Abstract
Background
The most commonly used types of phototherapy for treating psoriasis are narrow‐band ultraviolet B (NB‐UVB); broad‐band ultraviolet B (BB‐UVB), which includes selective (delivering radiation with a wavelength range of 305 to 325 nm) and conventional BB‐UVB (280 to 320 nm); and psoralen ultraviolet A photochemotherapy (oral or bath PUVA). There is substantial controversy regarding their efficacy when compared with each other.
Objectives
To assess the effects of narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen ultraviolet A photochemotherapy for psoriasis.
Search methods
We searched the following databases up to August 2013: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2013, Issue 7), MEDLINE (from 1946), and EMBASE (from 1974). We searched the following databases up to November 2012: CNKI (from 1974) and CBM (from 1978). We also searched trials registers and the OpenGrey database.
Selection criteria
We included all randomised controlled trials (RCTs) that compared NB‐UVB phototherapy with BB‐UVB or PUVA for treating psoriasis, which included chronic plaque psoriasis (CPP), guttate psoriasis (GP), and palmoplantar psoriasis (PPP).
Data collection and analysis
Two review authors independently conducted the study selection, 'Risk of bias' assessment, and data extraction.
Main results
We included 13 RCTs, with a total of 662 participants. We report the results of intention‐to‐treat analyses (ITT) here. Our primary outcomes of interest were as follows: Participant‐rated global improvement, Percentage of participants reaching Psoriasis Area and Severity Index (PASI) 75 (which meant equal to or more than 75% reduction in PASI score), Withdrawal due to side‐effects, and Clearance rate.
In one RCT of NB‐UVB compared with oral PUVA in participants with CPP, the difference in PASI 75 was not statistically significant (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.63 to 1.32; N = 51; low quality). In three other RCTs of CPP, the clearance rates were inconsistent because in one, there was no difference between the groups (RR 1.01, 95% CI 0.91 to 1.12; N = 54), and in the other two, the clearance rates were statistically significantly in favour of oral PUVA: RR 0.66, 95% CI 0.47 to 0.93; N = 93 and RR 0.75, 95% CI 0.59 to 0.96; N = 100, respectively. Pooled data from these three studies indicated that withdrawals due to adverse events were not significantly different between either group (RR 0.71, 95% CI 0.20 to 2.54; N = 247; low quality).
The evidence from the comparison of NB‐UVB with bath PUVA in terms of clearance rate for CPP was also inconsistent: Pooled data from two left‐right body comparison RCTs found no significant difference between the NB‐UVB and bath PUVA groups (RR 1.79, 95% CI 0.46 to 6.91; N = 92; low quality), while a parallel RCT favoured bath PUVA (RR 0.18, 95% CI 0.05 to 0.71; N = 36; low quality).
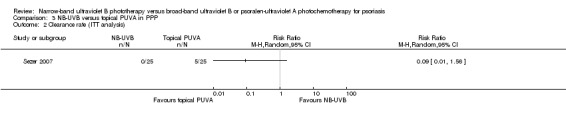
In participants with PPP, one RCT found there were no significant differences between NB‐UVB treated sides and topical PUVA treated sides in terms of clearance rate (RR 0.09, 95% CI 0.01 to 1.56; N = 50; low quality).
Two RCTs found NB‐UVB plus retinoid (re‐NB‐UVB) and PUVA plus retinoid (re‐PUVA) had similar effects for treating people with CPP or GP in terms of clearance rate (RR 0.93, 95% CI 0.79 to 1.10; N = 90; low quality).
One RCT in people with CPP found no significant differences between NB‐UVB and selective BB‐UVB in terms of clearance rate (RR 1.40, 95% CI 0.92 to 2.13; N = 100; low quality) and withdrawals due to adverse events (RR 3.00, 95% CI 0.32 to 27.87; N = 100; low quality).
No studies reported our primary outcomes for NB‐UVB compared with conventional BB‐UVB.
Authors' conclusions
Current evidence is very heterogeneous and needs to be interpreted with caution. The clearance rate between oral PUVA and NB‐UVB is inconsistent among the included studies. Evidence regarding NB‐UVB versus bath PUVA is also inconsistent. Re‐NB‐UVB and re‐PUVA are similarly effective for treating people with CPP or GP. In practice, NB‐UVB may be more convenient to use since exogenous photosensitiser is not required before phototherapy.
NB‐UVB is considered ineffective for PPP in clinical practice, and a small RCT did not detect a statistically significant difference between NB‐UVB and topical PUVA for clearing PPP. NB‐UVB seemed to be similar to selective BB‐UVB for clearing CPP.
Larger prospective studies are needed to confirm the long‐term safety of NB‐UVB.
Keywords: Humans, Photochemotherapy, Photochemotherapy/methods, Photosensitizing Agents, Photosensitizing Agents/therapeutic use, Psoriasis, Psoriasis/drug therapy, Psoriasis/pathology, Randomized Controlled Trials as Topic, Treatment Outcome, Ultraviolet Therapy, Ultraviolet Therapy/methods
Plain language summary
Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen ultraviolet A photochemotherapy for treating psoriasis
Psoriasis is a common, chronic inflammatory skin disease, with an estimated global prevalence ranging from 0.5% to 4.6%. Based on clinical features, psoriasis is generally divided into the following: chronic plaque psoriasis (CPP); psoriasis associated with psoriatic arthritis; and pustular, erythrodermic, or guttate psoriasis. We also considered psoriasis affecting the palms and soles (palmoplantar psoriasis, or PPP). Although psoriasis is rarely life‐threatening, it can affect a person's quality of life significantly.
Phototherapy is an essential treatment option for people with psoriasis. The most commonly used types of phototherapy are narrow‐band ultraviolet B (NB‐UVB), broad‐band ultraviolet B (BB‐UVB), and psoralen ultraviolet A photochemotherapy (PUVA). PUVA can be further divided into oral, bath, and topical PUVA according to the administrative route of psoralen. NB‐UVB delivers almost exclusively 311 nm radiation, whereas BB‐UVB can be divided into two types: selective BB‐UVB (305 to 325 nm radiation) and conventional BB‐UVB (280 to 320 nm radiation).
This review included 13 small randomised controlled trials (RCT), with a total of 662 participants. Most of these were of poor methodological quality.
For treating CPP, the clearance rate between the NB‐UVB and oral PUVA groups were inconsistent in three RCTs. In one, there was no difference between the groups, and in the other two, the clearance rate was in favour of oral PUVA. The evidence from the comparison of NB‐UVB with bath PUVA in terms of clearance rate was also inconsistent: Pooled data from two left‐right body comparison RCTs found no significant difference between the two groups, while another RCT favoured bath PUVA.
Two RCTs found NB‐UVB plus retinoid (re‐NB‐UVB) and PUVA plus retinoid (re‐PUVA) had similar effects for treating people with CPP or guttate psoriasis. One RCT found no significant differences between NB‐UVB and selective BB‐UVB for clearing CPP or in the number of withdrawals due to side‐effects.
In participants with PPP, one RCT found there were no statistically significant differences between NB‐UVB treated sides and topical PUVA treated sides in terms of clearance rate.
In summary, NB‐UVB may be preferred to oral or bath PUVA because it is more convenient to use. NB‐UVB seemed to be equal to selective BB‐UVB for clearing CPP. Evidence regarding NB‐UVB and conventional BB‐UVB is limited. The long‐term safety of NB‐UVB needs to be confirmed. The efficacy of NB‐UVB for clearing PPP needs to be confirmed in future studies.
Summary of findings
Summary of findings for the main comparison. NB‐UVB compared with oral PUVA for chronic plaque psoriasis.
| NB‐UVB compared with oral PUVA for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis Settings: ‐ Intervention: NB‐UVB Comparison: Oral PUVA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | 720 per 1000 | 655 per 1000 (454 to 950) | RR 0.91 (0.63 to 1.32) | 51 (1 study) | ⊕⊕⊝⊝ low¹, ² | This is the result of ITT analysis |
| Withdrawal due to side‐effects | 32 per 1000 |
50 per 1000 (7 to 82) |
RR 0.71 (0.20 to 2.54) | 247 (3 study) | ⊕⊕⊝⊝ low³ | This is the result of ITT analysis |
| Clearance rate | Study population | Not estimable | 0 (0) | See comment | The results of 3 small RCTs are contradictory. Because of the significant statistical heterogeneity, the data were not pooled | |
| See comment | See comment | |||||
| Moderate | ||||||
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ The study was of small sample size. ² The study was at high risk of bias. ³ All of the 3 studies were of small sample size and at high risk of bias, and the result was based on less than 300 participants.
Summary of findings 2. NB‐UVB compared with bath PUVA for chronic plaque psoriasis.
| NB‐UVB compared with bath PUVA for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis Settings: ‐ Intervention: NB‐UVB Comparison: Bath PUVA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bath PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 348 per 1000 | 623 per 1000 (160 to 1000) | RR 1.79 (0.46 to 6.91) | 92 (2 studies) | ⊕⊕⊝⊝ low¹ | 1. On the basis of studies performing left‐right body comparison. 2. This is the result of ITT analysis |
| Clearance rate | 611 per 1000 | 110 per 1000 (31 to 434) | RR 0.18 (0.05 to 0.71) | 36 (1 study) | ⊕⊕⊝⊝ low², ³ | 1. On the basis of the study performing comparison between participants. 2. This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ Both of the studies were of small sample size and at high risk of bias, and the result was based on less than 300 participants. ² The study was at high risk of bias. ³ The study was of small sample size, and the result was based on less than 300 participants.
Summary of findings 3. NB‐UVB compared with topical PUVA for palmoplantar psoriasis.
| NB‐UVB compared with topical PUVA for palmoplantar psoriasis | ||||||
| Patient or population: People with palmoplantar psoriasis Settings: ‐ Intervention: NB‐UVB Comparison: Topical PUVA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 200 per 1000 | 18 per 1000 (2 to 312) | RR 0.09 (0.01 to 1.56) | 50 (1 study) | ⊕⊕⊝⊝ low¹, ² | This is the result of ITT analysis |
| * Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ This study was at unclear risk of bias. ² The study was of small sample size, and the result was based on less than 300 participants.
Summary of findings 4. NB‐UVB plus retinoid compared with PUVA plus retinoid for chronic plaque or guttate psoriasis.
| NB‐UVB plus retinoid compared with PUVA plus retinoid for chronic plaque or guttate psoriasis | ||||||
| Patient or population: People with chronic plaque or guttate psoriasis Settings: ‐ Intervention: NB‐UVB plus retinoid Comparison: PUVA plus retinoid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PUVA plus retinoid | NB‐UVB plus retinoid | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | RR 0.89 (0.59 to 1.35) | 60 (1 study) | ⊕⊕⊝⊝ low¹, ² | This is the result of ITT analysis | |
| 633 per 1000 | 564 per 1000 (374 to 855) | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 756 per 1000 | 688 per 1000 (544 to 831) | RR 0.93 (0.79 to 1.10) | 90 (2 studies) | ⊕⊕⊝⊝ low², ³ | This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ This study was at high risk of bias. ² The studies were of small sample size, and the result was based on less than 300 people. ³ Both of the studies were at high risk of bias.
Summary of findings 5. NB‐UVB compared with selective BB‐UVB for chronic plaque psoriasis.
| NB‐UVB compared with selective BB‐UVB for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis Settings: ‐ Intervention: NB‐UVB Comparison: Selective BB‐UVB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Selective BB‐UVB | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 (0) | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | RR 3.00 (0.32 to 27.87) | 100 (1 study) | ⊕⊕⊝⊝ low¹, ² | This is the result of ITT analysis | |
| 20 per 1000 | 60 per 1000 (6 to 557) | |||||
| Moderate | ||||||
| Clearance rate | 400 per 1000 | 560 per 1000 (368 to 852) | RR 1.40 (0.92 to 2.13) | 100 (1 study) | ⊕⊕⊝⊝ low¹, ² | This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ The study was at unclear risk of bias. ² The study was of small sample size, and the result was based on less than 300 people.
Background
Please note that unfamiliar terms and abbreviations are listed in Table 6 ('Glossary of some important terms and abbreviations used').
1. Glossary of some important terms and abbreviations used.
| Medical term and abbreviations | Explanation |
| Apoptosis | The process of programmed cell death that occurs during growth and development of multicellular organisms. It is generally considered a part of normal cell aging, but it can also be a response to cellular injury |
| BB‐UVB | Broad‐band ultraviolet B |
| Collagenase | An enzyme that breaks the peptide bonds in collagen |
| CPP | Chronic plaque psoriasis |
| Cytokines | Small protein molecules that are secreted by cells of the nervous system or the immune system. They are used in intercellular communication |
| Defective maturation of epidermal keratinocytes | Incomplete formation of keratin (the horny material in nails) due to rapid growth of cells in the epidermal layer of the skin |
| Dilatation of dermal capillaries | Dilation of small blood vessels in the skin |
| Erythrodermic psoriasis | A subtype of psoriasis that affects nearly all body sites |
| Erythrogenic response | Redness of the skin caused by light exposure |
| Extensor aspects | An anatomical term ‐ when a joint bends, the parts of the skin on the opposite side of the joint are called the extensor aspects |
| Hyperkeratosis | Thickening of the stratum corneum (outermost layer of the skin) usually associated with an abnormality of the keratin and an increase of the granular layer of the skin |
| Hyperplasia | An increase in the number of cells |
| Hyperproliferation | An abnormally high rate of proliferation of cells by rapid division |
| Hypertriglyceridaemia | High levels of triglyceride fatty acids |
| ITT | Intention‐to‐treat: An ITT analysis is often recommended as the least biased way to estimate intervention effects in RCTs. The principals of ITT analysis are as follows: 1. keep participants in the intervention group to which they were randomised, regardless of the intervention they actually received; 2. measure outcome data on all participants; and 3. include all randomised participants in the analysis |
| MRA | Minimal residual activity |
| MOP | Methoxypsoralen |
| NB‐UVB | Narrow‐band ultraviolet B |
| Paronychia | Swelling of the skin over the nail |
| PASI | Psoriasis Area and Severity Index. The higher the score, the more severe the lesions are |
| PASI 75 | Equal to or more than 75% reduction in PASI score |
| PPP | Palmoplantar psoriasis |
| Psoralen | A compound that can be used as a kind of photosensitiser to improve the influence of natural or artificial light |
| PUVA | Psoralen plus ultraviolet A |
| Photosensitiser | Chemical treatments that are used to sensitise the skin and enhance the effect of light treatments |
| Pustular | Lesions containing purulent materials |
| QOL | Quality of life |
| Re‐NB‐UVB | NB‐UVB combined with retinoid |
| Re‐PUVA | PUVA combined with retinoid |
| Severity index of PPP | A tool developed by Hofer 2006 to evaluate the severity of palmoplantar psoriasis. The separate scores of erythema, scaling, pustulation, and infiltration for palms and soles were added to calculate the severity index (0 = absent; 1 = slight; 2 = moderate; 3 = marked; and 4 = very marked) |
| Xerophthalmia | Dryness of the eye, especially the cornea and conjunctiva |
| Xerosis | Extreme dryness of the skin |
Description of the condition
Description and epidemiology
Psoriasis is a common, chronic inflammatory skin disease, with an estimated global prevalence ranging from 0.5% to 4.6% (Lebwohl 2003). The typical lesions of psoriasis include well‐demarcated red plaques, with variable degrees of silvery thickening, and surface scale, particularly on the scalp, extensor aspects (backs of the elbows, fronts of the knees) of the limbs, and the trunk. Psoriatic arthritis, pustular psoriasis (a subtype of psoriasis with lesions containing purulent materials), or erythrodermic psoriasis (a subtype of psoriasis that affects nearly all body sites) may also be present. Among the various subtypes, psoriasis vulgaris is the most common form and accounts for more than 80% of psoriasis cases (Lebwohl 2003). The characteristic pathological changes of psoriasis present with hyperkeratosis (thickening of the stratum corneum, which is usually associated with an abnormality of the keratin and an increase of the granular layer), hyperplasia (increase in the number of cells) of the epidermis, inflammatory cell infiltration into the dermis and epidermis, and dilatation of dermal capillaries (dilated small blood vessels in the dermis). The diagnosis of psoriasis is mainly based on clinical features, and pathological changes are usually helpful to distinguish psoriasis from other diseases with a similar appearance.
Cause
The exact cause of psoriasis remains unclear. However, psoriasis appears to be a disorder of immune function (specifically involving the T set of lymphocytes), which causes an accelerated rate of cell turnover in the epidermal layer of the skin (Griffiths 1996). People seem to have a strong genetic predisposition to develop the condition. Certain medications (such as lithium, beta blockers, antimalarial drugs, and nonsteroidal anti‐inflammatory drugs) and infections are thought to be possible triggers.
Impact
Although psoriasis is rarely life‐threatening, the effect on a person's quality of life (QOL) can be profound, with a damaging effect on their self‐esteem, due to the long‐term nature of the disease, the persistent itching or pain of the skin, and the stigmatising effect of a disfiguring condition (De Korte 2004). It also seems to be associated with a significantly increased risk of cardiovascular disease (Gelfand 2006) and a variety of malignant diseases (Boffetta 2001; Gelfand 2003; Hannuksela‐Svahn 2000).
Description of the intervention
Management of psoriasis should depend upon a number of factors: These include the severity of the disease, associated diseases (comorbidities), education about the chronic nature of the disease, and realistic expectations about the effect of treatments, as well as the use of medication. Complete clearance of psoriasis may be unrealistic, so the main aim of treatment is to reduce disease activity with minimal side‐effects.
Interventions include topical therapy, ultraviolet light (phototherapy), systemic agents, and biological treatments. Those mildly affected can generally be treated adequately with topical medication, but 10% to 20% of those with moderate‐to‐severe psoriasis often depend upon phototherapy, systemic treatment, or combination therapy to achieve and sustain disease remission (Jensen 2010).
