ABSTRACT
Nontypeable Haemophilus influenzae (NTHi) is a major otitis media (OM) pathogen, with colonization a prerequisite for disease development. Most acute OM is in children <5 years old, with recurrent and chronic OM impacting hearing and learning. Therapies to prevent NTHi colonization and/or disease are needed, especially for young children. Respiratory viruses are implicated in driving the development of bacterial OM in children. We have developed an infant mouse model of influenza-driven NTHi OM, as a preclinical tool for the evaluation of safety and efficacy of clinical therapies to prevent NTHi colonization and the development of OM. In this model, 100% of infant BALB/cARC mice were colonized with NTHi, and all developed NTHi OM. Influenza A virus (IAV) facilitated the establishment of dense (1 × 105 CFU/mL) and long-lasting (6 days) NTHi colonization. IAV was essential for the development of NTHi OM, with 100% of mice in the IAV/NTHi group developing NTHi OM compared with 8% of mice in the NTHi only group. Histological analysis and cytokine measurements revealed that the inflammation observed in the middle ear of the infant mice with OM reflected inflammation observed in children with OM. We have developed the first infant mouse model of NTHi colonization and OM. This ascension model uses influenza-driven establishment of OM and reflects the clinical pathology of bacterial OM developing after a respiratory virus infection. This model provides a valuable tool for testing therapies to prevent or treat NTHi colonization and disease in young children.
KEYWORDS: otitis media, nontypeable Haemophilus influenzae (NTHi), infant mouse model, infection, influenza, preclinical model
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHi) is a Gram-negative bacterium that exhibits opportunistic pathogenic behavior in humans (1). Asymptomatic nasopharyngeal colonization with NTHi is common and transient in children, with carriage frequencies ranging from ~15% to 100% depending upon age and geographic region (2–5). NTHi can also cause a range of diseases including sinusitis, conjunctivitis, pharyngitis, meningitis, bacteraemia, pneumonia, and otitis media (OM) (6, 7). Globally, NTHi is the predominant pathogen associated with chronic OM with effusion, recurrent acute OM, or acute OM with failure to treat (8). Early and dense NTHi colonization is associated with the development of acute and recurrent OM (6, 9–13). Triggers for the development of asymptomatic NTHi colonization into OM are thought to involve virus-induced inflammation, which permits proliferation and dissemination of NTHi from the nasopharynx along the Eustachian tube and into the middle ear (14, 15).
The burden of OM is large, with over 700 million annual acute OM cases diagnosed globally, 30 million chronic cases, and over 21,000 OM-associated deaths annually. An estimated 51% of all OM is in children under 5 years of age (16). With such a high prevalence in the pediatric population, OM is the main reason that antibiotics are prescribed for pre-school children (17). Unfortunately, antibiotic treatment for OM is often ineffective (18).
Despite major efforts (19–22), there are no licensed vaccines or other therapies that have been proven to prevent NTHi colonization and/or infection (23–25). As development of preventative therapies for NTHi diseases including OM continues, relevant mouse models are important. However, intranasal inoculation of rodents with NTHi does not reliably result in development of NTHi OM (26). Therefore, NTHi OM animal models often involve direct inoculation of NTHi into the middle ear (either via the bulla or through the tympanic membrane) [reviewed in references (27, 28)], which does not accurately reflect the progression of disease in humans. NTHi OM ascension models, where intranasal delivery of a respiratory virus facilitates NTHi ascension from the nose to the ear have been established in chinchillas using adenovirus (29), and in adult mice using influenza (14, 26). These biologically relevant models of disease are most suitable for testing therapies to prevent or treat NTHi OM; especially as preventing dissemination of NTHi from the nose to the ear (or lung, meninges, and blood) is likely to be the most effective strategy for prevention of NTHi disease. As murine models are more accessible than chinchillas (cost, handling, availability, sourcing of animals, and animal-specific reagents), and NTHi OM is mostly a childhood infection, we adapted the adult mouse model of influenza-driven NTHi OM (14) into an infant mouse model. To our knowledge, this is the first infant animal model of NTHi colonization and OM. The model reflects real-world conditions and is suitable for preclinical testing of the safety and efficacy of therapies for prevention or treatment of acute NTHi OM in young children.
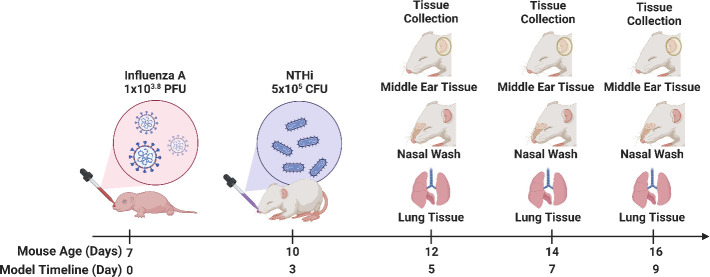
Male and female 7-day-old mice were intranasally administered either saline or influenza A virus (IAV). After 3 days, mice were intranasally administered either saline or NTHi. Nasal washes and middle ear and lung tissue samples were collected 5, 7, and 9 days post-treatment and analyzed for bacterial counts, viral titer, and inflammation.
RESULTS
Infant mice receiving both influenza and NTHi had dense and persistent NTHi colonization and were more likely to develop NTHi OM compared to mice that did not receive influenza
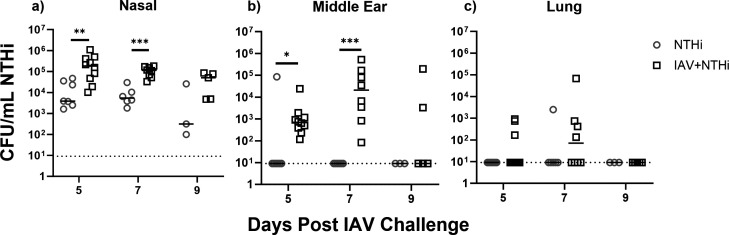
NTHi colonization was observed in the nasal wash of all mice in the IAV + NTHi and NTHi only groups on Days 5, 7, and 9 (Fig. 1a). NTHi was not detected in the nasal washes from the saline-treated control group or the IAV only group. Median NTHi titers in the nasal washes were 2 LOG higher in the IAV + NTHi challenged mice compared to the NTHi only group (P < 0.01 on Day 5, and P < 0.001 on Day 7; Fig. 1a). NTHi titers in the nasal wash had a median of 5.08 × 104 CFU/mL on Day 9 in the IAV + NTHi group (a titer that was never reached in the NTHi only group). In contrast, NTHi colonization was clearing by Day 9 in the NTHi only group.
