Abstract
Werner syndrome (WS) is a rare genetic disease in humans, caused by mutations in the WRN gene that encodes a protein containing helicase and exonuclease domains. WS is characterized by symptoms of accelerated aging in multiple tissues and organs, involving increased risk of cancer, heart failure, and metabolic dysfunction. These conditions ultimately lead to the premature mortality of patients with WS. In this study, using the null mutant flies (WRNexoΔ) for the gene WRNexo (CG7670), homologous to the exonuclease domain of WRN in humans, we examined how diets affect the lifespan, stress resistance, and sleep/wake patterns of a Drosophila model of WS. We observed that dietary restriction (DR), one of the most robust nongenetic interventions to extend lifespan in animal models, failed to extend the lifespan of WRNexoΔ mutant flies and even had a detrimental effect in females. Interestingly, the mean lifespan of WRNexoΔ mutant flies was not reduced on a protein-rich diet compared to that of wild-type (WT) flies. Compared to WT control flies, the mutant flies also exhibited altered responses to DR in their resistance to starvation and oxidative stress, as well as changes in sleep/wake patterns. These findings show that the WRN protein is necessary for mediating the effects of DR and suggest that the exonuclease domain of WRN plays an important role in metabolism in addition to its primary role in DNA-repair and genome stability.
Keywords: Werner syndrome, dietary restriction, lifespan, aging, Drosophila
Introduction
Werner syndrome (WS; OMIM# 277700) is an autosomal recessive disease that is characterized by accelerated aging with a high incidence of cancer, heart disease, low muscle mass, and metabolic syndromes including type II diabetes, dyslipidemia, and fatty liver (Oshima et al. 2017). Recent data show that the median life expectancy of a person with WS is 54 years old (Oshima et al. 2017). The leading causes of WS-associated death are cancer and myocardial infarction (Huang et al. 2006). WS is caused by mutations in the WRN gene (Huang et al. 2006; Oshima et al. 2017). The WRN protein is a member of the RecQ family of helicases and plays important roles in DNA structure and function including repair, replication, recombination, and telomere maintenance (Huang et al. 2006; Oshima et al. 2017). In humans, the WRN protein contains domains for a 3′ to 5′ ATP-dependent DNA helicase activity and a 3′ to 5′ DNA exonuclease activity. Over 80 different types of mutations in various regions of WRN gene has been discovered in WS patients, including substitution mutations within the exonuclease domain (Huang et al. 2006; Oshima et al. 2017; Cassidy et al. 2019). To date, several animal models of WS have been generated (reviewed in Harkema et al. (2016); Aumailley and Lebel (2021)). For example, mutant mice with only the helicase domain deleted (WrnΔhel/Δhel) and null mutant mice with both the helicase and exonuclease activities abolished (Wrn−/−) exhibited a reduction in mean lifespan and disease-free age ranging from 10 to 16.5% compared to wild-type (WT) mice (Lebel et al. 2003; Massip et al. 2010; Aumailley et al. 2015). These observations suggest that both domains are required for normal lifespan in mice and contribute to premature mortality when not fully functional. Unlike mice, the helicase and exonuclease domains of the human WRN protein are encoded by independent genes in worms and flies (Lee et al. 2004; Bolterstein et al. 2014). Loss-of-function mutant worms for wrn-1, the C. elegans ortholog of the helicase domain of the human WRN, also showed a strong reduction in lifespan (Dallaire et al. 2012). Although multiple animal studies have reported roles of the helicase domain of WRN protein in accelerated aging phenotypes and shortened lifespan, relatively less is known about the physiological functions of the exonuclease domain (Bolterstein et al. 2014). In Drosophila, the exonuclease domain of WRN protein is encoded by WRNexo (CG7670; FBgn0038608) (Boubriak et al. 2009; Bolterstein et al. 2014; Gramates et al. 2022; Ozturk-Colak et al. 2024). The deletion null mutant flies for WRNexo removing most of its gene sequences (WRNexoΔ) are fertile and viable despite some developmental defects (Bolterstein et al. 2014). The mutants recapitulate accelerated ageing phenotypes of WS with an increased tumor incidence and shortened lifespan, revealing critical roles of the exonuclease domain in WS (Cassidy et al. 2019). Interestingly, the mutants also display reduced fat contents, abnormal activity/sleep patterns, and reduced resistance to some environmental stresses such as starvation and thermal stress (Cassidy et al. 2019; Epiney et al. 2021). These observations suggest that the exonuclease domain also plays an important role in metabolism and systemic physiology beyond its primary function in genome stability.
Dietary restriction (DR) or caloric restriction (CR), reduction of food or calorie intake without causing malnutrition, is one of the most robust nongenetic interventions that delays aging and extends the lifespan of a variety of model organisms, including rhesus monkeys and fruit flies (Fontana and Partridge 2015). DR also promotes health by mitigating many age-related disease symptoms in both humans and animal models of human disease (Wu et al. 2022). As such, DR can be a potential nongenetic treatment option for WS patients. In rats, it was shown that CR increases the WRN protein level in the liver (Kahyo et al. 2008), functionally homologous to the fat body in Drosophila, suggesting that diet can regulate the expression of WRN. Importantly, the fat body plays critical roles in mediating lifespan extension by DR in flies (Bai et al. 2012; Banerjee et al. 2012). Yet, whether DR could ameliorate accelerated aging symptoms and delay premature mortality of WS has not been tested to date. Although the molecular mechanisms of DR are not fully understood, recent research has shown that nutrient sensing pathways play important roles in DR effects (Pignatti et al. 2020). In flies, a number of studies have shown that restriction of dietary yeast, the main protein source in the diet, is sufficient to extend lifespan (Grandison et al. 2009; Ja et al. 2009; Katewa et al. 2012; Gallinetti et al. 2013; Lee et al. 2014; Hoedjes et al. 2017), indicating that molecular pathways related to protein sensing and/or metabolism may limit the beneficial effects of DR. This also implies that DR could extend lifespan and delay accelerated aging processes of fly models of WS if their protein sensing mechanisms are not impaired.
Here, we investigate the physiological roles of the exonuclease activity of WRN in DR/diet-mediated lifespan, stress resistances, and sleep/activity patterns using WRNexoΔ null mutant flies (Bolterstein et al. 2014; Cassidy et al. 2019; Epiney et al. 2021). Our findings show that the WRNexoΔ mutant flies display a significantly altered physiological response to diet, including a deleterious effect on lifespan by DR in female flies, compared to WT flies. This work contributes to the understanding of the benefits and costs of dietary interventions in WS patients.
