Abstract
Background
Statin use in many studies is related to the improvement of a patients’ condition including reducing the risk of various malignancies. Herein, is a systematic review and meta-analysis to examine the evidence on the association between statin therapy and the risk of the occurrence of pancreatic cancer, mainly in terms of decreased risk of developing pancreatic cancer among patients using statin therapy in the long-term perspective.
Methods
PubMed, Web of Science, Scopus and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from database inception to December 1st, 2021. Random effect models were used to estimate summary odds ratios (OR) and the corresponding 95% confidence intervals (CI).
Results
A total of 26 studies comprising 2,797,186 patients were included. Polled analysis showed that pancreatic cancer occurrence in statin vs. no-statin group varied and amounted to 0.4% vs. 0.6% (OR = 0.83; 95% CI: 0.72–0.96; I2 = 84%; p = 0.01).
Conclusions
In summary, the present analysis shows that overall statins use is significantly associated with a reduction in risk of pancreatic cancer. However, these results were not confirmed for the randomized controlled trial subgroup. Further prospective studies are needed to confirm the current results.
Keywords: pancreatic cancer, pancreatic malignancy, statin, risk, systematic review, meta-analysis
Introduction
Due to the fact that pancreatic cancer is usually diagnosed in advanced stages, i.e., the presence of distant metastases is identified in more than 50% of patients at the time of diagnosis, this malignant neoplasm remains one with the worst prognosis [1]. Even considering the introduction of modern chemotherapeutic regimens (FOLFIRINOX, nab-paclitaxel with gemcitabine) and the development of pancreatic surgery — the 5-year survival rate remains low, especially compared to other solid tumors [2, 3]. Screening tests are only recommended in patients at very high risk of developing pancreatic cancer, for example, in certain genetic syndromes. Moreover, there is also no clear consensus on the type of screening (computed tomography, magnetic resonance imaging, endoscopic ultrasound) as well as the frequency of recommended testing [4]. The search for ways to reduce the risk of developing pancreatic cancer has led to providing rather general health-related recommendations, including a balanced diet, maintaining a healthy body weight, physical activity, or quitting smoking [5].
In addition, the search for relationships between pharmacotherapy (particularly long-term) and the risk of pancreatic cancer, particularly in the context of a reduced risk of developing this malignancy is highly warranted. Scientists have long highlighted the relationship between the use of acetylsalicylic acid (ASA) and a reduction in the risk of solid tumors, including pancreatic cancer — although the relationship is not as clear as it is in the case of, for example, colorectal cancer [6]. ASA has a pleiotropic effect, and the key to observing its impact on reducing the risk of pancreatic cancer is the length of its use [7]. Studies are also examining a connection between nonsteroidal anti-inflammatory drug (NSAIDs) use and the risk of pancreatic cancer [8]. Due to the fact that NSAIDs constitute a heterogeneous group of drugs and patients use anti-inflammatory drugs both chronically and sporadically — depending on the need and pain level — it is challenging to find any well-established relationship [9, 10].
On the other hand, due to the population potential and the fact that statins are used in long-term therapy, it is not surprising that scientists are interested in looking for evidence on the impact of their use on cancer risk [11]. Basic science research seems to indicate that there is a pancreatic carcinogenesis mechanism that can be influenced by statins [12]. From a clinical point of view, thanks to statins, one can obtain better control over the risk factors of pancreatic cancer, including the metabolic profile and obesity. Moreover, in patients diagnosed with pancreatic cancer, especially metastatic, statins appear to improve overall survival [13]. It may be related to the chemosensitizing properties of statins. Bearing in mind the evidence from basic science, preclinical studies in animal models as well as clinical observations, it is reasonable to conduct epidemiological observations aimed at demonstrating the relationship between long-term statin use and the risk of pancreatic cancer [14]. Conflicting results of observational studies, high heterogeneity of populations covered by epidemiological observations, and finally, different methodologies applied in studies make it difficult to analyze the available data objectively.
