Abstract
Dogs (Canis lupus familiaris) are the domestically bred descendant of wolves (Canis lupus). However, selective breeding has profoundly altered facial morphologies of dogs compared to their wolf ancestors. We demonstrate that these morphological differences limit the abilities of dogs to successfully produce the same affective facial expressions as wolves. We decoded facial movements of captive wolves during social interactions involving nine separate affective states. We used linear discriminant analyses to predict affective states based on combinations of facial movements. The resulting confusion matrix demonstrates that specific combinations of facial movements predict nine distinct affective states in wolves; the first assessment of this many affective facial expressions in wolves. However, comparative analyses with kennelled rescue dogs revealed reduced ability to predict affective states. Critically, there was a very low predictive power for specific affective states, with confusion occurring between negative and positive states, such as Friendly and Fear. We show that the varying facial morphologies of dogs (specifically non-wolf-like morphologies) limit their ability to produce the same range of affective facial expressions as wolves. Confusion among positive and negative states could be detrimental to human–dog interactions, although our analyses also suggest dogs likely use vocalisations to compensate for limitations in facial communication.
Keywords: Wolves, Domestic dogs, Facial expressions, Emotions, Domestication, Animal welfare
Subject terms: Zoology, Animal behaviour
Introduction
Successful communication is essential for highly social, group-living animals as it mediates important social behaviour, upholds social hierarchies and maintains strong social bonds1–5. The social communication of mammals has been well studied and includes a vast array of research on visual signalling6–20. Moreover, mammalian faces can convey a wealth of information via communicative signals15,16,18,19, and as a result, facial expressions are considered highly important for social communication amongst mammals18. Conveying information about one’s internal affective state is also essential for social animals as this allows for the selection of appropriate behavioural decisions to be made by receivers, in response to cues from others21–23. Affective states are forms of motivation such as emotions and moods24–33. The term ‘affect’ is used to describe states that have the property of valence (positive or negative)30,34. In non-human animals ‘affective states’ are based on contextualised, behavioural indicators (including body-language, vocalisations, and changes in social proximity) and consist of short-term emotion-like and long-term mood-like states22,30,34–38. One method of quantifying affective states is via the movements observed in facial expressions26,28,29,32,34,39. As a result, several approaches have been developed to quantify the facial expressions of different species, in particular Facial Action Coding Systems9,13,39–46.
One species that has frequently been used as a model for describing the social behaviour of group-living animals is the wolf (Canis lupus)47, and it has been long speculated that wolves use facial expressions to convey affective states6,8,48,49. Schenkel6 described more than 20 variations of wolf facial expressions, which he argued were associated with emotion-like affective states. Fox8 argued that wolves were capable of a broad range of facial expressions, which are used in varying social interactions and contexts. However, there have been no quantitative analyses of these suggested associations between facial expressions and affective states in wolves. It is thought that the head and facial feature morphologies of wolves aid the production of facial expressions that are key to successful social communication6,8. Combinations of facial features, including fur length and fur slope, mimic muscle movements, and the activities of the eyes, nose and ears, emphasise the appearance of the muzzle, lips, eyes, forehead and ears, which are the main conveyors of facial expressiveness6–8. The relative shape and position of the main conveyers of facial expressiveness are highly conserved across all wolves throughout the world50–53 (Fig. 1A) which highlights the adaptive value of facial communication in wolves. Quantifying these associations between facial expressions and affective states would provide a valuable tool for monitoring welfare in both wild and captive canids and provide the scope for cross-species comparisons to give insight into the evolution and adaptive value of affective states in Canidae.
Figure 1.
Illustration of the differences in the main conveyers of facial expressiveness between wolf and examples of domestic dog breeds. (A) Wolf (Canis lupus) portrait depicting typical head morphologies and facial patterning. Note erect ears, head shape, fur length and slope, and facial masking as a consequence of lighter coloured ‘eyebrows’, muzzle and cheek area. Photograph by ER Hobkirk. (B) ‘Wolf-like’ Finnish Lapphund dog, with head morphologies and facial patterning almost identical to that of the wolf. Photograph by SD Twiss. (C) Typical Rottweiler face with conspicuous brown eyebrows (red circle), set against a solid black background. Note flopped ears and broad head shape in comparison with the wolf. Image courtesy of the American Kennel Club. (D) Brachycephalic face of a Pug dog. Note flopped ears, bulging eyes and excessive wrinkling in comparison with the wolf. Image courtesy of the American Kennel Club. (E) Komondor dog with less distinct facial features due to fur type (dreadlocks), length and slope. Image courtesy of the American Kennel Club.
Like wolves, domestic dogs (Canis lupus familiaris) can produce facial expressions7 due to their complex facial musculature7,45,54 and have been shown to express affective states55. However, as a result of selective breeding, head morphologies and the associated main conveyers of facial expressiveness of many breeds of dogs have greatly diverged from those of their wolf ancestors56–58 (Fig. 1). While some breeds have retained a more ‘wolf-like’ appearance (Fig. 1B), other breeds differ markedly in head and facial feature morphologies. For example, Rottweillers and Pugs have flopped ears, brachycephalic faces (short, broad skulls and shortened muzzles) and pendulous lips (Fig. 1C and D). The eyes of the Pug are also relatively larger in proportion to the size of its head, and the forehead of the Pug is greatly wrinkled compared to the wolf (Fig. 1A). In addition, the visibility of the main conveyers of facial expressiveness of dogs such as the Komondor is greatly diminished as they are mostly hidden beneath their dreadlock fur (Fig. 1E). To date there has been little quantitative analyses of the associations between domestic dog facial expressions and affective states21,33,54,55,59. Therefore, in this study our aim was to first identify discrete facial expression movements within wolves and domestic dogs, and then determine whether combinations of different facial movements correlated with specific affective states. We also predicted that the various head and facial morphologies found across different dog breeds would limit their abilities to successfully produce the range of affective facial expressions observed in wolves. The facial expressions of captive, human-habituated wolves and kennelled rescue dogs were quantified during behavioural events using the Dog Facial Action Coding System (DogFACS)45 supplemented with records of Additional Facial Movements (AFM, Table S3). We then tested whether the observed facial expressions mapped onto the affective states exhibited by the wolves and dogs during these behavioural events. Where facial expressions were ambiguously associated with one or more affective state, we investigated the potential reasons for this by comparison with facial morphological differences.
Results
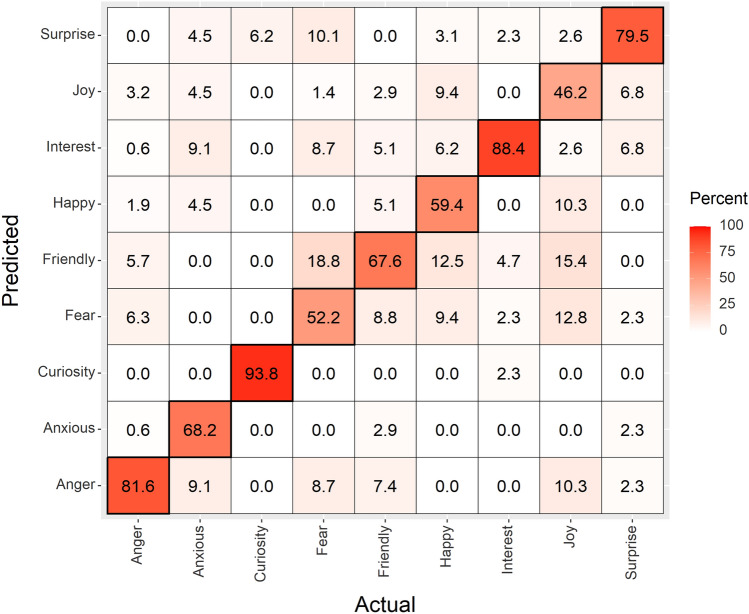
We used linear discriminant analysis (LDA) to conduct a supervised classification60 of facial movements (DogFACS and AFM codes), to identify how well each directly observed affective state could be predicted based upon the combinations of DogFACS and AFM codes recorded for each event. Predicted wolf affective states based upon LDA of facial movements exhibited substantial agreement with the independently allocated affective state classifications based on direct observation of the videos (71%, Fig. 2). Precision values within individual cells of the confusion matrix range between moderate (lowest precision value for the affective state Joy = 46%) and almost perfect agreement (highest precision; Curiosity = 94%). In addition, where confusion occurs within the matrix (values other than True Positives), the values do not exceed 20% (‘slight agreement’ according to criteria set by Landis & Koch61).
