Abstract
Aquatic ecosystems are large contributors to global methane (CH4) emissions. Eutrophication significantly enhances CH4-production as it stimulates methanogenesis. Mitigation measures aimed at reducing eutrophication, such as the addition of metal salts to immobilize phosphate (PO43−), are now common practice. However, the effects of such remedies on methanogenic and methanotrophic communities—and therefore on CH4-cycling—remain largely unexplored. Here, we demonstrate that Fe(II)Cl2 addition, used as PO43- binder, differentially affected microbial CH4 cycling-processes in field experiments and batch incubations. In the field experiments, carried out in enclosures in a eutrophic pond, Fe(II)Cl2 application lowered in-situ CH4 emissions by lowering net CH4-production, while sediment aerobic CH4-oxidation rates—as found in batch incubations of sediment from the enclosures—did not differ from control. In Fe(II)Cl2-treated sediments, a decrease in net CH4-production rates could be attributed to the stimulation of iron-dependent anaerobic CH4-oxidation (Fe-AOM). In batch incubations, anaerobic CH4-oxidation and Fe(II)-production started immediately after CH4 addition, indicating Fe-AOM, likely enabled by favorable indigenous iron cycling conditions and the present methanotroph community in the pond sediment. 16S rRNA sequencing data confirmed the presence of anaerobic CH4-oxidizing archaea and both iron-reducing and iron-oxidizing bacteria in the tested sediments. Thus, besides combatting eutrophication, Fe(II)Cl2 application can mitigate CH4 emissions by reducing microbial net CH4-production and stimulating Fe-AOM.
Keywords: bioremediation, Fe-AOM, freshwater sediment, geoengineering, Methanogenesis, mitigation
Fe(II)Cl2 application combat eutrophication and reduce CH4 emissions through increased Fe-dependent anaerobic CH4-oxidation.
Introduction
Aquatic ecosystems are responsible for half of the global CH4 emissions (Rosentreter et al. 2021). CH4 release from organic sediments is strongly driven by eutrophication (Davidson et al. 2018), which remains a significant and ongoing worldwide problem (Beaulieu et al. 2019). Eutrophication increases autochthonous organic matter production, which precludes substrate limitation and promotes methanogenesis, and hence is estimated to increase CH4 emissions by 30%–90%, making eutrophic waters hotspots for CH4 emission (Attermeyer et al. 2016, Beaulieu et al. 2019). Besides nutrient-load reduction, geo-engineering techniques are increasingly used to combat eutrophication. These include techniques using PO43− (P)-binding compounds such as Fe(II)Cl2, lanthanum-modified bentonite clay, and aluminum-modified zeolite (Jančula and Maršálek 2011). However, there is little insight into how eutrophication remediation strategies affect aerobic and anaerobic CH4-cycling microorganisms, and how these affect CH4 emissions (Jančula and Maršálek 2011, Nijman et al. 2022).
Therefore, in this study, we tested the effect of the P-binding agent Fe(II)Cl2 on microbial CH4-cycling in field experiments and batch incubations. In oxic layers of Fe-rich sediments the majority of the phosphorus (P) is bound to ferric iron (Fe(III)) (Parsons et al. 2017). Once the bound Fe(III)-P reaches the anoxic zone of the sediment, bound P is released from the Fe(III)-P complex, as a consequence of the reduction and accompanying dissolution of the Fe particles (Cooke et al. 1993). Subsequently, in the anoxic zone of aquatic sediments, in the presence of Fe(II), P is known to form vivianite (Fe(II)3(PO4)2•8 H2O) through authigenesis. Vivianite is a hydrated ferrous phosphate mineral that immobilizes phosphate (Walpersdorf et al. 2013, Rothe et al. 2014, Liu et al. 2018, Heinrich et al. 2021). However, when exposed to alternative electron acceptors (e.g. O2, NO3−, NO2, SO42−) up to 50% of the Fe(II) present in vivianite can be oxidized to poorly crystalline mixed-valence or ferric Fe(III)-P molecules, increasing the bioavailable Fe(III) in the sediment (Nielsen and Nielsen 1998, Miot et al. 2009, Kusunoki et al. 2015, Rothe et al. 2016). Additionally, ferrous Fe-salts like Fe(II)Cl2 that are directly applied in the sediments can be directly oxidized with O2 or NO3− (Benz et al. 1998, Nielsen and Nielsen 1998, Oikonomidis et al. 2010). Fe(II)Cl2 application could therefore, through direct Fe(II)Cl2 oxidation or through vivianite oxidation, substantially increase the bioavailable Fe(III) concentrations in the sediment.
CH4 formation (methanogenesis) is a microbial process commonly taking place in anoxic sediments (Segers 1998). CH4 can be oxidized to CO2 by methanotrophic microorganisms, either aerobically, using O2 as electron acceptor, or anaerobically, using different alternative electron acceptors (e.g. NO3−, Fe(III), Mn4+ or SO42−) depending on their availability (Segers 1998). Anaerobic oxidation of CH4 (AOM) can oxidize up to 50–90% of the CH4 formed in natural aquatic sediments and is suggested to be among the main processes involved in lowering the natural aquatic CH4 emissions (Norði et al. 2013, Segarra et al. 2015, Weber et al. 2016, Vigderovich et al. 2022). In Fe(III) rich freshwater sediments, Fe-dependent anaerobic CH4-oxidation (Fe-AOM) (Equation 1), has been found to oxidize up to 15% of the produced CH4 (Sivan et al. 2011, Ettwig et al. 2016, Scheller et al. 2016), by exploiting soluble and nanophase Fe(III) as electron acceptors (Norði et al. 2013).