The above-mentioned factors, have mainly contradictory results in epidemiological observations and have led to the necessity of conducting a systematic review of the literature and a meta-analysis — which findings may be valuable in designing other prospective scientific studies. Thus, the present study conducted a systematic review and meta-analysis to examine the evidence on the association between statin therapy and the risk of occurrence of pancreatic cancer, mainly in terms of decreased risk of developing pancreatic cancer among patients using statin therapy in the long-term perspective.
Methods
The current study was designed as a systematic review and meta-analysis. It was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement [15]. The study protocol has been deposited in the PROSPERO database prior to the start of the study. No protocol changes were made during the study. Due to the nature of the study (meta-analysis), the bioethical commission approval was not required.
Literature search
A computerized literature search of PubMed, Web of Science, Scopus and Cochrane Central Register of Controlled Trials (CENTRAL) was performed from each databases’ inception to December 1st, 2021. To increase the probability of identifying all relevant articles, a specific research equation was formulated for each database, using the following keywords: “pancreatic malignancy” OR “pancreatic cancer” OR “pancreatic neoplasm” AND “statin” OR “autorvastatin” OR “fluvastatin” OR “cerivastatin” OR “lovastatin” OR “resuvastatin” OR “pravastatin” OR “simvastatin”. Additionally, the reference list of the eligible trial and relevant review articles were crosschecked to identify additional pertinent studies.
Inclusion and exclusion criteria
Studies that were included in this meta-analysis had to fulfill the following PICOS criteria: 1) Participants, patients were 18 years old or older; 2) Intervention, treatment with statin; 3) Comparison, treatment without statin; 4) Outcomes, pancreatic cancer occurrence; 5) Study design: retrospective and prospective trials published in English. Studies were excluded if they were reviews, animal studies, case reports, letters, conference or poster abstracts, or articles not containing original data.
Data extraction
Two reviewers (K.S. and L.S.) independently extracted the following information from each included article. From studies that met the inclusion eligibility criteria, the following data were extracted into predefined Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA, USA): a) Study characteristic (i.e.: first author, year of publication, country, study design); b) Participant characteristics (i.e.: number of participants, age, sex); c) Main study outcomes (i.e.: incidence of pancreatic cancer in each study group). Potential disagreements were resolved by discussion with third reviewer (K.J.F.).
Risk of bias
Two reviewers (K.S. and L.S.) independently assessed the risk of bias using the Cochrane “Risk of Bias” tool. The RoB-2 tool was used to assess the risk of bias among randomized controlled trials (RCT) [16], and ROBINS-I tool for non-randomized trials [17], respectively. Any disagreements between the two reviewers in the evaluation process were resolved by discussion with third reviewer (M.J.J.). The risk of bias assessments was visualized using the Robvis application [18].
Statistical analysis
The meta-analysis was conducted using the Review Manager, version 5.4EN (RevMan; The Cochrane Collaboration, Oxford, UK). A p value less than 0.05 was accepted as statistically significant. For each study, event numbers in relation to the pancreatic cancer occurrence were collected. The pooled results are presented as odds ratios (OR) and 95% confidence intervals (CI). Random-effects models were used as they considered both sampling variance within the different trials and the variation in the underlying effect across studies. The quality of the heterogeneity was assessed by means of the Cochran’s Q and I2 statistics. Heterogeneity was determined with the I2 statistic, in which the results range from 0% to 100%. Heterogeneity was interpreted as not observed when I2 = 0%, low when I2 = 25%, medium when I2 = 50%, and high when I2 = 75% [19].