Figure 2.
Confusion matrix, showing the observed (actual) versus predicted affective states for wolf facial expressions (n = 559). Values within each true positive tile (diagonal) display the precision percentages per affective state. Overall precision = 71%.
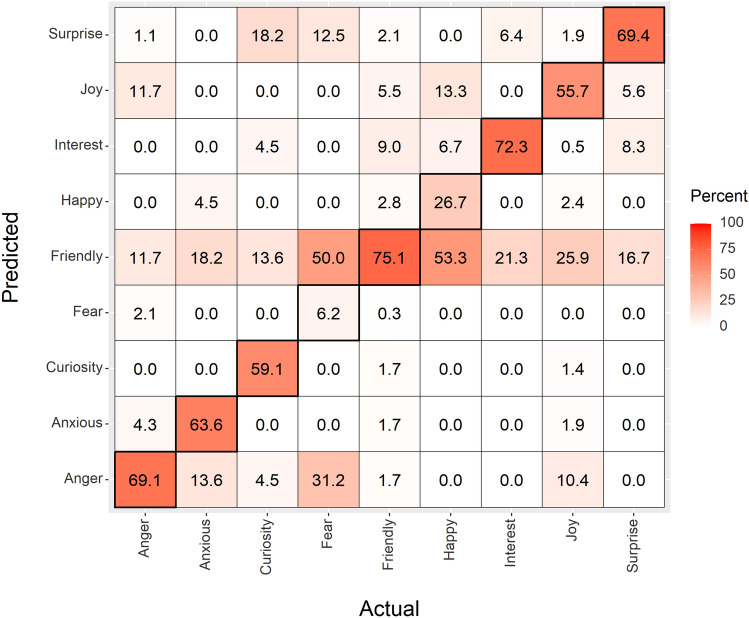
The confusion matrix for dog affective states (Fig. 3) also shows substantial agreement (albeit lower than that for wolves at 65%) between LDA predicted and observed affective states. However, precision for specific cells within the matrix for dogs is considerably reduced compared to that of wolves, ranging from 6% for the Fear affective state to a maximum of 75% for Friendly. Where confusion occurs, it is typically greater compared to wolves; for example, 31% confusion between Fear and Anger. The affective state termed Friendly has substantial overall precision, with 75% of the events classed as Friendly by both direct observation and the LDA classification. However, 12–53% of the events classed as other affective states (both positive and negative states, Table 1) by direct observation were incorrectly categorised as Friendly based upon the LDA classification of facial movements (Fig. 3). The level of disagreement in Fig. 3 will be dependent upon the particular dogs and breeds included in the analysis, and the range of affective states expressed by each. However, iterated simulations repeating the LDA with each individual (and each breed in further simulations) removed from the analysis in turn revealed no increase in levels of agreement, only more disagreement in some cases (see supplementary material and Table S7a and b). Therefore the 65% agreement in our dog confusion matrix appears to be a maximal estimate.
Figure 3.
Confusion matrix, showing the observed (actual) versus predicted affective states for dog facial expressions (n = 753). Values within each true positive tile (diagonal) display the precision percentages per affective state. Overall precision = 65%.
Table 1.
Primary, short-term, emotion-like affective states with corresponding descriptors.
| Primary affective state and positive (+ ve) or negative (− ve) classifications | Descriptors | Source |
|---|---|---|
| Anger (− ve) | Aggressive interactions; can be offensive or defensive; often results in decreased social proximity, unless full conflict occurs | 63–66 |
| Anxious (− ve) | Focal canid displays signs of distress (e.g. vocalisations such as whimpering), often in response to uncertain anticipation; social proximity is neither increased nor decreased | 64–67 |
| Curiosity (+ ve) | Focal canid fully approaches ‘emotive’ stimuli (e.g. familiar sounds, such as dog squeak toy) and becomes fixated on it for an extended period (> five seconds) | 64,66,68 |
| Fear (− ve) | Associated with aggressive interactions and sudden shocks by novel stimuli, e.g. the approach of an unfamiliar social interactant; social proximity is often decreased as focal canid attempts to escape from social interactant or novel stimuli | 63,64,66 |
| Friendly (+ ve) | Associated with submissive behaviour (e.g. lowered body posture) toward social interactant (who often has a higher social rank); social interactant may be familiar or unfamiliar (human social interactants only); social proximity is increased. This affective state can occur during positive interactions (as active submission) and negative interactions (as passive submission) | 64,66,69 |
| Happy (+ ve) | Focal canid is receiving tactile attention from social interactant, e.g. grooming or petting; social proximity is increased | 64–66 |
| Interest (+ ve) |
Focal canid approaches social interactant or inanimate object, though makes no attempt to fully interact; social proximity is initially increased but maintained at approximately one body length from social interactant/inanimate object Note: Focal canid may increase or decrease social proximity if social interactant attempts to interact with focal canid |
64,66,70 |
| Joy (+ ve) | Excitable interactions, e.g. play or copulation; social proximity is increased | 63,64,66,67 |
| Surprise (+ ve/ − ve) | Focal canid reacts to sudden shocks to the sensory system, in particular auditory, visual and tactile stimuli; focal canid is momentarily fixated on stimuli (< five seconds) and often becomes immobile (‘freezes’); proximity to stimuli is neither increased nor decreased | 63,64,66,71 |
Descriptors and positive and negative classifications are derived from contextual information gathered from video footage of canid social interactions and reactions to external stimuli. Source indicates the primary literature for comparison of descriptors and positive and negative classifications. Friendly was classified as positive as focal canids were unharmed during interactions involving this affective state. Surprise was classified as positive or negative.
The confusion seen in Fig. 3 consists of 262 incorrectly predicted affective states out of 753 dog facial expressions analysed. Table 2 shows how these 262 incorrect classifications distribute across dogs with different morphological facial features. Traits that were most associated with confusion within the dog matrix were brachycephalic and mesocephalic (medium proportioned skulls) heads, which together were associated with nearly 80% of the incorrectly predicted affective states, and flopped and semi-flopped ears, which were present in 84% of the incorrectly predicted events (Table 2). Flews (pendulous lips) were present in 40% of the cases of confusion within the dog matrix. Table 2 also shows that dolichocephalic (wolf-like morphology, long length skulls with long muzzles) accounted for 22% of incorrectly predicted affective states, however these dolichocephalic dogs also had non-wolf-like facial morphologies such as flopped and semi-flopped ears.
Table 2.
Domestic dog morphological facial features and the corresponding number of dogs and percentage of entries within the incorrectly predicted (confused, n = 262) affective states seen in Fig. 3 (dog confusion matrix).
| Morphological features | n, number of dogs | % of entries |
|---|---|---|
| Head shape | – | – |
| Brachycephalic | 103 | 39 |
| Mesocephalic | 102 | 39 |
| Dolichocephalic* | 57 | 22 |
| Ear position | – | – |
| Flopped | 149 | 57 |
| Semi-flopped | 70 | 27 |
| Erect* | 43 | 16 |
| Face | – | – |
| Flews | 104 | 40 |
| Ectropion | 40 | 15 |
| Neutral abnormalities | 18 | 7 |
*wolf-like morphological facial features.
Table 3 shows the frequency and percentage of occurrence for each facial movement per affective state for wolves. Any occurrence of 10% or more was considered to represent movements involved in signalling affective states (10% rule of thumb61,62). The affective states of Anxious, Curiosity, Fear, Happy, Interest, Joy and Surprise constitute relatively unique combinations of key facial movements (ranging between four movements for Joy and 12 movements for Fear) with little overlap, whereas Anger and Friendly constitute a wide range of facial movements (29 for Anger and 27 for Friendly) with a large degree of overlap. However, despite this overlap key differences still exist. These include the absence of AU118 (Fisher’s exact test p < 0.001), EAD101 (Fisher’s exact test p < 0.001), EAD102 (Fisher’s exact test p = 0.07), and JSNAP (Fisher’s exact test p < 0.001) for Friendly, and the absence of AD55 (Fisher’s exact test p = 0.00) and AD40 (Fisher’s exact test p = 0.06) for Anger.
Table 3.
Frequency and percentage (in brackets) of occurrence of DogFACS and Additional Facial Movement (AFM) codes per wolf affective state.
Accepted percentages (10% and above62,) are highlighted in grey, n = 559.
