Abstract
OBJECTIVES:
We assessed the association of preexisting diabetes mellitus with all-cause mortality and organ support receipt in adult patients with sepsis.
DESIGN:
Population-based cohort study.
SETTING:
Ontario, Canada (2008–2019).
POPULATION:
Adult patients (18 yr old or older) with a first sepsis-related hospitalization episode.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The main exposure of interest was preexisting diabetes (either type 1 or 2). The primary outcome was all-cause mortality by 90 days; secondary outcomes included receipt of invasive mechanical ventilation and new renal replacement therapy. We report adjusted (for baseline characteristics using standardization) risk ratios (RRs) alongside 95% CIs. A main secondary analysis evaluated the potential mediation by prior metformin use of the association between preexisting diabetes and all-cause mortality following sepsis. Overall, 503,455 adults with a first sepsis-related hospitalization episode were included; 36% had preexisting diabetes. Mean age was 73 years, and 54% of the cohort were females. Preexisting diabetes was associated with a lower adjusted risk of all-cause mortality at 90 days (RR, 0.81; 95% CI, 0.80–0.82). Preexisting diabetes was associated with an increased risk of new renal replacement therapy (RR, 1.53; 95% CI, 1.46–1.60) but not invasive mechanical ventilation (RR, 1.03; 95% CI, 1.00–1.05). Overall, 21% (95% CI, 19–28) of the association between preexisting diabetes and reduced risk of all-cause mortality was mediated by prior metformin use.
CONCLUSIONS:
Preexisting diabetes is associated with a lower risk of all-cause mortality and higher risk of new renal replacement therapy among adult patients with sepsis. Future studies should evaluate the underlying mechanisms of these associations.
Keywords: all-cause mortality, diabetes, invasive mechanical ventilation, metformin, renal replacement therapy, sepsis
KEY POINTS
Question: Is preexisting diabetes associated with worse outcomes among adult patients with sepsis?
Findings: In this population-based cohort study of adult patients with sepsis, the adjusted risk ratio of all-cause mortality at 90 days for preexisting diabetes was 0.81 (95% CI, 0.80–0.82).
Meaning: Preexisting diabetes is associated with a lower risk of all-cause mortality among adult patients with sepsis.
Sepsis is a complex and life-threatening organ dysfunction in response to infection; representing a significant global health burden and one of the leading causes of morbidity and mortality worldwide (1–4). Sepsis is responsible for an estimated 11 million deaths annually, accounting for approximately one in five deaths globally (3). The short-term effects of sepsis include increased mortality rates, recurrent infections, and the need for ICU admission and life-sustaining therapies (1, 5–11).
The interaction between preexisting diabetes and sepsis in relation to clinical outcomes is an area of considerable interest, given the ever-increasing prevalence of diabetes worldwide and the potential associated changes in inflammation and immune functions (12–18). Overall, there is uncertainty about the interplay of chronic hyperglycemia, inflammation, and modifications in the immune response with sepsis-associated changes such as immune suppression, endothelial dysfunction, and acute organ failure (19). The clinical consequences of these interactions (and of chronic medication use) remain incompletely characterized.
For example, adult patients with diabetes might have a higher likelihood of developing new infections, sepsis, and associated long-term outcomes (e.g., cardiovascular disease) (15, 19, 20). However, there is conflicting evidence on the effects of preexisting diabetes on the short-term outcomes following sepsis (14, 19, 21, 22). When compared with patients without diabetes, patients with preexisting diabetes appear to face an increased risk of acute kidney injury and new renal replacement therapy with similar (or lower) rates of all-cause mortality and acute respiratory failure (13, 14, 21–25). Notably, previous studies have been limited by: 1) short follow-up, 2) a restricted number of endpoints, 3) focusing solely on sepsis survivors, or 4) the lack of population-based data. Furthermore, no study has evaluated potential mechanisms for these associations via mediation analysis.
Hence, this study aimed to investigate whether preexisting diabetes was associated with short-term outcomes in adult patients with sepsis, including all-cause mortality and the receipt of organ support measures, using a population-based cohort study in the province of Ontario. We hypothesized that preexisting diabetes would be associated with a higher risk of acute kidney injury requiring renal replacement therapy but not of respiratory failure requiring invasive mechanical ventilation or all-cause mortality.
