ABSTRACT
Traditional folk treatments for the prevention and management of urinary tract infections (UTIs) and other infectious diseases often include plants and plant extracts that are rich in phenolic compounds. These have been ascribed a variety of activities, including inhibition of bacterial interactions with host cells. Here, we tested a panel of four well-studied phenolic compounds—caffeic acid phenethyl ester (CAPE), resveratrol, catechin, and epigallocatechin gallate—for the effects on host cell adherence and invasion by uropathogenic Escherichia coli (UPEC). These bacteria, which are the leading cause of UTIs, can bind and subsequently invade bladder epithelial cells via an actin-dependent process. Intracellular UPEC reservoirs within the bladder are often protected from antibiotics and host defenses and likely contribute to the development of chronic and recurrent infections. In cell culture-based assays, only resveratrol had a notable negative effect on UPEC adherence to bladder cells. However, both CAPE and resveratrol significantly inhibited UPEC entry into the host cells, coordinate with attenuated phosphorylation of the host actin regulator Focal Adhesion Kinase (FAK or PTK2) and marked increases in the numbers of focal adhesion structures. We further show that the intravesical delivery of resveratrol inhibits UPEC infiltration of the bladder mucosa in a murine UTI model and that resveratrol and CAPE can disrupt the ability of other invasive pathogens to enter host cells. Together, these results highlight the therapeutic potential of molecules like CAPE and resveratrol, which could be used to augment antibiotic treatments by restricting pathogen access to protective intracellular niches.
IMPORTANCE
Urinary tract infections (UTIs) are exceptionally common and increasingly difficult to treat due to the ongoing rise and spread of antibiotic-resistant pathogens. Furthermore, the primary cause of UTIs, uropathogenic Escherichia coli (UPEC), can avoid antibiotic exposure and many host defenses by invading the epithelial cells that line the bladder surface. Here, we identified two plant-derived phenolic compounds that disrupt activation of the host machinery needed for UPEC entry into bladder cells. One of these compounds, resveratrol, effectively inhibited UPEC invasion of the bladder mucosa in a mouse UTI model, and both phenolic compounds significantly reduced host cell entry by other invasive pathogens. These findings suggest that select phenolic compounds could be used to supplement existing antibacterial therapeutics by denying uropathogens shelter within host cells and tissues and help explain some of the benefits attributed to traditional plant-based medicines.
KEYWORDS: UPEC, urinary tract infection, invasion, FAK, phenolic, actin, Salmonella, Shigella, UTI, bladder
INTRODUCTION
Plants produce thousands of phenolic compounds, which are defined as secondary metabolites comprised of at least one aromatic ring with one or more hydroxyl groups (1, 2). These diverse molecules can serve a variety of functions, which include the protection of plants from ultraviolet radiation, oxidative stress, herbivores, and microbial pathogens (2–4). The dietary consumption of plant phenolic compounds is linked to an array of health benefits ranging from antitumorigenesis to antimicrobial effects (2, 5–7). Especially interesting are reports that phenolic and polyphenolic compounds derived from cranberry (Vaccinium macrocarpon) and other botanical sources may help protect against urinary tract infections (UTIs) in some individuals (8–12). These infections, which are most often caused by strains of uropathogenic Escherichia coli (UPEC), are exceptionally common and prone to recur (13). About one-quarter of women will have at least one recurrent UTI (rUTI) within 6 months of a primary infection, and many individuals suffer multiple rUTIs per year. The rampant dissemination and amplification of antibiotic-resistant UPEC strains and other uropathogenic bacteria over the past two decades have greatly complicated the treatment of UTIs and stimulated widespread interest in alternate, supplemental therapies (14–18).
There have been multiple clinical studies aimed at defining the effects of cranberry on UTI, but results have been mixed and difficult to compare due to heterogeneity in the types and quantities of cranberry products used, variations in study population characteristics, and disparate means of defining UTI [e.g., (19–22)]. Despite these complications, recent systemic reviews and meta-analyses of published studies concluded that the consumption of cranberry products could significantly lower the risk of UTI in patients with a history of rUTIs (23–25). However, this conclusion is controversial. Oftentimes, bacteria that cause a rUTI are similar, or identical, to the bacteria that were responsible for the initial UTI (26–28). These and other observations suggest that environmental or in-host bacterial reservoirs may repetitively seed symptomatic UTIs in some people. Studies in mice and humans indicate the existence of UPEC reservoirs both within the gut and within the host cells that comprise the mucosal surfaces of the genitourinary tract (26, 28–33).
By using adhesive organelles known as type 1 pili to bind key host receptors, UPEC can trigger actin cytoskeletal rearrangements that promote the envelopment and internalization of bound bacteria [reviewed in reference (26)]. Within bladder epithelial cells, bacteria that are not immediately expelled can either enter the cytosol and rapidly proliferate to form large but transitory intracellular bacterial communities, or the pathogens can establish small and seemingly quiescent, long-lived reservoirs within endosomal compartments. Once in place, intracellular UPEC reservoirs are well protected from host defenses and multiple frontline antibiotics and are consequently difficult to eradicate (26, 29, 32, 34–39). The inhibition of host cell invasion by UPEC could short circuit cycles of rUTI that may be caused, in some individuals, by the repeated resurgence of intracellular bacterial reservoirs.
Several phenolic compounds derived from cranberry can inhibit UPEC adherence to host cells in vitro, but few have been examined for their effects on host cell invasion by uropathogenic bacteria (8, 11, 12, 40–42). A class of polyphenols known as proanthocyanidins (PACs), which are found in cranberry and many other plants, are well-studied inhibitors of UPEC adherence to host cells and can interfere with bacterial invasion of intestinal epithelial and HeLa cells (43–49). Within the gut, PACs may inhibit host cell invasion by both inducing bacterial aggregation and by disrupting the actin cytoskeleton (47, 48). PACs may also impact UPEC colonization of the host via effects on bacterial stress response pathways, motility, biofilm development, iron metabolism, and toxin expression (42, 49–52). However, PACs likely have limited direct effects on either host cells or UPEC within the urinary tract, as these compounds are not well absorbed within the intestinal tract following consumption and are extensively metabolized by the gut microbiota (53–57). Some PAC-derived metabolites are absorbed within the gut and can later be detected in urine where in vitro assays suggest that they may protect against UTI by multiple mechanisms, including the inhibition bacterial adhesion to host cells (55). It is not yet clear if any of these PAC-derived metabolites can also impact bladder cell invasion independent of effects on bacterial adherence.
In this study, we probed the anti-invasion properties of four well-studied plant-derived phenolics: caffeic acid phenethyl ester (CAPE), resveratrol, catechin, and epigallocatechin gallate (EGCG). These phenolics are similar to many compounds found in extracts from cranberry and a variety of other medicinal plants and have been linked, at least tentatively, with protection against UTI (10, 53, 56, 58–63). Results presented here show that select phenolics can inhibit host cell invasion by UPEC, as well as other invasive pathogens. This inhibitory effect correlates with suppressed activation of Focal Adhesion Kinase (FAK), a key host regulator of F-actin dynamics.
RESULTS
CAPE and resveratrol inhibit host cell invasion by UPEC
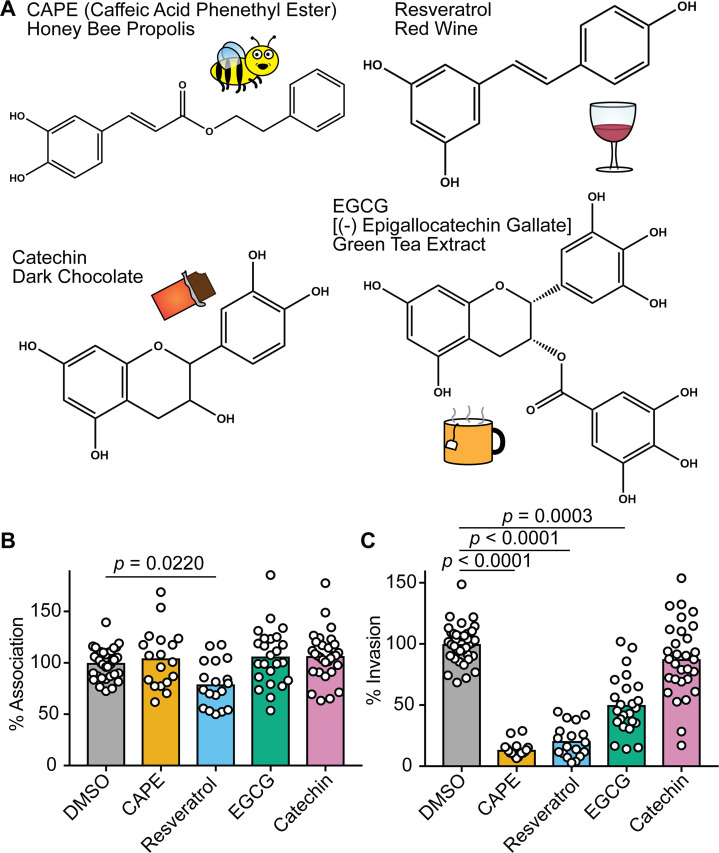
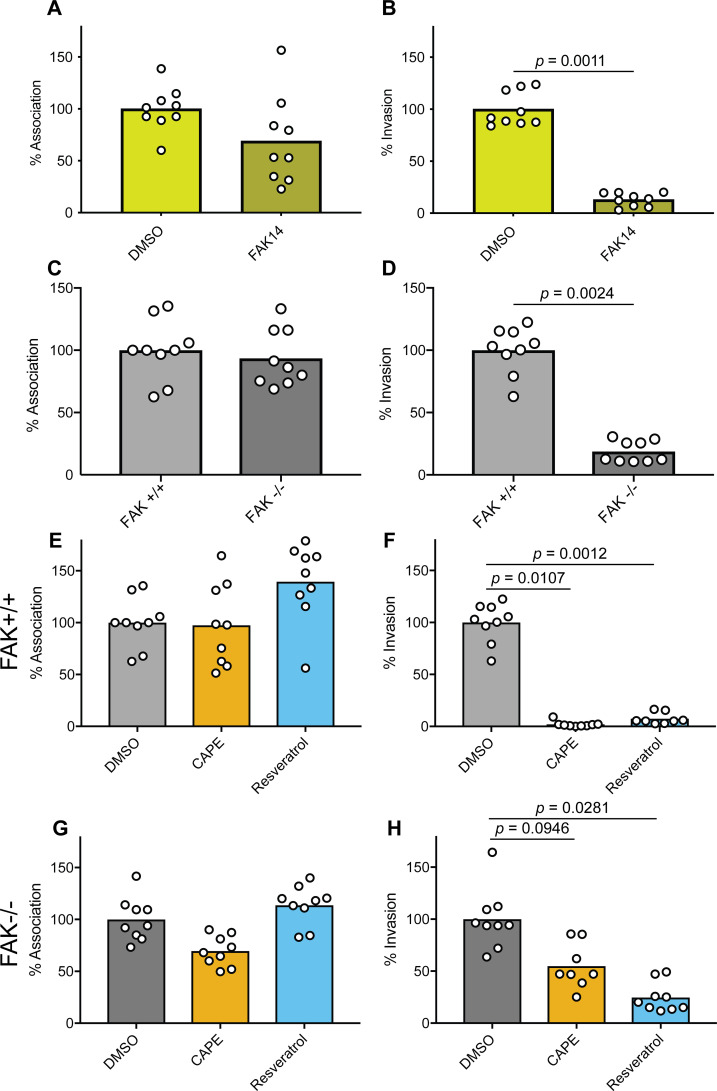
The structures of CAPE, resveratrol, catechin, and EGCG, as well as representative sources of each of these phenolics, are shown in Fig. 1A. To examine the potential effects of these compounds on UPEC-host cell interactions, we utilized standard cell association and gentamicin protection invasion assays with the reference UPEC isolate UTI89 and the human bladder epithelial cell (BEC) line designated 5637 (64). BECs were treated with each compound or carrier alone (dimethyl sulfoxide, DMSO) for 1 h prior to infection and maintained in the culture medium throughout the 2-h cell association assays. During the course of these assays, the BEC monolayers remained alive and intact based on trypan blue exclusion assays. None of the tested phenolic compounds altered the viability of UTI89, as determined by dilution plating (Fig. S1), and only resveratrol caused a significant reduction in the numbers of cell-associated (intra- and extracellular) bacteria (Fig. 1B). In contrast, CAPE, resveratrol, and EGCG treatments significantly decreased the ability of UTI89 to invade the BECs relative to controls treated with only DMSO (Fig. 1C).
