Abstract
Objective.
During 2000–2014, age-standardized five-year net survival for cervical cancer was 63–64% in the United States. Using data from CONCORD-3, we analyzed cervical cancer survival trends by race, stage and period of diagnosis.
Methods.
Data from 41 state-wide population-based cancer registries on 138,883 women diagnosed with cervical cancer during 2001–2014 were available. Vital status was followed up until December 31, 2014. We estimated age-standardized five-year net survival, by race (Black or White), stage and calendar period of diagnosis (2001–2003, 2004–2008, 2009–2014) in each state, and for all participating states combined.
Results.
White women were most commonly diagnosed with localized tumors (45–50%). However, for Black women, localized tumors were the most common stage (43.0%) only during 2001–2003. A smaller proportion of Black women received cancer-directed surgery than White women.
For all stages combined, five-year survival decreased between 2001–2003 and 2009–2014 for both White (64.7% to 63.0%) and Black (56.7% to 55.8%) women. For localized and regional tumors, survival increased over the same period for both White (by 2–3%) and Black women (by 5%). Survival did not change for Black women diagnosed with distant tumors but increased by around 2% for White women.
Conclusions.
Despite similar screening coverage for both Black and White women and improvements in stage-specific survival, Black women still have poorer survival than White women. This may be partially explained by inequities in access to optimal treatment. The results from this study highlight the continuing need to address the disparity in cervical cancer survival between White and Black women in the United States.
Keywords: Cervical cancer, Survival, Inequalities, Cancer stage, Race
1. Introduction
Cervical cancer incidence and mortality have declined steadily over the past few decades due to the introduction of and improvements in routine screening programs in the United States [1]. During 1999–2015, age-standardized annual incidence rates of invasive cervical cancer decreased on average by 1.6% per year, though the speed of decline varied by age, race/ethnic group and geographical region. The largest decline was for women aged 20–24 years, while incidence was stable for women aged 35–39 years [1]. In 2017, Black women had one of the highest annual incidence rates of cervical cancer, with 8.3 new cases per 100,000 women, despite experiencing the largest decline in incidence during 1999–2015. The annual incidence rate for White women was 7.3 per 100,000 women [1,2].
The CONCORD program established global surveillance of cancer survival trends in 2015 [3]. The third cycle of the CONCORD program included data for more than 37.5 million cancer patients diagnosed during 2000–2014 in the populations covered by 322 population-based cancer registries from 71 countries worldwide. CONCORD-3 revealed wide international variations in age-standardized 5-year net survival from cervical cancer, ranging from around 50% to 70% for women diagnosed during 2010–2014. In the United States, 5-year survival declined slightly over time from 64.3% (95% CI: 63.7%–64.8%) in 2000–2004 to 62.6% (95% CI: 62.0%–63.1%) in 2010–2014. Similar patterns in survival were seen in other high-income countries with intensive screening programs. Achieving a high proportion of women screened does not always result in improved survival at the population level. Slower-growing tumors, which may have higher survival than fast-growing tumors, are more easily detected during screening. Thus, through the treatment and surgical removal of these curable, often pre-invasive tumors, countries with intensive screening programs can report higher proportions of women with more aggressive disease that is generally not detected through screening [4]. In addition to including women who have tumors that are more difficult to detect during a preclinical phase due to a faster growth rate, the cancer patient population in countries with established screening programs can also include women who have never been screened due to various factors and those who have been screened but did not receive the appropriate follow-up after screening. Therefore, in countries with established screening programs, it is not surprising to see a fall in the incidence of invasive cervical cancer, increasing proportions of regional and distant-stage tumors that are more difficult to treat, and decreasing survival for all stages combined.
The United States Preventive Services Task Force (USPSTF) recommends routine cervical cancer screening for women aged 21–65 years. There has been a shift in cervical cancer screening techniques from cytology-based screening alone to the inclusion of HPV-based screening tests over recent years. The USPSTF recommends either cervical cytology screening alone every 3 years, primary high-risk HPV testing every 5 years, or co-testing with cervical cytology and primary high-risk HPV testing every 5 years for women aged 30–65 years. For women aged 21–29 years, only cervical cytology screening every 3 years is recommended, due to the high prevalence of HPV infection in this age group [5].
In the United States, in 2015, 83% of women aged 21–65 years reported having had a Pap test within the past 3 years or a Pap test with HPV testing within the past 5 years, which is below the Healthy People 2020 target of 93% and lower than in 2000 [6,7]. The proportion of women reporting that they were up to date with their cervical cancer screening varied by age, race/ethnic group, education level, income and insurance status [7].
Using data from CONCORD-3, this study evaluates cervical cancer survival by race, stage and state in the United States for women diagnosed during 2001–2014 [8].
2. Methods
Data from 41 state-wide population-based cancer registries that had participated in CONCORD-3 [4] were included, covering 85% of the US population in 2014. We collected data on 138,883 women (15–99 years) who were diagnosed with a tumor of the cervix (International Classification of Diseases for Oncology, 3rd edition topography codes C53.0-C53.1 and C53.8-C53.9) [9] during 2001–2014 and were followed up for their vital status until December 31, 2014. Only primary, invasive tumors (ICD-O-3 behavior code 3) were included in survival analyses. If a woman was diagnosed with two or more primary, invasive tumors of the cervix during the same time period, only the first record was included. Benign and in situ tumors were excluded.
