Abstract
We have previously found that genes of the CfrBI restriction–modification (R-M) system from Citrobacter freundii are oriented divergently and that their promoter regions overlap. The overlapping promoters suggest regulation of gene expression at the transcriptional level. In this study the transcription regulation of CfrBI R-M genes was analyzed in vivo and in vitro in Escherichia coli. It was shown that in the presence of CfrBI methyltransferase (M·CfrBI), cell galactokinase activity decreases 10-fold when the galactokinase gene (galK) is under the control of the cfrBIM promoter and increases 20-fold when galK is under the control of the cfrBIR promoter. The CfrBI site, proven to be unique for the entire CfrBI R-M gene sequence, is located in the –35 cfrBIM promoter region and is in close vicinity of the –10 cfrBIR promoter region. A comparison of the cfrBIM and the cfrBIR promoter activities in the in vitro transcription system using methylated and unmethylated DNA fragments as templates demonstrated that the efficiency of CfrBI R-M gene transcription is regulated by enzymatic modification at the N-4-position of cytosine bases of the CfrBI site by M·CfrBI. From the results of the in vivo and in vitro experiments we suggest a new model of gene expression regulation in type II R-M systems.
INTRODUCTION
Over 3000 restriction–modification (R-M) systems in almost all the major groups of prokaryotic organisms have been found to date (1). The structural organization of a number of R-M genes which have been cloned and sequenced is diverse (2). Some R-M genes are located on plasmids (3–6) which means regulation of R-M genes is essential, not only to prevent host DNA autorestriction but also for R-M systems entering a new host. Although the existence of R-M gene regulation is quite evident, the mechanism underlying such regulation remains almost completely unknown. It has been shown that the regulation of the PvuII (7) and BamHI (8,9) R-M systems is performed by small proteins, whose genes are located in the intergenic region of the genes for restriction endonuclease (ENase) and methyltransferase (MTase). Apart from the activator protein PvuIIC (7) of the ENase gene, the gene pvuIIW was also described (10,11), and their function is the subject of intensive study. Some m5-MTases such as M·EcoRII (12,13), M·SsoII (14) and M·MspI (15), beside MTase activity, display an additional repressor activity localized at the N-terminus. The N-terminal parts of these protein molecules contain the so-called ‘helix–turn–helix’ (HTH) motif typical of prokaryotic DNA binding proteins. In these cases the R-M system regulation mechanism is based on the specific MTase interaction with its own promoter, i.e. the expression of the respective MTase is autoregulated at the transcriptional level. Thus, the mechanism of control of R-M gene expression differs from system to system.
This paper is devoted to regulation of the CfrBI R-M system. It presents evidence that the CfrBI site located in the cfrBIR–cfrBIM intergenic region plays a key role in the regulation of expression of these genes.
MATERIALS AND METHODS
Construction of the pFD51 derivatives carrying various parts of the cfrBIR and cfrBIM promoter sequences
Plasmid pBGM5 (16) carrying the total nucleotide sequence of the CfrBI R-M system (DDBJ/EMBL/GenBank accession no. X57945) was used as a template for PCR synthesis of DNA fragments containing the intergenic region. Plasmid variants of pFD51 (17) where the galactokinase gene (galK) was under the control of the cfrBIM promoter were referred to as pMet with an appropriate number. Similarly, plasmids with various parts of the cfrBIR promoter were named as pRes. The nucleotide sequences of the primers used to clone DNA fragments containing the intergenic region follow (the CfrBI site is underlined and nucleotide substitutions are shown in lower case).
