Abstract
Congenital anomalies and defects of the skull base and calvarium encompass a broad and complex spectrum of pathologies. The clinical presentation is highly variable, and these anomalies may be discovered incidentally in asymptomatic individuals. Radiological assessment plays a pivotal role in precisely characterizing these abnormalities, facilitating the diagnostic process, and assisting in any preoperative preparation.
Keywords: congenital skull base anomalies, dural diverticulum, craniopharyngeal canal, encephalocele, dermal sinus tract, sinus pericranii
Introduction
Paediatric skull base and calvarial anomalies and defects represent a subset of congenital craniofacial abnormalities. They can arise from the incomplete closure or persistence of embryonic structures during development,1–2 as well as from abnormalities such as aplasia or the abnormal positioning of normally formed structures.1 In this article, we will categorize these anomalies based on their location, including those affecting the anterior, middle, and posterior cranial fossa (Table 1), as well as anomalies involving the scalp and skull (Table 2). Additionally, the clinical relevance and surgical implications of these anomalies are also briefly discussed in this article.
Table 1.
Summary of skull base anomalies and defects.
| Anatomical location | Types of anomaly | Imaging features |
|---|---|---|
| Anterior cranial fossa | Immature cribriform plate development | Developmental phase with cartilaginous cribriform plate before it ossifies |
| Dermal sinus tract | Extending to enlarged foramen caecum, restricted diffusion on DWI | |
| Nasal cerebral heterotopia | Dysplastic abnormally located brain tissue extruding into the nasal cavity or nasal region | |
| Nasofrontal encephalocele | Brain protrusion through the fonticulus frontalis | |
| Middle cranial fossa | Type II craniopharyngeal canal | Medium-sized canal with an associated inferiorly displaced pituitary gland |
| Trans-sphenoidal cephalocele | Large craniopharyngeal canal accompanied by a cephalocele | |
| Sphenoid wing dysplasia | Hypoplasia or defect of the sphenoid wing; a diagnostic criterion for NF1 | |
| Foramen Vesalii | Normal anatomy. Venous foramen anteromedial to foramen ovale housing an emissary vein | |
| Posterior cranial fossa | Chiari III malformation | Herniation of dysmorphic cerebellum, brainstem through a dorsal occipitocervical defect |
Table 2.
Summary of calvarial anomalies and defects.
| Types of anomaly | Imaging features | Associated finding or clinical implication |
|---|---|---|
| Parieto-occipital encephalocele | Herniation of brain tissue through defect in the calvarium | Associated with cortical anomalies, abnormalities in the venous sinuses |
| Parietal foramina | Focal nonossification of the medial parietal calvarium | Can be associated with bulging with Valsalva if large |
| Atretic cephalocele | Protruded involuted tissue connected to dura by fibrous stalk | Associated with malformations/anomalies; for example, holoprosencephaly, callosal dysgenesis |
| Sinus pericranii | Subcutaneous extracranial venous structure communicating with dural venous sinuses | Can be part of dominant venous drainage pathway (this must be excluded prior to surgical closure) |
| Cutis aplasia | Absence or ulceration of portion of skin | Severe cases have calvarial defects that can expose veins and meninges and increase risk of thrombosis and infection |
| Calvarial dermoid/epidermoid | Restricted diffusion on DWI, may have enhancing rim | The degree/depth of intracranial extent has implications for surgical planning |
Anterior cranial fossa
The fonticulus frontalis and foramen caecum are temporary embryologic openings in the fronto-naso-ethmoidal region, which should normally close during development. In the fully matured anterior skull base, a small vestigial foramen remains as the remnant of the foramen caecum. However, when proper closure fails to occur, a persistent opening referred to as a dural diverticulum can form. This persistent opening can serve as a gateway for various pathological conditions, including nasal dermoid sinus tracts, cephaloceles, and nasal cerebral heterotopia.1,3
Immature cribriform plate development
An immature cribriform plate is a normal phase of development and is not considered a true defect. In newborns, the cribriform plate is cartilaginous, and therefore it appears lucent on CT. Ossification of the cartilage typically commences at around 2 months of age, and it proceeds posteriorly to anteriorly. By the age of approximately 2 years, most of the ossification should be complete. In an older patient, the cribriform plate development is considered immature if it is not completely ossified (as demonstrated in Figure 1). However, it is important to note that a cartilaginous gap may still be present at the foramen caecum in some cases.1,3
Figure 1.
