Summary
Objective:
Independent of weight status, rapid weight gain has been associated with underlying brain structure variation in regions associated with food intake and impulsivity among pre-adolescents. Yet, we lack clarity on how developmental maturation coincides with rapid weight gain and weight stability.
Methods:
We identified brain predictors of 2-year rapid weight gain and its longitudinal effects on brain structure and impulsivity in the Adolescent Brain Cognitive DevelopmentSM Study®. Youth were categorized as Healthy Weight/Weight Stable (WSHW, n = 527) or Weight Gainers (WG, n = 221, >38lbs); 63% of the WG group were healthy weight at 9-to-10-years-old.
Results:
A fivefold cross-validated logistic elastic-net regression revealed that rapid weight gain was associated with structural variation amongst 39 brain features at 9-to-10-years-old in regions involved with executive functioning, appetitive control and reward sensitivity. Two years later, WG youth showed differences in change over time in several of these regions and performed worse on measures of impulsivity.
Conclusions:
These findings suggest that brain structure in pre-adolescence may predispose some to rapid weight gain and that weight gain itself may alter maturational brain change in regions important for food intake and impulsivity. Behavioural interventions that target inhibitory control may improve trajectories of brain maturation and facilitate healthier behaviours.
Keywords: biomarker, eating disorders, MRI, paediatric obesity
1 |. INTRODUCTION
Rapid weight gain is defined as abnormal growth within a short period and contributes to childhood obesity risk and exacerbated metabolic consequences.1–4 However, apart from infancy, rapid weight gain later in development is poorly understood. The neural mechanisms that may contribute to weight stability versus cause others to experience rapid (or excessive) weight gain independent of their weight status (i.e., healthy weight vs. overweight/obese) remain elusive. Given the current obesity epidemic in youth,5 it is imperative to understand the mechanisms driving weight stability versus rapid weight gain during childhood. The 10-year longitudinal Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®) permits a closer investigation into whether neural differences precede or result from excessive weight gain among 9-to-10-year-old children. Furthermore, we can learn how neural differences correlate with behaviour and potentially identify targets of future interventions.
The predictors and long-term effects associated with abnormal rapid weight gain during infancy are well-documented. Correlates of rapid weight gain during infancy include, being born small6 or large7 for gestational age, prenatal stressors such as substance exposure, preterm delivery, maternal obesity and undernutrition.6,8,9 Rapid weight gain during infancy has been associated with later obesity risk10 and medical comorbidities, such as reduced lung function,1 early menarche2 and cardiometabolic risk.3,4 Rapid weight gain during puberty,11,12 while less studied than in infancy, may be more consequential for cardiometabolic health,13 highlighting the need for further study in this age range.
Generally, weight gain occurs in response to a surplus of calories (i.e., overeating). Although reasons for overeating and obesity are multifactorial, the brain plays a key role as it controls food intake via homeostatic and hedonic control pathways.14,15 Within this realm, obesity in children has been correlated with altered brain structure,16 resting-state functional connectivity,17,18 brain activity during a working memory task19 and altered brain responses in reward and inhibitory control regions to pictures of food,20,21 suggesting that aberrations in brain structure and function may be for a marker for and contribute to overeating. We have previously shown that there are brain regions in 9-to-10-year-old children that predict 1-year rapid weight gain,19 but it is not known whether weight gain itself may affect trajectories of change in brain structure because of the rapid weight gain.
The current study assessed the longitudinal relationship between brain structure and weight gain, independent of weight status, in a cohort of youth classified as either Healthy Weight/Weight Stable (WSHW) or Weight Gainers (WG) over a 2-year period in development. We first assessed if brain regions predictive of 1-year weight gain19 showed continued structural variation after weight gain onset. However, because rapid weight gain can be temporally sensitive (e.g., some youth may gain weight within one year and stop, while other youth will continue to show multiyear rapid weight gain trajectories), we also assessed if there were other areas of structural variation at baseline that was predictive of youth who would have sustained, 2-year weight gain and how these regions changed after 2-years of weight gain. To contextualize extreme weight gain in terms of observable behaviour, we investigated neurocognitive metrics focusing on reward and inhibitory control, as deficits in these decision-making processes have been linked to both overeating and obesity.20–23 A greater understanding of the relationship between rapid weight gain and brain structure may permit more accurate identification of children at risk for obesity, thereby allowing for interventions to prevent risky eating behaviours before they start.
2 |. METHODS
2.1 |. Study design
Data were curated from the ABCD Study® (3.0 data release), which is a 21-site 10-year longitudinal cohort study aimed to assess neurocognitive development from 9-to-20-years-old. A general overview of the ABCD Study® has been published elsewhere.24–27 Here, we focused on anthropometric data from the baseline appointment (9-to-10-years-old) and the one- and two-year follow-up assessments, as well as the neuroimaging data collected at baseline and the two-year follow-up (ages 11-to-12-years-old). Data were available for the entire sample at baseline and 1-year follow-up (nbaseline = 11 878; nyear1 = 11 235) and contained half of the participants’ data for the 2-year follow-up (nyear2 = 6571 youth). Data collection for the baseline assessment occurred between 2016 and 2018.
2.2 |. Exclusion criteria
Details of exclusion criteria during screening for participation have been previously published28 and include magnetic resonance imaging (MRI) contraindications (e.g., metal implants), not being fluent in English, a history of major neurological disorders (e.g., low functioning autism), premature birth <28 weeks, infant hospitalization >30 days after birth and disinterest in committing to a longitudinal study.
The current manuscript excluded youth from the analyses if they met the following at any of the time points (e.g., baseline, year 1, or year 2): (1) underweight (according to the Center for Disease Control’s [CDC’s] age-sex-height-weight-specific growth curves);29 (2) took medications known to alter food intake or the metabolic processing of food (e.g., antipsychotics, antidepressants, insulin); (3) met criteria for neurological, psychiatric or learning disabilities (e.g., attention deficit hyperactive disorder); (4) met diagnostic criteria for eating disorders (e.g., anorexia, bulimia, binge eating disorder) as assessed by the caregiver-reported Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS);30 (5) mislabelled sex-assigned at birth combined with a mismatch with sex-specific pubertal questionnaires or transgendered youth (i.e., due to sex-specific effects on brain function); (6) missing covariate data; (7) height measurement error (e.g., decrease in height over time) (see Table S1 for details); or (8) youth with weight loss to avoid those with restrictive eating habits (e.g., dieting). MRI quality control was performed by the ABCD Study’s® Data Analytics, Informatics and Resource Center. Tabulated exclusion criteria were provided for the user to apply.
2.3 |. Anthropometrics
Annually, height and weight were measured twice (and then automatically averaged), by a trained research assistant, to the nearest 0.1in and 0.1 lb; a third measurement was collected if there was a large discrepancy. Height and weight were converted into BMI (kg/m2) and BMI z-scores (BMIz) and percentiles according to the CDC’s sex-age-height-weight-specific cut-offs29 per CDC-provided SAS code.
