ABSTRACT
Enterococci are common commensal bacteria that colonize the gastrointestinal tracts of most mammals, including humans. Importantly, these bacteria are one of the leading causes of nosocomial infections. This study examined the role of colonic macrophages in facilitating Enterococcus faecalis infections in mice. We determined that depletion of colonic phagocytes resulted in the reduction of E. faecalis dissemination to the gut-draining mesenteric lymph nodes. Furthermore, we established that trafficking of monocyte-derived CX3CR1-expressing macrophages contributed to E. faecalis dissemination in a manner that was not reliant on CCR7, the conventional receptor involved in lymphatic migration. Finally, we showed that E. faecalis mutants with impaired intracellular survival exhibited reduced dissemination, suggesting that E. faecalis can exploit host immune cell migration to disseminate systemically and cause disease. Our findings indicate that modulation of macrophage trafficking in the context of antibiotic therapy could serve as a novel approach for preventing or treating opportunistic infections by disseminating enteric pathobionts like E. faecalis.
KEYWORDS: Enterococcus faecalis, colon, dissemination, macrophages, CX3CR1, CCR2, gut-associated lymphoid tissue, commensal, migration, pathobiont
INTRODUCTION
Enterococci are Gram-positive commensal bacteria found in the gastrointestinal tract of most mammals. Although enterococci are typically non-pathogenic, they are intrinsically resistant to cephalosporin antibiotics, and treatment with cephalosporins can lead to opportunistic infections in humans and mice (1, 2). Currently, enterococci are the third leading cause of infectious endocarditis, with Enterococcus faecalis contributing to >90% of reported enterococcus-related cases (3, 4). Furthermore, the rising prevalence of vancomycin-resistant enterococci, predominantly Enterococcus faecium, has made enterococci a leading cause of hospital-acquired infections in the United States (5). Nevertheless, most individuals colonized with enterococci, including E. faecalis or E. faecium, do not succumb to infections (6, 7). This highlights the generally commensal nature of enterococci but suggests a gap in our understanding of the preconditions for opportunistic infections by these pathobionts. To date, the mechanisms by which commensal enterococci subvert host immunity to become pathogenic remain poorly understood.
The co-evolution between the host and commensals has resulted in immune mechanisms designed to tolerate, and even benefit from, gut-colonizing bacteria. Antigen-presenting cells (APCs) like macrophages and dendritic cells (DCs) of the gut-associated lymphoid tissue (GALT) selectively sample luminal microorganisms to educate the adaptive immune system and maintain homeostasis. For instance, the CX3CR1-dependent extension of transepithelial dendrites into the intestinal lumen or the passage of bacteria via goblet cells and microfold cells to underlying APCs represents coordinated processes of bacterial translocation across the epithelial barrier (8–12). Upon antigen uptake and processing, APCs migrate to the gut-draining mesenteric lymph nodes (MLN) where immunological tolerance to commensal antigen is generated (13–15).
A growing body of evidence suggests that APC migration can contribute to the transport of live bacteria from the gut to the MLNs, a process that may serve homeostatic functions. For instance, delivery of live Enterobacter cloacae to the MLNs by CD11c-expressing leukocytes promotes the production of IgA that protects mice from excessive microbial dissemination (16). Moreover, the presence of commensal bacteria in the MLNs at a steady state has been shown to induce low levels of circulating IgG that provides cross-reactive protection against challenges with lethal doses of E. coli or Salmonella (17). Although the mechanism by which these commensals arrived in the MLNs was not addressed in that study, the possible contribution from intestinal APCs should not be overlooked.
Many bacterial species are inherently well suited for intracellular survival within phagocytes (18–21) which likely contributes to their inadvertent transport to the MLN via migratory APCs. This phenomenon is well described in Salmonella typhimurium infections (22–25), whereby Salmonella subverts APC killing mechanisms while it is trafficked to the MLNs. This type of APC-mediated infection has also been observed with other pathogenic microbes like Listeria monocytogenes in the gut and Staphylococcus aureus in the skin (18, 26), suggesting that APC migration is a common mechanism by which pathogens gain access to peripheral tissues and cause disease. While several intracellular survival-related pathways have been described in enterococcal species (19, 27–30), the role that intracellular APC survival plays in enterococcal dissemination from the gut remains poorly understood.
In the present study, we leveraged experimental mouse models to investigate the hypothesis that intestinal APCs contribute to enterococcal infections. We established that colonic phagocytes are required for robust E. faecalis dissemination to the colon-draining MLN (cMLN). We also demonstrated that E. faecalis dissemination relied on CCR2-dependent recruitment of CX3CR1+ macrophage precursors, but not lymphatic migration of CCR7+ conventional DCs. Importantly, this dissemination was shown to rely on intracellular survival mechanisms employed by E. faecalis in both mono-colonized germ-free (GF) and ceftriaxone-treated mice. Our observations show that intracellular survival within colonic macrophages contributes to E. faecalis escape from the gut and dissemination into systemic circulation.
RESULTS
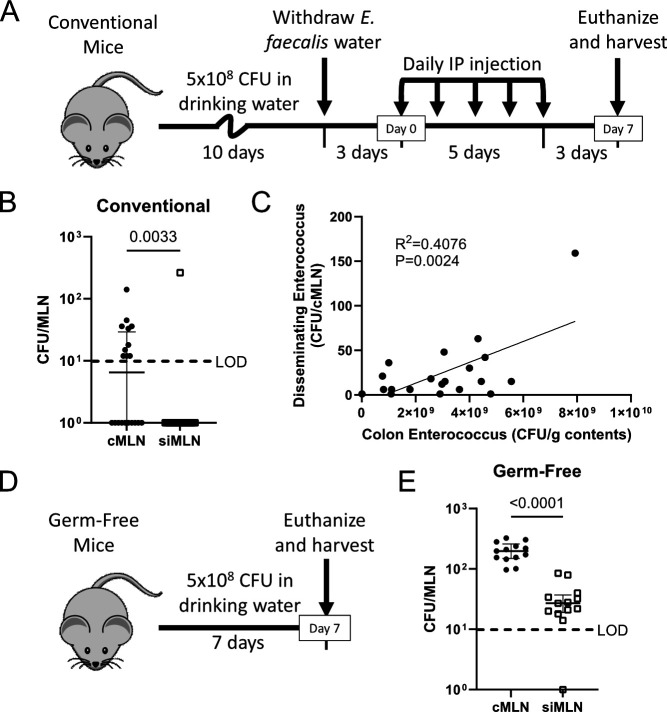
E. faecalis disseminate from the colon to the colon-draining mesenteric lymph node
The MLNs consist of several individual lymph nodes that drain distinct regions of the GI tract (31). We previously reported that ceftriaxone-induced dysbiosis led to E. faecalis overgrowth and subsequent dissemination to the MLNs (32); however, we did not distinguish between cMLN and small intestine-draining MLNs (siMLNs). Others have shown that broad microbial depletion using antibiotics led to dissemination of colonic bacteria, including E. faecalis, to the cMLN (12). Therefore, we adapted our previously reported ceftriaxone-induced E. faecalis dissemination model (2, 32) (Fig. 1A) to test whether E. faecalis dissemination was restricted to the cMLN in ceftriaxone-treated mice. We determined that siMLN dissemination was rarely observed and that the majority of E. faecalis disseminates to the cMLN (Fig. 1B), suggesting that ceftriaxone-mediated escape occurs from the colon. Furthermore, we observed a significant correlation between colonic E. faecalis abundance and its dissemination to the cMLN which was not observed when comparing with the contents from the distal small intestine (SI) or cecum (Fig. 1C; Fig. S1A through C). This confirms previous speculations put forth by us and others that enterococcal abundance within the gut contributes to its dissemination (2, 33). To determine whether E. faecalis dissemination requires antibiotic intervention, we colonized GF mice with E. faecalis and assessed dissemination after 7 days (Fig. 1D). While, in this case, E. faecalis was detected in the siMLN of GF mice, we observed significantly more dissemination to the cMLN (Fig. 1E). This suggests that E. faecalis preferentially disseminates from the colon to the cMLN, irrespective of antibiotics or the presence of competing microbiota.
