Abstract
Objective This study aimed to evaluate morphological features of the anterior clinoid process (ACP) and the optic strut (OS) in Chiari malformation Type I (CM-I).
Methods The study universe consisted of computed tomography images of 41 CM-I patients and 45 normal subjects. Comparison of the parameters for CM-I and the control group was performed with the Student's t -test. A “ p < 0.05” was accepted as the significance level.
Results ACP length was smaller in CM-I than the control group ( p < 0.001). In contrast to ACP length, ACP angle ( p < 0.001), OS length ( p = 0.022), and the distance between ACP and OS ( p = 0.020) were found greater in CM-I in comparison to the control group ( p < 0.05). ACP width ( p = 0.233) and OS width ( p = 0.376) were similar in both groups. ACP pneumatization in CM-I group was found as 12.20%, whereas in the control group as 8.90%. Two different types about the pneumatization were identified in CM-I group (Type 1: 4.9% and Type 2: 7.3%), whereas three different types in the control group (Type 1: 3.3%, Type 2: 4.4%, and Type 3: 1.1%). Relative to ACP, three different types about OS position were identified in CM-I group (Type C: 31.70%, Type D: 64.60%, and Type E: 3.70%) and the control group (Type C: 7.80%, Type D: 64.40%, and Type E: 27.80%).
Conclusion Shorter ACP, wide-angled ACP, longer OS, and more anteriorly located OS were found in CM-I group compared with the normal group. Our findings showed that the pneumatization of ACP was not affected by CM-I.
Keywords: anterior clinoidectomy, pneumatization, anterior clinoid process, optic strut, Chiari malformation Type I
Introduction
The optic canal, a part of the sphenoid bone, is a funnel-shaped structure formed by four walls, superior (the lesser wing's superior or anterior root), medial (the sphenoid bone body), inferior (the optic strut, OS: the lesser wing's posterior root), and lateral (the anterior clinoid process, ACP). 1 2 Lesions originating from these anatomical structures (e.g., optic glioma or anterior clinoidal meningioma) may result in compression of the optic nerve. 1 2 3 4 5 For decompression, transsphenoidal approaches are recommended if lesions arise from the medial or inferior wall, whereas transcranial approaches are recommended if lesions arise from the lateral or superior wall. 1 2 5 6 7 8 During implementation of these approaches for decompression, OS and/or ACP are entirely or partly removed. 1 3 4 6 9 Furthermore, these two bony structures are of great importance for neurosurgeons as reference points to ensure intraoperative orientation. 6 10 11 12 In this context, detailed knowledge about morphological features of ACP and OS is needed to surgeons for carrying out a successful operation. 1 7 13 14
Some authors stated that significant alterations in patients with Chiari malformation Type I (CM-I) should be expected not only in the posterior cranial fossa but also in the entire skull base, probably on account of a mesodermal defect. 15 16 For example, Sgouros et al 15 determined a longer anterior cranial fossa in pediatric subjects with CM-I in comparison to the control group. Nwotchouang et al 16 found 38% greater volume of the sphenoid sinus of CM-I patients compared with normal subjects. In the light of these information, we think that location, size, and shape of anatomical structures (ACP, OS, the prechiasmatic sulcus, the pterygoid canal, the optic canal, etc.) around the sellar area are significantly affected by CM-I, especially due to the increased sphenoid sinus volume. Changes in morphologies of OS and ACP may affect intraoperative surgical orientation, surgical technique choice, and positioning patient's head to prevent complications (e.g., the ophthalmic artery or optic nerve injury). 12 17 18 19 20 However, available information pool containing surgical procedure examinations, morphometric analysis, classifications, and detailed anatomical definitions is mainly conducted on normal subjects. Therefore, the main aim of this computed tomography (CT) study is to determine whether morphologies of ACP or OS are changed in patients with CM-I in comparison to healthy samples.
Materials and Methods
Study Population
In line with the criteria ( Table 1 ), patient folders (including information as follows: sex, age, radiographic images, complaints, diagnosis, treatment procedures, and hospital admission/discharge dates) were examined. The study universe consisted of two different populations as follows: (1) CM-I group and (2) control group (an age–sex-matched set).
Table 1. The inclusion and exclusion criteria for study population.
| Criteria | CM-I group | Control group |
|---|---|---|
| Inclusion criteria | Patients with CM-I | Patients without malformations (syndromic or genetic) |
| Patients without a history of surgical intervention around ACP | Patients without fractures, infections, tumors | |
| Patients with good quality CT images | Patients without a history of surgical intervention around ACP | |
| Patients without a history of medical treatment related to ACP | ||
| Patients with good quality CT images | ||
| Exclusion criteria | Patients with the other types of Chiari malformation | Patients with malformations (syndromic or genetic) |
| Patients with a history of surgical intervention around ACP | Patients with fractures, infections, tumors | |
| Patients with low-quality CT images | Patients with a history of surgical intervention around ACP | |
| Patients with a history of medical treatment related to ACP | ||
| Patients with low-quality CT images |
Abbreviations: ACP, anterior clinoid process; CM-I, Chiari malformation Type I; CT, computed tomography.
Computed Tomography Protocol
CT images of patients' heads were obtained with a 64-row multidetector scanner (Aquillion 64, matrix: 512 × 512, field of view: 240 mm, pixel size: 0.46 mm, 0.5-mm thick slices, 230 mA, 120 kV, 0.3-mm interval; Toshiba Medical Systems, Tokyo, Japan). The raw data were transferred to a workstation for creating three-dimensional images and then reformatted in axial, coronal, and sagittal planes.
Measured Parameters
The parameters determined to detect morphologies of ACP and OS were as follows: the length (ACP-L), width (ACP-W), and angle (ACP-A) of ACP, the length (OS-L) and width (OS-W) of OS, and the distance between ACP and OS (Dis-ACP-OS). The axial plane images were used to measure ACP-L, ACP-W, ACP-A, and Dis-ACP-OS, whereas the coronal plane images were utilized to measure OS-L and OS-W ( Figs 1 and 2 ). The explanations of the parameters were as follows 18 21 22 23 :
Fig. 1.

The photographs show the location of ACP and OS. ACP, anterior clinoid process; DS, dorsum sella; HF, hypophysial fossa; OS, optic strut; SS, sphenoid sinus.
Fig. 2.

The photographs show the parameters. a = ACP-A, b = ACP-L, c = ACP-W, d = Dis-ACP-OS, e = OS-L, and f = OS-W.
ACP-L: the length between the tip and base of ACP
ACP-W: the basal width of ACP at the optic canal's medial border
ACP-A: the angle between the lateral and medial borders of ACP
OS-L: the length of the inferior wall of the optic canal between ACP and the sphenoid bone body
OS-W: the width between the anterior and posterior borders of OS at the middle level
Dis-ACP-OS: the length between ACP tip and the posterior border of OS
Classification of Anterior Clinoid Process Pneumatization
Considering Abuzayed et al 23 's study, ACP pneumatization was classified as three types as follows: Type 1, less than 50% pneumatization; Type 2, more than 50% pneumatization but not totally; and Type 3, total pneumatization ( Fig. 3 ).