Fig 1.
NTHi counts (CFU/mL) in (a) nasal wash, (b) middle ear tissue, and (c) lung tissue on Days 5, 7, and 9, post-IAV challenge. Nontypeable Haemophilus influenzae (NTHi) density in log10 CFU/mL from (a) nasal wash, (b) middle ear tissue homogenate, and (c) lung tissue homogenate from mice on Days 5, 7, and 9, pos-influenza A (IAV) or saline treatment on Day 0, and NTHi challenge on Day 3. Each dot represents an individual mouse. Horizontal bars depict the median NTHi density, and the dotted line represents the limit of quantification by culture. Open circles represent NTHi titers in the NTHi only treatment group, open squares represent NTHi titers in the IAV + NTHi treatment group. *P < 0.05; **P < 0.01; ***P < 0.001 when compared between treatment groups at the same time point using the Mann-Whitney test.
In the middle ear tissue, 18/18 (100%) mice in the IAV + NTHi challenge group developed OM on Day 5 or Day 7 compared with 1/13 (7.69%) in the NTHi only group (Fig. 1b). Median NTHi titers in the middle ear tissue homogenate were below the limit of detection in the NTHi only group on all days. For the IAV + NTHi groups, the middle ears contained 6.95 × 102 CFU/mL NTHi (IQR: 3.5 × 102 to 1.35 × 103 CFU/mL) on Day 5 and peaked on Day 7 with 2.08 × 104 CFU/mL NTHi (IQR: 1.50 × 103 to 1.46 × 105 CFU/mL) (P < 0.05 and P < 0.001, respectively). By Day 9, OM was resolving in the IAV + NTHi challenge group with 2/5 mice with NTHi detected in the middle ear tissue. The highest median NTHi titer in middle ear tissue homogenate was on Day 7 of the model (4 days post-NTHi challenge IAV + NTHi group), at 2.08 × 104 CFU/mL (IQR: 1.50 × 103 to 1.46 × 105 CFU/mL).
Low titers of NTHi were detected in the lungs from 7/18 mice in the IAV + NTHi group and 1/13 mice in the NTHi only group on Days 5 and 7 (Fig. 1c). Only the IAV + NTHi group on Day 7 had a median titer above the limit of detection—7.12 × 101 CFU/mL (IQR: 9.12 to 6.75 × 104 CFU/mL). NTHi was not detected in the samples collected from saline-treated control or IAV only challenged mice (median titer below limit of detection). No difference was observed between male and female infant mice for NTHi titers detected in any specimen (Fig. S1).
No weight loss was observed in the infant NTHi OM mouse model
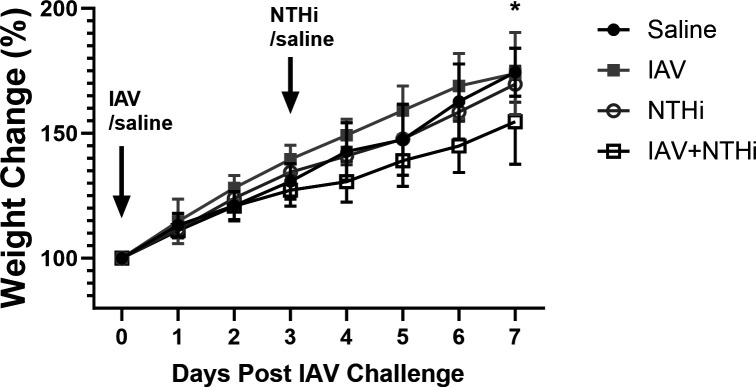
Any weight loss in infant mice is a significant adverse event, from which they are unlikely to recover. No weight loss was observed in this model using an NTHi challenge dose of 5 × 105 CFU (Fig. 2). The IAV + NTHi group had the least weight gain in comparison with the saline control group and the IAV only-treated group (Fig. 2). By Day 7, there was a difference in mean weight gain between the saline only-treated group and the IAV + NTHi group (74.42 ± 9.68% vs 54.63 ± 17.06%, respectively, P < 0.05).
Fig 2.
Weight of mice in NTHi OM model percent weight change (mean ± SD) of infant mice intranasally challenged with saline, IAV, NTHi, or IAV + NTHi. Numbers of mice per group decreased over time as samples were collected (Days 1–5, n ≥ 6/group; Days 6 and 7, n ≥ 3/group). *P < 0.05 when compared between saline and IAV + NTHi-treatment groups on Day 7.
While weight loss, or any other clinical symptom, was not observed with a 5 × 105 CFU NTHi challenge dose, a higher NTHi dose of 5 × 106 CFU did result in up to 16% weight loss (Fig. S2a). This was considered too severe an illness for an infant OM model, risking mismothering and mice not surviving until the model ended. In addition, the log-fold increase in NTHi challenge dose did not result in increased NTHi tires in the nasal washes or middle ear of mice on Day 5 or Day 7 of the model (Fig. S2b and c). Thus, an NTHi challenge dose of 5 × 105 CFU was selected for this model [which is 2 LOG lower than the adult mouse NTHi OM model challenge dose of 5 × 107 CFU (14)].
