Abstract
Background:
Takotsubo syndrome has been reported in patients with COVID-19, although minimal data are available. This investigation assessed the incidence and impact of takotsubo syndrome on patients hospitalized with COVID-19.
Methods:
A retrospective cohort study was conducted using International Statistical Classification of Diseases, Tenth Revision, codes to identify patients with a primary diagnosis of COVID-19 with or without takotsubo syndrome in the National Inpatient Sample 2020 database. Outcomes between groups were compared after propensity score matching for patient and hospital demographics and comorbidities.
Results:
A total of 211,448 patients with a primary diagnosis of COVID-19 were identified. Of these, 171 (0.08%) had a secondary diagnosis of takotsubo syndrome. Before matching, patients with COVID-19 and takotsubo syndrome, compared with patients without takotsubo syndrome, were older (68.95 vs 64.26 years; P < .001); more likely to be female (64.3% vs 47.2%; P < .001); and more likely to have anxiety (24.6% vs 12.8%; P < .001), depression (17.5% vs 11.4%; P = .02), and chronic obstructive pulmonary disease (24.6% vs 14.7%; P < .001). The takotsubo syndrome group had worse outcomes than the non–takotsubo syndrome group for death (30.4% vs 11.1%), cardiac arrest (7.6% vs 2.1%), cardiogenic shock (12.9% vs 0.4%), length of hospital stay (10.7 vs 7.5 days), and total charges ($152,685 vs $78,468) (all P < .001). After matching and compared with the non–takotsubo syndrome group (n = 508), the takotsubo syndrome group (n = 170) had a higher incidence of inpatient mortality (30% vs 14%; P < .001), cardiac arrest (7.6% vs 2.8%; P = .009), and cardiogenic shock (12.4% vs 0.4%; P < .001); a longer hospital stay (10.7 vs 7.6 days; P < .001); and higher total charges ($152,943 vs $79,523; P < .001).
Conclusion:
Takotsubo syndrome is a rare but severe in-hospital complication in patients with COVID-19.
Keywords: COVID-19, takotsubo cardiomyopathy, mortality
Key Points
Takotsubo syndrome complicates 0.08% of cases of patients hospitalized with COVID-19.
Patients with both COVID-19 and TTS have worse in-hospital outcomes than patients without TTS.
Takotsubo syndrome is a rare, severe in-hospital complication in patients with COVID-19.
Introduction
COVID-19 had affected more than 621 million individuals by October 2022.1 Infection with the etiologic virus SARS-CoV-2 can cause various complications, including those in the cardiovascular system. In more than 20% of hospitalized patients with COVID-19, cardiac troponin levels are elevated, suggesting acute cardiac injury.2 Other COVID-19–related cardiovascular complications include myocardial infarction, cardiac arrest, atrial fibrillation, myocarditis, and pericarditis.3-6
During the COVID-19 pandemic, takotsubo syndrome (TTS) was widely reported.7-9 Takotsubo syndrome is an acute transient heart failure syndrome characterized by transient systolic ventricular dysfunction accompanied by wall-motion abnormalities.10,11 It was traditionally considered rare and benign, but its hospital and long-term mortality rates are similar to those of acute coronary syndrome.12 Although the coexistence of TTS and COVID-19 has been reported, the incidence of TTS and its effects on the prognosis of patients with COVID-19 are not well established.
This study aimed to assess the incidence and impact of TTS on patients hospitalized with COVID-19 using the latest data from the National Inpatient Sample (NIS) database.
Patients and Methods
Data Sources
The association between COVID-19 and TTS outcomes was examined by extracting data from the NIS 2020 database, which comprises more than 7 million hospital stays each year. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes were used to classify in-hospital diagnoses since 2016. Starting in 2020, hospitalizations related to COVID-19 have been identified by any listed ICD-10-CM diagnosis code of U071 (COVID-19) on the discharge record.13 Supplemental Table I shows the ICD-10-CM codes used in this study. Institutional review board approval was not required for this study because data in the NIS database are deidentified.
Study Population and Covariates
All patients in the 2020 NIS database with the primary diagnosis of COVID-19 were studied. Patients who had no discharge status were excluded. Patient and hospital demographics (age; sex; race; geographic location; household income; primary payer; and hospital type, region, and size by number of beds); common cardiovascular comorbidities (eg, smoking, hypertension); and other reported risk factors for TTS (including hyperlipidemia,14 chronic obstructive pulmonary disease,15,16 anxiety,17 depression,18 obesity,19 diabetes,20 and chronic kidney disease21) were selected as covariates.
