Abstract
Background
In recent years, mRNA-based vaccines with promising safety and functional characteristics have gained significant momentum in cancer immunotherapy. However, stable immunological molecular subtypes of lung adenocarcinoma (LUAD) and novel tumor antigens for LUAD mRNA vaccine development remain elusive. Therefore, a novel approach is urgently needed to identify suitable LUAD subtypes and potential tumor antigens.
Methods
The Cancer Genome Atlas (TCGA), the Genotype Tissue Expression (GTEx), and Gene Expression Omnibus (GEO) databases were utilized to retrieve gene expression data. The LUAD Immunological Multi-Omics Classification (LIMOC) system was developed using seven machine learning (ML) algorithms by performing integrative immunogenomic analysis of single-cell and bulk tissue transcriptome profiling. Subsequently, a panel of approaches was applied to identify novel tumor antigens.
Results
First, the LIMOC system was construct to identify three subtypes: LIMOC1, LIMOC2, and LIMOC3. Second, we identified CHIT1, LILRA4, and MEP1A as novel tumor antigens in LUAD; these genes were up-regulated, amplified, and mutated, and showed a positive association with APC infiltration, making them promising candidates for designing mRNA vaccines. Notably, the LIMOC2 subtype had the worst prognosis and could benefit most from mRNA immunization. Furthermore, we performed a comprehensive in silico screening of approximately 2000 compounds and identified Sorafenib and Azacitidine as potential subtype-specific therapeutic agents.
Conclusions
Overall, our study established a robust LIMOC system and identified CHIT1, LILRA4, and MEP1A as promising tumor antigen candidates for development of anti-LUAD mRNA vaccines, particularly for the LIMOC2 subtype.
Keywords: mRNA vaccine, Single-cell RNA-sequencing, Machine learning, Novel tumor antigens, Subtype-specific therapeutic agents
Graphical Abstract
Highlights
-
•
Construct the Immunological Multi-Omics Classification (LIMOC) system.
-
•
Identify three hitherto undocumented tumor antigens (CHIT1, LILRA4, and MEP1A).
-
•
Develop a novel in silico screening strategy and identify Sorafenib and Azacitidine as potential therapeutic agents.
1. Introduction
Lung adenocarcinoma (LUAD) is the most common type of lung cancer, affecting roughly 40% of patients [1]. While smoking is a major risk factor, LUAD also occurs in non-smokers, particularly women [2], [3]. Despite advancements in treatment options like chemotherapy, radiotherapy, targeted therapy, and surgery, LUAD remains associated with high morbidity and poor prognosis [4]. Immune checkpoint therapy has revolutionized cancer treatment, but its effectiveness is limited to a specific patient population. Therefore, developing strategies to improve LUAD survival is critical.
The success of mRNA-based COVID-19 vaccines has ignited significant interest in developing mRNA vaccines for cancer, building upon the established potential of mRNA vaccines as a new immunotherapy approach [5]. Preclinical and clinical studies have demonstrated the effectiveness of mRNA vaccine therapies in melanoma [6], prostate cancer [7], and breast cancer [8]. Notably, Sebastian et al. showed that the RNActive® vaccination CV9201 could trigger an antigen-specific immune response and extend treatment-free life for over a year in five stage IV patients [9]. Additionally, another study reported a well-tolerated immune response elicited in late-stage castration-resistant prostate cancer patients by an mRNA vaccine [10]. However, LUAD presents a unique challenge due to its high heterogeneity and complex microenvironment. Currently, there are no reported novel candidate antigens or targeted mRNA vaccines specifically designed for LUAD. Fortunately, advancements in next-generation sequencing and computational analysis offer powerful tools to identify novel immune subtypes, tumor antigens, and screen compounds with potential subtype-specific efficacy.
This project aimed to identify immune subtypes in LUAD, discover novel subtype-specific tumor antigens for mRNA vaccine development, and pinpoint potential subtype-specific therapeutic agents. We achieved this by integrating immunogenomic analysis of single-cell and bulk tissue transcriptome profiling, identifying three immune subtypes with distinct clinical, molecular, and cellular characteristics. Additionally, we discovered three novel tumor antigens for mRNA vaccine design and employed an integrative strategy to identify potential subtype-specific therapeutic agents. These findings have the potential to lay the groundwork for mRNA vaccine development and provide new insights for precision oncology.
2. Materials and methods
2.1. Data collection and processing
The Cancer Genome Atlas (TCGA) [11] (https://tcga-data.nci.nih.gov/) and the Genotype Tissue Expression (GTEx)[12] (https://www.gtexportal.org/) databases were utilized to collect for gene expression data of 574 LUAD samples and 286 LUAD samples, respectively. The values have been normalized using TPM (transcripts per kilobase million) and shown as log 2 (TPM + 0.001). Additionally, immune-related genes were downloaded from ImmPort [13] (https://www.immport.org/shared/home) (n = 1793) and InnateDB [14] (https://www.innatedb.com/) (n = 1226) databases, and listed in Table S1–2. After combining these datasets and removing duplicates, 2660 immune-related genes were available for further analysis.
The DNA methylation profile, CNV data, as well as somatic mutation data were collected from the XENA database [15] (https://xenabrowser.net/), FireBrowse[16] (http://firebrowse.org/) and TCGAbiolinks [17] package, respectively. Moreover, survival data of TCGA patients was obtained from a previously published study[18]. In total, 431 primary LUAD samples with complete transcriptome expression, DNA methylation, CNV, somatic mutation, and survival data were available (listed in Table S3). Five independent GEO datasets (GSE19188 [19], GSE30219 [20], GSE37745 [21], GSE50081 [22], GSE72094 [23]) were included, each with survival information. Expression data and relevant clinical data for these datasets were downloaded using the GEOquery package [24]. Probe IDs were mapped to gene symbols using the corresponding annotation files. For genes with multiple probes, a single expression value was generated by averaging the values. The first four GEO datasets (GSE19188, GSE30219, GSE37745, and GSE50081) were generated using the Affymetrix Human Genome U133 Plus 2.0 Array platform. Batch effects were removed using the sva package [25], and these datasets were then merged into a single meta-cohort. Finally, GSE72094 was used as a separate set for independent validation (Table S4).
2.2. Construction of the LIMOC system
The LIMOC system was constructed using immunological multi-omics data with seven state-of-art clustering algorithms, including SNF [26], PINSPlus [27], NEMO [28], COCA [29], ConsensusClustering[30], CIMLR [31], and MoCluster [32]. For DNA methylation data, we focused on probes located at CpG promoter islands and used the median β value for each gene. Similarly, in the mutation data, a gene in the mutation matrix was designated as mutated (1) if it contained at least one non-synonymous variant, while wild-type genes were designated as 0. Based on immune-related genes, we extracted multi-omics data, including transcriptome expression, DNA methylation, somatic mutations, and CNV data. Finally, the immunological multi-omics data used for subtyping comprised 1858 mRNA genes, 752 methylated genes, 2100 mutation genes, and the top 2000 most variable copy number regions identified based on median absolute deviation.
The seven state-of-the-art multi-omics integrated clustering algorithms were independently used for integrative clustering. To improve clustering robustness, we adopted a consensus ensemble approach. To define the consensus, we created a consensus matrix (N × N) that stores the percentage of clustering algorithms in which any two samples were grouped together. Meanwhile, let M(x) denote the (N × N) connectivity matrix corresponding to an integrative clustering algorithm(x). The entries of this matrix were defined as follows:
Finally, the consensus matrix m is calculated by summing the connectivity matrices of all algorithms and then dividing by the total number of algorithms (NMU) used:
In addition, we defined a new distance measurement (1-M) based on the consensus matrix and conducted hierarchical tree construction to define the final subtypes. To classify samples in the external cohorts (the meta-cohort and GSE72094 cohort) into the LIMOC groups, we first identified the top 100 differentially expressed genes, ranked by log2FoldChange, for each LIMOC subtype. These biomarkers were selected based on a significance threshold (adjusted P-value < 0.05) and ensured they did not overlap with any biomarkers identified for other subtypes. We then performed nearest template prediction (NTP) analysis[33] to classify the subtypes in the external cohorts. Furthermore, we compared the LIMOC system's performance with other state-of-the-art LUAD subtyping methods published by research teams led by Song [34], Wei [35], Deng [36], Li [37], and Thorsson [38].
2.3. Calculations of cell abundance and pathway enrichment in microenvironments
To identify specific characteristics of the three subtypes, we analyzed microenvironmental cell infiltration. Two gene signatures, CIBERSORT [39] and MCP-counter[40], [41]) were used to compile gene lists associated with 24 microenvironmental cell types (364 genes total). The GSVA package [42] was employed for gene set variation analysis (GSVA), while the estimate package [43] was used to calculate immune and stromal scores. Additionally, we compared the patterns of metabolic reprogramming and canonical oncogenic pathways across the three subtypes [44], [45].
2.4. Mutation landscape analysis
We analyzed the mutation landscape using the maftools package [46]. We started by removing 100 FLAGs and used default settings for other parameters. Subsequently, the deconstructSigs package[47] was used to evaluate mutational signatures. This analysis identified four mutational signatures: SBS1 (age-related), SBS2, SBS5 (ERCC2 mutation-related), and SBS13 (APOBEC activity-related). Additionally, the percentage of the copy number-altered genome was calculated using the copy number segment data [48], [49].
