Abstract
Background
Transarterial embolization (TAE) for acute lower gastrointestinal bleeding (LGIB) can be technically challenging due to the compromise between achieving haemostasis and causing tissue ischaemia. The goal of the present study is to determine its technical success, rebleeding, and post-embolization ischaemia rates through meta-analysis of published literature in the last twenty years.
Methods
PubMed, Embase, and Cochrane Library databases were queried. Technical success, rebleeding, and ischaemia rates were extracted. Baseline characteristics such as author, publication year, region, study design, embolization material, percentage of superselective embolization were retrieved. Subgroup analysis was performed based on publication time and embolization agent.
Results
A total of 66 studies including 2121 patients who underwent embolization for acute LGIB were included. Endoscopic management was attempted in 34.5%. The pooled overall technical success, rebleeding, post-embolization ischaemia rates were 97.0%, 20.7%, and 7.5%, respectively. Studies published after 2010 showed higher technical success rates (97.8% vs 95.2%), lower rebleeding rates (18.6% vs 23.4%), and lower ischaemia rates (7.3% vs 9.7%). Compared to microcoils, NBCA was associated with a lower rebleeding rate (9.3% vs 20.8%) at the expense of a higher post-embolization ischaemia rate (9.7% vs 4.0%). Coagulopathy (P = .034), inotropic use (P = .040), and malignancy (P = .002) were predictors of post-embolization rebleeding. Haemorrhagic shock (P < .001), inotropic use (P = .026), malignancy (P < .001), coagulopathy (P = .002), blood transfusion (P < .001), and enteritis (P = .023) were predictors of mortality. Empiric embolization achieved a similarly durable haemostasis rate compared to targeted embolization (23.6% vs 21.1%) but a higher risk of post-embolization ischaemia (14.3% vs 4.7%).
Conclusion
For LGIB, TAE has a favourable technical success rate and low risk of post-embolization ischaemia. Its safety and efficacy profile has increased over the last decade. Compared to microcoils, NBCA seemed to offer a more durable haemostasis rate at the expense of higher ischaemia risk. Due to the heterogeneity of currently available evidence, future prospective and comparative studies are warranted.
Advances in knowledge
(1) Acute LGIB embolization demonstrate a high technical success rate with acceptable rate of rebleeding and symptomatic ischaemia rates. Most ischaemic stigmata discovered during routine post-embolization colonoscopy were minor. (2) Although NBCA seemed to offer a more durable haemostasis rate, it was also associated with a higher risk of ischaemia compared to microcoils. (3) Coagulopathy, malignant aetiology, and inotropic use were predictors of rebleeding and mortality. (4) Routine post-embolization endoscopy to assess for ischaemia is not indicated.
Keywords: lower gastrointestinal hemorrhage, embolization, vascular and interventional radiology
Introduction
Lower gastrointestinal bleeding (LGIB), defined as bleeding from the Ligament of Treiz to the anus, may result from a variety of aetiologies such as diverticulosis, vascular malformation, and neoplasm. The incidence of LGIB in the United States is approximately 33 per 100,000.1,2 While most LGIB can be managed conservatively with medical management alone, endoscopic treatment has been traditionally considered a first-line intervention.3 In the setting of hemodynamic instability, inadequate bowel preparation, brisk bleeding preventing colonoscopic localization of bleeding source, and/or failed colonoscopic treatment, radiologic transcatheter arterial embolization (TAE) may be utilized for both diagnostic and therapeutic purposes.4,5 However, unlike the upper gastrointestinal tract, the LGI circulation is deficient of collateral arterial supply and more prone to developing post-embolization ischaemia.6 In contemporary times, however, the adoption of co-axial microcatheters allowing superselective embolization has been shown to render a lower infarction rate in recently published studies when compared with techniques from the late 20th century. In addition, while interventional radiologists have become more experienced with microcoils, utilization with newer liquid embolic agents such as N-butyl-2-cyanoacrylate (NBCA) glue and Onyx (Micro Therapeutics, Inc., Irvine, CA) has also been described. Thus, in order to provide an updated overview of LGI embolization for the treatment of acute LGIB within the last twenty years, the present meta-analysis aims to characterize the efficacy of preventing rebleeding and risk of ischaemia with this technique.
Methods
Institutional Review Board Approval was not required for this meta-analysis, which complied with the Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement (PRISMA).
Search strategy and study selection
PubMed, Embase, and the Cochrane Library were queried for studies published up to April 2021. The following keywords were used: “lower gastrointestinal,” “bleeding,” “colonic,” “hemorrhage,” “embolization,” and “embolotherapy.”
A total of 552 studies were identified initially and subjected to the screening process (Figure 1).
Figure 1.
Flow diagram showing the screening Process.
The following inclusion criteria were adopted:
Studies using TAE to treat acute lower gastrointestinal bleeding.
Studies that reported rebleeding and/or ischaemia rates.
Randomized-controlled trial (RCT), retrospective cohort studies, prospective cohort studies, and case series with >5 samples size.
No language restriction.
Studies published after 2000
Exclusion criteria were as follows:
Animal or nonhuman studies.
Studies with a sample size of fewer than 5 in the empiric embolization group.
Letter/editorial, review, meta-analysis, or case reports.
Studies published prior to 2000.
Endnote X8 (Clarivate Analytics, Philadelphia, PA) was used to identify and delete duplicates. The titles and abstracts of the studies were screened initially. Then, full texts of the remaining studies were reviewed to finalize the list that eventually subjected to quantitative analysis.
Data collection
The following baseline characteristics from each were extracted: author name, year of publication, institution/country where the study was performed, study design, number of patients, and follow-up length. Primary outcomes of interest were rates of rebleeding and ischaemia. Secondary outcomes include technical success rate, rebleeding-related mortality, and ischaemia-related surgery. Rebleeding rate was further analysed based on early (<30 days) and delayed (>30 days) rebleed. Treatment of rebleeding was also queried (repeated TAE, surgery, colonoscopic, and conservative management). Ischaemia rate was analysed in terms of symptomatic ischaemia (pain), colonoscopic ischaemia, defined as ischaemic findings discovered on routine post-embolization colonoscopy, and overall ischaemia (symptomatic, colonoscopic, discovered during surgery, and/or on computed tomography). Two researchers extracted the data from the original studies. Any disagreement was discussed and arbitrated by a third author. Risk of bias assessment was assessed using the National Health’s Institute Quality Assessment tool (Supplementary Table S1).
Statistical analysis
Quantitative analyses were performed with STATA 15.1 statistical software (STATA Corp., College Station, TX, United States). Meta-analysis was conducted with the -metan function.7 Rates were pooled with the -metaprop function and reported in 95% CI.8 Odds ratios were pooled. Heterogeneity I2 was calculated. Random-effect model was implemented in the present study to achieve a conservative estimation. Subgroup analysis of rebleeding and ischaemia rates was performed based on NBCA versus microcoils and studies published before vs after 2010. Predictor analysis was performed using reported odds ratio (OR) with 95% CI if a given variable was analysed by two or more studies.
Results
Baseline
A total of 544 unique studies were subjected to the screening process (Figure 1). A total of 66 studies were included in the meta-analysis, encompassing 2121 patients who underwent embolization for acute LGIB9–73 (Table 1). Only one study (1/66, 1.5%) was prospective, while the rest were retrospective (65/66, 98.5%). The studies were mainly conducted in United States (22/66, 33.3%), Korea (8/66, 12.1%), and Australia (7/66, 10.6%). Based on available data from 17 studies, 172/498 (34.5%) patients underwent attempted endoscopic management prior to embolization (Table 1). In 42/66 (63.6%) studies, superselective embolization, defined as embolization of third-order branch vessels, was performed in all patients.
Table 1.
