Abstract
The Eucalyptus genus, characterized by its imposing stature and fragrant foliage, has been a source of fascination for humanity over the centuries. The focus of the present investigation was directed towards the essentials oils (EOs) of five Eucalyptus trees cultivated in Tunisia. The GC-MS analysis unveiled unique compositional profiles, a finding substantiated by both Hierarchical Clustering Analysis (HCA) and Principal Component Analysis (PCA) conducted on the leaves EOs. These analyses resulted in the formation of discrete HCA clades, delineating 23 significant components. Notably, the percentage of eucalyptol emerged as the pivotal factor demarcating the separation between three distinct groups. The statistical analysis revealed a dose-dependent relationship in both phytotoxicity evaluation and antibacterial activity. The EOs from Eucalyptus loxophleba and E. salubris exhibited the highest phytotoxicity, inhibiting radical elongation and germination of various seeds, especially Sinapis arvensis and Raphanus sativus. The antimicrobial assessment demonstrated significant inhibitory effects of the EOs on bacterial strains, with MIC values spanning from 14 to exceeding 50 mg/ml. The EOs also affected biofilm formation and cellular metabolism, displaying varied efficacy among different Eucalyptus species against some bacterial strains. The EOs exhibited selective inhibition against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-amylase, and α-glucosidase. E. campaspe EO showed the highest AChE activity, while E. loxophleba and E. salubris EOs were most potent toward α-amylase. E. loxophleba EO demonstrated notable activity against α-glucosidase. Overall, these findings provide important data about the diverse biological activities of Eucalyptus EOs, suggesting potential applications in agriculture, medicine, and pharmacy.
Keywords: Eucalyptus, Essentials oil, Antibiofilm, Phytotoxic, Cholinesterases, α-amylase, α-glucosidase
1. Introduction
The use of eucalyptus essential oils (EOs) is increasingly gaining prominence in research, sparking sustained interest due to the chemical richness and varied biological activities associated with these aromatic extracts [1,2]. Their compositions rich in monoterpenes and sesquiterpenes give these EOs a distinctive olfactory signature while offering a broad range of biological properties [3].
The interest in eucalyptus species stems from their chemical diversity, providing a myriad of industrial applications ranging from construction to paper production, honey harvesting, and rubber extraction [4]. However, recent attention has shifted towards exploring the biological potential of their EOs, paving the way for in-depth studies on their anti-inflammatory [5], antioxidant [6], antitumoral properties [7], among others.
Recently, several Eucalyptus species wereinvestigated for their potential phytotoxic potential against weed plants and crops: Danna and coworkers showed that E. gunnii EO inhibited germination of Portulaca oleracea, Raphanus sativus, and Solanum lycopersicum seeds and affect radical growth in S. lycopersicum and E. pulverulenta EO was active against germination of P. oleracea and R. sativus and radical elongation of Lepidium sativum and Lolium multiflorum [8]). Polito and colleagues demonstrated phytotoxicity of E. griffithsii, E. hemiphloia, E. lesouefii, E. longicornis, E. pyriformis, E. viminalis, and E. wandoo EOs against germination and radical elongation of R. sativus, L. multiflorum, and Sinapis arvensis seeds [9]. Instead Flores-Macías et al. studied phytotoxic potential of E. globulus essential oil against Avena fatua and Amaranthus hybridus two herbicide-resistant weeds [10]. Moreover, E. falcata, E. sideroxylon and E. citriodora EOs phytotoxicity was studied by Amri and coworkers against plants weed (S. arvensis, Phalaris canariensis) and durum wheat crop (Triticum durum) [11].
E. brevifolia, E. camaldulensis, E. extensa, E. globulus, E. lehmannii, E. leptophylla, E. patellaris, and E. woollsiana EOs were studied for their antidiabetic propriety evaluating their activity against amylase and glucosidase [[12], [13], [14]], but only in our previous study on EOs from E. gunni and E. pulverulenta, α-amylase activity was studied in relation to phytotoxic activity as enzyme involved in germination process [8].