Immature cribriform plate development. Coronal (A) and axial (B) CT images demonstrate a wide apparent gap (arrow) at the central anterior skull base above the nasal cavity where the osseous plate of the cribriform plate would be seen in an older patient.
Nasal dermoid/dermal sinus tract
This condition arises from the persistence of a neuroectodermal remnant due to the incomplete involution of the dural diverticulum, which remains present through the foramen caecum or fonticulus frontalis during development. Epithelial tissue trapped along this pathway has the potential to develop into a dermoid cyst containing hair and glandular tissues.1–2 Patients with this condition may exhibit a skin dimple with or without a tuft of hair, a nasoglabellar mass, or a mass within the nasal cavity causing congestion.
CT may show a bifid crista galli or abnormally large foramen caecum at the site of the lesion and defect. MRI evaluation should include a fat-suppressed contrast-enhanced sequence, which helps delineate the lesion/tract course (demonstrated in Figure 2A). Restricted diffusion on DWI (diffusion-weighted imaging) is sensitive and specific for a dermoid (shown in Figure 2B). Both CT and MRI studies are valuable for identifying potential intracranial communication, which could serve as a gateway for infection causing meningitis.1,3 It is crucial to avoid misinterpreting the fatty marrow signal in the crista galli as a dermoid lesion during the assessment.
Figure 2.
Nasal dermoid. Postcontrast fat-saturated sagittal T1W image (A) demonstrates a midline oval cystic subcutaneous lesion with thin rim enhancement representing a nasal dermoid cyst (arrowhead). A contiguous enhancing dermal sinus tract (curved arrow) traverses the foramen caecum and connects to an intracranial dermoid cyst (arrow) with thin rim enhancement and (Image (B), sagittal DWI-ADC map) internal restricted diffusion with low ADC signal (arrowhead).
Nasal cerebral heterotopia
Nasal cerebral heterotopia is commonly referred to as nasal glioma, but it is not a neoplastic lesion. It may present as a subcutaneous mass along the nose or glabella, or patients may present with congestion from a nasal cavity mass causing remodelling of the nasal cavity structures.
On MRI, the lesion might have T2-weighted hyperintense signal of gliosis (demonstrated in Figure 3B), or it could be isointense to brain. It may be connected to the intracranial cavity by a thin stalk. Peripheral enhancement may be present due to adjacent mucosa (demonstrated in Figure 3C).3 There should be no restricted diffusion (shown in Figure 3D) in contrast to epidermoid lesions.
Figure 3.
Nasal cerebral heterotopia. Coronal CT reformatted image (A) and coronal fat-saturated T2W image (B) demonstrate a discrete heterogeneously T2W hyperintense mass (arrow in (A) and (B)) in the left nasal cavity producing remodelling and expansion of the left nasal cavity and nasal septal deviation to the right (arrowhead, (A)). Postcontrast fat-saturated sagittal T1W image (C) demonstrates no central enhancement but thin linear enhancement of adjacent nasal mucosa (arrow). Axial DWI (D) demonstrates no restricted diffusion (arrowhead).
Nasofrontal encephalocele
Nasofrontal encephalocele is a specific type of encephalocele occurring from herniation of intracranial contents through the fonticulus frontalis. On physical examination, a mass is typically observed in the nasoglabellar region with a variable skin covering that might exhibit hyperpigmentation. Notably, this lesion’s size may fluctuate in response to crying and Valsalva manoeuvres.1,3
Nasofrontal encephalocele (shown in Figure 4) is 1 of 3 types of fronto-ethmoidal (sincipital) cephaloceles. The other two types are naso-ethmoidal (through the foramen caecum) and naso-orbital. The nasofrontal type is the most common variant.1,3 The size of the encephalocele may increase with time, which predisposes patients to CSF leak and meningitis.
Figure 4.
Nasofrontal encephalocele. Sagittal CT reformatted image (A) and axial T2W image (B) demonstrate a large volume of partially dysplastic bifrontal brain parenchyma (arrow in (A) and (B)) herniating through a large bone defect (arrowhead, (A)) in the region of where the developmental fonticulus frontalis (between the frontal and nasal bones) failed to ossify and close.
