Abstract
Transposable elements (TEs) are repetitive DNA that can create genome structure and regulation variability. The genome of Rhizophagus irregularis, a widely studied arbuscular mycorrhizal fungus (AMF), comprises ∼50% repetitive sequences that include TEs. Despite their abundance, two-thirds of TEs remain unclassified, and their regulation among AMF life stages remains unknown. Here, we aimed to improve our understanding of TE diversity and regulation in this model species by curating repeat datasets obtained from chromosome-level assemblies and by investigating their expression across multiple conditions. Our analyses uncovered new TE superfamilies and families in this model symbiont and revealed significant differences in how these sequences evolve both within and between R. irregularis strains. With this curated TE annotation, we also found that the number of upregulated TE families in colonized roots is 4 times higher than in the extraradical mycelium, and their overall expression differs depending on the plant host. This work provides a fine-scale view of TE diversity and evolution in model plant symbionts and highlights their transcriptional dynamism and specificity during host–microbe interactions. We also provide Hidden Markov Model profiles of TE domains for future manual curation of uncharacterized sequences (https://github.com/jordana-olive/TE-manual-curation/tree/main).
Keywords: mobile elements, genome evolution, arbuscular mycorrhizal fungi, gene expression, plant pathogens, repetitive DNA, transcriptome
Introduction
Arbuscular mycorrhizal fungi (AMF) are an ancient group of plant symbionts capable of colonizing thousands of different species. The AMF provides vitamins and minerals from the soil to the plants and receives carbohydrates and lipids in return (Martin et al. 2017). This relationship may have been established since plants first conquered the land (Delaux and Schornack 2021), and there is evidence showing that AMF improve plant growth and ecosystem productivity (Bonfante and Anca 2009). More recently, studies reported that these symbionts can retain atmospheric carbon, indicating they play a significant role in global carbon sequestration (Hawkins et al. 2023; Horsch et al. 2023). Nonetheless, despite their relevance for plant fitness and long-term evolution, AMF show low morphological variability with no apparent plant specificity (Corradi and Brachmann 2017).
In addition to being ecologically and agriculturally important, AMF harbors peculiar cellular features. Their spores and hyphae always carry thousands of nuclei, and to date, no stage where 1 or 2 nuclei coexist in one cell has ever been observed (Kokkoris et al. 2020). Furthermore, despite their longevity, no sexual reproduction has been formally observed in these organisms. However, evidence has now shown that AMF strains can either carry 1 (AMF homokaryons) or 2 nuclear (AMF heterokaryons) genotypes in their cells, a genetic characteristic found in sexually multinucleated strains of ascomycetes and basidiomycetes, suggesting that sexual reproduction does exist in these prominent symbionts (Ropars et al. 2016; Sperschneider et al. 2023).
The low morphological diversity of AMF masks remarkable differences in gene content and structure in this group. For example, species vary in genome size and gene counts (Morin et al. 2019), and the variation in genome size is significantly correlated with the abundance of transposable elements (TEs; Mathu et al. 2022). Recent studies based on chromosome-level assemblies of Rhizophagus irregularis also showed this model species carries 2 different (A/B) compartments, which are reminiscent of the “2-speed genome” structure previously reported in plant pathogens (Yildirir et al. 2022; Sperschneider et al. 2023). Overall, the A-compartment is gene-rich and is mostly composed of euchromatin, whereas the B-compartment contains a high concentration of TEs and is mainly composed of heterochromatin (Winter et al. 2018). Accordingly, in AMF, the A-compartment carries most “core genes”—i.e. genes shared by all members of the same species—and shows significantly higher gene expression, while the B-compartment has a higher density in repetitive DNA, as well as in secreted proteins and candidate effectors involved in the molecular dialogs between the partners of mycorrhizal symbiosis (Yildirir et al. 2022).
TEs are repetitive DNA classified into retrotransposons (class I), which use RNA molecules as intermediate for “copy-and-paste”, and DNA transposons (class II), which spread through “cutting-and-pasting” the DNA (Wicker et al. 2007). Each class is divided into orders and superfamilies based on pathways of transposition and phylogenetic relationships (Wicker et al. 2008; Seberg and Petersen 2009), and thus classifying TEs is important to describe the evolution of the genome and infer its impact on the biology of any organism (Wells and Feschotte 2020; Rech et al. 2022). For example, by modifying chromatin status and attracting transcription factors, these elements can also promote the regulation of gene expression (Bourque et al. 2018).
In the model, AMF R. irregularis, about 50% of the genome is composed of TEs (Yildirir et al. 2022; Manley et al. 2023; Sperschneider et al. 2023). Their higher abundance in the B-compartment is linked to higher rates of rearrangements (Sperschneider et al. 2023), and it was proposed that elevated TE expression in germinating spores may lead to new expansions of TEs in this AMF species (Dallaire et al. 2021). Despite recent findings, key questions regarding the diversity and evolution of TEs, as well as their role in mycorrhizal interactions, remain unanswered. For example, approximately two-thirds of TEs remain unclassified, making it difficult to infer their function in AMF genome biology and evolution (Miyauchi et al. 2020; Dallaire et al. 2021). Similarly, because analyses of TE expression have so far centered on germinating spores, it is unknown how these elements are controlled during host colonization, and whether some show host-specific regulation. The present study addresses these questions by providing an improved classification of TE families in all R. irregularis strains with chromosome-level assemblies and by investigating their expression among multiple hosts.
Materials and methods
Curation and classification of TE families
We used chromosome-level assemblies of 5 homokaryotic (4401, A1, B3, C2, and DAOM197198; Yildirir et al. 2022) and 4 heterokaryotic strains (A4, A5, G1, and SL1; Sperschneider et al. 2023; Supplementary Table 1) as a source to build repeat libraries. The curation for nonmodel species followed the most recommended guides (Wells and Feschotte 2020; Goubert et al. 2022). Firstly, repeat libraries were generated using RepeatModeler2.0.3 (Flynn et al. 2020) with the -LTRstruct mode for detecting Long Terminal Repeats sequences implemented by LTRharvest and LTR_retriever. The libraries from all strains were merged to create a single reference for the curation.