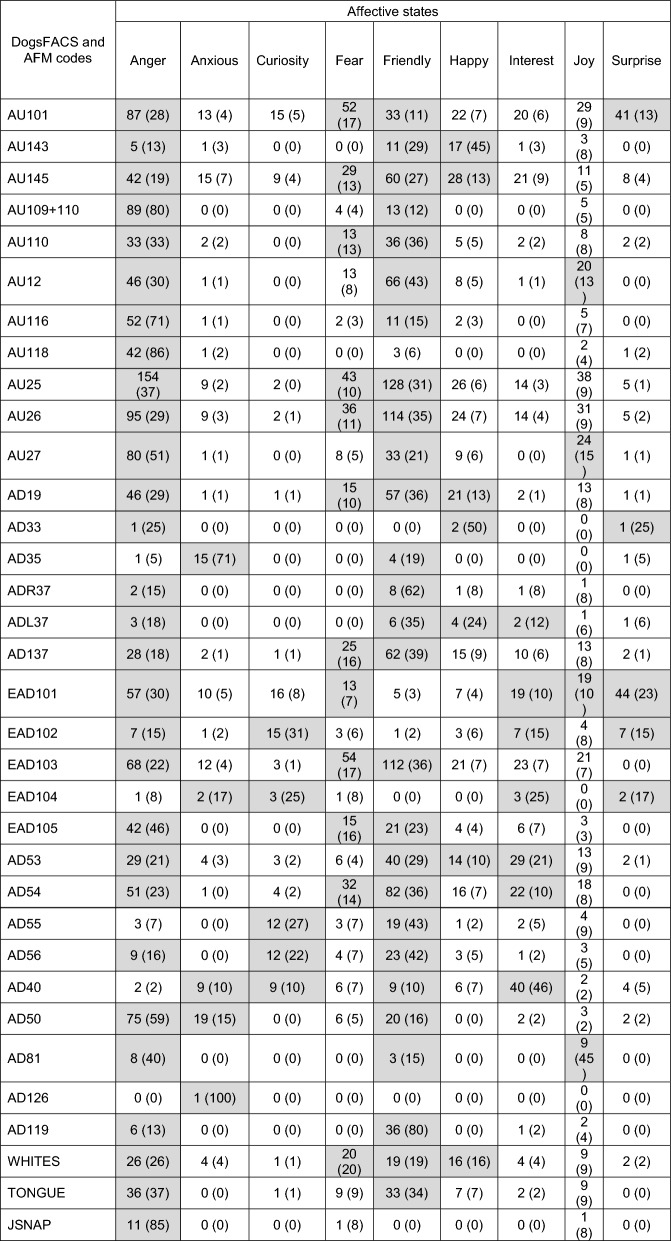
Table 4 shows the frequency and percentage of occurrence for each facial movement per affective state for domestic dogs. Using the 10% rule of thumb61,62 Anger and Friendly comprise the greatest range of facial movements, similar to the patterns observed for wolves in Table 3. However, for dogs Joy also encompasses a wide range of facial movements, in fact all but one facial movement (AD119, licking) are observed in events associated with Joy. Table 4 shows extensive overlap of facial movements between Anger, Friendly and Joy. Conversely, there are no facial movements that attain the 10% threshold for the affective state of Fear, and the remaining affective states comprise very few facial movements that meet the threshold, ranging between just one (for Happy and Interest) and three (for Curiosity and Surprise). In addition, Table 4 illustrates the 55 similarities (blue numbers) and 82 differences (red numbers) in the use of facial movements by dogs compared to wolves. This suggests that domestic dogs only in part, produce facial expressions like wolves, and do so for a limited range of affective states.
Table 4.
Frequency and percentage (in brackets) of occurrence of DogFACS and Additional Facial Movement (AFM) codes per domestic dog affective state.
Accepted percentages (10% and above62), are highlighted in grey, Blue numbers represent similarities between dogs and wolves (Table 3) in the use of facial movements for each affective state. Red numbers represent differences of dogs and wolves in the use of facial movements for each affective state. n = 753. See Table S2 for breed-type information.
Although our analysis focused on visual signalling, throughout our video decoding we also noted when vocalisations (AD50, Table S3) occurred during events. It was found that dogs vocalise more often than wolves when socially interacting and reacting to ‘emotive’ stimuli (dogs; n = 298/753 (40%), wolves; n = 137/559 (25%); X2 = 32.9, p < 0.00001). This increased use in vocalisations suggests that auditory communication may be a more important aspect of communication for conspecific interactions among domestic dogs compared to interaction between wolves. Within the events that comprise the correctly predicted affective states of Fig. 3 (n = 491) dogs were found to use vocalisations 29% of the time. However, vocalisation use increased to 35% within the events that were not classified correctly according to the predicted affective state (n = 262). The various types of vocalisations used in the context of different affective states for dogs and wolves are detailed in the supplementary material (Tables S4, S5 and S6).
Discussion
Here we show that distinct combinations of facial movements relate to specific affective states in wolves and dogs. However, while wolf affective states can be identified very well via facial movements, there is less clarity among domestic dogs. Our results show a greater degree of confusion within the dog matrix with reduced overall precision compared to that for wolves. The precise level of disagreement between actual and predicted affective states will be dependent upon the particular dogs and breeds included in an analysis of this type, although our simulations indicate that our estimate of the extent of confusion within the dog matrix is in fact quite conservative. However, it is not the reduced overall agreement that is the most critical difference between wolves and dogs, but rather, where the confusion exists within the matrices that is most revealing. Overall, these data suggest that dogs are limited in their ability to produce facial expressions for a wide range of affective states.
Domestication has resulted in the morphologies of many species diverging greatly from that of their wild, ancestral counterparts72,73. As a result, domesticated mammals generally possess a range of morphological traits that visibly distinguish them from their wild counterparts, in particular elements of head allometry72,73. The divergent head and facial feature morphologies of some domestic dog breeds likely constrain their ability to produce facial expressions that unambiguously convey specific affective states, with head shape and ear position being the most limiting. The brachycephalic and mesocephalic head morphologies seen in many dog breeds have muzzles that are proportionally shorter relative to the overall skull length than those of wolves (dolichocephalic heads), and therefore have facial features that are compacted together74. Movements involving the muzzle, nose, lips and tongue appear important for the successful production of affective facial expressions in wolves, but these movements are less frequently observed in dogs. For example, wolves use the facial movement ‘nose wrinkler and upper lip raiser’ (AU109 + 110) 80% of the time in the context of Anger (89 out of 111 events, Table 3), but domestic dogs (across breed-types) only use this movement 34% of time in the same context (25 out of 73 events, Table 4). Similarly, wolves sniff (AD40) 46% of the time in the context of Interest (40 out of 87 events, Table 3), but dogs sniff only 17% of the time in the context of Interest (47 out of 278 events, Table 4). The mimic muscles, especially those around the muzzle, lips and nose of brachycephalic and mesocephalic dogs, have much less space to develop to the same size as those seen in dolichocephalic heads. Therefore, some muscles are likely too small to produce the full range of movements needed to produce appropriate facial expressions.
It should be noted that the mimic muscles of wolves and dogs are the same except for the levator anguli oculi medialis muscle (LAOM). Kaminski et al.54 found that domestic dogs raise their inner brows (AU101) more frequently and with higher intensity than wolves do due to the presence of a larger, ‘fully developed’ LAOM in dogs compared to wolves. Kaminski et al.54 suggest that the ‘fully developed’ LAOM in dogs is the result of the necessity for dogs to communicate with humans. However, wolf-human interactions are unlikely to represent a context in which wolves are likely to use AU101. Wolves do not interact with humans in the same way that dogs do, and unlike dogs, wolves essentially perceive humans as heterospecifics75–81. The LAOM of wolves is comprised of relatively limited muscle tissue and a tendon, which Kaminski et al.54 claim produced the low frequency and low intensity of AU101 seen in wolves. While we do not dispute that an ‘under-developed’ LAOM likely results in decreased intensity of AU101, we do argue that the presence of a tendon with relatively limited muscle instead of the ‘fully developed’ LAOM that Kaminski et al.54 observed in some dog breeds (5 out of 6 breeds examined) may provide adequate, frequent movement of AU101 in wolves as tendons can enhance muscle performance82. Moreover, it is suggested that wolves see the world ‘faster’ than humans do, in that they have a greater sensitivity to motion, and are therefore able to make finer temporal use of visual information52. If wolves see the world ‘faster’, we argue that their use of AU101 is subtle, yet adequate for communication within wolf–wolf social interactions. Analysis of wolf facial movements requires the use of slow-motion video footage to fully allow movements to be detected by humans. By contrast, dogs exhibit a more exaggerated use of AU101, hence the larger, ’fully developed’ LAOM, allowing humans to detect the movement with the naked eye in real-time. While Kaminski et al.54 highlight a fascinating response to domestication with regards to the importance of AU101 for dog–human communication, their findings unfortunately do not directly address the importance of AU101 in wolf-wolf social communication.