2.5. Regulon analysis
The RTN package [50], [51] was used to reconstruct transcriptional regulatory networks involving 23 regulator genes and 68 chromatin remodeling-associated genes (Tables S5-S6). Spearman rank-order correlation and mutual information analysis were employed to assess potential regulatory connections between regulator genes and their downstream target genes. Subsequently, statistically significant associations were identified using a stringent false discovery rate (FDR) threshold of less than 0.00001. When the subset of either positive or negative targets was below the ‘minRegulonSize’ of 15, the spare targets were removed from largely unbalanced regulons. At this step, we obtained an activity matrix for each patient. Finally, we used the 'limma' package to perform differential activity analysis between the three subtypes.
2.6. Analysis of Single-cell RNA-sequencing data
We obtained raw single-cell RNA-sequencing data from GSE131907 [52], encompassing 44 LUAD patients. Prior to the analysis, we performed quality control steps to exclude low-quality cells. Specifically, we excluded cells expressing fewer than 500 genes, having a mitochondrial gene proportion exceeding 20%, or containing a hemoglobin gene proportion greater than 1%. Subsequently, the Seurat package's [53] SCTransform function was used to normalize the data. To further explore the data, we employed the RunPCA function and t-SNE algorithm for dimensionality reduction and cell clustering. Following clustering, we annotated each cell based on the corresponding annotation file. Finally, we utilized the CellChat package [54], to predict intercellular communication patterns across all identified cell types.
2.7. Identification of potential LUAD tumor antigens
We used the limma package [55] to identify the upregulated genes in LUAD samples, combining the TCGA and the GTEx datasets (|log2 (fold change)| > 1, P < 0.05). Next, the karyoploteR package [56] was used to visualize the chromosomal locations of these upregulated genes. We further calculated the altered genome fraction, tumor mutational counts, and copy number alterations for each sample. The intersection of these candidate genes yielded potential tumor antigens for LUAD. To assess their prognostic value for overall survival (OS), we performed Cox regression analysis on these potential tumor antigens. Additionally, we analyzed the correlation between the abundance of antigen-presenting cells (APCs) and the expression of each tumor antigen.
2.8. Clinical samples and immunohistochemistry (IHC)
The study was approved by the Ethics Committee of Shanghai Outdo Biotech Company. We obtained tissue microarray (TMA) slides containing 98 lung adenocarcinoma tissues and 82 adjacent normal lung tissues (HLugA180Su08) from a commercial source (Shanghai Outdo Biotech Company). Ten tissue samples (4 adjacent normal and 6 lung adenocarcinoma tissues) with tissue flaking or missing follow-up data were excluded. Antibodies against LILRA4 (21432–1-AP), CHIT1 (21432–1-AP), and MEP1A (24640–1-AP) were purchased from Proteintech (Wuhan, China). Immunohistochemical (IHC) staining was performed as previously described [57], [58]. Staining intensity and the percentage of positive cells were quantified for each sample. The positive cell percentage was scored on a five-point scale: 0 (negative), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (>75%). Staining intensity was scored on a four-point scale: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The IHC score was calculated using the following formula: H-Score = positive percentage score × intensity score.
2.9. siRNA transfection
siRNAs targeting CHIT1, LILRA4, and MEP1A were synthesized by GenePharma (Shanghai, China). The sequences of these siRNAs are listed in Table S7. Transfection with these siRNAs was performed using Trans-Mate (GenePharma) according to the manufacturer's protocol. After transfection, cell viability of A549 cells was measured using the CCK-8 assay at 1, 3, and 5 days.
2.10. Drug therapeutic response analysis
Drug response data in human cancer cell lines were gathered from three sources: the Cancer Therapeutics Response Portal (CTRP v.2.0; 481 compounds across 835 cell lines), Genomics of Drug Sensitivity in Cancer (GDSC; 297 compounds across 969 cell lines), and Profiling Relative Inhibition Simultaneously in Mixtures (PRISM; 1448 compounds across 482 cell lines)[59], [60], [61]. All three datasets used the area under the dose-response curve (AUC) as a metric for drug sensitivity, with lower AUC values indicating greater sensitivity to treatment. Before data imputation, we excluded drugs with missing values in more than 20% of cell lines. The k-nearest neighbors (KNN) method was then used to impute the remaining missing values. This resulted in 680 cancer cell lines and 354 drugs retained in CTRP, 962 cell lines and 278 drugs in GDSC, and 480 cell lines and 1285 drugs in PRISM. Expression data for the cancer cell lines were obtained from the Broad Institute-Cancer Cell Line Encyclopedia project (CCLE) [62]. The pRRophetic package[63], which utilizes a built-in ridge regression model, was employed to predict drug responses in clinical samples. Default parameters were used to predict drug sensitivity for each LUAD sample. Following the acquisition of predicted drug response data for LUAD patients, a differential sensitivity analysis was conducted among the three subtypes.
2.11. Cell viability assay
NCI-H1395, NCI-H2347, HCC827, and Calu-3 cells were plated in 96-well plates at a density of 5000 cells/well and incubated overnight. The cells were then incubated with a range of concentrations of Sorafenib and Azacitidine for an additional 48 h respectively. Following incubation, the culture medium was replaced with fresh serum-free media containing 10% Cell Counting Kit-8 solution (CCK-8, meilunbio, Dalian, China) and incubated for another 2 h at 37 °C in a humidified incubator. Finally, the absorbance of the culture medium was measured at 450 nm using a Cytation 5 (BioTek, America) microplate reader.
2.12. Statistical analysis
All statistical and bioinformatics analyses were performed using R software. Continuous data were compared using the Student's t-test or Mann-Whitney test, as appropriate. The analysis of different clinical outcomes was based on Kaplan-Meier plots and Cox regression analysis. Multi-omics integration analysis was performed using the MOVICS package[64]. Statistical significance was determined at a P-value threshold of less than 0.05. Key scripts and training data for the analysis are available on Github (https://github.com/saisaitian/LIMOC-system-in-LUAD).
3. Results
3.1. Construction of LIMOC system and identification of immune subtypes
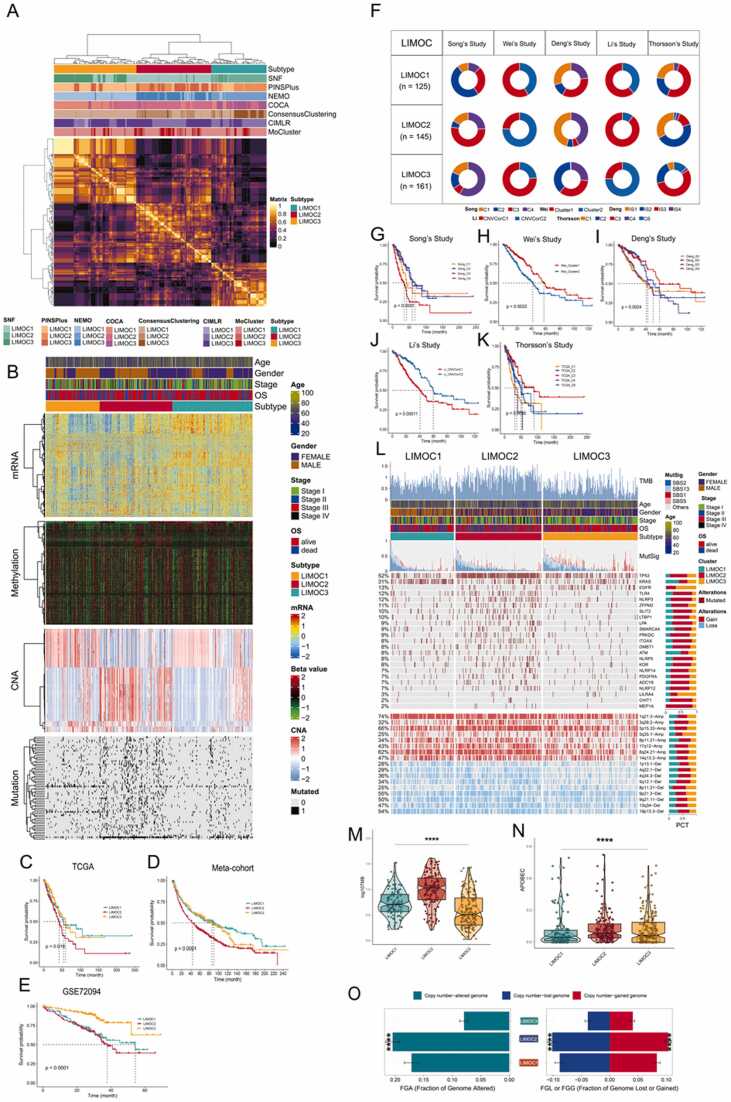
Immunologic subtypes may influence the effectiveness of immunotherapy and guide patient selection for mRNA vaccination [65], [66]. To identify these subtypes, we employed seven leading-edge clustering algorithms to build the LIMOC system. This analysis yielded three robust consensus subtypes named LIMOC1, LIMOC2, and LIMOC3, identified using the clustering prediction index and gaps-statistical analysis (Fig. 1A, Fig. S1A). Fig. 1B displays the obtained multi-omics data in LUAD. We then investigated the association between survival and LUAD subtypes. Patients in LIMOC2 exhibited a significantly shorter median OS compared to those in LIMOC1 and LIMOC3 (Fig. 1C, P < 0.05). Next, we used NTP to classify samples in the meta-cohort and GSE72094 cohort into LIMOC groups based on subtype-specific genes (Table S8, Fig. S1B-C). Interestingly, similar results were observed in both cohorts (Fig. 1D-E, P < 0.0001), with all LIMOC2 patients showing the worst clinical outcomes. Furthermore, we explored the association between our classification and previously reported molecular classifications by research teams led by Song, Wei, Deng, Li, and Thorsson (Fig. 1F-K). As shown, all these approaches displayed a close link with prognosis. Notably, the enrichment of poor prognosis subtypes within LIMOC2 suggests the accuracy of our classification system. For example, in Song et al.'s study, most LIMOC2 patients belonged to the C3 group, which had the worst prognosis, consistent with our finding that LIMOC2 patients have the poorest outcome (Fig. 1G, P < 0.0001). Similar results were observed in studies conducted by Wei, Deng, Li, and Thorsson (Fig. 1H-K), further supporting the accuracy of our system.
