google Scholar]
- 27.Nemec P, Rybnickova S, Fabian P, Fojtik Z, Soucek M. Idiopathic retroperitoneal fibrosis: an unusual cause of low back pain. Clin Rheumatol. 2008;27(3):381–384. doi: 10.1007/s10067-007-0736-5'. [DOI] [PubMed] [Google Scholar]
- 28.Sebastian S, Whitelaw DA. An unusual cause of chronic backache. QJM. 2015;108(5):405–407. doi: 10.1093/qjmed/hcs191. [DOI] [PubMed] [Google Scholar]
- 29.Chavez JM, Gonzalez PG. Suspected lumbar compartment syndrome: a rare cause of low back pain after strenuous exercise. Spine J. 2013;13(10):1409–1410. doi: 10.1016/j.spinee.2013.07.476. [DOI] [PubMed] [Google Scholar]
- 30.Hasturk AE, Basmaci M, Canbay S, Vural C, Erten F. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J. 2012;21(Suppl 4):S400–S403. doi: 10.1007/s00586-011-1847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogias E, Kircher A, Deininger MH, Psarras N, Keck T, Schäfer A-O, et al. A very rare cause of low-back pain and sciatica: deep vein thrombosis due to absence of the inferior vena cava mimicking the clinical and radiological signs of lumbar disc herniation. J Neurosurg Spine. 2011;15(2):164–167. doi: 10.3171/2011.4.SPINE10636. [DOI] [PubMed] [Google Scholar]
- 32.Seidahmed M, Abdel Rahman MO, Salih Abdulhadi A. Alkaptonuria: A rare cause of recurrent severe back pain in the emergency department. Journal of Emergency Medicine, Trauma and Acute Care 2012. Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L370286616&from=export.
- 33.Tieppo Francio V, Barndt B, Schappell JB, Allen T, Towery C, Davani S. Rare extraspinal cause of acute lumbar radiculopathy. BMJ Case Rep 2018; 2018. 10.1136/bcr-2018-224818. [DOI] [PMC free article] [PubMed]
- 34.Tseng Y-C, Wu Y-C, Chiu S-K, Hsu C-H. Low back pain: A rare presentation of klebsiella pneumoniae liver abscess. J Med Sci (Taiwan) 2015;35(3):128–30. doi: 10.4103/1011-4564.158691. [DOI] [Google Scholar]
- 35.Maselli F, Palladino M, Barbari V, Storari L, Rossettini G, Testa M. The diagnostic value of Red Flags in thoracolumbar pain: a systematic review. Disabil Rehabil. 2022;44(8):1190–1206. doi: 10.1080/09638288.2020.1804626. [DOI] [PubMed] [Google Scholar]
- 36.Henschke N, Maher CG, Ostelo RWJG, Vet HCW de, Macaskill P, Irwig L. Red flags to screen for malignancy in patients with low-back pain. Cochrane Database Syst Rev 2013(2):CD008686. 10.1002/14651858.CD008686.pub2. [DOI] [PMC free article] [PubMed]
- 37.Chenot J-F. Rückenschmerz: gezielte Anamnese und klinische Untersuchung. Dtsch Med Wochenschr. 2018;143(21):1556–1563. doi: 10.1055/a-0634-8084. [DOI] [PubMed] [Google Scholar]
- 38.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 39.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18(2):e1230. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med. 2013;2(5):38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barresi V, Cerasoli S, Vitarelli E, Donati R. Spinal intradural müllerianosis: a case report. Histol Histopathol. 2006;21(10):1111–4. doi: 10.14670/HH-21.1111. [DOI] [PubMed] [Google Scholar]
- 42.National Guideline Centre (UK). Low Back Pain and Sciatica in Over 16s: Assessment and Management. London: National Institute for Health and Care Excellence (NICE); 2016. PMID: 27929617. [PubMed]
- 43.Toprover M, Krasnokutsky S, Pillinger MH. Gout in the Spine: Imaging, Diagnosis, and Outcomes. Curr Rheumatol Rep. 2015;17(12):70. doi: 10.1007/s11926-015-0547-7. [DOI] [PubMed] [Google Scholar]
- 44.Mahmud T, Basu D, Dyson PHP. Crystal arthropathy of the lumbar spine: a series of six cases and a review of the literature. J Bone Joint Surg Br. 2005;87(4):513–517. doi: 10.1302/0301-620X.87B4.15555. [DOI] [PubMed] [Google Scholar]
- 45.Yen P-S, Lin J-F, Chen S-Y, Lin S-Z. Tophaceous gout of the lumbar spine mimicking infectious spondylodiscitis and epidural abscess: MR imaging findings. J Clin Neurosci. 2005;12(1):44–46. doi: 10.1016/j.jocn.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Suk K-S, Kim K-T, Lee S-H, Park S-W, Park Y-K. Tophaceous gout of the lumbar spine mimicking pyogenic discitis. Spine J. 2007;7(1):94–99. doi: 10.1016/j.spinee.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Yehia B, Flynn J, Sisson S. Crystal clear. Am J Med. 2008;121(6):488–490. doi: 10.1016/j.amjmed.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Fontenot A, Harris P, Macasa A, Menon Y, Quinet R. An initial presentation of polyarticular gout with spinal involvement. J Clin Rheumatol. 2008;14(3):188–189. doi: 10.1097/RHU.0b013e318177a6b2. [DOI] [PubMed] [Google Scholar]
- 49.Nygaard HB, Shenoi S, Shukla S. Lower back pain caused by tophaceous gout of the spine. Neurology. 2009;73(5):404. doi: 10.1212/WNL.0b013e3181b04cb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad I, Tejada JG. Spinal gout: A great mimicker - A case report and literature review. Neuroradiol J. 2012;25(5):621–5. doi: 10.1177/197140091202500518. [DOI] [PubMed] [Google Scholar]
- 51.Komarla A, Schumacher R, Merkel PA. Spinal gout presenting as acute low back pain. Arthritis Rheum. 2013;65(10):2660. doi: 10.1002/art.38069. [DOI] [PubMed] [Google Scholar]
- 52.Saripalli K, Baskar S. Tophaceous gouty arthropathy of the lumbar spine. Clin Med (Lond) 2014;14(6):683–684. doi: 10.7861/clinmedicine.14-6-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Mello FM, Helito PVP, Bordalo-Rodrigues M, Fuller R, Halpern ASR. Axial gout is frequently associated with the presence of current tophi, although not with spinal symptoms. Spine (Phila Pa 1976) 2014;39(25):E1531–6. doi: 10.1097/BRS.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 54.Cardoso FN, Omoumi P, Wieers G, Maldague B, Malghem J, Lecouvet FE et al. Spinal and sacroiliac gouty arthritis: Report of a case and review of the literature. Acta Radiologica Short Reports 2014; 3(8). Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L603715171&from=export. [DOI] [PMC free article] [PubMed]
- 55.Lu H, Sheng J, Dai J, Hu X. Tophaceous gout causing lumbar stenosis: A case report. Medicine (Baltimore) 2017;96(32):e7670. doi: 10.1097/MD.0000000000007670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Li Q, Cai L, Liu W. Lumbar spinal stenosis attributable to tophaceous gout: Case report and review of the literature. Ther Clin Risk Manag. 2017;13:1287–93. doi: 10.2147/TCRM.S145906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Da Ribeiro CP, Peliz AJ, Barbosa M. Tophaceous gout of the lumbar spine mimicking a spinal meningioma. Eur Spine J. 2018;27(4):815–819. doi: 10.1007/s00586-016-4831-7. [DOI] [PubMed] [Google Scholar]
- 58.Qin D-A, Song J-F, Li X-F, Dong Y-Y. Tophaceous Gout of Lumbar Spine with Fever Mimicking Infection. Am J Med. 2018;131(9):e353–e356. doi: 10.1016/j.amjmed.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Alqatari S, Visevic R, Marshall N, Ryan J, Murphy G. An unexpected cause of sacroiliitis in a patient with gout and chronic psoriasis with inflammatory arthritis: a case report. BMC Musculoskelet Disord. 2018;19(1):126. doi: 10.1186/s12891-018-2044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou Y, Li Y, Liu J, Zhang B, Gu R. Gouty spondylodiscitis with lumbar vertebral body retrolisthesis: A case report. Medicine (Baltimore) 2019;98(7):e14415. doi: 10.1097/MD.0000000000014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Xu G, Hu Q, Zhao T, Bi Q, Huang Y, et al. Percutaneous transforaminal endoscopic decompression for the treatment of intraspinal tophaceous gout: A case report. Medicine (Baltimore) 2020;99(21):e20125. doi: 10.1097/MD.0000000000020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujishiro T, Nabeshima Y, Yasui S, Fujita I, Yoshiya S, Fujii H. Pseudogout attack of the lumbar facet joint: a case report. Spine (Phila Pa 1976) 2002;27(17):E396–8. doi: 10.1097/00007632-200209010-00028. [DOI] [PubMed] [Google Scholar]
- 63.Gadgil AA, Eisenstein SM, Darby A, Cassar Pullicino V. Bilateral symptomatic synovial cysts of the lumbar spine caused by calcium pyrophosphate deposition disease: a case report. Spine (Phila Pa 1976) 2002;27(19):E428–31. doi: 10.1097/00007632-200210010-00024. [DOI] [PubMed] [Google Scholar]
- 64.Namazie MRbM, Fosbender MR. Calcium pyrophosphate dihydrate crystal deposition of multiple lumbar facet joints: a case report. J Orthop Surg (Hong Kong). 2012;20(2):254–6. doi: 10.1177/230949901202000225. [DOI] [PubMed] [Google Scholar]
- 65.Shen G, Su M, Liu B, Kuang A. A Case of Tophaceous Pseudogout on 18F-FDG PET/CT Imaging. Clin Nucl Med. 2019;44(2):e98–e100. doi: 10.1097/RLU.0000000000002308. [DOI] [PubMed] [Google Scholar]
- 66.Peicher K, Maalouf NM. Skeletal Fluorosis Due to Fluorocarbon Inhalation from an Air Dust Cleaner. Calcif Tissue Int. 2017;101(5):545–548. doi: 10.1007/s00223-017-0305-0. [DOI] [PubMed] [Google Scholar]
- 67.Shetty S, Nayak R, Kapoor N, Paul TV. An uncommon cause for compressive myelopathy. BMJ Case Rep 2015;2015. 10.1136/bcr-2014-208640. [DOI] [PMC free article] [PubMed]
- 68.Ludwig V, Fordice S, Lamar R, Martin WH, Delbeke D. Unsuspected skeletal sarcoidosis mimicking metastatic disease on FDG positron emission tomography and bone scintigraphy. Clin Nucl Med. 2003;28(3):176–179. doi: 10.1097/01.RLU.0000053528.35645.70. [DOI] [PubMed] [Google Scholar]
- 69.Ashamalla M, Koutroumpakis E, McCarthy L, Hegener P, Grimm R, Mehdi S. Osseous Sarcoidosis Mimicking Metastatic Cancer on Positron Emission Tomography. J Oncol Pract. 2016;12(7):697–698. doi: 10.1200/JOP.2016.012807. [DOI] [PubMed] [Google Scholar]
- 70.Rice CM, Beric V, Love S, Scolding NJ. Neurological picture. Vertebral sarcoidosis mimicking metastases. J Neurol Neurosurg Psychiatry. 2011;82(2):188. doi: 10.1136/jnnp.2009.204636. [DOI] [PubMed] [Google Scholar]
- 71.Valencia MP, Deaver PM, Mammarappallil MC. Sarcoidosis of the thoracic and lumbar vertebrae, mimicking metastasis or multifocal osteomyelitis by MRI: case report. Clin Imaging. 2009;33(6):478–481. doi: 10.1016/j.clinimag.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Packer CD, Mileti LM. Vertebral sarcoidosis mimicking lytic osseous metastases: development 16 years after apparent resolution of thoracic sarcoidosis. J Clin Rheumatol. 2005;11(2):105–108. doi: 10.1097/01.rhu.0000158538.29753.b8. [DOI] [PubMed] [Google Scholar]
- 73.Barazi S, Bodi I, Thomas N. Sarcoidosis presenting as an isolated extradural thoracolumbar lesion mimicking tumour. Br J Neurosurg. 2008;22(5):690–691. doi: 10.1080/02688690802020605. [DOI] [PubMed] [Google Scholar]
- 74.Khalatbari MR, Moharamzad Y. Brown tumor of the spine in patients with primary hyperparathyroidism. Spine (Phila Pa 1976). 2014;39(18):E1073–9. doi: 10.1097/BRS.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 75.Hoshi M, Takami M, Kajikawa M, Teramura K, Okamoto T, Yanagida I, et al. A case of multiple skeletal lesions of brown tumors, mimicking carcinoma metastases. Arch Orthop Trauma Surg. 2008;128(2):149–154. doi: 10.1007/s00402-007-0312-0. [DOI] [PubMed] [Google Scholar]
- 76.Yu H-I, Lu C-H. Sacroiliitis-like pain as the initial presentation of primary hyperparathyroidism. Arch Osteoporos. 2012;7:315–318. doi: 10.1007/s11657-012-0072-5. [DOI] [PubMed] [Google Scholar]
- 77.Anastasilakis AD, Polyzos SA, Karathanasi E, Efstathiadou Z. Coincidence of severe primary hyperparathyroidism and primary hypothyroidism in a postmenopausal woman with low bone mass–initial conservative management. J Musculoskelet Neuronal Interact. 2011;11(1):77–80. [PubMed] [Google Scholar]
- 78.Wiederkehr M. Brown tumor complicating end-stage kidney disease. Clinical Nephrology - Case Studies. 2020;8(1):72–9. doi: 10.5414/CNCS110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ordahan B, Uslu K, Uğurlu H. Osteomalacia due to vitamin D deficiency: A case report. Turk Osteoporoz Dergisi. 2020;26(2):143–5. doi: 10.4274/tod.galenos.2020.26056. [DOI] [Google Scholar]
- 80.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine (Phila Pa 1976) 2003;28(2):177–9. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 81.Johansen JV, Manniche C, Kjaer P. Vitamin D levels appear to be normal in Danish patients attending secondary care for low back pain and a weak positive correlation between serum level Vitamin D and Modic changes was demonstrated: A cross-sectional cohort study of consecutive patients with non-specific low back pain. BMC Musculoskelet Disord 2013; 14. Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L52473078&from=export. [DOI] [PMC free article] [PubMed]
- 82.Straube S, Derry S, Straube C, Moore RA. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database of Systematic Reviews 2015; (5). 10.1002/14651858.CD007771.pub3. [DOI] [PMC free article] [PubMed]
- 83.Rkain H, Bouaddi I, Ibrahimi A, Lakhdar T, Abouqal R, Allali F, et al. Relationship between vitamin D deficiency and chronic low back pain in postmenopausal women. Current Rheumatology Reviews. 2013;9(1):63–7. doi: 10.2174/1573397111309010011. [DOI] [PubMed] [Google Scholar]
- 84.Baykara B, Dilek B, Nas K, Ulu MA, Batmaz I, Çaǧlayan M, et al. Vitamin D levels and related factors in patients with chronic nonspecific low back pain. Journal of Musculoskeletal Pain. 2014;22(2):160–9. doi: 10.3109/10582452.2014.883025. [DOI] [Google Scholar]
- 85.Rehman S, Sharif N, Rahman S. Frequency of vitamin d deficiency and hypocalcemia in patients presenting with low back pain to a tertiary care hospital. J Med Sci (Peshawar) 2020;28(1):42–5. [Google Scholar]