Middle cranial fossa
Rathke’s pouch is a transient structure located at the central base of the middle cranial fossa. It normally undergoes involution as part of embryonic development. However, if this involution process is unsuccessful, it can lead to the formation of the craniopharyngeal canal, an abnormal connection between the nasopharynx and the sella. Depending on the size of the canal, it can be categorized into 3 types—type I: small canal measuring less than 1.5 mm in size; type II: medium-sized canal with an associated inferiorly displaced pituitary gland; and type III: large canal accompanied by a cephalocele.4
Type II craniopharyngeal canal (associated with morning glory disc anomaly)
Craniopharyngeal canals can be associated with congenital syndromes such as morning glory disc anomaly. MGDA (morning glory disc anomaly) is a congenital optic nerve anomaly with funnelling at the posterior pole of the ocular globe and optic disc (as shown in Figure 5A), and with a central glial tuft of tissue at the nerve head. This is usually a clinical ophthalmologic diagnosis, which triggers a search for associated cranial anomalies. MRI/MRA is routinely performed to rule out the possible associations, which can include skull base defects (persistent craniopharyngeal canal shown in Figure 5B) and arterial steno-occlusive disease. Hypothalamic-pituitary dysfunction may occur in these patients if there is a type II craniopharyngeal canal due to stretching of these structures from inferior displacement of the pituitary gland.3–4
Figure 5.
Type II craniopharyngeal canal associated with morning glory disc anomaly. Axial T2W image (A) demonstrates posterior funnelling of the right globe with tuft of tissue adjacent to distal end of the optic nerve (arrowhead). Sagittal T1W image (B) demonstrates a smooth congenital defect in the floor of the sella (arrow), with the anterior pituitary gland appearing to extend inferiorly into the defect and contiguous with nasopharyngeal mucosa.
Trans-sphenoidal cephalocele (type III persistent craniopharyngeal canal)
A trans-sphenoidal cephalocele occurs through a large persistent craniopharyngeal canal (shown in Figure 6), and hypothalamic-pituitary dysfunction is common due to the stretching of these structures as they herniate inferiorly. It can also be complicated by spontaneous CSF (cerebrospinal fluid) leak, and there is an increased risk for infection and nasopharyngeal airway narrowing. It is also important to note that attempting a surgical procedure in the nasopharynx without prior knowledge of this lesion can pose a risk of damaging the contents of the cephalocele.1,4
Figure 6.
Trans-sphenoidal cephalocele. Sagittal T1W images ((A) and (B)) of the same patient from 6 and 9 years of age demonstrate a gradually enlarging persistent craniopharyngeal canal and frank cephalocele (arrow), containing a stretched pituitary gland and optic chiasm.
Sphenoid wing dysplasia
Sphenoid wing dysplasia manifests as a defect or hypoplasia of the lesser or greater sphenoid wing (shown in Figure 7B). It can potentially result in the herniation of the temporal lobe into the orbit (shown in Figure 7A) and the infratemporal fossa (shown in Figure 7C). It is 1 of the 7 diagnostic criteria for clinical diagnosis of neurofibromatosis type I. It is almost always associated with plexiform neurofibroma of the orbital/periorbital/infratemporal regions (shown in Figure 7D).3 Erosion of the bone over time from an optic glioma may also contribute to this.
Figure 7.
Axial (A) and coronal (C) CT images demonstrate herniation of the right middle cranial fossa contents including the temporal lobe and extra-axial fluid space into the right orbit (arrow, (A)) and into the right infratemporal fossa/right pterygopalatine fossa (arrowhead, (C)). Axial CT image bone window (B) demonstrates marked remodelling and erosion of the right sphenoid greater wing (arrow). Coronal T2W fat-saturated image (D) demonstrates an irregular heterogeneous infiltrative mass in the right infratemporal fossa (arrow) consistent with a plexiform neurofibroma.
