Abstract
Background
Localized lymphoblastic lymphoma is rare in pediatric patients. We report the 5-year event-free survival (EFS) and overall (OS) for children and adolescents with localized lymphoblastic lymphoma (LL) treated on a uniform regimen based on Children’s Cancer Group (CCG) leukemia therapy (COG A5971).
Procedure
From June 2000 to October 2005, the study enrolled 60 patients > 12 months old with Murphy stages I or II lymphoblastic lymphoma. Central review confirmed 56 eligible patients. Treatment consisted of 24 months of CCG BFM without day 28 intrathecal methotrexate in maintenance therapy or prophylactic cranial radiation.
Results
Most patients had pre-B immunophenotype (75%). At a median follow-up of 5.9 years (range 1.4 – 9.3 years), the 5-year EFS was 90% (95% Confidence Interval [CI], 78 – 96%) and the 5-year OS was 96% (95% CI, 84 – 99%). Stage (I vs. II), immunophenotype, elevated LDH > institutional normal, or primary site did not impact outcome. Five relapses occurred - none in the CNS and none in patients with pre-T lymphoblastic disease. Patients tolerated treatment well with no toxic deaths.
Conclusion
Outcomes of pediatric patients with localized LL treated with 2 years of intensive ALL-type therapy was excellent and is similar to the outcome for standard risk ALL treated less intensively. CNS prophylaxis was adequate with limited intrathecal methotrexate and no radiation. Future studies should identify biologic prognostic factors or biomarkers for pediatric patients with LL, explore less intensive treatment for patients with localized disease, and explore novel immunophenotype directed therapies.
Keywords: lymphoblastic lymphoma, localized, pediatric
INTRODUCTION
Children and adolescents with lymphoblastic lymphoma (LL) present with localized disease much less frequently than disseminated disease [1]. The best treatment for patients with localized LL is unknown, with historical data confounded by small numbers of patients and different therapies.
LSA2L2 (cyclophosphamide, vincristine, methotrexate, prednisone, daunomycin, cytarabine, thioguanine, asparaginase, hydroxyurea, and carmustine) became an early gold standard for treating all stages of LL based on single institution studies [2,3]. Several international cooperative groups modified this therapy including the Children’s Cancer Group (CCG), the French (Malignant Lymphoma Therapy) LMT, and the Italian Association of Pediatric Hematology and Oncology (AIEOP) [4-7]. The Berlin-Frankfurt-Muenster group (BFM) historically treated children with localized lymphoblastic lymphoma on trials geared towards specific immunophenotype [8-11].
The largest published study of localized LL (n=50) tested two durations of CHOP therapy (cyclophosphamide, hydroxydaunomycin, vincristine and prednisone) and no radiation to the primary site of disease. The 5-year EFS in patients with LL was inferior (63%) to other histological subtypes (88%). Fifty-seven percent of children with localized LL who received 9 weeks of therapy relapsed, whereas thirty percent of children receiving 8 months of chemotherapy with or without irradiation relapsed [12].
The Children’s Oncology Group (COG) trial A5971 enrolled patients from 2000 to 2005 and included all newly diagnosed patients with lymphoblastic lymphoma. Patients with Murphy stage I and II were non-randomly assigned therapy on a modified Children’s Cancer Study Group (CCG BFM) acute lymphoblastic leukemia regimen, as previously described [13]. This report describes the clinical features and the outcome of children and adolescents with localized LL uniformly treated on the A5971 trial.
METHODS
Patients
From June 15, 2000 to October 7, 2005, 60 newly diagnosed patients over 12 months of age enrolled on COG A5971 with localized LL (Murphy stages I and II) and with either T or B cell immunophenotype. Staging procedures included: bilateral bone marrow aspirates and biopsies, lumbar puncture, CT of chest, abdomen, and pelvis, frontal and lateral chest x-ray, and gallium scan if available, or PET later in the conduct of the study. Patients with bone symptoms underwent a technitium bone scan. Response assessment included a gallium or PET scan and CT after two weeks of therapy. The end of induction and end of consolidation assessment included a gallium (or PET) scan, and repeat CT scans positive at diagnosis. Institutions repeated bone scans, if positive, per institutional standards. The protocol required repeat gallium or PET scans, once negative, only in the presence of residual disease on CT scan. Stage I bone disease was defined as a single lesion; Stage II bone disease as more than 1 bone lesion on the same side of the diaphragm or a single bone lesion with involved regional lymph nodes. Beginning January 13, 2003, the upper age limit increased from 22 to 30 years to open the trial to young adults. Two hundred thirty COG institutions participated in the study. All enrolling sites obtained Institutional Review Board approval. Institutional investigators obtained informed consent for all participants.
