Abstract
Objectives Postoperative cerebrospinal fluid (Po-CSF) leak is still a challenging complication of endoscopic endonasal skull base surgery. However, data describing the predictive factors of Po-CSF leak in pure pituitary adenomas is lacking. Aim of this study is to determine the risk factors of Po-CSF leak in a pituitary adenoma group operated via pure transsellar endoscopic approach.
Design This is a retrospective cohort study.
Setting A single-center academic hospital.
Participants Patients operated for a pituitary adenoma between 2015 and 2021 and followed up until June 2022 were included.
Main Outcome Measures Demographics, comorbidities, imaging, and outcome were recorded. Univariate and multivariate logistic regression analyses were used to determine the risk factors of Po-CSF leak.
Results Of the total 170 patients with a mean age of 47.5 ± 13.8 (min: 15; max: 80), 11 (6.5%) had Po-CSF leak. Univariate analysis revealed age, diabetes mellitus (DM), and tumor volume as predictors of Po-CSF leak. According to the receiver operating characteristic analysis, 7.5 cm 3 of tumor volume was found to be a good cutoff value with a sensitivity of 82% and a specificity of 75%. Hence, multivariable logistic regression model adjusted by age showed that a tumor volume of > 7.5 cm 3 (odds ratio [OR]: 22.9; 95% confidence interval [CI]: 3.8–135.9, p = 0.001) and DM (OR: 8.9; 95% CI: 1.7–46.5; p = 0.010) are strong independent risk factors of Po-CSF leak in pure endoscopic endonasal pituitary surgery.
Conclusion Besides younger age and DM, a cutoff value for tumor volume > 7.5 cm 3 is the most remarkable risk factor for Po-CSF leak in pure endoscopic pituitary surgery. These patients should carefully be assessed preoperatively and potential preemptive surgical strategies should be taken into consideration to avoid complications.
Keywords: pituitary, cerebrospinal fluid leak, risk factors, endoscopic endonasal
Introduction
Pituitary adenomas are the third most common intracranial lesions with an overall estimated incidence of 17.1%. 1 The development of endoscopic surgical techniques coupled with technological advancements has revolutionized the management of pituitary tumors over the past three decades. Endoscopic transsphenoidal route provides excellent visualization, avoids brain retraction, and offers less morbidity and mortality rates. Although the complication rates of endoscopic transsphenoidal surgery are low, they still can be challenging. Improvements in skull base reconstruction techniques reduced the rate of postoperative cerebrospinal fluid (Po-CSF) leaks but they are still one of the most common complications with a reported incidence of 1.4 to 16.9. 2 Besides the higher frequency, CSF leaks are also related with life-threatening consequences. Prospective identification of the risk factors of Po-CSF leak may reduce complication rates and improve prognosis. Thus, it is crucial to identify the patients with an increased risk of Po-CSF leak in the preoperative period. Several studies addressed the risk factors in mixed skull base surgery groups; however, it is hard to make a conclusion in these heterogeneous groups including endoscopic expanded approach for skull base lesions. The pituitary surgery patients are quite different in terms of CSF leaks and the current literature on this data are lacking. Therefore, in this study we aim to determine the potential risk factors of Po-CSF leak in a homogenous group of endoscopic pituitary surgery.
Methods
Demographics
This is a retrospective cohort study including patients operated for a pituitary adenoma between 2015 and 2021. All patients were operated via endoscopic endonasal route by a single endoscopic skull base team led by a neurosurgeon (N.E.C.). This study was approved by the Institutional Research Ethics Committee (November 5, 2021, Number 66) of Cukurova University Faculty of Medicine. This study did not require written informed consent due to its noninvasive and retrospective design. Patients with histologically confirmed pituitary adenomas were included. Patients with a pathology other than pituitary adenomas, recurrent surgeries, and the ones operated via extended approach were excluded. Demographic data including age at surgery, sex, relevant medical history including comorbidities, previous surgery or radiation, and preoperative clinical symptoms were recorded. All patients were followed up until June 2022.