Fig 1.
E. faecalis disseminate from the colon to the cMLN. (A) Experimental timeline for ceftriaxone-induced E. faecalis dissemination model. (B) The cMLN and siMLNs were collected separately, homogenized, and plated on selective agar to enumerate E. faecalis dissemination. (C) Colon contents were collected and plated to enumerate colonic E. faecalis abundance (x-axis) and plotted against cMLN dissemination (y-axis). (D) Experimental timeline for germ-free E. faecalis dissemination model. (E) E. faecalis dissemination to the cMLN and siMLN of germ-free mice was quantified as in A. (B and E) Median and interquartile range are reported, and P values were calculated using a Mann-Whitney test. Data are pooled from three independent experiments, using three to five mice each. (C) Data are pooled from four independent experiments using five mice per group. R2 and P values were calculated using a Pearson correlation analysis. LOD, limit of detection (10 CFU/organ); P > 0.05 not reported.
Ceftriaxone treatment does not elicit gross immunological changes in the colon
We previously reported that ceftriaxone does not lead to intestinal permeability or pathology (2). Others have determined that certain antibiotics induce goblet cell-associated passages that facilitate bacterial translocation and promote colonic inflammation (12). To further clarify ceftriaxone’s effects on intestinal immune responses, we compared colonic immune cell populations of ceftriaxone- and saline-treated mice. Our results indicate that ceftriaxone does not elicit gross immunological changes in colonic lymphocytes or APCs (S2A and B). Furthermore, we did not detect changes in any of the colonic lamina propria non-B cell APC subsets, including CD11c+ DCs, CD103+ DCs, CX3CR1-int macrophage/DCs, and CX3CR1-hi macrophages (S2C), subsets previously reported to migrate under various conditions (15, 16, 24, 34–36). Together, these data suggest that ceftriaxone treatment induces E. faecalis dissemination in the absence of changes in lymphocyte or APC populations.
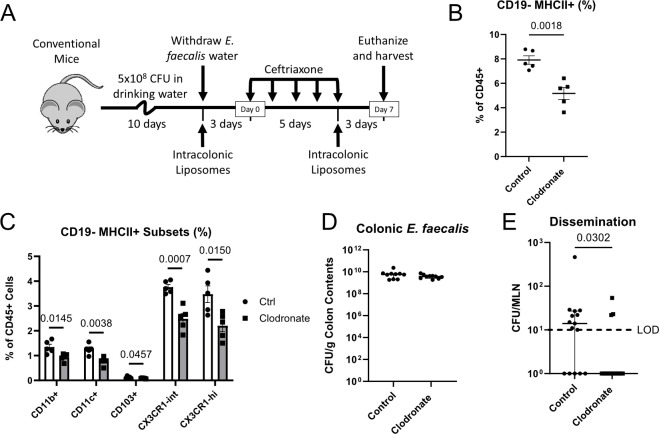
Depleting colonic phagocytes abrogates E. faecalis dissemination
APCs have been widely studied for their involvement in preventing bacterial invasion at mucosal surfaces. However, APCs have also been reported to contribute to the dissemination of commensals and pathogens (12, 16, 22–25). To investigate the role of intestinal APCs in E. faecalis dissemination, we depleted colonic phagocytes by rectally administering clodronate liposomes (37, 38) in the context of our dissemination model (Fig. 2A). We note that colons from mice treated with clodronate consistently yielded higher total cell numbers (S3A), making comparisons between absolute cell numbers difficult to interpret. Nonetheless, rectal clodronate clearly reduced colonic CD45+ MHCII+ CD3− CD19− APC frequencies, but not CD45+ CD3− CD19+ B cells or CD45+ MHCII− CD3+ CD19− T cells (Fig. 2B; Fig. S3B through F). Within the non-B cell APC pool, we observed broad depletion of nearly all APC subsets (Fig. 2C; Fig. S3G), indicating that these cells display phagocytic capabilities in the colon. While colonic E. faecalis abundance was unaffected by clodronate treatment (Fig. 2D), we observed reduced E. faecalis dissemination in mice whose colonic phagocytes were depleted (Fig. 2E). Since none of the assayed MHCII− myeloid cell subsets was reduced following clodronate administration (S3H), we deduced that one or more of the non-B cell MHCII+ APC subsets are responsible for promoting E. faecalis dissemination during ceftriaxone treatment.
Fig 2.
Clodronate-mediated depletion of colonic phagocytes reduces E. faecalis dissemination. (A) Experimental timeline for intracolonic liposome administration within our standard E. faecalis dissemination model. (B) Flow cytometric analysis of colonic non-B cell APCs (CD45+ CD3− CD19− MHCII+) or relevant APC subsets (C) as a percentage of CD45+ cells obtained from control (Ctrl) and clodronate-treated mice. (D) Colon contents from control or clodronate-treated mice were plated on selective agar to enumerate E. faecalis abundance. (E) MLNs from control or clodronate-treated mice were homogenized and plated on selective agar to enumerate E. faecalis dissemination. (B–D) Mean and standard error of the mean are reported, and statistical significance was determined using an unpaired t-test. Data are representative of three independent experiments using 5 mice (B and C) or 10 mice (D) per group. (E) Median and interquartile range are reported, and P values were calculated using a Mann-Whitney test. Data are pooled from three independent experiments, using four to five mice per group. LOD, limit of detection (10 CFU/organ); P > 0.05 not reported.
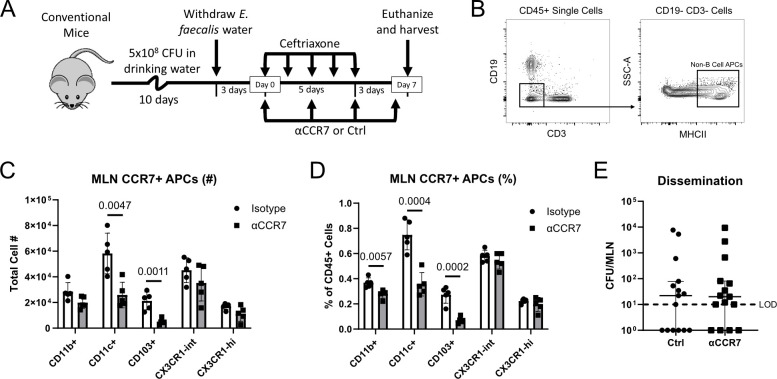
E. faecalis dissemination does not rely on CCR7+ DC migration to the MLNs
Intestinal DC migration from the lamina propria to the MLNs has been shown to contribute to the dissemination of both commensals and pathogens under various circumstances (12, 16, 22–25). This migration was consistently shown to rely on CCR7, the canonical chemokine receptor involved in lymphatic migration of DCs (36, 39). Thus, we sought to test whether CCR7-dependent DC migration was responsible for E. faecalis dissemination to the MLN. To address this question, we administered anti-CCR7 antibodies (αCCR7) in the context of our E. faecalis dissemination model (Fig. 3A). Flow cytometry of MLN APCs (Fig. 3B) revealed that CCR7 blockade selectively reduced CD11c+ and CD103+ DCs in the MLN (Fig. 3C and D), supporting the role of CCR7 in recruiting CD11c+ and CD103+ DCs from the gut to the MLN. Unexpectedly, however, treatment with αCCR7 did not prevent E. faecalis dissemination (Fig. 3E), suggesting that CCR7-mediated DC migration is not responsible for E. faecalis delivery to the MLN.