Fig. 3.
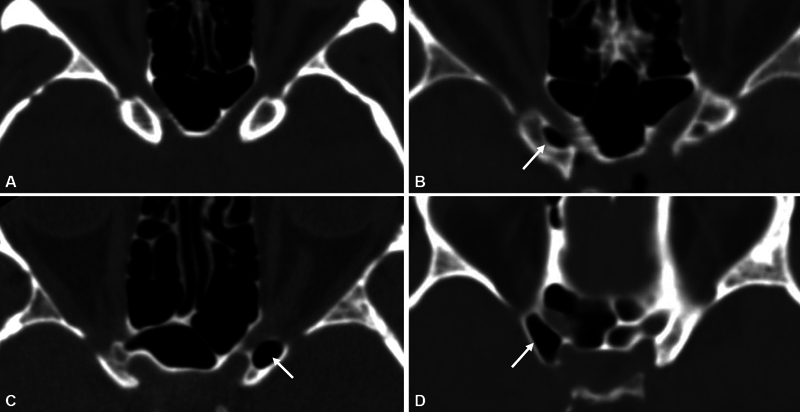
The photographs show the pneumatization degree of ACP. ( A ) No pneumatization, ( B ) Type 1, ( C ) Type 2, and ( D ) Type 3.
Classification of the Position of Optic Strut Relative to Anterior Clinoid Process
Considering Lee et al 18 's study, the location of OS according to ACP was classified as six types as follows: Type A, anterior to ACP root; Type B, anterior ⅕ of ACP; Type C, anterior ⅖ of ACP; Type D, anterior ⅗ of ACP; Type E, anterior ⅘ of ACP; and Type F, posterior ⅕ of ACP.
Statistical Analysis
All measurements were performed by two independent investigators with over 10 years of experience. The first researcher measured the parameters, and the dataset was used to perform statistical evaluations. A second researcher repeated the measurements twice in randomly selected cases (20% of all subjects) to check the reproducibility of measurements. The interobserver reproducibility was tested with the intraclass correlation coefficients (ICC), whereas the intraobserver reproducibility with the paired sample t -test. Using SPSS for the Windows version 22.0 package program (IBM, Armonk, New York, United States), the statistical analyses were performed. The normality check for the measured parameters was performed by Shapiro–Wilk test. Each ACP or OS was counted as an independent case, and comparison of the parameters for CM-I and the control group was performed with the independent sample t -test ( Table 2 ). To understand whether there is an individual difference, the averages of the right and left ACPs and OSs in each patient were taken, and the comparison of the two groups was made by the independent sample t -test ( Table 3 ). Comparisons of the measured parameters for male–female and right–left sides were performed with the independent sample t -test and the paired sample t -test, respectively ( Table 4 ). Distribution of ACP pneumatization and OS position types in CM-I and the control group was evaluated with the chi-square test ( Tables 5 and 6 ). A “ p < 0.05” was accepted as the significance level.
Table 2. Comparison of Chiari malformation Type I and the control group.
| Parameters | CM-I ( N = 82) | Control ( N = 90) | p |
|---|---|---|---|
| ACP-L | 11.12 ± 2.09 | 12.79 ± 2.43 | <0.001 |
| ACP-W | 5.55 ± 0.83 | 5.71 ± 0.93 | 0.233 |
| ACP-A | 27.23 ± 5.65 | 21.86 ± 4.71 | <0.001 |
| OS-L | 4.49 ± 0.87 | 4.23 ± 0.59 | 0.022 |
| OS-W | 3.08 ± 0.78 | 2.97 ± 0.72 | 0.376 |
| Dis-ACP-OS | 6.04 ± 0.92 | 5.60 ± 1.46 | 0.020 |
Abbreviations: ACP, anterior clinoid process; ACP-A, angle of ACP; ACP-L, length of ACP; ACP-W width of ACP; CM-I, Chiari malformation Type I; Dis-ACP-OS, distance between ACP and OS; OS, optic strut; OS-L, length of OS; OS-W, width of OS.
Note: N = numbers of sides.
Table 3. Comparison of Chiari malformation Type I and the control group according to the averages of the two anterior clinoid processes and optic strut in each subject.
| Parameters | CM-I ( N = 41) | Control ( N = 45) | p |
|---|---|---|---|
| ACP-L | 11.12 ± 2.05 | 12.79 ± 2.38 | 0.001 |
| ACP-W | 5.55 ± 0.79 | 5.71 ± 0.89 | 0.380 |
| ACP-A | 27.23 ± 5.51 | 21.86 ± 4.68 | <0.001 |
| OS-L | 4.49 ± 0.85 | 4.23 ± 0.56 | 0.095 |
| OS-W | 3.08 ± 0.72 | 2.98 ± 0.69 | 0.508 |
| Dis-ACP-OS | 6.04 ± 0.85 | 5.60 ± 1.44 | 0.091 |
Abbreviations: ACP, anterior clinoid process; ACP-A, angle of ACP; ACP-L, length of ACP; ACP-W width of ACP; CM-I, Chiari malformation Type I; Dis-ACP-OS, distance between ACP and OS; OS, optic strut; OS-L, length of OS; OS-W, width of OS.
Note: N = numbers of subjects.
Table 4. Sex and side comparisons for Chiari malformation Type I and the control group.
| Parameters | Females ( N = 50) | Males ( N = 32) | p | Right ( N = 41) | Left ( N = 41) | p | |
|---|---|---|---|---|---|---|---|
| CM-I | ACP-L | 11.32 ± 2.03 | 10.81 ± 2.18 | 0.291 | 11.18 ± 2.08 | 11.06 ± 2.13 | 0.806 |
| ACP-W | 5.31 ± 0.69 | 5.91 ± 0.92 | 0.001 | 5.71 ± 0.85 | 5.38 ± 0.79 | 0.078 | |
| ACP-A | 27.17 ± 5.97 | 27.34 ± 5.20 | 0.894 | 27.06 ± 5.59 | 27.40 ± 5.78 | 0.787 | |
| OS-L | 4.31 ± 0.90 | 4.78 ± 0.76 | 0.017 | 4.46 ± 0.79 | 4.52 ± 0.96 | 0.740 | |
| OS-W | 3.03 ± 0.80 | 3.14 ± 0.77 | 0.538 | 3.10 ± 0.77 | 3.06 ± 0.81 | 0.816 | |
| Dis-ACP-OS | 5.98 ± 0.93 | 6.13 ± 0.91 | 0.478 | 6.03 ± 0.97 | 6.05 ± 0.88 | 0.916 | |
| Parameters | Females ( N = 52) | Males ( N = 38) | p | Right ( N = 45) | Left ( N = 45) | p | |
| Control | ACP-L | 12.86 ± 2.53 | 12.70 ± 2.31 | 0.768 | 12.76 ± 2.41 | 12.83 ± 2.47 | 0.888 |
| ACP-W | 5.66 ± 1.05 | 5.77 ± 0.73 | 0.582 | 5.80 ± 0.94 | 5.62 ± 0.91 | 0.348 | |
| ACP-A | 22.35 ± 5.61 | 21.19 ± 3.02 | 0.250 | 21.77 ± 4.74 | 21.95 ± 4.73 | 0.852 | |
| OS-L | 4.12 ± 0.63 | 4.38 ± 0.50 | 0.039 | 4.23 ± 0.58 | 4.23 ± 0.60 | 0.893 | |
| OS-W | 3.05 ± 0.73 | 2.88 ± 0.69 | 0.274 | 2.98 ± 0.75 | 2.97 ± 0.69 | 0.953 | |
| Dis-ACP-OS | 5.31 ± 1.44 | 5.99 ± 1.42 | 0.030 | 5.51 ± 1.41 | 5.69 ± 1.52 | 0.554 |
Abbreviations: ACP, anterior clinoid process; ACP-A, angle of ACP; ACP-L, length of ACP; ACP-W width of ACP; CM-I, Chiari malformation Type I; Dis-ACP-OS, distance between ACP and OS; OS, optic strut; OS-L, length of OS; OS-W, width of OS.