In addition to head shape and muzzle length limitations, flopped and (to a lesser degree) semi-flopped ears were also associated with much of the confusion seen within the dog matrix. Ear movements appear to be important for the production of affective facial expressions in wolves (Table 3), but again there is a reduction in the use of ear movements across dog breed-types (Table 4). For example, wolves use the movement ‘ears forward’ (EAD101) 30% of the time in the context of Anger (57 out of 190 events, Table 3), while dogs use the same ear movement 12% of the time in the context of Anger (28 out of 235 events, Table 4). Similarly, wolves use the movement ‘ears adductor’ (EAD102) 31% of the time in the context of Curiosity (15 out of 48 events, Table 3), and again dogs only use this movement 12% in the same context (22 out of 191 events, Table 4). Waller et al.45 reported that only dogs with erect (wolf-like) ears could produce the DogFACS movement ‘ears rotator’ (EAD104), which demonstrates that departure from wolf-like head and facial feature morphologies can impair the ability of dogs to produce certain facial movements, which is reflected in the reductions of ear movements used by dogs in this study.
Flews (pendulous lips) seem to contribute to much of the confusion within the dog matrix, which is likely due to flews reducing the visibility of some facial movements. For example, Waller et al.45 reported that flews reduced the visibility of the facial movement ‘jaw drop’ (AU26), and we observed that the movement ‘tongue show’ (AD19) was difficult to discern in dogs with flews, which is reflected in the results. For example, jaw drop is used by wolves in the context Anger and Fear, 29% (95 out of 330 events) and 11% (36 out of 330 events) of the time (respectively, Table 3), but across dog breed-types this movement is only observed 7% of the time (34 out of 488 events) in the context of Anger, and a mere 2% of the time (8 out of 488 events) in the context of Fear (Table 4). Tongue show is used by wolves in the context of Anger 29% of the time (46 out of 157 events, Table 3), but dogs used this movement only 5% of the time in the same context (13 out of 241 events, Table 4). Therefore, these findings suggests that dogs are not just limited in their range of facial expressions due to smaller, compacted muscles (as seen with brachycephalic and mesocephalic dogs), but they are also limited due to exaggerated facial features such as long flopped ears and flews that obscure the use of mimic muscles and therefore, facial movements.
Neutral abnormalities (deformations of the main conveyers of facial expressiveness) and ectropion (drooping eyelids) contributed to only a small percentage of confusion within the dog matrix. However, few dogs in this study had neutral abnormalities (n = 6), which means their effect on the results when compared to all other dogs in this research is minimal. The inclusion of more dogs with neutral abnormalities would give a better indication whether such facial features impede ability to successfully produce consistent affective facial expressions. Ectropion causes the constant exposure of the whites of the eyes (sclera), and the DogFACS system uses the exposure of the sclera to determine eye movements. Eye movements were not analysed in this research, therefore, we are unable to comment directly on how ectropion affects the successful production of affective facial expressions in domestic dogs. However, our results do show that in the context of Friendly and Joy, dogs (across breed-types) expose their sclera (WHITES, Table S3) more than wolves (Tables 3 and 4), which suggests ectropion does indeed impede the production of affective facial expressions in domestic dogs.
The varying head and facial feature morphologies of dogs (in particular, non-wolf-like morphologies) limit their ability to produce the same range of affective facial expressions as their wolf ancestors. However, our research also provides preliminary evidence that domestic dogs may compensate for their limited range in affective facial expressions by using vocalisations. Our analysis of vocalisations found that dogs vocalise more often than wolves when socially interacting and reacting to ‘emotive’ stimuli, suggesting that dogs with limiting facial morphologies may compensate by using more vocalisations to convey their affective states. Certainly, domestic dogs are known to be very vocal in comparison to wolves and will bark for a range of reasons, such as play, defence, threat, pain and loneliness83,84. It has been argued that dog vocalisations have been artificially selected for the purpose of inter-specific communication with humans (for working purposes)84. However, our results raise the possibility that domestic dogs may use vocalisations for intra-specific communication where facial expressions are not adequate.
In addition to morphological constraints on facial expression, there are other factors that may reduce the ability of domestic dogs to convey affective states via facial expressions. For example, kennel environments can impact on the behaviour of domestic dogs85–87 especially, kennel environments that lack housing of dogs in groups, have a lack of dog–human contact and a lack of enrichment (such as toys)85,86. However, most of the dogs we used were housed together in groups, they were provided with regular human contact (in preparation for adoption) and they were provided with enrichment in the form of toys. Although behavioural data derived from studies of shelter dogs should be considered with caution due to potential for aberrant behaviour patterns, here we minimised this risk by selecting only individuals deemed suitable for adoption. Nevertheless, a kennel environment can never fully mimic a human home environment that domestic dogs generally live in. Therefore, a comparison of facial expressions across affective states in both kennel and home environments should be considered in future work. Likewise, the past histories of the dogs used are unknown, therefore it is possible the ontogenetic process of enculturation of the dogs used may have resulted in some dogs learning atypical social signalling88–90. Therefore, knowing the past histories of the dogs used would be beneficial to help explain unusual findings or indeed, allow one to select dogs that are well-versed in their social abilities and who display typical behaviours. Raising domestic dogs from puppies to adults would allow their past histories to be fully studied and documented and this should be considered for future work.
A key outcome from our study is that we observed considerable confusion between positive and negative affective states for domestic dogs, a pattern that was not seen in our analyses of wolf facial expressions. For example, among dogs, 50% of events allocated an affective state of Fear by direct observation, were classed as Friendly based upon LDA of facial movements. This implies that domestic dogs (across breed-types) are inconsistent in the way they convey affective states via facial expressions. Such high levels of confusion between positive and negative affective states is potentially detrimental to dog–dog and dog–human communication91. For example, many dogs that are fearful can become ‘fear aggressive’ and will bite to defend themselves from potential threats92,93. If a dog or human was to mistakenly perceive that another dog was displaying a Friendly affective state, when in fact it was displaying Fear, this may lead to dog–dog conflict, or the human being bitten. Therefore, it is important for dog welfare and dog bite prevention for humans to correctly identify the affective states of dogs.
Conclusion
We have provided the first quantitative evidence that shows distinct combinations of facial movements relate to specific affective states in wolves. Further, we show that divergent head and facial feature morphologies among dog breeds can impair their ability to produce affective facial expressions relative to their wolf ancestors. It is well known that selective breeding has led to a wealth of physical health problems in many domestic dog breeds94–101. However, here we provide evidence that such selective breeding also generates social communicative limitations in domestic dogs.
Methods
Study sites and subjects
Observations were conducted at two facilities; The UK Wolf Conservation Trust (UKWCT, Beenham UK, 51.42 N, − 1.15 W) and Dogs Trust Darlington (Sadberge UK, 54.56 N, − 1.47 W). Observations at the UKWCT were conducted between February 15th 2016 and March 4th 2016, on weekdays between 0900 and 1700 h (GMT), amounting to 15 days in total. Observations were conducted at Dogs Trust Darlington between August 9th 2016 and November 11th 2016, on weekdays between 1100 and 1700 h (BST), amounting to 21 days in total. The UKWCT provided 10 wolves, five female and five male, which included different sub-species (details in supplementary information Table S1) that were habituated to the presence of humans. Dogs Trust Darlington provided 64 domestic dogs; 43 standard-breeds and 21 cross-breeds (details in supplementary information Table S2). All dogs were adults and consisted of both females (n = 21) and males (n = 43). Wolves and dogs were housed in small packs of two to three individuals and were free to roam about their enclosures and interact with pack mates and humans during data collection. All dogs used in this study were individuals assessed as suitable for adoption by the Dogs Trust, and no data were collected from dogs deemed to exhibit behavioural issues.