MATERIALS AND METHODS
Data Sources and Study Population
The study cohort was created using population-based provincial health administrative databases contained at ICES, an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze healthcare and demographic data, without consent, for health system evaluation and improvement. These datasets were linked using unique encoded identifiers. Our study was developed in accordance with the amended Declaration of Helsinki and this report follows the Strengthening the Reporting of Observational Studies in Epidemiology (26). The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
This study included adults (18 yr old or older) in the province of Ontario, Canada, with a first sepsis-related hospitalization between April 2008 and March 2019; the study dates were chosen to optimize data completeness. We did not perform a priori sample size calculations. Sepsis was identified using a previously validated algorithm (see details in Table S1, http://links.lww.com/CCX/B334) (27). To analyze in-hospital outcomes (such as organ support) and avoid potential reverse causation, those patients with an elective admission or a sepsis episode not categorized as either a most responsible or admitting diagnosis were excluded. The start of follow-up (i.e., index date) was defined as the date of hospital admission. Follow-up was until primary outcome occurrence up to a maximum of 90 days.
Main Exposure and Outcomes of Interest
Our exposure of interest was an antecedent diagnosis of (i.e., preexisting) diabetes at the time of sepsis hospitalization, defined using a previously validated algorithm with International Classification of Diseases, 10th Revision, with Canadian Enhancements (ICD-10-CA) codes (28). This algorithm has high sensitivity and specificity (uses data from inpatient and outpatient encounters, as well as prescription information) but does not differentiate between type 1 and type 2 diabetes mellitus (Table S1, http://links.lww.com/CCX/B334). The primary outcome of interest was all-cause mortality at 90 days. Secondary outcomes of interest included all-cause mortality at 30 days, receipt of invasive mechanical ventilation, and new receipt of renal replacement therapy (organ support was measured during index hospitalization). We also collected information on ICU admission, presence of septic shock, total length of stay, glycated hemoglobin, and severity of illness measured at the time of ICU admission (i.e., multiple organ dysfunction score) (29). Table S1 (http://links.lww.com/CCX/B334) describes specific coding strategies (and their accuracy) used to define sepsis and main variables of interest; details can be found elsewhere (12, 20, 27, 30, 31).
Statistical Analysis
Patients’ demographic, clinical, and hospital-level characteristics were summarized using proportions for categorical variables and mean and sd or median and interquartile range for continuous variables, as appropriate. Baseline characteristics of patients with or without diabetes were compared using standardized mean differences (SMDs). SMDs greater than 10% were considered relevant (32). Cumulative incidence is reported for in-hospital outcomes and all-cause mortality at 30 and 90 days across groups. Unadjusted and adjusted risk ratios (RRs) alongside 95% CIs are presented for all outcomes of interest and organ support measures. We report crude measures of association (based on modified Poisson models) since adjusted measures might be difficult to conceptualize (e.g., the association of preexisting diabetes with outcomes of interest while “adjusting” for preexisting conditions that may have been caused by the disease itself, such as cardiovascular comorbidity). We also used multivariable modified Poisson models or standardization to estimate adjusted measures of association. Specifically, standardization was used to estimate the association of preexisting diabetes with main outcomes of interest when compared with a population without a diabetes diagnosis but with a similar distribution of baseline comorbidities as in the overall study sample; we derived adjusted RR and constructed 95% CIs using nonparametric bootstrapping (33). Following usual recommendations, we used subject matter knowledge for covariate selection in all models (i.e., including variables considered to be potential confounders or a proxy of an unmeasured confounder, and avoiding potential instruments) (34, 35). The vector of potential confounders included baseline demographics (e.g., age, sex, rural setting), social determinants of health (e.g., income quintile, material deprivation), comorbidity burden at baseline (e.g., Charlson comorbidity index (36), chronic kidney disease, coronary heart disease, congestive heart failure), and frailty (defined using a validated algorithm) (37).
Secondary and Sensitivity Analyses
Our main secondary analysis evaluated the potential role of prior metformin use in explaining the association between diabetes and short-term mortality following sepsis. Prior studies have shown a potential reduction in the risk of all-cause death with prior metformin use in adult patients admitted with sepsis (38). Hence, we first evaluated the association between prior metformin use (within 90 d of hospitalization and compared with no metformin use during this timeframe) and short-term outcomes while restricting to patients with sepsis and diabetes. We then performed a mediation analysis to describe whether the association between preexisting diabetes and 90-day mortality was either due to a direct (i.e., not mediated by metformin) or an indirect path through the prior use of metformin (39, 40). These analyses were restricted to adult patients with sepsis who were older than 65 years old because there is no prescription information available in ICES for patients younger than this age cutoff. Further details can be found in the Supplementary appendix (http://links.lww.com/CCX/B334).