Fig 3.
Inhibiting DC recruitment to the MLNs does not have impact on E. faecalis dissemination. (A) Experimental timeline for anti-CCR7 (αCCR7) administration within our standard E. faecalis dissemination model. (B) Representative gating strategy for identifying non-B cell APCs in the MLN by flow cytometry. (C and D) Flow cytometric analysis of CCR7-expressing APCs (CD45+ CD3− CD19− MHCII+ CCR7+) within the MLNs of control (isotype) or αCCR7-treated mice. Y-axis represents the total cell number (C) or the percentage of CD45+ cells (D). (E) Effects of αCCR7 treatment on E. faecalis dissemination in ceftriaxone-treated mice. (C and D) Data are representative of two independent experiments, using five mice per group. Mean and standard error of the mean are reported. Statistical significance was determined using an unpaired t-test. (E) Data are pooled from three independent experiments, using five mice per group. Median and interquartile range are reported. Statistical significance was determined using a Mann-Whitney test. LOD, limit of detection (10 CFU/organ); P > 0.05 not reported.
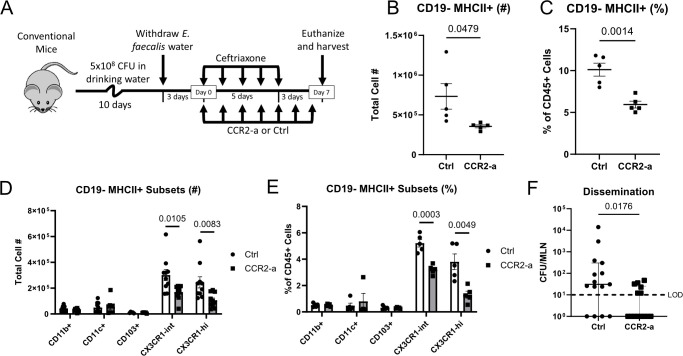
CCR2-dependent CX3CR1+ macrophages facilitate E. faecalis dissemination
Our observation that colonic phagocyte depletion reduced E. faecalis dissemination suggests that one or more macrophage or DC subsets facilitate E. faecalis translocation across the epithelial barrier and/or transport to the colon-draining MLN. Since abrogating CCR7-dependent trafficking to the MLN did not have impact on E. faecalis dissemination, we ruled out conventional DC migration as a potential mechanism. Thus, we hypothesized that intestinal macrophages were responsible for E. faecalis escape from the colon. Most intestinal macrophages are supplied by the constant recruitment of blood monocytes in a CCR2-dependent manner (40–43). To test whether monocyte-derived macrophages contributed to E. faecalis dissemination, we leveraged a chemical CCR2 antagonist, RS102895 (CCR2-a), which has been previously shown to disrupt monocyte recruitment and subsequently decrease colonic macrophage populations (44). Treatment with CCR2-a in the context of our dissemination model (Fig. 4A) markedly reduced non-B cell APCs in the colon (Fig. 4B and C). More specifically, we observed a significant decrease in number and frequency of CX3CR1-int and CX3CR1-hi APCs (Fig. 4D and E), consistent with previous work showing that CCR2-dependent monocytes give rise to CX3CR1-expressing macrophages (40, 43). Interestingly, we found that mice treated with CCR2-a exhibited significantly less E. faecalis dissemination when compared with injection controls (Fig. 4F). Together, these experiments indicate that monocyte-derived CX3CR1+ macrophages facilitate E. faecalis dissemination during ceftriaxone-mediated dysbiosis.
Fig 4.
CCR2 antagonism reduces E. faecalis dissemination. (A) Experimental timeline for CCR2 antagonism (CCR2-a) within our standard E. faecalis dissemination model. (B and C) Flow cytometric analysis of colonic APCs (CD45+ CD3− CD19− MHCII+) and relevant APC subsets (D and E) obtained from Ctrl or CCR2-a-treated mice. Y-axis represents the total cell number (B and D) or the percentage of CD45+ cells (C and E). (F) Effects of CCR2-a on E. faecalis dissemination in ceftriaxone-treated mice. (B–E) Data are representative of three independent experiments, using five mice per group. (D) Data are pooled from two experiments to account for variability in cell yields. Mean and standard error of the mean are reported. Statistical significance was determined using an unpaired t-test. (F) Data are pooled from three independent experiments, using five mice per group. Median and interquartile range are reported. Statistical significance was determined using a Mann-Whitney test. LOD, limit of detection (10 CFU/organ); P > 0.05 not reported.
E. faecalis require oxidative stress resistance for intracellular survival but not GI tract colonization
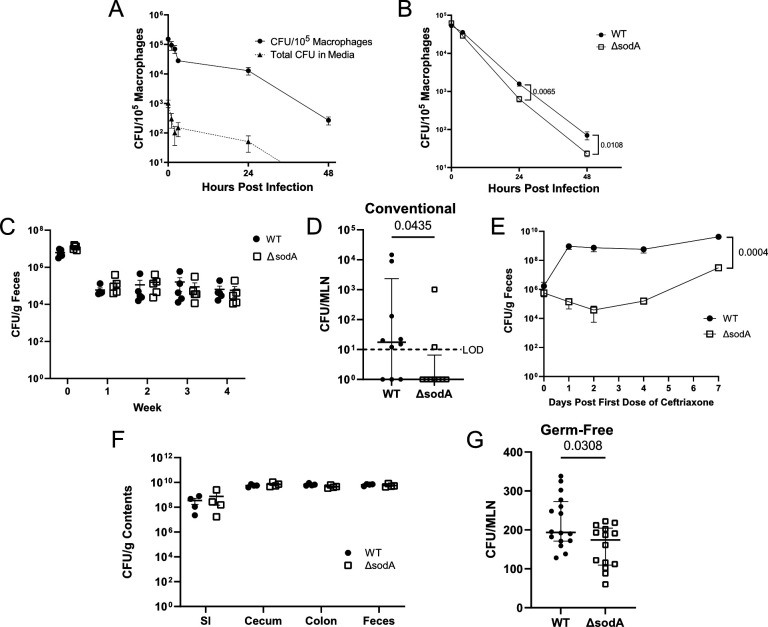
Since E. faecalis is known to express the machinery needed for intracellular survival in macrophages (19, 27–30), we hypothesized that intracellular APC survival serves as a mechanism of E. faecalis dissemination. We confirmed these findings by co-culturing J774.A1 macrophages with E. faecalis [OG1RF (45, 46)] and performing a gentamicin-vancomycin protection assay to evaluate intracellular E. faecalis persistence over time. We showed that E. faecalis survives within J774 macrophages for up to 2 days, consistent with previous reports from various in vitro macrophage systems (19, 27–30). To ensure that these observations were not due to extracellular persistence or growth despite the presence of antibiotics, the culture media were plated to enumerate extracellular CFU. We observed minimal extracellular E. faecalis (<200 CFU) at 2 hours and undetectable extracellular CFU by 48 hours, confirming that E. faecalis persistence was due to intracellular survival (Fig. 5A).
Fig 5.