Phototherapy is an essential therapeutic option for people with psoriasis and has been used for more than 75 years. The most commonly used types of phototherapy are photochemotherapy using psoralen ultraviolet A (PUVA) and ultraviolet B (UVB) therapy.
Therapy with PUVA is administered by the use of a photosensitiser prior to exposure to the phototherapy. The photosensitiser, psoralen, is administered either orally, or in bath water, or as a cream (or a gel) before exposure to long wavelength (320 to 400 nm) ultraviolet A (UVA) radiation. In consequence, PUVA is divided into oral PUVA, bath PUVA, and topical PUVA. With oral PUVA, different psoralens may be applied, such as 8‐methoxypsoralen (8‐MOP) and 5‐methoxypsoralen (5‐MOP). The psoralen 8‐MOP is the only available orally prescribed psoralen in the United States; it takes about one to three hours to reach peak concentration in the skin, so is usually administered at least two hours before UV irradiation. The most common side‐effect of PUVA is nausea that develops shortly after ingestion. Many people withdraw from PUVA therapy because of severe nausea. For those who cannot tolerate 8‐MOP, 5‐MOP is an alternative choice, which is more commonly used in Europe (Braun 2000; Jensen 2010; Menter 2010). Trimethylpsoralen, which is used for bath PUVA, is largely used in Scandinavia, whereas 8‐MOP in a hydrophilic water or oil emulsion is used for topical PUVA (Jensen 2010; Menter 2010).
Therapy with PUVA has been proven to be effective for most forms of psoriasis and induces complete or partial remission in 79% to 90% of those with psoriasis (De Gruijl 1996; Lauharanta 1997; Morison 1998). Unfortunately, current evidence shows a clear correlation between cumulative PUVA exposure and an increased risk of skin cancer and premature ageing of the skin (Lauharanta 1997; Lowe 1997; Stern 1988). Therefore, the British Association of Dermatologists' guideline on biological interventions for psoriasis recommended that PUVA should be limited to 150 lifetime treatments, to decrease the risk of skin cancer (Smith 2009). However, a combined analysis of two cohort studies with 944 participants treated with bath PUVA "found no increase in the risk of squamous cell carcinoma after a mean follow‐up of 14.7 years", suggesting that bath PUVA is possibly safer than oral PUVA (Naldi 2010).
UVB (spectrum light 280 to 320 nm) has been used to treat psoriasis for at least 90 years (Anderson 1984). There are several types of UVB radiation in clinical practice:
conventional broad‐band UVB (BB‐UVB) lamps, which deliver radiation in the range of 280 to 320 nm;
selective BB‐UVB, which has peaks at 305 to 325 nm; and
narrow‐band UVB (NB‐UVB) lamps, which deliver almost exclusively 311 nm radiation (Braun 2000; Ibbotson 2004).
Conventional BB‐UVB has been proven to cause the clearance of psoriasis within six weeks, but the use of it is limited by burning (Boer 1980). Selective BB‐UVB was also effective in treating psoriasis (Parrish 1981). Phototherapy with NB‐UVB was developed in the 1980s. It is emitted through Philips TL01 lamps and consists of a subset of the UVB spectrum between 311 and 313 nm. A study conducted by Parrish and Jaenicke demonstrated that the peak action spectrum for clinical antipsoriatic efficacy was between 308 and 312 nm (Fischer 1976; Parrish 1981). In this way, NB‐UVB can theoretically achieve an optimal response while minimising the erythrogenic (redness of the skin) response to non‐therapeutic wavelengths. In fact, several small‐scale clinical studies (Coven 1997; Storbeck 1993; van Weelden 1988; Walters 1999) have shown an improved response of psoriasis to NB‐UVB compared with conventional BB‐UVB.
There is controversy regarding the risk of skin cancer with NB‐UVB. Young 1995 summarised data from murine studies and reported NB‐UVB might be two to three times more carcinogenic per minimal erythema dose (MED) than conventional BB‐UVB. However, one systematic review (Pasker‐de 1999) estimated that "the excess annual risk of non‐melanoma skin cancer associated with UVB was likely to be less than 2%". Another systematic review found that UVB did not increase the risk of skin cancer during about 25 years' follow up (Lee 2005). Likewise, no increased risk of cancer was identified in 3867 people treated by NB‐UVB in Scotland (Hearn 2008). Most recently, Archier 2012 found a lack of robust evidence of the carcinogenic risk of NB‐UVB because of limited prospective studies.
Sometimes UVB or PUVA is combined with retinoids (e.g. etretinate and acitretin) to treat psoriasis. Retinoids have been established as an effective systemic therapy for psoriasis since the 1970s. They can be used as monotherapy or combined at low doses with UVB or PUVA for treating psoriasis. Etretinate was widely used initially; however, acitretin, the free acid of etretinate and its active metabolite, has replaced etretinate for treating psoriasis because of its more favourable pharmacokinetic profile (Saurat 1999). Generally, retinoids combined with NB‐UVB or PUVA are abbreviated as re‐NB‐UVB or re‐PUVA, respectively.
How the intervention might work
It has been found that UV exposure can affect cell signalling, favour development of T‐helper 2 (Th2) immune responses, and reduce both the number and function of antigen‐presenting Langerhans cells (Zanolli 2000).
Ultraviolet light in the UVA part of the spectrum is successfully used in the treatment of psoriasis, based on its ability to reduce mast cells and induce type I collagenase activity. Psoralen is used as a photosensitiser in PUVA therapy. Once psoralen is activated by UVA, "it crosslinks DNA strands, preventing replication of keratinocytes and inducing the death of activated T‐cells in the skin" (Coven 1999). The significant effects of PUVA may be due to its immunosuppressive properties. The immunosuppressive mechanisms of PUVA mainly involve the following: decreasing the antigen‐presenting capacity of epidermal Langerhans cells and the numbers and functional activity of T‐helper cells and messenger RNA (mRNA) encoding for proinflammatory cytokines IL‐6, IL‐8, and TNF‐α. They may also involve inhibition of cell proliferation, reduction of the percentage of CD3+ peripheral T lymphocytes producing IFN‐gamma and IL‐2, and induction of an anergy (failure of response) of type 1 activity in peripheral lymphocytes (Aubin 1998; Ashworth 1989; Borroni 1991; Kozenitzky 1992; Neuner 1994).
The exact mechanism of action of UVB is not fully understood. The proposed mechanism may cause apoptosis (cell death) of lymphocytes and epidermal cells, as well as immunosuppressive and anti‐inflammatory effects (Aufiero 2006). It has been demonstrated that the peak action spectrum for clinical efficacy is between 308 and 312 nm, while the maximal erythrogenic response occurs around 297 nm (Fischer 1976; Parrish 1981). With NB‐UVB, because the peak spectrum is at 311 nm, significant antipsoriatic efficacy can be achieved with a limited erythrogenic response.
The mechanism of the therapeutic effect of retinoids when combined with UVB or PUVA is also not yet fully understood. Pretreatment with retinoids can reduce "both desquamation and infiltration of psoriatic plaques", and in consequence might raise "the possibility of increased penetration of ultraviolet light" (Jensen 2010).
Why it is important to do this review
There have been many studies, of variable methodological quality, comparing the efficacy of different types of phototherapy. Some indicate that PUVA is more effective than BB‐UVB radiation (Brenner 1983; Boer 1984; Honigsmann 1977; Morison 1995); others demonstrate that NB‐UVB provides faster clearing of psoriasis, less burning reactions, and longer periods of remission than BB‐UVB phototherapy (Coven 1997; Green 1988; Storbeck 1993). NB‐UVB is also more convenient because no exogenous photosensitiser is needed before phototherapy. Recently, while some authors have claimed that NB‐UVB therapy has similar efficacy to PUVA (Markham 2003), other authors (Dawe 2003; Gordon 1999; Tahir 2004) have found different results.
No systematic review has been conducted to summarise the evidence of the effects of NB‐UVB phototherapy compared with BB‐UVB or PUVA photochemotherapy for psoriasis. Therefore, we aimed to summarise results from randomised controlled trials (RCTs) to provide reliable evidence for clinicians and for those with psoriasis.
Objectives
To assess the effects of narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen ultraviolet A photochemotherapy for psoriasis.
Methods
Criteria for considering studies for this review
Types of studies
We included any RCT involving NB‐UVB phototherapy versus BB‐UVB or PUVA photochemotherapy for psoriasis. We excluded quasi‐randomised trials.
Types of participants
We included any individual with a diagnosis of any type of psoriasis, regardless of age, race, gender, or the severity of their lesions.
Types of interventions
Any NB‐UVB phototherapy compared with BB‐UVB or PUVA photochemotherapy, either as a single or combination therapy. The following comparisons were performed:
NB‐UVB versus oral PUVA;
NB‐UVB versus bath PUVA;
NB‐UVB versus topical PUVA;
NB‐UVB combined with retinoids (re‐NB‐UVB) versus PUVA combined with retinoids (re‐PUVA);
NB‐UVB versus selective BB‐UVB;
NB‐UVB versus conventional BB‐UVB; and
NB‐UVB combined with dithranol versus BB‐UVB combined with dithranol.
Types of outcome measures
Primary outcomes
Participant‐rated global improvement.
Percentage of participants reaching Psoriasis Area and Severity Index (PASI) 75 (which meant equal to or more than 75% reduction in PASI score).
Withdrawal due to side‐effects.
Clearance rate. (Clearance was defined as no lesions of psoriasis or minimal residual activity (MRA)).
Secondary outcomes
The Physician's Global Evaluation score.
Dermatology Life Quality Index (DLQI).
Number of treatments to clearance.
Cumulative UV dose to clearance.
Time to clearance.
Clearance lasting six months.
PASI score reduction (before and after treatment).
Time to PASI 75.
Relapse rate.
Duration of remission.
Withdrawal due to poor response.
Clinical improvement.
Reduction of peripheral T cells.
Tolerability.
Adverse events.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 8 August 2013:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 7, 2013, in The Cochrane Library using the search strategy in Appendix 2;
MEDLINE via OVID (from 1946) using the strategy in Appendix 3; and
EMBASE via OVID (from 1974) using the strategy in Appendix 4.
We searched the following databases up to 27 November 2012:
CNKI (China National Knowledge Infrastructure, from 1974) using the strategy in Appendix 5; and
CBM (Chinese Biomedical Database, from 1978) using the strategy in Appendix 6.
Searching other resources
Trials registers
We searched the following trials registers using the strategy in Appendix 7 on 27 November 2012:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/ trialsearch).
Chinese Clinical Trial Registry (www.chictr.org).
Reference lists
We scanned the references of all included trials and relevant systematic reviews or meta‐analyses to identify further relevant trials.
Conference proceedings
We handsearched abstracts from the following dermatological conference proceedings for further RCTs up to November 2012:
World Congress of Dermatology (from 1980);
International Congress of Dermatology (from 1980); and
European Academy of Dermatology and Venereology (from 1980).
Unpublished literature
We searched the OpenGrey database (www.opengrey.eu) for grey literature using the search strategy in Appendix 7.
We were not able to contact authors to obtain unpublished trials, as we had planned, because of time and resource constraints.
Adverse effects
We did not perform a separate search for adverse effects of the target interventions. We considered data on adverse effects from the included studies we identified.
Data collection and analysis
Selection of studies
Two review authors (XMC and YC) independently scanned the titles and abstracts of all articles identified from the searches according to our inclusion and exclusion criteria. For all initially selected articles, we obtained the full text; thereafter, two review authors (XMC and MY) independently assessed them to see whether they were eligible for inclusion.
We listed the studies that were excluded and the reasons for their exclusion in the review. During this process, we resolved discrepancies by discussion with MZ, who acted as an arbitrator.
Data extraction and management
Two review authors (XMC and MY) extracted the data from the included studies separately. We documented the process of resolving discrepancies in this review. We used the standard data extraction form recommended by the Cochrane Skin Group and recorded information about the following areas:
general information (authors, title, source, year of publication, language of publication, trial numbers);
trial characteristics (design; manner of recruitment; inclusion and exclusion criteria; duration of intervention period; reason for, and number of, dropouts and withdrawals);
participants (baseline characteristics of participants in all groups, such as gender, age, psoriasis severity, and baseline health‐related quality of life (HRQoL) scores);
interventions (any intervention in both study and control groups); and
outcomes (specific outcomes reported, assessment instrument used, adverse events).
We tried to contact trial authors for more information where necessary. One of us (MY) checked and entered the data into Review Manager (RevMan). Another author (XMC) double‐checked the data. We resolved disagreements by discussion within the review team.
Assessment of risk of bias in included studies
Two authors (XMC and MY) independently assessed the methodological quality of the included studies. We settled discrepancies by discussion within the review team. We used The Cochrane Collaboration's tool for assessing risk of bias, which forms part of the 'Characteristics of included studies' tables (Higgins 2011), and we addressed the following issues:
(a) was there adequate sequence generation?; (b) was allocation adequately concealed?; (c) was knowledge of the allocated interventions adequately prevented during the study?; (d) were incomplete outcome data adequately addressed?; (e) were reports of the study free of suggestion of selective outcome reporting?; and (f) was the study apparently free of other problems that could put it at a risk of bias?
We documented our judgements for each item and the reasons for our judgements in the 'Risk of bias' table for each included study within the review.
Where necessary, we attempted to contact trial authors for more information.
Measures of treatment effect
According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we defined measures of treatment effects as follows.
Dichotomous data
We presented dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CIs) for individual trials. We discussed the main outcomes of each study and, if possible, pooled feasible data.
Continuous data
For continuous variables, such as the score of life quality index, we used the mean difference and 95% CI, unless different scales were used in the trials, in which case we used a standardised mean difference (SMD) and 95% CI to summarise the data.
Unit of analysis issues
Simple parallel RCTs
The unit of analysis was individual participants.
Cluster RCTs
In the protocol, we stated that if we identified cluster RCTs, we would try to re‐analyse these trials by calculating the effective sample sizes according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and if possible, we would calculate an estimate of the intracluster coefficient (ICC), using external estimates obtained from similar trials. We would not pool data from cluster RCTs with those from parallel RCTs. However, we found no eligible cluster trials.
Cross‐over RCTs
In the protocol, we stated that if we identified cross‐over RCTs, we would only extract and analyse data from the first period (Higgins 2011). We would not pool data from cross‐over RCTs with those from parallel RCTs. However, we found no eligible cross‐over RCTs.
Multiple intervention groups within a trial
No relevant trial was included in this review. If we identify relevant trials for future updates of this review, we will deal with them as we planned in the published protocol.
Multiple body parts receiving the same intervention
No relevant trial was included in this review. If we identify relevant trials for future updates of this review, we will deal with them as we planned in the published protocol.
Multiple body parts receiving different interventions
In some included trials, the left and right sides of the body were randomly allocated into different groups and to receive different interventions. In this regard, the unit of analysis was half‐body.
Dealing with missing data
First, we attempted to contact the trial authors to get more information where necessary. If this did not succeed, we considered participants with missing outcomes as treatment failures for dichotomous outcomes. In the case of participant dropout, we conducted intention‐to‐treat (ITT) analyses for primary outcomes.
For continuous outcomes, we only extracted and analysed the available data. In addition, we explored the impact of missing data on the treatment effect by using sensitivity analyses, where possible. In future updates, if there were missing continuous data, we would state the whole process of dealing with the missing data and its potential impact on the results of the review in the Discussion section of our review.
Assessment of heterogeneity
We evaluated the level of clinical heterogeneity by comparing the differences between the trials in the administration of therapy, the type of comparators used, and the characteristics of the study population. If an appropriate level of clinical homogeneity existed, we analysed the level of statistical heterogeneity using the Chi² test on N‐1 degrees of freedom, with an alpha of 0.1 used for statistical significance and the I² statistic. I² statistic values of 25%, 50%, and 75% correspond to low, medium, and high levels of heterogeneity (Higgins 2011). If heterogeneity existed, we attempted to probe the reasons for it and advised caution in the interpretation of our results.
Assessment of reporting biases
If we had identified sufficient RCTs, we would have used funnel plots to test for publication bias. However, we could not use funnel plots to test for publication bias, because for each outcome, there were insufficient studies to perform it (Higgins 2011).
Data synthesis
We pooled data using the random‐effects model, unless there were less than three trials without clinical heterogeneity ‐ in which case, we used the fixed‐effect model. If we identified substantial heterogeneity, we reported the results qualitatively.
Subgroup analysis and investigation of heterogeneity
Because of insufficient information, we could only perform subgroup analysis to detect the potential heterogeneity induced by study design (e.g. some studies performed left‐right body comparisons, while others performed comparisons between participants) in some outcomes.
Sensitivity analysis
In the protocol, we stated that we would perform sensitivity analyses, where possible, but we were unable to carry this out because of insufficient data.
Results
Description of studies
See the 'Characteristics of included studies', 'Characteristics of excluded studies', and 'Studies awaiting classification' tables.
Results of the search
Our electronic search retrieved 1798 references excluding duplicates. After scanning the titles and abstracts, we identified 25 references as potentially relevant, which we retrieved in full text. Among these, 17 references referring to 13 RCTs met the inclusion criteria. One reference (Nazari 2005) was published in Turkish, and we are waiting for a translation. It is listed in Characteristics of studies awaiting classification. We excluded the remaining seven references. We identified no further reports by screening the reference lists of all included RCTs, relevant systematic reviews or meta‐analyses, and dermatological conference proceedings. We present the screening process in Figure 1.
1.