Baseline characteristics and clinical outcomes of included studies.
| Study | Design | Region | Prior colonoscopy | Material | Technical success | Early rebleeding rate | Late rebleeding rate | Overall rebleeding rate | Rebleeding management | Deaths attributed to rebleed | Post-embolization ischaemia | Surgery to treat ischaemia | Deaths attributed to ischaemia | Superselective embolization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbas9 | Retrospective | New Zealand | NA | Coil, Gelfoam | NA | NA | NA | NA | NA | NA | 1/15 | 1/1 | 0/15 | <100% |
| Adusumilli10 | Retrospective | Australia | NA | Coil: 48 Gelfoam: 13 Coil+Gelfoam: 10 |
61/71 | NA | NA | 11/61 | TAE: 3/11 Surgery: 5/11 Conservative: 3/11 |
0/11 | 1/61 | 0/1 | 0/61 | NA |
| Ahmed11 | Retrospective | USA | NA | Coil: 20 | 20/20 | NA | NA | 4/13 | Surgery: 1/4 Conservative: ¾ |
0/4 | 3/13 | 0/3 | 0/13 | 100.00% |
| Ahmed12 | Retrospective | USA | 14/26 | Coil: 32 | 32/38 | 9/32 | 4/32 | 13/32 | Endoscopy: 1/13 Surgery: 6/13 Conservative: 6/13 |
3/13 | 1/32 | 0/1 | 0/32 | 100.00% |
| Ali13 | Retrospective | Pakistan | NA | Coil, Particles, Gelfoam | NA | 3/67 | NA | 3/67 | TAE: 1/3 Conservative: 2/3 |
0/3 | 1/67 | 1/1 | 0/67 | NA |
| Bandi14 | Retrospective | USA | NA | Particles: 28 Coil: 4 Particles +Coil: 4 Gelfoam: 3 |
35/48 | NA | NA | 12/33 | TAE: 1/12 Surgery: 8/12 Conservative: 3/12 |
0/12 | 6/35 | 0/6 | 0/35 | 100.00% |
| Buagnam15 | Retrospective | Thailand | 15/38 | Gelfoam: 27 Particles: 6 Coil: 2 Coil+ Particles: 2 |
35/38 | NA | NA | 10/35 | TAE: 1/10 Surgery: 6/10 Conservative: 3/10 |
1/10 | 5/38 | 2/5 | 1/35 | 100.00% |
| Burgess16 | Retrospective | Australia | 3/15 | Particles: 9 Gelfoam: 2 Coil: 2 Combination: 2 |
14/15 | 8/14 | NA | 8/14 | TAE: 2/6 Conservative: 4/6 |
2/8 | 4/14 | 1/4 | 0/14 | 100.00% |
| Chan17 | Retrospective | Singapore | NA | Gelfoam: 14 Coil: 10 Mixed: 3 |
26/26 | NA | NA | 9/26 | TAE: 1/9 Surgery: 3/9 Conservative: 5/9 |
0/9 | 0/26 | 0 | 0/26 | 100.00% |
| Charbonnet18 | Retrospective | Switzerland | NA | Coil, Particles | NA | NA | NA | NA | NA | NA | 0/8 | 0 | 0/8 | NA |
| Debarros19 | Retrospective | USA | 6/27 | Particles: 10 Coil: 16 Gelfoam: 1 |
NA | NA | NA | 6/27 | TAE: 1/6 Surgery: 5/6 |
0/6 | 2/27 | 0/2 | 0/27 | 100.00% |
| Defreyne20 | Retrospective | Belgium | NA | Particles: 9 Glue 1 Particles +Coil: 1 |
11/11 | 1/11 | NA | 1/11 | NA | 0/1 | 0/11 | 0 | 0/11 | 100.00% |
| Eriksson21 | Retrospective | USA | NA | Coil: 40 | NA | NA | NA | 10/40 | Surgery: 5/10 Conservative: 5/10 |
0/10 | 0/40 | 0 | 0/40 | NA |
| Evangelista22 | Retrospective | USA | NA | Coil: 11 Coil+ Particles: 3 Particles: 2 Coil+Gelfoam: 1 |
15/17 | NA | NA | 2/15 | TAE: 1/2 Surgery: 1/2 |
0/2 | 1/12 | 0/1 | 0/12 | 100.00% |
| Frodsham23 | Retrospective | USA | 5/14 | NBCA: 14 | 14/14 | 3/14 | NA | 3/14 | Surgery: 2/3 Conservative: 1/3 |
0/3 | 0/14 | 0 | 0/14 | 100.00% |
| Funaki73 | Retrospective | USA | NA | Coil: 25 | 25/27 | 3/25 | 2/25 | 5/25 | Endoscopy: 1/5 Surgery: 2/5 Conservative: 2/5 |
0/5 | 4/25 | 2/4 | 2/25 | 100.00% |
| Gady24 | Retrospective | USA | NA | NA | NA | NA | NA | 3/10 | Surgery: 3/3 | 0/3 | 1/10 | 1/1 | 0/10 | NA |
| Gillippse25 | Prospective | Australia | NA | NA | NA | NA | NA | 9/38 | TAE: 2/8 Surgery: 6/8 |
1/9 | 0/38 | 0 | 0/38 | 100.00% |
| Heinna26 | Retrospective | Japan | 10/10 | Gelfoam: 2 Coil: 5 Coil+Gelfoam: 3 |
NA | 1/10 | 0/10 | 1/10 | NA | NA | 4/10 | 0/4 | 0/10 | 100.00% |
| Hermie72 | Retrospective | Belgium | NA | Particles: 15 NBCA: 12 Coil: 27 Particles +Coil: 5 |
47/47 | NA | NA | 12/66 | TAE: 4/12 Endoscopy: 6/12 Surgery: 1/12 Conservative: 1/12 |
3/12 | 4/66 | 1/4 | 0/66 | NA |
| Horiguchi27 | Retrospective | Japan | NA | Coil: 6 Gelfoam: 5 Coil+gelfoam: 3 |
NA | NA | NA | NA | NA | NA | 0/14 | 0 | 0/14 | 100.00% |
| Huang28 | Retrospective | Taiwan | NA | NBCA: 27 | 27/27 | 3/27 | 1/27 | 4/27 | NA | NA | 0/27 | 0 | 0/27 | 100.00% |
| Hur29 | Retrospective | Korea | NA | NBCA : 84 Gelfoam: 20 Particles +Gelfoam: 1 Coil: 2 Blood clot: 1 |
108/112 | 15/108 | NA | 15/108 | TAE: 9/15 Endoscopy: 1/15 Surgery: 2/15 Conservative: 3/15 |
0/15 | 5/108 | 4/5 | 1/108 | <100.00% |
| Kariya30 | Retrospective | Japan | 6/6 | Coil: 2/6 NBCA: 3/6 Gelfoam: 2/6 |
6/6 | 1/5 | NA | 2/5 | TAE: 1/1 | 1/2 | NA | NA | NA | NA |
| Kickuth31 | Retrospective | Switzerland | 14/20 | Coil: 16 Particles: 2 Coil+ Particles: 2 |
20/20 | 1/20 | 1/20 | 2/20 | Surgery: 1/2 Conservative: 1/2 |
0/2 | 1/20 | 1/1 | 0/20 | 100.00% |
| Kim32 | Retrospective | Korea | NA | NA | NA | 12/75 | NA | 12/75 | TAE: 3/6 Surgery: 2/6 Conservative: 1/6 |
6/12 | NA | NA | NA | NA |
| Kim33 | Retrospective | USA | NA | Coil, Particles | NA | 0/11 | 0/11 | 0/11 | NA | NA | 1/11 | NA | 0/11 | 100.00% |
| Kim34 | Retrospective | Korea | NA | Coil: 4/72 NBCA: 43/72 Gelfoam: 25/72 |
72/74 | NA | NA | 7/57 | TAE: 2/7 Endoscopy: 2/7 Surgery: 3/7 |
0/7 | 15/72 | 7/15 | 3/72 | 100.00% |
| Kinoshita35 | Retrospective | Japan | 17/17 | Coil: 17 | 17/17 | 0/17 | 1/17 | 1/17 | Conservative: 1/1 | 0/1 | NA | NA | NA | 100.00% |
| Kodani37 | Retrospective | Japan | NA | NBCA: 16 | 16/16 | 1/16 | NA | 1/16 | Conservative: 1/1 | 0/1 | 8/16 | 1/8 | 0/16 | 100.00% |
| Koehler39 | Retrospective | Austria | NA | Coil, Gelfoam, Liquid | 25/25 | 6/25 | NA | 6/25 | TAE: 3/6 Surgery: 3/6 |