Patients with COVID-19 were then allocated into 1 of 2 groups: those with TTS and those without TTS. The detailed patient selection process is shown in Figure 1. The impct of COVID-19 on TTS was assessed by analyzing a group of patients with TTS but without COVID-19.
Fig. 1.
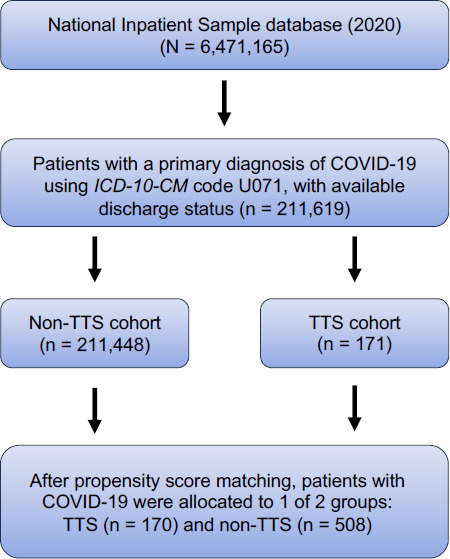
Flowchart of the selection process for the final patient sample shows that inclusion criteria were applied to the National Inpatient Sample 2020 database. All eligible patients were matched 1:3 based on propensity scoring to generate the TTS vs non-TTS comparison cohorts.
ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; TTS, takotsubo syndrome.
Outcomes
The main outcomes of interest were in-hospital mortality rate and severe in-hospital complications, including cardiogenic shock; cardiac arrest; ventricular arrhythmias (ventricular tachycardia, ventricular flutter, and ventricular fibrillation); acute respiratory failure; acute kidney injury (AKI); and mechanical ventilation. Hospital length of stay (LOS) and total admission costs were also examined.
Statistical Analysis
Mean (SD) values were used to express continuous variables, and percentages were used to express categorical variables. Continuous variables were analyzed using the t test, and categorical variables were analyzed using χ2 tests. P ≤ .05 was considered significant. All data analysis and statistical processes were performed using R, version 3.6.1, statistical software (R Foundation for Statistical Computing).
Selection bias was reduced in the unmatched cohort by conducting a propensity score matching analysis, matching patients in the TTS and the non-TTS groups in a 1:3 target ratio. A multivariate logistic regression model was adjusted for patient and hospital demographics (age; sex; race; geographic location; household income; primary payer; and hospital type, region, and size by number of beds) and for common cardiovascular comorbidities. This method has been used in previous studies.22 In-hospital outcomes between the groups were compared before and after covariate adjustment to demonstrate the effect of TTS on in-hospital outcomes of COVID-19.
Results
Baseline Characteristics
In the NIS database, 211,619 patients with a primary diagnosis of COVID-19 were identified: 171 (0.08%) patients with TTS and 211,448 (99.92%) patients without TTS. Baseline characteristics are provided in Supplemental Table II for the unmatched populations and in Table I for the matched cohorts.
TABLE I.
Baseline Characteristics of the Matched Cohort
| Variable | Patients with COVID-19 without TTSa (n = 508) | Patients with COVID-19 and TTSa (n = 170) | P value |
|---|---|---|---|
| Age, mean (SD), y | 68.93 (15.89) | 68.94 (12.99) | .99 |
| Sex, No. (%) | .62 | ||
| Male | 195 (38.4) | 61 (35.9) | |
| Female | 313 (61.6) | 109 (64.1) | |
| Unknown | 0 (0.0) | 0 (0.0) | |
| Race, No. (%) | .85 | ||
| Asian or Pacific Islander | 22 (4.3) | 8 (4.7) | |
| Black | 60 (11.8) | 22 (12.9) | |
| Hispanic | 89 (17.5) | 25 (14.7) | |
| Native American | 0 (0.0) | 0 (0.0) | |
| White | 319 (62.8) | 107 (62.9) | |
| Other | 12 (2.4) | 4 (2.4) | |
| Unknown | 6 (1.2) | 4 (2.4) | |
| Patient location, No. (%) | .99 | ||
| “Central” counties of metropolitan areas with populations of ≥1 million people | 172 (33.9) | 60 (35.3) | |
| “Fringe” counties of metropolitan areas with populations of ≥1 million people | 139 (27.4) | 42 (24.7) | |
