Abstract
Introduction
Many breast cancer patients suffer from fear of cancer recurrence (FCR). However, effective physical intervention for FCR has been scarce. Previous studies have confirmed that repetitive transcranial magnetic stimulation (rTMS) can help improve patients' anxiety, depression, fear, and stress level. Therefore, this study aims to assess the efficacy of rTMS in the treatment of FCR in breast cancer patients and explore its underlying neural mechanism.
Methods and analysis
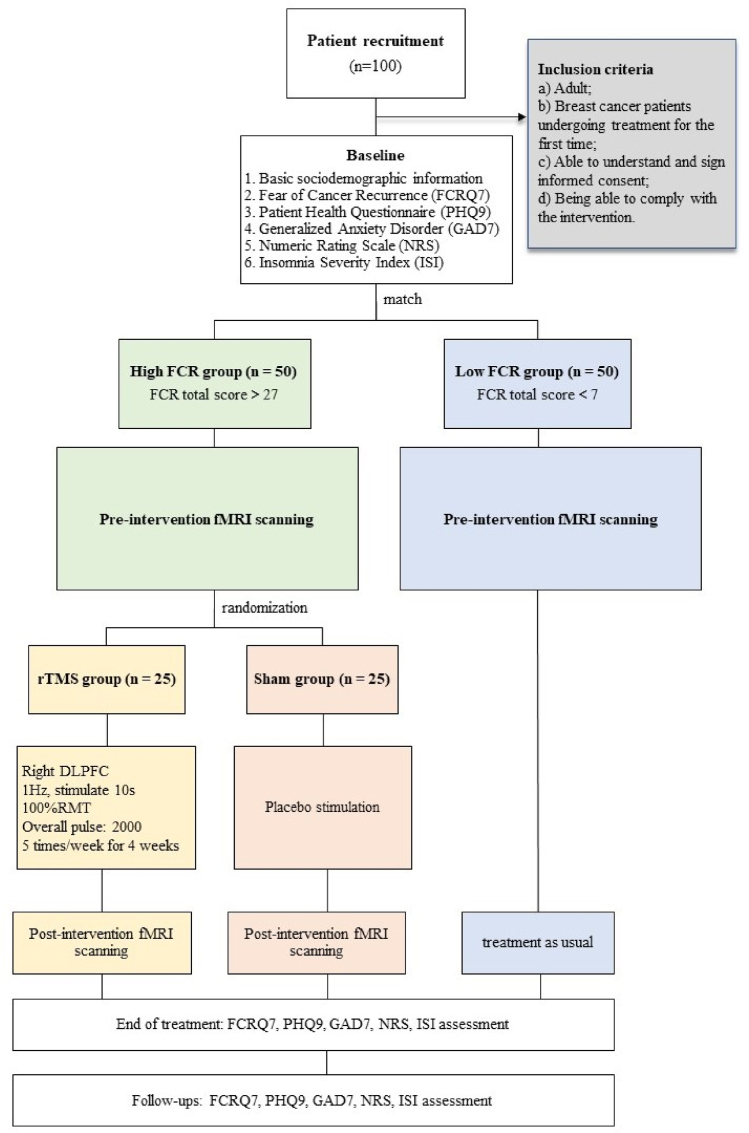
and analysis: Fifty breast cancer patients with high FCR (FCR total score >27), and fifty age- and gender-matched patients with low FCR (FCR total score <7) will be recruited to participate in this study. Patients in the high FCR group will be randomly assigned to receive 4-week low-frequency rTMS targeting the right dorsolateral prefrontal cortex (rDLPFC) + treatment as usual (TAU) (n = 25), or to receive sham stimulation + TAU (n = 25). Patients in the low FCR group will only receive TAU. All participants will take a baseline fMRI scan to examine the local activities and interactions of brain activity between the prefrontal cortex (DLPFC), amygdala and hippocampus. Fear of Cancer Recurrence Questionnaire (FCRQ7), Patient Health Questionnaire (PHQ9), Generalize Anxiety Disorder (GAD7), Numeric Rating Scale (NRS), and Insomnia Severity Index (ISI7) will be used to measure an individual's FCR, depression, anxiety, pain, and insomnia symptoms at week 0 (baseline), week 4 (the end of intervention), week 5 (1 week post-treatment), week 8 (1 month post-treatment), and week 16 (3 months post-treatment). Participants in the high FCR group will receive a post-treatment fMRI scan within 24 h after intervention to explore the neural mechanisms of rTMS treatment. The primary outcome of the study, whether the rTMS intervention is sufficient in relieving FCR in breast cancer patients, is measured by FCRQ7. Additionally, task activation, local activity and functional connectivity of the DLPFC, amygdala and hippocampus will be compared, between high and low FCR group, and before and after treatment.
Discussion
Studies have shown that low-frequency rTMS can be used to treat patient's FCR. However, there is a lack of relevant evidence to support the efficacy of rTMS on FCR in cancer patients, and the neural mechanisms underlying the effects of rTMS on FCR need to be further investigated.