Instead, for that concern activity against cholinesterases E. brevifolia, E. extensa, E. lehmannii, E. leptophylla, E. patellaris, and E. woollsiana EOs were studied as a possible coadjutant in treatment of Alzheimer disease [12]. Instead, only E. globulus and E. citriodora EOs were analyzed for their anti-cholinesterases activity related to their insecticide property.
Among the many intriguing properties of eucalyptus EOs, their ability to combat biofilms, notoriously resistant to antimicrobial agents, has emerged as a promising area of research [12,15]. Their chemical diversity provides an arsenal of substances that can play an important role in the fight against biofilms, opening innovative prospects in various sectors, including pharmacy, cosmetics, and agri-food.
In this context, the aim of this study was to thoroughly determine the chemical composition and enzymatic activity, phytotoxic effects, and antibacterial potential of the EOs extracted from some Tunisian Eucalyptus species, including E. amplifolia Naudin, E. bicolor A. Cunn. ex T. Mitch., E. campaspe S. Moore., E. loxophleba Benth., and E. salubris F. Muell. Phytotoxic activity was assessed on the germination and radical growth of seeds from Sinapis arvensis L., Lolium multiflorum Lam., Raphanus sativus L., and Cucumis sativus L. Moreover, the EOs were assayed for their activity against α-amylase and α-glucosidase, two key enzymes in germination process, and against cholinesterases, target of many pesticides. Lastly, the inhibitory potential in against the biofilm formation in both Gram-positive (Staphylococcus aureus subsp. aureus, Pseudomonas aeruginosa and Listeria monocytogenes) and Gram-negative bacteria (Acinetobacter baumannii and Escherichia coli) was evaluated.
2. Materials and methods
2.1. Chemical reagent
All chemical reagents used for assays were bought from Merck, Darmstadt, Germania.
2.2. Botanical resources
The plant leaves of E. amplifolia Naudin, E. bicolor A. Cunn. ex T. Mitch., E. campaspe S. Moore., E. loxophleba Benth., and E. salubris F. Muell. were collected from different arboretums as mentioned in Table 1. Plants were identified by Prof. Vincenzo De Feo. Voucher specimens (DF/725/2021 for E. amplifolia, DF/824/2021 for E. bicolor and DF/765/2021 for E. campaspe, DF/753/2021 for E. loxophleba, DF/814/2021 for E. salubris) were deposited in the Herbarium of the Pharmaceutical Botany Chair at the University of Salerno. To obtain representative samples, a selection of almost five individual trees were made for each species, and were amalgamated. Subsequently, the homogenized samples from diverse species were transferred to a greenhouse, where they underwent a controlled shade drying process until a uniform weight was attained.
Table 1.
Arboretum, Harvest period and bioclimatic condition.
| Arboretum(governorate) | Period of harvesting | Bioclimatic condition | |
|---|---|---|---|
| E. loxophleba | Hajeb Layoun (Kairouan) | April 2021 | Semi-arid upper (with moderate winters) |
| E. bicolor | Djebel Mansour (Zaghouan) | Mars 2021 | semi-arid Upper and middle |
| E. amplifolia | Mrifeg (Bizerte) | May 2022 | Upper humid |
| E. campaspe | Henchir El Naam (Siliana) | February 2021 | Semi-arid Upper and middle |
| E. salubris | El Hnaya (Sousse) | May2022 | semi-arid Lower |
2.3. Isolation and analysis of eucalyptus essential oils
Three hundred grams of each Eucalyptus species were ground in a Waring blender and then subjected to hydrodistillation, utilizing a Clevenger apparatus for 3 h, according to the European Pharmacopoeia [16]. The resulting oils were collected, dehydrated with anhydrous sodium sulfate and preserved in amber vials at 4 °C until subsequent studies.
The EO yield (w/v, %) was calculated according to the following equation:
where W0 is the dried plant weight distilled and VEO is the EO volume obtained.