Intracellular E. faecalis survival supports dissemination to the MLN. (A) J774.A1 macrophages were co-cultured with E. faecalis for 1 hour, and an antibiotic protection assay (150 µg/mL gentamicin, 100 µg/mL vancomycin) was performed to evaluate intracellular survival over time (solid line, circles). Total CFU obtained from culture media was assessed to control for extracellular E. faecalis (dashed line, triangles). (B) Intracellular survival of E. faecalis that lack MN-dependent superoxide dismutase (ΔsodA) (open squares), compared with OG1RF wild type (WT) (closed circles). (C) Mice were colonized with WT or ΔsodA by suspending the bacteria in drinking water for 10 days, and fecal abundance was enumerated weekly as CFU per gram feces. (D and E) Mice were colonized with WT or ΔsodA and treated with ceftriaxone as in Fig. 1A. MLNs were harvested to enumerate E. faecalis dissemination (D), and feces was collected throughout the experiment to track intestinal E. faecalis abundance (E). (F and G) Germ-free mice were colonized as in Fig. 1D. (F) Contents from the SI, cecum, colon, and feces were collected to enumerate colonization. (G) MLNs were harvested to enumerate E. faecalis dissemination. (A and B) Data are pooled from two independent experiments performed in triplicate. Mean and standard error of the mean are reported, and statistical significance was determined using an unpaired t-test. (C, E, F) Data are representative of two independent experiments, using five mice per group, and P values were calculated using unpaired t-tests. (D and G) Median and interquartile range are reported, and P values were calculated using a Mann-Whitney test. Data are pooled from three (D) or four (G) independent experiments, using three to five mice per group. LOD, limit of detection (10 CFU/organ); P > 0.05 not reported.
Previous studies have identified genes that are important for E. faecalis survival within macrophages (19, 27–30). One such gene encodes manganese-containing superoxide dismutase (sodA), which catalyzes the conversion of superoxide into hydrogen peroxide and is critical for resistance to oxidative stress within the phagolysosome of macrophages (30). We leveraged an intracellular survival-deficient strain which lacks sodA [OG1RF ΔsodA (47)], to assess whether E. faecalis persistence within APCs contributes to its dissemination in vivo. First, we confirmed that deletion of sodA rendered E. faecalis more susceptible to macrophage killing using the in vitro system described above. We observed a modest, albeit significant reduction in ΔsodA mutant survival at both 24 and 48 hours post-infection when compared with the WT isogenic strain, OG1RF (Fig. 5B). Next, we assessed whether ΔsodA could persist long term within the GI tract of mice. To do this, we colonized mice as in Fig. 1A with either WT or ΔsodA and tracked colonization persistence in the absence of ceftriaxone treatment by enumerating fecal E. faecalis abundance weekly. We found that both WT and ΔsodA strains stably colonized mice at ~105 CFU/g feces (Fig. 5C), suggesting that the ΔsodA mutant does not exhibit colonization defects.
SodA-mediated intracellular survival is required for E. faecalis dissemination to the MLN
To test our hypothesis that intracellular APC survival supports E. faecalis dissemination, we colonized mice with WT or ΔsodA and treated them with ceftriaxone as in Fig. 1A to induce E. faecalis dissemination. Upon ceftriaxone treatment, ΔsodA exhibited reduced dissemination when compared with WT (Fig. 5C), suggesting that ΔsodA does not disseminate as efficiently. Although our previous work showed that this ΔsodA strain does not exhibit ceftriaxone resistance defects (47), we observed an unexpected decrease in ΔsodA fecal abundance during ceftriaxone treatment that was not observed in the WT strain (Fig. 5E). This initial dip was followed by a rebounding increase above colonization baseline at the time of euthanasia but was nonetheless confounding, as previous studies suggest that E. faecalis dissemination is correlated to its intestinal abundance (2, 33). To address this, we turned to GF mice which have been shown to permit bacterial dissemination to the MLNs during early stages of colonization (48). We colonized GF mice with WT or ΔsodA strains as in Fig. 1D and assessed intestinal colonization and dissemination to the MLNs after 7 days. Although similar fecal and intestinal abundance was observed between strains throughout the experiment (Fig. 5F), ΔsodA dissemination was attenuated compared with WT (Fig. 5G). This provides additional support for our hypothesis that intracellular survival within colonic macrophages contributes to E. faecalis dissemination to the MLNs.
DISCUSSION
Bacteria have developed numerous mechanisms that support their colonization, survival, and pathogenesis within host organisms. To date, no clear consensus exists regarding the mode of bacterial passage through systemic circulation. Planktonic, free-floating bacteria within the blood or lymph are immediately opsonized by immunoglobulins and complement proteins that immobilize, perforate, and target invading bacteria for phagocytic uptake. Thus, the development of strategies that support intracellular survival within migratory cells provides bacteria with protection from the hostile environment of the circulatory system. In this study, we investigated how intracellular survival of the commensal pathobiont, E. faecalis, contributes to its dissemination during dysbiosis. Despite previous evidence that E. faecalis effectively survive intracellularly (19, 27–30), no studies have been conducted to test whether this contributes to E. faecalis dissemination from the gut to extraintestinal sites. In this study, we leveraged the intracellular survival-deficient E. faecalis strain, ΔsodA to show, for the first time, that intracellular survival contributes to E. faecalis escape from the gut and dissemination to the MLNs. These data provide support for our hypothesis that intracellular survival within migratory APCs is a mechanism by which E. faecalis disseminate to the MLNs (Fig. 6).
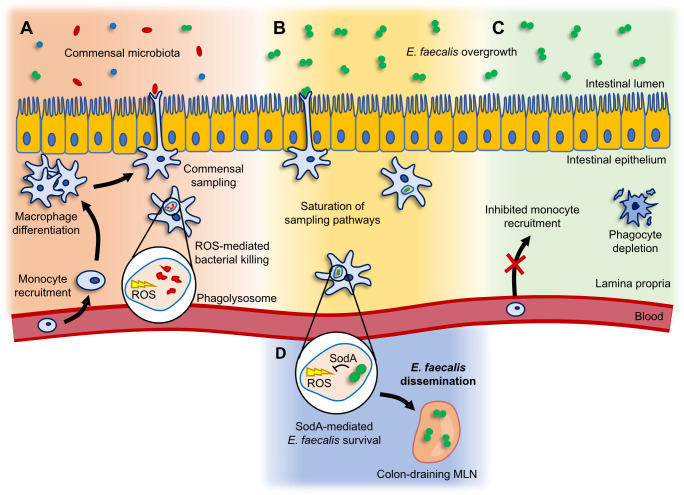
Fig 6.
Working model for phagocyte-mediated E. faecalis dissemination. (A) Monocytes are recruited from the blood and develop into macrophages that sample luminal bacteria during homeostatic conditions. (B) Upon antibiotic-induced E. faecalis overgrowth in the colon, the commensal sampling pathways become saturated by E. faecalis. (C) Disruption of monocyte recruitment or depletion of phagocytes ablates the commensal sampling processes and prevents E. faecalis translocation across the epithelial barrier. (D) SodA-mediated intracellular survival allows E. faecalis to disseminate to the colon-draining MLN.
Bacterial dissemination often occurs during states of intestinal inflammation or dysbiosis (2, 12, 17, 24, 33). Various mechanisms have been described that explain how bacteria translocate across the intestinal epithelium (9–12, 49, 50) (Fig. 6A); however, our understanding of the processes that contribute to the subsequent dissemination of bacteria to the MLNs and other extraintestinal organs remains unclear. Here, we sought to examine the role that phagocytic APCs play in E. faecalis dissemination (Fig. 6B). We found that global phagocyte depletion led to reduced E. faecalis dissemination to the MLNs, suggesting that APCs play a role in promoting E. faecalis escape from the gut. More specifically, we demonstrated that monocyte-derived CX3CR1+ macrophages are involved in E. faecalis dissemination during ceftriaxone treatment (Fig. 6C). Surprisingly, we found that the migration of CD11c+ and CD103+ bona fide DCs to the MLN did not contribute to E. faecalis dissemination. It is important to note that the treatment regimens used to manipulate phagocytes in this study do not completely ablate phagocytes or their migration. Furthermore, the differences in drug administration route, frequency, timing, and concentration should also be taken into consideration, as these variables likely influence the viability and migration of distinct APC subsets. Nonetheless, our observations raise questions regarding alternative mechanisms of APC migration and subsequent microbial trafficking that have yet to be described.