| Counties in metropolitan areas with populations of 250,000-999,999 people | 77 (15.2) | 27 (15.9) | |
| Counties in metropolitan areas with populations of 50,000-249,999 people | 46 (9.1) | 15 (8.8) | |
| Micropolitan counties | 42 (8.3) | 14 (8.2) | |
| Nonmetropolitan or micropolitan counties | 28 (5.5) | 11 (6.5) | |
| Not reported | 4 (0.8) | 1 (0.6) | |
| Mean household income, No. (%) | .87 | ||
| $1-$42,999 | 115 (22.6) | 40 (23.5) | |
| $43,000-$53,999 | 169 (33.3) | 55 (32.4) | |
| $54,000-$70,999 | 144 (28.3) | 43 (25.3) | |
| $71,000 or more | 75 (14.8) | 30 (17.6) | |
| Unknown | 5 (1.0) | 2 (1.2) | |
| Primary payer, No. (%) | .49 | ||
| Medicare | 338 (66.5) | 111 (65.3) | |
| Medicaid | 56 (11.0) | 13 (7.6) | |
| Private insurance, including health maintenance organizations | 93 (18.3) | 35 (20.6) | |
| Self-pay | 9 (1.8) | 4 (2.4) | |
| No charge | 0 (0.0) | 0 (0.0) | |
| Other | 12 (2.4) | 7 (4.1) | |
| Unknown | 0 (0.0) | 0 (0.0) | |
| Hospital type, No. (%) | .98 | ||
| Rural | 44 (8.7) | 14 (8.2) | |
| Urban nonteaching | 75 (14.8) | 26 (15.3) | |
| Urban teaching | 389 (76.6) | 130 (76.5) | |
| Hospital region, No. (%) | .99 | ||
| Northeast | 101 (19.9) | 36 (21.2) | |
| Midwest | 169 (33.3) | 55 (32.4) | |
| South | 148 (29.1) | 49 (28.8) | |
| West | 90 (17.7) | 30 (17.6) | |
| Hospital size, by No. of beds, No. (%) | .97 | ||
| Small | 116 (22.8) | 38 (22.4) | |
| Medium | 103 (20.3) | 36 (21.2) | |
| Large | 289 (56.9) | 96 (56.5) | |
| Patient comorbidities, No. (%) | |||
| Smoking | 147 (28.9) | 50 (29.4) | .98 |
| Hypertension | 152 (29.9) | 56 (32.9) | .52 |
| Diabetes | 202 (39.8) | 69 (40.6) | .92 |
| Hyperlipidemia | 261 (51.4) | 83 (48.8) | .63 |
| Obesity | 122 (24.0) | 40 (23.5) | .98 |
| Anxiety | 117 (23.0) | 41 (24.1) | .85 |
| Depression | 91 (17.9) | 29 (17.1) | .89 |
| Obstructive sleep apnea | 33 (6.5) | 10 (5.9) | .92 |
| Chronic kidney disease | 122 (24.0) | 39 (22.9) | .86 |
| Chronic obstructive pulmonary disease | 122 (24.0) | 41 (24.1) | .99 |
| Anemia | 157 (30.9) | 49 (28.8) | .68 |
| Cancer | 21 (4.1) | 6 (3.5) | .90 |
TTS, takotsubo syndrome.
P ≤ .05 was considered statistically significant.
Total percentages may exceed 100% due to rounding.
Before propensity score matching, patients with COVID-19 and TTS were older than patients with COVID-19 who did not have TTS (mean [SD] age, 68.95 [12.95] years vs 64.26 [16.84] years; P < .001) and more likely to be female (64.3% vs 47.2%; P < .001). Patients with COVID-19 and TTS also had higher rates of comorbidities, including anxiety (24.6% vs 12.8%; P < .001), depression (17.5% vs 11.4%; P = .02), chronic obstructive pulmonary disease (24.6% vs 14.7%; P < .001), and anemia (29.2% vs 20.0%; P < .001). Hypertension was more prevalent in patients with COVID-19 who did not have TTS (41.6% vs 33.3%; P = .03). The distribution of smoking, diabetes, hyperlipidemia, and obesity was similar between the groups (Supplemental Table II).
Socioeconomic factors, including race, medical service primary payers, and studied hospital size, also varied substantially. There was a higher proportion of White (63.2% vs 50.9%) and Asian or Pacific Islander (4.7% vs 3.1%) patients in the TTS group than in the non-TTS group. More patients with TTS were on Medicare (65.5% vs 51.8%). Differences in hospital types were also found; midwestern hospitals had more patients with COVID-19 and TTS than they had patients with only COVID-19 (32.7% vs 23.2%), as did institutions with higher bed numbers (56.7% vs 45.5%).
