Abstract
Over the past decades, the age of first-time fathers has been steadily increasing due to socio-economic pressures. While general mechanisms of aging are subject to intensive research, male reproductive aging has remained an understudied area, and the effects of increased age on the male reproductive system are still only poorly understood, despite new insights into the potential dire consequences of advanced paternal age for the health of their progeny. There is also growing evidence that reproductive aging is linked to overall health in men, but this review focuses mainly on pathophysiological consequences of old age in men, such as low sperm count and diminished sperm genetic integrity, with an emphasis on mechanisms underlying reproductive aging. The steady decline of NAD levels observed in aging men represents one of the emerging concepts in that regard. Because it offers some mechanistic rationale explaining the effects of old age on the male reproductive system, some of the NAD-dependent functions in male reproduction are briefly outlined in this review. The overview also provides many questions that remain open about the basic science of male reproductive aging.
In brief:
In light of the increasing age of first-time fathers, this article summarizes the current scientific knowledge base on reproductive aging in the male, including sperm quality and health impacts for the offspring. The emerging role of NAD decline in reproductive aging is highlighted.
Introduction
The age of first-time parents in developed countries has significantly risen in recent years, a delay that has been coupled to increased infertility problems, along with growing concern for the long-term health and well-being of children of older parents (Khandwala et al., 2017, 2018; Chan and Robaire, 2022; Eisenberg, 2022; Aitken, 2023). The reproductive system drastically declines with age, with far-reaching implications for an individual’s overall health, fertility and, as recent research suggests, even their children’s health (Kaufman et al., 2019; Frungieri et al., 2021; Matzkin et al., 2021; Meyer and Meyer-Ficca, 2021).
In men, the overall age-related health decline is accompanied by late-onset hypogonadism (LOH), defined by reduced testosterone levels, a key indicator of male health maintenance (Ide 2023), with consequences for somatic health and germ cell production (Zirkin and Tenover, 2012; Golan et al., 2015). Diminished testosterone levels in LOH markedly affect physical, metabolic and mental health, and are associated with sarcopenia, reduced sexual activity, cognitive function and overall vitality (Bhasin et al., 2018; Zitzmann, 2020; Ide, 2023). Low testosterone is also associated with elevated depression scores in elderly men (Seidman et al., 2002), and contributes to deteriorating liver function, lipid metabolism, bone density, and hemoglobin synthesis (Bagatell and Bremner, 1996).
Spermatogenesis depends on appropriately high levels of testicular testosterone, which is synthesized by Leydig cells in the testis, and sufficient numbers of healthy Sertoli cells. Both cell types decrease with increasing age in humans, resulting in impaired spermatogenesis (Mularoni et al., 2020), lowered sperm counts, and worsening semen and sperm quality, including increased sperm morphological defects, DNA damage, and epigenetic alterations (Schmid et al., 2007; Oliveira et al., 2014; Kleshchev et al., 2023). This age-related rise in genetic and epigenetic sperm abnormalities causes increased incidences of pregnancy loss, congenital defects and single gene mutations in humans (reviewed in (Yatsenko and Turek, 2018), with similar observations made in animal models (Serre and Robaire, 1998). Recent research results have made it clear that gamete quality has long-term consequences for offspring health, described by the parental origin of health and disease (POHaD), akin to the Developmental Origin of Health and Disease (DOHaD) concept, which is based on the realization that developmental circumstances during early development and the prenatal phase shape individual long-term health (Caballero-Campo et al., 2018; Batra et al., 2022).
This growing awareness of how important sperm quality is for optimal offspring health contrasts with our still limited understanding of the molecular processes that govern male gamete production in general (Kimmins et al., 2023), and, relevant for our aging society, the aging-associated physiological changes in the male reproductive system. The general aging process is thought to be driven by a set of molecular and cellular changes that occur during aging. These changes, referred to as the “hallmarks of aging”, include decreased genomic stability, mitochondrial function and telomere maintenance, as well as increased epigenetic alterations, deregulated nutrient sensing, and increased inflammation (López-Otín et al., 2023). Deteriorating homeostasis of these processes appears to be the main unifying factor, but specific insights into mechanisms of male reproductive aging are still scarce. The finding that blood and tissue levels of the coenzyme nicotinamide adenine dinucleotide (NAD) become gradually lower as humans age (Massudi et al., 2012) argues that NAD deficiency is one of the hallmarks of aging. Because NAD is involved in many biochemical processes central to tissue and energy homeostasis, insufficient availability of NAD in the aging male is emerging as an important factor in reproductive aging.
This review gives an overview over current insights into age-related changes that occur in males, with a focus on testicular function and fertility, consequences of increasing age for sperm quality, and health risks for offspring of elderly fathers. Some current concepts regarding molecular mechanisms, including those related to diminished NAD availability in aging men, that might be involved in diminished sperm quality and the increase in health problems seen in elderly men and their offspring, are presented.
The Aging Testis
Age-associated reproductive decline is both a species- and gender-specific process (Santiago et al., 2019). In women, this process is associated with menopause, a well-documented phenomenon that results in complete cessation of ovulation. In men, reproductive decline, or andropause, occurs gradually with age with greater individual variation and complete termination of reproductive capacity is rare. Male reproductive aging is not fully understood but appears to be a combination of both structural and molecular alterations in the reproductive system, including changes in the sensitivity of the hypothalamic-pituitary axis, likely due to age-related diseases and/or environmental impacts over time (Choubey et al., 2019; Santiago et al., 2019). The reproductive system is not only needed for species procreation, but the associated sex hormones also contribute to a healthy metabolism and longevity (Wu et al., 2008; Martins Da Silva and Anderson, 2022). Therefore, understanding the underlying mechanisms of testicular aging will contribute to improved reproductive success and offspring health, as well as to the development of therapeutic strategies to attenuate potential age-related diseases in men, as is laid out in the following sections.
Male Hormonal Control and Aging
The hypothalamic-pituitary-testicular (HPT) axis is needed for the maintenance of male development and reproduction, namely steroidogenesis and spermatogenesis, and appearance of secondary sex characteristics (Gunes et al., 2016; Almeida et al., 2017; Martins Da Silva and Anderson, 2022). Briefly, under normal conditions gonadotropin-releasing hormone (GnRH), a key regulator of the reproductive axis, is secreted in pulses from the hypothalamus to the anterior pituitary gland stimulating the release of two gonadotropic hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH), into the bloodstream, which, in turn, regulate gametogenesis in the gonads. LH release induces testosterone synthesis in Leydig cells, whereas FSH causes Sertoli cells to produce androgen binding protein (ABP) and inhibin B, molecules essential for spermatogenesis and establishment of a negative-feedback loop on the HPT axis.
Age-related changes to the HPT axis are complex, multifaceted, and difficult to accurately measure in men (Almeida et al., 2017) (section 1 in Fig. 1). For instance, while there is large individual variation in serum testosterone levels, observational studies have reported that the population mean declines with age even in the absence of disease. This suggests diminished hypothalamic secretion of GnRH, although impossible to directly measure in humans (Wu et al., 2008; Sharma et al., 2015; Almeida et al., 2017; Sokanovic et al., 2018; Kaufman et al., 2019). Testosterone is the major anabolic hormone in men and low testosterone not only negatively affects testicular and reproductive functions but is also associated with mood changes, osteoporosis, and sarcopenia, contributing to reduced quality of life in elderly men. Low serum testosterone is due to reduced synthesis of the steroid hormone by the Leydig cells, even in the presence of the appropriate levels of LH, which is likely a consequence of both HPT axis impairment and decreased Leydig cell number (Wu et al., 2008; Kaufman et al., 2019). Additional factors, including impaired testicular perfusion and reduced testosterone release into the blood stream, further contribute to low serum testosterone levels (Santiago et al., 2019). Obesity is a known confounding factor with significant effects on testosterone levels independent of chronological age. Furthermore, while testosterone levels typically decline with age, levels of estradiol, a testosterone metabolite formed by aromatase activity and the major biologically active estrogen, have been reported to be less affected (Vermeulen et al., 2002). Aromatase activity also increases with age and obesity in males, where research has demonstrated that weight loss in the elderly is associated with a significant decrease in total and free estradiol levels and a corresponding increase in serum testosterone. Increased estradiol levels can also impact the LH feedback loop in males, which, in turn, may directly affect testosterone production by the Leydig cells during aging. Overall, concomitant with low testosterone levels, aging men typically have increased serum levels of LH and FSH and lowered estradiol and inhibin B, indicating an age-related disruption in the HPT axis feedback loop in otherwise healthy individuals (Wu et al., 2008; Sharma et al., 2015; Gunes et al., 2016; Almeida et al., 2017; Kaufman et al., 2019; Santiago et al., 2019).
Figure 1. Impact of advanced age on hormonal control, testes morphology and function, and sperm production.
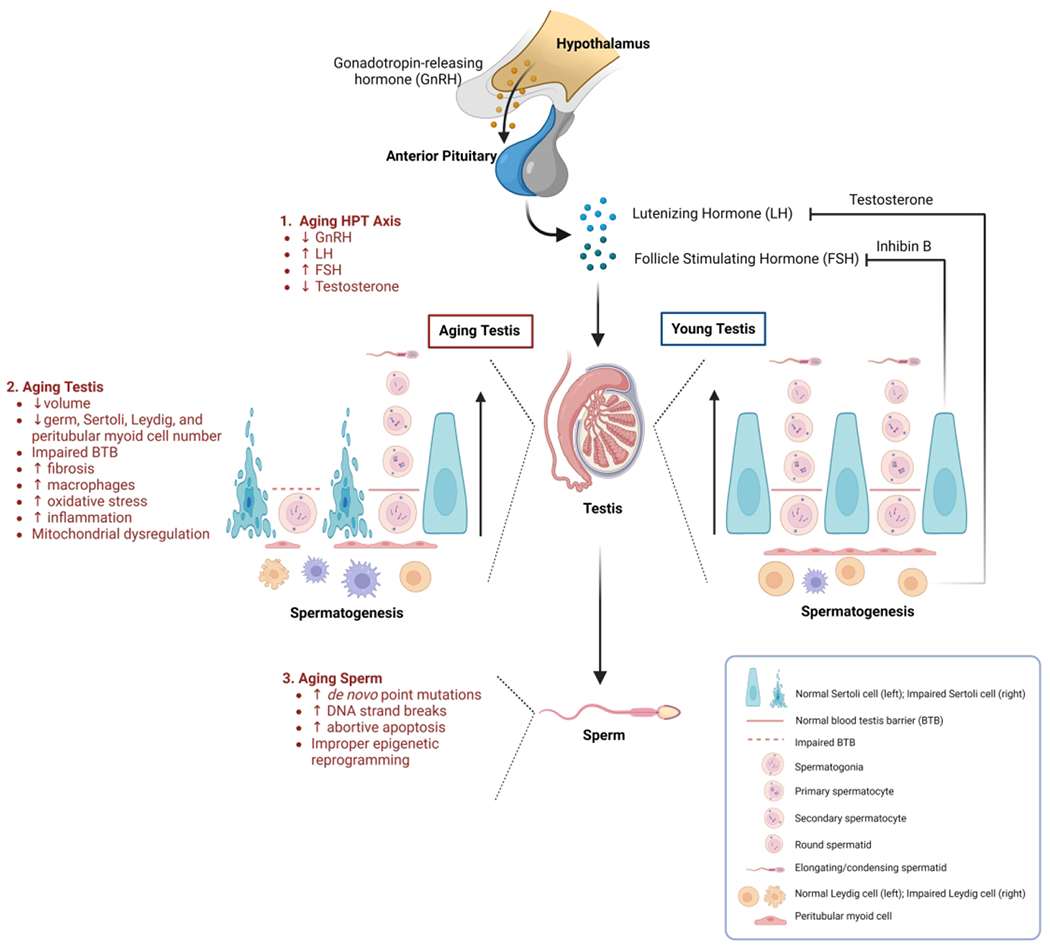
In aging men, the hypothalamic-pituitary-testicular (HPT) axis and associated negative-feedback loop is impaired, resulting in an overall hormonal imbalance and diminished testosterone production compared to younger men (1). Altered testicular structure, morphology, and a disruption in spermatogenesis, accompanied by elevated oxidative stress and inflammation, are characteristic of aging testis (2). Aging sperm can also display severe DNA abnormalities and inadequate epigenetic reprogramming (3).