Although it is accepted that CD103+ APCs are bona fide migratory DCs within the GALT, controversy surrounds the migratory potential of CX3CR1-expressing cells. Some researchers have suggested that CX3CR1-int APCs of the GALT should also be considered bona fide DCs, as they were shown to descend from DC progenitors, effectively prime naïve T cells, and continuously migrate in lymph (35, 51). In contrast, others have shown that CX3CR1-int APCs are monocyte derived but support the claims that they are migratory (52, 53). These studies also explicitly classify CX3CR1-hi APCs as non-migratory, tissue-resident macrophages. A separate study contradicts this assertion, showing that, during Salmonella infection, CX3CR1-hi APCs could be obtained from afferent lymphatics draining to the MLNs, suggesting that these cells do have the capacity to migrate (24). Importantly, these studies were conducted using disparate models of intestinal inflammation and antibiotic-induced dysbiosis, suggesting that their observations may be context dependent. Furthermore, their differential use of CD11b and CD11c to analyze specific subpopulations of APCs highlights the complexity of the migratory APC system in the GALT and the need for better characterization of intestinal APC subsets. Our findings that monocyte-derived macrophages contribute to E. faecalis dissemination may provide support for the stance that CX3CR1-expressing APCs are migratory. However, more direct experimentation would be required to confirm whether these cells traffic to the MLN during ceftriaxone-induced dysbiosis or whether their involvement in E. faecalis dissemination is the result of sampling at the epithelial interface.
Our study also provides insight into the poorly understood dynamics of commensal dissemination. Our observation that E. faecalis dissemination occurs in GF mice in the absence of antibiotic treatment supports previous reports that Enterococcus dissemination is the consequence of its overabundance in the gut (2, 33). We propose that E. faecalis dissemination is the result of homeostatic commensal sampling mechanisms performed by the host, which become saturated upon expansion of E. faecalis populations (Fig. 6B). As such, we expect that low levels of E. faecalis dissemination occurs at a steady state, albeit to a degree that is undetectable by methods of culturing or 16S sequencing. During states of dysbiosis, however, when E. faecalis is overrepresented in the gut, disseminating E. faecalis become readily detectable. We hypothesize that this is a general phenomenon of commensal bacteria and is not restricted to E. faecalis. We also expect bacterial taxa that are proficient in intracellular survival to be more likely to disseminate and persist within extraintestinal organs following translocation across the gut epithelium (Fig. 6D).
Mounting evidence suggests that the presence of live bacteria within organized lymphatic tissues may serve homeostatic functions by modulating B cell activity. For instance, commensal Alcaligenes spp. induce the production of antigen-specific IgA, which aids their stable colonization of Peyer’s patches (54). This cooperation provides a unique and preferred niche for Alcaligenes while offering host protection through IgA production. DC-mediated commensal delivery to the MLN also promotes the induction of IgA (16), and the presence of Gram-negative commensal bacteria in MLNs can elicit the production of IgG which confers cross-reactive protection against infection by pathogens like Salmonella (17). These studies suggest that the host may permit controlled colonization of secondary lymphoid organs in the GALT to modulate homeostatic humoral immunity and protect against disease. We propose that antibiotic-induced E. faecalis dissemination is the consequence of these permissive systems and that monocyte-derived CX3CR1-expressing cells are important arbiters of these processes. Currently, we cannot definitively conclude whether macrophage-mediated E. faecalis dissemination is the result of coordinated translocation across the epithelium, the direct transport of E. faecalis to the MLN, or both. Indeed, CX3CR1+ cells have the capacity to extend transepithelial dendrites that facilitate bacterial translocation across the epithelium (9). Furthermore, CX3CR1-hi APCs have been implicated in trafficking bacteria directly to the MLN in a CCR7-dependent manner (24). However, our data indicate that CCR7 is dispensable for this process, suggesting that CX3CR1-expressing APCs may rely on another receptor for chemotaxis to the MLN. Future studies should investigate the milieu of migratory APC subsets that may be responsible for bacterial transport, placing emphasis on the chemokine receptors that guide them.
Here, we provided evidence that monocyte-derived macrophages facilitate the dissemination of a common commensal pathobiont, E. faecalis. We showed that this dissemination is mostly restricted to the colon-draining MLN and likely occurs during states of E. faecalis overgrowth. We also clearly show that intracellular E. faecalis survival is crucial for its dissemination to the MLN. This study builds on a growing body of evidence that implicates migratory host cells in the delivery of live bacteria to the MLNs. While these mechanisms likely support homeostatic maintenance within the GALT, they can also be exploited by intracellular pathogens to gain access to extraintestinal organs and cause disease. We expect that many enterococcal infections originate in this way, particularly in immunocompromised hosts, or when intestinal homeostasis is disrupted by antibiotics. Our work suggests that inhibition of macrophage recruitment in the context of antibiotic therapy could reduce the risk of opportunistic infections by limiting extraintestinal escape of pathobionts like enterococci. Improving our understanding of the migratory APC network within the GALT will likely shed light on other strategies for preventing or treating infections by disseminated enteric pathogens.
MATERIALS AND METHODS
Animals
Five- to 6-week-old male C57BL/6J mice were obtained from the Jackson Laboratory (room RB08, JAXWEST facility, Sacramento, CA). Mice were acclimatized for 1 week prior to conducting experiments. Mice were fed a standard chow diet (PicoLab laboratory rodent diet) and de-chlorinated reverse osmosis water ad libitum. All mice were maintained under specific pathogen-free conditions throughout the course of the experiments, and mice that were colonized by experimental E. faecalis strains were housed in an ABSL2 facility. Five- to 10-week-old male and female germ-free mice were obtained from an in-house breeding colony and housed using the Innorack IVC Mouse 3.5 and Innovive IVC Rodent Caging System (Innovive) that allow isolation and colonization of groups of mice while otherwise maintaining gnotobiotic status. Germ-free mice were maintained on sterilized 2020SX Teklad Global Soy Protein-Free Extruded Rodent Diet (Envigo) and provided autoclaved Milli-Q water (IQ 7000, MilliporeSigma) ad libitum. All mice were humanely euthanized by CO2 asphyxiation followed by cervical dislocation.
Bacterial strains
Laboratory strains of E. faecalis used in this study are as follows: OG1RF (spontaneous rifampin-resistant derivative of OG1) (45), CK135 (spontaneous rifampin-resistant derivative of OG1 that also confers enhanced cephalosporin resistance) (55), and HH29 (ΔsodA) (in-frame deletion of the sodA locus from OG1RF that confers oxidative stress-sensitivity) (30, 47).
Colonization of mice with E. faecalis strains
Cultures of E. faecalis were grown to stationary phase overnight in Mueller-Hinton broth (Difco), supplemented with 50 µg/mL rifampicin (Chem-Impex), at 37°C, and shaking at 200 rpm. Cultures were pelleted by centrifugation at 4,000 relative cetrifugal force (rcf) for 10 minutes, and the pellets were washed with autoclaved Milli-Q water two times to remove excess media and antibiotics. Final pellets were resuspended in autoclaved Milli-Q water, and bacterial cell concentration was determined by measuring the optical density at 600 nm with a spectrophotometer (NanoDrop 2000 spectrophotometer; Thermo Scientific). Colonization was performed as described previously (2, 32). Briefly, mice were fed 5 × 108 CFU/mL of bacteria in 300 mL of drinking water which was replaced every 3–4 days. For conventional mouse colonization experiments, E. faecalis-containing water was removed and replaced by normal drinking water 10 days following the start of feeding. For germ-free mouse experiments, mice were maintained on E. faecalis-containing water for 7 days prior to euthanasia.
