Abstract
Objective
This study aimed to establish the first-ever MRI classification of uterosacral ligament (USL) involvement in deep infiltrating endometriosis (DIE), based on reliable preoperative MRI features correlated with positive predictive values (PPVs) determined through histopathological analysis.
Methods
Twenty-two women underwent surgery with histopathology due to symptoms highly suggestive of endometriosis. The 22 preoperative MRIs were analysed retrospectively, blinded to histopathology, and a classification of the preoperative aspect of USLs linked to PPVs was designed.
Results
According to their aspects, 6 radiological types of USL were identified. The “L-category” corresponded to linear types with regular or irregular margins, including types 1, 2, 3A, and 3B. The “N-category” corresponded to haemorrhagic or nodular types, including types 4, 5A, 5B, and 6. For the L-category, PPVs ranged from 75% to 88%, depending on the USL radiological type. For the N-category, PPVs were 100% for each type. In women with endometriosis symptoms, MRI underestimated USL involvement, especially for type 1. Among the 6 uteri with lateral deviation, only one false-positive result concerning the stretched USL was induced.
Conclusions
In women with endometriosis symptoms, our MRI classification identified 2 USL categories, corresponding to 2 kinds of PPV; in these symptomatic patients, a normal MRI does not rule out a DIE diagnosis.
Advances in knowledge
Our MRI classification of USL involvement in endometriosis may be used as a non-invasive staging of the disease, making it much clearer for clinicians and patients. Hence, we are able to propose a suitable diagnostic and therapeutic procedure for each radiological type.
Keywords: deep infiltrating endometriosis, DIE, uterosacral ligament, USL, MRI classification, Hôtel-Dieu, HTD
Introduction
Endometriosis is a common gynaecological condition that affects approximately 10% of women of reproductive age; in selected populations, such as patients with pelvic pain or infertile women, the prevalence may attain 70%.1 Endometriosis is histologically defined as the presence of endometrial glands and stroma outside of the uterus.2
Endometriosis can be classified as ovarian (i.e., endometriomas), peritoneal (i.e., superficial), or deep infiltrating endometriosis (DIE), with the latter defined as the presence of a lesion infiltrating 5 mm or more into the peritoneum.3
Deep infiltrating endometriosis may involve uterosacral ligaments (USLs), torus uterinum, posterior vaginal cul-de-sac, Douglas pouch, rectosigmoid colon, rectovaginal septum, and bladder.4
Women with peritoneal endometriosis can be asymptomatic; on the other hand, DIE is frequently associated with typical symptoms, the 6 “Ds”: dysmenorrhoea, dyspareunia, dyschezia, dysuria, dysfertility, inveterata pelvis dolor. Diagnostic accuracy of physical examination or transvaginal sonography for DIE is low.5,6
The gold standard for the diagnosis of endometriosis is considered to be laparoscopy with biopsy which allows histological confirmation of suspicious lesions.7 Laparoscopy has a few limitations such as the need for general anaesthesia and the poor assessment of subperitoneal lesions, small ovarian implants, and lesions hidden by adhesions. Laparoscopy itself may induce iatrogenic adhesions.
This difficulty in the staging assessment has allowed the development of MRI in this indication, and despite some limitations, it is currently considered the best examination for detecting deep endometriotic lesions due to its high contrast resolution and its harmlessness.8 A questioning-based clinical score has been developed, that reliably predicts the likelihood of endometriosis.9 Using this score, clinicians may then refer women to specialized radiologists to allow a non-surgical diagnosis of endometriosis. If excision surgery is planned, MRI will allow precise mapping of deep lesions. MRI also allows post-therapeutic monitoring as well as the differential diagnosis of endometriotic lesions.10–12
DIE more frequently involves the USLs with a prevalence of approximately 70% in patients with endometriosis.13,14 Up to now, no real consensus has been established on the imaging anatomy of USLs. On MR T2-weighted images (T2WI), normal USLs are not visible15 or are depicted as thin, regular, semicircular hypointense cords that originate from the lateral aspect of the uterine cervix and the vaginal vault and course dorsocranially toward the sacrum.16 USL endometriosis is depicted as nodularity within the ligament or as unilateral or bilateral hypointense thickening of the ligament, with regular or irregular margins.15,16
Currently, in the absence of an MRI classification, radiologists’ assessment of USL endometriosis is subjective. Due to the heterogeneity of radiology reports and the wide variation in report terminologies, results for some radiologists may be skewed. To address this issue, we have considered developing an MRI classification system for USLs that would prove beneficial for both radiologists and clinicians. This system would provide a structured framework for interpreting images, enabling standardization and uniformity in reporting. Consequently, it would enhance communication among health professionals and promote advancements in education and research.
To date, no study has been designed with the primary objective of determining the accuracy of MRI in the detection of DIE by weighting its diagnostic value according to the features of the USL based on histological correlations. No MRI classification currently exists, although it is considered the best imaging technique for detecting endometriotic lesions. Thus, the purpose of the present study was to classify USL features depending on their PPVs based on histological correlations and to build the very first preoperative MRI classification.