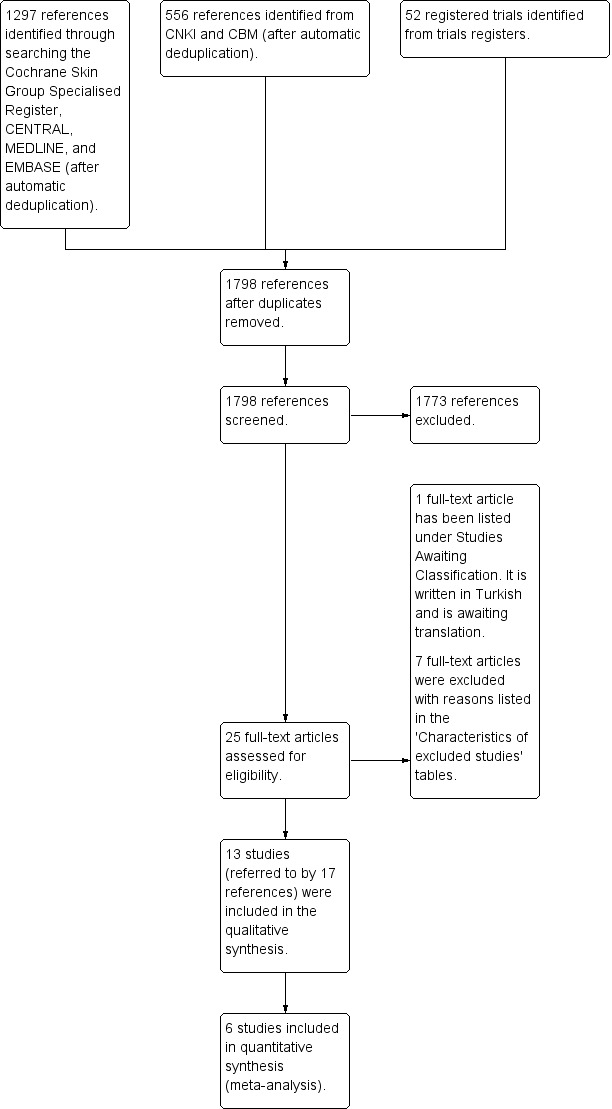
Study flow diagram
Included studies
In this review, we included 13 RCTs, with a total of 662 participants. More information about these 13 studies (Chauhan 2011; Dawe 2003; Gordon 1999; Green 1992; Kirke 2007; Larko 1989; Markham 2003; Özdemir 2008; Salem 2010; Sezer 2007; Snellman 2004; Storbeck 1993; Yones 2006) is available in the 'Characteristics of included studies' tables.
Design
Some of the included RCTs (Chauhan 2011; Green 1992; Gordon 1999; Kirke 2007; Markham 2003; Özdemir 2008; Salem 2010; Yones 2006) performed comparisons between participants, whereas others (Dawe 2003; Larko 1989; Sezer 2007; Snellman 2004; Storbeck 1993) performed within‐patient comparisons (left‐right body comparison).
Sample sizes
With regard to the size of the individual trials, participant numbers ranged from 18 to 100.
Setting
The included RCTs were published from 1989 to 2011. Five of them were conducted in the UK (Dawe 2003; Gordon 1999; Green 1992; Kirke 2007; Yones 2006); two, in Turkey (Özdemir 2008; Sezer 2007); the remaining RCTs were conducted in India (Chauhan 2011), Ireland (Markham 2003), Sweden (Larko 1989), Egypt (Salem 2010), Finland (Snellman 2004), and Germany (Storbeck 1993), respectively.
Participants
Most of the included studies recruited adults (≥18 years of age) except for two RCTs (Salem 2010; Storbeck 1993), which recruited participants aged from 13 to 63 years and 17 to 66 years, respectively. In addition, another RCT (Green 1992) did not report the age of the participants.
Most of the included RCTs (Chauhan 2011; Dawe 2003; Gordon 1999; Kirke 2007; Markham 2003; Özdemir 2008; Snellman 2004; Yones 2006) focused on chronic plaque psoriasis (CPP), and one RCT (Sezer 2007) paid attention to palmoplantar psoriasis (PPP), while the remaining RCTs (Green 1992; Larko 1989; Salem 2010; Storbeck 1993) included people with different kinds of psoriasis.
Interventions
The following comparisons were identified:
NB‐UVB versus oral PUVA (Chauhan 2011; Gordon 1999; Markham 2003; Yones 2006);
NB‐UVB versus bath PUVA (Dawe 2003; Salem 2010; Snellman 2004);
NB‐UVB versus topical PUVA (Sezer 2007);
re‐NB‐UVB versus re‐PUVA (Green 1992; Özdemir 2008);
NB‐UVB versus selective BB‐UVB (Kirke 2007);
NB‐UVB versus conventional BB‐UVB (Larko 1989; Storbeck 1993); and
NB‐UVB + dithranol versus conventional BB‐UVB + dithranol (Storbeck 1993).
In most included trials, NB‐UVB was performed three times weekly, except in two trials (Gordon 1999; Yones 2006), which carried out NB‐UVB twice a week. In addition, BB‐UVB was conducted three to five times weekly (Kirke 2007; Larko 1989; Storbeck 1993); bath PUVA, two (Dawe 2003) or three (Salem 2010; Snellman 2004) times weekly; oral PUVA was performed two (Gordon 1999; Green 1992; Markham 2003; Yones 2006) or three (Chauhan 2011; Özdemir 2008) times weekly; and topical PUVA was conducted three times weekly (Sezer 2007).
Outcomes
Outcome measurements were very variable. For example, some included RCTs reported "complete clearance" as their primary outcome, whereas others applied "minimal residual activity (MRA)" or clearance; some applied Psoriasis Area and Severity Index (PASI) score reduction to assess the improvement of psoriasis, whereas others presented the percentage of participants who achieved PASI 75 (which meant equal to or more than 75% reduction in PASI score). It was noteworthy that most of these outcomes were on the basis of judgement from clinicians and were subjective and relatively imprecise. Only one trial (Özdemir 2008) reported the tolerability of the treatment assessed by the participants themselves. Another trial (Yones 2006) assessed the participants' QOL, which is often omitted in clinical practice.
Excluded studies
We excluded seven studies. Our reasons for exclusion are shown in the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
We applied The Cochrane Collaboration's tool for assessing risk of bias. Figure 2 and Figure 3 illustrate the overall risk of bias.
2.
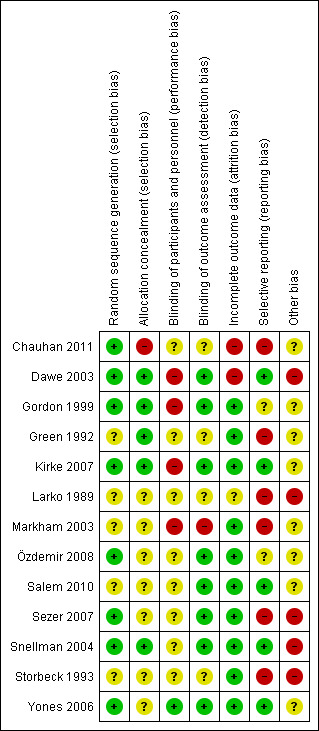
'Risk of bias' summary: Review authors' judgements about each 'Risk of bias' item for each included study
3.
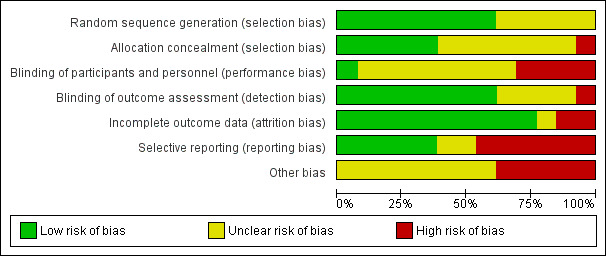
'Risk of bias' graph: Review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
Randomisation and concealment of allocation are thought to be essential components of a RCT to minimise bias. All included trials were reported as "randomised"; however, in five of them (Green 1992; Larko 1989; Markham 2003; Salem 2010; Storbeck 1993), no further methodological details were given, so we assessed these as having an 'unclear' risk of bias for this domain. In four of the included RCTs (Dawe 2003; Sezer 2007; Snellman 2004; Storbeck 1993), randomisation was conducted within participants; in other words, the left and right side of the participant's body were randomly allocated into different groups.
Seven included RCTs (Larko 1989; Markham 2003; Özdemir 2008; Salem 2010; Sezer 2007; Storbeck 1993; Yones 2006) did not explicitly report whether allocation concealment was performed or not, so we assessed these as having an unclear risk of bias for this domain. Another RCT (Chauhan 2011) clearly stated that "the random allocation list was not concealed," so we assessed this as at a high risk of bias for this domain.
Blinding
We evaluated blinding of participants and personnel and blinding of outcome assessment separately. We applied the former to check performance bias, whereas the latter was to check detection bias. Only one RCT (Yones 2006) performed blinding of participants and personnel. The reason may be that different devices and therapy schemes are needed to perform different types of UV irradiation, and consequently, it is hard to mask phototherapists and participants. In addition, eight RCTs (Dawe 2003; Gordon 1999; Kirke 2007; Özdemir 2008; Salem 2010; Sezer 2007; Snellman 2004; Yones 2006) performed blinding of the outcome assessment.
Incomplete outcome data
We labelled 10 of the 13 included studies as 'low risk of bias' in this regard. In most of the included trials, the rate of dropouts was lower than 20%, and the reasons were clearly reported and the withdrawals distributed equally between the groups. To be more specific, the rate of discontinuation in the included studies ranged from 0% (Green 1992; Storbeck 1993) to 36% (Dawe 2003). It was less than 10% in five RCTs (Gordon 1999; Green 1992; Salem 2010; Storbeck 1993; Yones 2006), 10% to 20% in six RCTs (Chauhan 2011; Kirke 2007; Markham 2003; Özdemir 2008; Sezer 2007; Snellman 2004), and more than 20% in one RCT (Dawe 2003). In Chauhan 2011, 16% of the participants discontinued the trial, and when assessing "time to relapse", only 57% of the participants were available for analysis. We assessed this study at 'high risk of bias'. One RCT (Larko 1989) did not report the rate of discontinuation, so we assessed this as unclear.
An intention‐to‐treat (ITT) analysis is often recommended as the least biased way to estimate intervention effects in RCTs (Higgins 2011). Three included RCTs (Dawe 2003; Kirke 2007; Snellman 2004) applied ITT analyses. In Dawe 2003, 10 (36%) participants discontinued the study, which might have induced significant attrition bias. As a result, we labelled this trial as 'high risk of bias', although ITT analyses were applied.
Selective reporting
Almost all included trials had no preliminarily published protocol or were not registered in any clinical trial database, except for one (Kirke 2007). In five trials (Dawe 2003; Kirke 2007; Salem 2010; Snellman 2004; Yones 2006), all outcomes described in their methods section were reported appropriately with statistical data in the results section, and in consequence, we labelled them as 'low risk of bias'. In two trials (Gordon 1999; Özdemir 2008), there was insufficient information to make a judgement. We labelled four RCTs (Larko 1989; Markham 2003; Sezer 2007; Storbeck 1993) at 'high risk of bias' where some outcomes were not supported by statistical data. Chauhan 2011 did not report in their results section some outcomes described in their methods section, and in Green 1992, the authors reported mean and range in the main outcomes, but not P values or 95% CIs, so we labelled these two studies as at 'high risk of bias' for this domain.
Other potential sources of bias
Five trials (Dawe 2003; Larko 1989; Sezer 2007; Snellman 2004; Storbeck 1993) conducted randomisation within participants, and as a result, withdrawal of one half‐body for any reason inevitably caused withdrawal of the other half. In addition, because each participant received both treatment regimens, the treatment to one side might have affected the other. These effects might have induced other potential biases. In the other eight trials, there was insufficient information to make a judgement.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
We made the decision to move one of our prespecified secondary outcomes to primary outcome 4 and rename it 'clearance rate'. We also added further outcomes to our secondary outcomes. We have explained our reasoning for making this change to our published protocol in the Differences between protocol and review section.
Please read this section with the following summaries:
Table 1: NB‐UVB compared with oral PUVA for chronic plaque psoriasis;
Table 2: NB‐UVB compared with bath PUVA for chronic plaque psoriasis;
Table 3: NB‐UVB compared with topical PUVA for palmoplantar psoriasis;
Table 4: NB‐UVB plus retinoid compared with PUVA plus retinoid for chronic plaque or guttate psoriasis; and
Table 5: NB‐UVB compared with selective BB‐UVB for chronic plaque psoriasis.
1. NB‐UVB compared with oral PUVA for chronic plaque psoriasis
Primary outcomes
1) Participant‐rated global improvement
No included RCTs addressed this outcome for this comparison.
2) Percentage of participants reaching PASI 75
Only one trial (Chauhan 2011) reported the percentage of participants with chronic plaque psoriasis (CPP) who reached PASI 75. Seventeen of 21 (80.9%) participants in the NB‐UVB group compared with 18 of 22 (81.8%) participants in the oral PUVA group reached PASI 75; the difference was not statistically significant (RR 0.99, 95% CI 0.74 to 1.32; N = 43; Analysis 1.1). Chauhan 2011 did not perform ITT analysis. As mentioned in the Methods section, we considered participants with missing outcomes as treatment failures for dichotomous outcomes and conducted ITT analysis. The result indicated that no significant difference was identified between NB‐UVB and oral PUVA groups (RR 0.91, 95% CI 0.63 to 1.32; N = 51; Analysis 1.2).
1.1. Analysis.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 1 PASI 75.
1.2. Analysis.
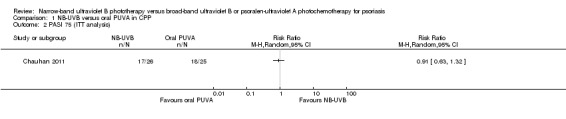
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 2 PASI 75 (ITT analysis).
3) Withdrawal due to side‐effects
Pooled data from three trials (Gordon 1999; Markham 2003; Yones 2006) indicated that withdrawals due to adverse events were not significantly different between the NB‐UVB group and the oral PUVA group in participants with CPP (RR 0.69, 95% CI 0.19 to 2.43; N = 231; Analysis 1.3). The ITT analysis revealed a similar result (RR 0.71, 95% CI 0.20 to 2.54; N = 247; Analysis 1.4).
1.3. Analysis.
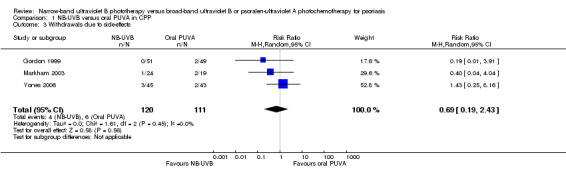
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 3 Withdrawals due to side‐effects.
1.4. Analysis.
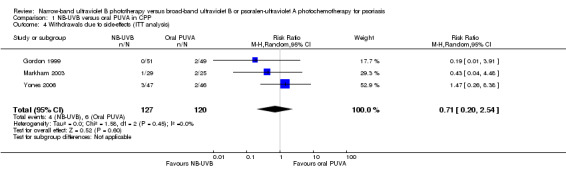
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 4 Withdrawals due to side‐effects (ITT analysis).
4) Clearance rate
Three trials (Gordon 1999; Markham 2003; Yones 2006) compared NB‐UVB to oral PUVA with respect to clearance rate in participants with CPP. Because we identified statistically significant heterogeneity between the three studies (I² statistic = 91%), we did not pool the data. Among them, Yones 2006 did not perform ITT analysis, and the result showed that the clearance rate was 51.1% in the NB‐UVB group and 79.1% in the oral PUVA group (RR 0.65, 95% CI 0.47 to 0.89; N = 88; Analysis 1.5). We conducted ITT analysis using the data of Yones 2006 and found a very similar result: The clearance rate was 48.9% in the NB‐UVB group and 73.9% in the oral PUVA group (RR 0.66, 95% CI 0.47 to 0.93; N = 93; Analysis 1.6). Gordon 1999 performed ITT analysis and found that the clearance rate was 62.7% in the NB‐UVB group and 83.7% in the oral PUVA group (RR 0.75, 95% CI 0.59 to 0.96; N = 100; Analysis 1.6). Markham 2003 also performed ITT analysis; however, there was no significant difference between the NB‐UVB and the oral PUVA groups with respect to clearance rate (96.6% versus 96%; RR 1.01, 95% CI 0.91 to 1.12; N = 54; Analysis 1.6).
1.5. Analysis.
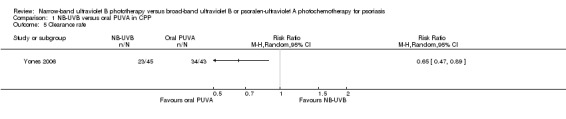
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 5 Clearance rate.
1.6. Analysis.
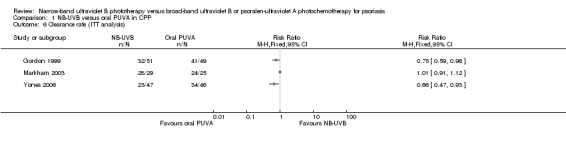
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 6 Clearance rate (ITT analysis).
Secondary outcomes
1) The Physician's Global Evaluation score
No included RCTs addressed this outcome for this comparison.
2) Dermatology Life Quality Index (DLQI)
Yones 2006 reported DLQI as an outcome in participants with CPP, which is a simple practical tool for assessing the QOL of people with skin diseases (Finlay 1994). The reduction of DLQI scores was statistically significantly greater in the oral PUVA group than in the NB‐UVB group (the Mann‐Whitney test, Z = ‐2.4, P = 0.02). In other words, the participants' QOL in the oral PUVA group was improved more than in the NB‐UVB group.
3) Number of treatments to clearance
Three included trials (Gordon 1999; Markham 2003; Yones 2006) reported this outcome in participants with CPP. We could not perform meta‐analysis because of insufficient data. Gordon 1999 showed the median number of treatments to clearance was 25.3 for NB‐UVB and 16.7 for oral PUVA (P < 0.001). Markham 2003 reported the median number of treatments to clearance was 25.5 for NB‐UVB and 19 for oral PUVA (the Mann‐Whitney test, P = 0.03). Yones 2006 found the median number of treatments to clearance was 28.5 for NB‐UVB and 17 for oral PUVA, and the difference was statistically significant (the Mann‐Whitney test, Z = ‐3.7, P < 0.01).