Imaging
All patients underwent contrast-enhanced magnetic resonance imaging (MRI) in the preoperative period and within postoperative 48 hours. Also, contrast-enhanced MRI studies were obtained at third month and first year follow-up. Tumor volumes were calculated by two independent investigators according to the previously defined ellipsoid volume assessment method. 3 Cavernous sinus invasion was defined with the Knosp classification. 4 Beside suprasellar extension, the Hardy classification was used to assess parasellar invasion. The extent of resection was evaluated on MRI performed within 48 hours following surgery and the patients were classified as partial or complete resection. 5
Endocrinological Evaluation
Endocrine functions of the pituitary were evaluated by blood levels of free thyroxine (T4), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), growth hormone (GH), age and gender-adjusted insulin-like growth factor-1 (IGF-1), estradiol (for women), testosterone (for men), cortisol, adrenocorticotropic hormone (ACTH), and prolactin (PRL). If the patient had endocrinological symptoms and GH excess, oral glucose tolerance test was performed for definitive diagnoses of acromegaly. The same endocrine function tests were performed on the first day after surgery.
Tumors originally were classified as hormone negative adenomas (nonfunctioning) and the other silent adenomas related with immunostaining for all pituitary hormones (silent GH), corticotroph (ACTH), lactotroph (PRL), gonadotroph (FSH + LH), and tyrotroph (TSH) adenomas). Nonfunctioning adenoma group consisted of the tumors classified as null-cell adenomas.
Serum GH levels were measured as a chemiluminescence immunometric assay using the IMMULITE 2000 (Siemens; ng/mL). Serum IGF-1 measurement was performed with a solid-phase enzyme-labeled chemiluminescence immunometric assay using the IMMULITE 2000 (Siemens; ng/mL). Calibration was up to 1600 ng/mL (World Health Organization National Institute for Biological Standards and Control first International Reference Reagent [NIBSC first IRR] 87/518). Serum levels of PRL, TSH, free thyroxin, ACTH, FSH, LH, estradiol, and testosterone were analyzed using an immunoassay kit (DxI 800, Beckman Coulter; ng/mL). The plasma cortisol measurements were performed by human enzyme-linked immunosorbent assay methods. 6
Surgical Technique
All patients were operated by a single endoscopic skull base team led by the same neurosurgeon (N.E.C.). We perform mononostril endoscopic endonasal approach and use computed tomography- and MRI-based neuronavigation system for all patients (NAVIENT- ClaroNav Kolahi Inc., Toronto, Canada) for the resection. 7 We use FLOSEAL (FLOSEAL Hemostatic Matrix, Baxter International Inc.) for hemostasis. According to the intraoperative CSF leak severity, we fill the cavity with autologous fat graft and repair the sellar floor in a multilayer fashion with fascia lata graft held in place with TISSEL (Baxter International Inc.). Pedicled nasoseptal flaps which were prepared in advance were used when needed. We use lumbar drains in case of significant intraoperative CSF leakage. Lumbar drain was left open in 5 to 7 mL/h for 3 to 5 days. Prophylactic lumbar drains were not used.
CSF Leak and Management
Intraoperative CSF leaks were determined according to intraoperative records and graded by Conger's system. 8 For patients with grade 0 and 1 intraoperative leakage, we performed multilayer closure with fascia lata graft while pedicled nasoseptal flap and lumbar external drainage was used in grade 2 and 3 leakage patients. Po-CSF leakage was diagnosed clinically with the tilt test. 9 Patients with intraoperative CSF leak and lumbar drainage were closely monitored during early postoperative period. If any postoperative rhinorrhea develops in a patient without intraoperative leakage, lumbar drainage was used afterwards. In case of persistent rhinorrhea in a 3 to 5 days' period despite existing lumbar drainage system, reoperation was performed. All patients with lumbar drain were also closely followed up in case of any meningitis with CSF microscopic evaluation.
Outcome
All surgical specimens were examined according to standardized immunohistochemical staining methods. Postoperative complications other than CSF leak, length of hospital stay, and in-hospital mortality were analyzed. All patients were followed up postoperatively at third month and yearly with clinical, endocrinological, and radiological examination.