 |
(1) |
Fe-AOM is mediated by anaerobic CH4-oxidizing archaea (ANME), which belong to three distinct clades (ANME 1–3) (Weber et al. 2017). Members of the ANME-1 clade are highly diverse, and fall in the order of Methanophagales. ANME-2 is comprised of three families, all in the order of Methanosarcinales, of which one is observed in freshwaters; the Methanoperedenaceae (ANME-2d), and two are observed in marine systems: Methanocomedenaceae and Methanogasteraceae. ANME-3 is closely related to known methanogens, and represents a novel genus within the family of Methanosarcinaceae (Chadwick et al. 2022).
Since Fe(II)Cl2 addition potentially leads to elevated Fe(III) concentrations in the sediment, it is hypothesized that this could lead to a significant change in the contribution of Fe-AOM to the CH4 cycle in the long term, lowering both internal PO43− concentrations and in situ CH4 emissions.
Here we aim to unravel the effects of Fe(II)Cl2 addition on the aquatic carbon cycle. With this study we generate new insights into how bioremediation techniques -besides combating eutrophication- can help in reducing aquatic CH4 emissions, contributing to climate-smart water management.
Methodology
Field description
To evaluate the effect of Fe(II)Cl2 on CH4 dynamics, an in-situ enclosure experiment was established inside an eutrophic pond. The pond is located at the property of Water Authority ‘Brabantse Delta’, Breda, The Netherlands (51°33′45.5″N 4°46′58.7″E). The pond consists of an open water system, which is connected to a surrounding channel system and encompasses a water surface area of roughly 14 500 m2, with an average water depth of 1.1 m (Kang et al. 2023). On average, the total water N and P concentrations range from 0.46 to 4 mg N L−1 and 0.03–0.22 mg P L−1 and the bioavailable P fraction (top 6 cm sediment) was on average 0.91 mg g dw-1. More details on site description can be found in Kang et al. (2023).
Experimental setup
In March 2020, a total of 8 transparent Perspex cylindrical enclosures (1.05 m diameter, 1.30 m height and a total volume of 865 L, open at the top and bottom) were firmly pushed into the ponds’ sediment (approximately 0.2 m deep) (Kang et al. 2023). The enclosures were positioned in line, at a 30 cm interval, and the top-edge of the enclosures extended approximately 25 cm above the water surface. After placement (9th March 2020), the enclosures were left to settle for approximately 1.5 months. On the 8th of April 2020 the enclosures were randomly treated with Fe(II)Cl2 (N=4) or left as a control (N=4) (Kang et al. 2023). While the control enclosures were left undisturbed, the Fe(II)Cl2 treatment received 19.1 g of crystalline Fe(II)Cl2 (CAS-Nr.: 13478–10–9, Honeywell) that was dissolved in 200 ml anoxic acetate buffer, to obtain a 0.48 M Fe(II)Cl2-solution (pH = 4.2). In total 20 ml of this solution was directly injected into the upper 6 cm of the sediment at 10 different spots, which were randomly chosen from a 16-squared grid placed on top of each enclosure (Kang et al. 2023). The applied dosage of Fe(II)Cl2 was based on the amount of bioavailable P in the first 6 cm of the sediment, to target a 1.5 molar ratio (Fe: P), and is further described in Kang et al. (2023). In the Fe(II)-treated sediments, Fe(III) accounted for between 66% and 91% of the total Fe, in the control it was 81% (Kang et al. 2023).
Field measurements
To quantify the effect of the Fe(II)Cl2 treatment on the CH4 emissions from the pond enclosures, we measured CH4 emissions (totaling 5 sampling occasions) from the 15th of September 2021 (17 months after Fe(II)Cl2 addition) until the 10th of November 2021. We measured CH4 emission after over a year of Fe(II)Cl2 application because in general differences in long-term ecosystem effects become apparent after a complete growing season, due to indirect effects of P-binding agents on the pond-community, for example by changing macrophyte development (Nijman et al. 2022). During the measuring period, O2 concentrations 1 cm above the sediment-water interface ranged between 0.2–6.3 mg L−1 for control enclosures, and between 0.1 and 8.2 mg L−1 in Fe(II)Cl2-treated enclosures. Additionally, the diffusive CH4 emissions from the enclosures were measured biweekly. To limit potential CH4-emission variation caused by measurement time, all measurements were performed around midday, between 11:00 and 14:00 h, hence we did not account for possible diel variability. Fluxes were measured using the floating chamber method, and ebullition was measured by permanently installed bubble traps (Almeida et al. 2016). The bubble traps consisted of up-side down funnels (surface area of 0.033 m2) connected to glass (1000 ml) collection bottles filled with pond water as exemplified in Almeida et al. (2016). During the study duration no bubbles were caught, indicating ebullition was of minor importance. The diffusive flux was measured using a transparent closed floating chamber (height 30 cm, diameter 28.8 cm) that was placed on top of the water column of the enclosure. The chamber was connected to an Ultra-portable greenhouse gas analyzer (UGGA—Los Gatos ®) that was used to quantify the change in CH4 concentration over a period of 3 min, using a closed loop system (Almeida et al. 2016). After placing the chamber, we waited for approximately 1 min for the flux to become stable, to ensure we were not underestimating or overestimating the CH4 flux. The UGGA has an operating range of 0.01–100 ppm for CH4, with a 10-second response time, and a measuring frequency of 1 Hz. Although wind speed is a known driver of water-air fluxes of CH4, due to its effect on turbulent mixing, wind effects were limited because of the enclosure-edges prevented wind-driven wave action. The flux was calculated using equation 2.
