Abstract
Background:
Approximately two-thirds of adults are genetically predisposed to decreased lactase activity after weaning, putting them at risk of lactose intolerance. However, symptoms are a poor marker of lactose maldigestion.
Aims:
We assessed association between self-reported lactose intolerance and intestinal lactase, lactose intake, and the small intestinal microbiome.
Methods:
Patients 18–75 years presenting for upper endoscopy were recruited prospectively. Observational study participants completed a lactose intolerance symptom questionnaire and reported lactose intake. Post-bulbar biopsies were obtained to measure lactase activity and assess the small intestinal mucosal microbiome. We compared intestinal lactase between patients with and without lactose intolerance. We assessed associations between lactose intolerance symptoms and lactase and lactose intake. We examined associations of small bowel microbial composition with self-reported lactose intolerance and symptoms.
Results:
Among 34 patients, 23 (68%) reported lactose intolerance. Those with lactose intolerance had higher total symptom scores, more frequent bowel urgency, and more bowel movements after consuming dairy. The proportion of individuals with abnormal lactase activity did not differ by lactose intolerance status. Median lactase levels were correlated with total lactose intolerance symptom scores (p=0.038) and frequency of bowel urgency (p=0.012). Daily lactose intake did not differ between groups. In 19 patients, we observed significant associations of small intestinal microbiome beta diversity with stool consistency after consuming dairy (p=0.03).
Conclusions:
Intestinal lactase is associated with lactose intolerance symptoms and bowel urgency in adults but does not distinguish the clinical phenotype entirely. Studying other contributing factors (microbiota, diet) may further clarify the pathophysiology of lactose intolerance.
Keywords: small intestine, duodenum, disaccharide, food intolerance, maldigestion
Introduction:
Lactose intolerance is common; approximately two-thirds of the population undergoes a programmed reduction in lactase activity as children.[1–5] Non-persistence of lactase is the wild type. The small intestine epithelium is unable to absorb lactose without it first being cleaved into its component monosaccharides – galactose and glucose – by lactase-phlorizin hydrolase (colloquially lactase).[6] If the brush border lactase enzyme has low activity, lactose is not digested in the small bowel and passes into the colon. In the lumen of the colon, undigested lactose undergoes bacterial fermentation, forms short chain fatty acids (primarily acetate), generates lactic acid, frees hydrogen, and can produce gastrointestinal symptoms.[6–8] Additionally, lactose creates an osmotic effect. Lactose intolerance is defined by the National Institutes of Health (NIH) as “the onset of gastrointestinal symptoms following a single-dose challenge of ingested lactose by an individual with lactose maldigestion, which are not observed when the person ingests an indistinguishable placebo.”[3]
Classical symptoms of lactose intolerance include bloating, cramping, borborygmi, flatus, diarrhea, and fecal urgency.[9–11] Symptom severity may be influenced by patient- and intake-specific factors.[12] Residual lactase expression, dose, dilution, transit, colonic flora, and sensitivity to products of fermentation are all variables that impact lactose intolerance.[12–16] Not all individuals who maldigest lactose exhibit symptoms.[17]
Despite the high symptomatic prevalence of lactose intolerance and the large number of individuals at risk of maldigestion, no specific symptom complaint has been shown to predict lactose malabsorption, and self-reported dairy intolerance is neither sensitive nor specific.[18] In blinded clinical trials, the correlation between biochemical maldigestion and symptomatic intolerance is poor; the clinical diagnosis necessitates both.[17,19] Rates of subjective intolerance are high in individuals with irritable bowel syndrome.[20,21] Furthermore, genetic testing (commonly a single nucleotide substitution that involves the LCT gene C/T-13910 variant on chromosome 2) for lactase persistence does not provide symptom information.[22,23] In populations where non-persistence by genotyping approaches 100%, only 45% self-report lactose intolerance.[17] Breath testing is cumbersome and conducted in an artificial testing environment given the large amount of lactose ingestion typically used to induce measurable hydrogen. The positive predictive value of about 40–66% and negative predictive value of about 44–82% arguably limits the utility of this diagnostic technique.[17,24] In 2010, the NIH consensus concluded that a majority of individuals with lactose maldigestion do not have clinical lactose intolerance, while many with self-perceived lactose intolerance do not have lactose maldigestion.[3]
Residual lactase levels vary among maldigesters, yet there is very limited evidence describing the relationship between residual intestinal lactase and clinical symptoms of lactose intolerance.[25–29] Additionally, other aspects that could influence the development of lactose intolerance (i.e. the intestinal microbiome) are incompletely characterized. We aimed to examine the association between intestinal lactase levels and self-reported lactose intolerance in a pilot study. We further explored associations between the small intestinal mucosal microbiome and symptoms of lactose intolerance. Our hypothesis was that degree of hypolactasia would be positively correlated with severity of self-reported symptoms of lactose intolerance – lower lactase levels would result in more severe gastrointestinal symptoms.