The unique library was submitted to TEclass, which separates the sequences into the order level: nonLTR, LTR, or DNA (Abrusán et al. 2009). This step helped us to distinguish orders with similar protein domains, such as DIRS (nonLTR) and Crypton (DNA). Tirvish, a tool from genome-tools (http://genometools.org/), was used to detect Terminal Inverted Repeats (TIRs) in DNA transposons elements (for order DNA/TIR) with the parameter -mintirlength 8. Hidden Markov Model (hmm) profiles of specific TE superfamilies or order domains were generated from a combination of conserved regions described in Supplementary Table 2. The sequences for each domain were first aligned using MAFFT (Nakamura et al. 2018), converted to the Stockholm format using esl-reformat, and finally submitted to hmmbuild (version 3.1b2) to generate the hmm profiles (Eddy 2011).
Lastly, we provided hmm profiles for detecting elements with reverse transcriptase (LINE, DIRS, PLE, LTR, Bel, Copia, and Gypsy), specific transposase superfamilies (Academ, CMC, Ginger, KDZ, Kolobok, MULE, Merlin, Novsib, P, PIF-Harbinger, PiggyBac, Plavaka, Sola-1, Sola-2, Sola-3, Tc-Mariner, Transib, Zator, and hAT) and tyrosine recombinase (DIRS and Crypton; https://github.com/jordana-olive/TE-manual-curation/tree/main/TE-domains). The open reading frames from the reference library were generated using the tool getorf with 200 amino acids as the minimum size (https://www.bioinformatics.nl/cgi-bin/emboss/help/getorf). The hmmrsearch (version 3.1b2) was used to find the sequences with TE domains using the abovementioned procedure and by selecting the best scores using HmmPy.py (https://github.com/EnzoAndree/HmmPy). Approximately 8% of R. irregularis genomes consist of high copy number genes, known as expanded genes (e.g. Sel1, BTB, Kelch, protein kinase, TPR). These expanded genes may also harbor (partial) TE insertions (Dallaire et al. 2021), and were thus removed from downstream analyses to avoid biases due to the chimeric nature of their TEs. The models can be accessed at https://github.com/jordana-olive/TE-manual-curation/tree/main/expanded-genes.
Class II elements (DNA/TIRS) were retained in the final library based on the following criteria: identified as DNA by TEclass, had a match with a transposon domain from hmmsearch, harbored a TIR sequence, and a size ranging between 1 and 17 kb. Sequences with TIRS, ranging between 50 bp and 1 kb and lacking transposase domains, were classified as MITEs (Miniature Inverted-repeat Transposable Elements). Following the hmmrsearch, certain elements exhibited similarity with more than one domain from different orders due to close relationships. To achieve the most accurate classification, these sequences were analyzed using phylogenetics to determine their evolutionary relationships. For this work, the protein sequences corresponding to these elements were aligned using MAFFT (Nakamura et al. 2018), and submitted to RAxML (Stamatakis 2014) to produce a phylogenetic tree using the PROTGAMMA model with 1,000 bootstraps. The best tree resolution was visualized using ggtree in R (Yu et al. 2017). The final classification of sequences aligning with more than one domain was based on their relationships, clustering elements from the same orders together, as illustrated in Supplementary Fig. 1.
Throughout the curation process, TE sequences classified by RepeatModeler were retained only if accurately identified by the program TEclass (Abrusán et al. 2009) and if the respective transposition domain was identified within their sequence. For newly identified sequences that were previously labeled as “unknown” by RepeatModeler, the classification was determined based on TEclass (Abrusán et al. 2009), the presence of a transposition domain, and their relationship to known sequences based on the phylogenetic reconstruction. The final library and models are available on https://github.com/jordana-olive/TE-manual-curation/tree/main and can be applied in any other dataset to custom TE characterization.
Repeat landscapes of genomes and compartments
We ran the RepeatMasker (version 4.1.2-p1; Smit et al. 2015) for all strains, with the parameters -a and -s, using the curated library as the reference (-lib option). The repeat landscapes were generated from modified createlandscape.pl and calculedivergence.pl scripts, provided in RepeatMasker files. These scripts calculate the divergence levels between the alignment of each TE sequence and the consensus family in the reference library. The landscapes also were generated to the A- and B-compartments, which are currently available only for the strains DAOM197198, A1, C2, A4, and A5 (Yildirir et al. 2022; Sperschneider et al. 2023). To assess whether the distribution of TEs varies across compartments, we extracted the percentage values from the landscapes of each Kimura bin, and then conducted a paired t-test using R. A significant difference between the landscapes was considered when P < 0.05.
TE and gene expression analysis
We evaluate the expression of genes and TEs using available RNA-seq from different tissues [germinating spores (Dallaire et al. 2021), intraradical mycelium (IRM), arbuscules (ARB; Zeng et al. 2018), and extraradical mycelium (ERM; Tsuzuki et al. 2016)], and mycorrhized roots from different plant hosts colonized by DAOM197198 [Allium schoenoprasum, Medicago truncatula, Nicotiana benthamiana (Zeng et al. 2018), and Brachypodium distachyon (Kamel et al. 2017)]. The accession numbers to the data are available in Supplementary Table 3. The reads were filtered using Trimmomatic (Bolger et al. 2014) and aligned to the DAOM197198 reference genome using Bowtie2 (Langmead and Salzberg 2012). The read count was accessed by TEtranscripts (Jin et al. 2015) guided by the TE annotation performed in this study and gene annotation executed by Yildirir et al. (2022). Using DESeq2 (Love et al. 2014), for each host condition and tissue, the differential expression was generated comparing germinated spores as control. A transcript was considered differentially expressed when Padj (adjusted P-value) is ≤0.05.
TE nearby gene correlation
TE location and gene expression correlation were analysed using available RNA-seq from DAOM197198 generated through Oxford Nanopore Technology (ONT) sequencing (Manley et al. 2023). The long ONT-RNA-seq reads were filtered and trimmed using pychopper (https://github.com/epi2me-labs/pychopper; 7.98% did not pass the quality parameters and were discarded). Nucleotide correction was performed based on self-clustering using isONTcorrect (Sahlin and Medvedev 2021). The filtered and corrected reads were aligned to the DAOM197198 genome using hisat2 and annotated using stringtie (Pertea et al. 2015) guided by the TE annotation performed in this study and gene annotation executed by Yildirir et al. (2022). In the same way, stringtie generated the counts of the transcripts used in the expression analysis. For detecting TEs upstream of genes, we extracted the genomic regions up to −1,000 to the transcript start position and then intersected with TE annotation using bedtools (Quinlan and Hall 2010). The Pearson correlation method in R was employed to assess the coexpression between genes and their upstream TE pairs.