Video collection
Video clips of wolves (n = 559) and dogs (n = 753) engaged in spontaneous social interactions or reactions to external ‘emotive’ stimuli (both referred to as ‘events’) were recorded ad-hoc using a hand-held Canon Legria HFR36-D video camera (51 × zoom). Average duration of each event was < 10 s. Social interactions commenced when eye-contact was made between a focal canid and one or more social interactants, with the focal canid becoming immediately focussed upon the interactant(s). Interactions ceased when the focal canid and social interactant(s) dispersed, and eye-contact was lost. External stimuli consisted of easily identifiable auditory and visual stimuli, specifically wind generated sounds, overhead aircraft, and novel objects placed around the study sites (by staff for various public engagement events). Other external stimuli were created using pre-recorded sounds randomly played to wolves and dogs by ERH, on a Nokia Lumia 820 mobile phone. Sounds used were one of four naturally occurring animal vocalisations (rabbit distress call, fawn distress call, squirrel alarm call, domestic dog puppy whines) and one unnatural sound (dog squeak toy). Reactions to stimuli were analysed when a focal canid reacted immediately (within less than one second) to an external stimulus and became focussed upon the direction of the stimulus origin (the focal canid attempted to make eye-contact with the stimulus). The end of an event was defined by the cessation of the external stimulus and the focal canid averting its gaze from the stimulus origin.
Assessment of affective state for each event
For each event ERH identified a single primary, short-term, emotion-like affective state (Table 1) based on subjective appraisal of key descriptors (sound stimuli were used to specifically evoke Curiosity, Interest and Surprise affective states). Each of these primary affective states are described in previous literature and have been observed in non-human animals6,21,49,52,63,65,67,68,70,102–110. However, the descriptions of these primary affective states vary among authors. Therefore, we produced descriptors (derived from contextual information of the video footage obtained, Table 1) to identify the motivation (the functional response) of the focal canid for each event. The affective state Friendly was also included as this affective state has been qualitatively described, but not quantified, in wolves and dogs52,69,104,111. In addition, the ability to identify and assess both positive and negative affective states in non-human animals is necessary to fully evaluate the psychological well-being and health of an individual animal112. Therefore, each primary affective state investigated in this research was also classified as either positive or negative (Table 1). Although the affective state Friendly can be classified as positive or negative69,71, for the purposes of this research, Friendly was categorised as positive as focal canids were unharmed during interactions involving this affective state (Table 1). The affective state Surprise was classified as both positive or negative (Table 1)71. The reliability of affective state classifications was independently verified. Seven independent observers were tasked to identify the primary affective state in a random sample of video clips representing one example of each of the affective states identified in both wolf and dog events (amounting to nine dog interactions and nine wolf interactions in total). Inter-observer concordance analyses113–116 of affective states was performed using the R117 package ‘raters’118, showing substantial inter-rater agreement at 70% (according to the Kappa statistic61) for all affective states. Inter-observer concordance analyses of positive and negative classifications of affective states showed almost perfect agreement at 82% (according to the Kappa statistic61).
Quantification of facial expressions observed during events
DogFACS45 was used to decode video footage of wolf and dog facial expressions. DogFACS comprises a list of 43 codes (see www.animalfacs.com, and supplementary material Table S3) that correspond to specific facial landmarks that move in association with the underlying mimic muscles45. We also noted the occurrence of three Additional Facial Movements (AFM); jaw snapping, tongue flicking, and ‘whites of eyes visible’ (see supplementary material Table S3 for full definitions). Jaw snapping and tongue flicking involve movement of the face and have been reported to occur in wolves8,52, therefore they were included in our analyses. ‘Whites of eyes visible’ was included as sclera visibility during facial communication was observed frequently in our videos, suggesting importance for communication45,119,120. All videos were decoded by ERH (certified DogFACS coder) in slow motion (0.25 × playback speed, using AVS video editor 7.2.), which allowed both obvious and very subtle facial movements to be detected. Although developed for use with domestic dogs, DogFACS was used also to decode wolf facial expressions on the basis that the mimic muscles of wolves and dogs are the same except for the levator anguli oculi medialis muscle (LAOM) being more ‘fully developed’ in dogs compared to wolves54. All DogFACS codes that occurred within a single facial expression were recorded once as either ‘on’ or ‘off’ (producing binary data). This method was employed to objectively record the range of facial movements in each facial expression, allowing individual wolf/dog facial expressions to be quantified. This yielded a database of all facial movements observed during each event. For the DogFACS and AFM coding ERH conducted intra-rater reliability coding of 20% of the original video footage processed (263 video clips) one year after her original coding54. Using Wexler’s agreement, overall intra-rater reliability resulted in almost perfect agreement at 98%13,45.
Previous studies coding facial expressions using DogFACS have not exceeded the use of more than one or two codes per facial expression45,54,55. Here we use all relevant codes with the following exceptions. During events canids would alter their gaze and head orientation (in the sagittal plane) only to maintain eye contact with social interactants and reaction stimuli, consequently, all DogFACS eye movements and left and right head movements were removed from subsequent data analyses. DogFACS ‘Body shake’ was also removed from data analyses as this code is predominantly a body movement with associated involuntary head and facial movements. In addition, DogFACS ‘other’ Action Descriptors (ADs) are associated with the visibility of focal canid faces in video footage, and not with facial movements. Therefore, ‘other’ ADs were removed from analyses, as videos lacking canid face visibility were not decoded.
Testing whether specific combinations of facial action codes predict affective states
We employed linear discriminant analysis (LDA)60 with leave-one-out cross-validation using the R package ‘MASS’121 to conduct a supervised classification of the DogFACS and AFM data, to identify how well each affective state could be predicted based upon the combinations of DogFACS and AFM codes recorded for each event. We produced separate confusion matrices for the wolf and dog data to examine how well combinations of facial movements map onto the identified affective states60. Overall precision (positive predictive value: ratio of correct positive predictions to total predicted positives) of the confusion matrices provided a measure of the general agreement between observed affective state and predictions based on facial movements for wolves and dogs61. Examination of individual cells within the matrices provided insights into which affective states were poorly defined by specific combinations of facial expressions, and which were well defined.
The level of agreement within matrix cells was categorised according to Cohen’s kappa61,113–116 using the R package ‘raters’. Levels of agreement were categorised according to Landis & Koch (1977; < 0% = Poor, 0–20% = Slight, 21–40% = Fair, 41–60% = Moderate, 61–80% = Substantial, 81–100% = Almost perfect)61. Where confusion did occur in the matrix for dogs, events that were incorrectly allocated to affective states were examined in more detail. The morphologies of the dogs involved in these incorrect classifications were tabulated to identify potential links between morphological divergences (from wolves) and the inability to classify affective states based upon facial expressions. Finally, using a 10% rule of thumb as a measure of acceptance61,62, the DogFACS and AFM codes per affective state were examined to determine which codes pertained to each affective facial expression for both wolves and dogs.
The level of disagreement in our confusion matrix for dog affective states will to some extent be dependent upon the particular breeds of dog and individuals used, and the range of affective states expressed by each. Therefore, to examine the effect of individual and breed on the levels of disagreement between actual and predicted affective state in our confusion matrix for dogs we conducted the following simulations. We iteratively re-ran our linear discriminant analysis (LDA) with the same data but removing each individual in turn. We then examined the overall level of agreement between actual and predicted affective state allocations in the resulting confusion matrices in comparison to the level of agreement reported for our LDA utilising the full data set.
Significance
We demonstrate that identifiable combinations of facial movements relate to nine specific affective states in wolves, whereas divergent head and facial feature morphologies among domestic dog breeds limit their ability to produce the same affective facial expressions. It is well known that selective breeding has led to a wealth of physical health problems in many domestic dog breeds. Here we show that selective breeding also generates social communicative limitations in dogs, potentially impacting dog–human interactions. Quantifying associations between facial expressions and affective states may provide a foundation for monitoring welfare in wild and captive canids and allows for cross-species comparisons to yield insight into the emotional evolution in Canidae.
Ethical approval
All data collection consisted of non-invasive behavioural observations at the UK Wolf Conservation Trust (UKWCT) and Dogs Trust Darlington, approval for data collection was granted by Dogs Trust and UKWCT. All observational protocols were approved by Durham University’s Animal Welfare Ethical Review Board (AWERB), and all procedures complied with BIAZA and Dogs Trust ethical guidelines.
Supplementary Information
Acknowledgements
We wish to thank the UK Wolf Conservation Trust and Dogs Trust Darlington for allowing access to their canids. We also wish to thank Dr Isla Fishburn of Kachina Canine Communication (UK), Dr Nicholas Hole, Dr Gillian Campling, Jillian Lynn and Helen Luke for their support during this project. Finally, we would like to thank our funders, the Grevillea Trust, the Thriplow Charitable Trust and Ustinov College.