Pathology Review methods
Central pathology review consisted of examination of morphology and immunophenotypic and any available genetic data from the original diagnostic biopsy to confirm the submission diagnosis and assign phenotypic lineage. Study pathologists reviewed morphology on submitted slides and performed centralized immunophenotyping including staining with a limited panel of commercially available antibodies to confirm phenotype that included CD3, CD43, CD45RO, CD79a, CD20, and terminal deoxytidyltransferase (TdT) (Supplemental material). Four micron sections underwent immunoperoxidase staining by standard methods using heat induced epitope retrieval (HIER) for most antibodies and an automated stainer (ES, Ventana Medical Systems, Tucson, AZ) (Supplemental material). All steps took place at 40°C. Appropriate positive and negative controls were performed for each antibody. A case was scored as positive if >50% of the tumor cells stained for the antibody evaluated. In most cases, the tumor cells showed relatively uniform expression of the antigens tested with >90% expression. Two hematopathologists (SLP, MAL) independently reviewed all cases and immunoperoxidase stains. They reviewed discrepant diagnoses at a multi-headed microscope to reach a consensus. When no tissue was available for central pathology review, the review pathologists used the initial institutional pathology reports and immunophenotypic studies to confirm eligibility.
Treatment
Patients with localized disease received non-randomized treatment on CCG BFM without the day 28 intrathecal methotrexate doses during maintenance therapy, as previously described [13]. Treatment consisted of 5 phases: induction, consolidation, interim maintenance, delayed intensification, and maintenance. Total duration of therapy was 24 months from diagnosis. Details of the treatment regimen are included in Supplementary material online. Patients received 175 mg/m2 of anthracycline and 3 grams/m2 of cyclophosphamide. Toxicities were graded by the NCI CTC version 2.0.
Response criteria
The study used the product of the two largest diameters of the tumor to define response. Patients underwent a rapid response assessment after two weeks of induction therapy. Institutions repeated positive studies at the end of induction and consolidation. Disappearance of all clinical evidence of disease by physical examination, imaging, or a negative biopsy of lesions showing residual abnormality by imaging, constituted a complete remission (CR1). A 55% reduction in tumor area with a negative radionuclide scan was defined as CR2. A decrease in tumor area of 30% constituted a partial response (PR). An increase of more than 25% in tumor area or new disease at a previously uninvolved site was progressive disease (PD). Relapsed disease was tumor at any previously uninvolved site in patients who had a prior complete response. Response not meeting criteria for CR1, CR2, PR, PD, or relapse represented stable disease.
Statistical Analysis
Event-free survival (EFS) was calculated from the time of registration to the first occurrence of progression, relapse, death, or date last seen for patients who remain alive and disease-free. Overall survival (OS) was calculated from the time of registration to death from any cause or date last seen for patients who remain alive. Both the event-free and overall survival distributions were estimated using the Kaplan-Meier method. The log-rank test was used to compare estimated survival distributions by patient characteristics.
RESULTS
Patient Characteristics
From June 2000 to October 2005, 60 patients with localized disease enrolled on the COG A5971 study and 56 received protocol therapy. Central review found four patients ineligible: one with a peripheral T-cell lymphoma, one with Burkitt lymphoma, one with leukemia, and one with Stage III disease. Table I shows the patient characteristics of the 56 eligible patients. The head and neck area accounted for 66% of the primary sites. In the head and neck cases, nodal disease comprised 49% and skin/subcutaneous tissue 41%. The remaining head and neck involvement consisted of salivary glands, gingiva, tonsil, and sinus. One was not specified. Bone primaries represented 18% of cases. Seven of 56 (13%) patients had a LDH above the institutional upper limit of normal (ULN). The mean age at diagnosis is 7.4 years (Standard Deviation, 5.0 years; range 1.4 – 24.5 years). Seven (13%) of the patients were over the age of 15 years at enrollment. Almost 60% of patients are male. The median duration of follow-up for surviving patients is 5.9 years (range 1.4 – 9.3 years).
Table I.