Ceftriaxone treatment
Mice were maintained on E. faecalis-free drinking water for 3 days prior to performing injections. All injections were performed intraperitoneally (IP) using insulin syringes (BD Biosciences). Ceftriaxone (ceftriaxone for injection USP; Apotex Corp.) was suspended in saline (0.9% sterile-preservative free; Phoenix) to generate a 25 mg/mL solution. Mice were injected with 100 µL ceftriaxone solution (2.5 mg/injection) once daily for 5 consecutive days. Control mice received equal volumes of sterile saline. Mice were euthanized 3 days following the final ceftriaxone injection.
Intracolonic clodronate liposome administration
Prior to intracolonic administration, mice were anesthetized by IP injection of 100 µL ketamine/xylazine solution (12 mg/mL ketamine, 1.6 mg/mL xylazine). Once anesthetized, mice were placed on a warm heating pad covered with clean dressing. One hundred microliters of clodronate-containing liposomes or control liposomes (Liposoma) was administered by inserting a steel blunt-tipped gavage needle (small-bore) approximately 3 cm into the rectum. Mice were returned to their respective cage which was maintained on a heat pad until all mice recovered from anesthesia. Intracolonic liposome administration was performed on the final day of E. faecalis colonization and on the final day of ceftriaxone treatment (7 days apart). Mice received no more than 200 µL of IP-injected volume per day.
CCR7 blockade
CCR7 blockade was adapted from previous studies (56). Briefly, anti-CCR7 monoclonal antibodies (αCCR7) (eBiosciences) were suspended to 5 mg/mL in sterile phosphate-buffered saline (PBS) (pH 7.4). Mice were injected with 100 µL of αCCR7 solution every other day starting on the first day of ceftriaxone treatment (four total injections). Control mice received equal volumes of rat IgG2a kappa isotype control antibody (eBiosciences) suspended to 5 mg/mL in sterile PBS (pH 7.4). Mice received no more than 200 µL of injected volume per day.
CCR2 antagonism
CCR2 antagonism was performed using the chemical antagonist RS 102895 hydrochloride (CCR2-a) (Tocris Bioscience) (44). CCR2-a was suspended in dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific) and diluted to 2 mg/mL in sterile saline (10% DMSO). Mice received 100 µL IP injections of CCR2-a daily for 7 consecutive days (prepared fresh each day). Daily injections were needed to sufficiently reduce macrophage populations, consistent with the short half-life of RS102895 (57). Control mice received equal volumes of 10% DMSO in sterile saline. For experiments where CCR2-a and ceftriaxone were both administered, CCR2-a treatments began on the first day of ceftriaxone injection and continued as described until the day of euthanasia. Mice received no more than 200 µL of injected volume per day.
Tissue collection for enumeration of E. faecalis colonization and dissemination
E. faecalis was enumerated from fresh fecal samples or representative intestinal samples by dilution plating on brain-heart infusion (BHI) agar (BD Biosciences), supplemented with 200 µg/mL rifampicin, and incubated at 37°C overnight to enumerate E. faecalis CFU/g sample. To enumerate E. faecalis dissemination, MLNs were sterilely collected in 500 µL ice-cold PBS (pH 7.4). MLNs were homogenized using a tissue homogenizer (Omni-International, TH-115), and the homogenate was plated on BHI agar, supplemented with 200 µg/mL rifampicin, using sterile glass plating beads, and incubated at 37°C overnight to enumerate E. faecalis CFU/organ.
In vitro J774 macrophage co-culture with E. faecalis
J774A.1 macrophages (ATCC) were cultured at 37°C, 5% CO2 in 100-mm tissue culture-treated petri dishes (CELLTREAT) containing 10 mL of filter-sterilized feed medium [Dulbecco’s modified Eagle medium (DMEM) containing 2 mM glutamine (Gibco) and 10% FBS (Premium, Bio-Techne) and supplemented with penicillin-streptomycin (1×) (Gibco)], replacing media as needed (~every 4 days). Prior to performing co-culture experiments, cell lines were passaged a minimum of three times. Adherent cells were lifted using cell scrapers (Sarstedt) before transferring to a fresh petri dish containing 10 mL feed medium. For co-culture experiments, ~80% confluent macrophage cultures were washed with Dulbecco’s phosphate-buffered saline (DPBS) as before and harvested using cell scrapers. Cell suspensions were pelleted by centrifugation at 600 rcf and counted via hemocytometer. 105 macrophages were seeded into 10-mm, 12-well tissue culture-treated plates (Corning) containing 1 mL of feed medium and allowed to adhere for 16 hours. E. faecalis strains were cultured and washed as described above and suspended to 106 CFU/mL in antibiotic-free feed medium. The antibiotic-containing media were aspirated from each well, and the macrophages were gently washed twice with DPBS before 1 mL of E. faecalis-containing media (106 CFU, MOI = 10) was added to each well. The co-culture plates were centrifuged at room temperature for 5 minutes at 600 rcf to enhance E. faecalis contact with adherent macrophages before incubating at 37°C, 5% CO2 for 1 hour. The E. faecalis-containing media were then removed, and macrophages were washed three times with DPBS before adding 1 mL of feed medium containing 150 µg/mL gentamicin and 100 µg/mL vancomycin to rapidly eliminate extracellular E. faecalis. Cultures were maintained at 37°C, 5% CO2 prior to each timepoint where the media from respective wells were collected and plated on BHI agar supplemented with 200 µg/mL rifampicin to enumerate extracellular E. faecalis growth. Macrophages were then washed twice with DPBS and lysed with 500 µL of saponin solution (10 µg/mL saponin in DPBS) while scraping with the back end of a pipette tip. Macrophage lysate was pelleted in a microcentrifuge tube at 20,000 rcf for 5 minutes, washed with DPBS, and similarly plated to enumerate intracellular E. faecalis CFU.
Cell isolation
Lamina propria
Colons were excised, dissected longitudinally, and the luminal contents were removed by shaking in 50-mL conicals containing PBS and transferred to 10 mL ice cold R10 media [10% FBS, 10 mM HEPES, 1% non-essential amino acids (100×), 1% sodium pyruvate (100×), and 220 µM β-ME (added fresh) in RPMI 1640 + GlutaMAX (1×) (Gibco)]. Tissues were cut into ~2-cm pieces and transferred to a 50-mL conical containing 40 mL HBSS (no Ca, Mg, Phenol Red) containing 10% FBS, 5 mM EDTA, 1 mM DTT, and 10 mM HEPES and incubated at 37°C, shaking at 300 rpm for 30 minutes to remove epithelial cells. Tissues were washed three times with wash media (10% FBS in DMEM) and mechanically dissociated by mincing thoroughly with a razor blade. Dissociated tissue was transferred to 20 mL of RPMI 1640 + GlutaMAX containing 10% FBS, 0.5 mg/mL Collagenase D (Millipore Sigma), 50 µg/mL DNase I (Worthington), and 25 µg/mL trypsin inhibitor and incubated at 37°C, shaking at 300 rpm for 45 minutes. Tissues were passed through a 100-µm nylon mesh cell strainer (Falcon) while periodically washing with wash media before pelleting cell suspensions at 600 rcf for 5 minutes at 4°C. Cells were passed through a 70-µm nylon mesh cell strainer and pelleted as above before being resuspended in 1 mL FACS buffer (10% FBS in DPBS) containing αCD16/32 Fc block (BD) (1:100). Cells were incubated for 5–10 minutes at room temperature, and 1–10 million cells were transferred to a 96-well U bottom plate (Greiner Bio-One), pelleted at 600 rcf for 10 minutes at 4°C, and resuspended in 100 µL of antibody cocktail in FACS buffer.