We also performed several sensitivity analyses to assess the robustness of our findings. These analyses make significant changes in either the exposure definition, population selection, or analytical strategy. First, we refitted our main estimates (both unadjusted and adjusted) while not restricting to patients with sepsis as a main or admitting diagnosis. Second, we report estimates after performing 1:1 matching of patients with diabetes to patients without diabetes (41). Matching was based on a disease risk score (i.e., propensity score for diabetes) using a multivariable logistic regression model that included the same covariates as the standardization described above. RRs are reported with CIs constructed using the sandwich estimator to account for the matching procedure. Third, since metabolic control may impact sepsis-associated outcomes, we refitted our analysis changing the exposure from a binary variable to the following three levels (following common thresholds in clinical practice): no diabetes, diabetes with a glycated hemoglobin less than or equal to 7%, and diabetes with a glycated hemoglobin of more than 7% (42). We also changed the comparator and exposure groups based on glycated hemoglobin as follows: 1) no diabetes without HbA1c measurement, 2) no diabetes with HbA1c lower than 5.7%, 3) no diabetes with HbA1c between 5.7% and 6.5%, 4) not previously diagnosed (i.e., not coded) diabetes but with HbA1c equal or higher than 6.5%, 5) preexisting diabetes with HbA1c lower than 6.5%, 6) preexisting diabetes with HbA1c measurement equal or higher than 6.5%. For both these analyses, we used the last available glycated hemoglobin level. Fourth, we refitted our main analysis while restricting to patients admitted to the ICU because these patients may face a higher degree of poor short-term outcomes with a differential impact of preexisting diabetes. Fifth, we assessed the impact of prior length from diabetes diagnosis on clinical outcomes. Finally, we refitted our estimates while restricting the study sample to patients with either pneumonia or urosepsis as source of infection.
All analyses were performed at ICES using SAS Enterprise Guide version 7.1 (Cary, NC).
RESULTS
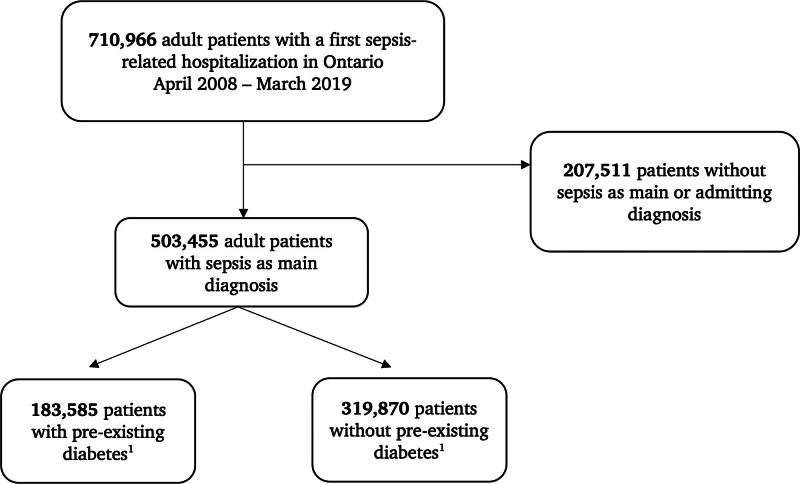
Overall, 710,966 adult patients presented with a first episode of a sepsis-related hospitalization during the study period in the province of Ontario (2008–2019; Fig. 1). Of these, 503,455 had sepsis as either main or admitting diagnosis and were included in the present analysis, of which 183,585 had preexisting diabetes upon hospital admission. Mean glycated hemoglobin for those patients with sepsis and diabetes was 7.1% (sd: 1.6; 80% of patients had at least one measurement available). Mean glycated hemoglobin for those patients with sepsis and without a diabetes diagnosis was 5.7% (sd: 0.5; 49% of patients had at least one measurement available). Table 1 summarizes the baseline characteristics of study patients. Mean age of patients was 73 years, and more than half (54%) were females. Main comorbidities were hypertension (73%), chronic pulmonary disease (36%), and congestive heart failure (24%); 6% of patients had chronic kidney disease.
Figure 1.
Flowchart of study patients. Preexisting diabetes is defined using the Ontario diabetes database.
TABLE 1.
