Abstract
BACKGROUND
Whether video laryngoscopy as compared with direct laryngoscopy increases the likelihood of successful tracheal intubation on the first attempt among critically ill adults is uncertain.
METHODS
In a multicenter, randomized trial conducted at 17 emergency departments and intensive care units (ICUs), we randomly assigned critically ill adults undergoing tracheal intubation to the video-laryngoscope group or the direct-laryngoscope group. The primary outcome was successful intubation on the first attempt. The secondary outcome was the occurrence of severe complications during intubation; severe complications were defined as severe hypoxemia, severe hypotension, new or increased vasopressor use, cardiac arrest, or death.
RESULTS
The trial was stopped for efficacy at the time of the single preplanned interim analysis. Among 1417 patients who were included in the final analysis (91.5% of whom underwent intubation that was performed by an emergency medicine resident or a critical care fellow), successful intubation on the first attempt occurred in 600 of the 705 patients (85.1%) in the video-laryngoscope group and in 504 of the 712 patients (70.8%) in the direct-laryngoscope group (absolute risk difference, 14.3 percentage points; 95% confidence interval [CI], 9.9 to 18.7; P<0.001). A total of 151 patients (21.4%) in the video-laryngoscope group and 149 patients (20.9%) in the direct-laryngoscope group had a severe complication during intubation (absolute risk difference, 0.5 percentage points; 95% CI, −3.9 to 4.9). Safety outcomes, including esophageal intubation, injury to the teeth, and aspiration, were similar in the two groups.
CONCLUSIONS
Among critically ill adults undergoing tracheal intubation in an emergency department or ICU, the use of a video laryngoscope resulted in a higher incidence of successful intubation on the first attempt than the use of a direct laryngoscope. (Funded by the U.S. Department of Defense; DEVICE ClinicalTrials.gov number, NCT05239195.)
More than 1.5 million critically ill adults undergo tracheal intubation in a setting other than an operating room each year in the United States.1,2 Failure to room each year in the United States.1,2 Failure to intubate the trachea on the first attempt occurs in 20 to 30% of tracheal intubations performed in the emergency department or intensive care unit (ICU) and is associated with an increased risk of life-threatening complications.3–5
Two types of laryngoscopes are commonly used to perform tracheal intubation: a direct laryngoscope and a video laryngoscope. A direct laryngoscope consists of a handle, a blade, and a light. To use a direct laryngoscope, a clinician displaces the patient’s tongue and epiglottis with the blade, visualizes the vocal cords through the mouth (direct laryngoscopy), and then passes an endotracheal tube through the vocal cords. A video laryngoscope includes the same components as those of a direct laryngoscope but is also equipped with a camera positioned in the distal half of the blade that transmits images to a screen.6 With the aid of the video screen to visualize the vocal cords (indirect laryngoscopy), a clinician can guide an endotracheal tube through the vocal cords without a direct line of sight from the mouth.
Although approximately 80% of the intubations that are performed in the emergency department and ICU in current clinical care worldwide are performed with a direct laryngoscope,5 the use of video laryngoscopes has increased over time.7,8 International guidelines on the performance of tracheal intubation in critically ill adults state that the use of either a video laryngoscope or a direct laryngoscope is acceptable.9,10 Several single-center trials and a moderate-sized multicenter trial have been conducted to compare the outcomes when a video laryngoscope is used with the outcomes when a direct laryngoscope is used among critically ill adults undergoing tracheal intubation.11–19 These trials showed differing results, including better outcomes with a video laryngoscope,11,12 better outcomes with a direct laryngoscope,13,14 and no significant differences in outcomes between the two types.15–19 Whether the results of trials that evaluated the use of a video laryngoscope in the operating room apply to intubation in critically ill adults in the emergency department and ICU is uncertain.20
To determine the effect of using a video laryngoscope as compared with a direct laryngoscope on the incidence of successful tracheal intubation on the first attempt in critically ill adults in the emergency department and ICU, we conducted the Direct versus Video Laryngoscope (DEVICE) trial. We hypothesized that the use of a video laryngoscope would result in a higher incidence of successful intubation on the first attempt.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted a pragmatic, multicenter, unblinded, randomized, parallel-group trial in which the use of a video laryngoscope was compared with the use of a direct laryngoscope for tracheal intubation in critically ill adults. The trial was initiated by the investigators and approved by the institutional review board at Vanderbilt University Medical Center, with secondary concurrence by the Defense Health Agency Office of Research Protections of the U.S. Department of Defense. The requirement for written informed consent was waived; details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. The trial was registered at ClinicalTrials.gov before initiation and was overseen by an independent data and safety monitoring board. The protocol and statistical analysis plan, available at NEJM.org, were published before the conclusion of enrollment.21 The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
TRIAL SITES AND PATIENT POPULATION
The trial was conducted at 17 sites, including 7 emergency departments and 10 ICUs in 11 medical centers across the United States. Critically ill adults (age, ≥18 years) undergoing orotracheal intubation with the use of a laryngoscope were eligible. Patients were excluded if they were known to be pregnant, were known to be prisoners (i.e., were incarcerated or involuntarily detained), or had an immediate need for tracheal intubation that precluded randomization, or if the clinician performing the procedure (referred to as the “operator”) determined that the use of a video laryngoscope or a direct laryngoscope on the first attempt was either necessary or contraindicated. Details of the trial sites and a complete list of the inclusion and exclusion criteria are provided in the Supplementary Appendix.
RANDOMIZATION
Patients were randomly assigned in a 1:1 ratio to undergo intubation with a video laryngoscope or with a direct laryngoscope. Randomization was performed with the use of permuted blocks of variable size and was stratified according to trial site. The trial-group assignments were placed in sequentially numbered opaque envelopes and remained concealed until after enrollment. Given the nature of the intervention, clinicians and research personnel were aware of the trial-group assignments after randomization.
INTERVENTIONS
For patients assigned to the video-laryngoscope group, the operator was instructed to use a video laryngoscope on the first attempt at laryngoscopy. A video laryngoscope was defined as a laryngoscope with a camera and a video screen. The trial protocol did not specify the brand of video laryngoscope or the shape of the blade; both were selected by the operator. Operators were instructed to view the video screen while they performed laryngoscopy and inserted the endotracheal tube.
For patients assigned to the direct-laryngoscope group, the operator was instructed to use a direct laryngoscope on the first attempt at laryngoscopy. A direct laryngoscope was defined as a laryngoscope without a camera or a video screen. The trial protocol did not specify the brand of direct laryngoscope or the blade shape (e.g., curved [Macintosh] or straight [Miller]).
All other aspects of the procedure were at the discretion of the treating clinicians, including the type of laryngoscope used on subsequent attempts. At all the trial sites, a stylet or bougie was routinely used during the first tracheal intubation attempt, and waveform capnography or colorimetric end-tidal carbon dioxide detection was used to confirm that the endotracheal tube was in the correct position. Additional details are provided in the Supplementary Appendix.