Author contributions
E. R. H. conceived the research, collected field data, carried out the statistical analyses and drafted the manuscript. S. D. T. coordinated the project and edited the manuscript. All authors gave final approval for publication.
Funding
ERH was supported by funding from the Grevillea Trust, the Thriplow Charitable Trust and the Ustinov Postgraduate Research Fund.
Data availability
Data and code are provided as electronic supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61110-6.
References
- 1.Smuts BB, Watanabe JM. Social relationships and ritualized greetings in adult male baboons (Papio cynocephalus anubis) Int. J. Primatol. 1990;11:147–172. doi: 10.1007/BF02192786. [DOI] [Google Scholar]
- 2.McGregor PK, Peake TM. Communication networks: social environments for receiving and signalling behaviour. Acta Ethol. 2000;2:71–81. doi: 10.1007/s102110000015. [DOI] [Google Scholar]
- 3.Whitham JC, Maestripieri D. Primate rituals: The function of greetings between male Guinea baboons. Ethology. 2003;109:847–859. doi: 10.1046/j.0179-1613.2003.00922.x. [DOI] [Google Scholar]
- 4.Smith JE, et al. Greetings promote cooperation and reinforce social bonds among spotted hyaenas. Anim. Behav. 2011;81:401–415. doi: 10.1016/j.anbehav.2010.11.007. [DOI] [Google Scholar]
- 5.Fernald RD. Communication about social status. Curr. Opin. Neurobiol. 2014;28:1–4. doi: 10.1016/j.conb.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkel R. Expression-studies of wolves. Behaviour. 1947;1:81–129. doi: 10.1163/156853948X00065. [DOI] [Google Scholar]
- 7.Bolwig N. Facial expression in primates with remarks on a parallel development in certain carnivores (a preliminary report on work in progress) Behaviour. 1964;22:167–192. doi: 10.1163/156853964X00012. [DOI] [Google Scholar]
- 8.Fox MW. A comparative study of the development of facial expressions in canids; wolf, coyote and foxes. Behaviour. 1970;36:49–73. doi: 10.1163/156853970X00042. [DOI] [Google Scholar]
- 9.Ekman P, Friesen WV. Facial Action Coding System. Consulting Psychologists Press; 1978. [Google Scholar]
- 10.Cheney DL, Seyfarth RM. Précis of how monkeys see the world. Behav. Brain Sci. 1992;15:135–147. doi: 10.1017/S0140525X00067911. [DOI] [Google Scholar]
- 11.East ML, Hofer H, Wickler W. The erect ‘penis’ is a flag of submission in a female dominated society: Greetings in Serengeti spotted hyenas. Behav. Ecol. Sociobiol. 1993;33:355–370. doi: 10.1007/BF00170251. [DOI] [Google Scholar]
- 12.Parr LA, de Waal FB. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- 13.Ekman P, Friesen WV, Hager JC. The Facial Action Coding System. Research Nexus; 2002. [Google Scholar]
- 14.Tomonaga M, et al. Development of social cognition in infant chimpanzees (Pan troglodytes): Face recognition, smiling, gaze, and the lack of triadic interactions. Jpn. Psychol. Res. 2004;46:227–235. doi: 10.1111/j.1468-5584.2004.00254.x. [DOI] [Google Scholar]
- 15.Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat. Rev. Neurosci. 2005;6:641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- 16.Burrows AM, Waller BM, Parr LA, Bonar CJ. Muscles of facial expression in the chimpanzee (Pan troglodytes): Descriptive, comparative and phylogenetic contexts. J. Anat. 2006;208:153–167. doi: 10.1111/j.1469-7580.2006.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitman RL, Durban JW. Cooperative hunting behavior, prey selectivity and prey handling by pack ice killer whales (Orcinus orca), type B, in Antarctic Peninsula waters. Mar. Mamm. Sci. 2012;28:16–36. doi: 10.1111/j.1748-7692.2010.00453.x. [DOI] [Google Scholar]
- 18.Somppi S, Tornqvist H, Hanninen L, Krause CM, Vainio O. How dogs scan familiar and inverted faces: An eye movement study. Anim. Cognit. 2014;17:793–803. doi: 10.1007/s10071-013-0713-0. [DOI] [PubMed] [Google Scholar]
- 19.Thunstrom M, Kuchenbuch P, Young C. Concealing of facial expressions by a wild Barbary macaque (Macaca sylvanus) Primates. 2014;55:369–375. doi: 10.1007/s10329-014-0423-5. [DOI] [PubMed] [Google Scholar]
- 20.Henkel S, Lambides AR, Berger A, Thomsen R, Widdig A. Rhesus macaques (Macaca mulatta) recognize group membership via olfactory cues alone. Behav. Ecol. Sociobiol. 2015;69:2019–2034. doi: 10.1007/s00265-015-2013-y. [DOI] [Google Scholar]
- 21.Albuquerque N, et al. Dogs recognize dog and human emotions. Biol. Lett. 2016;12:20150883. doi: 10.1098/rsbl.2015.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briefer EF, Tettamanti F, McElligott AG. Emotions in goats: Mapping physiological, behavioural and vocal profiles. Anim. Behav. 2015;99:131–143. doi: 10.1016/j.anbehav.2014.11.002. [DOI] [Google Scholar]
- 23.Parr LA, Waller BM, Fugate J. Emotional communication in primates: Implications for neurobiology. Curr. Opin. Neurobiol. 2005;15:716–720. doi: 10.1016/j.conb.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Conscious. Cogn. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Scherer KR. What are emotions? And how can they be measured? Soc. Sci. Inf. (Paris) 2005;44:695–729. doi: 10.1177/0539018405058. [DOI] [Google Scholar]
- 26.Mendl M, Burman OH, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc. Royal Soc. B. 2010;277:2895–2904. doi: 10.1098/rspb.2010.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panksepp J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. Rev. 2011;35:1791–1804. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolensek N, Gehrlach DA, Klein AS, Gogolla N. Facial expressions of emotion states and their neuronal correlates in mice. Science. 2020;368:89–94. doi: 10.1126/science.aaz946. [DOI] [PubMed] [Google Scholar]
- 30.Mendl M, Paul ES. Animal affect and decision-making. Neurosci. Biobehav. Rev. 2020;112:144–163. doi: 10.1016/j.neubiorev.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Bremhorst A, Mills DS, Stolzlechner L, Würbel H, Riemer S. ‘Puppy dog eyes’ are associated with eye movements, not communication. Front. Psychol. 2021;12:568935. doi: 10.3389/fpsyg.2021.568935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zych AD, Gogolla N. Expressions of emotions across species. Curr. Opin. Neurobiol. 2021;68:57–66. doi: 10.1016/j.conb.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremhorst A, Mills DS, Würbel H, Riemer S. Evaluating the accuracy of facial expressions as emotion indicators across contexts in dogs. Anim. Cogn. 2022;25:121–136. doi: 10.1007/s10071-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Kleef GA, Côté S. The social effects of emotions. Annu. Rev. Psychol. 2022;73:629–658. doi: 10.1146/annurev-psych-020821-010855. [DOI] [PubMed] [Google Scholar]
- 35.Desire L, Boissy A, Veissier I. Emotions in farm animals: A new approach to animal welfare in applied ethology. Behav. Process. 2002;60:165–180. doi: 10.1016/S0376-6357(02)00081-5. [DOI] [PubMed] [Google Scholar]
- 36.Paul ES, Harding EJ, Mendl M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005;29:469–491. doi: 10.1016/j.neubiorev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Siniscalchi M, Lusito R, Vallortigara G, Quaranta A. Seeing left-or right-asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013;23:2279–2282. doi: 10.1016/j.cub.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Burza LB, Bloom T, Trindade PHE, Friedman H, Otta E. Reading emotions in Dogs’ eyes and Dogs’ faces. Behav. Process. 2022;202:104752. doi: 10.1016/j.beproc.2022.104752. [DOI] [PubMed] [Google Scholar]
- 39.Cohn JF, Ambadar Z, Ekman P. Ch. 13: Observer-Based Measurement of Facial Expression with the Facial Action Coding System. In: Coan JA, Allen JJB, editors. The Handbook of Emotion Elicitation and Assessment. Oxford University Press; 2007. pp. 203–221. [Google Scholar]
- 40.Vick SJ, Waller B, Parr L, Smith Pasqualini MC, Bard KA. A cross species comparison of facial morphology and movement in humans and chimpanzees using FACS. J. Nonverbal Behav. 2007;31:1–20. doi: 10.1007/s10919-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr LA, Waller BM, Burrows AM, Gothard KM, Vick SJ. Brief communication: MaqFACS: A muscle-based facial movement coding system for the rhesus macaque. Am. J. Phys. Anthropol. 2010;143:625–630. doi: 10.1002/ajpa.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caeiro CC, Waller BM, Burrows AM. CatFACS: The Cat Facial Action Coding System Manual. University of Portsmouth; 2013. [Google Scholar]
- 43.Caeiro CC, Waller BM, Zimmermann E, Burrows AM, Davila-Ross M. OrangFACS: A muscle-based facial movement coding system for orangutans (Pongo spp.) Int. J. Primatol. 2013;34:115–129. doi: 10.1007/s10764-012-9652-x. [DOI] [Google Scholar]
- 44.Waller BM, Lembeck M, Kuchenbuch P, Burrows AM, Liebal K. GibbonFACS: A muscle-based facial movement coding system for hylobatids. Int. J. Primatol. 2012;33:809–821. doi: 10.1007/s10764-012-9611-6. [DOI] [Google Scholar]
- 45.Waller BM, et al. Paedomorphic facial expressions give dogs a selective advantage. PloS One. 2013;8:e82686. doi: 10.1371/journal.pone.0082686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wathan J, Burrows AM, Waller BM, McComb K. EquiFACS: The equine facial action coding system. PloS One. 2015;10:e0131738. doi: 10.1371/journal.pone.0131738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mech LD. Alpha status, dominance, and division of labor in wolf packs. Can. J. Zool. 1999;77:1196–1203. doi: 10.1139/z99-099. [DOI] [Google Scholar]
- 48.Zimen E. The Wolf: A Species in Danger. Delacorte press; 1981. [Google Scholar]
- 49.Abrantes R. The Evolution of Canine Social Behaviour. 2. Wakan Tanka Publishers; 2005. [Google Scholar]
- 50.Mech LD. Canis lupus. Mamm. Species. 1974;37:1–6. doi: 10.2307/3503924. [DOI] [Google Scholar]
- 51.Nowak, R.M. North American quaternary Canis. Monograph no 6. Museum of Natural History, University of Kansas, Lawrence, p. 154 (1979).