Materials and methods
Fly stocks and maintenance
WRNexo Δ null mutants (w1118/w1118; +/+; WRNexoΔ/TM6, Sb, GFP) and their isogenic w1118 control flies were generously provided by Elyse Bolterstein (Bolterstein et al. 2014; Cassidy et al. 2019; Epiney et al. 2021). The mutant flies were backcrossed four times to w1118 in 2014 (Bolterstein et al. 2014) and again in 2019 (Cassidy et al. 2019) in the Bolterstein lab. The work presented in the manuscript was performed during 2021 and 2022. Flies were expanded in vials (23 mm × 95 mm) containing ∼ 5 mL of a standard cornmeal-based medium for larval growth (Bloomington formula: Nutri-Fly, Genesee Scientific, Cat#: 66-113) adapted from the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu). All flies for growth/expansion and experiments were maintained at 25°C under a 12 h:12 h light–dark cycle with 40∼60% relative humidity conditions. For all experiments performed, ∼48-h cohorts after eclosion were collected and allowed an additional one day of posteclosion mating on the cornmeal-based medium before being separated by sex and genotype (homozygous WRNexoΔ mutants) on light CO2 anesthesia. Separated flies were then transferred by sex to respective sucrose-yeast (SY) experimental food vials with fixed 5% [w/v] sucrose (Genesee Scientific, Cat#: 62-112) and varying yeast (Brewer's yeast, MP Biomedicals) concentrations (1%, 5%, 20% [w/v]) (Bass et al. 2007). For relative comparison, 1%, 5%, 20% yeast diets were denoted as 1Y malnutrition (Mal) diet, 5Y (DR) diet, and 20Y control (Con) diet, respectively. The nutritional contents, including the amounts of total protein and carbohydrates in the Bloomington formula and the SY diets, are described in Supplementary Table 1. For yeast supplementation experiment in Fig. 4, yeast paste was prepared from a 1:1.25 [w/v] yeast (Brewer's yeast, MP Biomedicals) to water ratio. Experimental food vials were prepared in batches, wrapped with plastic bags to prevent dehydration, and stored in 4°C refrigerators until use. Only fresh food vials less than two weeks old were used for all assays.
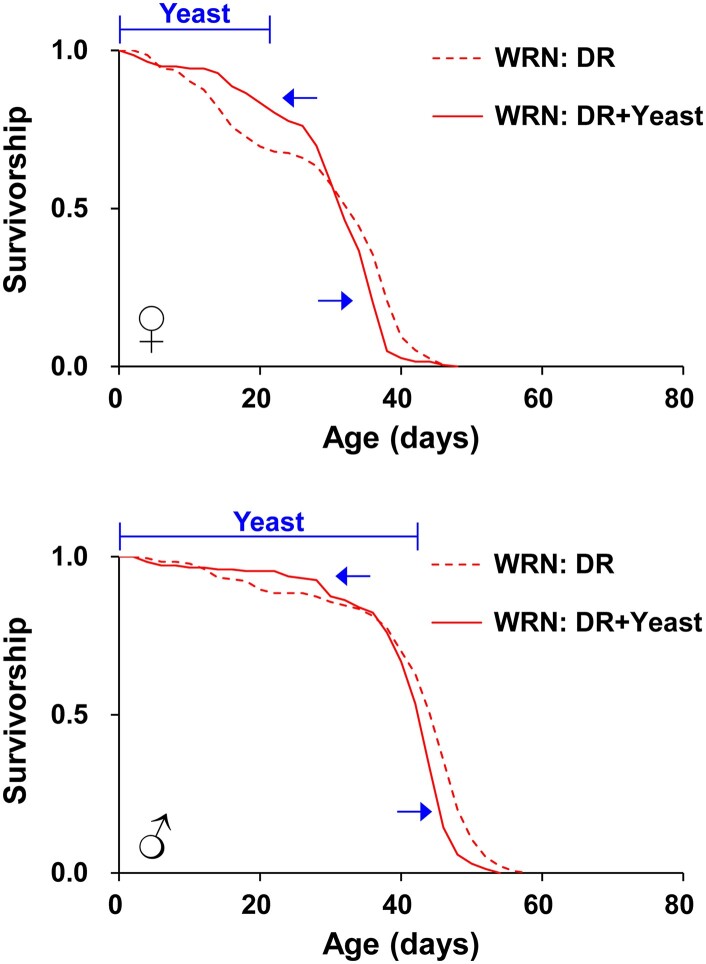
Fig. 4.
Effects of yeast supplementation on lifespan of WT and WRN mutant flies. Survivorship of female a) and male b) flies of WT and WRN mutants. DR: 5% yeast with 5% sucrose, DR + Yeast: DR diet supplemented with yeast paste (Brewer's yeast mixed with water in 2.5:1 ratio). Yeast paste was supplemented fresh on the side of lifespan vials every 2 days when dead flies were counted. Blue bars labelled “Yeast” at the top of the graphs represent the duration of time for yeast supplementation. Blue arrows indicate representative time points when yeast supplementation increased the survival of the WRN mutants. Refer to Material and methods and Supplementary Data 4 for descriptive statistical analysis and sample sizes.
Lifespan assays
Seven to ten replicate vials with up to ∼25 flies per replicate vial were set up for each diet, genotype, and sex. Flies were transferred to fresh vials every 2–3 days (usually 2 days) for Fig. 1 and every 2 days for Fig. 4. At each transfer, dead flies were counted and recorded on physical data forms before statistical analysis using JMP 14 (SAS Inc.) statistical package and the online analysis pipeline OASIS2 (https://sbi.postech.ac.kr/oasis2/) (Han et al. 2016). Lost or dead/damaged flies stuck on the cotton ball during transfer were censored from analysis. For Fig. 4, a half spatula-full of refrigerated yeast paste (less than one week old after preparation) was smeared on the side of DR/5Y food vials a few hours before each transfer. To measure accurate mortality pattern in Fig. 4, transfer was performed at the same time of day (between 8 AM and 10 AM).
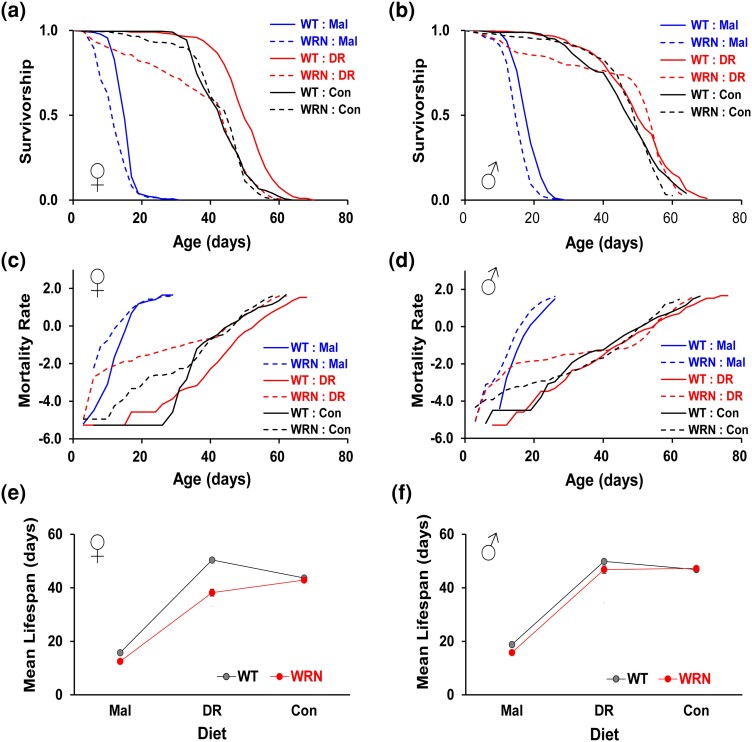
Fig. 1.
Effects of yeast-restriction diets on the lifespan of WT and WRN mutant flies. a,b) Survivorship. c,d) Mortality rate H(t): the risk of death at a given time (t) calculated using the following formula: H(t) = −log(S(t)), where S(t) represents the survival function estimated with the Kaplan–Meier estimator. e,f) Mean lifespan response to three different yeast-restriction diets. a,c,e): Males; b,d,f): Females. Mal (1Y): 1% yeast with 5% sucrose, DR (5Y): 5% yeast with 5% sucrose, Con (20Y): 20% yeast with 5% sucrose. Refer to the main text and Supplementary Data 1 for descriptive statistical analysis and sample sizes.
