Abstract
Amino-terminal (Nt-) acetylation (NTA) is a common protein modification, affecting approximately 80% of all human proteins. The human essential X-linked gene, NAA10, encodes for the enzyme NAA10, which is the catalytic subunit in the N-terminal acetyltransferase A (NatA) complex. There is extensive genetic variation in humans with missense, splice-site, and C-terminal frameshift variants in NAA10. In mice, Naa10 is not an essential gene, as there exists a paralogous gene, Naa12, that substantially rescues Naa10 knockout mice from embryonic lethality, whereas double knockouts (Naa10-/Y Naa12-/-) are embryonic lethal. However, the phenotypic variability in the mice is nonetheless quite extensive, including piebaldism, skeletal defects, small size, hydrocephaly, hydronephrosis, and neonatal lethality. Here we replicate these phenotypes with new genetic alleles in mice, but we demonstrate their modulation by genetic background and environmental effects. We cannot replicate a prior report of "maternal effect lethality" for heterozygous Naa10-/X female mice, but we do observe a small amount of embryonic lethality in the Naa10-/y male mice on the inbred genetic background in this different animal facility.
Introduction
Targeting 40% of the human proteome, NatA, the major N-terminal acetyltransferase (NAT) complex, acetylates Ser-, Ala-, Gly-, Thr-, Val-, and Cys- N-termini following cleavage of the initiator methionine [1, 2]. The canonical human NatA consists of two main subunits, the catalytic subunit N-α-acetyltransferase 10 (NAA10) (Ard1) and the auxiliary subunit NAA15 (Nat1) and engages with a regulatory subunit, HYPK [3–5]. N-terminal (Nt-) acetylation (NTA) is one of the most common protein modifications, occurring co- and post-translationally [6, 7]. Approximately 80% of cytosolic proteins are N-terminally acetylated in humans and ~50% in yeast [1], while NTA is less common in prokaryotes and archaea [6].
NTA is catalyzed by a set of enzymes, the NATs, which transfer an acetyl group from acetyl coenzyme A (Ac-CoA) to the free α-amino group of a protein’s N-terminus. To date, eight distinct NATs (NatA–NatH) have been identified in metazoan (NatA-F and NatH) and plant (NatG) species that are classified based on different conserved subunit compositions and substrate specificities [2, 7, 8]. NTA has been implicated in steering protein folding, stability or degradation, subcellular targeting, and complex formation [9–13]. Particularly, Naa10-catalyzed N-terminal acetylation has been reported to be essential for development in many species [14–18] and although NatA is not essential in S. cerevisiae, depletion of Naa10 or Naa15 has strong effects, including slow growth and decreased survival when exposed to various stresses [19, 20]. In addition, it has been recently shown that mice have a compensating enzyme Naa12, which prevents embryonic lethality in the Naa10 knockouts [21], but a similar gene has not been found in humans. Furthermore, given that NAA10 was also identified in screens for essential genes in human cell lines [22, 23], it seems unlikely that an unknown NAA10-like paralogous gene exists in humans, other than the already known NAA11 [24, 25].
Ogden syndrome (OS), was first reported and named in 2011 after the location of the first affected family residing in Ogden, Utah, USA [26, 27]. In that first family, five males were born to four different mothers and all the males died in early infancy, with a range of cardiac, phenotypic variation and other defects, including lethal cardiac arrhythmias. The underlying genetic defect was characterized as a single missense change coding for Ser37Pro in the X-linked gene, NAA10, and was confirmed in a second independent family in California, USA, with three males that also died during infancy. The identical variant was recently reported in a third family [28], and a fourth family [29]. There is a S. cerevisiae model for the Naa10 Ser37Pro mutant, in which that variant impairs NatA complex formation and leads to a reduction in both NatA catalytic activity and functionality [30, 31]. Furthermore, OS patient-derived cells have impaired in vivo NTA of a few NatA substrates [32].
Since the initial discovery of OS in 2011, multiple groups have reported additional variants either in NAA10 in both males and females [33–46] or in the heterodimeric protein partner encoded by NAA15 [35, 47–50]. The genetic landscape of variation in NAA10 and NAA15 in humans has been presented recently with many more cases of Ogden syndrome (OS) (also known as “NAA10-related neurodevelopmental syndrome”), and “NAA15-related neurodevelopmental syndrome” [51].
Previous publications have reported that one of the phenotypes observed in Naa10 knockout mice is low body weight compared to their wildtype littermates [21, 52]. A recent analysis in humans analyzed these growth defects in greater detail, which showed extensive weight fluctuations, along with the recommendation that OS individuals not tracking above the failure to thrive range past one year of age might be considered for G-tube placement to avoid prolonged growth failure [53]. The current study aims to characterize the development, growth and phenotypes of various mice deficient in Naa10, beginning with embryogenesis, thus extending and expanding on our prior findings [21].
Results
Independently generated knockdown (KD) and knockout mice for Naa10 demonstrate similar phenotypes
Twice we attempted to develop a mouse model for Ogden Syndrome with a Ser37Pro missense mutation found in the first family identified with this syndrome [26]. The first attempt included the use of a minigene for exons 2–3, which was inserted in an intron in Naa10, that could theoretically then be activated upon inversion of the cassette by crossing to Cre-expressing animals (see S1 Fig in S1 File for gene targeting design). Unfortunately, both Western blotting and quantitative PCR revealed that the insertion of this minigene in the intron between exons 3 and 4, regardless of its inversion status, led to >95% knockdown of expression of Naa10 (S2 and S3 Figs in S1 File). As such, the interpretation of any results in this mouse model for the Ogden Syndrome Ser37Pro mutation are complicated by the knockdown of Naa10. We refer to these mice as male Naa10mini/Y, female Naa10mini/mini, male Naa10invS37P/Y, and female Naa10 invS37P/ invS37P. When tabulating the Mendelian ratios, with genotyping performed after weaning around four weeks of age, there was a slight deviation (22%) from the predicted Mendelian ratio (25%) for the male Naa10mini/Y mice (S1 Table in S1 File) and a much larger deviation (13%) for the male Naa10invS37P/Y mice (S2 Table in S1 File), which suggests the possibility that the S37P mutation does indeed exert a hypomorphic effect on NatA function. However, the confound for any interpretation of these data is the presence of severe knockdown of overall Naa10 protein expression, given that the minigene apparently has some effect on RNA expression or stability (S2B Fig in S1 File).
The phenotypes of these Naa10 knockout mice are similar to previously published identifying characteristics including piebaldism, hydrocephaly, cardiac defects, homeotic anterior transformation, and urogenital anomalies [21]. Although there was complete penetrance for piebaldism in the male mice, there was variable amounts of this, which was quantified to show the high variability of this phenotype in this allelic series of mutant mice (S4 Fig in S1 File). Piebaldism was also present and quantified in female Naa10mini/mini and female Naa10 invS37P/ invS37P mice, with no obvious correlation between the amount of piebaldism and the genotype, age, or weight of the mice (S5 Fig in S1 File). Piebaldism in heterozygous females was only very rarely seen (on the order of 1–2 animals among >40 animals per allele). Another phenotype identified with complete penetrance was bilateral supernumerary ribs (14 pairs of rib instead of 13) in all male Naa10mini/Y, female Naa10mini/mini, male Naa10invS37P/Y, and female Naa10 invS37P/ invS37P (S3 Table in S1 File). This extra pair of ribs linking to the sternum transforms the T8 vertebrae into an anterior T7-like phenotype. Many of these mice also had four instead of the usual three sternebrae, which were sometimes fused. Cervical vertebrae fusion was also demonstrated in these mice, particularly involving C1 and C2, suggesting possible anteriorization of C2 into a C1-like phenotype (S4 Table in S1 File). These phenotypes are identical to what was described in the Naa10-/y mice [21].
Bone density of calvarias was measured using computerized tomography (CT) scanning (S6 Fig in S1 File), showing no difference from wild type, except in a few of the Naa10-/y mice where hydrocephaly developed, accompanied by dilatation of the skull over time with thinning of the calvarium. As such, the published calvarial bone density phenotype reported in three-day old Naa10-/Y mice [54] does not remain by adulthood. Femur bone density did show a small but statistically significant decrease in the male Naa10-/y mice and Naa10 invS37P/ y mice, compared to the Naa10+/y and Naa10mini/y mice (S7B Fig in S1 File), and the female heterozygous Naa10+/invS37P also had slightly decreased femur bone density compared to Naa10+/+ and Naa10+/- mice (S7D Fig in S1 File). However, these data are limited by the fact that these measurements were taken in mice at all ages, and the overall number of mice was small in some groups (e.g., n = 3 for Naa10+/invS37P) (S7A and S7C Fig in S1 File). Furthermore, the Naa10-/Y mice were still on a somewhat mixed genetic background at the time of these experiments, as the number of backcrosses to C57BL/6J had only reached about 12–13 backcrosses at that time. Future experiments should ideally repeat this with a larger number of mice at one age timepoint, ideally on an inbred C57BL/6J genetic background (>20 backcrosses or mice generated at the outset from zygotes from an inbred background).
The second attempt to generate Naa10 S37P/ y mice included four rounds of microinjection (see S5 Table in S1 File) of CRISPR reagent mix including guide RNA and oligonucleotide donor into zygotes obtained from the mating of B6D2F1 females (i.e., 50% C57BL/6J, 50% DBA/2J (D2)) females to inbred C57BL/6J males (Jackson Laboratories, Bar Harbour, ME, USA). The guide RNA was produced and validated from Horizon (Perkin Elmer, USA), using a Cel1-nuclease assay, and the most active guide was selected, including the targeting cr-RNA sequence and the tracrRNA portion. Despite screening 156 offspring from these four injections, only indels were obtained, with no evidence of homologous recombination with the oligonucleotide donor to generate the desired Ser37Pro missense mutation. Two of the indels, namely a one base pair deletion (Δ668) and a seven base pair deletion (Δ668–674) were successfully transmitted to the next generation by backcrossing to C57BL/6J mice. Western blotting and qPCR confirmed knockout of Naa10 due to the frameshifts introduced by the indels (S8 Fig in S1 File). Breeding of these mice on this mixed genetic background did not show any obvious embryonic or neonatal lethality for the Δ668 male mice or the Δ668–674 male mice, as they were born and genotyped in the first week of life with no deviation from the expected 25% Mendelian ratios (S6 and S7 Tables in S1 File). Although most of these knockout mice had piebaldism, some of them did not (6 out of 81 Δ668 male mice and 10 out of 36 Δ668–674 male mice without piebaldism). The Naa10 indel mice also have bilateral supernumerary ribs (14 pairs of rib instead of 13), and a majority of these mice also had four instead of the usual three sternebrae, which were sometimes fused (S3 Table in S1 File), as previously reported [21]. Cervical vertebrae fusion was also demonstrated in these mice (S4 Table in S1 File). Out of all mice that were generated in the above and other matings, hydrocephaly did develop in the Naa10 Δ668 male mice (23/62, or 37%) and in the Naa10 Δ668–674 male mice (16/45, or 36%), which is similar to the previously reported rate around 40% [21] and substantially higher than the rate of 1% in wild type mice on the C57BL/6J genetic background.