A PerkinElmer Sigma-115 gas chromatograph (PerkinElmer, Waltham, MA, USA) endowed with a flame ionization detector (FID) and a data handling processor was used for analytical gas chromatography. The separation was carried out using a HP-5 MS fused-silica capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness, Agilent, Roma, Italy). Column temperature: 40 °C, with 5 min initial hold, and then to 270 °C at 2 °C/min, 270 °C (20 min); injection mode, splitless (1 μL of a 1:1000 n-hexane solution); injector and detector temperatures 250 °C and 290 °C, respectively. The analysis was also conducted using a fused silica HP Innowax polyethylene glycol capillary column (50 m, 0.20 mm i.d., 0.25 μm film thickness, Agilent, Roma, Italy). Helium was the carrier gas (1.0 ml/min).
GC/MS analyses were achieved on an Agilent 6850 Ser. II apparatus (Agilent, Roma, Italy), endowed with a fused silica DB-5 capillary column (30 m, 0.25 mm i.d., 0.33 m film thickness, Agilent, Roma, Italy), coupled to an Agilent Mass Selective Detector MSD 5973; ionization energy voltage 70 eV; electron multiplier voltage energy 2000 V. Column temperature: 40 °C, with 5 min initial hold, and then to 270 °C at 2 °C/min, 270 °C (20 min); injection mode, splitless (1 μL of a 1:1000 n-hexane solution); injector and detector temperatures 250 °C and 290 °C, respectively. Mass spectra (MS) were scanned in the range 40–500 amu, scan time 5 scans/s.
Many constituents were identified by GC by comparing their Kovats retention indices (Ri), which were calculated relative to the retention times (tR) of n-alkanes (C10–C35), to either those in the literature [[17], [18], [19]] and mass spectra on both columns, or those of single compounds available in our laboratories via the NIST 17 and Wiley libraries [20]. Peak area normalization was used to determine the component relative concentrations. No response factors were calculated.
2.4. Phytotoxic activity
Phytotoxic activity was investigated concerning the germination and radical growth of Lolium multiflorum Lam., Sinapis arvensis L., Raphanus sativus L., and Cucumis sativus L. seeds selected for their known and reliable germination characteristics.
S. arvensis seeds were collected from wild populations in Sidi Ismail, Beja, Tunisia on June 2021. L. multiflorum seeds were bought from Fratelli Ingegnoli s.p.a. (Milano, Italy), whereas R. sativus and C. sativus seeds were purchased from Blumen group s.r.l. (Bologna, Italy).
The seeds were sterilized in 95 % ethanol for 15 s and then placed in Petri dishes (Ø = 90 mm) lined with three layers of Whatman filter paper.
Following sterilization, the Petri dishes received 7 ml treatments: double distilled water (utilized as control to verify seed viability), a water–acetone mixture (99.5:0.5, v/v) as the other control (due to essential oils being dissolved in this solution owing to their lipophilicity), or essential oils at concentrations of 1000, 500, 250, and 100 μg/ml.
Control experiments exclusively utilizing water–acetone exhibited no notable distinctions compared to the water control.
Germination conditions were maintained at 20 ± 1 °C, with a natural photoperiod. Seed germination was monitored in Petri dishes every 24 h, considering a seed germinated when root became evident [21]. After 120 h for Raphanus sativus and Sinapis arvensis seeds, and after 168 h for Lolium multiflorum and Cucumis sativus seeds, radicle growth effects were measured in centimeters. Each experiment was replicated three times, employing Petri dishes containing 10 seeds each.
2.5. Anti-enzymatic activities
2.5.1. Cholinesterases inhibition
The cholinesterase activity was determined by Ellman's assay [22] with some modifications. Briefly, in a total of 1 ml, 415 μL of Tris-HCl buffer 0.1 M (pH 8), 10 μL of a buffer solution of EOs (in methanol) at different concentrations (100, 10, 1, and 0.1 mg/ml, and 25 μL of a solution containing 0.28 U/mL of AChE (or BChE) were incubated for 15 min at 37 °C. Then, a solution of acetylthiocholine iodide (AChI) or butyrylthiocholine iodide.