Note: N = numbers of sides.
Table 5. Distribution of anterior clinoid process pneumatization in Chiari malformation Type I and the control group.
| ACP pneumatization | CM-I | Control | Total | p |
|---|---|---|---|---|
| ACP pneumatization absence | 72 (87.80%) | 82 (91.10%) | 154 | 0.619 |
| ACP pneumatization presence | 10 (12.20%) | 8 (8.90%) | 18 | |
| Total | 82 | 90 | 172 |
Abbreviations: ACP, anterior clinoid process; CM-I, Chiari malformation Type I.
Table 6. Distribution of optic strut location types in Chiari malformation Type I and the control group.
| Types | CM-I | Control | Total | p |
|---|---|---|---|---|
| Type C | 26 (31.70%) | 7 (7.80%) | 33 | <0.001 |
| Type D | 53 (64.60%) | 58 (64.40%) | 111 | |
| Type E | 3 (3.70%) | 25 (27.80%) | 28 | |
| Total | 82 | 90 | 172 |
Abbreviation: CM-I, Chiari malformation Type I.
Results
CM-I group consisted of CT images of 41 patients (average age: 20.24 ± 15.14 y; sex: 25 females and 16 males) admitted to the university hospital between 2010 and 2022. In addition, CT images of 45 normal subjects (average age: 21.16 ± 18.68 y; sex: 26 females and 19 males) admitted to the university hospital with different grievances (blow to head, falling from high, etc.) in 2021 were included to the investigation as the control group. Our findings were as follows:
The intraobserver ( p > 0.05) and interobserver (ICC score = 0.940 − 0.990 for the parameters, p < 0.001) analyzes displayed that the reproducibility was excellent.
Compared with CM-I, ACP-L was greater in the control group ( p < 0.001), but ACP-A ( p < 0.001), OS-L ( p = 0.022), and Dis-ACP-OS ( p = 0.020) were smaller. ACP-W ( p = 0.233) and OS-W ( p = 0.376) were similar in both groups ( Table 2 ).
Considering the averages of the two ACPs and OS in each subject, compared with CM-I, ACP-L was greater in the control group ( p = 0.001), but ACP-A was smaller ( p < 0.001). The other parameters were similar in both groups ( p > 0.05; Table 3 ).
In CM-I group, ACP-W ( p = 0.011) and OS-L ( p = 0.017) were smaller in females than males, but in the control group, OS-L ( p = 0.039) and Dis-ACP-OS ( p = 0.030) were smaller in females than males ( Table 4 ). All parameters were similar statistically on both sides for CM-I and the control group ( p > 0.05; Table 4 ).
In CM-I group, ACP pneumatization was identified in 10 (12.20%) sides. The pneumatization in the control group was determined in eight (8.90%) sides. Distribution rate of ACP pneumatization relative to both groups was presented in Table 5 , which displayed that the pneumatization was not affected by CM-I ( p = 0.619).
Two different types about the pneumatization of ACP were identified in CM-I group (Type 1: 4 sides, 4.9% and Type 2: 6 sides, 7.3%), whereas three different types in the control group (Type 1: 3 sides, 3.3%; Type 2: 4 sides, 4.4%; and Type 3: 1 side, 1.1%).
Three different types about OS position relative to ACP were identified in CM-I group (Type C: 26 sides, 31.70%; Type D: 53 sides, 64.60%; and Type E: three sides, 3.70%), similar to the control group (Type C: 7 sides, 7.80%; Type D: 58 sides, 64.40%; and Type E: 25 sides, 27.80%). Distribution rate of OS position types relative to both groups was presented in Table 6 , which displayed that the location of OS was affected by CM-I ( p < 0.001).
Discussion
The prevalence of CM-I, the downward herniation of the cerebellar tonsil through the foramen magnum, is presented as 0.24 to 3.6% in the literature. 24 Mutations in some genes such as COL4A1, SLC4A9, and OLFML2A, or chromosomal regions such as 11p15.4 may be associated with the pathogenesis of CM-I. 25 Deviations in the development of occipital somite arising from the paraxial mesoderm are considered the main cause of CM-I. 26 The most affected area in CM-I is the bony components of the posterior fossa, of which volume is approximately 23% smaller in comparison to normal subjects. 26 27 Overcrowding of the hindbrain caused by shrinking volume results in a wide variety of signs (gait instability, pain, hoarseness, hearing loss, vertigo, etc.) in CM-I. 26 27 28 29 30 31 However, Sgouros et al 15 found longer anterior fossa in children with CM-I compared with the control group and claimed that a mesodermal defect in such cases results in significant alterations not only in the posterior fossa but also in the entire skull base. For instance, Nwotchouang et al 16 observed 38% increased sphenoid sinus volume in CM-I (9.3 ± 3.0 cm 3 ) compared with the control group (6.7 ± 1.9 cm 3 , p < 0.001). On the other hand, after finding enlarged pituitary gland in magnetic resonance imaging scans of some adult patients with Chiari malformation Type II (CM-II), Patel et al 32 studied systematically on the sella morphology in 21 patients for the first time in the literature. They observed longer tuberculum sella, taller pituitary gland without any pathology, shallow sella, and shorter dorsum sella in CM-II compared with the normal group; thus, they reported that normal pituitary gland, which appears to be slightly taller owing to the shallow sella, may be mistakenly interpreted as pituitary enlargement (e.g., pituitary adenoma) in CM-II patients. 32 Considering the observations in these studies, we think that dimensions and positions of all structures (including ACP and OS) around the sellar region in CM-I or CM-II patients show great differences relative to healthy subjects.