Results
A curated database reveals new TE families in R. irregularis
Using RepeatModeler and RepeatMasker, recent analyses of R. irregularis chromosome-level datasets indicated that strains of this species carry an average of 50% of TEs (Yildirir et al. 2022; Sperschneider et al. 2023). However, only one-third of their repeat content could be classified, and thus, on average, 30% of all available genomes are composed of unclassified TE sequences (Miyauchi et al. 2020; Yildirir et al. 2022; Manley et al. 2023; Sperschneider et al. 2023). To address this, we used chromosome-level assemblies from five homokaryons and 4 heterokaryons R. irregularis strains to generate curated repeat libraries. When all genomes are considered, out of a total of 9,257 TE sequences identified by RepeatModeler, only 2,369 (∼25%) can be considered well-defined, bona-fide nonredundant consensus sequences harboring transposition domains. The notable reduction in TE numbers is due to noncurated datasets containing highly degenerated TE without domains (relics), and repeats that cannot be classified with current knowledge of TE evolution.
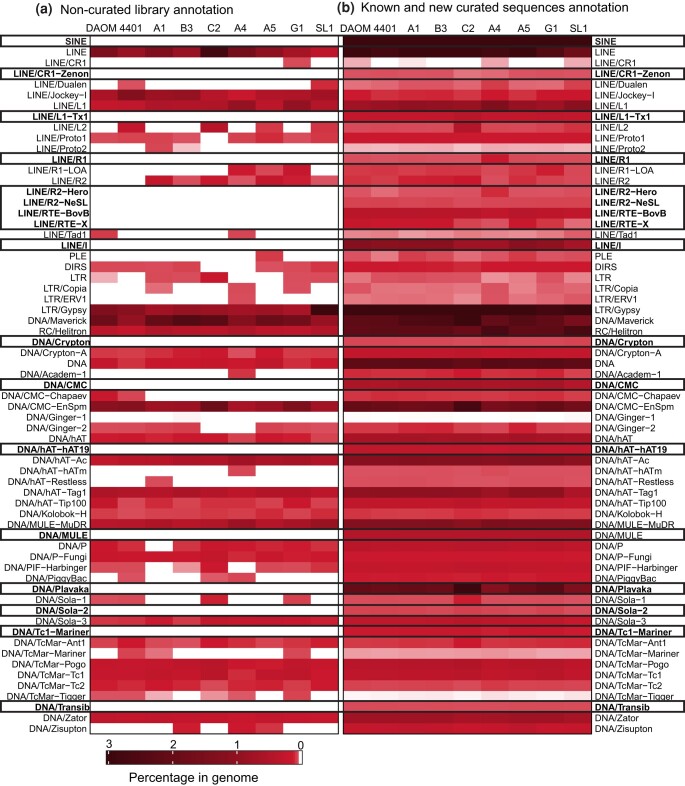
In the final curated library, 1,458 sequences belong to families previously classified by RepeatModeler (K, Fig. 1a), while 636 sequences represent newly identified families (N, Fig. 1a) of the following orders: SINE, LINE, LTR, DNA/TIRS, DNA/Crypton, Maverick, and RC/Helitron (Fig. 1a). Following curation, in the DAOM197198 genome, sequences belonging to these orders increased in number by 4-fold on average, and similar results were obtained for all investigated R. irregularis strains (Fig. 1b). We also assessed the influence of the manually curated library on the repeat landscape of the DAOM197198 genome (see Fig. 1c–e). Following TE curation, the percentage of the DAOM197198 genome represented by classified TE increased from 12% (Fig. 1, c and d) to 36% (Fig. 1e).
Fig. 1.
A description of the curated TE library. a) The proportion of known (detected by RepeatModeler, in light color, K) and novel (in bold colors, N) curated sequences. b) RepeatModeler and curated library annotation comparison across the strains. The y-axis represents the R. irregularis strains, while the x-axis shows the relative abundance of the TEs in the genome from the homology-based annotation (RepeatModeler, left) and the current annotation (curation, right). c–e) Repeat landscape of DAOM197198 using different libraries as a source for annotation. Each plot shows the sequence divergence from its consensus (x-axis) in relation to the number of copies in the genome (y-axis). Newer insertions are shown in the left peaks, while the older and more degenerate insertions are on the right side of the graph. c) Annotation performed using the noncurated library from RepeatModeler, which depicts the unknown elements (in gray). d) Only noncurated TEs detected by RepeatModeler, which comprehends 12% of the repeat content. e) Landscape generated with the final curated library.
The newly curated R. irregularis TE library now includes novel families of non-LTR retrotransposons: SINE, LINE/CR1-Zenon, LINE/L1-Tx1, LINE/L1-R1, LINE/R2-Hero, LINE/RTE-BovB, and LINE/I, which were detected based on phylogenetic analyses (Supplementary Fig. 1). A novel superfamily of DNA transposons—e.g. Transib—and novel families belonging to known AMF TE superfamilies were also detected, including Crypton, CMC, hAT-19, MULE, Sola-2, Tc1-Mariner, and Plavaka (Fig. 2). The Plavaka family, which is part of the CACTA/CMC/EnSpm superfamily, is particularly prevalent in DAOM197198, A1, B3, C2, A5, and G1. This family has already been identified in fungi (Iyer et al. 2014; Muszewska et al. 2019), but it is not deposited in publicly available repeat databases, which is why RepeatModeler could not detect this family.
Fig. 2.
The annotation of TE families in R. irregularis strains. The heatmaps show the percentage of families in each strain considering the RepeatMasker annotation. a) The percentage of TE families in each genome using the noncurated library. b) Annotation using the curated library with known and novel TE families. Blank areas mean absence of certain families in that annotation, while the black rectangles highlight the new families found in the curation.
Repeat landscapes differ among and within R. irregularis strains
In the R. irregularis isolate DAOM197198, TEs are mobilized and retained differently across the genome (Dallaire et al. 2021), with 2 main waves of expansions. We accessed the distribution of TEs in the other strains based on the Kimura substitution calculation, which estimates the level of divergence of annotated TE compared with its consensus in the reference library. A lower number of Kimura substitution indicates high similarity between annotated sequences and their consensus, suggesting recent insertion events, while a higher number indicates greater evolutionary distance and likely older TE insertions.
