Abstract
Background
Growth hormone (GH) has been proposed as an adjunct in in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles, especially in women with poor ovarian response. However, it is unclear whether GH supplementation is effective in women with poor embryonic development in the previous IVF cycle. The aim of this study was to evaluate the effectiveness of GH supplementation in IVF/ICSI cycles in women with poor embryonic development in the previous cycle.
Methods
This is a retrospective cohort study from a public fertility center in China, in which we performed propensity score-matching (PSM) for female age and AFC in a ratio of 1:1. We compared the cumulative live birth rate per started cycle, as well as a series of secondary outcomes. We included 3,043 women with poor embryonic development in the previous IVF/ICSI cycle, of which 1,326 had GH as adjuvant therapy and 1,717 had not. After PSM, there were 694 women in each group.
Results
After PSM, multivariate analyses showed the cumulative live birth rate to be significantly higher in the GH group than the control group [N = 694, 34.7% vs. N = 694, 27.5%, risk ratio (RR): 1.4 (95%CI: 1.1–1.8)]. Endometrial thickness, number of oocytes retrieved, number of embryos available, and number of good-quality embryos were significantly higher in the GH group compared to controls. Pregnancy outcomes in terms of birth weight, gestational age, fetal sex, preterm birth rate, and type of delivery were comparable. When we evaluated the impact of GH on different categories of female age, the observed benefit in the GH group did not appear to be significant. When we assessed the effect of GH in different AFC categories, the effect of GH was strongest in women with an AFC5-6 (32.2% versus 19.5%; RR 2.0; 95% CI 1.2–3.3).
Conclusions
Women with poor embryonic quality in the previous IVF/ICSI cycles have higher rates of cumulative live birth with GH supplementation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12958-024-01223-9.
Keywords: Growth hormone, Cumulative live birth rate, IVF, Propensity score matching, Embryo quality
Introduction
In vitro fertilization (IVF) is the cornerstone of modern infertility treatment, with an average live birth rate of 30% per transfer, resulting in cumulative live birth rates as high as 70% per started cycle. Female age and subsequent poor oocyte quality, however, is the main limiting factor of IVF success. Indeed, poor embryo quality results in low success rates [1, 2]. Improvement of embryo quality is therefore likely to improve clinical outcomes.
Growth hormone (GH) has been reported to be able to enhance the functional mitochondria in oocytes [3]. In vitro studies have shown that GH plays an important role in the proliferation of the theca cells [4]. Theoreticality, exogenous GH acts on insulin-like growth factor (IGF) receptors of the ovaries to increase steroidogenesis and oocyte maturation [5, 6].
We previously showed co-treatment with GH in women with normal ovarian response with poor embryo quality could increase clinical pregnancy rate (64.78% vs. 59.33%) [7]. Several reviews have suggested that GH supplementation improves IVF outcomes in poor responders [8, 9]. While some studies have demonstrated that pre-treatment of GH could potentially enhance pregnancy, implantation, and live birth rates, others have refuted the efficacy of GH as an adjuvant in infertility treatment due to the lack of significant increase in live birth rates. A recent Cochrane review therefore suggested there was insufficient evidence regarding the effect of adjuvant GH for routine use in IVF [10].
In view of this evidence gap, we studied the effects of GH supplementation in women with poor embryonic quality in previous cycles.
Materials and methods
Study design
We performed a retrospective, single-center cohort study in the Assisted Reproductive Center of Northwest Women’s and Children’s Hospital, Xi’an, China. The study protocol was approved by the Ethics Committee of Northwest Women’s and Children’s Hospital (No. 2022007).
We studied women treated between January 2017 and December 2020. Women were eligible if they met the following criteria: (1) undergoing a second IVF/ICSI cycle with a failure to achieve pregnancy in the first attempt; (2) no top-quality embryos on day 3 (grade I or II) in the first cycle [11]; and (3) age 20–45 years old. Exclusion criteria were: (1) hyperthyroidism or hypothyroidism; (2) hyperplasia of mammary glands; (3) history of malignant tumor; (4) diabetes mellitus; (5) inclusion in this study in a previous cycle.
Ovarian stimulation protocols
Ovarian stimulation could be with GnRH agonist or GnRH antagonist protocols, as has been described in detail elsewhere [12]. Briefly, for the GnRH agonist protocol, pituitary down-regulation began during the mid-luteal phase of the previous menstrual cycle with the GnRH agonist at a dose of 0.1–0.05 mg/day for 14 days. Recombinant follicle-stimulating hormone (rFSH) was started at 150–225 IU/day for ovarian stimulation. The dose of rFSH could be adjusted up to 300 IU/day based on ovarian response. Recombinant luteinizing hormone (rLH) could be added at the discretion of the treating physician.