Additional evidence suggests that hypothalamic functions are regulated, at least in part, through NAD, a coenzyme involved in numerous redox reactions and energy metabolism, as well as sirtuins, which are NAD-consuming deacetylases. The limited availability of NAD with increasing age likely contributes to hypothalamic dysregulation (Roh and Kim, 2020). Recent research demonstrated that in a mouse model that recapitulates body-wide NAD deficiency in humans, young male NAD-deficient mice displayed testicular shrinkage and impaired spermatogonial proliferation and differentiation with reduced sperm counts, similar to that observed in old mice (Meyer-Ficca et al., 2022). When NAD was increased back to young levels, testis weights recovered in the animals and normal spermatogenesis was restored, confirming the importance of NAD in healthy testicular function. This study suggests that low NAD levels may link metabolic changes as a hallmark of aging with declining male fertility. However, further research is required to better understand the complex changes of the HPT axis that are associated with testicular decline in the aging male, and the underlying mechanisms at work.
Spermatogenesis and Aging
The testicular parenchyma comprises highly convoluted seminiferous tubules, in which spermatogenesis occurs, and the surrounding interstitial compartment with Leydig cells, for steroidogenesis, peritubular myoid cells, as a major component of the tubule wall, and further blood vessels with endothelial cells, and immune cells (Kaur et al., 2014; Santiago et al., 2019). The seminiferous epithelium within the tubules contains Sertoli cells and developing germ cells. Tight junctions between adjacent Sertoli cells constitute the blood-testis barrier (BTB) that protects germ cells from the immune system. The germ cells develop in highly organized stratified layers that are formed by consecutive divisions of spermatogonial stem cells (SSC) located at the basement membrane, followed by their differentiation to primary and secondary meiotic cells (spermatocytes). After meiosis, haploid germ cells differentiate from round to elongated spermatids, and finally form testicular spermatozoa in the tubule lumen.
Testicular volume has been reported to get smaller with age in both humans and some rodent models, including mice and rats (Syed and Hecht, 2001; Anjum et al., 2012; Choubey et al., 2019; Santiago et al., 2019; Meyer-Ficca et al., 2022) (section 2 in Fig. 1). Histological and ultrastructural studies in humans have reported age-related aberrations in testicular structure, including abnormal seminiferous tubule size, thickening of the basal membrane, fibrosis, vacuolization of Sertoli cells with diminished inter-Sertoli tight junctions, decreased germ-, Sertoli-, Leydig- and peritubular myoid cell numbers, mitochondrial dysregulation, and atherosclerosis of testicular arteries (Jiang et al., 2014; Kaufman et al., 2019). Such degenerative changes have been attributed to changes in apoptosis and cell proliferation in the aged testis, as well as the breakdown of inter-Sertoli tight junctions suggesting damage to the BTB, which all may contribute to abnormal spermatogenesis (Jiang et al., 2014). Furthermore, steroid hormone alterations during aging have been proposed as a potential cause for these types of damage as well. Recently, Pohl et al. evaluated the age-related effects on spermatogenesis, spermatogonial dynamics, and Sertoli cells in a cohort of men with normal spermatogenesis. They described similar tubular structures and subpopulations of undifferentiated spermatogonia across age groups, but found an increased proportion of proliferating spermatogonia in older men (Pohl et al., 2019). This increased spermatogonial proliferation led to decreased spermatogenic efficiency, with fewer post-meiotic cells (round spermatids) being produced per active spermatogonium. Additionally, changes in the nucleoli of Sertoli cells were evaluated as an indicator of cellular metabolic aging. Here, the size of the nuclei and nucleoli in Sertoli cells, as well as the number of nucleoli per Sertoli cell nucleus, increased with age, potentially indicating altered protein synthesis rates. Overall, these age-related changes in germ cell dynamics and Sertoli cells are consistent with stem cell exhaustion and altered stress responses.
In addition to forming the BTB, Sertoli cells are crucial for spermatogenesis through the secretion of transport proteins and regulatory factors (Syed and Hecht, 2001). In aging testes, the expression of various Sertoli cell proteins declines, coinciding with fewer spermatids associated with Sertoli cells. Thus, the loss of germ cells during aging could be due to changes in the Sertoli cells’ ability to support germ cell development or to intrinsic properties of aging germ cells. Efforts to evaluate the effects of aging Sertoli cells on spermatogenesis in Brown Norway rats revealed a selective loss of gene expression in germ cells and Sertoli cells in regressed testes, specifically a decline in the expression of von Ebner’s-like protein and serotonin receptor protein, suggesting that cellular signaling breakdown contributes to impaired spermatogenesis and testicular regression (Syed and Hecht, 2001).
There is an interrelationship between changes in tight junctions, the p38 mitogen activated protein kinase (MAPK) / Matrix metalloproteinase 9 (MMP9) pathway, and autophagy in Sertoli cells during aging. This has been investigated in male Sprague-Dawley rats, where researchers found that the structure and function of tight junctions progressively degenerate with age leading to spermatogenesis disorders, likely due to activation of the p38 MAPK/MMP9 pathway in Sertoli cells (Ma et al., 2022). Activation of the p38 MAPK pathway can disrupt the tight junction barrier and decrease the expression of BTB proteins that are important for normal spermatogenesis (Ma et al., 2022). MMP9, a tissue-degrading protease, has also been associated with the degradation of tight junction proteins and its expression and activity can be activated by the p38 MAPK pathway. Autophagy is known as the process of degrading cellular materials in lysosomes, which can serve as a protective mechanism by removing damaged organelles and providing energy but may also contribute to cell damage if overactivated (Madeo et al., 2010; Matzkin et al., 2021; Wang et al., 2022). Functional autophagy has been shown to improve cell survival and fitness, while removal or silencing of essential autophagy genes often leads to cell death. Furthermore, reduced autophagy has been found to be associated with accelerated aging, and aging-related diseases, while enhanced autophagy is involved in protecting against the aging process. In the testis, autophagy is an important catabolic pathway involved in various physiological processes and spermatogenesis, including cell residual bodies disposal, regulation of testosterone synthesis, structural reconstruction, growth, and development (reviewed in Ma et al., 2018; Zhu et al., 2019; Wang et al., 2022; Yan et al., 2022; Raee et al., 2023). Specifically, autophagy in Sertoli cells is essential for normal spermatogenesis and its impairment has been linked to disrupted BTB integrity. Additionally, Ma et al., showed that autophagy was also impaired in Sertoli cells with increasing age, as evident by decreased numbers of autophagic vesicles in the cytoplasm, accompanied by vacuoles, a swollen endoplasmic reticulum, and disordered mitochondria (Ma et al., 2022). These results suggest that the disruption of tight junctions, through activation of the p38 MAPK/MMP9 pathway and diminished autophagy in Sertoli cells, contribute to degeneration of spermatogenesis during aging. Overall, these findings implicate age-associated morphological and structural deterioration of the BTB in testicular aging and shed light on the mechanisms underlying the aging-related decline in spermatogenesis and testicular function.
Testicular Metabolism and Aging
Recent reports have elucidated testicular metabolome changes that occur with aging and declining reproductive potential (Jarak et al., 2018; Choubey et al., 2019; Meyer-Ficca et al., 2022; Fang et al., 2023). Using a nuclear magnetic resonance-based metabolomics approach, testicular metabolic profiles of 3 to 24-month-old Wistar rats revealed age-associated decreases in antioxidant-defense-related metabolites that are normally important for maintaining the spermatogenesis microenvironment by limiting oxidative stress, specifically betaine, creatine, and glutathione, in addition to changes in amino acid, nucleotide, and phospholipid synthesis (Jarak et al., 2018). The expression levels of lactate dehydrogenase (LDH) and monocarboxylate transporter also decreased in the testis of older rats, indicating age-related changes in lactate metabolism and potential lactate accumulation in Sertoli cells with restricted availability to developing germ cells.
Furthermore, adiponectin, an adiposity hormone that is involved in regulating spermatogenesis, steroidogenesis, and maintaining insulin sensitivity in the testis, along with expression of its two receptors, AdipoRl and AdipoR2, was found to be significantly decreased in the testis of aged (> 65-week-old) mice (Choubey et al., 2019). The reduction of adiponectin/adiponectin-receptor numbers was also associated with a significant decline in testicular mass, testosterone synthesis and insulin receptor expression, potentially leading to metabolic aberrations, while exogenous adiponectin administration enhanced glucose transport, increased insulin sensitivity, and reduced oxidative stress, improving both testicular function and stimulation of spermatogenesis. The findings suggest that adiponectin plays a role in normal testicular function and that decreased adiponectin may contribute to impaired spermatogenesis during aging.
Impaired steroidogenesis seems to correlate with reduced testicular glucose concentrations in aging mice (Anjum et al., 2012). Interestingly, testosterone synthesis was not significantly impacted using an Acquired Niacin-Deficiency (ANDY) mouse model, where removal of niacin from the diet results in body-wide NAD-deficiency that leads to impaired spermatogenesis reminiscent of premature aging over time (Meyer-Ficca et al., 2022). However, the levels of retinol, a precursor molecule for retinoic acid (RA) synthesis that is essential for spermatogonial proliferation and differentiation, were significantly elevated in young NAD-deficient mice and in old mice, in comparative metabolomics analyses. Under normal conditions, retinol is first oxidized to retinal by retinol dehydrogenase (RDH10) and then to RA by aldehyde dehydrogenases (ALDHA1/2/3), all of which are NAD-dependent enzymes. Here, testicular retinol and NAD concentrations were highly significantly inversely correlated, suggesting that the observed testicular decline in young ANDY and old mice may, at least in part, be caused by an impaired rate-limiting step in the RA synthesis pathway due to insufficient NAD availability.
The testicular interstitial fluid (TIF) surrounding the seminiferous tubules contains important proteins and hormones that contribute to testicular cell regulation (Han et al., 2023). Using a mouse model, age-related changes in the testicular microenvironment were reported to also be reflected in the molecular profiles of the TIF (Li et al., 2021; Han et al., 2023). Specifically, evaluation of the TIF proteome of young (3-month-old), middle-aged (13-month-old), and old (18-month-old) mice using metabolomics techniques revealed age-related differentially expressed proteins (DEPs) associated with actin cytoskeleton organization, intrinsic apoptotic signaling pathways, and regulation of protein transport, among others (Li et al., 2021). Additionally, multi-omics analysis of transcripts in TIF-exosomes integrated with metabolomics of whole TIF of young (3-month-old) and aged (21-month-old) mice identified several age-related differentially expressed genes (DEGs). Gene ontology (GO) analysis of DEGs revealed primarily age-related changes in oxidative stress and metabolism (including “oxidation-reduction”, “response to oxidative stress”, “tricarboxylic acid cycle”, “fatty acid beta-oxidation”) (Han et al., 2023). Finally, metabolomics analysis of TIF in aged compared to young mice demonstrated significantly enriched pathways including purine and histidine metabolism, pantothenate and coenzyme A biosynthesis, as well as arachidonic acid metabolism. Taken together, these findings suggest that metabolic changes play a significant role in the aging process with key affected processes related to decreased energy metabolism, impaired antioxidant capacity, dysregulation in lipid metabolism, and increased oxidative stress.
Transcriptional Changes in the Aging Testis Implicate Increased Inflammation
Whole-transcriptome RNA-sequencing (RNA-seq) analysis has been used to profile potential transcriptomic changes underlying male reproductive aging (Han et al., 2021; Huang et al., 2022a). When testicular expression patterns of mRNAs and long-noncoding RNAs (lncRNAs) were evaluated in 3-, 6-, 12- and 18-month-old mice, modest age-related transcriptional changes were observed (Han et al., 2021). Notably, genes related to steroid hormone synthesis by Leydig cells in the testis, including Cyp17a1, Cyp11a1, and Klk1b27, were affected by age, which in turn could affect gene regulation in male germ cells. Additionally, gene enrichment analyses showed that upregulated DEGs in aged (21-month-old) compared to young (3-month-old) mouse testes were enriched for functions related to immune response activity, most notably “positive regulation of cytokine production”, and identified the immune-associated gene Pla2g2d as a candidate biomarker for male reproductive aging (Huang et al., 2022a).