Our analysis revealed that the patterns of repeat landscapes based on Kimura substitution levels differ among R. irregularis strains. Specifically, in most strains, expansions of TEs are recent, as highlighted by the high number of elements with Kimura substitutions ranging from 0 to 5, supporting the findings that new TE expansion bursts exceed older TE insertions in DAOM197198 (Dallaire et al. 2021; Fig. 3). However, other strains exhibit different TE distributions. For example, strains A4 and SL1 carry a larger proportion of TEs with older Kimura substitution levels, indicating strain-specific patterns of TE emergence, evolution, and retention. Remarkably, the genome of C2 has a much larger proportion of younger, and thus likely more active TEs, and it is plausible that this feature is linked to the larger genome size of this strain compared with relatives—i.e. the C2 genome is 162 Mb compared with an average of 147 Mb for other R. irregularis strains (Fig. 3).
Fig. 3.
Repeat landscape of R. irregularis strains. a–h) Each plot shows the sequence divergence from its consensus (x-axis) in relation to the number of copies in the genome (y-axis). Newer insertions are shown in the left peaks, while the older and more degenerate insertions are on the right side of the graph. The strains with recent burst of TE expansions are indicated with the green arrow, while the ones representing early TE degeneration are highlighted with red arrow.
The A- and B-compartments were recently identified in the chromosome-level assembly of 3 homokaryons (DAOM197198, A1, and C2; Yildirir et al. 2022) and 2 heterokaryon (A4 and A5; Sperschneider et al. 2023) strains. Based on available data, we observed that the distribution of TEs based on Kimura substitution levels also differs significantly between the A- and B-compartment in all strains (Fig. 4). Specifically, the proportion of TEs with Kimura substitutions between 10 and 20—i.e. older TEs—is always higher in the B-compartment (P < 0.05), indicating that these elements are maintained over time at higher levels in this portion of the genome. In contrast, old and degenerated TEs appear to be rapidly eliminated in the A-compartment (Fig. 4).
Fig. 4.
a–e) The repeat landscapes in R. irregularis strains with annotated A- and B-compartments. The arrows point to the divergence between the compartments. f–g) Coding genes and upstream TE correlation of expression in DAOM197198. Each point represents a coding gene-upstream TE pair, where the y-axis is the TE expression (logTPM) and the x-axis is the coding gene expression (logTPM). The line represents the regression, surrounded by the confidence interval.
TE and gene expression are positively correlated within the B-compartment
The localization of TEs near genes can impact their expression (Bourque et al. 2018; Drongitis et al. 2019). We investigated whether the presence of TEs upstream of genes is associated with gene expression in compartments by identifying the expression of TEs located within 1,000 nucleotides upstream of genes using long-read RNA data from the DAOM197198 strain (Manley et al. 2023). We found no correlation between the expression of TEs and coding genes located in the A-compartment (r = 0.21, P < 0.0001; Fig. 4f); however, a positive and significant correlation exists for genes and TEs in the B-compartment (r = 0.84, P < 0.0001; Fig. 4g). This finding suggests that TEs and genes in the B-compartment are being coexpressed.
TEs are significantly more expressed in colonized roots compared with ERM
To obtain additional insights into the biology of TEs, we investigated their regulation during host colonization using R. irregularis DAOM197198 RNA-seq data from multiple tissues, including the micro-dissected cells of ARB and IRM, and ERM (Fig. 5a) in symbiosis with M. truncatula roots (Fig. 5b–d).
Fig. 5.
The expression of TEs in R. irregularis through the life stages of colonization. a) A schematic view of the different life stages compared with spores. b–d) Volcano plots of expressed TEs in each colonization stage, evidencing the number of downregulated and upregulated sequences on the left (DOWN) and right (UP), respectively. The ones expressed but not significantly different from spores are shown in the center as NO. e) The number of upregulated TEs in each condition. Inside the parentheses are the number of total upregulated TEs in that specific condition. The gray circle size is according to the number of upregulated sequences. f) AGO-like gene (Rhiir2092) expression under different conditions. Colonization stages collected by laser dissection of ARB and IRM and ERM and mycorrhized roots. The t-test compared all the conditions with spores, where *P < 0.05, **P < 0.001, and ns is no significance. The box limits represent the range of 50% of the data, the central line marks the median, and the vertical lines out of the boxes are the upper and lower values. normTPM, normalized transcripts per million.
The number of upregulated TEs is more than 4 times higher in IRM and ARB than in the ERM in the same host (Fig. 5b–d). TEs significantly upregulated in colonized tissue (ARB and IRM) include DNA/TIRS, LTR, LINE, and SINES orders. Among these, LTR/Gypsy, LINE, CMC-EnSpm, Plavaka, hAT-Tag1, hAT-Ac, and Helitron are the superfamilies with more expressed families (Supplementary Table 4).
One mechanism to control TE mobilization is through RNAi and AGO proteins (Kelleher et al. 2018). Typically, fungi possess 1–4 AGO genes per genome (Chang et al. 2012). However, R. irregularis harbors 40 copies of AGO-like genes, and of these 25 contain all typical AGO core domains (e.g. PIWI, PAZ, MID, and N-terminal) with some exhibiting signs of expression (Silvestri et al. 2019). Among these AGO-like genes, only one (Rhiir2092, JGI 1582012) presents significant expression—i.e. more than >100 transcripts per million in the samples, while the remainder exhibit minimal to no expression. We find that the Rhiir2092 gene is significantly more expressed in ERM (P < 0.05) compared with the other conditions (Fig. 5, b and f). Specifically, for this gene, ARB and IRM laser-dissected cells have significantly reduced expression, which again differs from the significantly higher expression of TEs under these conditions (P < 0.05; Fig. 5, b and f).
TE regulation changes with different hosts and correlates with host genome size
Analyses of RNA-seq data roots from multiple hosts colonized by DAOM197198 (A. schoenoprasum, M. truncatula, N. benthamiana, and B. distachyon), also reveals that TE expression differs significantly among hosts, with some families being expressed only in 1 of 4 hosts (Fig. 6 and Supplementary Table 4). For example, a total of 404 families are upregulated during colonization with A. schoenoprasum roots (Fig. 6a), including both families from class II elements (e.g. Sola-1, hATm, Academ-1, and DIRS) and class I elements (e.g. CR1-Zenon and DIRS) that are only upregulated with this host. In N. benthamiana and M. truncatula roots, the symbiont upregulates a smaller number of TE families compared with A. schoenoprasum, 251 and 197, respectively, while B. distachyon was the condition with lowest TE upregulation overall (116 families; Fig. 6d). The differences in TE expression among hosts are not linked to the variability in expression of AMF AGO-like gene—i.e. hosts with highest TE expression do not always have low AGO-like expression and vice versa.
