Abstract
Breast cancer screening programmes frequently detect early, good prognosis breast cancers with significant treatment burden for patients, and associated health-cost implications. Emerging evidence suggests a role for minimally invasive techniques in the management of these patients enabling many women to avoid surgical intervention. Minimally invasive techniques include vacuum-assisted excision, cryoablation, and radiofrequency ablation. We review published evidence in relation to the risks and benefits of each technique and discuss ongoing trials. Data to date are promising, and we predict a trend towards minimally invasive treatment for early, good-prognosis breast cancer as technical skills, suitability criteria, and follow-up protocols are established.
Keywords: breast cancer, breast screening, minimally invasive surgery, vacuum-assisted excision, cryotherapy, radiofrequency ablation
Background
In the United Kingdom, women aged 50 to <71 years are invited for screening mammography every three years. The risks and benefits of breast screening have long been controversial, resulting in the publication of the UK Independent Panel Report on Breast Cancer Screening in 2012.1 This review examined published literature and concluded that for every three cases of breast cancer diagnosed and treated, one breast cancer death was prevented. Although the incidence of breast cancer was increasing prior to screening, the introduction of mammographic screening programmes resulted in a marked increase in the incidence of smaller (<2 cm) tumours, with a smaller decrease seen in the incidence of larger (≥2 cm) tumours.2 Further analysis of mortality trends suggests that women with smaller tumours are more likely to be over-diagnosed rather than having earlier detection of tumours which are destined to become large, with “overdiagnosis” defined as a diagnosis of a tumour which never becomes clinically apparent during a patient’s lifetime. Overdiagnosis may be due to either non-progressive disease, or due to indolent cancers which progress so slowly that competing causes of death negate their clinical significance, with estimates of the contribution of each cause varying with age. The consequence of the “overdiagnosis” of such small tumours is a treatment burden resulting from surgical and adjuvant treatments which may have minimal survival benefit. However, it is not currently possible to reliably identify individual tumours as overdiagnosed.
The reduction in breast cancer mortality seen in the decades since the initiation of screening may be attributable to improvements in systemic therapy rather than being entirely due to earlier detection of disease.3 Smaller tumours showing biologically favourable characteristics (oestrogen/progesterone receptor positive, HER2 negative tumours) are associated with almost 100% survival at 10 years.4 Our understanding of tumour biology and associated prognosis have been further improved by the introduction of molecular assays. For example, the Oncotype Dx 21 gene assay was developed in 2004 and is used to calculate the risk of distant recurrence at 10 years.5 The test assigns a recurrence score between 0 and 100, and patients are categorized into three groups—low (<18), intermediate (18-30), and high risk (≥31). The TAILORx trial demonstrated patients with low recurrence scores had a similarly excellent prognosis irrespective of tumour size.6 It is clear therefore that the tools exist to identify those small tumours with favourable biology and a good prognosis, many of which are likely to be screen detected.
It is therefore necessary to tailor treatment, with its attendant morbidities, appropriately to the individual tumour. There are published data to suggest that patients who are diagnosed in a screening programme receive less intense treatment than those patients never screened (or not recently), even after correcting for overdiagnosis.7 Given the potential advantages of this in reducing the treatment burden for the individual as well as healthcare provider costs, there is an urgent need to consider optimizing treatment and minimizing the burden for patients with small, good-prognosis screen-detected tumours. This includes the consideration of alternative, minimally invasive treatment pathways for such cancers.
Surgical treatment for screen detected breast cancers
Current clinical guidelines advise the same treatment pathways for screen detected and symptomatic cancers. Patients undergo surgical excision (with or without localization techniques) and axillary staging. Most screen detected breast cancers are managed with breast conserving surgery. Associated morbidity is low but not insignificant—complications include surgical site infection (1.4%), haematoma (2.6%), seroma (11.5%),8 and up to 28.3% of patients report poor cosmetic outcomes with associated psychosocial morbidity following breast conserving surgery.9 Furthermore, following histopathological assessment of resection margins, up to 25% of women return to theatre for a repeat procedure to achieve clear margins. Such re-operations add to associated morbidity as well as resulting in increased healthcare costs.10 Close or involved margins (≤2 mm) are associated with local and/or distal recurrence,11 however, 30%-65% of women demonstrate no residual disease on re-excision,12 and this does not impact survival outcomes.13 Axillary staging has additional risks of shoulder stiffness (17.1%-29.8%), lymphoedema (5.9%-23.6%), and chronic pain (21.7%-32.9%).14 The ACOSOG Z0011 trial15 found low-burden axillary disease can be treated by systemic therapy, avoiding completion axillary dissection without adversely impacting breast cancer mortality, and recent results from the SOUND trial have shown non-inferiority of no axillary surgery in a clinically node-negative axilla compared to sentinel lymph node biopsy with 98% disease-free survival at 5 years.16
Minimally invasive treatment options
With increased detection of early, good prognosis breast cancers, we need alternative treatment modalities to offer patients which reduce both treatment intensity and toxicity whilst maintaining optimal disease control. There is no prospective evidence that open surgical intervention of these tumours is required. Numerous minimally invasive techniques have been described—in this review, we are going to focus on vacuum-assisted excision (VAE), cryoablation, and radiofrequency ablation (RFA).