Stress assays
For both starvation and oxidative stress assays, separated flies (∼48 h cohorts followed by one day of post eclosion mating) were aged for 9–10 days in 5Y and 20Y food vials until the day of the assays. Due to an extremely high early mortality particularly in WRNexoΔ mutants, the 1Y malnutrition diet was not tested for stress response. Flies (6 replicates with 10–20 flies in each replicate) of each condition were transferred to starvation vials (1.5% agar [Genesee Scientific, Cat#: 66-103] as water source) and oxidative stress vials with paraquat (Methyl viologen dichloride hydrate; Sigma, Cat#: 856177) solution (10 mM paraquat in 10% [w/v] sucrose solution). For each oxidative stress vial, one piece of Kimwipe (Kimberly-Clark Professional) soaked with 2 mL of the paraquat solution was placed into the vial, then two round filter papers (23 mm, Whatman) were pushed into the vial until the filter papers were damp with paraquat solution. The excess paraquat solution was wiped off the sides of the vial. To control artifacts caused by potentially different feeding rhythms of flies in different conditions, flies for oxidative stress were first wet-starved for 5 h in starvation vials (1.5% agar) before transferred to paraquat solution vials. For both stress assays, the number of dead flies in each vial was counted every 2–8 h until all of the flies were dead.
Sleep/activity measurement
Monitoring of locomotor activity and sleep (defined as inactivity for 5 or more minutes) was assayed using the Drosophila Activity Monitoring (DAM, Trikinetics) system following established protocols (Chiu et al. 2010; Pfeiffenberger et al. 2010a, 2010b). Briefly, the DAM2 monitors contain 32 channels in which glass tubes containing food and flies are loaded. Each channel records the fly's movements continuously by shining an infrared beam through the center of the glass tube and recording the number of beam breaks. Flies aged for ∼7 days on each diet were loaded into glass tubes containing corresponding diet after light CO2 anesthetization. The activity and sleep of single flies were monitored for a period of ∼3 days, resulting in a total duration of diet treatment ∼10 days at the end of sleep measurement, which is comparable to the flies used for stress assays. To remove potential effects of CO2 anesthesia influencing the sleep and activity of flies, the first experimental day (day 8) was discarded from sleep/activity analysis. Also, flies with less than 10 beam breakings during the last experimental day, which is usually an indication of damaged flies due to moisture condensation in the glass tubes, were also removed from analysis. Activity and sleep profiles and parameters were generated with the Counting Macro program (Pfeiffenberger et al. 2010b).
Statistics
Descriptive survival statistics (such as mean lifespan and mortality rates) and pair-wise comparison of survival curves between groups (log-rank test) for lifespan and stress resistance assays (Figs. 1, 2 and 4; Supplementary Data 1, 2 and 4) were obtained using OASIS2 (https://sbi.postech.ac.kr/oasis2/) (Han et al. 2016) and JMP 14 (SAS Inc.) statistical package. Statistical significance for sleep parameters (Fig. 3) was determined using a two-way ANOVA following the Tukey HSD posthoc test.
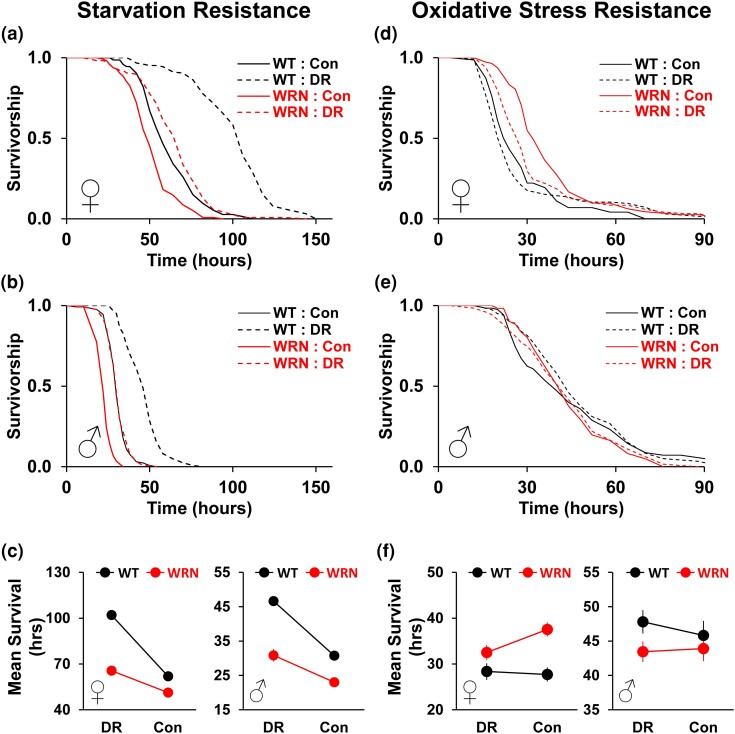
Fig. 2.
Effects of yeast-restriction diets on the stress response of WT and WRN mutant flies. a–c) Starvation Resistance. d–f) Oxidative Stress Resistance. a,d) Survivorship of females. b,e) Survivorship of males. c,f) Mean survival changes in response to diets. DR (5Y): 5% yeast with 5% sucrose, Con (20Y): 20% yeast with 5% sucrose. Refer to the main text and Supplementary Data 2 for descriptive statistical analysis and sample sizes.
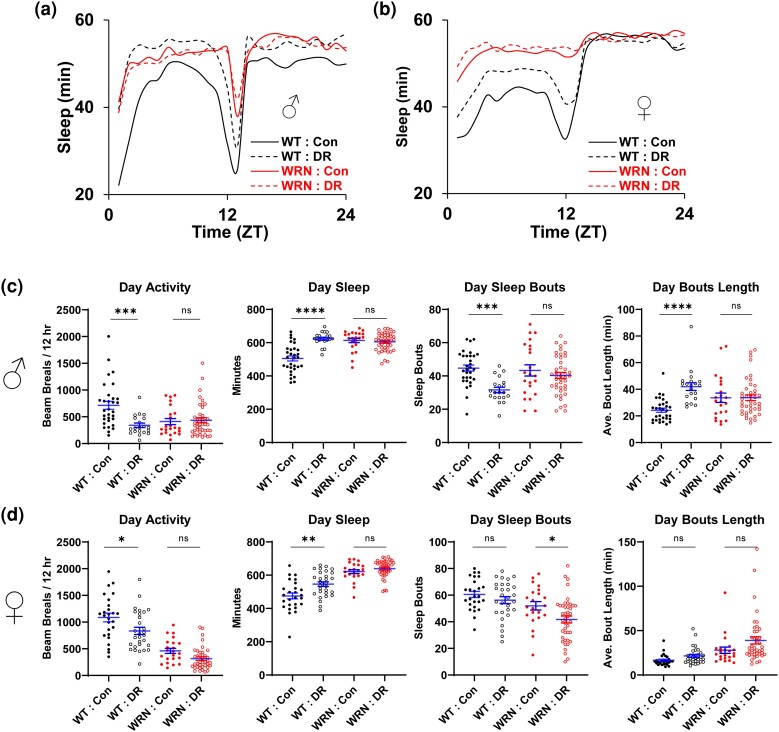
Fig. 3.