Methods
Study population
After institutional ethical board approval, our study population included retrospectively 22 women, aged 20-39 years (mean age 27), who underwent MRI in our university hospital for clinical suspicion of DIE upon variable symptoms before surgery from October 2008 to November 2016. All patients presented with pain symptoms (dysmenorrhoea, dyspareunia, and chronic pelvic pain) for more than 6 months. All patients underwent laparoscopic examination and surgery. The diagnosis of endometriosis involving a USL was proved in histopathology.
MRI protocol
MRIs were performed on 1.5T systems (Achieva®, Philips Healthcare, Best, the Netherlands; Aera®, Siemens Healthcare, Erlangen, Germany; Signa®, GE Healthcare, Chicago, IL, USA) using a phased array pelvic coil. Vagina was filled with ultrasound gel. Rectal filling was performed only in case of catamenial dyschezia. Protocols included sagittal and axial spin echo T2WI, axial spin echo T1-weighted images (T1WI) with and without fat saturation (Fat-Sat), sagittal and axial spin echo T1WI without Fat-Sat after intravenous injection of 0.1 mmol/kg body weight of gadoterate meglumine (Dotarem®, Guerbet, France). Thin-section sagittal and axial T2WI sequences (slice thickness 3 mm; slice spacing 0.6 mm) were used to improve the capability of conventional MRI to assess USL endometriosis.
Image analysis
MRIs were retrospectively analysed by 3 independent radiologists (30, 7, and 4 years of experience, respectively), blinded to the final histopathological diagnosis.
MRIs were reviewed, and USLs were sorted by their morphology to identify different patterns.
Findings were recorded by consensus.
Histopathological correlation
Diagnosis of all endometriosis cases was established at pathological examination, with macroscopy and microscopy.
Statistical analysis
MRI classification and histopathological reports of each case were retrospectively analysed, and the PPVs of each type were calculated. The PPV was calculated as the number of positive lesions in histopathology divided by the number of lesions assigned to each type. A cross-over approach was used for comparison and calculation of PPVs: the 2 USLs in the same patient could be classified on MRI, but one of them could be normal on histopathology (gold standard) and therefore considered a false positive.
Results
The 6 types of USL
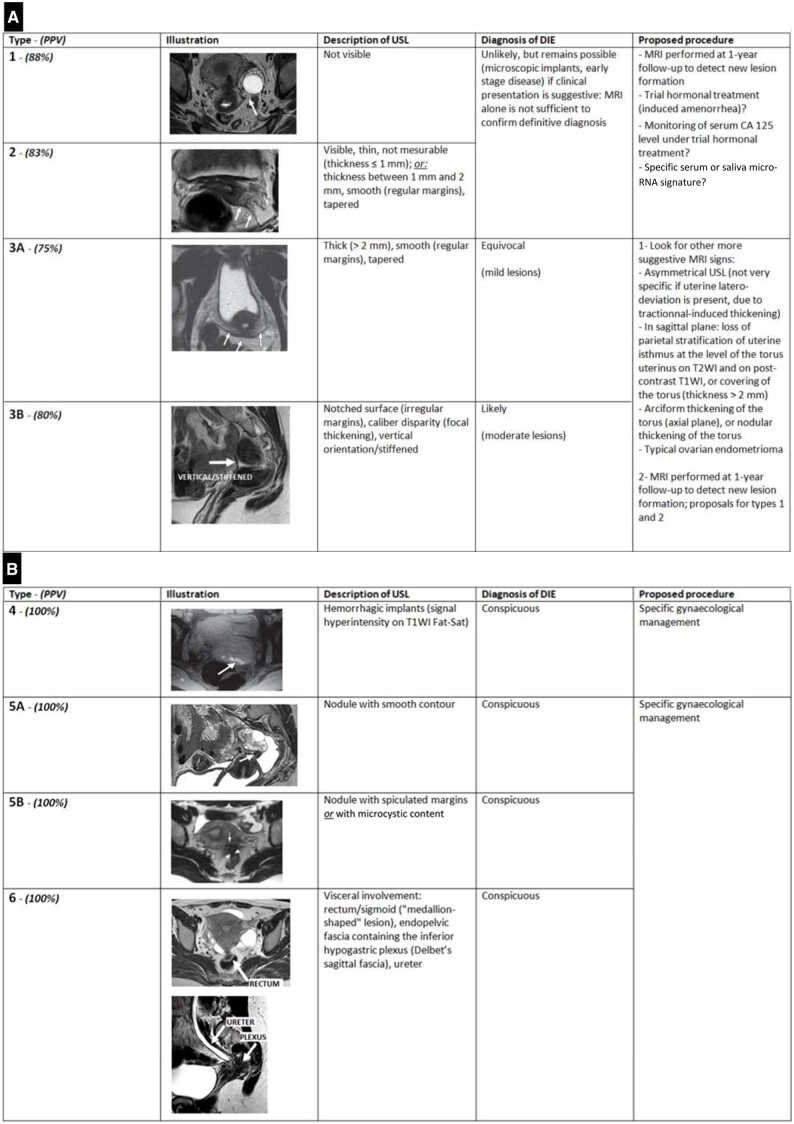
The “L-category” corresponded to linear types, with regular or irregular margins, and included types 1, 2, 3A, and 3B.