- 86.Bahinipati J, Mohapatra RA. Serum magnesium and Vitamin D in patients presenting to the orthopedics out-patient department with chronic low back pain. Biomed Pharmacol J. 2020;13(1):347–52. doi: 10.13005/bpj/1894. [DOI] [Google Scholar]
- 87.Mirzashahi B, Najafi A, Farzan M, Tafakhori A. Neglected alkaptonuric patient presenting with steppage gait. Arch Bone Jt Surg. 2016;4(2):188–91. [PMC free article] [PubMed] [Google Scholar]
- 88.Alkasem W, Boissiere L, Obeid I, Bourghli A. Management of a pseudarthrosis with sagittal malalignment in a patient with ochronotic spondyloarthropathy. Eur Spine J. 2019;28(10):2283–2289. doi: 10.1007/s00586-019-06020-2. [DOI] [PubMed] [Google Scholar]
- 89.Bozkurt S, Aktekin L, Uğurlu FG, Balci S, Sezer N, Akkus S. An Unusual Cause of Myelopathy: Ochronotic Spondyloarthropathy With Positive HLA B27. Am J Phys Med Rehabil. 2017;96(11):e206–e209. doi: 10.1097/PHM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 90.Capkin E, Karkucak M, Yayli S, Serdaroğlu M, Tosun M. Ochronosis in differential diagnosis of patients with chronic backache: a review of the literature. Rheumatol Int. 2007;28(1):61–64. doi: 10.1007/s00296-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 91.Al-Mahfoudh R, Clark S, Buxton N. Alkaptonuria presenting with ochronotic spondyloarthropathy. Br J Neurosurg. 2008;22(6):805–807. doi: 10.1080/02688690802226368. [DOI] [PubMed] [Google Scholar]
- 92.Grasko JM, Hooper AJ, Brown JW, McKnight CJ, Burnett JR. A novel missense HGD gene mutation, K57N, in a patient with alkaptonuria. Clinica Chimica Acta. 2009;403(1–2):254–6. doi: 10.1016/j.cca.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed S, Shah Z, Ali N. Chronic low backache and stiffness may not be due ankylosing spondylitis. J Pak Med Assoc. 2010;60(8):681–683. [PubMed] [Google Scholar]
- 94.Effelsberg NM, Hügle T, Walker UA. A metabolic cause of spinal deformity. Metabolism. 2010;59(1):140–143. doi: 10.1016/j.metabol.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 95.Amiri AH, Rafiei A. Alkaptonuria in a middle-aged female. Caspian J Intern Med. 2012;3(4):554–6. [PMC free article] [PubMed] [Google Scholar]
- 96.Etzkorn K, Oliver AM. Not just another case of low back pain. BMJ Case Rep 2014; 2014. 10.1136/bcr-2014-204085. [DOI] [PMC free article] [PubMed]
- 97.Ahlawat SK, Cuddihy M-T. 71-year-old woman with low back pain. Mayo Clin Proc. 2002;77(8):849–852. doi: 10.4065/77.8.849. [DOI] [PubMed] [Google Scholar]
- 98.Takeyachi Y, Yabuki S, Arai I, Midorikawa H, Hoshino S, Chiba K, et al. Changes of low back pain after vascular reconstruction for abdominal aortic aneurysm and high aortic occlusion: a retrospective study. Surg Neurol. 2006;66(2):172–6. doi: 10.1016/j.surneu.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 99.Sahutoglu T, Artim Esen B, Aksoy M, Kurtoglu M, Poyanli A, Gul A. Clinical course of abdominal aortic aneurysms in Behçet disease: a retrospective analysis. Rheumatol Int. 2019;39(6):1061–1067. doi: 10.1007/s00296-019-04283-y. [DOI] [PubMed] [Google Scholar]
- 100.Metcalfe D, Sugand K, Thrumurthy SG, Thompson MM, Holt PJ, Karthikesalingam AP. Diagnosis of ruptured abdominal aortic aneurysm: a multicentre cohort study. Eur J Emerg Med. 2016;23(5):386–390. doi: 10.1097/MEJ.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 101.Aydogan M, Karatoprak O, Mirzanli C, Ozturk C, Tezer M, Hamzaoglu A. Severe erosion of lumbar vertebral body because of a chronic ruptured abdominal aortic aneurysm. Spine J. 2008;8(2):394–396. doi: 10.1016/j.spinee.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Bhogal RH, Nayeemuddin M, Akhtar I, Grainger M, Downing R. Continued lumbar spinal erosion after repair of chronic contained rupture of a mycotic abdominal aortic aneurysm. Surgical Infections. 2008;9(4):475–80. doi: 10.1089/sur.2007.054. [DOI] [PubMed] [Google Scholar]
- 103.Caynak B, Onan B, Sanisoglu I, Akpinar B. Vertebral erosion due to chronic contained rupture of an abdominal aortic aneurysm. J Vasc Surg. 2008;48(5):1342. doi: 10.1016/j.jvs.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 104.Copetti R. Severe vertebral erosion by chronic contained rupture of an abdominal aortic aneurysm. Acta Med Acad. 2017;46(2):169–170. doi: 10.5644/ama2006-124.202. [DOI] [PubMed] [Google Scholar]
- 105.Dobbeleir J, Fourneau I, Maleux G, Daenens K, Vandekerkhof J, Nevelsteen A. Chronic contained rupture of an abdominal aortic aneurysm presenting as a Grynfeltt lumbar hernia. A case report. Acta Chir Belg. 2007;107(3):325–327. doi: 10.1080/00015458.2007.11680067. [DOI] [PubMed] [Google Scholar]
- 106.Moos JM, Rowell S, Dawson DL. Symptomatic 7-cm abdominal aortic aneurysm in an otherwise healthy 31-year-old woman. Vasc Endovascular Surg. 2009;43(6):622–626. doi: 10.1177/1538574409335920. [DOI] [PubMed] [Google Scholar]
- 107.Nguyen T-T, Le N-T, Doan Q-H. Chronic contained abdominal aortic aneurysm rupture causing vertebral erosion. Asian Cardiovasc Thorac Ann. 2019;27(1):33–35. doi: 10.1177/0218492318773237. [DOI] [PubMed] [Google Scholar]
- 108.van Wyngaarden JJ, Ross MD, Hando BR. Abdominal aortic aneurysm in a patient with low back pain. J Orthop Sports Phys Ther. 2014;44(7):500–507. doi: 10.2519/jospt.2014.4935. [DOI] [PubMed] [Google Scholar]
- 109.Bogie R, Willigendael EM, de Booij M, Meesters B, Teijink JAW. Acute thrombosis of an abdominal aortic aneurysm: a short report. Eur J Vasc Endovasc Surg. 2008;35(5):590–592. doi: 10.1016/j.ejvs.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 110.de Boer NJ, Knaap SFC, de Zoete A. Clinical detection of abdominal aortic aneurysm in a 74-year-old man in chiropractic practice. J Chiropr Med. 2010;9(1):38–41. doi: 10.1016/j.jcm.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharif MA, Soong CV, Lee B, McCallion K, Hannon RJ. Inflammatory Infrarenal Abdominal Aortic Aneurysm in a Young Woman. J Emerg Med. 2008;34(2):147–50. doi: 10.1016/j.jemermed.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 112.Diekerhof CH, Reedt Dortland RWH, Oner FC, Verbout AJ. Severe erosion of lumbar vertebral body because of abdominal aortic false aneurysm: report of two cases. Spine (Phila Pa 1976) 2002;27(16):E382–4. doi: 10.1097/00007632-200208150-00026. [DOI] [PubMed] [Google Scholar]
- 113.Lombardi AF, Cardoso FN, Da Rocha FA. Extensive Erosion of Vertebral Bodies Due to a Chronic Contained Ruptured Abdominal Aortic Aneurysm. J Radiol Case Rep. 2016;10(1):27–34. doi: 10.3941/jrcr.v10i1.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahn HJ, Kwon SH, Park HC. Abdominal aortic aneurysm rupture with vertebral erosion presenting with severe refractory back pain in Behçet's disease. Ann Vasc Surg. 2010;24(2):254.e17–9. doi: 10.1016/j.avsg.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Hocaoglu S, Kaptanoglu E, Hocaoglu S. Low-back pain in geriatric patients: remember abdominal aortic aneurysm! J Clin Rheumatol. 2007;13(3):171–172. doi: 10.1097/RHU.0b013e318065489c. [DOI] [PubMed] [Google Scholar]
- 116.Crawford CM, Hurtgen-Grace K, Talarico E, Marley J. Abdominal aortic aneurysm: an illustrated narrative review. J Manipulative Physiol Ther. 2003;26(3):184–195. doi: 10.1016/S0161-4754(02)54111-7. [DOI] [PubMed] [Google Scholar]
- 117.Anderson LA. Abdominal aortic aneurysm. J Cardiovasc Nurs. 2001;15(4):1–14. doi: 10.1097/00005082-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 118.Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85(1003):268–273. doi: 10.1136/pgmj.2008.074666. [DOI] [PubMed] [Google Scholar]
- 119.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 120.Kumar Y, Hooda K, Li S, Goyal P, Gupta N, Adeb M. Abdominal aortic aneurysm: pictorial review of common appearances and complications. Ann Transl Med. 2017;5(12):256. doi: 10.21037/atm.2017.04.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang T, Boyle JR, Dixon AK, Varty K. Inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2005;29(4):353–362. doi: 10.1016/j.ejvs.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 122.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 123.Rogers RL, McCormack R. Aortic disasters. Emerg Med Clin North Am. 2004;22(4):887–908. doi: 10.1016/j.emc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Patel SN, Kettner NW. Abdominal aortic aneurysm presenting as back pain to a chiropractic clinic: a case report. J Manipulative Physiol Ther. 2006;29(5):409.e1–7. doi: 10.1016/j.jmpt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Henderson MM. A 67-year-old man with increasing severe lower back pain since the night before. J Emerg Nurs. 2003;29(1):9–11. doi: 10.1067/men.2003.8. [DOI] [PubMed] [Google Scholar]
- 126.Jiménez Viseu Pinheiro JF, Blanco Blanco JF, Pescador Hernández D, García García FJ. Vertebral destruction due to abdominal aortic aneurysm. Int J Surg Case Rep. 2015;6:296–9. doi: 10.1016/j.ijscr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mechelli F, Preboski Z, Probaski Z, Boissonnault WG. Differential diagnosis of a patient referred to physical therapy with low back pain: abdominal aortic aneurysm. J Orthop Sports Phys Ther. 2008;38(9):551–557. doi: 10.2519/jospt.2008.2719. [DOI] [PubMed] [Google Scholar]
- 128.Tan TXZ, Balakrishnan T, Lam MHH, Chui YY, Cheng LT-E. A Case of Hoarseness with Acute Back Pain - Cardiovocal Syndrome Revisited. J Radiol Case Rep. 2019;13(7):21–8. doi: 10.3941/jrcr.v13i7.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walker ST, Pipinos II, Johanning JM, Vargo CJ. Contained Rupture of an Abdominal Aortic Aneurysm With Extensive Vertebral Body and Retroperitoneal Space Destruction. J Comput Assist Tomogr. 2017;41(5):839–842. doi: 10.1097/RCT.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 130.Juković M, Koković T, Nikolić D, Ilić D, Till V. LOWER BACK PAIN–SILENT SYMPTOM OF CHRONIC INFRARENAL ABDOMINAL ANEURYSM RUPTURE. Med Pregl. 2016;69(3–4):115–117. doi: 10.2298/mpns1604115j. [DOI] [PubMed] [Google Scholar]
- 131.Alshafei A, Kamal D. Chronic Contained Abdominal Aortic Aneurysm Rupture Mimicking Vertebral Spondylodiscitis: A Case Report. Ann Vasc Dis. 2015;8(2):113–115. doi: 10.3400/avd.cr.15-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barros M, Lozano F, Almazán A, Arias R. Angio-Behçet with vertebral erosion: an exceptional Behçet's complication and literature review. Joint Bone Spine. 2004;71(6):577–579. doi: 10.1016/j.jbspin.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384. doi: 10.1136/bmj.d1384. [DOI] [PubMed] [Google Scholar]
- 134.Al-Koteesh J, Masannat Y, James NVM, Sharaf U. Chronic contained rupture of abdominal aortic aneurysm presenting with longstanding back pain. Scott Med J. 2005;50(3):122–123. doi: 10.1177/003693300505000310. [DOI] [PubMed] [Google Scholar]
- 135.Arici V, Rossi M, Bozzani A, Moia A, Odero A. Massive vertebral destruction associated with chronic rupture of infrarenal aortic aneurysm: case report and systematic review of the literature in the English language. Spine (Phila Pa 1976) 2012;37(26):E1665–71. doi: 10.1097/BRS.0b013e318273dc66. [DOI] [PubMed] [Google Scholar]
- 136.Gandini R, Chiocchi M, Maresca L, Pipitone V, Messina M, Simonetti G. Chronic contained rupture of an abdominal aortic aneurysm: from diagnosis to endovascular resolution. Cardiovasc Intervent Radiol. 2008;31(Suppl 2):S62–S66. doi: 10.1007/s00270-007-9154-y. [DOI] [PubMed] [Google Scholar]
- 137.García Martos Á, de Los Riscos Álvarez M, Fernández-Espartero C. Aneurisma abdominal: una causa infrecuente de dolor lumbar. Reumatol Clin (Engl Ed) 2018;14(5):307–8. doi: 10.1016/j.reuma.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Horowitz M, Gayer G, Itzchak Y, Rapoport MJ. To biopsy or not to biopsy: An 82-year-old patient with a retroperitoneal mass and severe low back pain. Eur J Intern Med. 2006;17(1):55–6. doi: 10.1016/j.ejim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 139.Kim NS, Kang SH, Park SY. Coexistence of expanding abdominal aortic aneurysm and aggravated intervertebral disc extrusion. Korean J Anesthesiol. 2013;65(4):345–8. doi: 10.4097/kjae.2013.65.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lai C-C, Tan C-K, Chu T-W, Ding L-W. Chronic contained rupture of an abdominal aortic aneurysm with vertebral erosion. CMAJ. 2008;178(8):995–996. doi: 10.1503/cmaj.070332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lucas C, Costa J, Paixão J, Silva F, Ribeiro P, Rodrigues A. Low back pain: A pain that may not be harmless. European Journal of Case Reports in Internal Medicine 2018; 5(3). Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L621677053&from=export. [DOI] [PMC free article] [PubMed]
- 142.Nakano S, Okauchi K, Tsushima Y. Chronic contained rupture of abdominal aortic aneurysm (CCR-AAA) with massive vertebral bone erosion: computed tomography (CT), magnetic resonance imaging (MRI) and fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) findings. Jpn J Radiol. 2014;32(2):109–112. doi: 10.1007/s11604-013-0271-z. [DOI] [PubMed] [Google Scholar]
- 143.Seçkin H, Bavbek M, Dogan S, Keyik B, Yigitkanli K. Is every chronic low back pain benign? Case report. Surg Neurol. 2006;66(4):357–60. doi: 10.1016/j.surneu.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 144.Terai Y, Mitsuoka H, Nakai M, Goto S, Miyano Y, Tsuchiya H, et al. Endovascular Aneurysm Repair of Acute Occlusion of Abdominal Aortic Aneurysm with Intra-Aneurysmal Dissection. Ann Vasc Surg. 2015;29(8):1658.e11–1658.e14. doi: 10.1016/j.avsg.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 145.Chieh JJ, Brevetti LS, Scholz PM, Graham AM, Ciocca RG. Multiple isolated aneurysms in a case of "burned out" Takayasu aortitis. J Vasc Surg. 2003;37(5):1094–1097. doi: 10.1067/mva.2003.158. [DOI] [PubMed] [Google Scholar]