A healthy pool of proliferating SSCs is essential for male fertility and diminishing numbers of SSCs have been observed during aging (Dong et al., 2022). When age-dependent changes in SSC function were evaluated in mice, aged (15-month-old) undifferentiated spermatogonia displayed aberrant expression of meiosis-related genes, possibly mediated by Taf7l, compared to young (6-month-old) undifferentiated spermatogonia (Liao et al., 2021). Additionally, Ddit4, which mainly functions to inhibit the mammalian target of the rapamycin (mTOR) pathway that is important for regulating proper stem cell function, was enriched in aged undifferentiated spermatogonia, potentially serving as a direct link between SSC deterioration and aging. Besides SSCs, round spermatids are also particularly vulnerable to errors and de novo mutations with advanced age. Using a Brown Norway rat model, researchers investigated gene expression dysregulation in round spermatids in young (4-6 months) and old (18-20 months) male rats using bulk RNA-seq (Fice and Robaire, 2023). They found that age-related DEGs included genes involved in sperm-egg fusion, embryonic development, folate metabolism, spermatogenesis progression, and sperm motility. GO analysis showed that enriched pathways in aged rats related to oxidoreductase activity and immune signaling, suggesting age-dependent disruptions in cellular inflammation and immune responses in the reproductive system.
Advancements in RNA-sequencing technologies have allowed for the interrogation of age-associated testicular decline at the single-cell and single-nucleus level. Development of a single-cell transcriptomic atlas of old (24-month) compared to young (2-month) mouse testis uncovered that age-related DEGs correlated to an imbalance of differentiated and undifferentiated SSCs, as well as an increase in the number of proinflammatory macrophages. Specifically, these were subtype3 macrophages that are involved in mononuclear cell proliferation and T cell activation in the inflammatory microenvironment in the aged testis (Zhang et al., 2023). The study also highlights increased production of reactive oxygen species (ROS) and the presence of oxidative stress markers in the aged testis, which may contribute to apoptosis and compromised steroidogenesis likely through dampening of the Nrf2 pathway that primarily involves cellular antioxidant defense (Zhao et al., 2021). These results coincide with the oxidation-inflammatory theory of aging, or “inflammaging”, including testicular aging, where there is an imbalance between pro-inflammatory (i.e., Cyclooxygenase-2 (COX2), prostaglandin D2 (PGD2), NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α)) and anti-inflammatory molecules (i.e., Superoxide dismutase (Sod), Catalase, glutathione peroxidase 1 (Gpx1), peroxiredoxin 4 (Prx4), glutathione s-transferase mu 5 (Gstm5), and glutathione), which is reviewed in more detail elsewhere in the literature (Frungieri et al., 2018; Matzkin et al., 2021).
Using a non-human primate model, Huang et al., established single-nucleus transcriptomic profiles revealing that Sertoli cells were the testis cell type most susceptible to aging, followed by immature Leydig cells and peritubular myoid cells (Huang et al., 2022b). Specifically, these authors reported dysregulation of genes encoding tight junction components and downregulation of androgen receptor (AR) expression in Sertoli cells, suggesting disruption of the BTB and compromised sex hormone responses in aged testis. This corroborates earlier observations of decreases in BTB function in aging rats that used different STA-Put purified cells and microarrays (Paul and Robaire, 2013). Notably, Wilms’ Tumor 1 (WT1), a transcription factor essential for normal Sertoli cell function, was also found to be downregulated in an age-associated manner, leading to the proposal that WT1 silencing triggers accelerated senescence in Sertoli cells. In humans, single-cell RNA-seq of young (17-22 years) and old (62-76 years) cadaver-derived testis tissues displayed age-related dysregulation of spermatogenesis, Sertoli cell metabolism, testosterone production by Leydig cells, and apoptosis in peritubular cells, with exacerbated results in elderly men with a body mass index (BMI) >30 (Nie et al., 2022). In summary, these findings suggest that molecular mechanisms underlying testicular aging include disruptions in cellular metabolism, increased oxidative stress, and inflammation, which are all considered hallmarks of aging (López-Otín et al., 2023).
The Role of NAD in the Aging Process
A number of metabolic, redox, and antioxidant pathways involve NAD as an essential coenzyme, along with its reduced form, NADH. NAD and NADH levels decrease with age (Johnson and Imai, 2018; Strømland et al., 2021). In addition to its roles in cellular metabolism and redox reactions, NAD is also an important substrate that is cleaved by a diverse group of enzymes, including poly(ADP-ribose) polymerases (PARPs) and sirtuins, involved in several aspects of cellular homeostasis. CD38 is an NADase and cyclic ADP-ribose synthase expressed mostly on the surface of immune cells where it has immunomodulatory functions (Hogan et al., 2019). PARP enzymes are multifunctional enzymes involved in many processes including DNA repair, the maintenance of genomic stability and telomere function (Beneke and Bürkle, 2007). Sirtuins are NAD-dependent histone deacetylases and ADP-ribose transferases with known roles in the aging process (Houtkooper et al., 2012). Why NAD levels become lower as humans age is not fully understood, but recent research indicates that increased activity of NAD-consuming enzymes, such CD38, deplete NAD levels as individuals age (Malavasi et al., 2008; Chini et al., 2020). Both, CD38 and PARP enzymes are involved in inflammation, oxidative stress management, DNA repair, and other processes associated with aging, and their elevated activity is in line with the general concept of “inflammaging”. A further link between NAD levels and cellular aging is the bidirectional relationship between NAD and autophagy, with autophagy being regulated by activity of NADases, and autophagy in turn preserving NAD levels (Wilson et al., 2023). Given this wide range of molecular connections, a decline in NAD has far-reaching consequences for mitochondrial function, inflammation, autophagy, epigenetic regulation, and DNA maintenance, among others, which are known hallmarks of aging (Amjad et al., 2021; Covarrubias et al., 2021; Navas and Carnero, 2021; Stock and Liu, 2021; López-Otín et al., 2023; Chini et al., 2024) (Fig. 2a). Multiple organs experience an age-dependent NAD decline (Fang et al., 2017), including the testis (Meyer-Ficca et al., 2022), and changes consistent with hallmarks of aging have been described in male reproductive and testicular aging (see Fig. 2b). That spermatogenesis fails in NAD-deficient mice provides supportive evidence of that hypothesis (Meyer-Ficca et al., 2022). Some functions of NAD-dependent enzymes and processes in male reproduction are outlined in this review, as well as elsewhere in the literature (Quesada et al., 1996; Lankenau et al., 1999; Dantzer et al., 2006; Coussens et al., 2008; Meyer-Ficca et al., 2011a, b, 2015, 2022; Celik-Ozenci and Tasatargil, 2013; Ihara et al., 2014; Rato et al., 2016; Meyer and Meyer-Ficca, 2021; Matoba et al., 2024), but more research is needed to elucidate the molecular links and roles of NAD-dependent processes in male reproductive aging in more detail.
Figure 2. Hallmarks of aging in the context of male reproductive aging.
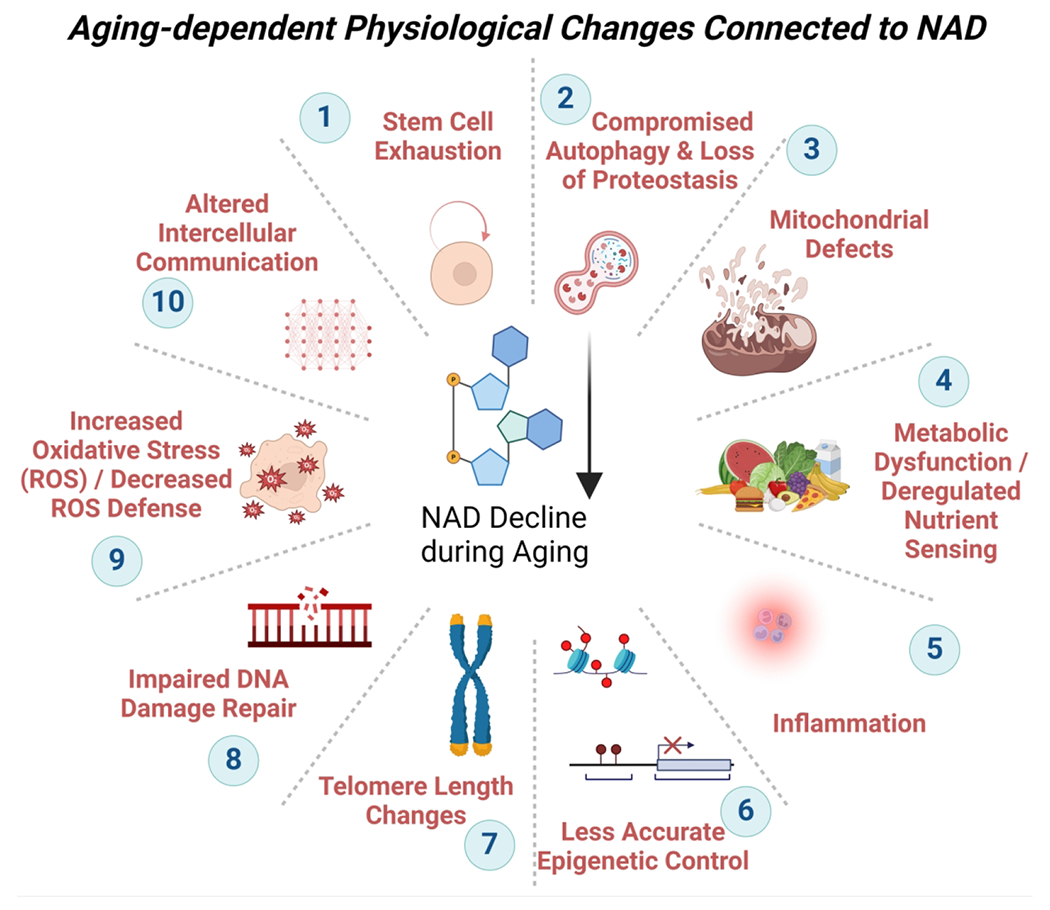
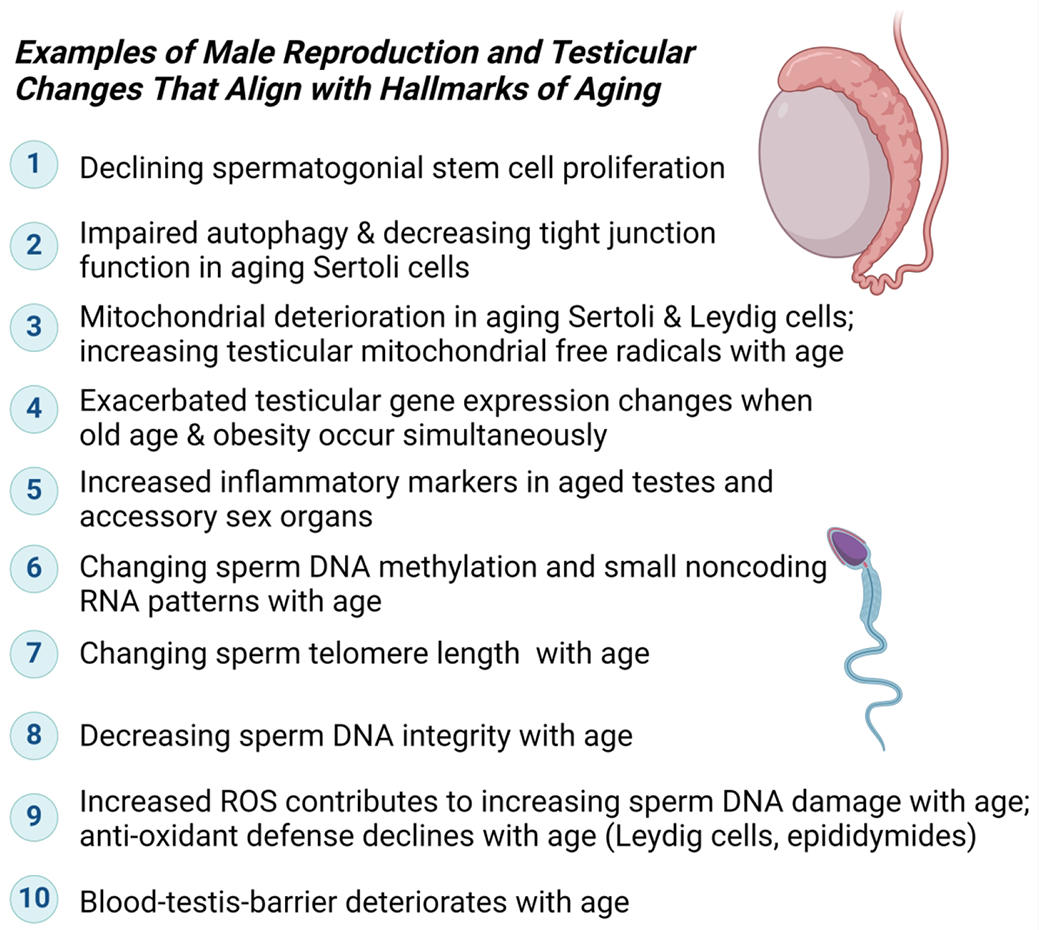
a. NAD is central to most metabolic processes, and many physiological changes during aging are intricately connected to NAD-dependent enzymes and adequate NAD levels (for a recent overview see (Lautrup et al., 2024). b. Some examples of research reports and reviews connecting individual hallmarks to male reproductive aging are listed: (1) Declining stem cell proliferation (Ryu et al., 2006; Zhang et al., 2006; Ozawa et al., 2023); (2) Compromised autophagy (Zhao et al., 2019; Ma et al., 2022; Nie et al., 2022; Wang et al., 2022; Raee et al., 2023); (3) mitochondrial defects (Paniagua et al., 1991; Sokanovic et al., 2018; Garza et al., 2022); (4) metabolic dysfunction (Kanatsu-Shinohara et al., 2019; Nie et al., 2022); (5) inflammation (Frungieri et al., 2018; Matzkin et al., 2021; Nie et al., 2022; Aitken, 2023);(6) loss of epigenetic control (Jenkins et al., 2014; Suvorov et al., 2020; Pilsner et al., 2022; Ashapkin et al., 2023; Bernhardt et al., 2023); (7) telomere length variation (Eisenberg and Kuzawa, 2018; Fice and Robaire, 2019; Amir et al., 2020; Aitken, 2023); (8) impaired DNA damage repair (Singh et al., 2003; Albani et al., 2019; Gonzalez et al., 2022); (9) increased reactive oxygen species and impaired ROS defense (Mueller et al., 1998; Nguyen-Powanda and Robaire, 2020); (10) altered intracellular communication and loss of blood-testis-barrier (Paniagua et al., 1991; Levy et al., 1999; Ma et al., 2022). Note that individual hallmarks are frequently interconnected to each other. Some consequences of NAD decline and / or absence of NAD-dependent enzymes for testicular and male reproductive health have been described (Dantzer et al., 2006; Meyer-Ficca et al., 2011a, 2015, 2022; Ihara et al., 2014; Chung et al., 2021), but more studies are needed to establish individual molecular links and their roles during aging.