Results
Search results and characteristics of studies
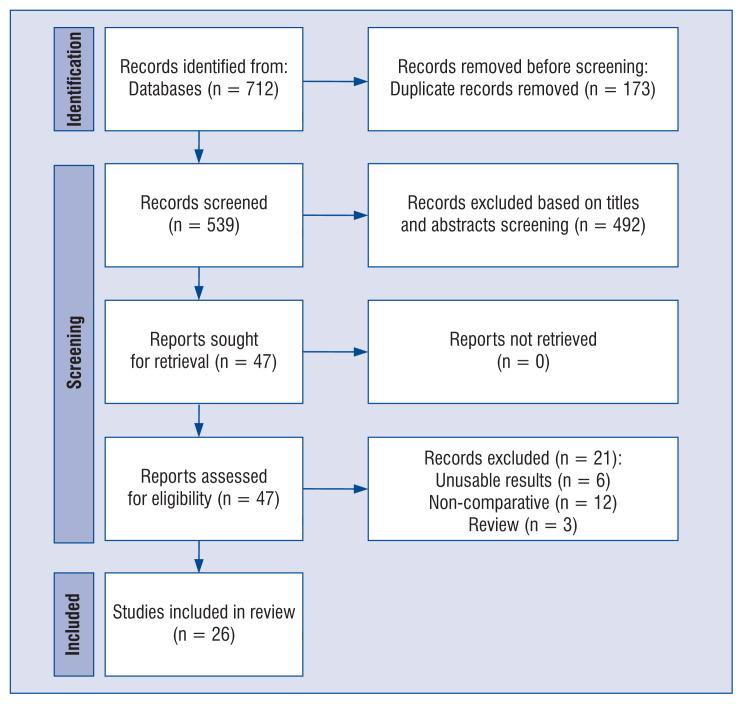
As illustrated in Figure 1, 712 studies were identified in the literature search, and 47 were selected for full-text review. A total of 26 studies [20–45] met the inclusion criteria and were included in the analysis, comprising 2,797,186 patients. A manual search did not identify any new eligible studies. Baseline data and other details are shown in Supplementary Table S1. Two studies were randomized controlled trials [26, 40] and other trials were non-RCTs [20–25, 27–39, 41–45]. The results of the assessment of risk of bias among the 4 included studies are provided in Supplementary Figures S1–S4.
Figure 1.
Flow diagram showing stages of database searching and study selection as per Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) guideline.
Meta-analysis
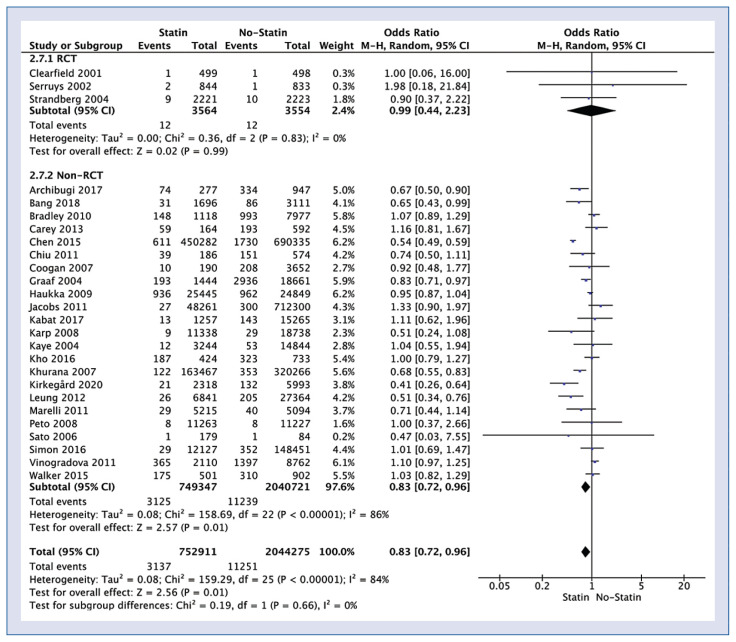
Twenty-six studies reported impact of statin use on pancreatic cancer occurrence. Polled analysis of those trials showed that pancreatic cancer occurrence in statin vs. no-statin group varied and amounted to 0.4% vs. 0.6% (OR = 0.83; 95% CI: 0.72–0.96; I2 = 84%; p = 0.01; Fig. 2). Sub-analysis showed that pancreatic cancer occurrence in statin vs. no-statin group in RCT was at the same level 0.3% (OR = 0.99; 95% CI: 0.44–2.23; I2 = 0%; p = 0.99), but in the non-RCT pancreatic cancer occurrence was 0.4% in the statin group, and 0.6% in the non-statin group (OR = 0.83; 95% CI: 0.72–0.96; I2 = 86%; p = 0.01).