As these new strains of knockdown or knockout mice recapitulated the phenotype of the already established Naa10 knockout (KO) lines [21], it was decided to not maintain these lines, so the minigene and indel strains were euthanized, with sperm cryopreservation at Cold Spring Harbor Laboratory (CSHL), available upon request.
Validation of a specific Naa10 antibody and demonstration of heterozygous expression in female Naa10 mice
Prior Western blotting using the Naa10 antibody obtained from Abcam always demonstrated a cross-reactive band of the same molecular weight as Naa10, which can be seen in the Western blot data in S2 and S3 Figs in S1 File. We hypothesized that this cross-reactive band might be the recently discovered mouse Naa12 [21]. A new rabbit monoclonal anti-Naa10 antibody is now available from Cell Signaling Technologies, Danvers, MA, USA, #13357, and this antibody was made with synthetic peptide corresponding to residues surrounding Asp204 of human NAA10. This is a region that diverges from mouse Naa12 [21] so there should not be any cross-reactivity to Naa12. This was confirmed by Western blotting from tissues isolated from male mice. Biological replicates (n = 8) were obtained consisting of Naa10-/Y (n = 4) and Naa10+/Y (n = 4) animals (S9 Fig in S1 File). Per genotype, the mean Naa10 signal normalized to total protein detected in Naa10–/Y was <10% of the Naa10+/Y animals, a significant difference between genotypes (2-way ANOVA, F statistic = 14.52 on 3 and 12 DF, *P < 0.05).
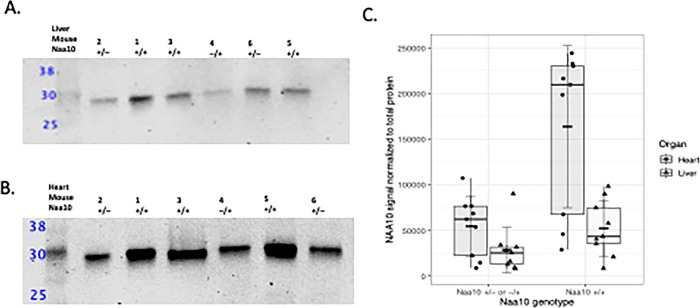
Using this Naa10-specific antibody, we quantified the relative amounts of Naa10 in heterozygous female mice (Fig 1; S10 and S11 Figs in S1 File). For quantification, Naa10 signal was normalized to total protein in each lane of a gel; post-transfer membranes were stained for total protein and scanned to verify transfer and equal loading before proceeding with Naa10 immunostaining. Liver and heart tissue lysates were prepared from Naa10-/+ and/or Naa10+/- (n = 3) and Naa10+/+ (n = 3) mice obtained from our animal colony. The former heterozygous genotypes differ based on the Naa10 knockout allele’s parent-of-origin. Based on the lack of maternal or paternal effect lethality in the embryonic and postnatal data (discussed below), we grouped the two Naa10 heterozygous mutant genotypes together. Western blots using samples from these were repeated in triplicate. Replicate analysis indicates the mean normalized Naa10 signal in Naa10+/- or Naa10-/+ animals is approximately 38% that of Naa10+/+ animals, a statistically significant difference (2-way ANOVA, F-statistic = 12.52 on 3 and 32 DF, *P < 0.05, R-Studio, Boston, MS, USA). According to post hoc Tukey testing, the main source of variation in mean NAA10 signal is attributable to genotype differences; organ type is not a significant source of variation, though it participates in a significant interaction with genotype. Notably, the reduction in normalized Naa10 signal was more variable compared to the Naa10-/Y dataset. However, for the Naa10-/Y dataset, one biological replicate was examined once in each organ, whereas each organ sample in the heterozygous mutant dataset was replicated three times. An earlier experiment (S11 Fig in S1 File) used liver lysates from a separate set of mice (n = 6) composed of Naa10–/+ (n = 3) and C57 females (n = 3); the mutant line [54] is now genetically inbred, with over 20 backcrosses to C57BL/6J. Compared to wild-type liver lysate, Naa10 levels are substantially reduced (>50%) in heterozygous liver, though this is a non-significant result due to variability (Welch’s 2-sample t-test, t = -1.6784, df = 9.1762, P = 0.1252). Heterozygous female mice can undergo random X-chromosome inactivation, which can lead to Naa10 variability within and between tissues.
Fig 1. Western blot analysis of liver and heart lysates from Naa10 heterozygous mutant female mice.
Biological replicates (n = 6) were obtained from Naa10+/–or Naa10–/+ mice (N = 3) and Naa10+/+ mice (N = 3). Liver and heart lysates were obtained from each mouse for immunoblotting; each liver and heart sample were replicated three times. Blots were stained for total protein post-transfer; after total protein stain removal, blots were incubated with anti-NAA10 MAb and anti-rabbit secondary). A) Representative western blot of NAA10 in liver lysates. B) Representative western blot of NAA10 in heart lysates. C) Quantification of NAA10 signal normalized to total protein. Short black crossbar indicates mean NAA10 signal normalized to total protein (±SD, 2-way ANOVA, F-statistic = 12.52 on 3 and 32 DF, *P < 0.05).
Effects of environment, genetic background, and parent-of-origin
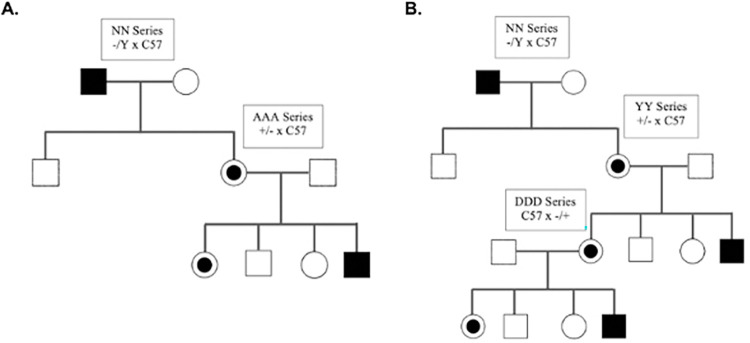
Given our findings related to genetic background on the phenotypes in the mice discussed above, we analyzed the phenotypes of Naa10 mice after being backcrossed for 20 generations with C57BL/6J mice; however, this analysis was complicated by the change of colony venue in March 2019, when the backcross was at the 15th generation. Once the backcross was completed to 20 generations, the embryonic dissections and live births occurred in this new environment. A second aspect of the experiment was meant to address whether Naa10 is associated with "maternal effect lethality", as a different group argued that maternal inheritance of the Naa10 knockout allele can have an effect with possible embryonic lethality for ~20% of heterozygous Naa10-/X females [52]. They used a nomenclature where Naa10-/X mice inherit the null allele maternally, whereas Naa10X/- female mice inherit the null allele paternally. We will use Naa10-/X and Naa10-/+ interchangeably in the remainder of this manuscript. We have previously presented data that minimizes this effect and suggested lethality might be due to other unidentified factors, such as decreased maternal care of offspring [21]. To address this further, several new breedings were undertaken, as detailed in Fig 2A and 2B. For example, embryos with maternal grandfather inherited Naa10 knockout are designated as the AAA mating series, whereas embryos with maternal grandmother inherited Naa10 knockout alleles are designated as the DDD mating series (with these letter combinations assigned based on internal lab databases).
Fig 2. Matings used to generate offspring and embryos.
The Naa10 knockout allele is inherited paternally in the NN series, whereas it is inherited maternally in the YY series. For embryo dissections AAA and DDD series, the allele is maternally inherited for both, but the origin of the allele is from the grandfather for the embryos from the AAA series and from the grandmother for the embryos in the DDD series. These letter combinations (like NN, YY, AAA, DDD) are arbitrary and assigned based on internal lab databases.
The Mendelian ratios for embryos from the AAA and DDD matings with the predicted genotypes are shown in Table 1, along with the data combined for AAA and DDD matings. The embryonic age ranged from E9.5 to E16.5, with the bulk of these matings falling between E9.5 to E13.5, as confirmed by Theiler staging comparison. There was some small degree of embryonic lethality (~20–25%) for Naa10-/y mice in both mating series, which differs from its absence in a somewhat mixed genetic background of mice bred in an animal facility in Korea, after at least 6 backcrosses to C57BL/6J [21]. Mendelian ratio calculations suggest neonatal lethality exist within both AAA and DDD combined populations where the observed percentage of Naa10 KO males deviated from the expected 25% to only 16.9% (Table 1).
Table 1. Genotypes of E9.5-E13.5 embryos from different mating series.
| Naa10x/- female mice crossed to C57BL/6J male mice (AAA series) | ||||||
|
Genotype
(Expected Mendelian %) |
Naa10
+/y
(25%) |
Naa10
-/y
(25%) |
Naa10
+/+
(25%) |
Naa10
-/+
(25%) |
Unable to genotype | Resorptions |
| Embryos + resorptions (n = 98) | 19 (19.6%) | 16 (16.5%) | 24 (24.7%) | 28 (28.6%) | 1 (1.0%) | 10 (10.3%) |
| Naa10-/x female mice crossed to C57BL/6J male mice (DDD series) | ||||||
|
Genotype
(Expected Mendelian %) |
Naa10
+/y
(25%) |
Naa10
-/y
(25%) |
Naa10
+/+
(25%) |
Naa10
-/+
(25%) |
Unable to genotype | Resorptions |
| Embryos + resorptions (n = 75) | 21 (28.00%) | 13 (17.33%) | 17 (22.67%) | 17 (22.67%) | 0 (0%) | 7 (10.29%) |
| Heterozygous Naa10x/- or Naa10-/x female mice crossed to C57BL/6J male mice (AAA + DDD series) | ||||||
|
Genotype
(Expected Mendelian %) |
Naa10
+/y
(25%) |
Naa10
-/y
(25%) |
Naa10
+/+
(25%) |
Naa10
-/+
(25%) |
Unable to genotype | Resorptions |
| Embryos + resorptions (n = 173) | 40 (23.3%) | 29 (16.9%) | 41 (23.8%) | 45 (26.2%) | 1 (0.58%) | 17 (11.0%) |
Expected and observed Mendelian ratio of genotypes in offspring from crosses, with paw tattoos and tails taken around day 6 of life.
