Abstract
Glioblastoma (GBM) is the most prevalent malignant tumor of the central nervous system. The prognosis of GBM is grim, with a median overall survival of 14.6 months and only 6.9% of patients surviving 5 years after the initial diagnosis. Despite poor outcomes, standard therapy of surgical resection, radiotherapy, chemotherapy, and tumor-treating fields has remained largely unchanged. The introduction of immune checkpoint inhibitors (ICI) has been a paradigm shift in oncology, with efficacy across a broad spectrum of cancer types. Nonetheless, investigations of ICIs in both newly diagnosed and recurrent GBM have thus far been disappointing. This lack of clinical benefit has been largely attributed to the highly immunosuppressive nature of GBM. However, immunotherapy still holds promise for the treatment of GBM, with combinatorial strategies offering hope for potentially overcoming these current limitations. In this review, we discuss the outcomes of clinical trials employing ICIs in patients with GBM. Afterward, we review ICI combination strategies and how these combinations may overcome the immunosuppressive microenvironment of GBM in the context of preclinical/clinical evidence and ongoing clinical trials.
Keywords: immunotherapy, combination therapy, ICI, vaccine therapy, oncolytic virotherapy
INTRODUCTION
Glioblastoma (GBM) is the most common malignant central nervous system (CNS) tumor, with an incidence of 3.26 cases per 100,000 individuals annually.[1] Treatment of newly diagnosed GBM involves maximal safe surgical resection followed by radiotherapy (RT) and temozolomide (TMZ) chemotherapy with or without tumor-treating fields (TTFields).[2] Epigenetic silencing of DNA-repair gene O6-methylguanine–DNA methyltransferase (MGMT) is associated with improved overall survival (OS) and increased benefit from chemotherapy with alkylating agent TMZ.[3] The addition of TTFields to adjuvant TMZ has also been associated with additional survival benefits.[4] Regardless, the prognosis of GBM remains grim, with a median OS of 14.6 months and only 6.9% of patients surviving 5 years after initial diagnosis.[1]
The introduction of immune checkpoint inhibitors (ICIs) has been a breakthrough in cancer therapy, with efficacy demonstrated across a variety of solid tumors.[5] Checkpoint-driven inhibitory pathways typically function as brakes for the adaptive immune system, dampening effector immune responses.[6] Cancer cells often use these pathways to evade the immune system. ICIs prevent the transduction of these inhibitory signals, allowing the immune system to mount an antitumor response.[7] ICIs have demonstrated efficacy for a variety of solid tumors, including melanoma, lung cancer, and renal cell carcinoma.[8–13]
Despite these great strides, ICI investigations in GBM to date have been disappointing. This article reviews the results of clinical trials using ICIs in patients with GBM and examines ongoing strategies for combining ICIs with other treatment modalities. Through this evaluation, we will discuss hypotheses for ICI failure and the future of this promising therapy area in GBM patients.
IMMUNOLOGIC PROPERTIES OF GLIOBLASTOMA
Historically, the CNS has been considered an immune-privileged environment, being immunologically isolated from the rest of the body. However, studies have demonstrated a functional lymphatic system running parallel to the dural venous sinuses, which permits immunologic access to the CNS.[14] The bridging of the systemic immune system and the CNS is further necessitated, given that there exist numerous immune-mediated CNS disorders.[15–17] Given the potential of the immune system to mount a response within the CNS, immunotherapy has been hypothesized as a promising treatment modality for brain tumors. However, the immune system of the CNS differs significantly from that of other sites. The sterile CNS environment is devoid of naïve lymphocytes, circulating monocytes, or dendritic cells.[18] Microglia serve as the primary resident immune cells in the CNS and are responsible for activating the innate immune system if necessary.[19] Moreover, when naïve lymphocytes gain access to the CNS, those primed against CNS antigens undergo anergy, favoring an immune-suppressed, proneuronal environment.[20,21] The successful development of immunotherapeutics for GBM requires generating a robust antitumor immune response while overcoming T-cell anergy and tolerance.
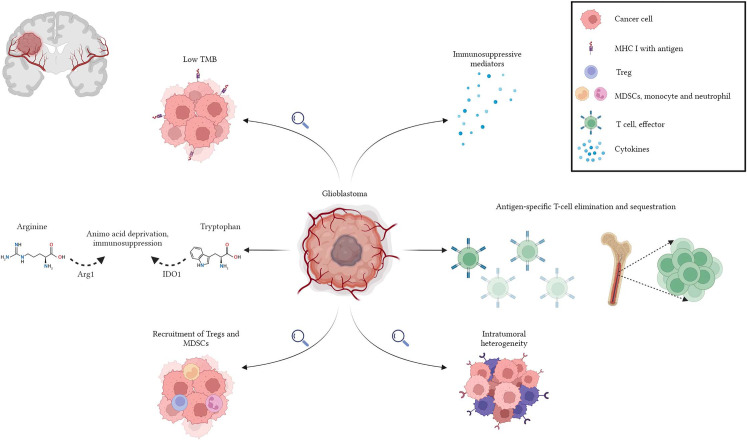
GBM is an immunologically cold tumor due to various factors that enhance its ability to evade the immune system.[22–25] GBM has a low tumor mutational burden (TMB), which reduces the number of possible neoantigens that the immune system can target.[26–28] GBM expresses the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO), which converts tryptophan to kynurenines.[29] Depleted tryptophan levels and the accumulation of immunomodulatory metabolites, kynurenines, have been shown to induce T-cell apoptosis, increase immunosuppressive programming, and death of tumor antigen-presenting dendritic cells.[30] Systemic T-cell lymphopenia is another characteristic finding in GBM patients. Lymphopenia is driven by the tumor-imposed loss of sphingosine-1-phosphate receptor 1 (S1P1) from the T-cell surface, which functions for T-cell trafficking, and its loss results in T-cell sequestration in the bone marrow.[31] This lymphopenia can be further augmented by RT and TMZ chemotherapy. Common adaptive resistance mechanisms shared by malignancies, such as the recruitment of T-regulatory cells (Tregs) and tumor-associated macrophages, are particularly pronounced in GBMs due to the complex interplay between tumor cells and their microenvironment.[32–34] GBM also generates high levels of soluble immunosuppressive mediators, such as TGF-β, interleukin-10 (IL-10), IL-7, and prostaglandin E2, suppressing effector T-cell activity.[35,36] Several subtypes (proneural, neural, classical, and mesenchymal) are defined to classify GBM to estimate its molecular and clinical characteristics.[37] Nevertheless, this effort is hindered by the presence of different molecular subtypes within the same tumor and the rapid outgrowth of resistant clones subsequent to the selective destruction of treatment-susceptible ones. This intratumoral heterogeneity and molecular plasticity of GBM represent another resistance mechanism.[38] Prospective immunotherapies for GBM must overcome these challenges (Fig. 1).
Figure 1.
Glioblastoma is an immunologically “cold” tumor due to several intrinsic and adaptive resistance mechanisms favoring immune evasion capacity.