(BChI) 1.83 mM (75 μL) and 475 μL of 5,5 -dithiobis (2-nitrobenzoic acid) (DTNB) was added, and the final mixture was placed for 30 min at 37 °C. The absorbance was read at 405 nm in a spectrophotometer (Thermo Scientific Multiskan GO, Monza, Italy). Galantamine was used as the positive control.
2.5.2. α-Amylase inhibition assay
Amylase activity was carried out using a modified version of the Bernfeld method [23] Essential oil (EO) at varying concentrations (100 μL) was mixed with amylase (10 U/mL) and sodium phosphate buffer (pH = 6.9). Then, the solutions were placed at 37 °C for 10 min at the end soluble starch solution (1 %) was added, and placed for additional 20 min at 37 °C. The reaction was blocked with 3,5-dinitrosalicyclic acid (DNSA) solution (96 mM), and boiled at 100 °C for 10 min and then cooling with 600 μL of distilled water. The absorbance was measured at 540 nm using a UV Spectrophotometer (Thermo Fischer Scientific, Vantaa, Finland).
3. 3α-glucosidase inhibition assay
The evaluation of α-glucosidase inhibitory activity was carried out according to a previously reported method [24] with some modifications. In brief, a 96-multiwell plate was utilized, where 0.1 M phosphate buffer at pH 7.0 (150 μL) was introduced into each well. Subsequently, 10 μL of EOs, dissolved in MeOH to achieve various concentrations, were sequentially added.
The reaction started with 15 μL of α-glucosidase (1 U/mL) in each well, followed by placement at 37 °C. After 5 min, 75 μL of 4-nitrophenyl α-d-glucopyranoside substrate (2.0 mM) was added, and the plate was incubated for an additional 10 min at 37 °C. Absorbance was read at 405 nm using a UV Spectrophotometer (Thermo Fischer Scientific, Vantaa, Finland).
Acarbose served as the positive control, and the negative control recorded absorbance using phosphate buffer instead of the sample. Enzyme inhibition was calculated, with results expressed as IC50 values. The experiments were triplicated. The percentage of enzyme activity inhibition for cholinesterases, α-amylase, and α-glucosidase was determined using the formula:
A0 is the absorbance of the control without the sample, and A1 is the absorbance of the sample. The concentration providing 50 % inhibition (IC50) was obtained by plotting the inhibition percentage against sample concentrations.
3.1. Antimicrobial activity
3.1.1. Microbial strains and cultivations conditions
The cultures of bacterial strains were obtained from the Leibniz Institute DSMZ (Germany), and were Acinetobacter baumannii ATCC 19606 and Escherichia coli DSM 8579, as Gram-negative strains and Staphylococcus aureus subsp. aureus Rosebach ATCC 25923, Pseudomonas aeruginosa DSM 50071 and Listeria monocytogenes ATCC 7644 as Gram-positive strains. The cultivation was performed in Luria broth at 37 °C for 18 h, except for A. baumannii, which was cultured at 35 °C.
3.1.2. Minimum inhibitory concentration (MIC)
To maintain sterility, EOs and dimethyl sulfoxide (DMSO) were subjected to ultrafiltration before being included in the study Stock of EOs, were dissolved in sterile DMSO to achieve a concentration of 500 mg/ml. MIC determination, performed in 96-multiwell microplates, employed a modified resazurin method [25]. Final serially descending concentration of EOs (diluted in DMSO) used in this study were 50.00 (dilution factor: 10), 25.00, (dilution factor: 20) and 12.50 mg/ml (dilution factor: 40), which were used for each strain. A resazurin solution (270 mg in 40 ml sterilized deionized water) was prepared. Thirty-five μL of 3.3 × strength iso-sensitized broth, 10 μL of bacterial suspension (1.5 × 107 CFU/ml), 10 μL of resazurin solution, and diluted samples of EOs were supplemented, to reach the specific EOs concentration and a final volume/well of 250 μL with different volumes of sterile Luria Bertani broth. The initial row received iso-sensitized broth, bacterial suspension, resazurin solution, and DMSO to reach a final volume/well of 250 μL with sterile Luria Bertani broth. The plates were subsequently sealed. Incubation at 37 °C (35 °C for A. baumannii) for 24 h preceded visual observation for color changes. MIC was the lowest EO concentration preventing a color shift from dark purple to pink.