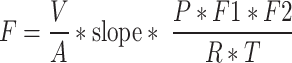 |
(2) |
Where F is the gas flux (mg m−2 d−1), V is chamber volume (L), A is the area of the chamber surface (m2), ‘slope’ is the CH4 concentration change over time (ppm s−1); P is the atmospheric pressure (atm), T is the temperature in (oK); R is the gas constant = 8.205746 × 10−5 m3 atm mol−1 K−1; F1 is the molecular weight of CH4 (16 g mol−1); F2 is the conversion from seconds to days.
At the end of the experiment, 3 sediment cores (diameter 6 cm, height 60 cm) were collected in each enclosure using a UWITEC sediment corer (UWITEC, Mondsee, Austria). The sediment of one core was sliced every 1 cm and analyzed for total P and total Fe. The other cores were used for batch incubations.
Incubation experiments
Four sets of incubation experiments were performed (Fig. 1). To evaluate the effect of the Fe(II)Cl2 treatment in the enclosures, enclosure sediments were incubated under oxic and anoxic conditions allowing for rate measurements of net CH4-production and CH4-oxidation. First, the aerobic top layer of the sediment cores (first 2 cm), as observed by a change in sediment color from light (oxidized) to black (anoxic), was used to quantify the aerobic CH4-oxidation rate, and subsequently to determine sediment moisture content by drying the sediments for 3 days at 70°C. Next, another intact sediment core was transferred to an anaerobic chamber (<10 ppm O2), where the top 2 cm was removed. The layer between 2 and 6 cm sediment-depth was used to quantify sediment CH4-production and potential Fe-dependent anaerobic CH4-oxidation (incubations 2, 3 and 4).
Figure 1.
Schematic overview of the sampling design. Where 8 sediment cores originating from the enclosures were sliced for either oxic incubations or anoxic incubations, following different treatments. Source: Partly created using Canva by the authors.
To measure potential aerobic CH4-oxidation rates, 5 g of sediment + 10 ml of oxygenated (∼7 mg L−1) enclosure water was incubated under atmospheric (oxic) conditions in 120 ml gastight serum bottles. After sediment transfer, the bottles were capped and injected with 1.2 ml (= 1%) 99% CH4 gas, as in Nijman et al. (2022). The sediments were incubated at 18°C and mixed continuously using a gyratory shaker (105 r/m). The CH4 concentration in the headspace was measured directly after injection of 1.2 ml (=1%) CH4, and daily at 10:00, 13:00, 17:00 h during 4 days, using a gas chromatograph equipped with flame ionization detection (Hewlett Packard HP 5890 Series II Gas Chromatograph, Agilent Technologies, California, USA). To ensure the incubation bottles did not become anoxic, we measured O2 concentrations in all the bottles using a Microx TX3 oxygen microsensor (flat-broken needle tip, Presens, Germany), at the end of the incubation experiments.
To determine net CH4-production rates (= CH4-production—anaerobic CH4-oxidation), direct anaerobic CH4-oxidation rates and potential Fe-AOM (all corrected for 82% sediment moisture content), we incubated 25 g of sediment (2–6 cm depth) + 15 ml anoxic enclosure water in 140 ml gastight serum bottles under fully anoxic conditions. Anoxia was confirmed using Microx TX3 oxygen microsensors (flat-broken needle tip, Presens, Germany) in all bottles. For these anoxic incubations, different treatments were used: 1) ‘H2O’– containing only sediment and water, used to determine the net CH4-production under control conditions; 2) ‘CH4’– supplemented with 10 ml of 99% 13CH4; and 3) ‘CH4 + Fe’—supplemented with 10 ml of 99% 13CH4 and 3 mM ferrihydrite (Fe(III)2O3·0.5H2O) to determine potential rates of Fe-AOM. The bottles were kept in a climate-controlled room at 18°C in the dark. Throughout 129 days, approximately once per month the total CH4, 13CO2 and 12CO2 concentrations of the headspace were determined repeatedly by gas chromatography coupled to mass spectrometry (Trace DSQ II, Thermo Finnigan, Austin TX, USA). The concentration of CH4 was quantified as described for aerobic CH4-oxidation. Due to simultaneous CH4-production and CH4-consumption in anoxic incubations, the AOM potential was monitored by the increase in 13/12CO2 rather than the change in CH4 concentration. To test for the potential of Fe-AOM in these incubations, we simultaneously measured the dissolved Fe(II), total Fe(II) and total Fe in water and sediment using a colorimetric ferrozine assay (Schaedler et al. 2018).