Propensity score matching was used to create 2 groups matched for baseline characteristics (P > .05) (Table I): patients with COVID-19 but without TTS (n = 508) and patients with both COVID-19 and TTS (n = 170). All variables in this study had standardized mean differences less than 0.1 (Supplemental Table III).
In-Hospital Complications
Before matching, patients with COVID-19 and TTS had a higher incidence of complications, including cardiac arrest (7.6% vs 2.1%; P < .001), cardiogenic shock (12.9% vs 0.4%; P < .001), ventricular arrhythmia (4.1% vs 1.7%; P = .04), AKI (46.8% vs 25.1%; P < .001), and mechanical ventilation (35.1% vs 9.3%; P < .001), than did patients with COVID-19 who did not have TTS (Table II). These differences were also observed after propensity score matching, except in the case of ventricular arrhythmia (2.8% vs 3.5%; P = .80). In the matched groups, patients with COVID-19 and TTS had a higher burden of cardiac arrest (7.6% vs 2.8%; P = .009), cardiogenic shock (12.4% vs 0.4%; P < .001), AKI (46.5% vs 27.6%; P < .001), and mechanical ventilation (34.7% vs 10.0%; P < .001). The adjusted odds ratio for in-hospital complications after matching is shown in Table III. A higher incidence of TTS was found in patients who did not have COVID-19 (1.29%). Patients with TTS in the cohort without COVID-19 had a lower incidence of the following inhospital outcomes: cardiac arrest (4.29%), cardiogenic shock (7.04%), ventricular arrhythmia (6.99%), AKI (25.21%), acute respiratory failure (32.11%), and mechanical ventilation (17.73%) (Supplemental Table IV).
TABLE II.
In-Hospital Outcomes
| Variable | Unmatched cohort | Propensity-matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Patients with COVID-19 without TTS (n = 211,448) | Patients with COVID-19 and TTS (n = 171) | P value | Patients with COVID-19 without TTS (n = 508) | Patients with COVID-19 and TTS (n = 170) | P value | Standardized mean difference | |
| Outcomes | |||||||
| Death, No. (%) | 23,404 (11.1) | 52 (30.4) | <.001 | 71 (14.0) | 51 (30.0) | <.001 | 0.394 |
| Cardiac arrest, No. (%) | 4,473 (2.1) | 13 (7.6) | <.001 | 14 (2.8) | 13 (7.6) | .009 | 0.222 |
| Cardiogenic shock, No. (%) | 818 (0.4) | 22 (12.9) | <.001 | 2 (0.4) | 21 (12.4) | <.001 | 0.505 |
| Ventricular arrhythmia, No. (%) | 3,691 (1.7) | 7 (4.1) | .04 | 14 (2.8) | 6 (3.5) | .80 | 0.044 |
| AKI, No. (%) | 53,065 (25.1) | 80 (46.8) | <.001 | 140 (27.6) | 79 (46.5) | <.001 | 0.399 |
| Acute respiratory failure, No. (%) | 117,962 (55.8) | 91 (53.2) | .55 | 299 (58.9) | 90 (52.9) | .21 | 0.119 |
| Mechanical ventilation, No. (%) | 19,729 (9.3) | 60 (35.1) | <.001 | 51 (10.0) | 59 (34.7) | <.001 | 0.62 |
| LOS, mean (SD), d | 7.45 (8.07) | 10.70 (12.95) | <.001 | 7.57 (7.40) | 10.73 (12.99) | <.001 | 0.299 |
| Total hospital-related cost, mean (SD), $ | 78,468.44 (149,660.09) | 152,685.12 (279,543.46) | <.001 | 79,523.29 (122,804.79) | 152,942.81 (280,348.92) | <.001 | 0.339 |
AKI, acute kidney injury; LOS, length of stay; TTS, takotsubo syndrome.
P ≤ .05 was considered statistically significant.
TABLE III.