Sperm Quality and Aging
The consequences of advanced parental age on reproductive outcome and effects on offspring have traditionally been studied with a strong focus on maternal age. More recently, that has changed to include more research on the effects of paternal age on reproduction and offspring health as well. Paternal age has been implicated in various adverse reproductive outcomes, including elevated frequency of spontaneous pregnancy loss, reduced fertility, decreased semen quality, and a noticeable increased incidence of autosomal dominant diseases, such as achondroplasia and Apert syndrome, as well as of complex syndromes, like autism (Wyrobek et al., 2006; Sharma et al., 2015). Furthermore, at the molecular level, male age has been associated with a rising frequency of spermatozoa with functional defects, including low mitochondrial potential, abnormal chromatin compaction, increased lipid peroxidation, and associated markers of sperm apoptosis (Condorelli et al., 2020). Cohort studies in men indicate an age-related reduction in overall sperm quality (reviewed in Yatsenko and Turek, 2018), including increased frequency of aneuploidies, chromosomal structural aberrations, including duplications, inversions, and translocations, along with elevated DNA fragmentation and de novo point mutations (Martin and Rademaker, 1987; Lowe et al., 2001; Sloter et al., 2007; Yatsenko and Turek, 2018). Of note, the majority of chromosomal abnormalities in sperm are structural aberrations with some increased incidence in XY-chromosome non-disjunctions, while autosomal aneuploidies are far more common in oocytes (Martin, 2008).
Selfish Spermatogonial Selection Hypothesis and Sperm De Novo Mutations
The “selfish spermatogonial selection” hypothesis describes a process by which mutations in SSCs, important for normal spermatogenesis, dysregulate critical cell cycle pathways, such as the rat sarcoma (RAS) pathway. In this model, proliferation of mutation-carrying SSCs is favored and results in the expansion of mutant clonal lines within the testes (Goriely et al., 2013), which, over time, will increasingly skew the mutational profile of sperm, resulting in an enrichment of de novo mutations in the offspring of older fathers. On average, SSCs divide by mitosis every 16 days to sustain ongoing normal sperm production. For example, this means that SSCs in a 20-year-old male will have undergone less than 150 replications, while those of a 50-year-old male have divided more than 840 times. Whole genome sequencing of parents and their offspring confirmed that approximately 80% of de novo mutations are linked to paternal origin. An accumulation of about two point mutations per year were identified, resulting in a doubling of paternally-derived mutations every 16.5 years, aligning with the “selfish spermatogonial selection” hypothesis (Goriely et al., 2013; Janecka et al., 2017).
Sperm Morphology and Aging
Sperm morphology results from spermatid to spermatozoa differentiation and comprises the development of the flagellum and the acrosome, for proper motility and oocyte fertilization, respectively. While there are various distinct, species-specific sperm head morphologies, intra-species head morphology displays minute variation to ensure successful oocyte fertilization (Teves and Roldan, 2022). Several studies have documented evidence for a correlation between advanced male age (>30-40 years old) and increased frequency of abnormal sperm morphology, including head shape (Bujan et al., 1988; Van Den Hoven et al., 2015; Kumar et al., 2017; Ulubay et al., 2022; Kleshchev et al., 2023). Other studies do not find a clear correlation between increasing age and morphological decline (Chen et al., 2022), and describe additional factors that are independent of male age like abstinence time and environmental exposures that might contribute to observed abnormal sperm morphology (Auger et al., 1995; Andolz et al., 1999). Clearly, more research will be necessary to better understand these effects and to reconcile conflicting results.
Aging Correlates with Increasing Sperm Mutations and DNA Strand Breaks
It is well documented that several sperm and semen factors are affected by age (section 3, Fig. 1). For example, the sperm DNA fragmentation index (DFI) markedly increases with male age, while semen volume, sperm concentration, and progressive motility all decline (Wyrobek et al., 2006; Chen et al., 2022; Gonzalez et al., 2022). Furthermore, advanced age is a driver of single-nucleotide and insertion-deletions mutations transmitted through the male germline (reviewed in Aitken and Lewis, 2023). Potentially compounding the problem, normal apoptosis during spermatogenesis decreases with age, possibly indicating a loss of “sperm quality control” and reduced ability to remove damaged sperm. Specifically, Singh et al. found reduced spermatid apoptosis in combination with significantly higher numbers of sperm DNA breaks in men aged 36-57 years old compared to 20-35 years old (Singh et al., 2003). Such “abortive apoptosis” may result in persistent immature or abnormal sperm with damaged or poorly condensed chromatin (Sharma et al., 2015).
Origin of Sperm DNA Breaks
During spermatogenesis, DNA repair mechanisms promote sperm DNA integrity, including mitotic division of SSCs, meiotic recombination in spermatocytes, and during post-meiotic nuclear condensation of spermatid nuclei. The latter two processes are germline-specific and require the formation of transient physiological endogenous DNA strand breaks (Smith and Haaf, 1998; Meyer et al., 2017). The origin of persistent DNA damage in sperm is still unclear, but likely includes one or several causes related to abortive apoptosis that failed to eliminate damaged spermatocytes and spermatids, unrepaired DNA strand breaks resulting from improper chromatin condensation during spermatid differentiation, and DNA damage from exposure to damaging agents like ROS. During spermiogenesis, (O’Donnell, 2014), the haploid genome must be tightly packaged into the future sperm head, which is 6-7 times more condensed than the somatic nucleus (Champroux et al., 2016). To achieve this extraordinary compaction, most somatic histones are removed from the condensing spermatid nucleus and replaced with small protamines in a complex process that also facilitates relaxation of supercoiled DNA associated with the protamine-based chromatin structure of the mature sperm (reviewed in (Moritz and Hammoud, 2022)). Normal mature sperm only retain about 1% of histones in mice and 10-15% in humans. The process of spermatid chromatin condensation requires the formation of transient DNA strand breaks, which is at least in part mediated by topoisomerase 2 beta (TOP2B) (Leduc et al., 2008). The efficient repair of DNA strand breaks requires the activity of PARP enzymes to form poly(ADP-ribose) from NAD, including during TOP2B-mediated spermatid DNA relaxation (Meyer-Ficca et al., 2011b). Interference with the tightly controlled process of spermatid nuclear remodeling impairs spermatid condensation and results in abnormally increased retention of histones in the sperm nucleus (Meyer-Ficca et al., 2011a, b). Low tissue NAD levels associated with increased paternal age may therefore provide a plausible mechanistic explanation for the increase in poorly condensed chromatin in sperm of older men. Furthermore, altered ratios of protamines and improper incorporation of protamines are associated with fertility defects (Lismer and Kimmins, 2023).
The tightly condensed sperm genome is transcriptionally inactive and relatively well protected from DNA damage (Singh et al., 2003). Despite the tightly controlled chromatin condensation mechanisms during spermiogenesis described above, DNA damage can persist in mature sperm. As post-meiotic haploid cells, spermatids cannot use DNA repair mechanisms that rely on DNA recombination (Singh et al., 2003). The age-related increase in abortive apoptosis thus permits more sperm with persistent DNA damage and poor chromatin condensation to persist (Singh et al., 2003).
DNA damage in sperm may also result from increased exposure to ROS and resulting oxidative DNA damage. Elevated ROS can originate endogenously, for example from dysfunctional mitochondria, or by exposure to external ROS sources. Mitochondrial dysfunction with increased ROS production is a known hallmark of general aging (Amorim et al., 2022) and testicular aging (Nguyen-Powanda and Robaire, 2020). Indeed, a large portion of sperm DNA fragmentation appears to stem from oxidative stress (Sharma et al., 2015). This is of interest since levels of oxidative stress adducts were elevated in sperm of a large proportion of infertile men, together with an age-related trend to increased oxidative stress adducts and DFI (Vaughan et al., 2020).
Oxidative stress results from an imbalance between ROS and cellular antioxidant capacity. It can either directly damage the DNA or cause indirect damage, e.g. when hydroxyl radicals induce DNA lesions which, in turn, activate sperm caspases and endonucleases that cause DNA double-strand breaks (Sakkas and Alvarez, 2010). Exposure to oxidative stressors can further damage the sperm plasma membrane and initiate lipid peroxidation leading to loss of sperm plasma membrane integrity and resulting in decreased sperm motility, premature acrosome reaction and decreased ability to interact with and penetrate an oocyte (Vaughan et al., 2020). Seminal fluid contains antioxidants, and lower semen antioxidant capacity correlates with infertility (Mahfouz et al., 2009; Pahune, 2013). With increasing age, the trend to increased levels of oxidative stress adducts (Vaughan et al., 2020) is not compensated by an increase in antioxidant enzymes levels, such as superoxide dismutase, peroxidases and catalase (Bibi et al., 2023), potentially resulting in an imbalance in oxidative stress exposure and antioxidant defense, leading to increasing levels of oxidative stress adducts, and possibly an increased DFI with increasing age (Vaughan et al., 2020).
Increased Paternally-Derived De Novo Mutation Rate due to Diminishing DNA Repair Capacity in Aging Fertilized Oocytes
Between fertilization and the initiation of zygotic S-phase, only some DNA damage in the paternal genome can be repaired in the zygote by the oocyte-derived DNA repair machinery (Aitken, 2022). Specifically, oxidative damage in the form of 8-hydroxyguanosine (8-OHdG) lesions can escape repair due to insufficient amounts of the maternally derived base excision repair enzyme 8-oxoguanine DNA glycosylase (OGG1). Since S-phase can still be initiated, such unrepaired DNA can persist (Lord and Aitken, 2015). The ability of the oocyte to repair damaged DNA from fertilizing sperm depends on the extent of damage, but maternal age may also play a role (Horta et al., 2020). For instance, decreased DNA repair efficiency of the oocyte with age has been observed in mice undergoing in vitro fertilization (IVF) and in humans undergoing intracytoplasmic sperm injection (ICSI) suggesting that the oocytes’ DNA repair capacity may be compromised (Horta et al., 2020; Setti et al., 2021). Diminished DNA repair by the oocyte, coupled with a lack of DNA repair in mature sperm, can lead to persistent de novo mutations in the F1 generation, which is referred to as the post-meiotic oocyte collusion hypothesis (Aitken, 2022).