16S rRNA sequencing
To explore the microbial potential for Fe-AOM we extracted DNA from the original in situ enclosure sediment which was used for the anaerobic incubations (2–6 cm) and from the anoxic batch incubations at the end of the 130-day incubation period. DNA was extracted using the PowerSoil DNA extraction kit (DNeasy PowerSoil Pro Kit, QIAGEN, Hilden, Germany), according to the manufacturer's protocol. The concentration of DNA was quantified using Qubit® 2.0 Fluorometer with DNA HS kits (Life Technologies, Carlsbad, CA, USA). 16S rRNA gene amplicon sequencing was performed by Macrogen (Amsterdam, The Netherlands) using the Illumina MiSeq Next Generation Sequencing platform*. Paired-end libraries were constructed using the Illumina Herculase II Fusion DNA Polymerase Nextera XT Index Kit V2 (Illumina, Eindhoven, Netherlands). The primers used for bacterial amplification were Bac341F (5′-CCTACGGGNGGCWGCAG-3′; (Herlemann et al. 2011) and Bac806R (5′-GGACTACHVGGGTWTCTAAT-3′; (Caporaso et al. 2012). Archaeal amplification was performed with primers Arch349F (5′-GYGCASCAGKCGMGAAW-3′) and Arch806R (5′-GGACTACVSGGGTATCTAAT-3′; (Takai and Horikoshi 2000). Analysis of the 16S rRNA sequencing output files was performed within R version 3.5.1 (Team 2013) using the DADA2 pipeline (Callahan et al. 2016). After checking the reads quality of the samples, the length of the reads were trimmed using the following parameter: truncLen=c(280 200). Taxonomic assignment of the reads was up to the species level when possible, using the Silva non-redundant database version 132 (Yilmaz et al. 2014). Count data were normalized to relative abundances. Further data analysis and the creation of amplicon sequence variant (ASV) tables at different taxonomical levels were performed using the phyloseq and microbiome packages (McMurdie and Holmes 2013).
*All raw sequencing data have been deposited at the read sequence archive (SRA) database of the NCBI under the BioProject ID PRJNA966799.
Statistical approach
All statistical tests were executed in R, developed by the R Core Team (2013). We tested the data for normal distribution and assumptions for every test executed. To test for differences in CH4 emissions between treatments in the field, a linear model (LM) was used on the log-transformed data (CH4 emissions being the dependent variable, and treatments + date the independent variables). Additionally, to test for differences between dissolved Fe(II) concentrations and changes in ratio 13/12CO2 between treatments, an analysis of variance (two-way ANOVA) combined with Tukey's post-hoc test was used. Furthermore, the difference in net CH4-production rates and aerobic CH4-oxidation rates between treatments was tested using a Welch's T-test for unequal variance and a Student's T-test, respectively. To visualize and test linear relationships for the increase in 13/12CO2 we used a LM (13/12CO2 being the dependent variable, and days of incubation * treatment * treatment the independent variables). Potential CH4-oxidation and CH4-production rates were calculated using the slope of the linear part of the potential CH4-oxidation curve divided by the absolute dry weight (g) of the incubated sediment, and corrected for time. Only the linear part of the slope was used to minimize the effects of substrate limitation and effects of (by)product inhibition on the calculation of potential CH4-oxidation rates.
Results and discussion
Fe(II)Cl2 addition effectively lowered PO43- concentrations in the enclosures, lowering the PO43- concentration from 2.2 ± 0.4 to 1 ± 0.4 µM in the surface water, and considerably boosted Fe concentrations in sediment and water (Table 1). This is in line with previous findings, where under favorable Fe: P ratios and redox conditions Fe addition successfully locked P into lake sediments (Wang and Jiang 2016). Here, in Fe(II)Cl2-treated enclosures, up to 91% of the Fe was present in the form of Fe(III) (Kang et al. 2023).
Table 1.
Average nitrate and trace metal concentrations in surface water and the sediment top layer (5 cm) for control and Fe(II)Cl2-treated enclosures at the end of the enclosure experiment (Nov 2021).
| Water | Sediment | ||||||
|---|---|---|---|---|---|---|---|
| Enclosure | PO43− µM | NO3− µM | Mn µM | Total Fe µM | SO42− µM | Mn mM kg−1 DW | Total Fe mM kg−1 DW |
| Control | 2.2±0.42 | 0.3±0.25 | 8±1.3 | 61±15 | 14.5±6 | 5.1±2.3 | 180±52 |