3.1.3. Biofilm inhibitory activity
To evaluate the inhibitory impact of essential oils on mature biofilm, 96-well microtiter plates with flat bottoms were employed. Bacterial cultures adjusted to a 0.5 McFarland standard [26] were dispensed into each well (10 μL), incubated for 24 h at 37 °C (35 °C for A. baumannii), and subsequently exposed to 5–10 or 20 μl/mL of EOs in a final volume of 250 μL Luria-Bertani broth. Following an additional 24-h incubation, planktonic cells were eliminated, sessile cells were fixed, stained with crystal violet, and the absorbance at λ = 540 nm was read using a Cary Varian spectrophotometer. Biofilm inhibitory activity was determined as a percentage relative to the control. Triplicate experiments were performed for reliability [27].
3.1.4. Metabolic activity evaluation using MTT colorimetric method
The metabolic activity of bacterial cells within the EOs-influenced biofilm was assessed utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method [27].
After a 24-h bacterial incubation with removal of planktonic cells, two EO concentration ranges, namely (5 and 10 μl/mL) and (10 and 20 μl/mL), were introduced. Following an additional 24-h incubation, planktonic cells were eliminated, and a solution of 150 μL phosphate-buffered saline (PBS) and 30 μL 0.3 % MTT was added. Microplates were placed for 2 h at 37 °C (35 °C for A. baumannii). Subsequently, the MTT solution was eliminated, and two washing steps with 200 μL sterile physiological solution were carried out. Finally, 200 μL of dimethyl sulfoxide (DMSO) were added to suspend the formazan crystals, and absorbance was read at λ = 570 nm using a Cary Varian spectrophotometer.
3.2. Statistical analysis
Experiments were triplicated. Phytotoxic assay data were analyzed using GraphPad Prism 6.0 (two-way ANOVA, Dunnett's multiple comparisons test, p < 0.05). Anti-enzymatic and antibacterial activities were assessed using SPSS 26 (one-way ANOVA, Tukey's post-hoc test, p < 0.05).
4. Results
4.1. Yield and chemical composition of essential oils
The EOs yields from the leaves of different Eucalyptus species are presented in Table 1, ranging from 1.47 % to 4.5 %. The order of representation, from highest to lowest, is as follows: E. salubris (4.51 %) > E. loxophleba (3.14 %) > E. campaspe (2.8 %) > E. amplifolia (1.63 %) > E. bicolor (1.47 %).
The exploration of essential oils (EOs) unveiled a varied composition among different Eucalyptus species (Table 2).
Table 2.
Percent composition of the EOs.