Influenza A virus was detected in nasal washes, lung tissue, and in some middle ear specimens
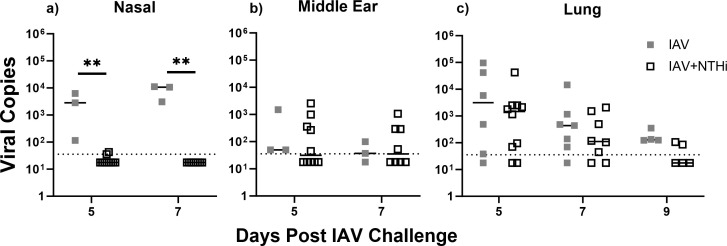
IAV was detected by qRT-PCR in all of the nasal washes tested in the IAV only-treated mice (n = 6 tested; n = 3 on Day 5 and n = 3 on Day 7) but not the IAV + NTHi group (n = 18; n = 10 on Day 5 and n = 8 on Day 7), with only 2/18 mice in the IAV + NTHi group with a positive IAV PCR, albeit at the limit of detection (Fig. 3a). The median IAV titer in the nasal washes of the IAV group was 2.83 × 103 (IQR: 1.15 × 102 to 6.29 × 103) on Day 5 and 1.06 × 104 (IQR: 1.11 × 104 to 3.06 × 103) on Day 7, higher in comparison with median IAV titers below the limit of detection for the IAV + NTHi group on both days, P < 0.01 (Fig. 3a). In the middle ear tissue, IAV was detected some mice in each of the IAV only and IAV + NTHi groups, with median IAV titers just above or at the lowest limit of detection (Fig. 3b). IAV was detected in the lung tissue of most mice, with the IAV only group having higher median IAV titers than the IAV + NTHi group on Days 5, 7, and 9 (Fig. 3c). On Day 9, IAV tires were only measured in the lung tissue and not the nasal wash and middle ear. Of note is the pattern of reduced IAV titers in the presence of NTHi for the nasal washes (Fig. 3a).
Fig 3.
Influenza A virus titers in the nasal wash, middle ear tissue, and lung tissue post-IAV challenge. Influenza A Virus (IAV) titers from (a) nasal wash, (b) middle ear tissue homogenate, and (c) lung tissue homogenate of mice on Day 5, Day 7 (and Day 9, lungs only) post-IAV or IAV + NTHi challenge. Each square represents an individual mouse. Horizontal bars depict the median IAV titer, and the dotted line represents the limit of quantification. **P < 0.01 when compared between treatment groups at the same time point using the Mann-Whitney test.
Immune mediator responses in infant model
Immune mediators IL-1β, IL-6, IL-10, IFN-γ, and KC were measured in the nasal wash and supernatant from the middle ear and lung tissue homogenate (when enough volume was available after NTHi and IAV counts were conducted; n ≥ 3) (Fig. 4). All cytokines except for IL-10 were detectable in at least one of the sample types. Low or baseline titers were measured for IL-1β and IFN-γ in all tissue types (Fig. S3). Titers for IL-6 and KC in all tissue types are shown in Fig. 4b through g. IAV only and IAV + NTHi challenged mice had increased IL-6 in their nasal washes on Days 5 and 7 in comparison to the saline-treated group [Day 5 IAV vs saline: 7.55 (IQR: 5.25–54.48) pg/mL vs 0.34 (IQR: 0.34–0.34) pg/mL, respectively, P < 0.05; Day 5 IAV + NTHi vs saline: 125.9 (54.11–306.1) pg/mL vs 0.34 (0.34–0.34) pg/mL, respectively, P < 0.001] (Fig. 4b). By Day 7, the IL-6 response was still higher in nasal washes of the IAV and IAV + NTHi groups in comparison with the saline only group [Day 7 IAV: 125.9 (45.51–226.8) pg/mL, Day 7 IAV + NTHi: 72.72 (38.79–151.6) pg/mL vs Day 7 saline: 1.13 (0.34–2.41) pg/mL, P = 0.06 and P < 0.01, respectively]. The nasal wash KC responses were similar to those observed for IL-6 (Fig. 4c), with increased KC titers in the IAV and IAV + NTHi groups on Days 5 and 7 in comparison with the saline group [Day 5 IAV only = 25.61 (16.10–66.81) pg/mL, IAV + NTHi = 62.57 (25.76–135.6) pg/mL vs saline 1.04 (1.04–4.51) pg/mL, P < 0.05 and P < 0.001, respectively, Day 7 IAV only = 54.52 (39.31–117.5) pg/mL, IAV + NTHi = 140.5 (60.69–162.2) pg/mL vs saline 13.40 (2.32–21.66) pg/mL; P = 0.06 and P < 0.01, respectively]. The IAV + NTHi group had increased KC in the middle ear tissue by Day 7 compared to the saline group [81.15 (50.13–86.73) pg/mL vs 33.99 (33.54–36.90) pg/mL, respectively, P < 0.05].
Fig 4.