pMet3: 5′-CCCAAGCTTGATCTGTTACCATACAAC-3′,
5′-CGGGATCCCCATGGACATAGTAAAAATG-3′;
pMet4: 5′-CCCAAGCTTAACCTGCTATCTTAGC-3′,
5′-CGGGATCCCCATGGACATAGTAAAAA-3′;
pMet6: 5′-CCCAAGCTTCTTAGCATCTCATTTTT-3′,
5′-CGGGATCCCCATGGACATAG-3′;
pMet11: 5′-CCCAAGCTTGATCTGTTACCATACAAC-3′,
5′-CGGGATCCtCATGGACATAGTAAAAATG-3′;
pMet15: 5′-CCCAAGCTTGATCTGTTACCATACAAC-3′,
5′-CGGGATCCCCATGGtaATAGTAAAAATG-3′;
pRes7: 5′-CGGGATCCGATCTGTTACCATACAAC-3′,
5′-CCCAAGCTTCCATGGACATAGTAAAAATG-3′;
pRes8: 5′-CGGGATCCAACCTGCTATCTTAG-3′,
5′-CCCAAGCTTCCATGGACATAGTAAAAATG-3′;
pRes9: 5′-CGGGATCCGATCTGTTACCATACAAC-3′,
5′-CCCAAGCTTCCATGGtaATAGTAAAAATG-3′;
pRes14: 5′-CGGGATCCAACCTGCTATCTTAG-3′,
5′-CCCAAGCTTtCATGGACATAGTAAAAATG-3′.
The nucleotides were substituted using PCR-dependent site-directed mutagenesis. The BamHI and HindIII sites were introduced into the primer sequences. The conditions for PCR reactions (30 cycles) were: 94°C for 30 s, 55°C for 30 s and 72°C for 25 s. The amplified DNA fragments were subcloned into the pFD51 plasmid at the BamHI and HindIII sites in both orientations. The pMet23 and pRes27 plasmids were derived from the pMet3 and pRes7 plasmids, respectively, which were hydrolyzed at the CfrBI site and their protruding ends were filled in with Klenow fragment. The plasmid pXB4 carrying the native M·CfrBI gene was constructed from plasmid pBGM5. A 1.25 kb HaeII-d fragment of pHSG415 (18) with the chloramphenicol acetyltransferase gene (cat) was inserted into the MluI site of pBGM5 and then a 0.3 kb BglII–XbaI fragment was deleted. The resulting recombinant DNAs were analyzed by restriction mapping and the cloned DNA sequences were tested as described (19). The isolation of plasmid DNA, restriction analysis and gene cloning were performed as described (20).
Determination of galactokinase activity
The Escherichia coli HB101 and HB101/pXB4 strains were transformed by plasmids of the pMet and pRes series. Expression of galK was estimated either visually from colony color on MacConkey plates containing 1% galactose or directly by determination of galactokinase activity as nmol of galactose phosphorylated per min per ml of cell suspension at OD650 = 1 (17).
Formation of the promoter–protein complex
To study promoter–protein binding, 0.2 µg of the purified M·CfrBI (containing an N-terminal His6 sequence as an affinity tag) was incubated with DNA fragments (1 µg) in 10 µl buffer (50 mM Tris–HCl pH 7.5, 7 mM β-mercaptoethanol, 150 mM NaCl). The reaction mixture was applied on 6% polyacrylamide gel after 20 min incubation at room temperature.
In vitro transcription
In vitro transcription experiments were carried out as described (21). The purified 50 ng DNA fragment was incubated with E.coli RNA polymerase (100 nM) in 20 mM Tris–HCl (pH 8.0) containing 100 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 50 µg BSA and 5% glycerol in a 20 µl reaction vol for 20 min at 37°C. A 2 µl mixture of four ribonucleotide triphosphates (500 µM UTP plus 2 mM each of ATP, GTP and CTP) containing 0.5 µCi [α-32P]UTP and 20 µg heparin was then added and incubation continued for another 10 min. The reaction was terminated with 200 µl cold stop solution (10 mM EDTA and 1 µg tRNA). The resulting RNA transcripts were phenol/chloroform extracted and ethanol precipitated. The pellets were redissolved in 10 µl formamide loading buffer, heated at 90°C for 5 min and the RNA fragments were analyzed by 7% PAGE under denaturating conditions using urea.