A thorough understanding of the sellar region's complex anatomy is considered as one of the substantial ways to refrain technical challenge during cure of pathological entities (optic glioma, meningiomas, aneurysms, etc.). 1 5 8 33 Owing to proximity of ACP and OS with neurovascular structures (e.g., the ophthalmic artery, oculomotor nerve, trochlear nerve, internal carotid artery, and optic nerve), the risk of iatrogenic injuries during operation (e.g., anterior clinoidectomy) should be considered by neurosurgeons. 6 9 11 17 23 33 34 35 Morphologies of OS and ACP may affect intraoperative surgical orientation, surgical technique choice, and positioning patient's head. 12 17 18 19 20 21 23 36 37 For example, Cecen et al 21 divided ACP into different types considering ACP-L, ACP-W, and ACP-A. They suggested that the wide, short, and wide-angled configuration (16.5%, 40 cases) may call for an easier surgery, whereas the narrow, long, and narrow-angled configuration (17.8%, 43 cases) for a more complex surgical processing. 21 Hereby, Cecen et al 21 proposed preoperative radiologic analysis to identify different configurations to prevent iatrogenic injuries. Furthermore, ACP pneumatization degree is of major significance for neurosurgeons in preventing complications (e.g., opening of sinuses). 23 38 To reduce mortality–morbidity rates, some authors suggested that a detailed morphological dataset (containing surgical procedure examinations, morphometric analysis, classifications, and detailed anatomical definitions) about ACP and OS may be helpful for surgeons. 1 7 10 21 23 39 Nevertheless, the current literature is mainly focused on subjects without any malformations. In this context, our data conducted on CM-I patients may be useful for neurosurgeons to obtain a successful and efficient outcome.
ACP-W was measured as 5.55 ± 0.83 mm for CM-I and 5.71 ± 0.93 for the control group ( p = 0.233). OS-W was measured as 3.08 ± 0.78 mm for CM-I and 2.97 ± 0.72 for the control group ( p = 0.376). Our ACP-W and OS-W values for both groups were compatible with previous studies ( Table 7 ), 10 12 18 19 21 22 39 40 41 42 43 44 45 46 47 48 49 in where the mean data range was given as 5.46 to 12.40 mm for ACP-W and as 2.40 to 3.88 mm for OS-W. Our data displayed that these two parameters were not affected by CM-I. However, ACP-L was smaller in CM-I (11.12 ± 2.09 mm) than the control group (12.79 ± 2.43 mm; p < 0.001). Our average values for both groups were compatible with previous studies ( Table 7 ), 10 12 18 19 21 22 39 40 41 42 44 45 46 47 48 49 50 in where the mean data range was presented between 5.30 and 12.50 mm. In contrast to ACP-L value, ACP-A, OS-L, and Dis-ACP-OS were found greater in CM-I than the control group. Our ACP-A values for CM-I (27.23 ± 5.65 degrees) and the control group (21.86 ± 4.71 degrees) were distinctly smaller in comparison to literature data ( Table 7 ), 21 49 in where the mean data range was presented between 39.67 and 42.56 degrees. The main reason for this situation may be the use of different reference points during ACP-A measurement. OS-L was measured as 4.49 ± 0.87 mm for CM-I and 4.23 ± 0.59 for the control group ( p = 0.022). Dis-ACP-OS was measured as 6.04 ± 0.92 mm for CM-I and 5.60 ± 1.46 for the control group ( p = 0.020). Our values of these parameters for both groups were compatible with previous studies ( Table 7 ), 10 18 41 43 48 49 50 in where the mean data range was given as 3.94 to 6 mm for OS-L and as 5.80 to 13.84 mm for Dis-ACP-OS. On the other hand, the wide range between average values given in the previous articles may be owing to differences in study populations (e.g., age, sex, region, and side) or methods (CT, cone-beam computed tomography, digital caliper, etc.). 10 20 21 40 46 49 51 For instance, Adanir et al 49 stated that ACP-L and ACP-W increased and ACP-A narrowed in the transition from fetal life to adulthood, considering Beger et al 20 's fetal study (ACP-L: 3.67 ± 0.82 mm, ACP-W: 4.65 ± 0.94 mm, and ACP-A: 59.57 ± 8.03 degrees). Cecen et al 21 observed statistically greater ACP-L and ACP-W values on patients (CT values) in comparison to dry skulls (measurement with digital caliper). We did not find differences between the measurements in terms of side, but Lee et al 18 observed greater ACP-L and ACP-W values on the right side compared with the left side. In this study, ACP-W in CM-I group, Dis-ACP-OS in the control group, and OS-L in both groups were smaller in females than males. Adanir et al 49 observed smaller Dis-ACP-OS, ACP-L, ACP-W, and ACP-A values in females than males. Moreover, Sabanciogullari et al 46 showed smaller ACP-L and ACP-W in females compared with males.
Table 7. The literature data related to the measured parameters.
| Study | Region | Specimen | N | Age | Technique | ACP-L | ACP-W | ACP-A | OS-L | OS-W | Dis-ACP-OS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andaluz et al 40 | United States | Cadaver | 20 | Adult | Anatomic | 8.67 ± 2.63 | 6.57 ± 1.68 | – | – | – | – |
| Boyan et al 10 | Türkiye | Dry skull | 68 | Unknown | Anatomic | 11.50 ± 1.90 | 12.40 ± 2.10 | – | – | – | 6.90 ± 1.60 |
| Cecen et al 21 | Türkiye | Dry skull | – | Adult | Anatomic | 12.50 ± 3.71 | 10.93 ± 2.25 | 42.09 ± 9.20 | – | – | – |
| Patient | 242 | 18–84 y | CT scan | 10.74 ± 3.35 | 8.05 ± 1.74 | 39.67 ± 12.64 | – | – | – | ||