Baseline Characteristics of Adult Patients With a First Sepsis-Related Hospitalization With or Without Preexisting Diabetes in Ontario (2008–2019)
| Baseline Characteristic | All Adult Patient With Sepsis (N = 503,455) | Patients With Preexisting Diabetes (N = 183,585) | Patients Without a Diabetes Diagnosis (N = 319,870) | Standardized Mean Differences |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (yr), mean (sd) | 73.3 (15.8) | 74.9 (12.6) | 72.5 (17.3) | 0.16 |
| Female sex (%) | 54.3 | 50.8 | 56.3 | 0.11 |
| Rural setting (%) | 13.3 | 12.9 | 13.5 | 0.02 |
| Income quintile (%)a | ||||
| 1 | 25.0 | 26.9 | 24.0 | 0.07 |
| 2 | 21.7 | 22.4 | 21.4 | 0.02 |
| 3 | 19.2 | 19.2 | 19.2 | 0.00 |
| 4 | 17.5 | 16.8 | 17.9 | 0.03 |
| 5 | 16.0 | 14.2 | 17.0 | 0.08 |
| Material deprivation (%)b | ||||
| Quintile 1–3 | 52.1 | 49.1 | 53.7 | 0.09 |
| Quintile 4–5 | 46.6 | 49.2 | 45.1 | 0.08 |
| Baseline comorbidities | ||||
| Charlson score, median (IQR)c | 0 (0–2) | 1 (0–3) | 0 (0–1) | 0.61 |
| Frailty index–mean (sd)d | 0.2 (0.1) | 0.2 (0.1) | 0.1 (0.1) | 0.80 |
| Hypertension (%) | 72.6 | 85.6 | 65.1 | 0.49 |
| Atrial fibrillation (%) | 7.2 | 8.7 | 6.4 | 0.09 |
| Coronary heart disease (%) | 10.1 | 14.1 | 7.8 | 0.20 |
| Stroke (%) | 4.2 | 5.3 | 3.5 | 0.09 |
| Congestive heart failure (%) | 23.6 | 31.8 | 18.9 | 0.30 |
| Venous thromboembolism (%) | 1.5 | 1.5 | 1.5 | 0.00 |
| Chronic liver disease (%) | 2.4 | 2.8 | 2.2 | 0.04 |
| Chronic kidney disease (%) | 5.9 | 10.9 | 3.0 | 0.32 |
| Chronic pulmonary disease (%) | 36.4 | 38.1 | 35.4 | 0.06 |
| Dementia (%) | 17.5 | 18.2 | 17.1 | 0.03 |
| Active malignancy (%) | 13.8 | 12.7 | 14.4 | 0.05 |
| Previous hospitalizations, median (IQR) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 0.23 |
| Source of infection | ||||
| Pneumonia as source of infection (%) | 44.0 | 41.8 | 45.2 | 0.07 |
| Urosepsis (%) | 41.2 | 43.5 | 39.9 | 0.07 |
IQR = interquartile range, SMD = absolute standardized mean difference.
Missing for less than 1% of patients.
Missing for 1% of patients.
Based on the Deyo adaptation.
Based on the preoperative frailty index derived by McIsaac et al (37).
Adult patients with sepsis and preexisting diabetes were older and had a higher burden of comorbid conditions at baseline (Table 1); the prevalence of hypertension, coronary heart disease, chronic kidney disease, and congestive heart failure was higher in patients with sepsis and preexisting diabetes than in patients with sepsis and no diabetes. Finally, patients with preexisting diabetes had higher Charlson and frailty scores at baseline (Table 1). Table 2 and Table S2 (http://links.lww.com/CCX/B334) summarize the characteristics of the hospital stay for patients with or without preexisting diabetes. Patients with sepsis and preexisting diabetes had comparable: 1) hospital lengths of stay, 2) incidence of septic shock, 3) ICU admission, and 4) severity of illness as measured by the multiple organ dysfunction score.
TABLE 2.
Characteristics of Hospital Stay
| Hospital Stay Characteristics | Preexisting Diabetes | |
|---|---|---|
| Yes (N = 183,585) | No (N = 319,870) | |
| ICU admission (%) | 21.5 | 18.9 |
| Multiple organ dysfunction score, mean (sd) | 4.2 (3.1) | 4.0 (3.2) |
| Septic shock (%) | 4.6 | 4.1 |
| Length of stay, d, median (interquartile range) | 7 (4–14) | 7 (4–14) |
Organ Support Measures and All-Cause Mortality
Table 3 shows the unadjusted results for all outcomes of interest. When compared with patients without a diabetes diagnosis at baseline, patients with preexisting diabetes were more likely to receive new renal replacement therapy (RR, 1.66; 95% CI, 1.60–1.73) and invasive mechanical ventilation (RR, 1.08; 95% CI, 1.06–1.10). Diabetes was not associated with an increased risk of all-cause mortality at either 90 (RR, 1.00; 95% CI, 0.99–1.01) or 30 days (RR, 0.98; 95% CI, 0.97–1.00).
TABLE 3.
Association of Preexisting Diabetes With Outcomes of Interest in Adult Patients With Sepsis (Unadjusted Estimates)
| Outcome of interest | Crude Absolute Risk Preexisting Diabetes |
Crude Risk Ratio (95% CI)a | |
|---|---|---|---|
| Yes (N = 183,585) | No (N = 319,870) | ||
| Organ support measures | |||
| Invasive mechanical ventilation (%) | 8.2 | 7.6 | 1.08 (1.06–1.10) |
| New renal replacement therapy (%) | 2.4 | 1.4 | 1.66 (1.60–1.73) |
| 30-d and 90-d outcomes | |||
| All-cause mortality at 30 d (%) | 13.9 | 14.1 | 0.98 (0.97–1.00) |
| All-cause mortality at 90 d (%) | 21.4 | 21.5 | 1.00 (0.99–1.01) |
Based on a modified Poisson regression model with diabetes as a binary indicator (i.e., yes vs. no).