RESULTS AND DISCUSSION
Determination of the minimal DNA region with the functionally active cfrBIM gene promoter
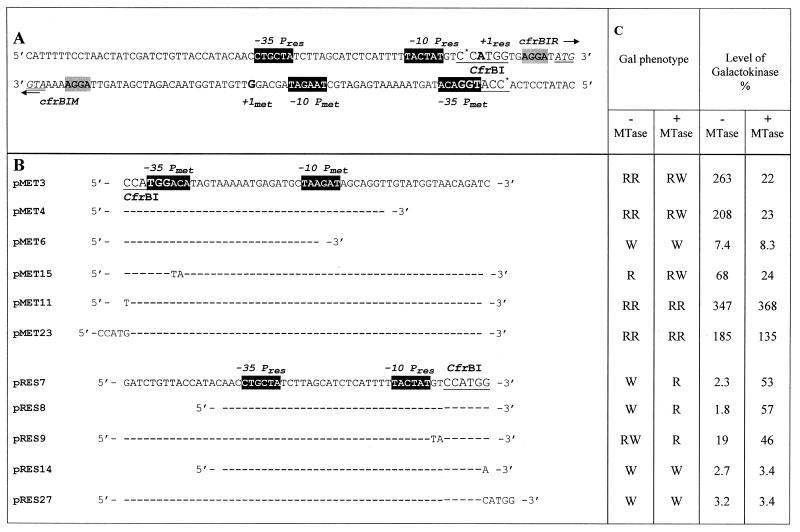
The cfrBIR and cfrBIM genes were previously shown to be located on opposite DNA strands and separated by 76 bp (16). Analysis of the intergenic region yielded identification of nucleotide sequences for both genes which were typical of bacterial promoters (Table 1A). To test the theoretically proposed boundaries of the gene promoters, the PCR-constructed DNA fragments representing the varying in length intergenic region were subcloned into the specific plasmid pFD51. The transcription efficiency was determined from galactokinase activity in cells harboring recombinant plasmids. The 40 bp DNA fragment (pMet4) covering the consensus –35 and –10 cfrBIM promoter sequences provided a level of galactokinase synthesis almost as high as that produced by the plasmid pMet3 containing a 55 bp DNA fragment of the intergenic region (Table 1B and C). It should be mentioned that the 55 bp fragment includes the intergenic region but lacks the Shine–Dalgarno (SD) sequences of both genes. The plasmid pMet6 contains a DNA fragment with a partially deleted –10 promoter region of cfrBIM. The galactokinase activity in the pMet6-harboring cells was about 35 times lower than that in cells containing plasmids with the intact promoter region (Table 1B and C). These results show that the 40 bp DNA fragment includes the functionally active cfrBIM promoter, which is 2–2.5 times more efficient than the well studied lacUV5 promoter (PlacUV5). It is noteworthy that the maximal galactokinase activity for the pRes-harboring cells (i.e. for cells transformed by pRes7 or pRes8) was only ∼2% of the galactokinase activity determined in the cells harboring plasmids with the cfrBIM promoter (Table 1B and C). In this case the level of galactokinase activity was comparable to that observed in the pFD51-harboring cells (data not shown).
Table 1. Effect of M.CfrBI on the cfrBIM and cfrBIR gene promoter activities in the in vivo model system.
(A) Nucleotide sequence of the CfrBI R-M intergenic region (16). Black boxes with light letters, the –35 and –10 promoter regions; indices res and met, elements belonging to cfrBIM and cfrBIR promoters, respectively; arrows, direction of transcription from these promoters; underlined text in larger typeface, the enzyme recognition site; *, methylated cytosine residues; double underlined text, ATG codons for each R-M gene; +1 (bold letters A and G), transcription start sites; grey shaded boxes, SD sequences.
(B) Plasmid constructs utilized to study CfrBI R-M gene promoter activities. DNA fragments of various lengths containing the intergenic region were cloned in both orientations into plasmid pFD51 at the BamHI and HindIII sites. The resulting plasmids with galK controlled by the cfrBIM promoter are referred to as pMet. Plasmids with various parts of the cfrBIR promoter are referred to as pRes. Only mutated bases in plasmids pMet11, pMet15, pMet23, pRes9, pRes14 and pRes 27 are shown.