Foramen Vesalii
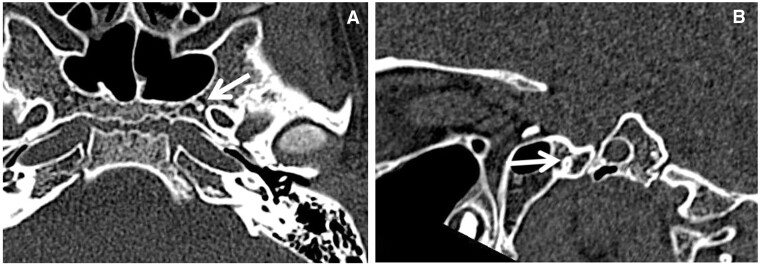
The foramen Vesalii is situated in the greater wing of the sphenoid bone (shown in Figure 8), anteromedial to the foramen ovale. Within it, there is an emissary vein that provides a connection between the cavernous sinus and the pterygoid venous plexus.5 This foramen can serve as a conduit for extracranial infection to spread into the intracranial compartment. It is crucial to identify the foramen Vesalii during neurosurgical procedures to prevent unintentional puncture and the subsequent risk of haemorrhage.5
Figure 8.
Foramen Vesalii. Axial CT image (A) demonstrates a small osseous foramen (foramen Vesalii) anteromedial to the foramen ovale. Its obliquely vertical course is better demonstrated on the sagittal plane (B).
Posterior cerebral fossa
Chiari III malformation
Chiari III malformation is an occipitocervical cephalocele that typically contains dysplastic cerebellum and occasionally brainstem protruding through a dorsal defect in the upper cervical vertebra (shown in Figure 9). Additionally, individuals with an occipitocervical cephalocele may experience midline anomalies, such as malformation of the corpus callosum. Furthermore, there can be traction on the spinal cord leading to tethered cord syndrome, along with the development of a syrinx in the spinal cord.6
Figure 9.
Occipitocervical cephalocele. Sagittal T2W image of brain and cervical spine (A) demonstrates a large occipitocervical cephalocele, which contains dysplastic cerebellum (black arrow), traction on the brainstem (black arrowhead), marked widening of the central canal (white arrow), and dysgenesis of posterior corpus callosum (white arrowhead). Axial T2W image (B) demonstrates dysraphism (black arrow) in the cervical spine and postsurgical changes to the dura from prior repair (arrowhead).
The occipitocervical cephalocele is usually discovered on prenatal ultrasound. Patients may exhibit difficulty breathing and swallowing at birth (brainstem-related dysfunction), seizures, and developmental delay. Chiari III malformation carries poor long-term prognosis. The patient is not a surgical candidate if the volume of tissue in the herniated sac exceeds that of the intracranial portion.6
Anatomical variants and congenital anomalies of the calvarium are usually asymptomatic and detected incidentally on radiologic examinations. These anomalies frequently present with focal defects in the bone, which can be clearly visualized on CT. The use of MRI is particularly beneficial for assessing for associated intracranial malformations/anomalies and other pathology.
Parieto-occipital encephalocele
Cephaloceles are often accompanied by additional anomalies, including cortical malformations and abnormalities in the venous sinuses. Notably, a dural venous sinus may extend into the cephalocele (shown in Figure 10), a crucial detail with significant implications for surgical planning. Therefore, the recommended imaging protocol should include venous imaging, and preferably for children this should include magnetic resonance venography (MRV) and a contrast-enhanced T1-weighted 3D sequence such as spoiled gradient recalled echo (SPGR) or magnetization-prepared rapid gradient-echo (MPRAGE).
Figure 10.