DATA COLLECTION
A trained observer who was not involved in the performance of the intubation collected data on the primary outcome (by recording the number of times a laryngoscope blade, a bougie, and an endotracheal tube entered the patient’s mouth), the duration of intubation, and the lowest oxygen saturation and lowest systolic blood pressure observed during the interval between induction of anesthesia and 2 minutes after intubation.
The operator reported a subjective assessment of the anticipated difficulty of tracheal intubation (easy, moderate, or difficult) before randomization. Immediately after intubation, the operator reported the Cormack–Lehane grade of laryngeal view (with grades ranging from 1 [view of most of the vocal cords] to 4 [epiglottis not visible]),22 the reasons for failure to intubate on the first attempt (if applicable), procedural complications (esophageal intubation, injury to the teeth, or aspiration), and the number of previous intubations the operator had performed. Trial personnel reviewed the medical record to collect data on baseline characteristics, periprocedural care, and clinical outcomes.
OUTCOMES
The primary outcome was successful intubation on the first attempt, defined as the placement of an endotracheal tube in the trachea with a single insertion of a laryngoscope blade into the mouth and either a single insertion of an endotracheal tube into the mouth or a single insertion of a bougie into the mouth followed by a single insertion of an endotracheal tube into the mouth.23 The single prespecified secondary outcome was the occurrence of severe complications between induction and 2 minutes after intubation. Severe complications were defined as severe hypoxemia (peripheral oxygen saturation, <80%), severe hypotension (systolic blood pressure, <65 mm Hg), new or increased use of vasopressors, cardiac arrest, or death. Additional details regarding the trial outcomes are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
Details regarding the determination of the sample size have been reported previously21 and are included in the Supplementary Appendix. Assuming an incidence of successful intubation on the first attempt of 80% in the direct-laryngoscope group,24–26 90% statistical power, and a two-sided alpha level of 0.05, we calculated that a sample of 1920 patients would need to be enrolled to detect an absolute difference of 5 percentage points between the groups in the incidence of successful intubation on the first attempt. To ensure adequate power if data were missing in up to 4% of the patients, we planned to enroll a total of 2000 patients (1000 per group). A single interim analysis was planned to be performed after 1000 patients had been enrolled; a P value threshold of ≤0.001 for the difference between the groups in the primary outcome was used as the value that justified stopping the trial at the time of the interim analysis.
The primary analysis was an unadjusted, intention-to-treat comparison of the primary outcome in the two groups that was performed with the use of the chi-square test. The primary analysis included all the patients who underwent randomization, except for those who were withdrawn from the trial because they were identified after intubation as being prisoners. Sensitivity analyses included the following: an adjusted analysis in which a generalized linear mixed-effects model with a random effect for trial site and fixed effects for prespecified baseline covariates was used; an analysis in which patients who received the nonassigned laryngoscope on the first laryngoscopy attempt were classified as not having had successful intubation on the first attempt; an analysis in which patients with missing data from the independent observer for the primary outcome were excluded; and an analysis that included only patients in whom the operator had performed a similar percentage of previous intubations with a video laryngoscope as with a direct laryngoscope (defined as having used a video laryngoscope in 25% to 75% of previous intubations).
In accordance with published guidelines,27 we examined whether prespecified baseline variables modified the effect of trial-group assignment on the primary outcome using a generalized linear mixed-effects model with a random effect for trial site and fixed effects for trial group, the proposed effect modifier, and the interaction between the trial group and the proposed effect modifier. Details of this analysis are provided in the Supplementary Appendix.
Between-group differences in secondary and exploratory outcomes are reported as point estimates and 95% confidence intervals. The widths of the confidence intervals were not adjusted for multiplicity and should not be used to infer definitive differences in treatment effects between the two groups. All the analyses were performed with the use of R software, version 4.1.2 (R Foundation for Statistical Computing).
RESULTS
INTERIM ANALYSIS
On November 17, 2022, trial enrollment was stopped at the recommendation of the data and safety monitoring board because the prespecified stopping criterion for efficacy had been met. Among the 1000 patients with data that were included in the interim analysis, successful intubation on the first attempt occurred in 425 of 494 patients (86.0%) in the video-laryngoscope group and in 365 of 506 patients (72.1%) in the direct-laryngoscope group (P<0.001). Complete details of the interim analysis are provided in the Supplementary Appendix, and characteristics of the patients are provided in Table S1 in the Supplementary Appendix.
PATIENTS
Between March 19, 2022, and November 17, 2022, a total of 1947 patients were assessed for eligibility, of whom 1420 (72.9%) were enrolled. The reasons for exclusion from the trial are listed in Figure S1. Three patients who were identified after enrollment as being prisoners were excluded from subsequent data collection and analysis. The remaining 1417 patients were included in the primary analysis. The median age was 55 years, and 69.7% of the patients underwent intubation in an emergency department. The most common indications for tracheal intubation were altered mental status (in 45.3% of the patients) and acute respiratory failure (in 30.4%). A total of 705 patients (49.8%) were assigned to the video-laryngoscope group and 712 patients (50.2%) to the direct-laryngoscope group (Table 1 and Tables S2 through S6). The representativeness of the patients is shown in the Supplementary Appendix.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Video Laryngoscope (N = 705) | Direct Laryngoscope (N = 712) |
|---|---|---|
| Median age (IQR) — yr | 54 (36–66) | 55 (39–67) |
| Female sex — no. (%) | 240 (34.0) | 258 (36.2) |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic White | 360 (51.1) | 346 (48.6) |
| Non-Hispanic Black | 166 (23.5) | 167 (23.5) |
| Hispanic | 101 (14.3) | 94 (13.2) |
| Other | 61 (8.7) | 84 (11.8) |
| Not reported | 17 (2.4) | 21 (2.9) |
| Median body-mass index (IQR)‡ | 26.3 (22.7–31.4) | 26.5 (23.0–31.6) |
| Location of intubation — no. (%) | ||
| Emergency department | 495 (70.2) | 493 (69.2) |
| Intensive care unit | 210 (29.8) | 219 (30.8) |
| Active conditions — no. (%)§ | ||
| Sepsis or septic shock | 188 (26.7) | 216 (30.3) |
| Traumatic injury | 171 (24.3) | 167 (23.5) |
| Cardiac arrest before intubation | 48 (6.8) | 65 (9.1) |
| Median APACHE II score (IQR)¶ | 16 (11–22) | 16 (11–22) |
| Primary indication for intubation — no. (%)∥ | ||
| Altered mental status | 318 (45.1) | 324 (45.5) |
| Acute respiratory failure | 215 (30.5) | 216 (30.3) |
| Emergency procedure | 41 (5.8) | 51 (7.2) |
| Cardiac arrest | 38 (5.4) | 47 (6.6) |
| Other | 93 (13.2) | 74 (10.4) |
| Anticipated difficulty of intubation — no. (%)** | ||
| Easy | 232 (32.9) | 223 (31.3) |
| Moderate | 317 (45.0) | 331 (46.5) |
| Difficult | 67 (9.5) | 62 (8.7) |
| Not reported | 89 (12.6) | 96 (13.5) |
IQR denotes interquartile range.