We defined three calendar periods of diagnosis (2001–2003, 2004–2008 and 2009–2014) to monitor trends in survival over time and to account for changes in data collection methods for SEER Summary Stage 2000, which occurred from January 1, 2004 [10].
We estimated age-standardized five-year net survival by race (Black or White), SEER Summary Stage 2000 and calendar period in each state, and for all participating states combined, using the Pohar Perme estimator [11]. Net survival is the probability of a cancer patient to survive their cancer up to a given time since diagnosis, e.g. one or five years, after controlling for competing risks of death (background mortality), which are higher in older adults. To account for the differences in background mortality between states, racial groups and over time, we used life tables of all-cause mortality that were specific to each county, single year of age, sex, calendar year, socio-economic status and race (all races combined, Black, and White).
We categorized stage at diagnosis according to SEER Summary Stage 2000 [12] (localized, regional, distant and unknown). Stage data were available for all three calendar periods for all states except Washington, which did not submit any data for 2009–2014.
We used the cohort approach to estimate net survival for women diagnosed during 2001–2003 and 2004–2008, because at least five years of follow-up data were available for all women by the end of 2014. The cohort of patients is defined by the year or calendar period of diagnosis (e.g., 2001–2003), and followed for the same length of time (e.g., 5 years). The cohort approach is considered the gold standard for survival estimation as all patients included in the analysis can be followed for the full duration of the survival analysis [13,14]. For women diagnosed during 2009–2014, we used the complete approach, because five years of follow-up data were not available for all women. The complete approach can be used to estimate survival for patients who have been diagnosed more recently but who have not had the opportunity to be followed up for the full amount of time by the end of the study (in this case, December 31, 2014). The follow-up time for women diagnosed during 2009–2014, therefore, varies between one and five years [10].
We produced survival estimates for 5 age groups (15–44, 45–54, 55–64, 65–74 and 75–99 years) and obtained age-standardized estimates for all ages combined using the International Cancer Survival Standard (ICSS) weights [15].
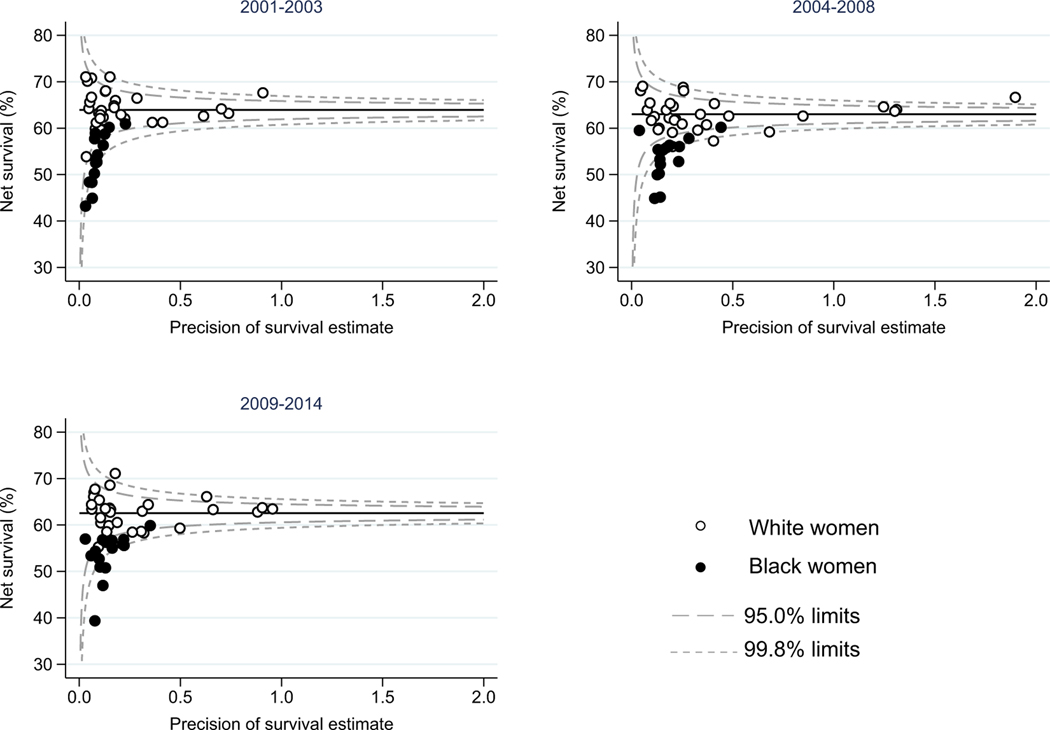
Funnel plots of age-standardized net survival were produced for each calendar period. The funnel plots show how much each race- and state-specific estimate varies from the US pooled estimate (“target” estimate), given the precision of each estimate. The pooled US estimate for all races combined, represented by the horizontal line, is the “target” estimate for this analysis. It was not possible to produce robust age-standardized estimates for all three calendar periods of diagnosis for every state and race combination, thus data from 35 states were included for White women and data from 15 states for Black women.
