Abstract
Background:
Our objective was to discover novel urinary biomarkers of antibiotic-associated nephrotoxicity using an ex-vivo human microphysiological system (MPS) and to translate these findings to a prospectively enrolled cystic fibrosis (CF) population receiving aminoglycosides and/or polymyxin E (colistin) for a pulmonary exacerbation.
Methods:
We populated the MPS with primary human kidney proximal tubule epithelial cells (PTECs) from three donors and modeled nephrotoxin injury through exposure to 50 μg/mL polymyxin E for 72 hours. We analyzed gene transcriptional responses by RNAseq and tested MPS effluents. We translated candidate biomarkers to a CF cohort via analysis of urine collected prior to, during and two weeks after antibiotics and patients were followed for a median of 3 years after antibiotic use.
Results:
Polymyxin E treatment resulted in a statistically significant increase in the pro-apoptotic Fas gene relative to control in RNAseq of MPS: fold-change=1.63, FDR q-value=7.29×10−5. Effluent analysis demonstrated an acute rise of soluble Fas (sFas) concentrations that correlated with cellular injury. In 16 patients with CF, urinary sFas concentrations were significantly elevated during antibiotic treatment, regardless of development of AKI. Over a median of three years of follow up, we identified seven cases of incident chronic kidney disease (CKD). Urinary sFas concentrations during antibiotic treatment were significantly associated with subsequent development of incident CKD (unadjusted relative risk=2.02 per doubling of urinary sFas, 95% CI=1.40, 2.90, p<0.001).
Conclusions:
Using an ex-vivo MPS, we identified a novel biomarker of proximal tubule epithelial cell injury, sFas, and translated these findings to a clinical cohort of patients with CF.
Keywords: Nephrotoxicity, chronic kidney injury, urinary biomarkers
INTRODUCTION:
Life expectancy in cystic fibrosis (CF) is near 50 years, thanks to novel therapies and improved diagnostics.1 This remarkable progress is attenuated by an increasing prevalence of comorbidities. In CF, the prevalence of chronic kidney disease (CKD) is 2.3%, double that of the general population when adjusted for age, and prevalence doubles with every 10-year increase in age.2 One major risk factor for CKD is repeated administration of nephrotoxic antibiotics, such as aminoglycosides and polymyxin E (colistin), to treat pulmonary exacerbations. Persons with CF often receive higher doses and longer courses of nephrotoxic antibiotics due to the accelerated rate of drug clearance. Nephrotoxic antibiotics have also been associated with acute kidney injury (AKI) in CF.3,4
Serum creatinine is the main clinical biomarker to diagnose AKI. However, changes in serum creatinine is an imperfect method to diagnose AKI as elevation in serum creatinine is poorly sensitive to kidney injury. Furthermore, a rise in serum creatinine often lags behind injury by as much as 72 hours.5 Serum creatinine is an especially poor biomarker for patients with CF, as serum levels are often abnormally low at baseline due to decreased muscle mass and metabolic derangement.6 These shortcomings of serum creatinine can lead to overestimation of kidney health in patients with CF and late detection of kidney damage.
We and others have developed a three-dimensional (3D) MPS to model the ex-vivo environment of the kidney.7,8 This system incorporates human primary renal epithelial cells in a 3D, gas-permeable hydrogel. Flow within the device is directed through the tubular chambers or via engineered side ports. In contrast to 2D cell culture systems, the MPS model retains polarized expression and function of proteins essential for reabsorption and secretion, responds to physiological stimuli and performs critical biochemical synthetic activities. In 2018, Weber et al. pioneered use of such an MPS in work measuring injury markers and transcriptional responses of human kidney PTECs response to polymyxin B.8 This investigation extends on the work of Weber et al., as we use an ex-vivo MPS that was treated with polymyxin E (colistin). The polymyxins have a different amino acid in position 6 and while polymyxin B is administered directly as the active antibiotic, polymyxin E is administered as a prodrug and requires conversion in vivo.9 Polymyxin E has considerably greater clinical experience with its use as an IV and inhaled formulation in CF and studies suggest that risk of nephrotoxicity is significantly greater with polymyxin E compared to polymyxin B.10–12 Due to these differences, we chose to primarily study polymyxin E associated nephrotoxicity in the MPS.
Due to the limitations in diagnosing kidney injury using serum creatinine, we sought to identify prognostic biomarkers of antibiotic-associated kidney injury in patients with CF. The research was completed in two stages. First, through analyses of RNA sequencing of PTECs and effluent from the MPS, we identified candidate biomarkers of PTEC injury. These candidate biomarkers guided the second stage of the investigation, which sought to associate the biomarkers with the development of AKI and CKD in a prospectively enrolled cohort of patients with CF who were receiving nephrotoxic antibiotic therapy.
METHODS:
Tissue Acquisition and Cell Isolation
Human PTECs were isolated from cortical kidney tissues collected at the University of Washington Medical Center under a UW Institutional Review Board approved human protocol (# STUDY00001297). Tissue was kept at 4 ºC at all times and bathed in media containing antimicrobial compounds. Tissues were minced thoroughly in DMEM/F12 media containing Type IV Collagenase and shaken in a 37 ºC incubator for 45 min then neutralized by the addition of 1:1 horse serum followed by centrifugation and resuspension of the supernatant into T75 flasks containing DMEM/F12 media supplemented with insulin, transferrin, selenium, hydrocortisone and antifungal/antibiotics.