Race and ethnic group were reported by the patients or their surrogates as part of clinical care and were obtained from the electronic health record by research personnel and grouped into fixed categories.
Data on body-mass index (the weight in kilograms divided by the square of the height in meters) were missing for 33 patients (2.3%): 20 in the videolaryngoscope group and 13 in the direct-laryngoscope group.
Data were abstracted from the electronic health record and grouped into prespecified categories. Patients could have had more than one active condition.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71, with higher scores indicating a greater severity of illness.
Data were abstracted from the electronic health record.
The anticipated difficulty of intubation was a subjective, global clinical assessment made by the operator before randomization.
OPERATORS
A total of 387 unique operators performed an intubation during the trial, with each operator performing a median of 2 intubations (interquartile range, 1 to 4). In total, 91.5% of the intubations were performed by an emergency medicine resident or a critical care fellow. Operators had performed a median of 50 previous tracheal intubations (interquartile range, 25 to 92). The median proportion of previous intubations that operators had performed with the use of a video laryngoscope was 0.69 (interquartile range, 0.50 to 0.80) (Fig. S2). Complete details of the clinical specialty, level of training, and previous experience of the operators performing the tracheal intubations are provided in Table 2 and Table S7.
Table 2.
Characteristics of the Operator and Intubation Procedure.
| Characteristic | Video Laryngoscope (N = 705) | Direct Laryngoscope (N = 712) |
|---|---|---|
| Operator * | ||
| Clinical specialty — no. (%) | ||
| Emergency medicine | 496 (70.4) | 497 (69.8) |
| Critical care medicine | 177 (25.1) | 182 (25.6) |
| Anesthesiology | 18 (2.6) | 25 (3.5) |
| Other† | 14 (2.0) | 8 (1.1) |
| Level of training — no. (%) | ||
| Resident physician | 513 (72.8) | 502 (70.5) |
| Fellow physician | 164 (23.3) | 173 (24.3) |
| Attending physician | 9 (1.3) | 18 (2.5) |
| Other clinician‡ | 19 (2.7) | 19 (2.7) |
| Median no. of previous intubations performed (IQR) | 50 (25–90) | 50 (26–99) |
| Proportion of previous intubations performed with a video laryngoscope — no./total no. (%)§ | ||
| <0.25 | 44/704 (6.2) | 34/711 (4.8) |
| 0.25 to 0.75 | 398/704 (56.5) | 429/711 (60.3) |
| >0.75 | 262/704 (37.2) | 248/711 (34.9) |
| Intubation Procedure | ||
| Preoxygenation received — no. (%) | 702 (99.6) | 711 (99.9) |
| Median oxygen saturation at induction (IQR)¶ | 100 (97–100) | 100 (98–100) |
| Median systolic blood pressure at induction (IQR) — mm Hg∥ | 130 (111–150) | 129 (110–148) |
| Sedative medication administered for induction — no./total no. (%) | 668/695 (96.1) | 676/705 (95.9) |
| Neuromuscular blocking medication administered — no./total no. (%) | 668/696 (96.0) | 677/706 (95.9) |
| Laryngoscope — no. (%) | ||
| Direct** | 0 | 704 (98.9) |
| Video†† | 705 (100) | 8 (1.1) |
| Standard geometry blade | 607 | 5 |
| Hyperangulated blade | 98 | 3 |
| Cormack–Lehane grade of view — no. (%)‡‡ | ||
| 1 | 538 (76.3) | 318 (44.7) |
| 2 | 141 (20.0) | 244 (34.3) |
| 3 | 19 (2.7) | 97 (13.6) |
| 4 | 7 (1.0) | 53 (7.4) |
| Instrument used on first laryngoscopy attempt — no. (%) | ||
| Endotracheal tube with stylet | 389 (55.2) | 339 (47.6) |
| Bougie | 297 (42.1) | 335 (47.1) |
| No attempt to intubate on first laryngoscopy attempt§§ | 19 (2.7) | 37 (5.2) |
| Not reported | 0 | 1 (0.1) |
A total of 387 unique operators performed an intubation during the trial, with each operator performing a median of 2 intubations (IQR, 1 to 4).
The other specialty category included internal medicine, combined emergency medicine and internal medicine, pediatric emergency medicine, and paramedicine.
The other clinician category included certified registered nurse anesthetists, physician assistants, and nurse practitioners.
The proportion of previous intubations performed with a video laryngoscope was calculated by dividing the number of intubations the operator had performed with a video laryngoscope by the total number of intubations the operator had performed with either a video laryngoscope or a direct laryngoscope. Values range from 0.0 (all previous intubations were performed with a direct laryngoscope) to 1.0 (all previous intubations were performed with a video laryngoscope).
Data on oxygen saturation at the time of induction of anesthesia were missing for 121 patients (8.5%): 57 in the video-laryngoscope group and 64 in the direct-laryngoscope group.
Data on systolic blood pressure at induction were missing for 208 patients (14.7%): 108 in the video-laryngoscope group and 100 in the direct-laryngoscope group.
Among the 704 patients who underwent intubation with a direct laryngoscope, 696 (98.9%) underwent procedures that were performed with a Macintosh blade and 8 (1.1%) underwent procedures that were performed with a Miller blade.
Video laryngoscopes with a standard geometry blade included Storz C-MAC (used in 428 patients), McGrath MAC (in 96 patients), and GlideScope MAC (in 88 patients). Video laryngoscopes with a hyperangulated geometry blade included GlideScope LoPro (used in 61 patients), GlideScope GVL (in 14 patients), Storz C-MAC D-blade (in 23 patients), and McGrath X blade (in 3 patients). No video laryngoscopes with channeled blades were used.
The operator assessed the view of the larynx on the first laryngoscopy attempt with the use of the Cormack–Lehane grading scale; grades range from 1 (view of most of the vocal cords) to 4 (epiglottis not visible).
Cases in which neither a stylet nor a bougie was used on the first laryngoscopy attempt are cases in which the laryngoscope blade was removed from the mouth without any attempt to intubate the trachea.
LARYNGOSCOPY AND TRACHEAL INTUBATION
On the first laryngoscopy attempt, a video laryngoscope was used in all 705 patients (100.0%) in the video-laryngoscope group, and a direct laryngoscope was used in 704 of the 712 patients (98.9%) in the direct-laryngoscope group. A view of most of the vocal cords (grade 1 on the Cormack–Lehane grading scale) was reported in 76.3% of the patients in the video-laryngoscope group, as compared with 44.7% of the patients in the direct-laryngoscope group (absolute risk difference, 31.6 percentage points; 95% confidence interval [CI], 26.7 to 36.6) (Table 2 and Fig. S3). Additional characteristics of the intubation procedure are shown in Table 2 and Tables S8 through S11.