Patient characteristics and outcome by stage, immunophenotype, LDH, primary site and age.
| Variable | Number (%) | 5-year OS (%) (95% CI) | 5-year EFS (%)(95% CI) | |||
|---|---|---|---|---|---|---|
| Stage | ||||||
| I | 31 | (54) | 96% | (76%-99%) | 89% | (70%-96%) |
| II | 20 | (36) | 94% | (65%-99%) | 89% | (64%-97%) |
| p-value | 0.41 | 0.98 | ||||
| Immunophenotype | ||||||
| Pre-B | 42 | (75) | 94% | (79%-99%) | 90% | (74%-96%) |
| Pre-T | 8 | (16) | 100% | 100% | ||
| p-value | 0.46 | 0.39 | ||||
| LDH | ||||||
| </= ULN | 49 | (87) | 95% | (82%-99%) | 89% | (75%-95%) |
| > ULN | 7 | (13) | 100% | 100% | ||
| p-value | 0.53 | 0.39 | ||||
| Primary Site | ||||||
| Bone | 10 | (18) | 100% | 90% | (47%-99%) | |
| Head & Neck | 37 | (66) | 96% | (77%-99%) | 91% | (74%-97%) |
| Non-head & Neck | 7 | (13) | 83% | (27%-97%) | 83% | (27%-97%) |
| p-value | 0.48 | 0.85 | ||||
| Age | ||||||
| < 10 years | 35 | (63) | 94% | (77%-98%) | 91% | (75%-97%) |
| ≥ 10 years | 21 | (37) | 100% | 90% | (64%-97%) | |
| p-value | 0.95 | 0.77 | ||||
Pathology Review
Of the 56 eligible cases, 35 had fixed tissue slides available for retrospective central pathology review, which confirmed a precursor B-cell phenotype for 29 cases (83%), and a precursor T-cell phenotype for five cases (14%). One reviewed case was unable to be assigned a definitive immunophenotype with the limited staining panel used in this protocol as it co-expressed both CD3 (T-cell marker) and CD79a (B-cell marker). Retrospectively, this case underwent further immunophenotyping with additional antibodies and showed positivity for CD2, CD5 and CD7 but was negative for CD19, confirming a T-cell phenotype with aberrant co-expression of CD79a, as is well described in the literature [14]. Fifteen additional cases underwent chart review of institutional pathology and immunophenotyping without central pathology review or immunophenotypic analysis. Of the total 50 reviewed cases, 42 cases exhibited precursor B phenotype (84%) and eight cases precursor T phenotype (16%). Six cases were not reviewed (11% of the total eligible patients) due to a lack of submitted materials or reports.
Responses
Disease response was as follows: 22/39 (56%) achieved a CR1 or CR2 (55% tumor size reduction with negative radionuclide scan) at 2 weeks, 33/49 (67%) at the end of induction, and 32/43 (74%) at the end of consolidation. No progressions occurred during induction or consolidation therapy.
Survival
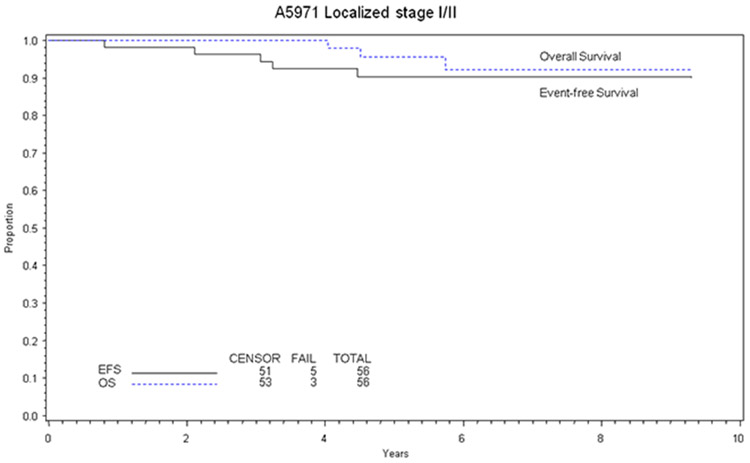
The 5-year event-free survival for patients with localized disease was 90% (95% Confidence IntervaI [CI], 78-96%) and the 5-year overall survival was 96% (95% CI, 84-99%) (Figure 1). There was no significant difference between overall or event-free survival by Stage (I vs. II) or immunophenotype (Table I). There was no statistically significant difference in event-free or overall survival for patients with elevated LDH, primary site, or age (Table I). There were too few failures to determine if response correlated with death or failure.