Age-Related Changes in the Sperm Epigenome
In addition to the paternal genome, sperm also transmit distinct paternal epigenetic marks to their offspring. The collective sperm epigenome is encoded by molecular mechanisms, such as DNA methylation patterns, position and posttranslational marks on the small percentage of histones that remain in mature sperm, as well as non-coding RNAs (Dahlen et al., 2023). This sperm epigenome is sensitive to the father’s physical environment and can be modified over time by factors like diet and external environmental exposures (Grover and Jenkins, 2020).
Sperm DNA Methylation
Age-related changes to the epigenome, especially the DNA methylome, are being explored as indicators of biological age, so-called epigenetic clocks (Horvath and Raj, 2018; Duan et al., 2022). Male germ cells undergo epigenetic reprogramming during their differentiation to sperm, and have similar age-dependent DNA methylation changes in sperm CpG islands, likely from age-related methylation errors in spermatogonia (Yatsenko and Turek, 2018; Pilsner et al., 2022). Akin to the somatic epigenetic clock concept, correlations between the chronological age of males and their sperm DNA methylome were used to develop a sperm epigenetic clock (Pilsner et al., 2022). Like chronological age, higher biological sperm methylome age was associated with increased time to pregnancy, indicating reduced fecundity in older fathers. Age-related changes in sperm methylation patterns include regions of hypermethylated CpG islands, frequently in the distal gene regions, and hypomethylated CpG islands near gene transcription start sites (Cao et al., 2020). It has been proposed that 20-80% of CpG sites are ‘dynamic CpGs’, which are sensitive to environmental exposures (Lismer and Kimmins, 2023). Environmental exposures can change the epigenome, and the overall sum of exposures increases with age, likely contributing to the observed changed in DNA methylation patterns. Since DNA methylation marks are mitotically stable, methylation changes can persist and accumulate over a male’s lifespan (Janecka et al. 2017). Epigenetic reprogramming after fertilization removes most of these DNA methylation marks, but some marks from the paternal germline, including imprinted genes, are maintained in the developing embryo where they can interfere with normal development and result an increased risk of offspring disorders (Janecka et al. 2017).
Sperm Histones
In addition to DNA methylation, histones that are retained in sperm provide epigenetic information encoded by their amount, specific positions within the genome, and posttranslational modifications (PTMs). Histones tend to remain, for example, in genes which are highly transcribed during spermatogenesis and are often enriched in genes with functional importance for early embryonic gene expression. Histone PTMs poise such genes for appropriate expression needed for successful embryonic development (Arpanahi et al., 2009; Hammoud et al., 2009; Miller et al., 2010; Jenkins and Carrell, 2011; Erkek et al., 2013; Ihara et al., 2014). A fraction of sperm histones remain at intergenic and repeat-rich regions like pericentromeric chromosome regions, and are therefore likely important for sperm nuclear architecture and condensation (Meyer-Ficca et al., 2013; Carone et al., 2014; Samans et al., 2014). The extent to which increasing paternal age influences the sperm’s histone code is not yet well understood. Some studies show that aging changes the patterns of testicular histone markings (Xie et al., 2018; Tatehana et al., 2020). For example, Tatehana et al. described an age-related loss of both permanent repressive (H3K9me3) and activating marks (H3K4me2), with increased temporary repressive signaling marks (H3K27me2/3) in testicular germ cells, including elongating spermatids. Such age-related changes in histone modifications could change embryonic gene expression and offspring development if able to persist in sperm and are transmitted to the embryo.
Sperm Noncoding RNAs
Noncoding RNAs (ncRNAs), including microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), and long noncoding RNAs (IncRNAs), represent another complex layer of sperm epigenetic information. Besides their roles in testicular function and male fertility (Joshi and Rajender, 2020), sperm-borne ncRNAs are important for early embryonic development (Conine et al., 2018; Alves et al., 2019), and sperm-borne miRNA signatures are currently being discussed as predictors of male fertilization potential (Alves et al., 2020). The composition of sperm ncRNAs varies between immature testicular sperm and mature sperm, since sperm take up epididymis-derived ncRNA-containing exosomes during the epidydimal transit (Sharma et al., 2018), which provides a means of adjusting sperm ncRNAs to environmental factors like stress. A connection between aging and changes in sperm ncRNA content is recently emerging. In normal fertile men, aging changed sperm miRNAs that target genes in pathways regulating stem cell pluripotency and metabolic pathways (Zhao et al., 2023) as well as sperm circular RNAs (circRNAs) (Zhou et al., 2023). Interestingly, environmental exposure also had an impact on age-related sperm ncRNA changes (Chu et al., 2020; Suvorov et al., 2020).
Consequences of Increasing Paternal Age for Offspring Health
Epidemiologic studies identified increased paternal age as a risk factor for developing diseases like cancer, metabolic, cardiovascular and neurological diseases in offspring (Siddeek et al., 2018) (Fig. 3). Such findings were confirmed in animal models, and additional challenges like dietary deficiencies and exposures to toxicants intensified the observed effects.
Figure 3. Timeline of the negative consequences associated with advanced paternal age on sperm.
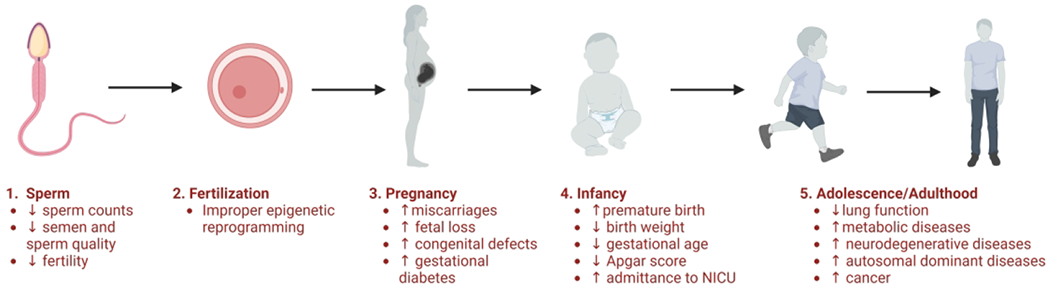
(1), fertilization (2), pregnancy (3), infancy (4), and adolescence/adulthood (5).
Sperm DNA Lesion and Offspring Viability
Sperm with extensive DNA double-strand breaks frequently are able fertilize an oocyte, but then fail to support embryo development (Mateo-Otero et al., 2022). Extensive amounts of sperm DNA damage that persist after fertilization frequently result in failed pregnancies (Larson-Cook et al., 2003; Sakkas and Alvarez, 2010; Simon et al., 2019; Musson et al., 2022). In various animal studies, sperm DNA damage led to a range of abnormal reproductive problems ranging from pregnancy loss to offspring with chromosomal abnormalities, as well as more subtle delayed effects like neurological diseases or cancer predisposition. Of note in this context, embryonic DNA repair mechanisms are different from, and much less understood, mechanisms regulating somatic cell DNA repair, and how damaged sperm DNA is repaired after fertilization is a topic of ongoing research (Khokhlova et al., 2020; Aitken and Bakos, 2021). Additional evidence suggests unrepaired sperm DNA lesions contribute to de novo point mutations and chromosomal structural aberrations in offspring of unaffected parents, and that the risk of such abnormalities increases with higher paternal age (Aitken and Bakos, 2021).
Examples of Paternal Age Effects on Offspring Health
How important paternal age at conception is for offspring health was shown in a cohort study of >40,000 live births conducted between 2007-2016 in the United States (Khandwala et al., 2018). Infants born to fathers aged >45 had lower gestational ages, higher chances of a premature birth, 18% higher chance of seizures, and a 14% greater risk of low birth weight. Infants born to fathers >55 years further had a higher incidence of low Apgar scores (<8) and an increased risk of needing additional medical care, including ventilation assistance and neonatal intensive care, compared to infants born to younger men. Additionally, Gau et al. found that increasing paternal age was linked to decreased lung function in school age children in a progressive way. Furthermore, prenatal exposure to environmental tobacco smoke and not being breastfed as infants exacerbated the adverse health effects of advanced paternal age on childhood lung function (Gau et al., 2022). While the underlying biological mechanisms are unknown, the authors speculate that telomere lengths could be a factor, given that telomere length is associated with lung function in children and adults. Telomere length is paternally inherited and depends on telomere length in spermatozoa, and increased paternal age results in offspring inheriting longer telomeres (Aitken, 2023). Increasing telomere length is potentially associated with increased risk of melanoma, lung adenocarcinoma, and several other cancers (Aitken, 2023). Therefore, increased sperm telomere length in older fathers may influence offspring lung function, and possibly increase cancer risks.
Autism and Schizophrenia
The most common neurodevelopmental disorders associated with advanced paternal age are schizophrenia and autism. Both depend on familial history, and their correlation with advanced paternal age is supported by a large body of literature. Schizophrenia and autism risks in offspring begin to increase with paternal age in the late 30s to early 40s. At conception, men in their 40s are 2-3 times more likely to father a child with schizophrenia than fathers in their 20s (Janecka et al., 2017). While the underlying mechanisms are not entirely clear, an age-related increase in de novo point mutations passed on through the sperm could contribute to this effect.
To overcome the limitation of retrospective epidemiologic human studies, animal models are used to gain insights into the mechanisms that connect advanced paternal age and offspring health, because they permit multigenerational studies, allow for a tightly controlled environment, and provide opportunity for experimental replicates and targeted interventions. Two example animal models used for that are the Brown Norway rat and the Swiss albino mouse. The Brown Norway rat is a good aging model because of its low disease rate and because it displays testicular phenotypic changes similar to aging humans (Serre and Robaire, 1998). Testicular changes in aging rats include decreased spermatogenesis and steroidogenesis, thickening of the epithelial basement membrane and accumulation of the aging indicator lipofuscin. Important effects of advanced paternal age on offspring in the Brown Norway rat include increased preimplantation loss and early neonatal death, and decreased fetal weight.
Autism spectrum disorders are characterized by deficits in social interaction, repetitive behavior, impaired communication, and restricted interests (Sampino et al., 2014). Sampino et al. used Swiss albino mice to study autism-like behavior in pups from aged fathers using tests accessing reflex, behavioral tendencies, social behaviors, explorative behavior, and vocalization. In these studies, F1 and F2 generations born from old fathers showed an increase in repetitive behavior, social deficits, and communication impairment compared to controls animals.
Conclusions
Progress in research directed at understanding male reproductive aging has allowed for important new insights into underlying mechanisms and consequences of aging in recent years, some of which are summarized in this review, such as the emerging role of paternal NAD metabolism. Yet, the field remains woefully understudied, despite the emerging realization that paternal age has overall significant negative impacts on the health of our children. The large number of open questions regarding the inheritance of sperm epigenetic marks, including the origin and consequences of age-related sperm DNA damage, and the impact of declining reproductive health on men’s health itself reflects the current deficit of information that needs to be addressed, because first-time fathers tend to become increasingly older in our modern time.
In this context it is worthwhile to consider the emerging pace-of-life concept that aims to integrate insights from reproductive biology and aging research with evolutionary biology. It is based on the general idea that resources in an organism are allocated to the different life functions (like growth, reproduction, and maintenance of the soma) in a manner that depends on the normal pace-of-life of the species’ and the individuals’, providing optimal evolutionary success by aligning reproductive timeframe, parenting and lifespan (Yuan et al., 2023). Slow metabolism, slow growth, and late puberty frequently are associated with delayed aging, as was seen in comparisons between different species, and also for individuals within one species (Arnqvist et al., 2022; Bartke, 2022; Yuan et al., 2023).