Adjusted Odds Ratios for In-Hospital Outcomes After Propensity Score Matching
| Outcome | Odds ratio | 95% CI | Control odds | Treatment odds | P value |
|---|---|---|---|---|---|
| Death | 2.64 | 1.75-3.99 | 0.16 | 0.43 | <.001 |
| Cardiac arrest | 35.66 | 8.27-153.83 | 0.00 | 0.14 | <.001 |
| Cardiogenic shock | 2.92 | 1.34-6.35 | 0.03 | 0.08 | <.001 |
| Ventricular arrhythmia | 1.29 | 0.49-3.41 | 0.03 | 0.04 | .61 |
| Acute kidney injury | 2.28 | 1.59-3.27 | 0.38 | 0.87 | <.001 |
| Acute respiratory failure | 0.79 | 0.55-1.12 | 1.43 | 1.13 | .18 |
| Mechanical ventilation | 4.97 | 3.1-7.31 | 0.11 | 0.53 | <.001 |
P ≤ .05 was considered statistically significant.
Mortality, LOS, and Total Cost
In the unmatched cohort (Table II), patients with COVID-19 and TTS had a higher rate of in-hospital mortality than patients with COVID-19 only (30.4% vs 11.1%; P < .001) as well as a longer mean [SD] LOS (10.70 [12.95] days vs 7.45 [8.07] days; P < .001) and higher mean [SD] total charges ($152,685.12 [$279,543.46] vs $78,468.44 [$149,660.09]; P < .001). As in the case of in-hospital complications, these differences remained significant after propensity matching. The TTS group had higher mortality rates (30.0% vs 14.0%; P < .001), a longer mean [SD] LOS (10.73 [12.99] days vs 7.57 [7.40] days; P < .001), and higher mean [SD] total charges ($152,942.81 [$280,348.92] vs $79,523.29 [$122,804.79]; P < .001) than the non-TTS group. Lower in-hospital mortality rates (7.31%), shorter mean [SD] LOS (6.98 [9.58] days), and lower mean [SD] total charges ($112,881.40 [$227,113.50]) were seen in patients without COVID-19 who had TTS (Supplemental Table IV).
Discussion
To the authors' knowledge, this is the first study of the incidence and impact of TTS in patients hospitalized with COVID-19. This study found that TTS is a rare complication in patients hospitalized with COVID-19 but that it results in higher rates of in-hospital mortality and complications (cardiac arrest, cardiogenic shock, and acute kidney failure), longer LOS, and higher hospitalization costs for this population than it does for hospitalized patients with COVID-19 who did not have TTS (Fig. 2).
Fig. 2.
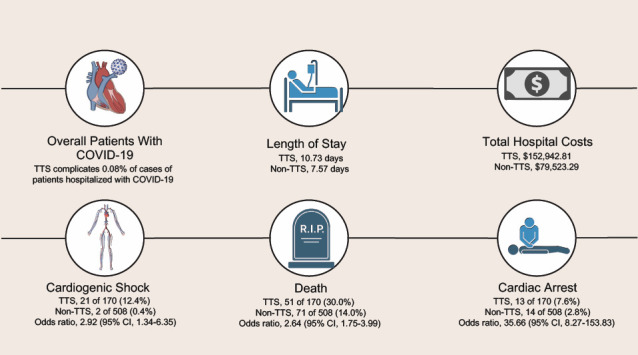
This infographic shows the effects of TTS in patients hospitalized with COVID-19.
TTS, takotsubo syndrome.
The study's findings of higher rates of mortality, cardiac arrest, and cardiogenic shock in patients with COVID-19 and TTS support those seen in previous studies in which worse clinical outcomes were reported when patients with COVID-19 had increased cardiovascular risks, including hypertension, arrhythmia, and heart failure.23,24 These results are also in accordance with a previous case report–based study whose TTS cohort had a higher mortality rate (33.9%) than its non-TTS group (15%).25 Although the exact mechanisms of the link between TTS and worse clinical outcomes in COVID-19 remain unidentified, 3 factors could be involved.26 First, an overactive immune response exacerbates the progression of TTS. In SARS-CoV-2 infections, vascular leakage and endothelial cell death trigger a cytokine storm, resulting in the release of proinflammatory cytokines and chemokines into the circulatory system.27 These inflammatory events hinder cardiac function and increase the risk of TTS onset and worse clinical outcomes.28 Second, the cytokine storm can stimulate and enhance signaling in the sympathetic nervous system, which could result in catecholamine-induced myocardial stunning, a predisposing factor for TTS.29,30 This perturbation in the neurologic system could help explain the unexpected higher mortality rate. Finally, microvascular dysfunction, a previously identified risk factor of TTS, has been seen in COVID-19 pathology, which may connect TTS with worse COVID-19 outcomes.31
A higher incidence of AKI was found in patients with COVID-19 and TTS. Coronary angiography, which is commonly used to diagnose TTS, may have adverse contrast-induced effects on kidney function.32 The bidirectional interconnections between the heart and the kidneys may also contribute to the onset of AKI.33 During TTS, high levels of blood-borne catecholamines and uncontrolled stimulation of the autonomic nervous system can result in a sympathoadrenal attack on the secondary organs, such as the kidneys.34
Patients with COVID-19 and TTS had higher total hospital-related costs and a longer LOS. Similar findings have been reported in patients with COVID-19 and other cardiovascular comorbidities.35,36 Because patients hospitalized with COVID-19 have more comorbidities and systemic inflammatory responses, TTS can complicate the overall treatment plan, which could increase the patient's LOS and related costs. The supportive care involved in treating TTS can add to the financial burden for patients with COVID-19.