Figure 2.
Forest plot of pancreatic cancer occurrence rate among statin vs. non-statin groups. The center of each square represents the weighted risk ratios for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results.
When matched data were included in pooled analysis occurrence on pancreatic cancer 0.4% in the statin group compared to 0.5% for the non-statin group (OR = 0.85; 95% CI: 0.71–0.95; I2 = 85%; p = 0.01).
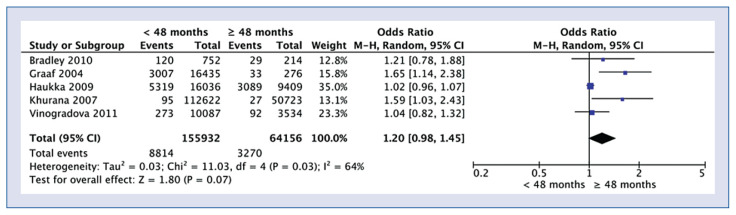
The analysis of the effect of the duration of taking statins showed no statistically significant differences with the incidence of cancer in the group of patients who took statins < 48 months and ≥ 48 months, respectively (5.7% vs. 5.1%; OR = 1.20; 95% CI: 0.98–1.45; I2 = 64%; p = 0.07; Fig. 3).
Figure 3.
Forest plot of pancreatic cancer occurrence rate among patients who are taking statin less than 48 months and more than 48 months, respectively. The center of each square represents the weighted risk ratios for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results.
Discussion
This meta-analysis indicates the protective role of statins in the prevention of pancreatic cancer. Although due to the high heterogeneity of the studies included in the meta-analysis and the observational nature of the studies — especially in the case of case-control studies (retrospective analysis), strong recommendations regarding the use of statins in the prevention of pancreatic cancer are impossible to provide. Similar conclusions as in the present meta-analysis can be found in the meta-analysis published in 2019, which included 26 studies [46]. Archibugi et al. [47] also showed that long-term use of statins (especially atorvastatin) might be associated with a significant reduction in the risk of developing pancreatic cancer (OR 0.70; 95% CI: 0.60–0.82; p < 0.001). Previous observations, however, indicated that the protective effect of statins on the development of pancreatic cancer is more questionable, especially at doses routinely used in the treatment of lipid disorders. The use of higher doses has not been routinely recommended due to the patients’ worries of side effects [48]. However, it is changing nowadays with the new guidelines and new treatment goals. Finally, our meta-analysis should be understood to be taking into consideration the difference between the pancreatic cancer occurrence between two subcategories: i) RCTs and; ii) Observational studies. Results obtained from observational studies are at a higher risk of bias compared to data obtained from RCTs. A sub-analysis was conducted to minimize bias in our meta-analysis. In addition, the previous meta-analyzes were created over 2 years ago, therefore, considering the significant development of medical sciences, it seems necessary to conduct a new analysis [45, 47]. Moreover, the high heterogeneity of the studies included in the analysis and the slight difference in effect additionally strengthen the need for another meta-analysis. In the correspondence accompanying this paper, it was suggested that it would be important to conduct research aimed at investigating the association between statins and other drugs used simultaneously on the prognosis in pancreatic cancer. One should also pay attention to the dose-response relationship — a research hypothesis could be made that higher doses of statins should show a greater protective effect [49, 46].