IMMUNE CHECKPOINT INHIBITORS IN GLIOBLASTOMA
The most widely used checkpoint inhibitor targets include cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death 1 ligand (PD-L1).[39] CTLA-4 is expressed on both CD4+ and CD8+ T cells and engages with CD80 and CD86 on antigen-presenting cells. This engagement inhibits T-cell response and proliferation. Anti–CTLA-4 antibodies bind CTLA-4 and, in turn, prevent the engagement of CTLA-4 and CD80/CD86, thereby enhancing antitumor immunity.[40] Ipilimumab and tremelimumab are Food and Drug Administration–approved anti–CTLA-4 ICIs.[8,41]
PD-1 is another immune checkpoint and is expressed by activated T lymphocytes, natural killer cells, B lymphocytes, dendritic cells, and macrophages.[42] Its ligand, PD-L1, is commonly overexpressed by tumor cells and tumor-infiltrating leukocytes (TILs) as an adaptive resistance mechanism against antitumoral immunity.[43] As the PD1/PD-L1 axis inhibits T-cell activation, proliferation, survival, and cytotoxic secretion within the tumor microenvironment (TME), its inhibition through PD-1 or PD-L1 inhibitors is hypothesized to promote T-cell activation.[42] Currently approved PD-1 inhibitors include pembrolizumab, nivolumab, cemiplimab, and dostarlimab, whereas currently approved PD-L1 inhibitors are durvalumab, avelumab, and atezolizumab.[44]
Although CTLA-4 and PD/PD-L1 are the cornerstones of ICI treatment, additional agents and inhibitory pathways are currently being explored. These include antibodies targeting lymphocyte activation gene-3 (LAG-3 or CD223), killer inhibitory receptors, T-cell immunoglobulin and mucin-3 (TIM-3), T-cell ITIM Domain (TIGIT), and V-domain Ig suppressor of T-cell activation.[45–49]
Single Immune Checkpoint Inhibitor Administration
Several studies investigated ICI therapy in GBM for patients with newly diagnosed and recurrent tumors (Table 1).
Table 1.
Comparison of large-scale studies of anti PD-1 in glioblastoma
| Trial Name | Study Type | Drug Name | Newly Diagnosed or Recurrent | Treatment Arms | Study Endpoints | N | PFS, mo* (median, 95% CI) | OS, mo* (median, 95% CI) | ORR, %* (median, 95% CI) |
OS Based on PD-L1 Status, mo** (median) |
|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate 143[50] | Open-label, randomized, prospective, phase III | Nivolumab | Recurrent | Nivolumab 3 mg/kg every two weeks Bevacizumab 10 mg/kg every two weeks |
Primary end point: OS Secondary end points: OS rate at 12 months, PFS, ORR Exploratory end points: OS based on MGMT status and baseline corticosteroid use |
369 | 1.5, 1.5–1.6 3.5, 2.9–4.6 |
9.8, 8.2–11.8 10, 9.0–11.8 |
7.8%, 4.1–13.3 23.1%,16.7–30.5 |
- |
| CheckMate 498[52] | Open-label, randomized, prospective, phase III | Nivolumab | Newly diagnosed, unmethylated MGMT promoter | RT 60 Gy + nivolumab 240 mg every 2 weeks for eight cycles, then 480 mg every 4 weeks RT+ TMZ (Stupp protocol) |
Primary end point: OS Secondary end points: Investigator-assessed PFS, OS at 24 months Exploratory end points: OS based on tumor PD-L1 expression |
560 | 6, 5.7–6.2 6.2, 5.9–6.7 |
13.4, 12.6–14.3 14.9, 13.3–16.1 |
7.8%, 3.6–14.2 7.2%, 3.2–13.7 |
PD-L1 ≥ %1 12.6 PD-L1 < %1 13.8 |
| CheckMate 548[55] | Single-blinded, randomized, prospective, phase III | Nivolumab | Newly diagnosed, methylated MGMT promoter |
RT+ TMZ + nivolumab 240 mg every 2 weeks for eight cycles, then 480 mg every 4 weeks RT+ TMZ + placebo |
Primary end point: PFS by blinded independent central review, OS, OS for those w/o corticosteroid Secondary end points: OS rate at 12 and 24 mos, investigator-assessed PFS Exploratory end points: OS based on tumor PD-L1 expression |
716 | 10.6, 8.9–11.8 10.3, 9.7–12.5 |
28.9, 24.4–31.6 32.1, 29.4–33.8 |
- | PD-L1 ≥ %1 29.8 PD-L1 < %1 28.7 PD-L1 ≥ %5 29.2 PD-L1 < %5 28.9 |
*The upper lines of each cell correspond to the nivolumab arms.
**OS based on PD-L1 status are demonstrated for the nivolumab arms.
ORR, overall response rate; PFS, progression-free survival; OS, overall survival; MGMT, O6-methylguanine–DNA methyltransferase; PD-1, programmed death 1; PD-L1, programmed cell death 1 ligand; RT, radiotherapy TMZ, temozolomide; w/o, without, CI, confidence interval.
CheckMate 143
The efficacy of anti–PD-1 ICI nivolumab compared with anti-VEGF monoclonal antibody bevacizumab in patients with first recurrence of GBM was explored in CheckMate 143.[50] Patients were randomized in 1:1 fashion to treatment with nivolumab or bevacizumab. Of patients, 369 were randomized, and 182 patients in the nivolumab arm and 165 in the bevacizumab arm received allocated treatment. There was no statistical difference in the risk of death (hazard ratio [HR], 1.04; 95% CI, 0.83–1.30; p = 0.76) or median OS (mOS), 9.8 months (95% CI, 8.2–11.8) with nivolumab versus 10 months (95% CI, 9.0–11.8) with bevacizumab (p = 0.76). Therefore, the study did not meet the primary endpoint of improved OS with nivolumab compared with bevacizumab. Further, progression-free survival (PFS) and objective response rate (ORR) favored bevacizumab. Median PFS was 1.5 months (95% CI, 1.5–1.6) with nivolumab and 3.5 months (95% CI, 2.9–4.6) with bevacizumab (HR, 1.97; 95% CI, 1.57–2.48; p < 0.001). ORR in evaluable patients in the nivolumab group (n = 153) and bevacizumab group (n = 156) were 7.8% (95% CI, 4.1–13.3) and 23.1% (95% CI, 16.7–30.5), respectively. However, caution must be taken when interpreting PFS and ORR results of the study. PFS was assessed using response assessment for neuro-oncology criteria, which does not account for the possibility of immunotherapy-related pseudoprogression. Immunotherapy response assessment for neuro-oncology was later developed for this purpose.[51] In addition, bevacizumab therapy can create pseudoresponses due to transient reduction in enhancement and cerebral edema, skewing ORR data in favor of the antiangiogenic without associated OS benefit.[51]
Of note, in an exploratory post hoc subgroup analysis, patients with MGMT methylated tumors with no baseline corticosteroid use receiving nivolumab had 17.0-months mOS compared with 10.1-months mOS observed for patients with similar tumors treated with bevacizumab.[50] This finding suggested that a subset of patients may benefit from checkpoint inhibitor monotherapy despite overall negative trial results.