Cell Seeding in Nortis Device
Nortis single- or triple- channeled MPS devices were seeded with PTECs trypsinized from confluent T25 flasks. Nortis devices contain a 0.12 mm x 6 mm long lumen constructed in Type I rat collagen (Ibidi, Madison, WI) and coated with human derived Type IV collagen (5 μg/mL, BD Biosciences, Bedford, MA) to facilitate cell attachment. Cells were counted and pelleted into a suspension of approximately 20 × 106 cells/mL followed by the injection of 1–2 μL of cells into the injection port. Cells were given at least four hours to attach before initiating flow at 0.5 μL/min and allowed to recover and populate the lumen for at least five days before treatments. Details of the preparation and cell seeding of the Nortis chip can be found in previous publications.7,8 Cells were maintained as described previously or supplemented with 50 μg/mL of polymyxin E (Sigma Aldrich, CAS# 1264–72-8) for 72 hours.
Live/dead Staining Methods
Viability demonstrated using live/dead staining. The LIVE/DEAD Viability/Cytotoxicity Kit and Hoechst 33342 stain (Life Technologies) were used to distinguish viable cells from dead cells according to the manufacturer’s specifications. Briefly, calcein AM (final concentration 2 μM), EthD-1 (final concentration 4 μM), and Hoechst 33342 (final concentration 0.1 μg/ml) were diluted in prewarmed D-PBS. MPS chips were perfused at 5 μl/min via a luminal port of a kidney MPS for 20 minutes and then incubated for 10 minutes at 37°C. After the staining procedure, chips were imaged using fluorescent microscopy to visualize live (green stained cells) and dead cells (red-stained cells) with nuclei marker Hoechst 33342 (blue-stained cells). Details of RNA extraction, sequencing and effluent biomarker analyses can be found in the Online Supplement.
CF Antibiotic Associated Acute Kidney Injury (CFA-AKI)
CFA-AKI is a single-center prospective cohort of patients with CF who were initiating antibiotic therapy with intravenous (IV) polymyxin E, aminoglycoside, or both for treatment of a CF pulmonary exacerbations. One patient provided consent who was prescribed an IV aminoglycoside but ultimately did not receive an aminoglycoside or polymyxin E. Recruitment began in 2018 and ended in 2020 prior to the COVID-19 pandemic. Participants were recruited from outpatient CF clinics and inpatients from the University of Washington hospital Montlake Campus. Inclusion criteria were age >18 years and receipt of either IV polymyxin E, aminoglycoside (tobramycin, gentamicin, or amikacin) or both polymyxin E and an aminoglycoside. Exclusion criteria for entry into the cohort included: previous lung transplantation, stage V CKD or chronic dialysis, treatment with IV antibiotics for a pulmonary exacerbation within one month prior to study enrollment. The study was approved by the University of Washington Institutional Review Board (STUDY00006502).
CFA-AKI Biospecimens
The protocol included urine collection prior to initiation of IV antibiotics (Day 0) and then first void morning urine collection on days 1, 2, 3, 7 and 14. Participants returned 28 days after study enrollment to collect urine and blood. Urine was collected into sterile specimen cups and transported on ice until centrifugation at 3000g for 10 min at room temperature. Peripheral blood was collected into ethylenediaminetetraacetic acid (EDTA) anti-coagulant tubes and plasma was isolated by centrifugation at 3000 g for 10 min at room temperature.
Urinary biomarker measurements were chosen a priori given literature supporting their use as AKI biomarkers and from findings from the MPS experiments. We measured soluble Fas and four other biomarkers that have been studied in AKI, which included kidney injury molecule-1 (KIM-1), neutrophil gelatinase associated lipocalin (NGAL), uromodulin (UMOD), epidermal growth factor (EGF). All biomarkers were measured in duplicate using the Meso Scale Discovery (MSD) platform (Meso Scale Diagnostics). Laboratory personnel were blinded to clinical data. Average inter-assay coefficients of variation for urinary biomarkers were low (Table S1). Urinary biomarkers were normalized to urine creatinine to account for urine dilution.
Serum creatinine was collected from the electronic medical record during antibiotic treatment and up to a median of 3 years after study enrollment. AKI was defined as an increase in serum creatinine of >50% or >0.3 mg/dL above the baseline creatinine. The lowest outpatient creatinine within the last year prior to study enrollment was used for the baseline. Prevalent CKD was defined as a study enrollment baseline estimated glomerular filtration rate (eGFR) less than 90 mL/min/1.73m2. Incident or progression of CKD was defined as new CKD (as defined as a eGFR of less than 90 mL/min/1.73m2 for three months or longer) or in patients with prevalent CKD an advance in stage on the KDIGO classification system.13 In a sensitivity analysis, we also incorporated microalbuminuria in the definition of CKD as per KDIGO classification.
Statistical Analyses
RNA Sequencing and Effluent Biomarkers
Quality of the raw reads (FASTQ files) was assessed using the FASTX toolkit (http://hannonlab.cshl.edu/fastx_toolkit/), and the sequences were then aligned to the GRCh38 genome using the STAR aligner (v2.6.1d). Read counts per gene were estimated using the Bioconductor Rsubread package.14 Treatment comparisons were made by first fitting a weighted analysis of variance (ANOVA) model using the limma-voom pipeline from the Bioconductor limma package, blocking on subject to account for subject-specific differences.15,16 Comparisons between treatments were made using empirical Bayes adjusted contrasts, while including a further 1.2-fold constraint between groups using the limma treat function.17,18 Differentially expressed genes were defined as those genes with evidence for a >1.2-fold difference between groups at a false discovery rate (FDR) < 0.05.19 Pairwise comparisons using Student’s T-test were completed for effluent biomarkers from baseline to 24 hours and baseline to subsequent timepoints at 48 and 72 hours.