(C) The E.coli strains HB101 (–MTase) and HB101/pXB4 (+MTase) were transformed by pMet and pRes plasmids. The colony color on the MacConkey agar medium containing 1% galactose (Gal phenotype) was visually differentiated as bright red (RR), red (R), pale red (RW) and white (W), respectively. The galactokinase activity was expressed as a percentage of the activity determined for cells of the E.coli strain HB101/pFD100 (PlacUV5), which was 70 U (17). Each value represents the average of the determinations done in triplicate.
In vivo study of CfrBI R-M gene regulation
To study the MTase effect on transcription efficiency (shown previously for the EcoRII and SsoII R-M systems), the transcription experiments were carried out in the presence of M·CfrBI whose gene was cloned into a pFD51-compatible plasmid providing for constitutive synthesis of M·CfrBI. The introduction of such a plasmid (pXB4) into the cell was accompanied by an increase in galactokinase synthesis of ∼20-fold for the pRes7- and pRes8-harboring cells and a >10-fold decrease for the cells harboring pMet3 and pMet4 (Table 1B and C). This suggests that M·CfrBI is a regulatory protein that decreases the level of cfrBIM gene expression and increases the level of cfrBIR expression.
The galactokinase activity measurements for pRes8-harboring cells confirmed the theoretically proposed boundaries of the cfrBIR promoter (Table 1B and C). The results show that the maximal activities of the cfrBIM and cfrBIR promoters are in a 5:1 ratio. This ratio is in line with the coincidence of the cfrBIM and cfrBIR promoter sequences with the consensus sequences of bacterial promoters.
From the assumption that M·CfrBI acts as an autorepressor, the deletion of the putative operator sequence would rule out the possibility of M·CfrBI binding and, consequently, the repression of its own synthesis. Galactokinase activity in strains containing the plasmid pMet4, which lacks the major part of the putative operator sequence, differs only slightly from that found for pMet3 (Table 1B and C). Further, gel shift experiments on M·CfrBI binding to methylated and unmethylated DNA fragments containing the intergenic region revealed no specific DNA–protein complexes for any DNA fragments (data not shown). This suggests that M·CfrBI is incapable of specific binding to the regulatory region of its own gene. Thus, the above data present clear evidence for distinction of regulation of gene expression in the CfrBI R-M system from that described for the EcoRII, SsoII and MspI R-M systems where MTase acts as a repressor of its own synthesis.
Efficiency of transcription from the cfrBIR and cfrBIM gene promoters depends on cytosine methylation at the CfrBI site
A peculiar feature of the CfrBI R-M intergenic region is the existence of the enzyme recognition site which is unique for the entire gene sequence. The CfrBI site is located in the –35 cfrBIM promoter region and is in close vicinity to that of the –10 cfrBIR (Table 1A). Specifically, 3 nt (TGG) of the CfrBI site (5′-CCATGG-3′) belong to the –35 cfrBIM promoter region (5′-TGGACA-3′). Further, the CfrBI site is separated from the –10 cfrBIR promoter region by only 2 nt. Two sets of experiments have been carried out to test the possible role of this site in cfrBIM and cfrBIR expression regulation.
The first set of experiments was designed to study promoter activity in the presence of M·CfrBI under conditions which ruled out DNA modification at the CfrBI site. The latter necessitated changes in the CfrBI DNA sequence without affecting the functionally active promoter-essential regions. Thus, the CfrBI site sequence (5′-CCATGG-3′) was replaced by 5′-TCATGG-3′. The C→T substitution affected neither the –35 cfrBIM promoter region nor that of the –10 cfrBIR. The plasmids containing the mutated CfrBI site were referred to as pMet11 and pRes14 (Table 1B). This substitution caused no marked changes in R-M gene promoter activities. However, these activities no longer depended on the presence of M·CfrBI (Table 1C).