CheckMate 498
The CheckMate 498 trial compared the efficacy of nivolumab and RT with conventional chemoradiotherapy (TMZ + RT) in patients with newly diagnosed MGMT unmethylated GBM.[52] Patients were randomized 1:1 to receive nivolumab + RT or TMZ + RT. Tumor-sample sections were also retrospectively assessed for PD-L1 expression. Of patients, 560 (280 patients in each arm) with newly diagnosed MGMT unmethylated GBM were randomized. Of 560 patients, 278 in the nivolumab arm and 275 in the TMZ arm received allocated treatment. The mOS was 13.4 months (95% CI, 12.6–14.3) in the nivolumab arm and 14.9 (95% CI, 13.3–16.1) months in the TMZ arm (p = 0.0037). The primary endpoint was not met, as TMZ + RT was associated with superior mOS compared with nivolumab + RT. Therefore, the study indicated that immunotherapy with nivolumab is not a suitable replacement for chemotherapy with TMZ for patients with MGMT unmethylated GBM. The 24-month OS rates were 10.3% (95% CI, 6.8–4.6) in the nivolumab arm and 21.2% (95% CI, 16.4–26.5) in the TMZ arm. The 24-month OS rates were 10.3% in the RT + nivolumab arm and 21.2% in the RT + TMZ. Hegi et al[53] found that the 24-month OS rates for patients with MGMT unmethylated GBM were less than 2% for the RT-only arm and 13.8% for the RT + TMZ arm. Therefore, an increase in 24-month OS rates since 2005 could be attributed to increased second-line treatment options in GBM. Moreover, patients seem to derive benefit from TMZ even with MGMT unmethylated tumors. Median PFS was 6.0 months (95% CI, 5.7–6.2) in nivolumab arm versus 6.2 months (95% CI, 5.9–6.7) in TMZ arm. The 12-month PFS rate was 5.7% (95% CI, 3.2–9.1) in the nivolumab arm and 17.7% (95% CI, 13.3–22.7) in the TMZ arm. ORR was 7.8% (9/116; 95% CI, 3.6–14.2) in nivolumab arm and 7.2% (8/111; 95% CI, 3.2–13.7) in TMZ arm. Although the 6-month PFS rates were similar between arms, the 12-month PFS rates were 5.7% in nivolumab arm and 17.7% in TMZ arm. Considering response assessment for neuro-oncology criteria were applied to evaluate the PFS for CheckMate 498, the potential for misinterpretation of pseudoprogression linked to immunotherapy is present regarding PFS data. Among patients with baseline PD-L1 expression greater than or equal to 1%, mOS was 12.6 months (n = 104; 95% CI, 11.3–14.2) in the nivolumab arm and 15.5 months (n = 125; 95% CI, 13.2–17.2) in the TMZ arm. The mOS in patients with PD-L1 less than 1% was 13.8 months (n = 171; 95% CI, 13.0–14.6) in the nivolumab arm and 14.7 months (n = 155; 95% CI, 12.6–16.0) in the TMZ arm. Therefore, PD-L1 expression status did not predict survival benefit with nivolumab. This result highlights the need for better strategies to overcome the mechanisms of immune evasion in GBM.[28,31,54]
CheckMate 548
CheckMate 548 evaluated the efficacy of nivolumab in combination with standard-of-care RT and TMZ in patients with newly diagnosed MGMT methylated or indeterminate GBM.[55] Patients were randomly assigned (1:1) to the following two treatment arms: RT + TMZ in combination with nivolumab or RT + TMZ with placebo. PD-L1 expression status was evaluated with two different cut-off values, those being expressions greater than or equal to 1% and greater than or equal to 5%. Of patients, 716 were randomized, and 709 received allocated treatment. Of 709 patients, 355 were in the nivolumab arm, and 354 were in the placebo arm. Median PFS was similar between arms; the mPFS of the nivolumab arm was 10.6 months (95% CI, 8.9–11.8) compared with 10.3 months (95% CI, 9.7–12.5) with the placebo arm per blinded independent central review. The mPFS was 14.1 months for the nivolumab arm (95% CI, 12.6–16.6) compared with 15.2 months for the placebo arm (95% CI, 13.1–17.1) per investigator assessment. Among all patients, the mOS was 28.9 months (95% CI, 24.4–31.6) in the nivolumab arm and 32.1 months (95% CI, 29.4–33.8) in the placebo arm. Among patients without baseline corticosteroid use, the mOS was 33.0 months (95% CI, 31.0–35.1) in the nivolumab arm and 31.3 months (HR, 1.1; 95% CI, 0.9-1.4) in the placebo arm. CheckMate 548 did not meet its primary or secondary endpoints, as improved PFS or OS was not observed in the overall patient population or the population without baseline corticosteroid use.
The 12-month OS rates in all patients were 82.7% (95% CI, 78.3–86.3) in the nivolumab arm and 87.7% (95% CI, 83.8–90.8) in the placebo arm. The 24-month OS rates were 55.9% (95% CI, 50.5–61.0) in the nivolumab arm and 63.3% (95% CI, 58.0–68.2) in the placebo arm. Among patients without baseline corticosteroid use, the 12-month OS rates were 85.5% (95% CI, 80.4–89.4) in the nivolumab arm and 89.9% (95% CI, 85.5–93.0) in the placebo arm. The 24-month OS rates were 60.9% (95% CI, 54.4–66.8) and 67.1% (95% CI, 61.0–72.6%), respectively.
The mOS was 29.8 months (95% CI, 23.3–34.6) in the nivolumab arm and 31.0 months (95% CI, 26.5–34.5) in the placebo arm for patients with PD-L1 greater than equal to 1%, compared with 29.2 months (95% CI, 21.8–42.9) in the nivolumab arm and 31.8 months (95% CI, 28.8–33.8) in the placebo arm for patients with PD-L1 greater than or equal to 5%. PD-L1 expression status was not associated with benefit from nivolumab therapy in CheckMate 498 and CheckMate 548 studies. A greater than or equal to 1% PD-L1 expression threshold was used in CheckMate 498, whereas greater than or equal to 1% and 5% PD-L1 expression thresholds were used in CheckMate 548. It is conceivable that the cut-offs used in these trials do not adequately identify potential responders.[56] While PD-L1 expression has been associated with responsiveness to PD-1 inhibition, this is inconsistent. According to studies involving solid malignancies, such as melanoma, renal cell carcinoma, bladder carcinoma, and lung carcinoma, PD-L1 positivity is associated with a higher ORR.[57] However, a significant proportion of PD-L1–negative patients still derive benefit from PD-1 pathway blockade.[57,58] PD-L1 expression level alone may not sufficiently predict response to ICI in GBM.
Given negative results from large-scale clinical trials in newly diagnosed and recurrent GBM with nivolumab, administration of anti-PD1 alone or in combination with standard-of-care chemoradiation is unlikely to change patient outcomes. Novel strategies and more nuanced approaches are needed.
Neoadjuvant Administration
Neoadjuvant administration of ICI for melanoma has demonstrated enhanced T-cell response as well as clinical benefit in several phase II studies.[59–61] Neoadjuvant ICI has also been evaluated in the setting of recurrent GBM (rGBM) in several clinical trials. In the study conducted by Cloughesy et al,[62] patients were randomized to either with pembrolizumab 14 ± 5 days before resection (n = 19), then continued immunotherapy or to start immunotherapy after resection (n = 16). Patients in the neoadjuvant arm had significantly extended mOS compared with patients who randomized to receive adjuvant pembrolizumab (mOS 417 vs 228.5 days, respectively; p = 0.04).[62] Also, focal upregulation of PD-L1 expression in the TME and decreased PD-1 expression on peripheral blood T cells were observed more frequently in the neoadjuvant pembrolizumab group, suggesting neoadjuvant administration of PD-1 blockade augments local and systemic antitumor immune responses.[62]
Schalper et al[63] investigated the neoadjuvant administration of ICI in patients with GBM through a phase II single-arm clinical trial involving 30 patients (27 with rGBM and 3 newly diagnosed patients). Here, patients received a single preoperative dose of nivolumab followed by postoperative ICI. The mPFS was 4.1 months, whereas mOS was 7.3 months for the 29 patients comprising the study cohort. However, investigators compared pre- and postnivolumab tissue specimens of those 27 rGBM patients and observed a higher immune cell infiltration and augmented T-cell receptor clonal diversity among TILs, supporting a local immunomodulatory effect of nivolumab.[63] Owing to the limited number of participants and the absence of a comparator arm, caution should be applied when interpreting these results. Together, both studies demonstrated enhanced local immunomodulatory effects on tumor samples related to neoadjuvant administration of ICIs.[62,63]
In the study by Groot et al,[64] with a cohort of 15 operable rGBM patients, five received two doses of neoadjuvant pembrolizumab in a single-arm phase II clinical trial. Here, mOS was 20 months and mPFS was 4.5 months for the study cohort. Of note, there was no increase in the number of CD8+ T cells in the TME after pembrolizumab treatment. Additionally, there was a substantial infiltration of immunosuppressive CD68+ macrophages. In contrast to the aforementioned two studies, the findings of this study indicate that pembrolizumab monotherapy failed to induce a robust immune response against tumors.