Fig. 6.
The differential expression of TEs in DAOM197198 during symbiosis with different plant hosts compared with germinated spores. a–d) Volcano plots showing the downregulated and upregulated TEs in relation to spores on the left (DOWN) and right (UP) respectively, considering Padj < 0.05. In the center are the ones with no significant difference in expression in relation to the control (NO). e) The number of overexpressed TE families in each host. f) AGO-like gene (Rhiir2092) expression under colonization with different hosts. The t-test was performed comparing all the conditions with spores, where *P < 0.05, **P < 0.001, and ns is no significance. The box limits represent the range of 50% of the data, the central line marks the median, and the vertical lines out of the boxes are the upper and lower values. normTPM, normalized transcripts per million. g) Correlation between the repeat content of the host genome (in base pairs) and the number of families overexpressed in the AMF during the symbiosis. The repeat size of the hosts was calculated based on the most recent genome assembly and annotation: Allium sp. (Liao et al. 2022), Brachypodium (International Brachypodium Initiative 2010 ), carrot (Iorizzo et al. 2016), chicory (Fan et al. 2022), Lotus japonicus (Kamal et al. 2020), M. truncatula (Pecrix et al. 2018), and N. benthamiana (Kurotani et al. 2023).
Given the observed variation in TE expression in the AMF, we wondered how the plant host influences the differential expression of these superfamilies. A significant and positive correlation (r = 0.91, P = 0.001) between the repeat content of the host genome and the number of overexpressed families in the symbiont. For example, A. schoenoprasum (14 Gb) is the host with the most TE content and its colonized roots have the highest number of TEs upregulated in the AMF. By contrast, B. distachyon (272 Mb) has the lowest TE content and has significantly lower TE upregulation in the symbiont.
It is noteworthy that, in contrast to fungi, the repeat content of plant species has been well characterized using available tools, because most repeat databases have been curated using plant genome information (Flynn et al. 2020; Storer et al. 2021). As such, it is very unlikely that the significant correlation we observed was skewed by the noncurated nature of the plant genomes we used, particularly given that these findings are consistent among multiple plant hosts and conditions.
Discussion
An improved view of TE family diversity and evolution in a model plant symbiont
Through curation of R. irregularis repeat libraries, we first improved the proportion of annotated families in these model plant symbionts and uncovered novel sequences representing the largest proportion of their repetitive sequences. Our work also revealed how R. irregularis strains differ in TE retention and deletion over time within 2 genome compartments. For example, our findings indicate that strains with the largest genome sizes (C2) show a combination of a higher rate of TE emergence and retention of these elements. We also uncovered notable differences in how TEs evolve within each strain, with some (A1, B3) having much higher proportions of very young TEs compared with others (SL1, A4) that carry levels of Kimura substitution rates indicative of early repeat degeneration and fewer cases of recent expansion.
TE retention rates are different between compartments
By investigating the degree to which TEs accumulate mutations over time, a significant distinction emerged between A/B compartments. Specifically, all A-compartment landscapes show a continuing invasion of novel/young TE insertions, and the high methylation present in this compartment (Yildirir et al. 2022), and/or purifying selection, might be needed for their control and rapid removal, as evidenced by the lower TE density in this compartment. By contrast, these insertions accumulate in the B-compartment, leading to notable inflation of these elements over time, as shown by the stable TE density along the axis that defines the Kimura substitution rates. The accumulation of TEs in the B-compartment might be linked with their domestication (Zhang et al. 2019; Modzelewski et al. 2022) and/or the emergence of new functions, a view supported by the significant positive correlations we observed in the expression of TEs and genes and by similar findings from plant pathogens (Fouché et al. 2020, 2022).
TE regulation and evolution further underpin similarities between AMF and known fungal pathogens
Obvious similarities between the genomes of AMF and those of plant pathogens have been known for some time (Mathieu et al. 2018; Reinhardt et al. 2021). These include enrichments in TEs, and genomes subdividing into highly diverging regions dense in effector genes and TEs, and more conserved ones composed of core genes and low repeat density.
In the plant pathogen Verticillium dahliae, TEs often locate in proximity to highly expressed pathogenicity-related genes within fast-evolving adaptive genomic regions (Torres et al. 2021). These regions are reminiscent of R. irregularis B-compartments (Reinhardt et al. 2021; Yildirir et al. 2022), which are also enriched in TEs and secreted proteins that promote symbiosis with different hosts (Teulet et al. 2023). As such, the significant correlation we observed between TEs and genes specific to the B-compartment may mirror identical processes in AMF and plant pathogens.
The increased upregulation of genes in close proximity to TEs during the colonization stages in the B-compartment could also indicates a TE control for derepressing those regions of the genome (Oggenfuss and Croll 2023). Indeed, it has been proposed that TE-effector regulation is well-timed, i.e. both are expressed during the infection and repressed in the absence of the host (Fouché et al. 2022). In filamentous fungi, the variability of TEs can also allow for escaping mechanisms of recognition by the plant immunity system (Fouché et al. 2020, 2022), and it is thus possible that in AMF the expression of TEs could aid plant–symbiont communication during colonization.
What drives TE upregulation and host specificity during colonization?
TE expression is active in germinating spores (Dallaire et al. 2021), but our study shows their expression in colonized roots is much higher still. In this context, we observed that an AGO-like gene exhibits significantly higher expression in ERM compared with colonized roots (Silvestri et al. 2019). AGO proteins are known TE regulators (Thomson et al. 2015), and thus our results suggest that RNAi is one of the key factors implicated in the regulation of TEs across the stages of the mycorrhizal symbiosis— i.e. downregulation in ERM, and upregulation in planta . In fungi, it is expected that 1–4 AGO proteins regulate TEs (Chang et al. 2012; Lax et al. 2020). However, the genome of R. irregularis unexpectedly contains an expanded set of 40 copies of this gene (Silvestri et al. 2019). The remaining AGO-like genes, which exhibit minimal or no expression, may be implicated in other functions (Silvestri et al. 2019). This could be attributed to these copies being incomplete or carrying additional domains (Silvestri et al. 2019). Notably, the expression of this AGO-like gene did not vary significantly among hosts, and thus other factors could be responsible for the host-specific variation in the TE expression that we observed.