2.4 |. Weight stability assessment
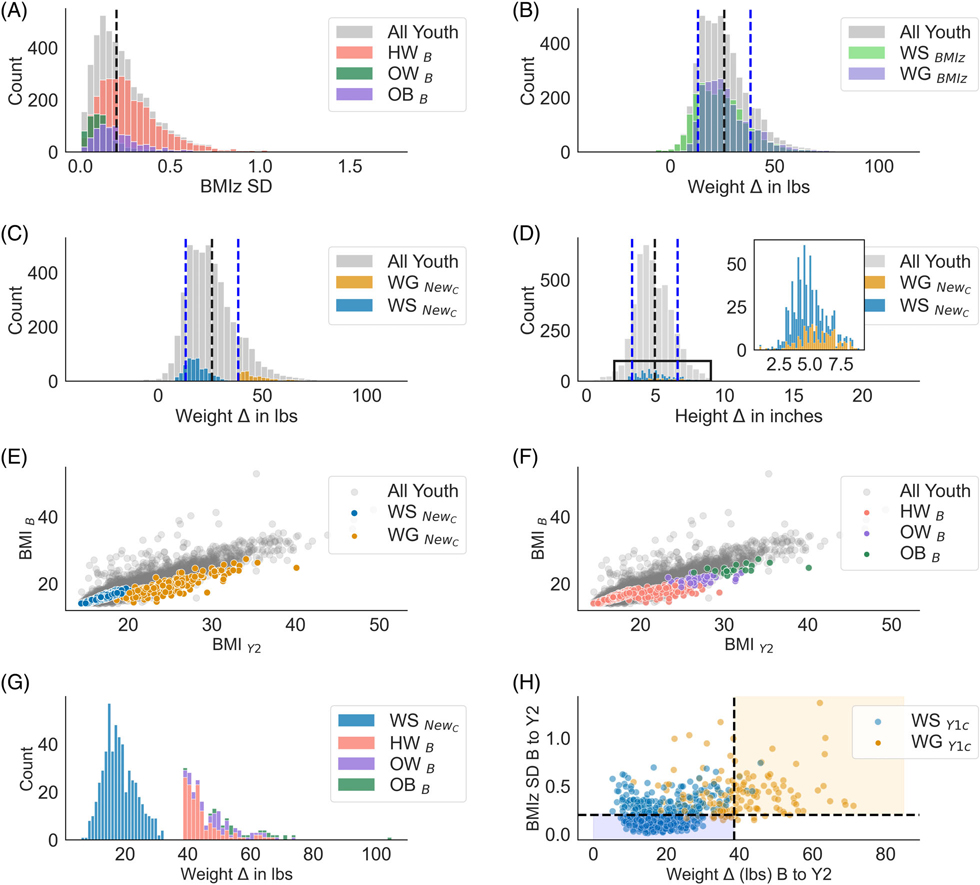
To evaluate excessive weight gain beyond normative development, youth were split into two groups based on three years of anthropometric data: WSHW and WG. Groups were defined based on clinical cut-offs and weight gain criteria. Clinical cut-offs for weight stability have used BMI z-score standard deviation (SD) criterion,31–34 in which an SD <0.2 is considered weight stable, and an SD ≥ 0.2 is considered not weight stable (Figure 1A). This clinical cut-off for weight stability was calculated across three time points (e.g., baseline, year1 and year2 assessments). Since BMI z-scores are poor indicators of weight gain over time35 and have several methodological limitations (see Hendrickson et al., (2021)36 and Palmer et al., (2021)37), and the clinical criterion for weight gain (BMIz SD ≥ 0.2) did not necessarily capture rapid weight gain (Figure 1B), we added a broader weight criterion (1SD above the mean weight gain; see below) to better identify youth who were rapidly gaining weight. The WSHW youth had a BMI z-score SD <0.2 and BMI percentiles <70% at all time points. This percentile cut-off was arbitrary but made to limit the number of youths who may transition to overweight at a later point. In contrast, WG youth had a SD ≥ 0.2 and gained ≥ 38.3 pounds from baseline to the year-two follow-up (1SD above the mean, Mweight gain = 25.7 ± 12.6lbs; Figure 1C). Figure 1D illustrates that a growth spurt (i.e., height change) was not influencing group dichotomization. Additionally, the criterion captured youth across a range of BMIs (Figure 1E) and weight classes (Figure 1F,G). Out of all youth with eligible data, 748 met criteria for being WSHW or WG (see Figure S1 for a flow chart).
FIGURE 1.
(A) Distribution of BMI z-score (BMIz) standard deviation (SD) for all youth and colour coded by baseline (B) weight class (e.g., healthy weight [HW], overweight [OW], obese [OB]). Black dashed line = the clinical cut-off for weight stability as described in the literature.31,32 For Panels (B, C and D), black dashed line = entire sample mean; blue dashed lines = ±1 SD for the entire sample. (B) The clinical weight stability cut-off does not adequately classify rapid weight gainers (colour coded). There were a substantial number of youths who gained more than 1 SD (i.e., ≥ 38 lbs) above the mean (25.0 ± 12.7 lbs) that met both the weight-stable and weight-gain criterion. (C) Weight stability redefined (NewC=WG: ≥ 38lbs + BMIz SD ≥ 0.2; WSHW: BMIz SD < 0.2 + BMI %ile <70); Weight-gain averages by group: M WSHW = 18.2 ± 5.1 lbs; MWG = 47.6 ± 8.8 lbs (D) Height-change distributions for the entire sample (M = 4.91 ± 1.7 inches) and by stability group with the new criteria (WSHW M = 4.7 ± 1.3 inches; MWS = 5.6 ± 2.4 inches). Height did not confound the weight stability classification. (E, F) The distribution of raw BMI (unadjusted for age, sex, height and weight) from baseline to year 2 (y2) colour coded by weight stability and weight class group. The weight stability criterion selected a subset of the youth across a range of BMIs. (G) The weight stability criterion colour coded by baseline weight status. At baseline, 62% of the WG group were classified as having a healthy weight at baseline, while 17% remained HW at year 2 but were still classified as weight gainers. (H) Not all youth identified as WSHW/WG in our previous report19 met the criteria at year 2 (WG = 32.5%, WSHW = 39%). Y1c = Year 1 classification. Coloured boxes = the youth who met Y1 and Y2 criteria, versus white areas = youth classified previously as WSHW /WG at Y119 but not at Y2. Black dashed lines indicate the cut-off for weight stability defined in this manuscript. The In our previous report, we found 18 regions that were predictive of which youth would experience 1-year weight gain19 (Table S4) and abscissa for all subplots is on different scales.
Only 32.5% of WG youth and 39% WSHW at the 1-year follow-up published previously19 were included in analyses here. Reasons for not being included in the current analysis are: some youth with 1-year rapid weight gain19 did not show continued weight gain trajectory over the 1-years (Figure 1H, Table S2 and Figure S2), or some youth initially identified as WSHW at the 1-year follow-up were no longer WSHW at the 2-year follow-up (Figure 1H).
2.5 |. Pubertal assessment
Puberty was assessed via caregiver and self-report sex-specific questionnaires. Scores were converted into sex-specific Tanner staging categories38 and averaged across caregiver and youth reports (1 = Prepubertal, 2 = Early puberty; 3 = Mid puberty; 4 = Late puberty; 5 = Post-pubertal).