0/6 | 0/25 | 0 | 0/25 | NA |
| Koh38 | Retrospective | USA | 18/68 | Particles: 15 Coil: 20 Particles +coil: 33 |
NA | 6/68 | 6/68 | 12/68 | TAE: 4/12 Surgery: 5/12 Conservative: 3/12 |
0/12 | 4/17 | 1/4 | 0/17 | <100% |
| Kuo40 | Retrospective | USA | 3/22 | Coil: 22 | 22/22 | NA | NA | 3/22 | Endoscopy: 2/3 Surgery: 1/3 |
0/3 | 1/22 | 0/1 | 0/22 | 100.00% |
| Kwak41 | Retrospective | Korea | 17/17 | Coil: 17 | 17/17 | NA | NA | 15/17 | Surgery: 1/1 | 1/15 | 0/17 | 0 | 0/17 | 100.00% |
| Kwon42 | Retrospective | Korea | NA | NBCA: 6 | 6/6 | 0/6 | 0/6 | 0/6 | NA | NA | 1/6 | 0/1 | 0/6 | <100.00% |
| Kwon42 | Retrospective | Korea | NA | Coil: 8 Gelfoam: 38 NBCA 81 |
127/134 | NA | NA | 31/111 | TAE: 6/31 Endoscopy: 10/31 Surgery: 7/31 Conservative: 8/31 |
13/31 | 23/124 | 10/23 | 3/124 | 66.00% |
| Lee43 | Retrospective | Korea | 41/52 | Coil: 6 Gelatin: 8 Particles: 5 histoacryl; 15 Coil+gelatin: 7 Coil+ Particles: 5 Coil+histoacryl: 5 Gelfoam+ Particles: 1 |
52/52 | 14/52 | NA | 14/52 | TAE: 3/14 Endoscopy: 2/14 Surgery: 2/14 Conservative 7/14 |
0/14 | 2/52 | NA | 0/2 | <100% |
| Lipof44 | Retrospective | USA | 10/73 | Particles: 50 Coil: 10 Coil+ Particles: 9 Gelfoam: 3 Coil+Gelfoam: 2 Coil+Glue: 1 |
NA | 12/73 | 8/73 | 20/73 | TAE: 5/20 Surgery: 12/20 Conservative: 3/20 |
0/20 | 5/75 | 4/5 | 1/75 | 100.00% |
| Luchefeld45 | Retrospective | USA | NA | NA | NA | NA | NA | 2/16 | NA | 0/2 | NA | NA | NA | NA |
| Lv46 | Retrospective | China | NA | Gelfoam: 16 Coil: 4 Coil+ Particles: 6 |
26/31 | 1/26 | NA | 1/26 | NA | NA | 0/26 | 0 | 0/26 | 100.00% |
| Maleux47 | Retrospective | Belgium | 20/39 | Coil: 17 Particles: 10 Coil+ Particles: 10 Gelfoam: 1 NBCA: 1 |
NA | 6/39 | 2/39 | 8/39 | TAE: 2/5 Surgery: 3/5 |
3/8 | 4/39 | 4/4 | 0/39 | NA |
| Mejaddam48 | Retrospective | USA | NA | Coil, Particles, Gelfoam | 21/22 | NA | NA | 4/21 | NA | NA | 3/21 | 1/3 | NA | 100.00% |
| Mensel49 | Retrospective | Germany | NA | Coil: 6 | NA | NA | NA | NA | NA | NA | 5/6 | 5/5 | 1/6 | 100.00% |
| Neuman50 | Retrospective | USA | 11/23 | Coil, Gelfoam | NA | 5/23 | NA | 5/23 | TAE: 1/5 Surgery: 2/5 Conservative: 2/5 |
0/5 | 5/23 | 1/5 | 0/23 | 100.00% |
| Noh51 | Retrospective | Korea | NA | Coil, Gelfoam | 88/89 | 61/96 | NA | 61/96 | NA | NA | 15/96 | 9/15 | 0/96 | 93.00% |
| Nykanen52 | Retrospective | Finland | 10/53 | Coil: 33 Gelfoam: 8 Particles: 1 Coil+ Particles: 6 Coil+Gelfoam: 3 Particles +gelfoam; 2 |
53/55 | 12/53 | 2/53 | 14/53 | TAE: 1/13 Endoscopy: 1/13 Surgery: 5/13 Conservative: 6/13 |
1/14 | 9/53 | 6/9 | 1/53 | 100.00% |
| d’Othée36 | Retrospective | USA | NA | Coil: 17 | 17/19 | 5/17 | NA | 5/17 | TAE: 1/5 Surgery: 2/5 Conservative: 2/5 |
0/5 | 2/17 | 2/2 | 0/17 | 100.00% |
| Pannatier53 | Retrospective | Switzerland | NA | Coil: 34/41 NBCA: 1/41 Gelfoam: 15/41 |
NA | NA | NA | 11/41 | TAE: 3/11 Endoscopy: 1/11 Surgery: 5/11 Conservative: 2/11 |
0/11 | 3/41 | 2/3 | 0/41 | 46.80% |
| Patel54 | Retrospective | USA | 6/10 | Coil, Gelfoam | NA | 2/10 | 0/10 | 2/10 | Endoscopy: 1/2 Surgery: 1/2 |
0/2 | 0/10 | 0 | 0/10 | 100.00% |
| Pham55 | Retrospective | Australia | NA | Microcoi: 6 Gelfoam+microcoil: 2 |
NA | 8/18 | NA | 8/18 | TAE: 1/8 Conservative: 7/8 |
0/8 | 0/18 | 0/18 | 0/18 | 100.00% |
| Rossetti56 | Retrospective | Switzerland | NA | Coil, Particles, Silk threads | 24/24 | 0/24 | 1/24 | 1/24 | Endoscopy: 1/1 | 0/1 | 5/24 | 4/24 | 1/24 | 100.00% |
| Self57 | Retrospective | Australia | NA | Coil: 19 | NA | NA | NA | 0/19 | NA | NA | NA | NA | NA | NA |
| Senadeera58 | Retrospective | New Zealand | NA | Coils: 77 | 72/74 | NA | NA | 14/72 | TAE: 1/14 Surgery: 4/14 Conservative: 9/14 |
0/14 | 4/72 | 2/4 | 1/72 | 100.00% |
| Sheth59 | Retrospective | India | NA | Coil: 16 Particles: 23 Coil+ Particles: 13 |
NA | NA | NA | 9/52 | Surgery: 6/9 Conservative: 3/9 |
0/9 | 7/51 | 0/7 | 0/51 | 100.00% |
| Shi60 | Retrospective | China | NA | Coils: 34 | NA | NA | NA | NA | NA | NA | 0/34 | 0 | 0/34 | 100.00% |
| Sildiroglu61 | Retrospective | USA | NA | Coil: 15 Gelfoam+ Particles: 1 Thrombin: 1 Coil+Gelfoam: 10 |
28/31 | 8/28 | 14/28 | 22/28 | NA | NA | NA | NA | NA | NA |
| Silver62 | Retrospective | USA | NA | Particles, Coil, Gelatin | 11/18 | NA | NA | NA | NA | NA | 7/11 | 3/7 | 5/11 | 100.00% |
| Tan63 | Retrospective | Singapore | NA | Coil: 23 Coil+ Particles: 3 Coil+Gelfoam: 1 Particles: 2 Gelfoam: 1 Vasopressin: 2 |
31/32 | NA | NA | 7/31 | TAE: 1/6 Endoscopy: 1/6 Surgery: 4/6 |
1/7 | 1/31 | 1/1 | 0/31 | 100.00% |
| Tan64 | Retrospective | Australia | NA | Coil: 16 Gelfoam: 9 Particles: 9 |
27/27 | NA | NA | 2/27 | TAE: 2/2 | 0/2 | 1/27 | 1/1 | 1/1 | 100.00% |
| Teng65 | Retrospective | China | 7/26 | Coil: 22 | 22/26 | 1/22 | 2/21 | 3/22 | TAE: 1/3 Conservative: 2/3 |
0/3 | 2/22 | 1/2 | 0/22 | 100.00% |
| Urbano66 | Retrospective | Spain | 11/31 | Onyx: 28 Onyx+coil: 2 |
30/31 | 1/30 | 1/30 | 2/30 | Conservative: 2/2 | 0/2 | 2/30 | 0/2 | 0/30 | 100.00% |
| Waugh67 | Retrospective | Australia | NA | Coil, Gelfoam, Particles | 26/27 | NA | NA | NA | NA | NA | 4/26 | 1/4 | 2/26 | 100.00% |
| Yap68 | Retrospective | USA | NA | Coil: 13 Particles: 5 Coil+gelfoam: 1 |
NA | 2/18 | NA | 2/18 | TAE: 1/2 Surgery: 1/2 |
0/2 | 0/18 | 0 | NA | 0/18 |
| Yata69 | Retrospective | Japan | NA | NBCA: 15 NBCA+coil: 4 NBCA+gelfoam: 1 NBCA+gealfoam+coil: 1 |