3. Results
For all races combined, there was a decrease in the proportion of women diagnosed with localized tumors from 50.1% during 2001–2003 to 43.3% during 2009–2014 (Table 1). Consistently, the proportion of regional tumors increased from 32.4% in 2001–2003 to 36.5% in 2009–2014, and from 9.5% in 2001–2003 to 14.3% in 2009–2014 for distant tumors. The proportion of unknown stage tumors decreased from 8.1% in 2001–2003 to 5.9% in 2009–2014.
Table 1.
Number (%) of women (15–99 years) diagnosed with cervical cancer during 2001–2014, by SEER Summary Stage at diagnosis, race and calendar period of diagnosis.
| 2001–2003 |
2004–2008 |
2009–2014 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women | White women | Black women | All women | White women | Black women | All women | White women | Black women | ||||||||||
| Summary Stage 2000 | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| Localized | 16,605 | 50.1 | 13,326 | 51.4 | 2269 | 43.0 | 24,623 | 45.9 | 19,711 | 47.2 | 3217 | 38.4 | 22,527 | 43.3 | 17,695 | 44.7 | 3260 | 36.4 |
| Regional | 10,731 | 32.4 | 8166 | 31.5 | 1946 | 36.9 | 19,534 | 36.4 | 14,892 | 35.6 | 3395 | 40.5 | 18,974 | 36.5 | 14,200 | 35.8 | 3596 | 40.1 |
| Distant | 3154 | 9.5 | 2403 | 9.3 | 582 | 11.0 | 6291 | 11.7 | 4813 | 11.5 | 1174 | 14.0 | 7441 | 14.3 | 5542 | 14.0 | 1523 | 17.0 |
| Unknown | 2678 | 8.1 | 2009 | 7.8 | 477 | 9.0 | 3249 | 6.1 | 2370 | 5.7 | 592 | 7.1 | 3076 | 5.9 | 2186 | 5.5 | 578 | 6.5 |
| Total | 33,168 | – | 25,904 | – | 5274 | – | 53,697 | – | 41,786 | – | 8378 | – | 52,018 | – | 39,623 | – | 8957 | – |
The distribution of stage at diagnosis was more favorable for White women than Black women in each calendar period of diagnosis. For White women, localized tumors were the most common in each calendar period (51.4%, 47.2% and 44.7% for 2001–2003, 2004–2008 and 2009–2014, respectively). For Black women, localized tumors were the most common from 2001 to 2003 (43.0%), while regional tumors were the most common during 2004–2008 (40.5%) and 2009–2014 (40.1%). The proportions of distant and unknown stage were consistently higher in Black women than in White women in all time periods (1.7%, 2.5% and 3.0% higher in 2001–2003, 2004–2008 and 2009–2014, respectively for distant tumors and 1.2%, 1.4% and 1.0% for tumors of unknown stage).
Age-standardized five-year net survival for all stages and races combined fell slightly from 64.0% (95% CI: 63.4–64.7) in 2001–2003 to 62.4% (61.8–63.1) in 2009–2014 (Table 2).
Table 2.
Age-standardized five-year net survival (NS,%) for women (15–99 years) diagnosed with cervical cancer during 2001–2014 by SEER Summary Stage 2000, race and calendar period of diagnosis.
| 2001–2003 |
2004–2008 |
2009–2014 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women | White women | Black women | All women | White women | Black women | All women | White women | Black women | ||||||||||
| Summary Stage 2000 | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI | No. | 95% CI |
| All | 64.0 | 63.4 – 64.7 | 64.7 | 63.9 – 65.4 | 56.7 | 55.1 – 58.3 | 63.1 | 62.6 – 63.6 | 63.6 | 63.0 – 64.2 | 55.5 | 54.2 – 56.8 | 62.4 | 61.8 – 63.1 | 63.0 | 62.2 – 63.7 | 55.8 | 54.3 – 57.4 |
| Localized | 84.7 | 83.7 – 85.7 | 85.4 | 84.2 – 86.5 | 78.6 | 76.0 – 81.1 | 86.1 | 85.2 – 86.9 | 86.6 | 85.7 – 87.6 | 79.3 | 77.2 – 81.4 | 86.9 | 85.6 – 88.2 | 86.9 | 85.4 – 88.5 | 83.6 | 80.8 – 86.5 |
| Regional | 53.1 | 52.0 – 54.2 | 53.6 | 52.3 – 54.8 | 46.8 | 44.2 – 49.3 | 56.1 | 55.2 – 56.9 | 56.5 | 55.5 – 57.4 | 50.1 | 48.1 – 52.1 | 56.4 | 55.3 – 57.5 | 56.7 | 55.4 – 58.0 | 51.7 | 49.2 – 54.2 |
| Distant | 17.1 | 15.7 – 18.5 | 17.4 | 15.8 – 19.0 | 13.8 | 10.8 – 16.8 | 163 | 15.3 – 17.3 | 16.7 | 15.5 – 17.8 | 14.0 | 11.8 – 16.1 | 18.7 | 17.4 – 20.0 | 193 | 17.9 – 20.8 | 13.7 | 11.2 – 16.2 |
| Unknown | 58.4 | 56.3 – 60.6 | 58.7 | 56.3 – 61.1 | 50.1 | 45.0 – 55.2 | 55.2 | 53.3 – 57.1 | 54.3 | 52.1 – 56.5 | 48.1 | 43.6 – 52.6 | 55.0 | 52.7 – 57.3 | 53.6 | 50.9 – 56.4 | 51.0 | 46.1 – 55.9 |
For all stages combined, survival decreased over time for both White and Black women. For White women, survival decreased slightly from 64.7% (95% CI: 63.9–65.4) in 2001–2003 to 63.0% (62.2–63.7) in 2009–2014. A similar decrease was seen for Black women, with survival decreasing from 56.7% (55.1–58.3) in 2001–2003 to 55.8% (54.3–57.4) in 2009–2014 (Table 2).