Methods:
Experimental Design and Study Participants:
We conducted a prospective observational pilot study from August 2019 to October 2020. Inclusion criteria were those aged 18–75 presenting to our ambulatory endoscopy suite. Exclusion criteria were as follows: known bleeding, history of eosinophilic esophagitis, inflammatory bowel disease, chronic pancreatitis, pancreatic insufficiency, celiac disease, peptic duodenitis, antibiotic use within the last 30 days, major abdominal surgery except for appendectomy or cholecystectomy (operation must have been >6 months before enrollment), prior radiation therapy to the abdomen, use of tobacco products within the last 3 months, milk allergy, and currently pregnant or breastfeeding. Individuals presenting for upper endoscopy for any indication were recruited prospectively from a single academic center. Eligibility was confirmed based on review of the medical records and patient medications. Each participant completed an 8-item lactose intolerance questionnaire assessing frequency and severity of symptoms and a lactose assessment tool (LAT) to estimate daily lactose intake.[30] The LAT lists 42 dairy-containing foods. Responses were used to calculate daily lactose intake in grams using the United States Department of Agriculture National Nutrient database (www.nal.usda.gov/fnic/foodcomp/search). Written, informed consent was obtained prior to enrollment of each patient and this study was approved by the Indiana University Institutional Review Board. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. Given the pilot nature of the research, no specific recruitment goals were set.
Endoscopy and Processing of Study Specimens:
Subjects underwent upper endoscopy (esophagogastroduodenoscopy) following an overnight fast. Four post-bulbar biopsies were taken using standard biopsy forceps. Two samples were stored without any supporting media and transported to the laboratory on ice for immediate disaccharidase analysis. Two additional samples were submerged in Allprotect ® Tissue Reagent (Qiagen) for immediate stabilization and archived at −80 degrees Celsius within two hours of collection for future analysis. Lactase was measured at the Indiana University Pathology Laboratory using quantitative spectrophotometry and assessed as both a dichotomous (normal/low activity) and continuous (level) outcome. We defined adult-type hypolactasia, or a low level, as ≤10.0 μmol/min/g protein.[31] Mucosa-associated microbiome was characterized by 16S rRNA-based sequencing on an Illumina MiSeq instrument (Illumina Inc, San Diego, CA).
Mucosa-associated microbiome was characterized by 16S rRNA-based sequencing. Briefly, mucosal biopsy samples were thawed on ice. Total nucleic acids were extracted using the DNeasy tissue kit (Qiagen, Venlo, The Netherlands). DNA quality and concentration were measured using automated electrophoresis (Agilent Tapestation) and fluorescent dsDNA assay on a Qubit device (Life Technologies Corporation, Carlsbad, CA, USA). The 16S rRNA gene V4 region was PCR amplified (NEXTflex® 16S V4 Amplicon-Seq Kit; Bioo Scientific, Austin, TX, USA). Amplicon libraries were pooled and sequenced using an Illumina MiSeq (Illumina, San Diego, California, USA). Negative controls were processed in parallel to monitor for contamination. Sequence Variants were generated within QIIME2 2018.8 and assigned taxonomic classification using release 132 of the SILVA database.
Study endpoints:
We assessed the primary outcome of hypolactasia in patients with and without self-reported lactose intolerance (LI). Self-reported LI was binary and determined based on participant response to question: do you experience any abdominal symptoms after consuming milk or lactose-based products. Secondary outcomes included lactase activity levels, symptom severity, and average dietary lactose intake. We further explored associations of the small intestine mucosal microbiome with lactose intolerance.
Statistical Analysis:
Data were summarized using percentages for categorical variables and medians ± interquartile range (IQR) or mean ± standard deviation (SD) for continuous variables with skewed or normal distributions, respectively. Study endpoints were compared between cases (self-reported LI) and controls (no LI) using the Wilcoxon rank sum test or two-sample t-test for continuous variables, Pearson’s chi-square or Fischer’s exact test for categorical variables, and the Cochran-Armitage test for ordinal variables (number of bowel movements per day, bowel urgency, stool consistency). We assessed associations of lactase levels and lactose intake with symptom severity after consuming dairy products using the Spearman correlation. Missing data including questionnaire responses of “don’t know” and one instance where a participant did not provide responses to 50% of the questions were omitted from the particular analysis.
Statistical analysis of microbiome results was performed to examine associations of overall microbial composition(α diversity by Faith’s Phylogenetic Distance; Shannon Diversity; Pielou eveness and β diversity by weighted and unweighted Unifrac distances) and individual taxa with self-reported lactose intolerance and with severity and frequency of symptoms using the Wilcoxon rank sum test, Spearman correlation, and permutational multivariate analysis of variance. P-values for the evaluation of relative abundance of individual taxa were adjusted using the False Discovery Rate (FDR) method.
Analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC) the R software environment ([https://www.r-project.org/]). Two-sided hypothesis tests were utilized with a p-value of <0.05 considered to be statistically significant.
Results:
Study Participants:
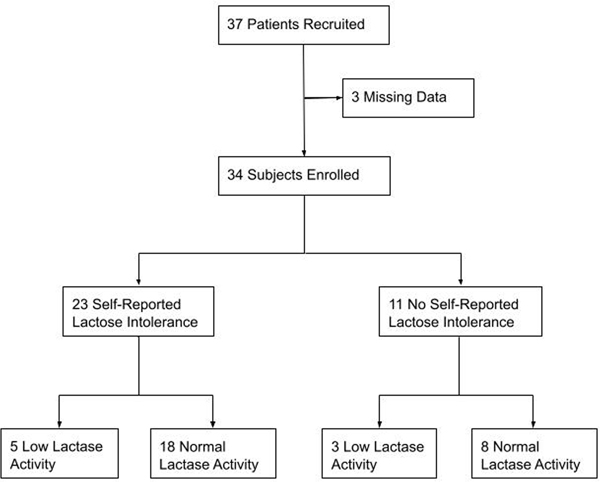
A total of 37 subjects consented to participate in the pilot study. Two subjects with missing lactose intolerance questionnaire data and one subject with no disaccharidase testing data were excluded from analysis (Figure 1). Overall, 23 (68%) participants reported lactose intolerance by self-report based on response to yes/no question (do you experience any abdominal symptoms after consuming milk or lactose-based products?). Demographic information is shown in Table 1.