21/21 | 1/21 | 0/20 | 1/21 | TAE: 1/1 | 0/1 | 4/21 | 0 | 0/21 | NA |
| Yi70 | Retrospective | USA | NA | Coil: 19 Coil+Gelfoam: 7 |
26/27 | NA | NA | 13/26 | Surgery: 10/13 Conservative: 3/13 |
0/13 | 1/26 | 1/1 | 1/26 | 100.00% |
| Zhao71 | Retrospective | China | NA | NBCA: 7 | 7/7 | 1/7 | NA | 1/7 | Surgery: 1/1 | 0/1 | NA | NA | NA | NA |
Technical success
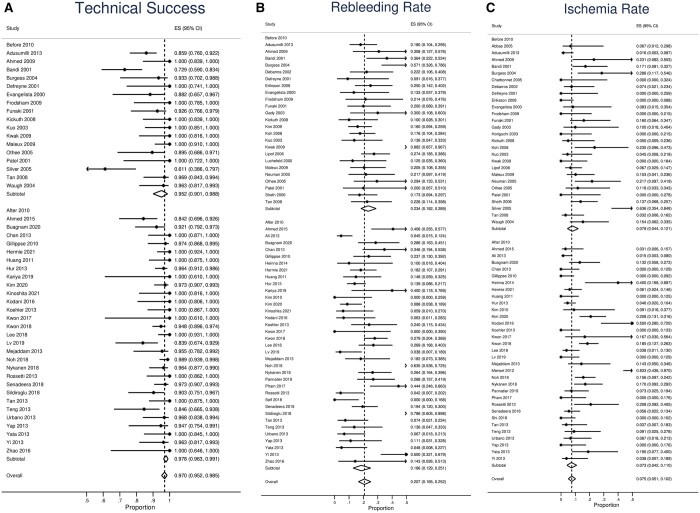
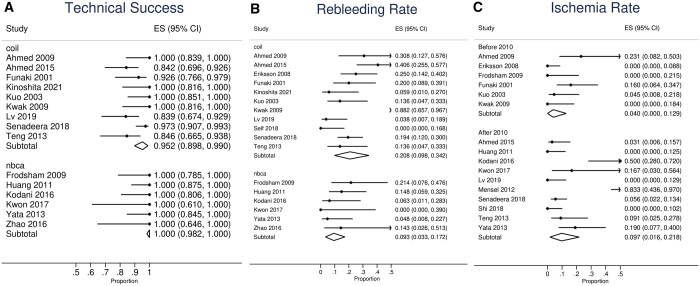
Technical success rate was reported in 46 studies (Table 1). The pooled overall technical success rate was 97.0% (95%CI, 95.2%-98.5%, Figure 2A and Table 2). Reasons for technical failure were specified in 35 patients. Reasons for technical failure included (Supplementary Table S2): 55.6% (20/36) were due to anatomical challenges prohibiting catheterization of the bleeding vessel; 22.2% (8/36) were attributed to vasospasm; and failure to achieve superselective catheterization accounted for 13.1%(4/36). Foci could not be identified in 4/36 (13.1%) cases and thus embolization was not performed. Based on publication time (Figure 2A), studies published before and after 2010 showed pooled technical success rates of 95.2% (95%CI, 90.1%-98.8%) and 97.8% (95%CI, 96.3%-99.1%), respectively. Patients who had coil embolization demonstrated a technical success rate of 95.2% (95CI: 89.8-99.0%), while that of NBCA was 100% (95%CI, 98.2%-100.0%, Figure 3A).
Figure 2.
Subgroup analysis by publication time. (A) Technical success. (B) Rebleeding rate. (C) Ischaemia rate.
Table 2.
Pooled clinical outcomes.
| Variables | Pooled value | Sample size | Study no. |
|---|---|---|---|
| Technical success | 97.0% (95%CI, 95.2%-98.5%) | 1568 | 46 |
| Rebleeding (overall) | 20.7% (95%CI, 16.5%-25.2%) | 1985 | 59 |
| Early rebleed | 14.8% (95%CI, 9.9%-20.5%) | 1144 | 36 |
| Delayed rebleed | 6.9% (95%CI, 3.5%-11.0 %) | 571 | 21 |
| Ischaemia (overall) | 7.5% (95%CI, 5.1%-10.2%) | 1911 | 59 |
| Ischaemia requiring surgery | 2.3 % (95%CI, 1.2%-3.7%) | 1849 | 57 |
| Mortality related ischaemia | 0.1% (95%CI, 0%-0.5%) | 1869 | 56 |
Figure 3.
Subgroup analysis by embolization material. (A) Technical success. (B) Rebleeding rate. (C) Ischaemia rate.
Rebleeding rate
Among 59 studies, the pooled overall rebleeding rate was 20.7% (95%CI, 16.5%-25.2%, Table 2, Figure 2B). Early rebleeding (<30 days) occurred in 14.8% (95%CI, 9.9%-20.5%, Table 2), whereas delayed rebleeding occurred in 6.9% (95%CI, 3.5%-11.0%). Among 451 patients who encountered rebleeding, mortality occurred in 36 (8.0%). Treatment method of rebleeding was reported in 346 patients. Conservative management was sufficient in 99/346 (28.6%); 77/346 (22.3%) rebleeding cases were successfully treated by repeated TAE. Surgery was necessary in 139/346 (40.2%, Table 3). Endoscopic treatment was performed in 31/346 (9.0%). Based on publication time, the overall rebleeding rate of studies published after 2010 was 18.6% (95%CI, 12.9%-25.1%), compared to 23.4% (95%CI, 18.2%-28.9%) of studies prior to 2010 (Figure 2B). Patients who were embolized by microcoils had a rebleeding rate of 20.8% (95%CI, 9.8%-34.2%), while that of NBCA was 9.3% (3.3%-17.2%, Figure 3B).
Table 3.
Treatment of rebleeding.
| Treatment of rebleeding | Number/total (percent) |
|---|---|
| Repeated TAE | 77/346 (22.3) |
| Endoscopic | 31/346 (9.0) |
| Surgery | 139/346 (40.2) |
| Conservative | 99/346 (28.6) |
Ischaemia rate
Among 59 studies, the pooled overall ischaemia rate was 7.5% (95%CI, 5.1%-10.2%, Table 2, Figure 2C). Only 2.3% (95%CI, 1.2%-3.7%, Table 2) patients experienced major ischaemia necessitating surgical resection, whereas ischaemia-related deaths occurred in 0.1% (95%CI, 0%-0.5%) of all patients with post-embolization ischaemia. Among 17 studies that performed routine colonoscopy (Supplementary Table S3), ischaemia findings were noted in 46/245 (18.8%) patients; 6.5% (3/46) of those with positive findings required surgery. Studies published after 2010 had an overall ischaemia rate of 7.3% (95%CI, 4.2%-11.0%), in contrast to the 7.9% (4.4-12.1%) observed among studies before 2010 (Figure 2C). Patients who received microcoils had an overall ischaemia rate of 4.0% (95%CI, 0%-12.9%) whereas that of NBCA was 9.7% (95%CI, 1.6%-21.8%, Figure 3C).
Predictors of clinical outcomes
Coagulopathy (OR: 2.09[1.06-4.12], P = .034), inotropic use (2.26 [1.04-4.91], P = .040), and malignancy (OR: 7.34 [2.07-26.05], P = .002) were significant predictors of rebleeding, whereas shock (OR: 1.51 [0.88-2.57], P = .131), recent gastrointestinal surgery (OR: 1.38 [0.59-3.25], P = .459), the use of NBCA vs other agents (OR: 2.00 [0.82-4.85], P = .125), SMA branch bleeding origin (OR: 0.78 [0.34-1.76], P = .544) were not (Table 4 and Supplementary Figure S1A). In terms of mortality (Table 4 and Supplementary Figure 1B), the presence of shock (OR: 1.44 [1.20-1.72], P < .001), inotropic use (OR: 1.24[1.03-1.51], P = .026), malignancy (OR: 18.14 [5.76-57.11], P < .001), coagulopathy (OR: 3.02 [1.53-5.98], P = .002), red-blood cell transfusion (OR: 1.41 [1.26-1.58], P < .001), enteritis as a cause of bleeding source (OR: 0.78 [0.63-0.97], P = .023) were significant predictors of mortality. Recent gastrointestinal surgery was not (OR: 1.86 [0.85-3.90], P = .123).
Table 4.