Figure 1.
Overall and event-free survival at 5 years calculated by the method of Kaplan-Meier.
Relapse
Five patients relapsed, four with pre-B immunophenotype and one with unknown immunophenotype. No pre-T immunophenotype patients experienced an event. One relapse occurred on therapy (9 months, local recurrence at the site of a Stage I bone primary) and one patient relapsed in the bone marrow a month after therapy completion (primary site, scalp). None of the other nine patients with bone primaries relapsed. Three relapses occurred later: at 37 months, 38 months, and 54 months from diagnosis. All three had lymph node primaries, two in the head and neck region. Sites of late relapse included local (n=1), isolated marrow (n=1), and local with dissemination including nodal, bone, and bone marrow (n=1). The five relapsed patients demonstrated the following response evaluations: 2/2 had CR1 or CR2 (3 not evaluated) at 2 weeks, 3/5 were CR1 or CR2, 1/5 PR, and 1 SD at the end of induction, and 3/4 CR1 or CR2, and 1/4 SD at the end of consolidation. Salvage was poor; four of the five relapsed patients are deceased. The fifth is lost to follow-up.
Toxicity of treatment
Overall, patients tolerated therapy well. No deaths from toxicity occurred. Grade 3 and 4 toxicities occurred in less than 10% of patients during induction therapy, with the exception of grade 3 or 4 elevation of ALT, which occurred in 17.5%. Consolidation therapy produced grade 3 or 4 hematologic toxicity (28% for neutropenia, 79% for anemia, and 61% for thrombocytopenia) and 35% grade 3 or 4 febrile neutropenia. Delayed intensification produced a similar toxicity profile to consolidation. Two patients developed osteonecrosis.
Two secondary malignancies occurred in the 56 patients. One patient with primary disease in a cervical lymph node developed a pelvic Ewing’s sarcoma 4 years after diagnosis (alive at last follow up) and another patient with a neck lymph node primary developed secondary acute myelogenous leukemia 5 years after diagnosis after treatment for relapse (deceased). If the statisticians counted the two secondary malignancies as events, the 5-year EFS was 88% (95% CI, 76 – 95%).
DISCUSSION
This represents the largest series of children and adolescents with localized LL treated on uniform therapy. Historically, investigators used two underlying treatment approaches for this population: LSA2L2 and BFM.
In the original single institution study, LSA2L2 produced 88% 5-year overall and event-free survival in patients with localized disease (n=8) [2,3]. Two sequential studies by the CCG using a modified LSA2L2 produced a long-term event-free survival of 84% [4,5]. Eight patients with localized LL treated with a LSA2L2 backbone augmented with ten additional doses of methotrexate at 3 grams/m2 by the French (LMT81) demonstrated EFS at 29 months of 73% with no relapses [6]. From 1992 to 1997, the Italian Association of Pediatric Hematology and Oncology treated children with lymphoblastic lymphoma on a modified LSA2L2 protocol including 3 grams/m2 methotrexate and maintenance therapy of oral 6-mercaptopurine and oral methotrexate. Patients with localized disease received therapy for 11 months. Five children with localized disease enrolled and their 5-year EFS was 100% [7].
The European Organization for Research and Treatment of Cancer (EORTC) treated 14 children with localized pre-T LL using 24 months of Acute Lymphoblastic Leukemia (ALL) type therapy (BFM backbone). For Stage I patients, they demonstrated a 6-year EFS of 86% (13.2% Standard Error) and for Stage II patients, 64% (21.0% Standard Error) [8]. Eleven children with localized pre-T LL enrolled on BFM NHL 86 and 90. They received intensive induction and consolidation therapy with maintenance therapy extending 24 months from diagnosis. The patients with Stage I or II disease did not receive a delayed intensification phase. Of the six evaluable patients on BFM NHL 86, two relapsed, but none of the four patients on BFM NHL 90 relapsed [9,10]. Ten patients with localized pre-B LL enrolled on BFM studies receiving either pre-B ALL therapy (n=7) or B-NHL therapy (n=3). One patient relapsed after receiving pre-B ALL type therapy [11]. None of twenty-one patients with stages I and II pre-B LL treated on LMT 96, EORTC 58881, and EORTC 58951 relapsed or progressed given therapy with a delayed intensified phase. The 5-year EFS was 93% (95%CI, 77-98%) and 5-year OS was 96% (95%CI, 82-99%) for combined stages I, II, and III [15]. Our study showing that a 24-month regimen of BFM based therapy used for patients with ALL produces a 5-year EFS and OS of 90% and 96% respectively, compares favorably with prior studies [2-6,8,9,11,12,16,17].