Figure 1.
Flow Chart of Study Participants
Table.
Participant characteristics by self-reported lactose intolerance (LI)
| Total (n=34) | No LI (n=11) | LI (n=23) | |
|---|---|---|---|
| Age, mean (SD) | 41.4 (13.6) | 46.0 (14.8) | 39.2 (12.7) |
| Females | 27 (79.4%) | 9 (81.8%) | 18 (78.3%) |
| Caucasian | 30 (88.2%) | 9 (81.8%) | 21 (91.3%) |
| Probiotic use | 1 (3.0%) | 0 (0%) | 1 (4.3%) |
| Body mass index (kg/m 2 ), mean (SD) | 33.1 (10.5) | 29.8 (8.4) | 34.7 (11.1) |
| Total LI symptom score, median (IQR) * | 12.0 (4.0–24.0) | 0.0 (0.0–4.0) | 22.0 (11.0–29.0) |
| Frequency of bowel urgency after consuming dairy products * | |||
| Never | 8 (24.2%) | 5 (45.5%) | 3 (13.6%) |
| Rarely | 7 (21.2%) | 4 (36.4%) | 3 (13.6%) |
| Sometimes | 7 (21.2%) | 1 (9.1%) | 6 (27.3%) |
| Most of the time | 4 (12.1%) | 1 (9.1%) | 3 (13.6%) |
| Always | 7 (21.2%) | 0 (0%) | 7 (31.8%) |
| Bowel movements per day when consuming dairy products # | |||
| 0 | 8 (25.8%) | 4 (40.0%) | 4 (19.0%) |
| 1 | 5 (16.1%) | 3 (30.0%) | 2 (9.5%) |
| 2 | 6 (19.4%) | 2 (20.0%) | 4 (19.0%) |
| 3 | 7 (22.6%) | 0 (0%) | 7 (33.3%) |
| 4+ | 5 (16.1%) | 1 (10.0%) | 4 (19.0%) |
| Stool consistency | |||
| Usually constipation (type 1,2) | 4 (13.8%) | 1 (10.0%) | 3 (15.8%) |
| Usually type 3 | 4 (13.8%) | 2 (20.0%) | 2 (10.5%) |
| Usually type 4 | 8 (27.6%) | 6 (60.0%) | 2 (10.5%) |
| Usually type 5 | 5 (17.2%) | 0 (0%) | 5 (26.3%) |
| Usually diarrhea (type 6,7) | 8 (27.6%) | 1 (10.0%) | 7 (36.8%) |
| Lactase level, median (IQR) | 26.6 (10.3–44.0) | 32.3 (9.9–44.0) | 25.3 (10.3–44.7) |
| Normal disaccharidase activity | 26 (76.5%) | 8 (72.7%) | 18 (78.3%) |
p<0.01
p=0.04; all other p-values non-significant; p-values are based on the two-sample t-test or Wilcoxon rank sum test for continuous variables, Cochran-Armitage trend test for ordinal variables (bowel urgency, bowel movements, stool consistency), and Pearson’s chi-square test or Fisher’s exact test for categorical variables
Lactase Activity:
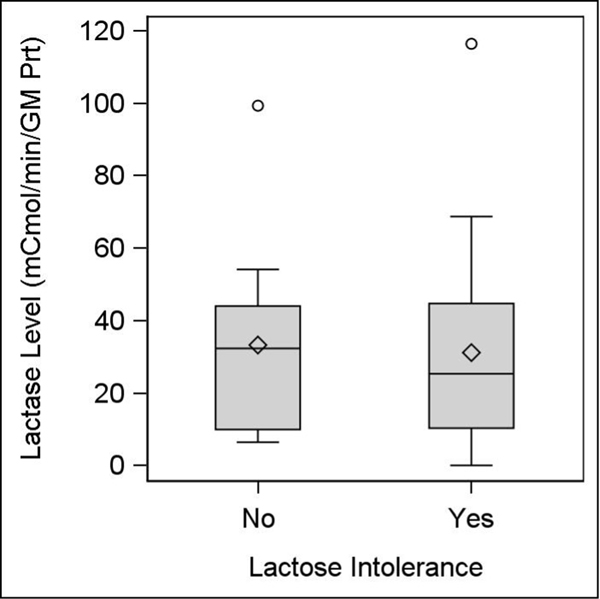
There were 8 subjects (23.5%) who had low lactase levels: 3 of 11 (27.3%) without and 5 of 23 (21.7%) with self-reported lactose intolerance (Table 1). There were no significant differences in the proportion of individuals with low lactase levels or in median lactase levels between groups (Figure 2). Median levels for both those with and without self-reported lactose intolerance were above the laboratory cutoff for hypolactasia (10 μmol/min/g protein).
Figure 2.
Boxplots of disaccharidase levels by self-reported lactose intolerance
°There were 2 notable outliers for lactase level, one for each group.