- 146.Defraigne JO, Sakalihasan N, Lavigne JP, van Damme H, Limet R. Chronic rupture of abdominal aortic aneurysm manifesting as crural neuropathy. Ann Vasc Surg. 2001;15(3):405–411. doi: 10.1007/s100160010069. [DOI] [PubMed] [Google Scholar]
- 147.Dorrucci V, Dusi R, Rombolà G, Cordiano C. Contained rupture of an abdominal aortic aneurysm presenting as obstructive jaundice: report of a case. Surg Today. 2001;31(4):331–332. doi: 10.1007/s005950170154. [DOI] [PubMed] [Google Scholar]
- 148.Sakai T, Katoh S, Sairyo K, Higashino K, Hirohasm N, Yasui N. Extension of contained rupture of an abdominal aortic aneurysm into a lumbar intervertebral disc. Case report. J Neurosurg Spine. 2007;7(2):221–226. doi: 10.3171/SPI-07/08/221. [DOI] [PubMed] [Google Scholar]
- 149.Whitwell GS, Vowden P. An unusual presentation of a ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2002;23(5):465–466. doi: 10.1053/ejvs.2002.1630. [DOI] [PubMed] [Google Scholar]
- 150.Kamano S, Yonezawa I, Arai Y, Iizuka Y, Kurosawa H. Acute abdominal aortic aneurysm rupture presenting as transient paralysis of the lower legs: a case report. J Emerg Med. 2005;29(1):53–55. doi: 10.1016/j.jemermed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 151.Tsuchie H, Miyakoshi N, Kasukawa Y, Nishi T, Abe H, Takeshima M, et al. High prevalence of abdominal aortic aneurysm in patients with chronic low back pain. Tohoku J Exp Med. 2013;230(2):83–86. doi: 10.1620/tjem.230.83. [DOI] [PubMed] [Google Scholar]
- 152.Hanschke D, Eberhardt E. Giant splenic artery aneurysm: A case report. Gefasschirurgie. 2002;7(2):70–3. doi: 10.1007/s00772-002-0204-0. [DOI] [Google Scholar]
- 153.Iihoshi S, Miyata K, Murakami T, Kaneko T, Koyanagi I. Dissection aneurysm of the radiculomedullary branch of the artery of Adamkiewicz with subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2011;51(9):649–652. doi: 10.2176/nmc.51.649. [DOI] [PubMed] [Google Scholar]
- 154.Matsumoto T, Ishizuka M, Iso Y, Kita J, Kubota K. Mini-Laparotomy for Superior Mesenteric Artery Aneurysm Due to Takayasu's Arteritis. Int Surg. 2015;100(4):765–9. doi: 10.9738/INTSURG-D-14-00127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakamura T, Ueno T, Arai A, Iwamura M, Midorikawa H, Murakami K, et al. Subarachnoid Hemorrhage Caused by Ruptured Aneurysm of the Artery of Adamkiewicz: a Case Report. J Stroke Cerebrovasc Dis. 2020;29(11):105224. doi: 10.1016/j.jstrokecerebrovasdis.2020.105224. [DOI] [PubMed] [Google Scholar]
- 156.Nogueira RG, Kasper E, Walcott BP, Nahed BV, Redjal N, Coumans J-V, et al. Lateral sacral artery aneurysm of the lumbar spine: hemorrhage resulting in cauda equina syndrome. J Neurointerv Surg. 2010;2(4):399–401. doi: 10.1136/jnis.2009.002154. [DOI] [PubMed] [Google Scholar]
- 157.Takebayashi K, Ishikawa T, Murakami M, Funatsu T, Ishikawa T, Taira T, et al. Isolated Posterior Spinal Artery Aneurysm Presenting with Spontaneous Thrombosis After Subarachnoid Hemorrhage. World Neurosurg. 2020;134:544–547. doi: 10.1016/j.wneu.2019.11.118. [DOI] [PubMed] [Google Scholar]
- 158.Bell DL, Stapleton CJ, Terry AR, Stone JR, Ogilvy CS. Clinical presentation and treatment considerations of a ruptured posterior spinal artery pseudoaneurysm. J Clin Neurosci. 2014;21(7):1273–1276. doi: 10.1016/j.jocn.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Bushby N, Wickramasinghe SYBT, Wickramasinghe DNS. Lumbosacral plexopathy due to a rupture of a common Iliac artery aneurysm. Emerg Med Australas. 2010;22(4):351–353. doi: 10.1111/j.1742-6723.2010.01310.x. [DOI] [PubMed] [Google Scholar]
- 160.Ferrero E, Viazzo A, Ferri M, Robaldo A, Piazza S, Berardi G, et al. Management and urgent repair of ruptured visceral artery aneurysms. Ann Vasc Surg. 2011;25(7):981.e7–11. doi: 10.1016/j.avsg.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 161.Caglar YS, Torun F, Pait G, Bagdatoglu C, Sancak T. Ruptured aneurysm of the posterior spinal artery of the conus medullaris. J Clin Neurosci. 2005;12(5):603–605. doi: 10.1016/j.jocn.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 162.Gonzalez LF, Zabramski JM, Tabrizi P, Wallace RC, Massand MG, Spetzler RF. Spontaneous spinal subarachnoid hemorrhage secondary to spinal aneurysms: diagnosis and treatment paradigm. Neurosurgery. 2005;57(6):1127–31. doi: 10.1227/01.neu.0000186010.34779.10. [DOI] [PubMed] [Google Scholar]
- 163.Knol J, Ceuppens H. Emergency aorto-iliac aneurysm surgery with low mortality and morbidity. Acta Chir Belg. 2002;102(6):445–449. doi: 10.1080/00015458.2002.11679349. [DOI] [PubMed] [Google Scholar]
- 164.Wu J, Zafar M, Qiu J, Huang Y, Chen Y, Yu C, et al. A systematic review and meta-analysis of isolated abdominal aortic dissection. J Vasc Surg. 2019;70(6):2046–2053.e6. doi: 10.1016/j.jvs.2019.04.467. [DOI] [PubMed] [Google Scholar]
- 165.Johnson J. A 49-year-old man with vague complaints of back pain. J Emerg Nurs. 2008;34(4):345–347. doi: 10.1016/j.jen.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 166.Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191–196. doi: 10.1016/j.jemermed.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 167.Itoga NK, Wu T, Dake MD, Dalman RL, Lee JT. Acute Type B Dissection Causing Collapse of EVAR Endograft and Iliac Limb Occlusion. Ann Vasc Surg. 2018;46:206.e1–206.e4. doi: 10.1016/j.avsg.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Korkut M, Bedel C. Aortic dissection or spontaneous renal artery dissection, a rare diagnosis? CEN Case Reports. 2020;9(3):257–9. doi: 10.1007/s13730-020-00469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sixsmith DM. Case studies in acute aortic dissection: strategies to avoid a catastrophic outcome. J Healthc Risk Manag. 2005;25(2):15–18. doi: 10.1002/jhrm.5600250206. [DOI] [PubMed] [Google Scholar]
- 170.Takahashi S, Komatsu S, Ohara T, Takewa M, Toyama Y, Yutani C, et al. Detecting intimal tear and subintimal blood flow of thrombosed acute aortic dissection with ulcer-like projections using non-obstructive angioscopy. J Cardiol Cases. 2018;18(5):164–7. doi: 10.1016/j.jccase.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kalko Y, Kafa U, Başaran M, Köşker T, Ozçalişkan O, Yücel E, et al. Surgical experiences in acute spontaneous dissection of the infrarenal abdominal aorta. Anadolu Kardiyol Derg. 2008;8(4):286–290. [PubMed] [Google Scholar]
- 172.Hsu R-B, Ho Y-L, Chen RJ, Wang S-S, Lin F-Y, Chu S-H. Outcome of medical and surgical treatment in patients with acute type B aortic dissection. Ann Thorac Surg. 2005;79(3):790–4. doi: 10.1016/j.athoracsur.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 173.Li Y, Yang N, Duan W, Liu S, Yu S, Yi D. Acute aortic dissection in China. Am J Cardiol. 2012;110(7):1056–1061. doi: 10.1016/j.amjcard.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 174.Falconi M, Oberti P, Krauss J, Domenech A, Cesáreo V, Bracco D, et al. Different clinical features of aortic intramural hematoma versus dissection involving the descending thoracic aorta. Echocardiography. 2005;22(8):629–635. doi: 10.1111/j.1540-8175.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- 175.Asouhidou I, Asteri T. Acute aortic dissection: be aware of misdiagnosis. BMC Res Notes. 2009;2:25. doi: 10.1186/1756-0500-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Januzzi JL, Marayati F, Mehta RH, Cooper JV, O'Gara PT, Sechtem U, et al. Comparison of aortic dissection in patients with and without Marfan's syndrome (results from the International Registry of Aortic Dissection) Am J Cardiol. 2004;94(3):400–402. doi: 10.1016/j.amjcard.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 177.Nallamothu BK, Mehta RH, Saint S, Llovet A, Bossone E, Cooper JV, et al. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am J Med. 2002;113(6):468–471. doi: 10.1016/s0002-9343(02)01254-8. [DOI] [PubMed] [Google Scholar]
- 178.Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109(24):3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 179.Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD) Circulation. 2003;108(Suppl 1):II312–7. doi: 10.1161/01.cir.0000087386.07204.09. [DOI] [PubMed] [Google Scholar]
- 180.Bossone E, Pyeritz RE, O'Gara P, Harris KM, Braverman AC, Pape L, et al. Acute aortic dissection in blacks: insights from the International Registry of Acute Aortic Dissection. Am J Med. 2013;126(10):909–915. doi: 10.1016/j.amjmed.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 181.Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111(8):1063–1070. doi: 10.1161/01.CIR.0000156444.26393.80. [DOI] [PubMed] [Google Scholar]
- 182.Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126(11 Suppl 1):S91–S96. doi: 10.1161/CIRCULATIONAHA.111.084541. [DOI] [PubMed] [Google Scholar]
- 183.Vagnarelli F, Corsini A, Lorenzini M, Pacini D, Ferlito M, Bacchi Reggiani L, et al. Acute heart failure in patients with acute aortic syndrome: pathophysiology and clinical-prognostic implications. Eur J Heart Fail. 2015;17(9):917–924. doi: 10.1002/ejhf.325. [DOI] [PubMed] [Google Scholar]
- 184.Jansen Klomp WW, Brandon Bravo Bruinsma GJ, Peelen LM, Nierich AP, Grandjean JG, van ’t Hof A. Clinical recognition of acute aortic dissections: Insights from a large single-centre cohort study. Neth Heart J. 2017;25(3):200–6. doi: 10.1007/s12471-016-0921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, Cooper JV, et al. Differences in clinical presentation, management, and outcomes of acute type a aortic dissection in patients with and without previous cardiac surgery. Circulation. 2004;110(11 Suppl 1):II237–42. doi: 10.1161/01.CIR.0000138219.67028.2a. [DOI] [PubMed] [Google Scholar]
- 186.Imamura H, Sekiguchi Y, Iwashita T, Dohgomori H, Mochizuki K, Aizawa K, et al. Painless acute aortic dissection. - Diagnostic, prognostic and clinical implications. Circ J . 2011;75(1):59–66. doi: 10.1253/circj.cj-10-0183. [DOI] [PubMed] [Google Scholar]
- 187.Li D, Zhang H, Cai Y, Jin W, Chen X, Tian L, et al. Acute type B aortic intramural hematoma: treatment strategy and the role of endovascular repair. J Endovasc Ther. 2010;17(5):617–621. doi: 10.1583/10-3125.1a. [DOI] [PubMed] [Google Scholar]
- 188.León Ayala IA, de, Chen Y-F. Acute aortic dissection: an update. Kaohsiung J Med Sci. 2012;28(6):299–305. doi: 10.1016/j.kjms.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372(9632):55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 190.Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest. 2002;122(1):311–328. doi: 10.1378/chest.122.1.311. [DOI] [PubMed] [Google Scholar]
- 191.Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA. 2016;316(7):754–763. doi: 10.1001/jama.2016.10026. [DOI] [PubMed] [Google Scholar]
- 192.Nienaber CA, Sievers H-H. Intramural hematoma in acute aortic syndrome: more than one variant of dissection? Circulation. 2002;106(3):284–285. doi: 10.1161/01.cir.0000023453.90533.82. [DOI] [PubMed] [Google Scholar]
- 193.Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628–635. doi: 10.1161/01.CIR.0000087009.16755.E4. [DOI] [PubMed] [Google Scholar]
- 194.Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJE, Thompson MM. The diagnosis and management of aortic dissection. BMJ. 2011;344:d8290. doi: 10.1136/bmj.d8290. [DOI] [PubMed] [Google Scholar]
- 195.Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112(24):3802–3813. doi: 10.1161/CIRCULATIONAHA.105.534198. [DOI] [PubMed] [Google Scholar]
- 196.Vilacosta I, San Román JA. Acute aortic syndrome. Heart. 2001;85(4):365–368. doi: 10.1136/heart.85.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vilacosta I, Aragoncillo P, Cañadas V, San Román JA, Ferreirós J, Rodríguez E. Acute aortic syndrome: a new look at an old conundrum. Heart. 2009;95(14):1130–1139. doi: 10.1136/hrt.2008.153650. [DOI] [PubMed] [Google Scholar]
- 198.Siegal EM. Acute aortic dissection. J Hosp Med. 2006;1(2):94–105. doi: 10.1002/jhm.69. [DOI] [PubMed] [Google Scholar]
- 199.Furui M, Ohashi T, Hirai Y, Kageyama S. Congenital pericardial defect with ruptured acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2012;15(5):912–914. doi: 10.1093/icvts/ivs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sen I, D'Oria M, Weiss S, Bower TC, Oderich GS, Kalra M, et al. Incidence and natural history of isolated abdominal aortic dissection: A population-based assessment from 1995 to 2015. J Vasc Surg. 2021;73(4):1198–1204.e1. doi: 10.1016/j.jvs.2020.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ho HH, Cheung CW, Jim MH, Miu KM, Siu CW, Lam YM, et al. Type A aortic intramural hematoma: clinical features and outcomes in Chinese patients. Clin Cardiol. 2011;34(3):E1–5. doi: 10.1002/clc.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nathan DP, Boonn W, Lai E, Wang GJ, Desai N, Woo EY, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55(1):10–15. doi: 10.1016/j.jvs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 203.Tsai S-H, Lin Y-Y, Hsu C-W, Chen Y-L, Liao M-T, Chu S-J. The characteristics of acute aortic dissection among young Chinese patients: a comparison between Marfan syndrome and non-Marfan syndrome patients. Yonsei Med J. 2009;50(2):239–244. doi: 10.3349/ymj.2009.50.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang W, Duan W, Xue Y, Wang L, Liu J, Yu S, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg. 2014;148(6):2995–3000. doi: 10.1016/j.jtcvs.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 205.Hughes KE, Seguin C, Felton B, Hughes MJ, Castle D. Acute Aortic Dissection Presenting as Bilateral Lower Extremity Paralysis: A Case Report. J Emerg Med. 2016;51(4):450–453. doi: 10.1016/j.jemermed.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 206.Ahmed M. Acute infra-renal aortic dissection presenting as back pain and transient paralysis of the lower limbs. Int J Surg Case Rep. 2012;3(2):39–41. doi: 10.1016/j.ijscr.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lech C, Swaminathan A. Abdominal Aortic Emergencies. Emerg Med Clin North Am. 2017;35(4):847–867. doi: 10.1016/j.emc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 208.Corvera JS. Acute aortic syndrome. Ann Cardiothorac Surg. 2016;5(3):188–93. doi: 10.21037/acs.2016.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hsu K-C, Tsai S-H, Kao H-W, Chu S-J, Hsu C-W. Acute aortic dissection presenting with acute lower-back pain following sexual intercourse. J Intern Med Taiwan. 2008;19(5):418–21. [Google Scholar]