For example, if the biologically optimal reproductive time in humans is in their 20s to early 30s, adequate resources are allocated to optimally maintain the germline cells during this phase as well as healthy bodies capable of parenting the next generation. The significantly delayed reproduction, as it is currently observed in many developed countries, might shift reproduction out of the optimal period of life and into a phase when maintenance of germ cells is not optimal anymore, and further when somatic aging of the parents’ generation commences during a time that child-rearing is ongoing. During the traditional post-reproductive time point in life, fewer resources are dedicated to maintaining germline cells pristine, just as physiological changes associated with aging set in. Reproducing at later points in life therefore results in a misalignment between the time of peak sperm quality and the time in life when males are ready to become fathers. As a consequence, sperm with impaired quality might contribute more frequently to the next generation of children. Even though the relative speed of metabolism, reproduction and aging appears to be coupled with each other and genetically controlled, environmental factors that change growth and metabolic rates can influence an individual’s pace-of-life, for example, changing the timing of puberty, fertility rate and subsequently, when the aging process commences. More insights into the molecular mechanisms linking these life-stage specific processes might in the future permit the development of intervention strategies that delay puberty and aging in order to adjust the observed culturally delayed reproductive phase with the extended lifespan seen in humans, and to restore optimal reproductive success.
Funding
The authors gratefully acknowledge support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number HD100970 (to RGM) and HD103027 (to MMF) and by the National Institute on Aging of the National Institutes of Health under award number AG069745 (to MMF), and support by a Utah Agricultural Experiment Station grant, Utah State University (UTA01403 and UTA01721, to RGM).
Footnotes
Declaration in interest
The authors declare no conflicts of interest.
References
- Aitken RJ (2022) Role of sperm DNA damage in creating de-novo mutations in human offspring: the ‘post-meiotic oocyte collusion’ hypothesis. Reproductive BioMedicine Online 45 109–124. [DOI] [PubMed] [Google Scholar]
- Aitken RJ (2023) Male reproductive ageing: a radical road to ruin. Human Reproduction (Oxford, England) 38 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ and Bakos HW (2021) Should we be measuring DNA damage in human spermatozoa? New light on an old question. Human Reproduction 36 1175–1185. [DOI] [PubMed] [Google Scholar]
- Aitken RJ and Lewis SEM (2023) DNA damage in testicular germ cells and spermatozoa. When and how is it induced? How should we measure it? What does it mean? Andrology 11 1545–1557. [DOI] [PubMed] [Google Scholar]
- Albani E, Castellano S, Gurrieri B, Arruzzolo L, Negri L, Borroni EM and Levi-Setti PE (2019) Male age: negative impact on sperm DNA fragmentation. Aging 11 2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S, Rato L, Sousa M, Alves MG and Oliveira PF (2017) Fertility and Sperm Quality in the Aging Male. Current Pharmaceutical Design 23. [DOI] [PubMed] [Google Scholar]
- Alves MBR, De Arruda RP, De Bern THC, Florez-Rodriguez SA, Sá Filho MFD, Belleannée C, Meirelles FV, Da Silveira JC, Perecin F and Celeghini ECC (2019) Sperm-borne miR-216b modulates cell proliferation during early embryo development via K-RAS. Scientific Reports 9 10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves MBR, Celeghini ECC and Belleannée C (2020) From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential. Frontiers in Cell and Developmental Biology 8 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Vakonaki E, Tsiminikaki K, Tzatzarakis M, Michopoulou V, Flamourakis M, Kalliantasi K, Karzi V, Fragkiadaki P, Renieri E et al. (2020) Sperm telomere length: Diagnostic and prognostic biomarker in male infertility (Review). World Academy of Sciences Journal. [Google Scholar]
- Amjad S, Nisar S, Bhat AA, Shah AR, Frenneaux MP, Fakhro K, Haris M, Reddy R, Patay Z, Baur J et al. (2021) Role of NAD+ in regulating cellular and metabolic signaling pathways. Molecular Metabolism 49 101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM and Sinclair DA (2022) Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nature Reviews Endocrinology 18 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolz P, Bielsa MA and Vila J (1999) Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period. Human Reproduction (Oxford, England) 14 731–735. [DOI] [PubMed] [Google Scholar]
- Anjum S, Krishna A, Sridaran R and Tsutsui K (2012) Localization of Gonadotropin-Releasing Hormone (GnRH), Gonadotropin-Inhibitory Hormone (GnIH), Kisspeptin and GnRH Receptor and Their Possible Roles in Testicular Activities From Birth to Senescence in Mice. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 317 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G, Rönn J, Watson C, Goenaga J and Immonen E (2022) Concerted evolution of metabolic rate, economics of mating, ecology, and pace of life across seed beetles. Proceedings of the National Academy of Sciences 119 e2205564119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, Saida M, Steger K, Tedder P and Miller D (2009) Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Research 19 1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashapkin V, Suvorov A, Pilsner JR, Krawetz SA and Sergeyev O (2023) Age-associated epigenetic changes in mammalian sperm: implications for offspring health and development. Human Reproduction Update 29 24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F and Jouannet P (1995) Decline in semen quality among fertile men in Paris during the past 20 years. The New England Journal of Medicine 332 281–285. [DOI] [PubMed] [Google Scholar]
- Bagatell CJ and Bremner WJ (1996) Androgens in Men — Uses and Abuses. New England Journal of Medicine 334 707–715. [DOI] [PubMed] [Google Scholar]
- Bartke A (2022) Somatotropic Axis, Pace of Life and Aging. Frontiers in Endocrinology 13 916139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra V, Norman E, Morgan HL and Watkins AJ (2022) Parental Programming of Offspring Health: The Intricate Interplay between Diet, Environment, Reproduction and Development. Biomolecules 12 1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke S and Bürkle A (2007) Poly(ADP-ribosyl)ation in mammalian ageing. Nucleic Acids Research 35 7456–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt L, Dittrich M, Prell A, Potabattula R, Drummer C, Behr R, Hahn T, Schorsch M, Müller T and Haaf T (2023) Age-related methylation changes in the human sperm epigenome. Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC and Yialamas MA (2018) Testosterone Therapy in Men With Hypogonadism: An Endocrine Society* Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 103 1715–1744. [DOI] [PubMed] [Google Scholar]
- Bibi R, Jahan S, Kafeel Qureshi S, Razak S, Afsar T, Almajwal A, Kafeel Qureshi M, Hammadeh ME and Amor H (2023) Analysis of sperm chromatin packaging and reproductive biomarker to evaluate the consequence of advanced male age. Frontiers in Endocrinology 14 1092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujan L, Mieusset R, Mondinat C, Mansat A and Pontonnier F (1988) Sperm morphology in fertile men and its age related variation. Andrologia 20 121–128. [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Lin W, Simbulan R, Liu X, Feuer S, Donjacour A and Rinaudo PF (2018) Advanced Paternal Age Affects Sperm Count and Anogenital Distance in Mouse Offspring. Reproductive Sciences (Thousand Oaks, Calif) 25 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Shao X, Chan P, Cheung W, Kwan T, Pastinen T and Robaire B (2020) High-resolution analyses of human sperm dynamic methylome reveal thousands of novel age-related epigenetic alterations. Clinical Epigenetics 12 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Hung J-H, Hainer SJ, Chou M-T, Carone DM, Weng Z, Fazzio TG and Rando OJ (2014) High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm. Developmental Cell 30 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik-Ozenci C and Tasatargil A (2013) Role of poly(ADP-ribose) polymerases in male reproduction. Spermatogenesis 3 e24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champroux A, Torres-Carreira J, Gharagozloo P, Drevet JR and Kocer A (2016) Mammalian sperm nuclear organization: resiliencies and vulnerabilities. Basic and Clinical Andrology 26 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PTK and Robaire B (2022) Advanced Paternal Age and Future Generations. Frontiers in Endocrinology 13 897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-X, Li H-Y, Lin Y-H, Huang Z-Q, Huang P-Y, Da L-C, Shi H, Yang L, Feng Y-B and Zheng B-H (2022) The effect of age and abstinence time on semen quality: a retrospective study. Asian Journal of Andrology 24 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, Gomez LS, Hogan KA, Tarragó MG, Puranik AS et al. (2020) CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nature Metabolism 2 1284–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CCS, Cordeiro HS, Tran NLK and Chini EN (2024) NAD metabolism: Role in senescence regulation and aging. Aging Cell 23 e13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey M, Ranjan A, Bora PS, Baltazar F, Martin LJ and Krishna A (2019) Role of adiponectin as a modulator of testicular function during aging in mice. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1865 413–427. [DOI] [PubMed] [Google Scholar]
- Chu C, Yu L, Henry-Berger J, Ru Y-F, Kocer A, Champroux A, Li Z-T, He M, Xie S-S, Ma W-B et al. (2020) Knockout of glutathione peroxidase 5 down-regulates the piRNAs in the caput epididymidis of aged mice. Asian Journal of Andrology 22 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J-Y, Chen H and Zirkin B (2021) Sirt1 and Nrf2: regulation of Leydig cell oxidant/antioxidant intracellular environment and steroid formation†. Biology of Reproduction 105 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli RA, La Vignera S, Barbagallo F, Alamo A, Mongioì LM, Cannarella R, Aversa A and Calogero AE (2020) Bio-Functional Sperm Parameters: Does Age Matter? Frontiers in Endocrinology 11 558374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Sun F, Song L, Rivera-Pérez JA and Rando OJ (2018) Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Developmental Cell 46 470–480.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens M, Maresh JG, Yanagimachi R, Maeda G and Allsopp R (2008) Sirt1 Deficiency Attenuates Spermatogenesis and Germ Cell Function. PLoS ONE 3 e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A and Verdin E (2021) NAD+ metabolism and its roles in cellular processes during ageing. Nature Reviews Molecular Cell Biology 22 119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen CR, Amat S, Caton JS, Crouse MS, Diniz WJDS and Reynolds LP (2023) Paternal effects on fetal programming. Animal Reproduction 20 e20230076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer F, Mark M, Quenet D, Scherthan H, Huber A, Liebe B, Monaco L, Chicheportiche A, Sassone-Corsi P, de Murcia G et al. (2006) Poly(ADP-ribose) polymerase-2 contributes to the fidelity of male meiosis I and spermiogenesis. Proceedings of the National Academy of Sciences of the United States of America 103 14854–14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Chen C, Zhang J, Gao Y, Zeng X and Zhang X (2022) Testicular aging, male fertility and beyond. Frontiers in Endocrinology 13 1012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Fu Q, Sun Y and Li Q (2022) Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Research Reviews 81 . [DOI] [PubMed] [Google Scholar]
- Eisenberg ML (2022) Can a father be too old? Fertility and Sterility 118 999–1000. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA and Kuzawa CW (2018) The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences 373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkek S, Hisano M, Liang C-Y, Gill M, Murr R, Dieker J, Schübeler D, van der Vlag J, Stadler MB and Peters AHFM (2013) Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa . Nature Structural & Molecular Biology 20 1236. [DOI] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP and Bohr VA (2017) NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends in Molecular Medicine 23 899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Chen S, Jin X, Liu S, Cao X and Liu B (2023) Metabolomics in aging research: aging markers from organs. Frontiers in Cell and Developmental Biology 11 1198794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fice HE and Robaire B (2019) Telomere Dynamics Throughout Spermatogenesis. Genes 10 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fice HE and Robaire B (2023) Aging affects gene expression in spermatids of Brown Norway rats. Experimental Gerontology 173 112086. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Calandra RS, Bartke A and Matzkin ME (2018) Ageing and inflammation in the male reproductive tract. Andrologia 50 e13034. [DOI] [PubMed] [Google Scholar]
- Frungieri MB Calandra RS Bartke A and Matzkin ME (2021) Male and female gonadal ageing: its impact on health span and life span. Mechanisms of Ageing and Development 197 111519. [DOI] [PubMed] [Google Scholar]