In another study, 27 patients underwent neoadjuvant nivolumab administration 24 hours before the surgery, followed by intraoperative ipilimumab ± nivolumab injection in the brain tissue lining the resection cavity and received adjuvant nivolumab cycles.[65] The mPFS was 11.7 weeks, and mOS was 38 weeks (95% CI, 27–49, p < 0.003), with a 6-month, 1-year, and 2-year OS rate of 74.1% (95% CI, 57–90), 40.7% (95% CI, 22–59), and 27% (95% CI, 9–44), respectively. Although a tendency toward superior OS in patients with the longest survival and improved 1-, 2-, and 3-year survival estimates were highlighted, a comparison of the study population was performed with a historic cohort.[65] Therefore, further clinical trials with expanded patient numbers to pursue neoadjuvant combinations of ICIs in GBM are needed. Selected clinical trials examining neoadjuvant ICIs in GBM are listed in Supplemental Table S1.
Combination with Radiotherapy
RT has been historically viewed as an immunosuppressive modality, as treatment regimens with larger irradiation fields and higher radiation doses almost invariably cause cytopenia.[66] However, an additional phenomenon called the abscopal effect was also observed, where, following tumor irradiation, patients would also demonstrate regression of nonirradiated tumor metastases. This led to the hypothesis that localized radiation may be able to trigger systemic antitumoral immunity.[67] The abscopal effect is believed to arise from a host of intratumoral changes, which may contribute to increased tumor sensitivity to immune-mediated clearance. These changes include “immunogenic cell death” by releasing damage-associated molecular patterns, cytokine upregulation, increasing major histocompatibility complex class I expression on tumor cells, and augmenting antigen presentation.[68–71]
As a relatively old and rare entity defined decades ago, the abscopal effect gained attention in the era of ICIs. In the study conducted by Zeng et al,[72] the combination of PD-1 blockade and stereotactic radiosurgery resulted in long-term survival in a mouse orthotopic glioblastoma model. Belcaid et al[73] further demonstrated that the combination of CTLA-4 blockade and T-cell costimulatory receptor 4-1BB activation with focal RT improved survival and TIL density in an orthotopic mouse model of glioma. However, the aforementioned CheckMate studies included six weeks of RT combined with nivolumab therapy every 2 weeks, yet did not demonstrate efficacy.[52,55] This lack of efficacy despite preclinical achievements in murine models might be explained by differences in irradiation, dosing, or physiological differences between animal models and human patients.[74]
There is clinical evidence for benefit when combining ICIs with RT for tumors other than GBM, although timing remains a controversial topic. For example, in a retrospective analysis of patients with brain metastases, the combination of stereotactic radiosurgery and ICIs was associated with enhanced efficacy, particularly when administered concurrently.[75] However, in a prospective study, melanoma patients with brain metastasis had better responses and clinical outcomes with a sequential combination, specifically RT followed by ICI.[76] Field size, number of treatment fractions, dose per fraction, and timing are the variables considered most likely to influence efficacy.[77,78] Accordingly, these variables must be prospectively examined in GBM patients. Currently, clinical trials NCT037436626 and NCT049773757 are evaluating ICI before irradiation, NCT054232108 concurrent administration, and NCT028667479 irradiation before ICI in patients with GBM (Supplemental Table S1).[79–82]
Multiple Immune Checkpoint Inhibitors in Glioblastoma
The immune system possesses several checkpoint pathways, which play distinct roles within discrete cell types and locations. For example, PD-1 is expressed on mature T cells within the TME, PD-L1 is expressed on antigen-presenting cells, and CTLA-4 is typically expressed on T cells present in the lymph nodes.[7] Considering this differential checkpoint expression, as well as primary and acquired resistance to ICIs, combination therapy targeting multiple checkpoint pathways is a promising strategy to improve treatment efficacy.[83] This hypothesis has been demonstrated to improve survival for melanoma patients who were treated with both CTLA-4 and PD-1 inhibitors as compared with ICI monotherapy. This breakthrough finding has subsequently paved the way for using ICI combinations in other cancer types.[9,84]
Ipilimumab was combined with nivolumab in clinical trial NCT04396860 for newly diagnosed MGMT unmethylated GBM patients; however, this study did not meet the predetermined protocol-specified phase II primary endpoint and was closed without proceeding to the phase III portion.[85] Given the ineffectiveness of nivolumab in GBM patients and the fact that dual CTLA-4 and PD-1 inhibition is efficacious in tumors already responsive to ICI monotherapy, this result may not be unexpected.[86]
This combination is currently being tested in GBM patients with the additional strategy of targeting patients with high TMB. A higher TMB correlates with increased ICI responsiveness across many cancer types as a result of an increased quantity of tumoral neoantigens that the immune system can target.[87] It has been reported that this correlation between increased TMB and enhanced survival with ICI does not exist in GBM.[87] On the contrary, in rGBM patients, a very low TMB is associated with markedly higher inflammation and prolonged survival after ICI.[88] Although GBM generally has a low TMB, two main patient populations, those with de novo mutations in DNA polymerase and/or mismatch repair defects and those with posttreatment mutations after administration of RT and TMZ, have been shown to have higher TMB.[89] Considering that de novo mutations in DNA polymerase and/or mismatch repair defects are very rare in GBM, a higher TMB in GBM patients reflects prior exposure to the alkylating agent TMZ and RT, which can promote the expansion of less immunogenic subclonal mutations. Currently, two clinical trials are examining ICI effectiveness in patients with rGBM and high TMB (NCT02658279, with ipilimumab, and NCT04145115, with the combination of ipilimumab and nivolumab).[90,91]
T-cell exhaustion refers to a progressive loss of effector functions within a previously activated T cell.[6] Exhaustion develops because of chronic antigenic stimulation, negative costimulatory signaling, and exposure to chronic inflammation.[92] Multiple checkpoint molecule expression on TILs is related to a more exhausted phenotype. For example, PD-1+ Tim-3+ and PD-1+ Lag-3+ TILs exhibit more severe dysfunction compared with TILs expressing only PD-1 or neither receptor.[93] Exhaustion signature of TILs in GBM is severe due to the highly expressed checkpoint molecules PD-1, TIGIT, Tim-3, and Lag-3.[94] Also, the upregulation of checkpoint molecules other than PD-1, such as TIM-3 and LAG-3, has been conferred as a resistance mechanism to classical checkpoint blockade with anti-PD1 and PDL-1.[95] Therefore, simultaneous inhibition of several checkpoints with PD-1 seems to be a promising strategy in GBMs. Currently, several early-phase clinical trials are testing whether combined inhibition of several checkpoint targets with anti-PD1 will lead to survival benefit (Table S1).
Combination with Laser Interstitial Thermal Therapy
Laser interstitial thermal therapy (LITT) is a minimally invasive surgical treatment modality that uses precisely directed light energy to induce tissue hyperthermia and apoptosis.[96] Supraphysiological hyperthermia generated by LITT enhances antitumor immunogenicity by releasing intracellular tumoral components upon cellular destruction, including DNA, RNA, heat shock proteins, and tumoral antigens.[97] Also, blood–brain barrier disruption due to LITT improves the trafficking of both tumoral components and immune cells, contributing to antitumor immunogenicity.[97] LITT holds a further advantage as this therapy is less dependent on corticosteroids, which are tapered within days after the procedure.[97,98] Therefore, LITT is proposed to be an optimal candidate for combination with ICIs. According to the preliminary results of phase I clinical trial NCT02311582, the combination of pembrolizumab and LITT in patients with rGBM (n = 7) or anaplastic astrocytoma (n = 2) has been demonstrated to be safe.[99] Also, a case series of three patients with rGBM using LITT combined with pembrolizumab showed promising results with a PFS of 33, 12, and 7 months and an OS of 12 and 40 months, with the third patient still alive at the time of the study’s end, greater than 29 months at data cut-off.[100] Ongoing clinical trials combining LITT and ICI for patients with rGBM will provide further information (Table S1).