Mesenteric lymph nodes
The colonic- and small intestinal-draining MLNs were excised from the mesenteric fat using curved forceps. MLNs from a single mouse were pooled in 3 mL ice cold R10 media. MLNs were mechanically disrupted using the back end of a syringe plunger and passed through a 100-µm nylon mesh cell strainer while periodically washing with 12 mL ice cold R10 media. Cells were pelleted at 600 rcf for 5 minutes at 4°C, resuspended in 1 mL FACS buffer containing αCD16/32 Fc block, and incubated for 5–10 minutes at room temperature. 3 × 106 cells were transferred to a 96-well U bottom plate, pelleted at 600 rcf for 10 minutes at 4°C, and resuspended in 100 µL of antibody cocktail in FACS buffer.
Flow cytometry
FACS buffer containing fluorescence-conjugated antibodies was used to stain single-cell suspensions at 4°C for 30 minutes, unless otherwise stated. The following conjugated antibodies were used: CD3 (17A2), CD19 (6D5), I-Ab (AF6-120.1), CX3CR1 (SA011F11), CD103 (2E7), CCR2 (SA203G11), CCR7 (4B12), CD11b (M1/70), CD11c (N418), Ly6C (HK1.4), CD64 (X54-5/7.1), CD169 (3D6.112), CD14 (M14-23), Tim4 (RMT4-54), and CSF1R (AFS98) from BioLegend. When staining for CCR7, single-cell suspensions were incubated with staining cocktail for 1 hour at 23°C. Following incubation, cells were pelleted at 600 rcf for 5 minutes at 4°C, washed twice with 200 µL of FACS buffer, then resuspended in 200 µL of FACS buffer, and passed through a 30-µm nylon mesh cell strainer (Falcon) into a FACS tube, using 300 µL of FACS buffer to wash the filter (500 µL final volume). Flow cytometry was performed using the BD LSRFortessa (X20), and data were analyzed using FlowJo (V10.8.1) software.
Statistics
Statistical analysis was performed using GraphPad Prism 9, and corresponding statistical methods were reported in the figure legends. Statistical significance for studies that assessed E. faecalis dissemination was determined using a Mann-Whitney test. For all other experiments comparing two groups, statistical significance was determined using an unpaired t-test. Correlation analysis was performed using a Pearson correlation analysis and simple linear regression.
ACKNOWLEDGMENTS
We would like to thank Rajrupa Chakraborty for her early contributions in developing our E. faecalis dissemination model. We thank Matthew Mortenson for contributing to the optimization of macrophage co-culture systems and Khadijah Dhoondia and Ashley Bauer for assisting with flow cytometry and E. faecalis dissemination experiments. We thank Jennifer Ziegelbauer and the Center for Microbiome Research Gnotobiotic Core Facility for their expert support in the maintenance of gnotobiotic mice. We also thank Benedetta Bonacci and the Versiti Flow Cytometry Core for the assistance with FACS. Finally, we thank Mary Holtz for the general laboratory support and organization.
This work was supported by National Institutes of Health R35 MIRA 2210596.
Conceptualization was done by the following: Kevin Jennings and Nita Salzman. Data collection was done by the following: Kevin Jennings, Michael Hayward, and Kaitlin Johnson. Data analysis was done by the following: Kevin Jennings. Supervision was done by the following: Nita Salzman and Christopher Kristich. Writing—draft preparation was done by the following: Kevin Jennings. Writing—review and editing was done by the following: Kevin Jennings, Kaitlin Johnson, Michael Hayward, Christopher Kristich, and Nita Salzman.
Contributor Information
Nita H. Salzman, Email: nsalzman@mcw.edu.
Nancy E. Freitag, University of Illinois Chicago, Chicago, Illinois, USA
ETHICS APPROVAL
All protocols have been approved by the committee for animal care and use at the Medical College of Wisconsin.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00006-24.
Figures S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Sood S, Malhotra M, Das BK, Kapil A. 2008. Enterococcal infections & antimicrobial resistance. Indian J Med Res 128:111–121. [PubMed] [Google Scholar]
- 2. Chakraborty R, Lam V, Kommineni S, Stromich J, Hayward M, Kristich CJ, Salzman NH. 2018. Ceftriaxone administration disrupts intestinal homeostasis, mediating noninflammatory proliferation and dissemination of commensal enterococci. Infect Immun 86:e00674-18. doi: 10.1128/IAI.00674-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council . 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the american heart association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 4. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG. 2016. Infective endocarditis. Nat Rev Dis Primers 2:16059. doi: 10.1038/nrdp.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raza T, Ullah SR, Mehmood K, Andleeb S. 2018. Vancomycin resistant enterococci: a brief review. J Pak Med Assoc 68:768–772. [PubMed] [Google Scholar]
- 6. Ramos S, Silva V, Dapkevicius M de L, Igrejas G, Poeta P. 2020. Enterococci, from harmless bacteria to a pathogen. Microorganisms 8:1118. doi: 10.3390/microorganisms8081118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Kersh TA, Marie MA, Al-Sheikh YA, Al-Agamy MH, Al Bloushy AA. 2016. Prevalence and risk factors of early fecal carriage of Enterococcus faecalis and Staphylococcus spp and their antimicrobial resistant patterns among healthy neonates born in a hospital setting in central Saudi Arabia. Saudi Med J 37:280–287. doi: 10.15537/smj.2016.3.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rios D, Wood MB, Li J, Chassaing B, Gewirtz AT, Williams IR. 2016. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol 9:907–916. doi: 10.1038/mi.2015.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258. doi: 10.1126/science.1102901 [DOI] [PubMed] [Google Scholar]
- 10. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. 2012. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483:345–349. doi: 10.1038/nature10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367. doi: 10.1038/86373 [DOI] [PubMed] [Google Scholar]
- 12. Knoop KA, McDonald KG, Kulkarni DH, Newberry RD. 2016. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65:1100–1109. doi: 10.1136/gutjnl-2014-309059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spahn TW, Weiner HL, Rennert PD, Lügering N, Fontana A, Domschke W, Kucharzik T. 2002. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol 32:1109–1113. doi: [DOI] [PubMed] [Google Scholar]
- 14. Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL. 2001. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol 31:1278–1287. doi: [DOI] [PubMed] [Google Scholar]
- 15. Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 203:519–527. doi: 10.1084/jem.20052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macpherson AJ, Uhr T. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665. doi: 10.1126/science.1091334 [DOI] [PubMed] [Google Scholar]
- 17. Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Núñez G. 2016. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44:647–658. doi: 10.1016/j.immuni.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3:e1409. doi: 10.1371/journal.pone.0001409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun 67:2160–2165. doi: 10.1128/IAI.67.5.2160-2165.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moliva MV, Campra N, Ibañez M, Cristofolini AL, Merkis CI, Reinoso EB. 2022. Capacity of adherence, invasion and intracellular survival of Streptococcus uberis biofilm-forming strains. J Appl Microbiol 132:1751–1759. doi: 10.1111/jam.15362 [DOI] [PubMed] [Google Scholar]
- 21. Pellon A, Barriales D, Peña-Cearra A, Castelo-Careaga J, Palacios A, Lopez N, Atondo E, Pascual-Itoiz MA, Martín-Ruiz I, Sampedro L, Gonzalez-Lopez M, Bárcena L, Martín-Mateos T, Landete JM, Prados-Rosales R, Plaza-Vinuesa L, Muñoz R, de Las Rivas B, Rodríguez JM, Berra E, Aransay AM, Abecia L, Lavín JL, Rodríguez H, Anguita J. 2021. The commensal bacterium Lactiplantibacillus plantarum imprints innate memory-like responses in mononuclear phagocytes. Gut Microbes 13:1939598. doi: 10.1080/19490976.2021.1939598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vazquez-Torres A, Jones-Carson J, Bäumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808. doi: 10.1038/44593 [DOI] [PubMed] [Google Scholar]