- Garza S Chen L Galano M Cheung G Sottas C Li L, Li Y, Zirkin BR and Papadopoulos V (2022) Mitochondrial dynamics, Leydig cell function, and age-related testosterone deficiency. The FASEB Journal 36. [DOI] [PubMed] [Google Scholar]
- Gau C-C, Lee H-J, Lu H-Y, Wu C-Y, Huang H-Y, Tsai H-J and Yao T-C (2022) Association of advanced paternal age with lung function at school age. Respiratory Research 23 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan R, Scovell JM and Ramasamy R (2015) Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. The Aging Male 18 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DC, Ory J, Blachman-Braun R, Nackeeran S, Best JC and Ramasamy R (2022) Advanced Paternal Age and Sperm DNA Fragmentation: A Systematic Review . The World Journal of Men’s Health 40 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A, McGrath JJ, Hultman CM, Wilkie AOM and Malaspina D (2013) “Selfish Spermatogonial Selection”: A Novel Mechanism for the Association Between Advanced Paternal Age and Neurodevelopmental Disorders. American Journal of Psychiatry 170 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover MM and Jenkins TG (2020) Transgenerational Epigenetics. Urologic Clinics of North America 47 219–225. [DOI] [PubMed] [Google Scholar]
- Gunes S, Hekim GNT, Arslan MA and Asci R (2016) Effects of aging on the male reproductive system. Journal of Assisted Reproduction and Genetics 33 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT and Cairns BR (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Hong S-H, Lee S-J, Hong S-P and Cho C (2021) Transcriptome Analysis of Testicular Aging in Mice. Cells 10 2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Yao J, Chen W, Zhuang J, Bian J, Ouyang B, Sun X, Deng C, Xie Y and Yang Q (2023) Altered transcriptomic and metabolomic profiles of testicular interstitial fluid during aging in mice. Theriogenology 200 86–95. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Chini CCS and Chini EN (2019) The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Frontiers In Immunology 10 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta F, Catt S, Ramachandran P, Vollenhoven B and Temple-Smith P (2020) Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Human Reproduction 35 529–544. [DOI] [PubMed] [Google Scholar]
- Horvath S and Raj K (2018) DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics 19 371–384. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E and Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nature Reviews Molecular Cell Biology 13 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li X, Sun X, Yao J, Gao F, Wang Z, Hu J, Wang Z, Ouyang B, Tu X et al. (2022a) Anatomical Transcriptome Atlas of the Male Mouse Reproductive System During Aging. Frontiers In Cell and Developmental Biology 9 782824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Zuo Y, Zhang C, Sun G, Jing Y, Lei J, Ma S, Sun S, Lu H, Zhang X et al. (2022b) A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein & Cell pwac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H (2023) The impact of testosterone in men’s health. Endocrine Journal 70 655–662. [DOI] [PubMed] [Google Scholar]
- Ihara M, Meyer-Ficca ML, Leu NA, Rao S, Li F, Gregory BD, Zalenskaya IA, Schultz RM and Meyer RG (2014) Paternal poly (ADP-ribose) metabolism modulates retention of inheritable sperm histones and early embryonic gene expression. PLoS Genetics 10 e1004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M, Mill J, Basson MA, Goriely A, Spiers H, Reichenberg A, Schalkwyk L and Fernandes C (2017) Advanced paternal age effects in neurodevelopmental disorders—review of potential underlying mechanisms. Translational Psychiatry 7 e1019–e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarak I, Almeida S, Carvalho RA, Sousa M, Barros A, Alves MG and Oliveira PF (2018) Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochimica Et Biophysica Acta. Molecular Basis of Disease 1864 3388–3396. [DOI] [PubMed] [Google Scholar]
- Jenkins TG and Carrell DT (2011) The paternal epigenome and embryogenesis: poising mechanisms for development. Asian Journal of Andrology 13 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, Aston Kl, Pflueger C, Cairns BR and Carrell DT (2014) Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility. PLoS Genetics 10 e1004458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zhu W-J, Li J, Chen Q-J, Liang W-B and Gu Y-Q (2014) Quantitative histological analysis and ultrastructure of the aging human testis. International Urology and Nephrology 46 879–885. [DOI] [PubMed] [Google Scholar]
- Johnson S and Imai S-I (2018) NAD + biosynthesis, aging, and disease. F1000Research 7 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M and Rajender S (2020) Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reproductive Biology and Endocrinology 18 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Yamamoto T, Toh H, Kazuki Y, Kazuki K, Imoto J, Ikeo K, Oshima M, Shirahige K, Iwama A et al. (2019) Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proceedings of the National Academy of Sciences 116 16404–16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J-M, Lapauw B, Mahmoud A, T’Sjoen G and Huhtaniemi IT (2019) Aging and the Male Reproductive System. Endocrine Reviews 40 906–972. [DOI] [PubMed] [Google Scholar]
- Kaur G, Thompson LA and Dufour JM (2014) Sertoli cells--immunological sentinels of spermatogenesis. Seminars in Cell & Developmental Biology 30 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandwala YS, Zhang CA, Lu Y and Eisenberg ML (2017) The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Human Reproduction 32 2110–2116. [DOI] [PubMed] [Google Scholar]
- Khandwala YS, Baker VL, Shaw GM, Stevenson DK, Lu Y and Eisenberg ML (2018) Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ k4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova EV, Fesenko ZS, Sopova JV and Leonova El (2020) Features of DNA Repair in the Early Stages of Mammalian Embryonic Development. Genes 11 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmins S, Anderson RA, Barratt CLR, Behre HM, Catford SR, De Jonge CJ, Delbes G, Eisenberg ML, Garrido N, Houston BJ et al. (2023) Frequency, morbidity and equity — the case for increased research on male fertility. Nature Reviews Urology. [DOI] [PubMed] [Google Scholar]
- Kleshchev M, Osadchuk L and Osadchuk A (2023) Age-Related Changes in Sperm Morphology and Analysis of Multiple Sperm Defects. Frontiers In Bioscience-Scholar 15 12. [DOI] [PubMed] [Google Scholar]
- Kumar N, Singh AK and Choudhari AR (2017) Impact of age on semen parameters in male partners of infertile couples in a rural tertiary care center of central India: A cross-sectional study. International Journal of Reproductive Biomedicine 15 497–502. [PMC free article] [PubMed] [Google Scholar]
- Lankenau S, Burkle A and Lankenau DH (1999) Detection of poly(ADP-ribose) synthesis in Drosophila testes upon gamma-irradiation. Chromosoma 108 44–51. [DOI] [PubMed] [Google Scholar]
- Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET and Evenson DP (2003) Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertility and Sterility 80 895–902. [DOI] [PubMed] [Google Scholar]
- Lautrup S, Hou Y, Fang EF and Bohr VA (2024) Roles of NAD+ in Health and Aging. Cold Spring Harbor Perspectives in Medicine 14 a041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc F, Maquennehan V, Nkoma GB and Boissonneault G (2008) DNA damage response during chromatin remodeling in elongating spermatids of mice. Biology of Reproduction 78 324–332. [DOI] [PubMed] [Google Scholar]
- Levy S, Serre V, Hermo L and Robaire B (1999) The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. Journal of Andrology 20 356–365. [PubMed] [Google Scholar]
- Li X, Yao J, Hu J, Deng C, Xie Y and Wang Z (2021) iTRAQ-based proteomics of testicular interstitial fluid during aging in mice. Theriogenology 175 44–53. [DOI] [PubMed] [Google Scholar]
- Liao J, Suen HC, Luk ACS, Yang L, Lee AWT, Qi H and Lee T-L (2021) Transcriptomic and epigenomic profiling of young and aged spermatogonial stem cells reveals molecular targets regulating differentiation. PLOS Genetics 17 e1009369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lismer A and Kimmins S (2023) Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nature Communications 14 2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M and Kroemer G (2023) Hallmarks of aging: An expanding universe. Cell 186 243–278. [DOI] [PubMed] [Google Scholar]
- Lord T and Aitken RJ (2015) Fertilization stimulates 8-hydroxy-2’-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Developmental Biology 406 1–13. [DOI] [PubMed] [Google Scholar]
- Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A and Wyrobek AJ (2001) Frequency of XY Sperm Increases with Age in Fathers of Boys with Klinefelter Syndrome. The American Journal of Human Genetics 69 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhou Y, Zhu Y-C, Wang S-Q, Ping P and Chen X-F (2018) Lipophagy Contributes to Testosterone Biosynthesis in Male Rat Leydig Cells. Endocrinology 159 1119–1129. [DOI] [PubMed] [Google Scholar]
- Ma Q, You X, Zhu K, Zhao X, Yuan D, Wang T, Dun Y, Wu J, Ren D, Zhang C et al. (2022) Changes in the tight junctions of the testis during aging: Role of the p38 MAPK/MMP9 pathway and autophagy in Sertoli cells. Experimental Gerontology 161 111729. [DOI] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N and Kroemer G (2010) Can autophagy promote longevity? Nature Cell Biology 12 842–846. [DOI] [PubMed] [Google Scholar]
- Mahfouz R, Sharma R, Sharma D, Sabanegh E and Agarwal A (2009) Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertility and Sterility 91 805–811. [DOI] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T and Aydin S (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiological Reviews 88 841–886. [DOI] [PubMed] [Google Scholar]
- Martin RH (2008) Meiotic errors in human oogenesis and spermatogenesis. Reproductive BioMedicine Online 16 523–531. [DOI] [PubMed] [Google Scholar]
- Martin RH and Rademaker AW (1987) The effect of age on the frequency of sperm chromosomal abnormalities in normal men. American Journal of Human Genetics 41 484–492. [PMC free article] [PubMed] [Google Scholar]
- Martins Da Silva S and Anderson RA (2022) Reproductive axis ageing and fertility in men. Reviews in Endocrine and Metabolic Disorders 23 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B and Guillemin GJ (2012) Age-Associated Changes In Oxidative Stress and NAD+ Metabolism In Human Tissue. PLoS ONE 7 e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo-Otero Y, Llavanera M, Recuero S, Delgado-Bermúdez A, Barranco I, Ribas-Maynou J and Yeste M (2022) Sperm DNA damage compromises embryo development, but not oocyte fertilisation in pigs. Biological Research 55 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba H, Fujii C, Maruyama K, Kawakubo M, Momose M, Sano K, Imamura H, Kurihara H and Nakayama J (2024) Sirt3 regulates proliferation and progesterone production in Leydig cells via suppression of reactive oxygen species. Endocrinology bqae017. [DOI] [PubMed] [Google Scholar]
- Matzkin ME, Calandra RS, Rossi SP, Bartke A and Frungieri MB (2021) Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells 10 3114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RG and Meyer-Ficca ML (2021) Metabolism in Male Reproductive Aging. Advances in Geriatric Medicine and Research 3 e210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RG, Ketchum CC and Meyer-Ficca ML (2017) Heritable sperm chromatin epigenetics: a break to remember. Biology of Reproduction 97 784–797. [DOI] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Ihara M, Lonchar JD, Meistrich ML, Austin CA, Min W, Wang ZQ and Meyer RG (2011a) Poly(ADP-ribose) Metabolism Is Essential for Proper Nucleoprotein Exchange During Mouse Spermiogenesis. Biology of Reproduction 84 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Lonchar JD, Ihara M, Meistrich ML, Austin CA and Meyer RG (2011b) Poly(ADP-ribose) polymerases PARP1 and PARP2 modulate topoisomerase II beta (TOP2B) function during chromatin condensation in mouse spermiogenesis. Biology of Reproduction 84 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Lonchar JD, Ihara M, Bader JJ and Meyer RG (2013) Alteration of poly(ADP-ribose) metabolism affects murine sperm nuclear architecture by impairing pericentric heterochromatin condensation. Chromosoma 122 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Ihara M, Bader JJ, Leu NA, Beneke S and Meyer RG (2015) Spermatid head elongation with normal nuclear shaping requires ADP-ribosyltransferase PARP11 (ARTD11) in mice. Biology of Reproduction 92 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Zwerdling AE, Swanson CA, Tucker AG, Lopez SA, Wandersee MK, Warner GM, Thompson KL, Chini CCS, Chen H et al. (2022) Low NAD+ Levels Are Associated With a Decline of Spermatogenesis in Transgenic ANDY and Aging Mice. Frontiers in Endocrinology 13 896356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Brinkworth M and Iles D (2010) Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction (Cambridge, England) 139 287–301. [DOI] [PubMed] [Google Scholar]