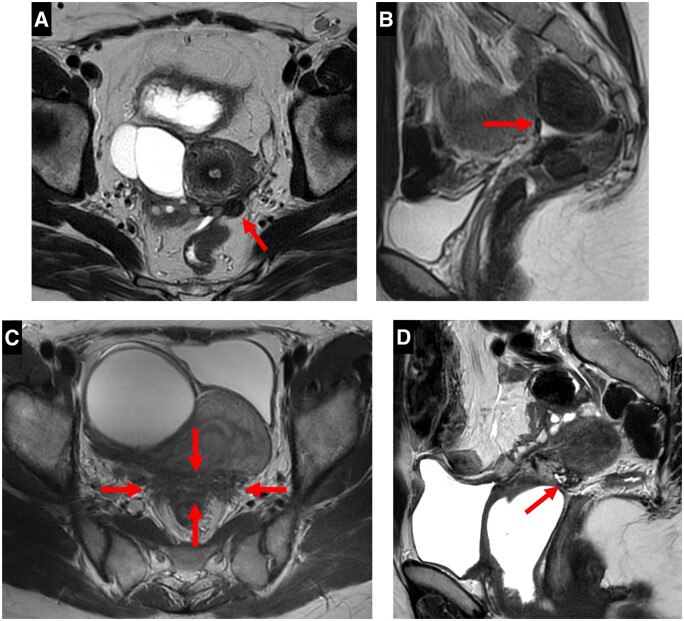
A type 1 USL was not visible on MRI scans despite a positive histopathological examination (Figure 1).
Figure 1.
Pelvic MRI of a symptomatic patient with a positive USL at pathology: axial T2WI showing a type 1 USL, not visible on scans (arrow).
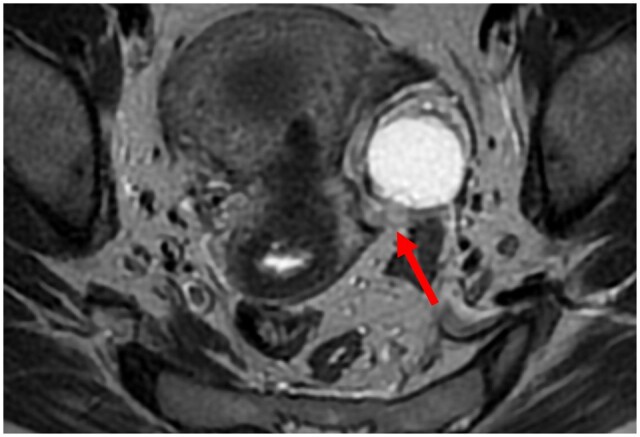
A type 2 USL was visible but either not measurable (i.e., thickness ≤ 1 mm) or measurable with a mild thickening ranging from 1 to 2 mm included; a type 2 USL displayed a smooth surface with regular margins and usually had a longitudinally tapering shape (Figure 2).
Figure 2.
Pelvic MRI of a symptomatic patient with a positive USL at pathology: (A, B) axial T2WI showing a type 2 USL (arrow) with a mild thickening ranging from 1 to 2 mm included (A) and a smooth surface (B).
A type 3A USL was thick (> 2 mm), smooth, and usually tapered. A type 3B USL displayed a notched surface with irregular margins, a calibre disparity with focal thickening, or a steep vertical orientation, or looked stiffened (Figure 3).
Figure 3.
Pelvic MRI scans of symptomatic patients with a positive USL at pathology: (A-D). (A) axial T2WI showing a type 3A USL (arrow) with a thick (> 2 mm) calibre, a smooth surface and a tapering shape. (B) Sagittal T2WI showing a type 3B USL with a notched and irregular surface (arrows). (C) Axial T2WI showing a type 3B right USL with a calibre disparity alternating a proximal thickening > 2 mm (arrow) and a distal thinness (arrowhead). (D) Sagittal T2WI showing a type 3B USL with a steep vertical orientation and a stiffened appearance (arrow).
The “N-category” corresponded to haemorrhagic or nodular types and included types 4, 5A, 5B, and 6.
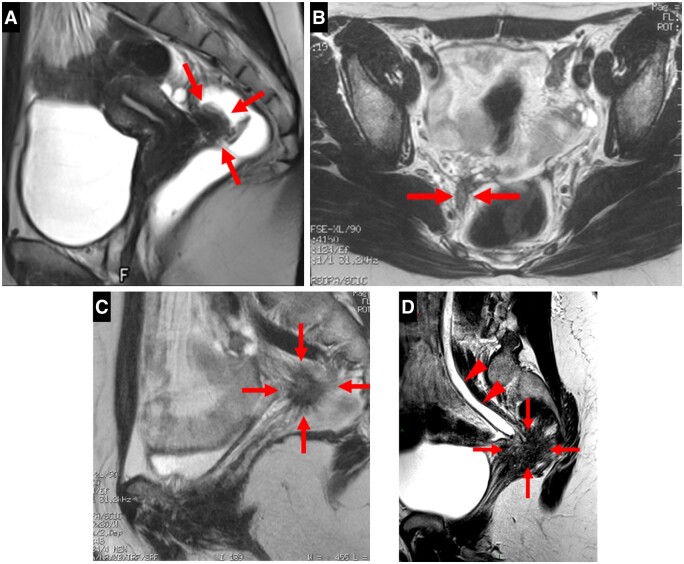
A type 4 USL displayed haemorrhagic implants appearing as hyperintense spots on fat-suppressed T1WI (Figure 4).