Fig 1.
Phenolic compounds can inhibit host cell invasion by UPEC. (A) Skeletal structures of the phenolic compounds used in this study are depicted, with key dietary sources indicated via text and illustrations. (B and C) BECs were pretreated with CAPE (25 µg/mL), resveratrol (22.9 µg/mL), EGCG (25 µg/mL), catechin (25 µg/mL), or carrier alone (0.1% DMSO) for 1 h prior to infection with UTI89. Cells were then incubated for 2 h in the continued presence of the compounds, followed by a final 2-h incubation in medium containing gentamicin. Graphs indicate relative numbers of (B) cell-associated bacteria present prior to the addition of gentamicin and (C) intracellular, gentamicin-protected bacteria calculated as a fraction of the cell-associated bacteria. Data are normalized to DMSO-treated controls, with bars denoting mean values from at least three independent experiments performed in triplicate. P values were determined by Student’s t tests.
BEC invasion by UPEC does not require de novo host transcription or translation
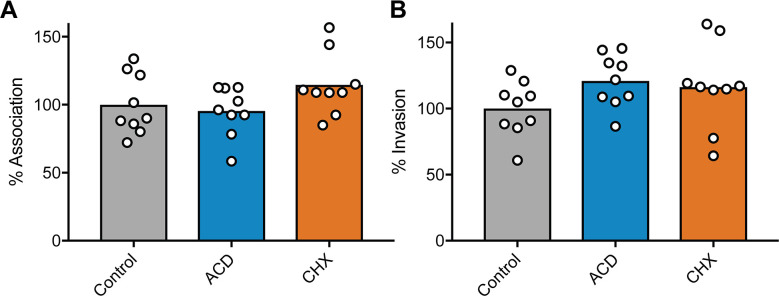
Previous studies indicated that CAPE, resveratrol, and ECGC can each inhibit activation of the host transcription factor NF-κB, which controls the expression of numerous genes, including many associated with inflammation and host responses to infection (65–67). With this information, we reasoned that if the inhibitory effects of CAPE, resveratrol, and ECGC on UPEC invasion of BECs were attributable to the repression of NF-κB activation, then preventing downstream host transcriptional or translational processes should also interfere with UPEC entry into BECs. To test this idea, BECs were treated with actinomycin D (ActD) or cycloheximide (CHX), which ablate host transcription and translation, respectively (68). Neither drug impaired the ability of UTI89 to bind to or invade BECs (Fig. 2), indicating that the anti-invasion effects of CAPE, resveratrol, and ECGC are not due to the disruption of host transcription or translation downstream of NF-κB or other host transcription factors.
Fig 2.
Host cell invasion by UPEC does not require active host transcription or protein synthesis. BECs were treated with ActD (5 µg/mL), CHX (26 µM), or carrier (ethanol) alone for 30 min and then infected in the continued presence of the inhibitors with UTI89 for 2 h followed by a 2-h incubation in medium containing gentamicin. Graphs show levels of (A) host cell-associated bacteria and (B) intracellular, gentamicin-protected bacteria, with bars indicating mean values. Data from three independent experiments performed in triplicate are expressed relative to controls that were treated with carrier (EtOH) alone. P values, as calculated by Student’s t tests, were all ≥0.28.
CAPE and resveratrol inhibit FAK phosphorylation and increase focal adhesion numbers
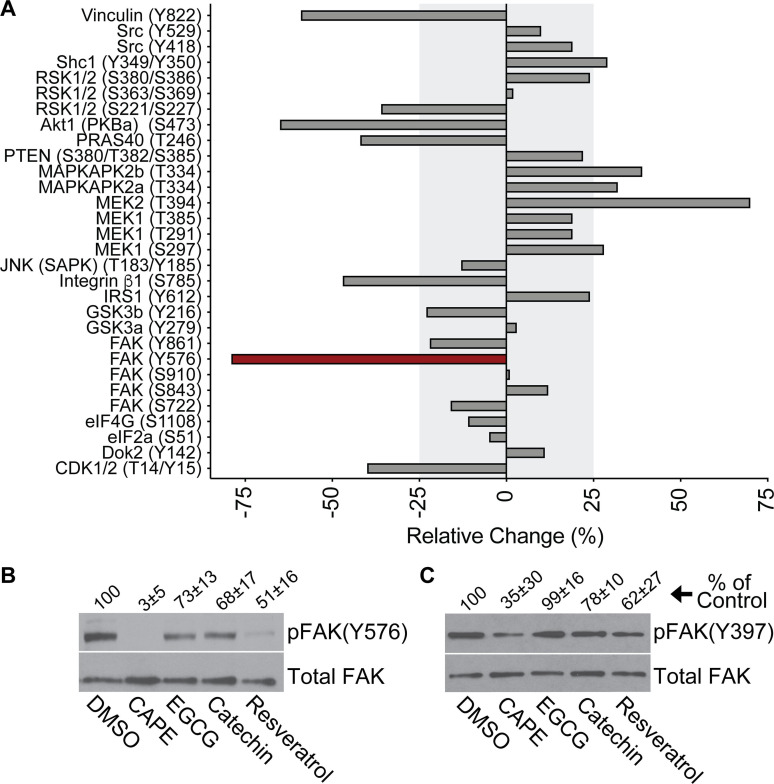
Binding of the type 1 pilus-associated adhesin FimH to mannosylated glycoprotein receptors, including α3 and β1 integrins, activates host signaling cascades that drive the actin-dependent envelopment and internalization of bound UPEC (26). To examine the phenolic effects on host signaling processes that might affect UPEC entry into BECs, we utilized an antibody microarray approach (Kinexus) to quantify changes in the phosphorylation of specific host proteins. For this assay, we focused on CAPE, which had the greatest inhibitory effect on UPEC invasion (see Fig. 1C). Following a 15-min infection with UTI89, phosphorylated residues within several host factors that were previously linked with UPEC invasion were notably reduced by >25% in CAPE-treated BECs relative to those that were treated with carrier alone (Fig. 3A) (26, 69–72). These factors included the FimH receptor β1 integrin, Akt (protein kinase B), vinculin, and FAK (protein tyrosine kinase 2), with phosphorylation of tyrosine 576 in FAK being the most diminished. Western blot analyses confirmed that CAPE treatment ablated FAK phosphorylation at Y576 [denoted as pFAK(Y576)] within UTI89-infected BECs and showed that resveratrol had a similar, though less pronounced, effect (Fig. 3B). ECGC and catechin had more subtle, but still discernable, effects on pFAK(Y576).
Fig 3.
CAPE and resveratrol ablate phosphorylation of FAK at Y576. (A) Graph shows results from a Kinetworks Phospho-Site screen (KPSS 7.0), in which phosphorylation levels of each of the indicated residues (in parentheses) were quantified in CAPE- and DMSO-treated BECs after a 15-min infection with UTI89. Differences between samples are presented as percentages of the DMSO-treated, UTI89-infected controls: [(CAPE-treated – DMSO-treated)/DMSO-treated * 100]. Shaded areas denote relative changes of 25% or less, and the red bar highlights FAK(Y576) as the phospho-site most altered by CAPE treatment in this analysis. (B and C) BECs were treated with carrier alone (0.1% DMSO), CAPE (25 µg/mL), EGCG (25 µg/mL), catechin (25 µg/mL), or resveratrol (22.9 µg/mL) for 1 h prior to a 15-min infection with UTI89 in the continued presence of each reagent. BEC lysates were then collected, resolved by SDS-PAGE, and probed by western blot analysis to assess (B) pFAK(Y576) and (C) pFAK(Y397) levels relative to total FAK in each sample. Mean values from at least three independent assays are denoted above the representative blots as a percentage (±SD) of the DMSO-treated controls.
FAK acts downstream of integrin receptors, working in concert with various signaling and scaffolding factors to modulate actin rearrangements and the maturation and turnover of focal adhesions (FAs) (73). These dynamic structures mediate actin-dependent host cell adherence and spreading processes, and a number of FA-associated factors, including FAK itself, are hijacked by UPEC and other pathogens to gain entry into host cells (69, 71, 74). Integrin interactions with extracellular matrix proteins lead to the autophosphorylation of FAK at Y397, which in turn stimulates the recruitment and activation of SH2-domain-containing proteins such as phosphatidylinositol 3-kinase (PI3K) and Src kinase (73). Src then phosphorylates multiple sites within FAK, including Y576, which is required for maximal activation of FAK and the proper regulation of FA dynamics (73, 75). CAPE, more so than the other phenolic compounds that we tested, diminished phosphorylation of FAK(Y397) within UTI89-infected BECs (Fig. 3C), but this effect was less robust than what was observed with pFAK(Y576) (Fig. 3B).