| E. amplifolia | E. bicolor | E. campaspe | E. loxophleba | E. salubris | KIa | KIb | Identificationc | |
|---|---|---|---|---|---|---|---|---|
| 1-Pentanol,2-ethyl- | – | – | – | 0.1 | – | 830 | 1, 2 | |
| 4-Methyl-1,6-heptadien-4-ol | – | 14.1 | – | – | – | 832 | 1, 2 | |
| α-Pinene | 4.3 | 6.4 | 7.0 | 2.9 | 1.8 | 857 | 1012 | 1, 2, 3 |
| Octane,4,5-diethyl- | – | 0.2 | – | – | – | 859 | 1, 2 | |
| Camphene | 0.4 | 0.2 | 0.3 | 0.2 | – | 868 | 1075 | 1, 2, 3 |
| Thuja-2,4(10)-diene | 0.1 | – | – | – | – | 875 | 1122 | 1, 2 |
| β-Pinene | – | 0.2 | 0.2 | 4.8 | – | 890 | 1120 | 1, 2 |
| Myrcene | – | 0.1 | – | – | – | 893 | 1166 | 1, 2 |
| α-Phellandrene | – | 0.5 | 0.1 | 0.4 | 0.1 | 918 | 1177 | 1, 2, 3 |
| α-Terpinene | – | 0.1 | – | 0.3 | 0.5 | 930 | 1170 | 1, 2, 3 |
| p-Cymene | – | – | – | 2.8 | 6.0 | 938 | 1250 | 1, 2 |
| Eucalyptol | 68.5 | 32.4 | 72.1 | 43.3 | 70.8 | 943 | 1210 | 1, 2, 3 |
| 1,3,8-p-Menthatriene | 3.3 | – | – | 0.4 | 4.8 | 948 | 1411 | 1, 2 |
| γ-Terpinene | – | 0.1 | 0.1 | 0.8 | – | 971 | 1221 | 1, 2, 3 |
| Fenchone | – | – | 0.1 | – | – | 994 | 1340 | 1, 2 |
| p-Mentha-2,4(8)-diene | – | – | – | 0.3 | – | 996 | 1639 | 1, 2 |
| 6-Camphenone | – | 0.1 | 0.1 | – | – | 998 | 1, 2 | |
| p-Cymenene | – | – | – | 0.6 | – | 999 | 1438 | 1, 2 |
| Linalool | – | – | – | 0.4 | – | 1009 | 1506 | 1, 2, 3 |
| 3-methylbutyl 3-methylbutanoate | – | 0.2 | – | 0.7 | – | 1014 | 1, 2 | |
| Thujone | – | – | – | 0.6 | – | 1018 | 1430 | 1, 2, 3 |
| exo-Fenchol | 0.7 | 0.3 | 1.0 | – | 0.4 | 1019 | 1570 | 1, 2, 3 |
| trans-p-Ment-2-en-1-ol | – | – | – | 0.5 | – | 1025 | 1, 2 | |
| 6-Campholenal | – | 0.1 | 0.2 | 0.9 | – | 1028 | 1496 | 1, 2 |
| Isogeraniol | – | – | 0.1 | – | – | 1036 | 1, 2 | |
| trans-Pinocarveol | 1.1 | 2.3 | 2.9 | 5.4 | 2.3 | 1040 | 1664 | 1, 2 |
| Camphor | – | 0.2 | 0.3 | – | – | 1044 | 1519 | 1, 2, 3 |
| neo-Isopulegol | – | 0.2 | 0.3 | – | – | 1046 | 1, 2 | |
| Sabina ketone | – | – | – | 1.0 | – | 1052 | 1651 | 1, 2 |
| Pinocarvone | 0.2 | 0.6 | 0.7 | 1.3 | – | 1065 | 1576 | 1, 2 |
| Borneol | 2.2 | 0.4 | 2.1 | 0.7 | 0.7 | 1067 | 1715 | 1, 2, 3 |
| Terpinen-4-ol | – | 0.3 | 0.6 | 3.7 | – | 1078 | 1636 | 1, 2, 3 |
| cis-Sabinene hydrate | – | – | – | – | 2.5 | 1083 | 1460 | 1, 2 |
| Cryptone | – | – | 0.3 | 7.2 | – | 1085 | 1659 | 1, 2 |
