INTRODUCTION
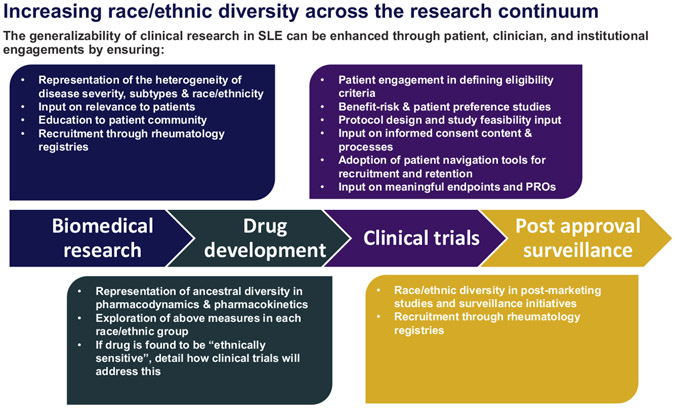
Biomedical and clinical research can have a major impact on the eradication of health disparities, which unfortunately remain common among a wide range of chronic conditions.1-3 There is a significant need to address disparities in systemic lupus erythematosus (SLE), and this is increasingly recognized within the SLE research and clinical community as a research priority.4-7 There are different ways to address this issue. One important means is to ensure that the inclusion of race/ethnic minorities in SLE clinical trials is adequate. In a review of 193 clinical trials for SLE with at least 1 site in the United States, we found that although race/ethnic minorities comprise nearly 70% of estimated prevalent SLE cases (43% Black, 16% Hispanic, and 13% Asian), they comprise only 49% of clinical trial participants (14% Black, 21% Hispanic, and 10% Asian).8 The study also found that the representation of Black individuals among trial enrollees has decreased since 2006 to 2011, whereas the representation of other race/ethnic minorities has increased.8 However, the lack of diversity in clinical trials is not unique to SLE.9 To provide a broader context, we draw extensively from oncology where the issue of diversity in clinical trials has a long history, and we highlight parallels to SLE. We recommend that clinical trials in SLE move beyond only increasing race/ethnic diversity to ensuring adequately powered subgroup analyses due to the preponderance of evidence for race/ethnic differences in response to therapeutic agents in SLE. In this article, we further argue that focusing diversity efforts on clinical trial recruitment alone is insufficient because this ignores decision-making that occurs upstream and downstream of clinical trials that may adversely impact these efforts. We present a framework (Fig. 1) for improving generalizability of clinical research in SLE through patient-level, clinician-level, and institutional-level engagements across the research continuum.
Fig. 1.
Enhancing diversity in the research pipeline: a democratizing framework.
HISTORICAL AND CONTEMPORARY TRENDS REGARDING DIVERSITY IN CLINICAL TRIALS
In the United States, the problem of lack of race/ethnic diversity is multidimensional, shaped by social determinants of health. This also limits the generalizability of trial results and can have unintended consequences, such as preventing minorities from adequately benefiting from scientific advances stemming from clinical trials. In addition to our finding of the underrepresentation of race/ethnic minorities in SLE randomized controlled trials (RCTs), ample evidence exists for this in other medical subspecialties. In an attempt to quantify the generalizability of trials for the US population, Loree and colleagues10 reported on the following mismatch: between the proportion of different races represented in trials for US Food and Drug Administration (FDA) approval of oncology drugs for specific indications with the proportion of different races (pertaining to incidence and mortality) among patients with a specific type of cancer versus the US population. There was consistent underrepresentation of Black and Hispanic patients in pivotal FDA approval studies between July 2008 and June 2018, with White, Asian, Black, and Hispanic patients representing 76%, 18%, 3%, and 6% of trial enrollees in oncology, respectively.10 Similar to our findings in SLE RCTs, Loree and colleagues10 found that the proportion of Black enrollees decreased or stayed the same over time (4% to 3% between 2008 and 2018), suggesting that this decline in representation may not be unique to SLE.