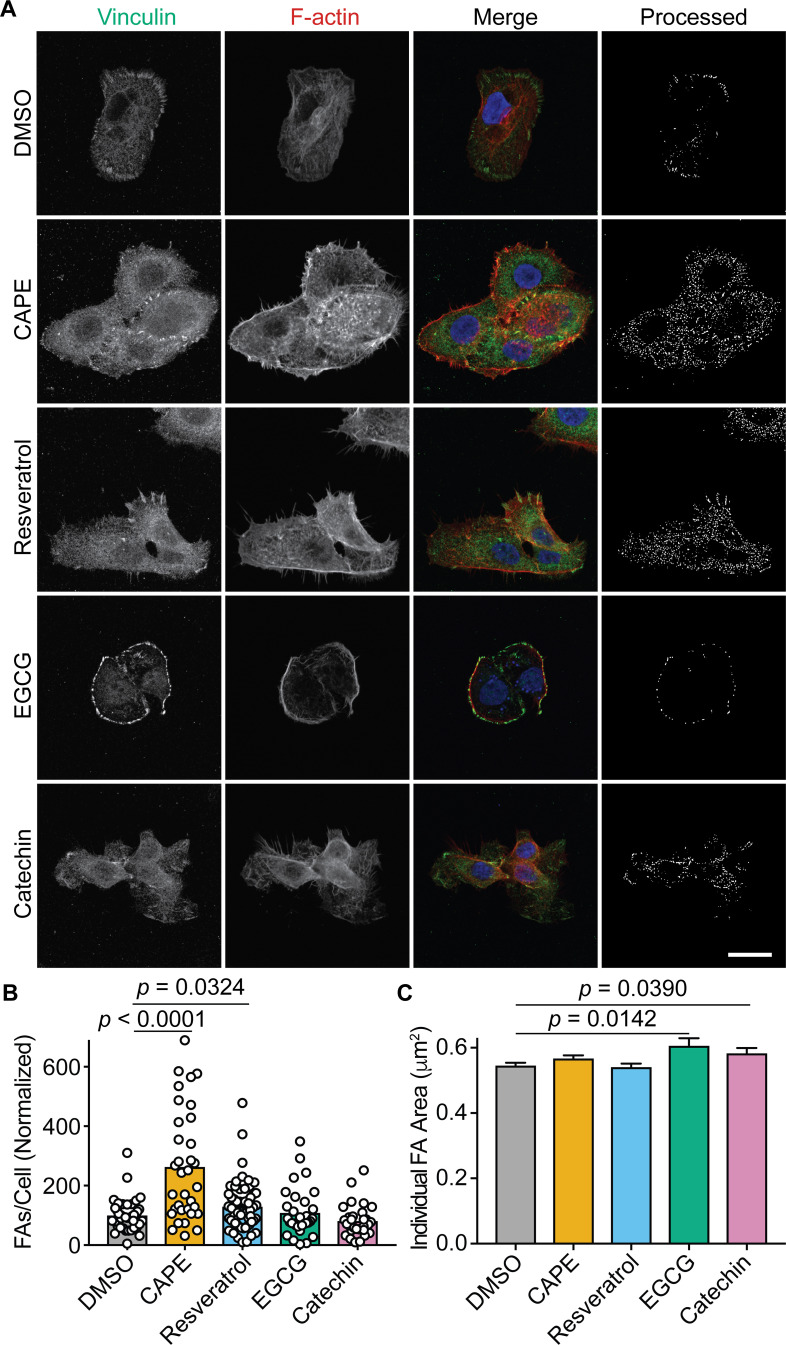
In fibroblasts, the deletion of FAK increases the numbers of FA-like structures due to diminished turnover of integrin-linked adhesion sites (73, 76). By preventing full activation of FAK, we hypothesized that CAPE and resveratrol (and to a lesser extent EGCG and catechin) would partially mirror the effects of a FAK deletion and alter FA numbers. To test this possibility, uninfected BECs were treated with each phenolic compound individually or with carrier (DMSO) alone for 3 h and then processed for imaging by fluorescence confocal microscopy. Labeling of vinculin was used to visualize and quantify FAs, as previously described (77), and the BECs were counterstained to highlight nuclei and F-actin (representative images are shown in Fig. 4A). CAPE and resveratrol treatments both significantly increased the numbers of FAs per cell (Fig. 4A and B) while EGCG and catechin slightly, but significantly, elevated the average size of the FAs (Fig. 4C). Together, these observations indicate that CAPE and resveratrol (more so than EGCG and catechin) can interfere with FAK activation and the turnover of FA-like complexes.
Fig 4.
CAPE and resveratrol increase focal adhesion numbers. (A) Confocal microscopy images of BECs that were treated for 3 h with carrier alone (DMSO), CAPE (25 µg/mL), resveratrol (22.9 µg/mL), EGCG (25 µg/mL), or catechin (25 µg/mL) and then fixed and stained for vinculin (green), F-actin (red), and nuclei (blue). Single-channel and merged images are indicated. The final panel in each row shows the cell images after processing to highlight focal adhesions for quantification. Scale bar, 10 µm. At least 30 cells from three independent experiments were processed to determine focal adhesion (B) numbers and (C) areas following the indicated treatments. Bars denote mean values (±SEM in C). P values were calculated relative to DMSO-treated controls by Student’s t tests.
The anti-invasion effects of CAPE are primarily due to FAK inhibition
Previously, the importance of FAK as a mediator of host cell invasion by UPEC was demonstrated using FAK-null (FAK−/−) mouse embryonic fibroblasts (MEFs) and siRNA with BECs (69). Building on this work, we treated BECs with a pharmacological inhibitor of FAK (FAK inhibitor 14, FAK14), which selectively blocks autophosphorylation of Y397 (78). We found that the treatment of BECs with FAK14 markedly reduced UTI89 internalization but did not significantly alter the levels of host cell-associated bacteria (Fig. 5A and B) nor bacterial viability in the culture medium (Fig. S1). These results echo those obtained using CAPE-, resveratrol-, and, to a lesser extent, EGCG-treated BECs (Fig. 1B and C).
Fig 5.
FAK inhibition and deletion mirror the effects of CAPE and resveratrol on host cell invasion by UPEC. (A and B) BECs or (C–H) FAK+/+ and FAK−/− MEFs were treated with FAK14 (10 µg/mL), CAPE (25 µg/mL), resveratrol (22.9 µg/mL), carrier (DMSO) alone, or left untreated, as indicated, for 1 h prior to and during a 2-h infection with UTI89. Monolayers were then washed and processed to determine total numbers of host cell-associated bacteria or incubated for an additional 2-h period with gentamicin to eradicate extracellular bacteria. Graphs show relative levels of (A, C, E, and G) host cell-associated bacteria and (B, D, F, and H) intracellular, gentamicin-protected bacteria. Data were normalized to DMSO-treated controls or to wild-type (FAK+/+) MEFs, as applicable, with bars representing mean values from three independent experiments carried out in triplicate. P values were determined by Student’s t tests.
Because CAPE and other phenolics can alter the phosphorylation patterns of multiple host factors [see Fig. 3A, e.g., (79–87)], we reasoned that the inhibitory effects of CAPE and resveratrol on UPEC invasion may not be entirely attributable to FAK inactivation. To address this possibility, we employed wild-type (FAK+/+) and FAK-null MEFs in combination with CAPE and resveratrol. As expected, UTI89 entry into the FAK-null MEFs was greatly impaired, though the bacteria bound the wild-type and FAK−/− host cells at similar levels (Fig. 5C and D). Treatment of the wild-type MEFs with either CAPE or resveratrol mirrored the effects seen with BECs (see Fig. 1B and C), suppressing host cell invasion by UTI89 while causing no significant changes in the total numbers of host cell-associated bacteria (Fig. 5E and F). Next, we asked if CAPE or resveratrol could further inhibit UTI89 entry into FAK-null MEFs, which are already by and large refractory to host cell invasion by this pathogen (see Fig. 5D). Treatment of the FAK-null MEFs with CAPE reduced the numbers of bound and internalized bacteria by an average of 30% and 45%, respectively, but these effects were not significant in comparison with DMSO-treated controls (Fig. 5G and H). In contrast, resveratrol significantly inhibited UTI89 entry into the FAK-null MEFs by about 75% (Fig. 5H) without altering the total numbers of bound bacteria (Fig. 5G). Together, these results support the hypothesis that both CAPE and resveratrol interfere with host cell invasion via inhibitory effects on FAK. However, it appears that resveratrol and, to a lesser degree, CAPE can also limit invasion by disrupting other as-yet undefined host cell activities, independent of effects on FAK.
CAPE and resveratrol impede host cell entry by distinct intracellular pathogens
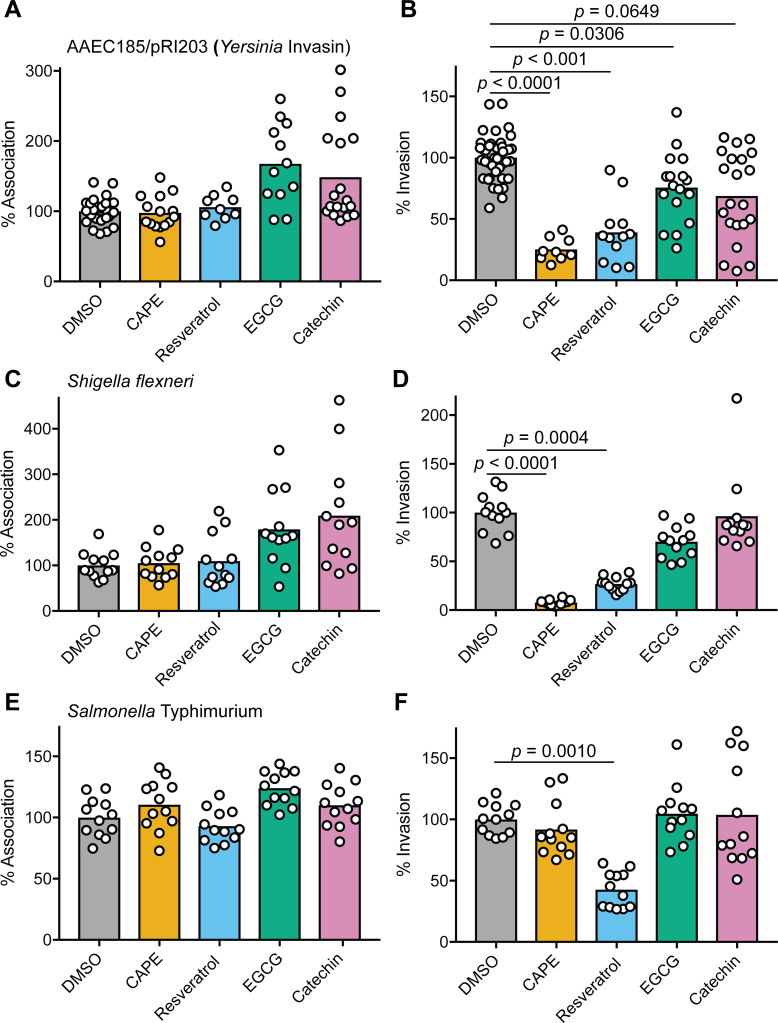
Many microbial pathogens, in addition to UPEC, can invade non-phagocytic host cells via actin-dependent processes that are facilitated by FAK (74). To determine if CAPE, resveratrol, EGCG, or catechin affect host cell entry by other invasive pathogens, we employed our standard cell association and invasion assays with Salmonella enterica serovar Typhimurium, Shigella flexneri, and a non-pathogenic surrogate for Yersinia pseudotuberculosis (AAEC185/pRI203). The latter is a type 1 pilus-negative K-12 E. coli strain that expresses the Y. pseudotuberculosis invasin protein, which promotes actin- and FAK-dependent host cell entry by binding integrin receptors (88, 89). None of the tested phenolics significantly altered the numbers of host cell-associated AAEC185/pRI203, though the numbers of adherent bacteria recovered from EGCG- and catechin-treated host cells trended higher and had a greater spread (Fig. 6A). As seen with UTI89, CAPE, resveratrol, and, to a lesser extent, EGCG significantly impeded host cell invasion by AAEC185/pRI203 (Fig. 6B). Similar results were obtained with S. flexneri (Fig. 6C and D), which mobilizes multiple type III secretion system effectors that engage integrin receptors and associated host factors to promote FAK phosphorylation coordinate with actin rearrangements that drive bacterial internalization (90).