Clinical Cohort
Urinary biomarker concentrations were compared at baseline, during antibiotic treatment and 28 days after study enrollment. During antibiotic treatment, we used the maximum urinary biomarker for sFas, KIM-1, and NGAL, as these are expected to rise with kidney injury, and the minimum urinary biomarker for EGF and uromodulin, as these are expected to decrease during kidney injury. We then summarized the biomarker fold change during antibiotics from baseline by the ratio of geometric means at these two time points. Finally, we estimated the association of the biomarker value during antibiotic treatment with the long-term development of CKD, via Poisson relative risk regression with robust Huber-White standard errors and an offset term for the time at risk, estimating the relative risk of CKD per doubling in the biomarker. We examined both unadjusted models and models adjusted for age and baseline biomarker value, respectively. Analyses were performed using R 4.2.1 (R Foundation for Computing, Vienna, Austria).
RESULTS:
MPS Gene Expression Profiles after Polymyxin E Exposure
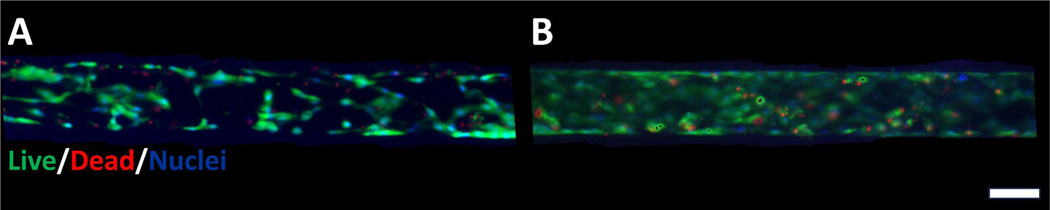
To identify the optimal dose of polymyxin E to test in the MPS, we completed dose escalation studies. PTECs cultured in the MPS after polymyxin E exposure of 50 μg/mL demonstrated intact tubular integrity and cell viability (Figure 1). In comparison, higher concentrations of polymyxin E exposure (75 μg/mL) resulted in cell blebbing and loss of tubule integrity (Supplement Figure 1).
Figure 1.
Viability of proximal tubule epithelial cells (PTECs) in human kidney 3D MPS.
Images demonstrate minimal increase in histologic cell death/injury or gross morphology with 50 μg/mL of polymyxin E treatment at 72 hours. Thus, transcriptomic signatures are early markers of PTEC injury. (A) Control MPS and (B) Polymyxin-E treated MPS live/dead staining (calcein AM and ethidium bromide, respectively). Scale bar is 100 micrometers
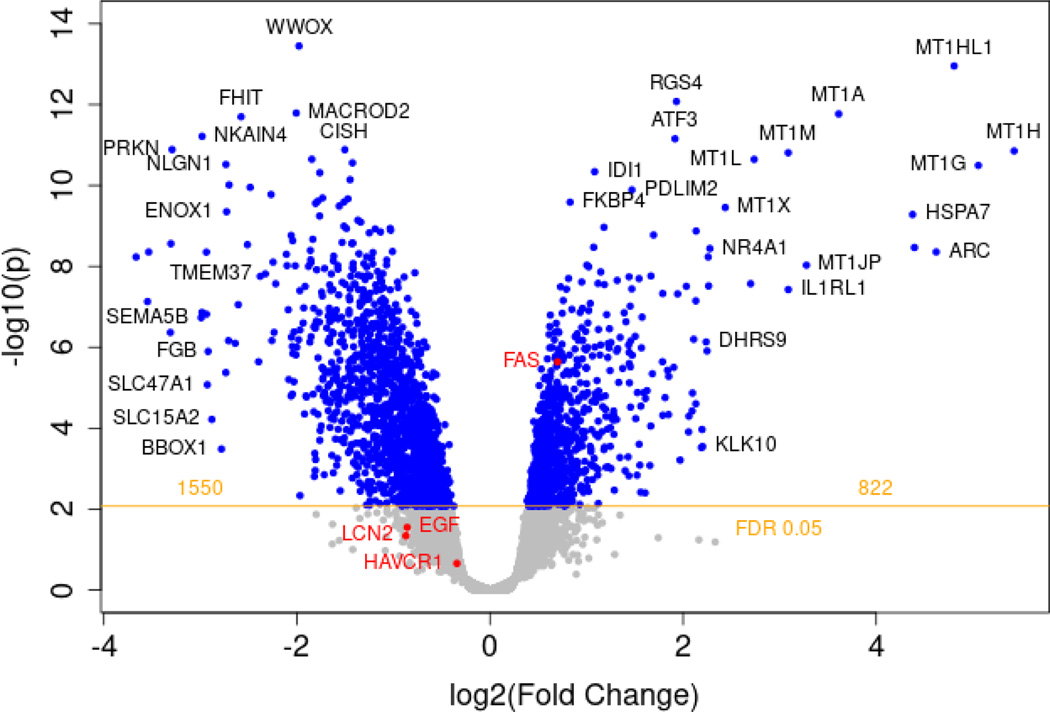
Next, MPS were populated from PTECs from three different donors with 16 MPS controls and 19 MPS treated with polymyxin E. To assess global transcriptional changes to polymyxin E exposure to PTECs in MPS devices, we employed RNAseq to identify differentially expressed genes after 72 hours of polymyxin E or control exposure. At an FDR <0.01 and 20%-fold change, 2,353 differently expressed genes were identified with polymyxin E exposure relative to controls (Figure 2). At an FDR of 0.05, the number of differently expressed genes increased to 7,641. A number of different pathways were upregulated including genes involved in oxidative stress and apoptosis. Among the differentially expressed genes, Fas gene expression was highly statistically significant. We observed an upregulation of Fas expression (fold-change 1.63, FDR q-value=7.29×10−5) with polymyxin E treatment. Other members of the ‘death receptor’ superfamily were also upregulated during polymyxin E treatment, including TNFSF15, TNFSF9 and heat shock proteins, suggesting that pathways of apoptosis and inflammation were dysregulated. Activation of Fas/FasL signaling has been previously shown among a number of cell types after polymyxin E exposure20. Since research assays are available to quantify soluble Fas concentrations and the biological plausibility, we focused on the Fas/FasL pathway.