2.6 |. Demographic assessments
Caregiver-reported child’s race/ethnicity, date of birth and sex at birth were obtained at the baseline visit (see Supplemental Materials for details).
2.7 |. Prenatal assessments
Caregivers reported preterm delivery (yes/no/refuse), weeks born premature, birth weight, prenatal tobacco exposure before and after pregnancy confirmation (yes/no/refuse) and prenatal alcohol exposure before and after pregnancy confirmation (yes/no/refuse).
2.8 |. Kiddie schedule for affective disorders and schizophrenia for school-age youth (KSADS)
The KSADS assessed psychiatric illnesses (including eating disorders, such as binge eating, anorexia and bulimia) via caregiver report. In sum, 32 Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) child psychiatric diagnoses were created including codes for present, remission and lifetime diagnoses and converted into 0 (absence of diagnosis) or 1 (definitive diagnosis). KSAD diagnoses for eating disorders were used as exclusion criteria (see Table S3 for details).
2.9 |. Cognitive assessments
Youth completed a modified and original Behavioural Inhibition System/Behavioural Approach System (BIS/BAS) questionnaire, which is used to assess trait-based reward and inhibitory control. Youth also completed the Urgency, Premeditation, Perseverance, Sensation Seeking and Positive Urgency (UPPS-P) Impulsive Behaviour Scale, which assess impulsivity (see Table S3 for details).
2.10 |. Neuroimaging acquisition and preprocessing
MRI data were collected with 29 scanners: Details on data acquisition and analyses are published elsewhere.26,39 The current manuscript focuses on structural MRI data, collected with the T1-weighted and Diffusion Tensor Imaging (DTI) acquisitions. Cortical data were parcellated with Freesurfer using the Destrieux Atlas (148 regions of interest [ROI]). Volumetric data were parcellated before surface projection using an atlas of 16 ROIs. Structural data consisted of cortical thickness (mean thickness per ROI), surface area (total surface area per ROI) and subcortical volume. Fractional anisotropy (FA) and mean diffusivity (MD) white-matter ROI estimates sub-adjacent to each cortical were extracted from full-shell DTI images. Subcortical estimates for each FA and MD ROI reflect a mixture of both white- and gray-matter estimates.
2.11 |. Statistics
2.11.1 |. Mixed models ROI analysis
In our previous report, we found 18 regions that were predictive of which youth would experience 1-year weight gain19 (Table S4) and here we examined how weight gain was related to changes in these regions over 2-years. Multiple linear mixed models were conducted in Python with the pymer4 package40 to determine the relationship between weight gain and change in brain structure. The mixed models corrected for BMI, sex, age, puberty, highest household education, race/ethnicity, scan year (i.e., baseline, two-year follow-up) and caregiver report of prenatal exposure to tobacco and alcohol. Models that included FA and MD included a motion estimate, while models with cortical data included intracranial volume as a covariate. Random effects were modelled to account for variability in scanners across the ABCD Study® sites and within-subject variation. Sibling relations were not included because 98% (n = 731) of youth in our analyses were singletons. The dependent variables consisted of ROIs associated with previously predicting 1-year weight gain (n = 18 regions).19 Categorical variables were dummy coded, with the reference variable set to the largest n per category. Pairwise comparisons were corrected for multiple comparisons with the Tukey’s approach. Main effects (WSHW vs. WG) and the Group * Time interactions were corrected for multiple comparisons using the Benjamini-Hochberg approach across each modality (e.g., cortical thickness, surface area, FA, MD and subcortical volume). Correction was conducted separately for the ROIs associated with 1-year and 2-year weight gain. Statistical reporting for categorical predictors in the main text, Tables, and Figures are reported using the F-statistic using pymer4’s anova function.
2.11.2 |. Mixed models cognition analysis
Mixed models were also run using the aforementioned method but, in this case, cognition was the dependent variable. Mixed models were run separately for each subscale of the UPPS (n = 5) and BIS/BAS (n = 4). Models controlled for the aforementioned covariates: age, sex, BMI, puberty, race/ethnicity, highest caregiver education, time (e.g., baseline, two-year follow-up) and caregiver report of prenatal exposure to tobacco and alcohol. Benjamini-Hochberg correction was applied to Group and Group × Time interactions separately for each questionnaire.
2.12 |. Identification of brain regions associated with weight gain over a 2-year period by utilizing an elastic-net regression
Previously, we used a five-fold elastic net regression to identify brain regions at baseline that were predictive of youth who had 1-year weight gain.19 Here, we used the same approach in which a five-fold cross-validation (80% train, 20% test) logistic elastic-net regression was employed with the Brain Predictability toolbox Python package41 (see Supplemental Materials for more details) to identify ROIs at baseline that were indicative of sustained, 2-year weight gain. Like our previous report, the elastic net was allowed to choose from an array of brain and non-brain features at baseline, but the outcome was youth who had 2-year weight gain. These results were used in comparison to our previously published findings,19 to determine if there were differences in baseline brain regions that were predictive of 1-year versus 2-year weight gain. We ran three elastic-net regressions to get the independent effects of brain only, nonbrain only (i.e., covariates like age, sex, puberty, race/ethnicity, highest household education, scanner ID) and a model that combined both brain and nonbrain features. Only models that included brain and nonbrain features are reported in the main manuscript, while the results from the additional models are reported in the Table S7. To assess change over time in regions that were newly identified as predictive of two-year weight gain, we ran mixed models as previously described.
3 |. RESULTS
3.1 |. Sample characteristics
The WSHW group consisted of 527 youth (MWS = 18.2 ± 5.1 lbs). The WG group consisted of 221 youth (MW = 47.7 ± 9.1 lbs; Figure 1C,D, Table 1), and 63.3% were classified as having a healthy weight at baseline (Table 1). Despite having significant weight gain (i.e., clinically unstable. +≥38 lbs), 17.6% remained of a healthy weight at the 2-year follow-up (Figure 1G).
TABLE 1.