Fig. 1.
Recognition of the LIMOC system in the LUAD cohort and comparison between the LIMOC system and other state-of-the-art LUAD subtyping. (A) Consensus ensemble derived from different multi-omics integrative clustering analyses. (B) Visualization of immunological multi-omics data in LUAD. (C) Differential overall survival outcome in three LIMOCs in TCGA, log-rank test. (D) Differential overall survival outcome in three LIMOCs in the meta-cohort, log-rank test. (E) Differential overall survival outcome in three LIMOCs in GSE72094, log-rank test. (F) Contribution of subtypes obtained by other methods in LIMOC subtypes. (G-K) Differential overall survival outcome from (G)Song’s Study, (H)Wei’s Study, (I)Deng’s Study, (J)Li’s Study, and (K)Thorsson’s study, log-rank test. (L) The genetic change landscape by subtype is shown. The contributions of APOBEC-relevant mutational signatures (i.e., SBS2 + SBS13) are used to order samples descendingly. From top to bottom, TMB, the relative contribution of four mutational signatures, and differentially modified genes and CNAs are illustrated. The proportion of each subtype is given in the right bar charts. (M-N) The contribution of the TMB (M) and APOBEC (N) to the specific alteration is shown. (O) Distribution of fraction genome changes (FGA) and gain/loss (FGA/FGG). The mean standard error of the mean is displayed in bar charts. For multiple comparisons, the Kruskal–Wallis rank sum test was used to determine statistical P values.
3.2. Relationship between mutational status and immunological subtypes
Given its established link to immunotherapy efficacy [67], we assessed the tumor mutational burden (TMB) across the three subtypes. LIMOC2 displayed a significantly higher TMB (Fig. 1L-M) and harbored more mutations associated with APOBEC signatures (Fig. 1N). Notably, LIMOC2 exhibited the most mutations among frequently altered genes, including TP53, NLRP3, TLR4, and RB1 (Table S9). As TP53 was the most frequently mutated gene, we investigated how its exclusion impacted the LIMOC classification system. Figure S2A shows that removing TP53 altered the LIMOC classification, highlighting its critical role. Furthermore, TP53 ranked first in our analysis of mutated gene importance (Fig. S2B). We also observed higher chromosomal instability in LIMOC2, evident by a significantly greater burden of copy number alterations (CNAs) (P < 0.001) (Fig. 1O). Within LIMOC2, copy number gains were primarily located in the 8q24.21 region, which was not amplified in other subtypes. Conversely, the Ch9p21.3 region, known to be susceptible to inactivation during cell immortalization, exhibited a deletion specifically in the LIMOC2 subtype [68].
3.3. Association between immune subtypes of LUAD and regulon analysis
Transcriptome regulatory networks play a critical role in tumor development and proliferation. We, therefore, evaluated the activity of 68 chromatin remodeling regulons and 23 other regulons to identify potential differences between the subtypes. Differential activity analysis revealed distinct regulatory patterns across the three subtypes. Notably, LIMOC2 displayed evidence of regulation by the human Fox gene family, while patients in LIMOC3 appeared to be under the influence of androgen receptor (AR) and estrogen receptor 2 (ESR2) activation (Fig. 2A, Fig. S3A, and Fig. S4A). We further constructed a signature of ten oncogenic pathways and calculated enrichment scores using the GSVA approach. This analysis indicated activation of the cell cycle and PI3K oncogenic pathways in LIMOC2 patients (Fig. 2B-C, Fig. S3B-C, and Fig. S4B-C). Activation of the cell cycle pathway is known to induce the expression of replication stress-related proteins involved in DNA damage response [69]. Additionally, the PI3K/AKT signaling pathway has been shown to promote cell metastasis through LAMA2 regulation and influence survival in LUAD [70]. Recent studies have shown that NRF2 suppresses the DNA-sensing innate immune response while increasing the COX2/PGE2 response, thereby promoting an immunologically cold microenvironment [71]. Similarly, our findings demonstrated that the LIMOC2 subtype was linked to activate NRF2 oncogenic pathway in meta-cohort and GSE72094, suggesting the low immune infiltration of LUAD might be driven by the functional disorders of oncogenic pathways. In addition, LIMOC3 had a higher score of TGF-β and stromal fraction, suggesting that the LIMOC3 phenotype is both immunological “hot” and immunosuppressive which could hinder the active immune reaction.
Fig. 2.
Differential activity of regulons and pathways across LIMOC system in TCGA cohort. (A) Diagram illustrating potential regulators and 23 regulators involved in chromatin remodeling in a heatmap. (B) Heatmap of 10 differentially activated oncogenic pathways. (C) Boxplot of 10 differentially activated oncogenic pathways. (D) Heatmap of subtype-specific metabolism signaling pathways. * P < 0.05; * * P < 0.01; * **P < 0.001; ns not significant.
Analysis of different metabolic pathway activation revealed distinct patterns across the subtypes (Fig. 2D, Fig. S3D, and Fig. S4D). LIMOC1 displayed activation of arginine biosynthesis, ascorbate and aldrate metabolism, and pyruvate metabolism pathways. In contrast, LIMOC2 exhibited activation of pyrimidine metabolism, pyrimidine biosynthesis, and vitamin B6 metabolism. Finally, LIMOC3 patients showed strong activation of linoleic acid metabolism and arachidonic acid metabolism pathways. These findings suggest that the three subtypes exhibit unique metabolic reprogramming patterns, potentially contributing to their distinct molecular profiles and clinical outcomes.
3.4. The clinical, cellular, and molecular features of every subtype of LUAD
We investigated the clinical characteristics of each LUAD subtype (LIMOC1, LIMOC2, and LIMOC3), including age, gender, and tumor stage (Table S10). Our analysis revealed an association between age and gender with subtype distribution. Patients belonging to the LIMOC2 subtype were predominantly older males and exhibited a significantly shorter overall survival (Fig. S5). Next, we assessed the abundance of 24 immune cell types within the TCGA, meta-cohort, and GSE72094 cohorts using the ssGSEA algorithm. The results are presented as heatmaps in Fig. 3A-C. LIMOC2 displayed a higher number of activated memory CD4+ T cells, whereas LIMOC3 exhibited a greater abundance of CD8+ T cells, CD4+ naïve T cells, macrophages, Tregs, monocytes, neutrophils, and other immune cells. Notably, the LIMOC3 subtype showed a particularly strong enrichment for M2 macrophages, neutrophils, and Tregs compared to the other subtypes (Fig. 3D, Fig. 3G, and Fig. 3J). Immune checkpoints (ICPs) is a mechanism that can prevent the immune system from recognizing and attacking cancer cells[72]. Interestingly, the LIMOC3 subtype displayed a higher expression of ICPs compared to the other subtypes (Fig. 3E, H, and K). Given the critical role of immunogenic cell death (ICD) modulators in stimulating anti-tumor immunity[73], we explored the expression levels of these modulators in the three LIMOC subtypes. We analyzed 21 ICD modulators across the three cohorts and found that over half were significantly upregulated in LIMOC2, including ANXA1, CALR, CXCL10, EIF2A, EIF2AK1, EIF2AK2, HMGB1, and PANX1 (Fig. 3F, I, and L).
Fig. 3.
Differential immune profile across LIMOC system. (A) This heatmap displays the immunological profile of the TCGA-LUAD cohort, with the top panel displaying the expression of immune checkpoint genes. The levels of enrichment for 24 microenvironment cell types are shown at the bottom of the heatmap. The top of the heatmap shows immune enrichment and stromal enrichment. (B) Similar analysis in meta-cohort. (C) Similar analysis in GSE72094. (D-F) Boxplot of the abundance of immune cells (D), ICP (E) and ICD modulators (F) as discriminated by various subtypes in the TCGA. (G-I) Similar analysis in meta-cohort. (J-L) Similar analysis in GSE72094.
3.5. The molecular characteristics of three subtypes at the single-cell level
To gain deeper insights into the LIMOC system within LUAD tissues, we analyzed a single-cell RNA sequencing dataset (GSE131907). Following quality control filtering, the dataset yielded approximately 27,578 unique transcripts and 191,736 cells from the 41 samples. Based on the associated annotation files, we generated a comprehensive single-cell atlas and quantified the cell type proportions to characterize the cellular composition (Fig. 4A). As shown in Fig. 4B, immune cells comprised the largest population, followed by epithelial cells. To identify malignant cells within the dataset, we performed further annotation and ultimately isolated 18,913 malignant cells. We then employed NTP for LUAD subtyping based on the normalized expression of classification biomarkers in each malignant cell. This analysis classified 6891, 6085, and 5937 cells as LIMOC1, LIMOC2, and LIMOC3, respectively. Notably, t-distributed stochastic neighbor embedding (t-SNE) visualization revealed distinct clusters for the malignant cells, reflecting their subtype and sample origin (Fig. 4C-E). We further calculated the percentage of each LIMOC subtype within each tissue sample origin. This analysis revealed a significant enrichment of LIMOC1 tumor cells in mBrain, LIMOC2 tumor cells in tL/B, and LIMOC3 tumor cells in mLN (Fig. 4F). These findings also suggest greater intratumoral heterogeneity within aggressive tumors.