Effects of yeast-restriction diets on the activity and sleep patterns of WT and WRN mutant flies. Flies were fed on DR (5% yeast with 5% sucrose) or Con (20% yeast with 5% sucrose) diets for ∼7 days in vials before sleep measurements were initiated using the DAM system. Sleep was monitored for about 3 days on each diet. ZT0: light on, ZT12: light off. The first day of sleep data was excluded from the analysis due to CO2 anesthesia. a,b) Twenty-four-hour sleep profiles (average sleep duration per hour plotted in minutes sleep) for male (a) and female (b) flies of WT and WRN mutants. ZT 0-12 and ZT 12-24 correspond to the 12 h of the day/light and the night/dark phases, respectively. c,d) Activity and sleep parameters for male (c) and female (d) flies of WT and WRN mutants during the light/day phase. Activity: The number of fly beam breaks recorded in the DAM machine. Sleep: The average total sleep time in minutes, calculated as the product of sleep bout numbers and sleep bout length in minutes. Sleep bouts: The total number of sleep episodes, defined as periods of >5 min of inactivity. Bout length: The average sleep bout length. Asterisks indicate P values resulting from a 2-way ANOVA followed by the Tukey multiple comparisons post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Refer to the main text and Supplementary Data 3 for descriptive statistical analysis, sample sizes, and the analysis for the night/dark phase.
Results
DR fails to extend lifespan of WRNexoΔ mutant flies
A previous study showed that the median lifespan of WRNexoΔ mutant flies was reduced compared to that of WT flies by 44% in females and 19% in males (Cassidy et al. 2019). To explore how diet impacts the lifespan of WRNexoΔ mutant flies and also to test whether DR has the potential to delay premature aging and reverse shortened lifespan of the mutants, we used a protein-restriction DR protocol, where yeast (protein source) concentration is diluted while sugar (carbohydrate source) concentration is fixed (Bass et al. 2007; Min et al. 2007; Ja et al. 2009). Unlike the total dilution protocol where both yeast and sugar are diluted (Bass et al. 2007), the yeast-dilution DR regimen is known to be devoid of DR-independent mortality caused by desiccation (Ja et al. 2009) and sugar-induced obesity (van Dam et al. 2020). Therefore, our diet protocol accurately assesses the impacts of DR on the aging process and other health markers. To understand the range of beneficial and deleterious effects by diets, we employed a reaction norm approach (Tatar 2007) by using three serially diluted diets (1Y/Mal, 5Y/DR, 20Y/Con diets; see Materials and methods).
Overall, lifespan and mortality analyses revealed significant gene and diet effects in a sex-dependent manner. In the 1Y/Mal diet, lifespan was dramatically reduced compared to that of 20Y diet in both WT (female: 15.8 days vs 43.7 days; −63.9%, χ2 = 422.3, P < 0.001; male: 18.8 days vs 46.9 days; −59.9%, χ2 = 369.5, P < 0.001) and WRNexoΔ flies (female: 12.5 days vs 42.9 days; −70.8%, χ2 = 417.7, P < 0.001; male: 15.8 days vs 47.3 days; −66.5%, χ2 = 379.1, P < 0.001), presumably due to malnutrition caused by severe protein restriction in 1Y diet. In the 1Y diet, despite the significant decrease in lifespan of WT flies, the lifespan of WRNexoΔ flies mutant flies was even shorter than that of WT, indicating that the physiological processes in the mutants are still capable of responding to the diet even under malnutrition conditions (female: 15.8 days (WT) vs 12.5 days (WRNexoΔ); −20.6%, χ2 = 38.9, P < 0.001; male: 18.8 days (WT) vs 15.8 days (WRNexoΔ); −15.8%, χ2 = 41.5, P < 0.001) (Fig. 1 and Supplementary Data 1). Surprisingly, in protein-rich 20Y/Con diet, the mean lifespan of WRNexoΔ mutant flies was not reduced compared to that of WT flies (female: 43.7 days (WT) vs 42.9 days (WRNexoΔ), −1.7%, χ2 = 0.1, P = 0.705; male: 46.9 days (WT) vs 47.3 days (WRNexoΔ), 0.9%, χ2 = 0.3, P = 0.589) (Fig. 1 and Supplementary Data 1). These results show that the composition of food impacts the lifespan of WRNexoΔ mutant flies and suggest that macro- and/or micronutrients in yeast can slow down or even reverse the accelerated premature death of WRNexoΔ flies. As expected, DR/5Y diet significantly extended lifespan compared to Con diet in WT (female: 15.5%, χ2 = 62.2, P < 0.001; male: 6.4%, χ2 = 6.8, P = 0.009) (Fig. 1 and Supplementary Data 1). DR was more effective in increasing lifespan in females, which is consistent with previous reports that females typically display larger lifespan increases by DR in flies (Magwere et al. 2004). However, DR failed to extend lifespan of WRNexoΔ flies in both sexes (Fig. 1 and Supplementary Data 1). The mean lifespan of WRNexoΔ mutants in DR diet was even shorter than that of Con diet in both sexes with little to no statistical significance (female: −11%, χ2 = 0.9, P = 0.335; male: −0.9%, χ2 = 3.2, P = 0.074). Notably, similar to the previous report by Cassidy et al. (2019), the overall impact of the WRNexo mutation on mean lifespan was stronger in female mutants. Although male WRNexoΔ mutants displayed increased early mortality on the DR diet compared to male WT flies, their mean lifespan was comparable to that of WT flies. Furthermore, this increased early mortality of male WRNexoΔ mutants on the DR diet disappeared on the Con diet, implying that dietary yeast protects against early mortality in the mutants (Fig. 1 and Supplementary Data 1). Together, these results indicate that the lifespan responses to dietary yeast is altered in WRNexoΔ mutant flies, with a sexually dimorphic lifespan pattern. They also suggest that a functional WRNexo protein plays a role in the lifespan responses to dietary yeast and is required for DR-mediated lifespan extension in flies.
WRNexoΔ mutants display altered diet-dependent stress resistance
As DR was incapable of lifespan extension and was even deleterious for the lifespan of WRNexoΔ flies, leading to a higher rate of early mortality during the first ∼three weeks (Fig. 1, a and c), we hypothesized that the physiological and metabolic adaptations linked to lifespan extension by DR may also be altered or impaired in the mutants. To test this hypothesis, we first evaluated starvation resistance of WRNexoΔ flies along with their control flies after ∼10 days on DR and Con diets. The 1Y/Mal diet was not tested due to high mortality in both WT and mutants. Since DR administered with a reduction in dietary yeast, as per the protocol employed in this study, increases starvation resistance in WT flies (Katewa et al. 2012, 2016), given their lifespan responses to DR, we predicted that WRNexoΔ mutant flies would exhibit an altered starvation resistance pattern on these diets. In both sexes, WRNexoΔ mutants were more sensitive to starvation stress and died faster than WT flies in both DR (female: 102.0 h (WT) vs 65.6 h (WRNexoΔ); −35.7%, χ2 = 136.4, P < 0.001; male: 46.6 h (WT) vs 30.8 h (WRNexoΔ); −33.9%, χ2 = 148.0, P < 0.001) and Con (female: 63.0 h (WT) vs 51.3 h (WRNexoΔ); −35.7%, χ2 = 136.4, P < 0.001; male: 30.8 h (WT) vs 23.0 h (WRNexoΔ); −25.1%, χ2 = 93.8, P < 0.001) diets (Fig. 2a–c and Supplementary Data 2). This observation is largely consistent with a recent report (Epiney et al. 2021), although a standard cornmeal-based diet instead of SY diet was used in the study. As expected, DR significantly increased starvation resistance in WT female and male flies by 64.6% (mean survival: 62.0 h (Con) vs 102.0 h (DR), χ2 = 138.5, P < 0.001) and 51.6% (mean survival: 30.8 h (Con) vs 46.6 h (DR), χ2 = 149.6, P < 0.001), respectively (Fig. 2). In WRNexoΔ flies, however, DR resulted in a smaller increase in starvation resistance compared to that of WT. The increase of starvation resistance by DR was only 27.9% (mean survival: 51.3 h (Con) vs 65.6 (DR) hours, χ2 = 44.3, P < 0.001) and 30.8% (mean survival: 23.0 h (Con) vs 30.8 (DR) hours, χ2 = 30.9, P < 0.001) in female and male mutants, respectively. This pattern corresponds to the lifespan results in Fig. 1 and suggests that a functional WRNexo protein is required for the full benefit of DR to increase resistance to starvation stress.