Symptom Severity:
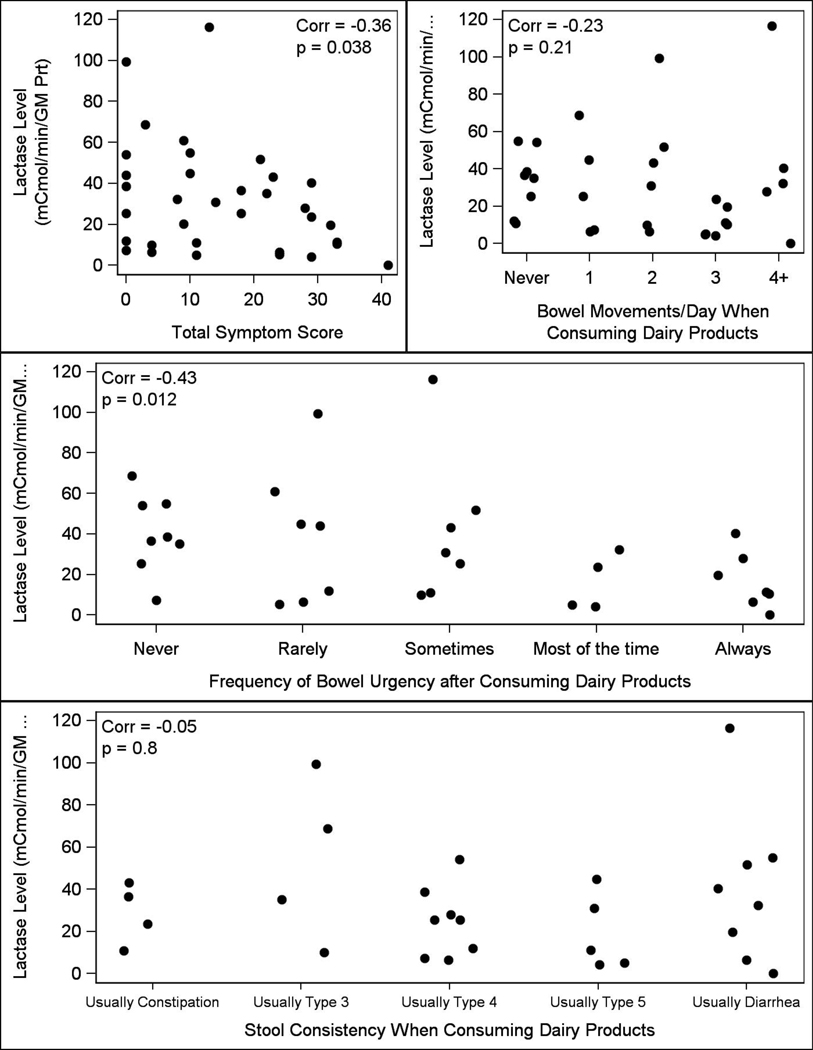
Our research showed differences in total symptom scores (p<0.001) as well as frequency (p=0.004) and urgency of bowel movements (p=0.039) following consumption of dairy products between groups (Table 1) with higher total scores, increased urgency, and more frequent bowel movements in those with self-reported lactose intolerance compared to those without LI. Stool consistency was not different between the groups. As shown in Figure 3, total symptom score (p=0.04) and bowel urgency (p=0.01) were inversely correlated with intestinal lactase levels; no significant correlations were observed between frequency (p=0.2) or stool consistency (p=0.8) and intestinal lactase levels.
Figure 3.
Scatter plot of lactase level against symptom severity with Spearman correlation
Daily Lactose Intake:
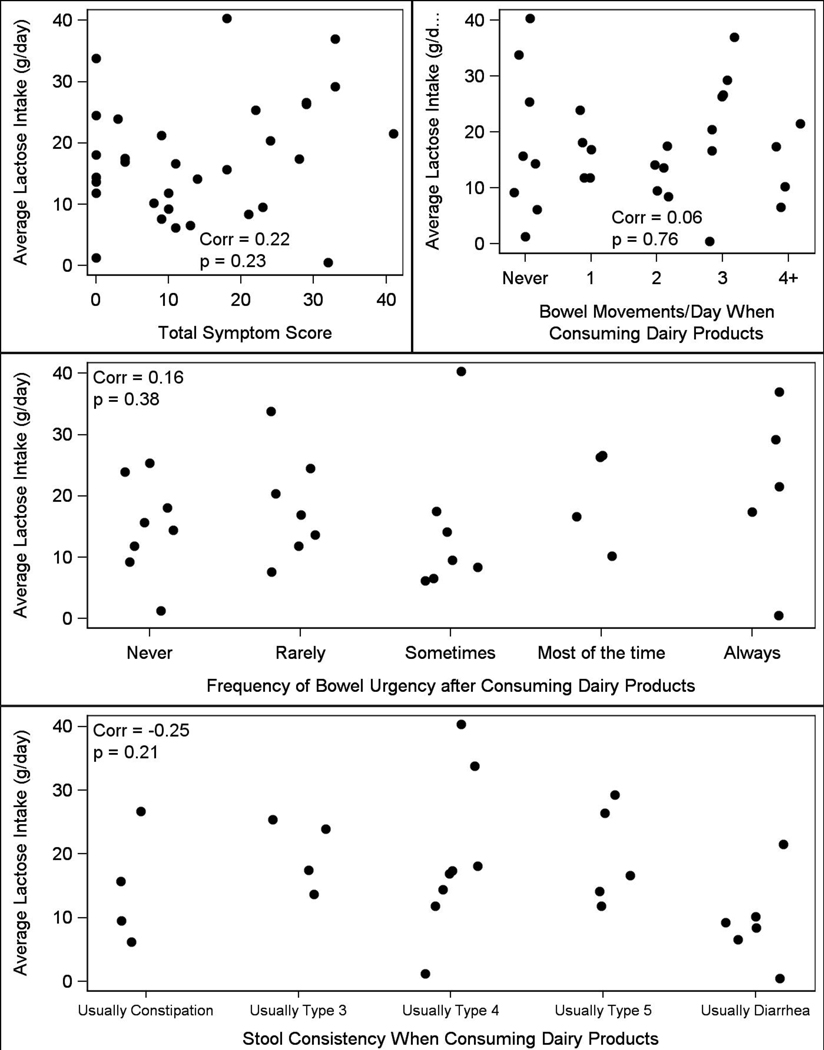
There were no significant differences in median [interquartile range] estimates of daily lactose intake in grams/day between those with (18.9 g/day [IQR 9.2–26.4]) and without (15.7 g/day [IQR 11.8–18.1]) self-reported lactose intolerance (p=0.61). There was no correlation between daily lactose intake and the total symptom score (p=0.23), frequency (p=0.76), urgency (p=0.38), or stool consistency (p=0.21) after consuming dairy products as shown in Figure 4.