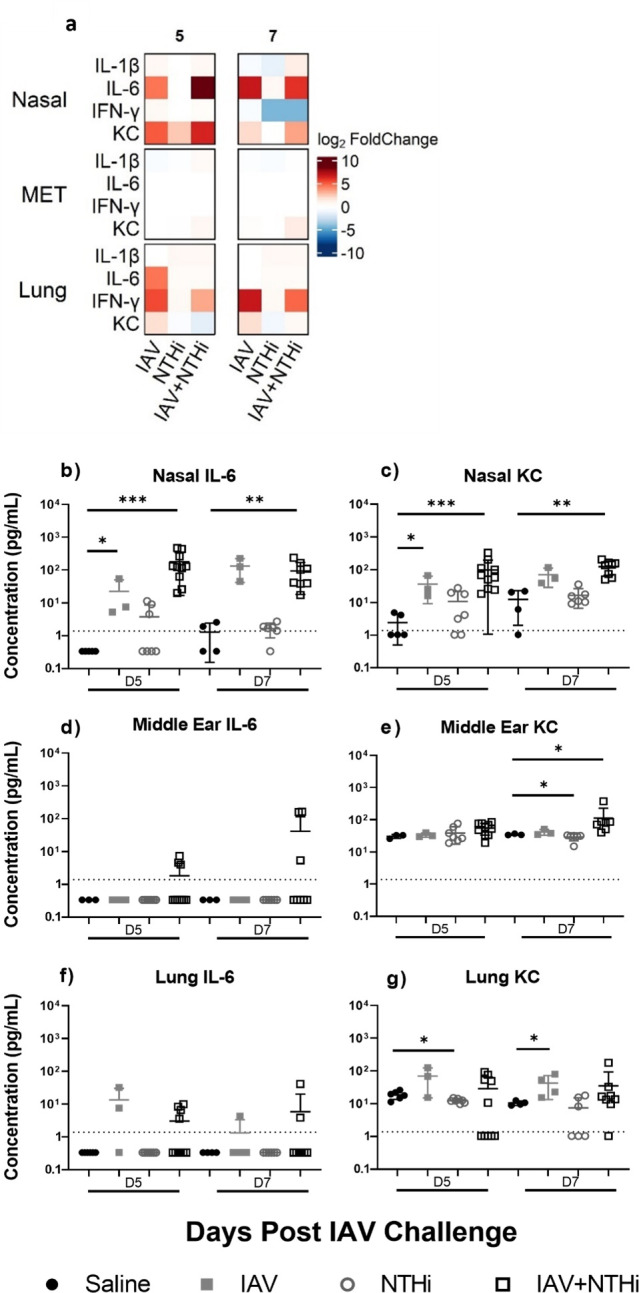
Immune mediator responses in infant NTHi OM model including IL-6 and KC titers in nasal wash, middle ear tissue, and lung on Days 5 and 7 post-IAV challenge. (a) Heat map representation of inflammatory mediator levels in nasal washes, and supernatant from middle ear tissue (MET) homogenate and lung tissue homogenate from mice on Days 5 and 7 post-influenza A virus (IAV) or saline treatment. Represented as log2 fold change over the saline control mediator response for each analyte for each treatment group. IL-6 and KC titers in nasal washes (b and c), middle ear tissue (d and e), and lung tissue (f and g) from mice on Days 5 and 7 post-IAV challenge or saline treatment. Each dot represents an individual mouse, horizontal bars depict median analyte titer, and the dotted line represents the limit of quantification for each analyte. *P < 0.05; **P < 0.01; ***P < 0.001 when compared between treatment groups at the same time point using the Mann-Whitney test.
Similar patterns were observed for KC in the lung tissue as in the nasal washes, with increased KC response in the lungs on Days 5 and 7 in the IAV only group compared with saline [Day 5 IAV only 68.67 (15.54–125.1] pg/mL vs saline 18.81 (14.05–23.07) pg/mL, P = 0.26 and Day 7 IAV 38.10 (17.39–72.91) pg/mL vs saline 10.16 (9.10–12.11) pg/mL, P < 0.05].
Of note is the absence of any cytokine signal in the specimens from the NTHi only group in comparison with the IAV only and IAV + NTHi groups (Fig. 4). Indeed, the IL-6 and KC responses in the NTHi only treatment group were often lower than the saline-treated group (Fig. 4). This was also observed for IFN-γ with decreased levels in the nasal wash of the NTHi only challenged mice compared to the saline only controls on Day 7 [9.59 (1.41–18.54) pg/mL vs 0.44 (0.44–0.44) pg/mL, P < 0.05 (Fig. S3b]. In the middle ear tissue and lungs, NTHi only challenged mice had a small but significant decrease in KC levels compared to saline-treated controls on Day 5 for lungs [18.81 (14.05–23.07) pg/mL compared to 12.46 (10.75–14.14) pg/mL] and Day 7 for middle ear tissue [33.99 (33.54–36.90) pg/mL compared to 31.63 (27.07–32.08) pg/mL] (P < 0.05 in all cases) (Fig. 4).
Histological assessment of middle ear tissue reveals evidence of inflammation and infection
Haematoxylin and eosin (H&E) staining of fixed middle ear tissue was used for histological assessment of the middle ear tissue (Fig. S4). Potential thickening of the middle ear epithelium was observed behind the tympanic membrane of IAV + NTHi-treated mice [mean ± SEM epithelial thickness for saline: 190.5 ± 24.55 µm (n = 2), IAV: 208.8 ± 20.08 µm (n = 3), NTHi: 142.6 ± 56.71 µm (n = 2), and IAV + NTHi 265.1 ± 23.26 µm (n = 3)]. (No statistical analysis was conducted due to small sample size). Infiltrate was observed in the middle ear space of IAV + NTHi-treated mice (Fig. S4f), which was not observed in H&E stained sections of middle ear tissue from the other treatment groups.
DISCUSSION
We have developed an infant mouse model of NTHi colonization and OM that reflects respiratory virus-driven bacterial OM in children (27, 30). Influenza challenge was essential for driving NTHi from the nose into the middle ear, as intranasal inoculation of NTHi only did not result in prolonged NTHi colonization or development of NTHi OM. Inflammation was observed in response to the development of NTHi OM in the nasal washes, and less so in the middle ear. Evidence of infiltration was observed in the ears of mice with NTHi OM. Disease development was the same between male and female mice, with no differences in clinical symptoms or bacterial/viral titers, permitting the use of both sexes in this model. The use of both sexes is important for providing safety and efficacy data for treatments that will be used in all children (in addition to meeting the principle of the 3Rs of animal research: reduce, refine, and replace).