Figure 4.

Pelvic MRI of a symptomatic patient with positive USLs at pathology: type 4 USLs with hyperintense spots on axial fat-suppressed T1WI representing punctate foci of haemorrhage (arrow).
A type 5 USL looked nodular with either a smooth contour for type 5A or spiculated margins for type 5B (Figure 5). Isolated microcystic content was possible for type 5B.
Figure 5.
Pelvic MRI scans of symptomatic patients with positive USLs at pathology: (A-D). Axial and sagittal T2WI showing nodularity (arrows) within type 5 USLs with regular (A, B) or irregular/spiculated (C) margins for types 5A and 5B, respectively. An example of a microcystic nodule for type 5B (D).
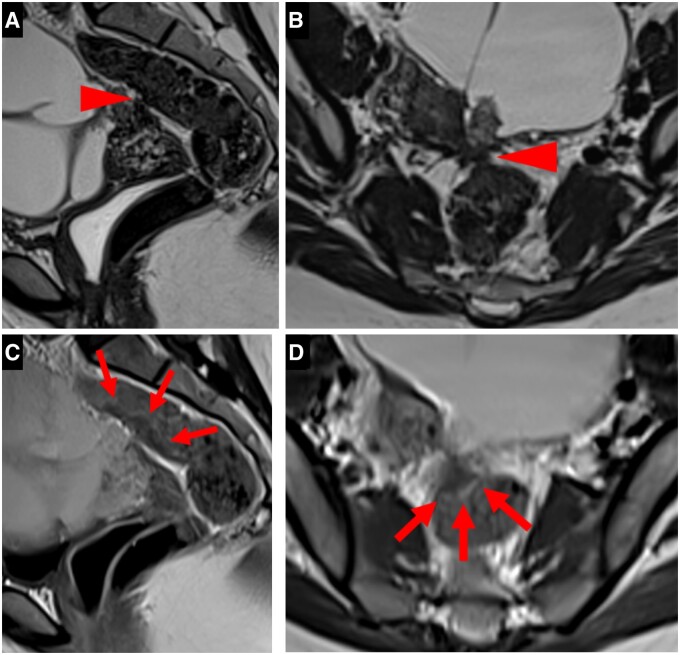
A type 6 USL was associated with a contiguous visceral involvement (Figure 6). Rectosigmoid wall infiltration usually appears as a “medallion-shaped” lesion with a spherically convex surface protruding into the lumen of the digestive tract. Endometriosis infiltration might affect a sacro-recto-genito-pubic lamina (also known as Delbet sagittal fascia) containing the inferior hypogastric plexus. Endometriotic lesions might affect the distal ureter; as a result, ureteral obstruction and hydronephrosis were common.
Figure 6.
Pelvic MRI scans of symptomatic patients with positive USLs at pathology: (A-D). Examples of type 6 USLs. (A) Sagittal T2WI showing an endometriotic lesion of the anterior rectal wall with a “medallion-shaped” appearance, protruding into the lumen of the digestive tract. (B) Axial and (C) sagittal T2WI showing a nodular infiltration of the right sacro-recto-genito-pubic lamina, this endometriotic lesion underlining the structure of the inferior hypogastric plexus. (D) Sagittal T2WI showing an endometriotic lesion affecting the distal ureter with consequential ureteral dilatation (arrowheads).
The respective PPVs of each type of USL
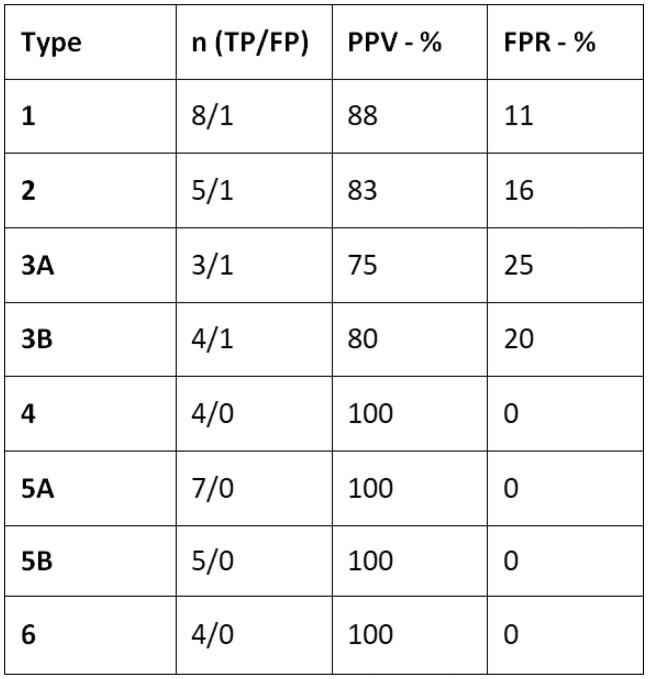
The PPVs of each type of the “L-category” were as follows: 88% for type 1, 83% for type 2, 75% for type 3A, and 80% for type 3B. The false-positive rates (FPRs) of each type were as follows: 11% (1/9) for type 1, 16% (1/6) for type 2, 25% (1/4) for type 3A, and 20% (1/5) for type 3B.