| Fe(II)Cl2 | 1±0.38 | 0.1±0.05 | 10±5.6 | 280±153 | 21±17 | 2.4±1.7 | 207±137 |
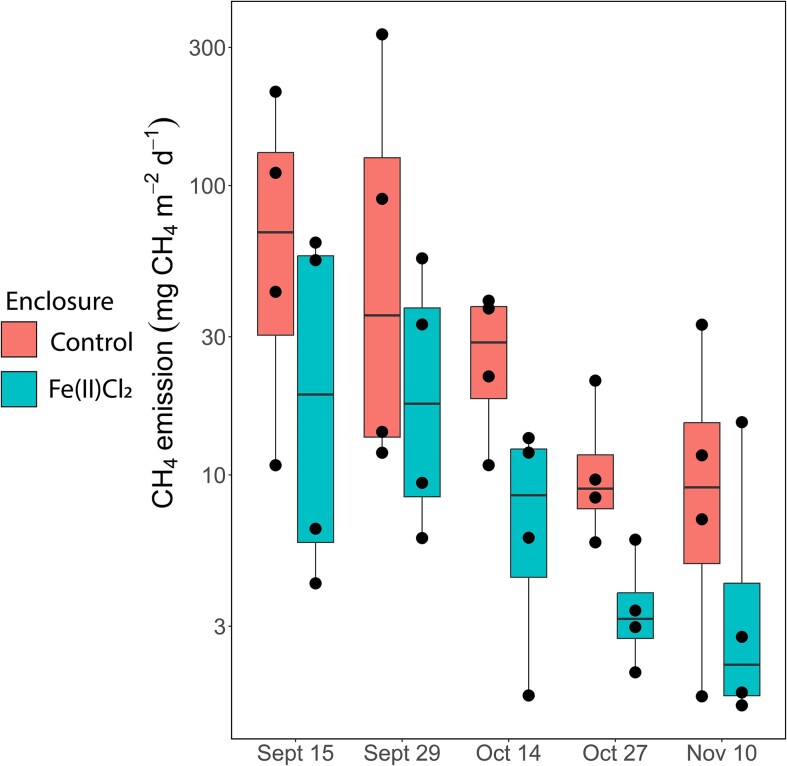
CH4 emissions of the Fe(II)Cl2-treated enclosures were on average 3.5 times lower than those of unamended (P<0.001, r2=0.43, df=34, F=6.9, LM; Fig. 2), where control CH4 emissions averaged 52 ± 78 mg CH4 m−2 d−1 and Fe(II)Cl2-treated enclosures 15 ±14 CH4 m−2 d−1, suggesting that Fe(II)Cl2 addition lowered CH4 emissions. Phosphorus control has previously been found to lower CH4 emissions through indirect effects of oligotrophication on the aquatic foodweb (Nijman et al. 2022). Additionally, we observed a general descending CH4 emission intensity over time (Fig. 2), which is likely due to seasonality, where in colder months CH4 emissions decreases. Here, we test if the effects on CH4 emission can also result from direct effects on microbial CH4-production and oxidation in the sediment.
Figure 2.
Water-atmosphere CH4 emissions from the enclosures in the field Y-axis is on log10 scale. Boxes show the median and first and third quartiles, whiskers indicate upper and lower quartiles, and dots indicate individual sampling points. CH4 emissions from the Fe(II)Cl2-treated enclosures (N=4) were significantly lower compared to controls (N=4) based on a linear model (LM) (P<0.001, r2=0.43, df=34, F=6.9).
The aerobic CH4-oxidation rates of Fe(II)Cl2-treated sediments (average: 2.4 ± 1.2 µmol CH4 g−1 d−1) were not significantly different than rates observed in the control-enclosure sediments (average: 3.6 ± 0.7 µmol CH4 g−1 d−1; P=0.34, df=5, t=1.1; T-test) (Fig. 3A), implying that aerobic CH4-oxidation played a minor role in lowering CH4 emissions from the Fe(II)Cl2-treated enclosures. However, sediments from the Fe(II)Cl2-treated enclosures showed ∼3 times lower net CH4-production rate compared to the control sediments (Fe(II)Cl2 average: 0.53 ± 0.13 µmol CH4 g−1 d−1, control average: 1.4 ± 0.52 µmol CH4 g−1 d−1, P=0.05, df=3.5 t=2.8, T-test, Fig. 3B and S1A). Hence, given that: (i) sediment and water samples from Fe(II)Cl2-treated enclosures showed enrichment in Fe(III) content, (ii) other potential electron acceptors were only found in low concentrations (Table 1, and in Kang et al. 2023), and (iii) potential aerobic CH4-oxidation rates were comparable between control and Fe(II)Cl2-treated enclosures, while in contrast net CH4-production rates—including potential anaerobic methane oxidation—were different, we hypothesized that Fe-AOM played an important role in lowering the in situ CH4 emissions.
Figure 3.
Batch incubation of sediments from the Fe(II)Cl2-treated enclosures (blue) and the control enclosures (red). (A) Aerobic CH4-oxidation potential (calculated from the linear part of the trend) per g. dry weight sediment per day (µmol CH4 g−1 d−1). No significant difference was observed between treatments (T-test, P=0.34, df=5, t=1.1). Boxes show the median and first and third quartiles, whiskers indicate upper and lower quartiles, dots indicate individual sampling points, and ‘x’ indicates the mean. (B) The net potential CH4-production rate of the sediment originating from different enclosures, expressed as µmol CH4 per g. dry weight sediment per day (µmol CH4 g−1 d−1). Sediments from control enclosures have a marginally significant higher net CH4-production rate, compared to the sediments from the Fe(II)Cl2-treatment (T-test, P=0.05, df=3.5, t=2.8).
We monitored AOM rates and Fe(III) reduction in incubations of enclosure sediments with and without Fe(III) addition. We found clear indications that the AOM-potential was higher in sediments originating from the Fe(II)Cl2-treated enclosures than in sediments originating from untreated controls, as seen by their significantly higher 13/12CO2 ratio after incubation with 13CH4 (average 13/12CO2 ratio at the end of the incubation period: control 0.22 ± 0.001, Fe(II)Cl2 0.29 ± 0.002) (Fig. 4A and S1B, Table S1). Moreover, the simultaneous addition of 13CH4 and Fe(III) to control sediments led to higher AOM-rates compared to the 13CH4-only addition, and also significantly boosted Fe(II)-production, suggesting that Fe-AOM is a key pathway (Fig. 4B and S1C). This finding is in line with a study on anaerobic methanotroph bioreactors inoculated with paddy soil, where ferrihydrite and 13CH4 addition also resulted in a rapid onset of 13CO2 production, indicating the occurrence of Fe-AOM (He et al. 2021).
Figure 4.