Statistical Analysis
Chi-square test was used to analyze categorical variables. Mann–Whitney U test was used to evaluate continuous variables between two independent groups. Receiver operating characteristic (ROC) analysis and area under the curve (AUC) statistics were used to assess various tumor volume cutoff points for risk variables of Po-CSF leak after endoscopic endonasal pituitary surgery. ROC analysis was used to determine the cutoff point using Youden's index. To identify independent risk factors, demographic, clinical, and laboratory characteristics were tested in univariate and multivariable logistic regression models. The model used stepwise entry variables from univariate analysis. Median, n , and % were reported. A value of 0.05 implied significance. SPSS 24.0 was used to analyze the data (IBM SPSS Statistics for Windows, Version 24.0. Armonk, New York, United States).
Results
Eighty male a total of 170 patients underwent surgery for a histopathologically confirmed pituitary adenoma were included. The mean age of patients at surgery was 47.5 ± 13.8 (15–80 years). Approximately one-third of the patients presented with visual impairment (31.8%). In addition, more than one-third had diabetes mellitus (DM) (34.1%). The cohort mostly consisted of macro- (74.7%) and giant adenomas (14.7%). Most of the patients were scored as Hardy ≥ 2 and Knosp ≥ 2. In addition, the vast majority of them were functional adenomas (78.8%).
Overall postoperative complication rate was 15.3%. Po-CSF leak was observed in 11 patients (6.5%). Patient demographics are presented in Table 1 .
Table 1. Characteristics of the total patients and comparison of the patients according to the presence of postoperative CSF leak.
| Total | Postoperative CSF | |||||||
|---|---|---|---|---|---|---|---|---|
| Leak (–) | Leak (+) | |||||||
| n | (%) | n | (%) | n | (%) | p | ||
| Age groups at surgery (y) | 15–34 | 25 | (14.7) | 20 | (80.0) | 5 | (20.0) | |
| 35+ | 145 | (85.3) | 139 | (95.9) | 6 | (4.1) | 0.003 | |
| Gender | Female | 90 | (52.9) | 84 | (93.3) | 6 | (6.7) | |
| Male | 80 | (47.1) | 75 | (93.8) | 5 | (6.3) | 0.912 | |
| Diabetes mellitus | No | 112 | (65.9) | 108 | (96.4) | 4 | (3.6) | |
| Yes | 58 | (34.1) | 51 | (87.9) | 7 | (12.1) | 0.033 | |
| Preoperative symptoms | Swollen hands and feet | 33 | (19.4) | 31 | (93.9) | 2 | (6.1) | |
| Visual impairment | 54 | (31.8) | 52 | (96.3) | 2 | (3.7) | ||
| Sexual dysfunction | 15 | (8.8) | 14 | (93.3) | 1 | (6.7) | ||
| Other | 68 | (40.0) | 62 | (91.2) | 6 | (8.8) | 0.726 | |
| Intraoperative CSF leak | No | 142 | (83.5) | 132 | (94.3) | 8 | (5.7) | |
| Yes | 28 | (16.5) | 27 | (90.0) | 3 | (10.0) | 0.387 | |
| Suprasellar extension | No | 65 | (38.2) | 62 | (95.4) | 3 | (4.6) | |
| Yes | 105 | (61.8) | 97 | (92.4) | 8 | (7.6) | 0.439 | |
| Adenoma size | Microadenoma | 18 | (10.6) | 17 | (94.4) | 1 | (5.6) | |
| Macroadenoma | 127 | (74.7) | 119 | (93.7) | 8 | (6.3) | ||
| Giant adenoma | 25 | (14.7) | 23 | (92.0) | 2 | (8.0) | 0.938 | |
| Pseudocapsule invasion | No | 146 | (85.9) | 136 | (93.2) | 10 | (6.8) | |
| Yes | 24 | (14.1) | 23 | (95.8) | 1 | (4.2) | 0.621 | |
| Knosp score | 0–1 | 72 | (42.4) | 69 | (95.8) | 3 | (4.2) | |