4) Cumulative UV dose to clearance
There is evidence that lower cumulative UV dose is relevant to lower risk of skin cancer (Godar 2003). In the study by Gordon 1999, in participants with CPP, the median cumulative UV dose to clearance was 35 J/cm² for NB‐UVB and 75.1 J/cm² for oral PUVA. However, the study authors did not clearly describe whether the difference between the two groups was statistically significant.
5) Time to clearance
In the study by Markham 2003, in people with CPP, the median time to clearance in the NB‐UVB group was 66 days, whereas it was 67 days in the oral PUVA group. The difference between the two groups did not reach statistical significance (P = 0.46).
6) Clearance lasting six months
In the study by Yones 2006 in people with CPP, more skin lesions in the oral PUVA group achieved clearance lasting six months, which was statistically significant compared with those in the NB‐UVB group (RR 0.51, 95% CI 0.28 to 0.94; N = 47; Analysis 1.7).
1.7. Analysis.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 7 Clearance lasting 6 months.
7) PASI score reduction (before and after treatment)
No included RCTs addressed this outcome for this comparison.
8) Time to PASI 75
In the study by Chauhan 2011, in participants with CPP, the mean time to PASI 75 was 9.9 weeks in both NB‐UVB and oral PUVA groups (mean difference (MD) 0.00, 95% CI ‐2.03 to 2.03; N = 43; Analysis 1.8).
1.8. Analysis.
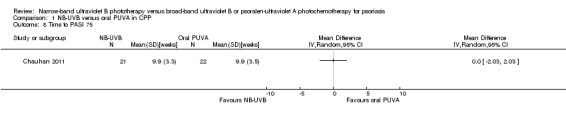
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 8 Time to PASI 75.
9) Relapse rate
Three included trials, which were conducted in participants with CPP, reported this outcome (Chauhan 2011; Gordon 1999; Markham 2003). Pooled data showed that the lesions in 36 of 90 (40%) participants who received NB‐UVB compared with 31 of 82 (37.8%) participants who received oral PUVA group relapsed at six months after treatment completion, but the difference between groups did not reach statistical significance (RR 1.08, 95% CI 0.74 to 1.58; N = 162; Analysis 1.9). These studies defined relapse as 50% of the original extent of the lesions.
1.9. Analysis.
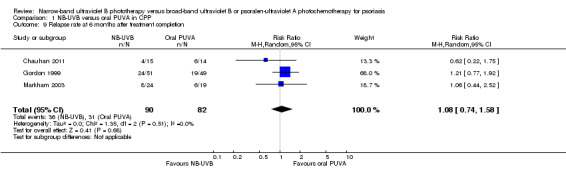
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 9 Relapse rate at 6 months after treatment completion.
10) Duration of remission
In the study by Markham 2003, the median duration of remission for participants with CPP was 288.5 days in the NB‐UVB group and 231 days in the oral PUVA group; however, the difference between groups was not statistically significant (P = 0.40). The study did not explicitly define remission.
11) Withdrawal due to poor response
In the study by Gordon 1999, in participants with CPP, withdrawals due to poor response were significantly more in the NB‐UVB group than in the oral PUVA group (29.4% versus 6%; RR 4.80, 95% CI 1.48 to 15.57; N = 100; Analysis 1.10).
1.10. Analysis.
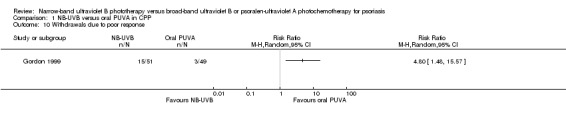
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 10 Withdrawals due to poor response.
12) Clinical improvement
No included RCTs addressed this secondary outcome for this comparison.
13) Reduction of peripheral T cells
No included RCTs addressed this secondary outcome for this comparison.
14) Tolerability
No included RCTs addressed this secondary outcome for this comparison.
15) Adverse events
Four RCTs (Chauhan 2011; Gordon 1999; Markham 2003; Yones 2006) addressed the following adverse events conducted in participants with CPP: erythema (in different degrees), pruritus, polymorphic light eruption (PMLE), nausea, and folliculitis (Analysis 1.11). They were generally slight and reversible. Chauhan 2011 indicated that the incidence of any adverse events was not significantly different between NB‐UVB and PUVA groups (RR 0.92, 95% CI 0.40 to 2.08; N = 43; Analysis 1.11, see Analysis 1.11.7).
1.11. Analysis.
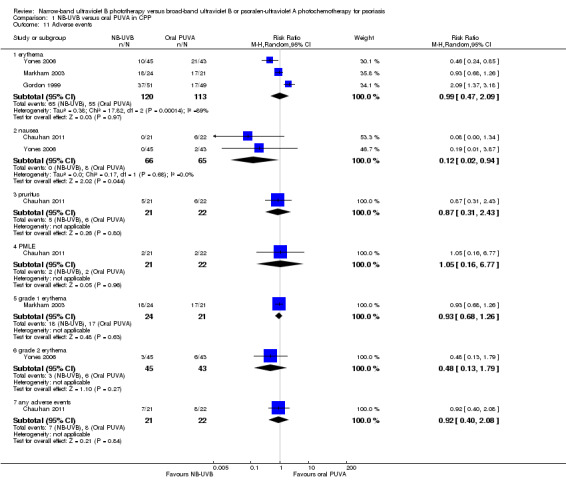
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 11 Adverse events.
Pooled data from three trials (Gordon 1999; Markham 2003; Yones 2006) indicated that the incidence of erythema was comparable between NB‐UVB and oral PUVA groups (RR 0.99, 95% CI 0.47 to 2.09; N = 233; Analysis 1.11, see Analysis 1.11.1). Similarly, no significant difference was identified between NB‐UVB and oral PUVA groups with respect to grade one erythema (Markham 2003; RR 0.93, 95% CI 0.68 to 1.26; N = 45; Analysis 1.11, see Analysis 1.11.5) and grade two erythema (Yones 2006; RR 0.48, 95% CI 0.13 to 1.79; N = 88; Analysis 1.11, see Analysis 1.11.6).
Pooled data from two trials (Chauhan 2011; Yones 2006) showed the incidence of nausea was significantly lower in the NB‐UVB group than in the oral PUVA group (0% versus 12.3%; RR 0.12, 95% CI 0.02 to 0.94; N = 131; Analysis 1.11, see Analysis 1.11.2).
Furthermore, Chauhan 2011 found that the incidence of pruritus was not significantly different between NB‐UVB and PUVA groups (23.8% versus 27.3%; RR 0.87, 95% CI 0.31 to 2.43; N = 43; Analysis 1.11, see Analysis 1.11.3), or between the NB‐UVB and PUVA groups with respect to PMLE (9% versus 9.5%; RR 1.05, 95% CI 0.16 to 6.77; N = 43; Analysis 1.11, see Analysis 1.11.4).
2. NB‐UVB compared with bath PUVA for chronic plaque psoriasis
Primary outcomes
Only one primary outcome was addressed for this comparison.
4) Clearance rate
Three trials (Dawe 2003; Salem 2010; Snellman 2004) compared NB‐UVB to bath PUVA in participants with CPP. Among them, Dawe 2003 and Snellman 2004 conducted left‐right body comparisons while Salem 2010 conducted comparisons between participants. Pooled data from Dawe 2003 and Snellman 2004 indicated that no significant difference between the two groups were identified (RR 2.03, 95% CI 0.29 to 14.06; N = 35; Analysis 2.1). However, Salem 2010 found that two of 16 participants (12.5%) in the NB‐UVB group compared with 11 of 18 participants (61.1%) in the bath PUVA groups achieved statistically significant clearance (RR 0.20, 95% CI 0.05 to 0.79; N = 34; Analysis 2.1). Because we identified moderate statistical heterogeneity between Dawe 2003 and Snellman 2004 (I² statistic = 74%), the pooled data should be interpreted with caution.
2.1. Analysis.
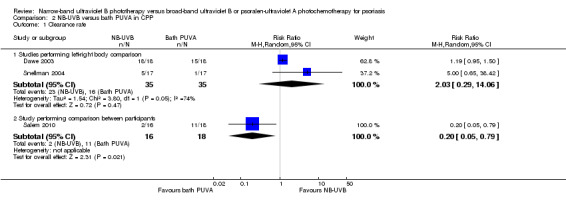
Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 1 Clearance rate.
Additionally, we conducted ITT analyses. The pooled data from Dawe 2003 and Snellman 2004 indicated that no significant difference between the two groups was identified (RR 1.79, 95% CI 0.46 to 6.91; N = 46; Analysis 2.2). Again, because of the moderate statistical heterogeneity between Dawe 2003 and Snellman 2004 (I² statistic = 52%), the pooled data should be interpreted with caution. However, the ITT analysis of Salem 2010 found that more participants in the bath PUVA group achieved clearance than those in the NB‐UVB group (RR 0.18, 95% CI 0.18 to 0.71; N = 36; Analysis 2.2).
2.2. Analysis.
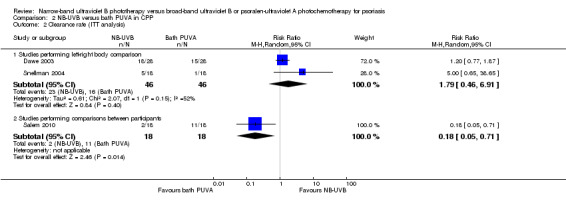
Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 2 Clearance rate (ITT analysis).
Secondary outcomes
The following four of our secondary outcomes were addressed for this comparison.
3) Number of treatments to clearance
Dawe 2003 showed that the median number of treatments to clearance for participants with CPP was 24.5 for NB‐UVB and 19 for bath PUVA, and the difference was statistically significant (P = 0.001).
7) PASI score reduction (before and after treatment)
Salem 2010 compared the PASI score reduction before and after therapy between groups in participants with CPP. The greater the reduction in score, the better the improvement in the lesions. The mean PASI score reduction was 11.71 in the NB‐UVB group and 22.51 in the bath PUVA group (MD ‐10.80, 95% CI ‐16.23 to ‐5.37; N = 34; Analysis 2.3), which was statistically in favour of bath PUVA. In the study by Dawe 2003, the median PASI score reduction was 20 in the NB‐UVB group and 17.5 in the bath PUVA group (P = 0.04).
2.3. Analysis.

Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 3 PASI score reduction.
13) Reduction of peripheral T cells
In the study by Salem 2010, in participants with CPP, the mean reduction (before‐after treatment values) of percentage of CD4+ T cells was significantly lower in NB‐UVB group than in the bath PUVA group (P = 0.03), but there was no significant difference between groups with respect to the mean change of CD8+ T cells (P = 0.27).
15) Adverse events
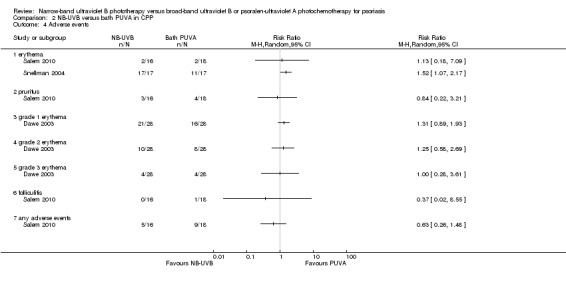
The following adverse events were addressed in three RCTs (Dawe 2003; Salem 2010; Snellman 2004) conducted in participants with CPP: erythema (in different degrees), pruritus, and folliculitis (Analysis 2.4).
2.4. Analysis.
Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 4 Adverse events.
Salem 2010 (N = 34) found no significant difference between groups with respect to the incidence of erythema (RR 1.13, 95% CI 0.18 to 7.09; N = 34; see Analysis 2.4.1), pruritus (RR 0.84, 95% CI 0.22 to 3.21; N = 34; see Analysis 2.4.2), and folliculitis (RR 0.37, 95% CI 0.02 to 8.55; N = 34; see Analysis 2.4.6).
The study by Dawe 2003 (N = 28) found no significant difference between groups in terms of grade one erythema (RR 1.31, 95% CI 0.89 to 1.93; see Analysis 2.4.3), grade two erythema (RR 1.25, 95% CI 0.58 to 2.69; see Analysis 2.4.4), and grade three erythema (RR 1.00, 95% CI 0.28 to 3.61; see Analysis 2.4.5).
However, Snellman 2004 (N = 17) found that erythema was more frequent in the NB‐UVB group than in the PUVA group (RR 1.52, 95% CI 1.07 to 2.17; see Analysis 2.4.1), which was statistically significant.
3. NB‐UVB compared with topical PUVA for palmoplantar psoriasis
Primary outcomes
Only one primary outcome was addressed for this comparison.
4) Clearance rate
Sezer 2007 conducted this within‐patient study on people with PPP. Compared with the topical PUVA treated sides, the NB‐UVB treated sides appeared harder to achieve clearance (0% versus 23.8%). However, the difference did not reach statistical significance (RR 0.09, 95% CI 0.01 to 1.55; N = 21; Analysis 3.1). Sezer 2007 did not perform ITT analysis. However, we carried out ITT analysis. The ITT analysis gave a very similar result (RR 0.09, 95% CI 0.01 to 1.56; N = 25; Analysis 3.2).
3.1. Analysis.
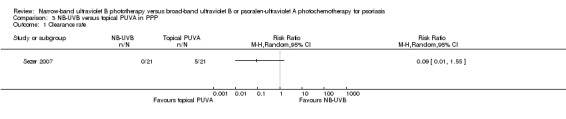
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 1 Clearance rate.
3.2. Analysis.
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 2 Clearance rate (ITT analysis).
Secondary outcomes
The following three of our secondary outcomes were addressed for this comparison.
9) Relapse rate
In the study by Sezer 2007, the skin lesions in 12 of 21 (57.1%) NB‐UVB treated sides compared with seven of 21 (33.3%) topical PUVA treated sides relapsed at nine weeks after treatment completion, but the difference was not statistically significant (RR 1.71, 95% CI 0.84 to 3.48; N = 21; Analysis 3.3). Relapse was defined as an increase in post‐treatment Severity Index scores of PPP (see Table 6).
3.3. Analysis.

Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 3 Relapse at 9 weeks after treatment completion.
12) Clinical improvement
Sezer 2007 compared the effect of NB‐UVB to topical PUVA in participants with PPP. The trial found that 42.9% of the sides treated with NB‐UVB achieved marked clinical improvement, while 71.4% of those sides treated with topical PUVA achieved marked clinical improvement, but the difference was not statistically significant (RR 0.60, 95% CI 0.34 to 1.05; N = 21; Analysis 3.4). In this study, marked clinical improvement was defined as those who had a reduction of 70% or more with respect to the baseline Severity Index scores at nine weeks.
3.4. Analysis.
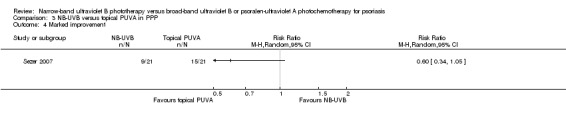
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 4 Marked improvement.
15) Adverse events
Sezer 2007 reported the following adverse events: phototoxicity, palmar hyperpigmentation, and mild xerosis. In this study, one participant dropped out because of a phototoxic reaction in the PUVA treated side. The incidence of palmar hyperpigmentation was significantly lower in the NB‐UVB treated side than in the PUVA treated side (0% versus 52.4%; RR 0.04, 95% CI 0.00 to 0.69; N = 21; Analysis 3.5). Mild xerosis was observed on both sides of the body and responded to emollients.
3.5. Analysis.
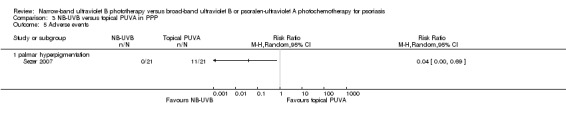
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 5 Adverse events.
4. NB‐UVB plus retinoid (re‐NB‐UVB) compared with PUVA plus retinoid (re‐PUVA) for chronic plaque or guttate psoriasis
Primary outcomes
Two of our primary outcomes were addressed for this comparison.
2) Percentage of participants reaching PASI 75
Only one trial (Özdemir 2008) addressed this comparison in participants with chronic plaque and guttate psoriasis. Özdemir 2008 found no significant difference between the two groups with respect to PASI 75 (RR 0.83, 95% CI 0.58 to 1.19; N = 52; Analysis 4.1). Özdemir 2008 also reported the result of ITT analysis: 17 of 30 (56.7%) participants in the retinoid NB‐UVB group compared with 19 of 30 (63.3%) in the retinoid PUVA group reached PASI 75, but the difference between groups was not statistically significant (RR 0.89, 95% CI 0.59 to 1.35; N = 60; Analysis 4.2).
4.1. Analysis.
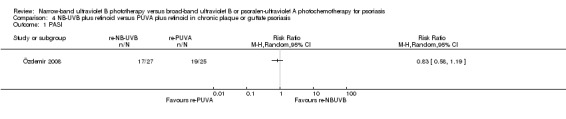
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 1 PASI.
4.2. Analysis.
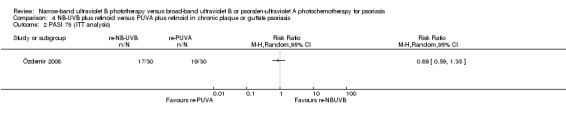
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 2 PASI 75 (ITT analysis).