Vacuum-assisted excision
Vacuum-assisted excision is a non-surgical technique used worldwide for both diagnosis and management of benign breast lesions and lesions of uncertain malignant potential. However, VAE is not currently in use for the treatment of breast cancer without clinical trials. Initially described for diagnosis only, vacuum-assisted biopsy (VAB) has evolved into VAE. A larger calibre needle (up to 7G) allows multiple cores to be taken with the needle remaining in situ after a single pass. This obtains an increased volume of tissue enabling histopathological assessment of an entire mass and provides additional information which cannot be achieved via fine-needle aspiration or core-needle biopsy. Using stereotactic or ultrasound guidance, the procedure is carried out under local anaesthetic in the outpatient setting (Figure 1). The technique is well accepted by patients—in a survey of 189 females, 90% of patients preferred VAB to surgical biopsy, noting that it took less time and provided ‘better cosmetic results’.17 It should be noted however that VAB may involve less extensive tissue sampling and a smaller bore needle than VAE (patients in this series had biopsies with 9G and 11G needles, whereas VAE might utilize 7G-11G needles). Consequently, VAE may be less well-tolerated than VAB, although there are no published data to support this hypothesis.
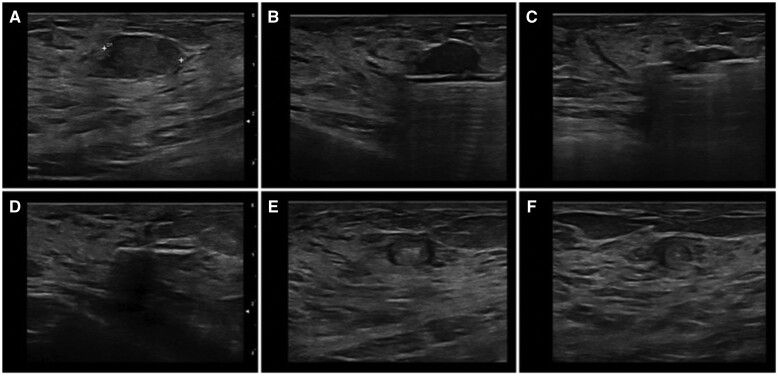
Figure 1.
Step-by-step ultrasound-guided vacuum-assisted excision of a fibroadenoma. (A) solid hypoechoic lesion, with regular shape and a circumscribed margin (B, C) US guided 8G vacuum-assisted excised (D) lesion has been completed removed and metallic marker clip release (E) axial follow-up scan at 12 months (F) longitudinal follow-up scan at 12 months.18
The use of VAE in the excision of benign breast lesions has been supported by the National Institute for Health and Care Excellence (NICE) since 2006.19 A meta-analysis of 26 studies investigating the efficacy and safety of VAE in benign lesions found complete resection rates following VAE of 93% (95% CI, 0.90-0.95) and recurrence rates of 39% (95% CI, 0.02-0.09). Complication rates following VAE of post-procedure haematoma, pain, and ecchymosis were reported as being 92% (95% CI, 0.07-0.13), 82% (95% CI, 0.05-0.13) and 75% (95% CI, 0.05-0.12) respectively, significantly higher than those for open surgical approaches.20 Lower rates of haematoma were reported in a further meta-analysis which suggested rates of around 2.6%-3.3% following open surgery. However, it is unclear whether the nature and severity of the complications reported in the series of the two techniques are comparable—as surgical series often only report complications requiring operative evacuation whereas the majority of haematomas following VAE are treated conservatively. For future studies evaluating VAE in comparison with surgery, it will be important to incorporate robust comparisons of complications between techniques, coupled with patient reported outcomes to assess acceptability and tolerability.
Building on this there are now substantial published data to support the safe use of VAE in the management of lesions of uncertain malignant potential (“B3 lesions”) with complete excision rates reported of up to 93.6%,18 post-VAE imaging confirming complete lesion removal in >90% cases.21 VAE is now included in the UK NHS Breast Screening Clinical Guidelines for management of B3 lesions.22
Vacuum-assisted excision is not currently in use for the treatment of either invasive or in situ breast cancer, although this is an area of interest and there are data to suggest that VAE may be able to fully excise small tumours.23 The ongoing UK SMALL trial is a prospective, randomized (2:1) phase III trial assessing VAE versus open surgery in patients with small, biologically favourable screen detected breast cancers.24,25 In the VAE arm, a clip is placed at the tumour bed, and completeness of excision is assessed radiologically. Any patients found to have a residual radiological disease or grade three pathology will be offered open surgical excision. All patients will have adjuvant radiotherapy to the breast and endocrine therapy as per local protocols with mammographic follow-up for 5 years. Co-primary outcomes of this study are non-inferiority rates of VAE in avoiding a second procedure to achieve complete resection (≤10% accepted), and local recurrence rates at 5 years. Secondary outcomes include psychological and aesthetic impact of VAE, and a full health economic analysis will also be undertaken (Table 1).
Table 1.