Table 4 shows the adjusted estimates for all outcomes of interest. When compared with patients without a diabetes diagnosis at baseline, patients with preexisting diabetes were more likely to receive new renal replacement therapy (RR, 1.53; 95% CI, 1.46–1.60) but not invasive mechanical ventilation (RR, 1.03; 95% CI, 1.00–1.05). Preexisting diabetes was associated with a reduction in the risk of all-cause mortality at both 90 (RR, 0.81; 95% CI, 0.82–0.82) and 30 days (RR, 0.79; 95% CI, 0.78–0.80).
TABLE 4.
Association of Preexisting Diabetes With Outcomes of Interest in Adult Patients With Sepsis (Adjusted Estimates)
| Outcome of interest | Standardized Absolute Riska Preexisting Diabetes | Adjusted Risk Ratio (95% CI) | |
|---|---|---|---|
| Yes (N = 183,585) | No (N = 319,870) | ||
| Organ support measures | |||
| Invasive mechanical ventilation (%) | 7.9 | 7.7 | 1.03 (1.00–1.05) |
| New renal replacement therapy (%) | 2.3 | 1.5 | 1.53 (1.46–1.60) |
| 30-d and 90-d outcomes | |||
| All-cause mortality at 30 d (%) | 12.1 | 15.4 | 0.79 (0.78–0.80) |
| All-cause mortality at 90 d (%) | 18.8 | 23.3 | 0.81 (0.80–0.82) |
Adjusted for all a priori considered potential confounders including age, sex, comorbidities, and frailty.
Secondary and Sensitivity Analysis
Our main secondary analysis analyzed the potential role of metformin in modifying outcomes following sepsis. Table S3 (http://links.lww.com/CCX/B334) presents the baseline characteristics of patients older than 65 years old, with preexisting diabetes and a prescription for an oral antidiabetic medication within 90 days of hospitalization. The prior use of metformin (compared with other oral agents; a sulfonylurea in 80% of the cases) was associated with a reduction in all-cause mortality at 90 days (odds ratio, 0.89; 95% CI, 0.80–0.99; Table S4, http://links.lww.com/CCX/B334). Table S5 (http://links.lww.com/CCX/B334) presents the summary for the mediation analysis; 21% (95% CI, 19–28) of the association of preexisting diabetes (compared with no diabetes diagnosis) with the (reduced) risk of 90-day mortality following sepsis in adult patients appeared to be mediated by metformin use.
Our results were robust to several sensitivity analyses. Estimates of the association between preexisting diabetes and all outcomes of interest were similar when performing 1:1 matching to patients without a previous diabetes diagnosis (Tables S6 and S7, http://links.lww.com/CCX/B334). Further, 1) restricting to patients with ICU admission, 2) changing the exposure to a three-level variable considering metabolic control as measured by the glycated hemoglobin, 3) restratifying the comparator and exposure groups based on glycated hemoglobin levels, and 4) including all sepsis hospitalizations yielded similar crude and adjusted estimates to our main analysis (Tables S8–S10, http://links.lww.com/CCX/B334). Table S11 http://links.lww.com/CCX/B334 shows the association between duration from diabetes diagnosis and outcomes of interest. Our estimates were robust when restricting to patients by source of infection; notably, preexisting diabetes was associated with an increased risk of receipt of invasive mechanical ventilation only among those patients with pneumonia (Table S12, http://links.lww.com/CCX/B334).
DISCUSSION
Our study shows the impact of preexisting diabetes on short-term outcomes following sepsis in adult patients. In a large population-based cohort, we found that despite a higher burden of comorbid disease and frailty at baseline, adult patients with sepsis and preexisting diabetes face a lower risk of all-cause mortality at 90 days when compared with patients with sepsis but without a diabetes diagnosis. Preexisting diabetes was associated with a significantly increased risk of new renal replacement therapy.