NTHi does not naturally colonize any animal other than humans (9). This is one of the difficulties of developing suitable NTHi OM animal models. Chinchillas are one of the most widely used OM animal models due to the relatively large size of their tympanic membranes and bulla, allowing for easy dissection and direct injection of the pathogen of interest into the middle ear space (27). While this makes model development easier, there are several downsides including that transbullar injection does not mimic natural disease progression in humans (30), and that chinchillas are available only in North and South America, limiting global use of the model (27). The previously developed adult ascension mouse model of IAV driven NTHi infection of the middle ear does mimic human disease progression and uses a widely available lab animal—BALB/c mice. However, only half of the adult mice develop NTHi OM in this model (14), which makes testing of therapeutics for disease prevention and treatment difficult and requires larger group sizes to take this into account. Using the infant NTHi OM model, all mice developed NTHi OM by Day 5, which was sustained by Day 7 with clearance around Day 9. Levels of IL-6 in the nasal wash and middle ear tissue were approximately three-fold lower in the infant mice compared with the adult model on Days 5 and 7 (14); however, KC levels were similar. This suggests that while the infant mice were mounting a protective innate immune response, it was not as strong as observed in the adult mice and may contribute to the increased susceptibility to NTHi-driven OM in infant mice (and young children). With a 100% disease rate and longer duration of infection, this makes the infant mouse model much more suited for testing vaccines and therapeutics. Additionally, the dense and prolonged NTHi colonization of the infant mice was stable at least to Day 9 (we did not measure any later), providing a promising feature for testing the therapeutic impact on NTHi colonization—the precursor for all NTHi disease development.
In terms of the immune responses in this model, we measured the innate response for each sample collection day. IAV challenge was the main driver of the measured innate inflammatory response, which is likely to facilitate NTHi proliferation and dissemination into the ear - as is proposed to occur in children (14, 15). In the nasal wash, both IL-6 and KC were significantly elevated but only in groups that had been challenged with IAV. In the middle ear tissue, both IAV and NTHi were required for an increased KC response. For the lungs, IAV was also the main driver of the increased KC response. Both IL-6 and KC are innate chemoattractants in mice, responsible for immune cell recruitment (31, 32), suggesting a strong innate response to intranasal IAV challenge in both the nasal wash and lungs, and that co-challenge with NTHi is required to extend that response to the middle ear tissue. The observation of a suppressed local innate immune response to NTHi aligns with previous reports of NTHi displaying immune evasion techniques (9), including suppression of cytokine signaling (14, 33).
While IAV facilitated NTHi colonization and dissemination into the ear, NTHi infection did not increase the IAV titers measured in the lungs. Instead, decreased IAV titers were observed in the nasal washes of co-challenged mice in comparison with the IAV only challenged mice, indicating that NTHi challenge may interfere or suppress IAV infection. The mechanisms behind this require further investigation.
The observation of middle ear epithelium thickening and infiltrate in the middle ear space are in line with previous reports in OM mouse models of NTHi-OM (transbullar) and pneumococcal-OM (intranasal ascension driven by influenza) (34, 35). Transbullar inoculation of NTHi resulted in thickened middle ear epithelium and increased immune cell infiltrate in the middle ear space in adult C57BL/6 mice (34). An IAV ascension model of pneumococcal-driven OM found increased infiltrate in the middle ear space of the infant mice (35). Similar results were observed in this infant mouse model for NTHi-driven OM, although small sample sizes mean no statistical testing could be performed.
Limitations of this infant NTHi OM model are (i) that only acute OM has been studied, not chronic or repeat OM [which is lacking across the whole OM research field (28)]; (ii) the model is labor intensive and requires an in-house breeding program and timed mating, and (iii) care must be taken to not overhandle the young mice to prevent rejection from the mothers.
In summary, we have developed an infant mouse model of viral-driven NTHi colonization and acute NTHi OM that can be used for development and testing of therapeutics targeted specifically at NTHi.
MATERIALS AND METHODS
Species, strains, and inoculum
Animals
All experiments were approved by the Telethon Kids Animal Ethics Committee prior to beginning the work (application: AEC #374). Specific pathogen-free mice (36 seven-week-old female BALB/cARC mice and 20 seven-week-old male BALB/cARC) were purchased from the Animal Resources Centre (Murdoch, WA, Australia) and housed at the Telethon Kids Institute under Physical Containment level 2 conditions and a 12:12 standard light/dark cycle. Standard mouse chow (Specialty Feeds, Glen Forrest, WA, Australia) and acidified water were freely accessible. After an acclimatization period of 1 week, male and female mice were housed together overnight for up to 2 weeks. Plugged female mice were separated and housed together until gestational day 14.5 (GD14.5) whereupon they were weighed. Mice weighing over 22 g were considered pregnant and housed in separate cages with extra bedding and red plastic huts provided for nesting. Starting at GD18.5, the presence of pups was checked daily to ascertain the day of birth. The average litter size was 3 for successful pregnancies, with a total of 79 pups from 24 litters from 8 rounds of breeding used in this study. An additional seven pups were used in NTHi dose optimization (see Fig. S2). To enable handling of manageable group sizes and control for day-to-day variability, litters of mice were distributed across treatment groups for each experiment (see Table S1 for litter allocation across experiments). When mice were 6 days old, their footpads were tattooed with green animal tattoo ink and tattoo device (Ketchum, Fine Science Tools, USA) using a 29-gauge needle to allow identification of individual mice. Mice were sexed by eye by trained personnel. Individual litters were assigned to one of four groups: saline-treated control, IAV challenge, NTHi challenge, or IAV + NTHi challenge. Multiple litters were used per treatment group.
Influenza
Influenza A/Mem71 virus (IAV) is a combined [A/Memphis/1/71 (H3N2) × A/Bellamy/42 (H1N1)] mouse-adapted H3N1 strain of influenza A (36) that causes mild to moderate disease symptoms in adult and infant mice (36, 37). IAV was subpassaged through Madin-Darby canine kidney (MDCK) cells (NBL-2, ATCC CCL-34) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Sydney, Australia), then harvested from tissue culture supernatant and stored at −80°C. Viral titers after thawing from −80°C storage were determined by plaque assay as previously described (38).