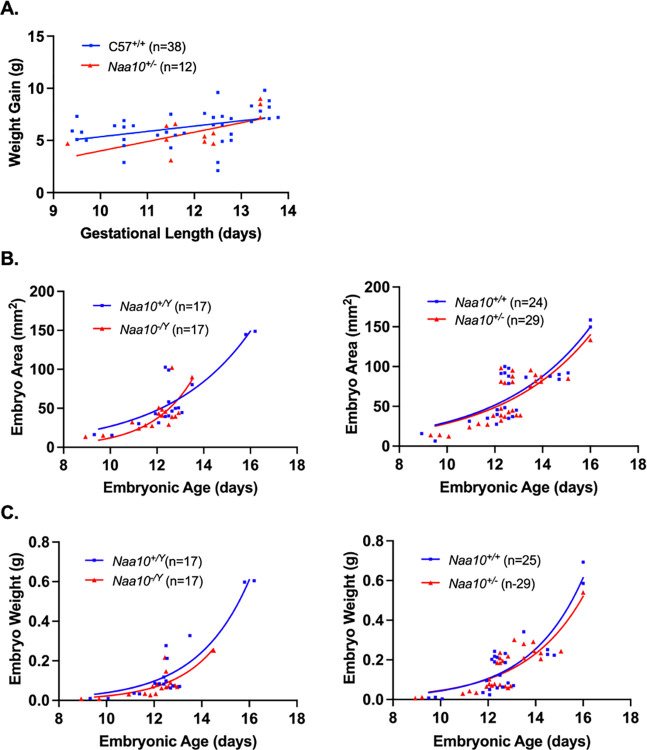
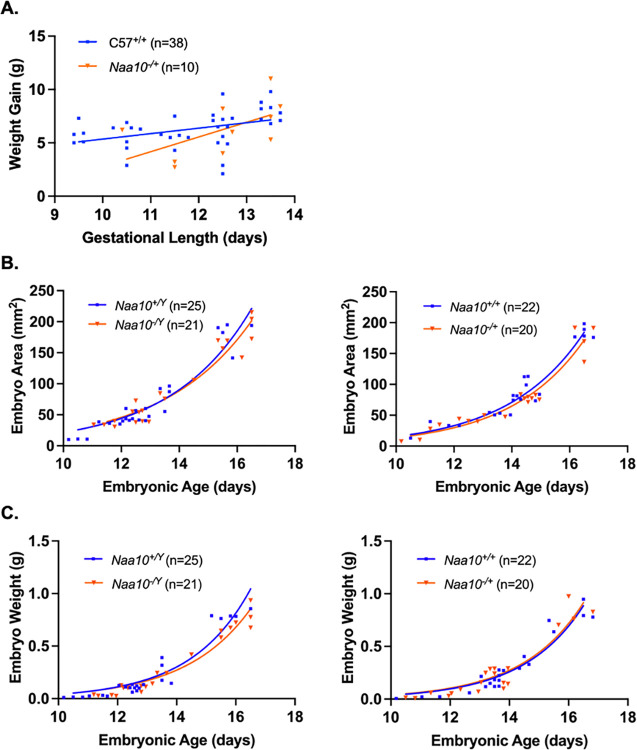
We next examined possible maternal effect lethality. The Naa10 allele of our AAA mating series is maternally inherited, with the mother of the dissected embryos having inherited the allele from her father (i.e., the maternal grandfather of the AAA embryos), as displayed in the breeding scheme. The number of our AAA embryos+resorptions (n = 98) versus (n = 16) is much larger than in the prior study [52] (Table 1). There is no statistically significant maternal effect lethality for the heterozygous Naa10-/X female mice (Table 2). Fig 3A shows weight gain of dams measured before their mating to their respective sire until the day of necropsy when embryos were harvested, dissected, and measured. t-tests comparing the weight gains of C57BL/6J and AAA dams at each length of pregnancy showed no significant statistical difference. Fig 3B and 3C show there is no statistically significant difference in either area or weight of wildtype embryos as compared to Naa10 knockout embryos in both males and females. Given the larger number of available embryos, within-litter analysis for E12.5 embryos was conducted, and Fig 4A and 4B show four litters only containing E12.5 embryos. The wildtype embryos were greater in area than the knockout mice; however, t-tests prove there was no statistical difference between the embryo areas and weights of the wildtype versus knockout embryos. Representative images of the embryos are shown in Fig 4C.
Table 2. Chi squared analyses.
| n-value | Degrees of Freedom | Critical Value | Chi Squared Value | Significance | |
|---|---|---|---|---|---|
| Chi Squared Analysis for all embryonic ages (Analyzing all four genotypes) | |||||
| Naa10+/- female mice crossed to C57BL/6J male mice (AAA series) | 115 | 3 | 7.81 | 6.89 | P>0.05 |
| Naa10-/+ female mice crossed to C57BL/6J male mice (DDD series) | 108 | 3 | 7.81 | 1.29 | P>0.05 |
| Naa10+/- or Naa10-/+ female mice crossed to C57BL/6J male mice (AAA + DDD series) | 223 | 3 | 7.81 | 5.84 | P>0.05 |
| Chi Squared Analysis for all embryonic ages (Analyzing only Naa10-/Y vs Naa10+/-and Naa10-/+) | |||||
| Naa10+/- female mice crossed to C57BL/6J male mice (AAA series) | 50 | 1 | 3.84 | 5.51 | *P<0.05 |
| Naa10-/+ female mice crossed to C57BL/6J male mice (DDD series) | 46 | 1 | 3.84 | 1.25 | P>0.05 |
| Naa10+/- or Naa10-/+ female mice crossed to C57BL/6J male mice (AAA + DDD series) | 96 | 1 | 3.84 | 5.18 | *P<0.05 |
| Chi Squared Analysis for E9.5-E13.5 embryos only (Analyzing all four genotypes) | |||||
| Naa10+/- female mice crossed to C57BL/6J male mice (AAA series) | 88 | 3 | 7.81 | 2.05 | P>0.05 |
| Naa10-/+ female mice crossed to C57BL/6J male mice (DDD series) | 75 | 3 | 7.81 | 2.78 | P>0.05 |
| Naa10+/- or Naa10-/+ female mice crossed to C57BL/6J male mice (AAA + DDD series) | 173 | 3 | 7.81 | 4.95 | P>0.05 |
| Chi Squared Analysis for E9.5-E13.5 embryos only (Analyzing only Naa10-/Y vs Naa10+/-and Naa10-/+) | P>0.05 | ||||
| Naa10+/- female mice crossed to C57BL/6J male mice (AAA series) | 44 | 1 | 3.84 | 3.28 | P>0.05 |
| Naa10-/+ female mice crossed to C57BL/6J male mice (DDD series) | 30 | 1 | 3.84 | 2.1 | P>0.05 |
| Naa10+/- or Naa10-/+ female mice crossed to C57BL/6J male mice (AAA + DDD series) | 74 | 1 | 3.84 | 4.65 | *P<0.05 |
Chi squared analyses calculated using critical value and p-value = 0.05.
Fig 3. Embryonic phenotypes of Naa10 knockout mice after 20 backcrosses, maternally inherited but originating from the maternal grandfather, and represented here as AAA.
(A) Naa10+/- and Naa10+/+ dams were weighed and compared at various lengths of gestation. However, there was no statistically significant difference between both simple linear regression lines that represent the weight gains of Naa10+/- and Naa10+/+ dams at their respective lengths of pregnancy (P>0.05). (B) Measured embryo surface areas of male (left) and female (right) versus embryonic age. The areas of Naa10-/Y and Naa10+/- embryos are not statistically different from those of wildtype (Naa10+/Y and Naa10+/+) embryos (P>0.05). (C) Embryo weights of male (left) and female (right) versus embryonic age were determined by measurement. The weights of Naa10-/Y and Naa10+/- embryos are not significantly different from those of wildtype embryos (P>0.05). Graphs B-C were produced using nonlinear exponential regression modeling.
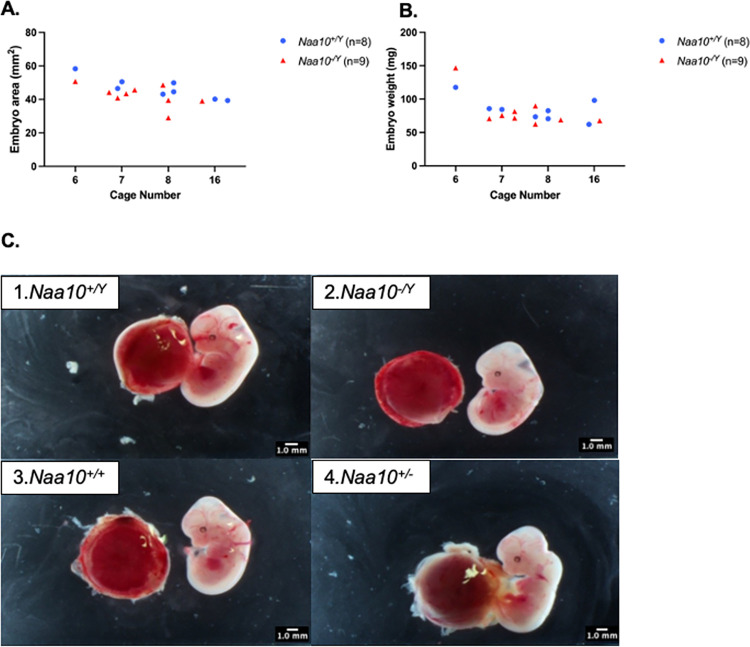
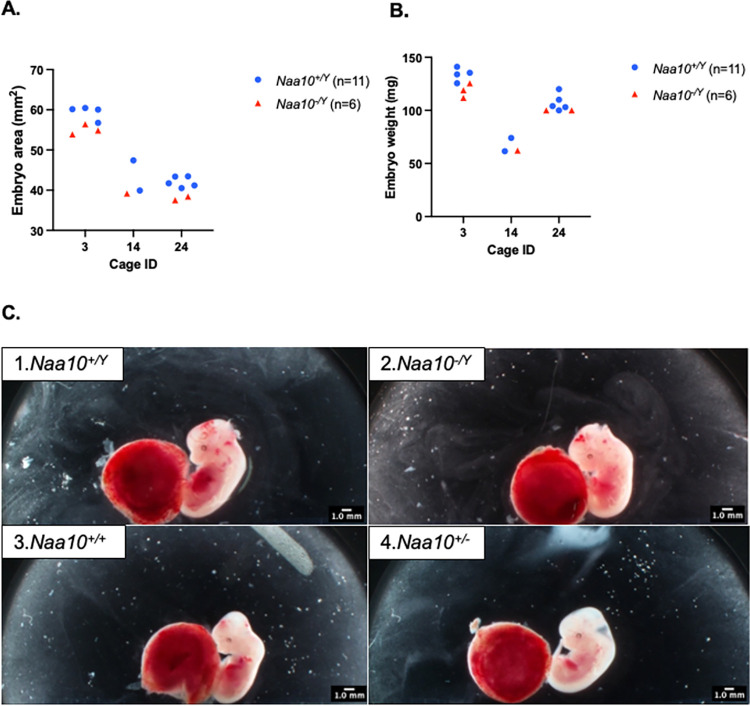
Fig 4. Within-litter analyses for embryos that inherited knockout for Naa10 maternally and from the maternal grandfather.
(A) Male embryo areas age E12.5 are graphed and grouped by litter, which were in cage #6, 7, 8 and 16). Two of the four litters contained more than one Naa10-/Y embryo. In all four litters, the embryo with the smallest area was the Naa10-/Y. (B) Male embryo weights age E12.5 are graphed and grouped by litter. Two out of four litters contained more than one Naa10-/Y embryo. In two out of four litters, the embryo with the lowest weight was the Naa10-/Y. (C) Pictures of E12.5 embryos from litter AAA8: Naa10+/Y (1), Naa10-/Y (2), Naa10+/+ (3), Naa10+/- (4).