Fig. 4.
The molecular characteristics of three subtypes in the single-cell RNA-sequencing dataset. (A) Cell distribution in scRNA-seq visualized by tSNE, colored by major cell type compartments. (B) Proportion of cell types in scRNA-seq. (C) Cell distribution in scRNA-seq visualized by tSNE, colored by malignant cells, (D) by subtypes, (E) by sample origin. (F) Distribution of three subtypes in the different sample origins. (G-H) The abundance of oncogenic pathways (G) and metabolic pathways (H) in three subtypes of scRNA-seq. (I-J) The expression of ICDs modulators and ICPs in three subtypes of scRNA-seq.
Next, GSVA was performed on the ten oncogenic pathways to calculate enrichment scores. Consistent with previous result, the cell cycle was activated in the LIMOC2 subtype and TGF-β pathways was activated in the LIMOC1 and LIMOC3 (Fig. 4G). Meanwhile, the metabolic reprogramming patterns of the three subtypes in malignant cells also showed similar trend (Fig. 4H). We further investigated the expression levels of ICPs and ICD modulators (Fig. 4I-J). As expected, LIMOC3 exhibited strong expression of ICPs, while nearly half of the ICD modulators were upregulated in LIMOC2, including CXCL10, EIF2AK2, HMGB1, and ANXA1. These findings further corroborate the validity of our previous results.
3.6. Decoding the intercellular communications underlying three subtypes
To construct a LUAD subtype-specific cell-cell communication atlas, we isolated 18,409 T lymphocytes, 4514 B lymphocytes, 633 endothelial cells, 8092 myeloid cells, 1003 NK cells, 1735 mast cells, and 1660 fibroblasts from patient primary tissue. The resulting cell-cell communication network is depicted in Fig. S6A. As expected, the LIMOC1 subtype exhibited the strongest outgoing signal, followed by LIMOC2, with LIMOC3 showing the weakest signal (Fig. S6B). We then employed a pattern recognition technique to identify global communication patterns. This method utilizes two metrics, cophenetic and silhouette coefficients, to determine the optimal number of patterns. In this study, we identified three outgoing communication patterns and five incoming communication patterns (Fig. S6C-D). Interestingly, regardless of the pattern considered, the three subtypes consistently clustered together. Furthermore, we identified 11 signaling pathways, including EGF, WNT, and TWEAK pathways, and 36 important ligand-receptor pairs specific to the three subtypes (Fig. S6E). Network centrality analysis of the inferred EGF signaling network revealed that the three LIMOC populations, especially LIMOC2, primarily act as receivers, likely influenced by signals from their own cells as well as myeloid, endothelial, mast, and NK cells. Additionally, all three LIMOC populations emerged as significant receivers within the TWEAK signaling pathway, primarily influenced by fibroblasts and myeloid cells (Fig. S6F-G).
3.7. Novel candidate antigen screening in LUAD
First, by integrating TCGA and GTEx data, 1897 upregulated genes potentially encoding tumor antigens were identified. The chromosomal distribution of the regulated genes is shown in Fig. 5A. Second, 12501 mutated genes were recognized and the top 20 of them are represented in Fig. 5B. Third, 15063 amplified genes were selected based on CNV data and the top 10 amplified genes are shown in Fig. 5C. Notably, TP53, TTN, and MUC16 were the most frequently mutated genes, while CDKN2A, CDKN2B, and SFTA3 were the most amplified. In the end, 935 high expressed, mutated, and amplified genes were recognized as promising candidate antigens after combining the expression, mutation, and copy number data. 3088 genes associated with OS in TCGA dataset were identified to survey the critical genes that might function as the best contenders for mRNA vaccine targets (Table S11). Furthermore, considering the role of APCs in immunity, we explored the relationship between gene expression and APC abundance. This analysis revealed 987 genes with a strong correlation to APC levels (Table S12). Finally, by intersecting all three analyses (expression, mutation/amplification, and APC correlation), we identified three candidate LUAD tumor antigens: CHIT1, LILRA4, and MEP1A (Fig. 5D). The detailed screening process is shown in Fig. S7. More importantly, the expression levels of the three candidate antigens (CHIT1, LILRA4, and MEP1A) showed a significant correlation with OS and progression-free survival (PFS) in LUAD patients (Fig. 5E-F, Fig. 5G-H, and Fig. 5I-J). This suggests that these genes play a crucial role in LUAD development and progression. To validate these findings at the protein level, we performed immunostaining on tissue microarrays (TMAs) containing human LUAD tissues. Consistent with our previous results, CHIT1, LILRA4, and MEP1A expression was significantly higher in LUAD tissues compared to adjacent normal tissues (Fig. 5K-P). Besides, the siRNA transfection experiments were also carried out, and it was found that knockdown of CHIT1, LILRA4, and MEP1A suppressed the proliferation of LUAD cell line A549, and the suppressive effects were time-dependent (Fig. 5Q). Five days after knockdown of CHIT1, LILRA4, and MEP1A, the cell viabilities of A549 were reduced to be 74.95 ± 4.67%, 86.03 ± 0.54%, and 78.95 ± 3.8%, respectively. Furthermore, we also assessed the knockdown efficiencies of siRNAs by qRT-PCR. As shown in Fig. S8, the knockdown efficiencies of siCHIT1, siLILRA4, and siMEP1A were determined to be 78 ± 0.83%, 68 ± 0.6%, and 73 ± 1.14%, respectively.
Fig. 5.
Identify the potential tumor antigens in LUAD and analyze the expression of the tumor antigens (CHIT1, LILRA4, and MEP1A) between the normal and tumor tissue. (A) The distribution of elevated genes across the chromosomes. (B) The state of mutation of the 20 most mutated genes for each LUAD sample. (C) The top 10 amplified genes in LUAD. (D) The overlap of therapeutic targets is linked to both the OS and APCs. (E, G, I) Kaplan-Meier curves of the association of CHIT1, LILRA4, and MEP1A with OS. (F, H, J) Kaplan-Meier curves of the association of CHIT1, LILRA4, and MEP1A with PFS. (K-M) Representative pictures showing that IHC signals of CHIT1(K), LILRA4(L), and MEP1A(M) was increased in tumor tissue compared with normal tissue. (N-P) Graph showing the CHIT1(N), LILRA4(O), and MEP1A(P) staining scores in lung adenocarcinoma tissues and adjacent normal tissues. (Q) The cell viabilities at different time points after knockdown of CHIT1, LILRA4, and MEP1A by siRNA of A549 cells.
Our findings collectively demonstrate that CHIT1, LILRA4, and MEP1A are not only upregulated and amplified in LUAD but also frequently mutated and associated with APC infiltration. These data strongly support our previous experimental results, suggesting that mRNA vaccines targeting these three genes could induce an anti-tumor immune response and eliminate cancer cells.
3.8. The correlation between APCs abundance and identified tumor antigens in LUAD
We first assessed the abundance of APCs in LUAD tumors using TIMER database. We then investigated the correlation between these APC abundnce and the expression of our candidate antigens, CHIT1, LILRA4, and MEP1A. Positive correlation was found between the expression of CHIT1, LILRA4, and MEP1A and the abundance of DCs (CHIT1: r = 0.62, LILRA4: r = 0.65, MEP1A: r = 0.25), B cells (CHIT1: r = 0.33, LILRA4: r = 0.4, MEP1A: r = 0.3), and macrophages (CHIT1: r = 0.24, LILRA4: r = 0.25, MEP1A: r = 0.25) (Fig. S9A-I). To validate these findings, we performed similar analyses in a meta-cohort and the GSE72094 cohort. In the results, CHIT1 and LILRA4 expression correlated positively with abundance of DCs (meta-cohort: CHIT1: r = 0.62, LILRA4: r = 0.39; GSE72094: CHIT1: r = 0.57, LILRA4: r = 0.58), B cells (meta-cohort: CHIT1: r = 0.32, LILRA4: r = 0.36; GSE72094: CHIT1: r = 0.13, LILRA4: r = 0.36) and macrophages (meta-cohort: CHIT1: r = 0.37, LILRA4: r = 0.19; GSE72094: CHIT1: r = 0.29, LILRA4: r = 0.16) (Fig. S10A-B, Fig. S10D-E, and Fig. S10G-H, Fig. S11A-B, Fig. S11D-E, and Fig. S11G- H). However, in the meta-cohort and GSE72094 cohort, the expression of MEP1A was not found to be significant correlated with the abundance of APCs (Fig. S10C, Fig. S10F, and Fig. S10I, Fig. S11C, Fig. S11F, and Fig. S11I). Given the synergistic roles played by helper stimulus molecules in immunity, the correlation analysis between CHIT1, LILRA4, and MEP1A and certain helper stimulus molecules like CD40, CD80, and CD86 was performed. The expressions of CHIT1 and LILRA4 exhibited a positive correlation with CD40 (CHIT1: r = 0.4, LILRA4: r = 0.42), CD80 (CHIT1: r = 0.48, LILRA4: r = 0.57), and CD86 (CHIT1: r = 0.47, LILRA4: r = 0.56) in the TCGA cohort (P < 0.05, Fig. S9J-K, Fig. S9M-N, and Fig. S9P-Q). These positive correlations were also observed in both the meta-cohort (CD40: CHIT1: r = 0.36, LILRA4: r = 0.24; CD80: CHIT1: r = 0.29, LILRA4: r = 0.27; CD86: CHIT1: r = 0.45, LILRA4: r = 0.28) (Fig. S10J-K, Fig. S10M-N, and Fig. S10P-Q) and the GSE72094 cohort (CD40: CHIT1: r = 0.42, LILRA4: r = 0.40; CD80: CHIT1: r = 0.35, LILRA4: r = 0.37; CD86: CHIT1: r = 0.37, LILRA4: r = 0.42) (Fig. S11J-K, Fig. S11M-N, and Fig. S11P-Q). In the TCGA cohort, the expression of CD80 (r = 0.22, P < 0.05) and CD86 (r = 0.27, P < 0.05) also showed a positive correlation with MEP1A expression, but not CD40 (r = 0.069, P > 0.05) (Fig. S9L, Fig. S9O, and Fig. S9R). Within the meta-cohort, MEP1A expression displayed a positive correlation with CD80 (r = 0.085, P = 0.02) but not with CD40 (r = −0.013, P = 0.71) or CD86 (r = −0.02, P = 0.59) (Fig. S10L, Fig. S10O, and Fig S10R). However, in the GSE72094 cohort, MEP1A expression did not exhibit significant correlations with CD40, CD80, or CD86 (Fig. S11L, Fig. S11O, and Fig S11R). Based on these results, it indicated that mRNA vaccines against these three antigens can active specialized APCs.