Plasmids pMet23 and pRes27 derived from plasmids pMet3 and pRes7 were constructed by filling in the CfrBI site with Klenow fragment. Similar to pMet11 and pRes14 plasmids, the produced insertion mutation affected neither the –35 cfrBIM promoter region nor the –10 cfrBIR promoter regions but altered the CfrBI site sequence, thereby preventing DNA methylation by M·CfrBI. The 4 nt insertion caused only a slight decrease in cfrBIM promoter activity (pMet23) and a slight increase in cfrBIR promoter activity (pRes27) (Table 1B and C). Gene promoter activity measurements done in the presence of M·CfrBI confirmed that the regulatory function is performed not by MTase itself, but by MTase-induced DNA modification at the CfrBI site. Presumably, the absence of cytosine modification makes the stronger cfrBIM promoter preferable for the binding of RNA polymerase. This interaction could make initiation of transcription from the cfrBIR promoter more difficult. In contrast, cytosine modification at the CfrBI site promotes RNA polymerase binding to the cfrBIR promoter. In this case, efficiency of transcription from the cfrBIM promoter drastically drops and is accompanied by an increase in the activity of the cfrBIR promoter.
It should be noted that the decrease in cfrBIM promoter activity can be caused either by DNA methylation at the CfrBI site or by activity-inhibiting mutations. Thus, the second set of experiments was designed to study R-M gene promoter activities in the case where the cfrBIM promoter activity has been mutationally reduced while cytosine modification at the CfrBI site is allowed. These experiments utilized plasmids pMet15 and pRes9 (Table 1B) with the modified –35 cfrBIM promoter region and the intact –10 cfrBIR promoter region. The activity caused by the cfrBIM mutant promoter (Table 1B and C) was about three times lower than that of the intact promoter. In contrast, the activity of the cfrBIR mutant promoter, as expected, increased ∼8-fold. In these experiments the activities of both the promoters depended on the presence of a plasmid carrying the cfrBIM gene since the CfrBI site remained intact. Thus, the co-transformation of pMet15 or pRes9 with plasmid pXB4 yielded activity of the cfrBIM promoter which was still low, while activity of the cfrBIR promoter was increased (Table 1C).
In vitro transcription from the cfrBIR and cfrBIM promoters
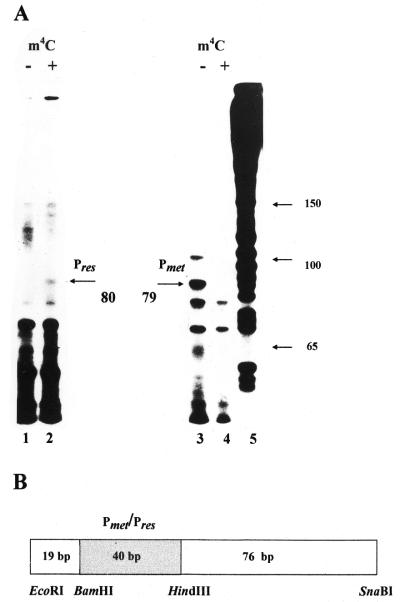
In vivo studies of the cfrBIM and cfrBIR promoter activities showed that DNA methylation at the CfrBI site had a marked effect on the efficiency of transcription from the gene promoters. In vitro experiments on transcription from the cfrBIM and cfrBIR promoters were carried out for direct studies of the effect of the methylated cytosine on the efficiency of transcription from these promoters. The 135 bp DNA fragments used as templates for E.coli RNA polymerase were isolated from plasmids pRes8 and pMet4. To obtain base-modified fragments, the respective plasmids were isolated from strains that also contained the cfrBIM-carrying plasmid. The position of the studied promoter was asymmetric relative to the fragment ends and the transcripts of interest were expected to exceed 76 nt in length (Fig. 1B). The reaction products were separated on 7% PAGE under denaturating conditions and analyzed by densitometric autoradiogram scanning. The 79 nt transcript synthesized from the cfrBIM promoter was effectively produced only when the DNA fragment with unmodified bases was used as a template (Fig. 1A, lane 3). No transcript from the cfrBIR promoter was detected when the DNA fragments with the unmodified cytosine were used (Fig. 1A, lane 1). However, the use of the DNA fragment with the methylated CfrBI site yielded a specific RNA fragment of 80 nt in length (Fig. 1A, lane 2). The transcript sizes allowed us to position the transcription start points for both genes (Table 1A). The intensity of the band corresponding to the transcript from the cfrBIM promoter was about six times higher than that of the band corresponding to the transcript produced from the cfrBIR promoter. This relationship is in good agreement with that estimated from measurements of galactokinase activity. Thus, in vitro transcription experiments provided additional evidence for the direct effect of cytosine methylation at the CfrBI site on the efficiency of transcription from R-M gene promoters.