4) Clearance rate
Özdemir 2008 and Green 1992 addressed this comparison in people with chronic plaque and guttate psoriasis; pooled data found no significant difference between those who were treated with re‐NB‐UVB and those who treated re‐PUVA in terms of clearance rate (RR 0.91, 95% CI 0.78 to 1.07; N = 82; Analysis 4.3). ITT analysis of the pooled data gave a very similar result (RR 0.93, 95% CI 0.79 to 1.10; N = 90; Analysis 4.4).
4.3. Analysis.
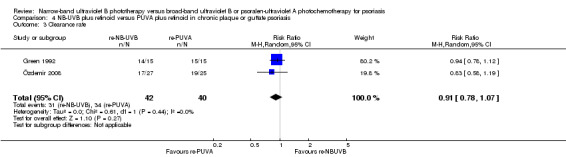
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 3 Clearance rate.
4.4. Analysis.
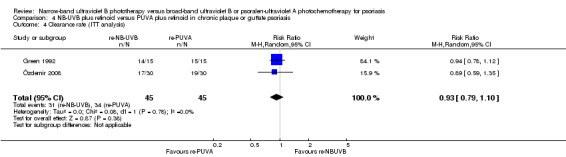
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 4 Clearance rate (ITT analysis).
Secondary outcomes
The following five of our secondary outcomes were addressed for this comparison.
7) PASI score reduction (before and after treatment)
In the study by Özdemir 2008, in participants with chronic plaque and guttate psoriasis, the mean PASI score reduction was not significantly different between the re‐NB‐UVB group and the re‐PUVA group (11.4 versus 12.6, P = 0.83).
9) Relapse rate
Green 1992 found no significant difference between re‐NB‐UVB and re‐PUVA with respect to relapse at six months after treatment completion (60% versus 46.7%; RR 1.29, 95% CI 0.65 to 2.54; N = 30; Analysis 4.5). Relapse was defined as a return of psoriasis to 50% or more of that at baseline.
4.5. Analysis.
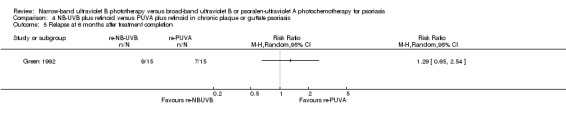
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 5 Relapse at 6 months after treatment completion.
12) Clinical improvement
Özdemir 2008 (N = 60) reported the percentage of participants who achieved a marked improvement (which was defined as 50% to 75% improvement in PASI score), moderate improvement (which referred to 25% to 50% improvement in PASI score), slight improvement (which referred to 5% to 25% improvement in PASI score), or no improvement (which was defined as less than 5% improvement in PASI score). Using ITT analyses, no significant differences were found between the re‐NB‐UVB group and the re‐PUVA group with respect to marked improvement (RR 1.00, 95% CI 0.28 to 3.63; Analysis 4.6, see Analysis 4.6.1), moderate improvement (RR 4.00, 95% CI 0.47 to 33.73; Analysis 4.6, see Analysis 4.6.2), slight improvement (RR 2.00, 95% CI 0.19 to 20.90; Analysis 4.6, see Analysis 4.6.3), or no improvement (RR 0.60, 95% CI 0.16 to 2.29; Analysis 4.6, see Analysis 4.6.4).
4.6. Analysis.
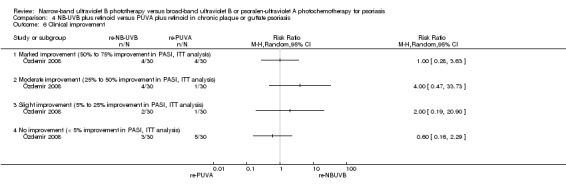
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 6 Clinical improvement.
14) Tolerability
Özdemir 2008 (N = 60) showed there was no significant difference in the tolerability of re‐NB‐UVB or re‐PUVA when assessed by the clinicians (RR 1.05, 95% CI 0.76 to 1.44; Analysis 4.7) or by the participants themselves (RR 1.05, 95% CI 0.73 to 1.53; Analysis 4.8).
4.7. Analysis.

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 7 Tolerability assessed as good or very good by observers (ITT analysis).
4.8. Analysis.

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 8 Tolerability assessed as good or very good by participants (ITT analysis).
15) Adverse events
Two RCTs (Green 1992; Özdemir 2008) addressed the following adverse events: erythema; pruritus; burning; diffuse hair loss; nausea; reversible hypertriglyceridaemia; dry lips, mouth, skin, and nose; joint pain; nose bleeding; taste loss; muscle pain; paronychia; xerophthalmia; nail fragility; headache; and gastrointestinal events.
In Analysis 4.9, no significant differences were identified between re‐NB‐UVB and re‐PUVA with respect to the incidence of erythema (RR 1.32, 95% CI 0.60 to 2.94; N = 52; see Analysis 4.9.1), diffuse hair loss (RR 1.00, 95% CI 0.07 to 14.55; N = 30; see Analysis 4.9.2), reversible hypertriglyceridaemia (RR 0.33, 95% CI 0.04 to 2.85; N = 30; see Analysis 4.9.3), withdrawal due to pruritus and burning (RR 3.00, 95% CI 0.13 to 68.26; N = 30; see Analysis 4.9.4), or nausea (RR 0.33, 95% CI 0.01 to 7.58; N = 30; see Analysis 4.9.5).
4.9. Analysis.
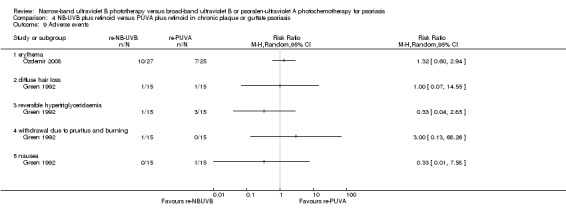
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 9 Adverse events.
5. NB‐UVB compared with selective BB‐UVB for chronic plaque psoriasis
Primary outcomes
The following two of our primary outcomes were addressed for this comparison.
3) Withdrawal due to side‐effects
Kirke 2007 found no significant difference between NB‐UVB and selective BB‐UVB with respect to withdrawals due to adverse events (RR 2.80, 95% CI 0.3 to 25.81; N = 85; Analysis 5.1); Kirke 2007 also performed ITT analysis and found a similar result (RR 3.00, 95% CI 0.32 to 27.87; N = 100; Analysis 5.2).
5.1. Analysis.
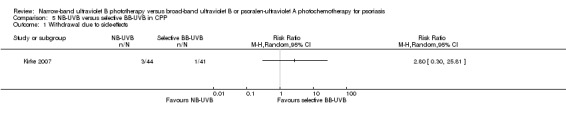
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 1 Withdrawal due to side‐effects.
5.2. Analysis.
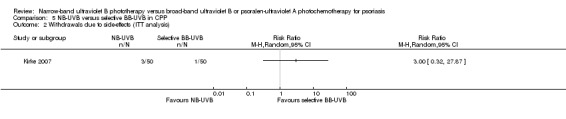
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 2 Withdrawals due to side‐effects (ITT analysis).
4) Clearance rate
Kirke 2007 conducted this comparison in people with CPP. The study found there was no significant difference between the two groups with respect to clearance rate (RR 1.30, 95% CI 0.89 to 1.92; N = 85; Analysis 5.3). Kirke 2007 also performed ITT analysis and found 28 of 50 (56%) participants who received NB‐UVB compared with 20 of 50 (40%) of those who received selective BB‐UVB achieved clearance, but the difference did not reach statistical significance (RR 1.40, 95% CI 0.92 to 2.13; N = 100; Analysis 5.4). Additionally, more participants with skin type III/IV achieved clearance than those with skin type I/II, irrespective of the type of irradiation (odds of clearance = 3.22, 95% CI 1.40 to 7.43).
5.3. Analysis.

Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 3 Clearance rate.
5.4. Analysis.
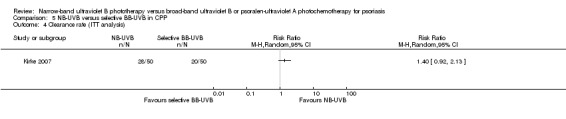
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 4 Clearance rate (ITT analysis).
Secondary outcomes
The following four of our secondary outcomes were addressed for this comparison.
3) Number of treatments to clearance
Based on Kirke 2007, the median number of treatments to clearance was 28.4 for NB‐UVB and 30.4 for selective BB‐UVB, but the difference did not reach statistical significance (P = 0.43). In addition, the authors reported that "patients with skin type III/IV cleared faster than patients with skin type I/II," regardless of the type of irradiation.
4) Cumulative UV dose to clearance
According to the Kirke 2007 trial conducted in participants with CPP, the median cumulative UV dose to clearance was 40.9 J/cm² for NB‐UVB and 39.9 J/cm² for selective BB‐UVB, but they did not report the relevant P value or 95% CI.
6) Clearance lasting six months
Based on a single outcome event in the study by Kirke 2007, no significant difference was found in clearance lasting six months after treatment completion between those in the NB‐UVB and selective BB‐UVB groups (5.3% versus 0%; RR 2.10, 95% CI 0.09 to 47.89; N = 32; Analysis 5.5).
5.5. Analysis.

Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 5 Clearance lasting 6 months.
15) Adverse events
Kirke 2007 (N = 100) reported the following adverse events: severe erythema (which caused the participants to miss treatments), PMLE, and pruritus (Analysis 5.6). There were no significant differences between NB‐UVB and selective BB‐UVB with respect to the incidence of severe erythema (RR 0.67, 95% CI 0.12 to 3.82; see Analysis 5.6.1), PMLE (RR 3.00, 95% CI 0.32 to 27.87; see Analysis 5.6.2), and pruritus (RR 0.20, 95% CI 0.01 to 4.06; see Analysis 5.6.3).
5.6. Analysis.
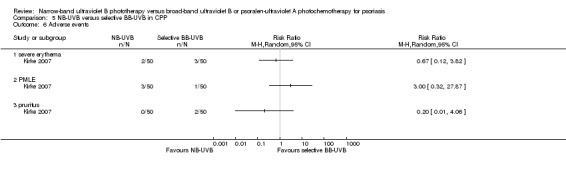
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 6 Adverse events.
6. NB‐UVB compared with conventional BB‐UVB in people with different types of psoriasis
Primary outcomes
No included studies addressed our primary outcomes for this comparison.
Secondary outcomes
Only the following two of our secondary outcomes were addressed for this comparison.
4) Cumulative UV dose to clearance
Two RCTs (Larko 1989; Storbeck 1993) addressed this outcome; both trials conducted half‐body irradiations by left‐right comparison. Because there were insufficient data available in Larko 1989, meta‐analysis could not be performed. In Storbeck 1993 (N = 10), the mean cumulative UV dose during the study that was statistically significant was 14.68 J/cm² with NB‐UVB and 1.427 J/cm² with conventional BB‐UVB (MD 13.25, 95% CI 7.11 to 19.39; Analysis 6.1). By contrast, in Larko 1989, the mean cumulative UV dose was 0.83 J/cm² with NB‐UVB and 4.8 J/cm² with conventional BB‐UVB (P value or 95% CI was not reported).
6.1. Analysis.

Comparison 6 NB‐UVB versus conventional BB‐UVB in different types of psoriasis, Outcome 1 Cumulative UV dose during the study.
7) PASI score reduction (before and after treatment)
Storbeck 1993 compared NB‐UVB with conventional BB‐UVB in 10 participants with different types of psoriasis. The total decrease of the PASI was significantly greater with NB‐UVB than with conventional BB‐UVB (P < 0.05).
7. NB‐UVB plus dithranol compared with conventional BB‐UVB plus dithranol in people with different types of psoriasis
Primary outcomes
No included studies addressed our primary outcomes for this comparison.
Secondary outcomes
Only the following two of our secondary outcomes were addressed for this comparison.
4) Cumulative UV dose to clearance
Storbeck 1993 also compared NB‐UVB plus dithranol with conventional BB‐UVB plus dithranol in 13 participants with different types of psoriasis. The mean cumulative UV dose during the study that was statistically significant was 10.93 J/cm² for NB‐UVB and 1.3 J/cm² for conventional BB‐UVB (MD 9.63, 95% CI 7.09 to 12.17; Analysis 7.1).
7.1. Analysis.

Comparison 7 NB‐UVB plus dithranol versus conventional BB‐UVB plus dithranol in different types of psoriasis, Outcome 1 Cumulative UV dose during the study.
7) PASI score reduction (before and after treatment)
Storbeck 1993 compared NB‐UVB plus dithranol with conventional BB‐UVB plus dithranol in 13 participants with different types of psoriasis. The total decrease of the PASI was statistically significantly greater with NB‐UVB than with conventional BB‐UVB (P < 0.05).
Discussion
Summary of main results
We included 13 RCTs, with 662 participants, in this review, and the main results are listed as follows.
NB‐UVB compared with oral PUVA in people with chronic plaque psoriasis: The percentage of participants reaching PASI 75 showed no statistically significant difference between the NB‐UVB group and the oral PUVA group, and the ITT analysis gave a similar result. Pooled data from three RCTs indicated that withdrawals due to adverse events were not significantly different between the NB‐UVB and the oral PUVA groups, and the ITT analysis gave a similar result. The clearance rate between groups was not consistent within the three included studies because in one, there was no difference between the groups, and in the other two, the clearance rate was statistically significantly in favour of oral PUVA. In one of these two studies, clearance was measured at six months, which was achieved by statistically significantly more participants in the oral PUVA group.
The median number of treatments to clearance was significantly lower in the oral PUVA group compared with NB‐UVB, but time to clearance was similar between the two groups. The cumulative UV dose to clearance, relapse rate at six months after treatment, and duration of remission were not significantly different between the groups. Moreover, the participants' QOL in the oral PUVA group was improved more than in the NB‐UVB group. Nausea was significantly higher in the oral PUVA group.
Narrow‐band UVB compared with bath PUVA in people with chronic plaque psoriasis: The evidence addressing this comparison was not consistent. Two RCTs, which performed left‐right body comparison, found no significant difference between the NB‐UVB and bath PUVA groups, while another RCT, which performed the comparison between participants, favoured bath PUVA. Intention‐to‐treat (ITT) analyses did not significantly change the results.
Narrow‐band UVB compared with topical PUVA in people with palmoplantar psoriasis: There were no significant differences between NB‐UVB treated sides and topical PUVA treated sides in terms of clearance rate, marked improvement rate, and relapse rate. The incidence of palmar hyperpigmentation was statistically significantly higher in the PUVA treated sides.
Retinoid NB‐UVB compared with retinoid PUVA in people with chronic plaque or guttate psoriasis: No significant difference was found between re‐NB‐UVB and re‐PUVA with respect to effectiveness, tolerability, and adverse events, irrespective of using the retinoids, etretinate or acitretin, as adjuvant therapy.
Narrow‐band UVB compared with selective BB‐UVB in people with chronic plaque psoriasis: No significant differences were found between those treated with NB‐UVB and those treated with selective BB‐UVB in terms of withdrawal due to side‐effects, clearance rate, number of treatments to clearance, cumulative UV dose to clearance, and adverse events.
Narrow‐band UVB compared with conventional BB‐UVB in people with different types of psoriasis: Based on one small RCT, NB‐UVB seemed to be more effective than conventional BB‐UVB. However, cumulative UV dose to clearance in both groups was not consistent between the two included RCTs.
Narrow‐band UVB plus dithranol compared with conventional BB‐UVB plus dithranol in people with different types of psoriasis: Based on a small RCT, NB‐UVB plus dithranol seemed to be more effective than conventional BB‐UVB plus dithranol. However, cumulative UV dose to clearance was higher in the NB‐UVB group than the BB‐UVB group.
Overall completeness and applicability of evidence
Most included RCTs in this review were conducted in adults with psoriasis, but one RCT (Salem 2010) enrolled participants aged more than 13 years, while another RCT (Green 1992) did not report the age of the participants. The results of this review should therefore be applied to adults as the literature regarding the use of phototherapy in paediatric patients with psoriasis is limited (Menter 2010). In addition, these RCTs either did not include or separately reported pregnant women, and in consequence, our review did not contribute to this specific population. However, a recent guideline reported that NB‐UVB has been used successfully in pregnant women with psoriasis and "should be considered first‐line therapy in pregnant women with plaque and guttate psoriasis who need a systemic approach to treatment" (Menter 2010). Moreover, because most of the included participants suffered from chronic plaque psoriasis (CPP), the evidence for guttate psoriasis and palmoplantar psoriasis was limited, and only one trial (NB‐UVB compared with selective BB‐UVB) included participants with erythrodermic psoriasis.
In recent years, NB‐UVB has replaced conventional BB‐UVB as the first‐line treatment for psoriasis and has been recommended by US and UK guidelines (Menter 2010; Smith 2009), respectively, or used in clinical practice. In the most recently published guideline (Paul 2012), neither conventional or selective BB‐UVB has been mentioned. In this review, we identified only two RCTs (Larko 1989; Storbeck 1993) that compared NB‐UVB with conventional BB‐UVB; both of the studies were of high risk of bias and small sample sizes. They gave contrasting results with respect to cumulative UV dose to clearance; however, Storbeck 1993 showed that NB‐UVB achieved a greater PASI score reduction than conventional BB‐UVB. It is noteworthy that many non‐RCTs (Coven 1997; Karvonen 1989; Picot 1992; Walters 1999) indicate that NB‐UVB is preferable to conventional BB‐UVB. The dosage and duration of phototherapy in different trials varied from each other, and no RCT so far has directly compared different dosing strategies and frequency of application.
Most included RCTs applied clearance, minimal residual activity (MRA), PASI, and clinical improvement as the main outcomes, which were subjective and measured by clinicians. Only one RCT (Yones 2006) assessed quality of life, an important outcome for people with psoriasis.