- 210.Stäubli M. Plötzliche Gehunfähigkeit. Ther Umsch. 2004;61(12):732–738. doi: 10.1024/0040-5930.61.12.732. [DOI] [PubMed] [Google Scholar]
- 211.Morris-Stiff G, Coxon M, Ball E, Lewis MH. Post coital aortic dissection: a case report. J Med Case Reports. 2008;2:6. doi: 10.1186/1752-1947-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu SD, Huang FJ, Yang JF, Li ZZ, Wang XY, Zhang ZG, et al. Endovascular repair of acute type B aortic dissection: early and mid-term results. J Vasc Surg. 2006;43(6):1090–1095. doi: 10.1016/j.jvs.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 213.Ozcakir N, Sherman SC, Kern K. Aortoenteric fistula. J Emerg Med. 2011;40(3):e61–e62. doi: 10.1016/j.jemermed.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 214.Kiyosue H, Matsumaru Y, Niimi Y, Takai K, Ishiguro T, Hiramatsu M, et al. Angiographic and Clinical Characteristics of Thoracolumbar Spinal Epidural and Dural Arteriovenous Fistulas. Stroke. 2017;48(12):3215–3222. doi: 10.1161/STROKEAHA.117.019131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kotsikoris I, Papas TT, Papanas N, Maras D, Andrikopoulou M, Bessias N, et al. Aortocaval fistula formation due to ruptured abdominal aortic aneurysms: a 12-year series. Vasc Endovascular Surg. 2012;46(1):26–29. doi: 10.1177/1538574411418842. [DOI] [PubMed] [Google Scholar]
- 216.Mehmood RK, Mushtaq A, Andrew DR, Miller GA. Clinical presentation of a missed primary aorto-enteric fistula. J Pak Med Assoc. 2007;57(12):616–618. [PubMed] [Google Scholar]
- 217.Patelis N, Giagkos G-C, Maltezos K, Klonaris C. Aortocaval fistula: An unusual complication of ruptured abdominal aortic aneurysm. BMJ Case Rep 2018; 2018. Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L623115130&from=export. [DOI] [PMC free article] [PubMed]
- 218.Maeda H, Umezawa H, Goshima M, Hattori T, Nakamura T, Nishii T, et al. Surgery for ruptured abdominal aortic aneurysm with an aortocaval and iliac vein fistula. Surg Today. 2007;37(6):445–448. doi: 10.1007/s00595-006-3429-9. [DOI] [PubMed] [Google Scholar]
- 219.Brightwell RE, Pegna V, Boyne N. Aortocaval fistula: current management strategies. ANZ J Surg. 2013;83(1–2):31–35. doi: 10.1111/j.1445-2197.2012.06294.x. [DOI] [PubMed] [Google Scholar]
- 220.Kopp R, Weidenhagen R, Hoffmann R, Waggershauser T, Meimarakis G, Andrassy J, et al. Immediate endovascular treatment of an aortoiliac aneurysm ruptured into the inferior vena cava. Ann Vasc Surg. 2006;20(4):525–528. doi: 10.1007/s10016-006-9061-8. [DOI] [PubMed] [Google Scholar]
- 221.Takazawa A, Sakahashi H, Toyama A. Surgical repair of a concealed aortocaval fistula associated with an abdominal aortic aneurysm: report of two cases. Surg Today. 2001;31(9):842–844. doi: 10.1007/s005950170062. [DOI] [PubMed] [Google Scholar]
- 222.Pevec WC, Lee ES, Lamba R. Symptomatic, acute aortocaval fistula complicating an infrarenal aortic aneurysm. J Vasc Surg. 2010;51(2):475. doi: 10.1016/j.jvs.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 223.Siepe M, Koeppe S, Euringer W, Schlensak C. Aorto-caval fistula from acute rupture of an abdominal aortic aneurysm treated with a hybrid approach. J Vasc Surg. 2009;49(6):1574–1576. doi: 10.1016/j.jvs.2008.12.074. [DOI] [PubMed] [Google Scholar]
- 224.Koch C. Spinal dural arteriovenous fistula. Curr Opin Neurol. 2006;19(1):69–75. doi: 10.1097/01.wco.0000200547.22292.11. [DOI] [PubMed] [Google Scholar]
- 225.Marcus J, Schwarz J, Singh IP, Sigounas D, Knopman J, Gobin YP, et al. Spinal dural arteriovenous fistulas: a review. Curr Atheroscler Rep. 2013;15(7):335. doi: 10.1007/s11883-013-0335-7. [DOI] [PubMed] [Google Scholar]
- 226.Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009;30(4):639–648. doi: 10.3174/ajnr.A1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Krings T, Mull M, Gilsbach JM, Thron A. Spinal vascular malformations. Eur Radiol. 2005;15(2):267–278. doi: 10.1007/s00330-004-2510-2. [DOI] [PubMed] [Google Scholar]
- 228.Klopper HB, Surdell DL, Thorell WE. Type I spinal dural arteriovenous fistulas: historical review and illustrative case. Neurosurg Focus. 2009;26(1):E3. doi: 10.3171/FOC.2009.26.1.E3. [DOI] [PubMed] [Google Scholar]
- 229.Nakazawa S, Mohara J, Takahashi T, Koike N, Takeyoshi I. Aortocaval fistula associated with ruptured abdominal aortic aneurysm. Ann Vasc Surg. 2014;28(7):1793.e5–9. doi: 10.1016/j.avsg.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 230.Ogura T, Sakamoto M, Yoshioka H, Torihashi K, Kambe A, Kurosaki M. Unusual Manifestation of Spinal Epidural Arteriovenous Fistula as Sudden Paraplegia. World Neurosurg. 2020;144:60–63. doi: 10.1016/j.wneu.2020.08.104. [DOI] [PubMed] [Google Scholar]
- 231.Verma K, Fennessy J, Huang R, Jabbour P, Rihn J. Spinal dural arteriovenous fistula presenting as a recurrent nucleus pulposus herniation. JBJS Case Connector 2015; 5(3). Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L611062770&from=export. [DOI] [PubMed]
- 232.Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004;100(4 Suppl Spine):385–91. doi: 10.3171/spi.2004.100.4.0385. [DOI] [PubMed] [Google Scholar]
- 233.Oldfield EH, Bennett A, Chen MY, Doppman JL. Successful management of spinal dural arteriovenous fistulas undetected by arteriography. Report of three cases. J Neurosurg. 2002;96(2 Suppl):220–229. doi: 10.3171/spi.2002.96.2.0220. [DOI] [PubMed] [Google Scholar]
- 234.Cinara IS, Davidovic LB, Kostic DM, Cvetkovic SD, Jakovljevic NS, Koncar IB. Aorto-caval fistulas: a review of eighteen years experience. Acta Chir Belg. 2005;105(6):616–620. doi: 10.1080/00015458.2005.11679788. [DOI] [PubMed] [Google Scholar]
- 235.Muralidharan R, Saladino A, Lanzino G, Atkinson JL, Rabinstein AA. The clinical and radiological presentation of spinal dural arteriovenous fistula. Spine (Phila Pa 1976) 2011;36(25):E1641–7. doi: 10.1097/BRS.0b013e31821352dd. [DOI] [PubMed] [Google Scholar]
- 236.Davidović LB, Marković MD, Jakovljević NS, Cvetković D, Kuzmanović IB, Marković DM. Unusual forms of ruptured abdominal aortic aneurysms. Vascular. 2008;16(1):17–24. doi: 10.2310/6670.2007.00042. [DOI] [PubMed] [Google Scholar]
- 237.Davidovic L, Dragas M, Cvetkovic S, Kostic D, Cinara I, Banzic I. Twenty years of experience in the treatment of spontaneous aorto-venous fistulas in a developing country. World J Surg. 2011;35(8):1829–1834. doi: 10.1007/s00268-011-1128-1. [DOI] [PubMed] [Google Scholar]
- 238.Davidovic LB, Kostic DM, Cvetkovic SD, Jakovljevic NS, Stojanov PL, Kacar AS, et al. Aorto-caval fistulas. Cardiovasc Surg. 2002;10(6):555–560. doi: 10.1016/s0967-2109(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 239.Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62(1):159–66. doi: 10.1227/01.NEU.0000311073.71733.C4. [DOI] [PubMed] [Google Scholar]
- 240.Rangel-Castilla L, Holman PJ, Krishna C, Trask TW, Klucznik RP, Diaz OM. Spinal extradural arteriovenous fistulas: a clinical and radiological description of different types and their novel treatment with Onyx. J Neurosurg Spine. 2011;15(5):541–549. doi: 10.3171/2011.6.SPINE10695. [DOI] [PubMed] [Google Scholar]
- 241.Gemmete JJ, Chaudhary N, Elias AE, Toma AK, Pandey AS, Parker RA, et al. Spinal dural arteriovenous fistulas: clinical experience with endovascular treatment as a primary therapy at 2 academic referral centers. AJNR Am J Neuroradiol. 2013;34(10):1974–1979. doi: 10.3174/ajnr.A3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.van Dijk JMC, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke. 2002;33(6):1578–1583. doi: 10.1161/01.str.0000018009.83713.06. [DOI] [PubMed] [Google Scholar]
- 243.Ruiz-Juretschke F, Perez-Calvo JM, Castro E, García-Leal R, Mateo-Sierra O, Fortea F, et al. A single-center, long-term study of spinal dural arteriovenous fistulas with multidisciplinary treatment. J Clin Neurosci. 2011;18(12):1662–1666. doi: 10.1016/j.jocn.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 244.Da Costa L, Dehdashti AR, TerBrugge KG. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009;26(1):E6. doi: 10.3171/FOC.2009.26.1.E6. [DOI] [PubMed] [Google Scholar]
- 245.Thron A. Spinale durale arteriovenöse Fisteln. Radiologe. 2001;41(11):955–960. doi: 10.1007/s001170170031. [DOI] [PubMed] [Google Scholar]
- 246.Calderon P, Heredero A, Pastor A, Higueras J, Hernandez J, Karagounis PA, et al. Successful removal of a floating thrombus in ascending aorta. Ann Thorac Surg. 2011;91(5):e67–e69. doi: 10.1016/j.athoracsur.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 247.Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting with flaccid paraplegia. Spine (Phila Pa 1976) 2011;36(15):E1042–5. doi: 10.1097/BRS.0b013e3181fee67f. [DOI] [PubMed] [Google Scholar]
- 248.Emanuela C, Francesco C, Massimiliano PA, Andrea V, Manicourt DH, Piccoli GB. Spontaneous Renal Artery Dissection in Ehler-Danlos Syndrome. Kidney Int Rep. 2019;4(11):1649–1652. doi: 10.1016/j.ekir.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Suntharalingam S, Ringelstein A, Forsting M, Sure U, van de Nes J, Gembruch O. Completely extradural intraspinal arteriovenous malformation in the lumbar spine: A case report. Journal of Medical Case Reports 2014; 8(1). Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L53202255&from=export. [DOI] [PMC free article] [PubMed]
- 250.Heldner MR, Arnold M, Nedeltchev K, Gralla J, Beck J, Fischer U. Vascular diseases of the spinal cord: a review. Curr Treat Options Neurol. 2012;14(6):509–520. doi: 10.1007/s11940-012-0190-9. [DOI] [PubMed] [Google Scholar]
- 251.Marshman LAG, David KM, Chawda SJ. Lumbar extradural arteriovenous malformation: case report and literature review. Spine J. 2007;7(3):374–379. doi: 10.1016/j.spinee.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 252.Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2017;89(7):1149–1154. doi: 10.1002/ccd.26977. [DOI] [PubMed] [Google Scholar]
- 253.McAree BJ, O'Donnell ME, Fitzmaurice GJ, Reid JA, Spence R, Lee B. Inferior vena cava thrombosis: A review of current practice. Vascular Medicine (United Kingdom) 2013;18(1):32–43. doi: 10.1177/1358863X12471967. [DOI] [PubMed] [Google Scholar]
- 254.Hammer A, Knight I, Agarwal A. Localized venous plexi in the spine simulating prolapse of an intervertebral disc: a report of six cases. Spine (Phila Pa 1976) 2003;28(1):E5–E12. doi: 10.1097/00007632-200301010-00025. [DOI] [PubMed] [Google Scholar]
- 255.Aoyama T, Hida K, Akino M, Yano S, Saito H, Iwasaki Y. Radiculopathy caused by lumbar epidural venous varix: case report. Neurol Med Chir (Tokyo) 2008;48(8):367–371. doi: 10.2176/nmc.48.367. [DOI] [PubMed] [Google Scholar]
- 256.Bozkurt G, Cil B, Akbay A, Türk CC, Palaoğlu S. Intractable radicular and low back pain secondary inferior vena cava stenosis associated with Budd-Chiari syndrome: endovascular treatment with cava stenting: case report and review of the literature. Spine (Phila Pa 1976) 2006;31(12):E383–6. doi: 10.1097/01.brs.0000219516.54500.97. [DOI] [PubMed] [Google Scholar]
- 257.Nowak P, Dietze L, Schlichter A, Jacoby N, Beckers KH. Beidseitige Becken- und Beinvenenthrombose bei einem 18-jährigen Patienten. Internist (Berl) 2008;49(2):228–231. doi: 10.1007/s00108-007-1992-9. [DOI] [PubMed] [Google Scholar]
- 258.Dudeck O, Zeile M, Poellinger A, Kluhs L, Ludwig W-D, Hamm B. Epidural venous enlargements presenting with intractable lower back pain and sciatica in a patient with absence of the infrarenal inferior vena cava and bilateral deep venous thrombosis. Spine (Phila Pa 1976) 2007;32(23):E688–91. doi: 10.1097/BRS.0b013e318158cf94. [DOI] [PubMed] [Google Scholar]
- 259.Vasco PG, López AR, Piñeiro ML, Gallego Rivera JI. Deep venous thrombosis caused by congenital inferior vena cava agenesis and heterozygous factor v Leiden mutation a case report. Int J Angiol. 2009;18(3):147–9. doi: 10.1055/s-0031-1278343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Aday AW, Sobieszczyk PS, Beckman JA. Absent at Birth: An Unusual Case of Deep Vein Thrombosis. Circulation. 2016;133(12):1209–1216. doi: 10.1161/CIRCULATIONAHA.115.020061. [DOI] [PubMed] [Google Scholar]
- 261.Williams JG, Phan H, Winston HR, Fugit RV, Graney B, Jamroz B, et al. A 27-Year-Old Man With Acute Severe Low Back Pain and Bilateral Leg Swelling That Prompted Renting a Wheelchair for Mobility. Chest. 2017;151(2):e35–e39. doi: 10.1016/j.chest.2016.08.1459. [DOI] [PubMed] [Google Scholar]