Before propensity score matching, unique socioeconomic features among patients with COVID-19 and TTS were identified. The race of the group was predominantly White, which is consistent with analysis from the American Heart Association COVID-19 Cardiovascular Disease Registry.37 The Asian population in the American Heart Association COVID-19 study notably had the worst cardiorespiratory severity of all races, which may explain the higher Asian and Pacific Islander ratio in the TTS group. As in the current study, the American Heart Association COVID-19 registry showed that Medicare was the major payer for patients with both COVID-19 and cardiovascular disorders. With regard to hospital size, findings from a Portuguese study38 supported the current study by showing that hospitals with more beds handled higher numbers of patients and more complicated cases of COVID-19.
The present study has several strengths. This study used a nationwide database that has a large number of registries to adequately power its results. It also used propensity score matching to avoid confounding factors and to minimize biased effects from baseline characteristics. The study's novel finding that morbidity and complication rates were higher in the TTS group than in the non-TTS group will help in developing considerations for the diagnosis and treatment pathway for patients with COVID-19 and different cardiovascular risks.
Study Limitations
The current study has several limitations. First, using codes from the ICD-10-CM to extract data may introduce bias. Second, the NIS database lacks some categories of information, such as treatment plans (eg, anti-inflammatory treatment, oxygen therapy, blood thinner prescriptions), laboratory data (including cardiac troponin levels, electrolytes, and full blood cell count), and diagnosis of disease features (symptoms, time of disease onset, and pathological presentation). The absence of this information may affect the understanding and analysis of patients with COVID-19 and TTS. Similarly, because the comorbidities in this study were assessed using ICD-10-CM codes in the NIS database, the severity of complications was unclear. Another limitation of this study was its potential for selection bias in covariate choice. Although the selection of covariates was based on results from the literature, the absence of specific ICD-10-CM codes for conditions such as history of TTS and heart failure may have led to incomplete adjustment for these factors, which may have affected the study's findings. Finally, the overlapping of clinical features between TTS and COVID-19 may result in the underdiagnosis and thus underreporting of TTS in the NIS database.
Conclusion
In this study, TTS was associated with worse in-hospital outcomes in patients with COVID-19. Patients with both COVID-19 and TTS had longer hospital LOS; higher total hospital-related costs; and higher rates of cardiac arrest, cardiogenic shock, and acute kidney failure than did patients with COVID-19 but without TTS. These findings suggest the need to optimize the current COVID-19 diagnosis plan, focusing on the early detection and prevention of TTS given the potentially atypical presentation of TTS in patients with COVID-19. The differential diagnosis of TTS and its corresponding symptoms, including shock and hemodynamic changes, should be considered in patients with COVID-19. Future studies should be conducted to create risk stratification tools to identify high-risk patients with COVID-19 who may develop TTS and to assess whether TTS treatment can reduce in-hospital complications in patients with COVID-19. Understanding the cross-talk between the disease processes of COVID-19 and TTS may also help improve current clinical protocols and develop new therapeutics.
Supplementary Material
Abbreviations and Acronyms
- AKI
acute kidney injury
- ICD-10-CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- LOS
length of stay
- NIS
National Inpatient Sample
- TTS
takotsubo syndrome
Article Information
Open Access: © 2024 The Authors. Published by The Texas Heart Institute®. This is an Open Access article under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC, https://creativecommons.org/licenses/by-nc/4.0/), which permits use and distribution in any medium, provided the original work is properly cited, and the use is noncommercial.