3.9. The relationship between APC markers and the three antigens in the LIMOC2 subtype
Patients with the LIMOC2 subtype exhibit higher TMB, somatic mutation rates, APOBEC activity, and CNVs. These factors may contribute to an increased number of tumor antigens and induce infiltration of APCs into the tumor microenvironment. Interestingly, previous studies have reported that individuals with overexpression of ICD modulators may experience greater benefit from mRNA vaccination, while patients with increased expression of ICPs may not [5], [65]. In the current study, the low expression of ICPs and elevated expression of ICD modulators in LIMOC2 further suggests its potential as a favorable target for mRNA vaccines. Additionally, LIMOC2 tumors display a lower TGF-β score and stromal fraction, indicative of an immune-activated phenotype. This suggests that patients with this subtype may have a more robust response to immunotherapy. Taken together, these findings suggest that patients belonging to the LIMOC2 subtype may benefit most from mRNA vaccination targeting CHIT1, LILRA4, and MEP1A. To further investigate this potential, we performed a correlation analysis between APC markers and the three candidate antigens specifically within the LIMOC2 subtype. The analysis revealed a positive correlation between the expression of CHIT1 and LILRA4 with the mRNA expression CD40 (CHIT1: r = 0.43, LILRA4: r = 0.49), CD80 (CHIT1: r = 0.57, LILRA4: r = 0.61), and CD86 (CHIT1: r = 0.54, LILRA4: r = 0.62) in the TCGA cohort (P < 0.05, Fig. 6A-B, Fig. 6D-E, and Fig. 6G-H). These positive correlations were also observed in both the meta-cohort (CD40: CHIT1: r = 0.28, LILRA4: r = 0.28; CD80: CHIT1: r = 0.24, LILRA4: r = 0.28; CD86: CHIT1: r = 0.39, LILRA4: r = 0.26) (Fig. S12A-B, Fig. S12D-E, and Fig. S12G-H) and GSE72094 cohort (CD40: CHIT1: r = 0.39, LILRA4: r = 0.40, CD80: CHIT1: r = 0.25, LILRA4: r = 0.29, CD86: CHIT1: r = 0.35, LILRA4: r = 0.39) (P < 0.05, Fig. S13A-B, Fig. S13D-E, and Fig. S13G-H). Collectively, these findings suggest that mRNA vaccines targeting CHIT1 and LILRA4 may be particularly effective in enhancing antigen presentation by APCs in the LIMOC2 subtype due to the strong positive correlations observed with costimulatory molecules. While MEP1A expression showed some positive correlations with CD40 (GSE72094, r = 0.22), CD80 (TCGA, r = 0.21; GSE72094, r = 0.20), and CD86 (TCGA, r = 0.22; GSE72094, r = 0.30) (Fig. 6F, I, Fig. S13C, Fig. S13F, and Fig. S13I), and no notable association was observed between MEP1A and CD40 (in TCGA and meta-cohort), CD80 (in meta-cohort), and CD86 (in meta-cohort) (Fig. 6C, Fig. S12C, Fig. S12F, and Fig. 12I). To summarize, the three antigens, especially for CHIT1 and LILRA4, had a close relationship with the APC markers in LIMOC2 subtype.
Fig. 6.
Correlation between APC markers and three antigens expression levels in LUAD with LIMOC2 subtype and functional enrichment analysis in TCGA. (A-C) Correlation of the expression levels between CD40 and CHIT1 (A), LILRA4 (B), and MEP1A (C). (D-F) Correlation of the expression levels between CD80 and CHIT1 (D), LILRA4 (E), and MEP1A (F). (G-I) Correlation between the expression levels between CD86 and CHIT1 (G), LILRA4 (H), and MEP1A (I). (J-L) KEGG analysis for categories associated with CHIT1 (J), LILRA4 (K), and MEP1A (L). (M-O) GO analysis for categories associated with CHIT1 (M), LILRA4 (N), and MEP1A (O).
3.10. Underlying biological roles of the three tumor antigens in LUAD
To elucidate the underlying mechanisms by which the three candidate antigens (CHIT1, LILRA4, and MEP1A) might function, we performed a correlation analysis. We further explored their potential biological roles by analyzing enriched pathways. CHIT1 displayed the strongest enrichment in immune-related pathways, including "Th17 cell differentiation", "chemokine signaling pathway", and "cytokine-cytokine receptor interaction" (Fig. 6J and M). Meanwhile, the most highly enriched biological pathways of LILRA4 included "regulation of immune effector process", "positive regulation of cytokine production", and "T cell activation" (Fig. 6K and N). MEP1A functional enrichment analysis yielded comparable results, with enriched pathways such as "B cell receptor signaling pathway" and "cytokine-cytokine receptor interaction" (Fig. 6L and O). These results suggested that CHIT1, LILRA4, and MEP1A were associated with immunologic biological processes, giving bioinformatic foundation for developing mRNA vaccines targeting these genes.
3.11. Screening and validation of subtype-specific therapeutic agents
Given the poor survival prognosis associated with the LIMOC2 subtype, exploring subtype-specific treatment options is crucial. In this study, we employed an integrated approach to identify potential therapeutic drugs for LIMOC2 patients (Fig. 7A). First, we downloaded data from three independent drug response datasets: CTRP, GDSC, and PRISM. Next, we utilized the calcPhenotype function within the pRRophetic package to estimate the sensitivity of each LUAD patient to various drugs across each dataset. This resulted in a matrix depicting each patient's sensitivity to each drug. Finally, we performed differential sensitivity analysis between the three subtypes using the limma package. This analysis identified several potential LIMOC2-specific drugs: 136 from CTRP, 66 from GDSC, and 269 from PRISM (Fig. 7B). We then defined LIMOC2-specific drugs as those significantly identified in at least two datasets, resulting in a final list of 28 potential therapeutic candidates (Fig. 7C).
Fig. 7.
Identification of subclass-specific agents. (A) A flowchart illustrating the process of subtype-specific drug identification. (B) LIMOC2 specific drugs identified in each dataset (CTRP, GDSC, and PRISM, Wilcoxon rank sum test, P < 0.05). (C) Venn diagram summarizing LIMOC2 specific agents. (D) Identification of most promising subclass-specific agents according to the evidence from multiple sources. (E-F) In vitro validation of Sorafenib (E) and Azacitidine (F) drug response between three subtypes using cell viability assay across 4 LUAD cell lines. Experiments were repeated at least three times.
While the identified 28 candidate drugs exhibit specific efficacy for the LIMOC2 subtype, the previous analyses are not sufficient to definitively establish their therapeutic potential for LUAD in general. To further narrow down the most promising candidates, we performed a multi-perspective analysis to comprehensively explore their therapeutic potential for LUAD (Fig. 7D). First, we conducted a systematic literature search on PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) to identify existing experimental and clinical evidence for the use of these candidate drugs in LUAD treatment. Next, we performed CMap analysis using the CMap database (https://clue.io/) to identify compounds with gene expression patterns that are opposite to those typically observed in LUAD. In this analysis, we focused on two LUAD cell lines (A549 and H1339) available in the CMap database. This analysis revealed two compounds, Sorafenib and Azacitidine, with CMap scores lower than − 85 in LUAD cell lines, suggesting their potential therapeutic effect for LUAD. Furthermore, we compared the estimated Area Under the Curve (AUC) values for Sorafenib and Azacitidine in the Prism dataset between the three LUAD subtypes using the Wilcoxon rank-sum test. As shown in Fig. S14, LIMOC2 patients displayed significantly higher estimated AUC values (P = 0.008) compared to the other subtypes, consistent with the CMap results.