Figure 2.
Volcano plot of all differentially regulated genes between polymyxin E and control.
RNAseq analyses shows differentially expressed genes after 72 hr of polymyxin E versus control exposure in MPS. MPS were populated from PTECs from three different donors with 16 MPS controls and 19 MPS treated with polymyxin E. 2,372 differently expressed genes were identified with polymyxin E exposure relative to controls. The increase or decrease in gene expression from control to polymyxin E is shown in the x-axis as a log2(fold change) value, and its corresponding significance value is shown on the y-axis as a -log10(p) value. Blue dots represent individual genes. Genes with a log2(fold change) greater than zero indicate an increase in expression. Greater significance is indicated by greater values on the y-axis, with a threshold of statistical significance indicated by the horizontal yellow line, with a calculated FDR of 0.05. The pro-apoptotic Fas gene annotated in red saw an increase in expression: log2 fold-change=0.70, FDR=7.29×10−5. Also highlighted in red are EGF, KIM-1 (HAVCR1), and NGAL (LCN2). UMOD was not detected.
MPS Effluent Analysis after Polymyxin E Exposure
The effluents of our 3D MPS devices were collected at 24 and 48 hours and served as a “pseudo” urine as it represents cell culture media that has flowed through lumens lined with PTECs that have been exposed to our treatment conditions. We collected MPS effluent after exposure to 50 μg/mL of polymyxin E from at least three different human donor sources and compared these values with the effluent biomarker concentrations of media only control MPS devices. After 24 hours of exposure polymyxin E treated MPS demonstrated significantly greater effluent sFas concentrations than control MPS (p<0.001) (Figure S2). At 48 hours of exposure the difference in effluent sFas concentrations between polymyxin E and control MPS was attenuated but remained statistically different (p=0.01).
CFA-AKI Clinical Cohort Baseline Characteristics
To translate biomarker discovery from the MPS to a clinical cohort, we prospectively enrolled participants with CF who were prescribed polymyxin E, aminoglycosides or both antibiotics to treat a CF pulmonary exacerbation. Among 16 participants with CF, the mean (SD) age was 33.8 (12.0) years and 9 (56%) participants were women. Seven (44%) individuals in the cohort had a history of diabetes mellitus with need for chronic insulin therapy and 1 (6%) had prevalent CKD. The mean duration of antibiotics was 13.9 days (range of 12 to 14 days). Nine patients (56%) received an aminoglycoside antibiotic, five (31%) polymyxin E, one patient (6%) received both and one patient (6%) was prescribed an aminoglycoside but ultimately received meropenem and aztreonam. During antibiotic therapy, two patients developed KDIGO Stage 1 AKI based on changes in serum creatinine. Despite the low rates of AKI during antibiotic therapy, seven (44%) patients developed CKD which included incident or progression of kidney disease during a median follow-up of 3 years after study enrollment (Table 1).
Table 1.
Patient Characteristics and Clinical Outcomes in the CFA-AKI cohort
| Patient Characteristics | N = 16 |
|---|---|
| Age, mean (SD), years | 33.8 (± 12.0) |
| Sex, male, n (%) | 7 (44 %) |
| Race, n (%) | |
| White | 14 (88%) |
| Black | 0 |
| American Indian or Alaska Native | 0 |
| Asian | 1 (6%) |
| Native Hawaiian or Other Pacific Islander | 1 (6%) |
| CF genotyping, n (%) | |
| ΔF508 homozygous | 7 (44%) |
| ΔF508 heterozygous | 7 (44%) |
| Other | 2 (13%) |
| BMI, mean (SD), kg/m2 | 23.2 (± 3.2) |
| Comorbidities, n (%) | |
| Prevalent CKD (eGFR < 90 mL/min/1.73 m2)A | 1 (6%) |
| Diabetes mellitus and/or chronic insulin therapy | 7 (44%) |
| Hypertension | 1 (6%) |
| eGFR, mean (SD), (mL/min/1.73 m2) | 124.5 (± 31.3) |
| FEV1, mean (SD), L | 1.59 (± 0.8) |
| CFTR modulator use prior to enrollment, n (%) B | 1 (6%) |
| Antibiotic course | |
| Antibiotic course by category, n (%) | |
| Aminoglycoside | 9 (56%) |
| Polymyxin | 5 (31%) |
| Both | 1 (6%) |
| OtherC | 1 (6%) |
| Days of antibiotic therapy, mean (range), days | 13.9 (12–14) |
| NSAID use during study period, n (%) | 4 (25%) |
| Outcomes | |
| Incident CKD from end of therapy to follow-up, n (%)D,E | 7 (44%) |
| Stage 2 | 3 (19%) |
| Stage 3a | 1 (6%) |
| Stage 3b | 1 (6%) |
| Stage 4 | 2 (13%) |
| Median duration to incident CKD, days (range) | 369 (104 – 751) |
| Mean duration to incident CKD, days (range) | 417 (104 – 751) |
| Death | 1 (6%) |
One patient met criteria for G2 CKD, a mildly decreased GFR of 60 to 89 mL/min/1.73 m2,, according to KDIGO guidelines. KDIGO. Summary of recommendation statements. Kidney Int 2013; 3 (Suppl):5.