For the GnRH antagonist protocol, rFSH was started on day 2 of the menstrual cycle, with similar doses of rFSH as the GnRH agonist protocol. GnRH antagonist, 0.25 mg/day was started when the dominant follicle reached 12–14 mm. When two or more follicles reached 17 mm, human chorionic gonadotropin (hCG) was given at a dose of 4,000 to 10,000 IU, and oocyte retrieval was performed 36 h later.
Growth hormone supplementation
The choice to use GH was based on the preference of the woman and her treating physician. Women in the GH group received 2 IU recombinant human GH (Jintropin, Gensci, China) daily, from the initial day of pituitary down-regulation for the GnRH agonist protocol or day 2 of the previous menstrual cycle for the GnRH antagonist protocol until the day of the hCG trigger. Otherwise, treatment of the groups was similar.
Embryo quality assessment
Embryo quality was assessed on day 3 at 72 h after oocyte retrieval. Embryos were scored according to a combination of blastomere number, blastomere size and fragmentation [13]. Briefly, embryos with 8–10 blastomeres, even homogeneous blastomeres < 10% cytoplasmic fragmentation were classified as grade I - embryos; embryos with 6–7 or > 10 blastomeres with even homogeneous blastomeres of no cytoplasmic fragmentation; or embryos with 8–10 blastomeres with even homogeneous blastomeres of 10%-20% cytoplasmic fragmentation were classified as grade II - embryos; embryos with 4–5 blastomeres with uneven and non-homogeneous blastomeres with 20%-50% cytoplasmic fragmentation were classified as grade III - embryos; embryos with fewer than 4 blastomeres with uneven and non-homogeneous blastomeres with > 50% cytoplasmic fragmentation were classified as grade IV—embryos (Supplementary Table 1). Only embryos classified as grade I, II, and III were available for transfer.
Embryos of grade I and II were regarded as top-quality embryos. For women with more than four top-quality cleavage embryos, all embryos were cultured to the blastocyst stage. A maximum of two embryos were transferred per transfer. The remaining embryos were frozen for future use. Women who were at risk of ovarian hyperstimulation syndrome (OHSS), women who presented with hydrosalpinx, and women who had high progesterone levels on hCG trigger day had frozen-thawed embryo transfer.
Luteal phase support and pregnancy confirmation
Luteal support was given with 600 mg of vaginal progesterone and 30 mg oral progesterone daily from the day of oocyte retrieval in the fresh cycle or the day of embryo transfer in the frozen-thawed embryo transfer cycle. A pregnancy tests using serum β-hCG was performed 14 days after embryo transfer. In case of a positive pregnancy test, transvaginal ultrasound was performed 5 weeks after embryo transfer to determine the number of gestational sacs and the fetal heartbeat.
Outcome measures
The primary outcome was cumulative live birth, defined as a live birth > 24 weeks of gestation, following the use of all fresh and frozen embryos derived from a single ovarian stimulation cycle. Secondary outcomes were biochemical pregnancy, clinical pregnancy, ongoing pregnancy, multiple pregnancy, miscarriage (defined as a pregnancy failure that occurs before 24 completed weeks of pregnancy) and ectopic pregnancy. We also assessed number of embryos, embryo quality and number of embryos available.
For women achieving live birth, we reported birth weight, fetal sex, gestational age at delivery in weeks, preterm birth (defined as delivery before 37 completed weeks of pregnancy) and type of delivery. All women in the study were followed-up until 2 years after oocyte retrieval.
Statistical analysis
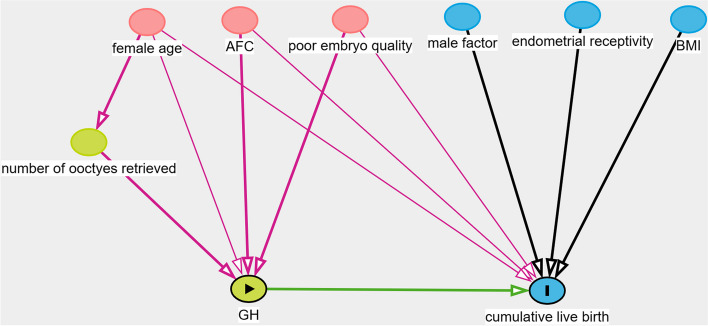
Propensity score matching (PSM) was performed to match the baseline characteristics of GH and control groups. Confounding was assessed by utilizing prior knowledge with the aid of directed acyclic graphs (DAG) (Fig. 1). The subsequent covariates were contemplated for incorporation in the ultimate model to match the GH group to the control group with a 1:1 ratio: female age, AFC, and embryo quality in previous cycle.