NTHi
Genetically modified spectinomycin-resistant nontypeable Haemophilus influenzae (NTHi 2866 Specr) was harvested mid-log growth phase as previously described (39), with the addition that 0.1 mg/mL spectinomycin was added to the culture media during growth to maintain resistance. This mutant strain was generated by allelic exchange of pseudogene R2866_1356 with a spectinomycin resistance cassette that was amplified from plasmid pR412 as previously described (14, 40).
Infectious challenges
At 7 days of age (Day 0 = IAV challenge), mice were intranasally challenged with either 5 µL of saline (phosphate-buffered saline, PBS) or 1 × 103.8 PFU IAV in 5 µL of saline, calculated using previously described dose changes between adult and weaning age mice (41). Three days after the first challenge, mice were challenged a second time with either 5 µL saline or 5 × 105 CFU NTHi in 5 µL of saline. Nasal washes and tissue samples were collected at Days 5, 7, and 9 days post-IAV challenge as outlined in Fig. 5. All challenges were performed without anesthetic due to the young age of the mice. Mice were monitored and weighed daily.
Fig 5.
Experimental timeline for infant model of NTHi OM. Experimental Day 0 (IAV challenge) occurs when the mouse is 7 days old. Mice were given an inoculation of influenza A (IAV) (1 × 103.8 PFU) or saline on Day 0. Three days following, mice were given an inoculation of NTHi (5 × 105 CFU) or saline. Samples were collected on Days 5, 7, and 9 post-IAV inoculation. This image was created using Biorender.com.
Specimen collection and processing
Mice were euthanized after 5 min in a small chamber containing 5% isoflurane (SomnoSuite, Ken Scientific). Nasal washes [50 µL saline lavaged into the nasal space through a 30-gauge Rycroft anterior chamber cannula (Sterimedix ophthalmic, IQmedical)], middle ear tissue (dissected from the skull) and whole lungs were collected (Fig. 5) immediately post-mortem and stored on ice. Samples were homogenized in saline (250 µL for middle ear tissue and 1 mL for lung tissue), serially diluted 10-fold, and spotted onto chocolate agar plates (PathWest, Perth, Australia) overlayed with spectinomycin (200 µL of 10 mg/mL spectinomycin) to select for NTHi Specr and incubated (37°C, 5% CO2) overnight. Viable counts were conducted by personnel not involved in the animal model to ensure blinding. The limit of detection (LOD) was 1.92 Log10 CFU/mL. For ease of statistical analysis, all values below the limit of detection were replaced with a value half that of the LOD.
Viral copy assessment
Influenza virus qPCR was performed as described previously (35). In brief, RNA was extracted using TRIzol (Invitrogen, USA), purified using an RNAeasy minielute kit (Qiagen, Germany), and treated with DNase (Turbo DNAse; Ambion, USA). Synthesis of cDNA was performed using random oligo(dT)18 (Roche) and Transcriptor High Fidelity cDNA synthesis kit (Roche, Switzerland). Quantitative PCR was performed using SYBR green (Quantance) and forward and reverse primers for the Influenza A/Mem71 Matrix gene (42) (Forward primer sequence 5′-AAGACCAATCTTGTCACCTCTGA-3′, Reverse Primer Sequence 5′-TCCTCGCTCACTGGGCA-3′). Quantitative viral copy number was determined using 10-fold dilutions of a plasmid containing the Influenza A/Mem71 matrix gene.
Immune mediator analysis
Aliquots of nasal wash, and homogenized middle ear and lung samples were centrifuged at 13,000 RPM for 10 min at 4°C. Supernatant was collected and filtered through 0.2 µm filters (MERK) before storage at −80°C. Once thawed, immune mediator response for IL-1β, IL-6, IL-10, IFN-γ, and KC was assessed using custom-generated multiplex kits from Bio-Rad (X-Plex kit, Bio-Rad, USA), following kit protocol. Analysis of multiplex results was performed using a Bioplex 200 with Bioplex Manager software V6.1.1. For ease of statistical analysis, all values below the limit of detection were replaced with a value half that of the lowest standard (limit of detection for IL-1β, IL-6, IFN-γ, and KC were 1.39, 0.67, 0.87, and 2.07 pg/mL, respectively).
Histology
Whole infant mouse heads obtained on Day 7 post-IAV challenge were fixed for at least 24 h in 10% formalin, prior to embedding in paraffin (n = 3 per group). Mouse heads were processed overnight for paraffin infiltration and embedded for horizontal sections beginning from the base of the skull. The head was cut until the outer ear canal was visible and then 10 µm sections were taken at intervals of 125 µm to isolate the middle and inner ear. Sections were then stained with H&E and slides where the tympanic membrane could be positively identified were selected for imaging using a Panoramic MIDI scanner and paired software (3DHISTECH Ltd.).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (V9.0.0). Analysis between groups was conducted using two-way Mann-Whitney testing methodologies due to the non-parametric nature of the data. All data are described as median or median (IQR) except for weight which is described as mean ± SD and epithelial thickness measurements described as mean ± SEM.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Jacob Young and the Telethon Kids Institute Bioresources team for their technical assistance in the breeding program and A/Prof Kirsty Short and Dr Keng Chew from the University of Queensland for measuring IAV titers. We also thank Dr Christian Tjiam and Edison Foo for assistance with the R code to prepare the heatmap in Fig. 4a and Dr Tamara Abel for technical assistance with the slidescanner.
Funding for this work was awarded to A/Prof Lea-Ann Kirkham by the Western Australian Department of Health and the Channel 7 Telethon Trust as a Western Australian Child Research Fund grant (2019–2020, Round 8). A/Prof Kirkham is supported by a fellowship from the Perron Foundation.
K.R.L. and C.M.G. conducted the majority of the sample collection, data collection, and analysis with assistance from K.M.M., N.M.S., K.S., K.C., and L.B. Experiments were designed by K.R.L., C.M.G., N.M.S., and L.-A.S.K. The study was conceived by L.-A.S.K., who together with E.J.S, D.S., A.F., and P.C.R. obtained funding to conduct this study. K.R.L. wrote the first draft of the manuscript with involvement from all authors prior to final submission.