The PPVs and the FPRs were 100% and 0%, respectively, for each type of the “N-category”.
Figure 7 presents a summary of the characteristics within the different groups for the 44 USLs analysed in the 22 patients.
Figure 7.
Summary of the characteristics within the different groups. Abbreviations: n = number of USLs; TP = true positives; FP = false positives; PPV = positive predictive value; FPR = false-positive rate.
All important data are summarized in Figure 8.
Figure 8.
Synoptic view of our MRI classification of USL involvement in endometriosis (also known as “the HTD classification”): (A, B). The “L-category” of USL (A) and the “N-category” of USL (B). Abbreviations: DIE = deep infiltrating endometriosis; T1WI = T1-weighted images; T2WI = T2-weighted images; USL = uterosacral ligament.
Discussion
We defined 2 radiological categories of USL, based on their MRI aspect, matching 2 different ranges of PPV. We have thus developed an MRI classification system for USLs using semi-objective descriptors for each plane of section in T2WI. Our system assigns a scale of 1 to 6 to indicate the possibilities of DIE.
The “L-category” corresponded to linear types, with regular or irregular margins, and included types 1, 2, 3A, and 3B. For the L-category, PPVs ranged from 75% to 88%, depending on the USL radiological type.
The “N-category” corresponded to haemorrhagic or nodular types and included types 4, 5A, 5B, and 6. For the N-category, PPVs were 100% for each type; hence, diagnostic confidence of endometriosis is very high in these symptomatic women.
The evolution of the radiological appearance of different USLs belonging to the “L-category” toward increased visibility on T2-weighted images could be explained by a histological phenomenon of adipose-fibrous transition. Indeed, adipose tissue is abundant in normal USLs17; while connective tissue is present, its density is less than that of adipose tissue. This histological observation could explain the non-visibility of type 1 USLs, likely to have a fatty consistency, as they are “drowned” in the T2 hypersignal of the surrounding pelvic visceral adipose tissue. The histological appearance of endometriotic USLs depends, among other factors, on the age of the lesions, which gradually evolve toward fibrosis.18 This transition to fibrosis of USLs fat makes them increasingly visible within the visceral adipose tissue as a fibrous T2 hyposignal, noticeable for type 2 USLs, and pronounced for type 3 USLs.
Our study has a few limitations. The gold standard is pathology with histological identification of ectopic endometrial tissue outside the uterus, composed of glands embedded within a cytogenetic chorion, in biopsy or surgical samples.19 However, extensive nonspecific fibrosis of USLs may follow the inflammatory phase of endometriotic lesions, thereby potentially hiding remnants of endometrial tissue on microscopic examination. This situation may lead to false-negative results in histopathology, whereas surgical macroscopic findings are highly evocative of endometriotic lesions.20,21
A confusion could possibly be made between type 3B and type 5A USLs. In short, a type 3B USL corresponds to a linear enlargement of the USL, more or less extensive, with irregular contours, while a type 5A USL corresponds to the presence of a very focal nodule on the USL, isolated, with regular contours.
Other classifications of endometriosis validated in the literature, in particular the American Fertility Society (AFS) classification and its derivatives, are based on surgical findings during laparoscopy in endometriosis-diagnosed women and may fail to fully evaluate subperitoneal involvement.22,23 In addition, these classifications aim to evaluate fertility alone in the presence of endometriotic lesions. The aim of our MRI classification is radically different, as it is exclusively diagnostic, based only on USL MRI aspects, without any other consideration of prognosis. Thus, our MRI classification of USL involvement in symptomatic women may be used as a non-invasive staging of endometriosis, making it much clearer for clinicians and patients. Hence, we are able to propose a suitable diagnostic and therapeutic procedure for each radiological type.
Linear types of USL may be found in asymptomatic women with no personal history of endometriosis.24 Hence, in women with endometriosis symptoms, many specific associated signs such as a nodular thickening of the torus or an ovarian endometrioma should be looked for in order to improve diagnostic confidence. Furthermore, in women with endometriosis symptoms, MRI underestimates USL involvement, especially for type 1 in which USLs are not visible and deemed to be normal.24 Hence, the threshold measurements mentioned in the literature are of no diagnostic value.10 In these patients with a normal MRI, endometriosis-associated pain may be due to endometriosis-induced chronic inflammatory microenvironment and/or perineural invasion in small endometriotic lesions.25
Interestingly, among the 6 uteri with lateral deviation, only one false-positive result concerning the stretched USL was induced. This is in contradiction with previous studies/common assumption evoking misleading “tractionnal thickening” of the stretched USL in this particular situation.26 The question that remains is how reliable a thickened USL is in a patient with a previous history of pelvic surgery or peritonitis, which may lead to nonspecific secondary fibrosis of the USL.