Ethics and dissemination
Ethical approval for the study has been obtained from the Ethics Committee of Guangdong Provincial People's Hospital (reference number: KY-N-2022-136-01). The results of the investigation will be published in scientific papers. The data from the investigation will be made available online if necessary.
Trial registration
NCT05881889 (ClinicalTrials.gov). Date of registration: May 31, 2023.
Keywords: Repetitive transcranial magnetic stimulation (rTMS), Fear of cancer recurrence (FCR), Randomized controlled trial (RCT)
Abbreviations
- rTMS
Repetitive Transcranial Magnetic Stimulation
- FCR
Fear of Cancer Recurrence
- RCT
Randomized Controlled Trial
- DLPFC
Dorsolateral Prefrontal Cortex
- TAU
Treatment as Usual
- PTSD
Post-Traumatic Stress Disorder
- FCRQ7
ear of Cancer Recurrence Questionnaire (7-item)
- GAD7
Generalize Anxiety Disorder (7-item)
- PHQ9
Patient Health Questionnaire (9-item)
- NRS
Numeric Rating Scale
- ISI7
Insomnia Severity Index (7-item)
- SD
Standard Deviations
- ITT
Intention to Treat
- GLMM
Generalized Linear Mixed Model
- REML:
Restricted Maximum Likelihood
1. Background
Breast cancer is the most common cancer in women [1]. Due to early diagnosis and effective treatments, life expectancy of breast cancer survivors is often extended [1]. Between 2011 and 2020, the mortality rate of breast cancer has decreased by an average of 1.3 % per year, with the five-year survival rate reaching 90.6 % [2]. At the same time, women with breast cancer suffer from a variety of psychological distresses, among which the fear of cancer recurrence (FCR) is one of the most common problems. FCR is defined as “Fear, worry or concern relating to the possibility that cancer will come back or progress” [3]. Studies have reported that up to 70 % of the breast cancer patients experience FCR [4,5]. FCR could be a chronic factor that seriously compromises breast cancer patients’ quality of life. It has been proved that breast cancer patients with high FCR were more likely to report repetitive body checking, over-screening and overtreatment, nonsensically and irrationally [6].
Anxiety and depression are also commonly reported psychological problems in breast cancer patients. The prevalence of anxiety and depression in breast cancer patients was reported to be at 41.9 % and 32.2 % respectively [1,7]. Luo et al., has reported that depression and anxiety symptoms were positively associated with high FCR in cancer patients [8], and a long-term research lasting 2 years confirmed that elevated anxiety and depression at baseline can help predict higher FCR [9]. Brent et al. found that cancer patients with more emotional distress were at a greater risk for Emergency Department visits and hospitalizations, experienced longer hospital stays, and accrued higher healthcare costs [10]. Treatment for psychological distresses has included drug therapy, physiotherapy and psychotherapy. Increasing number of studies have shown that non-drug intervention could help lessen psychological distress and promote quality of life of patients with cancer. For example, cancer pain could be alleviated through exercise, music, and meditation [[11], [12], [13]]. However, these interventions require long training and consistent adherence to the intervention, demanding considerable energy and time from the patients [[11], [12], [13]].
Repetitive transcranial magnetic stimulation (rTMS) is a safe, effective, noninvasive, and nonconvulsive neuromodulation therapy. rTMS can modulate the physiological dynamics across brain regions and networks, and has the potential to therapeutically modulate aberrant circuit properties across neuropsychiatric conditions with maladaptive circuit dynamics [14]. In 2018, Sriram et al., conducted transcranial microcurrent stimulation therapy for patients with advanced cancer and found that patient's anxiety, depression and insomnia symptoms were significantly improved after 4 weeks of intervention [15]. Similar studies were conducted by Tang et al., which showed that patients with advanced non-small cell lung cancer treated with rTMS had better pain relief, as well as better emotional state and quality of life [16]. rTMS has been considered as an effective treatment option for patients with anxiety and depression. However, there are limited studies that have evaluated the efficacy of rTMS for other neuropsychiatric disorders such as FCR among cancer patients.
Relevant reviews suggest that when low-frequency (frequency ≤1 Hz) rTMS is applied to the right dorsolateral prefrontal cortex (rDLPFC), patients' anxiety and depressive symptoms improved; and the longer the course of the treatment, the better the effect [17,18]. A randomized controlled trial (RCT) study evaluating the efficacy of rTMS (rDLPFC, 1 Hz, 110RMT, 1800 pulses) in the treatment of panic disorder combined with depression found that patient's symptoms improved after the 4-week treatment. During the subsequent 6 months of follow-up, patients showed continuous improvement in both panic disorder and depression [19]. Additionally, rTMS can increase the release of neurotransmitters such as 5-HT and brain-derived neurotrophic factor, and subsequently regulate the whole brain activity [17,18].