Figure 4.
Scatter plot of average daily lactose intake (grams) against symptom severity
Note that one subject had an average lactose intake of approximately 160 grams per day and was excluded from Figure 4 as an extreme outlier.
Microbiome Profile:
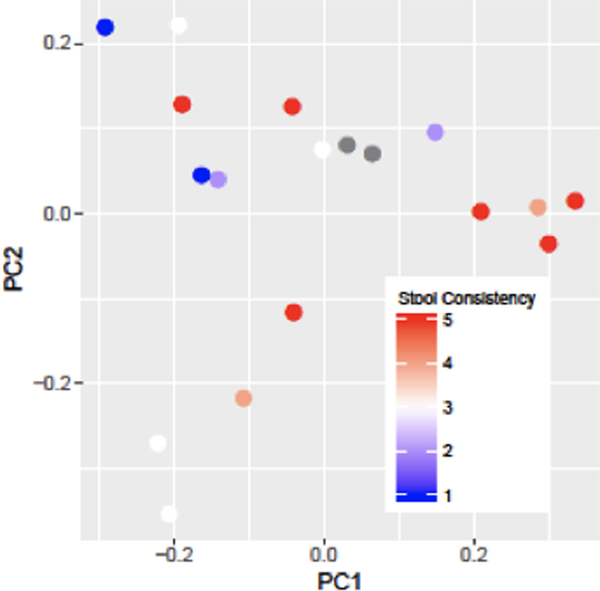
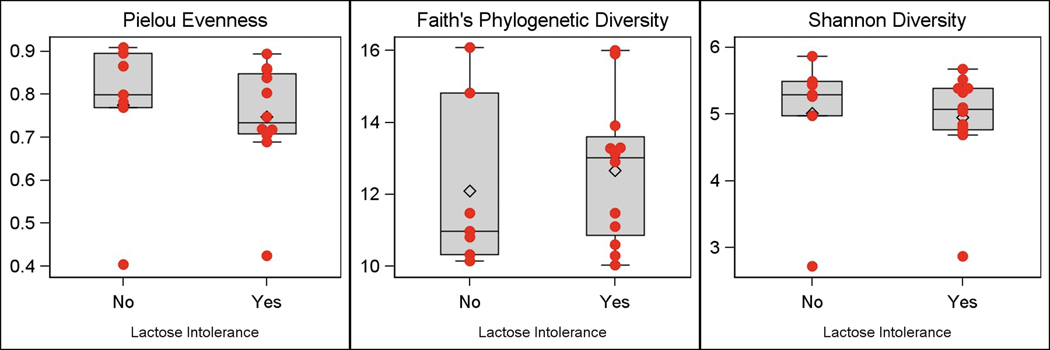
Microbiome results were obtained in a subset of 19 patients (63% [n=12] patients with self-reported lactose intolerance; 78% [n=15] females; 95% [n=18] Caucasian) with a mean (SD) age of 45.4 (14.9) years and mean body mass index of 30.5 (9.5) kg/m2. No participants were on probiotics. Analysis of 16S rRNA-based microbial community composition of the small intestinal mucosa at the amplicon sequence variant level revealed significant associations of small intestinal mucosal microbiome beta diversity (Figure 5) with stool consistency after consuming dairy (p=0.03, weighted UniFrac). There were no significant associations between small intestinal mucosal microbiome alpha diversity and lactose intolerance symptom assessments (total score, frequency of bowel urgency, number of bowel movements, stool consistency). Comparisons between patients with and without self-reported lactose intolerance showed no significant differences in small intestinal mucosal microbiome alpha diversity (Figure 6) or beta diversity. Analysis of individual microbial taxa identified 369 unique genera. Univariate genus-level analyses showed no significant associations of the relative abundance of individual genera with lactose intolerance group or symptoms after adjusting for FDR.
Figure 5.
Assessment of Small Intestine Mucosa-Associated Beta Diversity
Figure 6.
Assessment of Small Intestine Mucosa-Associated Alpha Diversity
Discussion:
In this prospective pilot study, we found that self-reported lactose intolerant patients had higher total symptom scores, greater bowel urgency, and a higher number of bowel movements after consuming dairy products. The proportion of individuals with low lactase activity did not differ by self-reported lactose intolerance status. Although median lactase levels were lower in those with self-reported lactose intolerance, the difference was not statistically significantly. More than a quarter of patients without self-reported lactose intolerance also exhibited abnormal intestinal lactase. In addition, self-reported intolerance and symptom severity did not impact participants’ intake of dairy products. Detailed symptom assessments, however, demonstrated significant correlations between intestinal lactase and total lactose intolerance symptom scores (p=0.038) and between intestinal lactase and frequency of bowel urgency (p=0.012). Our findings support the hypothesis that residual intestinal lactase variability contributes to lactose intolerance symptoms based on detailed symptom assessments; however, it does not distinguish the clinical phenotype alone. Furthermore, clinical phenotype did not clearly predict intake behaviors.