We highlight a few key time points in the modern era in which national policies for increasing the reporting and representation of race/ethnic minorities in clinical trials have implications for SLE. In 1993, the National Institutes of Health (NIH) Revitalization Act stated that women and minorities must be included in all NIH-funded clinical research, unless there is a justification approved by the NIH.11,12 An amendment was issued by the NIH in 2001 mandating that proposals for NIH-defined phase III clinical trials define processes for identifying differences in treatment responses among race/ethnic groups if the intervention effect is expected to differ among them.11,12 In 2017, NIH issued a policy revision requiring that applicable NIH-defined phase III clinical trials submit subgroup analyses by race/ethnicity and sex/gender to Clinicaltrials.gov.11,12 In 2018, Geller and colleagues13 published a review to investigate the contemporary levels of compliance with these guidelines in NIH-funded RCTs published in 14 leading US medical journals. They found that 85% of the published RCTs did not include race/ethnicity in the analysis and did not provide an explanation for this exclusion.13 When they compared this finding with their previous examination of these trends in 2004, 2009, and 2015, they found no statistically significant improvements in the lack of compliance with the guidelines between 2004 and 2018.13
DOES TREATMENT RESPONSE VARY BY RACE/ETHNICITY IN SYSTEMIC LUPUS ERYTHEMATOSUS?
Although there have certainly been advances in the treatment of SLE in the past decade, the therapeutic landscape has not changed as rapidly as hoped. Hydroxychloroquine remains the cornerstone of treatment, with glucocorticoids used for flares (with a goal to taper to a low dose as soon as is feasible), along with various immuno-suppressive agents that are used to induce and maintain remission, as well as facilitate steroid taper.14 Cyclophosphamide is used for severe SLE manifestations when other agents are not appropriate, as well as in refractory disease, a setting in which rituximab also can play a role.14 Despite increased understanding of disease pathogenesis, most SLE clinical trials of new targeted therapies have failed.15 One exception is belimumab, which is currently approved for use in patients with active, nonrenal, non–central nervous system SLE.16 Several other drugs have shown promise in recent clinical trials, although are not yet approved therapies.17-22
Our review did not ascertain whether SLE RCTs provided subgroup analyses by race/ethnicity in accordance with the NIH policy; however, there are many indications that treatment response in SLE may be heterogeneous by race/ethnicity based on our understanding of the epidemiology of the disease. Here, we present several mechanistic bases for race/ethnic differences in response to therapeutic agents in SLE. First, race/ethnic minorities share a disproportionate burden in risk of SLE, and the immunologic profile, clinical presentation, and overall prognosis differ by race/ethnicity.23 There are also differences in the immunologic profiles (in particular autoantibodies) by race/ethnicity. There are no reported race/ethnic differences in antinuclear antibody, although Black patients with SLE are known to have higher prevalence of positivity for specific autoantibodies, such as anti-Sm, anti-RNP and anti-dsDNA, compared with White patients.24 Taken together, these race/ethnic differences in SLE phenotypes suggest possible race/ethnic-dependent biological pathways that underlie the expression of disease.
Second, these disparities are also evident in mortality, where the rates are highest in Black individuals, followed by Hispanic and White individuals. SLE is also a significant cause of premature mortality for women of reproductive age. A recent study of death certificate data in the United States found that SLE was the fifth leading cause of death in Black and Hispanic 15-year-old to 24-year-old female patients, behind neoplasms, heart disease, infections, and pregnancy, a remarkable finding given that SLE is defined as a rare disease.25 Third, in a study of major treatment advancements in the context of SLE mortality rates, Singh and Yen26 found significant declines in mortality since the 1950s attributed to the improving understanding of the pathophysiology of the disease and advancements in treatment options. There are indications that race/ethnic minorities may have not fully benefited from these therapeutic advances.27
Fourth, one question that emerges from these disparities in mortality is whether these differences could be attributed to biological, genetic, environmental, and/or socioeconomic determinants. Although it could be argued that it might be more useful to infer genetic ancestry ascertaining (eg, through genome-wide association studies [GWAS]) instead of using race/ethnicity in understanding the pathophysiology of disease, this issue is complicated because both constructs capture different information.28 However, complex traits, and polygenic and environmental factors may be shaped by ancestry and social determinants of health.28 A major possible contributory factor is that the current treatment options for SLE may not be as appropriate for race/ethnic minorities and treatment guidelines are not optimized to address this issue. However, comparing studies that report differences in treatment response can be difficult due to differences in measurement and the changing nature of race/ethnic identity.23 Also, most studies showing differences in treatment response are often underpowered in post hoc analyses.23
There have been little to no “head-to-head” comparisons by race/ethnicity of heterogeneity in SLE treatment response in observational or experimental settings. In a review evaluating the evidence for race differences in the response to therapies for SLE, Litwic and colleagues23 reported that there are no major race/ethnic differences in the response to steroids, hydroxychloroquine, and azathioprine. However, this conclusion can be misleading because there have been no biomedical or clinical research studies evaluating whether there are differences in response to these medications by race/ethnicity. Future studies are needed to answer this question. Among therapeutic agents for induction therapy for lupus nephritis, intravenous cyclophosphamide (IVC) may be less effective in Black and Hispanic patients compared with White patients.29,30 For example, Dooley and colleagues29 reported that the 5-year renal survival for patients on IVC was 95% in non-Black patients, whereas it was 57% in Black patients. Mycophenolate mofetil for lupus nephritis appears to be more effective in Black and Hispanic patients compared with White patients.23,30 There is also some indication that mycophenolate mofetil is safer and causes fewer side effects in these groups.23,30,31 However, there are significant methodologic limitations to these studies, including sample size and reference groups (that include all non-Black patients) that limit the generalizability of their inferences.