- 262.Langer F, Dos Santos D, Suertegaray G, Haygert C. Bilateral Deep Vein Thrombosis Associated with Inferior Vena Cava Agenesis in a Young Patient Manifesting as Low Back Pain. Acta Med Port. 2017;30(4):333–7. doi: 10.20344/amp.7744. [DOI] [PubMed] [Google Scholar]
- 263.Adachi Y, Sakakura K, Okochi T, Mase T, Matsumoto M, Wada H, et al. A Pitfall in the Diagnosis of Bilateral Deep Vein Thrombosis in a Young Man. Int Heart J. 2018;59(2):451–454. doi: 10.1536/ihj.17-159. [DOI] [PubMed] [Google Scholar]
- 264.Ikeda S, Koga S, Yamagata Y, Koide Y, Kawano H, Mukae H, et al. Pulmonary thromboembolism caused by calcification in the inferior vena cava of a Japanese adult. Internal Medicine. 2019;58(13):1907–12. doi: 10.2169/internalmedicine.2259-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Umar M, Benish T, Camacho L. What is causing this patient's low back pain? JAAPA. 2019;32(9):53–56. doi: 10.1097/01.JAA.0000578800.23040.2d. [DOI] [PubMed] [Google Scholar]
- 266.Genevay S, Palazzo E, Huten D, Fossati P, Meyer O. Lumboradiculopathy due to epidural varices: two case reports and a review of the literature. Joint Bone Spine. 2002;69(2):214–217. doi: 10.1016/s1297-319x(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 267.Moonis G, Hurst RW, Simon SL, Zager EL. Intradural venous varix: a rare cause of an intradural lumbar spine lesion. Spine (Phila Pa 1976) 2003;28(20):E430–2. doi: 10.1097/01.BRS.0000085359.59829.44. [DOI] [PubMed] [Google Scholar]
- 268.Paksoy Y, Gormus N. Epidural venous plexus enlargements presenting with radiculopathy and back pain in patients with inferior vena cava obstruction or occlusion. Spine (Phila Pa 1976) 2004;29(21):2419–24. doi: 10.1097/01.brs.0000144354.36449.2f. [DOI] [PubMed] [Google Scholar]
- 269.Pennekamp PH, Gemünd M, Kraft CN, von Engelhardt LV, Lüring C, Schmitz A. Epidurale Varikose als seltene Ursache eines akuten radikulären Lumbalsyndroms mit kompletter Fussheber- und Fusssenkerparese-Kasuistik und Literaturübersicht. Z Orthop Ihre Grenzgeb. 2007;145(1):55–60. doi: 10.1055/s-2007-960503. [DOI] [PubMed] [Google Scholar]
- 270.Paldor I, Gomori JM, Lossos A, Yatsiv I, Cohen JE, Itshayek E. Intradural lumbar varix resembling a tumor: case report of a magnetic resonance imaging-based diagnosis. Spine (Phila Pa 1976) 2010;35(17):E864–6. doi: 10.1097/BRS.0b013e3181d6debb. [DOI] [PubMed] [Google Scholar]
- 271.Lee YS, Choi ES, Kim JO, Ji JH. A rare calcified thrombosis of the dilated epidural venous plexus presenting with lumbar radiculopathy: a case report. Spine J. 2011;11(2):e28–31. doi: 10.1016/j.spinee.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 272.Pacult MA, Henderson FC, Wooster MD, Varma AK. Sciatica Caused by Venous Varix Compression of the Sciatic Nerve. World Neurosurg. 2018;117:242–245. doi: 10.1016/j.wneu.2018.06.058. [DOI] [PubMed] [Google Scholar]
- 273.Im I-K, Son E-S, Du Kim H. Lumbar Epidural Varix Causing Radicular Pain: A Case Report and Differential Diagnosis of Lumbar Cystic Lesions. PM R. 2018;10(11):1283–1287. doi: 10.1016/j.pmrj.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 274.Hallan DR, McNutt S, Reiter GT, Thamburaj K, Specht CS, Knaub M. Dilated Epidural Venous Plexus Causing Radiculopathy: A Report of 2 Cases and Review of the Literature. World Neurosurg. 2020;144:231–7. doi: 10.1016/j.wneu.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 275.Wang R, Yan Y, Zhan S, Song L, Sheng W, Song X, et al. Diagnosis of ovarian vein syndrome (OVS) by computed tomography (CT) imaging: a retrospective study of 11 cases. Medicine (Baltimore) 2014;93(7):e53. doi: 10.1097/MD.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kalender M, Baysal AN, Ugur O, Gökmengil H. Spontaneous left iliac vein rupture — Case report. Acta Angiologica. 2016;22(1):20–2. doi: 10.5603/AA.2016.0005. [DOI] [Google Scholar]
- 277.Vermeulen K, Schwagten V, Menovsky T. Concomitant Intraspinal and Retroperitoneal Hemorrhage Caused by an Aneurysm on the Celiac Artery: A Case Report. J Neurol Surg Rep. 2015;76(1):e28–e31. doi: 10.1055/s-0034-1395491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Castillo JM, Afanador HF, Manjarrez E, Morales XA. Non-Traumatic Spontaneous Spinal Subdural Hematoma in a Patient with Non-Valvular Atrial Fibrillation During Treatment with Rivaroxaban. Am J Case Rep. 2015;16:377–81. doi: 10.12659/AJCR.893320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.McHaourab A, Evans G-H, Austin R. Spontaneous spinal subdural haematoma in a patient on apixaban. BMJ Case Rep 2019; 12(1). Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L626035482&from=export. [DOI] [PMC free article] [PubMed]
- 280.Joubert C, Gazzola S, Sellier A, Dagain A. Acute idiopathic spinal subdural hematoma: What to do in an emergency? Neurochirurgie. 2019;65(2–3):93–97. doi: 10.1016/j.neuchi.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 281.Yokota K, Kawano O, Kaneyama H, Maeda T, Nakashima Y. Acute spinal subdural hematoma: A case report of spontaneous recovery from paraplegia. Medicine (Baltimore) 2020;99(19):e20032. doi: 10.1097/MD.0000000000020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Braun P, Kazmi K, Nogués-Meléndez P, Mas-Estellés F, Aparici-Robles F. MRI findings in spinal subdural and epidural hematomas. Eur J Radiol. 2007;64(1):119–125. doi: 10.1016/j.ejrad.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 283.Baek BS, Hur JW, Kwon KY, Lee HK. Spontaneous spinal epidural hematoma. Journal of Korean Neurosurgical Society. 2008;44(1):40–42. doi: 10.3340/jkns.2008.44.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.DeSouza R-M, Uff C, Galloway M, Dorward NL. Spinal epidural hematoma caused by pseudogout: A case report and literature review. Global Spine J. 2014;4(2):105–8. doi: 10.1055/s-0033-1360722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Matsui H, Imagama S, Ito Z, Ando K, Hirano K, Tauchi R, et al. Chronic spontaneous lumbar epidural hematoma simulating extradural spinal tumor: a case report. Nagoya J Med Sci. 2014;76(1–2):195–201. [PMC free article] [PubMed] [Google Scholar]
- 286.Goyal G, Singh R, Raj K. Anticoagulant induced spontaneous spinal epidural hematoma, conservative management or surgical intervention—A dilemma? J Acute Med. 2016;6(2):38–42. doi: 10.1016/j.jacme.2016.03.006. [DOI] [Google Scholar]
- 287.Ismail R, Zaghrini E, Hitti E. Spontaneous Spinal Epidural Hematoma in a Patient on Rivaroxaban: Case Report and Literature Review. J Emerg Med. 2017;53(4):536–9. doi: 10.1016/j.jemermed.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 288.Massand MG, Wallace RC, Gonzalez LF, Zabramski JM, Spetzler RF. Subarachnoid hemorrhage due to isolated spinal artery aneurysm in four patients. American Journal of Neuroradiology. 2005;26(9):2415–9. [PMC free article] [PubMed] [Google Scholar]
- 289.Toi H, Matsubara S, Watanabe S, Yamashita T, Uno M. Paraspinal arteriovenous fistula presenting with subarachnoid hemorrhage and acute progressive myelopathy–case report. Neurol Med Chir (Tokyo) 2011;51(12):846–849. doi: 10.2176/nmc.51.846. [DOI] [PubMed] [Google Scholar]
- 290.Steele L, Raza MH, Perry R, Rane N, Camp SJ. Subarachnoid haemorrhage due to intracranial vertebral artery dissection presenting with atypical cauda equina syndrome features: case report. BMC Neurol. 2019;19(1):262. doi: 10.1186/s12883-019-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yamasaki T, Shirahase T, Hashimura T. Chronic expanding hematoma in the psoas muscle. Int J Urol. 2005;12(12):1063–5. doi: 10.1111/j.1442-2042.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 292.Fukuda C, Hirofuji E. Juxta-facet hematoma. J Orthop Sci. 2007;12(6):597–600. doi: 10.1007/s00776-007-1170-x. [DOI] [PubMed] [Google Scholar]
- 293.Taghipour M, Javadi S, Attaran Y, Bagheri MH. Intradural nerve root hematoma in the lumbar spine. A case report. Emerg Radiol. 2008;15(4):271–272. doi: 10.1007/s10140-007-0677-y. [DOI] [PubMed] [Google Scholar]
- 294.Li K-P, Zhu J, Zhang J-L, Huang F. Idiopathic retroperitoneal fibrosis (RPF): clinical features of 61 cases and literature review. Clin Rheumatol. 2011;30(5):601–605. doi: 10.1007/s10067-010-1580-6. [DOI] [PubMed] [Google Scholar]
- 295.Blanc G, Girard N, Alexandre C, Vignon E. Retroperitoneal fibrosis: a rare vascular and immune entity disclosed by chronic lombalgia. Joint Bone Spine. 2007;74(5):497–499. doi: 10.1016/j.jbspin.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 296.Drieskens O, Blockmans D, van den Bruel A, Mortelmans L. Riedel's thyroiditis and retroperitoneal fibrosis in multifocal fibrosclerosis positron emission tomographic findings. Clin Nucl Med. 2002;27(6):413–5. doi: 10.1097/00003072-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 297.Paetzold S, Gary T, Hafner F, Brodmann M. Thrombosis of the inferior vena cava related to Ormond's disease. Clin Rheumatol. 2013;32(SUPPL. 1):67–70. doi: 10.1007/s10067-010-1456-9. [DOI] [PubMed] [Google Scholar]
- 298.Reilly RF., Jr Retroperitoneal fibrosis presenting as acute renal failure. Nature clinical practice. Nephrology. 2005;1(1):55–59. doi: 10.1038/ncpneph0023. [DOI] [PubMed] [Google Scholar]
- 299.van Bommel E, Jansen I, Hendriksz TR, Aarnoudse A. Idiopathic retroperitoneal fibrosis: Prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore) 2009;88(4):193–201. doi: 10.1097/MD.0b013e3181afc420. [DOI] [PubMed] [Google Scholar]
- 300.Yachoui R, Sehgal R, Carmichael B. Idiopathic retroperitoneal fibrosis: clinicopathologic features and outcome analysis. Clin Rheumatol. 2016;35(2):401–407. doi: 10.1007/s10067-015-3022-y. [DOI] [PubMed] [Google Scholar]
- 301.Liu H, Zhang G, Niu Y, Jiang N, Xiao W. Retroperitoneal fibrosis: a clinical and outcome analysis of 58 cases and review of literature. Rheumatol Int. 2014;34(12):1665–1670. doi: 10.1007/s00296-014-3002-6. [DOI] [PubMed] [Google Scholar]
- 302.Palmisano A, Urban ML, Buzio C, Vaglio A. Treatment of idiopathic retroperitoneal fibrosis. Expert Opin Orphan Drugs. 2014;2(8):769–77. doi: 10.1517/21678707.2014.921615. [DOI] [Google Scholar]
- 303.Pipitone N, Vaglio A, Salvarani C. Retroperitoneal fibrosis. Best Pract Res Clin Rheumatol. 2012;26(4):439–448. doi: 10.1016/j.berh.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 304.Paravastu SCV, Murray D, Ghosh J, Serracino-Inglott F, Smyth JV, Walker MG. Inflammatory abdominal aortic aneurysms (IAAA): past and present. Vasc Endovascular Surg. 2009;43(4):360–363. doi: 10.1177/1538574409335915. [DOI] [PubMed] [Google Scholar]
- 305.Moroni G, Gallelli B, Banfi G, Sandri S, Messa P, Ponticelli C. Long-term outcome of idiopathic retroperitoneal fibrosis treated with surgical and/or medical approaches. Nephrol Dial Transplant. 2006;21(9):2485–2490. doi: 10.1093/ndt/gfl228. [DOI] [PubMed] [Google Scholar]
- 306.Ceresini G, Urban ML, Corradi D, Lauretani F, Marina M, Usberti E, et al. Association between idiopathic retroperitoneal fibrosis and autoimmune thyroiditis: a case-control study. Autoimmun Rev. 2015;14(1):16–22. doi: 10.1016/j.autrev.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 307.Gornik HL, Creager MA. Aortitis. Circulation. 2008;117(23):3039–3051. doi: 10.1161/CIRCULATIONAHA.107.760686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315–347. doi: 10.1146/annurev-pathol-012513-104708. [DOI] [PubMed] [Google Scholar]
- 309.Doshi A, Khosravi M, Marks DJB, Rodriguez-Justo M, Connolly JO, de Wolff JF. Back pain and acute kidney injury. Clin Med (Lond) 2013;13(1):71–74. doi: 10.7861/clinmedicine.13-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zen Y, Sawazaki A, Miyayama S, Notsumata K, Tanaka N, Nakanuma Y. A case of retroperitoneal and mediastinal fibrosis exhibiting elevated levels of IgG4 in the absence of sclerosing pancreatitis (autoimmune pancreatitis) Human Pathol. 2006;37(2):239–43. doi: 10.1016/j.humpath.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 311.Chiba K, Kamisawa T, Tabata T, Hara S, Kuruma S, Fujiwara T, et al. Clinical features of 10 patients with IgG4-related retroperitoneal fibrosis. Intern Med. 2013;52(14):1545–51. doi: 10.2169/internalmedicine.52.0306. [DOI] [PubMed] [Google Scholar]
- 312.Simone G, Leonardo C, Papalia R, Guaglianone S, Gallucci M. Laparoscopic ureterolysis and omental wrapping. Urology. 2008;72(4):853–858. doi: 10.1016/j.urology.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 313.Tritschler T, Bleisch J, Al Rifai A, Marques Maggio E, Müller S, Schorn R. Subakute lumbale Rückenschmerzen und akute Niereninsuffizienz bei einem 47-jährigen Mann. Internist (Berl) 2014;55(9):1096–1096-9. doi: 10.1007/s00108-014-3525-7. [DOI] [PubMed] [Google Scholar]
- 314.Brodmann M, Lipp RW, Aigner R, Pilger E. Positron emission tomography reveals extended thoracic and abdominal peri-aortitis. Vasc Med. 2003;8(2):127–128. doi: 10.1191/1358863x03vm472xx. [DOI] [PubMed] [Google Scholar]
- 315.Jois RN, Gaffney K, Marshall T, Scott DGI. Chronic periaortitis. Rheumatology (United Kingdom) 2004;43(11):1441–1446. doi: 10.1093/rheumatology/keh326. [DOI] [PubMed] [Google Scholar]
- 316.Maritati F, Corradi D, Versari A, Casali M, Urban ML, Buzio C, et al. Rituximab therapy for chronic periaortitis. Ann Rheum Dis. 2012;71(7):1262–1264. doi: 10.1136/annrheumdis-2011-201166. [DOI] [PubMed] [Google Scholar]
- 317.Vaglio A, Palmisano A, Ferretti S, Alberici F, Casazza I, Salvarani C, et al. Peripheral inflammatory arthritis in patients with chronic periaortitis: report of five cases and review of the literature. Rheumatology (Oxford) 2008;47(3):315–318. doi: 10.1093/rheumatology/kem328. [DOI] [PubMed] [Google Scholar]