Fig 6.
CAPE and resveratrol can inhibit host cell entry by other invasive bacteria. BECs were treated with the indicated phenolic compounds or DMSO alone for 1 h prior to infection with (A and B) AAEC185/pRI203, (C and D) S. flexneri (E and F), or S. Typhimurium. Monolayers were then incubated for 2 h in the continued presence of the compounds, followed by a 2-h incubation in the presence of gentamicin. Graphs show mean values of (A, C, and E) cell-associated and (B, D, and F) gentamicin-protected, intracellular bacteria from at least three independent experiments performed in triplicate. Data are expressed relative to DMSO-treated controls. P values were calculated using Student’s t tests.
S. Typhimurium can also use type III effectors to enter host cells via FAK- and actin-dependent processes, but the Salmonella effectors are distinct from those encoded by Shigella (91). Furthermore, though S. Typhimurium entry into host cells requires FAK, the kinase domain which contains the activating phospho-site Y576 is dispensable for host cell invasion by this pathogen (91). In our assays, none of the tested phenolics altered the levels of host cell-bound S. Typhimurium (Fig. 6E), and only resveratrol inhibited host cell invasion (Fig. 6F). Together, these findings indicate that the ability of CAPE, resveratrol, and EGCG to impede host cell invasion can vary markedly, dependent on the pathogen and its specific mechanism of entry.
Resveratrol inhibits UPEC invasion of the murine bladder mucosa
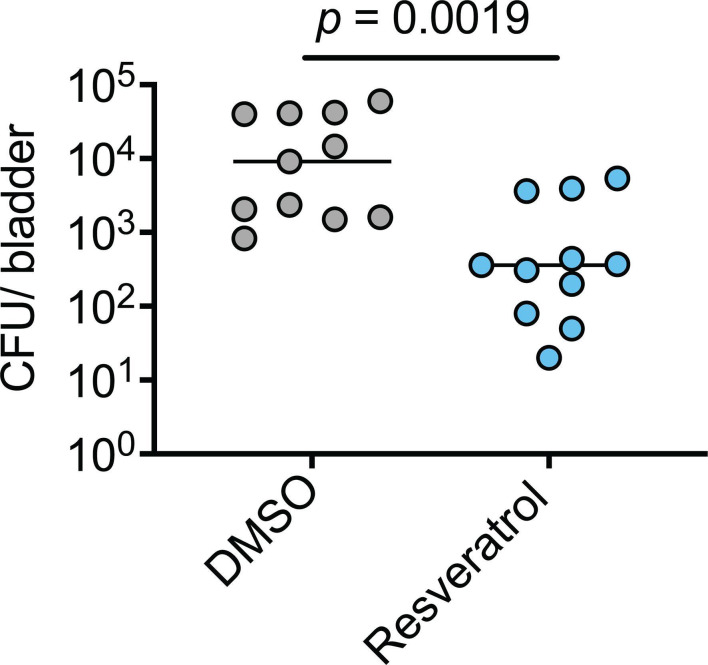
Next, we tested if resveratrol could interfere with UPEC invasion of host cells in an established mouse model of UTI (37). For this initial in vivo work, we focused on resveratrol as it was much more soluble than CAPE in both DMSO and in aqueous solutions and consequently less prone to precipitate out when introduced into the bladder (92). Adult female CBA/JCrHsd mice were inoculated via transurethral catheterization with ~107 CFU of UTI89 in PBS containing 300 µM resveratrol or just the carrier DMSO. After 1 h, the bladders were collected, rinsed, and treated with gentamicin to kill any extracellular bacteria. Over 25-fold fewer intracellular bacteria were recovered from the resveratrol-treated bladders relative to those treated with DMSO alone (Fig. 7). These results indicate that resveratrol has the capacity to effectively inhibit host cell invasion by UPEC within the murine bladder.
Fig 7.
Resveratrol inhibits bacterial invasion of the bladder mucosa. Adult female CBA/JCrHsd mice were inoculated via trans-urethral catheterization with 107 CFU of UTI89 suspended in PBS containing either DMSO or resveratrol (300 µM). Bladders were extracted 1 h later, and the numbers of intracellular, gentamicin-protected bacteria were determined. Bars indicate median values; n = 11 mice from two independent experiments. P value determined using the Mann-Whitney U test.
DISCUSSION
Results presented here show that the plant phenolics CAPE, resveratrol, and, to a lesser extent, EGCG can inhibit UPEC entry into BECs. These phenolics are similar to compounds derived from cranberry-associated PACs and other complex polyphenolic biomolecules like tannins, which are attributed with a variety of antimicrobial activities including bactericidal and antiadhesion effects (42, 45, 59, 93–95). In our assays, none of the tested phenolics interfered with bacterial viability (Fig. S1), and only resveratrol had a noticeable (though slight) inhibitory effect on UPEC adherence to BECs (Fig. 1B). Furthermore, we found that host cell invasion by UPEC did not require de novo host transcription or translation, indicating that the inhibitory effects of CAPE, resveratrol, and EGCG are not related to the ability of these phenolics to interfere with host transcription factors like NF-κB (Fig. 2). Rather, the more pronounced inhibitory effects of CAPE and resveratrol on UPEC entry into BECs were linked with the dysregulation of host actin dynamics via the suppression of FAK phosphorylation at Y576. The disruption of FAK signaling appears to be an effect of many plant-derived phenolic compounds [e.g., curcumin, enterolactone, and glabridin (85–87, 96–100)], and may help explain some of the reported benefits of these molecules for the prevention or treatment of infections, cancers, and other ailments.
Extracts from a variety of medicinal plants, including Citrus reticulata Blanco (mandarin seeds), Amaranthus caudatus (a flowering plant that thrives in temperate-tropical areas), Clinopodium bolivianum (an aromatic shrub from the Andes region of South America), and Lactuca indica (Vietnamese dandelion) have been shown to inhibit both UPEC adherence to and invasion of bladder cells in vitro (101–104). Like cranberry, these plants are rich in phenolic compounds (103, 105–108), but the specific extract components that inhibit UPEC entry into BECs were not defined. Mechanistically, these extracts did not have any direct antibacterial activities and instead appeared to interfere with the invasion process by downregulating host cell receptors for type 1 pili or by suppressing downstream cell signaling events (101–104).
In the case of L. indica extract, the inhibition of BEC invasion by UPEC was partially attributable to the attenuation of FAK phosphorylation at Y397 (104). The autophosphorylation of this site, as noted above, is a proximal step leading to the recruitment of other signaling factors like PI3K and Src kinase that precede full activation of FAK and the instigation of FAK-modulated actin cytoskeletal rearrangements (73). In our assays, the effects of CAPE and resveratrol on the phosphorylation of FAK(Y397) were much less pronounced than those observed with FAK(Y576) (see Fig. 3), suggesting that these two phenolic compounds act further downstream in the FAK activation pathway than L. indica extract. Our experiments with FAK-null cells confirm that FAK is a major, though likely not the sole, host cell target that explains the inhibitory effects of CAPE and resveratrol on BEC invasion by UPEC (Fig. 5). This conclusion is corroborated by observations showing that CAPE and resveratrol treatments both lead to marked increases in the numbers of FAs, coordinate with alterations in the actin cytoskeleton (see Fig. 4).
Our observations with S. Typhimurium, S. flexneri, and recombinant E. coli expressing the invasin protein from Y. pseudotuberculosis reveal that the anti-invasion effects of CAPE and, especially, resveratrol can extend beyond UPEC (see Fig. 6). Of note, FAK can modulate host cell entry by each of these microbes (88, 90, 91). However, the differential effects of CAPE and resveratrol on host cell entry by a pathogen like S. Typhimurium (see Fig. 6F) suggest that these phenolics can have additional, non-overlapping effects on host cell processes that promote invasion, independent of FAK. This possibility is supported by multiple reports indicating that both CAPE and resveratrol can disrupt various host factors and signaling cascades that might directly or indirectly impact host cell invasion and intracellular trafficking by bacterial pathogens [e.g., (109–114)]. Furthermore, results from our own phospho-site profiling screen (Fig. 3A) suggest that CAPE can alter the phosphorylation status of several host signaling factors, in addition to FAK. Among these is the dual-specificity protein kinase MEK2 (MAP2K2), the phosphorylation of which appears to be markedly elevated in CAPE-treated BECs. MEK2 is implicated as a regulator of various cellular processes, but its role as a modulator of host cell invasion by UPEC is not yet defined. The potential of plant-derived phenolic compounds to interfere with host cell invasion independent of effects on FAK is exemplified by luteolin, a secondary polyphenolic metabolite that is found in many fruits, vegetables, and medicinal herbs (115). Luteolin can limit UPEC entry into BECs by inhibiting host cAMP-phosphodiesterases, which in turn interferes with actin rearrangements driven by the activation of Rac1 GTPase.