Next, using the same rearing conditions as those used for assessing starvation resistance, we measured oxidative stress resistance. Despite shortened lifespan and reduced resistance to starvation stress, a previous study showed that WRNexoΔ females are more resistant to oxidative stress (5% H2O2) partially due to increased activities of antioxidant defense system (Epiney et al. 2021). Given the observation that the effects of DR on lifespan and starvation resistance were less pronounced in WRNexoΔ flies than WT flies, we hypothesized that WRNexoΔ flies might display greater sensitivity to oxidative stress when reared in DR diet compared to Con diet. To test this hypothesis, we chose paraquat (methyl viologen; 10 mM in 10% sucrose solution; see Materials and method) to induce oxidative stress, similar to its use in a previous study (Epiney et al. 2021). In males, neither genotype (WT vs WRNexoΔ) nor diet (Con vs DR) showed significant differences in survival after paraquat treatment (Fig. 2d–f and Supplementary Data 2). However, similar to the report by Epiney et al. (2021), female WRNexoΔ flies were more resistant to paraquat in both Con (27.7 h (WT) vs 37.5 h (WRNexoΔ); 35.4%, χ2 = 21.2, P < 0.001) and DR diets (28.4 h (WT) vs 32.5 h (WRNexoΔ); 14.7%, χ2 = 8.7, P = 0.003) (Fig. 2d–f and Supplementary Data 2). Notably, while mean survival of WT flies on paraquat was not significantly different between Con and DR (28.4 h (Con) vs 27.7 h (DR); χ2 = 0.1, P = 0.737), that of WRNexoΔ flies was lower in DR than in Con diet by ∼13% (37.5 h (Con) vs 32.5 h (DR); χ2 = 7.2, P = 0.007). This suggests that, unlike WT flies where the yeast concentration difference between DR and Con diets (5% vs 20%) does not lead to varying resistance to oxidative stress, lower yeast concentration in the DR diet reduces resistance in WRNexoΔ mutant flies. Therefore, similar to lifespan and starvation resistance results, the oxidative stress resistance pattern strongly indicates that WRNexoΔ flies have impaired physiological responses to diets.
WRNexoΔ mutants display altered diet-dependent sleep repatterning
In flies, the absence or quiescence of locomotive activity beyond a given time period (5 min), is considered sleep or rest (Pfeiffenberger et al. 2010b; Linford et al. 2012). This approach displays several key analogous features of mammalian sleep, including an increased arousal threshold, regulation by circadian clocks, and homeostatic needs (Allada et al. 2017). It has been successfully used to dissect the genetic and neuronal mechanisms of sleep. Similar to mammals, environmental changes such as in nutritional composition and value in the food affect locomotor activity and sleep patterns in flies (Catterson et al. 2010; Linford et al. 2012; Brown et al. 2020). Since WRNexoΔ mutant flies displayed altered lifespan and stress resistance responses to diets (Figs. 1 and 2), we hypothesized that the mutants' activity/sleep patterns in response to yeast dilution is also disrupted. After pretreating the flies on DR and Con diets for ∼7 days, we monitored time-dependent locomotor activity and sleep patterns of the flies on DR and Con diets for ∼2 days. We analyzed the total activity level (the number of flies' beam breaks in the DAM machine) and total sleep time (the product of sleep bout numbers and sleep bout length in minutes) separately for daytime and nighttime.
Compared to Con diet, DR diet significantly reduced the total activity of WT flies in both sexes (males: 713.8 [Con] vs 340.7 [DR], P < 0.001, females: 1086 [Con] vs 835.1 [DR], P = 0.0165), particularly during the daytime, which led to increased total sleep during the daytime (males: 505.2 min [Con] vs 623.6 min [DR], P < 0.0001, females: 475.1 min [Con] vs 546.3 min [DR], P = 0.0013) (Fig. 3 and Supplementary Data 3). This DR-dependent increase in daytime sleep in WT flies was primarily due to decreased sleep bout numbers (males: 44.7 [Con] vs 31.6 [DR], P < 0.001) with increased sleep bout lengths (males: 24.2 min [Con] vs 42.0 min [DR], P < 0.0001) particularly in males (Fig. 3c and Supplementary Data 3). In females, although the trend of change in sleep bout numbers and sleep bout length due to DR was similar to that in males, the differences between the Con and DR diets were not statistically significant (Fig. 3d and Supplementary Data 3).
As predicted, unlike WT flies, the repatterning of activity and sleep by dietary yeast was strongly suppressed in WRNexoΔ mutants. There were negligible differences between Con and DR diets in total activity (males: 411.1 [Con] vs 434.8 [DR], P = 0.992, females: 461.4 [Con] vs 316.5 [DR], P = 0.2944) and total sleep (males: 613.5 min [Con] vs 605.0 min [DR], P = 0.9565, females: 621.4 min [Con] vs 639.6 min [DR], P = 0.7567) during the daytime. In male mutants, unlike WT flies, neither sleep bout numbers nor sleep bout lengths were affected by DR (Fig. 3 and Supplementary Data 3). These observations indicate that WRNexoΔ mutant flies have lost the mechanisms to regulate activity and sleep in response to dietary yeast. Thus, these results also suggest that, in addition to altered baseline sleep in WRNexoΔ mutants as observed previously (Cassidy et al. 2019; Epiney et al. 2021), a functional exonuclease activity of WRN protein is required for diet-mediated sleep regulation.
Yeast supplementation delays accelerated mortality of WRNexoΔ mutants
Above concentrations that cause malnutrition, dietary yeast limits lifespan; the higher the yeast concentration, the shorter the lifespan (Bass et al. 2007; Katewa et al. 2016; Lin et al. 2018). Since DR did not extend lifespan of WRNexoΔ mutant flies, resulting in a significant increase in early mortality, we hypothesized that WRNexoΔ mutant flies require higher nutritional intake for survival during their early stages in adult compared to WT flies. To test this possibility, we examined the effect of additional dietary yeast on the lifespan of WRNexoΔ mutants. Flies were supplemented with a concentrated yeast paste (see Materials and methods) to DR diet for three weeks for females and six weeks for males (Fig. 4). The yeast paste was supplied on the side of food vial such that flies can consume both yeast paste and DR diet ad libitum. As predicted, yeast supplementation delayed and was protective against the early mortality observed in WRNexoΔ flies on the DR diet (Fig. 4; indicated by arrows). For example, at day 20, yeast supplementation increased survival of mutants on DR diet by 14 and 6% in females and males, respectively, while it had no obvious impacts on survival in WT flies. Similarly, the day of 25% mortality was delayed from day 18 (DR) to day 28 (yeast supplementation) in female WRNexoΔ flies. In male WRNexoΔ flies, the day of 25% mortality was day 40 for both groups, which is comparable to that of WT on DR diet (day 38). Overall, these data indicate that, despite the overall proaging effects of yeast in WT flies (Bass et al. 2007; Libert et al. 2007; Katewa et al. 2012), macro- and/or micronutrients in yeast during early life can at least partially reverse the accelerated aging process of WRNexoΔ flies. Given that protein is a major component of the dietary yeast in the yeast paste, our findings also raise the possibility that supplementing protein and/or specific amino acids might be able to delay the accelerated aging in WRNexoΔ flies.