- Moritz L and Hammoud SS (2022) The Art of Packaging the Sperm Genome: Molecular and Structural Basis of the Histone-To-Protamine Exchange. Frontiers in Endocrinology 13 895502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Hermo L and Robaire B (1998) The effects of aging on the expression of glutathione S-transferases in the testis and epididymis of the Brown Norway rat. Journal of Andrology 19 450–465. [PubMed] [Google Scholar]
- Mularoni V, Esposito V, Di Persio S, Vicini E, Spadetta G, Berloco P, Fanelli F, Mezzullo M, Pagotto U, Pelusi C et al. (2020) Age-related changes in human Leydig cell status. Human Reproduction 35 2663–2676. [DOI] [PubMed] [Google Scholar]
- Musson R, Gąsior Ł, Bisogno S and Ptak GE (2022) DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies. Human Reproduction Update 28 376–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas LE and Carnero A (2021) NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduction and Targeted Therapy 6 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Powanda P and Robaire B (2020) Oxidative Stress and Reproductive Function in the Aging Male. Biology 9 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Munyoki SK, Sukhwani M, Schmid N, Missel A, Emery BR, DonorConnect null, Stukenborg J-B, Mayerhofer A, Orwig KE et al. (2022) Single-cell analysis of human testis aging and correlation with elevated body mass index. Developmental Cell 57 1160–1176.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L (2014) Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 4 e979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JBA, Petersen CG, Mauri AL, Vagnini LD, Baruffi RLR and Franco JG (2014) The effects of age on sperm quality: an evaluation of 1,500 semen samples. JBRA Assisted Reproduction 18 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Mori H, Endo T, Ishikawa-Yamauchi Y, Motooka D, Emori C and Ikawa M (2023) Age-related decline in spermatogenic activity accompanied with endothelial cell senescence in male mice. iScience 26 108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahune PP (2013) The Total Antioxidant Power of Semen and Its Correlation with the Fertility Potential of Human Male Subjects. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua R, Nistal M, Sáez FJ and Fraile B (1991) Ultrastructure of the aging human testis. Journal of Electron Microscopy Technique 19 241–260. [DOI] [PubMed] [Google Scholar]
- Paul C and Robaire B (2013) Impaired Function of the Blood-Testis Barrier during Aging Is Preceded by a Decline in Cell Adhesion Proteins and GTPases. PLoS ONE 8 e84354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Saddiki H, Whitcomb BW, Suvorov A, Buck Louis GM, Mumford SL, Schisterman EF, Oluwayiose OA and Balzer LB (2022) Sperm epigenetic clock associates with pregnancy outcomes in the general population. Human Reproduction (Oxford, England) 37 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl E, Höffken V, Schlatt S, Kliesch S, Gromoll J and Wistuba J (2019) Ageing in men with normal spermatogenesis alters spermatogonial dynamics and nuclear morphology in Sertoli cells. Andrology 7 827–839. [DOI] [PubMed] [Google Scholar]
- Quesada P, Atorino L, Cardone A, Ciarcia G and Farina B (1996) Poly(ADPribosyl)ation system in rat germinal cells at different stages of differentiation. Experimental Cell Research 226 183–190. [DOI] [PubMed] [Google Scholar]
- Raee P, Tan SC, Najafi S, Zandsalimi F, Low TY, Aghamiri S, Fazeli E, Aghapour M, Mofarahe ZS, Heidari MH et al. (2023) Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view. Reproductive Biology and Endocrinology 21 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato L, Alves MG, Silva BM, Sousa M and Oliveira PF (2016) Sirtuins: Novel Players in Male Reproductive Health. Current Medicinal Chemistry 23 1084–1099. [DOI] [PubMed] [Google Scholar]
- Roh E and Kim M-S (2020) Hypothalamic NAD+-Sirtuin Axis: Function and Regulation. Biomolecules 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B-Y, Orwig KE, Oatley JM, Avarbock MR and Brinster RL (2006) Effects of Aging and Niche Microenvironment on Spermatogonial Stem Cell Self-Renewal. Stem Cells 24 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas D and Alvarez JG (2010) Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertility and Sterility 93 1027–1036. [DOI] [PubMed] [Google Scholar]
- Samans B, Yang Y, Krebs S, Sarode GV, Blum H, Reichenbach M, Wolf E, Steger K, Dansranjavin T and Schagdarsurengin U (2014) Uniformity of nucleosome preservation pattern in Mammalian sperm and its connection to repetitive DNA elements. Developmental Cell 30 23–35. [DOI] [PubMed] [Google Scholar]
- Sampino S, Juszczak GR, Zacchini F, Swiergiel AH, Modlinski JA, Loi P and Ptak GE (2014) Grandpaternal age and the development of autism-like symptoms in mice progeny. Translational Psychiatry 4 e386–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Silva JV, Alves MG, Oliveira PF and Fardilha M (2019) Testicular Aging: An Overview of Ultrastructural, Cellular, and Molecular Alterations. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 74 860–871. [DOI] [PubMed] [Google Scholar]
- Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D and Wyrobek AJ (2007) The effects of male age on sperm DNA damage in healthy non-smokers. Human Reproduction 22 180–187. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Araujo AB, Roose SP, Devanand DP, Xie S, Cooper TB and McKinlay JB (2002) Low Testosterone Levels in Elderly Men With Dysthymic Disorder. American Journal of Psychiatry 159 456–459. [DOI] [PubMed] [Google Scholar]
- Serre V and Robaire B (1998) Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertility and Sterility 70 625–631. [DOI] [PubMed] [Google Scholar]
- Setti AS, Braga DPDAF, Provenza RR, Iaconelli A and Borges E (2021) Oocyte ability to repair sperm DNA fragmentation: the impact of maternal age on intracytoplasmic sperm injection outcomes. Fertility and Sterility 116 123–129. [DOI] [PubMed] [Google Scholar]
- Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M and Turki RF (2015) Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reproductive Biology and Endocrinology: RB&E 13 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL and Rando OJ (2018) Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Developmental Cell 46 481–494.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddeek B, Mauduit C, Simeoni U and Benahmed M (2018) Sperm epigenome as a marker of environmental exposure and lifestyle, at the origin of diseases inheritance. Mutation Research 778 38–44. [DOI] [PubMed] [Google Scholar]
- Simon L, Emery B and Carrell DT (2019) Sperm DNA Fragmentation: Consequences for Reproduction. In Genetic Damage In Human Spermatozoa, pp 87–105. Eds Baldi E and Muratori M. Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Singh NP, Muller CH and Berger RE (2003) Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertility and Sterility 80 1420–1430. [DOI] [PubMed] [Google Scholar]
- Sloter ED, Marchetti F, Eskenazi B, Weldon RH, Nath J, Cabreros D and Wyrobek AJ (2007) Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertility and Sterility 87 1077–1086. [DOI] [PubMed] [Google Scholar]
- Smith A and Haaf T (1998) DNA nicks and increased sensitivity of DNA to fluorescence in situ end labeling during functional spermiogenesis. BioTechniques 25 496–502. [DOI] [PubMed] [Google Scholar]
- Sokanovic SJ, Capo I, Medar MM, Andric SA and Kostic TS (2018) Long-term inhibition of PDE5 ameliorates aging-induced changes in rat testis. Experimental Gerontology 108 139–148. [DOI] [PubMed] [Google Scholar]
- Stock AJ and Liu Y (2021) NAD-Linked Metabolism and Intervention in Short Telomere Syndromes and Murine Models of Telomere Dysfunction. Frontiers in Aging 2 785171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strømland Ø, Diab J, Ferrario E, Sverkeli U and Ziegler M (2021) The balance between NAD+ biosynthesis and consumption in ageing. Mechanisms of Ageing and Development 199 111569. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Pilsner JR, Naumov V, Shtratnikova V, Zheludkevich A, Gerasimov E, Logacheva M and Sergeyev O (2020) Aging Induces Profound Changes in sncRNA in Rat Sperm and These Changes Are Modified by Perinatal Exposure to Environmental Flame Retardant. International Journal of Molecular Sciences 21 8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed V and Hecht NB (2001) Selective Loss of Sertoli Cell and Germ Cell Function Leads to a Disruption in Sertoli Cell-Germ Cell Communication During Aging in the Brown Norway Rat1. Biology of Reproduction 64 107–112. [DOI] [PubMed] [Google Scholar]
- Tatehana M, Kimura R, Mochizuki K, Inada H and Osumi N (2020) Comprehensive histochemical profiles of histone modification in male germline cells during meiosis and spermiogenesis: Comparison of young and aged testes in mice. PLOS ONE 15 e0230930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME and Roldan ERS (2022) Sperm bauplan and function and underlying processes of sperm formation and selection. Physiological Reviews 102 7–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulubay M, Bahaettin M and Akdeniz E (2022) The effect of aging on semen parameters in normozoospermic men: A cross-sectional study. International Journal of Reproductive BioMedicine (IJRM). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hoven L, Hendriks JCM, Verbeet JGM, Westphal JR and Wetzels AMM (2015) Status of sperm morphology assessment: an evaluation of methodology and clinical value. Fertility and Sterility 103 53–58. [DOI] [PubMed] [Google Scholar]
- Vaughan DA, Tirado E, Garcia D, Datta V and Sakkas D (2020) DNA fragmentation of sperm: a radical examination of the contribution of oxidative stress and age in 16 945 semen samples. Human Reproduction 35 2188–2196. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Goemaere S and Van Pottelberg I (2002) Estradiol in elderly men. The Aging Male 5 98–102. [PubMed] [Google Scholar]
- Wang M, Zeng L, Su P, Ma L, Zhang M and Zhang YZ (2022) Autophagy: a multifaceted player in the fate of sperm. Human Reproduction Update 28 200–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kataura T, Korsgen ME, Sun C, Sarkar S and Korolchuk VI (2023) The autophagy–NAD axis in longevity and disease. Trends in Cell Biology 33 788–802. [DOI] [PubMed] [Google Scholar]
- Wu FCW, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A et al. (2008) Hypothalamic-Pituitary-Testicular Axis Disruptions in Older Men Are Differentially Linked to Age and Modifiable Risk Factors: The European Male Aging Study. The Journal of Clinical Endocrinology & Metabolism 93 2737–2745. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS and Evenson D (2006) Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proceedings of the National Academy of Sciences 103 9601–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Ryan DP, Pearson BL, Henzel KS, Neff F, Vidal RO, Hennion M, Lehmann I, Schleif M, Schröder S et al. (2018) Epigenetic alterations in longevity regulators, reduced life span, and exacerbated aging-related pathology in old father offspring mice. Proceedings of the National Academy of Sciences 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zhang Y, Wang Q and Yuan L (2022) Autophagy: A Double-Edged Sword in Male Reproduction. International Journal of Molecular Sciences 23 15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN and Turek PJ (2018) Reproductive genetics and the aging male. Journal of Assisted Reproduction and Genetics 35 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Hascup E, Hascup K and Bartke A (2023) Relationships among Development, Growth, Body Size, Reproduction, Aging, and Longevity – Trade-Offs and Pace-Of-Life. Biochemistry (Moscow) 88 1692–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ebata KT, Robaire B and Nagano MC (2006) Aging of Male Germ Line Stem Cells in Mice1. Biology of Reproduction 74 119–124. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xia S, Xiao W, Song Y, Tang L, Cao M, Yang J, Wang S, Li Z, Xu C et al. (2023) A single-cell transcriptomic landscape of mouse testicular aging. Journal of Advanced Research 53 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu X, Qu Y, Wang L, Geng D, Chen W, Li L, Tian Y, Chang S, Zhao C et al. (2019) The roles of p38 MAPK → COX2 and NF-κB → COX2 signal pathways in age-related testosterone reduction. Scientific Reports 9 10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Song L, Ma N, Liu C, Dun Y, Zhou Z, Yuan D and Zhang C (2021) The dynamic changes of Nrf2 mediated oxidative stress, DNA damage and base excision repair in testis of rats during aging. Experimental Gerontology 152 111460. [DOI] [PubMed] [Google Scholar]
- Zhao M-J, Zhang Y-N, Zhao Y-P, Chen X-B, Han B-S, Ding N, Gu Y-Q, Wang S-S, Ma J and Liu M-L (2023) Altered microRNA expression profiles of human spermatozoa in normal fertile men of different ages. Asian Journal of Andrology 25 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liu A, Ji H, Ji J, Sun J, Ling Z, Li G, Ling X, Xu L and Chen X (2023) Expression profiles of circular RNAs in spermatozoa from aging men. Molecular Biology Reports 50 8081–8088. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yin Q, Wei D, Yang Z, Du Y and Ma Y (2019) Autophagy in male reproduction. Systems Biology in Reproductive Medicine 65 265–272. [DOI] [PubMed] [Google Scholar]
- Zirkin BR and Tenover JL (2012) Aging and Declining Testosterone: Past, Present, and Hopes for the Future. Journal of Andrology 33 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann M (2020) Testosterone, mood, behaviour and quality of life. Andrology 8 1598–1605. [DOI] [PubMed] [Google Scholar]