Fig. 1.
Directed acyclic graphs in identification selection of covariates
Categorical variables were expressed as percentages and were compared using the chi-square test or Fisher’s exact test. Continuous variables were expressed as mean ± SD and were compared using Student’s t test and the Mann–Whitney U test. Multivariable logistic regression analyses were used to determine the adjusted risk ratios (aRR) and 95% confidence intervals (CIs) for dichotomous outcomes. In the multivariable analyses we adjusted for female age, male age, basal FSH, AFC, body mass index (BMI), infertility duration, and infertility type. Subgroup analysis was performed with quartiles in different female age groups and AFC groups before and after PSM. Subgroup factor (female age and AFC) in the Poisson regression model was used to test the treatment-covariate interaction.
Data were analyzed with the use of the statistical packages R (The R Foundation; http://www.r-project.org.version 3.4.3) and Empower (R) (http://www.empowerstats.net/en/, X&Y solutions, inc. Boston, Massachusetts). A P-value < 0.05 was supposed to indicate statistical significance.
Results
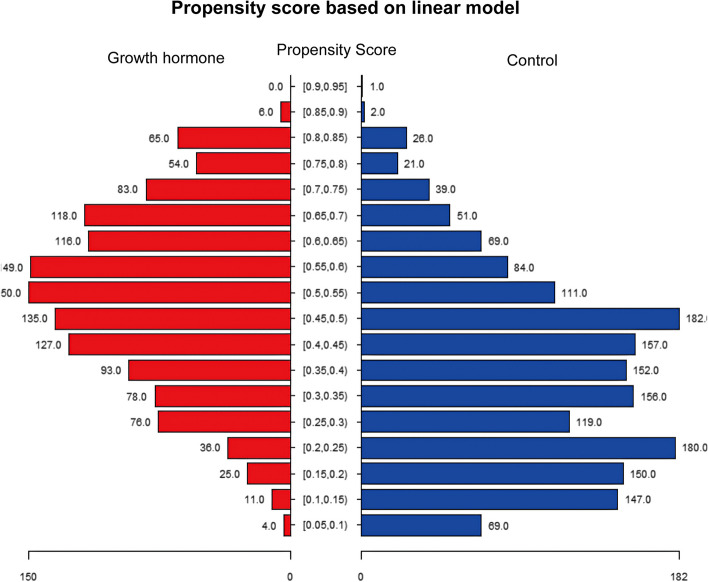
Between January 2017 and December 2020, 36,290 IVF/ICSI cycles were performed in our center. After assessing for eligibility, 3,043 women had a previous cycle without top-quality embryos on day 3 (grade I or II) and were eligible for the study. Of these women, 1,326 women were treated with GH and while 1,717 women did not use GH (Fig. 2). After PSM, 694 women treated with GH (intervention) could be matched to 694 women treated with regular IVF without GH supplementation (control). Propensity score in the two groups was shown in Fig. 3.
Fig. 2.
Flowchart of study cohort
Fig. 3.
Propensity score in two groups
Table 1 presents the demographic characteristics of women before and after PSM. After PSM, there was no statistically significant difference in demographic characteristics between the two groups, particularly regarding the embryo quality in previous cycle.
Table 1.
Demographic characteristics of women in the growth hormone and control groups
| Characteristic | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| Growth hormone (n = 1,326) | Control (n = 1,717) | P value | Growth hormone (n = 694) | Control (n = 694) | P value | |
| Female age (y) | 32.3 ± 4.7 | 34.6 ± 5.8 | < 0.001 | 33.0 ± 4.8 | 33.0 ± 4.8 | 0.99 |
| AFC | 10.5 ± 5.8 | 6.9 ± 5.3 | < 0.001 | 8.5 ± 5.1 | 8.5 ± 5.1 | 0.99 |
| Male age (y) | 33.9 ± 5.1 | 36.6 ± 6.5 | < 0.001 | 34.6 ± 5.3 | 35.1 ± 5.4 | 0.08 |
| Basal FSH (IU/L) | 8.8 ± 5.8 | 10.8 ± 7.5 | < 0.001 | 9.2 ± 6.2 | 9.9 ± 6.5 | 0.12 |
| BMI (kg/m2) | 22.5 ± 3.2 | 22.7 ± 3.2 | 0.11 | 22.5 ± 3.2 | 22.6 ± 3.3 | 0.31 |
| Infertility duration (y) | 4.0 ± 2.9 | 4.0 ± 3.4 | 0.05 | 4.0 ± 2.9 | 4.0 ± 3.1 | 0.89 |
| Primary infertility | 665 (50.2%) | 745 (43.4%) | < 0.001 | 334 (48.1%) | 331 (47.7%) | 0.87 |
| Oocytes retrieved previous cycle | 5.9 ± 4.4 | 5.5 ± 3.6 | 0.44 | 5.8 ± 4.1 | 5.9 ± 4.1 | 0.69 |
| Embryo quality in previous cycle | < 0.001 | 0.99 | ||||
| Grade III only | 39 (2.9%) | 248 (14.5%) | 17 (2.4%) | 17 (2.4%) | ||
| Grade III + IV | 995 (75.0%) | 831 (48.4%) | 179 (25.8%) | 179 (25.8%) | ||
| Grade IV only | 292 (22.0%) | 637 (37.1%) | 498 (71.8%) | 498 (71.8%) | ||
FSH follicle stimulating hormone, AFC antral follicle count, BMI body mass index, IVF in vitro fertilization
Endometrium was significantly thicker in the GH group (10.9 ± 2.7 versus 10.2 ± 3.1 mm, p-value < 0.001) (Table 2). Also, the number of oocytes retrieved (7.6 versus 6.6), the number of embryos available (3.3 versus 2.9), and number of good-quality embryos (1.8 versus 1.5) were higher after the use of GH.