To further validate the therapeutic potential of Sorafenib and Azacitidine, we performed small-scale in vitro experiments using four commonly used LUAD cell lines: NCI-H1395, NCI-H2347, HCC827, and Calu-3. Based on the NTP prediction results, NCI-H1395 and Calu-3 were attributed to the LIMOC1-like and LIMOC3-like subtypes, respectively, while HCC827 and NCI-H2347 were categorized as LIMOC2-like. Sorafenib and Azacitidine's cell viability assay results were more consistent with our predictions (Fig. 7E-F), demonstrating that the two compounds could be potential therapeutic agents for the treatment of LIMOC2 patients. Notably, two drug candidates have been previously reported in either experimental or clinical studies, demonstrating the robustness of our prediction [74], [75], [76], [77].
4. Discussion
LUAD, a subtype of NSCLC, presents a significant treatment challenge due to its biological and prognostic heterogeneity. Immunotherapy with PD-1/PD-L1 and CTLA-4 checkpoint inhibitors has shown limited success, highlighting the need for more effective treatment options. mRNA vaccines have emerged as a promising therapeutic strategy in cancer treatment [6], [7], [8]. Identifying suitable target antigens is crucial for developing successful prophylactic and therapeutic LUAD vaccines.
This study employed a multimodal analysis approach to construct the LIMOC system, resulting in the identification of three novel immune subtypes. Subsequently, scRNA-seq data analysis was used to validate these findings. Further investigation into intercellular communication revealed that all three LIMOC subtypes could interact with myeloid cells, NK cells, and fibroblasts. Certain pairs, such as MDK-(ITGA4 +ITGB1), MDK-NCL, MIF-(CD74 +CD44), and MIF-(CD74 +CXCR4), demonstrated significant regulation by the LIMOC system. Additionally, a landscape of LUAD somatic mutations, aberrantly expressed genes, and amplified genes, was constructed. This analysis led to the identification of numerous candidate antigen targets, with CHIT1, LILRA4, and MEP1A emerging as the most promising candidates for mRNA vaccine development. Furthermore, analysis of a LUAD tissue microarray revealed that CHIT1, LILRA4, and MEP1A expression was significantly higher in LUAD tissues compared to adjacent normal tissues. Functional studies demonstrated that the knockdown of CHIT1, LILRA4, and MEP1A could suppress the proliferation of the A549 LUAD cell line. Moreover, a substantial body of evidence supports the potential of these three tumor antigens as targets for mRNA vaccine development. First, CHIT1, a member of the 18-glycosyl hydrolase (GH) family, is the predominant chitinase found in human circulation and lungs [78]. This gene is expressed in various myeloid cells, including lung macrophages, mature monocyte-derived macrophages, neutrophils, and specific subpopulations of epithelial cells and tissue macrophages in both the intestine and lung. Notably, CHIT1 expression and activity are strongly correlated with the activation and differentiation state of macrophages [62]. Second, the LILRA4, also known as immunoglobulin-like transcript 7 (ILT-7) and CD85g, belongs to the leukocyte immunoglobulin-like receptor gene family. It functions as a surface receptor expressed exclusively on plasmacytoid dendritic cells (pDCs) [79], [80]. In a resting state, LILRA4 acts as a negative regulatory receptor on pDCs. However, upon stimulation by bacteria or viruses, it becomes significantly downregulated [81]. Additionally, LILRA4 cooperates with a signal adapter to activate signaling pathways regulated by the immunoreceptor tyrosine-based activation motif (ITAM) [82]. Notably, mutations in LILRA4 have been identified as associated with NSCLC metastasis, suggesting its potential as a biomarker and therapeutic target for NSCLC [83]. Third, MEP1A, encoding the metalloprotease Meprin α, belongs to the metzincin superfamily [84]. This gene exhibits abnormal expression in hepatocellular carcinoma (HCC) and acts as an independent prognostic factor, influencing both overall survival and recurrence rates in HCC patients. Additionally, MEP1A can regulate cytoskeletal events, induce epithelial-mesenchymal transition (EMT) in HCC cells, and enhance their invasive properties through pathways like the ERK/ZEB1 pathway [85]. Furthermore, MEP1A expression holds promise as a valuable biomarker for predicting disease outcomes, particularly for early-stage HCC patients. Functional enrichment analysis revealed distinct immune-related pathways associated with each candidate antigen. CHIT1 displayed strong enrichment in pathways like "Th17 cell differentiation", "cytokine-cytokine receptor interaction", and "chemokine signaling pathway". LILRA4 enrichment included "regulation of immune effector process", "positive regulation of cytokine production", and "T cell activation". MEP1A analysis yielded similar results, with enrichment in pathways like "cytokine-cytokine receptor interaction" and "B cell receptor signaling pathway". Interestingly, the LIMOC2 subtype exhibited an immune-activated phenotype with a low stromal percentage and TGF-β response score, potentially making it more susceptible to immune checkpoint inhibitors. Additionally, the low expression of ICPs and the elevated expression of ICD modulators in LIMOC2 further suggest its suitability for mRNA vaccine therapy. Taken together, these findings suggest that LIMOC2 patients may benefit most from mRNA vaccination targeting CHIT1, LILRA4, and MEP1A.
In conjunction with identifying candidate vaccine targets, we developed a novel in silico screening strategy to discover potential therapeutic drugs specifically for LIMOC2 patients, who exhibit the poorest clinical outcomes. This strategy involved an initial screen that leveraged data from hundreds of cancer cell lines to identify candidate drugs effective against LIMOC2. To further refine the candidates and maximize their therapeutic potential for LUAD, we additionally performed literature reviews and CMap analysis. This comprehensive approach ultimately led to the identification of two promising compounds: Sorafenib and Azacitidine. Interestingly, both Sorafenib and Azacitidine have documented immunomodulatory functions. Prior studies have shown that Sorafenib can enhance anti-tumor immunity in renal cell carcinoma (RCC) patients by reducing the population of CD4+CD25+FoxP3+ regulatory T cells (Tregs), independent of effects on peripheral effector T cells [86]. However, Sorafenib has also been found to decrease T cell priming, DC migration, and maturation, potentially inhibiting DC function [87]. Azacitidine, on the other hand, has demonstrated efficacy against tumors like human leukemia and lung cancer in preclinical and clinical models. It exhibits the potential to not only restore hematopoiesis but also reverse immune dysfunction in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Furthermore, studies have shown that Azacitidine can regulate CD4+ T cell differentiation in high-risk MDS patients, both in vitro and in vivo [88]. Collectively, the immunomodulatory properties of these two drugs indirectly support the validity of our computational approach and the reliability of our predicted results. Therefore, a combination therapy approach that incorporates an mRNA-based cancer vaccine alongside these drugs may offer a more favorable treatment strategy for LIMOC2 patients.
5. Conclusions
Overall, this study identified CHIT1, LILRA4, and MEP1A as promising novel tumor antigens for the development of mRNA vaccines against LUAD. Furthermore, we found that LIMOC2 patients, a distinct immune subtype, represent ideal candidates for immunotherapy with these mRNA vaccines. Additionally, we implemented a novel in silico screening strategy to discover potential therapeutic drugs specifically tailored to the LIMOC2 subtype. Collectively, our research offers a comprehensive approach for developing personalized medicine strategies for LUAD patients. This includes the development of mRNA vaccines targeting novel tumor antigens and the identification of subtype-specific therapeutic agents. These findings contribute valuable insights to the field of precision oncology and hold promise for improved patient outcomes.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC3502000), the National Natural Science Foundation of China (82004003, 82141203), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202004), the “Chen Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation, Shanghai Frontiers Science Center of TCM Chemical Biology, Shanghai Municipal Health Commission Project (20204Y0326), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY(2021-2023)-0401], and Wild Goose Array Project, Zhengzhou Center of PLAJLSF.
CRediT authorship contribution statement
Mengting Luo: Writing – review & editing, Writing – original draft, Resources, Data curation, Conceptualization. Xuyang Liao: Visualization, Methodology, Data curation, Conceptualization. Lijun Zhang: Methodology, Data curation, Conceptualization. Jienan Zhang: Resources, Data curation, Conceptualization. Jinbo Zhang: Methodology, Conceptualization. Yanan Li: Resources, Methodology, Data curation, Conceptualization. Jiangjiang Qin: Visualization, Data curation, Conceptualization. Xin Luan: Methodology, Data curation, Conceptualization. Weidong Zhang: Supervision, Project administration, Methodology, Funding acquisition. Saisai Tian: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We thank TCGA and GEO for using their data to enrich our analysis, the Home for Researchers editorial team (www.home-for-researchers.com) for the English check of this manuscript, and the biorender.com for drawing a workflow. In addition, we also thank Xiaoqi Wu (Genergy Biotechnology Shanghai Co., Ltd) for his help in bioinformatics analysis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2024.04.056.
Contributor Information
Saisai Tian, Email: saisai_tian@foxmail.com.
Xin Luan, Email: luanxin@shutcm.edu.cn.