It is worth highlighting here that the systematic reviews and meta-analyses conducted to date have focused mainly on determining the impact of long-term therapy on the prognosis of patients with diagnosed pancreatic cancer. One such meta-analysis showed a significantly better prognosis in patients diagnosed with pancreatic cancer (meta-hazard ratio [HR] = 0.75; 95% CI: 0.59–0.90; p < 0.001) compared to patients not receiving such treatment [50]. As in our meta-analysis, a significant limitation is the diversity of the population included in the review. A better prognosis of pancreatic cancer patients using statins has also been shown in another meta-analysis [51]. Similar observations were identified in another meta-analysis that included 14 studies. An interesting finding is a positive effect on outcomes in patients with the resectable disease, not seen in locally advanced or metastatic disease. This observation should encourage further research focused on reducing the risk of recurrence in patients undergoing treatment with the assumption of a radical cure [52]. The previously published systematic review of 2008 should be considered obsolete given new scientific evidence that has emerged since then [53]. In turn, the review that included studies describing the survival effects of both metformin and statins in patients diagnosed with pancreatic cancer was based on only 8 statin studies — although the article was published in 2018 [54]. These relationships, however, seem to be less potent than in the case of, for example, the influence of statins on progression of liver cirrhosis [55]. Newer data published recently from Norwegian registry pointed out, that statin users had lower mortality from pancreatic cancer (HR = 0.86, 95% CI: 0.76–0.97), and this association was more pronounced in users of hydrophilic (e.g., rosuvastatin) rather than lipophilic (e.g., atorvastatin) statins [56]. In a Japanese registry of 100,537 statin users vs. 326,033 non-statin users, after adjustments using inverse probability of treatment weighting, the statin exposure group was associated with a decreased incidence of pancreatic cancer (HR = 0.84; 95% CI: 0.72–0.99) [57]. It is striking, that this 14–16% relative reduction is almost identical in those 2 papers like in the present meta-analysis.
Further research should focus on the selection of populations in which statin use will be associated with a more significant reduction in the risk of developing pancreatic cancer compared to the general population. The starting point may be, in particular, the predictors that increase the risk of pancreatic cancer, especially the modifiable ones, e.g., a prospective observational study conducted among patients with nicotinism, the main modifiable risk factor for pancreatic cancer, next to obesity. Conducting a prospective clinical trial aimed at verifying the hypothesis that long-term statin use reduces the risk of developing pancreatic cancer would undoubtedly dispel doubts about the role of statins in the prevention of pancreatic cancer. Due to the long observation period necessary to demonstrate such a relationship, conducting a prospective clinical trial is highly difficult. It may turn out to be more convenient, as mentioned above, to design a clinical trial among a specified cohort of patients with a significantly increased risk of pancreatic cancer, e.g., in the family variant of this disease or specific genetic diseases.
Conclusions
The present analysis shows that overall statins use is significantly associated with a reduction in risk of pancreatic cancer. However, these results were not confirmed for the RCT sub-group. Further prospective studies are needed to confirm current results.
Supplementary Information
Acknowledgments
The study was supported by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari D, Tingstedt B, Andersson B, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929–1946. doi: 10.2217/fon-2016-0010. [DOI] [PubMed] [Google Scholar]
- 3.Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today. 2020;50(10):1117–1125. doi: 10.1007/s00595-020-02028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69(1):7–17. doi: 10.1136/gutjnl-2019-319352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang MJ, Dai JJ, Gu DN, et al. Aspirin in pancreatic cancer: chemopreventive effects and therapeutic potentials. Biochim Biophys Acta. 2016;1866(2):163–176. doi: 10.1016/j.bbcan.2016.08.002,. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Li Y, Liu L, et al. Aspirin use and pancreatic cancer risk: A systematic review of observational studies. Medicine (Baltimore) 2019;98(51):e18033. doi: 10.1097/MD.0000000000018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan XL, Reid Lombardo KM, Bamlet WR, et al. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res (Phila) 2011;4(11):1835–1841. doi: 10.1158/1940-6207.CAPR-11-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, Ma Y, Liu W, et al. Celecoxib combined with salirasib strongly inhibits pancreatic cancer cells in 2D and 3D cultures. Int J Med Sci. 2020;17(12):1795–1802. doi: 10.7150/ijms.47546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kho PF, Fawcett J, Fritschi L, et al. Nonsteroidal anti-inflammatory drugs, statins, and pancreatic cancer risk: a population-based case-control study. Cancer Causes Control. 2016;27(12):1457–1464. doi: 10.1007/s10552-016-0824-4. [DOI] [PubMed] [Google Scholar]