| Cheng et al 41 | China | Patient (R) | 102 | 19–70 y | CT scan | 10.76 ± 1.87 | – | – | 5.02 ± 0.98 | 3.67 ± 0.55 | – |
| Patient (L) | 102 | 10.43 ± 1.99 | – | – | 4.89 ± 0.76 | 3.88 ± 0.61 | – | ||||
| Dagtekin et al 39 | Türkiye | Dry skull (R) | 25 | Unknown | Anatomic | 9.7 (6.1–12.8) | 7.3 (5.6–11.3) | – | – | – | – |
| Dry skull (L) | 25 | 9.6 (6.3–11.8) | 7.2 (5.3–10.5) | – | – | – | – | ||||
| Gupta and Priya 50 | India | Dry skull (R) | 30 | Adult | Anatomic | 11.1 ± 1.49 | – | – | 3.94 ± 1 | – | – |
| Dry skull (L) | 30 | 11.61 ± 2.07 | – | – | 3.97 ± 1.3 | – | – | ||||
| Hayashi et al 43 | Japan | Cadaver | 66 | Adult | Anatomic | – | – | – | 5.4 (2.8–9.5) | 2.4 (0.3–4.2) | – |
| Hunnargi et al 44 | India | Dry skull (R) | 25 | Adult | Anatomic | 10.68 ± 1.90 | 12.4 ± 2.58 | – | – | – | – |
| Dry skull (L) | 25 | 9.96 ± 1.71 | 11.12 ± 1.81 | – | – | – | – | ||||
| Huynh-Le et al 22 | Japan | Cadaver | 55 | 18–91 y | Anatomic | 5.3 (4–6.8) | 5.6 (4–7.8) | – | 6 (2.8–8.9) | 3.1 (2–4.7) | 5.8 (2.3–8) |
| Kapur and Mehic 12 | Bosnia | Dry skull (MR) | 109 | 23–91 y | Anatomic | 9.9 ± 1.6 | 9.4 ± 1.4 | – | – | – | – |
| Dry skull (ML) | 109 | 9.3 ± 1.4 | 9.1 ± 1.7 | – | – | – | – | ||||
| Dry skull (FR) | 91 | 19–84 y | Anatomic | 9.3 ± 1.6 | 8.7 ± 1.5 | – | – | – | – | ||
| Dry skull (FL) | 91 | 8.9 ± 2 | 8.3 ± 2.1 | – | – | – | – | ||||
| Lee et al 18 | Korea | Dry skull | 120 | Adult | Anatomic | 9.18 ± 1.55 | 9.63 ± 1.49 | – | – | – | 1.3–9.3 |
| Lee et al 45 | Korea | Cadaver | 20 | Adult | Anatomic | 7.61 ± 1.50 | 10.82 ± 1.82 | – | – | – | – |
| Sabanciogullari et al 46 | Türkiye | Patient (MR) | 439 | 0–90 y | CT scan | 8.87 ± 2.62 | 11.08 ± 2.39 | – | – | – | – |
| Patient (ML) | 439 | 8.93 ± 2.64 | 10.98 ± 2.35 | – | – | – | – | ||||
| Patient (FR) | 592 | 0–90 y | CT scan | 8.32 ± 2.40 | 10.80 ± 2.27 | – | – | – | – | ||
| Patient (FL) | 592 | 8.34 ± 2.35 | 10.53 ± 2.07 | – | – | – | – | ||||
| Sibuor et al 47 | Kenya | Dry skull | 102 | Unknown | Anatomic | 10.92 ± 2.79 | 10.43 ± 2.67 | – | – | – | – |
| da Costa et al 42 | Brazil | Patient | 597 | 0–90 y | CT scan | 10.31 ± 2.1 | 7.7 ± 1.73 | – | – | – | – |
| Souza et al 48 | India | Dry skull (MR) | 13 | Unknown | Anatomic | 12.07 ± 1.77 | 10.30 ± 1.93 | – | – | – | 13.46 ± 1.21 |
| Dry skull (ML) | 13 | 12.46 ± 1.39 | 11.23 ± 1.57 | – | – | – | 13.84 ± 1.29 | ||||
| Dry skull (FR) | 14 | Unknown | Anatomic | 12.21 ± 1.69 | 10.64 ± 1.79 | – | – | – | 13.28 ± 0.95 | ||
| Dry skull (FL) | 14 | 12.28 ± 1.62 | 11.35 ± 1.34 | – | – | – | 12.85 ± 1.30 | ||||
| Suprasanna et al 19 | India | Patient | 95 | 18–84 y | CT scan | 11.20 ± 0.95 | 10.65 ± 0.79 | – | – | – | – |
| Beger et al 20 | Türkiye | Fetus | 48 | 16–28 gw | Anatomic | 3.67 ± 0.82 | 4.65 ± 0.94 | 59.57 ± 8.03 | 1.73 ± 0.48 | 1.55 ± 0.33 | 1.22 ± 0.60 |
| Adanir et al 49 | Türkiye | Adult subject | 800 | 36.49 y | CBCT | 10.56 ± 2.42 | 5.46 ± 1.31 | 42.56 ± 14.68 | – | – | 6.60 ± 1.50 |
| This study | Türkiye | CM-I | 82 | 20.24 y | CT | 11.12 ± 2.09 | 5.55 ± 0.83 | 27.23 ± 5.65 | 4.49 ± 0.87 | 3.08 ± 0.78 | 6.04 ± 0.92 |
| Control | 90 | 21.16 y | CT | 12.79 ± 2.43 | 5.71 ± 0.93 | 21.86 ± 4.71 | 4.23 ± 0.59 | 2.97 ± 0.72 | 5.60 ± 1.46 |
Abbreviations: ACP, anterior clinoid process; ACP-A, angle of ACP; ACP-L, length of ACP; ACP-W width of ACP; CBCT, cone-beam computed tomography; CM-I, Chiari malformation Type I; CT, computed tomography; Dis-ACP-OS, distance between ACP and OS; FL, female left; FR, female right; gw, gestational weeks; L, left; ML, male left; MR, male right; OS, optic strut; OS-L, length of OS; OS-W, width of OS; R, right.
Note: N = numbers of sides.
In CM-I group, three different types related to OS position relative to ACP were identified from the largest to the smallest as follows: Type D (64.60%) > Type C (31.70%) > Type E (3.70%). Similarly, three different types were identified in the control group as follows: Type D (64.40%) > Type E (27.80%) > Type C (7.80%). These findings proved that the location of OS relative to ACP was affected by CM-I ( p < 0.001). The attachment site of OS to ACP in CM-I is located more anteriorly in comparison to the control group. This is due to that smaller ACP-L (11.12 ± 2.09 mm for CM-I, 12.79 ± 2.43 mm for the control) and greater Dis-ACP-OS (6.04 ± 0.92 mm for CM-I and 5.60 ± 1.46 for the control) were observed in CM-I compared with the normal group. In this study, Type A, Type B, and Type F were not found in both groups. The incidence rates in both groups were incompatible with the previous adult data ( Table 8 ), 12 18 19 49 in where the incidence rates were given as 0 to 4.3% for Type A, as 3.16 to 14.5% for Type B, as 42 to 48.95% for Type C, as 27.5 to 37.7% for Type D, as 4.12 to 8.42% for Type E, and as 0 to 2.9% for Type F. Moreover, Beger et al 20 identified three different types in fetuses as follows: Type E (68.75%) > Type D (16.67%) > Type F (14.58%). Type C is the most common attachment area of OS to ACP in adult subjects, whereas Type E in fetal samples. Therefore, OS locates more posteriorly in fetuses compared with adult subjects. 20 Considering that the attachment site of OS to ACP may affect intraoperative surgical orientation, surgical technique choice, and positioning patient's head to prevent complications, 12 17 18 19 20 we think that neurosurgeons should not ignore the more anterior localization of OS in CM-I patients.