Footnotes
Presented at: Portions of this work were previously presented at the 22nd International Symposium on Recent Advances in Otitis Media conference, 19–21 June 2023, at Medical College of Wisconsin, Milwaukee, WI, USA.
Contributor Information
Lea-Ann S. Kirkham, Email: lea-ann.kirkham@telethonkids.org.au.
Igor E. Brodsky, University of Pennsylvania, Philadelphia, Pennsylvania, USA
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00453-23.
Fig. S1 to S4; Table S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Nørskov-Lauritsen N. 2014. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev 27:214–240. doi: 10.1128/CMR.00103-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giufrè M, Daprai L, Cardines R, Bernaschi P, Ravà L, Accogli M, Raponi M, Garlaschi ML, Ciofi degli Atti ML, Cerquetti M. 2015. Carriage of Haemophilus influenzae in the oropharynx of young children and molecular epidemiology of the isolates after fifteen years of H. influenzae type B vaccination in Italy. Vaccine 33:6227–6234. doi: 10.1016/j.vaccine.2015.09.082 [DOI] [PubMed] [Google Scholar]
- 3. Rahman T, de Gier C, Orami T, Seppanen EJ, Granland CM, Francis JP, Michael A, Yoannes M, Corscadden KJ, Ford RL, Martinovich KM, Jacoby P, van den Biggelaar AHJ, Lehmann D, Richmond PC, Pomat WS, Thornton RB, Kirkham L-AS. 2021. PCV10 elicits protein D IgG responses in Papua New Guinean children but has no impact on NTHi carriage in the first two years of life. Vaccine 39:3486–3492. doi: 10.1016/j.vaccine.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 4. Barbosa-Cesnik C, Farjo RS, Patel M, Gilsdorf J, McCoy SI, Pettigrew MM, Marrs C, Foxman B. 2006. Predictors for Haemophilus influenzae colonization, antibiotic resistance and for sharing an identical isolate among children attending 16 licensed day-care centers in Michigan. Pediatr Infect Dis J 25:219–223. doi: 10.1097/01.inf.0000202130.78540.28 [DOI] [PubMed] [Google Scholar]
- 5. Watson K, Carville K, Bowman J, Jacoby P, Riley TV, Leach AJ, Lehmann D, Kalgoorlie Otitis Media Research Project Team . 2006. Upper respiratory tract bacterial carriage in aboriginal and non-aboriginal children in a semi-arid area of Western Australia. Pediatr Infect Dis J 25:782–790. doi: 10.1097/01.inf.0000232705.49634.68 [DOI] [PubMed] [Google Scholar]
- 6. Slack MPE. 2017. The evidence for non-typeable Haemophilus influenzae as a causative agent of childhood pneumonia. Pneumonia (Nathan) 9:9. doi: 10.1186/s41479-017-0033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy TF, Bakaletz LO, Smeesters PR. 2009. Microbial interactions in the respiratory tract. Pediatr Infect Dis J 28:S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec [DOI] [PubMed] [Google Scholar]
- 8. Ngo CC, Massa HM, Thornton RB, Cripps AW. 2016. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One 11:e0150949. doi: 10.1371/journal.pone.0150949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foxwell AR, Kyd JM, Cripps AW. 1998. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev 62:294–308. doi: 10.1128/MMBR.62.2.294-308.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leach AJ, Boswell JB, Asche V, Nienhuys TG, Mathews JD. 1994. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr Infect Dis J 13:983–989. doi: 10.1097/00006454-199411000-00009 [DOI] [PubMed] [Google Scholar]
- 11. Smith-Vaughan H, Byun R, Nadkarni M, Jacques NA, Hunter N, Halpin S, Morris PS, Leach AJ. 2006. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 6:10. doi: 10.1186/1472-6815-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith-Vaughan HC, Binks MJ, Marsh RL, Kaestli M, Ward L, Hare KM, Pizzutto SJ, Thornton RB, Morris PS, Leach AJ. 2013. Dominance of Haemophilus influenzae in ear discharge from indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord 13:12. doi: 10.1186/1472-6815-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seppanen EJ, Thornton RB, North HJ, Corscadden KJ, Wiertsema SP, Vijayasekaran S, Coates HL, Jacoby P, Richmond PC, Kirkham L-AS. 2020. Bacterial reservoirs in the middle ear of otitis-prone children are associated with repeat ventilation tube insertion. Pediatr Infect Dis J 39:91–96. doi: 10.1097/INF.0000000000002541 [DOI] [PubMed] [Google Scholar]
- 14. Granland CM, Scott NM, Lauzon-Joset J-F, Langereis JD, de Gier C, Sutherland KMJ, Clark SL, Pickering JL, Thornton RB, Richmond PC, Strickland DH, Kirkham L-AS. 2020. Nasal delivery of a commensal Pasteurellaceae species inhibits nontypeable Haemophilus influenzae colonization and delays onset of otitis media in mice. Infect Immun 88:e00685-19. doi: 10.1128/IAI.00685-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. 2016. Otitis media. Nat Rev Dis Primers 2:16063. doi: 10.1038/nrdp.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. 2012. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 7:e36226. doi: 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vergison A, Dagan R, Arguedas A, Bonhoeffer J, Cohen R, Dhooge I, Hoberman A, Liese J, Marchisio P, Palmu AA, Ray GT, Sanders EAM, Simões EAF, Uhari M, van Eldere J, Pelton SI. 2010. Otitis media and its consequences: beyond the earache. Lancet Infect Dis 10:195–203. doi: 10.1016/S1473-3099(10)70012-8 [DOI] [PubMed] [Google Scholar]
- 18. Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. 2015. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015:CD000219. doi: 10.1002/14651858.CD000219.pub4 [DOI] [PubMed] [Google Scholar]