There is growing interest in the development of therapeutics that can ameliorate disease by targeting host factors that are highjacked by microbial pathogens rather than the pathogens themselves (116–118). If effective, such host-directed therapeutics are expected to help sidestep the growing challenge of antibiotic resistance. Results with resveratrol-treated mice (Fig. 7) indicate that this phenolic, or compounds with similar activities, could be valuable therapeutic options that can deny UPEC refuge within host cells. Without the ability to hide within host cells, UPEC would be more susceptible to clearance by host defenses and antibiotic treatments that are often ineffective against intracellular microbes (36, 38). Phenolic compounds derived from cranberry, if able to act within the urinary tract in a similar fashion to resveratrol, could help explain the potentially beneficial effects of consuming cranberry products by some individuals who suffer from rUTI (23–25). The benefits of such phenolics could vary dependent on the cause, or source, of the rUTIs. These recurrent infections may arise via repeated inoculation of the urinary tract by pathogens acquired from environmental sources, from bacterial reservoirs within the gut, or from the resurgence of intracellular populations that can persist within the vaginal or bladder mucosa (28–32). We speculate that inhibitors of UPEC invasion like CAPE and resveratrol might primarily aid the latter group, by interrupting cycles of intracellular persistence, growth, resurgence, and re-invasion of host cells within the genitourinary tract.
Though the use of resveratrol, CAPE, or functionally homologous compounds from cranberry or other sources as a means to combat UTI is an appealing notion, it should be tempered with an appreciation of the many obstacles associated with such an approach. Instillation of phenolic compounds by intravesical catheterization is not facile nor cost-effective, and agents delivered in this manner may not remain soluble or might not effectively penetrate target host cells within the mucosa. Furthermore, timing of this treatment approach may be complicated if the compounds need to be present prior to invasion, or re-invasion, of the mucosa by UPEC. Oral administration of anti-invasion phenolic compounds faces similar challenges, in addition to potential problems with absorption and modification by metabolic processes and microbes within the gut (40, 56, 119–121). Furthermore, the intake of very high amounts of plant-derived phenolics might have detrimental effects, such as iron depletion, liver and kidney toxicity, and irritation of the gastrointestinal tract (10, 12, 122–124). Despite these limitations, the work presented here highlights the potential therapeutic utility of plant-derived phenolic compounds as a means to inhibit host cell invasion by UPEC which, if optimized, could help disrupt cycles of rUTIs in some individuals.
MATERIALS AND METHODS
Bacterial strains, cell culture, and inhibitors
The UPEC cystitis isolate UTI89 was grown statically from frozen stocks for 24 h at 37°C in either lysogeny broth (LB, Difco) or modified M9 minimal medium to induce expression of type 1 pili (6 g L−1 Na2HPO4, 3 g L−1 KH2PO4, 0.5 g L−1 NaCl, 0.1 mM CaCl2, 1 g L−1 NH4Cl, 1 mM MgSO4, 0.1% Glucose, 0.0025% nicotinic acid, 0.2% casein amino acids, and 16.5 µg mL−1 thiamine) (29, 125). S. Typhimurium (SL1344) and S. flexneri (ATCC 12022) were cultured shaking at 37°C in LB overnight, then diluted 1:33 in fresh LB, and grown for an additional 3.5 h, as previously described (126). AAEC185/pRI203 was grown shaking in LB to stationary phase prior to usage (127).
The bladder carcinoma cell line 5637 (ATCC HTB-9) was cultured in RPMI1640 (HyClone) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone) in a 37°C humidified incubator with 5% CO2. FAK+/+ (ATCC CRL-2645) and FAK−/− (ATCC CRL-2655) MEFs were grown in DMEM (HyClone) supplemented with 10% heat-inactivated FBS.
FAK14 (a.k.a. Y15) was purchased from Cayman Chemical, while CAPE, resveratrol (specifically trans-resveratrol), EGCG, and catechin were from Sigma-Aldrich, Biomol, or Cayman Chemical. These compounds were prepared as 1,000× stocks in DMSO. Actinomycin D and cycloheximide were obtained from Sigma-Aldrich and solubilized in ethanol.
Bacterial cell association and invasion assays
Bacterial host cell association and invasion assays were performed using established protocols (69). Briefly, 5637 or MEF cells were seeded into 24-well tissue culture plates and grown for about 24 h to near confluency. Where indicated, cell monolayers were treated with CAPE (100.7 µM; 25 µg/mL), resveratrol (100 µM; 22.9 µg/mL), EGCG (54.5 µM; 25 µg/mL), catechin (86.1 µM; 25 µg/mL), FAK14 (10 µg/mL; 35 µM), or DMSO (carrier, 0.1% final concentration) in complete media for 1 h prior to infection. In other experiments, host cells were treated with actinomycin D (5 µg/mL), cycloheximide (26 µM), or an equal volume of ethanol (diluent) for 30 min prior to infection. Triplicate sets of host cells were then infected with UTI89 or AAEC185/pRI203 using a multiplicity of infection (MOI) of approximately 15, while an MOI of 100 was used with S. flexneri and S. Typhimurium. Plates were centrifuged at 600 × g for 5 min to accelerate and synchronize bacterial contact with the host cells. UTI89- and AAEC185/pRI203-infected monolayers were then incubated at 37°C in the continued presence of the compounds or carrier, washed four times with PBS containing Mg+2 and Ca+2 (PBS+2), and lysed in PBS with 0.4% Triton-X 100. Serial dilutions of these lysates were plated on LB agar to determine numbers of cell-associated bacteria. Alternatively, sets of triplicate wells were washed twice with PBS+2 and treated for 2 h with complete media containing gentamicin (100 µg/mL) to kill extracellular bacteria. Subsequently, monolayers were washed four times with PBS+2 and lysed and plated as noted above to quantify the numbers of surviving intracellular bacteria. Experiments with S. Typhimurium and S. flexneri used 30-min infection periods for the cell association assays, followed by 1-h incubations with gentamicin for the invasion assays. Results from the invasion assays were normalized by dividing the numbers of intracellular bacteria by the total number of cell-associated bacteria, accounting for any differences in host cell numbers. All assays were repeated at least three times in triplicate.
Potential effects of the phenolic compounds and FAK14 on bacterial growth and viability were assessed by adding bacteria to complete RPMI media in 24-well plates using the same times and drug concentrations as used for the cell association assays, but in the absence of host cells. Bacterial titers were then determined by plating serial dilutions of the media on LB agar. These assays were independently repeated three times. Trypan blue exclusion assays were used to assess host cell viability (128).
Signal transduction protein phospho-site profiling
Sub-confluent 5637 BEC monolayers in six-well plates were serum starved overnight, treated with CAPE (25 µg/mL) or DSMO alone for 1 h, infected with UTI89 (MOI ~15), and centrifuged at 600 × g for 5 min. After an additional 15-min incubation at 37°C in the continued presence of CAPE or DSMO, wells were washed three times with PBS+2 and then lysed on ice with cold buffer containing 50 mM Tris (pH 7.4), 1 mM NaCl, 1% NP-40, complete protease inhibitor cocktail (Roche Applied Science), 1 mM PMSF, 1 mM NaF, 0.4 mM orthovanadate, 5 µM leupeptin, and 1 mM aprotinin. Protein concentrations were determined using a BCA reagent system (Pierce). Lysates were diluted in 4× Kinexus sample buffer to a final concentration of 0.8 µg/µL and shipped to Kinexus (Vancouver, Canada) for multi-immunoblotting analysis using the Kinetworks signal transduction protein phospho-site profiling service (KPSS 7.0 Profile).
Western blot analysis
Nearly confluent BEC monolayers cultured in 12-well plates were serum starved overnight, treated with the specified phenolic compounds or 0.1% DMSO alone for 1 h, and infected with UTI89 from M9 cultures using an MOI of about 25. The cell culture medium was not exchanged when adding either the compounds or during the infection process. After a 5-min spin at 600 × g, the plates were incubated for 15 min at 37°C, washed three times with PBS+2, and then lysed in ice-cold RIPA buffer supplemented complete protease inhibitor cocktail (Roche Applied Science), 1 mM PMSF, 1 mM NaF, and 0.4 mM orthovanadate. Equivalent protein amounts (as determined by BCA assays; Pierce) were resolved by SDS-PAGE, transferred to Immobilon PVDF-FL membrane (Millipore), and processed for western blot analysis. Membranes were incubated with phospho-site-specific rabbit anti-pFAK(Y576) (1:200; Upstate Biotechnology), mouse anti-pFAK(Y397) (1:1,000; BD Biosciences), or mouse anti-FAK (1:500; BD Biosciences) primary antibodies and then probed and imaged using IRDye-labeled secondary antibodies and an Odyssey DLx instrument (LI-COR Biosciences). For quantification, membranes were re-probed with the different antibodies following treatments with stripping buffer (Thermo Scientific). Phospho-site-specific band intensities, minus background values, were measured using ImageJ software (129) and normalized to total FAK levels in each sample.
Visualization and quantification of focal adhesions
5637 BECs were seeded onto 12-mm diameter coverslips in 24-well plates and grown overnight until nearly confluent. Cells were treated with CAPE (25 µg/mL), resveratrol (22.9 µg/mL), EGCG (25 µg/mL), catechin (25 µg/mL), or DMSO (carrier, 0.1%) alone in complete RPMI medium for 3 h, washed three times with PBS+2, and then fixed for 20 min with 3.7% paraformaldehyde dissolved in PBS. After three washes in PBS, cells were blocked and permeabilized using PBS containing 1% powered milk, 3% bovine serum albumin, and 0.1% saponin. The cells were then labeled using primary mouse anti-vinculin antibody (1:100; Sigma-Aldrich) and donkey anti-mouse Alexa Fluor 555-conjugated secondary antibody (1:400; Abcam). F-Actin and nuclei were stained using Oregon Green 488-conjugated phalloidin (1:200; Thermo Fisher) and Hoechst (1:1,000; Sigma-Aldrich), respectively. Coverslips were mounted in FluorSave (Calbiochem) and imaged using a Nikon A1 series confocal microscope with NIS Elements software.
Quantitative analysis of vinculin-positive focal adhesions was performed as previously described, with slight modifications (77). Briefly, using the Fiji processing package with ImageJ software, the background for each image of vinculin-stained cells was subtracted and local contrast enhanced using the CLAHE plugin (130). Next, a mathematical exponential was utilized via the Exp function to further reduce background, and brightness and contrast were adjusted automatically. A Gaussian filter was applied using the Log3D plugin with sigma X = 1.5 and sigma Y = 1.5. An automatic threshold function was then used to create binary images in which pixels were assigned to either a background or foreground signal. Particles (representing focal adhesions) within the binary images were enumerated and sized using the ANALYZE PARTICLES command in ImageJ, with the size parameter set at 14.5-infinity.