Table 2.
Characteristics in IVF cycles of two groups before and after propensity score matching
| Characteristic | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| Growth hormone (n = 1,326) | Control (n = 1,717) | P value | Growth hormone (n = 694) | Control (n = 694) | P value | |
| Protocol | 0.509 | 0.67 | ||||
| GnRH agonist | 922 (53.70%) | 728 (54.90%) | 367 (52.9%) | 359 (51.7%) | ||
| GnRH antagonist | 795 (46.30%) | 598 (45.10%) | 327 (47.1%) | 335 (48.3%) | ||
| Change in protocol after IVF failure | 448 (33.8%) | 547 (31.9%) | 0.26 | 237 (34.1%) | 232 (33.4%) | 0.78 |
| Total gonadotropin dosage (IU) | 2541.2 ± 944.1 | 2540.6 ± 1142.0 | 0.63 | 2629.3 ± 968.6 | 2633.6 ± 1075.5 | 0.94 |
| Total gonadotropin duration (days) | 10.1 ± 2.5 | 9.4 ± 3.0 | < 0.01 | 10.0 ± 2.6 | 9.8 ± 2.6 | 0.15 |
| E2 level on hCG day | 3202.4 ± 2452.7 | 2164.2 ± 2093.7 | < 0.01 | 2763.1 ± 2148.0 | 2675.1 ± 2287.8 | 0.46 |
| Endometrial thickness (mm) | 11.2 ± 2.6 | 9.7 ± 3.1 | < 0.01 | 10.9 ± 2.7 | 10.2 ± 3.1 | < 0.01 |
| Number of oocytes retrieved | 9.0 ± 5.6 | 5.4 ± 5.1 | < 0.01 | 7.6 ± 5.0 | 6.6 ± 5.2 | < 0.01 |
| Fertilization type | < 0.01 | 0.17 | ||||
| IVF | 677 (51.9%) | 889 (55.6%) | 367 (54.0%) | 337 (51.0%) | ||
| ICSI | 590 (45.3%) | 694 (43.4%) | 297 (43.7%) | 315 (47.7%) | ||
| IVF + ICSI | 37 (2.8%) | 15 (0.9%) | 16 (2.4%) | 9 (1.4%) | ||
| Number of embryos available | 3.7 ± 3.0 | 2.5 ± 2.5 | < 0.01 | 3.3 ± 2.8 | 2.9 ± 2.8 | < 0.01 |
| Number of good-quality embryos | 2.0 ± 2.3 | 1.3 ± 1.8 | < 0.01 | 1.8 ± 2.1 | 1.5 ± 2.0 | < 0.01 |
| Number of transfers | 1.2 ± 0.8 | 1.0 ± 0.7 | < 0.01 | 1.2 ± 0.7 | 1.1 ± 0.8 | 0.27 |
| Freeze-all cycles | 306 (23.1%) | 391 (22.8%) | 0.84 | 161 (23.2%) | 154 (22.2%) | 0.65 |
| Embryo type of first transfer | < 0.01 | 0.67 | ||||
| Cleavage embryos | 807 (74.0%) | 1039 (80.3%) | 419 (75.4%) | 416 (76.5%) | ||
| Blastocyst embryos | 283 (26.0%) | 255 (19.7%) | 137 (24.6%) | 128 (23.5%) | ||
IVF in vitro fertilization, ICSI intracytoplasmic sperm injection
The cumulative live birth rate in the GH was significantly higher after PSM than control [34.7% vs. 27.5%, RR: 1.4, 95% confidence interval (95%CI) (1.1–1.8)] (Table 3). Secondary outcomes including live birth of first transfer (24.8% vs. 18.7%, RR: 1.4 (1.1, 1.8)), biochemical miscarriage (44.1% vs. 35.2%, RR: 1.5 (1.2–1.8)), clinical pregnancy (40.5% vs. 32.1%, RR: 1.4 (1.1–1.8)), and ongoing pregnancy (34.7% vs. 27.7%, RR: 1.4 (1.1–1.8)) were all higher after use of GH. For women achieving live birth, birth weight of singleton and twins, gestation delivery in weeks, fetal sex of singleton and twins, type of delivery were comparable.