Combination with Small Molecule Inhibitors
IDO is an enzyme responsible for tryptophan catabolism and has been shown to modulate T-cell behavior.[29,30] Its activity is associated with the recruitment of immunosuppressive Tregs, whereas its deficiency is associated with increased antitumor T-cell activity. This has been confirmed to be true in GBM as well.[30,101] The IDO1 inhibitor, epacadostat, was examined in a phase II study for patients with rGBM (NCT03532295).[102] Cohort A, the arm without epacadostat, received retifanlimab, an anti–PD-1 monoclonal antibody, bevacizumab, and hypofractionated RT and reached its primary endpoint with an OS at 9 months of 71.4% (95%CI, 46.7–86.1), along with OS of 12.2 months (95%CI, 7.3–not reached) and PFS of 9.9 months (95%CI, 5.5–not reached). As of July 2023, cohort B, which adds epacadostat to the regimen, is enrolling.[102] In addition, the safety of nivolumab and BMS-986205, another IDO1 inhibitor, has been demonstrated in newly diagnosed GBM patients, and a phase II/III trial is being planned.[103]
Poly(ADP-ribose) polymerase (PARP) is a member of the PARP enzyme family, and it contributes to DNA repair and the maintenance of genomic stability upon binding to single-stranded DNA breaks.[104] Olaparib, a PARP inhibitor, inhibits DNA repair pathways and results in genomic instability, increased TMB and immunogenicity, and an increase in the number of TILs, all of which may contribute to the efficacy of ICIs.[105] Currently, a clinical trial combining pembrolizumab with olaparib is testing these effects on rGBM patients.[106]
Histone deacetylases are responsible for the posttranslational modification of chromatin histones, cell cycle progression, cell survival, and differentiation.[107] Their inhibition is associated with the inhibition of cell proliferation, tumorolysis, and the induction of antitumor immune response, making them a promising option for improving ICI efficacy in GBM.[108] A clinical trial combining pembrolizumab with vorinostat, a histone deacetylases inhibitor, is ongoing.[109]
Ongoing clinical trials combining ICIs with several other small molecule inhibitors are listed in Table S1.
Combination with Cytokine Therapy
Cytokines and chemokines play a critical role in GBM, as the immunosuppressive TME promotes the expression of suppressive mediators, which contributes to the immune tolerance of the tumor.[110] Clinical trials target these immune mediators to reverse and, potentially, break the tumor immune tolerance they engender. One such cytokine is IL-7, which has critical roles in B-cell maturation as well as proliferation, maturation, and survival of T cells.[111] The progressive suppression of the IL-7 receptor-mediated pathway is related to immune evasion in GBM.[112] Efineptakin alpha, a long-acting recombinant human IL-7, was demonstrated to be associated with increased survival in combination with RT and TMZ in a mouse glioma model.[113] This survival benefit was related to the reversal of iatrogenic lymphopenia induced by RT and TMZ because of IL-7–driven lymphocyte expansion, increased cytotoxic CD8 T cells, and decreased Tregs in the TME.[113] Early results of the clinical trial NCT03687957 demonstrated that efineptakin alpha is safe in glioma patients and increases absolute lymphocyte counts in a dose-dependent manner.[114] As of July 2023, a clinical trial combining pembrolizumab with efineptakin alpha in rGBM patients is ongoing (NCT05465954).[115]
Combination With Tumor Treating Fields
TTFields use alternating electric fields and interfere with mitosis. Through its electromagnetic power, which is absorbed by the mitotic furrow, TTFields reduce the proliferation of different glioma cell lines in a field strength- and frequency-dependent manner.[116] Also, TTFields induce immunogenic cell death similar to RT, leading to the activation of antitumor adaptive immunity, which makes TTFields a candidate for ICI combination to exert a synergistic effect.[117] The combination of TTFields, pembrolizumab, and TMZ has been evaluated in newly diagnosed GBM patients.[118] Twenty-six patients were enrolled in this phase II, single-arm, nonrandomized trial, and results were encouraging, as mPFS was 12.1 months whereas mOS was 25.2 months for 26 patients in the study, compared with 7.9 months and 15.9 months for matched controls, respectively.[118] While these results are promising, larger prospective and randomized trials are warranted.
Combination with Oncolytic Virotherapies
Another emerging strategy in the immunotherapy of GBM is the combination of oncolytic virotherapies with ICIs. Oncolytic virotherapies mediate antitumor activity through two distinct mechanisms. First, oncolytic viruses selectively infect and replicate within the tumor cells and result in the tumor cell lysis.[119] Second, tumor cell lysis results in the release of a wide range of tumor-associated antigens and damage-associated molecular patterns and enhances immune cell infiltration and TME remodeling.[120] Oncolytic virotherapy with adenovirus vector in mouse GBM models demonstrated upregulated expression of PD-1 and PD-L1 in tumor specimens and increased tumor-infiltrating CD8+ T cells after the treatment.[121] Combining controlled IL-12 gene therapy by use of an adenoviral vector Ad-RTS-hIL-12 with nivolumab was demonstrated to be well-tolerated.[122] As of July 2023, a phase II clinical trial of PD-1 inhibitor cemiplimab in combination with velemidex-controlled IL-12 gene therapy is ongoing (NCT04006119).[123] The comprehensive review of oncolytic virotherapies for GBM can be accessed for further reading.[124] Selected clinical trials are also listed in Table S1.
Combination with Vaccine-Based Therapies
Vaccine-based therapies aim to stimulate antitumor activity by exposing T cells to tumor-associated antigens.[125] Vaccines that target neoantigens include peptide and DNA vaccines, as well as dendritic cell-based (DC) cellular vaccines.[126] In addition, personalized vaccines are being investigated as a potential treatment for GBM by profiling the mutations of an individual’s tumor and eliciting T-cell immunity against multiple targets.[127] However, the high inter- and intratumoral heterogeneity of GBM, as well as the antigen escape phenomenon, loss, or downregulation of the target antigen, impede efficiency.[128] The ability of immune checkpoint blockade to facilitate the clonal expansion and maintenance of activity of neoantigen-specific T cells, which are stimulated by the vaccine, offers hope for a potential synergy between the vaccine and ICIs.[129,130] This synergy has been demonstrated in murine GBM models as ICI combined with the vaccine was related to survival benefit and enhanced immunity.[129,130]
Multiple DC vaccines have been developed to treat GBM, with several early-phase studies demonstrating their safety and potential efficacy.[131–133] Of these, DCVax-L uses an autologous tumor lysate. In a phase III nonrandomized trial involving 331 GBM patients, patients treated with DC vaccine had mOS of 19.3 months compared with 16.5 months in an external control group for newly diagnosed GBM (p = 0.002).[134] Among patients with rGBM, mOS for the DCVax-L group was 13.2 months compared with 7.8 months in the control group (p < 0.001). While these results are promising, the study has significant limitations due to the use of external controls without individual patient-level data. In addition, the primary endpoint of the study was changed from the initial design due to the high incidence of pseudoprogression reported by the authors.[134] Nevertheless, these promising results merit further investigation. The combination of ICI with DC-activated T-cell vaccines is one strategy that may further improve therapeutic efficacy.