This study confirmed observations made by others. The pattern of primary sites in pediatric localized LL in the A5971 reflects historical descriptions with frequent skin, subcutaneous, nodal, and bone involvement [3,5,6,11,12]. Ten patients had bone primaries. This report confirmed excellent outcome of localized bone LL [18,19]. This study also confirmed localized LL as predominantly a pre-B lymphoblastic disease with the median age and gender predilection more similar to pre-B ALL than pre-T LL [1]. The only other large study of localized LL occurred prior to routine immunophenotyping [12].
The outcome of patients with localized LL on A5971 was better than that of patients with disseminated LL treated contemporaneously [5-year EFS 82% (95% CI, 77% - 86%) and OS 85% (95% CI, 80% - 89%)] [20]. The outcomes were similar to those of children with standard risk pre-B ALL. Standard risk pre-B ALL patients undergoing the methotrexate randomization in interim maintenance on COG 1991 had a 5-year EFS and OS of 90% and 96% respectively [21]. Standard risk and medium risk patients treated on ALL-BFM 95 also had a similar outcome to these localized LL patients with a 6- year EFS of 90% and 80% respectively [22]. It is notable that no child with pre-T LL relapsed (n=8) and EFS for that group was 100% at 5 years. This contrasted to the EORTC report, but represented a very small number of patients [8].
Relapse occurred locally and a distant sites. In contrast to reports of excellent short term salvage rates in patients with relapsed localized LL, this study supported literature documenting the difficulty in salvaging patients with recurrent LL, with four of five deceased [4,8,12,23]. No CNS relapses occurred. CNS directed therapy consisted of intrathecal methotrexate, indicating this was adequate to prevent CNS relapse. The randomized portion of the A5971 trial demonstrated no difference in EFS between 5 gm/m2 methotrexate and intrathecal methotrexate [20]. Infusional methotrexate during interim maintenance proved superior in trials of acute lymphoblastic leukemia [21]. Methotrexate was used in the French LMT studies but not in the EORTC studies, with excellent outcome [15]. Its role in the treatment of localized disease is unknown.
The high-risk ALL therapy used in this study exposed patients to 175 mg/m2 of anthracycline and 3 grams/m2 of cyclophosphamide. The EORTC used 180 mg/m2 of anthracycline and 2 grams/m2 of cycophosphamide with excellent outcome in stage I and II disease [15]. BFM decreased the anthracycline exposure to 120 mg/m2 and cyclophosphamide to 2 grams/m2 in BFM 90, also with excellent results [9-11]. The current regimen in COG for standard risk ALL, based on CCG 1991, does not use anthracycline in induction or consolidation, for a total exposure of 75 mg/m2. It also eliminates cyclophosphamide in consolidation and exposes patients to only 1 gram/m2. It uses intravenous PEG-asparaginase and dexamethasone in induction. From historical data, decreasing duration of standard cytotoxic chemotherapy is not prudent in this patient population, but there is a precedent to decreasing exposure of select agents [12].
Establishing prognostic factors for outcome is important for a disease where salvage therapy is not associated with high cure rates. This study demonstrated that Stage I vs. II, LDH, primary site, and age (above and below 10 years) were not prognostic in localized disease, although the numbers of patients were small. The immunophenotypic analysis allowed by the protocol was limited to lineage assessment only and was not sufficiently extensive to allow for assignment of stage of blast differentiation, as advocated in a recent study by the European Childhood Lymphoma Pathology Panel [24]. Over 50% of patients undergoing radiographic evaluation after 2 weeks of therapy had at least a 55% reduction in tumor area (31% had complete resolution). Unfortunately, the numbers were too small to determine the predictive value of early radiographic disease response in the subset of patients with localized disease. A more detailed analysis of the imaging responses is pending for the A5971 study. It remains to be determined if initial response by gallium scan (or PET) will help predict those at risk of local recurrence.