- 11.Lai SW, Kuo YH, Liao KF. Statins and pancreatic cancer risk. J Gastroenterol. 2020;55(4):471–472. doi: 10.1007/s00535-020-01675-x. [DOI] [PubMed] [Google Scholar]
- 12.Carrer A, Trefely S, Zhao S, et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 2019;9(3):416–435. doi: 10.1158/2159-8290.CD-18-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Sachdev E, Robbins LA, et al. Statins and pancreatic cancer. Oncol Lett. 2017;13(3):1035–1040. doi: 10.3892/ol.2017.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HS, Lee SH, Lee HJ, et al. Statin use and its impact on survival in pancreatic cancer patients. Medicine (Baltimore) 2016;95(19):e3607. doi: 10.1097/MD.0000000000003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAc, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-ofbias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archibugi L, Piciucchi M, Stigliano S, et al. Exclusive and combined use of statins and aspirin and the risk of pancreatic cancer: a case-control study. Sci Rep. 2017;7(1):13024. doi: 10.1038/s41598-017-13430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang UC, Watanabe T, Bendtsen F. The relationship between the use of statins and mortality, severity, and pancreatic cancer in Danish patients with chronic pancreatitis. Eur J Gastroenterol Hepatol. 2018;30(3):346–351. doi: 10.1097/MEG.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 22.Bradley MC, Hughes CM, Cantwell MM, et al. Statins and pancreatic cancer risk: a nested case-control study. Cancer Causes Control. 2010;21(12):2093–2100. doi: 10.1007/s10552-010-9628-0. [DOI] [PubMed] [Google Scholar]
- 23.Carey FJ, Little MW, Pugh TFG, et al. The differential effects of statins on the risk of developing pancreatic cancer: a case-control study in two centres in the United Kingdom. Dig Dis Sci. 2013;58(11):3308–3312. doi: 10.1007/s10620-013-2778-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen MJ, Tsan YT, Liou JM, et al. Statins and the risk of pancreatic cancer in Type 2 diabetic patients: a population-based cohort study. Int J Cancer. 2016;138(3):594–603. doi: 10.1002/ijc.29813. [DOI] [PubMed] [Google Scholar]
- 25.Chiu HF, Chang CC, Ho SC, et al. Statin use and the risk of pancreatic cancer: a population-based case-control study. Pancreas. 2011;40(5):669–672. doi: 10.1097/MPA.0b013e31821fd5cd. [DOI] [PubMed] [Google Scholar]
- 26.Clearfield M, Downs JR, Weis S, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women. J Womens Health Gend Based Med. 2001;10(10):971–981. doi: 10.1089/152460901317193549. [DOI] [PubMed] [Google Scholar]
- 27.Coogan PF, Rosenberg L, Strom BL. Statin use and the risk of 10 cancers. Epidemiology. 2007;18(2):213–219. doi: 10.1097/01.ede.0000254694.03027.a1. [DOI] [PubMed] [Google Scholar]
- 28.Graaf MR, Beiderbeck AB, Egberts ACG, et al. The risk of cancer in users of statins. J Clin Oncol. 2004;22(12):2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Haukka J, Sankila R, Klaukka T, et al. Incidence of cancer and statin usage-record linkage study. Int J Cancer. 2010;126(1):279–284. doi: 10.1002/ijc.24536. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs EJ, Newton CC, Thun MJ, et al. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71(5):1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 31.Kabat GC, Kim MY, Chlebowski RT, et al. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control. 2018;29(1):13–24. doi: 10.1007/s10552-017-0991-y. [DOI] [PubMed] [Google Scholar]
- 32.Karp I, Behlouli H, Lelorier J, et al. Statins and cancer risk. Am J Med. 2008;121(4):302–309. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90(3):635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khurana V, Sheth A, Caldito G, et al. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas. 2007;34(2):260–265. doi: 10.1097/MPA.0b013e318030e963. [DOI] [PubMed] [Google Scholar]
- 35.Kirkegård J, Lund JL, Mortensen FV, et al. Statins and pancreatic cancer risk in patients with chronic pancreatitis: A Danish nationwide population-based cohort study. Int J Cancer. 2020;146(3):610–616. doi: 10.1002/ijc.32264. [DOI] [PubMed] [Google Scholar]