Predictor analysis of rebleeding and mortality rates. CI: confidence interval. NBCA: N-butyl cyanoacrylate. SMA: superior mesenteric artery. OR: odds ratio.
| Variables | Rebleeding |
Mortality |
||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | I 2 (%) | OR (95%CI) | P-value | I 2 (%) | |
| Shock | 1.51 (0.88-2.57) | .131 | 36.7 | 1.44 (1.20-1.72) | <.001 | 0.0 |
| Coagulopathy | 2.09 (1.06-4.12) | .034 | 0.0 | 3.02 (1.53-5.98) | .002 | 34.0 |
| Inotropic Use | 2.26 (1.04-4.91) | .04 | 0.0 | 1.24 (1.03-1.51) | .0026 | 72.7 |
| Recent GI Surgery | 1.38 (0.59-3.25) | .459 | 0.0 | 1.82 (0.85-3.9) | .123 | 34.0 |
| Malignancy | 7.34 (2.07-26.05) | .002 | 71.3 | 18.14 (5.76-57.11) | <.001 | 0.0 |
| Use of NBCA | 2.00 (0.82-4.85) | .125 | 0.0 | – | – | – |
| SMA branch as bleeding origin | 0.78 (0.34-1.76) | .544 | 89.4 | – | – | – |
| RBC Transfusion | – | – | – | 1.41 (1.26-1.58) | <.001 | 38.6 |
| Enteritis | – | – | – | 0.78 (0.63-0.97) | .023 | 0.0 |
Empiric embolization
Four studies performed and reported clinical outcomes of empiric embolization in angiographically negative cases (Figure 4). Burgess et al and Heianna et al relied on findings during RBC scintiography and endoscopic clipping as landmarks, respectively,16,26 whereas a combination of pre-angiographic CTA and colonoscopy were used by Nykanen et al.52 Hermie et al implemented intraprocedural CBCT.72 The rate of rebleeding between empiric and target groups was 23.6% (25/106) and 21.1% (8/38), respectively (OR: 0.96 [0.31-2.97], P = .947). The rate of post-embolization ischaemia was 14.3% (4/28) and 4.7% (5/106) for the two groups, respectively (OR 0.28 [0.07-1.19], P = .086).
Figure 4.
Targeted vs empiric embolization: odds ratio (OR) of ischaemia and rebleeding rates.
Discussion
Ischaemia following transarterial embolization for LGIB is known to occur more often compared to its upper GI counterpart, as the LGI system lacks collateral circulation to maintain perfusion to the embolized enteric segment. The risk of inducing local ischaemia must however be balanced against efforts to reduce the rate of rebleeding in LGIB. The current consensus to achieve this balance is to catheterize the bleeding arterial bed as selectively as possible (ie, “superselective”) prior to administration of embolic.74,75 Based on evidence from the last two decades, the pooled overall 20.7% (95%CI, 16.5%-25.2%,) and early rebleeding rate 14.8% (95%CI, 9.9%-20.5%) of the present study was similar to the 21.8% (93/427) rebleeding rate of TAE for upper GI bleeding in a published meta-analysis.76 Salvage treatment for rebleeding can be done through surgery, repeated TAE, endoscopic intervention, and medical management, resulting in a low rebleeding-related mortality. Notably, 40.2% of interventions directed towards rebleeding are surgery-based, highlighting the importance of the importance of surgical consultation as part of a multidisciplinary therapy. While the risk of ischaemia was 7.5% (95%CI, 5.1%-10.2%), escalation of care with the requirement of surgery from ischaemia was only 2.3% (95%CI, 1.2%-3.7%). Only 3/245 (1.2%) patients who underwent routine colonoscopy underwent surgery for ischaemic stigmata. It might be unnecessary to perform universal post-embolization colonoscopy for asymptomatic patients after TAE to evaluate signs of ischaemia, unless the goal is to identify the culprit of the LGIB. These findings suggest that current methods for embolization of LGIB are effective in achieving haemostasis and safe in terms of ischaemic complications.
Using the year of 2010 as a cut-off, the present study also demonstrated improved technical and clinical outcomes in recently published studies compared to older ones: whereas the technical success rates improved (95.2% [95%CI, 90.1%-98.8%] vs 97.8% [95%CI, 96.3%-99.1%]), the risks of post-embolization rebleeding and ischaemia down-trended (23.4% [95%CI, 18.2%-28.9%] vs 18.6% [95%CI, 12.9%-25.1%] and 9.7% [4.4-12.1%] vs 7.3% [95%CI, 4.2%-11.0%], respectively). The cause could be multifactorial, as superselective embolization beyond the year 2010 could be achieved with the increasing availability of microcatheters, embolization material (smaller, deployable, and softer coils, etc.), and imaging technologies such as cone-beam CT, as well as improved operator experience over time. Such technology advancement can also be reflected by the gradual adoption of empiric embolization in LGIB, which has been historically practiced only in UGIB cases.77,78 Based on the limited evidence from four comparative studies, both targeted and empiric embolization were associated with similar rates of post-embolization rebleeding (23.6% vs 21.1%). Three studies relied on pre-angiographical guidance, such as RBC scintigraphy and endoscopic clipping. Hermie et al, recently reported their use of intraoperative CBCT in identifying the culprit vessel in LGIB patients without contrast extravasation, suggesting similar efficacy in reducing rebleeding rate while minimizing major ischaemia rate.72 However, empiric embolization for LGIB is associated with higher ischaemia, though not statistically significant, which may be attributed to underpowering. Nonetheless, if clinically necessary, pre-embolization endoscopic marking, intraoperative CBCT, and hybrid CT-angiography suites may be implemented to increase embolization precision and decrease risks of ischaemia.
While there is no level-I evidence supporting the universal use of a specific embolization agent for acute LGIB, microcoils and NBCA glue are the most commonly utilized in literature. The advantage of microcoils is their visibility under fluoroscopy, low cost (pushable), and ease of use. It induces coagulation cascade at the local deployment site to achieve haemostasis by decreasing perfusion pressure to bleeding site. Compared to NBCA, interventional radiologists have historically had more experience with this embolic agent. In our previously published studies, we highlighted that microcoils should be deployed distally after achieving superselective catheterization to achieve a state of low local perfusion pressure precluding rebleeding while maintaining collateral flow to prevent ischaemia.12,75 By contrast, NBCA is a liquid agent whose monomers polymerize upon contact with the blood and follow the direction of blood flow to occlude the more distal vasculature.79 Yet, its use has also been debated due to risks of nontarget embolization, risks of microcatheter blockage, catheter tethering, and requirement of operator expertise.80 Preclinical data showed that a higher number of embolized vasa recta might result in end-organ infarction.81 This rationale was supported by findings in the present study: Whereas NBCA had a lower pooled rebleeding rate (9.3% [3.3-17.2%] vs 20.8% [95%CI, 9.8%-34.2%]), post-embolization ischaemia rate was lower in patients treated with microcoils (9.7% [95%CI 1.6%-21.8%] vs 4.0% [95%CI, 0%-12.9%]). Further, patient’s coagulation status has been a factor in deciding the use of coils versus glue, as the former relies on intact coagulation to thrombose the target vessel.12,23 However, no comparative cohort study was available comparing these two agents in the setting of LGIB. As such, the choice of microcoil vs NBCA is largely dependent on operator experience and availability in practice.
In comparison, particles and gel foam are less commonly used as the sole embolization agent, and therefore were not subjected to quantitative analysis in the present study. These liquid embolics are similarly more prone to nontarget embolization and ischaemic complication. Specifically, smaller polyvinyl particles may travel distally beyond the level of collateralization, leading to end-organ ischaemia.80 Ethylene-vinyl copolymer, another liquid embolization agent that is initially used to treat cerebrovascular AVMs as NBCA, is nonadhesive and allows discontinuous injection for operators to assess embolization progress and then achieve complete vascular filling. But it has pitfalls of longer preoperative preparation time and is typically more expensive.82 Only one study included in the present meta-analysis utilized Onyx for LGIB embolization, with a technical success rate of 30/31 (93.5%), 30-day rebleeding rate of 3/30 (10%), and no ischaemia during follow-up.66
With the limited number of studies that performed predictor analysis, coagulopathy, malignancy as a bleeding source, and inotropic support seemed to be associated with high rebleeding and mortality risks in acute LGIB embolization (Figure 3A and B). These findings were also observed in studies focusing on embolization for nonvariceal UGIB.83–85 In the setting of malignancy, TAE is merely providing symptomatic control of tumour progression, which will inevitably lead to rebleeding and mortality without surgical resection. Meanwhile, elective surgery can be contraindicated among patients with advanced disease burden or high comorbidities, so TAE may be the only intervention available at the time of LGIB presentation to decrease the chance of haemorrhage-related death. Thus, for patients who are surgical candidates, TAE should be implemented to bridge to nonurgent or elective surgery once patient stabilizes from acute bleeding. The fact that the presence of shock, blood transfusion, and inotropic use were predictors of mortality suggested the importance of maintaining hemodynamic stability for this patient group. Reversal of coagulopathy should also be considered to prevent rebleeding and lower mortality risk after TAE. In a meta-analysis on endoscopic clipping for LGIB, patients who were on anticoagulation demonstrated a seemingly higher pooled rebleeding rate compared to the overall rate.86 The effects of anticoagulant therapy in patients who underwent LGIB embolization are equivocal: Adusumilli et al reviewed 61 patients with colonic TAE and showed that the rebleeding rate was higher among patients who took aspirin (P = .02). However, Nykannen et al retrospectively analysed 53 successful LGIB embolization patients and did not find significant association between anticoagulation use (OR: 1.93 [95%CI, 0.52-7.24], P = .329), though the type of anticoagulation was poorly defined. Future research stratifying rebleeding risk based on anticoagulation type is necessary to determine the optimum time for anticoagulation therapy resumption after LGIB TAE.