Table 3.
Comparisons of cumulative clinical outcomes before and after propensity score matching
| Characteristic | Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Growth hormone (n = 1,326) | Control (n = 1,717) | aRR (95%CI)a | P value | Growth hormone (n = 694) | Control (n = 694) | aRR (95%CI)a | P value | |
| Cumulative live birth | 499 (37.6%) | 390 (22.7%) | 1.5 (1.2, 1.7) | < 0.01 | 241 (34.7%) | 191 (27.5%) | 1.4 (1.1, 1.8) | < 0.01 |
| Live birth of first transfer | 325 (24.51%) | 277 (16.13%) | 1.4 (1.2, 1.7) | < 0.01 | 172 (24.8%) | 130 (18.7%) | 1.4 (1.1, 1.8) | < 0.01 |
| Biochemical pregnancy | 624 (47.1%) | 521 (30.3%) | 1.5 (1.3, 1.8) | < 0.01 | 306 (44.1%) | 244 (35.2%) | 1.5 (1.2, 1.8) | < 0.01 |
| Clinical pregnancy | 578 (43.6%) | 472 (27.5%) | 1.50 (1.3, 1.8) | < 0.01 | 281 (40.5%) | 223 (32.1%) | 1.4 (1.1, 1.8) | < 0.01 |
| Ongoing pregnancy | 503 (37.9%) | 393 (22.9%) | 1.5 (1.3, 1.8) | < 0.01 | 241 (34.7%) | 192 (27.7%) | 1.4 (1.1, 1.8) | < 0.01 |
| Twin pregnancy | 99 (19.5%) | 55 (13.6%) | 1.5 (1.0, 2.1) | 0.05 | 50 (20.5%) | 31 (15.8%) | 1.4 (0.8, 2.3) | 0.21 |
| Miscarriage | 80 (6.0%) | 85 (5.0%) | 1.2 (0.9, 1.7) | 0.20 | 40 (5.8%) | 34 (4.9%) | 1.2 (0.8, 2.0) | 0.47 |
| Ectopic pregnancy | 5 (0.4%) | 2 (0.1%) | 3.4 (0.6, 19.0) | 0.17 | 3 (0.4%) | 1 (0.1%) | 3.0 (0.3, 30.7) | 0.62 |
| Birth weight (kg) | ||||||||
| Singleton | ||||||||
| Mean | 3.3 ± 0.6 | 3.3 ± 0.5 | NA | 0.20 | 3.3 ± 0.5 | 3.3 ± 0.5 | NA | 0.25 |
| Number of observations | 408 | 351 | 173 | 132 | ||||
| Low birth weight | 2.0 ± 0.3 | 2.1 ± 0.4 | NA | 0.36 | 2.1 ± 0.3 | 2.2 ± 0.3 | NA | 0.64 |
| Macrosomic infants | 4.2 ± 0.3 | 4.2 ± 0.2 | NA | 0.61 | 4.2 ± 0.3 | 4.2 ± 0.1 | NA | 0.60 |
| Twins | ||||||||
| Mean | 2.5 ± 0.4 | 2.4 ± 0.4 | NA | 0.68 | 2.5 ± 0.4 | 2.5 ± 0.4 | NA | 0.89 |
| Number of observations | 99 | 55 | 50 | 31 | ||||
| Low birth weight | 2.1 ± 0.4 | 2.1 ± 0.3 | NA | 0.92 | 2.1 ± 0.4 | 2.1 ± 0.3 | NA | 0.92 |
| Macrosomic infants | NA | NA | NA | NA | NA | NA | NA | NA |
| Gestation delivery in weeks | 38.3 ± 2.1 | 38.4 ± 2.1 | NA | 0.49 | 38.5 ± 1.9 | 38.3 ± 2.1 | NA | 0.29 |
| Fetal sex | ||||||||
| Singleton | NA | 0.81 | NA | 0.52 | ||||
| Female | 158 (48.0%) | 124 (49.0%) | 88 (50.9%) | 72 (54.5%) | ||||
| Male | 171 (52.0%) | 129 (51.0%) | 85 (49.1%) | 60 (45.5%) | ||||
| Twins | NA | 0.59 | NA | 0.89 | ||||
| Female twins | 29 (29.3%) | 12 (21.8%) | 12 (24.0%) | 6 (19.4%) | ||||
| Male twins | 26 (26.3%) | 15 (27.3%) | 15 (30.0%) | 10 (32.3%) | ||||
| Boy-girl twins | 44 (44.4%) | 28 (50.9%) | 23 (46.0%) | 15 (48.4%) | ||||
| Preterm birth | 91 (18.2%) | 63 (16.2%) | 1.19 (0.83, 1.72) | 0.35 | 35 (14.5%) | 34 (17.8%) | 0.8 (0.5, 1.4) | 0.36 |