Author Contributions: Pengyang Li, MD, MSc, and Ao Shi, PhD, contributed equally to the work. Pengyang Li was involved in study conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, study supervision, visualization, and the review and editing of the report. Ao Shi was involved in study conceptualization, data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, writing the original draft, and the review and editing of the report. Xiaojia Lu was involved in formal analysis, investigation, software, validation, and the review and editing of the report. Chenli Li was involved in formal analysis, study methodology, validation, visualization, and the review and editing of the report. Peng Cai was involved in data curation, formal analysis, investigation, study methodology, resources, software, writing the original draft, and review and editing of the report. Catherine Teng was involved in formal analysis and the review and editing of the report. Lingling Wu was involved in study investigation, methodology, validation, and the review and editing of the report. Yuan Shu was involved in procuring study resources, visualization, and the review and editing of the report. Su Pan was involved in the study investigation and the review and editing of the report. Richard A. F. Dixon was involved in the study investigation, methodology, and the review and editing of the report. Qi Liu was involved in the study conceptualization, project administration, resources, supervision, and the review and editing of the report. Bin Wang was involved in the acquisition of funds, project administration, resources, supervision, and the review and editing of the report.
Conflict of Interest Disclosure: None.
Funding/Support: None.
Acknowledgments: The authors thank Rebecca Bartow, PhD, of the Department of Scientific Publications at The Texas Heart Institute for her editorial assistance.
References
- 1.Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19) 2020. [Accessed March 14, 2023]. https://ourworldindata.org/covid-cases
- 2.Sandoval Y, Januzzi JL, Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi E, Sechi GM, Mare C, et al. Lombardia CARe researchers COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41(32):3045–3054. doi: 10.1093/eurheartj/ehaa508. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capaccione KM, Leb JS, D'Souza B, Utukuri P, Salvatore MM. Acute myocardial infarction secondary to COVID-19 infection: a case report and review of the literature. Clin Imaging. 2021;72:178–182. doi: 10.1016/j.clinimag.2020.11.030. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Shao W, Zhang J, et al. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID-19: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:720129. doi: 10.3389/fcvm.2021.720129. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2(9):1326–1330. doi: 10.1016/j.jaccas.2020.04.009. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez JMD, Nair G, Nanavaty P, Rao A, Marinescu K, Suboc T. COVID-19-associated takotsubo cardiomyopathy. BMJ Case Rep. 2020;13(12):e236811. doi: 10.1136/bcr-2020-236811. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Desai R, Gandhi Z, et al. Takotsubo syndrome in patients with COVID-19: a systematic review of published cases. SN Compr Clin Med. 2020;2(11):2102–2108. doi: 10.1007/s42399-020-00557-w. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst RT, Prasad A, Askew JW III, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3(6):641–649. doi: 10.1016/j.jcmg.2010.01.009. doi: [DOI] [PubMed] [Google Scholar]
- 11.Medeiros K, O'Connor MJ, Baicu CF, et al. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129(16):1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781. doi: [DOI] [PubMed] [Google Scholar]
- 12.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. doi: [DOI] [PubMed] [Google Scholar]
- 13.Healthcare cost and utilization project (HCUP) Agency for Healthcare Research and Quality. Feb 1, 2024. [Accessed March 14, 2023]. https://hcup-us.ahrq.gov/nisoverview.jsp [PubMed]