The present meta-analysis should be interpreted with caution. First, the study population is rather heterogenous: various embolization agents were used, and bleeding from different vascular territories was included. The aetiology of bleeding, such as tumour, diverticulosis, and vascular malformation, was analysed as a single entity among most individual studies. For example, post-embolization rebleeding may vary in LGIB patients with malignancy vs diverticulosis due to the nature of the disease, but subgroup analysis of these variables was not possible because individual studies did not specify clinical outcomes of these subgroups. Secondly, follow-up time was not consistent among individual studies, and clinical outcomes such as rebleeding are time-dependent. Only a few studies characterized clinical outcomes on a Kaplan-Meier curve. Further, long-term data was also limited. Thirdly, when comparing embolization materials, a majority of studies using mixed embolization materials were excluded because outcomes were not reported per each subgroup. Therefore, operators and groups who were family with both coils and glues were more likely to be excluded from the present analysis, which may introduce bias. Fourthly predictor analysis was limited by the number of available studies, resulting in underpowering and lack of statistical significance. Further, definition of outcome may vary among studies. For example, definition of technical success carries different meaning in selective and superselective embolization. Further, empiric embolization could be considered successful if embolization materials were successfully deployed, whereas other studies only included targeted embolization into consideration. Finally, nearly all studies were retrospective in nature and noncomparative, and as such considered to be low quality of evidence.
Conclusion
In conclusion, the present meta-analysis reviewed studies focusing on acute LGIB embolization within the last twenty years. This technique continues to demonstrate a high technical success rate with acceptable rate of rebleeding and a trend towards improved outcomes over time. Rebleeding-related mortality and symptomatic ischaemia rates were low. Most ischaemic stigmata discovered during routine post-embolization colonoscopy were minor. Although NBCA seemed to offer a more durable haemostasis rate, it was also associated with higher risk of ischaemia compared to microcoils. Coagulopathy, malignant aetiology, and inotropic use were predictors of rebleeding and mortality. Empiric embolization seemed to be as durable as targeted embolization regarding preventing rebleeding, and recent technology in CBCT might help operators to superselectively embolize the culprit vessel under negative angiographical extravasation setting and thus reducing post-embolization ischaemia. However, due to the heterogeneity of current available evidence, future prospective and comparative studies with stratification based on different variables are required for the standardization of this modern technique.
Supplementary Material
Acknowledgements
All authors have read and approved the submitted manuscript. The manuscript has not been submitted elsewhere nor published elsewhere in whole or in part. A portion of this project was presented as an oral presentation during Society of Interventional Radiology Annual Meeting 2022 at Boston, MA, USA.
Contributor Information
Qian Yu, Division of Interventional Radiology, Department of Radiology, University of Chicago, Chicago, IL, 60637, United States; Department of Surgery, Cleveland Clinic Florida, Weston, FL, 33331, United States.
Brian Funaki, Division of Interventional Radiology, Department of Radiology, University of Chicago, Chicago, IL, 60637, United States.
Osman Ahmed, Division of Interventional Radiology, Department of Radiology, University of Chicago, Chicago, IL, 60637, United States.
Supplementary material
Supplementary material is available at BJR online.
Funding
None declared.
Conflicts of interest
Osman Ahmed reported receiving personal fees from Argon Medical, Penumbra, Medtronic, Johnson & Johnson, and Boston Scientific and grants from Canon Medical, outside the submitted work. No other disclosures were reported. Brian Funaki reported receiving consulting fees from Balt Medical and Okami Medical.
References
- 1. Lanas A, Garcia-Rodriguez LA, Polo-Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633-1641. [DOI] [PubMed] [Google Scholar]
- 2. Barnert J, Messmann H.. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6(11):637-646. [DOI] [PubMed] [Google Scholar]
- 3. Marion Y, Lebreton G, Le Pennec V, Hourna E, Viennot S, Alves A.. The management of lower gastrointestinal bleeding. J Visc Surg. 2014;151(3):191-201. [DOI] [PubMed] [Google Scholar]
- 4. Strate LL, Gralnek IM.. Management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oakland K, Chadwick G, East JE, et al. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019;68(5):776-789. [DOI] [PubMed] [Google Scholar]
- 6. Pesapane F, Patella F, Borelli A, Angileri SA, Ierardi AM, Carrafiello G.. New advances in lower gastro-intestinal bleeding managment with embolotherapy. Eur Cong Radiol. 2017;2017. Doi: 10.1594/ecr2017/C-0438. [DOI] [Google Scholar]
- 7. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA.. Metan: fixed-and random-effects meta-analysis. The Stata Journal. 2008;8(1):3-28. [Google Scholar]
- 8. Nyaga VN, Arbyn M, Aerts M.. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abbas SM, Bissett IP, Holden A, Woodfield JC, Parry BR, Duncan D.. Clinical variables associated with positive angiographic localization of lower gastrointestinal bleeding. ANZ J Surg. 2005;75(11):953-957. 10.1111/j.1445-2197.2005.03582.x [DOI] [PubMed] [Google Scholar]
- 10. Adusumilli S, Gosselink M, Ctercteko G, et al. The efficacy of selective arterial embolization in the management of colonic bleeding. Tech Coloproctol. 2014;18(6):529-533. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed TM, Cowley JB, Robinson G, et al. Long term follow-up of transcatheter coil embolotherapy for major colonic haemorrhage. Colorectal Dis. Oct 2010;12(10):1013-1017. 10.1111/j.1463-1318.2009.01906.x [DOI] [PubMed] [Google Scholar]
- 12. Ahmed O, Jilani D, Sheth S, Giger M, Funaki B.. Long-term results of microcoil embolization for colonic haemorrhage: how common is rebleeding? Br J Radiol. 2015;88(1051):20150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ali M, Ul Haq T, Salam B, Beg M, Sayani R, Azeemuddin M.. Treatment of nonvariceal gastrointestinal hemorrhage by transcatheter embolization. Radiol Res Pract. 2013;2013:604328. 10.1155/2013/604328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D.. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001;12(12):1399-1405. 10.1016/s1051-0443(07)61697-2 [DOI] [PubMed] [Google Scholar]
- 15. Bua-Ngam C, Norasetsingh J, Treesit T, et al. Efficacy of emergency transarterial embolization in acute lower gastrointestinal bleeding: a single-center experience. Diagn Interv Imaging. 2017;98(6):499-505. 10.1016/j.diii.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 16. Burgess AN, Evans PM.. Lower gastrointestinal haemorrhage and superselective angiographic embolization. ANZ J Surg. 2004;74(8):635-638. 10.1111/j.1445-1433.2004.03109.x [DOI] [PubMed] [Google Scholar]
- 17. Chan DK, Soong J, Koh F, Tan KK, Lieske B.. Predictors for outcomes after super-selective mesenteric embolization for lower gastrointestinal tract bleeding. ANZ J Surg. 2016;86(6):459-463. 10.1111/ans.12762 [DOI] [PubMed] [Google Scholar]
- 18. Charbonnet P, Toman J, Bühler L, et al. Treatment of gastrointestinal hemorrhage. Abdom Imaging. 2005;30(6):719-726. 10.1007/s00261-005-0314-8 [DOI] [PubMed] [Google Scholar]
- 19. DeBarros J, Rosas L, Cohen J, Vignati P, Sardella W, Hallisey M.. The changing paradigm for the treatment of colonic hemorrhage: superselective angiographic embolization. Dis Colon Rectum. 2002;45(6):802-808. 10.1007/s10350-004-6301-2 [DOI] [PubMed] [Google Scholar]