The Naa10 allele in our DDD mating series is also inherited maternally, but with the mother of these embryos having inherited the allele from her mother, i.e., originating from the maternal grandmother. There is no maternal effect lethality for the heterozygous Naa10-/X female mice (Tables 1 and 2). Fig 5A shows the weight gain of DDD dams and weight gain of dams from the C57BL/6J line. The weight gain values are measured starting from mating date and day of dissection. T-tests comparing the weight gain of dams at each length of pregnancy showed no statistically significant difference (P>0.05). Fig 5B and 5C compare embryo areas and weights of Naa10 knockout male and female embryos compared to the area and weight values of wildtype embryos. There was no statistically significant difference (P>0.05). Within litter analyses were performed on our DDD embryos where there was no statistical difference caused by variation among different litters. Fig 6A shows three litters only containing E12.5 embryos. The smallest area was consistently measured in the Naa10 knockout embryo. Fig 6B shows the weights of the embryos in the three litters. In two of the three litters, the smallest weighing embryo was a Naa10 knockout. Fig 6C shows representative images of the DDD embryos.
Fig 5. Embryonic phenotypes of Naa10 knockout mice after 20 backcrosses, maternally inherited but originating from the maternal grandmother, and represented here as DDD.
(A) Naa10-/+ and Naa10+/+ dams were weighed and compared at various lengths of gestation. There was no statistically significant difference between both simple linear regression lines that represent the weight gains of Naa10+/- and Naa10+/+ dams at their respective length of pregnancy. (B) Measured embryo surface areas of male (left) and female (right) versus embryonic age. The areas of Naa10-/Y and Naa10-/+ embryos are not significantly different from those of wildtype embryos (P>0.05). (C) Embryo weights of male (left) and female (right) versus embryonic age were determined by measurement. The weights of Naa10-/Y and Naa10-/+ embryos are not statistically significantly different from those of wildtype (Naa10+/Y and Naa10+/+) embryos (P>0.05). Graphs B-C were produced using nonlinear exponential regression modeling.
Fig 6. Within-litter analyses for embryos that inherited knockout for Naa10 maternally and from the maternal grandmother.
(A) Male embryo areas age E12.5 are graphed and grouped by litter; two of the three litters contained more than one Naa10-/Y embryo. In all three litters, the embryo with the smallest area was the Naa10-/Y. (B) Male embryo weights age E12.5 are graphed and grouped by litter. Two out of three litters contained more than one Naa10-/Y embryo. In two out of three litters, the embryo with the lowest weight was the Naa10-/Y. (C) Pictures of E12.5 embryos from litter DDD14: Naa10+/Y (1), Naa10-/Y (2), Naa10+/+ (3), Naa10-/+ (4).
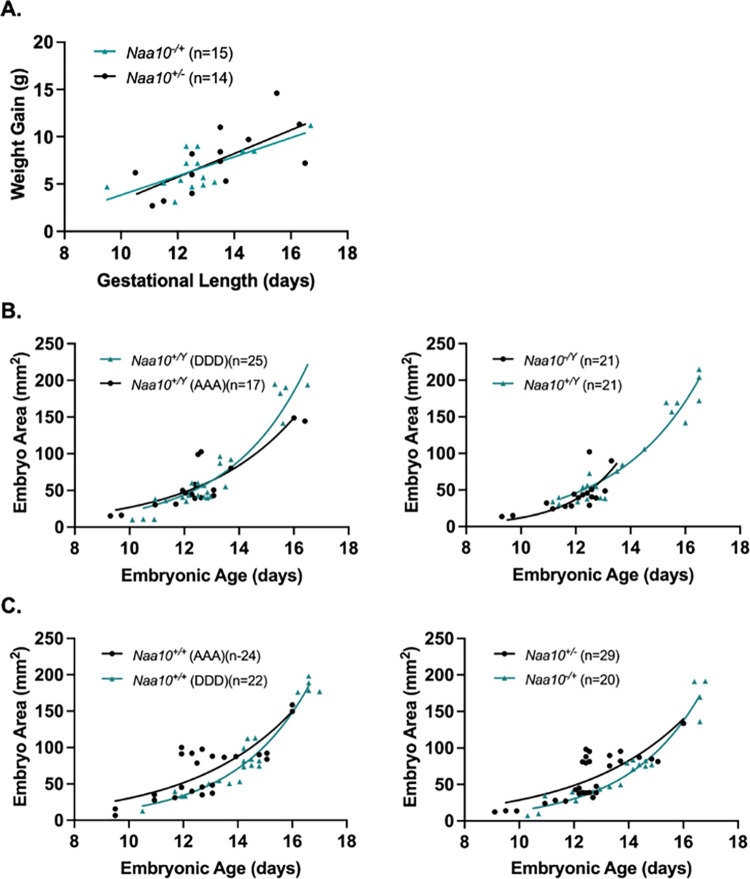
Fig 7A shows no difference in weight gain of the dams’ in both the AAA and DDD series matings. The measured embryo areas were not statistically different when analyzed by genotypes. Finally, data of the two breeding groups ranging from E9.5 to E13.5 were combined and shown at the bottom of Table 1. The difference in Mendelian ratios based on the number of heterozygous Naa10-/X female mice (n = 45) compared to the number of wild type male mice (n = 40) is not statistically significant.
Fig 7. Embryo area versus embryonic age for embryos with maternally inherited Naa10 knockout allele, where the allele originated from the maternal grandmother (DDD) or the maternal grandfather (AAA).
(A) Weight gain of Naa10-/+ (DDD dams) and Naa10+/- (AAA dams) at various lengths of gestation were compared. We observed no statistically significant difference between both simple linear regression lines that represent the weight gains of dams from both mating series at each length of pregnancy (P>0.05). (B) Left: Embryo area versus embryonic age for DDD vs. AAA Naa10+/Y males. Right: Embryo area versus embryonic age for DDD vs. AAA Naa10-/Y males. (C) Left: Embryo area versus embryonic age for DDD vs. AAA Naa10+/+ females. Right: Embryo area versus embryonic age for DDD Naa10-/+ vs. AAA Naa10+/- females. There are no statistically significant differences between the embryo areas for each genotype from each mouse line (P>0.05). Graphs B-C were produced using nonlinear exponential regression modeling.
Pleiotropic effects of Naa10 Knockout on postnatal mice
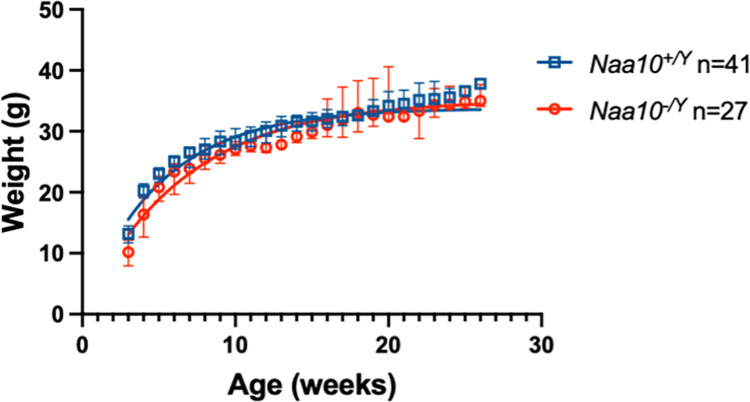
This current study continues our analysis of the original Naa10-/Y knockout strain of live born mice, as previously reported [21], but now genetically inbred with more than 20 backcrosses to C57BL/6J (Jackson Laboratories, Bar Harbour, ME). Surviving Naa10-/Y mice showed decreased body weight when compared to Naa10+/Y (WT) mice throughout the course of postnatal development. Fig 8 charts the body weights of male Naa10-/Y and Naa10+/Y mice from three to 20 weeks. As displayed in the Fig, Naa10-/Y mice consistently had reduced body weights compared to Naa10+/Y mice throughout the entire 18-week period where data was collected. The mean body weights of Naa10-/Y mice presented with lower than the mean body weight when compared to Naa10+/Y mice. To further assess whether these differences in body weights were significant, t-tests were performed comparing the two groups’ weight data each week. Analysis shows a statistically significant difference (*P<0.05) between the weights of Naa10+/Y and Naa10-/Y mice in 12 out of 18 weeks. Our observations show that the Naa10-/Y mice had significantly less body weight than the Naa10+/Y mice throughout most of their postnatal development with the exception of six out of the 18 weeks; specifically in weeks five and 16 through 20. During these six weeks there was no statistically significant difference (P>0.05); however, sample sizes were low which may have led to higher variability and lack of significance in these weeks. Fig 8 shows a phenotypic difference in body weight where the KO mouse is noticeably smaller than the WT littermate mouse. This is similar to the human condition, as some individuals with Ogden syndrome are small in weight and have short stature, whereas a few humans develop at typical size [53].
Fig 8. Phenotypic weight data of neonatal Naa10-/Y(KO) and Naa10+/Y(WT) mice after 20 backcrosses.
Male mice body weights were obtained from 3–20 weeks. Twelve out of 18 weeks showed a statistically significant difference between both nonlinear exponential regressions of body weights when comparing KO mice to wildtype littermates (*P<0.05).
Other phenotypic defects present in the Naa10 knockout mice include hydrocephalus, piebaldism, and hydronephrosis (Table 3), which are consistent with previous findings [21].
Table 3. Percentage of phenotypes observed in Naa10-/Y mice vs. C57BL/6J male mice.
| Phenotypes | Piebaldism | Hydrocephalus | Hydronephrosis | White digits and tail tips. |
|---|---|---|---|---|
| Percentage in Naa10-/Y mice | 97% (28/29) | 17% (5/29) | 27% (3/11) | 100% (29/29) |
| Percentage in male C57BL/6J mice | 0% |
1.6% (3/191) |
Never observed in routine necropsies |
0% |
S12A Fig in S1 File shows an Naa10-/Y mouse (left) with hydrocephaly as compared to a wildtype littermate with a normally developed skull as well as normal body composition. Hydrocephalus was observed in 17% (5/29) of Naa10-/Y mice. S12B Fig in S1 File displays piebaldism on the ventral abdomen of a Naa10-/Y mouse, which is a consistent phenotype seen in many Naa10-/Y mice. This phenotype was observed in 97% (28/29) of Naa10-/Y mice. The prior paper reported piebaldism in 100% of Naa10-/Y mice [21]. We believe that the one mouse we observed to lack piebaldism may have lost the hypopigmentation during maturation (discussed further below). S12C Fig in S1 File displays hydronephrosis in a Naa10-/Y mouse. The left kidney is noticeably enlarged, with the left kidney weighing 13.02 grams compared to the right kidney weighing 0.34 grams. S12C Fig in S1 File also shows that the Naa10-/Y mouse presenting with hydronephrosis also had an enlarged bladder. The hydronephrosis phenotype was observed in 27% (3/11) of surviving Naa10-/Y mice.
White digits and tails can be seen in the Naa10-/Y mice shown in S12A Fig (left) and S12B (left) in S1 File, as the Naa10-/Y mouse exhibits ventral abdominal piebaldism, white tail tips and digits, while its wildtype littermate does not. We observed this phenotype in 100% (29/29) of Naa10 knockout mice on this pure genetic background, displaying its complete penetrance on that background.