Figure 1.
In vitro transcription from the cfrBIM and cfrBIR gene promoters. (A) The 7% PAGE autoradiogram of mRNAs produced by in vitro transcription from a 135 bp DNA fragment. In vitro transcription was performed as described (21) using E.coli RNA polymerase. The transcripts from the DNA fragment containing the cfrBIR gene promoter are presented in lanes 1 and 2 and those from the cfrBIM gene promoter in lanes 3 and 4. Transcription products in the absence of m4C DNA modification at the CfrBI site (lanes marked by a minus) and in the presence of MTase (lanes marked by a plus) were analyzed. The mRNA fragments resulting from transcription initiated by the cfrBIR and cfrBIM promoters were 80 nt (lane 2) and 79 nt (lane 3), respectively. Lane 5 represents DNA fragments of known length resulting from the sequencing reaction. (B) Schematic representation of the DNA fragment utilized for studies on in vitro transcription from the CfrBI R-M gene promoters. The 135 bp EcoRI–SnaBI DNA fragment was derived from plasmids pMet4 and pRes8. The EcoRI–BamHI (19 bp) and HindIII–SnaBI (76 bp) DNA fragments were from plasmid pFD51; the shaded BamHI–HindIII (40 bp) DNA fragment containing either the cfrBIM or cfrBIR promoter was constructed from pMet4 or pRes8, respectively.
A new model of regulation of R-M gene activity
These results clearly show that cytosine modification at the CfrBI site plays a key role in regulation of cfrBIR and cfrBIM gene expression. We suggest that this modification alters the E.coli RNA polymerase affinity for R-M gene promoters and, consequently, the expression levels of these genes. In the absence of cytosine methylation at the CfrBI site, transcription of the cfrBIM gene is more effective than that of the cfrBIR gene. Accumulation of M·CfrBI results in modification of cell DNA including the recognition site located within the intergenic region. Methylation of this site causes a drastic reduction in cfrBIM promoter activity and, in contrast, cfrBIR promoter activity becomes much higher. The highly coordinated activity of cfrBIM and cfrBIR promoters regulated by DNA modification at the CfrBI site reflects the essence of regulation of R-M system genes as genes of antagonistic proteins.
It should be noted that regulation of the CfrBI R-M system has been studied in a heterologous background. However, Escherichia and Citrobacter are both representatives of the Enterobacteriaceae family and we believe that regulation in the original strain would be the same as in E.coli, but this has yet to be confirmed.
The function of MTase in the regulation of cfrBIR and cfrBIM gene expression is clearly different from that of MTases in known R-M systems (e.g. EcoRII, SsoII and MspI) which accomplish their regulatory function as autorepressor proteins. M·CfrBI belongs to the least abundant m4C MTases, which also include M·PvuII and M·BamHI. An analysis of the CfrBI R-M gene nucleotide sequences yielded no ORF coding for a regulatory C-protein which is characteristic of the PvuII and BamHI R-M systems (10). Thus, the mechanism of regulation described for the PvuII and BamHI R-M systems (7–9) differs from that found for the CfrBI R-M system.