- 20. Defreyne L, Vanlangenhove P, De Vos M, et al. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218(3):739-748. 10.1148/radiology.218.3.r01mr05739 [DOI] [PubMed] [Google Scholar]
- 21. Eriksson LG, Ljungdahl M, Sundbom M, Nyman R.. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19(10):1413-1418. 10.1016/j.jvir.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 22. Evangelista PT, Hallisey MJ.. Transcatheter embolization for acute lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2000;11(5):601-606. 10.1016/s1051-0443(07)61612-1 [DOI] [PubMed] [Google Scholar]
- 23. Frodsham A, Berkmen T, Ananian C, Fung A.. Initial experience using N-butyl cyanoacrylate for embolization of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20(10):1312-1319. 10.1016/j.jvir.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 24. Gady JS, Reynolds H, Blum A.. Selective arterial embolization for control of lower gastrointestinal bleeding: recommendations for a clinical management pathway. Curr Surg. 2003;60(3):344-347. 10.1016/s0149-7944(02)00749-3 [DOI] [PubMed] [Google Scholar]
- 25. Gillespie CJ, Sutherland AD, Mossop PJ, Woods RJ, Keck JO, Heriot AG.. Mesenteric embolization for lower gastrointestinal bleeding. Dis Colon Rectum. 2010;53(9):1258-1264. 10.1007/DCR.0b013e3181e10e90 [DOI] [PubMed] [Google Scholar]
- 26. Heianna J, Miyauchi T, Yamano H, Yoshikawa K, Hashimoto M, Murayama S.. Management of angiogram-negative acute colonic hemorrhage: safety and efficacy of colonoscopy-guided superselective embolization. Tech Coloproctol. 2014;18(7):647-652. [DOI] [PubMed] [Google Scholar]
- 27. Horiguchi J, Naito A, Fukuda H, et al. Morphologic and histopathologic changes in the bowel after super-selective transcatheter embolization for focal lower gastrointestinal hemorrhage. Acta Radiol. 2003;44(3):334-339. 10.1034/j.1600-0455.2003.00062.x [DOI] [PubMed] [Google Scholar]
- 28. Huang CC, Lee CW, Hsiao JK, et al. N-butyl cyanoacrylate embolization as the primary treatment of acute hemodynamically unstable lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2011;22(11):1594-1599. 10.1016/j.jvir.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 29. Hur S, Jae HJ, Lee M, Kim HC, Chung JW.. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. 2014;25(1):10-19. 10.1016/j.jvir.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 30. Kariya S, Nakatani M, Ono Y, et al. Provocative angiography for lower gastrointestinal bleeding. Jpn J Radiol. 2020;38(3):248-255. 10.1007/s11604-019-00909-0 [DOI] [PubMed] [Google Scholar]
- 31. Kickuth R, Rattunde H, Gschossmann J, Inderbitzin D, Ludwig K, Triller J.. Acute lower gastrointestinal hemorrhage: minimally invasive management with microcatheter embolization. J Vasc Interv Radiol. 2008;19(9):1289-1296.e2. 10.1016/j.jvir.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 32. Kim CY, Suhocki PV, Miller MJ Jr., Khan M, Janus G, Smith TP.. Provocative mesenteric angiography for lower gastrointestinal hemorrhage: results from a single-institution study. J Vasc Interv Radiol. 2010;21(4):477-483. 10.1016/j.jvir.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 33. Kim JH, Shin JH, Yoon HK, et al. Angiographically negative acute arterial upper and lower gastrointestinal bleeding: incidence, predictive factors, and clinical outcomes. Korean J Radiol. 2009;10(4):384-390. 10.3348/kjr.2009.10.4.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YS, Kwon JH, Han K, et al. Superselective transcatheter arterial embolization for acute small bowel bleeding: clinical outcomes and prognostic factors for ischemic complications. Acta Radiologica (Stockholm, Sweden: 1987). 62(5):574-583. 10.1177/0284185120936258 [DOI] [PubMed] [Google Scholar]
- 35. Kinoshita M, Kondo H, Hitomi S, et al. Ultraselective transcatheter arterial embolization with small-sized microcoils for acute lower gastrointestinal bleeding. CVIR Endovasc. 2021;4(1):28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. d’Othée BJ, Surapaneni P, Rabkin D, Nasser I, Clouse M.. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2006;29(1):49-58. 10.1007/s00270-004-0301-4 [DOI] [PubMed] [Google Scholar]
- 37. Kodani M, Yata S, Ohuchi Y, Ihaya T, Kaminou T, Ogawa T.. Safety and risk of superselective transcatheter arterial embolization for acute lower gastrointestinal hemorrhage with N-butyl cyanoacrylate: angiographic and colonoscopic evaluation. J Vasc Interv Radiol. 2016;27(6):824-830. 10.1016/j.jvir.2016.01.140 [DOI] [PubMed] [Google Scholar]
- 38. Koh DC, Luchtefeld MA, Kim DG, et al. Efficacy of transarterial embolization as definitive treatment in lower gastrointestinal bleeding. Colorectal Dis. 2009;11(1):53-59. 10.1111/j.1463-1318.2008.01536.x [DOI] [PubMed] [Google Scholar]
- 39. Köhler G, Koch OO, Antoniou SA, et al. Relevance of surgery after embolization of gastrointestinal and abdominal hemorrhage. World J Surg. 2014;38(9):2258-2266. 10.1007/s00268-014-2570-7 [DOI] [PubMed] [Google Scholar]
- 40. Kuo WT, Lee DE, Saad WE, Patel N, Sahler LG, Waldman DL.. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14(12):1503-1509. 10.1097/01.rvi.0000099780.23569.e6 [DOI] [PubMed] [Google Scholar]
- 41. Kwak HS, Han YM, Lee ST.. The clinical outcomes of transcatheter microcoil embolization in patients with active lower gastrointestinal bleeding in the small bowel. Korean J Radiol. 2009;10(4):391-397. 10.3348/kjr.2009.10.4.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwon JH, Han YH.. Efficacy and safety of superselective trans-catheter arterial embolization of upper and lower gastrointestinal bleeding using N-butyl-2-cyanoacrylate. Emerg Radiol. 2018;25(2):111-120. 10.1007/s10140-017-1552-0 [DOI] [PubMed] [Google Scholar]
- 43. Lee HH, Oh JS, Park JM, et al. Transcatheter embolization effectively controls acute lower gastrointestinal bleeding without localizing bleeding site prior to angiography. Scand J Gastroenterol. 2018;53(9):1089-1096. 10.1080/00365521.2018.1501512 [DOI] [PubMed] [Google Scholar]
- 44. Lipof T, Sardella WV, Bartus CM, Johnson KH, Vignati PV, Cohen JL.. The efficacy and durability of super-selective embolization in the treatment of lower gastrointestinal bleeding. Dis Colon Rectum. 2008;51(3):301-305. 10.1007/s10350-007-9149-4 [DOI] [PubMed] [Google Scholar]
- 45. Luchtefeld MA, Senagore AJ, Szomstein M, Fedeson B, Van Erp J, Rupp S.. Evaluation of transarterial embolization for lower gastrointestinal bleeding. Dis Colon Rectum. 2000;43(4):532-534. 10.1007/bf02237200 [DOI] [PubMed] [Google Scholar]
- 46. Lv LS, Gu JT.. Super-selective arterial embolization in the control of acute lower gastrointestinal hemorrhage. World J Clin Cases. 2019;7(22):3728-3733. 10.12998/wjcc.v7.i22.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maleux G, Roeflaer F, Heye S, et al. Long-term outcome of transcatheter embolotherapy for acute lower gastrointestinal hemorrhage. Am J Gastroenterol. 2009;104(8):2042-2046. 10.1038/ajg.2009.186 [DOI] [PubMed] [Google Scholar]
- 48. Mejaddam AY, Cropano CM, Kalva S, et al. Outcomes following "rescue" superselective angioembolization for gastrointestinal hemorrhage in hemodynamically unstable patients. J Trauma Acute Care Surg. 2013;75(3):398-403. 10.1097/TA.0b013e31829a8b7a [DOI] [PubMed] [Google Scholar]
- 49. Mensel B, Kühn JP, Kraft M, et al. Selective microcoil embolization of arterial gastrointestinal bleeding in the acute situation: outcome, complications, and factors affecting treatment success. Eur J Gastroenterol Hepatol. 2012;24(2):155-163. 10.1097/MEG.0b013e32834c33b2 [DOI] [PubMed] [Google Scholar]
- 50. Neuman HB, Zarzaur BL, Meyer AA, Cairns BA, Rich PB.. Superselective catheterization and embolization as first-line therapy for lower gastrointestinal bleeding. Am Surg. 2005;71(7):539-545. [PubMed] [Google Scholar]
- 51. Noh SM, Shin JH, Kim HI, et al. Clinical outcomes of angiography and transcatheter arterial embolization for acute gastrointestinal bleeding: analyses according to bleeding sites and embolization types. Korean J Gastroenterol. 2018;71(4):219-228. 10.4166/kjg.2018.71.4.219 [DOI] [PubMed] [Google Scholar]