| 2 + | 98 | (57.6) | 90 | (91.8) | 8 | (8.2) | 0.295 | |
| Hardy class | 0–2 | 84 | (49.4) | 80 | (95.2) | 4 | (4.8) | |
| 3+ | 86 | (50.6) | 79 | (91.9) | 7 | (8.1) | 0.371 | |
| Resection rate | Subtotal | 46 | (27.1) | 45 | (97.8) | 1 | (2.2) | |
| Total | 124 | (72.9) | 114 | (91.9) | 10 | (8.1) | 0.165 | |
| Postoperative complication | No | 144 | (84.7) | 138 | (95.8) | 6 | (4.2) | |
| Yes | 26 | (15.3) | 21 | (80.8) | 5 | (19.2) | 0.004 | |
| Cutoff for tumor volume | < 7.5 cm 3 | 122 | (71.8) | 120 | (98.4) | 2 | (1.6) | |
| ≥ 7.5 cm 3 | 48 | (28.2) | 39 | (81.3) | 9 | (18.8) | 0.001 | |
| Tumor volume | Median (min-max) | 2.5 | (1–30) | 8.5 | (5.0–20.9) | 2.7 | (1–30) | 0.001 |
| Median hospital stay (d) | Median (min-max) | 7 | (3–68) | 25 | (10.0–54) | 7 | (3–68) | 0.001 |
| Follow-up duration (mo) | Median (min-max) | 50 | (1–100) | 30 | (9.0–78) | 50 | (1–100) | 0.756 |
Abbreviation: CSF, cerebrospinal fluid.
When the management and outcome of these postoperative rhinorrhea patients were analyzed, 8 patients had only postoperative rhinorrhea while 3 patients had intra- and postoperative rhinorrhea which was managed with lumbar drainage. Just one patient was reoperated due to lumbar external drainage failure. Intraoperative CSF leak was observed in 28 patients (16.5%). Out of 28 patients of intraoperative CSF leak, 8 of them were successfully treated by pedicled nasoseptal flap plus lumbar drainage. The remaining 20 cases were managed by multilayered closure techniques; however, 3 of them persisted postoperatively and were analyzed in the Po-CSF leak group. Further complications were observed in 72.7% of 11 patients with Po-CSF leakage ( n = 8). Meningitis developed in 4 patients in whom one of them had additionally intraventricular hemorrhage. One patient with Cushing's disease was lost due to meningitis and hydrocephalus at the postoperative third month. Four patients had transient diabetes insipidus while one of them had pulmonary thromboembolism and one had atrial fibrillation additionally. Overall postoperative 30 days mortality rate was 1.2% ( n = 2). These two patients were not in the Po-CSF leak group.
When the patients with and without Po-CSF leak were compared, there was no significant difference in terms of gender, preoperative symptoms, adenoma size, immunohistochemical staining, and Hardy and Knosp scores. However, younger age, DM, and tumor volume were associated with increased risk of Po-CSF leak according to univariate analysis ( p < 0.05). As well, length of hospital stay was significantly higher in patients with Po-CSF leak ( p < 0.05) ( Table 1 ). ROC analysis revealed that the cutoff values of tumor volume have discriminative ability for presence of Po-CSF leak (AUC values were estimated as 0.79). The best cutoff value was found to be 7.5 cm 3 for tumor volume, with a sensitivity of 82% and a specificity of 75%. Fig. 1 shows the results of ROC analysis of tumor volume.
Fig. 1.
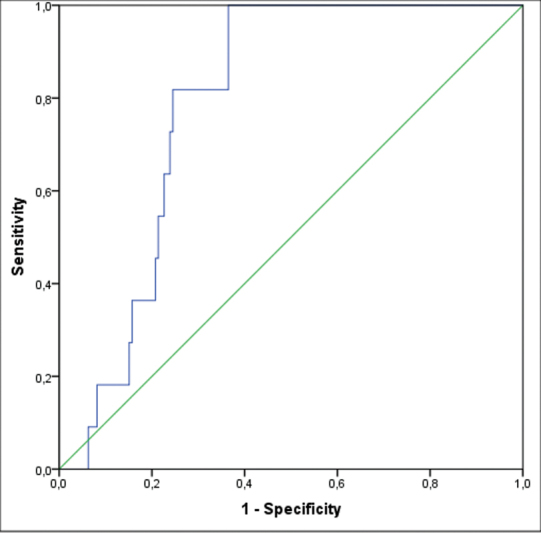
Receiver operating characteristic (ROC) curve (area under the curve [AUC] = 0.79) of the tumor volume according to the postoperative cerebrospinal fluid (CSF) leak after endoscopic endonasal pituitary surgery.