Survival was consistently higher in White women than in Black women in each calendar period of diagnosis. However, the disparity in survival between White and Black women may be narrowing over time – in absolute terms, survival was 8.0% higher for White women diagnosed during 2001–2003, but only 7.2% higher for White women diagnosed during 2009–2014. Fig. 1 provides a visual representation of the geographic variation in age-standardized 5-year net survival by race for each calendar period of diagnosis. The funnel plots show that for most states, survival is lower for Black women than for White women, with the survival for Black women falling below the pooled estimate for the United States for most states in each calendar period of diagnosis. The funnel plots also demonstrate that the geographic range in survival is wide for both Black and White women (Supplementary Table 1 and Supplementary Fig. 1). The difference in survival between Black and White women is wide, systematic and persistent over time.
Fig. 1.
Age-standardized five-year net survival (%) for women (aged 15–99 years) diagnosed with cervical cancer during 2001–2014.
The circles in the figure represent state-specific survival estimates. Open circles represent the state-specific estimate for White women and closed circles represent the state-specific estimate for Black women. The pooled (US) survival estimates for each calendar period are shown by the horizontal (solid) line with corresponding 95.0% and 99.8% control limits (dotted lines).
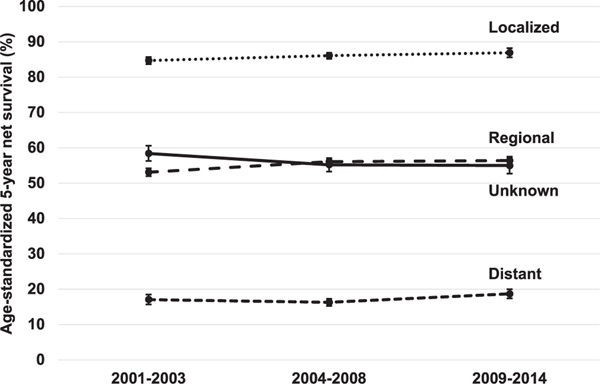
For all races combined, stage-specific survival was highest for women diagnosed during 2009–2014 for all stages, except tumors of unknown stage (Table 2). There were consistent improvements in survival for localized and regional tumors for all races combined, with survival increasing from 84.7% (95% CI: 83.7–85.7) in 2001–2003 to 86.9% (85.6–88.2) in 2009–2014 for localized tumors and from 53.1% (52.0–54.2) in 2001–2003 to 56.4% (55.3–57.5) in 2009–2014 for regional tumors. Survival from distant-stage tumors decreased slightly from 17.1% (15.7–18.5) in 2001–2003 to 16.3% (15.3–17.3) in 2004–2008, but then increased to 18.7% (17.4–20.0) in 2009–2014 (Fig. 2).
Fig. 2.
Trends in age-standardized five-year net survival (%) for women (aged 15–99 years) diagnosed during 2001–2014 with cervical cancer by SEER Summary Stage at diagnosis (all racial groups combined).