Mouse infections
Using established protocols approved by the University of Utah and Institutional Animal Care and Use Committee (IACUC), 8- to 9-week-old female CBA/JCrHsd mice (Harlan Laboratories) were inoculated via transurethral catheterization with 107 CFU of E. coli UTI89 in 50 µL PBS containing 300 µM resveratrol or DMSO. Mice were sacrificed 1 h post-catheterization and the bladders were harvested aseptically, quadrisected, and incubated for 30 min at 37°C in PBS with gentamicin (100 µg/mL) to kill extracellular bacteria. The bladder pieces were then washed three times with PBS and homogenized in PBS containing 0.025% Triton X-100. Serial dilutions of each homogenate were plated on LB agar to determine numbers of intracellular bacteria. A total of 11 mice from two independent experiments were tested for each treatment.
Statistics
For the mouse experiments, data distribution normality (Gaussian) was not assumed. Mann–Whitney U tests and unpaired two-tailed Student’s t tests were performed using Prism 9.0.0 (GraphPad Software). P values of less than or equal to 0.05 were considered significant.
ACKNOWLEDGMENTS
This work was funded in part by NIH grants GM134331, AI095647, and DK069526 to M.A.M. Additional support was provided by NIH Microbial Pathogenesis T32 training grant AI055434 to A.C.R., A.A.R., and D.S.E., NIH Hematology T32 training grant DK7115 to A.J.L., and a University of Utah Chevron Undergraduate Research Scholarship for women in STEM to A.A.M. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
This article is a direct contribution from Matthew A. Mulvey, a member of the Infection and Immunity Editorial Board, who arranged for and secured reviews by Harry L.T. Mobley, University of Michigan, and David G. Thanassi, Stony Brook University.
Contributor Information
Matthew A. Mulvey, Email: mulvey@biology.utah.edu.
Andreas J. Bäumler, University of California, Davis, Davis, California, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00080-24.
Impact of the phenolic compounds and FAK14 on bacterial viability.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Dai J, Mumper RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352. doi: 10.3390/molecules15107313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheynier V. 2012. Phenolic compounds: from plants to foods. Phytochem Rev 11:153–177. doi: 10.1007/s11101-012-9242-8 [DOI] [Google Scholar]
- 3. Chowdhary V. AS, Pandya RV, Tank JG. 2022. Physiological function of phenolic compounds in plant defense system. In Badria FA (ed), Phenolic compounds - chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications. IntechOpen. [Google Scholar]
- 4. Bhattacharya A, Sood P, Citovsky V. 2010. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719. doi: 10.1111/j.1364-3703.2010.00625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar N, Goel N. 2019. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst) 24:e00370. doi: 10.1016/j.btre.2019.e00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H, Qin W, Wu H, Chen S. 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21:1374. doi: 10.3390/molecules21101374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin B, Park W. 2018. Zoonotic diseases and phytochemical medicines for microbial infections in veterinary science: current state and future perspective. Front Vet Sci 5:166. doi: 10.3389/fvets.2018.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González de Llano D, Moreno-Arribas MV, Bartolomé B. 2020. Cranberry polyphenols and prevention against urinary tract infections: relevant considerations. Molecules 25:3523. doi: 10.3390/molecules25153523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marouf RS, Mbarga JAM, Ermolaev AV, Podoprigora IV, Smirnova IP, Yashina NV, Zhigunova AV, Martynenkova AV. 2022. Antibacterial activity of medicinal plants against uropathogenic Escherichia coli. J Pharm Bioallied Sci 14:1–12. doi: 10.4103/jpbs.jpbs_124_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maisto M, Iannuzzo F, Novellino E, Schiano E, Piccolo V, Tenore GC. 2023. Natural polyphenols for prevention and treatment of urinary tract infections. Int J Mol Sci 24:3277. doi: 10.3390/ijms24043277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das S. 2020. Natural therapeutics for urinary tract infections-a review. Futur J Pharm Sci 6:64. doi: 10.1186/s43094-020-00086-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guay DRP. 2009. Cranberry and urinary tract infections. Drugs 69:775–807. doi: 10.2165/00003495-200969070-00002 [DOI] [PubMed] [Google Scholar]
- 13. Murray BO, Flores C, Williams C, Flusberg DA, Marr EE, Kwiatkowska KM, Charest JL, Isenberg BC, Rohn JL. 2021. Recurrent urinary tract infection: a mystery in search of better model systems. Front Cell Infect Microbiol 11:691210. doi: 10.3389/fcimb.2021.691210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loubet P, Ranfaing J, Dinh A, Dunyach-Remy C, Bernard L, Bruyère F, Lavigne J-P, Sotto A. 2020. Alternative therapeutic options to antibiotics for the treatment of urinary tract infections. Front Microbiol 11:1509. doi: 10.3389/fmicb.2020.01509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaheen G, Akram M, Jabeen F, Ali Shah SM, Munir N, Daniyal M, Riaz M, Tahir IM, Ghauri AO, Sultana S, Zainab R, Khan M. 2019. Therapeutic potential of medicinal plants for the management of urinary tract infection: a systematic review. Clin Exp Pharmacol Physiol 46:613–624. doi: 10.1111/1440-1681.13092 [DOI] [PubMed] [Google Scholar]
- 16. Barea BM, Veeratterapillay R, Harding C. 2020. Nonantibiotic treatments for urinary cystitis: an update. Curr Opin Urol 30:845–852. doi: 10.1097/MOU.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 17. Sihra N, Goodman A, Zakri R, Sahai A, Malde S. 2018. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol 15:750–776. doi: 10.1038/s41585-018-0106-x [DOI] [PubMed] [Google Scholar]
- 18. Wawrysiuk S, Naber K, Rechberger T, Miotla P. 2019. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: a systemic review. Arch Gynecol Obstet 300:821–828. doi: 10.1007/s00404-019-05256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cimolai N, Cimolai T. 2007. The cranberry and the urinary tract. Eur J Clin Microbiol Infect Dis 26:767–776. doi: 10.1007/s10096-007-0379-0 [DOI] [PubMed] [Google Scholar]
- 20. Hisano M, Bruschini H, Nicodemo AC, Srougi M. 2012. Cranberries and lower urinary tract infection prevention. Clinics (Sao Paulo) 67:661–668. doi: 10.6061/clinics/2012(06)18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gbinigie OA, Spencer EA, Heneghan CJ, Lee JJ, Butler CC. 2020. Cranberry extract for symptoms of acute, uncomplicated urinary tract infection: a systematic review. Antibiotics (Basel) 10:12. doi: 10.3390/antibiotics10010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jepson R, Craig J, Williams G. 2013. Cranberry products and prevention of urinary tract infections. JAMA 310:1395–1396. doi: 10.1001/jama.2013.277509 [DOI] [PubMed] [Google Scholar]
- 23. Williams G, Hahn D, Stephens JH, Craig JC, Hodson EM. 2023. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 4:CD001321. doi: 10.1002/14651858.CD001321.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luís Â, Domingues F, Pereira L. 2017. Can cranberries contribute to reduce the incidence of urinary tract infections? A systematic review with meta-analysis and trial sequential analysis of clinical trials. J Urol 198:614–621. doi: 10.1016/j.juro.2017.03.078 [DOI] [PubMed] [Google Scholar]
- 25. Xia JY, Yang C, Xu DF, Xia H, Yang LG, Sun GJ. 2021. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: a systematic review and meta-analysis with trial sequential analysis. PLoS One 16:e0256992. doi: 10.1371/journal.pone.0256992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lewis AJ, Richards AC, Mulvey MA. 2016. Invasion of host cells and tissues by uropathogenic bacteria. Microbiol Spectr 4. doi: 10.1128/microbiolspec.UTI-0026-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis 172:440–445. doi: 10.1093/infdis/172.2.440 [DOI] [PubMed] [Google Scholar]
- 28. Thänert R, Reske KA, Hink T, Wallace MA, Wang B, Schwartz DJ, Seiler S, Cass C, Burnham C-A, Dubberke ER, Kwon JH, Dantas G. 2019. Comparative genomics of antibiotic-resistant uropathogens implicates three routes for recurrence of urinary tract infections. mBio 10:e01977-19. doi: 10.1128/mBio.01977-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brannon JR, Dunigan TL, Beebout CJ, Ross T, Wiebe MA, Reynolds WS, Hadjifrangiskou M. 2020. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat Commun 11:2803. doi: 10.1038/s41467-020-16627-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forde BM, Roberts LW, Phan MD, Peters KM, Fleming BA, Russell CW, Lenherr SM, Myers JB, Barker AP, Fisher MA, Chong TM, Yin WF, Chan KG, Schembri MA, Mulvey MA, Beatson SA. 2019. Population dynamics of an Escherichia coli ST131 lineage during recurrent urinary tract infection. Nat Commun 10:3643. doi: 10.1038/s41467-019-11571-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kerrn MB, Struve C, Blom J, Frimodt-Møller N, Krogfelt KA. 2005. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J Antimicrob Chemother 55:383–386. doi: 10.1093/jac/dki002 [DOI] [PubMed] [Google Scholar]
- 33. Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494 [DOI] [PubMed] [Google Scholar]
- 34. Schilling JD, Lorenz RG, Hultgren SJ. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun 70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma K, Dhar N, Thacker VV, Simonet TM, Signorino-Gelo F, Knott GW, McKinney JD. 2021. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. Elife 10:e66481. doi: 10.7554/eLife.66481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma K, Thacker VV, Dhar N, Clapés Cabrer M, Dubois A, Signorino-Gelo F, Mullenders J, Knott GW, Clevers H, McKinney JD. 2021. Early invasion of the bladder wall by solitary bacteria protects UPEC from antibiotics and neutrophil swarms in an organoid model. Cell Rep 36:109351. doi: 10.1016/j.celrep.2021.109351 [DOI] [PubMed] [Google Scholar]
- 37. Blango MG, Ott EM, Erman A, Veranic P, Mulvey MA. 2014. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One 9:e93327. doi: 10.1371/journal.pone.0093327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother 54:1855–1863. doi: 10.1128/AAC.00014-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu SC, Han XM, Shi M, Pang ZL. 2016. Persistence of uropathogenic Escherichia coli in the bladders of female patients with sterile urine after antibiotic therapies. J Huazhong Univ Sci Technolog Med Sci 36:710–715. doi: 10.1007/s11596-016-1649-9 [DOI] [PubMed] [Google Scholar]