Table 8. The literature data related to the location of optic strut according to anterior clinoid process.
| Study | Kapur and Mehic 12 | Suprasanna et al 19 | Lee et al 18 | Beger et al 20 | Adanir et al 49 | This study | |||
|---|---|---|---|---|---|---|---|---|---|
| Region | Bosnia and Herzegovina | India | Korea | Türkiye | Türkiye | Türkiye | |||
| Specimen | Dry skulls | Patients | Dry skulls | Fetuses | Healthy subjects | CM-I | Control | ||
| Age | 19–91 y | 40.73 ± 15.94 y | Adult | 21.54 ± 3.11 wk | 36.49 ± 15.91 y | 20.24 ± 15.14 y | 21.16 ± 18.68 y | ||
| Sex | 109 M, 91 F | 49 M, 46 F | Unknown | 14 M, 10 F | 200 M, 200 F | 16 M, 25 F | 19 M, 26 F | ||
| Number of sides | (Right) 200 | (Left) 200 | 190 | (Right) 69 | (Left) 69 | 48 | 800 | 82 | 90 |
| Techniques | Anatomic | CT scans | Anatomic | Anatomic | CBCT | CT | CT | ||
| Type A | 1.4% | 4.3% | 0 | 1.4% | 4.3% | 0 | 3.64% | 0 | 0 |
| Type B | 11.6% | 14.5% | 3.16% | 11.6% | 14.5% | 0 | 13.12% | 0 | 0 |
| Type C | 42% | 47.8% | 48.95% | 42% | 47.8% | 0 | 43.00% | 31.70% | 7.80% |
| Type D | 37.7% | 27.5% | 37.37% | 37.7% | 27.5% | 16.67% | 36.12% | 64.60% | 64.40% |
| Type E | 4.4% | 5% | 8.42% | 4.3% | 5.9% | 68.75% | 4.12% | 3.70% | 27.80% |
| Type F | 2.9% | 0.9% | 2.11% | 2.9% | 0 | 14.58% | 0 | 0 | 0 |
Abbreviations: CBCT, cone-beam computed tomography; CT, computed tomography; F, female; M, male.
ACP pneumatization in CM-I group was found as 12.20%, whereas in the control group as 8.90%. Distribution rate of ACP pneumatization relative to both groups displayed that the pneumatization was not affected by CM-I ( p = 0.619). The incidence rates in both groups were compatible with the literature data, 23 37 46 49 52 53 54 55 56 57 58 59 in where the pneumatization rate of ACP was presented as 4 to 29.3%. According to Abuzayed et al 23 's classification, two different types about the pneumatization were identified in CM-I group as follows: Type 2 (7.3%) > Type 1 (4.9%), and three different types were identified in the control group as follows: Type 2 (4.4%) > Type 1 (3.3%) > Type 3 (1.1%). The incidence ranges of Abuzayed et al 23 's types in this study were compatible with the literature ( Table 9 ), 23 41 49 60 in where the incidence ranges were given as 6.6 to 14% for Type 1, 3.4 to 12% for Type 2, and 2 to 5.23% for Type 3.
Table 9. Literature data related to anterior clinoid process pneumatization types reported by Abuzayed et al 23 .
| Study | Region | Sample | N | Age | Method | Side | NP | Type 1 | Type 2 | Type 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cheng et al 41 | China | Adult subjects | 204 | 46.34 (19–70) y | CTA | – | 87.7% | 6.9% | 3.4% | 2% |
| Abuzayed et al 23 | Türkiye | Adult subjects | 1296 | 41 ± 19.3 y | MDCT | – | 87.4% | 6.6% | 3.5% | 2.5% |
| Cetinkaya et al 60 | Türkiye | Patients | 200 | 13–78 y | MDCT | Right | 73% | 12.5% | 12% | 2.5% |
| 200 | Left | 70.5% | 14% | 11.5% | 4% | |||||
| Adanir et al 49 | Türkiye | Adult subjects | 800 | 36.49 ± 15.91 y | CBCT | – | 78.87% | 8.87% | 7% | 5.26% |
| This study | Türkiye | CM-I | 82 | 20.24 ± 15.14 y | CT | – | 87.8% | 4.9% | 7.3% | 0 |
| Control | 90 | 21.16 ± 18.68 y | CT | – | 91.2% | 3.3% | 4.4% | 1.1% |
Abbreviations: CBCT, cone-beam computed tomography; CM-I, Chiari malformation Type I; CTA, computed tomography angiography; MDCT, multidetector-row computed tomography; NP, no pneumatization.
Note: N = numbers of sides.
Conclusion
We observed shorter ACP, wide-angled ACP, and longer OS in CM-I patients. The attachment site of OS to ACP in CM-I was located more anteriorly in comparison to the control group; thus, the location of OS was affected by CM-I. However, the distribution rate of ACP pneumatization relative to both groups showed that the pneumatization was not affected by CM-I. In our opinion, the size and shape of ACP and OS, and positional relationship between these bony structures should be evaluated during preoperative radiologic examinations to safely remove ACP and/or OS.
Conflict of Interest None declared.
Authors' Contributions
Conceptualization: H.Ö., O.Ö., E.A., S.Ö.; methodology: H.Ö., S.Ö., O.Ö., E.A.; data collection: O.Ö., B.C.A.; analysis and interpretation of results: H.U., Aİ, O.B.; writing original draft: H.Ö., O.B., H.U., Aİ; critical revision of the manuscript: H.Ö., O.Ö., O.B.; approval of the final version of the manuscript: all authors.
Ethical Approval
The Clinical Research Ethics Committee of Ankara University approved this retrospective CT work ethically (confirmation date September 22, 2022, no. 108-521-22).
Informed Consent
Approval from the Institutional Review Board was obtained and in keeping with the policies for a retrospective review; informed consent was not required.