Demographics for the Weight Stable (WSHW) and Weight Gain (WG) groups
| WSHW (n = 527) | WG (n = 221) | p group | Other (n = 3869) | p all | ||
|---|---|---|---|---|---|---|
| Age [M (SD)] | Baseline | 119.6 (7.3) | 121.8 (7.2) | 0.001 | 119.7 (7.4) | 0.001 |
| Y2 | 143.5 (7.5) | 145.8 (7.6) | 0.001 | 143.5 (7.7) | 0.001 | |
| Puberty [M (SD)] | Baseline | 1.8 (0.7) | 2.3 (0.8) | <0.001 | 2.0 (0.8) | <0.001 |
| Y2 | 2.5 (0.9) | 3.2 (0.9) | <0.001 | 2.7 (1.0) | <0.001 | |
| BMI [M (SD)] | Baseline | 16.3 (0.9) | 19.3 (2.5) | <0.001 | 19.6 (4.0) | <0.001 |
| Y2 | 17.4 (1.1) | 24.7 (3.5) | <0.001 | 21.3 (4.5) | <0.001 | |
| Weight Δ in lbs [M (SD)] | 18.2 (5.1) | 47.7 (9.1) | <0.001 | 25.5 (12.1) | <0.001 | |
| Height Δ in inches [M (SD)] | 4.7 (1.3) | 5.6 (1.5) | <0.001 | 4.9 (1.7) | <0.001 | |
| Sex [n (%)] | Male | 281 (53.3) | 97 (43.9) | 0.023 | 2058 (53.2) | 0.026 |
| Female | 246 (46.7) | 124 (56.1) | 1811 (46.8) | |||
| Race [n (%)] | White | 397 (75.3) | 124 (56.1) | <0.001 | 2188 (56.6) | <0.001 |
| Black | 29 (5.5) | 36 (16.3) | 456 (11.8) | |||
| Hispanic | 52 (9.9) | 41 (18.6) | 777 (20.1) | |||
| Asian | 8 (1.5) | 1 (0.5) | 76 (2.0) | |||
| Other | 41 (7.8) | 19 (8.6) | 372 (9.6) | |||
| Highest Household Edu [n (%)] | <HS | 9 (1.7) | 11 (5.0) | <0.001 | 135 (3.5) | <0.001 |
| HS/GED | 13 (2.5) | 17 (7.7) | 281 (7.3) | |||
| Some College | 98 (18.6) | 89 (40.3) | 976 (25.2) | |||
| BA degree | 145 (27.5) | 45 (20.4) | 1072 (27.7) | |||
| Postgraduate degree | 262 (49.7) | 59 (26.7) | 1405 (36.3) | |||
| Baseline Weight Class [n (%)] | Healthy Weight | 527 (100.0) | 140 (63.3) | <0.001 | 2388 (61.7) | <0.001 |
| Overweight | 59 (26.7) | 716 (18.5) | ||||
| Obese | 22 (10.0) | 765 (19.8) | ||||
| Y2 Weight Class [n (%)] | Healthy Weight | 527 (100.0) | 39 (17.6) | <0.001 | 2345 (60.6) | <0.001 |
| Overweight | 89 (40.3) | 761 (19.7) | ||||
| Obese | 93 (42.1) | 763 (19.7) |
Note: Weight class was determined by the Center for Disease Control’s sex-age-height-weight-specific growth curves.29 pgroup = significant group differences between WG and WSHW. pall = significant differences between WG, WSHW and the rest of the sample. p-values represent significance testing for t-tests and chi-squared testing.
Abbreviations: BA, Bachelor’s degree; BMI, body mass index; GED, generalized education diploma; HS, high school; lbs., pounds; M = mean; SD, standard deviation; Δ, change score.
The WG group differed significantly from those in the WSHW group on all demographic variables (age, sex, BMI, puberty, race/ethnicity, parental highest education). Youth in the WG group were two months older (p < 0.001), had more advanced puberty (p < 0.001) and consisted of more females (p = 0.023). Additionally, the WG group had higher percentages of Black and Hispanic youth (p < 0.001) and lower percentages of parents with advanced education (p < 0.001; Table 1).
Mothers of youth in the WG group reported higher rates of short-term prenatal tobacco exposure (i.e., exposure during the first trimester, but discontinued post pregnancy confirmation; n = 40, 18.1%) compared to youth in the WSHW group (n = 52, 10%; p < 0.001). Although significantly different, only 7% of youth in the WG group were exposed to prenatal tobacco continuously (i.e., exposure during all three trimesters; n = 15) compared to youth in the WSHW group (n = 22, 4%; p = 0.03). However, youth in the WSHW group were more likely to be exposed to continuous alcohol exposure (n = 16, 3%), than those in the WG group (n = 5, 2%; p = 0.03, Table S5). Because youth in the WG group differed on these demographic factors, they were controlled for in the subsequent analyses.
3.2 |. Do regions identified as predictive of 1-year rapid weight gain19 show continued structural change over a 2-year period of sustained weight gain?
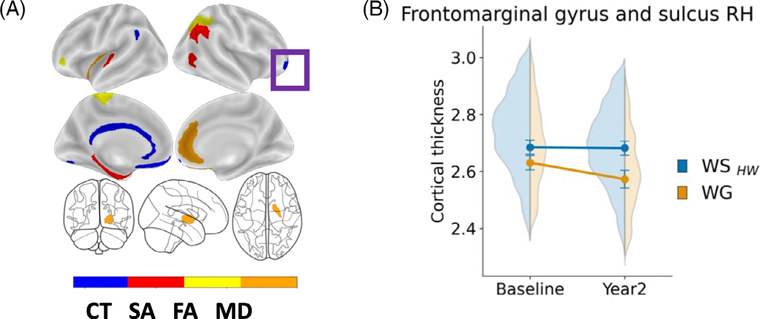
Mixed models revealed that while controlling for age, sex, BMI, puberty, race/ethnicity, education, intercranial volume and caregiver report of prenatal exposure to alcohol and tobacco, there was a main effect of Group (F[1, 1092.61] = 19.2, p < 0.001) and a Group × Time interaction (F[1, 1241.5] = 10.0, p < 0.001) in the right frontomarginal gyrus and sulcus (Table S6). Youth in the WG group had thinner cortices (M = 2.602 ± 0.025, 95% CI [2.545, 2.59], Figure 2A) in this region compared to those in the WSHW group (M = 2.684 ± 0.023, 95% CI [2.634, 2.736]) and showed greater acceleration of thinning in this region by year two (M = 2.573 ± 0.03, 95% CI [2.51, 2.644]; Figure 2B) than WSHW youth (M = 2.682 ± 0.03, 95% CI [2.652, 2.274]). For a complete list of main effects and interactions, please refer to Table S6.
FIGURE 2.
(A) Visualization of the brain regions by modality associated with weight gain (i.e., ≥20 lbs) at the 1-year period (previously identified in Adise et al., 2021).19 Purple box = significant longitudinal change in the frontomarginal gyrus (B) Frontromarginal gyrus and sulcus change over time. Significant interaction (p = 0.002) from the mixed model assessing how these regions changed over time weight gain onset. Mixed model effects were independent of age, sex, puberty, race/ethnicity, highest household education, BMI, time and caregiver report of prenatal exposure to alcohol and tobacco.
3.3 |. Are there underlying differences in brain structure at baseline that can predict group membership (e.g., WSHW/WG) two years later?