- 40. Liu H, Howell AB, Zhang DJ, Khoo C. 2019. A randomized, double-blind, placebo-controlled pilot study to assess bacterial anti-adhesive activity in human urine following consumption of a cranberry supplement. Food Funct 10:7645–7652. doi: 10.1039/c9fo01198f [DOI] [PubMed] [Google Scholar]
- 41. Scharf B, Schmidt TJ, Rabbani S, Stork C, Dobrindt U, Sendker J, Ernst B, Hensel A. 2020. Antiadhesive natural products against uropathogenic E. coli: what can we learn from cranberry extract?. J Ethnopharmacol 257:112889. doi: 10.1016/j.jep.2020.112889 [DOI] [PubMed] [Google Scholar]
- 42. Côté J, Caillet S, Doyon G, Sylvain J-F, Lacroix M. 2010. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr 50:666–679. doi: 10.1080/10408390903044107 [DOI] [PubMed] [Google Scholar]
- 43. Foo LY, Lu Y, Howell AB, Vorsa N. 2000. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod 63:1225–1228. doi: 10.1021/np000128u [DOI] [PubMed] [Google Scholar]
- 44. Rafsanjany N, Senker J, Brandt S, Dobrindt U, Hensel A. 2015. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against fimh-dominated uropathogenic Escherichia coli: a combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon. J Agric Food Chem 63:8804–8818. doi: 10.1021/acs.jafc.5b03030 [DOI] [PubMed] [Google Scholar]
- 45. Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. 2005. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 46. Howell AB. 2007. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res 51:732–737. doi: 10.1002/mnfr.200700038 [DOI] [PubMed] [Google Scholar]
- 47. Harmidy K, Tufenkji N, Gruenheid S. 2011. Perturbation of host cell cytoskeleton by cranberry proanthocyanidins and their effect on enteric infections. PLoS One 6:e27267. doi: 10.1371/journal.pone.0027267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polewski MA, Krueger CG, Reed JD, Leyer G. 2016. Ability of cranberry proanthocyanidins in combination with a probiotic formulation to inhibit in vitro invasion of gut epithelial cells by extra-intestinal pathogenic E. coli. J Functional Foods 25:123–134. doi: 10.1016/j.jff.2016.05.015 [DOI] [Google Scholar]
- 49. Roussel C, Chabaud S, Lessard-Lord J, Cattero V, Pellerin FA, Feutry P, Bochard V, Bolduc S, Desjardins Y. 2022. UPEC colonic-virulence and urovirulence are blunted by proanthocyanidins-rich cranberry extract microbial metabolites in a gut model and a 3D tissue-engineered urothelium. Microbiol Spectr 10:e0243221. doi: 10.1128/spectrum.02432-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Samarasinghe S, Reid R, Al-Bayati M. 2019. The anti-virulence effect of cranberry active compound proanthocyanins (PACs) on expression of genes in the third-generation cephalosporin-resistant Escherichia coli CTX-M-15 associated with urinary tract infection. Antimicrob Resist Infect Control 8:181. doi: 10.1186/s13756-019-0637-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ranfaing J, Dunyach-Remy C, Louis L, Lavigne JP, Sotto A. 2018. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) against the virulence of uropathogenic Escherichia coli. Sci Rep 8:10706. doi: 10.1038/s41598-018-29082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tapiainen T, Jauhiainen H, Jaakola L, Salo J, Sevander J, Ikäheimo I, Pirttilä AM, Hohtola A, Uhari M. 2012. Biofilm formation and virulence of uropathogenic Escherichia coli in urine after consumption of cranberry-lingonberry juice. Eur J Clin Microbiol Infect Dis 31:655–662. doi: 10.1007/s10096-011-1355-2 [DOI] [PubMed] [Google Scholar]
- 53. Feliciano RP, Krueger CG, Reed JD. 2015. Methods to determine effects of cranberry proanthocyanidins on extraintestinal infections: relevance for urinary tract health. Mol Nutr Food Res 59:1292–1306. doi: 10.1002/mnfr.201500108 [DOI] [PubMed] [Google Scholar]
- 54. Rajbhandari R, Peng N, Moore R, Arabshahi A, Wyss JM, Barnes S, Prasain JK. 2011. Determination of cranberry phenolic metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem 59:6682–6688. doi: 10.1021/jf200673h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tamargo A, Cueva C, Taladrid D, Khoo C, Moreno-Arribas MV, Bartolomé B, González de Llano D. 2022. Simulated gastrointestinal digestion of cranberry polyphenols under dynamic conditions. Impact on antiadhesive activity against uropathogenic bacteria. Food Chem 368:130871. doi: 10.1016/j.foodchem.2021.130871 [DOI] [PubMed] [Google Scholar]
- 56. Marín L, Miguélez EM, Villar CJ, Lombó F. 2015. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int 2015:905215. doi: 10.1155/2015/905215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Llano DG, Esteban-Fernández A, Sánchez-Patán F, Martínlvarez PJ, Moreno-Arribas MV, Bartolomé B. 2015. Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int J Mol Sci 16:12119–12130. doi: 10.3390/ijms160612119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee JH, Kim YG, Raorane CJ, Ryu SY, Shim JJ, Lee J. 2019. The anti-biofilm and anti-virulence activities of trans-resveratrol and oxyresveratrol against uropathogenic Escherichia coli. Biofouling 35:758–767. doi: 10.1080/08927014.2019.1657418 [DOI] [PubMed] [Google Scholar]
- 59. Reygaert W, Jusufi I. 2013. Green tea as an effective antimicrobial for urinary tract infections caused by Escherichia coli. Front Microbiol 4:162. doi: 10.3389/fmicb.2013.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Celik S, Gorur S, Aslantas O, Erdogan S, Ocak S, Hakverdi S. 2007. Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli-induced pyelonephritis in rats. Mol Cell Biochem 297:131–138. doi: 10.1007/s11010-006-9337-x [DOI] [PubMed] [Google Scholar]
- 61. Nemzer BV, Al-Taher F, Yashin A, Revelsky I, Yashin Y. 2022. Cranberry: chemical composition, antioxidant activity and impact on human health: overview. Molecules 27:27. doi: 10.3390/molecules27051503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. 2004. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem 52:4713–4719. doi: 10.1021/jf040095e [DOI] [PubMed] [Google Scholar]
- 63. Zhang K, Zuo Y. 2004. GC-MS determination of flavonoids and phenolic and benzoic acids in human plasma after consumption of cranberry juice. J Agric Food Chem 52:222–227. doi: 10.1021/jf035073r [DOI] [PubMed] [Google Scholar]
- 64. Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA. 2008. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell Microbiol 10:2553–2567. doi: 10.1111/j.1462-5822.2008.01229.x [DOI] [PubMed] [Google Scholar]
- 65. Sah DK, Khoi PN, Li S, Arjunan A, Jeong JU, Jung YD. 2022. (-)-Epigallocatechin-3-gallate prevents IL-1β-induced uPAR expression and invasiveness via the suppression of NF-κB and ap-1 in human bladder cancer cells. Int J Mol Sci 23:14008. doi: 10.3390/ijms232214008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A 93:9090–9095. doi: 10.1073/pnas.93.17.9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, Mao Q, Yang R. 2013. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 68:689–694. [PubMed] [Google Scholar]
- 68. Mulvey M, Brown DT. 1994. Formation and rearrangement of disulfide bonds during maturation of the Sindbis virus E1 glycoprotein. J Virol 68:805–812. doi: 10.1128/JVI.68.2.805-812.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eto DS, Jones TA, Sundsbak JL, Mulvey MA. 2007. Integrin-mediated host cell invasion by type 1–piliated uropathogenic Escherichia coli. PLoS Pathog 3:e100. doi: 10.1371/journal.ppat.0030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim WJ, Shea AE, Kim JH, Daaka Y. 2018. Uropathogenic Escherichia coli invades bladder epithelial cells by activating kinase networks in host cells. J Biol Chem 293:16518–16527. doi: 10.1074/jbc.RA118.003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19:2803–2812. doi: 10.1093/emboj/19.12.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martinez JJ, Hultgren SJ. 2002. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol 4:19–28. doi: 10.1046/j.1462-5822.2002.00166.x [DOI] [PubMed] [Google Scholar]
- 73. Mitra SK, Hanson DA, Schlaepfer DD. 2005. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68. doi: 10.1038/nrm1549 [DOI] [PubMed] [Google Scholar]
- 74. Murphy KN, Brinkworth AJ. 2021. Manipulation of focal adhesion signaling by pathogenic microbes. Int J Mol Sci 22:1358. doi: 10.3390/ijms22031358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hanks SK, Ryzhova L, Shin N-Y, Brábek J. 2003. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci 8:d982–96. doi: 10.2741/1114 [DOI] [PubMed] [Google Scholar]
- 76. Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci 113 ( Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673 [DOI] [PubMed] [Google Scholar]
- 77. Horzum U, Ozdil B, Pesen-Okvur D. 2014. Step-by-step quantitative analysis of focal adhesions. MethodsX 1:56–59. doi: 10.1016/j.mex.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. 2008. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the Y397 site of focal adhesion kinase decreases tumor growth. J Med Chem 51:7405–7416. doi: 10.1021/jm800483v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. 2001. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol 60:217–224. doi: 10.1124/mol.60.1.217 [DOI] [PubMed] [Google Scholar]
- 80. Hwang YS, Park KK, Chung WY. 2013. Epigallocatechin-3 gallate inhibits cancer invasion by repressing functional invadopodia formation in oral squamous cell carcinoma. Eur J Pharmacol 715:286–295. doi: 10.1016/j.ejphar.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 81. Yu HJ, Shin JA, Cho SD. 2023. Inhibition of focal adhesion kinase/paxillin axis by caffeic acid phenethyl ester restrains aggressive behaviors of head and neck squamous cell carcinoma in vitro. Arch Oral Biol 146:105611. doi: 10.1016/j.archoralbio.2022.105611 [DOI] [PubMed] [Google Scholar]
- 82. Tepedelen BE, Soya E, Korkmaz M. 2017. Epigallocatechin-3-gallate reduces the proliferation of benign prostatic hyperplasia cells via regulation of focal adhesions. Life Sci 191:74–81. doi: 10.1016/j.lfs.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 83. Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. 2003. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol 66:2281–2289. doi: 10.1016/j.bcp.2003.07.014 [DOI] [PubMed] [Google Scholar]
- 84. Zheng ZS, Xue GZ, Grunberger D, Prystowsky JH. 1995. Caffeic acid phenethyl ester inhibits proliferation of human keratinocytes and interferes with the EGF regulation of ornithine decarboxylase. Oncol Res 7:445–452. [PubMed] [Google Scholar]