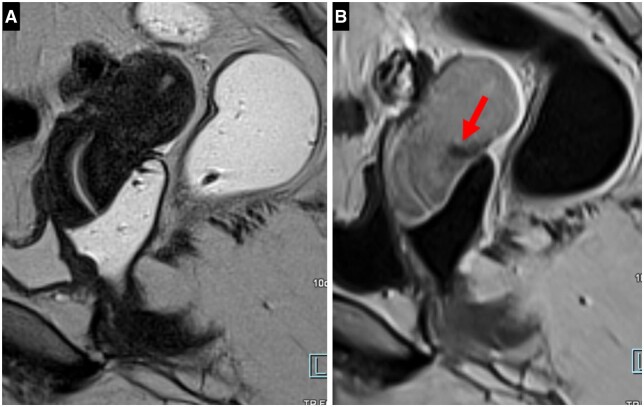
In our experience, an injection of a contrast agent is useful in case of uterine retroflexion. In the sagittal plane, in T2WI, the torus and the origin of the USLs are blurred by this retroflexion. In T1WI without fat suppression, the injection of a contrast agent makes it possible to underline the torus and to analyse it because it enhances much less than the adjacent myometrium due to the marked proliferation of smooth muscle cells and fibrosis (Figure 9). Similarly, in case of doubt about an endometriotic rectosigmoid invasive lesion on T2WI, especially if a rectal filling was not performed, a markedly enhanced mucosa underlining a rectosigmoid local wall thickening on post-contrast T1WI without fat suppression (“medallion-shaped” lesion) is a valuable aid to the diagnosis (Figure 10).
Figure 9.
Pelvic MRI scan of a symptomatic patient with torus involvement at pathology: (A, B). (A) Sagittal T2WI showing a uterus in a retroverted/retroflexed position resulting in a torus area that cannot be analysed. (B) Sagittal contrast-enhanced T1WI without Fat-Sat making the thickened torus visible (arrow).
Figure 10.
Pelvic MRI scan of a symptomatic patient with a rectosigmoid wall involvement (A-D). (A) Sagittal and (B) axial T2WI showing an anterior triangular attraction of the rectosigmoid toward the torus (arrowhead), without a clear visualization of an intestinal wall thickening due to the lack of rectal filling. (C) Sagittal and (D) axial contrast-enhanced T1WI without Fat-Sat making the “medallion-shaped” rectosigmoid wall thickening (arrows) visible thanks to a markedly enhancing juxta-lesional mucosa allowing for improved definition and distinction between the lesion itself and normal intestinal wall.
The data summarized in Figure 8 allow clinicians to offer their patients a personalized diagnostic and clinical approach based on the type of USL identified using our MRI classification.
The diagnostic options we proposed for the management of each type of USL described in our classification are based on a literature review.27,28 In dealing with the “L-category” of USL, clinicians need more arguments to reinforce their diagnostic confidence. Few diagnostic models have been developed based on the questioning of patients and physical examination, and they exhibit variable diagnostic values for DIE.29–31 Some authors have added biological criteria such as the estimation of cancer antigen 125 (CA-125) serum levels,32 concluding that CA-125 is useful for monitoring the medical or surgical treatment of this pathology, its serum level being well correlated with the therapeutic response. In symptomatic women, a CA-125 serum level ≥ 30 U/mL is highly specific for the diagnosis of endometriosis, which allows the start of a medical treatment, without the need to wait for laparoscopic diagnostic confirmation. However, a CA-125 serum level below 30 U/mL does not exclude the diagnosis; this situation requires further investigation.33 Using CA-125 ≥ 30 U/mL, this biomarker was significantly more sensitive for the diagnosis of moderate or severe endometriosis compared with minimal disease (63%, 95% CI 47%-77% versus 24%, 95% CI 19%-32%, P-value = .001). Unfortunately, a negative test (i.e., CA-125 < 30 U/mL) is unable to rule out endometriosis. The diagnostic use of micro-RNAs is considered by some.34,35
Hormonal treatments can be tried, and improvement in symptoms during follow-up visits would support endometriosis as a possible etiology1,36–40; the same will be true in the case of lesion reduction on a surveillance MRI scan performed 12 months after hormonal treatment initiation.37,40,41
Our MRI classification system requires further corroboration through prospective studies involving larger patient cohorts and a comparison with a control group of women experiencing chronic pelvic pain suggestive of endometriosis, but without endometriotic lesions identified during laparoscopic inspection. Considering this MRI classification, in order to preoperatively identify patients with a high likelihood of having DIE, particularly to enhance the management of chronic pelvic pain by enabling an early diagnosis, it may be feasible to design a scoring system that combines clinical, laboratory, and radiological parameters, as has already been done for other diseases.42 Based on our MRI classification, it should also be possible to develop an artificial intelligence algorithm for automated diagnosis in radiology practices.