Discussion
WS is caused by mutations in the human WRN gene, which contains two important functional domains, a RecQ-type helicase domain and an exonuclease domain. At present, there is no cure for WS, which highlights dietary and lifestyle interventions, such as DR, as potential options for increasing the life expectancy of individuals with WS. In this study, utilizing a deletion null mutant Drosophila (WRNexoΔ) for the WRNexo gene encoding a protein homologous to the exonuclease domain of the human WRN gene (Bolterstein et al. 2014; Cassidy et al. 2019; Epiney et al. 2021; Gramates et al. 2022; Ozturk-Colak et al. 2024), we demonstrate that a yeast-restriction DR regimen fails to extend the lifespan of a fly model of WS. We also show that WRNexoΔ mutants display significantly altered overall physiological (stress resistance) and behavioral (activity/sleep) responses to DR compared to those of WT control flies (Figs. 1–3). Therefore, our observations provide direct evidence that the WRNexo protein is necessary for a wide range of DR responses in WT flies. Together with recent studies showing its role in lipid metabolism (Cassidy et al. 2019; Epiney et al. 2021; Tian et al. 2024), our data also raise the possibility that the exonuclease domain of the WRN protein may be directly involved in nutrient sensing and metabolism, particularly amino acids given the type of DR regimen used in our study. However, our results may also indicate that DR affects the WRN protein's primary functions in genome stability and DNA-related molecular processes, including replication, repair, recombination, and transcription, thereby indirectly altering the DR response in lifespan and physiology observed in WRNexoΔ mutants. In fact, mutant flies deficient of DNA-repair system tend to accumulate second-site mutations, which might have affected the phenotypes of WRNexo mutants described in our study. While we cannot rule out the possibility that second-site mutations occurred in the WRNexo mutants, we favor the idea that potential second-site mutations do not significantly contribute to the main phenotypes reported in this manuscript. Previous studies have shown that DR is not linked to protection against somatic DNA damage, but it can still delay aging and extend lifespan in DNA-repair deficient mutant mice and flies that have accumulated massive somatic mutations (Edman et al. 2009; Vermeij et al. 2016). Furthermore, as presented in Fig. 1, homozygous male mutant flies for WRNexo exhibited lifespans almost comparable to those of control flies across different SY diets. We interpret this observation as indicating that the mutant flies, when maintained in a heterozygous state with balancer chromosomes, do not possess second-site mutations critically affecting lifespan.
Notably, the mean lifespan of female WRNexoΔ mutants on the DR diet (5Y) was even shorter than that of the protein-rich Con diet (20Y), primarily due to increased early mortality during the first ∼ two weeks (Fig. 1, a, c, and e). This observation indicates that nutrient-restricted diets such as the 5Y diet used in this study, which are beneficial in WT flies, can have deleterious effects in the fly model of WS. It also implies that WRNexoΔ mutant flies require more nutrients than the amount that confers lifespan extension in WT flies. In accordance with this, supplementation of protein-rich yeast pastes partially rescued the high early life mortality (Fig. 4). This finding provides additional evidence that certain nutrients present in dietary yeast can effectively decrease mortality in WRNexoΔ mutants. Considering that dietary yeast in our fly food serves as the primary source for protein (Bass et al. 2007; Linford et al. 2012), which is known to limit lifespan and also play as the major dietary component in DR-mediated lifespan modulation in flies (Bass et al. 2007; Libert et al. 2007; Katewa et al. 2012), we favor the idea that the molecular pathways involved in protein metabolism and sensing might be compromised in WRNexoΔ mutant flies. Indeed, our preliminary lifespan analysis on the Bloomington formula, which contains an even lower protein content (Supplementary Table 1) than the 5Y/DR diet, supports this idea. Unlike WT control flies, whose lifespan was longest on the 5Y/DR diet but decreased on both the protein-rich 20Y/Con and the malnutrition-like Bloomington formula, the lifespan of WRNexoΔ mutants linearly increased from its lowest on the Bloomington formula to the 20Y/Con diet (Supplementary Fig. 1). This confirms that protein supplementation beyond the DR diet is beneficial and rescues the shortened lifespan in the WRNexoΔ mutants. Furthermore, the lifespan of WRNexoΔ mutants on the Bloomington formula, although still reduced compared with that of WT control flies (Supplementary Fig. 1, P = 0.0231), was similar to that on the 1Y/malnutrition diet (Fig. 1 and Supplementary Fig. 1). Therefore, all these lifespan analyses using different protein concentrations (Fig. 1, and 4, Supplementary Fig. 1) support our main conclusion that nutritional value and composition, particularly dietary protein content, affect the lifespan of WRNexoΔ mutants.
However, it is also possible that the residual amounts of other macro- and micronutrients in dietary yeast, such as lipids and vitamins, could have contributed to the phenotypes of the mutants (Bahadorani et al. 2008; Zanco et al. 2021). For example, previous studies demonstrated that vitamin C and NAD+ could at least partially rescue the shortened lifespan of WRN mutants in some model organisms (Fang et al. 2019; Aumailley and Lebel 2021). A further study employing a defined medium that controls the concentrations of total protein and each amino acid, along with vitamin C or NAD+ supplementation, could provide a clearer answer to this possibility.
Elevated resistance against environmental stress is often associated with increased lifespan. While age functions as a confounding variable impacting stress resistance (Burger et al. 2007), the composition of diets, including yeast and sugar concentration, significantly affect the stress resistance of flies (Burger et al. 2007; Katewa et al. 2012; Strilbytska et al. 2021, 2022). For example, yeast-restriction DR increases survival during starvation stress, partly due to elevated lipid content (synthesized via lipogenesis) and enhanced utilization (broken down through lipolysis) (Katewa et al. 2012). Earlier studies indicates that WRNexoΔ mutants contain a significantly lower amount of body fat and are more sensitive to starvation stress compared to those of WT flies in both sexes. (Cassidy et al. 2019; Epiney et al. 2021). However, only female mutants unexpectedly exhibit increased resistance to oxidative stress, attributed to the upregulation of cellular antioxidant defense mechanisms (Cassidy et al. 2019; Epiney et al. 2021). Our observation is largely consistent with these findings: Regardless of diets, WRNexoΔ mutants exhibited greater sensitivity to starvation stress in both sexes, while displaying increased resistance to oxidative stress only in females compared to WT control flies (Fig. 2). With respect to the diet effects, although DR was still able to enhance the starvation resistance of WRNexoΔ mutant flies, the increase was much smaller than that was observed in WT flies in both sexes (Fig. 2, a and b). Unlike starvation stress resistance, diet showed minimal impact on oxidative stress resistance in both sexes of WT flies and in males of the mutants. Interestingly, DR negatively affected oxidative stress resistance in female mutants. This finding is consistent with an earlier study (Burger et al. 2007) and also comparable to their lifespan on DR and Con diets (Fig. 1). Taken together, although stress-specific with sexually dimorphic patterns, our data indicate that WRNexo plays a role in diet-dependent stress response. Also, considering the lifespan patterns of the mutants between DR and Con diets and compared to those of WT flies, these observations imply that the mutants' altered response to diets in starvation and oxidative stress resistance cannot fully explain their lifespan patterns.