Another vaccination strategy involves the development of personalized neoantigen-based vaccines informed by sequencing data from individual tumors. In prior studies, personalized vaccines have been shown to elicit polyfunctional neoantigen-specific CD4+ and CD8+ T-cell responses.[126,135] Efficacy of these approaches may be augmented by the addition of ICI. A phase I clinical trial combining a personalized neoantigens vaccine with pembrolizumab is currently ongoing, with preliminary results indicating an increase in effector T-cell function against the targets.[136] Selected clinical trials are listed in Table S1. In addition, a comprehensive review of vaccine therapies for GBM is available for further reading.[137]
Combination with Chimeric Antigen Receptor T-Cell Therapies
Chimeric antigen receptors (CARs) are synthetic receptors designed to direct T cells to recognize and eliminate cells expressing a specific target antigen. In CAR T-cell therapy, T lymphocytes collected from patients are modified to express a CAR, allowed to proliferate, and administered back to the patient to elicit a durable tumor-specific immune response.[138] Multiple CAR T-cell products have been studied in GBM and other high-grade gliomas. As an example, disialoganglioside GD2 is highly expressed in diffuse midline glioma, H3 K27-altered, which is a CNS World Health Organization grade 4 tumor.[139,140] In a prior phase I study, anti-GD2 CAR T-cell therapy was associated with three radiographic responses. However, to date, there have been no large-scale clinical trials demonstrating the efficacy of CAR T-cell therapy in GBM. This is in part due to limited persistence and low proliferation of effector immune cells in the TME, tumor heterogeneity, in which the target antigen may not be present on all tumor cells, CAR T-cell exhaustion, and the antigen escape phenomenon.[44,138] Combining ICIs with CAR T-cell therapies may overcome these limitations. In preclinical models of GBM, blocking PD1 immunosuppression was shown to enhance the activation of CAR T cells.[141] Currently, the clinical trial NCT04003649 is investigating whether IL13Ra2 CAR T cells are more effective alone or in combination with nivolumab and ipilimumab for treating recurrent and refractory GBM.[142] Similarly, clinical trial NCT03726515 investigates the combination of EGFRvIII CAR T cells and pembrolizumab.[143]
FUTURE DIRECTIONS
To date, ICI monotherapy has not shown efficacy for the treatment of newly diagnosed or recurrent GBM. The highly immunosuppressive nature of GBM due to a multitude of mechanisms, including the release of immunosuppressive cytokines, elimination of antigen-specific T cells, T-cell sequestration in the bone marrow, recruitment of regulatory T cells, abundance of MDSCs, and T-cell exhaustion collectively contribute to the inefficacy of ICI therapy in GBM. Low TMB, small number of neoantigens to elicit durable T-cell responses, tumor heterogeneity, and antigen escape also contribute to the limited efficacy of immunotherapy in GBM.[144]
It may be possible to overcome these limitations with combinatorial strategies targeting these mechanisms concurrently.[145] These strategies include enhancing T-cell response by neoadjuvant administering ICIs; activating antitumor adaptive immunity via RT, LITT, or TTFields-induced immunologic cell death; reversing the exhaustion signature of TILs by simultaneously inhibiting multiple checkpoints; reversing tumoral immune tolerance through cytokine therapies; facilitating tumor cell lysis and TME remodeling via oncolytic virotherapy; stimulating antitumor activity by exposing T cells to tumor-associated antigens via vaccine-based therapies or CAR T-cell therapies. ICIs are hypothesized to complement the antitumor immunity achieved through the aforementioned strategies by enhancing T-cell functions.
The unique challenges of GBM regarding trial design and interpretation in the context of immune-oncology need to be addressed to achieve success.[146] The efficacy of treatment modalities is limited by the anatomic location and the existence of the blood–brain barrier. Additionally, owing to the rarity of GBM, large randomized studies are frequently difficult to conduct. A better understanding of how discrete immunotherapies influence the microenvironment of GBM and its immunosurveillance mechanisms is mandatory. Moreover, improved methods for detecting disease progression in the context of immunotherapy are warranted, as indicated by the CheckMate 143 and CheckMate 498 studies. Advanced neuroimaging techniques, as well as detection and quantification of tumoral content through liquid biopsy, might be helpful for this purpose.[147] Last, conventional approaches to trial design and interpretation might not be compatible with immunotherapy. Therefore, to ascertain the efficacy of immunotherapy, it is necessary to redefine the time points for evaluation, establish clear enrollment criteria that include an immunologic baseline, and conduct endpoint analyses correlating with the immune response.
CONCLUSION
Despite limited successes to date with ICI monotherapy, immunotherapy still holds promise for the treatment of GBM. Individualized combination therapies may ultimately transform GBM care and improve patient outcomes. ICIs possess the potential to serve as the critical elements of these combinations.
Supplementary Material
Footnotes
Sources of Support: Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS); Conflict of Interest: None.
References
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5): v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352: 987–996. [DOI] [PubMed] [Google Scholar]
- 3. Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4: 296–307. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318: 2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18: 899–900. [DOI] [PubMed] [Google Scholar]
- 6. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18: 153–167. [DOI] [PubMed] [Google Scholar]
- 7. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381: 2020–2031. [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 12. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 14. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47: 707–717. [DOI] [PubMed] [Google Scholar]
- 16. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 17. Canessa A, Del Bono V, Miletich F, Pistoia V. Serum cytokines in toxoplasmosis: increased levels of interferon- in immunocompetent patients with lymphadenopathy but not in aids patients with encephalitis. J Infect Dis. 1992;165: 1168–1170. [DOI] [PubMed] [Google Scholar]
- 18. Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res. 2014;20: 3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176: 6802–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brabb T, von Dassow P, Ordonez N, et al. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Na SY, Hermann A, Sanchez-Ruiz M, et al. Oligodendrocytes enforce immune tolerance of the uninfected brain by purging the peripheral repertoire of autoreactive CD8+ T Cells. Immunity. 2012;37: 134–146. [DOI] [PubMed] [Google Scholar]
- 22. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22: 1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110: 2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19: 4951–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 26. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13: 84–88. [DOI] [PubMed] [Google Scholar]
- 27. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19: 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platten M, Nollen EAA, Röhrig UF, et al. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18: 379–401. [DOI] [PubMed] [Google Scholar]
- 30. Zhai L, Bell A, Ladomersky E, et al. Tumor cell IDO enhances immune suppression and decreases survival independent of tryptophan metabolism in glioblastoma. Clin Cancer Res. 2021;27: 6514–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dapash M, Hou D, Castro B, et al. The interplay between glioblastoma and its microenvironment. Cells. 2021;10: 2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished cd4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66: 3294–3302. [DOI] [PubMed] [Google Scholar]
- 34. Mirghorbani M, Van Gool S, Rezaei N. Myeloid-derived suppressor cells in glioma. Exp Rev Neurother. 2013;13: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 35. Tran TT, Uhl M, Ma JY, et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Cancer Genome Atlas Research Network . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- 41. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39: 2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10: 727–742. [PMC free article] [PubMed] [Google Scholar]
- 43. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439: 682–687. [DOI] [PubMed] [Google Scholar]
- 44. Sener U, Ruff MW, Campian JL. Immunotherapy in glioblastoma: current approaches and future perspectives. IJMS. 2022;23: 7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. In: Dranoff G, Ed. Cancer Immunology and Immunotherapy. (Current Topics in Microbiology and Immunology, 344). Springer Berlin Heidelberg; 2010:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benson DM, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res. 2014;2: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2: 393–398. [DOI] [PubMed] [Google Scholar]
- 48. Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8 + T cell effector function. Cancer Cell. 2014;26: 923–937. [DOI] [PubMed] [Google Scholar]
- 49. Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208: 577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6: 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16: e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Omuro A, Brandes AA, Carpentier AF, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 2023;25: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352: 997–1003. [DOI] [PubMed] [Google Scholar]
- 54. Mangani D, Weller M, Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol. 2017;130: 1–9. [DOI] [PubMed] [Google Scholar]
- 55. Lim M, Weller M, Idbaih A, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24: 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Festino L, Botti G, Lorigan P, et al. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76: 925–945. [DOI] [PubMed] [Google Scholar]
- 57. Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372: 320–330. [DOI] [PubMed] [Google Scholar]
- 59. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6: 1382–1399. [DOI] [PubMed] [Google Scholar]
- 60. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel SP, Othus M, Chen Y, et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med. 2023;388: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25: 470–476. [DOI] [PubMed] [Google Scholar]
- 64. de Groot J, Penas-Prado M, Alfaro-Munoz K, et al. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol. 2020;22: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Duerinck J, Schwarze JK, Awada G, et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: a phase I clinical trial. J Immunother Cancer. 2021;9: e002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grilli G, Nothdurft W, Fliedner TM. Radiation sensitivity of human erythropoietic and granulopoietic progenitor cells in the blood and in the bone marrow. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;41: 685–687. [DOI] [PubMed] [Google Scholar]
- 67. Mole RH. Whole body irradiation—radiobiology or medicine? BJR. 1953;26: 234–241. [DOI] [PubMed] [Google Scholar]
- 68. Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189: 558–566. [DOI] [PubMed] [Google Scholar]
- 70. Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (Hmg-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE. 2014;9: e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monjazeb AM, Schoenfeld JD. Radiation dose and checkpoint blockade immunotherapy: unanswered questions. Lancet Oncol. 2016;17: e3–e4. [DOI] [PubMed] [Google Scholar]
- 75. Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol. 2019;130: 104–112. [DOI] [PubMed] [Google Scholar]
- 76. Hassel JC, Schank TE, Smetak H, et al. Evaluation of radio-immunotherapy sequence on immunological responses and clinical outcomes in patients with melanoma brain metastases (ELEKTRA). OncoImmunology. 2022;11: 2066609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ko EC, Formenti SC. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther Adv Med Oncol. 2018;10: 175883591876824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rothschilds AM, Wittrup KD. What, why, where, and when: bringing timing to immuno-oncology. Trends Immunol. 2019;40: 12–21. [DOI] [PubMed] [Google Scholar]
- 79. Nivolumab with radiation therapy and bevacizumab for recurrent MGMT methylated glioblastoma. ClinicalTrials.gov identifier: NCT03743662.