Conclusions
Our MRI classification of USL involvement in symptomatic women performs well as a rule-in non-invasive staging method, facilitating expedited diagnosis and ensuring investigation and treatment can be confidently tailored for the management of endometriosis. Our MRI classification system allows standardization and uniformity of reporting, which is useful for clinicians to explain the severity of endometriosis in simple terms to patients.
Acknowledgements
We thank Dr Salim Benabadji (Paris, France), Dr Audrey Mansuet-Lupo (Paris, France), Dr Nathalie Pierre-Kahn (Paris, France), Dr Catherine Sciot (Paris, France). Preliminary results of this study were previously presented as a poster at the Hôtel-Dieu Gynecological Imaging Symposium 2020, and at the 2024 Annual Meeting of the European Congress of Radiology (ECR).
Contributor Information
Siegfried Hélage, Department of Radiology, Hôtel-Dieu de Paris (AP-HP), Paris 75004, France.
Lucas Rivière, Department of Radiology, Hôtel-Dieu de Paris (AP-HP), Paris 75004, France.
Jean-Noël Buy, Department of Radiology, Hôtel-Dieu de Paris (AP-HP), Paris 75004, France.
Corinne Bordonné, Department of Radiology, Hôtel-Dieu de Paris (AP-HP), Paris 75004, France.
Frédéric Préaux, Léonard de Vinci Medical Imaging, 43 rue Cortambert, Paris 75016, France.
Pierre-Alexandre Just, Department of Pathology, Hôpital Cochin (AP-HP), Paris 75014, France.
Nizar Aflak, Department of Gynecological Surgery, Hôpital Beaujon (AP-HP), Clichy 92110, France.
Pascal Rousset, Department of Radiology, Centre Hospitalier Lyon Sud, Hospices Civils de Lyon, Pierre Bénite 69495, France.
Élisabeth Dion, Department of Radiology, Hôtel-Dieu de Paris (AP-HP), Paris 75004, France.
Funding
None declared.
Conflicts of interest
None declared.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request in order to protect patient privacy.
References
- 1. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clement MD. Diseases of the peritoneum (including endometriosis). In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. Vol 1. Springer-Verlag; 2002. [Google Scholar]
- 3. Koninckx PR, Meuleman C, Demeyere S, et al. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55(4):759-765. [DOI] [PubMed] [Google Scholar]
- 4. Koninckx PR, Oosterlynck D, D'Hooghe T, Meuleman C.. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann N Y Acad Sci. 1994;734:333-341. [DOI] [PubMed] [Google Scholar]
- 5. Chapron C, Dubuisson J-B, Pansini V, et al. Routine clinical examination is not sufficient for diagnosing and locating deeply infiltrating endometriosis. J Am Assoc Gynecol Laparosc. 2002;9(2):115-119. [DOI] [PubMed] [Google Scholar]
- 6. Bazot M, Thomassin I, Hourani R, et al. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol. 2004;24(2):180-185. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy S, Bergqvist A, Chapron C, et al. ; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698-2704. [DOI] [PubMed] [Google Scholar]
- 8. Loubeyre P, Petignat P, Jacob S, et al. Anatomic distribution of posterior deeply infiltrating endometriosis on MRI after vaginal and rectal gel opacification. AJR Am J Roentgenol. 2009;192(6):1625-1631. [DOI] [PubMed] [Google Scholar]
- 9. Chapron C, Lafay-Pillet MC, Santulli P, et al. A new validated screening method for endometriosis diagnosis based on patient questionnaires. EClinicalMedicine. 2022;44:101263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinkel K, Chapron C, Balleyguier C, et al. Magnetic resonance imaging characteristics of deep endometriosis. Hum Reprod. 1999;14(4):1080-1086. [DOI] [PubMed] [Google Scholar]
- 11. Dumontier I, Roseau G, Vincent B, et al. Comparison of endoscopic ultrasound and magnetic resonance imaging in severe pelvic endometriosis. Gastroenterol Clin Biol. 2000;24(12):1197-1204. [PubMed] [Google Scholar]
- 12. Thomassin I, Bazot M, Detchev R, et al. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004;190(5):1264-1271. [DOI] [PubMed] [Google Scholar]
- 13. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422-469. [Google Scholar]
- 14. Chapron C, Fauconnier A, Vieira M, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. 2003;18(1):157-161. [DOI] [PubMed] [Google Scholar]
- 15. Bazot M, Gasner A, Ballester M, et al. Value of thin section oblique axial T2-weighted magnetic resonance images to assess uterosacral ligament endometriosis. Hum Reprod. 2011;26(2):346-353. [DOI] [PubMed] [Google Scholar]
- 16. Coutinho A Jr, Bittencourt LK, Pires CE, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. Radiographics. 2011;31(2):549-567. [DOI] [PubMed] [Google Scholar]
- 17. Campbell RM, Arbor A.. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59(1):1-12. [DOI] [PubMed] [Google Scholar]