HETEROGENEITY IN SYSTEMIC LUPUS ERYTHEMATOSUS AND THE CHALLENGE OF CLINICAL TRIALS AND BIOMEDICAL STUDIES
The heterogeneity of SLE presents a unique set of challenges in clinical trials and biomedical studies. For example, there are differences in the association of SLE with genetic factors by race/ethnicity. One recent GWAS identified 58 distinct non-HLA regions in White, 9 in Black, and 16 in Hispanic individuals.32 There was a lack of considerable overlap in the genetic regions by race/ethnicity.32 When evaluating how well the “White”-associated genetic factors predicted SLE, the odds ratio was 30, but when applied to the Black population the odds ratio was only 3, highlighting the differences in genetic association by race/ethnicity in SLE.32,33 In clinical trials, this heterogeneity leads to smaller, mostly homogeneous cohorts that often do not adequately represent the broad spectrum of the disease. In biomedical and biomarker studies, this challenge may be reflected in the lack of understanding of more severe and less prevalent subtypes. This issue is particularly important because one of the biggest benefits of “-omics” and biomarkers research is the possibility of discovering novel pathobiological pathways.34 Biomarkers discovered in homogeneous cohorts may only generalize to external cohorts similar to the original cohort and may be less useful in a cohort that differs significantly from the original cohort.34 Conversely, biomarkers discovered using heterogeneous cohorts may be more likely to generalize to a more comprehensive spectrum of disease subtypes.34 The proliferation of GWAS has been useful in discovering significant associations between genetic variants and biological traits. However, only 3% of the participants in the National Human Genome Research Institute GWAS catalog (the most comprehensive publicly available resource of human genetic association research) are of African ancestry.28 Although our review found a lack of representation of race/ethnic minorities in RCTs of patients with SLE, much is unknown about whether biomedical studies take advantage of the rich ancestral diversity of patients with SLE. Specifically, how representative of the spectrum of disease severity and subtypes are the samples/specimens used to develop biomarkers for SLE?
Examples of the consequences of the lack of diversity in biomedical studies are ample. For example, the first iteration of the human papillomavirus (HPV) vaccine covered 2 subtypes of the infection, however, Black women are 50% less likely to have HPV subtypes represented in those vaccines.35,36 Although the newest versions of the vaccines now protect against 9 HPV subtypes, the most common HPV subtypes found in Black women are still not covered by these new iterations of the vaccines.35 As a corollary, the identification and characterization of the molecular biological pathways distinctively driving refractory manifestations in racially and ethnically diverse populations that lead to higher mortality in minority groups have yet to be established in SLE. This means that the development of therapeutic agents that are more suitable for the phenotypes in race/ethnic minorities may be delayed. Future studies in these directions are warranted to develop clinically applicable preventive and therapeutic strategies for better SLE management.
The pharmacogenetic differences in the frequencies of variants associated with drug metabolism may translate to certain therapeutic agents being safer and more efficacious in some race/ethnic groups than others. For example, the CYP2D6 gene is responsible for the metabolism and elimination of 25% of commonly prescribed drugs.28 Race is a major determinant of variability in the CYP2D6 gene.28 Several GWAS have identified associations between treatment responses and clinically relevant genetic variants.28 The lack of efficacy of cyclophosphamide in Black patients with SLE may be due to the twofold higher level of toxic metabolites of this agent in Black patients compared with White patients.23 This issue is also reflected in the high rates of adverse events in clinical trials of cyclophosphamide in Black patients.23 Despite these findings, there is a surprising lack of evidence of the race/ethnic composition of drug development studies for SLE. The consequences of knowing little about the pharmacogenomics, pharmacodynamics, and pharmacokinetics of SLE drugs in race/ethnic minorities may translate to less effective (or in some cases, potentially harmful) dosage decisions for these populations. This may also lead to issues with medication adherence in race/ethnic minorities. If there is heterogeneity in pharmaco-dynamics and pharmacokinetics by race/ethnicity, efforts should be made to address this issue before planning the RCTs (see Fig. 1).