Discussion
NAA10, which encodes the catalytic subunit of the principal NAT complex (NatA) and is mutated in humans affected by OS, is likely an essential gene for eukaryotic development. Initially reported in yeast, other Naa10 (also known as Ard1) orthologues were later identified in humans and various model organisms, including D. melanogaster, D. rerio, A. thaliana, and M. musculus [55, 56]. In D. melanogaster, ovary-specific loss of Naa10 causes defective oogenesis in vnc mutants. The loss of Naa10 also contributes to cell growth and proliferation failure in wing discs [57]. Morpholino-mediated Naa10 knockdown in D. rerio increases developmental lethality; further, surviving zebrafish exhibit growth failure and other developmental abnormalities (e.g., bent axes, missing eyes, and missing tails) [58]. In addition to the apparent conservation of Naa10, the NTA profile of plants and animals are broadly similar which supports the necessity of this protein modification in eukaryotes [59, 60]. Naa10 knockout mouse lines also support the necessity of NatA-catalyzed NTA in mammalian development [21, 61, 62]. Previously, we showed that Naa12 complexes with Naa15 to form functional NatA in Naa10–/Y animals, which explains why these mice were born and were not completely embryonic lethal, at least in the mixed genetic background reported in that paper [21]. Confirming this, double mutants for Naa10 and Naa12 knockout mice were embyronic lethal [21].
The impact of genetic background is supported by our observation that additional null alleles (Naa10 Δ668 and Naa10 Δ668–674) on mixed genetic backgrounds (~ 75% C57BL/6J, 25% DBA/2J (D2)) have far less penetrance for piebaldism and much less (if any) neonatal lethality, whereas the piebaldism was fully penetrant after eight backcrosses to C57BL/6J for the previously published allele [21], along with the previously reported partial penetrance for ventricular septal defects (VSD), atrial septal defects (ASD), persistent truncus arteriosus (PTA) or double outlet right ventricle (DORV) of the mice who died in the first three days of life (n = 6/28, or 21%) [21]. We also previously reported that there was no embryonic lethality for the Naa10-/y mice after eight backcrosses to C57BL/6J, except for when the compensating enzyme Naa12 was knocked out [21]. The present study examined this on an inbred C57BL/6J genetic background (20 backcrosses) and in the new environment of the animal facility at IBR, where we did observe some embryonic lethality (~20–25% of Naa10-/Y male mice), even in the presence of Naa12. Phenotypic variation due to genetic background has been observed in several genetically engineered mouse models [63]. Alternatively, the transfer of all mice to a new animal colony and environment in March 2019 may have contributed to this embryonic lethality potentially through the effects of maternal stress on in utero development [64] or immune system dysfunction [65]. In support of this latter hypothesis, there was a parallel increase in neonatal lethality in the new colony observed for all mice, including the wild type C57BL/6J mice (unpublished data). Lastly, the gene targeting scheme presents the possibility that insertion of the neomycin cassette utilized to generate this strain of Naa10 KO mice may have resulted in dysregulation of off-target gene expression—a phenomenon documented in previously published literature [66–71], which could itself be modified by genetic background and environment, although this is not something that we have investigated for our mice.
We have previously noted that Naa10-/- mutants somewhat phenocopy Pax3 mutants [21]. Pax3 knockout mice, which model symptoms of Waardenburg syndrome, lack a transcription factor necessary for cardiac neural crest cell (cNCC) migration and proliferation in the pharyngeal arches and embryonic outflow tract. Without Pax3, inadequate NCC migration results in congenital heart defects attributable to improper remodeling of the arches and outflow tract [72–75]. Pax3+/- adults have 100% piebaldism, along with variable penetrance for neural crest (NC)-related persistent truncus arteriosus (PTA) or double outlet right ventricle (DORV) with concomitant ventricular septal defects VSDs [72, 73, 76, 77], congenital hydrocephalus [78], and/or skeletal defects due to abnormal somite morphogenesis [79, 80]. Of most relevance here, the phenotype of Pax3 nulls can be modulated by genetic background, in which it has been shown that those mice exhibit 100% mid-gestational lethality due to cardiac neural crest-related deficiencies on C57Bl/6J inbred genetic background [77]. As such, there is modulation of these neural crest-related phenotypes by genetic background. There is a long and very extensive literature regarding the modulation of phenotype by genetic background, some of which is summarized in a book chapter written by one of us [81, 82].
A prior study [52] found embryonic lethality in mice on a somewhat mixed genetic background, as that strain was used after "at least six generations of backcross with C57BL/6 mice", which was noted by the authors to be the sub-strain C57BL/6JNarl, first established at the Animal Center of National Research Institute from the Jackson Laboratories in 1995. However, as noted, the different environments of the animal facilities may play some role; therefore, embryonic lethality may be somehow more apparent even when the mice are not fully inbred. Also, the previous study [52] used the Cre/loxP system to generate the Naa10 KO mice, where a floxed Naa10 female mouse was crossed with the Ella-Cre transgenic male mouse expressing Cre recombinase for germ line deletion of loxP-flanked Naa10, whereas the Naa10-/y mice were made using standard gene-targeting methods without the use of Cre recombinase [21, 54]. It is not clear how Cre recombinase could impact the phenotype in the strain maintained at the Taiwan facility. It is notable that the other group did not comment on whether their mice have piebaldism or any skeletal defects, so it is not clear if the mice possess these phenotypes [52, 62].
We report that we cannot replicate the previously reported "maternal effect lethality" and we cannot find any correlation between the sizes of Naa10-null embryos and placental weight [52]. It is possible that additional study of earlier embryonic ages might reveal whether the embryonic lethality starts internally within the embryo or if it is mediated instead by some placental abnormality, as previously claimed [52]. Since this prior publication, there have been no other publications replicating these effects or the genomic imprinting findings of the paper. There is also still no crystal structure or any other structure showing that Naa10 has any direct DNA-binding domain or activity. The authors speculated in their paper that the various phenotypes of the mice (growth retardation, embryonic lethality, brain disorder, and maternal-effect lethality) might be caused by a previously unappreciated role for Naa10 in DNA methylation and genomic imprinting. Notably, that study was published prior to the discovery of Naa12 [21], and those results should be re-evaluated in the context of this compensating enzyme. The prior study utilized a small number of mice and samples for the various phenotypes in the mice such as "maternal effect lethality" and were able to only use two embryos of each genotype for the imprinting analyses [52]. It thus remains an open question in the field whether the various replicated phenotype findings in the mice (such as variable amounts of piebaldism, skeletal defects, small size, hydrocephaly, and hydronephrosis) are due to decreased amino-terminal acetylation of certain key proteins or whether some effect related to DNA methylation or genomic imprinting plays a role. Other groups have published that NAA10 might acetylate lysine side chains as a lysine acetyltransferase (KAT), although this is controversial [83]. Future studies are needed to identify acetylated proteins and to study their mechanism of action in both mouse models and humans.
Material and methods
Experimental animals
All experiments were performed in accordance with guidelines of International Animal Care and Use Committees (IACUC) of Cold Spring Harbor Laboratory (CSHL) and New York State Institute for Basic Research in Developmental Disabilities (IBR).Mice suffering from hydrocephalous or serve cases of ulcerative dermatitis, malocclusion, and microphthalmia due to genetics of the strain humane endpoint intervention was used. Humane endpoint intervention consisted of exposure to CO2 to render the animal unconscious, followed by cervical dislocation to ensure rapid passing. Potential breeding complications such as dystocia and severe fight wounds, partially cannibalized neonates, trampled neonates, loss of body condition, reproductive tumors and other causes of neonatal death, injured or traumatized animals are also sought humane endpoint intervention.
Mice were moved to the IBR facility in March 2019, after being housed at CSHL from 2015–2019. While at CSHL, they were housed as breeding pairs or were weaned and housed by sex in individually ventilated autoclaved caging (Ancare Ventilated Caging, Bellmore, NY). At IBR, animals are maintained in autoclaved cages and bedding with 1/8-inch corn cob bedding (The Andersons, Maumee, OH) and were fed a closed-formula, natural-ingredient, γ-irradiated diet (PicoLab Mouse Diet 5058, Purina LabDiet, St. Louis MO) ad libitum, and received tap municipal water in polysulfone bottles (Thoren). Mice receive a sterile supplement Love Mash Rodent Reproductive Diet (Bio-Serv; Flemington, NJ). A complete cage change was performed every 7–10 days within horizontal laminar flow cage change station (model Nu602-400Class II TypeNuaire, Plymouth, MN). The room was maintained on a 10:14-h light: dark cycle with a relative humidity of 30–70%, and room temperature ranging from 69–78°F. At IBR, since March 2019, mice were housed in a specific pathogen free room of a conventional animal facility in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition) [84]. Mice were housed as breeding pairs or were weaned and housed by sex in individually ventilated autoclaved caging (no. 5, Thoren Caging Systems, Hazelton, PA).
Rodent health monitoring assessment is performed three times a year. Mice in this colony are specific pathogen free for the following; astroviruses types 1 and 2, new world Hantaviruses, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, mouse hepatitis virus, ectromelia virus, mouse kidney parvovirus (murine chapparvovirus), mouse parvovirus, minute virus of mice, mouse rotavirus (epizootic diarrhea of infant mice virus), pneumonia virus of mice, reovirus, Sendai virus, Theiler meningoencephalitis virus; beta Strep Groups A, B, C and G, Bordetella bronchiseptica, Bordetella pseudohinzii, Campylobacter spp., ciliary-associated respiratory bacillus (F. rodentium),Corynbacterium kutscheri, Klebsiella oxytoca, Klebsiella pneumoniae, Mycoplasma pulmonis, Citrobacter rodentium, Rodentibacter spp., Chlamydia muridarum, Pseudomonas aeruginosa, Salmonella spp., Streptobacillus moniliformis, Proteus mirabilis, and Clostridium piliforme; Pneumocystis, Giardia spp. and Spironucleus muris; and fur mites (Myobia musculi, Myocoptes musculinis, and Radfordia affinis) and pinworms (Syphacia spp. and Aspiculuris spp.) all of which is based on multiplex polymerase chain reaction pooled from oral swabs, pelt swabs, and fecal pellets obtained directly (representing 10–15% of cages) and serologic panels from a subset (representing ~2% of cages). PCR and serology were performed by Charles River Laboratories (Wilmington, MA).
Generation and genotyping of Naa10 knockout mice
The Naa10 knockout (KO) mice were generated as previously described (Yoon, H., et al. [54]). The progeny was backcrossed to C57BL/6J for more than 20 generations. This was confirmed with genome scanning at the Jackson Laboratory. The stock of C57BL/6J was replenished annually from Jackson Laboratory (JAX) to avoid genetic drift from the JAX inbred line. Paw tattoo and tail genotyping was performed on day 5 or 6 of life, so as not disturb the litters and thus not increase the risk for maternal rejection of the litter. The primers used for Naa10 KO and Naa10tm1a genotyping were Naa10-F: 5’-cctcacgtaatgctctgcaa-3’, Naa10-neo-F: 5’-acgcgtcaccttaat-atgcg-3’, Naa10-R: 5’- tgaaagttgagggtgttgga-3’ (S8 Table in S1 File).
Generation and genotyping of Naa10 minigene mice
Standard methods were used to generate and select ES clones that were used for blastocyst microinjection and generation of chimeric mice. Chimeric mice were mated with C57BL/6 mice, and germ-line transmission of targeted alleles was detected by PCR. Primers used were: MG1F: 5’-GTCGACGGCTCAGCATGAAGA; Lox1: 5’-AGCTCCTATCGTCCTTTCCCTGC; SQ2: 5’-AACTATGGCCAGCTTGCTATG; and PT4: 5’-TCTCCAGTCTACCTCTACCAAACCC. Ser37Pro Genotyping PCR: MG1F-PT4, 980 bp product; Ser37Pro mutation activation PCR: Lox1-MG1F (730 bp) or Lox1-SQ2 (1150 bp).