The prefrontal cortex (PFC) is one of the most important brain regions in which emotional function is regulated [20]. The medial prefrontal cortex (mPFC) exerts inhibitory “top–down” control over amygdala activity, limiting its output and thus preventing inappropriate emotional expression [21]. If the prefrontal cortex fails to inhibit over-activation of the amygdala during fear stimulation, individuals are more likely to report high levels of fear [22]. Additionally, the hippocampus is the neural basis for encoding and consolidating fear memories, and studies in mice have shown that the development of fear memories is significantly associated with increased connections between the hippocampus and the amygdala. Therefore, weakening the connections between the hippocampus and the amygdala might help reduce fear memories [23].
In recent years, the interaction of the prefrontal cortex, amygdala, hippocampus and other brain regions has attracted increasing attention [21,23,24]. Future intervention targeting an individual's PFC, amygdala and hippocampus might be helpful in disrupting fear-memory reconsolidation and might help prevent the return of fear.
1.1. Research objectives
This study aims to 1) examine the efficacy of the 4-week low-frequency rTMS on FCR in breast cancer patients, and 2) explore the underlying neural mechanisms as well as examine the interactions of the prefrontal cortex (DLPFC), amygdala and hippocampus of FCR patients by fMRI scans between the high and low FCR group, and before and after the rTMS intervention. The primary hypothesis for the trial is: low-frequency rTMS treatment can alleviate FCR in breast cancer patients.
2. Methods
2.1. Participants
Fifty breast cancer patients with high FCR, and fifty age- and gender-matched breast cancer patients with low FCR (100 in total) will be recruited at Guangdong Provincial People's Hospital. To be included, participants must fulfil the following inclusion criteria: a) Adult; b) Breast cancer patients undergoing treatment for the first time; c) Able to understand and sign informed consent; and d) Being able to comply with the intervention. The exclusion criteria for this study are as follows: a) Minors; b) Having a diagnosis for a significant untreated mental or medical illness (e.g., consciousness disturbances, mania, acute phase of schizophrenia, major depressive disorder, etc.); c) Patients with recurrent cancer; and d) Hospice patients.
2.2. Research design
Fifty breast cancer patients with high FCR (FCR total score >7), and fifty age-and gender-matched patients with low FCR (FCR total score <7) will be recruited to participate in the study. Patients in the high FCR group will be randomly assigned to receive 4-week low-frequency rTMS + TAU (n = 25), or to receive sham stimulation + TAU (n = 25). Patients in the low FCR group will only receive TAU.
A written informed consent will be completed before participation in the study. All participants will be first asked to complete a personal information sheet. Fear of Cancer Recurrence Questionnaire (FCRQ7), Patient Health Questionnaire (PHQ9), Generalize Anxiety Disorder (GAD7), Numeric Rating Scale (NRS), and Insomnia Severity Index (ISI7) will be used to measure individual's FCR, depression, anxiety, pain, and insomnia symptoms from week 0 (baseline), week 4 (the end of intervention), week 5 (1 week post-treatment), week 8 (1 month post-treatment), and week 16 (3 months post-treatment).
At baseline, all participants will have a fMRI scan to examine the local activities and interactions between the prefrontal cortex, amygdala and hippocampus. Subsequently, Participants in the high FCR group will receive a post-treatment fMRI scan within 24 h after intervention to examine the underlying neural mechanism of rTMS. The research design is summarized in Fig. 1 for reference.
Fig. 1.
Study design.
2.3. Randomization
Participants with high FCR fulfilling the study criteria will be randomly allocated to rTMS + TAU group or sham + TAU group using simple randomization (computer-generated random numbers) [25]. The allocation ratio of this study will be 1:1 [26]. To reduce the risk of selection bias, allocation concealment will be implemented [26]. The randomization sequence will be kept by a third person who will not be involved in any other parts of this study. After a participant is formally enrolled and the baseline data collection is completed, the research assistants will contact the third person for the allocation. No allocation sequence will be revealed before a participant is ready for randomization [26].
2.4. Blinding
The study will be a single-blind label trial in which participants will know nothing about the assignment, however, it will be impossible for the intervention facilitator to be blinded. Data analysts will know nothing about group assignments and research assistants.
2.5. Assessment points
Assessments will be conducted at week 0 (baseline), week 4 (the end of intervention), week 5 (1 week post-treatment), week 8 (1 month post-treatment), and week 16 (3 months post-treatment).
2.6. Planned intervention
After baseline assessment, participants with high FCR will be randomly assigned to receive 4-week rTMS treatment + TAU, or sham stimulation + TAU. For patients in the rTMS group, we will first determine the intensity of the rTMS protocol by assessing the individual resting motor threshold (RMT). After determination of each individual's RMT, we will apply a single train of low-frequency rTMS over the DLPFC at 1 Hz for a total duration of 30 min (100 % RMT, 2000 pulses). For patients in the sham group, we will apply rTMS over the DLPFS in sham modality.