According to the multivariate logistic regression analysis, age, tumor volume, and DM were independent risk factors for Po-CSF leak. When the multivariate logistic regression model was adjusted by age, the incidence of Po-CSF leak for patients with tumor volume > 7.5 cm 3 increased 22.9-fold compared to those with tumor volume < 7.5 cm 3 (odds ratio [OR]:22.9; 95% confidence interval [CI]: 3.8–135.9; p = 0.001). Also, the risk of Po-CSF leak increased 8.9-fold (OR: 8.9; 95% CI: 1.7–46.5; p = 0.010) for those with DM ( Table 2 ).
Table 2. Multivariate analysis of possible risk factors of postoperative CSF leak after endoscopic endonasal pituitary surgery.
| B | OR | 95% CI | p | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Younger age (< 35 years old) | 2.742 | 15.5 | 2.6 | 91.1 | 0.002 |
| Tumour volume (> 7.5 cm 3 ) | 3.129 | 22.9 | 3.8 | 135.9 | 0.001 |
| Diabetes mellitus | 2.185 | 8.9 | 1.7 | 46.5 | 0.010 |
Abbreviations: CI, confidence interval; CSF, Cerebrospinal fluid; OR, odds ratio.
Discussion
In a large series of pure endoscopic endonasal pituitary surgery performed by the same team, we report a 6.5% Po-CSF leak rate. In addition, this study demonstrated for the first time that the higher tumor volume (> 7.5 cm 3 ), younger age, and DM are strong independent predictors of Po-CSF leak.
Endoscopic surgery revolutionized the surgical management of sellar lesions. Despite the well-known advantages, endoscopic pituitary surgery is not free of complications. Complication rates averaged 12%. 10 Besides the substantial rates, these complications have further significant impact on patient outcome. Herein, we reported an overall complication rate of 15.3%. However, we included all complications including minor ones such as short-term transient diabetes insipidus.
Po-CSF leak is one of the most common complications of endoscopic skull base surgery with an incidence of 1.3 to 16.9%. 10 11 12 The discrepancy of the incidence rates is probably due to the lack of standardized definitions of rhinorrhea, heterogeneous patient groups, and the other variables such as experience of the surgeon and the surgical technique used. There are several endoscopic surgical approaches such as transcribriform, transplanum-transtuberculum, and transorbital using the transsphenoidal corridor to manage anterior skull base lesions. 13 Several studies examined Po-CSF leak in mixed surgical groups. 2 However, transsellar approach is quite different in terms of Po-CSF leak development risk then the other expanded approaches. 14 In this context, very few studies indicated the risk factors of Po-CSF leak in the pituitary adenoma group operated via pure transsellar approach. In one of them, Xue et al reported the Po-CSF leak rate as 6% in a series of 216 patients. 15 By using the same surgical technique in a similar homogeneous group of patients, we found the rate of Po-CSF leak as 6.5%.
Regardless of the reported rates, Po-CSF leak has a strong impact on patients' prognosis in terms of total charge and length of hospital stay. 16 Thus, it is crucial to identify the risk factors to improve outcome. Several studies investigated Po-CSF leak variables including age, gender, comorbidities, pathology, surgical factors, and tumor size, volume, and extension. 11 14 17 18 19 20 21 22 23
Tumor size is a frequently reported risk factor of Po-CSF leak. 20 24 Macroadenomas were more likely to develop CSF leak than microadenomas. However, this retrospective study enrolled a mixed group of patients including pathologies other than pituitary adenomas. 20 Later, it has been shown that the tumor size and consistency are independent risk factors of intraoperative CSF leak in which tumor volume was not taken into consideration. 5 However, most of these studies investigated intraoperative CSF leak unlike ours. Herein, we found no significant difference among micro–macro and giant adenomas regarding Po-CSF leak.