References
- 1.Güler T M, Yılmazlar S, Özgün G. Anatomical aspects of optic nerve decompression in transcranial and transsphenoidal approach. J Craniomaxillofac Surg. 2019;47(04):561–569. doi: 10.1016/j.jcms.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Onofrey C B, Tse D T, Johnson T E et al. Optic canal decompression: a cadaveric study of the effects of surgery. Ophthalmic Plast Reconstr Surg. 2007;23(04):261–266. doi: 10.1097/IOP.0b013e3180cac220. [DOI] [PubMed] [Google Scholar]
- 3.Maurer J, Hinni M, Mann W, Pfeiffer N. Optic nerve decompression in trauma and tumor patients. Eur Arch Otorhinolaryngol. 1999;256(07):341–345. doi: 10.1007/s004050050160. [DOI] [PubMed] [Google Scholar]
- 4.Taha A N, Erkmen K, Dunn I F, Pravdenkova S, Al-Mefty O. Meningiomas involving the optic canal: pattern of involvement and implications for surgical technique. Neurosurg Focus. 2011;30(05):E12. doi: 10.3171/2011.2.FOCUS1118. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli M, Caroli M, Pluderi M et al. Endoscopic transsphenoidal optic nerve decompression: an anatomical study. Surg Radiol Anat. 2011;33(03):257–262. doi: 10.1007/s00276-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 6.Beer-Furlan A, Evins A I, Rigante L et al. Endoscopic extradural anterior clinoidectomy and optic nerve decompression through a pterional port. J Clin Neurosci. 2014;21(05):836–840. doi: 10.1016/j.jocn.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Yilmazlar S, Saraydaroglu O, Korfali E. Anatomical aspects in the transsphenoidal-transethmoidal approach to the optic canal: an anatomic-cadaveric study. J Craniomaxillofac Surg. 2012;40(07):e198–e205. doi: 10.1016/j.jcms.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Locatelli M, Di Cristofori A, Draghi R et al. Is complex sphenoidal sinus anatomy a contraindication to a transsphenoidal approach for resection of sellar lesions? Case series and review of the literature. World Neurosurg. 2017;100:173–179. doi: 10.1016/j.wneu.2016.12.123. [DOI] [PubMed] [Google Scholar]
- 9.Puzzilli F, Ruggeri A, Mastronardi L, Agrillo A, Ferrante L. Anterior clinoidal meningiomas: report of a series of 33 patients operated on through the pterional approach. Neuro-oncol. 1999;1(03):188–195. doi: 10.1093/neuonc/1.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyan N, Ozsahin E, Kizilkanat E, Tekdemir I, Soames R, Oğuz O. Surgical importance of the morphometry of the anterior clinoid process, optic strut, caroticoclinoid foramen, and interclinoid osseous bridge. Neurosurg Q. 2011;21:133–136. [Google Scholar]
- 11.Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58(06):824–831. doi: 10.3171/jns.1983.58.6.0824. [DOI] [PubMed] [Google Scholar]
- 12.Kapur E, Mehić A. Anatomical variations and morphometric study of the optic strut and the anterior clinoid process. Bosn J Basic Med Sci. 2012;12(02):88–93. doi: 10.17305/bjbms.2012.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beger O, Taghipour P, Çakır S et al. Fetal anatomy of the optic strut and prechiasmatic sulcus with a clinical perspective. World Neurosurg. 2020;136:e625–e634. doi: 10.1016/j.wneu.2020.01.125. [DOI] [PubMed] [Google Scholar]
- 14.Beger O, Ten B, Balcı Y et al. A computed tomography study of the prechiasmatic sulcus anatomy in children. World Neurosurg. 2020;141:e118–e132. doi: 10.1016/j.wneu.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Sgouros S, Kountouri M, Natarajan K.Skull base growth in children with Chiari malformation Type I J Neurosurg 2007107(3, Suppl):188–192. [DOI] [PubMed] [Google Scholar]
- 16.Nwotchouang B ST, Eppelheimer M S, Bishop P et al. Three-dimensional CT morphometric image analysis of the clivus and sphenoid sinus in Chiari malformation type I. Ann Biomed Eng. 2019;47(11):2284–2295. doi: 10.1007/s10439-019-02301-5. [DOI] [PubMed] [Google Scholar]
- 17.Kerr R G, Tobler W D, Leach J L et al. Anatomic variation of the optic strut: classification schema, radiologic evaluation, and surgical relevance. J Neurol Surg B Skull Base. 2012;73(06):424–429. doi: 10.1055/s-0032-1329626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H Y, Chung I H, Choi B Y, Lee K S. Anterior clinoid process and optic strut in Koreans. Yonsei Med J. 1997;38(03):151–154. doi: 10.3349/ymj.1997.38.3.151. [DOI] [PubMed] [Google Scholar]
- 19.Suprasanna K, Ravikiran S R, Kumar A, Chavadi C, Pulastya S. Optic strut and para-clinoid region—assessment by multidetector computed tomography with multiplanar and 3 dimensional reconstructions. J Clin Diagn Res. 2015;9(10):TC06–TC09. doi: 10.7860/JCDR/2015/15698.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beger O, Hamzaoğlu V, Özalp H et al. Anatomy of the anterior clinoid process in human fetuses. J Craniofac Surg. 2020;31(05):1469–1473. doi: 10.1097/SCS.0000000000006325. [DOI] [PubMed] [Google Scholar]
- 21.Cecen A, Celikoglu E, Is M, Kale A C, Eroğlu B T. Pre-operative measurement of the morphometry and angles of the anterior clinoid process (ACP) for aneurysm surgery. Int J Morphol. 2016;34:1333–1338. [Google Scholar]
- 22.Huynh-Le P, Natori Y, Sasaki T. Surgical anatomy of the anterior clinoid process. J Clin Neurosci. 2004;11(03):283–287. doi: 10.1016/j.jocn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Abuzayed B, Tanriover N, Biceroglu Het al. Pneumatization degree of the anterior clinoid process: a new classification Neurosurg Rev 20103303367–373., discussion 374 [DOI] [PubMed] [Google Scholar]
- 24.Kahn E N, Muraszko K M, Maher C O. Prevalence of Chiari I malformation and syringomyelia. Neurosurg Clin N Am. 2015;26(04):501–507. doi: 10.1016/j.nec.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 25.AvŞar T, ÇaliŞ Ş, Yilmaz B, Demİrcİ OtluoĞlu G, Holyavkİn C, KiliÇ T. Genome-wide identification of Chiari malformation type I associated candidate genes and chromosomal variations. Turk J Biol. 2020;44(06):449–456. doi: 10.3906/biy-2009-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aydin S, Hanimoglu H, Tanriverdi T, Yentur E, Kaynar M Y.Chiari type I malformations in adults: a morphometric analysis of the posterior cranial fossa Surg Neurol 20056403237–241., discussion 241 [DOI] [PubMed] [Google Scholar]
- 27.Schady W, Metcalfe R A, Butler P.The incidence of craniocervical bony anomalies in the adult Chiari malformation J Neurol Sci 198782(1-3):193–203. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86(01):40–47. doi: 10.3171/jns.1997.86.1.0040. [DOI] [PubMed] [Google Scholar]
- 29.Trigylidas T, Baronia B, Vassilyadi M, Ventureyra E C. Posterior fossa dimension and volume estimates in pediatric patients with Chiari I malformations. Childs Nerv Syst. 2008;24(03):329–336. doi: 10.1007/s00381-007-0432-4. [DOI] [PubMed] [Google Scholar]
- 30.Tubbs R S, McGirt M J, Oakes W J. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg. 2003;99(02):291–296. doi: 10.3171/jns.2003.99.2.0291. [DOI] [PubMed] [Google Scholar]
- 31.Sperling N M, Franco R A, Jr, Milhorat T H. Otologic manifestations of Chiari I malformation. Otol Neurotol. 2001;22(05):678–681. doi: 10.1097/00129492-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Patel D, Saindane A, Oyesiku N, Hu R. Variant sella morphology and pituitary gland height in adult patients with Chiari II malformation: potential pitfall in MRI evaluation. Clin Imaging. 2020;64:24–28. doi: 10.1016/j.clinimag.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Guthikonda B, Tobler W D, Jr, Froelich S C et al. Anatomic study of the prechiasmatic sulcus and its surgical implications. Clin Anat. 2010;23(06):622–628. doi: 10.1002/ca.21002. [DOI] [PubMed] [Google Scholar]
- 34.Abozed M, Alsulaiti G, Almannaei F, Raza A, El Beltagi A, Ayyad A. Anterior clinoid mucocele causing optic neuropathy: a case report and review of literature. eNeurologicalSci. 2017;7:57–59. doi: 10.1016/j.ensci.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beretta F, Sepahi A N, Zuccarello M, Tomsick T A, Keller J T.Radiographic imaging of the distal dural ring for determining the intradural or extradural location of aneurysms Skull Base 20051504253–261., discussion 261–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kier E L, Rothman S L.Radiologically significant anatomic variation of the developing sphenoid in humans. In: Bosma JF, ed. Symposium on the Development of the Basicranium. Bethesda: U.S. Department of Health Education, and Welfare, Public Health Service, National Institutes of Health,1976107–140.