These results highlight the complex interactions between preexisting diabetes and sepsis-associated outcomes (19, 20). Our findings that preexisting diabetes increases the risk of renal replacement therapy receipt during sepsis, but not necessarily of all-cause mortality are consistent with current evidence; previous reports, although conflicting, have shown an increased risk of acute renal failure but not invasive mechanical ventilation among adult patients with sepsis and diabetes (14, 19, 22–24). Our study adds to the current literature by providing population-based estimates, with additional adjustments for an extensive set of characteristics and comorbid conditions at baseline, thus increasing our overall confidence in these relationships. The increased risk of renal replacement therapy in patients with sepsis and diabetes—which appears clinically relevant and highlights the importance of close monitoring—might be related to the degree of chronic kidney disease at baseline (43). Notably, our results were robust when considering multiple sensitivity analyses including restricting to those patients who were admitted to the ICU. Conversely, the finding that patients with sepsis with or without preexisting diabetes receive in a similar proportion invasive mechanical ventilation may be explained by similar degrees of chronic pulmonary disease at baseline; however, the source of infection may further modify this association. This may also suggest that different pathways are at play when considering the different interactions between sepsis, preexisting diabetes, source and type of infection, and distinct end-organ damage (20). Further research is needed to better understand such underlying mechanisms.
The finding that preexisting diabetes might be associated with a reduced risk of all-cause mortality has been reported in previous studies evaluating adult patients with sepsis (14, 21, 24). Our study adds to the current evidence through its population-based design and consideration of key subgroups of patients. Notably, whether this association is a fundamentally causal one remains unclear, as its potential underpinning mechanisms. Such mechanisms warrant further study, given the 1) higher burden of comorbidity at baseline, and 2) association between diabetes and increased mortality in other settings (44). Hypothetical explanations for the mitigated effects on mortality may include: 1) closer monitoring or more aggressive treatment in patients with sepsis and diabetes, 2) differential impact of sepsis on the immune, coagulation, and inflammation systems in patients with and without diabetes (45), 3) different prehospital trajectories with closer monitoring or associated treatments that may impact subsequent outcomes, and 4) residual and unmeasured confounding.
Our secondary analysis evaluating the potential impact of metformin may also suggest that previous pharmacological treatments might affect the outcome pathway of patients with preexisting diabetes and sepsis. Prior studies have shown potential beneficial effects of metformin on the outcomes of patients with sepsis (46). Our study adds to such prior evidence by showcasing the potential mediation by a specific (prior) pharmacological treatment and its role in modifying clinical outcomes of sepsis. Notwithstanding the potential implications, our mediation analysis should be taken with caution given the likely existence of confounding and selection bias. Overall, our study also highlights potential avenues for further research. Future studies should seek to understand the mechanistic underpinnings of the presented associations, including the role of prior medication use and glycemic control before and during the infection episode.
Our study has several limitations. First, both sepsis and diabetes were defined using administrative algorithms, so misclassification is expected; however, this is likely nondifferential and toward the null value of no association (in the setting of a binary exposure) (47). Second, and as previously described, residual and unmeasured confounding are likely; however, we included an extensive set of potential confounders including baseline characteristics, comorbidity, and frailty indices that have been previously shown to be associated with sepsis-related outcomes. Notably, the frailty index used in our article has been validated for patients undergoing surgery, and largely depends on the burden of comorbidities; this may in turn result in residual confounding (37). In addition, the extent to which adjusted estimates should be prioritized over crude estimates in this setting remains unclear. For example, adjusting for baseline characteristics and comorbidities associated with diabetes may not reflect the reality most practitioners face when taking care of patients with preexisting diabetes (where this is associated with a higher degree of, e.g., chronic kidney disease). We have thus chosen to present both crude (to give an extent of the overall risk that can be expected in this population) and adjusted estimates (to further describe the specific role of diabetes while “controlling” for other comorbid and general conditions). Third, our analysis of organ support measures is also subject to misclassification and the competing event of all-cause in-hospital death (48, 49). Furthermore, our definition focused on invasive mechanical ventilation, and we did not consider noninvasive support strategies; hence, it remains possible that patients with diabetes have a higher degree of acute respiratory failure subject to noninvasive support. However, respiratory failure requiring invasive mechanical ventilation remains arguably the most relevant for both practitioners and patients. Fourth, we did not capture information on the withholding of life-sustaining therapies, which could affect the reported estimates for invasive respiratory support. Fifth, we have restricted our analysis to patients with sepsis-related hospitalization; to correctly weigh the overall burden of sepsis-associated outcomes in patients with diabetes, the potential increased risk of infection in this population needs to be taken into consideration (15). Sixth, our sepsis definition does not take into account the likely existence of subphenotypes (50). The degree to which different subphenotypes of sepsis may affect the relationship between preexisting diabetes and clinical outcomes remains unknown. Seventh, we did not have available data on in-hospital glycemic control and associated strategies which may also affect downstream clinical outcomes. Eighth, our mediation analysis is restricted to those patients older than 65 years old; the extent to which our findings apply to younger patients remains undefined. Finally, since the algorithm used to capture preexisting diabetes does not differentiate between type 1 and type 2, differences in sepsis-associated outcomes across these cannot be ruled out.