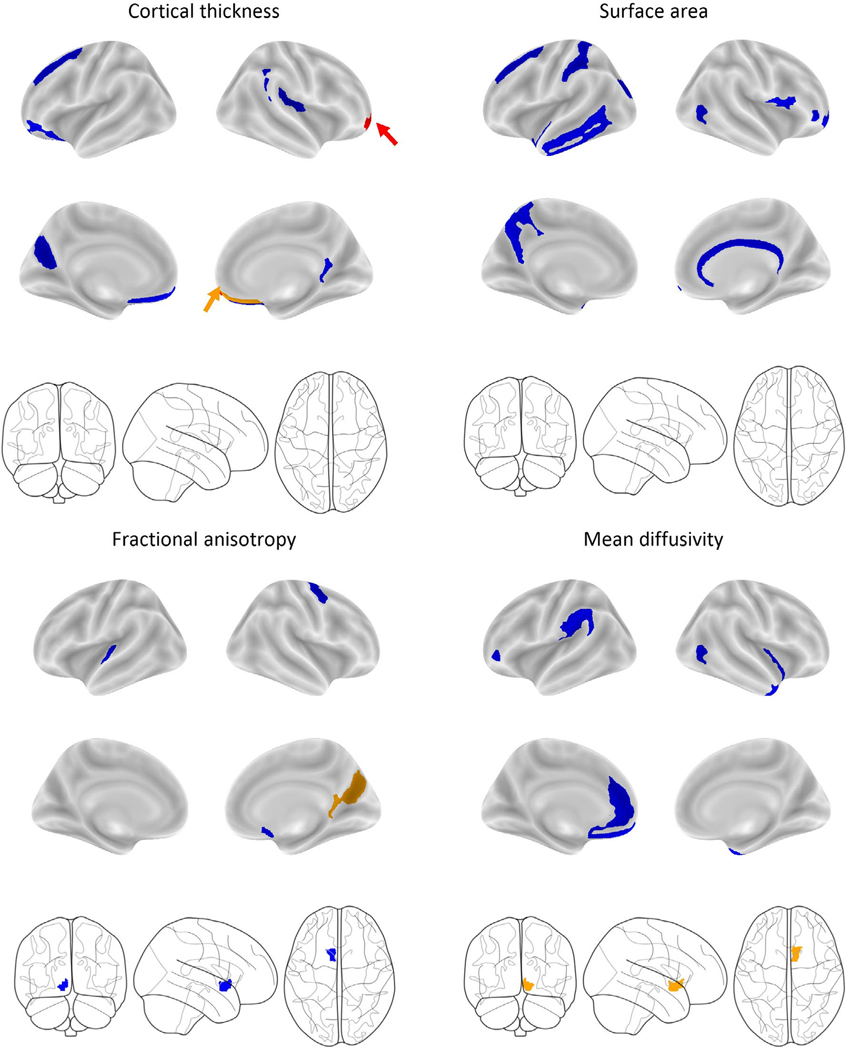
A logistic elastic-net regression identified 39 brain ROIs, along with baseline age, baseline puberty, highest household education and motion, as features that predicted WG group membership two years later (AUCtrain = 0.75, Matthews Correlation Coefficient [MCC] = 0.38; AUCtest = 0.68, MCC = 0.22). In the test set, 17 of 33 (52%) WG and 81 of 109 (74%) WSHW youth were correctly identified, while the overall balanced accuracy was 62.5%. The brain features included 10 cortical-thickness ROIs, 13 surface-area ROIs, 5 cortical-FA ROIs, 9 MD ROIs, 1 subcortical-FA ROI and 1 subcortical-MD ROI (see Table S4 and Figure S3, main text Table 2). ROIs identified for rapid weight gain over two years were largely non-overlapping (90%, n = 35) with those identified for 1-year rapid weight gain (Table 2).19 Results for the brain only and nonbrain only (i.e., covariates) are presented in the Table S7.
TABLE 2.
Results for the logistic elastic-net regression showing which baseline features predicted group membership (i.e., WSHW / WG) based on two years of sustained, rapid weight gain. In other words, these sets of features are associated with weight prior to weight onset and may be potential biomarkers of continued weight gain beyond a 1-year period.
| Feature | Beta weight |
|---|---|
| Cortical thickness | |
| Frontomarginal gyrus RHb | −0.09422 |
| Medial orbito-olfactory sulcus RH | −0.04753 |
| Orbital gyrus LH | −0.03948 |
| Parieto-occipital sulcus LH | −0.06094 |
| Posterior ramus of the lateral sulcus RH | −0.0668 |
| Posterior ventral cingulate gyrus RH | 0.012326 |
| Rectal gyrus LHb | −0.01181 |
| Rectal gyrus RH | −0.01684 |
| Sulcus intermedius primus (of Jensen) RH | 0.008321 |
| Superior frontal sulcus LH | −0.02883 |
| Surface area | |
| Anterior occipital sulcus RHb | −0.01295 |
| Frontomarginal gyrus RH | −0.00979 |
| Inferior circular insula sulcus RHb | 0.003891 |
| Inferior frontal opercular gyrus RH | 0.038942 |
| Inferior temporal gyrus LH | −0.02406 |
| Lateral orbital sulcus RH | 0.014423 |
| Middle temporal gyrus LH | −0.06602 |
| Pericallosal sulcus RH | 0.083268 |
| Planum polare of the superior temporal gyrus LH | −0.11852 |
| Postcentral sulcus LH | 0.03637 |
| Precuneus gyrus LH | −0.0761 |
| Superior frontal sulcus LH | 0.048763 |
| Superior occipital sulcus and transverse occipital sulcus LH | 0.011407 |
| Fractional anisotropy | |
| Accumbens area LH | 0.029662 |
| Anterior transverse temporal gyrus LH | −0.0033 |
| Parieto-occipital sulcus RH | −0.04135 |
| Posterior ventral cingulate gyrus RH | −0.01394 |
| Subcallosal gyrus RH | 0.044645 |
| Superior part of the precentral sulcus RH | −0.00169 |
| Mean diffusivity | |
| Accumbens area RH | −0.02754 |
| Anterior cingulate gyrus and sulcus LH | −0.07266 |
| Anterior occipital sulcus RH | −0.05326 |
| Long insular gyrus and central sulcus of the insula RH | −0.06025 |
| Rectal gyrus LH | −0.01869 |
| Subcallosal gyrus LH | −0.00035 |
| Superior circular insula sulcus LH | −0.01971 |
| Supramarginal gyrus LH | −0.01258 |
| Temporal pole RH | −0.01419 |
| Nonbrain features | |
| DTI Motion | 0.119316 |
| Some college | 0.093156 |
| Postgraduate education | −0.05622 |
| Baseline Age | 0.11139 |
| Baseline Puberty | 0.249004 |
Region of interest (ROI) labels are in accordance with the Destrieux atlas labels. G = gyrus; S = sulcus; L = left; R = right.
= brain regions that were predictive of one-year and two-year weight gain.
At baseline, WG youth had thinner cortices (80% of the predictive CT ROIs), decreased MD (100% of the predictive MD ROIs), and decreased FA (67% of the predictive FA ROIs) but greater surface area (64% of the predictive SA ROIs; see Figure S3 for a visual representation). Baseline age, puberty and motion during DTI were positive predictors of WG youth 2-years later, while household highest education was a negative predictor of group membership.
3.4 |. Do regions found at baseline to be predictive of WSHW/WG group membership 2-years later show additional longitudinal structural changes over time (i.e., after weight gain onset)?