Generation of Naa10 (Gm16286, UniProt: Q9CQX6) indel knockout mice
The mice were made using standard methods by microinjection of CRISPR reagent mix into zygotes obtained from the mating of B6D2F1 females (i.e., 50% C57BL/6J, 50% DBA/2J (D2)) females to inbred C57BL/6J males. The guide RNA was produced and validated from Horizon using a Cel1-nuclease assay, and the most active guide was selected, including the targeting cr-RNA sequence and the tracrRNA portion. The indels were transmitted by breeding again to inbred C57BL/6J males, and the resulting progeny were interbred on a mixed genetic background of approximately 12.5% DBA/2J (D2) / 87.5% C57BL/6J, for use in the reported experiments. Genomic DNA was isolated from paw and tail. DNA was screened for mutations using PCR and Surveyor assay [85], followed by Sanger sequencing of selected clones and the use of CRISP-ID [86] to identify putative deletions.
Quantitative PCR
Organs from mice were dissected >2 months after birth. 70–120 mg tissue (heart/kidney/liver) were lysed in 5 μl/mg tissue RIPA buffer (Sigma) with 1x Complete protease inhibitors and 1 U/μl Superase In (Thermo Scientific, Waltham, MA, USA) using Fisherbrand Pellet Pestle Cordless Motor. After homogenization debris was removed by centrifugation at 20.800 g for 10 min at 4°C. Protein concentration was determined using APA assay (Cytoskeleton Inc. Denver, CO, USA) and 50 μg total protein were separated on SDS-PAGE followed by western blot. Membranes were stained with anti-Naa10, anti-Naa15 and anti-GAPDH antibodies (all Abcam, Waltham, MA,USA). For RNA purification, 30 μl clarified lysate were mixed with 70 μl RNase free water and RNA isolated using the RNeasy Mini Kit (Qiagen, Germantown, MA, USA) according to the manufacturer’s recommendations, including on-column Dnase digest. 1 μg RNA was reverse transcribed using the TaqMan Reverse transcription kit and gene level detection performed using SYBR Green
Master Mix (all Thermo Scientific). Relative expression was normalized to GAPDH and ACTB. The following primer pairs were used:
Naa10 (exon2-4) CTCTTGGCCCCAGCTTTCTT & TCGTCTGGGTCCTCTTCCAT
Naa11 ACCCCACAAGCAAAGACAGTG & AGCGATGCTCAGGAAATGCTCT
GAPDH AGGTCGGTGTGAACGGATTTG & TGTAGACCATGTAGTTGAGGTCA
ACTB GGCTGTATTCCCCTCCATCG & CCAGTTGGTAACAATGCCATGT
Isolation and imaging of mouse embryos
Timed matings were performed by counting the number of days since the male and female were paired. The male mice were left in the cage for three days, prior to removal, giving a three-day window for embryogenesis. Theiler staging was performed for a precise gestational age. Pregnant mice were euthanized at several time points after conception. The embryos were isolated on ice, then washed three times in cold 1% PBS. Embryos were imaged using an Olympus SZX10 with Olympus CellSens imaging software (Center Valley, PA, USA). Both embryos and placentas were measured, weighed, then stored in 10% formalin buffered saline and then stored at four degrees Celsius. Scale bars were created for adult mouse and embryo dissections using software program Fiji by ImageJ (National Institute of Health, Public Domain, BSD-2)
Whole body CT scanning
CT scans were acquired on a Nanoscan PET/CT scanner at CSHL from Mediso using Nucline v2.01 software. All mice were kept sedated under isoflurane anesthesia for the duration of the scan. Scans were acquired with an X-ray tube energy and current of 70kVp and 280uA respectively. 720 projections were acquired per rotation, for 3 rotations, with a scan time of approximately 11 minutes, followed by reconstruction with a RamLak filter and voxel size 40x40x122μm. The relative mean bone density of the femur from these mice was measured in Hounsfield units using VivoQuant software (v2.50patch2). Briefly, the femur was accurately segmented from the image by first applying a ROI about the bone. Global thresholding, with a minimum of 1000 HU and a maximum of 8000 HU was then applied to accurately segment the femur from the initial ROI. For ex vivo analyses, mouse heads were fixed in 10% formalin buffered saline, followed by scanning and reconstruction with 1440 projections per revolution. Cranial volume was measured using VivoQuant software (v2.50patch2), using the spline tool to manually draw around the circumference of the cranium on multiple stepwise 2D slices.
Western blot
Adult mice were euthanized in a CO2 chamber, followed by cervical dislocation. Tissue was dissected and washed in 1% PBS before immediate processing or flash freezing in liquid nitrogen. Fresh or thawed tissue was lysed in RIPA buffer supplemented with protease inhibitor (#R0278, Sigma-Aldrich; #11836170001, Roche) and disrupted using a handheld cordless motorized microtube pestle. Lysate was cleared via centrifugation. Protein quantification was determined via Bradford assay using PrecisionRed Advanced Protein Assay reagent (#ADV01, Cytoskeleton, Denver, CO, USA). Lysate concentration was normalized before dilution in 2X Laemmli Sample buffer with 10% v/v 2-mercaptoethanol. Accordingly, reducing and denaturing SDS-PAGE was conducted on 10% resolving gel (#4561033, Bio-Rad, Hercules, CA, USA) in the Mini-PROTEAN Tetra Cell system. Resolved proteins were transferred to 0.2 μm nitrocellulose membrane (Amersham, Buckinghamshire, UK) using Towbin’s transfer buffer (100 V, 30 min). Membranes were dried at least 15 minutes before reactivating in TBS. Reactivated membranes were stained for total protein using REVERT 700 Total Protein stain kit and scanned wet on Odyssey Classic in the 700nm channel (LI-COR, Lincoln, NB, USA). S9 Table in S1 File.
After scanning, membranes were blocked in 5% non-fat dry milk (1 hr, RT). Blocked membranes were incubated with 1/500 anti-NAA10 Mab (#13357, Cell Signaling Technology, Danvas, MA, USA) diluted in blocking buffer supplemented with 0.1% Tween-20 (overnight, 4°C). After primary incubation, membranes were placed in TBS-T for 5 minutes (3 repetitions). Membranes were then incubated in 1/20,000 goat-anti-rabbit IR800 CW secondary antibody diluted in blocking buffer (1 hr, RT). Stained membranes were washed in TBS-T for 5 minutes (3 repetitions) and rinsed in TBS. Membranes were dried before scanning on the Odyssey Classic. NAA10 signal was normalized to total protein as indicated by REVERT 700 stains; normalized NAA10 signal was quantified in Empiria Studio (LI-COR). Hypothesis testing was conducted in RStudio. Western blot datasets and analyses are available on Github (https://github.com/ajgarcuny).
For Western blotting of the minigene and inverted Naa10 mice, 50–180 mg tissue (liver or heart) was lysed in RIPA buffer (Sigma) with Complete protease inhibitors (5 ul lysis buffer per mg tissue) using Fisherbrand Pellet Pestle Cordless Motor. After homogenization debris was removed by centrifugation at 20,800 g for 10 min at 4°C. Protein concentration was determined using APA assay (Cytoskeleton Inc.) and 50–100 μg total protein was separated on SDS-PAGE followed by western blot. Membranes were stained with anti-Naa10, anti-Naa15 and anti-GAPDH antibodies (all Abcam).
For Western blotting on the indel mice, three mice of each genotype (NAA10 wild type [wt], Δ668, or Δ668–674), tissue lysates were prepared from 30–80 μg of liver by mechanical lysis in RIPA buffer. Protein concentration was determined using a commercial version of the Bradford assay, and then normalized to 5 mg/ml in Laemmli SDS-PAGE Sample Buffer. Twenty microliters (i.e., 100 μg) was then loaded on each of two 10% SDS-PAGE gels. After running, gels were transferred to PVDF membranes and then blocked. After blocking, one membrane was incubated with rabbit anti-Naa10 (ab155687, Abcam, Walthan, USA) followed by goat anti-rabbit IRDye 680RD (926–68071, LI-COR, Lincoln, NB, USA), while the other was incubated with mouse anti-GAPDH (Abcam ab9484) followed by goat anti-mouse IRDye 800CW (LI-COR 926–32210). The membranes were then imaged on a LI-COR Odyssey scanner with the 700 and 800 nm channels.
Measurement of piebaldism
While at CSHL, photographs of anesthetized or euthanized mice with piebaldism were obtained using a digital camera with a ruler alongside each animal to facilitate measurement of the surface area of the spotting, using the software program ImageJ (National Institute of Health, Public Domain, BSD-2). For those mice photographed during anesthesia, they were continually sedated with isoflurane, and many of these mice underwent CT scanning. Mice were weighed at the time of photography. Data was plotted using GraphPad Prism 9.5.1 (San Diego,CA, USA) for Macintosh IOS.
Statistical analysis
Chi squared analyses were preformed analyzing maternal effect lethality using a statistical threshold of 0.05. For data comparisons of two groups, two-tailed unpaired student’s t-test or multiple unpaired t-tests were used to calculate statistical significances between means using GraphPad Prism version 9.5.1(528) (San Diego, CA, USA). All t-tests performed herein utilized a significance threshold of 0.05. For western blotting and total protein assays Welch’s two sample t-test and two-way ANOVAs were performed using R-Studio version 1.4.1564 (Boston, MS, USA) for Macintosh IOS. Asterisks denote statistical significance marked by *p<0.05, **p<0.01, ***p<0.0001. Simple linear regression models were used to analyze differences between length of gestation and weights of dams using GraphPad Prism version 9.5.1(528) (San Diego, CA, USA). For analyzing embryo weight, embryo area and embryonic age nonlinear exponential regression models were performed using GraphPad Prism version 9.5.1(528) (San Diego, CA, USA).
Data analysis
GraphPad Prism software version 9.5.1(528) (San Diego, CA, USA), Microsoft Excel version 16.72 (Redmond, WA, USA) and Fiji by ImageJ (National Institute of Health, Public Domain, BSD-2) were used.