- 18. Chapron C, Dubuisson JB, Tardif D, et al. Retroperitoneal endometriosis and pelvic pain: results of laparoscopic uterosacral ligament resection according to the rAFS classification and histopathologic results. J Gynecol Surg. 1998;14(2):51-58. [Google Scholar]
- 19. Walter AJ, Hentz JG, Magtibay PM, et al. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol. 2001;184(7):1407-1411. discussion 11–3. [DOI] [PubMed] [Google Scholar]
- 20. Bonte H, Chapron C, Vieira M, et al. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J Am Assoc Gynecol Laparosc. 2002;9(4):519-524. [DOI] [PubMed] [Google Scholar]
- 21. Marchino GL, Gennarelli G, Enria R, et al. Diagnosis of pelvic endometriosis with use of macroscopic versus histologic findings. Fertil Steril. 2005;84(1):12-15. [DOI] [PubMed] [Google Scholar]
- 22. Zanardi R, Del Frate C, Zuiani C, et al. Staging of pelvic endometriosis based on MRI findings versus laparoscopic classification according to the American Fertility Society. Abdom Imaging. 2003;28(5):733-742. [DOI] [PubMed] [Google Scholar]
- 23. Haas D, Shebl O, Shamiyeh A, et al. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet Gynecol Scand. 2012;92(1):3-7. [DOI] [PubMed] [Google Scholar]
- 24. Bazot M, Lafont C, Rouzier R, et al. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92(6):1825-1833. [DOI] [PubMed] [Google Scholar]
- 25. Liang Y, Liu D, Yang F, et al. Perineural invasion in endometriotic lesions contributes to endometriosis-associated pain. J Pain Res. 2018;11:1999-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Propst AM, Storti K, Barbieri RL.. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568-570. [DOI] [PubMed] [Google Scholar]
- 27. RANZCOG. Australian Clinical Practice Guideline for the Diagnosis and Management of Endometriosis. RANZCOG; 2021 [Google Scholar]
- 28. Kim MR, Chapron C, Römer T, et al. Clinical Diagnosis and Early Medical Management for Endometriosis: Consensus from Asian Expert Group. Healthcare (Basel). 2022;10(12):2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eskenazi B, Warner M, Bonsignore L, et al. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril. 2001;76(5):929-935. [DOI] [PubMed] [Google Scholar]
- 30. Ballard K, Lane H, Hudelist G, et al. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil Steril. 2010;94(1):20-27. [DOI] [PubMed] [Google Scholar]
- 31. Nnoaham KE, Hummelshoj L, Kennedy SH, et al. ; World Endometriosis Research Foundation Women's Health Symptom Survey Consortium. Developing symptom-based predictive models of endometriosis as a clinical screening tool: results from a multicenter study. Fertil Steril. 2012;98(3):692-701 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koninckx PR, Meuleman C, Oosterlynck D, et al. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;65(2):280-287. [PubMed] [Google Scholar]
- 33. Hirsch M, Duffy J, Davis CJ, et al. International Collaboration to Harmonise Outcomes and Measures for Endometriosis. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG. 2016;123(11):1761-1768. [DOI] [PubMed] [Google Scholar]
- 34. Moustafa S, Burn M, Mamillapalli R, et al. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol. 2020;223(4):557.e1-11. [DOI] [PubMed] [Google Scholar]
- 35. Ferrier C, Bendifallah S, Suisse S, et al. Saliva microRNA signature to diagnose endometriosis: A cost-effectiveness evaluation of the Endotest®. BJOG. 2023;130(4):396-406. [DOI] [PubMed] [Google Scholar]
- 36. Telimaa S, Puolakka J, Rönnberg L, et al. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13-23. [DOI] [PubMed] [Google Scholar]
- 37. Harada T, Momoeda M, Taketani Y, et al. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583-1588. [DOI] [PubMed] [Google Scholar]
- 38. Mabrouk M, Solfrini S, Frasca C, et al. A new oral contraceptive regimen for endometriosis management: preliminary experience with 24/4-day drospirenone/ethinylestradiol 3 mg/20 mcg. Gynecol Endocrinol. 2012;28(6):451-454. [DOI] [PubMed] [Google Scholar]
- 39. Leone Roberti Maggiore U, Remorgida V, Scala C, et al. Desogestrel-only contraceptive pill versus sequential contraceptive vaginal ring in the treatment of rectovaginal endometriosis infiltrating the rectum: a prospective open-label comparative study. Acta Obstet Gynecol Scand. 2014;93(3):239-247. [DOI] [PubMed] [Google Scholar]
- 40. Taniguchi F, Enatsu A, Ota I, et al. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. Eur J Obstet Gynecol Reprod Biol. 2015;191:116-120. [DOI] [PubMed] [Google Scholar]
- 41. Ferrero S, Leone Roberti Maggiore U, Scala C, et al. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch Gynecol Obstet. 2013;287(3):447-453. [DOI] [PubMed] [Google Scholar]
- 42. Hélage S, Revel MP, Alifano M, et al. A simple computed tomography scoring system to predict histological malignancy of solitary fibrous tumors of the pleura. BJMMR. 2015;5(10):1301-1308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request in order to protect patient privacy.