Out of the 39 baseline brain features associated with 2-year sustained weight gain, only six brain features (all right hemisphere) showed significant group differences in change over the two years (p’s < 0.001; Figure 3). Mean differences, confidence intervals and significance are reported in Table S7. The Supplemental Materials contain visual representations of these effects (Figures S4–S6). When compared to WSHW youth, over the 2-years, WG youth had greater reductions in cortical thickness in the right frontomarginal gyrus (aforementioned, t[4.4], p < 0.001, Figure S4A) and rectal gyrus (t[3.04], p = 0.001, Figure S4D), greater reductions in FA in the right parieto-occipital sulcus (t[3.66], p < 0.001) and posterior ventral cingulate gyrus (t[3.31], p = 0.001), and greater reductions in MD in the nucleus accumbens (t[4.42], p < 0.001). Effects were independent of age, sex, baseline puberty, BMI, race/ethnicity, highest household education, intracranial volume, caregiver report of prenatal exposure to alcohol and tobacco, and motion (for FA and MD). No other main effects or interactions were observed (Table S8 lists effects and interactions, corrected and uncorrected; Table S9 shows posthoc comparisons). Youth who had overweight/obese at baseline were removed from the analyses to confirm that the prediction model was not being driving by brain/BMI associations. Even with a reduced sample size (nWG = 140), the elastic net identified WSHW versus WGHW youth (AUCtest = 0.76, MCC = 0.34, balanced accuracy = 0.71, confusion matrix percent correct = 67%). Results did not change when participant groups were matched on age, puberty and caregiver education (Table S10).
FIGURE 3.
Visualization of the baseline brain features across each modality identified from the elastic-net regression that predicted youth in the WG group after 2-years of sustained, rapid weight gain. Colour schematics represent significant main effects and interactions (corrected for multiple comparisons for Group and Group * Time interaction) from the mixed model assessing how these regions changed over time weight gain onset. Mixed model effects were independent of age, sex, puberty, race/ethnicity, highest household education, BMI, time and caregiver report of prenatal exposure to alcohol and tobacco. Blue = no significant change. Orange = Significant main effect of Group. Red = Significant interaction between Group and Time. The arrows highlight areas difficult to see.
3.5 |. Behavioural differences between groups
Lastly, to effectively contextualize the behavioural relevance of neurostructural differences between WSHW and WG groups, we conducted a series of analyses to determine how the WSHW and WG groups differed with respect to impulsive behaviours.
3.5.1 |. BIS/BAS
No effects survived multiple-comparisons correction (Table S11). Results did not change when participant groups were matched on age, puberty and caregiver education (Table S12). Correlations between the BIS/BAS and brain structure are represented in Table S13.
3.5.2 |. UPPS-P
WG youth scored significantly higher on the lack of perseverance subscale (i.e., the tendency to quit when a task gets hard or boring) (F[1, 1020.12] = 7.6, p = 0.005, M = 7.3 ± 0.3, 95% CI [7.11, 8.35]) than those in the WSHW group (M = 7.11 ± 0.3, 95% CI [6.55, 7.67]; Table S14, Figure S7A). For positive urgency (i.e., the tendency to respond impulsively to positive affective states), there was a Group × Time interaction (F[1, 1195.06] = 11.0, p = 0.002, Figure S7C). At baseline, WG youth scored higher (M = 9.02 ± 0.3, 95% CI [8.26, 9.77]) compared to WSHW youth (M = 8.46 ± 0.3, 95% CI [7.71, 9.21], p = 0.03), but no differences were observed at the 2-year follow-up (MWSHW = 7.95 ± 0.371, 95% CI [7.18871], p = 0.2; MWG=7.43 ± 0.4, 95% CI [8.4, 9.06]). All main effects and interactions were independent of age, sex, BMI, baseline puberty, race/ethnicity, highest parental education and caregiver report of prenatal exposure to alcohol or tobacco. No other main effects or interactions were observed (Table S14). Results did not change when participant groups were matched on age, puberty and caregiver education (Table S15). Correlations between the UPPS and brain structure are presented in Table S13.
4 |. DISCUSSION
Much research has focused on understanding the metabolic consequences of rapid weight gain during infancy, but little is known about this weight gain phenomena later in development. Here, for the first time, we show that (1) there are different trajectories of rapid weight gain (i.e., short, one-year; sustained, two-year), (2) structural variation at 9-to-10-years-old can predict who will experience rapid weight over longer periods of time (2 years), and (3) the pattern of brain structural differences between WSHW and WG groups are largely non-overlapping for short- (i.e., 1-year) versus sustained (i.e., 2-years) weight gain. Furthermore, we show that trajectories of maturational change in some brain regions are altered by two-years of rapid weight gain after its onset. These findings are of considerable significance, as they show that individual differences in brain structure may predispose such individuals to sustained unhealthy weight gain over longer periods of time and that brain structure may change as a function of the excessive weight gain itself. Overall, our results attest to the power of studies like the ABCD Study® given its longitudinal design, permitting investigation of competing hypotheses/predictions, and its large demographically diverse sample, allowing for the analytical power to investigate relatively rare phenotypes/outcomes (e.g., rapid weight gain independent of diagnostic criteria for eating disorders).
Adolescence is a developmental period of risk for weight gain due to the effects of puberty on growth42 and the emergence of eating disorders.11,12 Our research highlights the importance of studying rapid weight gain during adolescence as we observed phenotypic differences in weight gain (i.e., short vs. sustained) that corresponded with different patterns of structural variation predicting which youth would experience rapid weight gain over a short (i.e., 1-year) and longer (i.e., 2-year) duration. When compared to 1-year weight gain,19 2-year weight gain was predicted by more widespread structural variation at 9-to-10-years-old in regions involved with executive functioning (e.g., frontomarginal and rectal gyri, and superior frontal sulcus), reward and appetitive control processing (e.g., nucleus accumbens, anterior cingulate), emotion regulation (e.g., anterior and posterior cingulate gyrus) and working memory (e.g., parieto-occipital sulcus).43–45 Decreased available resources (e.g., reduced cortical thickness, decreased FA) across more regions associated with decision-making and food intake, may make it increasingly hard for some youth to inhibit food intake, and thereby result in longer durations of rapid weight gain. Animal studies add support to this theory as neuroinflammation affects the brain prior to weight gain onset46 and is modulated by dietary intake.47 Therefore, it may be that greater structural variation prior to 2-year weight gain may be indicative of more neuroinflammation that coincides with increase caloric intake of high fat foods. This corresponds with our observed phenotypic differences suggesting that food intake patterns may differ amongst those with short and sustained weight gain. Additional longitudinal data will continue to add to our knowledge of the neural differences between these weight gain phenotypes. However, combined with our previous work,19 this demonstrates the predictive power of brain structure as a potential biomarker for identifying youth who are at risk for gaining weight over a short and relatively longer period of time. Additionally, these findings add to the literature that support the theory of neuroinflammation as one reason to explain structural brain changes in youth that experience weight gain over time.