PRIMARY OUTCOME
Successful intubation on the first attempt occurred in 600 of the 705 patients (85.1%) in the video-laryngoscope group and in 504 of the 712 patients (70.8%) in the direct-laryngoscope group (absolute risk difference, 14.3 percentage points; 95% CI, 9.9 to 18.7; P<0.001) (Fig. 1 and Table 3). Results were similar in the adjusted analyses and in all prespecified sensitivity analyses, including in the analysis that was limited to cases in which the number of previous intubations the operator had performed with a video laryngoscope was similar to the number performed with a direct laryngoscope (absolute risk difference, 13.5 percentage points; 95% CI, 7.7 to 19.4) (Tables S12 and S13).
Figure 1. Cumulative Incidence of Successful Intubation on the First Attempt.
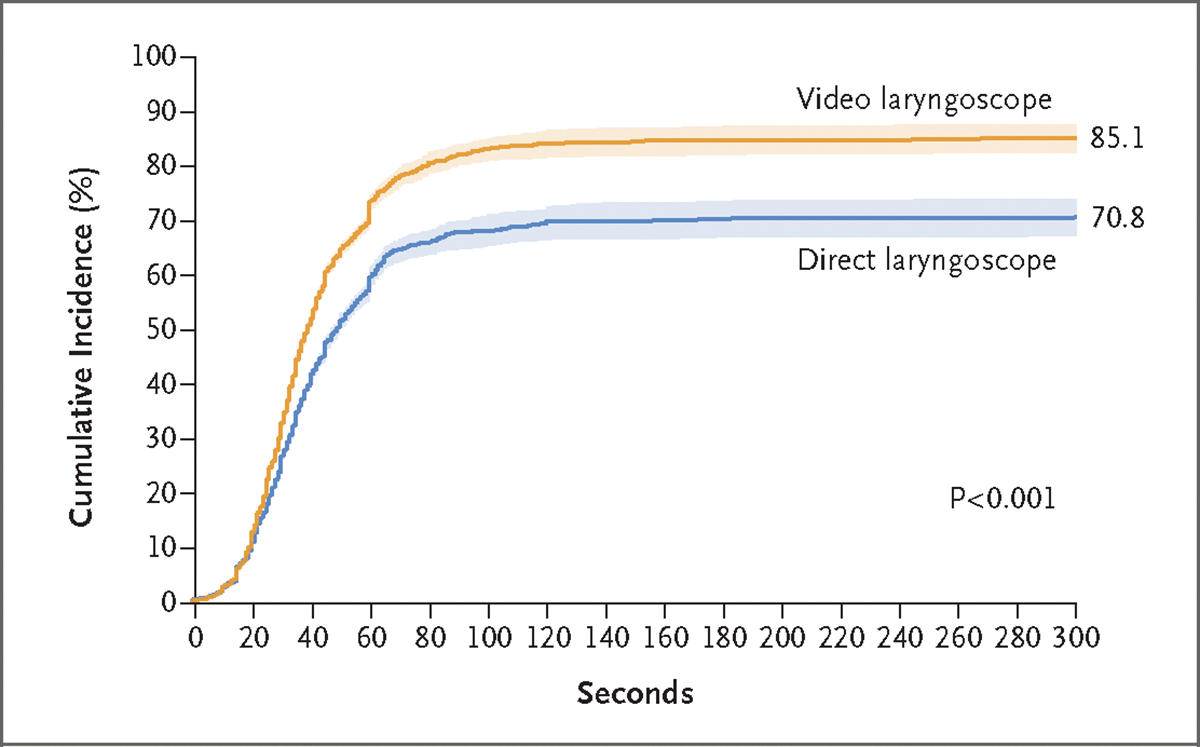
Shown are the cumulative incidence and 95% confidence intervals (shaded areas) for successful intubation on the first attempt among patients in each trial group relative to the time since the initial insertion of a laryngoscope blade into the mouth. Successful intubation on the first attempt occurred in 600 of 705 patients in the video-laryngoscope group and in 504 of 712 patients in the direct-laryngoscope group (absolute risk difference, 14.3 percentage points; 95% CI, 9.9 to 18.7; P<0.001 by the chi-square test).
Table 3.
Outcomes of Tracheal Intubation.
| Outcome | Video Laryngoscope (N = 705) | Direct Laryngoscope (N = 712) | Absolute Difference or Median Difference (95% CI)* |
|---|---|---|---|
| Primary outcome: successful intubation on first attempt — no. (%) | 600 (85.1) | 504 (70.8) | 14.3 (9.9 to 18.7)† |
| Secondary outcome: severe complication during intubation — no. (%)‡ | 151 (21.4) | 149 (20.9) | 0.5 (-3.9 to 4.9) |
| Peripheral oxygen saturation <80% — no./total no. (%)§ | 64/658 (9.7) | 69/659 (10.5) | −0.7 (−4.2 to 2.7) |
| Systolic blood pressure <65 mm Hg — no./total no. (%) | 20/624 (3.2) | 29/644 (4.5) | −1.3 (−3.6 to 1.0) |
| New or increased use of vasopressors — no. (%) | 91 (12.9) | 87 (12.2) | 0.7 (−2.9 to 4.3) |
| Cardiac arrest not resulting in death — no. (%) | 2 (0.3) | 0 | 0.3 (−0.3 to 0.8) |
| Cardiac arrest resulting in death — no. (%) | 1 (0.1) | 3 (0.4) | −0.3 (−1.0 to 0.4) |
| Exploratory procedural outcomes | |||
| Median duration of intubation (IQR) — sec¶ | 38 (26–60) | 46 (30–83) | −8 (−12 to −4) |
| Successful intubation on first laryngoscope blade insertion — no./total no. (%)∥ | 636/704 (90.3) | 546/706 (77.3) | 13.0 (9.1 to 16.9) |
| Successful intubation on first attempt without occurrence of a severe complication — no. (%)** | 484 (68.7) | 420 (59.0) | 9.7 (4.5 to 14.8) |
| Reason for intubation failure on first attempt — no. (%)†† | |||
| Inadequate view of vocal cords | 26 (3.7) | 123 (17.3) | −13.6 (−16.8 to −10.3) |
| Inability to insert an endotracheal tube or bougie | 49 (7.0) | 51 (7.2) | −0.2 (−3.0 to 2.6) |
| Other | 17 (2.4) | 24 (3.4) | −1.0 (−2.8 to 0.9) |
| Not reported | 23 (3.3) | 40 (5.6) | −2.4 (−4.6 to −0.1) |
| Exploratory safety outcomes — no. (%) | |||
| Esophageal intubation | 6 (0.9) | 9 (1.3) | −0.4 (−1.6 to 0.8) |
| Injury to teeth | 3 (0.4) | 2 (0.3) | 0.1 (−0.6 to 0.9) |
| Operator-reported aspiration | 7 (1.0) | 12 (1.7) | −0.7 (−2.0 to 0.6) |
| Exploratory clinical outcomes‡‡ | |||
| Median ICU-free days (IQR) | 20 (0–25) | 19 (0–24) | 1 (−1 to 3) |
| Median ventilator-free days (IQR) | 24 (0–26) | 23 (0–26) | 1 (0 to 2) |
| In-hospital death — no. (%) | |||
| Within 1 hr after randomization§§ | 15 (2.1) | 27 (3.8) | −1.7 (−3.6 to 0.2) |
| Within 28 days after randomization | 184 (26.1) | 191 (26.8) | −0.7 (−5.5 to 4.0) |
The widths of the confidence intervals for the secondary and exploratory outcomes were not adjusted for multiplicity and should not be used to infer definitive differences in treatment effects between the groups.