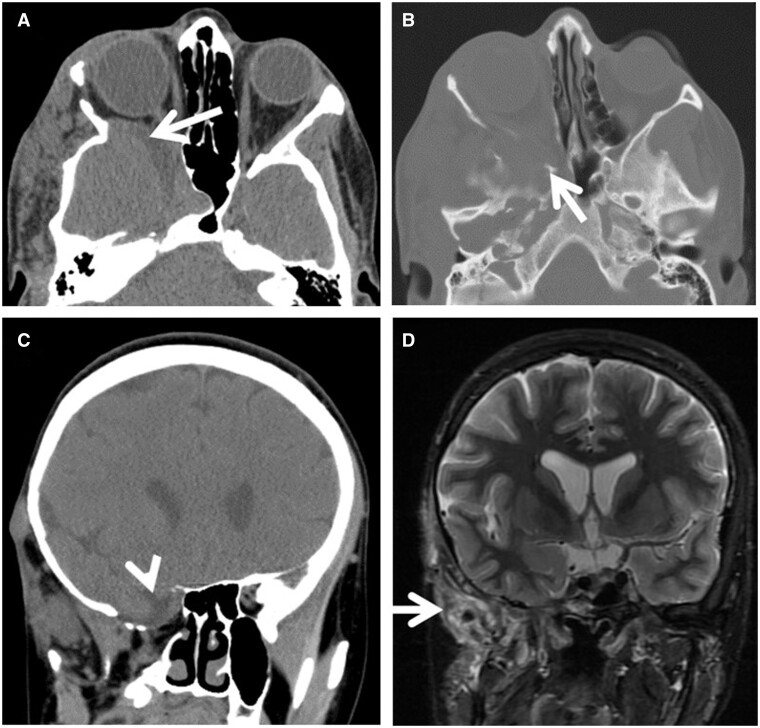
Axial T2W image (A) of brain demonstrates a large posterior parietal/occipital calvarial defect and encephalocele containing irregular dysplastic and cystic brain tissue (black arrow), prominent flow void from an indeterminate vessel (black arrowhead), and thickened irregular cortex in the posterior intracranial left parietal lobe consistent with polymicrogyria (white arrows). Sagittal T1W MPRAGE postcontrast image (B) demonstrates a long segment of the superior sagittal sinus extending into the herniated sac (arrow). There is conspicuous microcephaly.
The clinical outcome is related to size and volume of tissue herniated through the defect and the presence of associated anomalies. The herniated tissue may be dysplastic or cystic. CSF flow can be impaired with hydrocephalus resulting in macrocephaly in the majority of cases, but there is also microcephaly in a substantial minority.1,3
Parietal foramina
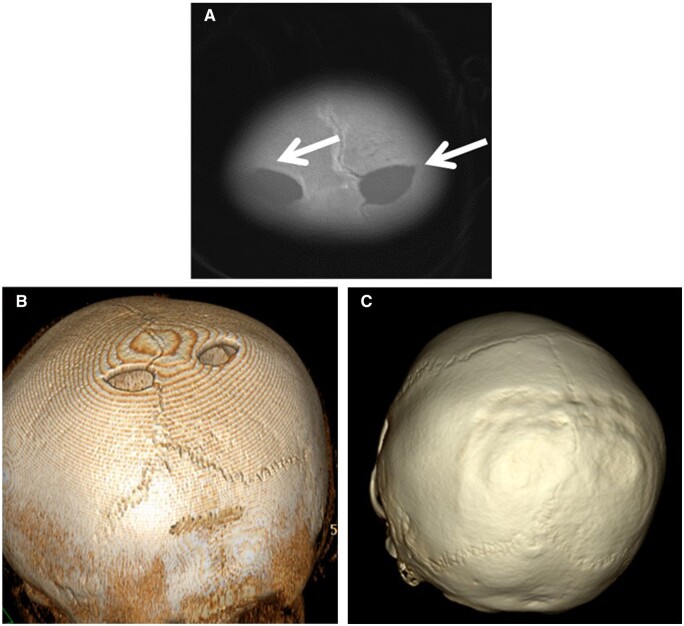
These are a common benign anatomic variant. Parietal foramina represent the most prevalent congenital skull defect, featuring a distinctive symmetric focal nonossification in the medial parietal bones (shown in Figure 11).7
Figure 11.
Axial CT bone window (A) and (B) 3D reconstructed images demonstrate symmetric discrete foramina (arrows) in the parasagittal high parietal bones. The patient and family requested elective surgical closure with particulate bone graft. CT 3D reconstructed image (C) several months postoperatively demonstrate well-integrated bone grafting and complete closure of the foramina.