Additionally, the risk of carcinogenesis as a result of phototherapy attracted the greatest concern by the participants and clinicians. Because of the limited duration of follow‐up, none of the included RCTs addressed this important issue. A clear relationship between cumulative PUVA exposure and an increased risk of skin cancer had been established (Naldi 2010; Paul 2012; Smith 2009), but there is controversy regarding the risk of skin cancer with NB‐UVB or BB‐UVB (Weischer 2004). Young 1995 summarised data from murine studies and reported that NB‐UVB might be two to three times more carcinogenic per minimal erythema dose (MED) than conventional BB‐UVB. However, the following systematic reviews of trials conducted in people with psoriasis (Hearn 2008; Lee 2005; Pasker‐de 1999) found that UVB did not increase the risk of skin cancer. Most recently, Archier 2012 found no robust evidence of carcinogenic risk of NB‐UVB because of limited prospective studies.
Quality of the evidence
The included trials were of varying methodological quality. In general, these trials did not fully follow good practice conduct and reporting guidelines, such as CONSORT (Consolidated Standards of Reporting Trials) (Schulz 2010). First, although all of the studies stated the participants (or half‐bodies) were randomly allocated, four of them (Green 1992; Markham 2003; Salem 2010; Storbeck 1993) did not explicitly report the methods of randomisation. Second, allocation concealment was not clearly mentioned in six trials (Markham 2003; Özdemir 2008; Salem 2010; Sezer 2007; Storbeck 1993; Yones 2006), while another trial (Chauhan 2011) explicitly stated that allocation concealment was not performed. Insufficient randomisation or allocation concealment might cause potential selection bias. Third, using clearance, minimal residual activity (MRA), or PASI score as the end points was subjective and relatively imprecise. Thus, it is important to blind the evaluating observers to treatment allocation and treatment supervision. One included trial (Markham 2003) did not apply blinding; the other three trials (Chauhan 2011; Green 1992; Storbeck 1993) did not report whether blinding was used or not. Lack of blinding might cause an overestimation of the effects. It should be noted that different types of phototherapies were performed in different irradiation devices. Besides, PUVA needs use of a photosensitiser (in oral, bath, or topical form), while NB‐UVB does not. Hence, it was hard to blind therapists and participants. Fourth, seven of 12 trials had more than 10% dropouts, but only two of them performed ITT analysis, which may be useful to maintain the unbiased group comparison supplied by randomisation. Lack of ITT analysis might lead to potential biases. Last but not least, the sample sizes of these included trials were generally small, which might compromise the value of the results. In a disease with poorly defined treatment outcome measures, small sample size might lead to an underpowered study.
Potential biases in the review process
We experienced some limitations during the review process. One published study (Nazari 2005) appeared to meet the inclusion criteria, but we have not yet been able to include or exclude this study. Requests for unpublished data from the authors of some included trials failed, and as a consequence, meta‐analysis could not be performed for some outcomes and comparisons. Therefore, the results of this review have to be interpreted with caution.
Agreements and disagreements with other studies or reviews
A systematic review (Archier 2012a), which was published most recently, included three RCTs (Gordon 1999; Markham 2003; Yones 2006) that compared NB‐UVB with PUVA in people with chronic plaque psoriasis. We included all three of these RCTs in our review. Archier 2012a did not include RCTs that compared NB‐UVB with bath PUVA, and the outcomes were slightly different to those in our review: They combined "clearance" with "clearance or MRA" as a single outcome, whereas we reported them separately. In addition, we reported more secondary outcomes; however, the authors drew a similar conclusion, which was that both PUVA and NB‐UVB were effective for treating CPP, but oral PUVA was more effective than NB‐UVB to "clear psoriasis, with fewer sessions, provided longer lasting clearance, and should therefore still be used in appropriate selected patients".
Authors' conclusions
Implications for practice.
Current available evidence is very heterogeneous and has to be interpreted or applied with caution.
According to current limited evidence, in people with chronic plaque psoriasis, oral PUVA, compared with NB‐UVB, leads to longer lasting clearance, a fewer number of treatments, and higher levels of QOL, but more nausea and a similar relapse rate at six months. The clearance rate between oral PUVA and NB‐UVB is contradictory among the included studies. Evidence regarding NB‐UVB versus bath PUVA is contradictory. Retinoids with NB‐UVB and retinoids with PUVA have a similar effect for treating people with chronic plaque or guttate psoriasis. However, the long‐term side‐effects of PUVA, especially the potential risk of carcinogenesis, need to be taken into account. In practice, NB‐UVB may be more convenient to use since exogenous photosensitiser is not required before phototherapy.
Although NB‐UVB is considered ineffective for palmoplantar psoriasis in clinical practice, a small included RCT did not detect a statistically significant difference in the efficacy of NB‐UVB and topical PUVA in clearing palmoplantar psoriasis. This needs to be investigated in the future.
NB‐UVB is more effective than or at least equal to selective BB‐UVB, irrespective of whether it is combined with dithranol.
Evidence regarding NB‐UVB and conventional BB‐UVB is limited and of poor quality; none of the included studies addressed the primary outcomes in this comparison.
Implications for research.
This review highlights the need for further high‐quality research regarding the use of NB‐UVB and PUVA for treating psoriasis. The following key points should be taken into account in future research: a big enough sample size to identify the presumptive difference, strict standardisation of the method of UV irradiation, and appropriate outcomes that matter to people (e.g. quality of life and the cost‐effectiveness of the therapy). Good practice guidelines (e.g. CONSORT) must be followed during the process of study design, implementation, and reporting. In addition, prospective studies regarding the carcinogenic risk of NB‐UVB therapy are urgently needed.
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2016 | Review declared as stable | There were no ongoing studies listed in the last published review, and a search of MEDLINE and PubMed in December 2014 did not find any relevant results. A new search in January 2016 did not reveal any new relevant trials. This review has been deemed stable as an update has not been considered necessary for two successive years. Our Information Specialist will run a new search in 2017 to re‐assess whether an update is needed. |
History
Protocol first published: Issue 12, 2011 Review first published: Issue 10, 2013
| Date | Event | Description |
|---|---|---|
| 23 December 2014 | Amended | There were no ongoing studies listed in the last published review, and a search of MEDLINE and PubMed in December 2014 did not find any relevant results. Thus, an update has not been considered necessary at this time. Our Trials Search Co‐ordinator will run a new search in 2015 to re‐assess whether an update is needed. |
Notes
There were no ongoing studies listed in the last published review, and a search of MEDLINE and PubMed in December 2014 did not find any relevant results. A new search in January 2016 did not reveal any new relevant trials. This review has been deemed stable as an update has not been considered necessary for two successive years. Our Information Specialist will run a new search in 2017 to re‐assess whether an update is needed.
Acknowledgements
The authors appreciate the kind help given by Hywel Williams, Finola Delamere, Elizabeth Doney, and Laura Prescott from the Cochrane Skin Group during the process of drafting the review.
The Cochrane Skin Group editorial base wishes to thank Luigi Naldi who was the Key Editor for this review; Matthew Grainge and Ching‐Chi Chi who were the Statistical and Methods Editors, respectively; the clinical referee, Robert Dawe and another clinical referee who wishes to remain anonymous; and the consumer referee, who also wishes to remain anonymous.
Appendices
Appendix 1. Skin Group Specialised Register search strategy
(psoria* or “palmoplantar* pustulosis” or “pustulosis palmaris et plantaris” or “pustulosis and palms and soles”) and (Phototherap* or Photochemotherap* or “light therap*” or “photodynamic therap*” or “photoradiation therap*” or Ultraviolet or BBUVB or NBUVB or “BB‐UVB” or “NB‐UVB” or “broad band uvb” or “broad band ultraviolet b” or “narrow band uvb” or “narrow band ultraviolet b” or psoralen or PUVA)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 (psoria*):ti,ab,kw or (palmoplantar* pustulosis):ti,ab,kw or (pustulosis palmaris et plantaris):ti,ab,kw or (pustulosis and palms and soles):ti,ab,kw #2 MeSH descriptor Psoriasis, this term only #3 (#1 OR #2) #4 MeSH descriptor Phototherapy, this term only #5 MeSH descriptor Ultraviolet Therapy, this term only #6 MeSH descriptor PUVA Therapy, this term only #7 MeSH descriptor Photochemotherapy, this term only #8 (photodynamic therap*):ti,ab,kw or (phototherap*):ti,ab,kw or (photochemotherap*):ti,ab,kw or (puva):ti,ab,kw or (ultraviolet):ti,ab,kw #9 (light therap*):ti,ab,kw or (photoradiation therap*):ti,ab,kw or (BBUVB):ti,ab,kw or (NBUVB):ti,ab,kw or (BB‐UVB or NV‐UVB):ti,ab,kw #10 (broad band uvb):ti,ab,kw or (broad band ultraviolet b):ti,ab,kw or (narrow band uvb):ti,ab,kw or (narrow band ultraviolet b):ti,ab,kw #11 (psoralen ultraviolet a):ti,ab,kw or (psoralen uva):ti,ab,kw #12 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 (#3 AND #12)
Appendix 3. MEDLINE (OVID) search strategy
1. exp Psoriasis/ or psoria$.mp. 2. palmoplantar$ pustulosis.mp. 3. pustulosis palmaris et plantaris.mp. 4. (pustulosis and palms and soles).mp. 5. 1 or 2 or 3 or 4 6. exp Phototherapy/ 7. exp Ultraviolet Therapy/ 8. exp PUVA Therapy/ 9. exp Photochemotherapy/ 10. photodynamic therap$.mp. 11. phototherap$.mp. 12. photochemotherap$.mp. 13. puva.mp. 14. ultraviolet.mp. 15. light therap$.mp. 16. photoradiation therap$.mp. 17. BBUVB.mp. 18. NBUVB.mp. 19. BB‐UVB.mp. 20. NB‐UVB.mp. 21. broad band uvb.mp. 22. broad band ultraviolet b.mp. 23. narrow band uvb.mp. 24. narrow band ultraviolet b.mp. 25. psoralen ultraviolet a.mp. 26. psoralen uva.mp. 27. or/6‐26 28. randomized controlled trial.pt. 29. controlled clinical trial.pt. 30. randomized.ab. 31. placebo.ab. 32. clinical trials as topic.sh. 33. randomly.ab. 34. trial.ti. 35. 28 or 29 or 30 or 31 or 32 or 33 or 34 36. (animals not (human and animals)).sh. 37. 35 not 36 38. 5 and 27 and 37
Appendix 4. EMBASE (OVID) search strategy
1. photodynamic therap$.ti,ab. 2. phototherap$.ti,ab. 3. photochemotherap$.ti,ab. 4. puva.ti,ab. 5. ultraviolet.ti,ab. 6. light therap$.ti,ab. 7. photoradiation therap$.ti,ab. 8. BBUVB.ti,ab. 9. NBUVB.ti,ab. 10. BB‐UVB.ti,ab. 11. NB‐UVB.ti,ab. 12. broad band uvb.ti,ab. 13. broad band ultraviolet b.ti,ab. 14. narrow band uvb.ti,ab. 15. narrow band ultraviolet b.ti,ab. 16. psoralen ultraviolet a.ti,ab. 17. psoralen uva.ti,ab. 18. exp phototherapy/ 19. exp PUVA/ 20. exp photochemotherapy/ 21. or/1‐20 22. exp PSORIASIS/ 23. psoria$.ti,ab. 24. palmoplantar$ pustulosis.ti,ab. 25. pustulosis palmaris et plantaris.ti,ab. 26. (pustulosis and palms and soles).ti,ab. 27. 22 or 23 or 24 or 25 or 26 28. random$.mp. 29. factorial$.mp. 30. (crossover$ or cross‐over$).mp. 31. placebo$.mp. or PLACEBO/ 32. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 33. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 34. (assign$ or allocat$).mp. 35. volunteer$.mp. or VOLUNTEER/ 36. Crossover Procedure/ 37. Double Blind Procedure/ 38. Randomized Controlled Trial/ 39. Single Blind Procedure/ 40. 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. 21 and 27 and 40
Appendix 5. CNKI search strategy (in Chinese)
#1 psoriasis/exp
#2 psoriasis or "palmoplantar pustulosis"
#3 #1 or #2
#4 Phototherapy/exp
#5 Ultraviolet Therapy/exp
#6 PUVA Therapy/exp
#7 Photochemotherapy/exp
#8 photodynamic therapy
#9 phototherapy
#10 photochemotherapy
#11 photoradiation therapy
#12. broad band ultraviolet
#13 narrow band ultraviolet
#14 psoralen ultraviolet
#15 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #3 and #15
Appendix 6. CBM search strategy (in Chinese)
#1 psoriasis/exp
#2 psoriasis or "palmoplantar pustulosis"
#3 #1 or #2
#4 Phototherapy/exp
#5 Ultraviolet Therapy/exp
#6 PUVA Therapy/exp
#7 Photochemotherapy/exp
#8 photodynamic therapy
#9 phototherapy
#10 photochemotherapy
#11 photoradiation therapy
#12. broad band ultraviolet
#13 narrow band ultraviolet
#14 psoralen ultraviolet
#15 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #3 and #15
Appendix 7. Trial registers and OpenGray database search strategy
(psoriasis or palmoplantar pustulosis) and (phototherapy or ultraviolet therapy or photochemotherapy or photoradiation therapy or PUVA or NB‐UVB or BB‐UVB or NBUVB or BBUVB)
Data and analyses
Comparison 1. NB‐UVB versus oral PUVA in CPP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PASI 75 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 PASI 75 (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Withdrawals due to side‐effects | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.19, 2.43] |
| 4 Withdrawals due to side‐effects (ITT analysis) | 3 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.20, 2.54] |
| 5 Clearance rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Clearance rate (ITT analysis) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Clearance lasting 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Time to PASI 75 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Relapse rate at 6 months after treatment completion | 3 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 10 Withdrawals due to poor response | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 erythema | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.09] |
| 11.2 nausea | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.94] |
| 11.3 pruritus | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.31, 2.43] |
| 11.4 PMLE | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.77] |
| 11.5 grade 1 erythema | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.26] |
| 11.6 grade 2 erythema | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.79] |
| 11.7 any adverse events | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.40, 2.08] |
Comparison 2. NB‐UVB versus bath PUVA in CPP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance rate | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Studies performing left‐right body comparison | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.29, 14.06] |
| 1.2 Study performing comparison between participants | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.79] |
| 2 Clearance rate (ITT analysis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Studies performing left‐right body comparison | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.46, 6.91] |
| 2.2 Studies performing comparisons between participants | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.71] |
| 3 PASI score reduction | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 erythema | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 pruritus | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 grade 1 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 grade 2 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 grade 3 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 folliculitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 any adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. NB‐UVB versus topical PUVA in PPP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Clearance rate (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse at 9 weeks after treatment completion | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Marked improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 palmar hyperpigmentation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PASI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 PASI 75 (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clearance rate | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.07] |
| 4 Clearance rate (ITT analysis) | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| 5 Relapse at 6 months after treatment completion | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Clinical improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Marked improvement (50% to 75% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Moderate improvement (25% to 50% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Slight improvement (5% to 25% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 No improvement (< 5% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Tolerability assessed as good or very good by observers (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Tolerability assessed as good or very good by participants (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 diffuse hair loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 reversible hypertriglyceridaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 withdrawal due to pruritus and burning | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. NB‐UVB versus selective BB‐UVB in CPP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to side‐effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Withdrawals due to side‐effects (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clearance rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Clearance rate (ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Clearance lasting 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 severe erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 PMLE | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 pruritus | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. NB‐UVB versus conventional BB‐UVB in different types of psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative UV dose during the study | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. NB‐UVB plus dithranol versus conventional BB‐UVB plus dithranol in different types of psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative UV dose during the study | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chauhan 2011.
| Methods | This was a randomised controlled trial conducted in India | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
51 participants were recruited; 43 of them completed the study Age: 35.7 ± 13.1 years Men: 35 Women: 8 |
|
| Interventions |
Group 1
Group 2
In both groups, no concomitant treatment was allowed except for emollients and antihistamines If no improvement in disease severity was observed after treatment for 6 weeks, the treatment was stopped and considered a treatment failure. The treatment protocol was continued until a participant achieved > 75% reduction in PASI or for up to 4 months, whichever was earlier |
|
| Outcomes |
|
|
| Notes | The trial included participants with skin types IV and V. In addition, the authors defined the following outcomes but did not report them: no response rate, mild improvement rate, and moderate improvement rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned using a computer‐generated random number table |
| Allocation concealment (selection bias) | High risk | Quote: "The random allocation list was not concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors did not clearly state whether blinding was used or not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not clearly state whether blinding was used or not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 (16%) participants discontinued the trial. When assessing "time to relapse", only 29 (57%) participants were available for analysis |
| Selective reporting (reporting bias) | High risk | The following outcomes were described in the methods section, but were not reported in the results section: no response rate, mild improvement rate, and moderate improvement rate |
| Other bias | Unclear risk | Insufficient information was available |
Dawe 2003.
| Methods | This was a randomised, controlled, single‐blind, within‐patient, side‐to‐side comparison trial conducted in the UK from September 1996 to May 1999 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
28 participants were included; 18 of them completed the study Age: 22 to 71 years Men: 17 Women: 11 |
|
| Interventions | The randomisation was performed within participants. Each half‐body (sagittal plane) was treated independently. The side allocated to NB‐UVB therapy was treated first, followed by bath water application of trimethoxypsoralen (TMP), and later on, UVA irradiation to the other side of the body. Hence, the unit of analysis was half of the participant's body Group 1
Group 2
Treatment was stopped when the participant was clear or after the fourth exposure following first documentation of minimal residual activity (MRA), whichever was earlier. Moreover, the authors set a maximum limit of 30 treatments to either side |
|
| Outcomes |
|
|
| Notes | Loss to follow up was very high (36%) in this trial. In addition, randomisation was performed within participants. The unit of analysis was the "half‐body". Furthermore, only participants with skin phototype I to III participated in this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistical book was consulted for a "random number" |
| Allocation concealment (selection bias) | Low risk | The random number was held by a departmental secretary who was not directly involved in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and nurse phototherapists in this study were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The observer was masked |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 (36%) participants were lost to follow up, although it reported findings for ITT analysis (full analysis set) and per‐protocol analysis set |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported in the results of the trial report. In addition, the mean, 95% CI, and P value were all reported for the main outcomes |
| Other bias | High risk | The included participants were atypical of the psoriasis participant population as a whole, because they were more likely to have been treated with PUVA before and appeared to have more treatment‐resistant psoriasis than non‐participants. In other words, the baseline in both groups seemed to be unequal. In addition, the study withdrawal was extremely high, and withdrawal of 1 body‐half for any reason inevitably caused withdrawal of the other half. Third, each participant received both treatment regimens, so the treatment to 1 side might have affected the other. All of these pitfalls might have induced other bias |
Gordon 1999.