P<0.001.
Patients could have had more than one severe complication.
Oxygen saturation was measured by pulse oximetry.
Data on the duration of intubation, which was defined as the number of seconds between the start of laryngoscopy and intubation of the trachea, were missing for 28 patients (2.0%): 9 patients in the video-laryngoscope group and 19 patients in the direct-laryngoscope group.
Successful intubation on the first laryngoscope blade insertion refers to successful tracheal intubation during the first laryngoscopy attempt, which was defined as a single insertion of the laryngoscope blade into the mouth, regardless of the number of times a bougie or an endotracheal tube was inserted into the mouth.
Successful intubation on the first attempt without the occurrence of a severe complication was defined as meeting the primary outcome and not the secondary outcome.
Reasons for intubation failure were reported by the operator. Patients could have had more than one reason.
Intensive care unit (ICU)–free days, ventilator-free days, and in-hospital death were assessed at 28 days, with follow-up data censored at the time of hospital discharge.
In-hospital death within 1 hour after randomization was a post hoc outcome.
Figure 2 shows the results of the primary outcome in prespecified subgroups. Figure S4 shows the heterogeneity of the treatment effect according to the operator’s total number of previous intubations. Among the operators who had performed fewer than 25 intubations, the absolute difference between the two groups in the incidence of successful intubation on the first attempt was 26.1 percentage points (95% CI, 15.4 to 36.8). Among the operators who had performed more than 100 intubations, the absolute difference was 5.9 percentage points (95% CI, −4.1 to 16.0). Additional analyses of effect modification are shown in Figures S5 through S8.
Figure 2. Subgroup Analyses of the Primary Outcome.
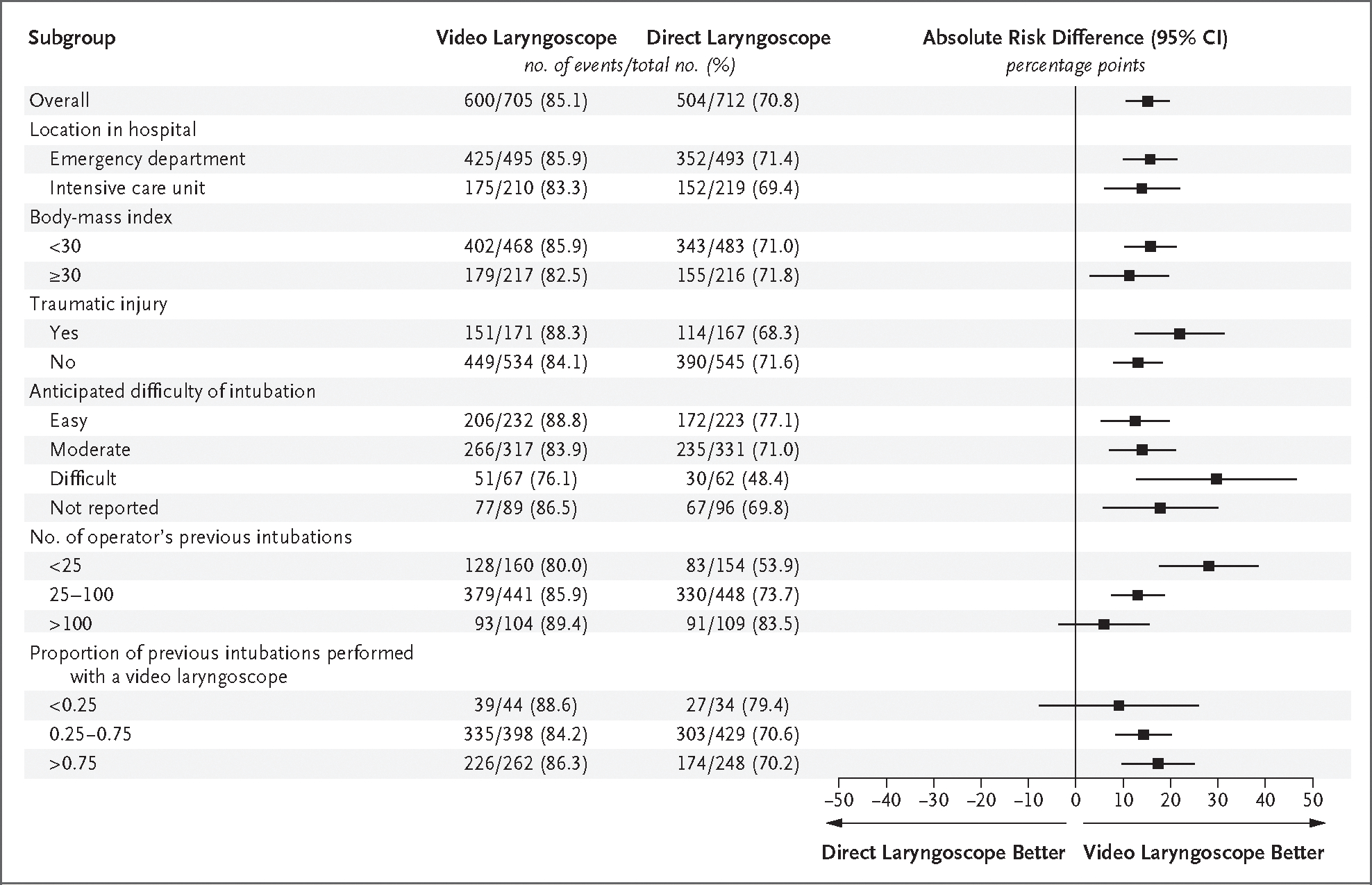
Shown are the absolute risk differences and 95% confidence intervals for the primary outcome (successful intubation on the first attempt) in the video-laryngoscope group as compared with the direct-laryngoscope group in each prespecified subgroup. Absolute risk differences were calculated with the use of a generalized linear mixed-effects model with a random effect for trial site and fixed effects for trial group, the proposed effect modifier, and the interaction between the trial group and the proposed effect modifier. Absolute risk differences of greater than 0 indicate a higher likelihood of successful intubation on the first attempt with use of a video laryngoscope. The body-mass index is the weight in kilograms divided by the square of the height in meters.