Most patients are asymptomatic, with the foramina most often being inconspicuous punctate incidental findings. However, if they are large, they may present with sensation of a palpable subcutaneous calvarial mass that bulges with crying or Valsalva.1,7
Atretic cephalocele
Atretic cephalocele is a type of involuted true cephalocele that remains connected to dura mater via a fibrous stalk. Clinically, it may manifest as a soft palpable scalp mass with overlying thin skin or alopecia, which enlarges with crying or Valsalva.1
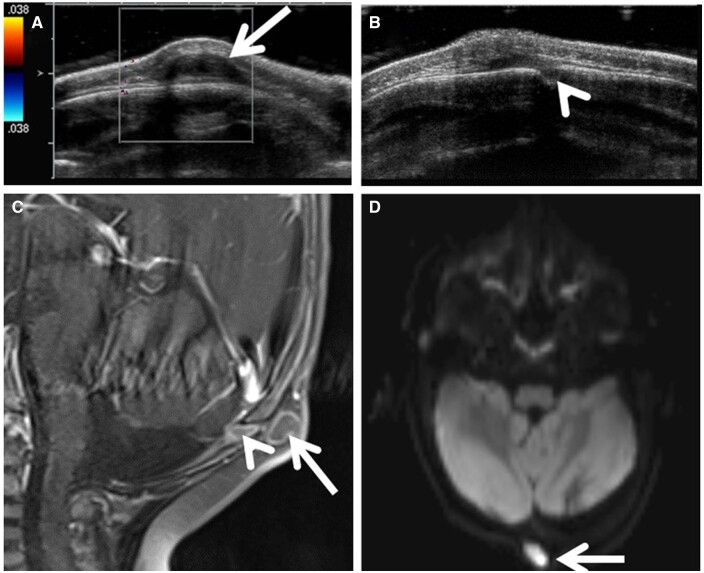
The imaging characteristics of this lesion include focal heterogeneous or cystic subcutaneous scalp tissue, presence of a contiguous intracranial falcine venous sinus, and adjacent fibrous tract (shown in Figure 12). It is important to look for other malformations/anomalies that can be associated with an atretic cephalocele, including holoprosencephaly, callosal dysgenesis, interhemispheric cysts, and eye anomalies, among others.1,7
Figure 12.
Focused ultrasound image (A) demonstrates a heterogenous scalp lesion with hypoechoic tract (arrow) extending deep through a calvarial defect and adjacent colour Doppler signal from the sagittal sinus (arrowhead). Sagittal fat-saturated T2W image (B) demonstrates the tract coursing intracranially through a bifid superior sagittal sinus, along a persistent falcine venous sinus (arrows), down to the level of a dysplastic tectum.
Sinus pericranii
Sinus pericranii is a prominent subcutaneous extracranial venous structure that communicates with the dural venous sinuses via transcalvarial emissary veins (shown in Figure 13A and B). It usually presents as a painless, nonpulsatile scalp mass and occasionally with overlying blue skin colour, and it can distend with crying or Valsalva.7
Figure 13.
Sinus pericranii. Sagittal left parietal paramidline ultrasound image (A) demonstrates a lobulated subcutaneous anechoic structure (arrow) abutting and superficial to the calvarium with (B) colour Doppler demonstrating vascular flow within and a small vascular channel (arrowhead) communicating with the superior sagittal sinus (arrow). A 3D MRV TOF reconstructed MIP image (C) confirms a minor emissary vein (black arrow) connecting the superior sagittal sinus (white arrow) with the palpable finding (sinus pericranii) and with a scalp vein extending anteriorly (arrowhead).
Before considering ligation or closure of a sinus pericranii, it is essential to assess the venous system to ensure that the sinus pericranii is not serving as the dominant venous drainage pathway. The evaluation should include MRV as a crucial step (shown in Figure 13C). Additionally, the use of contrast-enhanced T1W 3D imaging (eg, MPRAGE or SPGR) can also provide valuable information. In cases of complex or extensive lesions, a conventional angiogram may be necessary for a thorough evaluation. It is important to note that ultrasound is limited in its ability to evaluate intracranial structures.1,7
Cutis aplasia
Cutis aplasia presents as the absence or ulceration of a portion of scalp skin at birth in a localized or widespread area that is well demarcated. It may heal during gestation, subsequently appearing as a scar with focal alopecia; otherwise, it presents as ulceration. In severe cases, there can be involvement of the deep tissues with associated calvarial defects (shown in Figure 14); this may expose the dura and venous sinuses, significantly increasing the risk of complications such as meningitis, haemorrhage, or venous thrombosis.