- 36.Leung HWC, Chan ALF, Lo D, et al. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin Drug Saf. 2013;12(1):19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 37.Marelli C, Gunnarsson C, Ross S, et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol. 2011;58(5):530–537. doi: 10.1016/j.jacc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Peto R, Emberson J, Landray M, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359(13):1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Ajiki W, Kobayashi T, et al. PCS Study Group. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol. 2006;16(5):201–206. doi: 10.2188/jea.16.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287(24):3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 41.Simon MS, Desai P, Wallace R, et al. Prospective analysis of association between statins and pancreatic cancer risk in the Women’s Health Initiative. Cancer Causes Control. 2016;27(3):415–423. doi: 10.1007/s10552-016-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strandberg T, Pyörälä K, Cook T, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) The Lancet. 2004;364(9436):771–777. doi: 10.1016/s0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 43.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. doi: 10.1186/1471-2407-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker EJ, Ko AH, Holly EA, et al. Statin use and risk of pancreatic cancer: results from a large, clinic-based case-control study. Cancer. 2015;121(8):1287–1294. doi: 10.1002/cncr.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Liang M, Sun C, et al. Statin use and risk of pancreatic cancer: an updated meta-analysis of 26 studies. Pancreas. 2019;48(2):142–150. doi: 10.1097/MPA.0000000000001226. [DOI] [PubMed] [Google Scholar]
- 46.Kawada T. Statin use and pancreatic cancer: A risk assessment. Dig Liver Dis. 2019;51(5):749. doi: 10.1016/j.dld.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Archibugi L, Arcidiacono PG, Capurso G. Statin use is associated to a reduced risk of pancreatic cancer: A meta-analysis. Dig Liver Dis. 2019;51(1):28–37. doi: 10.1016/j.dld.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Bonovas S, Filioussi K, Sitaras NM. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am J Gastroenterol. 2008;103(10):2646–2651. doi: 10.1111/j.1572-0241.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 49.Archibugi L, Arcidiacono PG, Capurso G. Statin use and pancreatic cancer: a risk assessment. Authors? reply. Dig Liver Dis. 2019;51(5):750–751. doi: 10.1016/j.dld.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Jian-Yu E, Graber J, Lu SE, et al. Effect of metformin and statin use on survival in pancreatic cancer patients: a systematic literature review and meta-analysis. Curr Med Chem. 2018;25(22):2595–2607. doi: 10.2174/0929867324666170412145232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Rodriguez EA, Barkin JS, et al. Statin use shows increased overall survival in patients diagnosed with pancreatic cancer: a meta-analysis. Pancreas. 2019;48(4):e22–e23. doi: 10.1097/MPA.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 52.Tamburrino D, Crippa S, Partelli S, et al. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A metaanalysis. Dig Liver Dis. 2020;52(4):392–399. doi: 10.1016/j.dld.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Bonovas S, Filioussi K, Sitaras N. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am J Gas-troenterol. 2008;103(10):2646–2651. doi: 10.1111/j.1572-0241.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 54.Jian-Yu E, Graber J, Lu SE, et al. Effect of metformin and statin use on survival in pancreatic cancer patients: a systematic literature review and meta-analysis. Curr Med Chem. 2018;25(22):2595–2607. doi: 10.2174/0929867324666170412145232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu Y, Yang X, Liang H, et al. Comprehensive evaluation of effects and safety of statin on the progression of liver cirrhosis: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):231. doi: 10.1186/s12876-019-1147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Støer NC, Bouche G, Pantziarka P, et al. Use of non-cancer drugs and survival among patients with pancreatic adenocarcinoma: a nationwide registry-based study in Norway. Acta Oncol. 2021;60(9):1146–1153. doi: 10.1080/0284186X.2021.1953136. [DOI] [PubMed] [Google Scholar]
- 57.Saito K, Sato Y, Nakatani E, et al. Statin exposure and pancreatic cancer incidence: a Japanese regional population-based cohort study, the Shizuoka study. Cancer Prev Res (Phila) 2021;14(9):863–872. doi: 10.1158/1940-6207.CAPR-21-0123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.