A summary of the main completed and ongoing clinical trials for these three minimally invasive techniques.
| Study | Minimally invasive technique | Study type | Inclusion criteria | Status |
|---|---|---|---|---|
| SMALL24,25 | VAE | Phase III randomized-controlled trial |
|
|
| ACOSOG Z1072 (Alliance)30 |
Cryoablation | Phase II single-arm trial |
|
Results published 2016
|
| ICE331,33 | Cryoablation | Phase II single-arm trial |
|
Interim 3-year analysis published 2022
|
| FROST32 | Cryoablation | Phase II single-arm trial |
|
Follow up phase; results awaited |
| RAFAELO39 | Radiofrequency ablation (RFA) | Phase III single-arm trial |
|
Follow up phase; results awaited |
Cryoablation
Cryoablation uses the cytotoxic effects of cold to create tumour necrosis. The ablation process has three phases: first freeze; passive thawing; and second freeze. Freezing tissues at lethal temperatures causes increased intracellular osmolarity and cellular dehydration, whilst passive thawing causes cellular swelling and subsequent rupture. Indirect cryoablation also results in microthrombus formation and ischaemia. The second freeze is necessary to enhance the damaging effects of cold and expand the area of tumour necrosis—this is indicated for malignant lesions to create an “ice ball” extending at least 1 cm beyond the tumour margins. The procedure takes less than 45 min to complete, can be performed under ultrasound, CT, or MRI guidance (Figure 2), and typically requires less local anaesthetic compared to other minimally invasive techniques due to the synergistic cooling effect of the probe. Adverse side effects include low capability to limit ablation areas due to concerns of possible fat necrosis and/or infection as well as minor effects including ecchymosis, skin burns, and minor breast pain.26 Skin rash at the time of intervention is common, but this typically resolves within 8 h.27 In a systematic review of 161 patients, there were only three cases of seroma (1.9%), one case of skin retraction (0.6%), and one case of skin necrosis (0.6%), with satisfactory cosmesis reported in 99% cases.28 Following cryoablation, the damaged cancer cells remain in situ in the treated area—this is less likely to be accepted by patients with symptomatic disease and can impose challenges for surveillance imaging. Early detection of local recurrence is of paramount importance for disease-free survival and to date, there is no consensus on the best method of radiological follow-up for patients following cryoablation therapy.26
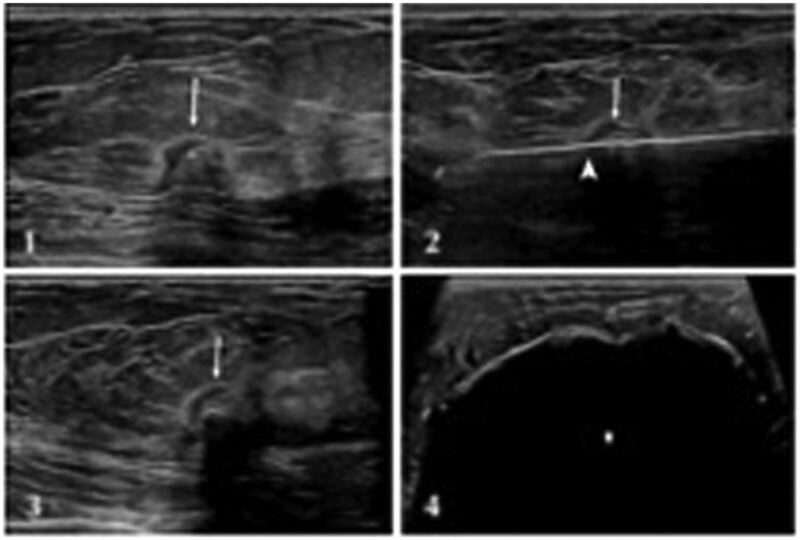
Figure 2.
(1) 1.1 cm grade II ER/HR+ HER2-intraductal carcinoma (2 and 3) long-axis and short-axis view of cryoprobe placement within the tumour [arrowhead denotes edge of tumour; caliper (+) denotes cryoprobe tip] (4) long-axis view of ice ball (*) enveloping the tumour. Adapted from Regen-Tuero et al.29
The use of cryotherapy in benign breast lesions and metastatic deposits is well described however substantive evidence supporting the use of cryoablation for invasive breast cancer is lacking. The most important trial to date exploring the use of cryoablation in the treatment of early-stage breast cancer was the ACOSOG Z1072 trial30 (Table 1). Patients with unifocal invasive ductal carcinoma ≤2 cm underwent cryoablation, followed by surgical resection of the primary tumour within 28 days. Of 87 cancers treated, ablation of the target lesion was successful in 92% of patients however residual invasive and/or in situ disease was identified in 24.1%. This was attributed to multifocal disease outside of the targeted cryoablation zone. Typically, this technique would not be advised in patients with ductal carcinoma in situ (DCIS) or lobular cancer due to its multifocal nature.