| Type of delivery | NA | 0.12 | NA | 0.29 | ||||
| Vaginal | 101 (20.2%) | 96 (24.6%) | 49 (20.3%) | 47 (24.6%) | ||||
| Cesarean section | 398 (79.8%) | 294 (75.4%) | 192 (79.7%) | 144 (75.4%) | ||||
aOR adjusted odds ratio, CI confidence interval, NA not available/applicable
aAdjusted for female age, male age, basal FSH, AFC, BMI, infertility duration, and infertility type
When we evaluated the impact of GH on different categories of female age, the observed benefit in the GH group did not appear to be significant (Table 4). When we assessed the effect of GH in different AFC categories, the effect of GH was strongest in women with an AFC5-6 (32.2% versus 19.5%; RR 2.0; 95% CI 1.2–3.3).
Table 4.
Cumulative live birth of women stratified according to female age and AFC after propensity score matching
| Subgroup | Before propensity score matching | Subgroup | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Growth hormone (n = 1326) | Control (n = 1717) | Growth hormone vs control RR (95%CI) | P for interaction | Growth hormone (n = 694) | Control (n = 694) | Growth hormone vs control RR | P for interaction | ||
| Female age | 0.57 | Female age | 0.93 | ||||||
| Quartile 1 (21–29) | 181 (48.9%) | 111 (32.8%) | 1.5 (1.3, 1.8) | Quartile 1 (23–29) | 76 (47.2%) | 59 (36.6%) | 1.5 (1.0, 2.4) | ||
| Quartile 2 (30–32) | 145 (36.9%) | 103 (27.0%) | 1.4 (1.1, 1.7) | Quartile 2 (30–31) | 56 (38.6%) | 47 (32.4%) | 1.3 (0.8, 2.1) | ||
| Quartile 3 (33–36) | 131 (38.2%) | 117 (28.1%) | 1.4 (1.1, 1.7) | Quartile 3 (32–35) | 71 (35.5%) | 54 (27.0%) | 1.5 (1.0, 2.3) | ||
| Quartile 4 (> 36) | 42 (19.1%) | 59 (10.2%) | 1.9 (1.3, 2.8) | Quartile 4 (> 35) | 38 (20.2%) | 31 (16.5%) | 1.3 (0.8, 2.2) | ||
| AFC | 0.31 | AFC | 0.14 | ||||||
| Quartile 1 (1–3) | 19 (19.4%) | 59 (11.6%) | 1.7 (1.0, 2.6) | Quartile 1 (1–4) | 32 (21.1%) | 20 (13.2%) | 1.8 (1.0, 3.2) | ||
| Quartile 2 (4–6) | 90 (30.5%) | 107 (20.2%) | 1.5 (1.2, 1.9) | Quartile 2 (5–6) | 48 (32.2%) | 29 (19.5%) | 2.0 (1.2, 3.3) | ||
| Quartile 3 (7–10) | 123 (33.2%) | 108 (29.8%) | 1.1 (0.9, 1.4) | Quartile 3 (7–10) | 73 (34.4%) | 74 (34.9%) | 1.0 (0.7, 1.5) | ||
| Quartile 4 (> 10) | 267 (47.4%) | 116 (36.7%) | 1.3 (1.1, 1.5) | Quartile 4 (> 10) | 88 (48.6%) | 68 (37.6%) | 1.6 (1.0, 2.4) | ||
AFC antral follicle count
Discussion
In this retrospective matched cohort study, we found that women with poor embryonic development in the previous cycle had an 8% higher cumulative live birth rate if they used GH in the new cycle. There were also more oocytes retrieved and more good quality embryos available after the treatment with GH.