- 52.Mech LD, Boitani L. Wolves, Behaviour, Ecology, and Conservation. The University of Chicago Press; 2003. [Google Scholar]
- 53.Hobkirk, E.R. Through the eyes of a wolf: Using non-invasive methods to quantify and classify the facial signalling of wolves (Canis lupus) and domestic dogs (Canis lupus familiaris). Masters thesis, Durham University (2019).
- 54.Kaminski J, Waller BM, Diogo R, Hartstone-Rose A, Burrows AM. Evolution of facial muscle anatomy in dogs. Proc. Natl. Acad. Sci. U.S.A. 2019;116:14677–14681. doi: 10.1073/pnas.1820653116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagasawa M, Kawai E, Mogi K, Kikusui T. Dogs show left facial lateralization upon reunion with their owners. Behav Process. 2013;98:112–116. doi: 10.1016/j.beproc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Scott JP. The evolution of social behavior in dogs and wolves. Am. Zool. 1967;7:373–381. doi: 10.1093/icb/7.2.373. [DOI] [Google Scholar]
- 57.Clutton-Brock J. Ch. 2: Origins of the Dog: Domestication and Early History. In: Serpell J, editor. The Domestic Dog, Its Evolution, Behaviour and Interactions with People. Cambridge University Press; 1995. pp. 8–20. [Google Scholar]
- 58.Goodwin D, Bradshaw JW, Wickens SM. Paedomorphosis affects agonistic visual signals of domestic dogs. Anim. Behav. 1997;53:297–304. doi: 10.1006/anbe.1996.0370. [DOI] [Google Scholar]
- 59.Albuquerque N, Guo K, Wilkinson A, Resende B, Mills DS. Mouth-licking by dogs as a response to emotional stimuli. Behav. Process. 2018;146:42–45. doi: 10.1016/j.beproc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Zheng F. Facial expression recognition based on LDA feature space optimization. Comput. Intell. Neurosci. 2022;2022:9521329. doi: 10.1155/2022/9521329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 62.Bennett V, Gourkow N, Mills DS. Facial correlates of emotional behaviour in the domestic cat (Felis catus) Behav. Process. 2017;141:342–350. doi: 10.1016/j.beproc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Plutchik R. The nature of emotions: Human emotions have deep evolutionary roots, a fact that may explain their complexity and provide tools for clinical practice. Am. Sci. 2001;89:344–350. doi: 10.1511/2001.28.344. [DOI] [Google Scholar]
- 64.Reimert I, Bolhuis JE, Kemp B, Rodenburg TB. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013;109:42–50. doi: 10.1016/j.physbeh.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Oatley K, Johnson-Laird PN. Cognitive approaches to emotions. Trends Cogn. Sci. 2014;18:134–140. doi: 10.1016/j.tics.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Paulus MP, et al. Latent variable analysis of positive and negative valence processing focused on symptom and behavioral units of analysis in mood and anxiety disorders. J. Affect. Disord. 2017;216:17–29. doi: 10.1016/j.jad.2016.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arapakis I., Jose J.M. & Gray P.D. Affective feedback: An investigation into the role of emotions in the information seeking process. In Proceedings of the 31st annual international ACM SIGIR conference on Research and development in information retrieval, 395–402 (2008).
- 68.Rutherford KM, Donald RD, Lawrence AB, Wemelsfelder F. Qualitative behavioural assessment of emotionality in pigs. Appl. Anim. Behav. Sci. 2012;139:218–224. doi: 10.1016/j.applanim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schenkel R. Submission: Its features and function in the wolf and dog. Am. Zool. 1967;7:319–329. doi: 10.1093/icb/7.2.319. [DOI] [Google Scholar]
- 70.Izard CE. Basic emotions, relations among emotions, and emotion-cognition relations. Psychol. Rev. 1992;99:561–565. doi: 10.1037/0033-295X.99.3.561. [DOI] [PubMed] [Google Scholar]
- 71.Noordewier MK, Breugelmans SM. On the valence of surprise. Cogn. Emot. 2013;27:1326–1334. doi: 10.1080/02699931.2013.777660. [DOI] [PubMed] [Google Scholar]
- 72.Wilkins AS, Wrangham RW, Tecumseh Fitch W. The “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics. 2014;197:795–808. doi: 10.1534/genetics.114.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen WC, van der Bijl W, Wheat CW. Morphology does not covary with predicted behavioral correlations of the domestication syndrome in dogs. Evol. Lett. 2020;4:189–199. doi: 10.1002/evl3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Packer RM, Hendricks A, Tivers MS, Burn CC. Impact of facial conformation on canine health: Brachycephalic obstructive airway syndrome. PloS One. 2015;10:e0137496. doi: 10.1371/journal.pone.0137496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miklosi A, et al. A simple reason for a big difference: Wolves do not look back at humans, but dogs do. Curr. Biol. 2003;13:763–766. doi: 10.1016/S0960-9822(03)00263-X. [DOI] [PubMed] [Google Scholar]
- 76.Range F, Viranyi Z. Tracking the evolutionary origins of dog-human cooperation: The “Canine cooperation hypothesis”. Front. Psychol. 2014 doi: 10.3389/fpsyg.2014.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Range F, Viranyi Z. Wolves are better imitators of conspecifics than dogs. PloS One. 2014;9:e86559. doi: 10.1371/journal.pone.0086559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Udell MA. When dogs look back: inhibition of independent problem-solving behaviour in domestic dogs (Canis lupus familiaris) compared with wolves (Canis lupus) Biol. Lett. 2015;11:20150489. doi: 10.1098/rsbl.2015.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall-Pescini S, Schwarz JF, Kostelnik I, Virányi Z, Range F. Importance of a species’ socioecology: Wolves outperform dogs in a conspecific cooperation task. PNAS. 2017;114:11793–11798. doi: 10.1073/pnas.170902711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazzaroni M, et al. The effect of domestication and experience on the social interaction of dogs and wolves with a human companion. Front. Psychol. 2020;11:785. doi: 10.3389/fpsyg.2020.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazzaroni M, et al. Village dogs match pet dogs in reading human facial expressions. Peer J. 2023;11:e15601. doi: 10.7717/peerj.15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002;133:1087–1099. doi: 10.1016/S1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 83.Yin S, McCowan B. Barking in domestic dogs: Context specificity and individual identification. Anim. Behav. 2004;68:343–355. doi: 10.1016/j.anbehav.2003.07.016. [DOI] [Google Scholar]
- 84.Pongrácz P, Molnár C, Miklósi Á. Barking in family dogs: An ethological approach. Vet. J. 2010;183:141–147. doi: 10.1016/j.tvjl.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Wells DL. A review of environmental enrichment for kennelled dogs, Canis familiaris. Appl. Anim. Behav. Sci. 2004;85:307–317. doi: 10.1016/j.applanim.2003.11.005. [DOI] [Google Scholar]
- 86.Taylor KD, Mills DS. The effect of the kennel environment on canine welfare: A critical review of experimental studies. Anim. Welf. 2007;16:435–447. doi: 10.1017/S0962728600027378. [DOI] [Google Scholar]