Less reports focused on tumor volume in pituitary adenoma patients. In one of them, Jakimovski et al showed that not only tumor size but also tumor volume are best predictors of intraoperative CSF leak. 25 Besides, in pituitary macroadenomas tumor volume predicted subtotal resection and postoperative morbidity. 26 Pérez-López et al found that previous sellar surgery, higher Knosp grade, and isointense T2-weighed signal on MRI decreased the chance of gross total resection (GTR). 27 Tumor volume, not Knosp grade predicted GTR of nonfunctioning pituitary adenomas in a recent research. 28 Although highlighting the key role of tumor volume rather than the size on GTR, these studies did not focus on the effect of volume on CSF leak. In addition, they were performed in mixed patient groups operated via different surgical techniques. To our knowledge, only two studies investigated the potential link between pituitary adenoma volume and Po-CSF leak to date. None of them found tumor volume as a risk factor for Po-CSF leak. Also, these studies were performed in heterogeneous groups in terms of pathology and surgical technique. 19 29 For the first time we demonstrated that tumor volume independently predicts Po-CSF leak. Furthermore, we defined a cutoff value of > 7.5 cm 3 to predict Po-CSF leak. We claim that tumor volume is a better descriptive parameter then the size because these tumors have irregular contours with lobular shapes. Suprasellar extension or larger tumors protruding through the diaphragma sella and even the arachnoid may explain higher CSF leakage and tumor volume correlation. Furthermore, surgeon's aggressive attempts to achieve GTR in larger tumors may increase CSF leakage rates. 5
In accordance with the literature, we defined that younger age is another significant risk factor of Po-CSF leakage. 14 17 19 Younger patients were more likely to experience postoperative leak probably due to the surgeon's aggressive effort for GTR in this otherwise healthier group. The fact that more conservative treatments were applied in older patients may be the explanation of higher complication rates in younger ages who undergo surgery more frequently.
DM is another independent risk factor for postoperative CSF leak. DM is a well-known predictor of poor outcome in neurosurgical practice. It is related with an increased risk of several complications and mortality. 30 31 DM increased CSF leakage 5.56 times in a randomized controlled trial of elective craniotomy patients. 32 Systemic inflammation and progressive vascular disease in DM are the well-stated factors for poor wound healing. DM disrupts normal angiogenesis, needed for wound healing. Poor wound healing in diabetics may be caused by increased vascular endothelial growth factor, a proangiogenic factor, and decreased pigment epithelium-derived factor, an anti-angiogenic signal. Thus, these factors, causing poor skull base defect healing, may explain greater CSF leakage in diabetics.
The limitations of the current study can largely be attributed to its retrospective design and limited number of patients. The data used in this study was collected from medical records and surgical videos. Thus, bias was within the bounds of possibility. Second, the calculation method of the tumor volume may be criticized. Some authors claimed that three-dimensional calculations performed with software analyses demonstrate more accurate results in terms of tumor volume. However, there are inconsistent results regarding these calculation methods. As well, our method is a simple, cheap, and widely accepted choice of volume calculation currently used worldwide in daily practice. Third, we did not focus on tumor consistency because its evaluation methods are not well defined in the literature. Being a more objective method than the surgeon's intraoperative judgment, the accuracy of tumor consistency prediction according to the MRI-T2 signal is only 70%. 33 Lastly, as a well described predictor for Po-CSF leak in previous studies, body mass index was not evaluated in this study. 14 19 We believe that rather than being a limitation, our cohort consisting of pure pituitary adenoma patients operated via endoscopic transsphenoidal approach is the strength of our study. The results of this study should certainly be highlighted with further prospective trials performed in larger groups.
Conclusion
This is the first study which indicates a clear association between tumor volume, younger age, DM, and Po-CSF leak in endoscopic endonasal pituitary surgery. In addition, we defined a tumor cutoff volume which indicates the increased risk of Po-CSF leak by ROC analyses. It is obvious that more reliable cutoff values can be obtained with larger cohorts. As a result, younger patients, those with higher tumor volumes, and those with DM should carefully be assessed preoperatively and potential preemptive surgical strategies such as preparation of a nasoseptal pedicled flap in advance should be taken into consideration to reduce Po-CSF leak and to improve prognosis.