- 37.Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K. Anatomical variations in pneumatization of the anterior clinoid process. J Neurosurg. 2007;106(01):170–174. doi: 10.3171/jns.2007.106.1.170. [DOI] [PubMed] [Google Scholar]
- 38.Nandapalan V, Watson I D, Swift A C. Beta-2-transferrin and cerebrospinal fluid rhinorrhoea. Clin Otolaryngol Allied Sci. 1996;21(03):259–264. doi: 10.1111/j.1365-2273.1996.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 39.Dagtekin A, Avci E, Uzmansel D et al. Microsurgical anatomy and variations of the anterior clinoid process. Turk Neurosurg. 2014;24(04):484–493. doi: 10.5137/1019-5149.JTN.8738-13.1. [DOI] [PubMed] [Google Scholar]
- 40.Andaluz N, Beretta F, Bernucci C, Keller J T, Zuccarello M.Evidence for the improved exposure of the ophthalmic segment of the internal carotid artery after anterior clinoidectomy: morphometric analysis Acta Neurochir (Wien) 200614809971–975., discussion 975–976 [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y, Wang C, Yang F, Duan Y, Zhang S, Wang J. Anterior clinoid process and the surrounding structures. J Craniofac Surg. 2013;24(06):2098–2102. doi: 10.1097/SCS.0b013e31829ae3af. [DOI] [PubMed] [Google Scholar]
- 42.da Costa M DS, de Oliveira Santos B F, de Araujo Paz D et al. Anatomical variations of the anterior clinoid process: a study of 597 skull base computerized tomography scans. Oper Neurosurg (Hagerstown) 2016;12(03):289–297. doi: 10.1227/NEU.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi N, Masuoka T, Tomita T, Sato H, Ohtani O, Endo S. Surgical anatomy and efficient modification of procedures for selective extradural anterior clinoidectomy. Minim Invasive Neurosurg. 2004;47(06):355–358. doi: 10.1055/s-2004-830121. [DOI] [PubMed] [Google Scholar]
- 44.Hunnargi S, Ray B, Pai S R, Siddaraju K S. Metrical and non-metrical study of anterior clinoid process in South Indian adult skulls. Surg Radiol Anat. 2008;30(05):423–428. doi: 10.1007/s00276-008-0346-1. [DOI] [PubMed] [Google Scholar]
- 45.Lee H W, Park H S, Yoo K S, Kim K U, Song Y J. Measurement of critical structures around paraclinoidal area: a cadaveric morphometric study. J Korean Neurosurg Soc. 2013;54(01):14–18. doi: 10.3340/jkns.2013.54.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabanciogullari V, Tastemur Y, Salk I, Dogruyol G, Cimen M. Assessment of dimensions of pneumatisation of the anterior clinoid process in middle Anatolian population by computed tomography. Folia Morphol (Warsz) 2018;77(03):558–563. doi: 10.5603/FM.a2018.0011. [DOI] [PubMed] [Google Scholar]
- 47.Sibuor W, Cheruiyot I, Munguti J, Kigera J, Gikenye G. Morphology of the anterior clinoid process in a select Kenyan population. Anat J Africa. 2018;7:1132–1137. [Google Scholar]
- 48.Souza A D, Ankolekar V H, Nayak N, Hosapatna M, Souza A S. Morphometric study of anterior clinoid process and optic strut and the ossification of carotico-clinoid ligament with their clinical importance. J Clin Diagn Res. 2016;10(04):AC05–AC07. doi: 10.7860/JCDR/2016/19316.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adanir S S, Ceylan E S, İnceoğlu A et al. Change in the location of the optic strut relative to the anterior clinoid process pneumatization. J Craniofac Surg. 2022;33(06):1924–1928. doi: 10.1097/SCS.0000000000008707. [DOI] [PubMed] [Google Scholar]
- 50.Gupta N, Priya A. Anterior clinoid process and optic strut-a morphometric study. J Evol Med Dent Sci. 2018;7:3577–3581. [Google Scholar]
- 51.Budu V, Mogoantă C A, Fănuţă B, Bulescu I. The anatomical relations of the sphenoid sinus and their implications in sphenoid endoscopic surgery. Rom J Morphol Embryol. 2013;54(01):13–16. [PubMed] [Google Scholar]
- 52.Arslan H, Aydinlioğlu A, Bozkurt M, Egeli E. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx. 1999;26(01):39–48. doi: 10.1016/s0385-8146(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 53.Bolger W E, Butzin C A, Parsons D S.Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery Laryngoscope 1991101(1 Pt 1):56–64. [DOI] [PubMed] [Google Scholar]
- 54.Citardi M J, Gallivan R P, Batra P S et al. Quantitative computer-aided computed tomography analysis of sphenoid sinus anatomical relationships. Am J Rhinol. 2004;18(03):173–178. [PubMed] [Google Scholar]
- 55.DeLano M C, Fun F Y, Zinreich S J. Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study. AJNR Am J Neuroradiol. 1996;17(04):669–675. [PMC free article] [PubMed] [Google Scholar]
- 56.Gean A D, Pile-Spellman J, Heros R C. A pneumatized anterior clinoid mimicking an aneurysm on MR imaging. Report of two cases. J Neurosurg. 1989;71(01):128–132. doi: 10.3171/jns.1989.71.1.0128. [DOI] [PubMed] [Google Scholar]
- 57.Senyuva C, Yücel A, Okur I, Cansiz H, Sanus Z. Free rectus abdominis muscle flap for the treatment of complications after neurosurgical procedures. J Craniofac Surg. 1996;7(04):317–321. doi: 10.1097/00001665-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi J A, Kawarazaki A, Hashimoto N. Intradural en-bloc removal of the anterior clinoid process. Acta Neurochir (Wien) 2004;146(05):505–509. doi: 10.1007/s00701-004-0249-9. [DOI] [PubMed] [Google Scholar]
- 59.Szmuda T, Sloniewski P, Baczalska A et al. The pneumatisation of anterior clinoid process is not associated with any predictors that might be recognised preoperatively. Folia Morphol (Warsz) 2013;72(02):100–106. doi: 10.5603/fm.2013.0017. [DOI] [PubMed] [Google Scholar]
- 60.Cetinkaya E A, Koc K, Kucuk M F, Koc P, Muluk N B, Cingi C. Calculation of an optic nerve injury risk profile before sphenoid sinus surgery. J Craniofac Surg. 2017;28(01):e75–e78. doi: 10.1097/SCS.0000000000003239. [DOI] [PubMed] [Google Scholar]