2.7. Assessment of safety
Studies have reported that the most common adverse effects of rTMS are scalp pain during stimulation and transient headache after stimulation, with the incidence of 35%–40 % and 25%–30 %, respectively [27]. These symptoms are gradually alleviated during treatment and are usually effective to over-the-counter analgesics [27]. In the current study, participant's anxiety, depression, FCR, insomnia, and pain symptoms will be continuously monitored by subjective measurements during treatment and at follow-ups. Any participant who reports worse symptoms after the completion of the intervention will be introduced to receive standard medication and/or psychological treatment.
2.8. Data collection
The research coordinator will obtain consent from all the eligible patients, and collect the baseline data which will include the sociodemographic and clinical characteristics such as gender, age, weight, height, religion, marital status, employment status, education level, financial condition, treatment(s), time since diagnosis, physical comorbidities, etc. Fear of Cancer Recurrence Questionnaire (FCRQ7), Generalize Anxiety Disorder (GAD7), Patient Health Questionnaire (PHQ9), Numeric Rating Scale (NRS), and Insomnia Severity Index (ISI7) will be used to measure individual's FCR, anxiety, depression, pain, and insomnia symptoms. Assessments will be conducted at week 0 (baseline), week 4 (the end of intervention), week 5 (1 week post-treatment), week 8 (1 month post-treatment), and week 16 (3 months post-treatment).
All participants will be contacted by the same research staff throughout the study period. Participants will be invited to scan the QR code through WeChat app to complete the assessment. If the research staff fails to receive a response from a patient after three attempts, the patient will then be marked as ‘loss of follow-up’.
2.8.1. fMRI data collection
2.8.1.1. Stimulation materials
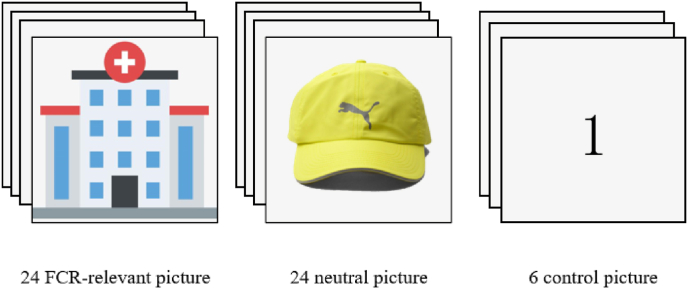
We will first select 30 FCR-relevant pictures (e.g., picture of cancer center, ward, cancer-related drugs, radiotherapy and chemotherapy equipment etc.) as stimuli. All pictures will be rated by four independent raters, and the pictures with consistent categories will be selected. Ten independent raters will then classify these pictures according to emotional categories and intensities, and 24 FCR-relevant pictures will then be finally selected. Meanwhile, 24 neutral pictures (e.g., a hat) will be selected from International Affective Picture System (IAPS). Notably, the size and brightness of all pictures will be adjusted as the same.
2.8.1.2. fMRI task
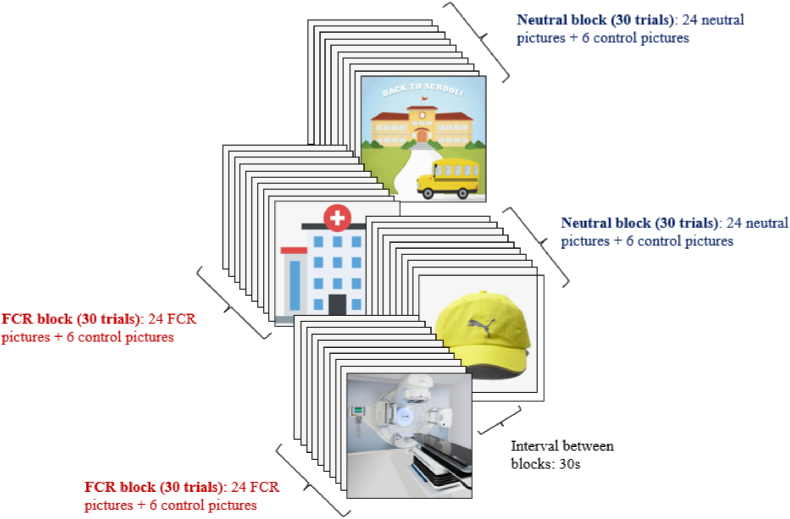
The fMRI task will be presented using block design. A total of 4 blocks (2 FCR and 2 neutral blocks) will be presented (each block owning 30 trials). FCR block and neutral block will be presented alternately, with an interval of 30s between blocks. In each FCR block, 24 trials will present with FCR-relevant pictures, and 6 trials will present with control pictures which will display a random digital number (i.e., number 1, 2, or 3). Similarly, in each neutral block, 24 neutral pictures will be displayed, and control pictures will be presented in 6 trials.
When FCR-relevant or neutral pictures are displayed on the screen (4 s), subjects will be simply asked to maintain attention on these pictures and not response to anything [28]. However, when control picture (the random digital number) is displayed, the participant will be asked to press the corresponding keycard (2 s). The inter-trial interval will be jittered between 5 ± 1 s. During the inter-trial interval (ITI), participants will view a white fixation cross on a black background. If a subject's accuracy response rate of the control trials is lower than 80 %, her data will be removed in the subsequent analysis.