In conclusion, preexisting diabetes is associated with a lower risk of short-term all-cause mortality among adult patients following sepsis. Preexisting diabetes acts as a significant risk factor for renal complications and the deployment of renal replacement therapy in patients with sepsis, while diabetes does not carry a relevant increased risk of invasive mechanical ventilation. Further studies should focus on understanding the underpinnings of these associations to then identify potential mitigation strategies.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Acute & Intensive Care Outcomes Research Network during the conduct of the present research. Sepsis Canada Network Executive Committee are as follows: Dr. Alison Fox-Robichaud, Dr. Osama Loubani, and Dr. Saad Salim.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Angriman and Scales were involved in material preparation and data analysis and wrote the first draft of the article. All authors contributed to the study conception, design, and data interpretation, and read and approved the final article. All authors commented on previous versions of the article.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care. This study also received funding from the Sepsis Canada Network (grant number 2021-1870). Parts of this material are based on data and information compiled and provided by Ontario MOH. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. Parts of this report are based on Ontario Registrar General (ORG) information on deaths, the original source of which is ServiceOntario. The views expressed therein are those of the author and do not necessarily reflect those of ORG or the Ministry of Public and Business Service Delivery. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada.
Dr. Scales holds operating grants from the Canadian Institute for Health Research. Dr. Lawler is supported by a Heart and Stroke Foundation of Canada National New Investigator Award. Dr. Shah is supported by the University of Toronto as the Novo Nordisk Research Chair in Equitable Care of Diabetes and Related Conditions. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
REFERENCES
- 1.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman TG, Simpson SQ, Sciarretta KL, et al. : Sepsis among Medicare beneficiaries: 1. The burdens of sepsis, 2012-2018. Crit Care Med 2020; 48:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herridge MS, Azoulay E: Outcomes after critical illness. N Engl J Med 2023; 388:913–924 [DOI] [PubMed] [Google Scholar]
- 6.Seymour CW, Gesten F, Prescott HC, et al. : Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaukonen KM, Bailey M, Suzuki S, et al. : Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014; 311:1308–1316 [DOI] [PubMed] [Google Scholar]
- 8.Farrah K, McIntyre L, Doig CJ, et al. : Sepsis-associated mortality, resource use, and healthcare costs: A propensity-matched cohort study*. Crit Care Med 2021; 49:215–227 [DOI] [PubMed] [Google Scholar]
- 9.Kosyakovsky LB, Angriman F, Katz E, et al. : Association between sepsis survivorship and long-term cardiovascular outcomes in adults: A systematic review and meta-analysis. Intensive Care Med 2021; 47:931–942 [DOI] [PubMed] [Google Scholar]
- 10.Shankar-Hari M, Ambler M, Mahalingasivam V, et al. : Evidence for a causal link between sepsis and long-term mortality: A systematic review of epidemiologic studies. Crit Care 2016; 20:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar-Hari M, Saha R, Wilson J, et al. : Rate and risk factors for rehospitalisation in sepsis survivors: Systematic review and meta-analysis. Intensive Care Med 2020; 46:619–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hux JE, Ivis F, Flintoft V, et al. : Diabetes in Ontario determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002; 25:512–516 [DOI] [PubMed] [Google Scholar]
- 13.Schuetz P, Castro P, Shapiro NI: Diabetes and sepsis: Preclinical findings and clinical relevance. Diabetes Care 2011; 34:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Cheng M: Impact of diabetes mellitus on outcomes of patients with sepsis: an updated systematic review and meta-analysis. Diabetol Metab Syndr 2022; 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Ashour W, Twells L, Valcour J, et al. : The association between diabetes mellitus and incident infections: A systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res Care 2017; 5:e00033, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley JA, Carey IM, Harris T, et al. : Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018; 41:2127–2135 [DOI] [PubMed] [Google Scholar]
- 17.Trevelin SC, Carlos D, Beretta M, et al. : Diabetes mellitus and sepsis: A challenging association. Shock 2017; 47:276–287 [DOI] [PubMed] [Google Scholar]
- 18.Balintescu A, Lind M, Franko MA, et al. : Glycemic control and risk of sepsis and subsequent mortality in type 2 diabetes. Diabetes Care 2022; 45:127–133 [DOI] [PubMed] [Google Scholar]
- 19.Costantini E, Carlin M, Porta M, et al. : Type 2 diabetes mellitus and sepsis: State of the art, certainties and missing evidence. Acta Diabetol 2021; 58:1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angriman F, Rosella L, Lawler P, et al. : Risk factors for major cardiovascular events in adult sepsis survivors: A population-based cohort study. Crit Care Med 2023; 51:471–483 [DOI] [PubMed] [Google Scholar]