Batch incubation of sediments from the Fe(II)Cl2-treated enclosures (blue) and the control enclosures (red). (A) Increase in 13/12CO2, with a significant difference between Fe(II)Cl2-treated sediments and control based on a LM (P<0.0001, r2=0.88, df=90, F=69.5). Shaded areas show the 95% confidence interval. (B) Concentrations of dissolved Fe(II) (mM) at the end of the experiment (day 129). Boxes show the median and first and third quartiles, whiskers indicate upper and lower quartiles, dots indicate individual sampling points, and X indicates the mean. Letters depict which groups significantly differ from each other based on a two-way ANOVA (P<0.01, df=2, F=6.3) combined with a Tukey post-hoc test (Table S2) (C) 16S rRNA gene sequencing data, “in situ” refers to the microbial community originating from the original enclosure sediment, whereas the other three samples are taken from the incubations at the end of the experiment from each serum bottle (day 130).
Microbial community analysis revealed the presence of Fe-cycling and CH4-cycling microorganisms in the control and Fe(II)Cl2-treated sediments, implying there is Fe-AOM-potential (Fig. 4C). The presence of Fe(II)-oxidizing bacteria supports the hypothesis that Fe(II) originating from Fe(II)Cl2 can be oxidized to Fe(III), providing an electron acceptor for Fe-AOM (Benz et al. 1998, Nielsen and Nielsen 1998, Oikonomidis et al. 2010). The archaeal community mainly consisted of methanogens, explaining the observed high CH4 emissions. Known CH-producing taxa such as Methanosarcinales and Methanomicrobia also include members capable of CH4-oxidation via reverse methanogenesis, possibly involved in Fe-AOM (Oni and Friedrich 2017).
Methanoperedens species known to mediate both nitrate-dependent AOM and Fe-AOM (Legierse et al. 2023) were also present, albeit at low abundance (below 0.06% in both treatments). Additionally, in both control and Fe(II)Cl2-supplemented enclosure sediments, the methanogen Methanomassiliicoccaceae, was abundant (control 5%, Fe(II)Cl2 7%), suggesting its involvement in Fe-AOM (He et al. 2021). Although the Fe(II)Cl2-treated sediments had a slightly higher abundance of Fe-cycling bacteria, the control sediments already contained Fe-oxidizers and Fe-reducers, indicating the functional capacity for Fe-AOM within the indigenous microbial community. This also explains the rapid—compared to Weber et al. (2017)—onset of Fe-AOM in our incubations. Furthermore, the relative abundance of sulphide-oxidizing phototrophic bacteria such as Chlorobium was substantially lower in the Fe(II)Cl2-treated sediments (2.5%, compared to the control 5.7%) which was likely related to the lower availability of sulphides that may have precipitated in the form of iron sulphide minerals.
At the end of our 130-day incubation experiment the microbial community did not differ much from the in-situ community composition. This implies that the structure and composition of bacterial and archaeal communities was persistent and represented environmental conditions. Additionally, we found that the microbial community in both sediments had the potential to reduce Fe(III), as suggested by the 16S rRNA sequencing, and indicated by the increase in Fe(II) throughout the incubation experiment (Fig. 5A). The Fe(II)-production rates were similar between H2O and 13CH4 treatment; which may be due to the active CH4 supply caused by the sediments’ high methanogenesis rates. Fe(III)-reducers, mainly affiliating with Geobacteraceae were present at similar abundances in both types of sediment (2.2% in the control and 2.6% in the FeCl2-treated enclosure), however, irrespective of the incubation treatment, in all the Fe(II)Cl2-treated sediments, the higher availability of Fe(III) minerals, resulted in a significantly higher concentration of total Fe(II) over time (LM, P=0.003, r2=0.80, df=91, F=37.7). However, part of the Fe(II) formation may also arise from anaerobic respiration of organic substrates. Additionally, we found that the headspace CH4 concentration correlated with total Fe(II) (P=<0.0001) concentrations, which is a proxy for Fe(III) reduction. Moreover, irrespective of the incubation treatment, total headspace CH4 concentration was lower in Fe(II)Cl2-treated sediments (Fig. 5B, LM, P=0.008, r2=0.72, df=91, F=25.8). This suggests that less CH4 is released when there is more Fe(III) reduction. This hypothesis is furthermore strengthened by the significant correlation between 13/12CO2 and total Fe(II) concentration, caused by Fe(III) reduction (LM, P=<0.0001). Fe(II)Cl2-treated sediments had a marginally higher 13/12CO2 compared to control sediments (Fig. 5C, LM, P=0.05, r2=0.76, df=90, F=30.1), as a result of more 13CH4 oxidation.
Figure 5.
Batch incubation of sediments from the Fe(II)Cl2-treated enclosures (blue) and the control enclosures (red). Shaded areas show the 95% confidence interval and the dots indicate individual sampling points (A) Increase in total Fe(II) concentrations (mM) throughout different times during the incubation experiment. The Fe(II)Cl2 treated sediments had significantly higher total Fe(II) concentrations (mM) over time (LM, P=0.003, r2=0.80, df=91, F=37.7). (B) The headspace CH4 concentrations correlated to total Fe(II) concentrations (mM) (P=<0.0001). Total headspace CH4 concentration were lower in Fe(II)Cl2-treated sediments (LM, P=0.008, r2=0.72, df=91, F=25.8). (C) The increase in 13/12CO2 correlated to total Fe(II) concentrations (P =<0.0001). Fe(II)Cl2-treated sediments had a marginally higher 13/12CO2 compared to control sediment (LM, P=0.05, r2=0.76, df=90, F=30.1).