Two ongoing trials in the United States are exploring this further: ICE3 (Cryoablation of Low Risk Small Breast Cancer)31 and FROST (Freezing Instead of Removal Of Small Tumours).32 Patients with unifocal primary invasive disease ≤1.5 cm undergo cryoablation treatment, with a follow-up ultrasound core biopsy at 6 months to confirm the absence of residual viable disease. Patients then receive a minimum of 5 years of endocrine treatment and serial breast imaging. Adjuvant radiotherapy is mandatory for patients aged 50-69 but optional for those ≥70 years in FROST, and as per local policies in ICE3. Any subjects found to have residual or recurrent disease will be offered standard surgical excision. The primary endpoint for both studies is 5 years local recurrence rate. ICE3 has released a three-year interim analysis with promising results: ipsilateral breast cancer recurrence following cryoablation was 2.06% and more than 95% of patients and 98% of clinicians reported satisfaction with cosmetic results.33 The optimal method of radiological follow-up is not clear; however, the FROST trial is carrying out mammography, ultrasound, and MRI for all patients which may advise guidelines on the best method of radiological follow-up post-cryoablation. FROST is due to report results later this year. Of note, both these trials are single-arm phase II studies and there are currently no randomized controlled trials exploring the use of cryotherapy in early breast cancer.
Radiofrequency ablation
Radiofrequency ablation uses low-frequency radio-waves with long wavelengths to generate heat around a needle electrode causing localized coagulative tissue necrosis. Cancer cells contain more water than normal cells with fragile neoplastic vasculature—their susceptibility to heat-induced thrombosis results in localized necrosis of malignant tissue with minimal destruction of surrounding healthy cells. RFA can be performed under local anaesthetic with ultrasound, CT, or MRI guidance—ablation is observed with the formation of echogenic microbubbles (Figure 3). Reported side effects include skin burns (<3% incidence), inflammation of the breast (2.5%), nipple retraction (0.5%), and pneumothorax (0.5%).34 Like cryotherapy, the treatment goal is to ablate the whole lesion plus a 1 cm tissue rim, which reduces its suitability for lesions close to the skin, nipple, chest wall, or implant. Heat is not supplied by the probe itself therefore a limited volume of tissue can be ablated at one time and/or multiple probes are required for larger lesions.
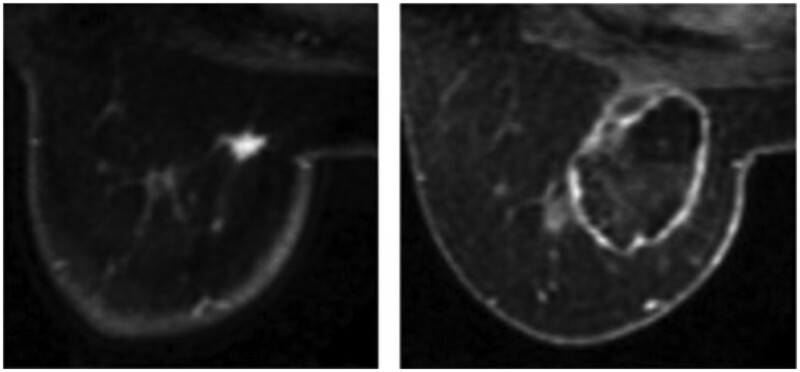
Figure 3.
(Left) MRI before RFA; (Right) MRI after RFA.34
Radiofrequency ablation is used for both treatment and palliation in numerous solid tumours including hepatocellular carcinoma, non-small cell lung cancer, and renal neoplasms.35 Technical success of RFA in the treatment of breast cancer was first described in 1999. Evidence suggests RFA is best used for smaller lesions ≤2 cm. Retrospective analysis of 386 patients across 10 institutes undergoing RFA found higher local recurrence rates in patients with initial tumour size >2 cm compared to those with ≤2 cm (10% versus 2.3%). Disease-free survival at 5 years following RFA was 97%, 94%, and 87% in patients with initial tumour sizes <1 cm, 1.1-2 cm, and >2 cm, respectively.36 These results are supported by a meta-analysis of 401 lesions ≤2 cm,34 however, there is an ongoing requirement for prospective studies to validate these findings and help guide clinical practice.
In a single-arm prospective study, Kinoshita et al treated 58 localized breast cancers ≤1 cm with RFA alone and followed them up with clinical examination, diagnostic imaging (ultrasound, MRI, and mammography), and VAB at 3, 6, and 12 months. All patients with suspected residual disease or incomplete ablation were offered surgical resection. Ninety percent of patients had a complete ablation rate, and there were no episodes of ipsilateral breast cancer or distal recurrence at a median follow-up of 1832 days.37 Garcia-Tejedor et al randomized 40 patients to RFA and lumpectomy, or lumpectomy alone, reporting positive margins in 55% of the control group, but only 20% of the RFA group. However, local breast inflammation and infection after surgery were higher in the RFA group (40% versus 5%, and 15% versus 0 respectively). No cases of ipsilateral recurrence were identified at a median follow-up of 25 months.38 These results led to the phase III, multicentre RAFAELO trial (Radiofrequency Ablation Therapy for Early Breast Cancer as Local Therapy).39 This is a single-arm study assessing the safety and efficacy of RFA in early-stage breast cancer; results will be compared with a previous randomized controlled study of breast conserving surgery. Women with localized breast cancer ≤1.5 cm undergo RFA and sentinel lymph node biopsy under general anaesthetic. Following adjuvant whole-breast radiotherapy, patients will have follow-up imaging and VAB at three months. If there is evidence of histological residual lesion, patients will undergo open surgical resection. The primary endpoint is 5-year ipsilateral disease-free survival, and secondary endpoints include overall survival, distal disease-free survival, adverse events of RFA, and tumour viability after RFA. A total of 372 patients were recruited to this trial making it the largest prospective trial assessing the use of RFA in early breast cancer to date. Results should be available later this year, but analysis of results should consider concurrent use of general anaesthesia and sentinel lymph node biopsy. To our knowledge, there are no large, prospective randomized-controlled trials exploring the use of RFA in early breast cancer.