Prior to and during study enrollment, one patient was on CFTR modulatory therapy, specifically, lumacaftor/ivacaftor.
One patient in the cohort received meropenem and aztreonam during the treatment period.
Chronic kidney disease classification based upon glomerular filtration rate (mL/min/1.73 m2), where: G1 is a normal eGFR of ≥ 90 mL/min/1.73 m2, G2 a mildly decreased eGFR of 60 to 89 mL/min/1.73 m2, G3a is a mildly to moderately decreased eGFR of 45 to 59 mL/min/1.73 m2, G3b is a moderately to severely decreased eGFR of 30 to 44 mL/min/1.73 m2, G4 is a severely decreased eGFR of 15 to 29 mL/min/1.73 m2 and G5 is kidney failure with a eGFR of <15 mL/min/1.73 m2, +/− dialysis. CKD was defined by an eGFR of less than 90 mL/min/1.73 m2 for three months or longer.
Incident or progression of CKD was determined through chart review in the years following the conclusion of the study period (i.e., 28-day period of urine and plasma collection concurrent with antibiotic therapy). Follow-up continued until patient death or lost to follow-up.
CF Urinary Biomarkers
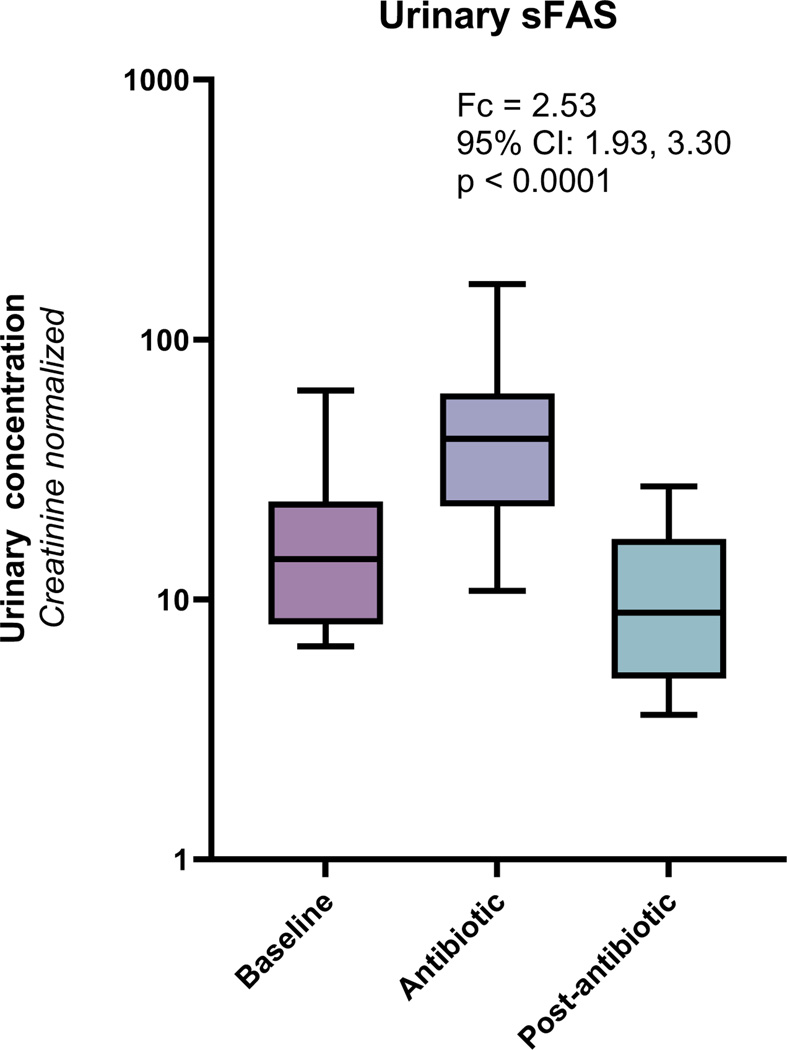
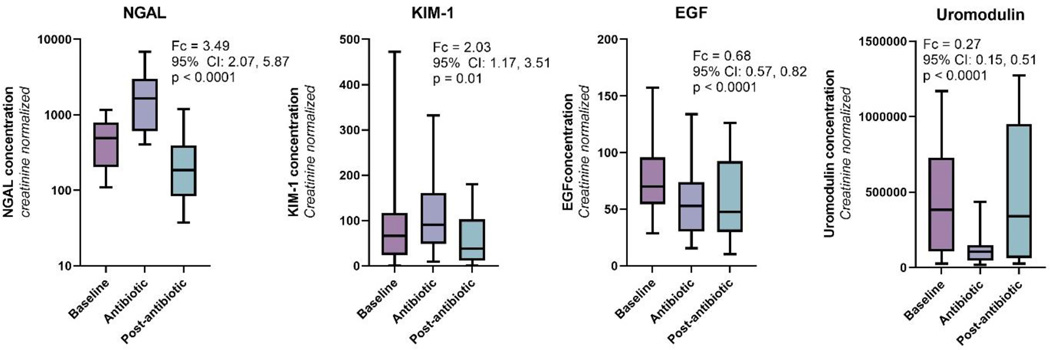
Among a total of 108 urine samples collected from 16 patients in the cohort (median of 7 urine samples per patient), we found that urinary sFas concentrations were significantly greater during antibiotic therapy compared to prior to antibiotics (fold change=2.53; 95% CI: 1.93, 3.30; p<0.001) (Figure 3 and Table S2). Two weeks after antibiotic administration, urinary sFas concentrations returned to baseline suggesting that the increase in sFas concentrations was transitory. We also measured other urinary biomarkers that have been previously studied in AKI. We found that two biomarkers of tubular injury, NGAL and KIM-1, were both significantly elevated during treatment with antibiotics. We also found that two biomarkers of kidney health, EGF and uromodulin, were significantly reduced during antibiotic administration (Figure 4). These changes in urinary biomarkers were also transitory and concentrations returned to baseline concentrations two weeks after antibiotic treatment.