3. Assessment tools
3.1. MRI scanning parameters
MRI scanning will be performed on a 3.0 T scanner (MR Philips, Ingenia CX). fMRI parameters: repetition time (TR) = 800 ms, echo time = 30 ms, slice thickness/gap = 2.4/2.4 mm, in-plane resolution = 220 × 220, field of view (FOV) = 100 × 100 mm, flip angle (FA) = 52°; parameters for 3D-T1-weighted structural imaging were: slices = 192, repetition time = 8 ms, echo time = 3 ms, slice thickness/gap = 1/1 mm, in-plane resolution = 256 × 256, field of view = 100 × 100 mm, flip angle = 8°.
3.2. Fear of Cancer Recurrence Questionnaire (FCRQ7)
This scale has been applied to large-scale FCR studies many times [29]. There are 7 items scored on 5 levels, the total score of each item is summed up. The score above 27 indicates that the patient has significant fear, which requires certain psychological intervention and treatment. The internal consistency of the Chinese version of the scale has reached 0.87 [30].
3.3. Patient Health Questionnaire (PHQ9)
The PHQ9 strictly conforms to the 9 symptomatic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), with each item scoring 0–3 points and the total value range 0–27 points [31]. PHQ9 can be used for both screening and evaluating the severity of depression. The results of PHQ9 are graded as follows: 0–4: no depression; 5–9: mild depression; 10–14: moderate depression; 15–19: moderate to severe depression; and 20–27: severe depression [32].
3.4. Generalized anxiety disorder (GAD7)
Similar to PHQ9, GAD7 is also a self-rating anxiety scale recommended by DSM-5 for screening and assessing the severity of anxiety in patients [33]. The total score is categorized as follows: 0–4: no generalized anxiety; 5–9: mild generalized anxiety; 10–14: moderate generalized anxiety; and 15–21: severe generalized anxiety [34].
3.5. Numeric rating scale (NRS)
Participant's somatic pain level will be assessed using the “0–10” numeric rating scale (NRS) (i.e., how would you rate your pain at its worst over the past 3 days?) [35], in which 0 means ‘no pain’ and 10 means ‘the worst pain’. Participants will be asked to select a number between 0 and 10, to indicate their current pain level. A total score of 4 or more indicates that the patient is currently suffering from pain [35].
3.6. Insomnia severity index (ISI)
The change in insomnia symptoms will be measured by the insomnia severity index (ISI) [36,37], which assesses the severity, nature, and impact of insomnia. The ISI is a 7-item self-report measure, ranging from 0 (no problem) to 4 (very severe problem). The resulting sum score of the ISI ranges from 0 to 28. Higher scores indicate more severe insomnia symptoms [36,37].
4. Statistical analysis
4.1. fMRI data analysis
4.1.1. Data processing
As we have mentioned above, if the accuracy rate of a subject in control trials is lower than 80 %, the subject will be removed from the subsequent fMRI data analysis. All the fMRI data processing will be performed using DPABI (https://rfmri.org/dpabi) and SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/).
The task fMRI data will be preprocessed as follows: 1) removing the first 3 vol; 2) slice timing; 3) realigned all volumes using all Friston-24 parameters; the subjects with headmotion higher than 3 mm translation or 3 rotations will be excluded. Normalizing to the MNI space using “New segment + DARTEL”; 4) co-registration to the T1 image; 5) normalizing to the MNI space; 5) smoothing using a Gaussian kernel with full width at half-maximum (FHWM) equals to 6 mm. Activation map of both the FCR condition and natural condition will be generated in the native space. The peak activation ROI will be defined as a sphere with 6 mm radius centered at activation peak point. Then, functional connectivity of the activation region of interest (ROI) will be conducted to estimate the connectivity patterns based on dynamic causal modeling (DCM).
The previous 6 preprocessing steps will be conducted as task fMRI except that resting state fMRI will be removed 10 volumes. After that, the fMRI images will be filtered with 0.01–0.1 Hz. The amplitude of low frequency fluctuation (ALFF)/Percent amplitude fluctuation (PerAF) and regional homogeneity (ReHo) will be used to estimate brain local activities. Moreover, the ROI-wise functional connectivity will be calculated to estimate the interactions among DLPFC, amygdala (AMYG) and hippocampus (HIP). Specifically, for the AMYG and HIP, the peak activation areas of these two brain regions will be binarized, and their overlaps with their corresponding templates (Brainnetome Atlas, http://atlas.brainnetome.org/) will be defined as AMYG and HIP region of interests (ROI). The overlap of the activation areas and previously reported left DLPFC will be selected as the left DLPFC, the right DLPFC will be defined as the overlap of the activation areas and the mirror of the left DLPFC [38,39].