Comparison of techniques
A meta-analysis of 1168 breast lesions compared technical success, technique efficacy, and complications associated with minimally invasive image-guided percutaneous ablation methods. RFA is the most used method (50%), with cryoablation used in only 13%. Pooled technical success was 96% (96% in radiofrequency and 95% in cryoablation), whilst pooled technique efficacy was suboptimal at 75% (67%-81%). Technique efficacy was significantly better in patients who underwent RFA and cryoablation compared to laser, microwaves, and high-intensity focused ultrasound, and complication rates were low across the techniques (6%-8%). Patient-reported outcomes were not considered in this review.40 Whilst this remains the largest published meta-analysis comparing different minimally invasive techniques, there are some limitations. The mean sample size was small (n = 24), the type of staining method used to assess the completeness of ablation at histology was not accounted for and in terms of reporting complication rates—there was an inhomogeneous description between the studies, thus patients were largely grouped into major and minor categories.
Manenti et al compared RFA (n = 40) and cryoablation (n = 40) in the treatment of early breast cancer. All patients subsequently underwent open surgery 30-45 days following ablation treatment and no episodes of local recurrence were identified at 18-month follow-up. Cryotherapy was considered the preferred method due to the analgesic effect of freezing with associated with better patient compliance.27 More recently, van de Voort et al have opened the THERMAC trial comparing the efficacy of RFA, microwaves, and cryoablation for early-stage breast cancer with the goal of selecting a technique for a subsequent phase III comparative study.41 There is currently no evidence, nor any ongoing clinical trials comparing percutaneous ablation techniques and VAE.
Discussion
When comparing minimally invasive techniques, it is important to acknowledge that whilst VAE will excise the tumour, both percutaneous ablation techniques leave a mass in situ. This may be difficult for patients, particularly those with palpable lesions, who will continue to complain of a lump, and pose challenges for both clinical and radiological follow-up. In addition, minimally invasive techniques do not allow for formal histopathological assessment of surgical margins. These considerations, together with concerns about the tolerability of minimally invasive approaches may mean that some patients would prefer one treatment approach over the other. It is important that future trials evaluating minimally invasive approaches explore patient preferences, as is taking place within the Qualitative Recruitment Intervention in the SMALL trial.24,25
Whilst close or involved margins are associated with local recurrence, we also know that a clear margin does not mean there is no residual disease in the breast, nor does this impact survival outcomes. Holland et al examined a series of patients undergoing mastectomy for clinically and/or radiologically unifocal disease.42 This series included a cohort of patients (18% of the total) with screen-detected disease. In 130 patients with T1 tumours, 17% of cases had tumour foci within 2 cm of the index lesion, with a further 42% having additional foci >2 cm from the reference tumour. There was no statistically significant difference in the incidence of multifocal tumours with reference to lesions </> 2 cm.
Improved imaging techniques and the use of contrast imaging modalities (such as magnetic resonance imaging and contrast-enhanced spectral mammography) may be valuable in identifying patients with such occult foci of disease.43,44 Patients with occult multifocal disease may not be suitable for such percutaneous approaches to treatment, and future studies of minimally invasive techniques may require assessing the role of contrast imaging for patient selection in this context.
However, in an era of effective systemic therapies, what is important is not necessarily the volume of tumour left behind but rather the clinical significance of such microscopic occult disease. In a large retrospective population-based cohort study of 31 199 patients, Vos et al report a local recurrence rate of 2.9% in patients with focally positive margins at breast conserving surgery compared to 2.3% in patients with negative margins (P = .099). In the context of radiotherapy, re-excision of positive margins was not associated with improved disease-free or overall survival when compared to omitting re-excision (87.1% versus 86%, adjusted HR 0.83 [95% CI, 0.59-1.17] and 92.1% versus 92.7%, adjusted HR 1.17 [95% CI, 0.87-1.59] respectively).13 Findings from the IMPORT LOW randomized controlled trial report very low local recurrence rates in low-risk breast cancer patients receiving adjuvant radiotherapy, and this study found non-inferiority survival outcomes for both reduced-dose and partial-breast radiotherapy compared to whole-breast radiotherapy at 5-year follow up (0.2%, 0.5%, and 1.1%, respectively, P = .003-.016).45 These findings support the hypothesis that minimally invasive treatment approaches in the context of standard multidisciplinary adjuvant therapies can achieve acceptable oncological outcomes.