There are some discrepant observations regarding the impacts of dietary sugar and yeast in fly food for sleep architecture (Catterson et al. 2010; Linford et al. 2012; Brown et al. 2020). Our data appear to align with one of the earlier studies (Catterson et al. 2010), revealing that dietary yeast tends to fragment sleep by increasing bout numbers while decreasing bout length, particular in male WT flies (Fig. 3, c and d). Although it is still not well understood whether lowered sleep quality caused by a protein-rich diet has a negative impact on lifespan or if yeast-driven repatterning of sleep contributes to DR-mediated lifespan modulation, our data indicate that the mechanisms by which flies sense the nutritional values of yeast to regulate sleep is strongly suppressed in WRNexoΔ mutant flies. It is worth noting that, unlike the lifespan patterns, the altered responses to dietary yeast in activity and sleep patterns in WT flies were more evident in males than in females. This raises the possibility that the repatterning of activity and sleep by DR is unlikely the key determinant of lifespan extension through DR.
Overall, using WRNexoΔ mutant flies, our data suggest previously uncharacterized roles of the exonuclease domain of the human WRN protein in response to diets to regulate lifespan, and stress response, and activity/sleep. Considering that the Drosophila WRNexo protein shares many molecular and biochemical characteristics of the human WRN protein (Brosh et al. 2001; Saunders et al. 2008; Boubriak et al. 2009; Mason et al. 2013; Cassidy et al. 2019; Epiney et al. 2021), we suggest that nutritional interventions and treatments for individuals with WS should be performed with care. Further studies aiming to identify molecular signatures, such as differentially regulated genes and metabolites, between WT and WRNexoΔ mutant flies on varied diets, investigating whether compromised genome stability in the mutants is influenced by diets, and determining the specific nutrients that impact the mutants' lifespan, hold significant importance for translational insights for WS patients.
Supplementary Material
Acknowledgments
We thank the Elyse Bolterstein lab for providing the mutant flies, and we also appreciate the technical assistance of Sarayu Alli, Nicholas Wright, Jason Ho, and Erica Hassoun in conducting lifespan and sleep assays. Additionally, we acknowledge the valuable feedback provided by Rafael Demarco, Lee Thompson, and Cynthia Corbitt on an earlier version of this manuscript.
Contributor Information
Eileen Sember, Department of Biology, University of Louisville, Louisville, KY 40292, USA.
Ranga Chennakesavula, Department of Biology, University of Louisville, Louisville, KY 40292, USA.
Breanna Beard, Department of Biology, University of Louisville, Louisville, KY 40292, USA.
Mubaraq Opoola, Department of Biology, University of Louisville, Louisville, KY 40292, USA.
Dae-Sung Hwangbo, Department of Biology, University of Louisville, Louisville, KY 40292, USA.
Data availability
Drosophila strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material available at G3 online.
Author contributions
Conceptualization: E.S. and D.H.; investigation: E.S., R.C., B.B., M.O., and D.H.; data curation: E.S., B.B., and D.H.; analysis: E.S., B.B., and D.H.; manuscript preparation: E.S. and D.H.; supervision, funding acquisition, and project administration: D.H. All authors have read and agreed to the published version of the manuscript.
Literature cited
- Allada R, Cirelli C, Sehgal A. 2017. Molecular mechanisms of sleep homeostasis in flies and mammals. Cold Spring Harb Perspect Biol. 9(8):a027730. doi: 10.1101/cshperspect.a027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley L, Garand C, Dubois MJ, Johnson FB, Marette A, Lebel M. 2015. Metabolic and phenotypic differences between mice producing a Werner syndrome helicase mutant protein and WRN null mice. PLoS One. 10(10):e0140292. doi: 10.1371/journal.pone.0140292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley L, Lebel M. 2021. The impact of vitamin C on different system models of Werner syndrome. Antioxid Redox Signal. 34(11):856–874. doi: 10.1089/ars.2020.8147. [DOI] [PubMed] [Google Scholar]
- Bahadorani S, Bahadorani P, Phillips JP, Hilliker AJ. 2008. The effects of vitamin supplementation on Drosophila life span under normoxia and under oxidative stress. J Gerontol A Biol Sci Med Sci. 63(1):35–42. doi: 10.1093/gerona/63.1.35. [DOI] [PubMed] [Google Scholar]
- Bai H, Kang P, Tatar M. 2012. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell. 11(6):978–985. doi: 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. 2012. Dsir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2(6):1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MDW. 2007. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 62(10):1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolterstein E, Rivero R, Marquez M, McVey M. 2014. The Drosophila Werner exonuclease participates in an exonuclease-independent response to replication stress. Genetics. 197(2):643–652. doi: 10.1534/genetics.114.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubriak I, Mason PA, Clancy DJ, Dockray J, Saunders RD, Cox LS. 2009. DmWRNexo is a 3′–5′ exonuclease: phenotypic and biochemical characterization of mutants of the Drosophila orthologue of human WRN exonuclease. Biogerontology. 10(3):267–277. doi: 10.1007/s10522-008-9181-3. [DOI] [PubMed] [Google Scholar]
- Brosh RM, Jr, von Kobbe C, Sommers JA, Karmakar P, Opresko PL, BroshRM, Jr. 2001. Werner syndrome protein interacts with human flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 20(20):5791–5801. doi: 10.1093/emboj/20.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EB, Shah KD, Faville R, Kottler B, Keene AC. 2020. Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. PLoS Genet. 16(3):e1008270. doi: 10.1371/journal.pgen.1008270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JM, Hwangbo DS, Corby-Harris V, Promislow DE. 2007. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. 6(1):63–71. doi: 10.1111/j.1474-9726.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Cassidy D, Epiney DG, Salameh C, Zhou LT, Salomon RN, Schirmer AE, McVey M, Bolterstein E. 2019. Evidence for premature aging in a Drosophila model of Werner syndrome. Exp Gerontol. 127:110733. doi: 10.1016/j.exger.2019.110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson JH, Knowles-Barley S, James K, Heck MM, Harmar AJ, Hartley PS. 2010. Dietary modulation of Drosophila sleep-wake behaviour. PLoS One. 5(8):e12062. doi: 10.1371/journal.pone.0012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. 2010. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J Vis Exp. 43:2157. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire A, Garand C, Paquel ER, Mitchell SJ, de Cabo R, Simard MJ, Lebel M. 2012. Down regulation of miR-124 in both Werner syndrome DNA helicase mutant mice and mutant Caenorhabditis elegans wrn-1 reveals the importance of this microRNA in accelerated aging. Aging (Albany NY). 4(9):636–647. doi: 10.18632/aging.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman U, Garcia AM, Busuttil RA, Sorensen D, Lundell M, Kapahi P, Vijg J. 2009. Lifespan extension by dietary restriction is not linked to protection against somatic DNA damage in Drosophila melanogaster. Aging Cell. 8(3):331–338. doi: 10.1111/j.1474-9726.2009.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epiney DG, Salameh C, Cassidy D, Zhou LT, Kruithof J, Milutinović R, Andreani TS, Schirmer AE, Bolterstein E. 2021. Characterization of stress responses in a Drosophila model of Werner Syndrome. Biomolecules. 11(12):1868. doi: 10.3390/biom11121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Lautrup S, Jensen MB, Yang B, SenGupta T, Caponio D, Khezri R, Demarest TG, Aman Y, et al. 2019. NAD+ augmentation restores mitophagy and limits accelerated aging in werner syndrome. Nat Commun. 10(1):5284. doi: 10.1038/s41467-019-13172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L. 2015. Promoting health and longevity through diet: from model organisms to humans. Cell. 161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinetti J, Harputlugil E, Mitchell JR. 2013. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 449(1):1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Agapite J, Attrill H, Calvi BR, Crosby MA, dos Santos G, Goodman JL, Goutte-Gattat D, Jenkins VK, Kaufman T, et al. 2022. FlyBase: a guided tour of highlighted features. Genetics. 220(4). doi: 10.1093/genetics/iyac035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 462(7276):1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Lee D, Lee H, Kim D, Son HG, Yang J-S, Lee S-JV, Kim S. 2016. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget. 7:56147–56152. doi: 10.18632/oncotarget.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema L, Youssef SA, de Bruin A. 2016. Pathology of mouse models of accelerated aging. Vet Pathol. 53(2):366–389. doi: 10.1177/0300985815625169. [DOI] [PubMed] [Google Scholar]