| Methods | This was a single‐blind, parallel, randomised, controlled trial conducted in the UK from July 1996 to September 1997 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
100 participants were included; 94 participants completed the study Age: 43.3 ± 12.9 years in the NB‐UVB group; 41.0 ± 11.2 in the PUVA group Gender: not reported |
|
| Interventions |
Group 1
Group 2
Participants whose skin failed to improve significantly after 16 treatments were withdrawn from the trial |
|
| Outcomes |
|
|
| Notes | NB‐UVB was performed twice weekly in this trial; this regimen might not be optimal, as there was evidence that NB‐UVB might be more effective when given more frequently. In addition, the trial included participants with skin type I to IV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was based on randomised permuted blocks within strata" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and phototherapists were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were made by a clinician, unaware of the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6 (6%) participants were lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information was available |
| Other bias | Unclear risk | Insufficient information was available |
Green 1992.
| Methods | This was a parallel, randomised, controlled trial conducted in the UK | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
45 participants were included and completed the study Age: not reported Men: 25 Women: 20 |
|
| Interventions |
Group 1
Group 2
Group 3
|
|
| Outcomes |
|
|
| Notes | This trial included 3 interventions. According to our preliminary protocol, only data regarding NB‐UVB and retinoids versus PUVA and retinoids were extracted and applied in this review. In addition, the trial did not report the participants' skin type | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: The detail of randomisation was not clear |
| Allocation concealment (selection bias) | Low risk | "A sealed code kept in the pharmacy" was used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information was available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost to follow up |
| Selective reporting (reporting bias) | High risk | The authors only reported mean and range in main outcomes, and relevant P value or 95% CI were not stated in the study. We failed to make contact with them to get more information |
| Other bias | Unclear risk | Insufficient information was available |
Kirke 2007.
| Methods | This was a randomised, controlled, single‐blind, parallel trial conducted in the UK from May 2003 to June 2005 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
100 participants were included in the study; 85 of them completed the study Age: 19 to 77 years Men: 45 Women: 55 |
|
| Interventions |
Group 1
Group 2
The initial treatment dose was 70% of the MED, and the dose was increased after alternate treatments by 40%, decreasing stepwise to 5% by the 18th treatment. If erythema developed during treatment, depending on the severity, planned dose increments were postponed or treatments were missed until the erythema resolved. Participants who cleared, and those who did not clear but received at least 16 exposures, were judged to have completed the trial Adjunctive therapy was restricted to emollients |
|
| Outcomes |
|
|
| Notes | The trial included participants with skin type I to IV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was "based on randomised permuted blocks within strata" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation used opaque, sequentially numbered, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and nurse phototherapists were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 (15%) participants discontinued the study. The reason for discontinuation was clearly stated in the study, and the withdrawals were distributed equally between the groups. Furthermore, ITT analysis was used |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the protocol were reported in the results of the trial report. In addition, the mean, 95% CI, and P value were all reported for the main outcomes |
| Other bias | Unclear risk | Insufficient information was available |
Larko 1989.
| Methods | This was a randomised, double‐blind, within‐patient trial conducted in Sweden | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
29 participants were included in this study. The author did not report how many participants completed this study The median age was 35 (range from 19 to 76 years) Men: unclear Women: unclear |
|
| Interventions | The NB‐UVB (TL‐01) and conventional BB‐UVB (TL‐12) treatments were assigned randomly to the left or right side. The maximum irradiation time was set to 30 minutes Group 1
Group 2
Adjunctive therapy was restricted to emollients |
|
| Outcomes |
|
|
| Notes | This was a left‐right comparison study. In addition, the trial did not report the participants' skin type | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the left and right sides of the participants received NB‐UVB (TL‐01) or conventional BB‐UVB (TL‐12), respectively in "randomized order", the method of randomisation was not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available, although the author stated that this study was a "double‐blind" study in the abstract. The method of blinding was not addressed in the report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information was available, although the author stated that this study was a "double‐blind" study in the abstract. The method of blinding was not addressed in the report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The author did not report relevant information |
| Selective reporting (reporting bias) | High risk | The author did not report a P value or 95% CI for most of the outcomes |
| Other bias | High risk | The unit of analysis was the half‐body. Withdrawal of 1 body‐half for any reason inevitably caused withdrawal of the other half. In addition, each participant received both treatment regimens; the treatment to 1 side might have affected the other. All of these pitfalls might have induced other bias |
Markham 2003.
| Methods | This was an open‐label, parallel, randomised, controlled trial conducted in Ireland from January 1999 to June 2000 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
54 participants were included; 45 participants completed the study Age: 27 to 52 years Men: 30 Women: 14 |
|
| Interventions |
Group 1
Group 2
The end point of the study was complete clearance of psoriasis |
|
| Outcomes |
|
|
| Notes | Some outcomes (e.g. grade 2 erythema, pruritus, subgroup analyses according to PASI score, etc) were not fully reported with statistical data. In addition, the trial included only participants with skin type I to III | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although participants were "randomly allocated to either treatment group", the method of randomisation was not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated that it was an "open trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only 1 outcome (namely "remission") was assessed by "a blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 9 (17%) participants discontinued the study, the reason for discontinuation was clearly reported, and the withdrawals were distributed equally between both groups |
| Selective reporting (reporting bias) | High risk | Some outcomes (e.g. grade 2 erythema, pruritus, subgroup analyses according to PASI score, etc) were not supported by statistical data |
| Other bias | Unclear risk | Insufficient information was available |
Salem 2010.
| Methods | This was a randomised controlled trial conducted in Egypt | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
36 participants were included for randomisation, and 34 of them completed the study Age: 13 to 63 years Men: 18 Women: 16 |
|
| Interventions |
Group 1
Group 2
Adjunctive therapy was restricted to emollients |
|
| Outcomes |
|
|
| Notes | PASI evaluation was of the lesions in the trunk and upper and lower extremities. In other words, facial or scalp psoriasis was not taken into account. In addition, PASI score was higher in the bath PUVA group than in the NB‐UVB group. Also, the trial included participants with skin type I to V | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...simple randomisation" Comment: There was no further information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessment of the disease severity before and after treatment was carried out by two dermatologists in an observer‐blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 (6%) participants withdrew from the study after randomisation. The reasons for withdrawal were reported and had no relation to the study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported in the results of the trial report. In addition, the mean, 95% CI, and P value were all reported for the main outcomes |
| Other bias | Unclear risk | Insufficient information was available |
Sezer 2007.
| Methods | This was a randomised, controlled, within‐patient, side‐to‐side comparison trial conducted in Turkey | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
25 participants were included; 21 of them completed the study Age: 19 to 75 years Men: 14 Women: 11 |
|
| Interventions | The NB‐UVB and PUVA treatments were assigned randomly to the left or right hand, foot, or both. The treatments in both groups were used 3 times weekly over 9 weeks Group 1
Group 2
Only topical emollients were allowed between treatment sessions in both groups |
|
| Outcomes |
|
|
| Notes | The unit of analysis was the half‐body. Additionally, the trial did not report the participants' skin type | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐based programme |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinical assessments were performed by a blinded investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (16%) participants dropped out. The reasons for dropouts were reported and unrelated to the study |
| Selective reporting (reporting bias) | High risk | Some outcomes (e.g. cumulative doses) were not fully reported. Standard deviation and P value were omitted |
| Other bias | High risk | The unit of analysis was the half‐body. Withdrawal of 1 body‐half for any reason inevitably caused withdrawal of the other half. In addition, each participant received both treatment regimens; the treatment to 1 side might have affected the other. All of these pitfalls might have induced other bias |
Snellman 2004.
| Methods | This was a randomised, controlled, single‐blind, within‐patient, side‐to‐side comparison trial conducted in Finland from September 2001 to March 2002 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
18 participants were enrolled; 17 of them completed the study Age: 46 ± 12 years Men: 13 Women: 4 |
|
| Interventions | Half‐bodies (left or right) of the included participants were randomly assigned to receive NB‐UVB or bath PUVA. A maximum of 30 treatments of each type of irradiation were given. After disappearance of psoriasis on either treatment side, that treatment was withdrawn, but the other was continued Group 1
Group 2
Adjunctive therapy was restricted to emollients and salicylic acid in white petrolatum |
|
| Outcomes |
|
|
| Notes | The unit of analysis was the half‐body. In addition, the trial included participants with skin type II to IV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was on the basis of "an automatically computed random number table" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes" were used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator was masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (18%) participants discontinued the study; 1 of them withdrew before any interventions or assessments were performed because of his busy schedule and was not analysed. The other 2 withdrew due to personal reasons and deterioration on the PUVA side, respectively. The latter 2 participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported appropriately in the results of the trial report |
| Other bias | High risk | The unit of analysis was the half‐body. Withdrawal of 1 body‐half for any reason inevitably caused withdrawal of the other half. In addition, each participant received both treatment regimens; the treatment to 1 side might have affected the other. All of these pitfalls might have induced other bias |
Storbeck 1993.
| Methods | This was a randomised, controlled, within‐patient, side‐to‐side comparison trial conducted in Germany from October 1989 to May 1990 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
23 participants were included and completed the study Age: 17 to 66 years Gender: not reported |
|
| Interventions |
Group 1
Group 2
Group 3
Group 4
Irradiation was performed 3 to 5 times weekly. The initial dose of both irradiation doses was 70% of the MED. Dose increments of 10% were applied every session if there was no erythema, 5% if there was slight erythema, while no increments were applied in the presence of moderate erythema. Dose decrements of 10% were applied every session if there was marked erythema. Irradiation was suspended if there was burning |
|
| Outcomes |
|
|
| Notes | The unit of analysis was the half‐body. The trial included participants with skin type I to IV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No related information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors did not mention whether the blinding method was used or not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not mention whether the blinding method was used or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants completed the study and were analysed and reported as well |
| Selective reporting (reporting bias) | High risk | Some outcomes were not supported by statistical data. For example, In 11 of 13 patients the Philips TL 01/100 W lamp proved to be more effective than the Sylvania lamp" |
| Other bias | High risk | The unit of analysis was the half‐body. Each participant received both treatment regimens; the treatment on 1 side might have affected the other, which might have induced other bias |
Yones 2006.
| Methods | This was a randomised, placebo‐controlled, double‐blind trial conducted in the UK from April 2002 to March 2005 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
93 participants were included; 88 of them completed the study Men: 64 Women: 9 |
|
| Interventions | Participants were randomly assigned to receive NB‐UVB and PUVA therapy Group 1
Group 2
Adjunctive therapy was restricted to emollients and aqueous cream. Treatment was terminated in the event of any of the following: clearance of psoriasis, absent or minimal improvement after 16 treatments or very slow progress thereafter, intolerance to therapy, or the completion of 30 treatments |
|
| Outcomes |
|
|
| Notes | The trial included participants with skin type I to VI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A sequentially numbered list" was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. It was not clear whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 5 (5%) participants discontinued the study. The withdrawals were distributed equally between both groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported appropriately in the results of the trial report |
| Other bias | Unclear risk | Insufficient information was available |
Özdemir 2008.
| Methods | This was a randomised, controlled, single‐blind, parallel trial conducted in Turkey from August 2005 to Decemeber 2006 | |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
60 participants were included; 52 of them completed the study Age: 37.2 ± 11.6 years in the NB‐UVB group; 36.1 ± 9.9 years in the PUVA group Men: 34 Women: 26 |
|
| Interventions | During the first week, participants in both groups received acitretin (0.3 to 0.5 mg/kg per day). NB‐UVB or PUVA were then started in the second week in the different groups, respectively Group 1
Group 2
During the study and the follow‐up period, additional therapy was restricted to the use of emollients that were applied once daily in the evening Treatments were discontinued when neither improvement nor exacerbation was seen after 6 weeks, or when severe side‐effects occurred or laboratory analyses showed abnormalities |
|
| Outcomes |
|
|
| Notes | The trial included participants with skin type II to V | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised assignment of the two treatments was performed by asking the patients to throw a dice without knowing the underlying allocation criteria" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 (13%) participants discontinued the study. The reasons for discontinuation were clearly reported, and the withdrawals were distributed equally between both groups. ITT analyses were performed for the main outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods section were reported in the results of the trial report |
| Other bias | Unclear risk | Insufficient information was available |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boer 1984 | This was a non‐randomised controlled trial |
| Coven 1997 | This was a non‐randomised controlled trial |
| Dayal 2010 | This was not a real randomised controlled trial. Participants who were recruited on Monday, Wednesday, or Friday received NB‐UVB, whereas those who were recruited on Tuesday, Thursday, or Saturday received PUVA |
| Malhotra 2010 | This was an abstract of a conference paper (not a RCT) |
| Roson 2005 | This was a quasi‐randomised trial |
| Tanew 1996 | This was an abstract of a conference paper; it was a non‐randomised controlled trial |
| Ul 2005 | The authors compared PUVA with UVB in this trial. They did not clearly define the type of UVB they used. Was it NB‐UVB, BB‐UVB, or both of them? We could not draw a conclusion from the paper. And we failed to make contact with the corresponding author to get more information |
Characteristics of studies awaiting assessment [ordered by study ID]
Nazari 2005.
| Methods | This was a randomised controlled trial conducted in Turkey |
| Participants | 32 participants with chronic plaque psoriasis were included |
| Interventions |
|
| Outcomes |
|
| Notes | The study was published in Turkish, and we are awaiting a translation |
Differences between protocol and review
We added some useful information to the background regarding the categories of psoriasis, and the treatments PUVA and BB‐UVB. These additional pieces of information might help readers understand the relevance of the contents more easily.
We moved one of our prespecified secondary outcomes, namely 'Percentage of participants who achieved complete clearance in the clinician's opinion', to a primary outcome, and we also renamed it 'Clearance rate'. The reason we did this is because 'clearance rate' is an important outcome for both clinicians and people with psoriasis, and during the process of working on the review, we identified some studies that reported 'complete clearance' and 'minimal residual activity (MRA)' as an independent outcome named 'clearance', and in our opinion, it was a reasonable change to make.
We also added further outcomes to our secondary outcomes, which we identified while working on the review, and we thought they might be valuable for users to make an optimal treatment choice.
In the protocol, we planned to search for information regarding adverse events from non‐RCTs. However, we did not carry out these further searches for three reasons:
The included RCTs revealed that phototherapy is generally well‐tolerated although some mild adverse events might exist.
The included RCTs had paid much attention to the adverse events.
According to the Cochrane Handbook for Systematic Reviews of Interventions, it is reasonable to use either identical or different eligibility criteria for selecting studies that address beneficial effects and adverse effects.
In addition, in the protocol, we planned to use an alpha of 0.05 for the Chi² test. However, during the process of drafting, we found that the number of trials included in meta‐analyses was few. In this case, we used a P value of 0.10 for the Chi² test.
Contributions of authors
Ming Yang and Xiaomei Chen are joint first authors as they contributed equally to this review. Min Zhang was the contact person with the editorial base, co‐ordinated contributions from the co‐authors, and wrote the final draft of the review. Xiaomei Chen, Yan Cheng, and Ming Yang screened papers against eligibility criteria. Xiaomei Chen and Yan Cheng appraised the quality of the papers. Xiaomei Chen and Ming Yang extracted data for the review and sought additional information about papers. Ming Yang entered data into RevMan. Ming Yang, Xiaomei Chen, and Guanjian Liu analysed and interpreted data. Xiaomei Chen and Ming Yang worked on the methods sections. Xiaomei Chen and Min Zhang drafted the clinical sections of the background. Yan Cheng was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. Min Zhang is the guarantor of the update.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
Dermatology Department, West China Hospital of Sichuan University, China.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
None known.