Our present work has several strengths. Firstly, our study reports cumulative live birth rate extends the application of GH to an improvement of embryo quality. Secondly, we had a large sample size and PSM was conducted to control the potential confounders which might have effects on the outcomes. Comparisons were not only performed after PSM but were also explored before PSM.
The main limitation of our study is that, due to its retrospective nature, though PSM was performed, individual differences may still have existed, possibly affecting the results. Thus, further randomized controlled trials on GH co-treatment in women with poor embryo quality in the previous IVF/ICSI cycle are needed.
GH can affect oocyte and folliculogenesis via insulin-like growth factor 1 (IGF-1) or by the direct action of GH [9]. GH could improve ovarian response to gonadotropin via IGF-1, increasing oocyte competence by improving the mitochondrial activity of oocytes and possibly increasing the DNA repair capacity in oocytes [14–17]. The mitochondrial DNA in cumulus granulosa cells is proven to be positively associated with embryo development competence [18, 19]. GH also plays important antioxidant functions in oocytes [3] and could decrease reactive oxygen species (ROS) production associated apoptosis and activate the PI3K/Akt signaling pathway in granulosa cells [20].
The most recent Cochrane review identified 55 randomized studies of growth hormone as an adjunct to IVF, of which 39 studies were not used for the review but classified as waiting further information [10, 21]. Among 16 remaining studies, the effect of GH was estimate to be odds ratio (OR) 1.32, 95% CI 0.40 to 4.43. It was inconclusive to ascertain the effectiveness of GH supplementation.
Our previous study already suggested an effect of GH co-treatment in improving clinical pregnancy in women with a normal ovarian response [7]. Poor embryo quality driven by increased maternal age has a detrimental effect on clinical outcomes [22]. The number of clinical interventions to overcome poor embryo quality driven by maternal age are limited, including pretreatment with coenzyme Q10, melatonin, and artificial oocyte activation. In fact, IVF with oocyte donation is the only treatment that overcomes the detrimental impact of maternal age, albeit at the expense of transferring the use of the own genetic material of the woman.
It can be speculated that GH supplementation may also benefit women with poor embryo quality in other subgroups of ovarian reserve. Women of different ages and ovarian reserve can suffer from poor embryo quality, however, which subgroup of women could benefit from GH supplementation is still not clear. As co-treatment of GH is expensive and beyond indication, it is, therefore, essential to justify the potentially effective patients who may benefit from it, by improving the cumulative live birth rate. Randomized controlled trials are needed to confirm the findings.
Conclusions
Our results suggest that women with poor embryonic development in the previous cycle could benefit from GH supplementation.
Supplementary Information
Acknowledgements
We thank all the physicians, scientists, and embryologists in our IVF clinic for their assistance with data collection as well the patients for participating in this study.
Abbreviations
- GH
Growth hormone
- IVF
In vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- PSM
Propensity score-matching
- RR
Risk ratio
- IGF
Insulin-like growth factor
- rFSH
Recombinant follicle-stimulating hormone
- rLH
Recombinant luteinizing hormone
- hCG
Human chorionic gonadotropin
- OHSS
Ovarian hyperstimulation syndrome
- AFC
Antral follicle count
- CI
Confidence interval
- BMI
Body mass index
- IGF-1
Insulin-like growth factor 1
- ROS
Reactive oxygen species
- OR
Odds ratio
Authors’ contributions
XTL, NL, DYW, JZS, TW and BWM designed this study. WW, LT, HYZ conducted the study and enrolled patients. NL contributed to data acquisition, analyses and data interpretation. XTL drafted the manuscript. TW and BWM revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the General Projects of Social Development in Shaanxi Province (No. 2022SF-565).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
The study received approval and was carried out in accordance with the approved guidelines of the Northwest women’s and children’s hospital Ethics Board, and informed consent was waived due to the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xitong Liu and Na Li contributed equally to this work.