- Hoedjes KM, Rodrigues MA, Flatt T. 2017. Amino acid modulation of lifespan and reproduction in Drosophila. Curr Opin Insect Sci. 23:118–122. doi: 10.1016/j.cois.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, Poot M, Rubin CD, Chen D-F, Yang C-C, Juch H, et al. 2006. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat. 27(6):558–567. doi: 10.1002/humu.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Zid BM, Mak EM, Brummel T, Benzer S. 2009. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci U S A. 106(44):18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahyo T, Mostoslavsky R, Goto M, Setou M. 2008. Sirtuin-mediated deacetylation pathway stabilizes werner syndrome protein. FEBS Lett. 582(17):2479–2483. doi: 10.1016/j.febslet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng XZ, Hall D, Davis S, Nelson CS, Brem RB, et al. 2016. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab. 23(1):143–154. doi: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, Kapahi P. 2012. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 16(1):97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel M, Lavoie J, Gaudreault I, Bronsard M, Drouin R. 2003. Genetic cooperation between the Werner syndrome protein and poly(ADP-ribose) polymerase-1 in preventing chromatid breaks, complex chromosomal rearrangements, and cancer in mice. Am J Pathol. 162(5):1559–1569. doi: 10.1016/S0002-9440(10)64290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. 2014. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat Commun. 5(1):3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yook JS, Han SM, Koo HS. 2004. A werner syndrome protein homolog affects C. elegans development, growth rate, life span and sensitivity to DNA damage by acting at a DNA damage checkpoint. Development. 131(11):2565–2575. doi: 10.1242/dev.01136. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. 2007. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 315(5815):1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lin WS, Yeh SR, Fan SZ, Chen LY, Yen JH, Fu T-F, Wu M-S, Wang P-Y. 2018. Insulin signaling in female Drosophila links diet and sexual attractiveness. FASEB J. 32(7):3870–3877. doi: 10.1096/fj.201800067R. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Chan TP, Pletcher SD. 2012. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 8(5):e1002668. doi: 10.1371/journal.pgen.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. 2004. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 59(1):B3–B9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Mason PA, Boubriak I, Robbins T, Lasala R, Saunders R, Cox LS. 2013. The Drosophila orthologue of progeroid human WRN exonuclease, DmWRNexo, cleaves replication substrates but is inhibited by uracil or abasic sites: analysis of DmWRNexo activity in vitro. Age (Dordr). 35(3):793–806. doi: 10.1007/s11357-012-9411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massip L, Garand C, Paquet ER, Cogger VC, O’Reilly JN, Tworek L, Hatherell A, Taylor CG, Thorin E, Zahradka P, et al. 2010. Vitamin C restores healthy aging in a mouse model for werner syndrome. FASEB J. 24(1):158–172. doi: 10.1096/fj.09-137133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. 2007. Counting calories in Drosophila diet restriction. Exp Gerontol. 42(3):247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima J, Sidorova JM, MonnatRJ, Jr. 2017. Werner syndrome: clinical features, pathogenesis and potential therapeutic interventions. Ageing Res Rev. 33:105–114. doi: 10.1016/j.arr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk-Colak A, Marygold SJ, Antonazzo G, Attrill H, Goutte-Gattat D, Jenkins VK, Matthews BB, Millburn G, dos Santos G, Tabone CJ, et al. 2024. FlyBase: updates to the Drosophila genes and genomes database. Genetics. iyad211. doi: 10.1093/genetics/iyad211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. 2010a. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) system. Cold Spring Harb Protoc. 2010(11):pdb.prot5518. doi: 10.1101/pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. 2010bprocessing sleep data created with the Drosophila Activity Monitoring (DAM) system. Cold Spring Harb Protoc. 2010(11):pdb.prot5520. doi: 10.1101/pdb.prot5520. [DOI] [PubMed] [Google Scholar]
- Pignatti C, D’Adamo S, Stefanelli C, Flamigni F, Cetrullo S. 2020. Nutrients and pathways that regulate health span and life span. Geriatrics (Basel). 5(4):95. doi: 10.3390/geriatrics5040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RD, Boubriak I, Clancy DJ, Cox LS. 2008. Identification and characterization of a Drosophila ortholog of WRN exonuclease that is required to maintain genome integrity. Aging Cell. 7(3):418–425. doi: 10.1111/j.1474-9726.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilbytska O, Strutynska T, Semaniuk U, Burdyliyk N, Bubalo V, Bubalo V, Lushchak O. 2022. Dietary sucrose determines stress resistance, oxidative damages, and antioxidant defense system in Drosophila. Scientifica (Cairo). 2022:7262342. doi: 10.1155/2022/7262342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilbytska O, Zayachkivska A, Strutynska T, Semaniuk U, Vaiserman A, Lushchak O. 2021. Dietary protein defines stress resistance, oxidative damages and antioxidant defense system in Drosophila melanogaster. Ukr Biochem J. 93(5):90–101. doi: 10.15407/ubj93.05.090. [DOI] [Google Scholar]
- Tatar M. 2007. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip Top Gerontol. 35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- Tian Y, Lautrup S, Law PWN, Dinh ND, Fang EF, Chan W-Y. 2024. WRN loss accelerates abnormal adipocyte metabolism in Werner syndrome. Cell Biosci. 14(1):7. doi: 10.1186/s13578-023-01183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam E, van Leeuwen LAG, Dos Santos E, James J, Best L, Lennicke C, Vincent AJ, Marinos G, Foley A, Buricova M, et al. 2020. sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila. Cell Metab. 31(4):710–725.e7. doi: 10.1016/j.cmet.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij WP, Dolle ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, Wu H, Roks AJM, Botter SM, van der Eerden BC, et al. 2016. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Gao ZJ, Yu X, Wang P. 2022. Dietary regulation in health and disease. Signal Transduct Target Ther. 7(1):252. doi: 10.1038/s41392-022-01104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanco B, Mirth CK, Sgro CM, Piper MD. 2021. A dietary sterol trade-off determines lifespan responses to dietary restriction in Drosophila melanogaster females. Elife. 10:e62335. doi: 10.7554/eLife.62335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drosophila strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material available at G3 online.