Figure 3.
Urinary sFas concentrations among 16 patients with CF at baseline, during antibiotics and then 2 weeks after antibiotic treatment
Box plots demonstrate that urinary sFas concentrations are significantly elevated during antibiotic treatment and return to baseline levels two weeks after completing of antibiotics. Urinary sFas concentrations are normalized for urinary creatinine to account for dilution of urinary samples. Fc is comparing sFas concentrations between baseline and antibiotic.
Figure 4.
Urinary biomarkers of epithelial cell injury are higher and biomarkers of kidney health are lower during nephrotoxic antibiotics.
Box plots demonstrate that concentrations of kidney injury biomarkers, NGAL and KIM-1, are elevated during antibiotic treatment and return to baseline levels two weeks after completing of antibiotics. Concentrations of the kidney health biomarkers, EGF and uromodulin, are decreased during antibiotic treatment. Urinary biomarker concentrations are normalized for urinary creatinine to account for dilution of urinary samples.
CF Urinary Biomarkers and Clinical Outcomes
Despite the increase in urinary biomarker concentrations, we found that the incidence of AKI based on changes in serum creatinine to be uncommon: 2 of 16 patients developed AKI during antibiotic administration. We also found that urinary biomarkers were not associated with development of AKI during antibiotic treatment, suggesting that patients with CF may have subclinical AKI during antibiotic therapy. Subclinical AKI is defined as elevated urinary biomarkers without an elevated serum creatinine.21 Thus, to determine if elevations in urinary sFas concentrations are associated with clinically meaningful long-term outcomes, we evaluated associations between urinary biomarkers and long-term CKD. Urinary sFas concentrations during antibiotic treatment were strongly associated with the development of CKD (unadjusted relative risk=2.28, 95% CI: 1.55 – 3.36; p<0.001) per doubling of sFas (Table 2). In models adjusted for age and biomarker concentrations prior to antibiotic therapy, sFas continued to be associated with CKD. We also found that EGF was strongly associated with CKD, while KIM-1, NGAL and uromodulin were not associated with CKD in this cohort. In a sensitivity analysis, we included microalbuminuria in the definition of CKD. While most patients in the clinical cohort did not have urinary albumin levels measured in the outpatient setting, we found one additional patient qualified for CKD with the inclusion of microalbuminuria. We also found that urinary sFas concentrations remained associated with the development of AKI (unadjusted relative risk = 2.09, 95% CI: 1.50 – 2.93; p<0.0001) (Table S3).
Table 2.
Association of urinary biomarkers with risk of CKD
| CKD | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for age and DM | Adjusted for age and baseline biomarker | ||||
| RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| sFAS | 2.28 (1.55, 3.36) | < 0.0001 | 1.62 (0.79, 3.32) | 0.19 | 1.63 (1.01, 2.62) | 0.046 |
| EGF | 0.25 (0.12, 0.53) | 0.0003 | 0.31 (0.10, 1.03) | 0.055 | 0.18 (0.06, 0.58) | 0.004 |
| KIM-1 | 1.26 (0.80, 2.00) | 0.32 | 1.00 (0.63, 1.57) | 0.99 | 0.72 (0.26, 1.96) | 0.52 |
| NGAL | 0.96 (0.60, 1.52) | 0.85 | 0.78 (0.33, 1.85) | 0.58 | 1.17 (0.65, 2.12) | 0.60 |
| Uromodulin | 0.98 (0.42, 2.28) | 0.96 | 1.53 (0.90, 2.57) | 0.11 | 1.12 (0.42, 2.98) | 0.81 |
RRs are per doubling in the maximum of the biomarker during antibiotic treatment (or minimum of biomarker, for EGF and uromodulin). Baseline biomarker refers to biomarker concentrations prior to starting antibiotic treatment.