After 2-years of weight gain onset, we observed continued structural change in some of the regions identified to be predictive of prospective two-year (but not one-year) weight gain. By 11-to-12-years-old, youth with two-years of rapid weight gain continued to present with significant differences from their WSHW youth counterparts in regions implicated in food intake and obesity43–45 such as the fronto-marginal and rectal gyrus, parieto-occipital sulcus, ventral gyrus and nucleus accumbens. Structural variation in these regions may send the wrong signals to the hypothalamus to trigger food intake47 that creates a predisposition to and contributes to weight gain maintenance via a cyclical pattern garnered in food intake facilitation and subsequent future weight gain. Because our data only covered a 2-year time span, future studies are needed to assess the role of structural variation and maintained rapid weight gain. Interestingly, no other regions that were predictive of 2-year weight gain showed sustained changes after weight gain, nor did baseline regions that were predictive of 1-year weight gain. There are several explanations for this: It may be that (1) a 2-year period during development is not long enough to observe detrimental consequences of weight gain on brain structure, (2) that differences in brain structure occurred earlier (or will occur later) in development, or (3) regions predictive of 1-year weight gain may be indicative of another phenotype of weight gain (i.e., short term) that may be subject to little variation over time. Although the neuroinflammatory effects of weight gain have been established in humans and animals,47 its temporal course is not clearly defined. Neuroinflammatory effects on brain structure may be moderated by diet and exercise,48,49 and normative developmental brain maturation and reorganization50,51 may adjust deleterious effects of weight gain on brain structure if the detrimental effects of neuroinflammation can be reversed. While these findings serve as a reference point, additional research is needed to understand how the brain changes over a prolonged period in response to short and sustained (i.e., multi-year) weight gain during this period of maturation.50
It is important to note, that there was little overlap between the brain regions that were previously identified as predicting 1-year versus the regions that were identified as predicting 2-years of weight gain. One explanation for this is that there may be two different phenotypes for weight gain that are distinguishable in the brain by machine-learning algorithms prior to any weight gain occurrence. This would confer with our findings that show that some youth gain a lot of weight over a 1-year period but then may plateau versus others who have sustained extreme weight gain (see Figure S1C,D). Moreover, brain regions previously identified at baseline as predictive of 1-year weight gain were fewer, and as noted, did not show continued structural change. Thus, it may be that these regions identified are sensitive to 1-year weight gain but not beyond, again strengthening the notion that there are different phenotypes of weight gain that can be predicted by the brain prior to weight gain. More longitudinal research is needed to understand how extreme weight gain trajectories are related to brain development over the course of adolescence.
The literature suggests that one reason for overeating may be rooted in deficits in impulsivity20,52 (i.e., the ability to control urges to eat). Our data support this possibility as WG youth presented with thinner cortices in regions that are involved with impulsivity (e.g., frontal cortex [i.e., frontomarginal gyrus, rectal gyrus]), while thickness of the rectal gyrus was also related to a lack of perseverance (Table S14). Additionally, WG youth were more likely to act impulsively during a positive mood (i.e., positive urgency) and scored higher on the lack of perseverance subscale of the UPPS. Therefore, this suggests that rapid weight gain may be partly explained by deficits in impulsivity regarding food intake decisions. However, the ABCD Study® did not measure objective food intake or how decisions may change in the presence of food. Therefore, more research is needed to understand how structural changes in inhibitory control regions relate to weight gain and impulsive food choices over time. Surprisingly, youth with 2-year WG did not differ from WS youth on any of the BIS/BAS subscales. The UPPS is more sensitive to impulsivity whereas the BIS/BAS largely assess reward responsiveness. Thus, one interpretation of our results could be that youth with 2-year WG may show greater impulsivity but not reward responsiveness. However, future research is needed to verify this.
5 |. STRENGTHS AND LIMITATIONS
To our knowledge, this was the first study to assess how the brain changes after 2-years of rapid weight gain in regions showing structural variation prior to weight gain. Because youth did not meet diagnostic criteria for binge eating or other eating disorders, these findings have relevance for understanding the brain’s role in rapid weight gain development, maintenance and its deleterious neurological and cognitive effects. However, the ABCD Study® did not collect measurements of emotional overeating or objectively measured food intake, which limits the inferences that can be made from the brain and weight-gain associations. While the WG youth differed significantly from those in the WSHW on several key demographics, these variables were added as covariates in the mixed models, though, we lacked statistical power to further explore these potential associations. Moreover, due to sample-size limitations, we were not able to investigate longitudinal phenotypic and neurological differences between youth who had short versus sustained weight gain. Fortunately, future releases of the ABCD Study® data may afford larger samples of these two phenotypes so that we can further understand how youth who experience shorter durations of weight gain (and then plateau) differ from those with sustained weight gain. While the elastic net chose the best model that was predictive of weight gain, it is possible that there are other regions that are also important but that were not selected for inclusion in the model. The elastic net offers a unique benefit because it can handle highly correlated data (like brain data). Multivariate approaches allow insight into how a combination of features are predictive of an outcome. However, a univariate approached (e.g., mixed models with a ROI) is more informative to assess change over time. Although each of these approaches offer strengths, it may be possible that other statistical analyses may offer additional insight into predictive features and change over time. Of note, the software used to run the elastic net regression differed slightly than our previous published report (e.g., MATLAB [a paid for closed-source software] vs. Python [an open-source, free software]). However, this likely had little to no impact on our findings. We also acknowledge that adolescence is a time of maturation, and thus, it may be that group differences were driven by greater rates of maturation and not necessarily a decrease in resources or cognitive decline. Our findings do offer some insight into this, as youth with sustained two-year weight gain did show behavioural differences in impulsivity. However, because these differences were also apparent at baseline and it is possible that other pre-existing conditions could have contributed to this, it is unclear if these associations are related to differences in brain development acceleration. Future data releases will provide greater insight into whether differences in accelerated brain development are more indicative of maturation or a clinical consequence that is associated with behavioural deficits. Lastly, information about parental weight, a strong predictor child obesity risk, was not collected by the ABCD Study®.
6 |. CONCLUSION
Adolescence is a period in development at risk for excess weight gain. Despite growing obesity trends, little is known about the causes and effects of rapid weight gain and its temporal nature. The current study sheds light on the predictive power of the brain as a biomarker for identifying youth with rapid weight gain trajectories who may present with subclinical eating disorders but do not yet meet diagnostic criteria. Moreover, a large percentage of youth were of a healthy weight prior to weight gain, but still exhibited patterns of structural variation that were associated with weight gain prediction and maintenance. These findings add to the literature suggesting that overeating may produce neuroinflammatory effects that cause brain structure variation prior to weight gain, suggesting that changes in the brain may be an early marker of obesity later in life. Two-year rapid weight gain was preceded by structural variation in brain regions associated with inhibitory control, emotion regulation and appetitive control, and these differences were maintained over a 2-year period. This suggests that structural variation in these regions may be important not only for initiation but for continuation of rapid weight gain trajectories throughout adolescence. Furthermore, these findings add to the growing body of literature aimed at understanding the causes and consequences of rapid weight gain during adolescence. Follow-up studies are needed to examine how these brain structures continue to differentially relate to rapid weight gain trajectories in adolescence.
Supplementary Material
ACKNOWLEDGEMENTS
SA wrote the manuscript and performed the analyses with the guidance of ES, AM, MH. SA conceptualized and curated the data with the help of ES. ES and MH provided funding for the project as part of the ABCD Study consortium. EK, AM and SZ contributed to interpretation and data analysis. All authors contributed feedback, and read, and approved the final manuscript. The ABCD Study consortium investigators designed and implemented the study and/or provided data but did not participate in the analyses or writing of this manuscript.