- 80. Patil CG. Trial of anti-PD-1 immunotherapy and stereotactic radiation in patients with recurrent glioblastoma. ClinicalTrials.gov identifier: NCT04977375.
- 81. Atezolizumab and pre-surgical brain radiation therapy for glioblastoma multiforme. ClinicalTrials.gov identifier: NCT05423210.
- 82. A study evaluating the association of hypofractionated stereotactic radiation therapy and durvalumab for patients with recurrent glioblastoma (STERIMGLI). ClinicalTrials.gov identifier: NCT02866747.
- 83. Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Testing the use of the immunotherapy drugs ipilimumab and nivolumab plus radiation therapy compared to the usual treatment (temozolomide and radiation therapy) for newly diagnosed MGMT unmethylated glioblastoma. ClinicalTrials.gov identifier: NCT04396860.
- 86. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gromeier M, Brown MC, Zhang G, et al. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun. 2021;12: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. A study testing the effect of immunotherapy (ipilimumab and nivolumab) in patients with recurrent glioma with elevated mutational burden. ClinicalTrials.gov identifier: NCT04145115.
- 91. Pembrolizumab (MK-3475) in patients with recurrent malignant glioma with a hypermutator phenotype. ClinicalTrials.gov identifier: NCT02658279.
- 92. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24: 4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7: 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sugiyama K, Sakai T, Fujishima I, et al. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg. 1990;54: 501–505. [DOI] [PubMed] [Google Scholar]
- 97. Lerner EC, Edwards RM, Wilkinson DS, Fecci PE. Laser ablation: heating up the anti-tumor response in the intracranial compartment. Adv Drug Deliv Rev. 2022;185: 114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Iorgulescu JB, Gokhale PC, Speranza MC, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res. 2021;27: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Campian J, Ghiaseddin A, Rahman M, et al. ATIM-45. Long term follow-up of a phase I/II study testing the toxicities and efficacy of pembrolizumab in combination with MRI-guided laser interstitial thermal therapy (LITT) in recurrent malignant gliomas. Neuro Oncol. 2019;21(Suppl 6): vi11–vi11. [Google Scholar]
- 100. Hwang H, Huang J, Khaddour K, et al. Prolonged response of recurrent IDH -wild-type glioblastoma to laser interstitial thermal therapy with pembrolizumab. CNS Oncol. 2022;11: CNS81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory t cells and negatively impacts survival. Clin Cancer Res. 2012;18: 6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Campian JL, Butt O, Huang J, et al. 278O Preliminary results of a phase II study of retifanlimab (PD-1 inhibitor) plus or minus epacadostat (IDO1 inhibitor) in combination with bevacizumab and hypofractionated radiotherapy for recurrent glioblastoma: NCT03532295. Ann Oncol. 2022;33: S666. [Google Scholar]
- 103. Lukas R, Sachdev S, Kumthekar P, et al. CTIM-12. A phase 1 trial of immunoradiotherapy with the ido enzyme inhibitor (BMS-986205) and nivolumab in patients with newly diagnosed mgmt promoter unmethylated IDHwt glioblastoma. Neuro Oncol. 2021;23(Suppl 6): vi51–vi52. [Google Scholar]
- 104. Virag L. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54: 375–429. [DOI] [PubMed] [Google Scholar]
- 105. Sim HW, Galanis E, Khasraw M. PARP inhibitors in glioma: a review of therapeutic opportunities. Cancers. 2022;14: 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wen P. Surgical pembro +/- olaparib w TMZ for rGBM. ClinicalTrials.gov identifier: NCT05463848.
- 107. Gallinari P, Marco SD, Jones P, et al. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17: 195–211. [DOI] [PubMed] [Google Scholar]
- 108. Liu JR, Yu CW, Hung PY, et al. High-selective HDAC6 inhibitor promotes HDAC6 degradation following autophagy modulation and enhanced antitumor immunity in glioblastoma. Biochem Pharmacol. 2019;163: 458–471. [DOI] [PubMed] [Google Scholar]
- 109. Pembrolizumab and vorinostat combined with temozolomide for newly diagnosed glioblastoma. ClinicalTrials.gov identifier: NCT03426891.
- 110. Yeo ECF, Brown MP, Gargett T, Ebert LM. The role of cytokines and chemokines in shaping the immune microenvironment of glioblastoma: implications for immunotherapy. Cells. 2021;10: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang C, Kong L, Kim S, et al. The role of IL-7 and IL-7R in cancer pathophysiology and immunotherapy. Int J Mol Sci. 2022;23: 10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tompa M, Kraboth Z, Galik B, et al. Epigenetic suppression of the IL-7 pathway in progressive glioblastoma. Biomedicines. 2022;10: 2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Campian JL, Ghosh S, Kapoor V, et al. Long-acting recombinant human interleukin-7, NT-I7, increases cytotoxic CD8 T cells and enhances survival in mouse glioma models. Clin Cancer Res. 2022;28: 1229–1239. [DOI] [PubMed] [Google Scholar]
- 114. rhIL-7-hyFc on increasing lymphocyte counts in patients with newly diagnosed non-severe lymphopenic gliomas following radiation and temzolomide. ClinicalTrials.gov identifier: NCT03687957.
- 115. Efineptakin alfa and pembrolizumab for the treatment of recurrent glioblastoma. ClinicalTrials.gov identifier: NCT05465954.
- 116. Berkelmann L, Bader A, Meshksar S, et al. Tumour-treating fields (TTFields): investigations on the mechanism of action by electromagnetic exposure of cells in telophase/cytokinesis. Sci Rep. 2019;9: 7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Voloshin T, Kaynan N, Davidi S, et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020;69: 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tran D, Ghiaseddin A, Chen D, et al. CTIM-05. Final results of 2-the-top: a pilot phase 2 study of TTfields (OPTUNE) plus pembrolizumab plus maintenance temozolomide (TMZ) in patients with newly diagnosed glioblastoma (NDGBM). Neuro Oncol. 2022;24(Suppl 7): vii60–vii60. [Google Scholar]
- 119. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14: 642–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Suryawanshi YR, Schulze AJ. Oncolytic viruses for malignant glioma: on the verge of success? Viruses. 2021;13: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Speranza MC, Passaro C, Ricklefs F, et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chiocca EA, Gelb AB, Chen CC, et al. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: an open-label, multi-institutional phase I trial. Neuro Oncol. 2022;24: 951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Study of Ad-RTS-hIL-12 + veledimex in combination with cemiplimab in subjects with recurrent or progressive glioblastoma. ClinicalTrials.gov identifier: NCT04006119.