- 318.Kawamoto M. Clinical diagnosis: retroperitoneal fibrosis. Ann Nucl Med. 2003;17(1):frontcover 68. doi: 10.1007/BF02988262. [DOI] [PubMed] [Google Scholar]
- 319.Oshiro H, Ebihara Y, Serizawa H, Shimizu T, Teshima S, Kuroda M, et al. Idiopathic retroperitoneal fibrosis associated with immunohematological abnormalities. Am J Med. 2005;118(7):782–786. doi: 10.1016/j.amjmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 320.Adler S, Lodermeyer S, Gaa J, Heemann U. Successful mycophenolate mofetil therapy in nine patients with idiopathic retroperitoneal fibrosis. Rheumatology (Oxford) 2008;47(10):1535–1538. doi: 10.1093/rheumatology/ken291. [DOI] [PubMed] [Google Scholar]
- 321.Brandt AS, Kamper L, Kukuk S, Haage P, Roth S. Associated findings and complications of retroperitoneal fibrosis in 204 patients: results of a urological registry. J Urol. 2011;185(2):526–531. doi: 10.1016/j.juro.2010.09.105. [DOI] [PubMed] [Google Scholar]
- 322.Corradi D, Maestri R, Palmisano A, Bosio S, Greco P, Manenti L, et al. Idiopathic retroperitoneal fibrosis: clinicopathologic features and differential diagnosis. Kidney Int. 2007;72(6):742–753. doi: 10.1038/sj.ki.5002427. [DOI] [PubMed] [Google Scholar]
- 323.Ilie CP, Pemberton RJ, Tolley DA. Idiopathic retroperitoneal fibrosis: the case for nonsurgical treatment. BJU Int. 2006;98(1):137–140. doi: 10.1111/j.1464-410X.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 324.Jansen I, Hendriksz TR, Han SH, Huiskes AWLC, van Bommel EFH. (18)F-fluorodeoxyglucose position emission tomography (FDG-PET) for monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Eur J Intern Med. 2010;21(3):216–221. doi: 10.1016/j.ejim.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 325.Kardar AH, Kattan S, Lindstedt E, Hanash K. Steroid therapy for idiopathic retroperitoneal fibrosis: dose and duration. J Urol. 2002;168(2):550–555. doi: 10.1016/S0022-5347(05)64677-0. [DOI] [PubMed] [Google Scholar]
- 326.Kermani TA, Crowson CS, Achenbach SJ, Luthra HS. Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomes. Mayo Clin Proc. 2011;86(4):297–303. doi: 10.4065/mcp.2010.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Nakajo M, Jinnouchi S, Tanabe H, Tateno R, Nakajo M. 18F-fluorodeoxyglucose positron emission tomography features of idiopathic retroperitoneal fibrosis. J Comput Assist Tomogr. 2007;31(4):539–543. doi: 10.1097/01.rct.0000284388.45579.05. [DOI] [PubMed] [Google Scholar]
- 328.van Bommel EFH, Siemes C, van der Veer SJ, Han SH, Huiskes AWLC, Hendriksz TR. Clinical value of gallium-67 SPECT scintigraphy in the diagnostic and therapeutic evaluation of retroperitoneal fibrosis: a prospective study. J Intern Med. 2007;262(2):224–234. doi: 10.1111/j.1365-2796.2007.01805.x. [DOI] [PubMed] [Google Scholar]
- 329.Vega J, Goecke H, Tapia H, Labarca E, Santamarina M, Martínez G. Treatment of idiopathic retroperitoneal fibrosis with colchicine and steroids: a case series. Am J Kidney Dis. 2009;53(4):628–637. doi: 10.1053/j.ajkd.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 330.Zen Y, Onodera M, Inoue D, Kitao A, Matsui O, Nohara T, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol. 2009;33(12):1833–1839. doi: 10.1097/pas.0b013e3181b72882. [DOI] [PubMed] [Google Scholar]
- 331.Salvarani C, Pipitone N, Versari A, Vaglio A, Serafini D, Bajocchi G, et al. Positron emission tomography (PET): evaluation of chronic periaortitis. Arthritis Rheum. 2005;53(2):298–303. doi: 10.1002/art.21074. [DOI] [PubMed] [Google Scholar]
- 332.Vaglio A, Corradi D, Manenti L, Ferretti S, Garini G, Buzio C. Evidence of autoimmunity in chronic periaortitis: a prospective study. Am J Med. 2003;114(6):454–462. doi: 10.1016/S0002-9343(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 333.Warnatz K, Keskin AG, Uhl M, Scholz C, Katzenwadel A, Vaith P, et al. Immunosuppressive treatment of chronic periaortitis: a retrospective study of 20 patients with chronic periaortitis and a review of the literature. Ann Rheum Dis. 2005;64(6):828–833. doi: 10.1136/ard.2004.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhou H-J, Yan Y, Zhou B, Lan T-F, Wang X-Y, Li C-S. Retroperitoneal fibrosis: a retrospective clinical data analysis of 30 patients in a 10-year period. Chin Med J (Engl) 2015;128(6):804–810. doi: 10.4103/0366-6999.152648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ha YJ, Jung SJ, Lee KH, Lee S-W, Lee S-K, Park Y-B. Retroperitoneal fibrosis in 27 Korean patients: single center experience. J Korean Med Sci. 2011;26(8):985–990. doi: 10.3346/jkms.2011.26.8.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang S, Chen M, Li C-M, Song G-D, Liu Y. Differentiation of Lymphoma Presenting as Retroperitoneal Mass and Retroperitoneal Fibrosis: Evaluation with Multidetector-row Computed Tomography. Chin Med J (Engl) 2017;130(6):691–697. doi: 10.4103/0366-6999.201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pipitone N, Salvarani C, Peter HH. Chronische Periaortitis. Internist (Berl) 2010;51(1):45–52. doi: 10.1007/s00108-009-2407-x. [DOI] [PubMed] [Google Scholar]
- 338.Hara N, Kawaguchi M, Takeda K, Zen Y. Retroperitoneal disorders associated with IGG4-related autoimmune pancreatitis. World J Gastroenterol. 2014;20(44):16550–8. doi: 10.3748/wjg.v20.i44.16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Burkhardt Soares S, Kukuk S, Brandt AS, Fehr A, Roth S. Retroperitoneale Fibrose. Urologe A. 2008;47(4):489–499. doi: 10.1007/s00120-008-1705-6. [DOI] [PubMed] [Google Scholar]
- 340.Caiafa RO, Vinuesa AS, Izquierdo RS, Brufau BP, Ayuso Colella JR, Molina CN. Retroperitoneal fibrosis: role of imaging in diagnosis and follow-up. Radiographics. 2013;33(2):535–552. doi: 10.1148/rg.332125085. [DOI] [PubMed] [Google Scholar]
- 341.Cronin CG, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol. 2008;191(2):423–431. doi: 10.2214/AJR.07.3629. [DOI] [PubMed] [Google Scholar]
- 342.Swartz RD. Idiopathic retroperitoneal fibrosis: a review of the pathogenesis and approaches to treatment. Am J Kidney Dis. 2009;54(3):546–553. doi: 10.1053/j.ajkd.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 343.Palmisano A, Vaglio A. Chronic periaortitis: a fibro-inflammatory disorder. Best Pract Res Clin Rheumatol. 2009;23(3):339–353. doi: 10.1016/j.berh.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 344.Vaglio A, Buzio C. Chronic periaortitis: a spectrum of diseases. Curr Opin Rheumatol. 2005;17(1):34–40. doi: 10.1097/01.bor.0000145517.83972.40. [DOI] [PubMed] [Google Scholar]
- 345.Vaglio A, Greco P, Corradi D, Palmisano A, Martorana D, Ronda N, et al. Autoimmune aspects of chronic periaortitis. Autoimmun Rev. 2006;5(7):458–464. doi: 10.1016/j.autrev.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 346.Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367(9506):241–251. doi: 10.1016/S0140-6736(06)68035-5. [DOI] [PubMed] [Google Scholar]
- 347.Vaglio A, Palmisano A, Corradi D, Salvarani C, Buzio C. Retroperitoneal fibrosis: evolving concepts. Rheum Dis Clin North Am. 2007;33(4):803–17. doi: 10.1016/j.rdc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 348.Vaglio A, Palmisano A, Alberici F, Maggiore U, Ferretti S, Cobelli R, et al. Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet. 2011;378(9788):338–346. doi: 10.1016/S0140-6736(11)60934-3. [DOI] [PubMed] [Google Scholar]
- 349.Vaglio A, Pipitone N, Salvarani C. Chronic periaortitis: a large-vessel vasculitis? Curr Opin Rheumatol. 2011;23(1):1–6. doi: 10.1097/BOR.0b013e328341137d. [DOI] [PubMed] [Google Scholar]
- 350.Vaglio A, Maritati F. Idiopathic Retroperitoneal Fibrosis. J Am Soc Nephrol. 2016;27(7):1880–1889. doi: 10.1681/ASN.2015101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Geoghegan T, Byrne AT, Benfayed W, McAuley G, Torreggiani WC. Imaging and intervention of retroperitoneal fibrosis. Australas Radiol. 2007;51(1):26–34. doi: 10.1111/j.1440-1673.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 352.Zeina A-R, Slobodin G, Gleb S, Naschitz JE, Loberman Z, Barmeir E. Isolated periaortitis: clinical and imaging characteristics. Vasc Health Risk Manag. 2007;3(6):1083–1086. [PMC free article] [PubMed] [Google Scholar]
- 353.Hadži-Djokić J, Pejčić T, Bašić D, Vukomanović I, Džamić Z, Aćimović M, et al. Idiopathic retroperitoneal fibrosis: A report on 15 patients. Vojnosanitetski Pregled. 2015;72(10):928–31. doi: 10.2298/VSP140426076H. [DOI] [PubMed] [Google Scholar]
- 354.Cavalleri A, Brunner P, Monticelli I, Mourou M-Y, Bruneton J-N. CT-guided biopsy in two cases of retroperitoneal fibrosis. Clin Imaging. 2008;32(3):230–232. doi: 10.1016/j.clinimag.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 355.Yang Q, Xu X, Zhu C. Low-Back Pain Due to Idiopathic Retroperitoneal Fibrosis. J Emerg Med. 2018;54(1):124–126. doi: 10.1016/j.jemermed.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 356.Famularo G, Palmisano A, Afeltra A, Buzzulini F, Versari A, Minisola G, et al. Retroperitoneal fibrosis associated with psoriasis: a case series. Scand J Rheumatol. 2009;38(1):68–69. doi: 10.1080/03009740802406187. [DOI] [PubMed] [Google Scholar]
- 357.Young PM, Peterson JJ, Calamia KT. Hypermetabolic activity in patients with active retroperitoneal fibrosis on F-18 FDG PET: report of three cases. Ann Nucl Med. 2008;22(1):87–92. doi: 10.1007/s12149-007-0077-0. [DOI] [PubMed] [Google Scholar]
- 358.Wu J, Catalano E, Coppola D. Retroperitoneal fibrosis (Ormond's disease): clinical pathologic study of eight cases. Cancer Control. 2002;9(5):432–437. doi: 10.1177/107327480200900510. [DOI] [PubMed] [Google Scholar]
- 359.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. The Lancet. 2002;359(9315):1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 360.Pizzini AM, Corrado S, Radighieri E, Ferretti G, Carani C, Papi G. Hashimoto's thyroiditis associated with idiopathic retroperitoneal fibrosis: case report and review of the literature. Int J Clin Pract. 2007;61(1):162–164. doi: 10.1111/j.1742-1241.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 361.Al-Hammouri FA, Khori FA, Abu Qamar AA, Nimate A, Kaabneh AB, Almajali A, et al. Management of Idiopathic Retroperitoneal Fibrosis, a Retrospective Study at Prince Hussein Urology and Organ transplantation center (PHUO) Jordan. Iran J Kidney Dis. 2019;13(4):251–256. [PubMed] [Google Scholar]
- 362.Fry AC, Singh S, Gunda SS, Boustead GB, Hanbury DC, McNicholas TA, et al. Successful use of steroids and ureteric stents in 24 patients with idiopathic retroperitoneal fibrosis: a retrospective study. Nephron Clin Pract. 2008;108(3):c213–c220. doi: 10.1159/000119715. [DOI] [PubMed] [Google Scholar]
- 363.Fairweather J, Jawad ASM. Immunoglobulin G4-related retroperitoneal fibrosis: a new name for an old disease. Urology. 2013;82(3):505–507. doi: 10.1016/j.urology.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 364.Tambyraja AL, Murie JA, Chalmers RTA. Ruptured inflammatory abdominal aortic aneurysm: insights in clinical management and outcome. J Vasc Surg. 2004;39(2):400–403. doi: 10.1016/j.jvs.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 365.Goldoni M, Bonini S, Urban ML, Palmisano A, de Palma G, Galletti E, et al. Asbestos and smoking as risk factors for idiopathic retroperitoneal fibrosis: a case-control study. Ann Intern Med. 2014;161(3):181–188. doi: 10.7326/M13-2648. [DOI] [PubMed] [Google Scholar]
- 366.Kasashima S, Zen Y. IgG4-related inflammatory abdominal aortic aneurysm. Curr Opin Rheumatol. 2011;23(1):18–23. doi: 10.1097/BOR.0b013e32833ee95f. [DOI] [PubMed] [Google Scholar]
- 367.Ichinose T, Yamase M, Yokomatsu Y, Kawano Y, Konishi H, Tanimoto K, et al. Acute myocardial infarction with myocardial perfusion defect detected by contrast-enhanced computed tomography. Intern Med. 2009;48(14):1235–1238. doi: 10.2169/internalmedicine.48.1753. [DOI] [PubMed] [Google Scholar]
- 368.Sederholm Lawesson S, Isaksson R-M, Thylén I, Ericsson M, Ängerud K, Swahn E. Gender differences in symptom presentation of ST-elevation myocardial infarction – An observational multicenter survey study. Int J Cardiol. 2018;264:7–11. doi: 10.1016/j.ijcard.2018.03.084. [DOI] [PubMed] [Google Scholar]
- 369.Berg J, Björck L, Dudas K, Lappas G, Rosengren A. Symptoms of a first acute myocardial infarction in women and men. Gend Med. 2009;6(3):454–462. doi: 10.1016/j.genm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 370.Culić V, Eterović D, Mirić D, Silić N. Symptom presentation of acute myocardial infarction: influence of sex, age, and risk factors. Am Heart J. 2002;144(6):1012–1017. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- 371.Kosuge M, Kimura K, Ishikawa T, Ebina T, Hibi K, Tsukahara K, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222–226. doi: 10.1253/circj.70.222. [DOI] [PubMed] [Google Scholar]
- 372.Løvlien M, Schei B, Hole T. Women with myocardial infarction are less likely than men to experience chest symptoms. Scand Cardiovasc J. 2006;40(6):342–347. doi: 10.1080/14017430600913199. [DOI] [PubMed] [Google Scholar]
- 373.Kinoshita H, Hashimoto M, Nishimura K, Kodama T, Matsuo H, Furukawa S, et al. Two cases of acute cholecystitis in which percutaneous transhepatic gallbladder aspiration (PTGBA) was useful. Kurume Med J. 2002;49(3):161–165. doi: 10.2739/kurumemedj.49.161. [DOI] [PubMed] [Google Scholar]