- 52. Nykänen T, Peltola E, Kylänpää L, Udd M.. Transcatheter arterial embolization in lower gastrointestinal bleeding: ischaemia remains a concern even with a superselective approach. J Gastrointest Surg. 2018;22(8):1394-1403. 10.1007/s11605-018-3728-7 [DOI] [PubMed] [Google Scholar]
- 53. Pannatier M, Duran R, Denys A, Meuli R, Zingg T, Schmidt S.. Characteristics of patients treated for active lower gastrointestinal bleeding detected by CT angiography: interventional radiology versus surgery. Eur J Radiol. 2019;120:108691. 10.1016/j.ejrad.2019.108691 [DOI] [PubMed] [Google Scholar]
- 54. Patel TH, Cordts PR, Abcarian P, Sawyer MA.. Will transcatheter embolotherapy replace surgery in the treatment of gastrointestinal bleeding?. Curr Surg. 2001;58(3):323-327. 10.1016/s0149-7944(01)00417-2 [DOI] [PubMed] [Google Scholar]
- 55. Pham T, Tran BA, Ooi K, et al. Super-selective mesenteric embolization provides effective control of lower GI bleeding. Radiol Res Pract. 2017;2017:1074804. 10.1155/2017/1074804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rossetti A, Buchs NC, Breguet R, Bucher P, Terraz S, Morel P.. Transarterial embolization in acute colonic bleeding: review of 11 years of experience and long-term results. Int J Colorectal Dis. 2013;28(6):777-782. 10.1007/s00384-012-1621-5 [DOI] [PubMed] [Google Scholar]
- 57. Self D, Reece M, Dilernia S.. Predicting the need for transfer and interventional angiography for patients with acute colonic haemorrhage in a regional setting. ANZ J Surg. 2019;89(4):E109-E112. [DOI] [PubMed] [Google Scholar]
- 58. Senadeera SC, Vun SV, Butterfield N, Eglinton TW, Frizelle FA.. Role of super-selective embolization in lower gastrointestinal bleeding. ANZ J Surg. 2018;88(9):E644-e648. 10.1111/ans.14441 [DOI] [PubMed] [Google Scholar]
- 59. Sheth R, Someshwar V, Warawdekar G.. Treatment of acute lower gastrointestinal hemorrhage by superselective transcatheter embolization. Ind J Gastroenterol 2006;25(6):290-294. [PubMed] [Google Scholar]
- 60. Shi ZX, Yang J, Liang HW, Cai ZH, Bai B.. Emergency transcatheter arterial embolization for massive gastrointestinal arterial hemorrhage. Medicine (Baltimore). 2017;96(52):e9437. 10.1097/md.0000000000009437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sıldıroğlu O, Muasher J, Bloom TA, et al. Acute lower gastrointestinal bleeding: predictive factors and clinical outcome for the patients who needed first-time mesenteric conventional angiography. Diagnostic and Interventional Radiology (Ankara, Turkey). 2018;24(1):23-27. 10.5152/dir.2018.15427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Silver A, Bendick P, Wasvary H.. Safety and efficacy of superselective angioembolization in control of lower gastrointestinal hemorrhage. Am J Surg. 2005;189(3):361-363. 10.1016/j.amjsurg.2004.11.024 [DOI] [PubMed] [Google Scholar]
- 63. Tan KK, Strong DH, Shore T, Ahmad MR, Waugh R, Young CJ.. The safety and efficacy of mesenteric embolization in the management of acute lower gastrointestinal hemorrhage. Ann Coloproctol. 2013;29(5):205-208. 10.3393/ac.2013.29.5.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan KK, Wong D, Sim R.. Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg. 2008;32(12):2707-2715. 10.1007/s00268-008-9759-6 [DOI] [PubMed] [Google Scholar]
- 65. Teng HC, Liang HL, Lin YH, et al. The efficacy and long-term outcome of microcoil embolotherapy for acute lower gastrointestinal bleeding. Korean J Radiol. 2013;14(2):259-268. 10.3348/kjr.2013.14.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Urbano J, Manuel Cabrera J, Franco A, Alonso-Burgos A.. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: feasibility and initial experience. J Vasc Interv Radiol. 2014;25(6):839-846. 10.1016/j.jvir.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 67. Waugh J, Madan A, Sacharias N, Thomson K.. Embolization for major lower gastrointestinal haemorrhage: five-year experience. Australas Radiol. 2004;48(3):311-317. 10.1111/j.0004-8461.2004.01313.x [DOI] [PubMed] [Google Scholar]
- 68. Yap FY, Omene BO, Patel MN, et al. Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dig Dis Sci. 2013;58(7):1976-1984. 10.1007/s10620-012-2547-z [DOI] [PubMed] [Google Scholar]
- 69. Yata S, Ihaya T, Kaminou T, et al. Transcatheter arterial embolization of acute arterial bleeding in the upper and lower gastrointestinal tract with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2013;24(3):422-431. 10.1016/j.jvir.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 70. Yi WS, Garg G, Sava JA.. Localization and definitive control of lower gastrointestinal bleeding with angiography and embolization. Am Surg. 2013;79(4):375-380. [PubMed] [Google Scholar]
- 71. Zhao Y, Li G, Yu X, Xie P.. Evaluation of superselective transcatheter arterial embolization with n-butyl cyanoacrylate in treating lower gastrointestinal bleeding: a retrospective study on seven cases. Gastroenterol Res Pract. 2016;2016:8384349. 10.1155/2016/8384349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hermie L, Dhondt E, Vanlangenhove P, De Waele J, Degroote H, Defreyne L.. Empiric cone-beam CT-guided embolization in acute lower gastrointestinal bleeding. Eur Radiol. 2020;31(4):2161-2172. [DOI] [PubMed] [Google Scholar]
- 73. Funaki B, Kostelic JK, Lorenz J, et al. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177(4):829-836. [DOI] [PubMed] [Google Scholar]
- 74. Valek V, Husty J.. Quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage. Cardiovasc Intervent Radiol. 2013;36(3):608-612. [DOI] [PubMed] [Google Scholar]
- 75. Funaki B. Superselective embolization of lower gastrointestinal hemorrhage: a new paradigm. Abdom Imaging. 2004;29(4):434-438. [DOI] [PubMed] [Google Scholar]
- 76. Tarasconi A, Baiocchi GL, Pattonieri V, et al. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14(1):3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M.. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol. 2013;36(4):970-977. [DOI] [PubMed] [Google Scholar]
- 78. Ichiro I, Shushi H, Akihiko I, Yasuhiko I, Yasuyuki Y.. Empiric transcatheter arterial embolization for massive bleeding from duodenal ulcers: efficacy and complications. J Vasc Interv Radiol. 2011;22(7):911-916. [DOI] [PubMed] [Google Scholar]
- 79. Loffroy R, Mouillot T, Bardou M, Chevallier O.. Current role of cyanoacrylate glue transcatheter embolization in the treatment of acute nonvariceal gastrointestinal bleeding. Expert Rev Gastroenterol Hepatol. 2020;14(10):975-984. [DOI] [PubMed] [Google Scholar]
- 80. Navuluri R, Kang L, Patel J, Van Ha T.. Acute lower gastrointestinal bleeding. Semin Intervent Radiol. 2012;29(3):178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jae HJ, Chung JW, Kim H-C, et al. Experimental study on acute ischemic small bowel changes induced by superselective embolization of superior mesenteric artery branches with N-butyl cyanoacrylate. J Vasc Interv Radiol. 2008;19(5):755-763. [DOI] [PubMed] [Google Scholar]
- 82. Guimaraes M, Wooster M.. Onyx (ethylene-vinyl alcohol copolymer) in peripheral applications. Semin Intervent Radiol. 2011;28(3):350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Koo HJ, Shin JH, Kim HJ, et al. Clinical outcome of transcatheter arterial embolization with N-butyl-2-cyanoacrylate for control of acute gastrointestinal tract bleeding. AJR Am J Roentgenol. 2015;204(3):662-668. [DOI] [PubMed] [Google Scholar]
- 84. Mohan P, Manov J, Diaz-Bode A, et al. Clinical predictors of arterial extravasation, rebleeding and mortality following angiographic interventions in gastrointestinal bleeding. J Gastrointestin Liver Dis. 2018;27(3). [DOI] [PubMed] [Google Scholar]
- 85. Loffroy R, Rao P, Ota S, De Lin M, Kwak B-K, Geschwind J-F.. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33(6):1088-1100. [DOI] [PubMed] [Google Scholar]
- 86. Chandrasekar VT, Desai M, Aziz M, et al. Efficacy and safety of over-the-scope clips for gastrointestinal bleeding: a systematic review and meta-analysis. Endoscopy. 2019;51(10):941-949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.