For fMRI metrics, two-way ANOVA will be conducted to estimate the group effects (real/sham), the intervention effects (pre-treatment/post-treatment) and their interaction effects on local activity (ReHo/PerAF/ALFF), ROIs-wise FCs and effective connectivity. Moreover, based on the significant group or intervention effects, ROIs were generated for the correlation analysis between fMRI and clinical scores using multilinear regression analysis.
4.1.2. Quantitative data analysis
Descriptive analyses will be conducted to obtain frequencies, means, and standard deviations (SD) (where appropriate). Intention to treat (ITT) will be used for the main efficacy analysis [40]. Independent t-test and non-parametrical analyses, where appropriate, will be applied to compare the differences between the two groups. Repeated measurement analysis will be used to compare the changes in symptoms (e.g., FCR total Score) following treatment and at follow-ups. Additionally, the effects of baseline categorical variables on the changes of FCR total scores (dependent variable) will be examined via generalized linear mixed model (GLMM) by restricted maximum likelihood (REML), with the fixed effects of time, age, marital status, education, baseline depression, anxiety and pain, and random effects of subjects [41]. The post hoc comparisons of estimated marginal means of FCRs over time will be estimated with Bonferroni method [26]. A two-side P value < 0.05 will be considered statistically significant. All the statistical analyses will be conducted with SAS software, version 9.4 (SAS Institute Inc., Cary. NC).
5. Ethics and dissemination
An informed consent form will be signed by all participants. Participants will be assured that their participation is completely voluntary, and that their information will be kept confidential. Only the research team will have access to the final data set and make the final decision to terminate the trial if necessary.
6. Patient and public involvement
Patients or the public will not be involved in the design, conduct, reporting and/or dissemination of our research.
7. Discussion
FCR is one of the most prevalent concerns cancer patients report. It can be a concern for cancer patients immediately after diagnosis or treatment; and has been shown to be stable for years for many patients [6,42]. FCR can lead to various negative consequences, such as increased psychological disturbances, poor treatment adherence, amplification of physical symptoms, and increased functional impairment [43]. A previous study showed that low-frequency rTMS could be used in humans to prevent the expression and return of fear, and rTMS treatment could be slightly more effective than psychotherapy and pharmacotherapy in patients with treatment-resistant depression [27]. A randomized controlled study also confirmed that rTMS can help improve patients' anxiety, and insomnia symptoms [44]. In addition, several studies have demonstrated that structural and functional abnormalities of the prefrontal cortex, amygdala and hippocampus play an important role in fear development and reprocessing [21,23,24]. The neuromechanism underlying the effects of rTMS on FCR warrants further investigation.
8. Limitation
Several limitations of this study should be acknowledged. First, the relatively small number of participants included may undermine the universality and representativeness of the results, thereby limiting the applicability of the conclusions. Second, the focus on breast cancer patients alone leads to potential selection bias, which may prevent the results from accurately reflecting the situation of patients with other types of cancer. In addition, as this study will be a single-blind label trial, it is impossible to blind the intervention facilitator. Finally, only self-report measurements will be used in this study. Further studies using objective measurements, such as actigraphy and polysomnography to assess sleep quality would be beneficial.
Funding source
This study was supported in part by the Medical Scientific Research Foundation of Guangdong Province (A2023004), the NSFC Pilot Funds of Guangdong Provincial People's Hospital (KY0120220783), and the Start-up Funds of Guangdong Provincial People's Hospital (KY0120211134).
Ethical approval
This study was performed in accordance with the Helsinki standard and the study's protocol was approved by Guangdong Provincial People's Hospital Research Ethics Committee (ref No: KY-N-2022-136-01).
Consent for publication
Not applicable.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Strengths and limitations
-
•
Participants in experiment group will be randomly allocated using computer-generated random numbers.
-
•
An independent researcher will implement randomization and treatment allocation through an automated online system.
-
•
Data analysists will be blinded to group allocation and to the research assistants.
CRediT authorship contribution statement
Wenjing Xu: Writing – original draft. Na Zhao: Writing – original draft. Wengao Li: Investigation, Formal analysis, Data curation. Lirong Qiu: Investigation, Formal analysis, Data curation. Xian Luo: Investigation, Formal analysis, Data curation. Yuanyuan Lin: Investigation, Formal analysis, Data curation. Wenjing Wang: Investigation, Formal analysis, Data curation. Samradhvi Garg: Writing – review & editing. Hengwen Sun: Writing – review & editing. Yuan Yang: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
None.
Contributor Information
Wenjing Xu, Email: 2634798850@qq.com.
Na Zhao, Email: nazhaon@163.com.
Wengao Li, Email: qqyo-yo@163.com.
Lirong Qiu, Email: qiu.lirong@outlook.com.
Xian Luo, Email: 2443180803@qq.com.
Yuanyuan Lin, Email: zjcnlyy@126.com.
Wenjing Wang, Email: wwjwwj2018@163.com.
Samradhvi Garg, Email: garg.sam41@yahoo.co.in.