One intriguing possibility is that TE expression in the hosts directly or indirectly influences TE expression in the AMF, as seen in multiple plant–microbe interactions in cross-talk regulations (Melayah et al. 2001; Weiberg et al. 2013; Ren et al. 2019; Wong-Bajracharya et al. 2022). In support of this view, we found a positive correlation between TE abundance in the host and TE expression in AMF. With mounting evidence of molecular cross-communication between the mycorrhizal partners, including RNA from the fungi interacting with mRNA from the hosts (Silvestri et al. 2019, 2020; Ledford et al. 2023), it is likely that molecular dialogs between mycorrhizal partners also result in the increased TE expression we observed in the fungal symbiont.
Supplementary Material
Contributor Information
Jordana Inácio Nascimento Oliveira, Department of Biology, Faculty of Sciences, University of Ottawa, Ottawa, ON, Canada K1N 6N5.
Nicolas Corradi, Department of Biology, Faculty of Sciences, University of Ottawa, Ottawa, ON, Canada K1N 6N5.
Data availability
The genomes and RNA-seq used in this study are described in Supplementary Tables 1 and 3. The ONT RNA-seq can be accessed at SRR21968700. The TE reference library and domains used in the curation are available at https://github.com/jordana-olive/TE-manual-curation/tree/main.
Supplemental material available at G3 online.
Funding
Our research is funded by the Discovery program (RGPIN2020-05643) and a Discovery Accelerator Supplements Program (RGPAS-2020-00033) of the Natural Sciences and Engineering Research Council of Canada. NC is a University of Ottawa Research Chair in Microbial Genomics. JINO is funded by Mitacs Accelerate Program (IT16902) from Mitacs and a Discovery Accelerator Supplements Program (RGPAS-2020-00033) from Natural Sciences and Engineering Research Council of Canada.
Author contributions
JINO and NC designed the study. JINO performed the experiments and analyzed the data. JINO and NC wrote the paper. NC mentored and supervised all the processes.
Literature cited
- Abrusán G, Grundmann N, DeMester L, Makalowski W. 2009. TEclass—a tool for automated classification of unknown eukaryotic transposable elements. Bioinformatics. 25(10):1329–1330. doi: 10.1093/bioinformatics/btp084. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P, Anca I-A. 2009. Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol. 63(1):363–383. doi: 10.1146/annurev.micro.091208.073504. [DOI] [PubMed] [Google Scholar]
- Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, et al. 2018. Ten things you should know about transposable elements. Genome Biol. 19(1):199. doi: 10.1186/s13059-018-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-S, Zhang Z, Liu Y. 2012. RNA interference pathways in fungi: mechanisms and functions. Annu Rev Microbiol. 66(1):305–323. doi: 10.1146/annurev-micro-092611-150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi N, Brachmann A. 2017. Fungal mating in the most widespread plant symbionts? Trends Plant Sci. 22(2):175–183. doi: 10.1016/j.tplants.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Dallaire A, Manley BF, Wilkens M, Bista I, Quan C, Evangelisti E, Bradshaw CR, Ramakrishna NB, Schornack S, Butter F, et al. 2021. Transcriptional activity and epigenetic regulation of transposable elements in the symbiotic fungus Rhizophagus irregularis. Genome Res. 31(12):2290–2302. doi: 10.1101/gr.275752.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaux P-M, Schornack S. 2021. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science. 371(6531):eaba6605. doi: 10.1126/science.aba6605. [DOI] [PubMed] [Google Scholar]
- Drongitis D, Aniello F, Fucci L, Donizetti A. 2019. Roles of transposable elements in the different layers of gene expression regulation. Int J Mol Sci. 20(22):5755. doi: 10.3390/ijms20225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol. 7(10):e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Wang S, Wang H, Wang A, Jiang F, Liu H, Zhao H, Xu D, Zhang Y. 2022. The genomes of chicory, endive, great burdock and yacon provide insights into Asteraceae palaeo-polyploidization history and plant inulin production. Mol Ecol Resour. 22(8):3124–3140. doi: 10.1111/1755-0998.13675. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Hubley R, Goubert C, Rosen J, Clark AG, Feschotte C, Smit AF. 2020. RepeatModeler2 for automated genomic discovery of transposable element families. Proc Natl Acad Sci USA. 117(17):9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouché S, Badet T, Oggenfuss U, Plissonneau C, Francisco CS, Croll D. 2020. Stress-driven transposable element de-repression dynamics and virulence evolution in a fungal pathogen. Mol Biol Evol. 37(1):221–239. doi: 10.1093/molbev/msz216. [DOI] [PubMed] [Google Scholar]
- Fouché S, Oggenfuss U, Chanclud E, Croll D. 2022. A devil's bargain with transposable elements in plant pathogens. Trends Genet. 38(3):222–230. doi: 10.1016/j.tig.2021.08.005. [DOI] [PubMed] [Google Scholar]
- Goubert C, Craig RJ, Bilat AF, Peona V, Vogan AA, Protasio AV. 2022. A beginner's guide to manual curation of transposable elements. Mob DNA. 13(1):7. doi: 10.1186/s13100-021-00259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins H-J, Cargill RIM, Van Nuland ME, Hagen SC, Field KJ, Sheldrake M, Soudzilovskaia NA, Kiers ET. 2023. Mycorrhizal mycelium as a global carbon pool. Curr Biol. 33(11):R560–R573. doi: 10.1016/j.cub.2023.02.027. [DOI] [PubMed] [Google Scholar]
- Horsch CCA, Antunes PM, Fahey C, Grandy AS, Kallenbach CM. 2023. Trait-based assembly of arbuscular mycorrhizal fungal communities determines soil carbon formation and retention. New Phytol. 239(1):311–324. doi: 10.1111/nph.18914. [DOI] [PubMed] [Google Scholar]
- International Brachypodium Initiative 2010. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature. 463(7282):763–768. doi: 10.1038/nature08747. [DOI] [PubMed] [Google Scholar]