Pilot results are consistent with prior studies demonstrating that genetic lactase non-persistence does not predict clinical phenotype, and serves as direct evidence that the mismatch between genotype and phenotype is not fully explained by variable gene expression, but is instead, a consequence of multiple factors.[32] In the effort to investigate additional patient-specific factors that may mediate the relationship between intestinal lactase and clinical symptoms, we examined the previously unstudied role of the small intestinal microbiome in a subset of patients. In this exploratory analysis, we observed associations between community composition of the small intestinal mucosal microbiota and stool consistency after consuming dairy products. While existing literature clearly links lactose intolerance with the colonic microbiome, our novel exploration of the small intestine-associated microbiome uncovered associations between beta diversity and stool consistency after dairy consumption, suggesting that the small intestinal microbiome may participate in lactose tolerance.[15,16,33]
The strengths of this pilot study include its prospective design and comprehensive data collection on each individual patient. Our study size of 34 was modest; however, we gathered in-depth patient-reported symptom assessments that focused on objective descriptions of stool symptoms utilizing the Bristol stool chart for consistency determinations, included data on dietary habits by calculating daily lactose intake, and conducted our study in the outpatient setting during routine esophagogastroduodenoscopy.[34] Given that it was a pilot study, a limitation was small sample size, which does not enable us to draw firm conclusions. Lactase determination was made based on biopsy from duodenum. Lactase expression exhibits a mosaic pattern; therefore the sample we collected may not be fully representative given this patchy expression in villous enterocytes.[28] It is reported that mean duodenal disaccharidase activity is approximately 40% less than jejunal activity which also may have affected our results;[35] however, post-bulbar duodenal biopsies have been shown to be sufficient to diagnose enzyme deficient states.[36,37] Baseline differences in BMI between the groups may have also accounted for some of the differences we noted. The female predominance in the study may have also influenced our data. Irritable bowel syndrome is a common condition that may have also impacted results, but was not the focus of our pilot study. Lastly, we did not perform breath testing to verify symptoms or confirm maldigestion which could have added data.
This pilot study provides important new insights on a common, yet incompletely characterized condition. Although the role of lactase non-persistence was recognized in the 1960s, the multifactorial pathophysiology of lactose intolerance remains only partially understood. The earliest efforts uncovering lactose intolerance and low intestinal lactase levels were quite small or conducted among patients with underlying diseases for which the findings would not be generalizable to the larger population.[27,29,38,39] A study of disaccharidase activity on surgical specimens in unselected adults undergoing surgery for duodenal ulcer or gastric cancer revealed 3 of 18 jejunal biopsies with lactase deficiency where all 3 subjects reported milk intolerance.[38] Another early study used peroral jejunal biopsies to show hypolactasia in 3 of 4 subjects with milk intolerance and 5 of 9 normal control subjects without symptoms also indicating that self-reported symptoms have poor reliability to predict intestinal lactase levels.[27] In a study of Crohn’s patients that included 24 healthy controls, the correlation between duodenal lactase levels and clinical symptoms in patients with Crohn’s disease was lacking with no difference in lactase levels between Crohn’s patients and healthy volunteers; associations of duodenal lactase and milk intolerance were not examined separately for controls.[40] Previous sizable research obtained peroral biopsies under fluoroscopy at the Ligament of Treitz (duodenum) in 25 lactase-deficient patients and 25 controls and symptoms were assessed.[26] The study highlighted that while all patients with hypolactasia reported symptoms in response to an oral lactose load, symptoms were also reported among some (n=3 of 25) normal subjects.[26] Numerous other studies that have examined adult-type hypolactasia have lacked symptom correlation.[36,41,42] Overall, our findings corroborate that the relationship between hypolactasia and symptoms is poor, but extend the findings to a larger population of adults without underlying gastrointestinal diseases.
Researchers were quick to recognize that lactase was not a dependable measure of lactose maldigestion, although the original studies were commonly conducted among selected patient groups and some conflicting reports have more recently been produced. Metz et al. found that among 8 patients with symptoms and abnormal breath tests, only 6 demonstrated hypolactasia on jejunal biopsies; associations between breath testing and hypolactasia were not studied in non-symptomatic individuals.[25] In a group of 21 patients with normal jejunal biopsies no statistically significant correlation could be found between intestinal lactase and lactose tolerance testing.[27] In a study of 16 patients with jejunal biopsies, there were 9 patients with hypolactasia and 72% of “non-absorbers” by their definition were symptomatic; patients were selected among a cohort of 60 individuals of whom many were hospitalized for various reasons.[43] In contrast, a more recent open-label study conducted among patients with self-reported lactose intolerance, lactase activity from duodenal biopsies and breath testing were found to be concordant in 83% of cases; duodenal lactase was more accurate than breath testing in predicting clinical response to a lactose-free diet.[37]
There has been limited information on the impact of lactose intolerance on actual lactose intake. One prior study collected dietary history along with intestinal biopsies in 27 adults in Italy and found that in a group of 6 persisters and 21 non-persisters, no difference in dietary history of milk and dairy intake existed.[28] We made similar observations; daily lactose intake did not differ by self-reported lactose intolerance and was low in the overall cohort. These pilot data suggest that dietary consumption of dairy products is influenced by other factors.