In 2019, the FDA issued draft guidelines to broaden clinical trials eligibility criteria and avoid unnecessary exclusions by improving recruitment for trial participants to reflect the population likely to use the drug.9 The inclusive practices recommendations include accounting for the serologic and immunologic markers before excluding patients with human immunodeficiency virus, hepatitis, and tuberculosis, and noted that patients can be stable on medication used to treat these underlying conditions and still be eligible for clinical trials.9 The guidelines also requested that trialists use evidence-based exclusions to limit the participation of individuals with renal, cardiac, and hepatic function and recommended that clinical trials include patients with mild organ dysfunction, for example.9 These recommendations may be useful for clinical trials in SLE in which eligibility may, in some cases, be considered stringent. In a study of the eligibility of lupus nephritis trials, Collinson and colleagues37 applied published trial eligibility criteria to a large registry of patients with SLE in the United Kingdom. They found that 51% of the registry did not satisfy the inclusion and exclusion criteria, making them ineligible for study entry.37 The extent to which this finding varies by race/ethnicity is unknown; however, overly stringent inclusion/exclusion criteria may have significant implications for the study of treatment options in more severe disease and in race/ethnic minorities.
ENHANCING DIVERSITY IN THE SYSTEMIC LUPUS ERYTHEMATOSUS RESEARCH PIPELINE: A DEMOCRATIZING FRAMEWORK
In this section, we propose potential solutions to the barriers to diversifying the research pipeline for SLE drugs identified in the previous section. The lack of diversity in biomedical and clinical trial research may be related to both provider and patient-related factors. Clinician-focused interventions should include increasing awareness and knowledge, addressing implicit bias (where present), and removing logistical hindrances.38 For example, some providers may have limited knowledge of available clinical trials and they may also have beliefs that race/ethnic minorities may not understand or adhere to trial protocols.38 Other barriers to recruiting race/ethnic minorities include limited time to talk to patients during their consultations and limited clinical trial sites within close proximity to the provider’s practice location.38 Finally, clinician communication may not be culturally and linguistically tailored to understanding the needs of the patients in their practice. To be clear, these are provider barriers that exist throughout medicine, and are not unique to rheumatologists. Nevertheless, to address these challenges, the American College of Rheumatology (ACR) developed Materials to Increase Minority Involvement in Clinical Trials (MIMICT), an education program for clinicians involved in SLE care. MIMICT connects clinical trial sites and clinicians to provide resources for discussing clinical trial opportunities with patients.38 Another ACR initiative, Lupus Clinical Trials Training (LuCTT), is a didactic program that aims to increase community health workers’ knowledge and skills to educate and support Black and Hispanic patients with SLE in navigating clinical trials and the health care system.38
Race/ethnic minorities may have limited access to clinicians involved in clinical trials. Patient-level barriers to participating in clinical trials include lack of access, opportunity, mistrust, health literacy, and cultural factors.38 Other access issues include lack of health insurance, lack of transportation to trial sites, frequency of blood draws, extra office visits, restrictive child care options, and inability to miss work.38 In addition, the historical exploitation of the Black community in clinical and biomedical research may have a lingering effect on recruitment of these patients into clinical trials.38-41 This may be attributed to medical mistrust and could be mitigated by having a more diverse clinical trials workforce, as only approximately 1% of rheumatologists in the United States are Black.42 Evidence suggests that racial concordance between doctor and patient matter for the utilization of preventive medicine.43
In the framework presented in this article, we highlight ways in which patient/community education and engagement needs to be prioritized when designing biomedical studies and interventions. Patient groups should be engaged across the research continuum: from formulating research areas that are relevant to patients to providing input on meaningful endpoints and patient-reported outcomes. To overcome patient-level barriers related to costs and logistics, efforts to recruit and retain race/ethnic minorities in RCTs may require labor-intensive measures and culturally appropriate and more personal contacts. One such initiative is patient navigation or the use of lay community health workers to educate patients about RCTs and provide individualized support for patients enrolled in these clinical trials.44 A study that evaluated the adoption of patient navigators for the recruitment and retention of Black patients in clinical trials at a cancer center in the United States found that 75% of patients receiving patient navigation support completed the RCT compared with 38% of patients without this intervention.44 Based on this compelling evidence, future studies should consider adopting the patient navigation model for SLE RCTs. In terms of institutional-level removal of barriers to race/ethnic minorities enrolling in RCTs, we suggest that NIH incentivize efforts to diversify RCTs to encourage trial sites to comply with current guidelines. Finally, to diversify the sampling frame for biomedical and clinical research, disease registries have been used to identify trial participants for multicenter RCTs, trials involving rare diseases or race/ethnic minorities.45 Trialists may consider querying the Rheumatology Informatics System for Effectiveness (RISE) Registry using inclusion/exclusion criteria to facilitate recruitment, thus democratizing the process and increasing efficiency and the probability of success.