Supporting information
(PDF)
(PDF)
Acknowledgments
GJL would like to thank the staff of the animal facility and/or transgenic core facility at CSHL (Leyi Li, Jodi Coblentz, Rachel Rubino, and Lisa Bianco) and IBR (Michael Parascando) for their assistance. Alison Sebold assisted with dissection and characterization of mouse vertebrae. We thank Dr. Goo Taeg Oh at Ewha Woman’s University (Seoul, Korea) for providing the original Naa10-/y mice to us.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work has been supported by Cold Spring Harbor Laboratory startup funds (G.J.L.), New York State Office for People with Developmental Disabilities (OPWDD) (G.J.L.), NIH NIGMS R35-GM-133408 (G.J.L.), The Research Council of Norway 249843 (T.A.), ISCIII Consolidation Program Grant (R.A.), Ministerio Español de Economía y Competitividad Torres Quevedo Program (PTQ-13-06466), Departamento de Desarrollo Económico del Gobierno de Navarra (0011-1383-2018-000011) (R.A.) and Fundación para la Investigación Médica Aplicada (FIMA) (R.A.).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A. 2009;106: 8157–8162. doi: 10.1073/pnas.0901931106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starheim KK, Gevaert K, Arnesen T. Protein N-terminal acetyltransferases: when the start matters. Trends Biochem Sci. 2012;37: 152–161. doi: 10.1016/j.tibs.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 3.Arnesen T, Anderson D, Baldersheim C, Lanotte M, Varhaug JE, Lillehaug JR. Identification and characterization of the human ARD1-NATH protein acetyltransferase complex. Biochem J. 2005;386: 433–443. doi: 10.1042/BJ20041071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnesen T, Starheim KK, Van Damme P, Evjenth R, Dinh H, Betts MJ, et al. The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation. Mol Cell Biol. 2010;30: 1898–1909. doi: 10.1128/MCB.01199-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb L, Marmorstein R. Structure of Human NatA and Its Regulation by the Huntingtin Interacting Protein HYPK. Structure. 2018;26: 925–935.e8. doi: 10.1016/j.str.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dörfel MJ, Lyon GJ. The biological functions of Naa10—from amino-terminal acetylation to human disease. Gene. 2015;567: 103–131. doi: 10.1016/j.gene.2015.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksnes H, Ree R, Arnesen T. Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases. Mol Cell. 2019;73: 1097–1114. doi: 10.1016/j.molcel.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polevoda B, Arnesen T, Sherman F. A synopsis of eukaryotic Nalpha-terminal acetyltransferases: nomenclature, subunits and substrates. BMC Proc. 2009;3 Suppl 6: S2. doi: 10.1186/1753-6561-3-S6-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ree R, Varland S, Arnesen T. Spotlight on protein N-terminal acetylation. Exp Mol Med. 2018;50: 90. doi: 10.1038/s12276-018-0116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shemorry A, Hwang C-S, Varshavsky A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol Cell. 2013;50: 540–551. doi: 10.1016/j.molcel.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dikiy I, Eliezer D. N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound α-synuclein and increases its affinity for physiological membranes. J Biol Chem. 2014;289: 3652–3665. doi: 10.1074/jbc.M113.512459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott DC, Monda JK, Bennett EJ, Harper JW, Schulman BA. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science. 2011;334: 674–678. doi: 10.1126/science.1209307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes WM, Mannakee BK, Gutenkunst RN, Serio TR. Loss of amino-terminal acetylation suppresses a prion phenotype by modulating global protein folding. Nat Commun. 2014;5: 4383. doi: 10.1038/ncomms5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Mijares M, Gall MD, Turan T, Javier A, Bornemann DJ, et al. Drosophila variable nurse cells encodes arrest defective 1 (ARD1), the catalytic subunit of the major N-terminal acetyltransferase complex. Dev Dyn. 2011;240: spcone–spcone. doi: 10.1002/dvdy.22559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Zhang J, Minnerly J, Kaul T, Riddle DL, Jia K. daf-31 encodes the catalytic subunit of N alpha-acetyltransferase that regulates Caenorhabditis elegans development, metabolism and adult lifespan. PLoS Genet. 2014;10: e1004699. doi: 10.1371/journal.pgen.1004699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linster E, Stephan I, Bienvenut WV, Maple-Grødem J, Myklebust LM, Huber M, et al. Downregulation of N-terminal acetylation triggers ABA-mediated drought responses in Arabidopsis. Nat Commun. 2015;6: 7640. doi: 10.1038/ncomms8640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Li R, Yu J, Ma S, Wu C, Li Y, et al. Protein N-terminal acetylation is required for embryogenesis in Arabidopsis. J Exp Bot. 2016;67: 4779–4789. doi: 10.1093/jxb/erw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Li S, Li L, Wu W, Ke X, Zou W, et al. Nα-acetyltransferase 10 and 15 are required for the correct initiation of endosperm cellularization in Arabidopsis. Plant Cell Physiol. 2018;59: 2113–2128. doi: 10.1093/pcp/pcy135 [DOI] [PubMed] [Google Scholar]
- 19.Mullen JR, Kayne PS, Moerschell RP, Tsunasawa S, Gribskov M, Colavito-Shepanski M, et al. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989;8: 2067–2075. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2551674 doi: 10.1002/j.1460-2075.1989.tb03615.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polevoda B, Sherman F. Composition and function of the eukaryotic N-terminal acetyltransferase subunits. Biochem Biophys Res Commun. 2003;308: 1–11. doi: 10.1016/s0006-291x(03)01316-0 [DOI] [PubMed] [Google Scholar]
- 21.Kweon HY, Lee M-N, Dorfel M, Seo S, Gottlieb L, PaPazyan T, et al. Naa12 compensates for Naa10 in mice in the amino-terminal acetylation pathway. Elife. 2021;10. doi: 10.7554/eLife.65952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blomen VA, Májek P, Jae LT, Bigenzahn JW, Nieuwenhuis J, Staring J, et al. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350: 1092–1096. doi: 10.1126/science.aac7557 [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350: 1096–1101. doi: 10.1126/science.aac7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang AL, Clark J, Chan WY, Rennert OM. Expression of human NAA11 (ARD1B) gene is tissue-specific and is regulated by DNA methylation. Epigenetics. 2011;6: 1391–1399. doi: 10.4161/epi.6.11.18125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnesen T, Betts MJ, Pendino F, Liberles DA, Anderson D, Caro J, et al. Characterization of hARD2, a processed hARD1 gene duplicate, encoding a human protein N-alpha-acetyltransferase. BMC Biochem. 2006;7: 13. doi: 10.1186/1471-2091-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rope AF, Wang K, Evjenth R, Xing J, Johnston JJ, Swensen JJ, et al. Using VAAST to identify an X-linked disorder resulting in lethality in male infants due to N-terminal acetyltransferase deficiency. Am J Hum Genet. 2011;89: 28–43. doi: 10.1016/j.ajhg.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon GJ. Personal account of the discovery of a new disease using next-generation sequencing. Interview by Natalie Harrison. Pharmacogenomics. 2011;12: 1519–1523. doi: 10.2217/pgs.11.117 [DOI] [PubMed] [Google Scholar]
- 28.Gogoll L, Steindl K, Joset P, Zweier M, Baumer A, Gerth-Kahlert C, et al. Confirmation of Ogden syndrome as an X-linked recessive fatal disorder due to a recurrent NAA10 variant and review of the literature. Am J Med Genet A. 2021;185: 2546–2560. doi: 10.1002/ajmg.a.62351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofman J, Hutny M, Chwialkowska K, Korotko U, Loranc K, Kruk A, et al. Case report: Rare among ultrarare-Clinical odyssey of a new patient with Ogden syndrome. Front Genet. 2022;13: 979377. doi: 10.3389/fgene.2022.979377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Damme P, Støve SI, Glomnes N, Gevaert K, Arnesen T. A Saccharomyces cerevisiae model reveals in vivo functional impairment of the Ogden syndrome N-terminal acetyltransferase NAA10 Ser37Pro mutant. Mol Cell Proteomics. 2014;13: 2031–2041. doi: 10.1074/mcp.M113.035402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dörfel MJ, Fang H, Crain J, Klingener M, Weiser J, Lyon GJ. Proteomic and genomic characterization of a yeast model for Ogden syndrome. Yeast. 2017;34: 19–37. doi: 10.1002/yea.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myklebust LM, Van Damme P, Stove SI, Dörfel MJ, Abboud A, Kalvik TV, et al. Biochemical and cellular analysis of Ogden syndrome reveals downstream Nt-acetylation defects. Hum Mol Genet. 2015;24: 1956–1976. doi: 10.1093/hmg/ddu611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bader I, McTiernan N, Darbakk C, Boltshauser E, Ree R, Ebner S, et al. Severe syndromic ID and skewed X-inactivation in a girl with NAA10 dysfunction and a novel heterozygous de novo NAA10 p.(His16Pro) variant—a case report. BMC Med Genet. 2020;21: 153. doi: 10.1186/s12881-020-01091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey JP, Støve SI, McGorrian C, Galvin J, Blenski M, Dunne A, et al. NAA10 mutation causing a novel intellectual disability syndrome with Long QT due to N-terminal acetyltransferase impairment. Sci Rep. 2015;5: 16022. doi: 10.1038/srep16022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H, Gottlieb L, Marchi E, Kleyner R, Bhardwaj P, Rope AF, et al. Phenotypic and biochemical analysis of an international cohort of individuals with variants in NAA10 and NAA15. Hum Mol Genet. 2019. doi: 10.1093/hmg/ddz111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McTiernan N, Gill H, Prada CE, Pachajoa H, Lores J, CAUSES study, et al. NAA10 p.(N101K) disrupts N-terminal acetyltransferase complex NatA and is associated with developmental delay and hemihypertrophy. Eur J Hum Genet. 2021;29: 280–288. doi: 10.1038/s41431-020-00728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popp B, Stove SI, Endele S, Myklebust LM, Hoyer J, Sticht H, et al. De novo missense mutations in the NAA10 gene cause severe non-syndromic developmental delay in males and females. Eur J Hum Genet. 2015;23: 602–609. doi: 10.1038/ejhg.2014.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ree R, Geithus AS, Tørring PM, Sørensen KP, Damkjær M, DDD study, et al. A novel NAA10 p.(R83H) variant with impaired acetyltransferase activity identified in two boys with ID and microcephaly. BMC Med Genet. 2019;20: 101. doi: 10.1186/s12881-019-0803-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Støve SI, Blenski M, Stray-Pedersen A, Wierenga KJ, Jhangiani SN, Akdemir ZC, et al. A novel NAA10 variant with impaired acetyltransferase activity causes developmental delay, intellectual disability, and hypertrophic cardiomyopathy. Eur J Hum Genet. 2018;26: 1294–1305. doi: 10.1038/s41431-018-0136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afrin A, Prokop JW, Underwood A, Uhl KL, VanSickle EA, Baruwal R, et al. NAA10 variant in 38-week-gestation male patient: a case study. Cold Spring Harb Mol Case Stud. 2020;6. doi: 10.1101/mcs.a005868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esmailpour T, Riazifar H, Liu L, Donkervoort S, Huang VH, Madaan S, et al. A splice donor mutation in NAA10 results in the dysregulation of the retinoic acid signalling pathway and causes Lenz microphthalmia syndrome. J Med Genet. 