However, there is considerable interest among researchers in studies examining de-escalation of such adjuvant therapies, particularly radiotherapy. Studies such as PRIMETIME,46,47 PRECISION,47 EXPERT,48 DEBRA,49 and IDEA50 are examining the omission of radiotherapy in biomarker-determined low-risk disease, and the PROSPECT trial used MRI to identify patients with true unifocal disease who could potentially avoid radiotherapy.51 Currently, such studies require patients to undergo standard surgery in order to de-escalate the radiotherapy component of their treatment. Such de-escalation may have benefits for patients in terms of reducing treatment burden, and to healthcare systems in terms of reducing resource use. The implementation of hypofractionated radiotherapy regimens, reducing treatment to 5 fractions, however, partly mitigates against these benefits. There are fewer studies evaluating the omission or de-escalation of endocrine therapy, although both LALEAST52 and LESS53 are evaluating shorter durations of adjuvant endocrine therapy in the setting of low-risk disease.
What is clear, however, is that patients may well have different preferences for treatment in line with their own beliefs, cultures, and circumstances. This is borne out by patient surveys which confirm that different patients wish to de-escalate different components of their therapy should it be safe to do so. A recent international patient survey demonstrated that 30% of patients would prefer to omit chemotherapy, 26% endocrine therapy, 17% surgery, and only 8% radiotherapy (unpublished data; Potter et al SABCS 2023). It is therefore important to generate data to support the de-escalation of each therapeutic modality to allow breast cancer patients to make informed choices regarding treatment.
Conclusions
Minimally invasive techniques may offer a suitable alternative to surgical excision for low-risk, early breast cancers, particularly those small, good-prognosis tumours likely to be overtreated currently, with the attendant morbidities that ensure. These techniques are less invasive for patients, deliver good results, and are a cost-effective intervention for healthcare providers. VAE offers the benefit of excising the lesion as whole, whilst cryoablation and RFA destroy cancer cells with localized effects, although all may have a role to play in the management of the low-risk disease. Furthermore, such approaches need to be viewed in the context of the multidisciplinary treatment of breast cancer and take into account the preferences of patients to de-escalate different treatment modalities. High-quality evidence from prospective studies including randomized controlled trials will be required before adopting these approaches into routine clinical practice.
Contributor Information
Mhairi Mactier, Golden Jubilee National Hospital, Clydebank G81 4DY, United Kingdom.
Stuart A McIntosh, Patrick G Johnston Centre for Cancer Research, Queen’s University Belfast, Belfast BT9 7AE, United Kingdom.
Nisha Sharma, Breast Unit, St James Hospital, Leeds LS9 7TF, United Kingdom.
Funding
None declared.
Conflicts of interest
S.M. reports honoraria from MSD, Roche, BD, Novartis, Lilly, and Astra Zeneca; conference travel and support from Roche, Lilly, and MSD, and institutional research funding from Novartis and Almac Diagnostic Services. S.M. reports grant funding from Cancer Research UK, the National Institute for Health Research (including as Chief Investigator for the SMALL trial) and Breast Cancer Now. The other authors have no conflicts to declare.
References
- 1. Marmot MG, Altman DG, Cameron DA, et al. The benefits and harms of breast cancer screening: An independent review. Br J Cancer. 2013;108(11):2205-2240. 10.1016/S0140-6736(12)61611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welch HG, Prorok PC, O'Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447. 10.1056/NEJMoa1600249 [DOI] [PubMed] [Google Scholar]
- 3. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer for the cancer intervention and surveillance modeling network (CISNET) collaborators. N Engl J Med. 2005;353(17):1784-1792. 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 4. Lannin DR, Wang S.. Are Small breast cancers good because they are small or small because they are good? N Engl J Med. 2017;376(23):2286-2291. 10.1056/NEJMsr1613680 [DOI] [PubMed] [Google Scholar]
- 5. Paik S, Shak S, Kim C, et al. A Multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 6. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005-2014. 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elder K, Nickson C, Pattanasri M, et al. Treatment intensity differences after early-stage breast cancer (esbc) diagnosis depending on participation in a screening program. Ann Surg Oncol. 2018;25(9):2563-2572. 10.1245/s10434-018-6469-7 [DOI] [PubMed] [Google Scholar]
- 8. Cil TD, Cordeiro E.. Complications of oncoplastic breast surgery involving soft tissue transfer versus breast-conserving surgery: an analysis of the NSQIP database. Ann Surg Oncol. 2016;23(10):3266-3271. 10.1245/s10434-016-5477-8 [DOI] [PubMed] [Google Scholar]