SUMMARY
Significant disparities exist in SLE regarding prevalence, disease severity, and mortality, with race/ethnic minorities being disproportionately affected. Despite these disparities, race/ethnic minorities are underrepresented within SLE research, whether basic science-related (eg, GWAS) or in the recruitment of patients for clinical trials of new therapeutic agents. Both provider and patient-related barriers to their participation likely play a role. Decreased race/ethnic minority involvement in SLE research has real-world implications, including less understanding of the disease itself and less applicability of approved therapies among this group of patients. Although the underrepresentation of race/ethnic minorities and barriers to their participation in research are not unique to SLE, members of the lupus research community have an obligation to narrow this gap going forward to ensure that future advances within the field are derived from and benefit a more representative group of patients.
KEY POINTS.
One important means of addressing disparities is to ensure that the inclusion of race/ethnic minorities in systemic lupus erythematosus (SLE) clinical trials is adequate.
There are many indications that treatment response in SLE may be heterogeneous by race/ethnicity based on our understanding of the epidemiology of the disease.
We recommend that clinical trials in SLE move beyond only increasing race/ethnic diversity to ensuring adequately powered subgroup analyses.
Diversity efforts should also be focused on biomedical studies to ensure that the pathophysiology of race/ethnic minorities are adequately represented.
We present a framework for improving generalizability of clinical research in SLE through patient-, clinician-, and institutional-level engagements across the research continuum.
Footnotes
DISCLOSURE
Y. Chaichian has received support from Gilead Sciences, AMPEL BioSolutions, Pfizer, GSK, and the Lupus Research Alliance. The other authors have nothing to disclose.
REFERENCES
- 1.Sue S, Dhindsa MK. Ethnic and racial health disparities research: issues and problems. Health Educ Behav 2006;33(4):459–69. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Special feature on racial and ethnic health disparities. Heal United States. 2015;3:216. Available at: https://www.cdc.gov/nchs/data/hus/hus15.pdf. [Google Scholar]
- 3.2015 National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy ∣ Agency for Health Research and Quality. Available at: https://www.ahrq.gov/research/findings/nhqrdr/nhqdr15/index.html. Accessed March 11, 2020. [Google Scholar]
- 4.Arntsen KA, Raymond SC, Farber KM. Lupus: Patient Voices Report on Externally-Led Patient-Focused Drug Development Meeting A Message of Gratitude. Available at: http://www.lupuspfdd.org/LupusPatientVoicesFINAL.pdf. [Google Scholar]
- 5.To bridge health disparities, diagnose lupus early & improve access - the rheumatologist. Available at: https://www.the-rheumatologist.org/article/to-bridge-health-disparities-diagnose-lupus-early-improve-access/?singlepage=1&theme=print-friendly. Accessed March 11, 2020.
- 6.Lupus Highlighted in New Congressional Report on Health Disparities ∣ Lupus Foundation of America. Available at: https://www.lupus.org/news/lupus-highlighted-in-new-congressional-report-on-health-disparities. Accessed March 11, 2020. [Google Scholar]
- 7.Lupus Grants - The Office of Minority Health. Available at: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=2&lvlid=62. Accessed March 11, 2020.
- 8.Falasinnu T, Chaichian Y, Bass MB, et al. The representation of gender and race/ethnic groups in randomized clinical trials of individuals with systemic lupus erythematosus. Curr Rheumatol Rep 2018;20(4):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fda Cder, Fox Stephanie. Enhancing the Diversity of Clinical Trial Populations-Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry DRAFT GUIDANCE. Available at: https://www.fda.gov/media/127712/download. [Google Scholar]
- 10.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 2019;5(10):e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research ∣ grants.nih.gov. Available at: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. Accessed March 11, 2020.