- 87.Kogan LR, Schoenfeld-Tacher R, Simon AA. Behavioral effects of auditory stimulation on kenneled dogs. J. Vet. Behav. Clin. Appl. Res. 2012;7:268–275. doi: 10.1016/j.jveb.2011.11.002. [DOI] [Google Scholar]
- 88.Serpell J, Jagoe JA. Ch. 6: Early Experience and The Development of Behaviour. In: Serpell J, editor. The Domestic Dog, Its Evolution, Behaviour and Interactions with People. Cambridge University Press; 1995. pp. 79–103. [Google Scholar]
- 89.Appleby DL, Bradshaw JW, Casey RA. Relationship between aggressive and avoidance behaviour by dogs and their experience in the first six months of life. Vet. Rec. 2002;150:434–438. doi: 10.1136/vr.150.14.434. [DOI] [PubMed] [Google Scholar]
- 90.Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2002;298:1634–1636. doi: 10.1126/science.10727. [DOI] [PubMed] [Google Scholar]
- 91.Sexton CL, et al. What is written on a dog’s face? Evaluating the impact of facial phenotypes on communication between humans and canines. Animals. 2023;13:2385. doi: 10.3390/ani13142385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galac S, Knol BW. Fear-motivated aggression in dogs: Patient characteristics, diagnosis and therapy. Anim. Welf. 1997;6:9–15. doi: 10.1017/S0962728600019357. [DOI] [Google Scholar]
- 93.Haug LI. Canine aggression toward unfamiliar people and dogs. Vet. Clin. North Am. Small Anim. 2008;38:1023–1041. doi: 10.1016/j.cvsm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Wood JLN, Lakhani KH, Dennis R. Heritability and epidemiology of canine hip dysplasia score in flat-coated retrievers and Newfoundlands in the United Kingdom. Prev. Vet. Med. 2000;46:75–86. doi: 10.1016/S0167-5877(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 95.Asher L, Diesel G, Summers JF, McGreevy PD, Collins LM. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet. J. 2009;182:402–411. doi: 10.1016/j.tvjl.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 96.Rooney NJ. The welfare of pedigree dogs: Cause for concern. J. Vet. Behav. Clin. Appl. 2009;4:180–186. doi: 10.1016/j.jveb.2009.06.002. [DOI] [Google Scholar]
- 97.Summers JF, Diesel G, Asher L, McGreevy PD, Collins LM. Inherited defects in pedigree dogs. Part 2: Disorders that are not related to breed standards. Vet. J. 2010;183:39–45. doi: 10.1016/j.tvjl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 98.Collins LM, Asher L, Summers J, McGreevy P. Getting priorities straight: Risk assessment and decision-making in the improvement of inherited disorders in pedigree dogs. Vet. J. 2011;189:147–154. doi: 10.1016/j.tvjl.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 99.Bellumori TP, Famula TR, Bannasch DL, Belanger JM, Oberbauer AM. Prevalence of inherited disorders among mixed-breed and purebred dogs: 27,254 cases (1995–2010) J. Am. Vet. Med. Assoc. 2013;242:1549–1555. doi: 10.2460/javma.242.11.1549. [DOI] [PubMed] [Google Scholar]
- 100.Dan GO, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PloS One. 2014;9:e90501. doi: 10.1371/journal.pone.0090501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farrell LL, Schoenebeck JJ, Wiener P, Clements DN, Summers KM. The challenges of pedigree dog health: Approaches to combating inherited disease. Canine Genet. Epidemiol. 2015;2:3–95. doi: 10.1186/s40575-015-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.King JE, Landau VI. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? J. Res. Pers. 2003;37:1–15. doi: 10.1016/S0092-6566(02)00527-5. [DOI] [Google Scholar]
- 103.Bekoff M. The Emotional Lives of Animals. New World Library; 2007. [Google Scholar]
- 104.Mech LD. The Wolf: The Ecology and Behaviour of an Endangered Species. 13. University of Minnesota Press; 2007. [Google Scholar]
- 105.Morris PH, Doe C, Godsell E. Secondary emotions in non-primate species? Behavioural reports and subjective claims by animal owners. Cogn. Emot. 2008;22:3–20. doi: 10.1080/02699930701273716. [DOI] [Google Scholar]
- 106.Miele M. The taste of happiness: Free-range chicken. Environ. Plan. A. 2011;43:2076–2090. doi: 10.1068/a43. [DOI] [Google Scholar]
- 107.Bloom T, Friedman H. Classifying dogs’ (Canis familiaris) facial expressions from photographs. Behav. Process. 2013;96:1–10. doi: 10.1016/j.beproc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 108.Haber G, Holleman M. Among Wolves. University of Alaska press; 2013. [Google Scholar]
- 109.Catia C, Kun G, Daniel M. Dogs and humans respond to emotionally competent stimuli by producing different facial actions. Sci. Rep. 2017;7:15525. doi: 10.1038/s41598-017-15091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Briefer EF. Vocal contagion of emotions in non-human animals. Proc. Royal Soc. B. 2018;285:20172783. doi: 10.1098/rspb.2017.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vas J, Topal J, Gacsi M, Miklosi A, Csanyi V. A friend or an enemy? Dogs’ reaction to an unfamiliar person showing behavioural cues of threat and friendliness at different times. Appl. Anim. Behav. Sci. 2005;94:99–115. doi: 10.1016/j.applanim.2005.02.001. [DOI] [Google Scholar]
- 112.Yeates JW, Main DCJ. Assessment of positive welfare: A review. Vet. J. 2008;175:293–300. doi: 10.1016/j.tvjl.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Caro TM, Roper R, Young M, Dank GR. Inter-observer reliability. Behaviour. 1979;69:303–315. doi: 10.1163/156853979X00520. [DOI] [Google Scholar]
- 114.Whitham JC, Wielebnowski N. Animal-based welfare monitoring: Using keeper ratings as an assessment tool. Zoo Biol. 2009;28:545–560. doi: 10.1002/zoo.20281. [DOI] [PubMed] [Google Scholar]
- 115.Garcia V.A., Junior C.F.C. & Marino-Neto J. Assessment of observers’ stability and reliability-A tool for evaluation of intra-and inter-concordance in animal behavioural recordings. In Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE, pp. 6603–6606 (2010). [DOI] [PubMed]
- 116.Phythian C, Michalopoulou E, Duncan J, Wemelsfelder F. Inter-observer reliability of qualitative behavioural assessments of sheep. Appl. Anim. Behav. Sci. 2013;144:73–79. doi: 10.1016/j.applanim.2012.11.011. [DOI] [Google Scholar]
- 117.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ (2017)
- 118.Giardiello, D., Quatto, P., Ripamonti, E. & Vigliani, S. A modification of Fleiss’ Kappa in case of nominal and ordinal variables. R package version 2.0.2. https://cran.r-project.org/web/packages/raters/raters.pdf (2022).
- 119.Mayhew JA, Gomez JC. Gorillas with white sclera: A naturally occurring variation in a morphological trait linked to social cognitive functions. Am. J. Primatol. 2015;77:869–877. doi: 10.1002/ajp.22411. [DOI] [PubMed] [Google Scholar]
- 120.Mearing AS, Burkart JM, Dunn J, Street SE, Koops K. The evolutionary drivers of primate scleral coloration. Sci. Rep. 2022;12:14119. doi: 10.1038/s41598-022-18275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are provided as electronic supplementary material.