- 85. Brownson DM, Azios NG, Fuqua BK, Dharmawardhane SF, Mabry TJ. 2002. Flavonoid effects relevant to cancer. J Nutr 132:3482S–3489S. doi: 10.1093/jn/132.11.3482S [DOI] [PubMed] [Google Scholar]
- 86. Chen PN, Chu SC, Kuo WH, Chou MY, Lin JK, Hsieh YS. 2011. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J Agric Food Chem 59:3836–3844. doi: 10.1021/jf1049408 [DOI] [PubMed] [Google Scholar]
- 87. Hung CF, Huang TF, Chiang HS, Wu WB. 2005. (-)-Epigallocatechin-3-gallate, a polyphenolic compound from green tea, inhibits fibroblast adhesion and migration through multiple mechanisms. J Cell Biochem 96:183–197. doi: 10.1002/jcb.20509 [DOI] [PubMed] [Google Scholar]
- 88. Bruce-Staskal PJ, Weidow CL, Gibson JJ, Bouton AH. 2002. Cas, Fak and Pyk2 function in diverse signaling cascades to promote Yersinia uptake. J Cell Sci 115:2689–2700. doi: 10.1242/jcs.115.13.2689 [DOI] [PubMed] [Google Scholar]
- 89. Alrutz MA, Isberg RR. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc Natl Acad Sci U S A 95:13658–13663. doi: 10.1073/pnas.95.23.13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Watarai M, Kamata Y, Kozaki S, Sasakawa C. 1997. Rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J Exp Med 185:281–292. doi: 10.1084/jem.185.2.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shi J, Casanova JE. 2006. Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and P130Cas. Mol Biol Cell 17:4698–4708. doi: 10.1091/mbc.e06-06-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Olgierd B, Kamila Ż, Anna B, Emilia M. 2021. The pluripotent activities of caffeic acid phenethyl ester. Molecules 26:1335. doi: 10.3390/molecules26051335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jubair N, R M, Fatima A, Mahdi YK, Abdullah NH. 2022. Evaluation of catechin synergistic and antibacterial efficacy on biofilm formation and acra gene expression of uropathogenic E. coli clinical isolates. Antibiotics (Basel) 11:1223. doi: 10.3390/antibiotics11091223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Subramanian M, Goswami M, Chakraborty S, Jawali N. 2014. Resveratrol induced inhibition of Escherichia coli proceeds via membrane oxidation and independent of diffusible reactive oxygen species generation. Redox Biol 2:865–872. doi: 10.1016/j.redox.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kaur A, Gabrani R, Dang S. 2019. Nanoemulsions of green tea catechins and other natural compounds for the treatment of urinary tract infection: antibacterial analysis. Adv Pharm Bull 9:401–408. doi: 10.15171/apb.2019.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Leu TH, Su SL, Chuang YC, Maa MC. 2003. Direct inhibitory effect of Curcumin on SRC and focal adhesion kinase activity. Biochemical Pharmacology 66:2323–2331. doi: 10.1016/j.bcp.2003.08.017 [DOI] [PubMed] [Google Scholar]
- 97. Hsu YL, Wu LY, Hou MF, Tsai EM, Lee JN, Liang HL, Jong YJ, Hung CH, Kuo PL. 2011. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res 55:318–327. doi: 10.1002/mnfr.201000148 [DOI] [PubMed] [Google Scholar]
- 98. Chikara S, Lindsey K, Borowicz P, Christofidou-Solomidou M, Reindl KM. 2017. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement Altern Med 17:30. doi: 10.1186/s12906-016-1512-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang Y, Han M, Sun X, Gao G, Yu G, Huang L, Chen Y. 2021. EGCG promotes neurite outgrowth through the Integrin β1/FAK/p38 signaling pathway after subarachnoid hemorrhage. Evid Based Complement Alternat Med 2021:8810414. doi: 10.1155/2021/8810414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Weyant MJ, Carothers AM, Dannenberg AJ, Bertagnolli MM. 2001. (+)-Catechin inhibits intestinal tumor formation and suppresses focal adhesion kinase activation in the min/+ mouse. Cancer Res 61:118–125. [PubMed] [Google Scholar]
- 101. Vollmerhausen TL, Ramos NL, Dzung DTN, Brauner A. 2013. Decoctions from Citrus reticulata Blanco seeds protect the uroepithelium against Escherichia coli invasion. J Ethnopharmacol 150:770–774. doi: 10.1016/j.jep.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 102. Mohanty S, Zambrana S, Dieulouard S, Kamolvit W, Nilsén V, Gonzales E, Östenson C-G, Brauner A. 2018. Amaranthus caudatus extract inhibits the invasion of E. coli into uroepithelial cells. J Ethnopharmacol 220:155–158. doi: 10.1016/j.jep.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 103. Mohanty S, Kamolvit W, Zambrana S, Sandström C, Gonzales E, Östenson C-G, Brauner A. 2017. Extract of Clinopodium bolivianum protects against E. coli invasion of uroepithelial cells. J Ethnopharmacol 198:214–220. doi: 10.1016/j.jep.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 104. Lüthje P, Dzung DN, Brauner A. 2011. Lactuca indica extract interferes with uroepithelial infection by Escherichia coli. J Ethnopharmacol 135:672–677. doi: 10.1016/j.jep.2011.03.069 [DOI] [PubMed] [Google Scholar]
- 105. Kumar D, Ladaniya MS, Gurjar M, Kumar S, Mendke S. 2021. Quantification of flavonoids, phenols and antioxidant potential from dropped Citrus reticulata Blanco fruits influenced by drying techniques. Molecules 26:4159. doi: 10.3390/molecules26144159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang H, Yang Y, Zhou Z. 2018. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J Integrative Agriculture 17:256–263. doi: 10.1016/S2095-3119(17)61664-2 [DOI] [Google Scholar]
- 107. Karamać M, Gai F, Longato E, Meineri G, Janiak MA, Amarowicz R, Peiretti PG. 2019. Antioxidant activity and phenolic composition of amaranth (Amaranthus caudatus) during plant growth. Antioxidants (Basel) 8:173. doi: 10.3390/antiox8060173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang H, Liang F-X, Kong X-P. 2008. Characteristics of the phagocytic cup induced by uropathogenic Escherichia coli. J Histochem Cytochem 56:597–604. doi: 10.1369/jhc.2008.950923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ferreira RS, Dos Santos NAG, Bernardes CP, Sisti FM, Amaral L, Fontana ACK, Dos Santos AC. 2019. Caffeic acid phenethyl ester (CAPE) protects PC12 cells against cisplatin-induced neurotoxicity by activating the AMPK/SIRT1, MAPK/Erk, and PI3k/Akt signaling pathways. Neurotox Res 36:175–192. doi: 10.1007/s12640-019-00042-w [DOI] [PubMed] [Google Scholar]
- 110. Yang N, Shi JJ, Wu FP, Li M, Zhang X, Li YP, Zhai S, Jia XL, Dang SS. 2017. Caffeic acid phenethyl ester up-regulates antioxidant levels in hepatic stellate cell line T6 via an Nrf2-mediated mitogen activated protein kinases pathway. World J Gastroenterol 23:1203–1214. doi: 10.3748/wjg.v23.i7.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alayev A, Doubleday PF, Berger SM, Ballif BA, Holz MK. 2014. Phosphoproteomics reveals resveratrol-dependent inhibition of Akt/mTORC1/S6K1 signaling. J Proteome Res 13:5734–5742. doi: 10.1021/pr500714a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang R, Lv Y, Miao L, Zhang H, Qu X, Chen J, Xu B, Yang B, Fu J, Tan C, Chen H, Wang X. 2021. Resveratrol attenuates meningitic Escherichia coli-mediated blood-brain barrier disruption. ACS Infect Dis 7:777–789. doi: 10.1021/acsinfecdis.0c00564 [DOI] [PubMed] [Google Scholar]
- 113. Chuu CP, Lin HP, Ciaccio MF, Kokontis JM, Hause RJ, Hiipakka RA, Liao S, Jones RB. 2012. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev Res (Phila) 5:788–797. doi: 10.1158/1940-6207.CAPR-12-0004-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L, Chopp M. 2009. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol 8:25–33. [PMC free article] [PubMed] [Google Scholar]
- 115. Shen X, Ren L, Teng Y, Zheng S, Yang X, Guo X, Wang X, Sha K, Li N, Xu G, Tian H, Wang X, Liu X, Li J, Huang N. 2014. Luteolin decreases the attachment, invasion and cytotoxicity of UPEC in bladder epithelial cells and inhibits UPEC biofilm formation. Food Chem Toxicol 72:204–211. doi: 10.1016/j.fct.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 116. Munguia J, Nizet V. 2017. Pharmacological targeting of the host-pathogen interaction: alternatives to classical antibiotics to combat drug-resistant superbugs. Trends Pharmacol Sci 38:473–488. doi: 10.1016/j.tips.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Varma DM, Zahid MSH, Bachelder EM, Ainslie KM. 2020. Formulation of host-targeted therapeutics against bacterial infections. Transl Res 220:98–113. doi: 10.1016/j.trsl.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. 2018. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov 17:35–56. doi: 10.1038/nrd.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gescher AJ, Steward WP. 2003. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev 12:953–957. [PubMed] [Google Scholar]
- 120. Feliciano RP, Mills CE, Istas G, Heiss C, Rodriguez-Mateos A. 2017. Absorption, metabolism and excretion of cranberry (poly)phenols in humans: a dose response study and assessment of inter-individual variability. Nutrients 9:268. doi: 10.3390/nu9030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Prior RL, Rogers TR, Khanal RC, Wilkes SE, Wu X, Howard LR. 2010. Urinary excretion of phenolic acids in rats fed cranberry. J Agric Food Chem 58:3940–3949. doi: 10.1021/jf9028392 [DOI] [PubMed] [Google Scholar]
- 122. Lambert JD, Sang S, Yang CS. 2007. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol 20:583–585. doi: 10.1021/tx7000515 [DOI] [PubMed] [Google Scholar]
- 123. Duda-Chodak A, Tarko T. 2023. Possible side effects of polyphenols and their interactions with medicines. Molecules 28:2536. doi: 10.3390/molecules28062536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mennen LI, Walker R, Bennetau-Pelissero C, Scalbert A. 2005. Risks and safety of polyphenol consumption. Am J Clin Nutr 81:326S–329S. doi: 10.1093/ajcn/81.1.326S [DOI] [PubMed] [Google Scholar]
- 125. Chen SL, Hung C-S, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103:5977–5982. doi: 10.1073/pnas.0600938103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Knodler LA, Finlay BB, Steele-Mortimer O. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem 280:9058–9064. doi: 10.1074/jbc.M412588200 [DOI] [PubMed] [Google Scholar]
- 127. Isberg RR, Voorhis DL, Falkow S. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769–778. doi: 10.1016/0092-8674(87)90335-7 [DOI] [PubMed] [Google Scholar]
- 128. Strober W. 2001. Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3:Appendix 3B [DOI] [PubMed] [Google Scholar]
- 129. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impact of the phenolic compounds and FAK14 on bacterial viability.