- 19. Galgani I, Annaratone M, Casula D, Di Maro G, Janssens M, Tasciotti A, Schwarz T, Ferguson M, Arora AK. 2022. Safety and immunogenicity of three doses of non-typeable Haemophilus influenzae-Moraxella catarrhalis (NTHi-Mcat) vaccine when administered according to two different schedules: a phase 2, randomised, observer-blind study. Respir Res 23:114. doi: 10.1186/s12931-022-02019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andreas S, Testa M, Boyer L, Brusselle G, Janssens W, Kerwin E, Papi A, Pek B, Puente-Maestu L, Saralaya D, Watz H, Wilkinson TMA, Casula D, Di Maro G, Lattanzi M, Moraschini L, Schoonbroodt S, Tasciotti A, Arora AK, Maltais F, NTHi-Mcat-002 study group . 2022. Non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine for the prevention of exacerbations in chronic obstructive pulmonary disease: a multicentre, randomised, placebo-controlled, observer-blinded, proof-of-concept, phase 2b trial. Lancet Respir Med 10:435–446. doi: 10.1016/S2213-2600(21)00502-6 [DOI] [PubMed] [Google Scholar]
- 21. Teo E, Lockhart K, Purchuri SN, Pushparajah J, Cripps AW, van Driel ML. 2017. Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev 6:CD010010. doi: 10.1002/14651858.CD010010.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novotny LA, Brockman KL, Mokrzan EM, Jurcisek JA, Bakaletz LO. 2019. Biofilm biology and vaccine strategies for otitis media due to nontypeable Haemophilus influenzae. J Pediatr Infect Dis 14:69–77. doi: 10.1055/s-0038-1660818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jalalvand F, Riesbeck K. 2018. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev Vaccines 17:503–512. doi: 10.1080/14760584.2018.1484286 [DOI] [PubMed] [Google Scholar]
- 24. Alderson MR, Murphy T, Pelton SI, Novotny LA, Hammitt LL, Kurabi A, Li J-D, Thornton RB, Kirkham L-AS. 2020. Panel 8: vaccines and immunology. Int J Pediatr Otorhinolaryngol 130:109839. doi: 10.1016/j.ijporl.2019.109839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarke C, Bakaletz LO, Ruiz-Guiñazú J, Borys D, Mrkvan T. 2017. Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage. Expert Rev Vaccines 16:1–14. doi: 10.1080/14760584.2017.1333905 [DOI] [PubMed] [Google Scholar]
- 26. Langereis JD, Stol K, Schweda EK, Twelkmeyer B, Bootsma HJ, de Vries SPW, Burghout P, Diavatopoulos DA, Hermans PWM. 2012. Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media. mBio 3:e00079-12. doi: 10.1128/mBio.00079-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davidoss NH, Varsak YK, Santa Maria PL. 2018. Animal models of acute otitis media – a review with practical implications for laboratory research. Eur Ann Otorhinolaryngol Head Neck Dis 135:183–190. doi: 10.1016/j.anorl.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 28. Bhutta MF, Thornton RB, Kirkham L-A, Kerschner JE, Cheeseman MT. 2017. Understanding the aetiology and resolution of chronic otitis media from animal and human studies. Dis Model Mech 10:1289–1300. doi: 10.1242/dmm.029983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki K, Bakaletz LO. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun 62:1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heikkinen T, Thint M, Chonmaitree T. 1999. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med 340:260–264. doi: 10.1056/NEJM199901283400402 [DOI] [PubMed] [Google Scholar]
- 31. Sawant KV, Sepuru KM, Lowry E, Penaranda B, Frevert CW, Garofalo RP, Rajarathnam K. 2021. Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: role of Cxcr2 activation and glycosaminoglycan interactions. J Leukoc Biol 109:777–791. doi: 10.1002/JLB.3A0820-207R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. 2002. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci 43:737–743. [PubMed] [Google Scholar]
- 33. Vikhe PP, Purnell T, Brown SDM, Hood DW. 2019. Cellular immune response against nontypeable Haemophilus influenzae infecting the preinflamed middle ear of the junbo mouse. Infect Immun 87:e00689-19. doi: 10.1128/IAI.00689-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim HW, Pak K, Kurabi A, Ryan AF. 2019. Lack of the hyaluronan receptor CD44 affects the course of bacterial otitis media and reduces leukocyte recruitment to the middle ear. BMC Immunol 20:20. doi: 10.1186/s12865-019-0302-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Short KR, Reading PC, Brown LE, Pedersen J, Gilbertson B, Job ER, Edenborough KM, Habets MN, Zomer A, Hermans PWM, Diavatopoulos DA, Wijburg OL. 2013. Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun 81:645–652. doi: 10.1128/IAI.01278-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reading PC, Morey LS, Crouch EC, Anders EM. 1997. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol 71:8204–8212. doi: 10.1128/JVI.71.11.8204-8212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, Turner SJ, Duca KA, Anderson GP. 2008. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res 9:53. doi: 10.1186/1465-9921-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baer A, Kehn-Hall K. 2014. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J Vis Exp:e52065. doi: 10.3791/52065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kirkham L-A, Corscadden KJ, Wiertsema SP, Currie AJ, Richmond PC. 2013. A practical method for preparation of pneumococcal and nontypeable Haemophilus influenzae inocula that preserves viability and immunostimulatory activity. BMC Res Notes 6:522. doi: 10.1186/1756-0500-6-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin B, Prudhomme M, Alloing G, Granadel C, Claverys JP. 2000. Cross‐regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol Microbiol 38:867–878. doi: 10.1046/j.1365-2958.2000.02187.x [DOI] [PubMed] [Google Scholar]
- 41. Larcombe AN, Foong RE, Bozanich EM, Berry LJ, Garratt LW, Gualano RC, Jones JE, Dousha LF, Zosky GR, Sly PD. 2011. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 5:334–342. doi: 10.1111/j.1750-2659.2011.00236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol 146:2275–2289. doi: 10.1007/s007050170002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4; Table S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.