We see the evolution of markers that may be predictive of systemic recurrence in pre-T LL and pre-B ALL [25,26]. Improved understanding of the biology of lymphoblastic disease is emerging [26-32]. Techniques to determine minimal residual disease may help predict patients at risk of marrow relapse [33]. Patients with localized disease did not undergo minimal residual disease testing on this study.
The number of children and adolescents diagnosed with localized LL limits the possibility of randomized phase III trials focusing on therapeutic questions such as the backbone therapy and CNS prophylaxis. Future trials should include a uniform backbone therapy based on current treatment for standard risk ALL, with close monitoring for therapeutic failures, testing biomarkers for recurrence, and integration of novel agents specifically targeting the T and B disease.
Supplementary Material
Acknowledgements:
Myron Chang Ph.D. Jim Anderson Ph.D., Jim Lynch Ph.D.
Research is supported by the Chair’s Grant U10 CA98543 and Statistics and Data Center Grant U10 CA98413 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH
A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://applications.childrensoncologygroup.org/admin/grantinfo.htm
Footnotes
Conflict of interest: The authors have none to declare.
Previously presented at the Third International Symposium on Childhood, Adolescent, and Young Adult Lymphoma, Frankfurt, Germany; June 2009.
REFERENCES
- 1.Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol 2005:131(1):39–49. [DOI] [PubMed] [Google Scholar]
- 2.Wollner N, Burchenal JH, Lieberman PH, et al. Non-Hodgkin's lymphoma in children. A comparative study of two modalities of therapy. Cancer 1976:37(1):123–134. [DOI] [PubMed] [Google Scholar]
- 3.Mora J, Filippa DA, Qin J, et al. Lymphoblastic lymphoma of childhood and the LSA2-L2 protocol: the 30-year experience at Memorial-Sloan-Kettering Cancer Center. Cancer 2003:98(6):1283–1291. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JR, Jenkin RD, Wilson JF, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin's lymphoma: a report of CCG-551 from the Childrens Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1993:11(6):1024–1032. [DOI] [PubMed] [Google Scholar]
- 5.Tubergen DG, Krailo MD, Meadows AT, et al. Comparison of treatment regimens for pediatric lymphoblastic non-Hodgkin's lymphoma: a Childrens Cancer Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1995:13(6):1368–1376. [DOI] [PubMed] [Google Scholar]
- 6.Patte C, Kalifa C, Flamant F, et al. Results of the LMT81 protocol, a modified LSA2L2 protocol with high dose methotrexate, on 84 children with non-B-cell (lymphoblastic) lymphoma. Medical and pediatric oncology 1992:20(2):105–113. [DOI] [PubMed] [Google Scholar]
- 7.Pillon M, Piglione M, Garaventa A, et al. Long-term results of AIEOP LNH-92 protocol for the treatment of pediatric lymphoblastic lymphoma: a report of the Italian Association of Pediatric Hematology and Oncology. Pediatric blood & cancer 2009:53(6):953–959. [DOI] [PubMed] [Google Scholar]
- 8.Uyttebroeck A, Suciu S, Laureys G, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. Eur J Cancer 2008:44(6):840–846. [DOI] [PubMed] [Google Scholar]
- 9.Reiter A, Schrappe M, Parwaresch R, et al. Non-Hodgkin's lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage--a report of the Berlin-Frankfurt-Munster Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1995:13(2):359–372. [DOI] [PubMed] [Google Scholar]
- 10.Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood 2000:95(2):416–421. [PubMed] [Google Scholar]
- 11.Neth O, Seidemann K, Jansen P, et al. Precursor B-cell lymphoblastic lymphoma in childhood and adolescence: clinical features, treatment, and results in trials NHL-BFM 86 and 90. Medical and pediatric oncology 2000:35(1):20–27. [DOI] [PubMed] [Google Scholar]
- 12.Link MP, Shuster JJ, Donaldson SS, et al. Treatment of children and young adults with early-stage non-Hodgkin's lymphoma. The New England journal of medicine 1997:337(18):1259–1266. [DOI] [PubMed] [Google Scholar]
- 13.Nachman J, Sather HN, Cherlow JM, et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: a report from the Children's Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1998:16(3):920–930. [DOI] [PubMed] [Google Scholar]
- 14.Pilozzi E, Pulford K, Jones M, et al. Co-expression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. J Pathol. Volume 186. England; 1998. p 140–143. [DOI] [PubMed] [Google Scholar]