- 14.Li P, Lu X, Teng C, et al. The association between hyperlipidemia and in-hospital outcomes in takotsubo cardiomyopathy. Diabetes Metab Syndr Obes. 2021;14:117–126. doi: 10.2147/DMSO.S282009. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfredini R, Fabbian F, Giorgi AD, et al. Heart and lung, a dangerous liaison—tako-tsubo cardiomyopathy and respiratory diseases: a systematic review. World J Cardiol. 2014;6(5):338–344. doi: 10.4330/wjc.v6.i5.338. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Lu X, Teng C, et al. The Impact of COPD on in-hospital outcomes in patients with takotsubo cardiomyopathy. Int J Chron Obstruct Pulmon Dis. 2020;15:2333–2341. doi: 10.2147/COPD.S267289. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzeroni D, Bini M, Castiglioni P, et al. Anxiety disorders and stressful events in takotsubo syndrome. Cardiol J. 2018;25(4):495–500. doi: 10.5603/CJ.a2017.0136. doi: [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Senecal C, Lewis B, et al. Natural history and predictors of mortality of patients with takotsubo syndrome. Int J Cardiol. 2018;267:22–27. doi: 10.1016/j.ijcard.2018.04.139. doi: [DOI] [PubMed] [Google Scholar]
- 19.Zalewska-Adamiec M, Malyszko J, Bachórzewska-Gajewska H, Tomaszuk-Kazberuk A, Dobrzycki SJ. Takotsubo syndrome—fatal prognosis of patients with low body mass index in 5-year follow-up. Arch Med Sci. 2019;16(2):282–288. doi: 10.5114/aoms.2019.87082. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiermaier T, Santoro F, El-Battrawy I, et al. Prevalence and prognostic impact of diabetes in takotsubo syndrome: insights from the international, multicenter GEIST registry. Diabetes Care. 2018;41(5):1084–1088. doi: 10.2337/dc17-2609. doi: [DOI] [PubMed] [Google Scholar]
- 21.Yassin AS, Adegbala O, Subahi A, et al. Clinical impact of advanced chronic kidney disease on outcomes and inhospital complications of takotsubo syndrome (broken-heart-syndrome): propensity-matched national study. Int J Cardiol. 2019;277:16–19. doi: 10.1016/j.ijcard.2018.09.098. doi: [DOI] [PubMed] [Google Scholar]
- 22.Li P, Li C, Mishra AK, et al. Impact of malnutrition on in-hospital outcomes in takotsubo cardiomyopathy. Nutrition. 2022;93:111495. doi: 10.1016/j.nut.2021.111495. doi: [DOI] [PubMed] [Google Scholar]
- 23.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth GA, Emmons-Bell S, Alger HM, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4(5):e218828. doi: 10.1001/jamanetworkopen.2021.8828. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah RM, Shah M, Shah S, Li A, Jauhar S. Takotsubo syndrome and COVID-19: associations and implications. Curr Probl Cardiol. 2021;46(3):100763. doi: 10.1016/j.cpcardiol.2020.100763. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueiredo Neto JA, Marcondes-Braga FG, Moura LZ, et al. Coronavirus disease 2019 and the myocardium. Arq Bras Cardiol. 2020;114(6):1051–1057. doi: 10.36660/abc.20200373. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadha S. ‘COVID-19 pandemic' anxiety-induced Takotsubo cardiomyopathy. QJM. 2020;113(7):488–490. doi: 10.1093/qjmed/hcaa135. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariyanna PT, Chandrakumar HP, Jayarangaiah A, et al. Apical takotsubo cardiomyopathy in a COVID-19 patient presenting with stroke: a case report and pathophysiologic insights. Am J Med Case Rep. 2020;8(10):350–357. doi: 10.12691/ajmcr-8-10-8. doi: [DOI] [Google Scholar]
- 31.Montone RA, Iannaccone G, Meucci MC, Gurgoglione F, Niccoli G. Myocardial and microvascular injury due to coronavirus disease 2019. Eur Cardiol. 2020;15:e52. doi: 10.15420/ecr.2020.22. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azzalini L, Candilio L, McCullough PA, Colombo A. Current risk of contrast-induced acute kidney injury after coronary angiography and intervention: a reappraisal of the literature. Can J Cardiol. 2017;33(10):1225–1228. doi: 10.1016/j.cjca.2017.07.482. doi: [DOI] [PubMed] [Google Scholar]
- 33.Shin MJ, Rhee H, Kim IY, et al. Clinical features of patients with stress-induced cardiomyopathy associated with renal dysfunction: 7 case series in single center. BMC Nephrol. 2013;14:213. doi: 10.1186/1471-2369-14-213. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madias JE. Reversible acute renal failure in patients with takotsubo syndrome. Int J Cardiol. 2016;220:356. doi: 10.1016/j.ijcard.2016.06.262. doi: [DOI] [PubMed] [Google Scholar]
- 35.Hamdan M, Badrasawi M, Zidan S, et al. Risk factors associated with hospitalization owing to COVID-19: a cross-sectional study in Palestine. J Int Med Res. 2021;49(12):3000605211064405. doi: 10.1177/03000605211064405. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pshenichnaya N, Lizinfeld I, Zhuravlev G. Factors influencing on hospitalization of COVID-19 patients with comorbidity. Int J Infect Dis. 2022;116(suppl):S39. doi: 10.1016/j.ijid.2021.12.094. doi: [DOI] [Google Scholar]
- 37.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seringa J, Pedreiras S, Freitas MJ, et al. Direct costs of COVID-19 inpatient admissions in a Portuguese tertiary care university centre. Port J Public Health. 2022;40(1):26–34. doi: 10.1159/000524368. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