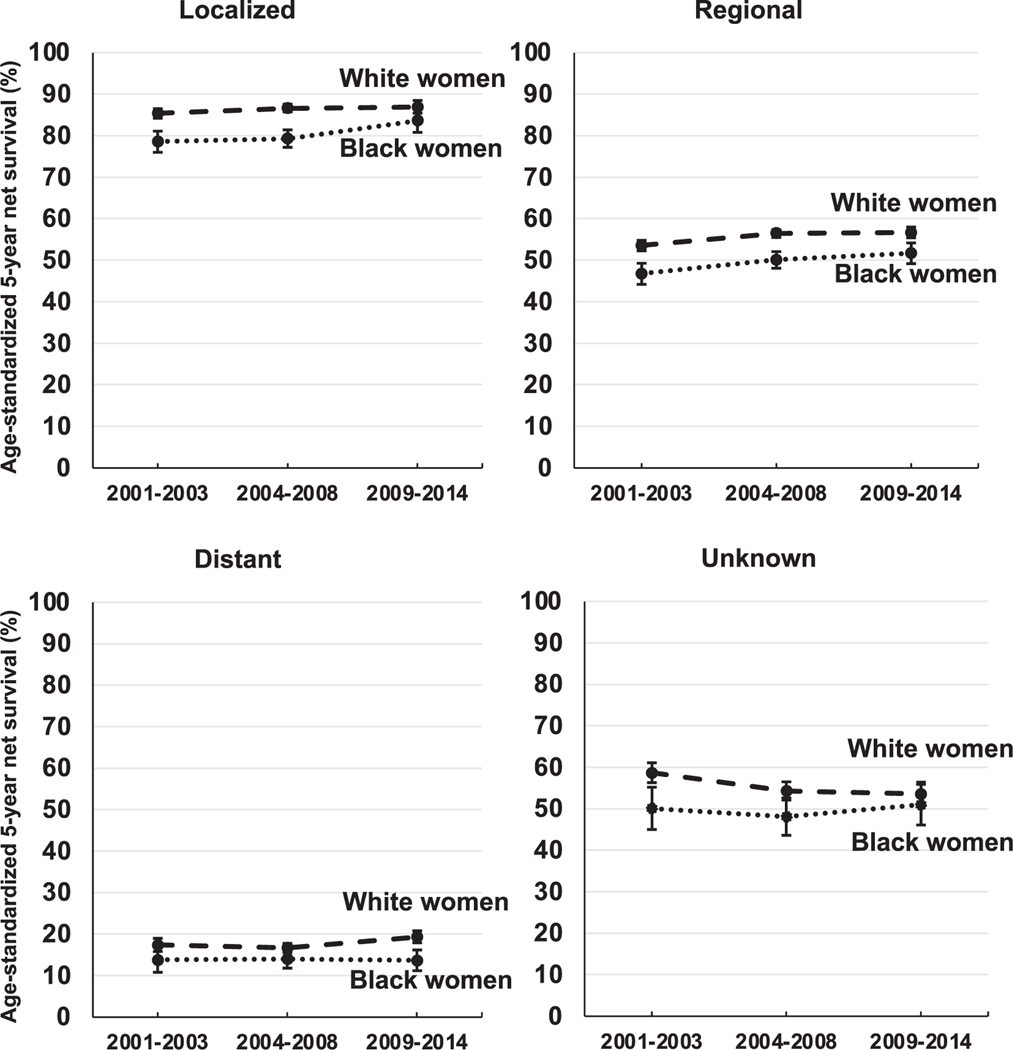
For localized and regional tumors, race- and stage-specific survival increased over time for both White and Black women (Fig. 3). While for both stages White women had consistently higher survival for each calendar period of diagnosis, the improvements over time were greater for Black women. Five-year survival from localized tumors increased in absolute terms by 5.0% over time for Black women (78.6%, 95% CI: 76.0–81.1 in 2001–2003 to 83.6%, 80.8–86.5 in 2009–2014), but only by 1.5% for White women (85.4%, 84.2–86.5 in 2001–2003 to 86.9%, 85.4–88.5 in 2009–2014). For regional tumors, survival increased 4.9% over time for Black women and 3.1% for White women. For distant tumors, however, survival did not change over time for Black women (13.8%, 10.8–16.8 in 2001–2003 and 13.7%, 11.2–16.2 in 2009–2014), but increased by 1.9% for White women. Survival for unknown stage tumors decreased for White women by 5.1% but increased slightly for Black women by 0.9%. The disparity in survival between White and Black women, thus, appears to narrow over time for localized tumors (from 6.8% in 2001–2003 to 3.3% in 2009–2014), regional tumors (from 6.8% in 2001–2003 to 5.0% in 2009–2014) and tumors of unknown stage (from 8.6% in 2001–2003 to 2.6% in 2009–2014), but widens for distant stage tumors (from 3.6% in 2001–2003 to 5.6% in 2009–2014).
Fig. 3.
Trends in age-standardized five-year net survival (%) for Black and White women (aged 15–99 years) diagnosed during 2001–2014 with cervical cancer, by SEER Summary Stage at diagnosis and race.
The planned first course of treatments (cancer-directed surgery, radiotherapy and/or systemic therapy) differed between White and Black women. For localized tumors, for which surgery is a common treatment, 84.2% of White women received cancer-directed surgery, while only 74.3% of Black women did so (Table 3). For regional and distant tumors, higher proportions of White women received surgery (36.0% and 19.1%, respectively) than Black women (26.8% and 15.1%, respectively).
Table 3.
Number (%) of women (15–99 years) diagnosed with cervical cancer during 2001–2014, by SEER Summary Stage at diagnosis, race and treatment.