- 12.Chen MS, Lara PN, Dang JHT, et al. Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the ground-work for improving minority clinical trial accrual. Cancer 2014;120:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geller SE, Koch AR, Roesch P, et al. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med 2018;93(4):630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78(6):736–45. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G, Isenberg DA. New therapies for systemic lupus erythematosus - past imperfect, future tense. Nat Rev Rheumatol 2019;15(7):403–12. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz DL, Furie R. Belimumab is approved by the FDA: what more do we need to know to optimize decision making? Curr Rheumatol Rep 2012;14(4):318–23. [DOI] [PubMed] [Google Scholar]
- 17.Vukelic M, Li Y, Kyttaris VC. Novel treatments in lupus. Front Immunol 2018;9:2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an anti–interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 2017;69(2):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63(12):3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382(3):211–21. [DOI] [PubMed] [Google Scholar]
- 21.Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392(10143):222–31. [DOI] [PubMed] [Google Scholar]
- 22.van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 2018;392(10155):1330–9. [DOI] [PubMed] [Google Scholar]
- 23.Litwic AE, Sriranganathan MK, Edwards CJ. Race and the response to therapies for lupus: how strong is the evidence? Int J Clin Rheumtol 2013;8(4):471–81. [Google Scholar]
- 24.Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology 2016;56(suppl_1):kew399. [DOI] [PubMed] [Google Scholar]
- 25.Yen EY, Singh RR. Brief report: lupus-an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol 2018;70(8):1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh RR, Yen EY. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus 2018;27(10):1577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen EY, Shaheen M, Woo JMP, et al. 46-year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: a nationwide population-based study. Ann Intern Med 2017;167(11):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature 2016;538(7624):161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dooley MA, Hogan S, Jennette C, et al. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Kidney Int 1997;51(4):1188–95. [DOI] [PubMed] [Google Scholar]
- 30.Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. - PubMed - NCBI. Available at: https://www.ncbi.nlm.nih.gov/pubmed/?term=19933596. Accessed March 11, 2020. [Google Scholar]
- 31.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20(5):1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langefeld CD, Ainsworth HC, Cunninghame Graham DS, et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun 2017;8:16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anjorin A, Lipsky P. Engaging African ancestry participants in SLE clinical trials. Lupus Sci Med 2018;5(1):e000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney TE, Khatri P. Generalizable biomarkers in critical care: toward precision medicine. Crit Care Med 2017;45(6):934–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal AC, Smith JS, Valea F, et al. HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA. Cancer Causes Control 2014;25(8):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.African American Women Are Less Likely to Benefit From HPV Vaccines for Cervical Cancer Prevention - The ASCO Post. Available at: https://www.ascopost.com/News/8703. Accessed March 11, 2020.
- 37.Collinson S, Parker B, Mccarthy E, et al. FRI0173 how well do clinical trials represent real world lupus nephritis patients? Ann Rheum Dis 2019;78:760, 1–760. [Google Scholar]
- 38.Sheikh SZ, Wanty NI, Stephens J, et al. The state of lupus clinical trials: minority participation needed. J Clin Med 2019;8(8):1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 2006;7(2):141–8. [DOI] [PubMed] [Google Scholar]
- 40.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008;112(2): 228–42. [DOI] [PubMed] [Google Scholar]
- 41.Gorelick PB, Harris Y, Burnett B, et al. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial: an interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc 1998;90(3):141–5. [PMC free article] [PubMed] [Google Scholar]
- 42.Battafarano DF, Ditmyer M, Bolster MB, et al. 2015 American College of Rheumatology Workforce Study: supply and demand projections of Adult Rheumatology Workforce, 2015–2030. Arthritis Care Res 2018;70(4):617–26. [DOI] [PubMed] [Google Scholar]
- 43.Alsan M, Garrick O, Graziani G. Does diversity matter for health? Experimental evidence from Oakland. Am Econ Rev 2019;109(12):4071–111. [Google Scholar]
- 44.Fouad MN, Acemgil A, Bae S, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract 2016;12(6):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan MH, Thomas M, MacEachern MP. Using registries to recruit subjects for clinical trials. Contemp Clin Trials 2015;41:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]