Methods of collection differed in earlier studies and endoscopy has changed significantly since the 1960s when lactase deficiency was originally recognized – particularly in terms of instruments, quality, and access. Lactase activity is greater in proximal jejunum than duodenum, but the duodenum is more accessible endoscopically.[44] Therefore, investigating the utility of duodenal lactase assessments in adults has important practical implications for clinical care.
Our pilot research further presents an opportunity to evaluate the role of detailed symptom assessments through the incorporation of data on stool urgency and total symptom score in defining a clinical phenotype for lactose intolerance.[45] Lactase activity correlated with certain symptoms of lactose intolerance (e.g. total symptoms and bowel urgency) but not self-identification of lactose intolerance. Our findings could inform the development and validation of a future questionnaire based on symptoms that could be combined with other methods of testing for lactase deficiency and maldigestion (blood, breath, intestinal biopsy) for diagnostic confirmation or as an improved screening tool to increase specificity in clinical practice. Importantly it will inform sample size calculations for future clinical trials in the field.
Additional studies may consider longitudinal analysis to evaluate peak or starting lactase levels in childhood with repeat measurements and symptom correlation over time to determine if change from baseline, as opposed to absolute value, may be more indicative of lactose intolerance; moderate change over time may permit adaption within the intestinal microbiome or other patient-specific factors that may minimize the effects of lactase non-persistence. While our understanding of the genetic underpinnings of lactose intolerance is adequate, factors that contribute to clinical manifestation of this enzymatic non-persistence and contribute to the behavioral changes such as diet and microbiome merit ongoing assessment.
Acknowledgements:
We would like to thank Christopher Hemmerich, MS, from the Center for Genomics and Bioinformatics at Indiana University, Bloomington, IN for critical bioinformatics support.
Acknowledgment of Grant Support:
AS is supported by NIH K23DK122015
Abbreviations:
- FDR
False Discovery Rate
- IQR
Interquartile Range
- LAT
Lactose Assessment Tool
- LI
Lactose Intolerance
- NIH
National Institutes of Health
- SD
Standard Deviation
Footnotes
Disclosure of Financial Arrangements:
Dr. Savaiano is a consultant for Dannon North America. He receives research funding from the a2 milk company. The other authors have no potential conflicts or disclosures to declare.
References
- 1.Itan Y, Jones BL, Ingram CJ, Swallow DM, Thomas MG. A worldwide correlation of lactase persistence phenotype and genotypes BMC Evol Biol. 2010;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutrition Brussow H., population growth and disease: a short history of lactose Environ Microbiol. 2013;15:2154–2161. [DOI] [PubMed] [Google Scholar]
- 3.Suchy FJ, Brannon PM, Carpenter TOet al. . NIH consensus development conference statement: Lactose intolerance and health NIH Consens State Sci Statements. 2010;27:1–27. [PubMed] [Google Scholar]
- 4.Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis Lancet Gastroenterol Hepatol. 2017;2:738–746. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Harvey CB, Hollox EJ et al. The genetically programmed down-regulation of lactase in children Gastroenterology. 1998;114:1230–1236. [DOI] [PubMed] [Google Scholar]
- 6.Diarrhea Sweetser S., malabsorption, and small-bowel disorders. In: Wittich C, ed.êds. Mayo Clinic Internal Medicine Board Review, City; Oxford University Press;2016:229–239. [Google Scholar]
- 7.Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technic N Engl J Med. 1971;284:1394–1398. [DOI] [PubMed] [Google Scholar]
- 8.Levitt MD, Ingelfinger FJ. Hydrogen and methane production in man Ann N Y Acad Sci. 1968;150:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Saha M, Parveen I, Shil BC et al. Lactose Intolerance and Symptom Pattern of Lactose Intolerance among Healthy Volunteers Euroasian J Hepatogastroenterol. 2016;6:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szilagyi A, Ishayek N. Lactose Intolerance, Dairy Avoidance, and Treatment Options Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilt TJ, Shaukat A, Shamliyan T et al. Lactose intolerance and health Evid Rep Technol Assess (Full Rep). 2010:1–410. [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson-Knodell CL, Krajicek EJ, Savaiano DA, Shin AS. Lactose Intolerance: A Concise Review to Skim the Surface Mayo Clin Proc. 2020;95:1499–1505. [DOI] [PubMed] [Google Scholar]
- 13.Savaiano DA, Ritter AJ, Klaenhammer TR et al. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial Nutr J. 2013;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaukat A, Levitt MD, Taylor BC et al. Systematic review: effective management strategies for lactose intolerance Ann Intern Med. 2010;152:797–803. [DOI] [PubMed] [Google Scholar]
- 15.Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance Am J Clin Nutr. 1996;64:232–236. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Y, Priebe MG, Vonk RJ et al. The role of colonic microbiota in lactose intolerance Dig Dis Sci. 2004;49:78–83. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Chu H, Cong Y et al. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices Neurogastroenterol Motil. 2015;27:1138–1146. [DOI] [PubMed] [Google Scholar]