| Treatment type | Localized |
Regional |
Distant |
Unknown |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women | White women | Black women | All women | White women | Black women | All women | White women | Black women | All women | White women | Black women | |||||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Cancer-directed surgery | ||||||||||||||||||||||||
| Yes | 52,804 | 82.8 | 42,691 | 84.2 | 6497 | 74 3 | 16,920 | 34.4 | 13,431 | 36.0 | 2392 | 26.8 | 3090 | 18.3 | 2440 | 19.1 | 495 | 15.1 | 1748 | 19.4 | 1399 | 21.3 | 241 | 14.6 |
| No | 9549 | 15.0 | 6986 | 13.8 | 2006 | 22.9 | 31,261 | 63.5 | 23,086 | 62.0 | 6290 | 70.4 | 13,411 | 79.4 | 10,043 | 78.7 | 2695 | 82.2 | 5235 | 58.1 | 3760 | 57.3 | 1051 | 63.8 |
| Unknown | 1402 | 2.2 | 1055 | 2.1 | 243 | 2.8 | 1058 | 2.1 | 741 | 2.0 | 255 | 2.9 | 385 | 2.3 | 275 | 2.2 | 89 | 2.7 | 2020 | 22.4 | 1406 | 21.4 | 355 | 21.6 |
| Radiotherapy | ||||||||||||||||||||||||
| Yes | 15,326 | 24.0 | 11,659 | 23.0 | 2835 | 32.4 | 37,088 | 753 | 27,840 | 74.7 | 6842 | 76.6 | 9756 | 57.8 | 7348 | 57.6 | 1886 | 57.5 | 1373 | 15.3 | 1002 | 15.3 | 313 | 19.0 |
| No | 39,547 | 62.0 | 31,749 | 62.6 | 4808 | 55.0 | 5881 | 11.9 | 4424 | 11.9 | 1078 | 12.1 | 4982 | 29.5 | 3710 | 29.1 | 1019 | 31.1 | 4667 | 51.8 | 3380 | 51.5 | 846 | 51.4 |
| Unknown | 8882 | 13.9 | 7324 | 14.4 | 1103 | 12.6 | 6270 | 12.7 | 4994 | 13.4 | 1017 | 11.4 | 2148 | 12.7 | 1700 | 13.3 | 374 | 11.4 | 2963 | 32.9 | 2183 | 33.3 | 488 | 29.6 |
| Systemic therapy | ||||||||||||||||||||||||
| Yes | 11,406 | 17.9 | 8771 | 17.3 | 2037 | 233 | 35,062 | 71.2 | 26,744 | 71.8 | 6158 | 68.9 | 10,691 | 63.3 | 8240 | 64.6 | 1891 | 57.7 | 1275 | 14.2 | 967 | 14.7 | 248 | 15.1 |
| No | 46,654 | 73.2 | 37,504 | 73.9 | 5850 | 66.9 | 11,251 | 22.8 | 8423 | 22.6 | 2123 | 23.8 | 5110 | 30.3 | 3740 | 29.3 | 1144 | 34.9 | 4848 | 53.8 | 3560 | 54.2 | 868 | 52.7 |
| Unknown | 5695 | 8.9 | 4457 | 8.8 | 859 | 9.8 | 2926 | 5.9 | 2091 | 5.6 | 656 | 73 | 1085 | 6.4 | 778 | 6.1 | 244 | 7.4 | 2880 | 32.0 | 2038 | 31.0 | 531 | 32.2 |
The bold value is simply to distinguish the percentages from the numbers.
A slightly higher proportion of Black women diagnosed with a regional tumor received radiotherapy (76.6% vs. 74.7% for White women), while a slightly higher proportion of White women received systemic therapy (71.8% vs. 68.9% for Black women). For distant tumors, for which systemic therapy is the standard treatment, there were large differences between White and Black women. For White women diagnosed with distant tumors, 64.6% received systemic therapy, compared with only 57.7% of Black women.
4. Discussion
This study included high-quality data from 41 population-based state registries covering 85% of the US population. Net survival estimates were produced using the same robust methods for each state, and life tables of background mortality that were specific to single year of age, race/ethnicity, county, county-level socioeconomic status and the calendar year of death. The results from this study on cervical cancer survival show a continuation of a slight decline in survival for both Black and White women over time, but it also highlights the continuing need to address the disparity in survival between Black and White women.
The distribution of stage at diagnosis changed over time, with more women diagnosed with regional and distant tumors in 2009–2014 than had been diagnosed at advanced stages in 2001–2003. There has been improvement in the reporting of stage at diagnosis, in that the proportion of tumors of unknown stage decreased by 2.2%. During 2001–2003, most cancer registries in the United States coded SEER Summary Stage 2000 directly from the medical record. However, starting from January 1, 2004, all registries derived SEER Summary Stage 2000 using the Collaborative Staging System [12,16]. However, Black women were diagnosed at more advanced stages than White women, regardless of the calendar period of diagnosis.
For women diagnosed during 2001–2014, age-standardized 5-year net survival from cervical cancer for all races combined remained relatively stable over time, showing a slight decrease from 64% to 62%. There were persistent racial differences in survival for all stages of diagnosis combined and at each specific stage. Survival for Black women was around 7–8% lower than for White women for all stages combined, with little improvement over time.
Stage at diagnosis is an important predictor of survival from cervical cancer, but the unfavorable stage distribution for Black women does not fully account for the differences in survival for all stages combined. The disparity in stage-specific survival between Black and White women appears to have narrowed slightly for localized, regional and tumors of unknown stage, but has widened for distant stage tumors. Despite greater improvements in survival for Black women with localized and regional tumors and tumors of unknown stage than for White women, Black women still have lower stage-specific survival for each stage.
Lower cervical cancer survival for Black women than White women is thus a combination of a higher proportion of tumors that are diagnosed at a more advanced stage and persistently lower survival at each stage of disease.