- Iorizzo M, Ellison S, Senalik D, Zeng P, Satapoomin P, Huang J, Bowman M, Iovene M, Sanseverino W, Cavagnaro P, et al. 2016. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat Genet. 48(6):657–666. doi: 10.1038/ng.3565. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Zhang D, de Souza RF, Pukkila PJ, Rao A, Aravind L. 2014. Lineage-specific expansions of TET/JBP genes and a new class of DNA transposons shape fungal genomic and epigenetic landscapes. Proc Natl Acad Sci U S A. 111(5):1676–1683. doi: 10.1073/pnas.1321818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tam OH, Paniagua E, Hammell M. 2015. TEtranscripts: a package for including transposable elements in differential expression analysis of RNA-seq datasets. Bioinformatics. 31(22):3593–3599. doi: 10.1093/bioinformatics/btv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal N, Mun T, Reid D, Lin J-S, Akyol TY, Sandal N, Asp T, Hirakawa H, Stougaard J, Mayer KFX, et al. 2020. Insights into the evolution of symbiosis gene copy number and distribution from a chromosome-scale Lotus japonicus Gifu genome sequence. DNA Res. 27(3):dsaa015. doi: 10.1093/dnares/dsaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel L, Tang N, Malbreil M, San Clemente H, Le Marquer M, Roux C, Frei dit Frey N. 2017. The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front Plant Sci. 8:124. doi: 10.3389/fpls.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher ES, Azevedo RBR, Zheng Y. 2018. The evolution of small-RNA-mediated silencing of an invading transposable element. Genome Biol Evol. 10(11):3038–3057. doi: 10.1093/gbe/evy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkoris V, Stefani F, Dalpé Y, Dettman J, Corradi N. 2020. Nuclear dynamics in the arbuscular mycorrhizal fungi. Trends Plant Sci. 25(8):765–778. doi: 10.1016/j.tplants.2020.05.002. [DOI] [PubMed] [Google Scholar]
- Kurotani KI, Hirakawa H, Shirasawa K, Tanizawa Y, Nakamura Y, Isobe S, Notaguchi M. 2023. Genome sequence and analysis of Nicotiana benthamiana, the model plant for interactions between organisms. Plant Cell Physiol. 64(2):248–257. doi: 10.1093/pcp/pcac168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax C, Tahiri G, Patiño-Medina JA, Cánovas-Márquez JT, Pérez-Ruiz JA, Osorio-Concepción M, Navarro E, Calo S. 2020. The evolutionary significance of RNAi in the fungal kingdom. Int J Mol Sci. 21(24):9348. doi: 10.3390/ijms21249348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford WC, Silvestri A, Fiorilli V, Roth R, Rubio-Somoza I, Lanfranco L. 2023. A journey into the world of small RNAs in the arbuscular mycorrhizal symbiosis. New Phytol. n/a:1–11. doi: 10.1111/nph.19394. [DOI] [PubMed] [Google Scholar]
- Liao N, Hu Z, Miao J, Hu X, Lyu X, Fang H, Zhou Y-M, Mahmoud A, Deng G, Meng Y-Q, et al. 2022. Chromosome-level genome assembly of bunching onion illuminates genome evolution and flavor formation in allium crops. Nat Commun. 13(1):6690. doi: 10.1038/s41467-022-34491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley BF, Lotharukpong JS, Barrera-Redondo J, Llewellyn T, Yildirir G, Sperschneider J, Corradi N, Paszkowski U, Miska EA, Dallaire A. 2023. A highly contiguous genome assembly reveals sources of genomic novelty in the symbiotic fungus Rhizophagus irregularis. G3 (Bethesda). 13(6):jkad077. doi: 10.1093/g3journal/jkad077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FM, Uroz S, Barker DG. 2017. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science. 356(6340):eaad4501. doi: 10.1126/science.aad4501. [DOI] [PubMed] [Google Scholar]
- Mathieu S, Cusant L, Roux C, Corradi N. 2018. Arbuscular mycorrhizal fungi: intraspecific diversity and pangenomes. New Phytol. 220(4):1129–1134. doi: 10.1111/nph.15275. [DOI] [PubMed] [Google Scholar]
- Mathu MC, Wang Y, Stajich JE, Kokkoris V, Villeneuve-Laroche M, Yildirir G, Corradi N. 2022. Early branching arbuscular mycorrhizal fungus Paraglomus occultum carries a small and repeat poor genome compared to relatives in the Glomeromycotina. Microbial Genomics. 8(4):000810. doi: 10.1099/mgen.0.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melayah D, Bonnivard E, Chalhoub B, Audeon C, Grandbastien MA. 2001. The mobility of the tobacco Tnt1 retrotransposon correlates with its transcriptional activation by fungal factors. Plant J. 28(2):159–168. doi: 10.1046/j.1365-313X.2001.01141.x. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Kiss E, Kuo A, Drula E, Kohler A, Sánchez-García M, Morin E, Andreopoulos B, Barry KW, Bonito G, et al. 2020. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat Commun. 11(1):5125. doi: 10.1038/s41467-020-18795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewski AJ, Gan Chong J, Wang T, He L. 2022. Mammalian genome innovation through transposon domestication. Nat Cell Biol. 24(9):1332–1340. doi: 10.1038/s41556-022-00970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin E, Miyauchi S, San Clemente H, Chen ECH, Pelin A, de la Providencia I, Ndikumana S, Beaudet D, Hainaut M, Drula E, et al. 2019. Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina. New Phytol. 222(3):1584–1598. doi: 10.1111/nph.15687. [DOI] [PubMed] [Google Scholar]
- Muszewska A, Steczkiewicz K, Stepniewska-Dziubinska M, Ginalski K. 2019. Transposable elements contribute to fungal genes and impact fungal lifestyle. Sci Rep. 9(1):4307. doi: 10.1038/s41598-019-40965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Yamada KD, Tomii K, Katoh K. 2018. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics. 34(14):2490–2492. doi: 10.1093/bioinformatics/bty121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggenfuss U, Croll D. 2023. Recent transposable element bursts are associated with the proximity to genes in a fungal plant pathogen. PLoS Pathog. 19(2):e1011130. doi: 10.1371/journal.ppat.1011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecrix Y, Staton SE, Sallet E, Lelandais-Brière C, Moreau S, Carrère S, Blein T, Jardinaud M-F, Latrasse D, Zouine M, et al. 2018. Whole-genome landscape of Medicago truncatula symbiotic genes. Nat Plants. 4(12):1017–1025. doi: 10.1038/s41477-018-0286-7. [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 33(3):290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech GE, Radío S, Guirao-Rico S, Aguilera L, Horvath V, Green L, Lindstadt H, Jamilloux V, Quesneville H, González J. 2022. Population-scale long-read sequencing uncovers transposable elements associated with gene expression variation and adaptive signatures in Drosophila. Nat Commun. 13(1):1948. doi: 10.1038/s41467-022-29518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Roux C, Corradi N, Di Pietro A. 2021. Lineage-specific genes and cryptic sex: parallels and differences between arbuscular mycorrhizal fungi and fungal pathogens. Trends Plant Sci. 26(2):111–123. doi: 10.1016/j.tplants.2020.09.006. [DOI] [PubMed] [Google Scholar]