❖ Joint first author
✢ Joint first author
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Chauhan 2011 {published data only}
- Chauhan PS, Kaur I, Dogra S, De D, Kanwar AJ. Narrowband ultraviolet B versus psoralen plus ultraviolet A therapy for severe plaque psoriasis: an Indian perspective. Clinical & Experimental Dermatology 2011;36(2):169‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dawe 2003 {published data only}
- Dawe RS, Cameron H, Yule S, Man I, Wainwright N J, Ibbotson S H, et al. A randomized controlled trial of narrowband ultraviolet B vs bath‐psoralen plus ultraviolet A photochemotherapy for psoriasis. British Journal of Dermatology 2003;148(6):1194‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gordon 1999 {published data only}
- Gordon PM, Diffey BL, Farr PM. A comparison of narrow‐band TL01 phototherapy and psoralen‐UVA photochemotherapy for psoriasis [Summary]. British Journal of Dermatology 1997;137(Suppl 50):15. [DOI: 10.1111/j.1365-2133.1997.tb01879.x] [DOI] [Google Scholar]
- Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow‐band TL‐01 phototherapy and PUVA photochemotherapy for psoriasis. Journal of the American Academy of Dermatology 1999;41(5 Pt 1):728‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Green 1992 {published data only}
- Green C, Lakshmipathi T, Johnson BE, Ferguson J. A comparison of the efficacy and relapse rates of narrowband UVB (TL‐01) monotherapy vs. etretinate (re‐TL‐01) vs. etretinate‐PUVA (re‐PUVA) in the treatment of psoriasis patients. British Journal of Dermatology 1992;127(1):5‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirke 2007 {published data only}
- Kirke SM, Lowder S, Lloyd J, Matthews JN, Farr PM. A randomized comparison of selective broadband UVB and narrowband UVB in the treatment of psoriasism [Abstract RF‐4]. The 85th BAD Annual Meeting 5‐8th July 2005, Glasgow, UK. British Journal of Dermatology 2005;153(Suppl 1):10. [DOI] [PubMed] [Google Scholar]
- Kirke SM, Lowder S, Lloyd JJ, Diffey BL, Matthews JN, Farr PM. A randomized comparison of selective broadband UVB and narrowband UVB in the treatment of psoriasis. Journal of Investigative Dermatology 2007;127(7):1641‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larko 1989 {published data only}
- Larko O. Treatment of psoriasis with a new UVB‐lamp. Acta Dermato‐Venereologica 1989;69(4):357‐9. [EMBASE: 1989165254] [PubMed] [Google Scholar]
Markham 2003 {published data only}
- Markham T, Rogers S, Collins P. A comparison of oral 8‐methoxypsoralen PUVA and narrowband UVB (TL‐OI) phototherapy in the management of chronic plaque psoriasis [Summary]. British Journal of Dermatology 2001;145(Suppl 59):25. [DOI: 10.1046/j.1365-2133.2001.012ff.x] [DOI] [Google Scholar]
- Markham T, Rogers S, Collins P. Narrowband UV‐B (TL‐01) phototherapy vs oral 8‐methoxypsoralen psoralen‐UV‐A for the treatment of chronic plaque psoriasis. Archives of Dermatology 2003;139(3):325‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Özdemir 2008 {published data only}
- Özdemir M, Engin B, Baysal I, Mevlitoglu I. A randomized comparison of acitretin‐narrow‐band TL‐01 phototherapy and acitretin‐psoralen plus ultraviolet A for psoriasis. Acta Dermato‐Venereologica 2008;88(6):589‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salem 2010 {published data only}
- Salem SA, Barakat MA, Morcos CM. Bath psoralen+ultraviolet A photochemotherapy vs. narrow band‐ultraviolet B in psoriasis: a comparison of clinical outcome and effect on circulating T‐helper and T‐suppressor/cytotoxic cells. Photodermatology, Photoimmunology & Photomedicine 2010;26(5):235‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sezer 2007 {published data only}
- Sezer E, Erbil AH, Kurumlu Z, Tastan HB, Etikan I. Comparison of the efficacy of local narrowband ultraviolet B (NB‐UVB) phototherapy versus psoralen plus ultraviolet A (PUVA) paint for palmoplantar psoriasis. Journal of Dermatology 2007;34(7):435‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Snellman 2004 {published data only}
- Snellman E, Klimenko T, Rantanen T. Randomized half‐side comparison of narrowband UVB and trimethylpsoralen bath plus UVA treatments for psoriasis. Acta Dermato‐Venereologica 2004;84(2):132‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Storbeck 1993 {published data only}
- Storbeck K, Holzle E, Schurer N, Lehmann P, Plewig G. Narrow‐band UVB (311 nm) versus conventional broad‐band UVB with and without dithranol in phototherapy for psoriasis. Journal of the American Academy of Dermatology 1993;28(2 Pt 1):227‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yones 2006 {published data only}
- Yones SS, Garibadinos T, Seed P, Csaszar M, Hawk J. A prospective randomized double blind trial of the efficacy of narrowband UVB (TL01) phototherapy and psoralen photochemotherapy (PUVA) in the treatment of chronic plaque psoriasis (CPP) [Abstract FC11.10]. 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):47. [DOI: 10.1111/j.1468-3083.2005.01310.x] [DOI] [Google Scholar]
- Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double‐blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen‐UV‐A therapy vs narrowband UV‐B therapy. Archives of Dermatology 2006;142(7):836‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boer 1984 {published data only}
- Boer J, Hermans J, Schothorst AA, Suurmond D. Comparison of phototherapy (UV‐B) and photochemotherapy (PUVA) for clearing and maintenance therapy of psoriasis. Archives of Dermatology 1984;120(1):52‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Coven 1997 {published data only}
- Coven TR, Burack LH, Gilleaudeau R, Keogh M, Ozawa M, Krueger JG. Narrowband UV‐B produces superior clinical and histopathological resolution of moderate‐to‐severe psoriasis in patients compared with broadband UV‐B. Archives of Dermatology 1997;133(12):1514‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Dayal 2010 {published data only}
- Dayal S, Mayanka, Jain VK. Comparative evaluation of NBUVB phototherapy and PUVA photochemotherapy in chronic plaque psoriasis. Indian Journal of Dermatology, Venereology & Leprology 2010;76(5):533‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malhotra 2010 {published data only}
- Malhotra V. Comparative efficacy of photochemotherapy and narrow band ultraviolet B radiation in the treatment of severe plaque‐type psoriasis [Summary PD‐9]. Conference: 90th Annual Meeting of the British Association of Dermatologists Manchester United Kingdom. Conference Start: 20100706 Conference End: 20100708. British Journal of Dermatology 2010;163(Suppl 1):134‐5. [EMBASE: 70234375] [Google Scholar]
Roson 2005 {published data only}
- Roson E, Garcia‐Doval I, Florez A, Cruces M. Comparative study of the treatment of psoriasis plaques with PUVA baths and narrow‐band UVA (311 NM). Actas Dermo‐Sifiliograficas 2005;96(6):371‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tanew 1996 {published data only}
- Tanew A, Fijan S, Honigsmann H. Halfside comparison study on narrow‐band UV‐B phototherapy versus photochemotherapy (PUVA) in the treatment of severe psoriasis [Abstract 212]. 1996 Annual Meeting Society for Investigative Dermatology . Journal of Investigative Dermatology 1996;106(4):841. [DOI: 10.1111/1523-1747.ep12346478] [DOI] [Google Scholar]
Ul 2005 {published data only}
- Ul Bari A, Iftikhar N, Ber Rahman S. Comparison of PUVA and UVB therapy in moderate plaque psoriasis. Journal of Pakistan Association of Dermatologists 2005;15(1):26‐31. [EMBASE: 2006474827] [Google Scholar]
References to studies awaiting assessment
Nazari 2005 {published data only}
- Nazari S, Ozarmagan G, Erzengin D, Akar U. A randomized comparison of narrow‐band UVB phototherapy and PUVA photochemotherapy in the management of plaque‐type psoriasis [Psoriasiste PUVA ve dar bant UVB tedavilerinin klinik etkinliklerinin karsilastirilmasi]. Turkderm Deri Hastaliklari ve Frengi Arsivi 2005;39(2):103‐8. [EMBASE: 2006204570] [Google Scholar]
Additional references
Anderson 1984
- Anderson TF, Waldinger TP, Voorhees JJ. UV‐B phototherapy. An overview. Archives of Dermatology 1984;120(11):1502‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Archier 2012
- Archier E, Devaux S, Castela E, Gallini A, Aubin F, Maitre M, et al. Carcinogenic risks of psoralen UV‐A therapy and narrowband UV‐B therapy in chronic plaque psoriasis: a systematic literature review. Journal of the European Academy of Dermatology and Venereology 2012;26(Suppl 3):22‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Archier 2012a
- Archier E, Devaux S, Castela E, Gallini A, Aubin F, Maitre M, et al. Efficacy of psoralen UV‐A therapy vs. narrowband UV‐B therapy in chronic plaque psoriasis: a systematic literature review. Journal of the European Academy of Dermatology and Venereology 2012;26(Suppl 3):11‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ashworth 1989
- Ashworth J, Kahan MC, Breathnach SM. PUVA therapy decreases HLA‐DR+ CDIa+ Langerhans cells and epidermal cell antigen‐presenting capacity in human skin, but flow cytometrically‐sorted residual HLA‐DR+ CDIa+ Langerhans cells exhibit normal alloantigen‐presenting function. British Journal of Dermatology 1989;120(3):329‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aubin 1998
- Aubin F, Humbert P. Immunomodulation induced by psoralen plus ultraviolet A radiation. European Journal of Dermatology 1998;8(3):212‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Aufiero 2006
- Aufiero BM, Talwar H, Young C, Krishnan M, Hatfield JS, Lee HK, et al. Narrow‐band UVB induces apoptosis in human keratinocytes. Journal of Photochemistry & Photobiology 2006;82(2):132‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boer 1980
- Boer J, Schothorst AA, Suurmond D. UV‐B phototherapy of psoriasis. Dermatologica 1980;161(4):250‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boffetta 2001
- Boffetta P, Gridley G, Lindelof B. Cancer risk in a population‐based cohort of patients hospitalized for psoriasis in Sweden. Journal of Investigative Dermatology 2001;117(6):1531‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borroni 1991
- Borroni G, Zaccone C, Vignati G, Fietta A, Gatti M, Brazzelli V, et al. Lymphopenia and decrease in the total number of circulating CD3+ and CD4+ T cells during 'long‐term' PUVA treatment for psoriasis. Dermatologica 1991;183(1):10‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Braun 2000
- Braun Falcon O, Plewig G, Wolff HH, Burgdorf WHC. Physical methods of therapy. In: Braun Falcon O, Plewig G, Wolff HH editor(s). Dermatology. Berlin: Springer‐Verlag, 2000:1767‐80. [Google Scholar]
Brenner 1983
- Brenner W, Jaschke E, Honigsmann H. UV‐B phototherapy in psoriasis [UV‐B‐Phototherapie der Psoriasis]. Zeitschrift fur Hautkrankheiten 1983;58(15):1113‐24. [MEDLINE: ] [PubMed] [Google Scholar]
Coven 1999
- Coven TR, Walters IB, Cardinale I, Krueger JG. PUVA‐induced lymphocyte apoptosis: mechanism of action in psoriasis. Photodermatology, Photoimmunology & Photomedicine 1999;15(1):22‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Gruijl 1996
- Gruijl FR. Long‐term side effects and carcinogenesis risk in UVB therapy. In: Honigsmann H, Jori G, Young AR editor(s). The Fundamental Bases of Photo‐therapy. Milan, Italy: OEMF, 1996:153‐70. [Google Scholar]
De Korte 2004
- Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. Journal of Investigative Dermatology Symposium 2004;9(2):140‐7. [EMBASE: 2004161605] [DOI] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clinical & Experimental Dermatology 1994;19:210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fischer 1976
- Fischer T. UV‐light treatment of psoriasis. Acta Dermato‐Venereologica 1976;56(6):473‐9. [EMBASE: 0978025023] [PubMed] [Google Scholar]
Fitzpatrick 1988
- Fitzpatrick TB. The validity and practicality of sunreactiveskin type‐I through type‐VI. Arch. Dermatol 1988;124:869–871. [DOI] [PubMed] [Google Scholar]
Gelfand 2003
- Gelfand JM, Berlin J, Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population‐based cohort study in the United Kingdom. Archives of Dermatology 2003;139(11):1425‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gelfand 2006
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296(14):1735‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Godar 2003
- Godar DE, Urbach F, Gasparro FP, Leun JC. UV doses of young adults. Photochem Photobiol 2003;77(4):453‐7. [DOI] [PubMed] [Google Scholar]
Green 1988
- Green C, Ferguson J, Lakshmipathi T, Johnson BE. 311 nm UVB phototherapy‐‐an effective treatment for psoriasis. British Journal of Dermatology 1988;119(6):691‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1996
- Griffiths CE, Voorhees JJ. Psoriasis, T cells and autoimmunity. Journal of the Royal Society of Medicine 1996;89(6):315‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannuksela‐Svahn 2000
- Hannuksela‐Svahn A, Pukkala E, Laara E, Poikolainen K, Karvonen J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. Journal of Investigative Dermatology 2000;114(3):587‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hearn 2008
- Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow‐band ultraviolet B phototherapy. British Journal of Dermatology 2008;159(4):931‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofer 2006
- Hofer A, Fink‐Puches R, Kerl H, Quehenberger F, Wolf P. Paired comparison of bath water versus oral delivery of 8‐methoxypsoralen in psoralen plus ultraviolet: A therapy for chronic palmoplantar psoriasis.. Photodermatol Photoimmunol Photomed 2006;22:1‐5. [DOI] [PubMed] [Google Scholar]
Honigsmann 1977
- Honigsmann H, Fritsch P, Jaschke E. UV‐therapy of psoriasis. Half‐side comparison between oral photochemotherapy (PUVA) and selective UV‐phototherapy (SUP) [UV‐Therapie der Psoriasis Halbseitenvergleich zwischen oraler Photochoemotherapie (PUVA) und selektiver UV‐Phototherapie (SUP)]. Zeitschrift fur Hautkrankheiten 1977;52(21):1078‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Ibbotson 2004
Jensen 2010
- Jensen P, Skov L, Zachariae C. Systemic combination treatment for psoriasis: a review. Acta Dermato‐Venereologica 2010;90(4):341‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karvonen 1989
- Karvonen J, Kokkonen E L, Ruotsalainen E. 311 nm UVB lamps in the treatment of psoriasis with the Ingram regimen. Acta Dermato‐Venereologica 1989;69(1):82‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Kozenitzky 1992
- Kozenitzky L, David M, Sredai B, Albeck M, Shohat B. Immunomodulatory effects of AS101 on interleukin‐2 production and T‐lymphocyte function of lymphocytes treated with psoralens and ultraviolet A. Photodermatology, Photoimmunology & Photomedicine 1992;9(1):24‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Lauharanta 1997
- Lauharanta J. Photochemotherapy. Clinics in Dermatology 1997;15(5):769‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lebwohl 2003
- Lebwohl M. Psoriasis. Lancet 2003;361(9364):1197‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2005
- Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. International Journal of Dermatology 2005;44(5):355‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowe 1997
- Lowe NJ, Chizhevsky V, Gabriel H. Photo(chemo)therapy: general principles. Clinics in Dermatology 1997;15(5):745‐52. [EMBASE: 1997295624] [DOI] [PubMed] [Google Scholar]
Menter 2010
- Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. Journal of the American Academy of Dermatology 2010;62(1):114‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morison 1995
- Morison WL. Combination of methoxsalen and ultraviolet B (UVB) versus UVB radiation alone in treatment of psoriasis: a bilateral comparison study. Photodermatology, Photoimmunology & Photomedicine 1995;11(1):6‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morison 1998
- Morison WL, Baughman RD, Day RM, Forbes PD, Hoenigsmann H, Krueger GG, et al. Consensus workshop on the toxic effects of long‐term PUVA therapy. Archives of Dermatology 1998;134(5):595‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naldi 2010
- Naldi L. Malignancy concerns with psoriasis treatments using phototherapy, methotrexate, cyclosporin, and biologics: facts and controversies. Clinics in Dermatology 2010;28(1):88‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuner 1994
- Neuner P, Charvat B, Knobler R, Kirnbauer R, Schwarz A, Luger TA, et al. Cytokine release by peripheral blood mononuclear cells is affected by 8‐methoxypsoralen plus UV‐A. Photochemistry & Photobiology 1994;59(2):182‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parrish 1981
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. Journal of Investigative Dermatology 1981;76(5):359‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pasker‐de 1999
- Pasker‐de Jong PC, Wielink G, Valk PG, Wilt GJ. Treatment with UV‐B for psoriasis and nonmelanoma skin cancer: a systematic review of the literature. Archives of Dermatology 1999;135(7):834‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paul 2012
- Paul C, Gallini A, Archier E, Castela E, Devaux S, Aractingi S, et al. Evidence‐based recommendations on topical treatment and phototherapy of psoriasis: systematic review and expert opinion of a panel of dermatologists. Journal of the European Academy of Dermatology and Venereology 2012;26(Suppl 3):1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Picot 1992
- Picot E, Meunier L, Picot‐Debeze MC, Peyron JL, Meynadier J. Treatment of psoriasis with a 311‐nm UVB lamp. British Journal of Dermatology 1992;127(5):509‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saurat 1999
- Saurat JH. Retinoids and psoriasis: novel issues in retinoid pharmacology and implications for psoriasis treatment. Journal of the American Acadmy Dermatology 1999;41(3 Pt 2):s2‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009
- Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. British Journal of Dermatology 2009;161(5):987‐1019. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stern 1988
- Stern RS, Lange R. Non‐melanoma skin cancer occurring in patients treated with PUVA five to ten years after first treatment. Journal of Investigative Dermatology 1988;91(2):120‐4. [EMBASE: 1988194616] [DOI] [PubMed] [Google Scholar]
Tahir 2004
- Tahir R, Mujtaba G. Comparative efficacy of psoralen ‐ UVA photochemotherapy versus narrow band UVB phototherapy in the treatment of psoriasis. Journal of the College of Physicians & Surgeons ‐ Pakistan 2004;14(10):593‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Weelden 1988
- Weelden H, Faille HB, Young E, Leun JC. A new development in UVB phototherapy of psoriasis. British Journal of Dermatology 1988;119(1):11‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walters 1999
- Walters IB, Burack LH, Coven TR, Gilleaudeau P, Krueger JG. Suberythemogenic narrow‐band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. Journall of American Academic Dermatology 1999;40(6 Pt 1):893‐900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weischer 2004
- Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Dermato‐Venereologica 2004;84(5):370‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Young 1995
- Young AR. Carcinogenicity of UVB phototherapy assessed. Lancet 1995;345(8962):1431‐2. [DOI: 10.1016/S0140-6736(95)92617-8] [DOI] [Google Scholar]
Zanolli 2000
- Zanolli MD, Camisa C, Feldman S, Gulliver W, Menter A. Psoriasis: the high notes on current treatment. Program of the American Academy of Dermatology. Nashville, TN, August 5, 2000.