References
- 1.Oron G, Son WY, Buckett W, Tulandi T, Holzer H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: a pilot study. Human reproduction (Oxford, England) 2014;29(7):1444–1451. doi: 10.1093/humrep/deu079. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Lian Y, Li M, Chen L, Liu P, Qiao J. Does IVF cleavage stage embryo quality affect pregnancy complications and neonatal outcomes in singleton gestations after double embryo transfers? J Assist Reprod Genet. 2014;31(12):1635–1641. doi: 10.1007/s10815-014-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weall BM, Al-Samerria S, Conceicao J, Yovich JL, Almahbobi G. A direct action for GH in improvement of oocyte quality in poor-responder patients. Reproduction (Cambridge, England) 2015;149(2):147–154. doi: 10.1530/REP-14-0494. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi J, Mizunuma H, Kikuchi N, Liu X, Andoh K, Abe Y, Yokota H, Yamada K, Ibuki Y, Hagiwara H. Morphological assessment of the effect of growth hormone on preantral follicles from 11-day-old mice in an in vitro culture system. Biochem Biophys Res Commun. 2000;268(1):36–41. doi: 10.1006/bbrc.1999.2072. [DOI] [PubMed] [Google Scholar]
- 5.Hull KL, Harvey S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. International journal of endocrinology. 2014;2014:234014. doi: 10.1155/2014/234014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ipsa E, Cruzat VF, Kagize JN, Yovich JL, Keane KN. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front Endocrinol. 2019;10:777. doi: 10.3389/fendo.2019.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Bai H, Xie J, Shi J. Growth hormone co-treatment on controlled ovarian stimulation in normal ovarian response women can improve embryo quality. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2019;35(9):787–791. doi: 10.1080/09513590.2019.1590545. [DOI] [PubMed] [Google Scholar]
- 8.Lattes K, Brassesco M, Gomez M, Checa MA. Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2015;31(7):565–568. doi: 10.3109/09513590.2015.1025378. [DOI] [PubMed] [Google Scholar]
- 9.Hart RJ, Rombauts L, Norman RJ. Growth hormone in IVF cycles: any hope? Curr Opin Obstet Gynecol. 2017;29(3):119–125. doi: 10.1097/GCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 10.Sood A, Mohiyiddeen G, Ahmad G, Fitzgerald C, Watson A, Mohiyiddeen L. Growth hormone for in vitro fertilisation (IVF) Cochrane Database Syst Rev. 2021;11(11):Cd000099. doi: 10.1002/14651858.CD000099.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Xue X, Zhang S, Li W, Zhao X, Ren W, Shi J. Effects of triploidy incidence on clinical outcomes for IVF-ET cycles in different ovarian stimulation protocols. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2015;31(10):769–773. doi: 10.3109/09513590.2015.1041907. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Shi W, Shi J. Natural cycle frozen-thawed embryo transfer in young women with regular menstrual cycles increases the live-birth rates compared with hormone replacement treatment: a retrospective cohort study. Fertil Steril. 2020;113(4):811–817. doi: 10.1016/j.fertnstert.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Xue X, Zhang S, Zhao W, Liu S, Zhou H, Wang M, Shi J. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril. 2012;97(6):1338–1342. doi: 10.1016/j.fertnstert.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Drakopoulos P, Pluchino N, Bischof P, Cantero P, Meyer P, Chardonnens D. Effect of Growth Hormone on Endometrial Thickness and Fertility Outcome in the Treatment of Women with Panhypopituitarism: A Case Report. J Reprod Med. 2016;61(1–2):78–82. [PubMed] [Google Scholar]
- 15.Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol. 1990;126(3):R1–4. doi: 10.1677/joe.0.126R001. [DOI] [PubMed] [Google Scholar]
- 16.Bachelot A, Monget P, Imbert-Bolloré P, Coshigano K, Kopchick JJ, Kelly PA, Binart N. Growth hormone is required for ovarian follicular growth. Endocrinology. 2002;143(10):4104–4112. doi: 10.1210/en.2002-220087. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Chen Q, Wang J, Huang G, Ye H. Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomized controlled trial. BMC Pregnancy Childbirth. 2020;20(1):310. doi: 10.1186/s12884-020-03004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogino M, Tsubamoto H, Sakata K, Oohama N, Hayakawa H, Kojima T, Shigeta M, Shibahara H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33(3):367–371. doi: 10.1007/s10815-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taugourdeau A, Desquiret-Dumas V, Hamel JF, Chupin S, Boucret L, Ferré-L'Hotellier V, Bouet PE, Descamps P, Procaccio V, Reynier P, May-Panloup P. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J Assist Reprod Genet. 2019;36(2):223–228. doi: 10.1007/s10815-018-1348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y, Luo S, Fan P, Zhu H, Li Y, Huang W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reproductive biology and endocrinology : RB&E. 2020;18(1):121. doi: 10.1186/s12958-020-00677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yovich JL, Stanger JD. Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reprod Biomed Online. 2010;21(1):37–49. doi: 10.1016/j.rbmo.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Cai J, Liu L, Xu Y, Liu Z, Chen J, Jiang X, Sun X, Ren J. Does the transfer of a poor quality embryo with a good quality embryo benefit poor prognosis patients? Reproductive biology and endocrinology : RB&E. 2020;18(1):97. doi: 10.1186/s12958-020-00656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.