- Ren B, Wang X, Duan J, Ma J. 2019. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science. 365(6456):919–922. doi: 10.1126/science.aav8907. [DOI] [PubMed] [Google Scholar]
- Ropars J, Toro KS, Noel J, Pelin A, Charron P, Farinelli L, Marton T, Krüger M, Fuchs J, Brachmann A. 2016. Evidence for the sexual origin of heterokaryosis in arbuscular mycorrhizal fungi. Nat Microbiol. 1(6):16033. doi: 10.1038/nmicrobiol.2016.33. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Medvedev P. 2021. Error correction enables use of Oxford Nanopore technology for reference-free transcriptome analysis. Nat Commun. 12(1):2. doi: 10.1038/s41467-020-20340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seberg O, Petersen G. 2009. A unified classification system for eukaryotic transposable elements should reflect their phylogeny. Nat Rev Genet. 10(4):276. doi: 10.1038/nrg2165-c3. [DOI] [PubMed] [Google Scholar]
- Silvestri A, Fiorilli V, Miozzi L, Accotto GP, Turina M, Lanfranco L. 2019. In silico analysis of fungal small RNA accumulation reveals putative plant mRNA targets in the symbiosis between an arbuscular mycorrhizal fungus and its host plant. BMC Genomics. 20(1):169. doi: 10.1186/s12864-019-5561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri A, Turina M, Fiorilli V, Miozzi L, Venice F, Bonfante P, Lanfranco L. 2020. Different genetic sources contribute to the small RNA population in the arbuscular mycorrhizal fungus Gigaspora margarita. Front Microbiol. 11:395. doi: 10.3389/fmicb.2020.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P. 2015. RepeatMasker Open-4.0. http://www.repeatmasker.org.
- Sperschneider J, Yildirir G, Rizzi YS, Malar C M, Mayrand Nicol A, Sorwar E, Villeneuve-Laroche M, Chen ECH, Iwasaki W, Brauer EK, et al. 2023. Arbuscular mycorrhizal fungi heterokaryons have two nuclear populations with distinct roles in host-plant interactions. Nat Microbiol. 8(11):2142–2153. doi: 10.1038/s41564-023-01495-8. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):28–36. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer J, Hubley R, Rosen J, Wheeler TJ, Smit AF. 2021. The Dfam community resource of transposable element families, sequence models, and genome annotations. Mob DNA. 12(1):2. doi: 10.1186/s13100-020-00230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulet A, Quan C, Evangelisti E, Wanke A, Yang W, Schornack S. 2023. A pathogen effector FOLD diversified in symbiotic fungi. New Phytol. 239(3):1127–1139. doi: 10.1111/nph.18996 [DOI] [PubMed] [Google Scholar]
- Thomson DW, Pillman KA, Anderson ML, Lawrence DM, Toubia J, Goodall GJ, Bracken CP. 2015. Assessing the gene regulatory properties of argonaute-bound small RNAs of diverse genomic origin. Nucleic Acids Res. 43(1):470–481. doi: 10.1093/nar/gku1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DE, Thomma BPHJ, Seidl MF. 2021. Transposable elements contribute to genome dynamics and gene expression variation in the fungal plant pathogen Verticillium dahliae. Genome Biol Evol. 13(7):evab135. doi: 10.1093/gbe/evab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S, Handa Y, Takeda N, Kawaguchi M. 2016. Strigolactone-induced putative secreted protein 1 is required for the establishment of symbiosis by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mol Plant Microbe Interact. 29(4):277–286. doi: 10.1094/MPMI-10-15-0234-R. [DOI] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin F-M, Zhao H, Zhang Z, Kaloshian I, Huang H-D, Jin H. 2013. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 342(6154):118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JN, Feschotte C. 2020. A field guide to eukaryotic transposable elements. Annu Rev Genet. 54(1):539–561. doi: 10.1146/annurev-genet-040620-022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, et al. 2007. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 8(12):973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, et al. 2008. Reply: a unified classification system for eukaryotic transposable elements should reflect their phylogeny. Nat Rev Genet. 9(5):411–412. doi: 10.1038/nrg2165-c2. [DOI] [PubMed] [Google Scholar]
- Winter DJ, Ganley ARD, Young CA, Liachko I, Schardl CL, Dupont P-Y, Berry D, Ram A, Scott B, Cox MP. 2018. Repeat elements organise 3D genome structure and mediate transcription in the filamentous fungus Epichloë festucae. PLoS Genet. 14(10):e1007467. doi: 10.1371/journal.pgen.1007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Bajracharya J, Singan VR, Monti R, Plett KL, Ng V, Grigoriev IV, Martin FM, Anderson IC, Plett JM. 2022. The ectomycorrhizal fungus Pisolithus microcarpus encodes a microRNA involved in cross-kingdom gene silencing during symbiosis. Proc Natl Acad Sci U S A. 119(3):e2103527119. doi: 10.1073/pnas.2103527119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirir G, Sperschneider J, Malar C M, Chen ECH, Iwasaki W, Cornell C, Corradi N. 2022. Long reads and Hi-C sequencing illuminate the two-compartment genome of the model arbuscular mycorrhizal symbiont Rhizophagus irregularis. New Phytol. 233(3):1097–1107. doi: 10.1111/nph.17842. [DOI] [PubMed] [Google Scholar]
- Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 8(1):28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- Zeng T, Holmer R, Hontelez J, Te Lintel-Hekkert B, Marufu L, de Zeeuw T, Wu F, Schijlen E, Bisseling T, Limpens E. 2018. Host- and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant J. 94(3):411–425. doi: 10.1111/tpj.13908. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cheng TC, Huang G, Lu Q, Surleac MD, Mandell JD, Pontarotti P, Petrescu AJ, Xu A, Xiong Y, et al. 2019. Transposon molecular domestication and the evolution of the RAG recombinase. Nature. 569(7754):79–84. doi: 10.1038/s41586-019-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomes and RNA-seq used in this study are described in Supplementary Tables 1 and 3. The ONT RNA-seq can be accessed at SRR21968700. The TE reference library and domains used in the curation are available at https://github.com/jordana-olive/TE-manual-curation/tree/main.
Supplemental material available at G3 online.