- 9. Wang HT, Barone CM, Steigelman MB, et al. Aesthetic outcomes in breast conservation therapy. Aesthet Surg J. 2008;28(2):165-170. 10.1016/j.asj.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 10. McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467-475. 10.1001/jama.2012.43 [DOI] [PubMed] [Google Scholar]
- 11. Bundred JR, Michael S, Stuart B, et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. BMJ. 2022;378:e070346. 10.1136/bmj-2022-07034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdulla HA, Rajab B, Hammad M, et al. Risk factors for positive margins in breast-conserving surgery. Cureus. 2023;15(5):e38399. 10.7759/cureus.38399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vos EL, Gaal J, Verhoef C, et al. Focally positive margins in breast conserving surgery: Predictors, residual disease, and local recurrence. Eur J Surg Oncol. 2017;43(10):1846-1854. 10.1016/j.ejso.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 14. Che Bakri NA, Kwasnicki RM, Khan N, et al. Impact of axillary lymph node dissection and sentinel lymph node biopsy on upper limb morbidity in breast cancer patients: a systematic review and meta-analysis. Ann Surg. 2023;277(4):572-580. 10.1097/SLA.0000000000005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926. 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gentilini O, Veronesi U.. Staging the axilla in early breast cancer: will imaging replace surgery? JAMA Oncol. 2015;1(8):1031-1032. 10.1001/jamaoncol.2015.2337 [DOI] [PubMed] [Google Scholar]
- 17. Eller A, Janka R, Lux M, et al. Stereotactic vacuum-assisted breast biopsy (VABB)—a patients’ survey. Anticancer Res. 2014;34(7):3831-3837. [PubMed] [Google Scholar]
- 18. Perretta T, Lamacchia F, Ferrari D, et al. Evaluation of ultrasound-guided 8-gauge vacuum-assisted excision system for the removal of US-detectable breast lesions. Anticancer Res. 2020;40(3):1719-1729. 10.21873/anticanres.14125 [DOI] [PubMed] [Google Scholar]
- 19. Nice. 2006. Image-guided vacuum-assisted excision biopsy of benign breast lesions Interventional procedures guidance. Accessed December 2023. www.nice.org.uk/guidance/ipg156
- 20. Yoo HS, Kang WS, Pyo JS, et al. Efficacy and safety of vacuum-assisted excision for benign breast mass lesion: A meta-analysis. Medicina (Kaunas). 2021;57(11):1260. 10.3390/medicina57111260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine RE, Israel PZ, Walker LC, et al. A prospective study of the removal rate of imaged breast lesions by an 11-gauge vacuum-assisted biopsy probe system. Am J Surg. 2001;182(4):335-340. 10.1016/s0002-9610(01)00723-1 [DOI] [PubMed] [Google Scholar]
- 22. Nhs. 2016. Breast Screening Programme Clinical guidance for breast cancer screening assessment Public Health England leads the NHS Screening Programme. Accessed December 2023. https://www.gov.uk/government/publications/breast-screening-clinical-guidelines-for-screening-management
- 23. Valadares CN, Couto HL, Soares AN, et al. Potential role of vacuum-assisted procedures in resecting breast cancers and highlighting selection criteria to support future trials. Front Oncol. 2023;13:1239574. 10.3389/fonc.2023.1239574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan J, Potter S, Sharma N, et al. The SMALL trial: a big change for small breast cancers. Clin Oncol (R Coll Radiol). 2019;31(9):659-663. 10.1016/j.clon.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 25. McIntosh S, Coles CE, Dodwell DJ, et al. SMALL: open surgery versus minimally invasive vacuum-assisted excision for small screen-detected breast cancer—A UK phase III randomised multi-centre trial. JCO. 2023;41(16_suppl):TPS625-TPS625. 10.1200/JCO.2023.41.16_suppl.TPS625 [DOI] [Google Scholar]
- 26. Pusceddu C, Paliogiannis P, Nigri G, et al. Cryoablation in the management of breast cancer: evidence to date. Breast Cancer (Dove Med Press). 2019;11:283-292. 10.2147/BCTT.S197406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manenti G, Scarano AL, Pistolese CA, et al. Subclinical breast cancer: minimally invasive approaches. our experience with percutaneous radiofrequency ablation vs. cryotherapy. Breast Care (Basel). 2013;8(5):356-360. 10.1159/000355707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanza E, Palussiere J, Buy X, et al. Percutaneous Image-Guided Cryoablation of Breast Cancer: A Systemic Review. J Vasc Interv Radiol. 2015;26(11):1652-1657.e1. 10.1016/j.jvir.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 29. Regen-Tuero HC, Ward RC, Sikov WM, et al. Cryoablation and immunotherapy for breast cancer: Overview and rationale for combined therapy. Radiol Imaging Cancer. 2021;3(2):e200134. 10.1148/rycan.2021200134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simmons RM, Ballman KV, Cox C, et al. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. 2016;23(8):2438-2445. 10.1245/s10434-016-5275-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov. 2014. Cryoablation of low risk small breast cancer—Ice3 trial. Accessed December 2023. https://clinicaltrials.gov/ct2/show/NCT02200705
- 32. Coronado G, Ho E, Holmes D.. Abstract OT2-01-04: freezing instead of resection of small breast tumors (FROST): a study of cryoablation in the management of early stage breast cancer. Cancer Res. 2018;78(4_Supplement):OT2-01-04. 10.1158/1538-7445.SABCS17-OT2-01-04 [DOI] [Google Scholar]