Weidong Zhang, Email: wdzhangy@hotmail.com.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Shi J., Hua X., Zhu B., Ravichandran S., Wang M., Nguyen C., et al. Somatic genomics and clinical features of lung adenocarcinoma: a retrospective study. Plos Med. 2016;13(12) doi: 10.1371/journal.pmed.1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J., Han X., Lin L., Chen J., Wang F., Ding Q., et al. Unraveling the expression patterns of immune checkpoints identifies new subtypes and emerging therapeutic indicators in lung adenocarcinoma. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/3583985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song P., Li W., Wu X., Qian Z., Ying J., Gao S., et al. Integrated analysis of single-cell and bulk rna-sequencing identifies a signature based on b cell marker genes to predict prognosis and immunotherapy response in lung adenocarcinoma. Cancer Immunol Immunother. 2022;71(10):2341–2354. doi: 10.1007/s00262-022-03143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Li Z., Cheang I., Li J., Zhou C. Rna-binding protein igf2bp1 associated with prognosis and immunotherapy response in lung adenocarcinoma. Front Genet. 2022;13 doi: 10.3389/fgene.2022.777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng X., Xu H., Yi X., Zhang T., Wei Q., Li H., et al. Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mrna vaccine. Mol Cancer. 2021;20(1):160. doi: 10.1186/s12943-021-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang L., Xu Z., Miao L., Huang L. Mrna vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol Ther. 2018;26(2):420–434. doi: 10.1016/j.ymthe.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rausch S., Schwentner C., Stenzl A., Bedke J. Mrna vaccine cv9103 and cv9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10(11):3146–3152. doi: 10.4161/hv.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Wang Y., Miao L., Liu Q., Musetti S., Li J., et al. Combination immunotherapy of muc1 mrna nano-vaccine and ctla-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebastian M., Papachristofilou A., Weiss C., Fruh M., Cathomas R., Hilbe W., et al. Phase ib study evaluating a self-adjuvanted mrna cancer vaccine (rnactive(r)) combined with local radiation as consolidation and maintenance treatment for patients with stage iv non-small cell lung cancer. Bmc Cancer. 2014;14:748. doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubler H., Scheel B., Gnad-Vogt U., Miller K., Schultze-Seemann W., Vom D.F., et al. Self-adjuvanted mrna vaccination in advanced prostate cancer patients: a first-in-man phase i/iia study. J Immunother Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomczak K., Czerwinska P., Wiznerowicz M. The cancer genome atlas (tcga): an immeasurable source of knowledge. Conte Oncol (Pozn) 2015;19(1A) doi: 10.5114/wo.2014.47136. A68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., et al. The genotype-tissue expression (gtex) project. Nat Genet. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya S., Dunn P., Thomas C.G., Smith B., Schaefer H., Chen J., et al. Immport, toward repurposing of open access immunological assay data for translational and clinical research. Sci Data. 2018;5 doi: 10.1038/sdata.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breuer K., Foroushani A.K., Laird M.R., Chen C., Sribnaia A., Lo R., et al. Innatedb: systems biology of innate immunity and beyond--recent updates and continuing curation. Nucleic Acids Res. 2013;41(Database issue):D1228–D1233. doi: 10.1093/nar/gks1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S., Liu X. The ucscxenatools r package: a toolkit for accessing genomics data from ucsc xena platform, from cancer multi-omics to single-cell rna-seq. J Open Source Softw. 2019;4(40):1627. doi: 10.21105/joss.01627. [DOI] [Google Scholar]
- 16.Lai W., Zhu W., Li X., Han Y., Wang Y., Leng Q., et al. Gtse1 promotes prostate cancer cell proliferation via the sp1/foxm1 signaling pathway. Lab Invest. 2021;101(5):554–563. doi: 10.1038/s41374-020-00510-4. [DOI] [PubMed] [Google Scholar]
- 17.Colaprico A., Silva T.C., Olsen C., Garofano L., Cava C., Garolini D., et al. Tcgabiolinks: an r/bioconductor package for integrative analysis of tcga data. Nucleic Acids Res. 2016;44(8) doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Lichtenberg T., Hoadley K.A., Poisson L.M., Lazar A.J., Cherniack A.D., et al. An integrated tcga pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400–416. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou J., Aerts J., den Hamer B., van Ijcken W., den Bakker M., Riegman P., et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. Plos One. 2010;5(4) doi: 10.1371/journal.pone.0010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rousseaux S., Debernardi A., Jacquiau B., Vitte A., Vesin A., Nagy-Mignotte H., et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 2013;5(186) doi: 10.1126/scitranslmed.3005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botling J., Edlund K., Lohr M., Hellwig B., Holmberg L., Lambe M., et al. Biomarker discovery in non-small cell lung cancer: integrating gene expression profiling, meta-analysis, and tissue microarray validation. Clin Cancer Res. 2013;19(1):194–204. doi: 10.1158/1078-0432.CCR-12-1139. [DOI] [PubMed] [Google Scholar]
- 22.Der S.D., Sykes J., Pintilie M., Zhu C.Q., Strumpf D., Liu N., et al. Validation of a histology-independent prognostic gene signature for early-stage, non-small-cell lung cancer including stage ia patients. J Thorac Oncol. 2014;9(1):59–64. doi: 10.1097/JTO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 23.Schabath M.B., Welsh E.A., Fulp W.J., Chen L., Teer J.K., Thompson Z.J., et al. Differential association of stk11 and tp53 with kras mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene. 2016;35(24):3209–3216. doi: 10.1038/onc.2015.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis S., Meltzer P.S. Geoquery: a bridge between the gene expression omnibus (geo) and bioconductor. Bioinformatics. 2007;23(14):1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 25.Leek J.T., Johnson W.E., Parker H.S., Jaffe A.E., Storey J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B., Mezlini A.M., Demir F., Fiume M., Tu Z., Brudno M., et al. Similarity network fusion for aggregating data types on a genomic scale. Nat Methods. 2014;11(3):333–337. doi: 10.1038/nmeth.2810. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen H., Shrestha S., Draghici S., Nguyen T. Pinsplus: a tool for tumor subtype discovery in integrated genomic data. Bioinformatics. 2019;35(16):2843–2846. doi: 10.1093/bioinformatics/bty1049. [DOI] [PubMed] [Google Scholar]
- 28.Rappoport N., Shamir R. Nemo: cancer subtyping by integration of partial multi-omic data. Bioinformatics. 2019;35(18):3348–3356. doi: 10.1093/bioinformatics/btz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoadley K.A., Yau C., Wolf D.M., Cherniack A.D., Tamborero D., Ng S., et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158(4):929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monti S., Tamayo P., Mesirov J., Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52:91–118. [Google Scholar]
- 31.Ramazzotti D., Lal A., Wang B., Batzoglou S., Sidow A. Multi-omic tumor data reveal diversity of molecular mechanisms that correlate with survival. Nat Commun. 2018;9(1):4453. doi: 10.1038/s41467-018-06921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng C., Helm D., Frejno M., Kuster B. Mocluster: identifying joint patterns across multiple omics data sets. J Proteome Res. 2016;15(3):755–765. doi: 10.1021/acs.jproteome.5b00824. [DOI] [PubMed] [Google Scholar]
- 33.Hoshida Y. Nearest template prediction: a single-sample-based flexible class prediction with confidence assessment. Plos One. 2010;5(11) doi: 10.1371/journal.pone.0015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y., Yan S., Fan W., Zhang M., Liu W., Lu H., et al. Identification and validation of the immune subtypes of lung adenocarcinoma: implications for immunotherapy. Front Cell Dev Biol. 2020;8:550. doi: 10.3389/fcell.2020.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Q., Jiang X., Miao X., Zhang Y., Chen F., Zhang P. Molecular subtypes of lung adenocarcinoma patients for prognosis and therapeutic response prediction with machine learning on 13 programmed cell death patterns. J Cancer Res Clin Oncol. 2023;149(13):11351–11368. doi: 10.1007/s00432-023-05000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng L., Long F., Wang T., Dai L., Chen H., Yang Y., et al. Identification of an immune classification and prognostic genes for lung adenocarcinoma based on immune cell signatures. Front Med. 2022;9 doi: 10.3389/fmed.2022.855387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B., Huang Z., Yu W., Liu S., Zhang J., Wang Q., et al. Molecular subtypes based on cnvs related gene signatures identify candidate prognostic biomarkers in lung adenocarcinoma. Neoplasia. 2021;23(7):704–717. doi: 10.1016/j.neo.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Y.T., et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen B., Khodadoust M.S., Liu C.L., Newman A.M., Alizadeh A.A. Profiling tumor infiltrating immune cells with cibersort. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meylan M., Becht E., Sautès-Fridman C., De Reyniès A., Fridman W.H., Petitprez F. Webmcp-counter: a web interface for transcriptomics-based quantification of immune and stromal cells in heterogeneous human or murine samples. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2020. doi: 10.1101/2020.12.03.400754.