- 21.Yende S, van der Poll T: Diabetes and sepsis outcomes—it is not all bad news. Crit Care 2009; 13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akinosoglou K, Kapsokosta G, Mouktaroudi M, et al. ; Hellenic Sepsis Study Group: Diabetes on sepsis outcomes in non-ICU patients: A cohort study and review of the literature. J Diabetes Complications 2021; 35:107765. [DOI] [PubMed] [Google Scholar]
- 23.Esper AM, Moss M, Martin GS: The effect of diabetes mellitus on organ dysfunction with sepsis: An epidemiological study. Crit Care 2009; 13:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Ren J, Wang G, et al. : Association between diabetes mellitus and outcomes of patients with sepsis: A meta-analysis. Med Sci Monit 2017; 23:3546–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CW, Kok VC, Tseng TC, et al. : Diabetic patients with severe sepsis admitted to intensive care unit do not fare worse than non-diabetic patients: A Nationwide Population-Based Cohort Study. PLoS One 2012; 7:e50729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 27.Jolley RJ, Quan H, Jette N, et al. : Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open 2015; 5:e009487–e009410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipscombe LL, Hwee J, Webster L, et al. : Identifying diabetes cases from administrative data: A population-based validation study. BMC Health Serv Res 2018; 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall JC, Cook DJ, Christou NV, et al. : Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652 [DOI] [PubMed] [Google Scholar]
- 30.Jolley RJ, Sawka KJ, Yergens DW, et al. : Validity of administrative data in recording sepsis: A systematic review. Crit Care 2015; 19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angriman F, Rosella L, Lawler P, et al. : Sepsis hospitalization and risk of subsequent cardiovascular events in adults: A population-based matched cohort study. Intensive Care Med 2022; 48:448–457 [DOI] [PubMed] [Google Scholar]
- 32.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernán MA, Robins JM: Causal inference: What if. Chapman & Hall/CRC, Boca Raton. 2020 [Google Scholar]
- 34.VanderWeele TJ: Principles of confounder selection. Eur J Epidemiol 2019; 34:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernán MA, Hernández-díaz S, Werler MM, et al. : Causal knowledge as a prerequisite for confounding evaluation. Am J Epidemiol 2002; 155:176–184 [DOI] [PubMed] [Google Scholar]
- 36.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619 [DOI] [PubMed] [Google Scholar]
- 37.McIsaac DI, Wong CA, Huang A, et al. : Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann Surg 2019; 270:102–108 [DOI] [PubMed] [Google Scholar]
- 38.Liang H, Ding X, Li L, et al. : Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Crit Care 2019; 23:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWeele TJ: Mediation analysis: A practitioner’s guide. Annu Rev Public Health 2016; 37:17–32 [DOI] [PubMed] [Google Scholar]
- 40.Angriman F, Godoy L, Lawler P: What is mediation analysis? Linking exposures and outcomes through intermediary mechanisms&lowast. JACC 2023:100746. Available at: http://www.elsevier.com/artworkinstructions [DOI] [PMC free article] [PubMed]
- 41.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivers NM, Jiang M, Alloo J, et al. : Diabetes Canada 2018 clinical practice guidelines: Key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician 2019; 65:14–24 [PMC free article] [PubMed] [Google Scholar]
- 43.Chu L, Fuller M, Jervis K, et al. : Prevalence of chronic kidney disease in type 2 diabetes: The Canadian REgistry of Chronic Kidney Disease in Diabetes Outcomes (CREDO) Study. Clin Ther 2021; 43:1558–1573 [DOI] [PubMed] [Google Scholar]
- 44.Lind M, Garcia-Rodriguez LA, Booth GL, et al. : Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: A population-based study. Diabetologia 2013; 56:2601–2608 [DOI] [PubMed] [Google Scholar]
- 45.Yende S, Kellum JA, Talisa VB, et al. : Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open 2019; 2:e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez H, Del Rio-Pertuz G, Priyanka P, et al. : Association of metformin use during hospitalization and mortality in critically ill adults with type 2 diabetes mellitus and sepsis. Crit Care Med 2022; 50:935–944 [DOI] [PubMed] [Google Scholar]
- 47.Lash TL, Fox MP, MacLehose RF, et al. : Good practices for quantitative bias analysis. Int J Epidemiol 2014; 43:1969–1985 [DOI] [PubMed] [Google Scholar]
- 48.Rojas-Saunero LP, Young JG, Didelez V, et al. : Considering questions before methods in dementia research with competing events and causal goals. Am J Epidemiol 2023; 192:1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angriman F, Ferreyro BL, Harhay MO, et al. : Accounting for competing events when evaluating long-term outcomes in survivors of critical illness. Am J Respir Crit Care Med 2023; 208:1158–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seymour CW, Kennedy JN, Wang S, et al. : Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019; 321:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.