- 374.Petersen EJ, Thurmond SM. Differential Diagnosis in a Patient Presenting With Both Systemic and Neuromusculoskeletal Pathology: Resident's Case Problem. J Orthop Sports Phys Ther. 2018;48(6):496–503. doi: 10.2519/jospt.2018.7652. [DOI] [PubMed] [Google Scholar]
- 375.van Dijk AH, Wennmacker SZ, de Reuver PR, Latenstein CSS, Buyne O, Donkervoort SC, et al. Restrictive strategy versus usual care for cholecystectomy in patients with gallstones and abdominal pain (SECURE): a multicentre, randomised, parallel-arm, non-inferiority trial. Lancet. 2019;393(10188):2322–2330. doi: 10.1016/S0140-6736(19)30941-9. [DOI] [PubMed] [Google Scholar]
- 376.Matsubara T, Sakurai Y, Miura H, Kobayashi H, Shoji M, Nakamura Y, et al. Complete obstruction of the lower common bile duct caused by autoimmune pancreatitis: Is biliary reconstruction really necessary? J Hepato-Biliary-Pancreat Surg. 2005;12(1):76–83. doi: 10.1007/s00534-004-0937-0. [DOI] [PubMed] [Google Scholar]
- 377.Nishimura T, Masaoka T, Suzuki H, Aiura K, Nagata H, Ishii H. Autoimmune pancreatitis with pseudocysts. J Gastroenterol. 2004;39(10):1005–1010. doi: 10.1007/s00535-004-1436-4. [DOI] [PubMed] [Google Scholar]
- 378.Rompianesi G, Hann A, Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database Syst Rev. 2017;4:CD012010. doi: 10.1002/14651858.CD012010.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, Irie H, et al. Japanese clinical guidelines for autoimmune pancreatitis. Pancreas. 2009;38(8):849–866. doi: 10.1097/MPA.0b013e3181b9ee1c. [DOI] [PubMed] [Google Scholar]
- 380.Hoffman RJ, Dhaliwal G, Gilden DJ, Saint S. Clinical problem-solving. Special cure. N Engl J Med. 2004;351(19):1997–2002. doi: 10.1056/NEJMcps040877. [DOI] [PubMed] [Google Scholar]
- 381.Kobayashi T, Casablanca NM, Harrington M. Pyeloduodenal fistula diagnosed with technetium-99m scintigraphy and managed with a conservative strategy. BMJ Case Rep 2018; 2018. Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L621327148&from=export. [DOI] [PMC free article] [PubMed]
- 382.Kuday Kaykisiz E, Yildirim MB, Dadali E, Kaykisiz H, Ünlüer EE. Different manifestation of a familiar diagnosis: From anuria to acute appendicitis. Am J Emerg Med. 2018;36(5):910.e5–910.e7. doi: 10.1016/j.ajem.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 383.Anaya A, Plantmason L, Dhaliwal G. Back attack. J Gen Intern Med. 2014;29(1):255–259. doi: 10.1007/s11606-013-2487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871–874. doi: 10.1007/s11606-008-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Khan RJK, Fick DP, Guier CA, Menolascino MJ, Neal MC. Acute paraspinal compartment syndrome. A case report. J Bone Joint Surg Am. 2005;87(5):1126–8. doi: 10.1002/14651858.CD012010.pub2. [DOI] [PubMed] [Google Scholar]
- 386.Wik L, Patterson JM, Oswald AE. Exertional paraspinal muscle rhabdomyolysis and compartment syndrome: a cause of back pain not to be missed. Clin Rheumatol. 2010;29(7):803–805. doi: 10.1007/s10067-010-1391-9. [DOI] [PubMed] [Google Scholar]
- 387.Paryavi E, Jobin CM, Ludwig SC, Zahiri H, Cushman J. Acute exertional lumbar paraspinal compartment syndrome. Spine (Phila Pa 1976) 2010;35(25):E1529–33. doi: 10.1097/BRS.0b013e3181ec4023. [DOI] [PubMed] [Google Scholar]
- 388.Karam MD, Amendola A, Mendoza-Lattes S. Case report: successful treatment of acute exertional paraspinal compartment syndrome with hyperbaric oxygen therapy. Iowa Orthop J. 2010;30:188–190. [PMC free article] [PubMed] [Google Scholar]
- 389.Kitajima I, Tachibana S, Hirota Y, Nakamichi K. Acute paraspinal muscle compartment syndrome treated with surgical decompression: a case report. Am J Sports Med. 2002;30(2):283–285. doi: 10.1177/03635465020300022301. [DOI] [PubMed] [Google Scholar]
- 390.Xu YM, Bai YH, Li QT, Yu H, Cao ML. Chronic lumbar paraspinal compartment syndrome: a case report and review of the literature. J Bone Joint Surg Br. 2009;91(12):1628–1630. doi: 10.1302/0301-620X.91B12.22647. [DOI] [PubMed] [Google Scholar]
- 391.Nathan ST, Roberts CS, Deliberato D. Lumbar paraspinal compartment syndrome. Int Orthop. 2012;36(6):1221–1227. doi: 10.1007/s00264-011-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Darai E, Ackerman G, Bazot M, Rouzier R, Dubernard G. Laparoscopic segmental colorectal resection for endometriosis: Limits and complications. Surgical Endoscopy. 2007;21(9):1572–7. doi: 10.1007/s00464-006-9160-1. [DOI] [PubMed] [Google Scholar]
- 393.Markham R, Luscombe GM, Manconi F, Fraser IS. A detailed profile of pain in severe endometriosis. J Endometriosis Pelvic Pain Dis. 2019;11(2):85–94 https://www.embase.com/search/results?subaction=viewrecord&id=L627201072&from=export.
- 394.Agrawal A, Shetty BJP, Makannavar JH, Shetty L, Shetty J, Shetty V. Intramedullary endometriosis of the conus medullaris: case report. Neurosurgery. 2006;59(2):E428. doi: 10.1227/01.NEU.0000223375.23617.DC. [DOI] [PubMed] [Google Scholar]
- 395.Hsieh M-F, Wu I, Tsai C-J, Huang S-S, Chang L-C, Wu M-S. Ureteral endometriosis with obstructive uropathy. Intern Med. 2010;49(6):573–6. doi: 10.2169/internalmedicine.49.2797. [DOI] [PubMed] [Google Scholar]
- 396.Kanthimathinathan V, Elakkary E, Bleibel W, Kuwajerwala N, Conjeevaram S, Tootla F. Endometrioma of the large bowel. Dig Dis Sci. 2007;52(3):767–769. doi: 10.1007/s10620-006-9623-1. [DOI] [PubMed] [Google Scholar]
- 397.Steinberg JA, Gonda DD, Muller K, Ciacci JD. Endometriosis of the conus medullaris causing cyclic radiculopathy. J Neurosurg Spine. 2014;21(5):799–804. doi: 10.3171/2014.7.SPINE14117. [DOI] [PubMed] [Google Scholar]
- 398.Generao SE, Keene KD, Das S. Endoscopic diagnosis and management of ureteral endometriosis. J Endourol. 2005;19(10):1177–1179. doi: 10.1089/end.2005.19.1177. [DOI] [PubMed] [Google Scholar]
- 399.Al-Khodairy A-WT, Bovay P, Gobelet C. Sciatica in the female patient: anatomical considerations, aetiology and review of the literature. Eur Spine J. 2007;16(6):721–31. doi: 10.1007/s00586-006-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Young K, Fisher J, Kirkman M. Women's experiences of endometriosis: a systematic review and synthesis of qualitative research. J Fam Plann Reprod Health Care. 2015;41(3):225–234. doi: 10.1136/jfprhc-2013-100853. [DOI] [PubMed] [Google Scholar]
- 401.Daraï E, Ballester M, Chereau E, Coutant C, Rouzier R, Wafo E. Laparoscopic versus laparotomic radical en bloc hysterectomy and colorectal resection for endometriosis. Surgical Endoscopy. 2010;24(12):3060–7. doi: 10.1007/s00464-010-1089-8. [DOI] [PubMed] [Google Scholar]
- 402.Stepniewska A, Grosso G, Molon A, Caleffi G, Perin E, Scioscia M, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26(1):112–116. doi: 10.1093/humrep/deq293. [DOI] [PubMed] [Google Scholar]
- 403.Byrne D, Curnow T, Smith P, Cutner A, Saridogan E, Clark TJ. Laparoscopic excision of deep rectovaginal endometriosis in BSGE endometriosis centres: a multicentre prospective cohort study. BMJ Open. 2018;8(4):e018924. doi: 10.1136/bmjopen-2017-018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Schliep KC, Mumford SL, Peterson CM, Chen Z, Johnstone EB, Sharp HT, et al. Pain typology and incident endometriosis. Hum Reprod. 2015;30(10):2427–2438. doi: 10.1093/humrep/dev147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Chudzinski A, Collinet P, Flamand V, Rubod C. Ureterovesical reimplantation for ureteral deep infiltrating endometriosis: A retrospective study. J Gynecol Obstet Hum Reprod. 2017;46(3):229–33. Verfügbar unter. https://www.embase.com/search/results?subaction=viewrecord&id=L616130655&from=export. [DOI] [PubMed]
- 406.Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2006;21(5):1243–1247. doi: 10.1093/humrep/dei491. [DOI] [PubMed] [Google Scholar]
- 407.Redwine DB, Wright JT. Laparoscopic treatment of complete obliteration of the cul-de-sac associated with endometriosis: long-term follow-up of en bloc resection. Fertil Steril. 2001;76(2):358–365. doi: 10.1016/s0015-0282(01)01913-6. [DOI] [PubMed] [Google Scholar]
- 408.Thomassin I, Bazot M, Detchev R, Barranger E, Cortez A, Darai E. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004;190(5):1264–1271. doi: 10.1016/j.ajog.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 409.Moore JS, Gibson PR, Perry RE, Burgell RE. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. 2017;57(2):201–205. doi: 10.1111/ajo.12594. [DOI] [PubMed] [Google Scholar]
- 410.Chiantera V, Abesadze E, Mechsner S. How to understand the complexity of endometriosis-related pain. J Endometr Pelvic Pain Dis. 2017;9(1):30–8. Verfügbar unter. https://www.embase.com/search/results?subaction=viewrecord&id=L616230958&from=export.
- 411.Kondo T. Ureteral polypoid endometriosis causing hydroureteronephrosis. Indian J Pathol Microbiol. 2009;52(2):246–248. doi: 10.4103/0377-4929.48934. [DOI] [PubMed] [Google Scholar]
- 412.Seyam R, Mokhtar A, Al Taweel W, Al Sayyah A, Tulbah A, Al Khudair W. Isolated ureteric endometriosis presenting as a ureteric tumor. Urol Ann. 2014;6(1):94–7. doi: 10.4103/0974-7796.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Uppal J, Sobotka S, Jenkins AL. Cyclic Sciatica and Back Pain Responds to Treatment of Underlying Endometriosis: Case Illustration. World Neurosurg. 2017;97:760.e1–760.e3. doi: 10.1016/j.wneu.2016.09.111. [DOI] [PubMed] [Google Scholar]
- 414.Troyer MR. Differential diagnosis of endometriosis in a young adult woman with nonspecific low back pain. Phys Ther. 2007;87(6):801–810. doi: 10.2522/ptj.20060141. [DOI] [PubMed] [Google Scholar]
- 415.Kumar S, Tiwari P, Sharma P, Goel A, Singh JP, Vijay MK, et al. Urinary tract endometriosis: Review of 19 cases. Urology Annals. 2012;4(1):6–12. doi: 10.4103/0974-7796.91613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Mu D, Li X, Zhou G, Guo H. Diagnosis and treatment of ureteral endometriosis: study of 23 cases. Urol J. 2014;11(4):1806–1812. [PubMed] [Google Scholar]
- 417.Carmignani L, Vercellini P, Spinelli M, Fontana E, Frontino G, Fedele L. Pelvic endometriosis and hydroureteronephrosis. Fertil Steril. 2010;93(6):1741–1744. doi: 10.1016/j.fertnstert.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 418.Ballard K, Lane H, Hudelist G, Banerjee S, Wright J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil Steril. 2010;94(1):20–27. doi: 10.1016/j.fertnstert.2009.01.164. [DOI] [PubMed] [Google Scholar]
- 419.Soliman AM, Coyne KS, Zaiser E, Castelli-Haley J, Fuldeore MJ. The burden of endometriosis symptoms on health-related quality of life in women in the United States: a cross-sectional study. J Psychosom Obstet Gynecol. 2017;38(4):238–48. doi: 10.1080/0167482X.2017.1289512. [DOI] [PubMed] [Google Scholar]
- 420.Jackson B, Telner DE. Managing the misplaced: approach to endometriosis. Can Fam Physician. 2006;52(11):1420–1424. [PMC free article] [PubMed] [Google Scholar]
- 421.Engemise S, Gordon C, Konje JC. Endometriosis. BMJ. 2010;340:c2168. doi: 10.1136/bmj.c2168. [DOI] [PubMed] [Google Scholar]
- 422.Ahmad M, Kumar A, Thomson S. The unique case of foot drop secondary to a large ovarian cyst. Br J Neurosurg. 2014;28(4):549–551. doi: 10.3109/02688697.2013.847174. [DOI] [PubMed] [Google Scholar]
- 423.Barresi V, Barns D, Grundmeyer Rd R, Rodriguez FJ. Cystic endosalpingiosis of lumbar nerve root: a unique presentation. Clin Neuropathol. 2017;36(3):108–13. doi: 10.5414/NP300984. [DOI] [PubMed] [Google Scholar]
- 424.Scheel AH, Frasunek J, Meyer W, Ströbel P. Cystic endosalpingiosis presenting as chronic back pain, a case report. Diagn Pathol. 2013;8:196. doi: 10.1186/1746-1596-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Murphy DR, Bender MI, Green G. Uterine fibroid mimicking lumbar radiculopathy: a case report. Spine (Phila Pa 1976) 2010;35(24):E1435–7. doi: 10.1097/BRS.0b013e3181e8ab84. [DOI] [PubMed] [Google Scholar]
- 426.Sharma MC, Sarkar C, Jain D, Suri V, Garg A, Vaishya S. Uterus-like mass of müllerian origin in the lumbosacral region causing cord tethering. Report of two cases. Neurosurg Spine. 2007;6(1):73–6. doi: 10.3171/spi.2007.6.1.73. [DOI] [PubMed] [Google Scholar]
- 427.Goff B. Symptoms associated with ovarian cancer. Clin Obstet Gynecol. 2012;55(1):36–42. doi: 10.1097/GRF.0b013e3182480523. [DOI] [PubMed] [Google Scholar]
- 428.Kinouani S, de Lary Latour H, Joseph J-P, Letrilliart L. Diagnostic strategies for urinary tract infections in French general practice. Med Mal Infect. 2017;47(6):401–408. doi: 10.1016/j.medmal.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 429.Soler JK, Corrigan D, Kazienko P, Kajdanowicz T, Danger R, Kulisiewicz M, et al. Evidence-based rules from family practice to inform family practice; the learning healthcare system case study on urinary tract infections. BMC Fam Pract. 2015;16:63. doi: 10.1186/s12875-015-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Derouiche A, El Attat R, Hentati H, Blah M, Slama A, Chebil M. Emphysematous pyelitis: epidemiological, therapeutic and evolutive features. Tunis Med. 2009;87(3):180–183. [PubMed] [Google Scholar]
- 431.Giesen LGM, Cousins G, Dimitrov BD, van de Laar FA, Fahey T. Predicting acute uncomplicated urinary tract infection in women: a systematic review of the diagnostic accuracy of symptoms and signs. BMC Fam Pract. 2010;11:78. doi: 10.1186/1471-2296-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Acute uncomplicated urinary tract infection in women. MeReC Bulletin 2006; 17(3):18–20. Verfügbar unter: https://www.embase.com/search/results?subaction=viewrecord&id=L351863799&from=export.