2014;51: 185–196. doi: 10.1136/jmedgenet-2013-101660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston JJ, Williamson KA, Chou CM, Sapp JC, Ansari M, Chapman HM, et al. NAA10 polyadenylation signal variants cause syndromic microphthalmia. J Med Genet. 2019; jmedgenet-2018. doi: 10.1136/jmedgenet-2018-105836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta AS, Saif HA, Lent JM, Couser NL. Ocular Manifestations of the NAA10-Related Syndrome. Case Rep Genet. 2019;2019: 8492965. doi: 10.1155/2019/8492965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunier C, Stove SI, Popp B, Gerard B, Blenski M, AhMew N, et al. Expanding the Phenotype Associated with NAA10-Related N-Terminal Acetylation Deficiency. Hum Mutat. 2016;37: 755–764. doi: 10.1002/humu.23001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maini I, Caraffi SG, Peluso F, Valeri L, Nicoli D, Laurie S, et al. Clinical Manifestations in a Girl with NAA10-Related Syndrome and Genotype–Phenotype Correlation in Females. Genes. 2021;12: 900. doi: 10.3390/genes12060900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McTiernan N, Tranebjærg L, Bjørheim AS, Hogue JS, Wilson WG, Schmidt B, et al. Biochemical analysis of novel NAA10 variants suggests distinct pathogenic mechanisms involving impaired protein N-terminal acetylation. Hum Genet. 2022. doi: 10.1007/s00439-021-02427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng H, Dharmadhikari AV, Varland S, Ma N, Domingo D, Kleyner R, et al. Truncating Variants in NAA15 Are Associated with Variable Levels of Intellectual Disability, Autism Spectrum Disorder, and Congenital Anomalies. Am J Hum Genet. 2018;102: 985–994. doi: 10.1016/j.ajhg.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritter A, Berger JH, Deardorff M, Izumi K, Lin KY, Medne L, et al. Variants in NAA15 cause pediatric hypertrophic cardiomyopathy. Am J Med Genet A. 2021;185: 228–233. doi: 10.1002/ajmg.a.61928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward T, Tai W, Morton S, Impens F, Van Damme P, Van Haver D, et al. Mechanisms of congenital heart disease caused by NAA15 haploinsufficiency. Circ Res. 2021;128: 1156–1169. doi: 10.1161/CIRCRESAHA.120.316966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Y, Xie H, Yang S, Shangguan S, Wang J, Jin C, et al. Possible Catch-Up Developmental Trajectories for Children with Mild Developmental Delay Caused by NAA15 Pathogenic Variants. Genes. 2022;13. doi: 10.3390/genes13030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyon GJ, Vedaie M, Besheim T, Park A, Marchi E, Gottlieb L, et al. Expanding the Phenotypic spectrum of Ogden syndrome (NAA10-related neurodevelopmental syndrome) and NAA15-related neurodevelopmental syndrome. medRxiv. 2022. doi: 10.1101/2022.08.22.22279061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CC, Peng SH, Shen L, Lee CF, Du TH, Kang ML, et al. The Role of N-alpha-acetyltransferase 10 Protein in DNA Methylation and Genomic Imprinting. Mol Cell. 2017. doi: 10.1016/j.molcel.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandomirsky K, Marchi E, Gavin M, Amble K, Lyon GJ. Phenotypic variability and gastrointestinal manifestations/interventions for growth in NAA10-related neurodevelopmental syndrome. Am J Med Genet A. 2023. doi: 10.1002/ajmg.a.63152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon H, Kim HL, Chun YS, Shin DH, Lee KH, Shin CS, et al. NAA10 controls osteoblast differentiation and bone formation as a feedback regulator of Runx2. Nat Commun. 2014;5: 5176. doi: 10.1038/ncomms6176 [DOI] [PubMed] [Google Scholar]
- 55.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325: 595–622. doi: 10.1016/s0022-2836(02)01269-x [DOI] [PubMed] [Google Scholar]
- 56.Whiteway M, Freedman R, Van Arsdell S, Szostak JW, Thorner J. The yeast ARD1 gene product is required for repression of cryptic mating-type information at the HML locus. Mol Cell Biol. 1987;7: 3713–3722. doi: 10.1128/mcb.7.10.3713-3722.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Mijares M, Gall MD, Turan T, Javier A, Bornemann DJ, et al. Drosophila variable nurse cells encodes arrest defective 1 (ARD1), the catalytic subunit of the major N-terminal acetyltransferase complex. Dev Dyn. 2010;239: 2813–2827. doi: 10.1002/dvdy.22418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ree R, Myklebust LM, Thiel P, Foyn H, Fladmark KE, Arnesen T. The N-terminal acetyltransferase Naa10 is essential for zebrafish development. Biosci Rep. 2015;35: 1–10. doi: 10.1042/BSR20150168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bienvenut WV, Sumpton D, Martinez A, Lilla S, Espagne C, Meinnel T, et al. Comparative large scale characterization of plant versus mammal proteins reveals similar and idiosyncratic N-α-acetylation features. Mol Cell Proteomics. 2012;11: 1–14. doi: 10.1074/mcp.M111.015131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huesgen PF, Alami M, Lange PF, Foster LJ, Schröder WP, Overall CM, et al. Proteomic amino-termini profiling reveals targeting information for protein import into complex plastids. PLoS One. 2013;8: e74483. doi: 10.1371/journal.pone.0074483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee C-C, Peng SH, Shen L, Lee CF, Du TH, Kang ML, et al. The Role of N-α-acetyltransferase 10 Protein in DNA Methylation and Genomic Imprinting. Mol Cell. 2017;68: 89–103.e7. doi: 10.1016/j.molcel.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee C-C, Shih Y-C, Kang M-L, Chang Y-C, Chuang L-M, Devaraj R, et al. Naa10p inhibits beige adipocyte-mediated thermogenesis through N-α-acetylation of Pgc1α. Mol Cell. 2019;76: 500–515.e8. doi: 10.1016/j.molcel.2019.07.026 [DOI] [PubMed] [Google Scholar]
- 63.Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol. 2009;530: 423–433. doi: 10.1007/978-1-59745-471-1_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seckl JR. Glucocorticoids, developmental “programming” and the risk of affective dysfunction. Prog Brain Res. 2008;167: 17–34. doi: 10.1016/S0079-6123(07)67002-2 [DOI] [PubMed] [Google Scholar]
- 65.Clark DA, Banwatt D, Chaouat G. Stress-triggered abortion in mice prevented by alloimmunization. Am J Reprod Immunol. 1993;29: 141–147. doi: 10.1111/j.1600-0897.1993.tb00579.x [DOI] [PubMed] [Google Scholar]
- 66.Pham CT, MacIvor DM, Hug BA, Heusel JW, Ley TJ. Long-range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci U S A. 1996;93: 13090–13095. doi: 10.1073/pnas.93.23.13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren S-Y, Angrand P-O, Rijli FM. Targeted insertion results in a rhombomere 2-specific Hoxa2 knockdown and ectopic activation of Hoxa1 expression. Dev Dyn. 2002;225: 305–315. doi: 10.1002/dvdy.10171 [DOI] [PubMed] [Google Scholar]
- 68.Scacheri PC, Crabtree JS, Novotny EA, Garrett-Beal L, Chen A, Edgemon KA, et al. Bidirectional transcriptional activity of PGK-neomycin and unexpected embryonic lethality in heterozygote chimeric knockout mice. Genesis. 2001;30: 259–263. doi: 10.1002/gene.1072 [DOI] [PubMed] [Google Scholar]
- 69.Meier ID, Bernreuther C, Tilling T, Neidhardt J, Wong YW, Schulze C, et al. Short DNA sequences inserted for gene targeting can accidentally interfere with off-target gene expression. FASEB J. 2010;24: 1714–1724. doi: 10.1096/fj.09-140749 [DOI] [PubMed] [Google Scholar]
- 70.Jin C, Kang H, Yoo T, Ryu JR, Yoo Y-E, Ma R, et al. The Neomycin Resistance Cassette in the Targeted Allele of Shank3B Knock-Out Mice Has Potential Off-Target Effects to Produce an Unusual Shank3 Isoform. Front Mol Neurosci. 2020;13: 614435. doi: 10.3389/fnmol.2020.614435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West DB, Engelhard EK, Adkisson M, Nava AJ, Kirov JV, Cipollone A, et al. Transcriptome Analysis of Targeted Mouse Mutations Reveals the Topography of Local Changes in Gene Expression. PLoS Genet. 2016;12: e1005691. doi: 10.1371/journal.pgen.1005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;124: 505–514. Available: https://www.ncbi.nlm.nih.gov/pubmed/9053326 doi: 10.1242/dev.124.2.505 [DOI] [PubMed] [Google Scholar]
- 73.Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 1997;36: 163–173. doi: 10.1016/s0008-6363(97)00172-7 [DOI] [PubMed] [Google Scholar]
- 74.Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131: 3367–3379. doi: 10.1242/dev.01197 [DOI] [PubMed] [Google Scholar]
- 75.Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, et al. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000;127: 1869–1878. doi: 10.1242/dev.127.9.1869 [DOI] [PubMed] [Google Scholar]
- 76.van den Hoff MJ, Moorman AF. Cardiac neural crest: the holy grail of cardiac abnormalities? Cardiovasc Res. 2000;47: 212–216. doi: 10.1016/s0008-6363(00)00127-9 [DOI] [PubMed] [Google Scholar]
- 77.Olaopa M, Zhou HM, Snider P, Wang J, Schwartz RJ, Moon AM, et al. Pax3 is essential for normal cardiac neural crest morphogenesis but is not required during migration nor outflow tract septation. Dev Biol. 2011;356: 308–322. doi: 10.1016/j.ydbio.2011.05.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou HM, Conway SJ. Restricted Pax3 Deletion within the Neural Tube Results in Congenital Hydrocephalus. J Dev Biol. 2016;4. doi: 10.3390/jdb4010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson DJ, Conway SJ, Copp AJ. Rib truncations and fusions in the Sp2H mouse reveal a role for Pax3 in specification of the ventro-lateral and posterior parts of the somite. Dev Biol. 1999;209: 143–158. doi: 10.1006/dbio.1999.9215 [DOI] [PubMed] [Google Scholar]
- 80.Dickman ED, Rogers R, Conway SJ. Abnormal skeletogenesis occurs coincident with increased apoptosis in the Splotch (Sp2H) mutant: putative roles for Pax3 and PDGFRalpha in rib patterning. Anat Rec. 1999;255: 353–361. Available: https://www.ncbi.nlm.nih.gov/pubmed/10411402 doi: [DOI] [PubMed] [Google Scholar]
- 81.Lyon GJ, O’Rawe J. Human genetics and clinical aspects of neurodevelopmental disorders. In: Mitchell K, editor. The Genetics of Neurodevelopmental Disorders. Wiley; ); 2015. doi: 10.1101/000687 [DOI] [Google Scholar]
- 82.Lyon GJ, O’Rawe J. Human genetics and clinical aspects of neurodevelopmental disorders. bioRxiv. 2014. doi: 10.1101/000687 [DOI] [Google Scholar]
- 83.Magin RS, March ZM, Marmorstein R. The N-terminal acetyltransferase Naa10/ARD1 does not acetylate lysine residues. J Biol Chem. 2016. doi: 10.1074/jbc.M115.709428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals: Eighth Edition. National Academies Press; 2011. Available: https://play.google.com/store/books/details?id=GyxkAgAAQBAJ [Google Scholar]
- 85.Qiu P, Shandilya H, D’Alessio JM, O’Connor K, Durocher J, Gerard GF. Mutation detection using Surveyor nuclease. Biotechniques. 2004;36: 702–707. doi: 10.2144/04364PF01 [DOI] [PubMed] [Google Scholar]
- 86.Dehairs J, Talebi A, Cherifi Y, Swinnen JV. CRISP-ID: decoding CRISPR mediated indels by Sanger sequencing. Sci Rep. 2016;6. doi: 10.1038/srep28973 [DOI] [PMC free article] [PubMed] [Google Scholar]