Conflict of Interest None declared.
Informed Consent
This study did not require written informed consent due to its non-invasive and retrospective design.
References
- 1.Ostrom Q T, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan J S.CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018 Neuro-oncol 202123(12, Suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobatto D J, de Vries F, Zamanipoor Najafabadi A H et al. Preoperative risk factors for postoperative complications in endoscopic pituitary surgery: a systematic review. Pituitary. 2018;21(01):84–97. doi: 10.1007/s11102-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kothari R U, Brott T, Broderick J P et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(08):1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 4.Knosp E, Steiner E, Kitz K, Matula C.Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings Neurosurgery 19933304610–617., discussion 617–618 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Yang Z, Wang X et al. Risk factors and management of intraoperative cerebrospinal fluid leaks in endoscopic treatment of pituitary adenoma: analysis of 492 patients. World Neurosurg. 2017;101:390–395. doi: 10.1016/j.wneu.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 6.[Anonymous] American Diabetes Association Professional Practice Committee . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45 01:S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 7.Baussart B, Declerck A, Gaillard S. Mononostril endoscopic endonasal approach for pituitary surgery. Acta Neurochir (Wien) 2021;163(03):655–659. doi: 10.1007/s00701-020-04542-z. [DOI] [PubMed] [Google Scholar]
- 8.Conger A, Zhao F, Wang X et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018;130(03):861–875. doi: 10.3171/2017.11.JNS172141. [DOI] [PubMed] [Google Scholar]
- 9.Hannan C J, Almhanedi H, Al-Mahfoudh R, Bhojak M, Looby S, Javadpour M. Predicting post-operative cerebrospinal fluid (CSF) leak following endoscopic transnasal pituitary and anterior skull base surgery: a multivariate analysis. Acta Neurochir (Wien) 2020;162(06):1309–1315. doi: 10.1007/s00701-020-04334-5. [DOI] [PubMed] [Google Scholar]
- 10.Berker M, Hazer D B, Yücel T et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012;15(03):288–300. doi: 10.1007/s11102-011-0368-2. [DOI] [PubMed] [Google Scholar]
- 11.Cavallo L M, Frank G, Cappabianca P et al. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121(01):100–113. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi T, Chaus F, Fogg L, Dasgupta M, Straus D, Byrne R W. Learning curve for the transsphenoidal endoscopic endonasal approach to pituitary tumors. Br J Neurosurg. 2016;30(06):637–642. doi: 10.1080/02688697.2016.1199786. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz T H, Fraser J F, Brown S, Tabaee A, Kacker A, Anand V K.Endoscopic cranial base surgery: classification of operative approaches Neurosurgery 20086205991–1002., discussion 1002–1005 [DOI] [PubMed] [Google Scholar]
- 14.Karnezis T T, Baker A B, Soler Z M et al. Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy Rhinol. 2016;6(11):1117–1125. doi: 10.1002/alr.21783. [DOI] [PubMed] [Google Scholar]
- 15.Xue H, Wang X, Yang Z, Bi Z, Liu P. Risk factors and outcomes of cerebrospinal fluid leak related to endoscopic pituitary adenoma surgery. Br J Neurosurg. 2020;34(04):447–452. doi: 10.1080/02688697.2020.1754336. [DOI] [PubMed] [Google Scholar]
- 16.Vimawala S, Chitguppi C, Reilly E et al. Predicting prolonged length of stay after endoscopic transsphenoidal surgery for pituitary adenoma. Int Forum Allergy Rhinol. 2020;10(06):785–790. doi: 10.1002/alr.22540. [DOI] [PubMed] [Google Scholar]
- 17.Boling C C, Karnezis T T, Baker A B et al. Multi-institutional study of risk factors for perioperative morbidity following transnasal endoscopic pituitary adenoma surgery. Int Forum Allergy Rhinol. 2016;6(01):101–107. doi: 10.1002/alr.21622. [DOI] [PubMed] [Google Scholar]