Abbreviations: sFas: soluble Fas; EGF: epidermal growth factor; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin
DISCUSSION:
Polymyxins and aminoglycosides undergo extensive net tubular reabsorption by PTECs and intracellular accumulation of the drug leads to kidney epithelial cell injury. Previous work has shown that one key mechanism of antibiotic nephrotoxicity is apoptosis, or programmed cell death.22 The Fas/FasL system is a key biologic pathway regulating apoptosis in kidney epithelial cells.23 Fas is a transmembrane protein constitutively expressed on renal tubular epithelial cells. Activation of Fas leads to caspase-3,7 dependent signaling, culminating in formation of apoptotic bodies and cell death.24 Soluble Fas (sFas) is a truncated form of Fas that is also constitutively released either through matrix metalloproteinase cleavage of membrane bound Fas or through alternative mRNA splicing.25 sFas is present circulating in plasma and urine and easily measurable with standard immunoassays. Thus, based on the findings in the MPS, we measured urinary sFas in a clinical cohort of patients with CF and found that elevations in sFas during antibiotic treatment were strongly associated with long-term risk of development or progression of CKD.
Patients with CF have a number of risk factors for development of kidney disease including nephrotoxic antibiotics, immunosuppressants, non-steroidal anti-inflammatory drugs, CF related diabetes, CFTR derangement in the tubules, and others.26 With these risks for kidney disease, it is paramount to identify biomarkers that can identify early clinical disease. The standard metric of kidney health, serum creatinine, is insensitive, nonspecific and lags behind the time of kidney injury.27 Several alternatives to serum creatinine have been assessed for their use as kidney health indicators, including serum and urinary biomarkers, such as blood urea nitrogen (BUN), blood cystatin C (CysC), and urinary NGAL and KIM-1. But these biomarkers remain seldom used in clinical practice, due to limited research and lack of Food and Drug Administration approval of testing protocols. The pro-apoptotic protein sFas is a promising candidate biomarker that has been associated with the development of AKI in our previous work and other studies.28,29 To date, this is the only investigation into the efficacy of urinary sFas as a biomarker of kidney injury in CF. These findings suggest that urinary sFas could be used as a sensitive and timely biomarker for the development of subclinical AKI in patients with CF receiving nephrotoxic antibiotics. Furthermore, the association of urinary sFas with incident CKD gives it potential utility in approximating CKD risk. Receipt of nephrotoxic antibiotics in CF may serve as a kidney ‘stress test’ and elevations of urinary sFas may suggest patients at high risk of development of CKD. While validation in multiple clinical cohorts is necessary, possible future use of urinary sFas may be as a marker for early detection of kidney injury in patients with CF, assessing CKD risk and guiding antibiotic dosing and duration.
This study has limitations such as the sample size of the clinical cohort was low. Additional factors, such as non-steroidal anti-inflammatory agents or immunosuppressants after lung transplant, may have also led to an increased risk of AKI. However, despite the small sample size, we were able to identify that urinary sFas concentrations are strongly associated with the risk of long-term CKD. Future studies in alternative clinical cohorts receiving nephrotoxic antibiotics are necessary to validate our findings and adjust for alternative risk factors for AKI. Second, polymyxin E was the main antibiotic used in the MPS experiments. Additional studies in non-polymyxin antibiotics, such as aminoglycosides and combination antibiotics may identify alternative urinary biomarkers for study. Third, the concentration of polymyxin E used in the MPS (50 μg/mL) was higher than steady state serum concentrations of polymyxins (range of 6 to 18 μg/mL)30. However, a number of studies have suggested accumulation of polymyxins in PTECs with estimates of 1000 fold higher concentrations in tubular cells than serum31,32. Fourth, for our primary analysis, CKD was defined by a change in eGFR based on serum creatinine. Another criterion that can qualify patients for CKD is urinary albumin concentrations. However, a minority of patients in our study had urinary studies completed as part of routine follow-up care to quantify urinary albumin levels. Nevertheless, the inclusion of urinary albumin led to one more patient qualifying for CKD that was not previously identified using changes in eGFR. In sensitivity analyses with CKD defined based on changes in eGFR, albuminuria or both, urinary sFas concentrations during treatment with antibiotics continued to be associated with development of CKD during follow-up.
In summary, we utilized a human kidney 3D MPS platform to discover novel biomarkers of epithelial cell injury due to nephrotoxic antibiotics. We identified upregulation of Fas gene expression with polymyxin E exposure in the MPS and a concordant increase in effluent sFas concentrations. We then translated findings that sFas may be an early marker of kidney epithelial cell injury and, in turn, kidney dysfunction to a pilot clinical cohort of patients with CF receiving nephrotoxic antibiotics. We found that despite low rates of AKI based on changes in serum creatinine, urinary sFas concentrations were more than 2-fold greater during antibiotic treatment compared to prior to antibiotics. Moreover, the increase in sFas concentrations during antibiotics were associated with risk of long-term CKD. Overall, these findings suggest that urinary sFas is a novel biomarker of subclinical kidney injury and may provide early signs prior to a rise in serum creatinine in patients with CF at risk of long-term kidney dysfunction.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge all the research personnel who supported the completion of this study and all the individuals with CF who participated in the study.
Funding:
This work was supported by the National Institutes of Health [grant number; P30 DK089507] and the Cystic Fibrosis Foundation [grant number; BHATRA18A0].
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest for the work.