Numerous publications have reported involvement of DNA adenine methylation in the regulation of many important processes in bacterial cells (22,23). Thus, the dependence of transcription from a number of promoters on Dam MTase has been described (24–29). In the case of the CfrBI R-M system studied here, cfrBIM and cfrBIR gene expression depends on m4C DNA methylation at the CfrBI site which is located in the gene promoter regions. It is noteworthy that in some R-M systems the recognition sites for the coding enzymes are also located in the regulatory regions of MTase and ENase genes. Specifically, two sites for PaeR7 are located in front of paeR7M (30). In the case of FokI R-M system, two FokI sites are located in front of fokIR and another pair of FokI sites is upstream of the fokIM gene (31). However, the involvement of these sites in the regulation of gene expression for the above systems has not been reported. Presumably, the mechanism of gene regulation for the PaeR7 and FokI R-M systems may be similar to the mechanism described here for the CfrBI R-M system.
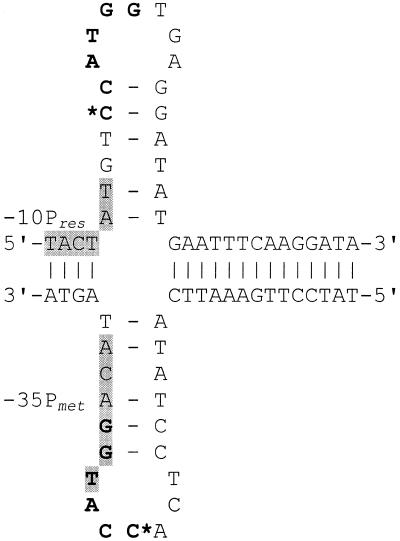
The CfrBI site is part of the inverted repeat sequence (Fig. 2). The formation of a cruciform structure suggested for such a sequence may be important for the binding of RNA polymerase to the cfrBIR promoter. In the absence of cytosine methylation at the CfrBI site, this structure may act as a potential terminator of cfrBIR transcription. It was shown that the methylation of adenine within the inverted repeats results in a lowered local stability of the cruciform structure, because the methylation of adenine at N6 directly perturbs the amine participation in hydrogen bonding to thymine (32). The methylation of cytosine by MTase CfrBI at N4 also affects the exocyclic amine forming hydrogen bonds with guanine and this should lead to lower stability of the CG base pair. So, the proposed cruciform structure may be considered as a possible additional regulator of gene expression control. Thus, the CfrBI R-M system is a promising model for further studies of gene expression control.
Figure 2.
A putative cruciform structure of the CfrBI-involving DNA sequence. The CfrBI site is shown in bold. *, cytosine undergoing m4-modification; shaded boxes, the –35 cfrBIM and –10 cfrBIR promoter regions. The PC/Gene (IntelliGenetics, Mountain View, CA) package of programs was used to analyze the nucleotide sequences.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Olga Ozoline (Institute of Cell Biophysics RAS, Pushchino) for kindly providing E.coli RNA polymerase and fruitful discussions. We are indebted to Dr Michael Sinev for helpful discussion and critical reading of the manuscript. This research was supported by the Russian Foundation for Basic Research (Project 98-04-49109).