- 23. Voedisch S, Koenecke C, David S, Herbrand H, Förster R, Rhen M, Pabst O. 2009. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect Immun 77:3170–3180. doi: 10.1128/IAI.00272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diehl GE, Longman RS, Zhang J-X, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. 2013. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494:116–120. doi: 10.1038/nature11809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hapfelmeier S, Müller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, Kirschning C, Jung S, Stallmach T, Kremer M, Hardt W-D. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in ΔinvG S. Typhimurium colitis. J Exp Med 205:437–450. doi: 10.1084/jem.20070633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu H, Prince JE, Brayton CF, Shah C, Zeve D, Gregory SH, Smith CW, Ballantyne CM. 2003. Host resistance of CD18 knockout mice against systemic infection with Listeria monocytogenes. Infect Immun 71:5986–5993. doi: 10.1128/IAI.71.10.5986-5993.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coburn PS, Baghdayan AS, Dolan GT, Shankar N. 2008. An AraC-type transcriptional regulator encoded on the Enterococcus faecalis pathogenicity island contributes to pathogenesis and intracellular macrophage survival. Infect Immun 76:5668–5676. doi: 10.1128/IAI.00930-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. La Carbona S, Sauvageot N, Giard J-C, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A. 2007. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, alkyl hydroperoxide reductase and thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol Microbiol 66:1148–1163. doi: 10.1111/j.1365-2958.2007.05987.x [DOI] [PubMed] [Google Scholar]
- 29. Verneuil N, Sanguinetti M, Le Breton Y, Posteraro B, Fadda G, Auffray Y, Hartke A, Giard JC. 2004. Effects of the Enterococcus faecalis hypR gene encoding a new transcriptional regulator on oxidative stress response and intracellular survival within macrophages. Infect Immun 72:4424–4431. doi: 10.1128/IAI.72.8.4424-4431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verneuil N, Mazé A, Sanguinetti M, Laplace J-M, Benachour A, Auffray Y, Giard J-C, Hartke A. 2006. Implication of (Mn)superoxide dismutase of Enterococcus faecalis in oxidative stress responses and survival inside macrophages. Microbiology (Reading) 152:2579–2589. doi: 10.1099/mic.0.28922-0 [DOI] [PubMed] [Google Scholar]
- 31. Houston SA, Cerovic V, Thomson C, Brewer J, Mowat AM, Milling S. 2016. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol 9:468–478. doi: 10.1038/mi.2015.77 [DOI] [PubMed] [Google Scholar]
- 32. Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH. 2015. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 526:719–722. doi: 10.1038/nature15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Archambaud C, Derré-Bobillot A, Lapaque N, Rigottier-Gois L, Serror P. 2019. Intestinal translocation of enterococci requires a threshold level of enterococcal overgrowth in the lumen. Sci Rep 9:8926. doi: 10.1038/s41598-019-45441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. 2009. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206:3101–3114. doi: 10.1084/jem.20091925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SWF. 2013. Intestinal CD103- dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 6:104–113. doi: 10.1038/mi.2012.53 [DOI] [PubMed] [Google Scholar]
- 36. Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 202:1063–1073. doi: 10.1084/jem.20051100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Rooijen N, Sanders A, van den Berg TK. 1996. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 193:93–99. doi: 10.1016/0022-1759(96)00056-7 [DOI] [PubMed] [Google Scholar]
- 38. Moreno SG. 2018. Depleting macrophages in vivo with clodronate-liposomes. Methods Mol Biol 1784:259–262. doi: 10.1007/978-1-4939-7837-3_23 [DOI] [PubMed] [Google Scholar]
- 39. Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, Seoh JY, Lipp M, Kiyono H, Miyasaka M. 2006. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol 176:803–810. doi: 10.4049/jimmunol.176.2.803 [DOI] [PubMed] [Google Scholar]
- 40. Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15:929–937. doi: 10.1038/ni.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat Am, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42:3150–3166. doi: 10.1002/eji.201242847 [DOI] [PubMed] [Google Scholar]
- 42. Cerovic V, Bain CC, Mowat AM, Milling SWF. 2014. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol 35:270–277. doi: 10.1016/j.it.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 43. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. 2013. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6:498–510. doi: 10.1038/mi.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao Q, Lin Y, Yue C, Wang Y, Quan F, Cui X, Bi R, Tang X, Yang Y, Wang C, Li X, Gao X. 2021. IL-6 deficiency promotes colitis by recruiting Ly6Chi monocytes into inflamed colon tissues in a CCL2-CCR2-dependent manner. Eur J Pharmacol 904:174165. doi: 10.1016/j.ejphar.2021.174165 [DOI] [PubMed] [Google Scholar]
- 45. Dunny GM, Brown BL, Clewell DB. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A 75:3479–3483. doi: 10.1073/pnas.75.7.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliver DR, Brown BL, Clewell DB. 1977. Analysis of plasmid deoxyribonucleic acid in a cariogenic strain of Streptococcus faecalis: an approach to identifying genetic determinants on cryptic plasmids. J Bacteriol 130:759–765. doi: 10.1128/jb.130.2.759-765.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Djorić D, Kristich CJ. 2015. Oxidative stress enhances cephalosporin resistance of Enterococcus faecalis through activation of a two-component signaling system. Antimicrob Agents Chemother 59:159–169. doi: 10.1128/AAC.03984-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shroff KE, Meslin K, Cebra JJ. 1995. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun 63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Owen RL. 1999. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer's patches--a personal and historical perspective. Semin Immunol 11:157–163. doi: 10.1006/smim.1999.0171 [DOI] [PubMed] [Google Scholar]
- 50. Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Kassel R, Newberry RD. 2017. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 8:400–411. doi: 10.1080/19490976.2017.1299846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, Luda K, Guilliams M, Lambrecht BN, Agace WW, Milling SW, Mowat AM. 2015. CCR2+CD103- intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol 8:327–339. doi: 10.1038/mi.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koscsó B, Kurapati S, Rodrigues RR, Nedjic J, Gowda K, Shin C, Soni C, Ashraf AZ, Purushothaman I, Palisoc M, Xu S, Sun H, Chodisetti SB, Lin E, Mack M, Kawasawa YI, He P, Rahman ZSM, Aifantis I, Shulzhenko N, Morgun A, Bogunovic M. 2020. Gut-resident CX3CR1hi macrophages induce tertiary lymphoid structures and IgA response in situ. Sci Immunol 5:eaax0062. doi: 10.1126/sciimmunol.aax0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. 2012. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37:1076–1090. doi: 10.1016/j.immuni.2012.08.026 [DOI] [PubMed] [Google Scholar]
- 54. Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, Kagiyama Y, Nochi T, Yuki Y, Fukuyama Y, Mukai A, Shinzaki S, Fujihashi K, Sasakawa C, Iijima H, Goto M, Umesaki Y, Benno Y, Kiyono H. 2010. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A 107:7419–7424. doi: 10.1073/pnas.1001061107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kristich CJ, Little JL. 2012. Mutations in the β subunit of RNA polymerase alter intrinsic cephalosporin resistance in enterococci. Antimicrob Agents Chemother 56:2022–2027. doi: 10.1128/AAC.06077-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Somovilla-Crespo B, Alfonso-Pérez M, Cuesta-Mateos C, Carballo-de Dios C, Beltrán AE, Terrón F, Pérez-Villar JJ, Gamallo-Amat C, Pérez-Chacón G, Fernández-Ruiz E, Zapata JM, Muñoz-Calleja C. 2013. Anti-CCR7 therapy exerts a potent anti-tumor activity in a xenograft model of human mantle cell lymphoma. J Hematol Oncol 6:89. doi: 10.1186/1756-8722-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitchell LA, Hansen RJ, Beaupre AJ, Gustafson DL, Dow SW. 2013. Optimized dosing of a CCR2 antagonist for amplification of vaccine immunity. Int Immunopharmacol 15:357–363. doi: 10.1016/j.intimp.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S3.