- 41.Petitprez F., Vano Y.A., Becht E., Giraldo N.A., de Reynies A., Sautes-Fridman C., et al. Transcriptomic analysis of the tumor microenvironment to guide prognosis and immunotherapies. Cancer Immunol Immunother. 2018;67(6):981–988. doi: 10.1007/s00262-017-2058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanzelmann S., Castelo R., Guinney J. Gsva: gene set variation analysis for microarray and rna-seq data. Bmc Bioinforma. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshihara K., Shahmoradgoli M., Martinez E., Vegesna R., Kim H., Torres-Garcia W., et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Vega F., Mina M., Armenia J., Chatila W.K., Luna A., La K.C., et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(2):321–337. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng J., Lu X., Jin C., Zhou Y., Ge Q., Zhou J., et al. Integrated multi-omics data reveals the molecular subtypes and guides the androgen receptor signalling inhibitor treatment of prostate cancer. Clin Transl Med. 2021;11(12) doi: 10.1002/ctm2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayakonda A., Lin D.C., Assenov Y., Plass C., Koeffler H.P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal R., Mcgranahan N., Herrero J., Taylor B.S., Swanton C. Deconstructsigs: delineating mutational processes in single tumors distinguishes dna repair deficiencies and patterns of carcinoma evolution. Genome Biol. 2016;17:31. doi: 10.1186/s13059-016-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakraborty G., Ghosh A., Nandakumar S., Armenia J., Mazzu Y.Z., Atiq M.O., et al. Fraction genome altered (fga) to regulate both cell autonomous and non-cell autonomous functions in prostate cancer and its effect on prostate cancer aggressiveness. Am Soc Clin Oncol. 2020 [Google Scholar]
- 49.Rizvi H., Sanchez-Vega F., La K., Chatila W., Jonsson P., Halpenny D., et al. Molecular determinants of response to anti-programmed cell death (pd)-1 and anti-programmed death-ligand 1 (pd-l1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson A.G., Kim J., Al-Ahmadie H., Bellmunt J., Guo G., Cherniack A.D., et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Audia J.E., Campbell R.M. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8(4) doi: 10.1101/cshperspect.a019521. a19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim N., Kim H.K., Lee K., Hong Y., Cho J.H., Choi J.W., et al. Single-cell rna sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun. 2020;11(1):2285. doi: 10.1038/s41467-020-16164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gribov A., Sill M., Lück S., Rücker F., Döhner K., Bullinger L., et al. Seurat: visual analytics for the integrated analysis of microarray data. Bmc Med Genom. 2010;3(1):6. doi: 10.1186/1755-8794-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C., et al. Inference and analysis of cell-cell communication using cellchat. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smyth G.K. Limma: linear models for microarray data. Springer. 2005:397–420. [Google Scholar]
- 56.Gel B., Serra E. Karyoploter: an r/bioconductor package to plot customizable genomes displaying arbitrary data. Bioinformatics. 2017;33(19):3088–3090. doi: 10.1093/bioinformatics/btx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu K., Cheng L., Zhu K., Wang J., Shu Q. The cancer/testis antigen hormad1 mediates epithelial-mesenchymal transition to promote tumor growth and metastasis by activating the wnt/beta-catenin signaling pathway in lung cancer. Cell Death Discov. 2022;8(1):136. doi: 10.1038/s41420-022-00946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang M., Wang L., Sun Z., Chen X., Wang H., Qin L., et al. E3 ligase trim15 facilitates non-small cell lung cancer progression through mediating keap1-nrf2 signaling pathway. Cell Commun Signal. 2022;20(1):62. doi: 10.1186/s12964-022-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., et al. A landscape of pharmacogenomic interactions in cancer. Cell. 2016;166(3):740–754. doi: 10.1016/j.cell.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees M.G., Seashore-Ludlow B., Cheah J.H., Adams D.J., Price E.V., Gill S., et al. Correlating chemical sensitivity and basal gene expression reveals mechanism of action. Nat Chem Biol. 2016;12(2):109–116. doi: 10.1038/nchembio.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corsello S.M., Nagari R.T., Spangler R.D., Rossen J., Kocak M., Bryan J.G., et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer. 2020;1(2):235–248. doi: 10.1038/s43018-019-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho S.J., Weiden M.D., Lee C.G. Chitotriosidase in the pathogenesis of inflammation, interstitial lung diseases and copd. Allergy, Asthma Immunol Res. 2015;7(1):14–21. doi: 10.4168/aair.2015.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geeleher P., Cox N., Huang R.S. Prrophetic: an r package for prediction of clinical chemotherapeutic response from tumor gene expression levels. Plos One. 2014;9(9) doi: 10.1371/journal.pone.0107468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu X., Meng J., Zhou Y., Jiang L., Yan F. Movics: an r package for multi-omics integration and visualization in cancer subtyping. Bioinformatics. 2020;36(22-23):5539–5541. doi: 10.1093/bioinformatics/btaa1018. [DOI] [PubMed] [Google Scholar]
- 65.Huang X., Tang T., Zhang G., Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mrna vaccine development. Mol Cancer. 2021;20(1):50. doi: 10.1186/s12943-021-01342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin H., Wang K., Xiong Y., Zhou L., Yang Y., Chen S., et al. Identification of tumor antigens and immune subtypes of glioblastoma for mrna vaccine development. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.773264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sha D., Jin Z., Budczies J., Kluck K., Stenzinger A., Sinicrope F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10(12):1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu X., Meng J., Su L., Jiang L., Wang H., Zhu J., et al. Multi-omics consensus ensemble refines the classification of muscle-invasive bladder cancer with stratified prognosis, tumour microenvironment and distinct sensitivity to frontline therapies. Clin Transl Med. 2021;11(12) doi: 10.1002/ctm2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branzei D., Foiani M. The checkpoint response to replication stress. Dna Repair (Amst) 2009;8(9):1038–1046. doi: 10.1016/j.dnarep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Liang J., Li H., Han J., Jiang J., Wang J., Li Y., et al. Mex3a interacts with lama2 to promote lung adenocarcinoma metastasis via pi3k/akt pathway. Cell Death Dis. 2020;11(8):614. doi: 10.1038/s41419-020-02858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jessen C., Kress J., Baluapuri A., Hufnagel A., Schmitz W., Kneitz S., et al. The transcription factor nrf2 enhances melanoma malignancy by blocking differentiation and inducing cox2 expression. Oncogene. 2020;39(44):6841–6855. doi: 10.1038/s41388-020-01477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmed A., Tait S.W.G. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006. doi: 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Awasthi N., Zhang C., Hinz S., Schwarz M.A., Schwarz R.E. Enhancing sorafenib-mediated sensitization to gemcitabine in experimental pancreatic cancer through emap ii. J Exp Clin Cancer Res. 2013;32(1):12. doi: 10.1186/1756-9966-32-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahut W.L., Scripture C., Posadas E., Jain L., Gulley J.L., Arlen P.M., et al. A phase ii clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14(1):209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 76.Rozati S., Cheng P.F., Widmer D.S., Fujii K., Levesque M.P., Dummer R. Romidepsin and azacitidine synergize in their epigenetic modulatory effects to induce apoptosis in ctcl. Clin Cancer Res. 2016;22(8):2020–2031. doi: 10.1158/1078-0432.CCR-15-1435. [DOI] [PubMed] [Google Scholar]
- 77.Grinblatt D.L., Sekeres M.A., Komrokji R.S., Swern A.S., Sullivan K.A., Narang M. Patients with myelodysplastic syndromes treated with azacitidine in clinical practice: the avida registry. Leuk Lymphoma. 2015;56(4):887–895. doi: 10.3109/10428194.2014.935366. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y., Xu D., Yao J., Wei Z., Li S., Gao X., et al. Inhibition of mir-155-5p exerts anti-fibrotic effects in silicotic mice by regulating meprin alpha. Mol Ther Nucleic Acids. 2020;19:350–360. doi: 10.1016/j.omtn.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao W., Bover L., Cho M., Wen X., Hanabuchi S., Bao M., et al. Regulation of tlr7/9 responses in plasmacytoid dendritic cells by bst2 and ilt7 receptor interaction. J Exp Med. 2009;206(7):1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho M., Ishida K., Chen J., Ohkawa J., Chen W., Namiki S., et al. Sage library screening reveals ilt7 as a specific plasmacytoid dendritic cell marker that regulates type i ifn production. Int Immunol. 2008;20(1):155–164. doi: 10.1093/intimm/dxm127. [DOI] [PubMed] [Google Scholar]
- 81.Hotter D., Sauter D., Kirchhoff F. Emerging role of the host restriction factor tetherin in viral immune sensing. J Mol Biol. 2013;425(24):4956–4964. doi: 10.1016/j.jmb.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 82.Palma G., De Laurenzi V., De Marco M., Barbieri A., Petrillo A., Turco M.C., et al. Plasmacytoids dendritic cells are a therapeutic target in anticancer immunity. Biochim Biophys Acta. 2012;1826(2):407–414. doi: 10.1016/j.bbcan.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Wu Y., Ni H., Yang D., Niu Y., Chen K., Xu J., et al. Driver and novel genes correlated with metastasis of non-small cell lung cancer: a comprehensive analysis. Pathol Res Pr. 2021;224 doi: 10.1016/j.prp.2021.153551. [DOI] [PubMed] [Google Scholar]
- 84.Sterchi E.E., Stocker W., Bond J.S. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Asp Med. 2008;29(5):309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ouyang H.Y., Xu J., Luo J., Zou R.H., Chen K., Le Y., et al. Mep1a contributes to tumor progression and predicts poor clinical outcome in human hepatocellular carcinoma. Hepatology. 2016;63(4):1227–1239. doi: 10.1002/hep.28397. [DOI] [PubMed] [Google Scholar]
- 86.Busse A., Asemissen A.M., Nonnenmacher A., Braun F., Ochsenreither S., Stather D., et al. Immunomodulatory effects of sorafenib on peripheral immune effector cells in metastatic renal cell carcinoma. Eur J Cancer. 2011;47(5):690–696. doi: 10.1016/j.ejca.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Hipp M.M., Hilf N., Walter S., Werth D., Brauer K.M., Radsak M.P., et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood, J Am Soc Hematol. 2008;111(12):5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 88.Fozza C., Corda G., Barraqueddu F., Virdis P., Contini S., Galleu A., et al. Azacitidine improves the t-cell repertoire in patients with myelodysplastic syndromes and acute myeloid leukemia with multilineage dysplasia. Leuk Res. 2015;39(9):957–963. doi: 10.1016/j.leukres.2015.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.