SECONDARY OUTCOME
A total of 151 patients (21.4%) in the video-laryngoscope group and 149 patients (20.9%) in the direct-laryngoscope group had a severe complication during intubation (absolute risk difference, 0.5 percentage points; 95% CI, −3.9 to 4.9). Further details are provided in Table 3 and Table S14.
EXPLORATORY OUTCOMES
Successful intubation on the first attempt without the occurrence of a severe complication was achieved in 484 patients (68.7%) in the videol-aryngoscope group and in 420 patients (59.0%) in the direct-laryngoscope group (absolute risk difference, 9.7 percentage points; 95% CI, 4.5 to 14.8). Failure to intubate the trachea on the first attempt because of an inadequate view of the vocal cords occurred in 26 patients (3.7%) in the video-laryngoscope group and in 123 patients (17.3%) in the direct-laryngoscope group (absolute risk difference, −13.6 percentage points; 95% CI, −16.8 to −10.3) (Table 3 and Table S15). Failure to intubate the trachea on the first attempt because of an inability to insert a bougie or an endotracheal tube with a stylet occurred in 49 patients (7.0%) in the video-laryngoscope group and in 51 patients (7.2%) in the direct-laryngoscope group (absolute risk difference, −0.2 percentage points; 95% CI, −3.0 to 2.6). The median time interval between the initiation of laryngoscopy and intubation of the trachea was 38 seconds (interquartile range, 26 to 60) in the video-laryngoscope group and 46 seconds (interquartile range, 30 to 83) in the direct-laryngoscope group (median difference, −8; 95% CI, −12 to −4) (Table 3). Additional procedural outcomes are shown in Table S16.
SAFETY OUTCOMES
The incidences of esophageal intubation, injury to the teeth, and aspiration were similar in the two groups (Table 3). Cricothyrotomy was not performed in any patients in the video-laryngoscope group and was performed in 1 patient in the direct-laryngoscope group (Table S17).
DISCUSSION
Among critically ill adults in this multicenter, randomized trial, the use of a video laryngoscope for tracheal intubation resulted in a higher incidence of successful intubation on the first attempt than the use of a direct laryngoscope. This finding may have important clinical implications because failure to intubate on the first attempt is associated with life-threatening complications,3–5 and in current clinical care worldwide, most critically ill adults undergo intubation with a direct laryngoscope rather than a video laryngoscope.5,28,29
The effect of video laryngoscopy as compared with direct laryngoscopy has been evaluated previously in small and moderate-sized trials involving patients in emergency departments16,17,30–34 and in ICUs.11,15,19,35–39 Among these trials, the only multicenter trial — in which 371 patients undergoing tracheal intubation in an ICU were enrolled — showed no significant difference between the two approaches in the incidence of successful intubation on the first attempt.19
Two main factors may explain the difference in findings between our trial and the previous multicenter trial. First, although both trials showed that the use of a video laryngoscope improved the view of the vocal cords, this beneficial effect was negated in the previous trial by the increased difficulty of inserting an endotracheal tube into the trachea among the patients in whom a video laryngoscope was used — a finding that was not seen in the current trial.40 The absence of an increased difficulty of inserting an endotracheal tube during video laryngoscopy in our trial may be explained by the consistent use of a stylet or bougie,25,41 which facilitates the insertion of an endotracheal tube into the trachea during laryngoscopy. These instruments were used in all the intubations in the current trial and in only 16% of the intubations in the previous trial. Second, since the publication of the results of the previous trial, the use of video laryngoscopes in emergency departments and ICUs has increased, in part because of recommendations made during the coronavirus disease 2019 pandemic with respect to increasing the distance between the patient’s mouth and the operator to potentially lower the risk of viral transmission.42,43 In previous trials, the limited experience of the clinicians in the use of a video laryngoscope may have complicated the comparison of outcomes when a video laryngoscope was used with outcomes when a direct laryngoscope was used.15 By contrast, most of the clinicians in our trial had performed at least as many previous intubations with a video laryngoscope as with a direct laryngoscope, a factor that facilitated a direct comparison of the two devices.
The difference in findings between the current trial and previous trials is not related to a lower incidence of successful intubation on the first attempt in the direct-laryngoscope group of the current trial; intubation with one laryngoscope blade insertion occurred in 77.3% of the patients in the direct-laryngoscope group of the current trial, as compared with 66 to 83% of those in previous trials.5,15,18,19 The benefit of video laryngoscope use in our trial is consistent with the results of previous trials that took place in operating rooms.20,44
In our trial, the use of a video laryngoscope appeared to increase the likelihood of successful intubation on the first attempt for operators at all levels of experience, but the between-group difference in the primary outcome appeared to be greatest among the least experienced operators (although the trial was not designed to make such comparisons and definitive conclusions may not be drawn). This finding could be the result of various factors, including the fact that operators who are less familiar with anatomical landmarks derive greater benefit from improved laryngeal visualization or the fact that the video screen allows a second clinician to provide real-time feedback.
Successful intubation on the first attempt is the most common outcome in emergency intubation research19,25,41,45 and has been consistently associated with a lower risk of life-threatening complications.3–5 In this trial, the use of a video laryngoscope resulted in a higher incidence of successful intubation on the first attempt than the use of a direct laryngoscope. The incidence of successful intubation on the first attempt without the occurrence of a severe complication also appeared to be higher in the video-laryngoscope group, and the median duration of intubation appeared to be lower; however, definitive conclusions may not be drawn. The trial was not powered to evaluate the effect of video laryngoscope use on hypoxemia, hypotension, or cardiac arrest.
Our trial has several strengths. The trial design included randomization to balance baseline characteristics; concealment of the trial-group assignment until enrollment to prevent selection bias; conduct of the trial in emergency departments and ICUs at multiple sites at which hundreds of operators used a variety of types of video laryngoscopes, all of which increased the generalizability of the trial; and the collection of trial outcome data by an independent observer to minimize observer bias. Adherence to the group assignment was excellent, and the percentage of patients with missing data for the primary outcome was low.
Our trial also has several limitations. Because operators selected the brand of video laryngoscope and the shape of the blade, the results of our trial cannot be used to determine the brand of video laryngoscope or the blade shape that leads to the best outcomes. Because 97% of the operators had performed fewer than 250 previous tracheal intubations, the findings may not apply to operators with more experience. All the intubations occurred in an emergency department or ICU; therefore, our findings cannot be used to inform the approach to tracheal intubation in the operating room. Finally, patients, clinicians, and trial personnel were aware of the trial-group assignments.
In this trial, among critically ill adults undergoing tracheal intubation in an emergency department or ICU, the use of a video laryngoscope resulted in a higher incidence of successful intubation on the first attempt than the use of a direct laryngoscope.