REFERENCES
- 1.MacKenzie T, Gifford AH, Sabadosa KA, et al. Longevity of Patients With Cystic Fibrosis in 2000 to 2010 and Beyond: Survival Analysis of the Cystic Fibrosis Foundation Patient Registry. Ann Intern Med. 2014;161(4):233–241. doi: 10.7326/M13-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quon BS, Mayer-Hamblett N, Aitken ML, Smyth AR, Goss CH. Risk Factors for Chronic Kidney Disease in Adults with Cystic Fibrosis. Am J Respir Crit Care Med. 2011;184(10):1147–1152. doi: 10.1164/rccm.201105-0932OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aloul M, Miller H, Alapati S, Stockton P a., Ledson M j., Walshaw M j. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39(1):15–20. doi: 10.1002/ppul.20138 [DOI] [PubMed] [Google Scholar]
- 4.Hmiel SP, Beck AM, De La Morena MT, Sweet S. Progressive Chronic Kidney Disease After Pediatric Lung Transplantation. Am J Transplant. 2005;5(7):1739–1747. doi: 10.1111/j.1600-6143.2005.00930.x [DOI] [PubMed] [Google Scholar]
- 5.Edelstein CL. Biomarkers of Acute Kidney Injury. Adv Chronic Kidney Dis. 2008;15(3):222–234. doi: 10.1053/j.ackd.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groeneweg M, Tan S, Boot AM, de Jongste JC, Bouquet J, Sinaasappel M. Assessment of nutritional status in children with cystic fibrosis: conventional anthropometry and bioelectrical impedance analysis. A cross-sectional study in Dutch patients. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2002;1(4):276–280. [DOI] [PubMed] [Google Scholar]
- 7.Weber EJ, Chapron A, Chapron BD, et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016;90(3):627–637. doi: 10.1016/j.kint.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber EJ, Lidberg KA, Wang L, et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight. 2018;3(24):e123673, 123673. doi: 10.1172/jci.insight.123673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y, Lee W, Kwa AL. Polymyxin B versus colistin: an update. Expert Rev Anti Infect Ther. 2015;13(12):1481–1497. doi: 10.1586/14787210.2015.1093933 [DOI] [PubMed] [Google Scholar]
- 10.Lim LM, Ly N, Anderson D, et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30(12):1279–1291. doi: 10.1592/phco.30.12.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nord NM, Hoeprich PD. POLYMYXIN B AND COLISTIN. A CRITICAL COMPARISON. N Engl J Med. 1964;270:1030–1035. doi: 10.1056/NEJM196405142702002 [DOI] [PubMed] [Google Scholar]
- 12.Justo JA, Bosso JA. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy. 2015;35(1):28–33. doi: 10.1002/phar.1493 [DOI] [PubMed] [Google Scholar]
- 13.Summary of Recommendation Statements. Kidney Int Suppl. 2013;3(1):5–14. doi: 10.1038/kisup.2012.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47(8):e47. doi: 10.1093/nar/gkz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. doi: 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipson B, Lee S, Majewski IJ, Alexander WS, Smyth GK. ROBUST HYPERPARAMETER ESTIMATION PROTECTS AGAINST HYPERVARIABLE GENES AND IMPROVES POWER TO DETECT DIFFERENTIAL EXPRESSION. Ann Appl Stat. 2016;10(2):946–963. doi: 10.1214/16-AOAS920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testing significance relative to a fold-change threshold is a TREAT - PubMed. Accessed December 19, 2022. https://pubmed.ncbi.nlm.nih.gov/19176553/ [DOI] [PMC free article] [PubMed]
- 19.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 20.Azad MAK, Finnin BA, Poudyal A, et al. Polymyxin B Induces Apoptosis in Kidney Proximal Tubular Cells. Antimicrob Agents Chemother. 2013;57(9):4329–4335. doi: 10.1128/AAC.02587-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care Lond Engl. 2012;16(3):313. doi: 10.1186/cc11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azad MAK, Finnin BA, Poudyal A, et al. Polymyxin B Induces Apoptosis in Kidney Proximal Tubular Cells. Antimicrob Agents Chemother. 2013;57(9):4329–4335. doi: 10.1128/AAC.02587-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Arduan A, Danoff TM, Kalluri R, et al. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol. 1996;271(6 Pt 2):F1193–1201. [DOI] [PubMed] [Google Scholar]
- 24.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710 [DOI] [PubMed] [Google Scholar]
- 25.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154(6):2706–2713. [PubMed] [Google Scholar]
- 26.Nazareth D, Walshaw M. A review of renal disease in cystic fibrosis. J Cyst Fibros. 2013;12(4):309–317. doi: 10.1016/j.jcf.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 27.Prigent A. Monitoring Renal Function and Limitations of Renal Function Tests. Semin Nucl Med. 2008;38(1):32–46. doi: 10.1053/j.semnuclmed.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Bhatraju PK, Robinson-Cohen C, Mikacenic C, et al. Circulating levels of soluble Fas (sCD95) are associated with risk for development of a nonresolving acute kidney injury subphenotype. Crit Care. 2017;21(1):217. doi: 10.1186/s13054-017-1807-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatraju P, Hsu C, Mukherjee P, et al. Associations between single nucleotide polymorphisms in the FAS pathway and acute kidney injury. Crit Care. 2015;19(1):368. doi: 10.1186/s13054-015-1084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins Revisited. Clin Microbiol Rev. 2008;21(3):449–465. doi: 10.1128/CMR.00006-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perazella MA. Pharmacology behind Common Drug Nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897–1908. doi: 10.2215/CJN.00150118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azad MAK, Roberts KD, Yu HH, et al. Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem. 2015;87(3):1590–1595. doi: 10.1021/ac504516k [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.