Disparities in access to treatment for cervical cancer may explain differences in survival between White and Black women. In the US Military Health Care System, a health care system with equal access to care, there was no difference in treatment received, or in survival, between Black and White women [17]. Given the difference in the proportion of White and Black women receiving cancer-directed surgery for localized or regional tumors, lower stage-specific survival for Black women in the US population may be partially explained by lack of optimal treatment. Treatment data may be under-ascertained in cancer registry data, particularly for radiotherapy and, to a lesser extent, systemic therapy [18,19], but the percentage of patients for whom receipt of radiotherapy was unknown was less than 15% overall, and higher for White women. For systemic therapy, the percentage of women with unknown receipt of treatment was less than 10%, but slightly higher for Black women.
While the proportion of women aged 21–65 years screened for cervical cancer in the United States is around 80%, this is still below the Healthy People 2020 target of 93% and there are disparities in screening for racial and ethnic minority groups [6,7]. The proportions of Black and White women screened are similar, however, there are disparities in follow-up treatment for Black women [20]. Screening is recommended every 3 or 5 years, depending on the woman’s age and the screening test used, until age 65 years. Although annual cervical cancer screening was withdrawn as a formal recommendation in 2003 by the USPSTF, it remains a common practice in the United States [21]. Preference for annual screening may deter some women from seeking screening, especially if they are uninsured. Insurance status is an important factor in receiving routine cervical cancer screening, regardless of race/ethnicity [22]. While routine screening is not recommended for women aged 65 years or older who have been screened previously and have had negative results, many older women are not being screened adequately. Given that the incidence rate of cervical cancer generally increases until 85 years, inadequate screening of older women – many of whom may be at higher risk or have no screening history – may contribute to poor cervical cancer survival for older women [23].
Data for women of other racial groups than White and Black were included in the data submissions, and estimates for all races and stages combined, but we could not produce robust survival estimates for non-Black or non-White races or ethnicities individually due to small numbers for women from these groups. Thus, there may be other racial disparities in cervical cancer survival that are unmeasured in this study.
Population-based cancer survival has been used routinely as a measure to assess the health care system’s deficits in managing the cancer burden equitably. Despite similar screening coverage for both Black and White women and improvements in stage-specific survival, Black women continue to have poorer survival than White women. This may be partially explained by inequities in access to adequate treatment. The results from this study highlight the continuing need to address the disparity in cervical cancer survival between White and Black women in the United States. Monitoring and updating the trends in cervical cancer survival by stage and race can inform the development of public health initiatives to eliminate racial disparities and improve cervical cancer survival for all women.
Supplementary Material
HIGHLIGHTS.
Slight decline in cervical cancer survival for both Black and White women.
Black women have poorer survival from cervical cancer than White women.
Black women have lower stage-specific survival than White women.
Acknowledgements
This project was supported by American Cancer Society; Centers for Disease Control and Prevention; Swiss Re; Swiss Cancer Research foundation; Swiss Cancer League; Institut National du Cancer; La Ligue Contre le Cancer; Rossy Family Foundation; US National Cancer Institute; and the Susan G Komen Foundation.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
US CONCORD Working Group
T Freeman, JT George (Alabama Statewide Cancer Registry); RM Avila, DK O’Brien (Alaska Cancer Registry); A Holt (Arkansas Central Cancer Registry); L Almon (Metropolitan Atlanta Registry); S Kwong, C Morris (California State Cancer Registry); R Rycroft (Colorado Central Cancer Registry); L Mueller, CE Phillips (Connecticut Tumor Registry); H Brown, B Cromartie (Delaware Cancer Registry); AG Schwartz, F Vigneau (Metropolitan Detroit Cancer Surveillance System); GM Levin, B Wohler (Florida Cancer Data System); R Bayakly (Georgia Cancer Registry); KC Ward (Georgia Cancer Registry; Metropolitan Atlanta Registry); SL Gomez, M McKinley (Greater Bay Area Cancer Registry); R Cress (Cancer Registry of Greater California); J Davis, B Hernandez (Hawaii Tumor Registry); CJ Johnson (Cancer Data Registry of Idaho); LP Ruppert (Indiana State Cancer Registry); S Bentler, ME Charlton (State Health Registry of Iowa); B Huang, TC Tucker* (Kentucky Cancer Registry); D Deapen, L Liu (Los Angeles Cancer Surveillance Program); MC Hsieh, XC Wu (Louisiana Tumor Registry); M Schwenn (Maine Cancer Registry); K Stern (Maryland Cancer Registry); ST Gershman, RC Knowlton (Massachusetts Cancer Registry); G Alverson, T Weaver (Michigan State Cancer Surveillance Program); J Desai (Minnesota Cancer Reporting System); DB Rogers (Mississippi Cancer Registry); J Jackson-Thompson (Missouri Cancer Registry and Research Center); D Lemons, HJ Zimmerman (Montana Central Tumor Registry); M Hood, J Roberts-Johnson (Nebraska Cancer Registry); CA Geiger, JR Rees (New Hampshire State Cancer Registry); KS Pawlish, A Stroup (New Jersey State Cancer Registry); C Key, C Wiggins (New Mexico Tumor Registry); AR Kahn, MJ Schymura (New York State Cancer Registry); S Radhakrishnan, C Rao (North Carolina Central Cancer Registry); LK Giljahn, RM Slocumb (Ohio Cancer Incidence Surveillance System); A Feld (Oklahoma Central Cancer Registry); KG Aird, T Beran (Oregon State Cancer Registry); JJ Rubertone, SJ Slack (Pennsylvania Cancer Registry); J Oh (Rhode Island Cancer Registry); TA Janes, SM Schwartz (Seattle Cancer Surveillance System); SC Chiodini, DM Hurley (South Carolina Central Cancer Registry); MA Whiteside (Tennessee Cancer Registry); S Rai, MA Williams (Texas Cancer Registry); K Herget, C Sweeney (Utah Cancer Registry); AT Johnson (Vermont Cancer Registry); MB Keitheri Cheteri, P Migliore Santiago (Washington State Cancer Registry); SE Blankenship, S Farley (West Virginia Cancer Registry); R Borchers, R Malicki (Wisconsin Department of Health Services); J Espinoza, J Grandpre (Wyoming Cancer Surveillance Program); HK Weir*, R Wilson (Centers for Disease Control and Prevention); BK Edwards*, A Mariotto (National Cancer Institute). *CONCORD Steering Committee.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2021.08.015.