- 18.Ajlan A, Achrol A S, Albakr A et al. Cavernous sinus involvement by pituitary adenomas: clinical implications and outcomes of endoscopic endonasal resection. J Neurol Surg B Skull Base. 2017;78(03):273–282. doi: 10.1055/s-0036-1598022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dlouhy B J, Madhavan K, Clinger J D et al. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116(06):1311–1317. doi: 10.3171/2012.2.JNS111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senior B A, Ebert C S, Bednarski K K et al. Minimally invasive pituitary surgery. Laryngoscope. 2008;118(10):1842–1855. doi: 10.1097/MLG.0b013e31817e2c43. [DOI] [PubMed] [Google Scholar]
- 21.Thawani J P, Ramayya A G, Pisapia J M, Abdullah K G, Lee J Y, Grady M S. Operative strategies to minimize complications following resection of pituitary macroadenomas. J Neurol Surg B Skull Base. 2017;78(02):184–190. doi: 10.1055/s-0036-1597276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallapiazza R, Bond A E, Grober Y et al. Retrospective analysis of a concurrent series of microscopic versus endoscopic transsphenoidal surgeries for Knosp Grades 0-2 nonfunctioning pituitary macroadenomas at a single institution. J Neurosurg. 2014;121(03):511–517. doi: 10.3171/2014.6.JNS131321. [DOI] [PubMed] [Google Scholar]
- 23.Gondim J A, Almeida J P, Albuquerque L A et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(02):174–183. doi: 10.1007/s11102-010-0280-1. [DOI] [PubMed] [Google Scholar]
- 24.Jang J H, Kim K H, Lee Y M, Kim J S, Kim Y Z. Surgical results of pure endoscopic endonasal transsphenoidal surgery for 331 pituitary adenomas: a 15-year experience from a single institution. World Neurosurg. 2016;96:545–555. doi: 10.1016/j.wneu.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Jakimovski D, Bonci G, Attia Met al. Incidence and significance of intraoperative cerebrospinal fluid leak in endoscopic pituitary surgery using intrathecal fluorescein World Neurosurg 201482(3-4):e513–e523. [DOI] [PubMed] [Google Scholar]
- 26.Hofstetter C P, Nanaszko M J, Mubita L L, Tsiouris J, Anand V K, Schwartz T H. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(03):450–463. doi: 10.1007/s11102-011-0350-z. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-López C, Palpán A J, Saez-Alegre M et al. Volumetric study of nonfunctioning pituitary adenomas: predictors of gross total resection. World Neurosurg. 2021;147:e206–e214. doi: 10.1016/j.wneu.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 28.DiRisio A C, Feng R, Shuman W H et al. The Knosp Criteria Revisited: 3-dimensional volumetric analysis as a predictive tool for extent of resection in complex endoscopic pituitary surgery. Neurosurgery. 2023;92(01):179–185. doi: 10.1227/neu.0000000000002170. [DOI] [PubMed] [Google Scholar]
- 29.Lee S J, Cohen J, Chan J, Walgama E, Wu A, Mamelak A N. Infectious complications of expanded endoscopic transsphenoidal surgery: a retrospective cohort analysis of 100 cases. J Neurol Surg B Skull Base. 2020;81(05):497–504. doi: 10.1055/s-0039-1696999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randhawa K S, Choi C B, Shah A D et al. Impact of diabetes mellitus on adverse outcomes after meningioma surgery. World Neurosurg. 2021;152:e429–e435. doi: 10.1016/j.wneu.2021.05.101. [DOI] [PubMed] [Google Scholar]
- 31.Caputo M P, Shabani S, Mhaskar R, McMullen C, Padhya T A, Mifsud M J. Diabetes mellitus in major head and neck cancer surgery: systematic review and meta-analysis. Head Neck. 2020;42(10):3031–3040. doi: 10.1002/hed.26349. [DOI] [PubMed] [Google Scholar]
- 32.Hutter G, von Felten S, Sailer M H, Schulz M, Mariani L. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial. J Neurosurg. 2014;121(03):735–744. doi: 10.3171/2014.6.JNS131917. [DOI] [PubMed] [Google Scholar]
- 33.Snow R B, Johnson C E, Morgello S, Lavyne M H, Patterson R H., Jr Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26(05):801–803. doi: 10.1097/00006123-199005000-00011. [DOI] [PubMed] [Google Scholar]