- 433.Pietrucha-Dilanchian P, Hooton TM. Diagnosis, Treatment, and Prevention of Urinary Tract Infection. Microbiol Spectr 2016; 4(6). 10.1128/microbiolspec.UTI-0021-2015. [DOI] [PubMed]
- 434.Germani S, Miano R, Forte F, Finazzi Agrò E, Virgili G, Vespasiani G. Acute lumbago and sciatica as first symptoms of focal xanthogranulomatous pyelonephritis. Urol Int. 2002;69(3):247–249. doi: 10.1159/000063938. [DOI] [PubMed] [Google Scholar]
- 435.Nakamura T, Kawagoe Y, Ueda Y, Koide H. Polymyxin B-immobilized fiber hemoperfusion with low priming volume in an elderly septic shock patient with marked endotoxemia. ASAIO J. 2005;51(4):482–484. doi: 10.1097/01.mat.0000169114.30787.0f. [DOI] [PubMed] [Google Scholar]
- 436.Roy C, Pfleger DD, Tuchmann CM, Lang HH, Saussine CC, Jacqmin D. Emphysematous pyelitis: findings in five patients. Radiology. 2001;218(3):647–650. doi: 10.1148/radiology.218.3.r01fe14647. [DOI] [PubMed] [Google Scholar]
- 437.Nakamura K, Kokubo H, Kato K, Aoki S, Taki T, Mitsui K, et al. Ammonium acid urate urinary stone caused by a low-caloric diet: a case report. Hinyokika Kiyo. 2002;48(8):483–486. [PubMed] [Google Scholar]
- 438.Godara R, Garg P, Karwasra RK. Prostatic calculi–a diagnostic dilemma. Trop Doct. 2003;33(2):105. doi: 10.1177/004947550303300218. [DOI] [PubMed] [Google Scholar]
- 439.Bajaj SK, Seitz JP, Qing F. Diagnosis of acute bacterial prostatitis by Ga-67 scintigraphy and SPECT-CT. Clin Nucl Med. 2008;33(11):813–815. doi: 10.1097/RLU.0b013e318187ef1f. [DOI] [PubMed] [Google Scholar]
- 440.Qiu Y, Liu Y, Ren W, Ren J. Prostatic cyst in general practice: A case report and literature review. Medicine (Baltimore) 2018;97(9):e9985. doi: 10.1097/MD.0000000000009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Rau M, Bärlocher C, Knechtle B. Erfolgreiche antibiotische Behandlung lumbaler Rückenschmerzen. Praxis (Bern 1994) 2018;107(9–10):535–44. doi: 10.1024/1661-8157/a002965. [DOI] [PubMed] [Google Scholar]
- 442.Mateos JJ, Lomena F, Velasco M, Horcajada JP, Ortega M, Fuertes S, et al. Diagnosis and follow-up of acute bacterial prostatitis and orchiepididymitis detected by In-111-labeled leukocyte imaging. Clin Nucl Med. 2003;28(5):403–404. doi: 10.1097/01.RLU.0000063693.10412.E1. [DOI] [PubMed] [Google Scholar]
- 443.Cahill TJ, Nagaratnam K, Browning R, Anthony S, Dwight J. A case of loin pain: a cause close to the heart. BMJ. 2012;345:e6644. doi: 10.1136/bmj.e6644. [DOI] [PubMed] [Google Scholar]
- 444.Maekawa M, Imaizumi T, Yamakawa T, Ito Y. Acute Renal Failure with Severe Loin Pain and Patchy Renal Vasoconstriction in a Patient without Hypouricemia. Provoked by Epileptic Seizure. Intern Med. 2017;56(15):2001–2005. doi: 10.2169/internalmedicine.56.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Ito K, Yamamoto T, Nishio H, Sawaya A, Murakami M, Kitagawa A, et al. Bacteremic kidney cyst infection caused by Helicobacter cinaedi. CEN Case Reports. 2016;5(2):121–4. doi: 10.1007/s13730-015-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Jensen V, Dargis R, Nielsen XC, Wiese L, Christensen JJ. Actinotignum schaalii and aerococcus urinae as etiology of infected kidney cyst: A diagnostic challenge. Open Microbiol J. 2020;14(1):247–51. doi: 10.2174/1874434602014010247. [DOI] [Google Scholar]
- 447.Mandai S, Kasagi Y, Kusaka K, Shikuma S, Akita W, Kuwahara M. Helicobacter cinaedi kidney cyst infection and bacteremia in a patient with autosomal dominant polycystic kidney disease. J Infect Chemother. 2014;20(11):732–734. doi: 10.1016/j.jiac.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 448.Atlas SJ, Deyo RA. Evaluating and managing acute low back pain in the primary care setting. J Gen Intern Med. 2001;16(2):120–131. doi: 10.1111/j.1525-1497.2001.91141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Cohen SP, Argoff CE, Carragee EJ. Management of low back pain. BMJ. 2008;337:a2718. doi: 10.1136/bmj.a2718. [DOI] [PubMed] [Google Scholar]
- 450.Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthop Clin North Am. 2004;35(1):7–16. doi: 10.1016/S0030-5898(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 451.Maher C, Underwood M, Buchbinder R. Non-specific low back pain. The Lancet. 2017;389(10070):736–747. doi: 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 452.Melcher C, Kanz K-G, Birkenmaier C. Pragmatic and effective coping with acute low back pain problems. Notfall und Rettungsmedizin. 2014;17(7):623–36. doi: 10.1007/s10049-014-1914-z. [DOI] [Google Scholar]
- 453.Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am. 2014;98(4):777–89. doi: 10.1016/j.mcna.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 454.Chou R. In the clinic. Low back pain. Ann Intern Med. 2014;160(11):ITC6–1. doi: 10.7326/0003-4819-160-11-201406030-01006. [DOI] [PubMed] [Google Scholar]
- 455.Reith W. Nichtspezifische Kreuzschmerzen und Chronifizierung. Radiologe. 2020;60(2):117–122. doi: 10.1007/s00117-019-00636-7. [DOI] [PubMed] [Google Scholar]
- 456.Schulte-Mattler WJ. Wenn der Schmerz ausstrahlt: Nur selten ist es ein Bandscheibenvorfall. MMW Fortschr Med. 2013;155(15):46–48. doi: 10.1007/s15006-013-2105-4. [DOI] [PubMed] [Google Scholar]
- 457.Jones LD, Pandit H, Lavy C. Back pain in the elderly: a review. Maturitas. 2014;78(4):258–262. doi: 10.1016/j.maturitas.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 458.Vlaeyen JWS, Maher CG, Wiech K, van Zundert J, Meloto CB, Diatchenko L, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52. doi: 10.1038/s41572-018-0052-1. [DOI] [PubMed] [Google Scholar]
- 459.Amirdelfan K, McRoberts P, Deer TR. The differential diagnosis of low back pain: a primer on the evolving paradigm. Neuromodulation. 2014;17(Suppl 2):11–17. doi: 10.1111/ner.12173. [DOI] [PubMed] [Google Scholar]
- 460.Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc Rev. 2008;16(1):39–46. doi: 10.1097/JSA.0b013e318163be67. [DOI] [PubMed] [Google Scholar]
- 461.Rückenschmerzen Ludwig J. Mit gezielten Griffen zur Diagnose. MMW Fortschr Med. 2010;152(21):45–8. doi: 10.1007/BF03366644. [DOI] [PubMed] [Google Scholar]
- 462.Zimmermann PG. Triage and differential diagnosis of patients with headaches, dizziness, low back pain, and rashes: a basic primer. J Emerg Nurs. 2002;28(3):209–15. doi: 10.1067/men.2002.125052. [DOI] [PubMed] [Google Scholar]
- 463.Stowell T, Cioffredi W, Greiner A, Cleland J. Abdominal differential diagnosis in a patient referred to a physical therapy clinic for low back pain. J Orthop Sports Phys Ther. 2005;35(11):755–764. doi: 10.2519/jospt.2005.35.11.755. [DOI] [PubMed] [Google Scholar]
- 464.Klineberg E, Mazanec D, Orr D, Demicco R, Bell G, McLain R. Masquerade: medical causes of back pain. Cleve Clin J Med. 2007;74(12):905–913. doi: 10.3949/ccjm.74.12.905. [DOI] [PubMed] [Google Scholar]
- 465.Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: Low back pain. Can Fam Physician. 2007;53(6):1058. [PMC free article] [PubMed] [Google Scholar]
- 466.Weiland T, Wessel K. Wie kläiren Sie akute Lumbago ab? MMW Fortschr Med. 2007;149(45):48–49. doi: 10.1007/BF03365192. [DOI] [PubMed] [Google Scholar]
- 467.Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J. 2010;10(6):514–529. doi: 10.1016/j.spinee.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 468.Verhagen AP, Downie A, Popal N, Maher C, Koes BW. Red flags presented in current low back pain guidelines: a review. Eur Spine J. 2016;25(9):2788–2802. doi: 10.1007/s00586-016-4684-0. [DOI] [PubMed] [Google Scholar]
- 469.Nagayama M, Yanagawa Y, Aihara K, Watanabe S, Takemoto M, Nakazato T, et al. Analysis of non-traumatic truncal back pain in patients who visited an emergency room. Acute Med Surg. 2014;1(2):94–100. doi: 10.1002/ams2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Borré DG, Borré GE, Aude F, Palmieri GN. Lumbosacral epidural lipomatosis: MRI grading. Eur Radiol. 2003;13(7):1709–1721. doi: 10.1007/s00330-002-1716-4. [DOI] [PubMed] [Google Scholar]
- 471.Chan J-Y, Chang C-J, Jeng C-M, Huang S-H, Liu Y-K, Huang J-S. Idiopathic spinal epidural lipomatosis - two cases report and review of literature. Chang Gung Med J. 2009;32(6):662–667. [PubMed] [Google Scholar]
- 472.Ishikawa Y, Shimada Y, Miyakoshi N, Suzuki T, Hongo M, Kasukawa Y, et al. Decompression of idiopathic lumbar epidural lipomatosis: diagnostic magnetic resonance imaging evaluation and review of the literature. J Neurosurg Spine. 2006;4(1):24–30. doi: 10.3171/spi.2006.4.1.24. [DOI] [PubMed] [Google Scholar]
- 473.Al-Khawaja D, Seex K, Eslick GD. Spinal epidural lipomatosis–a brief review. J Clin Neurosci. 2008;15(12):1323–1326. doi: 10.1016/j.jocn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 474.Duran E, Ilik K, Acar T, Yıldız M. Idiopathic Lumbar Epidural Lipomatosis Mimicking Disc Herniation: A Case Report. Acta Med Iran. 2016;54(5):337–338. [PubMed] [Google Scholar]
- 475.Min W-K, Oh C-W, Jeon I-H, Kim S-Y, Park B-C. Decompression of idiopathic symptomatic epidural lipomatosis of the lumbar spine. Joint Bone Spine. 2007;74(5):488–490. doi: 10.1016/j.jbspin.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 476.McCormick Z, Plastaras C. Transforaminal epidural steroid injection in the treatment of lumbosacral radicular pain caused by epidural lipomatosis: a case series and review. J Back Musculoskelet Rehabil. 2014;27(2):181–190. doi: 10.3233/BMR-130434. [DOI] [PubMed] [Google Scholar]
- 477.Botwin KP, Sakalkale DP. Epidural steroid injections in the treatment of symptomatic lumbar spinal stenosis associated with epidural lipomatosis. Am J Phys Med Rehabil. 2004;83(12):926–930. doi: 10.1097/01.PHM.0000143397.02251.56. [DOI] [PubMed] [Google Scholar]
- 478.Choi K-C, Kang B-U, Lee CD, Lee S-H. Rapid progression of spinal epidural lipomatosis. Eur Spine J. 2012;21(Suppl 4):408–12. doi: 10.1007/s00586-011-1855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lisai P, Doria C, Crissantu L, Meloni GB, Conti M, Achene A. Cauda equina syndrome secondary to idiopathic spinal epidural lipomatosis. Spine (Phila Pa 1976) 2001;26(3):307–9. doi: 10.1097/00007632-200102010-00017. [DOI] [PubMed] [Google Scholar]
- 480.Maillot F, Mulleman D, Mammou S, Goupille P, Valat J-P. Is epidural lipomatosis associated with abnormality of body fat distribution? A case report. Eur Spine J. 2006;15(1):105–108. doi: 10.1007/s00586-005-0955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Tiegs-Heiden CA, Murthy NS, Glazebrook KN, Skinner JA. Subfascial fat herniation: sonographic features of back mice. Skeletal Radiol. 2018;47(1):137–140. doi: 10.1007/s00256-017-2772-9. [DOI] [PubMed] [Google Scholar]
- 482.Ben-Galim P, Ben-Galim T, Rand N, Haim A, Hipp J, Dekel S, et al. Hip-spine syndrome: the effect of total hip replacement surgery on low back pain in severe osteoarthritis of the hip. Spine (Phila Pa 1976) 2007;32(19):2099–102. doi: 10.1097/BRS.0b013e318145a3c5. [DOI] [PubMed] [Google Scholar]
- 483.Nakamura J, Konno K, Orita S, Hagiwara S, Shigemura T, Nakajima T, et al. Distribution of hip pain in patients with idiopathic osteonecrosis of the femoral head. Mod Rheumatol. 2017;27(3):503–507. doi: 10.1080/14397595.2016.1209830. [DOI] [PubMed] [Google Scholar]
- 484.Buckland AJ, Miyamoto R, Patel RD, Slover J, Razi AE. Differentiating Hip Pathology From Lumbar Spine Pathology: Key Points of Evaluation and Management. Instr Course Lect. 2017;66:315–327. [PubMed] [Google Scholar]
- 485.Prather H, Cheng A, Steger-May K, Maheshwari V, van Dillen L. Hip and Lumbar Spine Physical Examination Findings in People Presenting With Low Back Pain, With or Without Lower Extremity Pain. J Orthop Sports Phys Ther. 2017;47(3):163–172. doi: 10.2519/jospt.2017.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Ceballos-Laita L, Estébanez-de-Miguel E, Jiménez-Rejano JJ, Bueno-Gracia E, Jiménez-Del-Barrio S. The effectiveness of hip interventions in patients with low-back pain: A systematic review and meta-analysis. Braz J Phys Ther. 2023;27(2):100502. doi: 10.1016/j.bjpt.2023.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Han CS, Hancock MJ, Downie A, Jarvik JG, Koes BW, Machado GC, et al. Red flags to screen for vertebral fracture in people presenting with low back pain. Cochrane Database Syst Rev. 2023;8(8):CD014461. doi: 10.1002/14651858.CD014461.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Verhagen AP, Downie A, Maher CG, Koes BW. Most red flags for malignancy in low back pain guidelines lack empirical support: a systematic review. Pain. 2017;158(10):1860–1868. doi: 10.1097/j.pain.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 489.Siddiq MAB, Clegg D, Hasan SA, Rasker JJ. Extra-spinal sciatica and sciatica mimics: a scoping review. Korean J Pain. 2020;33(4):305–317. doi: 10.3344/kjp.2020.33.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 491.Alan, Hammer Ian, Knight Anand, Agarwal. Localized Venous Plexi in the Spine Simulating Prolapse of an Intervertebral Disc Spine. 2003;28(1)E5–E12. 10.1097/00007632-200301010-00025. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.