FUNDING INFORMATION
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM Study® (https://abcdstudy.org/), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10 000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and National Institute on Drug Abuse and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123 and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators/. The ABCD Study® consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or other ABCD Study® consortium investigators. The ABCD Study® data repository grows and changes over time. The ABCD Study® data used in this report came from https://doi.org/10.15154/1503209.
Footnotes
CONFLICT OF INTEREST
No authors report conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Van Der Gugten AC, Koopman M, Evelein AMV, Verheij TJM, Uiterwaal CSPM, Van Der Ent CK. Rapid early weight gain is associated with wheeze and reduced lung function in childhood. Eur Respir J. 2012;39(2):403–410. [DOI] [PubMed] [Google Scholar]
- 2.Salgin B, Norris SA, Prentice P, et al. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int J Obes. 2015;39(6):939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arisaka O, Ichikawa G, Koyama S, Sairenchi T. Childhood obesity: Rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin Pediatr Endocrinol. 2020;29(4):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerkhof GF, Willemsen RH, Leunissen RWJ, Breukhoven PE, Hokken-Koelega ACS. Health profile of young adults born preterm: Negative effects of rapid weight gain in early life. J Clin Endocrinol Metabol. 2012;97(12):4498–4506. [DOI] [PubMed] [Google Scholar]
- 5.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 Key findings Data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;288:2015–2016. https://www.cdc.gov/nchs/data/databriefs/db288.pdf [Google Scholar]
- 6.Singhal A Long-term adverse effects of early growth acceleration or catch-up growth. Annal Nutr Metab. 2017;70(3):236–240. [DOI] [PubMed] [Google Scholar]
- 7.Bichteler A, Gershoff ET. Identification of children’s BMI trajectories and prediction from weight gain in infancy. Obesity. 2018;26(6):1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ion R, Bernal AL. Smoking and preterm birth. Reproductive Sciences. 2015;22:918–926. [DOI] [PubMed] [Google Scholar]
- 9.Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity. 2006;14(3):491–499. [DOI] [PubMed] [Google Scholar]
- 11.Klump KL. Puberty as a critical risk period for eating disorders: a review of human and animal studies. Horm Behav. 2013;64(2):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klump KL, Culbert KM, O’Connor S, Fowler N, Burt SA. The significant effects of puberty on the genetic diathesis of binge eating in girls. Int J Eat Disord. 2017;50(8):984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Tilling K, Martin RM, et al. Analysis of “sensitive” periods of fetal and child growth. Int J Epidemiol. 2019;48(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthoud HR, Münzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71(3):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent JS, Watts R, Adise S, et al. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr. 2019;174:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C, Stamatakis EA, Verdejo-Garcia A. Disrupted functional connectivity in adolescent obesity. NeuroImage. 2016;12:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black WR, Lepping RJ, Bruce AS, et al. Tonic hyper-connectivity of reward neurocircuitry in obese children. Obesity. 2014;22(7):1590–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adise S, Allgaier N, Laurent J, et al. Multimodal brain predictors of current weight and weight gain in children enrolled in the ABCD study®. Dev Cogn Neurosci. 2021;49:100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce AS, Holsen L, Chambers R, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34(10):1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce AS, Lepping RJ, Bruce JM, et al. Brain responses to food logos in obese and healthy weight children. J Pediatr. 2013;162(4):759–764.e2. [DOI] [PubMed] [Google Scholar]
- 22.Adise S, Geier CF, Roberts NJ, White CN, Keller KL. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite. 2018;128:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro ALB, Johnson SL, Sutton B, Legget KT, Dabelea D, Tregellas JR. Eating in the absence of hunger in young children is related to brain reward network hyperactivity and reduced functional connectivity in executive control networks. Pediatr Obes. 2019;14(6):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uban KA, Horton MK, Jacobus J, et al. Biospecimens and the ABCD study: rationale, methods of collection, measurement and early data. Dev Cogn Neurosci. 2018;32:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 2018;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074 [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 31.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Eng J Med. 2018;379(14):1303–1312. [DOI] [PubMed] [Google Scholar]
- 32.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents us preventive services task force recommendation statement. J Am Med Assoc. 2017;317(23):2417–2426. [DOI] [PubMed] [Google Scholar]
- 33.Wiegand S, Keller KM, Lob-Corzilius T, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. 2014;82(6):380–387. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention forweight management in children and adolescents evidence report and systematic review for the us preventive services task force. J Am Med Assoc. 2017;317(23):2427–2444. [DOI] [PubMed] [Google Scholar]
- 35.Vanderwall C, Eickhoff J, Randall Clark R, Carrel AL. BMI z-score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr. 2018;18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrickson MA, Pitt MB. Three areas where our growth chart conversations fall short—room to grow. JAMA Pediatr. 2021;1:123–124. [DOI] [PubMed] [Google Scholar]
- 37.Palmer CE, Sheth C, Marshall AT, et al. A comprehensive overview of the physical health of the adolescent brain cognitive development study cohort at baseline. Front Pediatr. 2021;9:734184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. [DOI] [PubMed] [Google Scholar]
- 39.Hagler DJ, Hatton SN, Makowski C, et al. Image processing and analysis methods for the Adolescent Brain Cognitive. bioRxiv. 2018:1–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jolly E Pymer4: connecting R and Python for linear mixed modeling. J Open Source Softw. 2018;3. https://joss.theoj.org/papers/10.21105/joss.00862.pdf [Google Scholar]
- 41.Hahn S, Yuan DK, Thompson WK, Owens M, Allgaier N, Garavan H. Brain Predictability toolbox: a Python library for neuroimaging-based machine learning. Bioinformatics. 2021;37(11):1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31(6 SUPPL):192–200. [DOI] [PubMed] [Google Scholar]
- 43.Rolls ET. Limbic Structures, Emotion, and Memory; 2017. doi: 10.1016/B978-0-12-809324-5.06857-7 [DOI] [Google Scholar]
- 44.Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2(2). doi: 10.1093/braincomms/fcaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson EL, Dewar CD, Solbakk AK, Endestad T, Meling TR, Knight RT. Bidirectional frontoparietal oscillatory systems support working memory. Curr Biol. 2017;27(12):1829–1835.e4. doi: 10.1016/j.cub.2017.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullins CA, Gannaban RB, Khan MS, et al. Neural underpinnings of obesity: the role of oxidative stress and inflammation in the brain. Antioxidants. 2020;9(10):1–21. doi: 10.3390/antiox9101018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajiluian G, Abbasalizad Farhangi M, Nameni G, Shahabi P, Megari-Abbasi M. Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr Neurosci. 2018;21(10):744–752. doi: 10.1080/1028415X.2017.1348436 [DOI] [PubMed] [Google Scholar]
- 49.Kang EB, Koo JH, Jang YC, et al. Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J Neuroendocrinol. 2016;28(5). doi: 10.1111/jne.12385 [DOI] [PubMed] [Google Scholar]
- 50.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36(3–4):147–160. [DOI] [PubMed] [Google Scholar]
- 51.Shulman EP, Smith AR, Silva K, et al. The dual systems model: review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7 (4):315–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.