References
- [1].Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB, Trends in Human Papillomavirus-Associated Cancers United States, 1999–2015, MMWR Morb Mortal Wkly Rep 67 (2018) 918–924, 10.15585/mmwr.mm16733a15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].U.S. Cancer Statistics Working Group, U.S. Cancer Statistics Data Visualizations Tool, based on November 2019 submission data (1999–2017), Available from URL www.cdc.gov/cancer/dataviz.
- [3].Allemani C, Weir HK, Carreira H, et al. , Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2), Lancet. 385 (2015) 977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allemani C, Matsuda T, Di Carlo V, et al. , Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries, Lancet. 391 (2018) 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Curry SJ, Krist AH, Owens DK, et al. , Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement, JAMA. 320 (2018) 674–686. [DOI] [PubMed] [Google Scholar]
- [6].Healthy People 2020, Cancer objectives, Available from URL https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives [accessed 1 October, 2019].
- [7].White A, Thompson TD, White MC, et al. , Cancer Screening Test Use United States, 2015, MMWR Morb. Mortal. Wkly Rep. 66 (2017) 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Benard VB, Watson M, Saraiya M, et al. , Cervical cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study, Cancer. 123 (Suppl. 24) (2017) 5119–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fritz AG, Percy C, Jack A, et al. , International Classification of Diseases for Oncology (ICD-O), World Health Organization, Geneva, 2000. [Google Scholar]
- [10].Allemani C, Harewood R, Johnson CJ, et al. , Population-based cancer survival in the United States: Data, quality control, and statistical methods, Cancer. 123 (2017) 4982–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pohar Perme M, Stare J, Estève J, On estimation in relative survival, Biometrics. 68 (2012) 113–120. [DOI] [PubMed] [Google Scholar]
- [12].Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, SEER Summary Staging Manual 2000: Codes and Coding Instructions. NIH Pub. No. 01–4969, Bethesda, MD, National Cancer Institute, 2001. [Google Scholar]
- [13].Cutler SJ, Ederer F, Maximum utilization of the life table method in analyzing survival, J. Chronic Dis. 8 (1958) 699–712. [DOI] [PubMed] [Google Scholar]
- [14].Esteve J, Benhamou E, Raymond L, Statistical methods in cancer research. Volume IV. Descriptive epidemiology, IARC Sci. Publ. (1994) 1–302. [PubMed] [Google Scholar]
- [15].Corazziari I, Quinn M, Capocaccia R, Standard cancer patient population for age standardising survival ratios, Eur. J. Cancer 40 (2004) 2307–2316. [DOI] [PubMed] [Google Scholar]
- [16].Surveillance E, and End Results Program, Collaborative Stage, Available from URL https://seer.cancer.gov/tools/collabstaging/ [accessed August, 2020].
- [17].Farley JH, Hines JF, Taylor RR, et al. , Equal care ensures equal survival for African-American women with cervical carcinoma, Cancer. 91 (2001) 869–873. [PubMed] [Google Scholar]
- [18].Healy MA, Morris AM, Abrahamse P, Ward KC, Kato I, Veenstra CM, The accuracy of chemotherapy ascertainment among colorectal cancer patients in the surveillance, epidemiology, and end results registry program, BMC Cancer 18 (2018) 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ, Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data, Cancer. 118 (2012) 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W, Adherence to guide-lines for follow-up of low-grade cytologic abnormalities among medically under-served women, Obstet. Gynecol. 105 (2005) 1323–1328. [DOI] [PubMed] [Google Scholar]
- [21].Cooper CP, Saraiya M, Cervical Cancer Screening Intervals Preferred by U.S. Women, Am. J. Prev. Med. 55 (2018) 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heintzman J, Hatch B, Coronado G, et al. , Role of Race/Ethnicity, Language, and Insurance in Use of Cervical Cancer Prevention Services Among Low-Income Hispanic Women, 2009–2013, Prev. Chronic Dis. 15 (2018), E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].White MC, Shoemaker ML, Benard VB, Cervical Cancer Screening and Incidence by Age: Unmet Needs Near and After the Stopping Age for Screening, Am. J. Prev. Med. 53 (2017) 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.