Hengwen Sun, Email: sunrise761114@foxmail.com.
Yuan Yang, Email: yangyuan@gdph.org.cn.
References
- 1.Yang Y, Sun H, Luo X, et al. Network connectivity between fear of cancer recurrence, anxiety, and depression in breast cancer patients[J] Journal of Affective Disorders. 2022;309:358–367. doi: 10.1016/j.jad.2022.04.119. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, epidemiology, and end results program. Cancer Statistics. 2022 https://seer.cancer.gov/index.html [Google Scholar]
- 3.Wei J, Xiangbo C, Gang N, et al. Application of cancer anxiety scale in detecting recurrence fear of breast cancer patients after surgery [J] (in Chinese) Chinese Journal of Modern Nursing. 2014;(36):4571–4573. [Google Scholar]
- 4.Thewes B, Butow P, Bell M L, et al. Fear of cancer recurrence in young women with a history of early-stage breast cancer: a cross-sectional study of prevalence and association with health behaviours[J]. Supportive Care in Cancer. Official Journal of the Multinational Association of Supportive Care in Cancer. 2012;20(11):2651–2659. doi: 10.1007/s00520-011-1371-x. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Lim JW. Cognitive behavioral therapy for reducing fear of cancer recurrence (FCR) among breast cancer survivors: a systematic review of the literature[J]. BMC Cancer. 2022 Feb 28;22(1):217. [DOI] [PMC free article] [PubMed]
- 6.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies[J] Journal of Cancer Survivorship: Research and Practice. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi SM, Rafiemanesh H, Aghamohammadi T, Badakhsh M, Amirshahi M, Sari M, Behnamfar N, Roudini K. Prevalence of anxiety among breast cancer patients: a systematic review and meta-analysis[J] Breast Cancer. 2020 Mar;27(2):166–178. doi: 10.1007/s12282-019-01031-9. [DOI] [PubMed] [Google Scholar]
- 8.Luo X, Li W, Yang Y, et al. High Fear of Cancer Recurrence in Chinese Newly Diagnosed Cancer Patients[J] Frontiers in Psychology. 2020;11:1287. doi: 10.3389/fpsyg.2020.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Pérez M, Schootman M, Aft RL, Gillanders WE, Jeffe DB. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer[J] Breast Cancer Res Treat. 2011 Nov;130(1) doi: 10.1007/s10549-011-1551-x. 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bt M, G D, Rb S, et al. Healthcare use and costs in adult cancer patients with anxiety and depression[J]. Depression and anxiety, 2020, 37(9). [DOI] [PMC free article] [PubMed]
- 11.Park S, Sato Y, Takita Y, et al. Mindfulness-Based Cognitive Therapy for Psychological Distress, Fear of Cancer Recurrence, Fatigue, Spiritual Well-Being, and Quality of Life in Patients With Breast Cancer-A Randomized Controlled Trial[J]. Journal of Pain and Symptom Management, 2020, 60(2): 381-389. [DOI] [PubMed]
- 12.Dolan N, Simmonds-Buckley M, Kellett S, Siddell E, Delgadillo J. Effectiveness of stress control large group psychoeducation for anxiety and depression: Systematic review and meta-analysis[J] Br J Clin Psychol. 2021 Sep;60(3):375–399. doi: 10.1111/bjc.12288. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez M, Pascoe M C, Yang G, et al. Yoga for depression and anxiety symptoms in people with cancer: A systematic review and meta-analysis[J] Psycho-Oncology. 2021;30(8):1196–1208. doi: 10.1002/pon.5671. [DOI] [PubMed] [Google Scholar]
- 14.Balderston N L, Beydler E M, Goodwin M, et al. Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety[J]. Translational Psychiatry, 2020, 10(1): 68. [DOI] [PMC free article] [PubMed]
- 15.Yennurajalingam S, Kang DH, Hwu WJ, et al. Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients With Advanced Cancer: A Preliminary Study[J] J Pain Symptom Manage. 2018 Feb;55(2):198–206. doi: 10.1016/j.jpainsymman.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Chen H, Zhou Y, et al. Analgesic Effects of Repetitive Transcranial Magnetic Stimulation in Patients With Advanced Non-Small-Cell Lung Cancer: A Randomized, Sham-Controlled, Pilot Study[J] Frontiers in Oncology. 2022;12:840855. doi: 10.3389/fonc.2022.840855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein W K, Noda Y, Barr M S, et al. Neurobiological predictors of response to dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation in depression: a systematic review[J] Depression and Anxiety. 2015;32(12):871–891. doi: 10.1002/da.22424. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018) [J] Clin Neurophysiol. 2020 Feb;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Aly M, Dagan Y, et al. Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression[J] Journal of Affective Disorders. 2013;144(1–2):153–159. doi: 10.1016/j.jad.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Motzkin J C, Philippi C L, Wolf R C, et al. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans[J] Biological Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu WZ, Zhang WH, Zheng ZH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety[J]. Nat Commun. 2020 May 6;11(1):2221. [DOI] [PMC free article] [PubMed]