- 33. Fine RE, Gilmore RC, Dietz JR, et al. Cryoablation without excision for low-risk early-stage breast cancer: 3-year interim analysis of ipsilateral breast tumor recurrence in the ICE3 trial. Ann Surg Oncol. 2021;28(10):5525-5534. 10.1245/s10434-021-10501-4 [DOI] [PubMed] [Google Scholar]
- 34. Xia LY, Hu QL, Xu WY.. Efficacy and safety of radiofrequency ablation for breast cancer smaller than 2 cm: a systematic review and meta-analysis. Front Oncol. 2021;11:651646. 10.3389/fonc.2021.651646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah DR, Green S, Elliot A, et al. Current oncologic applications of radiofrequency ablation therapies. World J Gastrointest Oncol. 2013;5(4):71-80. 10.4251/wjgo.v5.i4.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ito T, Oura S, Nagamine S, et al. Radiofrequency ablation of breast cancer: a retrospective study. Clin Breast Cancer. 2018;18(4):e495-e500. 10.1016/j.clbc.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 37. Kinoshita T, Yamamoto N, Fujisawa T, et al. Radiofrequency ablation for early-stage breast cancer: results from 5 years of follow-up in a prospective multicenter study. JCO. 2017;35(15_suppl):e12098. 10.1200/JCO.2017.35.15_suppl.e12098 [DOI] [Google Scholar]
- 38. García-Tejedor A, Guma A, Soler T, et al. Radiofrequency ablation followed by surgical excision versus lumpectomy for early stage breast cancer: a randomized phase II clinical trial. Radiology. 2018;289(2):317-324. 10.1148/radiol.2018180235. [DOI] [PubMed] [Google Scholar]
- 39. Kinoshita T, Ohtani S, Doihara H, et al. Multicenter study to evaluate the efficacy and standardize radiofrequency ablation therapy for early breast cancer (RAFAELO study). Ann Oncol. 2018;29(8):viii85-viii86. 10.1093/annonc/mdy270.263 [DOI] [Google Scholar]
- 40. Mauri G, Sconfienza LM, Pescatori LC, et al. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: a systematic review and meta-analysis. Eur Radiol. 2017;27(8):3199-3210. 10.1007/s00330-016-4668-9 [DOI] [PubMed] [Google Scholar]
- 41. Van De Voort EMF, Struik GM, Koppert LB, et al. Treatment of early-stage breast cancer with percutaneous thermal ablation, an open-label randomised phase 2 screening trial: Rationale and design of the THERMAC trial. BMJ Open. 2021;11(9):e052992. 10.1136/bmjopen-2021-052992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of tis, T1–2 breast carcinomas implications for clinical trials of breast‐conserving surgery. Cancer. 1985;56(5):979-990. [DOI] [PubMed] [Google Scholar]
- 43. Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR AM J Roentgenol. 2004;183(4):1149-1157. 10.2214/ajr.183.4.1831149 [DOI] [PubMed] [Google Scholar]
- 44. Jochelson MS, Lobbes MBI.. Contrast-enhanced mammography: state of the art. Radiology. 2021;299(1):36-48. 10.1148/radiol.2021201948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048-1060. 10.1016/S0140-6736(17)31145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kirwan CC, Coles CE, Bliss J, It’s PRIMETIME. postoperative avoidance of radiotherapy: biomarker selection of women at very low risk of local recurrence. Clin Oncol (R Coll Radiol). 2016;28(9):594-596. 10.1016/j.clon.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 47. Braunstein LZ, Wong J, Dillon DA, et al. Abstract OT1-12-02: preliminary report of the PRECISION trial (profiling early breast cancer for radiotherapy omission): a phase II study of breast-conserving surgery without adjuvant radiotherapy for favorable-risk breast cancer. Cancer Res. 2023;83(5_Supplement):OT1-12-02. 10.1158/1538-7445.SABCS22-OT1-12-02 [DOI] [Google Scholar]
- 48.ClinicalTrials.gov. 2017. EXamining PErsonalised Radiation Therapy for Low-risk Early Breast Cancer (EXPERT). Accessed December 2023. https://clinicaltrials.gov/study/NCT02889874
- 49. White JR, Anderson SJ, Harris EE, et al. NRG-BR007: a phase III trial evaluating de-escalation of breast radiation (DEBRA) following breast-conserving surgery (BCS) of stage 1, hormone receptor+, HER2-, RS ≤18 breast cancer. JCO. 2023;40(16_suppl):TPS613. 10.1200/JCO.2022.40.16_suppl.TPS613 [DOI] [Google Scholar]
- 50. Jagsi R, Griffith KA, Harris EE, et al. Omission of radiotherapy after breast-conserving surgery for women with breast cancer with low clinical and genomic risk: 5-year outcomes of IDEA. J Clin Oncol. 2023;42(4):390-398. 10.1200/JCO.23.02270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mann GB, Skandarajah AR, Zdenkowski N, et al. Postoperative radiotherapy omission in selected patients with early breast cancer following preoperative breast MRI (PROSPECT): primary results of a prospective two-arm study. Lancet. 2024;403(10423):261-270. 10.1016/S0140-6736(23)02476-5 [DOI] [PubMed] [Google Scholar]
- 52. Khalaf D, Pansegrau G, Levasseur N, et al. 2019. LA LEAST—luminal A, limited endocrine adjuvant systemic therapy. A trial of abbreviated hormone therapy for low risk hormone receptor positive, HER2 negative early breast cancer. Accessed December 2023. https://www.medifind.com/articles/clinical-trial/4454920
- 53. Deluche E, Michiels S, Fric D, et al. LESS—single-arm study to de-escalate adjuvant endocrine therapy duration in post- menopausal women with HR+ HER2- early-stage breast cancer at very low risk of metastasis. JCO. 2023;41(16_suppl):TPS615. 10.1200/JCO.2023.41.16_suppl.TPS615 [DOI] [Google Scholar]