REFERENCES
- 1.Roberts R.J. and Macelis,D. (1999) Nucleic Acids Res., 27, 312–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson G.G. (1991) Nucleic Acids Res., 19, 2539–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betlach M., Hershfield,V., Chow,L., Brown,W., Goodman,H.M. and Boyer,H.W. (1976) Fed. Proc., 35, 2037–2043. [PubMed] [Google Scholar]
- 4.Mise K. and Nakajima,K. (1984) Gene, 30, 79–85. [DOI] [PubMed] [Google Scholar]
- 5.Miyahara M. and Mise,K. (1993) Gene, 129, 83–86. [DOI] [PubMed] [Google Scholar]
- 6.Zakharova M.V., Pertzev,A.V., Kravetz,A.N., Beletskaya,I.V., Shlyapnikov,M.G. and Solonin,A.S. (1998) Biochim. Biophys. Acta, 1398, 106–112. [DOI] [PubMed] [Google Scholar]
- 7.Tao T. and Blumenthal,R.M. (1992) J. Bacteriol., 174, 3395–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ives C.L., Nathan,P.D. and Brooks,J.E. (1992) J. Bacteriol., 174, 7194–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohail A., Ives,C.L. and Brooks,J.E. (1995) Gene, 157, 227–228. [DOI] [PubMed] [Google Scholar]
- 10.Tao T., Bourne,J.C. and Blumenthal,R.M. (1991) J. Bacteriol., 173, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams G.M. and Blumenthal,R.M. (1995) Gene, 157, 193–199. [DOI] [PubMed] [Google Scholar]
- 12.Som S. and Friedman,S. (1993) EMBO J., 12, 4297–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Som S. and Friedman,S. (1994) Nucleic Acids Res., 22, 5347–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karyagina A., Shilov,I., Tashlitskii,V., Khodoun,M., Vasil’ev,S., Lau,P.C.K. and Nikolskaya,I. (1997) Nucleic Acids Res., 25, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Som S. and Friedman,S. (1997) J. Bacteriol., 179, 964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakharova M.V., Kravetz,A.N., Beletskaya,I.V., Repyk,A.V. and Solonin,A.S. (1993) Gene, 129, 77–81. [DOI] [PubMed] [Google Scholar]
- 17.Rak B. and Von-Reutern,M. (1984) EMBO J., 3, 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto-Gotoh T., Franklin,F.C., Nordheim,A. and Timmis,K.N. (1981) Gene, 16, 227–235. [DOI] [PubMed] [Google Scholar]
- 19.Sanger F., Nicklen,S. and Coulson,A.R. (1977) Proc. Natl Acad. Sci. USA, 74, 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis T., Fritsch,E.F. and Sambrook,J. (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Busby S., Kolb,A. and Minchin,S. (1994) In Geoff,G. (ed.), Methods in Molecular Biology, DNA–Protein Interaction, Principles and Protocols. Kneale Humana Press Inc., Ottowa, NJ, Vol. 30, pp. 397–411.
- 22.Marinus M.G. (1987) Annu. Rev. Genet., 21, 113–131. [DOI] [PubMed] [Google Scholar]
- 23.Barras F. and Marinus,M.G. (1989) Trends Genet., 5, 139–146. [DOI] [PubMed] [Google Scholar]
- 24.Marinus M.G. (1985) Mol. Gen. Genet., 200, 185–186. [DOI] [PubMed] [Google Scholar]
- 25.Braun R.E. and Wright,A. (1986) Mol. Gen. Genet., 202, 246–250. [DOI] [PubMed] [Google Scholar]
- 26.Roberts D., Hoopes,B.C., McClure,W.R. and Kleckner,N. (1985) Cell, 43, 117–130. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik P.K., Merlin,S. and Polisky,B. (1990) J. Bacteriol., 172, 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plasterk R.H., Vollering,M., Brinkman,A. and Van de Putte,P. (1984) Cell, 36, 189–196. [DOI] [PubMed] [Google Scholar]
- 29.Bolker M. and Kahmann,R. (1989) EMBO J., 8, 2403–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theriault G., Roy,P.H., Howard,K.A., Benner,J.S., Brooks,J.E., Waters,A.F. and Gingeras,T.R. (1985) Nucleic Acids Res., 13, 8441–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Looney M.C., Moran,L.S., Jack,W.E., Feehery,G.R., Benner,J.S., Statko,B.E. and Wilson,G.G. (1989) Gene, 80, 193–208. [DOI] [PubMed] [Google Scholar]
- 32.Murchie A.I.H. and Lilley,D.M.J. (1989) J. Mol. Biol., 205, 593–602. [DOI] [PubMed] [Google Scholar]