- 18.Jellema P, Schellevis FG, van der Windt DA, Kneepkens CM, van der Horst HE. Lactose malabsorption and intolerance: a systematic review on the diagnostic value of gastrointestinal symptoms and self-reported milk intolerance QJM. 2010;103:555–572. [DOI] [PubMed] [Google Scholar]
- 19.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance N Engl J Med. 1995;333:1–4. [DOI] [PubMed] [Google Scholar]
- 20.Varju P, Gede N, Szakacs Z et al. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: A meta-analysis Neurogastroenterol Motil. 2019;31:e13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Deng Y, Chu H et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome Clin Gastroenterol Hepatol. 2013;11:262–268 e261. [DOI] [PubMed] [Google Scholar]
- 22.Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Jarvela I. Identification of a variant associated with adult-type hypolactasia Nat Genet. 2002;30:233–237. [DOI] [PubMed] [Google Scholar]
- 23.Tishkoff SA, Reed FA, Ranciaro A et al. Convergent adaptation of human lactase persistence in Africa and Europe Nat Genet. 2007;39:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyerlein L, Pohl D, Delco F, Stutz B, Fried M, Tutuian R. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance Aliment Pharmacol Ther. 2008;27:659–665. [DOI] [PubMed] [Google Scholar]
- 25.Metz G, Jenkins DJ, Peters TJ, Newman A, Blendis LM. Breath hydrogen as a diagnostic method for hypolactasia Lancet. 1975;1:1155–1157. [DOI] [PubMed] [Google Scholar]
- 26.Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency N Engl J Med. 1975;293:1232–1236. [DOI] [PubMed] [Google Scholar]
- 27.Haemmerli UP, Kistler H, Ammann R et al. Acquired Milk Intolerance in the Adult Caused by Lactose Malabsorption Due to a Selective Deficiency of Intestinal Lactase Activity Am J Med. 1965;38:7–30. [DOI] [PubMed] [Google Scholar]
- 28.Maiuri L, Raia V, Potter Jet al. . Mosaic pattern of lactase expression by villous enterocytes in human adult-type hypolactasia Gastroenterology. 1991;100:359–369. [DOI] [PubMed] [Google Scholar]
- 29.Dahlqvist A, Hammond JB, Crane RK, Dunphy JV, Littman A. Intestinal Lactase Deficiency and Lactose Intolerance in Adults. Preliminary Report Gastroenterology. 1963;45:488–491. [PubMed] [Google Scholar]
- 30.Chey W, Sandborn W, Ritter AJ, Foyt H, Azcarate-Peril MA, Savaiano DA. Galacto-Oligosaccharide RP-G28 Improves Multiple Clinical Outcomes in Lactose-Intolerant Patients Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackenmueller SA, Grenache DG. Reference Intervals for Intestinal Disaccharidase Activities Determined from a Non-Reference Population J Appl Lab Med. 2016;1:172–180. [DOI] [PubMed] [Google Scholar]
- 32.Di Stefano M, Terulla V, Tana P, Mazzocchi S, Romero E, Corazza GR. Genetic test for lactase non-persistence and hydrogen breath test: is genotype better than phenotype to diagnose lactose malabsorption? Dig Liver Dis. 2009;41:474–479. [DOI] [PubMed] [Google Scholar]
- 33.Azcarate-Peril MA, Ritter AJ, Savaiano D et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals Proc Natl Acad Sci U S A. 2017;114:E367–E375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time Scand J Gastroenterol. 1997;32:920–924. [DOI] [PubMed] [Google Scholar]
- 35.Langman JM, Rowland R. Activity of duodenal disaccharidases in relation to normal and abnormal mucosal morphology J Clin Pathol. 1990;43:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keane R, O’Grady JG, Sheil J et al. Intestinal lactase, sucrase and alkaline phosphatase in relation to age, sex and site of intestinal biopsy in 477 Irish subjects J Clin Pathol. 1983;36:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furnari M, Bonfanti D, Parodi A et al. A comparison between lactose breath test and quick test on duodenal biopsies for diagnosing lactase deficiency in patients with self-reported lactose intolerance J Clin Gastroenterol. 2013;47:148–152. [DOI] [PubMed] [Google Scholar]
- 38.Auricchio S, Rubino A, Landolt M, Semenza G, Prader A. Isolated Intestinal Lactase Deficiency in the Adult Lancet. 1963;2:324–326. [DOI] [PubMed] [Google Scholar]
- 39.Klotz AP. Intestinal Lactase Deficiency and Diarrhea in Adults Am J Dig Dis. 1964;9:345–354. [DOI] [PubMed] [Google Scholar]
- 40.von Tirpitz C, Kohn C, Steinkamp M et al. Lactose intolerance in active Crohn’s disease: clinical value of duodenal lactase analysis J Clin Gastroenterol. 2002;34:49–53. [DOI] [PubMed] [Google Scholar]
- 41.Harvey CB, Wang Y, Hughes LA et al. Studies on the expression of intestinal lactase in different individuals Gut. 1995;36:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skovbjerg H, Gudmand-Høyer E, Fenger HJ. Immunoelectrophoretic studies on human small intestinal brush border proteins--amount of lactase protein in adult-type hypolactasia Gut. 1980;21:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuatrecasas P, Lockwood DH, Caldwell JR. Lactase Deficiency in the Adult. A Common Occurrence Lancet. 1965;1:14–18. [DOI] [PubMed] [Google Scholar]
- 44.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human J Clin Invest. 1957;36:1521–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casellas F, Varela E, Aparici A, Casaus M, Rodriguez P. Development, validation, and applicability of a symptoms questionnaire for lactose malabsorption screening Dig Dis Sci. 2009;54:1059–1065. [DOI] [PubMed] [Google Scholar]