- 124. Webb MJ, Sener U, Vile RG. Current status and challenges of oncolytic virotherapy for the treatment of glioblastoma. Pharmaceuticals. 2023;16: 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wilcox JA, Ramakrishna R, Magge R. Immunotherapy in glioblastoma. World Neurosurg. 2018;116: 518–528. [DOI] [PubMed] [Google Scholar]
- 126. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 128. Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 129. Antonios JP, Soto H, Everson RG, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1: e87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu CJ, Schaettler M, Blaha DT, et al. Treatment of an aggressive orthotopic murine glioblastoma model with combination checkpoint blockade and a multivalent neoantigen vaccine. Neuro Oncol. 2020;22: 1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Batich KA, Reap EA, Archer GE, et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23: 1898–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Inogés S, Tejada S, De Cerio ALD, et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med. 2017;15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ardon H, Van Gool SW, Verschuere T, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 2012;61: 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565: 240–245. [DOI] [PubMed] [Google Scholar]
- 136. Personalized neoantigen cancer vaccine w RT plus pembrolizumab for patients with newly diagnosed GBM. ClinicalTrials.gov identifier: NCT02287428.
- 137. Neth BJ, Webb MJ, Parney IF, Sener UT. The current status, challenges, and future potential of therapeutic vaccination in glioblastoma. Pharmaceutics. 2023;15: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23: 1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shen L, Li H, Bin S, et al. The efficacy of third generation anti-HER2 chimeric antigen receptor T-cells in combination with PD1 blockade against malignant glioblastoma cells. Oncol Rep. 2019;42: 1549–1557. [DOI] [PubMed] [Google Scholar]
- 142. IL13Ra2-CAR T cells with or without nivolumab and ipilimumab in treating patients with GBM. ClinicalTrials.gov identifier: NCT04003649.
- 143. CART-EGFRvIII + pembrolizumab in GBM. ClinicalTrials.gov identifier: NCT03726515.
- 144. Australian Pancreatic Cancer Genome Initiative, ICGC Breast Cancer Consortium, ICGC MMML-Seq Consortium, et al. Signatures of mutational processes in human cancer. Nature. 2013;500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Simonds EF, Lu ED, Badillo O, et al. Deep immune profiling reveals targetable mechanisms of immune evasion in immune checkpoint inhibitor-refractory glioblastoma. J Immunother Cancer. 2021;9: e002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chuntova P, Chow F, Watchmaker PB, et al. Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank. Neuro Oncol. 2021;23: 356–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ronvaux L, Riva M, Coosemans A, et al. Liquid biopsy in glioblastoma. Cancers (Basel). 2022;14: 3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Miller D. Neoadjuvant chemoimmunotherapy in recurrent glioblastoma. ClinicalTrials.gov identifier: NCT05700955.
- 149. Neoadjuvant PD-1 in newly diagnosed glioblastoma. ClinicalTrials.gov identifier: NCT04583020.
- 150. Pembrolizumab and standard therapy in treating patients with glioblastoma. ClinicalTrials.gov identifier: NCT03197506.
- 151. Anti-LAG-3 alone & in combination w/nivolumab treating patients w/recurrent GBM (Anti-CD137 Arm Closed 10/16/18). ClinicalTrials.gov identifier: NCT02658981.
- 152. AB154 combined with AB122 for recurrent glioblastoma. ClinicalTrials.gov identifier: NCT04656535.
- 153. ASP8374 + cemiplimab in recurrent glioma. ClinicalTrials.gov identifier: NCT04826393.
- 154. Trial of anti-Tim-3 in combination with anti-PD-1 and SRS in recurrent GBM. ClinicalTrials.gov identifier: NCT03961971.
- 155. Wen P. Surgical nivolumab and ipilimumab for recurrent GBM. ClinicalTrials.gov identifier: NCT04606316.
- 156. MK-3475 in combination with MRI-guided laser ablation in recurrent malignant gliomas. ClinicalTrials.gov identifier: NCT02311582.
- 157. Avelumab with laser interstitial therapy for recurrent glioblastoma. ClinicalTrials.gov identifier: NCT03341806.
- 158. Laser interstitial thermotherapy (LITT) combined with checkpoint inhibitor for recurrent GBM (RGBM). ClinicalTrials.gov identifier: NCT03277638.
- 159. Atezolizumab and cabozantinib for the treatment of recurrent glioblastoma. ClinicalTrials.gov identifier: NCT05039281.
- 160. A study to evaluate safety and efficacy of ACT001 and anti-PD-1 in patients with surgically accessible recurrent glioblastoma multiforme. ClinicalTrials.gov identifier: NCT05053880.
- 161. Glioblastoma treatment with irradiation and olaptesed pegol (NOX-A12) in MGMT unmethylated patients (GLORIA). ClinicalTrials.gov identifier: NCT04121455.
- 162. Retifanlimab and epacadostat in combination with radiation and bevacizumab in patients with recurrent gliomas. ClinicalTrials.gov identifier: NCT03532295.
- 163. Nivolumab, BMS-986205, and radiation therapy with or without temozolomide in treating patients with newly diagnosed glioblastoma. ClinicalTrials.gov identifier: NCT04047706.
- 164. LUMINOS-101: lerapolturev (PVSRIPO) and pembrolizumab in patients with recurrent glioblastoma. ClinicalTrials.gov identifier: NCT04479241.
- 165. A phase I/II study of pembrolizumab and M032 (NSC 733972), a genetically engineered HSV-1 expressing IL-12, in patients with recurrent/progressive and newly diagnosed glioblastoma multiforme, anaplastic astrocytoma, or gliosarcoma. ClinicalTrials.gov identifier: NCT05084430.
- 166. A phase II, multi-center, open-label study of a conditionally replicative adenovirus (DNX-2401) with pembrolizumab (KEYTRUDA®) for recurrent glioblastoma or gliosarcoma (CAPTIVE/KEYNOTE-192). ClinicalTrials.gov identifier: NCT02798406.
- 167. An open-label, phase I/II multicenter clinical trial of VXM01 in combination with avelumab in patients with progressive glioblastoma following standard treatment, with or without second surgery. ClinicalTrials.gov identifier: NCT03750071.
- 168. A pilot study to assess the safety and immunogenicity of a neoantigen-based personalized DNA vaccine with retifanlimab PD-1 blockade therapy in patients with newly diagnosed, unmethylated glioblastoma. ClinicalTrials.gov identifier: NCT05743595.
- 169. Neoadjuvant PD-1 antibody alone or combined with autologous glioblastoma stem-like cell antigens-primed DC vaccines (GSC-DCV) for patients with recurrent glioblastoma: a phase II, randomized controlled, double blind clinical trial. ClinicalTrials.gov identifier: NCT04888611.
- 170. Pembrolizumab and a vaccine (ATL-DC) for the treatment of surgically accessible recurrent glioblastoma. ClinicalTrials.gov identifier: NCT04201873.
- 171. Peereboom D. Phase II study of pembrolizumab plus SurVaxM for glioblastoma at first recurrence. ClinicalTrials.gov identifier: NCT04013672.
- 172. Radiation therapy plus temozolomide and pembrolizumab with and without HSPPC-96 in newly diagnosed glioblastoma (GBM). ClinicalTrials.gov identifier: NCT03018288.
- 173. First-in-human, phase 1b/2a trial of a multipeptide therapeutic vaccine in patients with progressive glioblastoma (ROSALIE). ClinicalTrials.gov identifier: NCT04116658.
- 174. Pembrolizumab in association with the multipeptide vaccine IMA950 adjuvanted with poly-ICLC for relapsing glioblastoma: a randomized phase I/II trial. ClinicalTrials.gov identifier: NCT03665545.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.