In conclusion, we show that Fe(II)Cl2-treatment of eutrophic sediments in an urban pond led to significantly decreased aquatic CH4 emissions, which was likely facilitated by a rapid onset of Fe-AOM, without substantially changing the native microbial community. This highlights that Fe-AOM can be important in freshwater sediments. Therefore, in sediments where Fe(III) is the main alternative electron acceptor, Fe(II)Cl2 application has the potential to combat eutrophication as well as CH4 emissions, contributing to climate-smart water management.
Supplementary Material
Acknowledgements
We would like to thank Germa Verheggen, Roy Peters from the RU-Aquatic Ecology and Environmental Biology Department and Sebastian Krosse, and Paul van der Ven from the RU-FNWI- General Instrumentation for their assistance with sediment and nutrient analysis. We would also like to thank Water Authority Brabantse Delta, Maíra Mucci, Li Kang, Sina Haasler, Leon Korving, Thomas Prot and Lulian Dugulan, who were involved in the conceptualization and realization of the enclosure experiment.
Contributor Information
Quinten Struik, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
José R Paranaíba, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Martyna Glodowska, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Sarian Kosten, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Berber M J W Meulepas, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Ana B Rios-Miguel, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Mike S M Jetten, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Miquel Lürling, Aquatic Ecology & Water Quality Management Group, Department of Environmental Sciences, Wageningen University, PO Box 47, 6700 AA, Wageningen, The Netherlands.
Guido Waajen, Water Authority Brabantse Delta, 4836 AA, Breda, The Netherlands.
Thomas P A Nijman, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Annelies J Veraart, Department of Ecology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ, Nijmegen, The Netherlands.
Author contributions
Quinten Struik (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing), José R. Paranaíba (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing), Martyna Glodowska (Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing), Sarian Kosten (Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing), Berber M. J. W. Meulepas (Data curation, Investigation, Writing – original draft), Ana B. Rios-Miguel (Formal analysis, Methodology, Software, Visualization, Writing – original draft), Mike S.M. Jetten (Funding acquisition, Investigation, Resources, Writing – original draft), Miquel Lurling (Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing), Guido Waajen (Conceptualization, Methodology, Resources, Writing – original draft), Thomas P.A. Nijman (Conceptualization, Writing – original draft, Writing – review & editing), and Annelies J. Veraart (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing)
Conflict of interest
None declared.
Funding
This research was partially financed by Netherlands Organisation for Scientific Research Applied and technical sciences (NWO-TTW) project n⁰ 18661, and in part supported by the Ministry of Education (OCW) through the Soehngen Institute of Anaerobic Microbiology Gravitation Grant SIAM N.W.O./OCW 024002002 and through ERC Synergy MARIX 8540088. Lastly, the enclosure study was made possible through a grant from the Netherlands Organization for Scientific Research (NWA Idea Generator 2019, project NWA.1228.191.285).
Data Availability
*All raw sequencing data have been deposited at the read sequence archive (SRA) database of the NCBI under the BioProject ID PRJNA966799
The remaining data will be uploaded in the repository, and will become available upon acceptance.
References
- Almeida RM, Nóbrega GN, Junger PC et al. High primary production contrasts with intense carbon emission in a eutrophic tropical reservoir. Front Microbiol. 2016;7:717. 10.3389/fmicb.2016.00717. (29 February 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attermeyer K, Flury S, Jayakumar R et al. Invasive floating macrophytes reduce greenhouse gas emissions from a small tropical lake. Sci Rep. 2016;6:20424. 10.1038/srep20424. (6 December 2022, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JJ, DelSontro T, Downing JA. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat Commun. 2019;10:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz M, Brune A, Schink B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch Microbiol. 1998;169:159–65. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick GL, Skennerton CT, Laso-Pérez R et al. Comparative genomics reveals electron transfer and syntrophic mechanisms differentiating methanotrophic and methanogenic archaea. PLoS Biol. 2022;20:e3001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke GD, Welch EB, Martin AB et al. Effectiveness of Al, Ca, and Fe salts for control of internal phosphorus loading in shallow and deep lakes. Hydrobiologia. 1993;253:323–35. [Google Scholar]
- Davidson TA, Audet J, Jeppesen E et al. Synergy between nutrients and warming enhances methane ebullition from experimental lakes. Nature Clim Change. 2018;8:156–60. [Google Scholar]
- Ettwig KF, Zhu B, Speth D et al. Archaea catalyze iron-dependent anaerobic oxidation of methane. P Natl Acad Sci USA. 2016;113:12792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhu Y, Feng J et al. Long-term effects of four environment-related iron minerals on microbial anaerobic oxidation of methane in paddy soil: a previously overlooked role of widespread goethite. Soil Biol Biochem. 2021;161:108387. [Google Scholar]