Supplementary Material
Acknowledgments
The views expressed are those of the authors and do not necessarily reflect the official views or policy of the Department of Defense or its components. The informed consent of the patients who participated in this trial was addressed as required by 32 CFR 219 and DODI 3216.02_AFI 40-402 with the use of a patient information sheet, and the institutional review board requirement for written informed consent was waived.
Supported by the U.S. Department of Defense through Defense Health Agency Restoral funding and the 59th Medical Wing of the U.S. Air Force.
Footnotes
Contributor Information
M.E. Prekker, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Hennepin County Medical Center, Minneapolis Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis.
B.E. Driver, Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis
S.A. Trent, Department of Emergency Medicine, Denver Health Medical Center, Denver Department of Emergency Medicine, University of Colorado School of Medicine, Aurora.
D. Resnick-Ault, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora
K.P. Seitz, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville
D.W. Russell, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine, Birmingham University of Alabama at Birmingham Heersink School of Medicine, and the Pulmonary Section, Birmingham Veterans Affairs Medical Center, Birmingham.
J.P. Gaillard, Department of Anesthesiology, Section on Critical Care, Atrium Health Wake Forest Baptist, North Carolina Department of Emergency Medicine, Atrium Health Wake Forest Baptist, North Carolina.
A.J. Latimer, Department of Emergency Medicine, University of Washington Harborview Medical Center, Seattle
S.A. Ghamande, Department of Medicine, Division of Pulmonary Disease, Critical Care, and Sleep Medicine, Baylor Scott and White Health, Temple, Texas
K.W. Gibbs, Section on Pulmonary, Critical Care, Allergy, and Immunology, Wake Forest School of Medicine, North Carolina
D.J. Vonderhaar, Department of Pulmonary and Critical Care Medicine, Ochsner Health, New Orleans
M.R. Whitson, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine Department of Emergency Medicine, Birmingham.
C.R. Barnes, Anesthesiology and Critical Care Medicine, University of Washington Harborview Medical Center, Seattle
J.P. Walco, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville
I.S. Douglas, Division of Pulmonary, Critical Care, and Sleep Medicine, Denver Health Medical Center, Denver Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Colorado School of Medicine, Aurora.
V. Krishnamoorthy, Winston-Salem, and the Department of Anesthesiology, Duke University School of Medicine, Durham, North Carolina
A. Dagan, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston
J.J. Bastman, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora
B.D. Lloyd, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville
S. Gandotra, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine
J.K. Goranson, Department of Emergency Medicine, Atrium Health Wake Forest Baptist, North Carolina
S.H. Mitchell, Department of Emergency Medicine, University of Washington Harborview Medical Center, Seattle
H.D. White, Department of Medicine, Division of Pulmonary Disease, Critical Care, and Sleep Medicine, Baylor Scott and White Health, Temple, Texas
J.A. Palakshappa, Section on Pulmonary, Critical Care, Allergy, and Immunology, Wake Forest School of Medicine, North Carolina
A. Espinera, Department of Pulmonary and Critical Care Medicine, Ochsner Health, New Orleans
D.B. Page, Department of Medicine, Division of Pulmonary, Allergy and Critical Care Medicine
A. Joffe, Anesthesiology and Critical Care Medicine, University of Washington Harborview Medical Center, Seattle
S.J. Hansen, Department of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Hennepin County Medical Center, Minneapolis
C.G. Hughes, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville
T. George, Division of Pulmonary, Critical Care, and Sleep Medicine, Denver Health Medical Center, Denver
J.T. Herbert, Winston-Salem, and the Department of Anesthesiology, Duke University School of Medicine, Durham, North Carolina
N.I. Shapiro, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston
S.G. Schauer, U.S. Army Institute of Surgical Research, Joint Base San Antonio
B.J. Long, 59th Medical Wing, U.S. Air Force, Fort Sam Houston, San Antonio, Texas
B. Imhoff, Department of Biostatistics, Vanderbilt University Medical Center, Nashville
L. Wang, Department of Biostatistics, Vanderbilt University Medical Center, Nashville
J.P. Rhoads, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville
K.N. Womack, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville
D.R. Janz, University Medical Center New Orleans and the Department of Medicine, Section of Pulmonary, Critical Care Medicine, and Allergy and Immunology, Louisiana State University School of Medicine, New Orleans
W.H. Self, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville.
T.W. Rice, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville.
A.A. Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora
J.D. Casey, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville
M.W. Semler, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville.
REFERENCES
- 1.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. hospitals, 2011. Rockville, MD: Agency for Healthcare Research and Quality, October 2013 (https://www.ncbi.nlm.nih.gov/books/NBK174682/pdf/Bookshelf_NBK174682.pdf). [PubMed] [Google Scholar]
- 2.Cairns C, Kang K, Santo L. National Hospital Ambulatory Medical Care Survey: 2018 emergency department summary tables. Washington, DC: National Center for Health Statistics; (https://www.cdc.gov/nchs/data/nhamcs/web_tables/2018-ed-web-tables-508.pdf). [Google Scholar]
- 3.Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg 2004;99:6 07–13. [DOI] [PubMed] [Google Scholar]
- 4.Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med 2013; 20:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russotto V, Myatra SN, Laffey JG, et al. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA 2021; 325:1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkow LC, Morey TE, Urdaneta F. The technology of video laryngoscopy. Anesth Analg 2018;126:1527–34. [DOI] [PubMed] [Google Scholar]
- 7.Groombridge CJ, Maini A, Olaussen A, Kim Y, Fitzgerald M, Smit DV. Unintended consequences: the impact of airway management modifications introduced in response to COVID-19 on intubations in a tertiary centre emergency department. Emerg Med Australas 2021;33:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins A, Stapleton S, Rodriguez G, Gonzalez RM, Baker WE. Emergency tracheal intubation in patients with COVID-19: a single-center, retrospective cohort study. West J Emerg Med 2021;22:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myatra SN, Shah A, Kundra P, et al. All India Difficult Airway Association 2016 guidelines for the management of unanticipated difficult tracheal intubation in adults. Indian J Anaesth 2016;60:885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgs A, McGrath BA, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth 2018;120:323–52. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Li N, Acquah SO, Kory PD. Comparison of video laryngoscopy versus direct laryngoscopy during urgent endotracheal intubation: a randomized controlled trial. Crit Care Med 2015;43:636–41. [DOI] [PubMed] [Google Scholar]