- 15.Ducassou S, Ferlay C, Bergeron C, et al. Clinical presentation, evolution, and prognosis of precursor B-cell lymphoblastic lymphoma in trials LMT96, EORTC 58881, and EORTC 58951. Br J Haematol 2011:152(4):441–451. [DOI] [PubMed] [Google Scholar]
- 16.Murphy SB, Hustu HO, Rivera G, et al. End results of treating children with localized non-Hodgkin's lymphomas with a combined modality approach of lessened intensity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1983:1(5):326–330. [DOI] [PubMed] [Google Scholar]
- 17.Jenkin RD, Anderson JR, Chilcote RR, et al. The treatment of localized non-Hodgkin's lymphoma in children: a report from the Children's Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1984:2(2):88–97. [DOI] [PubMed] [Google Scholar]
- 18.Lones MA, Perkins SL, Sposto R, et al. Non-Hodgkin's lymphoma arising in bone in children and adolescents is associated with an excellent outcome: a Children's Cancer Group report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002:20(9):2293–2301. [DOI] [PubMed] [Google Scholar]
- 19.Suryanarayan K, Shuster JJ, Donaldson SS, et al. Treatment of localized primary non-Hodgkin's lymphoma of bone in children: a Pediatric Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1999:17(2):456–459. [DOI] [PubMed] [Google Scholar]
- 20.Abromowitch M TA, Chang M, Perkins SL, Gross T, Weinstein HJ, Finlay J. High-Dose Methotrexate and Early Intensification ofTherapy Do Not Improve 3-Year EFS in Children and Adolescents with Disseminated Lymphoblastic Lymphoma. Results of the Randomized Arms of COG A5971. Blood 2008:112(11):Abstract 3610. [Google Scholar]
- 21.Matloub Y BB, Hunger SP, Angiolillo AL, Cole C, Thomson B, Devidas M, Heerema NA, La MK, Buckley PJ, Carroll WL, Winick N, Sather H, Nachman JB, Gaynon PS. Escalating Dose Intravenous Methotrexate without Leucovorin Rescue during Interim Maintenance is Superior to Oral Methotrexate for Children with Standard Risk Acute Lymphoblastic Leukemia (SR-ALL): Children's Concology Group Study 1991. Blood 2008:112(11):Abstract 9. [Google Scholar]
- 22.Moricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008:111(9):4477–4489. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt B, Reiter A, Landmann E, et al. Poor Outcome for Children and Adolescents With Progressive Disease or Relapse of Lymphoblastic Lymphoma: A Report From the Berlin-Frankfurt-Muenster Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009. [DOI] [PubMed] [Google Scholar]
- 24.Oschlies I, Burkhardt B, Chassagne-Clement C, et al. Diagnosis and immunophenotype of 188 pediatric lymphoblastic lymphomas treated within a randomized prospective trial: experiences and preliminary recommendations from the European childhood lymphoma pathology panel. Am J Surg Pathol 2011:35(6):836–844. [DOI] [PubMed] [Google Scholar]
- 25.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 2008:111(12):5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coustan-Smith E, Sandlund JT, Perkins SL, et al. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children's oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009:27(21):3533–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies SM, Borowitz MJ, Rosner GL, et al. Pharmacogenetics of minimal residual disease response in children with B-precursor acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 2008:111(6):2984–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004:306(5694):269–271. [DOI] [PubMed] [Google Scholar]
- 29.Raetz EA, Perkins SL, Bhojwani D, et al. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatric blood & cancer 2006:47(2):130–140. [DOI] [PubMed] [Google Scholar]
- 30.Feng H, Stachura DL, White RM, et al. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. Volume 18. United States: 2010 Elsevier Inc; 2010. p 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lones MA, Heerema NA, Le Beau MM, et al. Chromosome abnormalities in advanced stage lymphoblastic lymphoma of children and adolescents: a report from CCG-E08. Cancer genetics and cytogenetics 2007:172(1):1–11. [DOI] [PubMed] [Google Scholar]
- 32.Smock KJ, Nelson M, Tripp SR, et al. Characterization of childhood precursor T-lymphoblastic lymphoma by immunophenotyping and fluorescent in situ hybridization: a report from the Children's Oncology Group. Pediatric blood & cancer 2008:51(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabesan V, Cairo MS, Lones MA, et al. Assessment of minimal residual disease in childhood non-hodgkin lymphoma by polymerase chain reaction using patient-specific primers. Journal of pediatric hematology/oncology 2003:25(2):109–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.