- Heinrich L, Rothe M, Braun B et al. Transformation of redox-sensitive to redox-stable iron-bound phosphorus in anoxic lake sediments under laboratory conditions. Water Res. 2021;189:116609. [DOI] [PubMed] [Google Scholar]
- Herlemann DP, Labrenz M, Jürgens K et al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011;5:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jančula D, Maršálek B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere. 2011;85:1415–22. [DOI] [PubMed] [Google Scholar]
- Kang L, Haasler S, Mucci M et al. Comparison of dredging, lanthanum-modified bentonite, aluminium-modified zeolite, and FeCl2 in controlling internal nutrient loading. Water Res. 2023;244:120391. [DOI] [PubMed] [Google Scholar]
- Kusunoki A, Nanzyo M, Kanno H et al. Effect of water management on the vivianite content of paddy-rice roots. Soil Science and Plant Nutrition. 2015;61:910–6. [Google Scholar]
- Legierse A, Struik Q, Smith G et al. Nitrate-dependent anaerobic methane oxidation (N-DAMO) as a bioremediation strategy for waters affected by agricultural runoff. FEMS Microbiol Lett. 2023;370:fnad041. 10.1093/femsle/fnad041. (19 March 2024, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Izon G, Wang J et al. Vivianite formation in methane-rich deep-sea sediments from the South China Sea. Biogeosciences. 2018;15:6329–48. [Google Scholar]
- McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. 10.1371/journal.pone.0061217. (20 June 2023, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot J, Benzerara K, Morin G et al. Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology. 2009;7:373–84. [DOI] [PubMed] [Google Scholar]
- Nielsen JL, Nielsen PH. Microbial nitrate-dependent oxidation of ferrous iron in activated sludge. Environ Sci Technol. 1998;32:3556–61. [Google Scholar]
- Nijman TPA, Lemmens M, Lurling M et al. Phosphorus control and dredging decrease methane emissions from shallow lakes. Sci Total Environ. 2022;847:157584. [DOI] [PubMed] [Google Scholar]
- Norði K, Thamdrup B, Schubert CJ. Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment. Limnology & Oceanography. 2013;58:546–54. [Google Scholar]
- Oikonomidis I, Burrows LJ, Carliell-Marquet CM. Mode of action of ferric and ferrous iron salts in activated sludge. J Chem Technol Biotechnol. 2010;85:1067–76. [Google Scholar]
- Oni OE, Friedrich MW. Metal oxide reduction linked to Anaerobic methane oxidation. Trends Microbiol. 2017;25:88–90. [DOI] [PubMed] [Google Scholar]
- Parsons CT, Rezanezhad F, O'Connell DW et al. Sediment phosphorus speciation and mobility under dynamic redox conditions. Biogeosciences. 2017;14:3585–602. [Google Scholar]
- Rosentreter JA, Borges AV, Deemer BR et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat Geosci. 2021;14:225–30. [Google Scholar]
- Rothe M, Frederichs T, Eder M et al. Evidence for vivianite formation and its contribution to long-term phosphorus retention in a recent lake sediment: a novel analytical approach. Biogeosciences. 2014;11:5169–80. [Google Scholar]
- Rothe M, Kleeberg A, Hupfer M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth Sci Rev. 2016;158:51–64. [Google Scholar]
- Schaedler F, Lockwood C, Lueder U et al. Microbially mediated coupling of Fe and N cycles by nitrate-reducing Fe(II)-oxidizing bacteria in littoral freshwater sediments. Appl Environ Microb. 2018;84:e02013–17. 10.1128/AEM.02013-17. (20 June 2023, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller S, Yu H, Chadwick GL et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science (1979). 2016;351:703–7. [DOI] [PubMed] [Google Scholar]
- Segarra KEA, Schubotz F, Samarkin V et al. High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat Commun. 2015;6:7477. [DOI] [PubMed] [Google Scholar]
- Segers R. Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry. 1998;41:23–51. [Google Scholar]
- Sivan O, Adler M, Pearson A et al. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol Oceanogr. 2011;56:1536–44. [Google Scholar]
- Takai K, Horikoshi K. Rapid detection and quantification of members of the Archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microb. 2000;66:5066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: a Language and Environment for Statistical Computing. 2013. [Google Scholar]
- Vigderovich H, Eckert W, Elul M et al. Long-term incubations provide insight into the mechanisms of anaerobic oxidation of methane in methanogenic lake sediments. Biogeosciences. 2022;19:2313–31. [Google Scholar]
- Walpersdorf E, Koch CB, Heiberg L et al. Does vivianite control phosphate solubility in anoxic meadow soils?. Geoderma. 2013;193-194:189–99. [Google Scholar]
- Wang C, Jiang H-L. Chemicals used for in situ immobilization to reduce the internal phosphorus loading from lake sediments for eutrophication control. Crit Rev Environ Sci Technol. 2016;46:947–97. [Google Scholar]
- Weber HS, Habicht KS, Thamdrup B. Anaerobic methanotrophic archaea of the ANME-2d cluster are active in a low-sulfate, iron-rich freshwater sediment. Front Microbiol. 2017;8:619. 10.3389/fmicb.2017.00619. (12 April 2023, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber HS, Thamdrup B, Habicht KS. High sulfur isotope fractionation associated with Anaerobic oxidation of methane in a low-sulfate, iron-rich environment. Front Earth Sci. 2016;4:61. 10.3389/feart.2016.00061. (27 September 2022, date last accessed). [DOI] [Google Scholar]
- Yilmaz P, Parfrey LW, Yarza P et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucl Acids Res. 2014;42:D643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
*All raw sequencing data have been deposited at the read sequence archive (SRA) database of the NCBI under the BioProject ID PRJNA966799
The remaining data will be uploaded in the repository, and will become available upon acceptance.