Figure 14.
Cutis aplasia. Axial CT image (A) and CT 3D bone reconstructed image (B) demonstrate extensive symmetric absence of scalp tissue and calvarium (arrow) over the frontal and anterior parietal region. This is an especially severe case of cutis aplasia due to the marked depth and extent. CT 3D bone reconstructed image (C) demonstrates eventual postsurgical changes from cranioplasty with particulate bone graft that was performed with fasciocutaneous flap.
Calvarial dermoid/epidermoid
From an embryological perspective, epidermoid cysts are ectodermal inclusion cysts originating from implanted residual embryonic tissue. It is worth noting that dermoid cysts are distinct in composition, typically containing squamous epithelium, keratin debris, and dermal appendages. In contrast, epidermoid cysts lack dermal appendages. While epidermoid cysts often manifest in the region of the anterior fontanelle, they have the potential to develop anywhere in the body.7
Fat-suppressed contrast-enhanced MRI helps delineate the lesion and course of an associated sinus tract if present. Rim enhancement may be seen along the cyst and along a contiguous sinus tract, but there should be no nodular enhancement (shown in Figure 15C). Restricted diffusion in the lesion is a sensitive and specific finding for an epidermoid cyst (shown in Figure 15D). The degree and depth of intracranial extent have implications for surgical planning.
Figure 15.
Epidermoid cyst. Colour Doppler image (A) demonstrates a discrete hypoechoic subcutaneous lesion (arrow) overlying the occipital calvarium without any internal vascular flow. Image (B) demonstrates a contiguous component extending through a defect in the bone (arrowhead). Therefore, MRI was indicated to evaluate intracranial extent. Sagittal postcontrast fat-saturated T1W image (C) demonstrates rim enhancement of the lesion (arrow) and a contiguous cyst or tract extending deep to contact the dural surface (arrowhead). Axial DWI sequence (D) demonstrates restricted diffusion in the lesion (arrow).
Conclusions
Skull base and calvarial anomalies and defects are a group of pathology with variable clinical and radiological manifestations. The appropriate utilization of imaging is crucial in not only establishing a precise diagnosis but also identifying any related complications and facilitating pre-operative planning.
Contributor Information
Wen Wang, Radiology Department, University of Florida College of Medicine—Jacksonville, Jacksonville, FL 32209, United States.
Jeet Patel, Radiology Department, University of Florida College of Medicine—Jacksonville, Jacksonville, FL 32209, United States.
Funding
None declared.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1. Barkovich AJ, Raybaud CA.. Congenital malformations of the brain and skull. In: Barkovich AJ, Raybaud CA, eds. Pediatric Neuroimaging. 5th ed. Lippincott Williams & Wilkins; 2012:367-568. [Google Scholar]
- 2. Riley CA, Soneru CP, Overdevest JB, Otten ML, Gudis DA.. Pediatric sinonasal and skull base lesions. World J Otorhinolaryngol Head Neck Surg. 2020;6(2):118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osborn AG, ed. Anomalies of the skull and meninges. In: Osborn’s Brain: Imaging, Pathology, and Anatomy. Lippincott Williams & Wilkins; 2012:1072-1203. [Google Scholar]
- 4. Abele TA, Salzman KL, Harnsberger HR, Glastonbury CM.. Craniopharyngeal canal and its spectrum of pathology. AJNR Am J Neuroradiol. 2014;35(4):772-777. 10.3174/ajnr.a3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raval BB, Singh P, Rajguru J.. A morphologic and morphometric study of foramen vesalius in dry adult human skulls of Gujarat region. J Clin Diagn Res. 2015;9(2):AC04-AC07. 10.7860/jcdr/2015/11632.5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross JS, ed., Moore KR.. Chiari 3. In: Diagnostic Imaging: Spine. 3rd ed. Elsevier; 2015:62-63. [Google Scholar]
- 7. Khodarahmi I, Alizai H, Chalian M, et al. Imaging spectrum of calvarial abnormalities. Radiographics. 2021;41(4):1144-1163. 10.1148/rg.2021200198 [DOI] [PubMed] [Google Scholar]