- 12.Macke C, Gralla F, Winkelmann M, et al. Increased first pass success with C-MAC videolaryngoscopy in prehospital endotracheal intubation — a randomized controlled trial. J Clin Med 2020;9:2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trimmel H, Kreutziger J, Fertsak G, Fitzka R, Dittrich M, Voelckel WG. Use of the Airtraq laryngoscope for emergency intubation in the prehospital setting: a randomized control trial. Crit Care Med 2011;39:489–93. [DOI] [PubMed] [Google Scholar]
- 14.Trimmel H, Kreutziger J, Fitzka R, et al. Use of the GlideScope ranger video laryngoscope for emergency intubation in the prehospital setting: a randomized control trial. Crit Care Med 2016;44(7):e470–e476. [DOI] [PubMed] [Google Scholar]
- 15.Janz DR, Semler MW, Lentz RJ, et al. Randomized trial of video laryngoscopy for endotracheal intubation of critically ill adults. Crit Care Med 2016;44:1980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driver BE, Prekker ME, Moore JC, Schick AL, Reardon RF, Miner JR. Direct versus video laryngoscopy using the C-MAC for tracheal intubation in the emergency department, a randomized controlled trial. Acad Emerg Med 2016;23:433–9. [DOI] [PubMed] [Google Scholar]
- 17.Yeatts DJ, Dutton RP, Hu PF, et al. Effect of video laryngoscopy on trauma patient survival: a randomized controlled trial. J Trauma Acute Care Surg 2013;75:212–9. [DOI] [PubMed] [Google Scholar]
- 18.Kreutziger J, Hornung S, Harrer C, et al. Comparing the McGrath Mac Video Laryngoscope and direct laryngoscopy for prehospital emergency intubation in air rescue patients: a multicenter, randomized, controlled trial. Crit Care Med 2019;47:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lascarrou JB, Boisrame-Helms J, Bailly A, et al. Video laryngoscopy vs direct laryngoscopy on successful first-pass orotracheal intubation among ICU patients: a randomized clinical trial. JAMA 2017;317:483–93. [DOI] [PubMed] [Google Scholar]
- 20.Hansel J, Rogers AM, Lewis SR, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation. Cochrane Database Syst Rev 2022;4(4):C D011136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prekker ME, Driver BE, Trent SA, et al. DirEct versus VIdeo LaryngosCopE (DEVICE): protocol and statistical analysis plan for a randomised clinical trial in critically ill adults undergoing emergency tracheal intubation. BMJ Open 2023;13(1):e068978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984;39:1105–11. [PubMed] [Google Scholar]
- 23.Trent SA, Driver BE, Prekker ME, et al. Defining successful intubation on the first attempt using both laryngoscope and endotracheal tube insertions: a secondary analysis of clinical trial data. Ann Emerg Med 2023. April 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey JD, Janz DR, Russell DW, et al. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med 2019;380:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driver BE, Semler MW, Self WH, et al. Effect of use of a bougie vs endotracheal tube with stylet on successful intubation on the first attempt among critically ill patients undergoing tracheal intubation: a randomized clinical trial. JAMA 2021;326:2488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell DW, Casey JD, Gibbs KW, et al. Effect of fluid bolus administration on cardiovascular collapse among critically ill patients undergoing tracheal intubation: a randomized clinical trial. JAMA 2022;328:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schandelmaier S, Briel M, Varadhan R, et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020;192(32):E901–E906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green RS, Fergusson DA, Turgeon AF, et al. Device and medication preferences of Canadian physicians for emergent endotracheal intubation in critically ill patients. CJEM 2017;19:186–97. [DOI] [PubMed] [Google Scholar]
- 29.Seisa MO, Gondhi V, Demirci O, Died-rich DA, Kashyap R, Smischney NJ. Survey on the current state of endotracheal intubation among the critically ill: HEMAIR investigators. J Intensive Care Med 2018;33:354–60. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadi K, Ebrahimi M, Hashemian AM, Sarshar S, Rahimi-Movaghar V. GlideScope video laryngoscope for difficult intubation in emergency patients: a quasirandomized controlled trial. Acta Med Iran 2015;53:738–42. [PubMed] [Google Scholar]
- 31.Goksu E, Kilic T, Yildiz G, Unal A, Kartal M. Comparison of the C-MAC video laryngoscope to the Macintosh laryngoscope for intubation of blunt trauma patients in the ED. Turk J Emerg Med 2016;16:53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JW, Park SO, Lee KR, et al. Video laryngoscopy vs. direct laryngoscopy: which should be chosen for endotracheal intubation during cardiopulmonary resuscitation? A prospective randomized controlled study of experienced intubators. Resuscitation 2016;105:196–202. [DOI] [PubMed] [Google Scholar]
- 33.Sulser S, Ubmann D, Schlaepfer M, et al. C-MAC videolaryngoscope compared with direct laryngoscopy for rapid sequence intubation in an emergency department: a randomised clinical trial. Eur J Anaesthesiol 2016;33:943–8. [DOI] [PubMed] [Google Scholar]
- 34.Sanguanwit P, Yuksen C, Laowattana N. Direct versus video laryngoscopy in emergency intubation: a randomized control trial study. Bull Emerg Trauma 2021;9:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griesdale DEG, Chau A, Isac G, et al. Video-laryngoscopy versus direct laryngoscopy in critically ill patients: a pilot randomized trial. Can J Anaesth 2012;59:1032–9. [DOI] [PubMed] [Google Scholar]
- 36.Abdelgalel EF, Mowafy SMS. Comparison between Glidescope, Airtraq and Macintosh laryngoscopy for emergency endotracheal intubation in intensive care unit: randomized controlled trial. Egypt J Anaesth 2018;34:123–8. [Google Scholar]
- 37.Gao Y-X, Song Y-B, Gu Z-J, et al. Video versus direct laryngoscopy on successful first-pass endotracheal intubation in ICU patients. World J Emerg Med 2018;9:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dey S, Pradhan D, Saikia P, Bhat-tacharyya P, Khandelwal H, Adarsha KN. Intubation in the intensive care unit: C-MAC video laryngoscope versus Macintosh laryngoscope. Med Intensiva (Engl Ed) 2020;44:135–41. [DOI] [PubMed] [Google Scholar]
- 39.Dharanindra M A comparative study of King Vision video laryngoscope and Macintosh laryngoscope for intubation in the ICU. Indian J Crit Care Med 2020;24:Suppl 2:S38–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prekker ME, Trent SA, Lofrano A, et al. Laryngoscopy and tracheal intubation: does use of a video laryngoscope facilitate both steps of the procedure? Ann Emerg Med 2023. April 5 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaber S, Rollé A, Godet T, et al. Effect of the use of an endotracheal tube and stylet versus an endotracheal tube alone on first-attempt intubation success: a multicentre, randomised clinical trial in 999 patients. Intensive Care Med 2021;47:653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia 2020;75:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020;75:785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kriege M, Noppens RR, Turkstra T, et al. A multicentre randomised controlled trial of the McGrath Mac videolaryngoscope versus conventional laryngoscopy. Anaesthesia 2023;78:722–9. [DOI] [PubMed] [Google Scholar]
- 45.Nauka PC, Moskowitz A, Fein DG. Ap-praising first-pass success: during emergency airway management, what does it mean to be successful? Ann Am Thorac Soc 2023;20:21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