- 22.Phillips J, Morris A, Cushman F. How We Know What Not To Think[J]. Trends in Cognitive Sciences. 2019;23(12):1026–1040. doi: 10.1016/j.tics.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Kim W B, Cho J H. Encoding of contextual fear memory in hippocampal-amygdala circuit[J]. Nature Communications, 2020, 11(1): 1382. [DOI] [PMC free article] [PubMed]
- 24.Moustafa A A, Gilbertson M W, Orr S P, et al. A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals[J] Brain and Cognition. 2013;81(1):29–43. doi: 10.1016/j.bandc.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim CY, In J. Randomization in clinical studies[J]. Korean J Anesthesiol. 2019 Jun;72(3):221-232. [DOI] [PMC free article] [PubMed]
- 26.Yang Y, Luo X, Paudel D, et al. Effects of e-aid cognitive behavioural therapy for insomnia (eCBTI) to prevent the transition from acute insomnia to chronic insomnia: study protocol for a randomised controlled trial[J]. BMJ Open. 2019 Nov 18;9(11): e033457. [DOI] [PMC free article] [PubMed]
- 27.Downar J, Blumberger DM, Daskalakis ZJ. Repetitive transcranial magnetic stimulation: an emerging treatment for medication-resistant depression[J]. CMAJ. 2016 Nov 1;188(16):1175-1177. [DOI] [PMC free article] [PubMed]
- 28.Ren Z, Shi L, Wei D, et al. Brain Functional Basis of Subjective Well-being During Negative Facial Emotion Processing Task-Based fMRI[J]. Neuroscience. 2019 Dec 15;423:177-191. [DOI] [PubMed]
- 29.Humphris GM, Watson E, Sharpe M, et al. Unidimensional scales for fears of cancer recurrence and their psychometric properties: the FCR4 and FCR7[J] Health Qual Life Outcomes. 2018 Feb 9;16(1):30. doi: 10.1186/s12955-018-0850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Humphris G, Sun H, et al. Psychometric properties of the Chinese version Fear of Cancer Recurrence Questionnaire-7 (FCR-7) [J]. Professional Psychology: Research and Practice. 50, 376–383.
- 31.Kroenke K, Spitzer R L, Williams J B W, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review[J] General Hospital Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Chen Manman Shengli. Qu Shan. A diagnostic test of patient health questionnaire screening for depressive disorders in psychiatric outpatient clinics of general hospitals[J] (in Chinese) Chinese Journal of Mental Health. 2015;29(04):241–245. [Google Scholar]
- 33.Spitzer R L, Kroenke K, Williams J B W, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7[J] Archives of Internal Medicine. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q, He Y, Liu H, et al. Reliability and validity of the Chinese version of the Generalized Anxiety Scale in the outpatient population of traditional Chinese medicine [J] (in Chinese). Chinese Journal of Mental Health,2013,27(03):163-168.
- 35.Butt Z, Wagner L I, Beaumont J L, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice[J] Journal of Pain and Symptom Management. 2008;35(1):20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 36.Morin C M, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response[J] Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapou Ba, Gang Ck. Reliability and Validity of the Chinese Translation of Insomnia Severity Index and Comparison with Pittsburgh Sleep Quality Index[J] The Malaysian Journal of Psychiatry. 2013;22:3–9. [Google Scholar]
- 38.Zhao N, Yue J, Feng ZJ, et al. The Location Reliability of the Resting-State fMRI FC of Emotional Regions Towards rTMS Therapy[J] Neuroinformatics. 2022 Oct;20(4):1055–1064. doi: 10.1007/s12021-022-09585-4. [DOI] [PubMed] [Google Scholar]
- 39.Cash RFH, Cocchi L, Lv J, et al. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility[J] Hum Brain Mapp. 2021 Sep;42(13):4155–4172. doi: 10.1002/hbm.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alshurafa M, Briel M, Akl EA, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature[J] PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Qi H, Li W, et al. Predictors and trajectories of fear of cancer recurrence in Chinese breast cancer patients[J] J Psychosom Res. 2023 Mar;166:111177. doi: 10.1016/j.jpsychores.2023.111177. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Cameron J, Bedi C, et al. Fear of cancer recurrence trajectory during radiation treatment and follow-up into survivorship of patients with breast cancer[J]. BMC cancer. 2018;18(1):1002. doi: 10.1186/s12885-018-4908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehnert A, Vehling S, Scheffold K, et al. Prevalence of adjustment disorder, acute and posttraumatic stress disorders as well as somatoform disorders in cancer patients[J] Psychotherapie, Psychosomatik, Medizinische Psychologie. 2013;63(12):466–472. doi: 10.1055/s-0033-1347197. [DOI] [PubMed] [Google Scholar]
- 44.Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study[J] Brain Stimul. 2018;11(5):1103–1109. doi: 10.1016/j.brs.2018.05.016. Sep-Oct. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.