| trans-p-Mentha-1(7),8-dien-2-ol | – | 0.5 | 0.1 | – | – | 1088 | 1810 | 1, 2 |
| α-Terpineol | 0.5 | 1.0 | 3.3 | 2.2 | – | 1090 | 1662 | 1, 2, 3 |
| p-Cymen-8-ol | – | – | – | 0.9 | – | 1092 | 1828 | 1, 2 |
| Safranal | – | – | – | 1.2 | – | 1096 | 1648 | 1, 2 |
| Myrtenol | – | – | – | 1.1 | – | 1098 | 1791 | 1, 2 |
| Verbenone | – | – | 0.1 | 0.3 | – | 1100 | 1720 | 1, 2 |
| Iso-Dihydro carveol | – | – | 0.2 | 0.3 | – | 1105 | 1, 2 | |
| p-Cumic aldehyde | – | – | – | 5.1 | – | 1133 | 1753 | 1, 2 |
| Pulegone | – | – | 0.6 | 1.6 | – | 1144 | 1662 | 1, 2 |
| Phellandral | – | – | – | 2.1 | – | 1164 | 1720 | 1, 2 |
| p-Cymen-7-ol | – | – | – | 2.4 | – | 1195 | 2113 | 1, 2 |
| δ-Elemene | – | 0.1 | 0.1 | – | – | 1208 | 1479 | 1, 2 |
| Benzyl butanoate | – | 0.2 | 0.5 | – | – | 1225 | 1, 2 | |
| α-Terpinyl acetate | – | 1.0 | – | – | – | 1232 | 1685 | 1, 2 |
| Isoledene | – | 0.1 | – | – | – | 1251 | 1, 2 | |
| α-Copaene | 0.1 | 0.2 | – | – | – | 1262 | 1477 | 1, 2 |
| β-Patchoulene | 0.1 | – | – | – | – | 1271 | 1, 2 | |
| α-Panasinsene | 0.5 | 0.1 | – | – | – | 1272 | 1, 2 | |
| β-Longipinene | – | – | – | – | 0.1 | 1276 | 1, 2 | |
| Longifolene | – | – | – | – | 0.2 | 1283 | 1575 | 1, 2 |
| trans-Caryophyllene | – | 0.3 | – | – | 0.2 | 1284 | 1617 | 1, 2 |
| α-Gurjunene | 0.2 | 0.2 | – | – | – | 1288 | 1529 | 1, 2 |
| β-Gurjunene | – | 0.7 | – | – | 0.1 | 1304 | 1597 | 1, 2 |
| α-Guaiene | 0.4 | – | 0.2 | – | – | 1307 | 1600 | 1, 2 |
| Aromadendrene | – | 10.6 | – | – | 1.7 | 1308 | 1631 | 1, 2 |
| cis-Muurola-3,5-diene | 0.2 | – | – | – | 0.4 | 1311 | 1, 2 | |
| allo-Aromadendrene | 7.7 | – | 0.1 | – | 0.4 | 1312 | 1660 | 1, 2 |
| γ-Muurolene | – | – | – | – | 0.3 | 1337 | 1725 | 1, 2 |
| γ-Himachalene | – | – | – | – | 0.2 | 1339 | 1709 | 1, 2 |
| cis-β-Guaiene | 0.6 | – | – | – | – | 1349 | 1664 | 1, 2 |
| Viridiflorene | 2.6 | – | – | – | 1.2 | 1350 | 1696 | 1, 2 |
| 9-epi-β-Caryophyllene | – | 2.0 | 0.1 | – | – | 1352 | 1, 2 | |
| dehydro-Aromadendrene | – | 0.1 | – | – | – | 1366 | 1, 2 | |
| trans-β-Guaiene | 3.3 | – | – | – | – | 1371 | 1532 | 1, 2 |
| γ-Cadinene | – | – | – | – | 0.2 | 1384 | 1763 | 1, 2 |
| 7-epi-α-Selinene | 0.3 | – | – | – | – | 1389 | 1, 2 | |
| Cadina-3,9-diene | – | 0.1 | – | – | – | 1391 | 1, 2 | |
| δ-Cadinene | – | – | – | – | 1.2 | 1397 | 1756 | 1, 2 |
| trans-Cadina-1(6),4-diene | – | 0.1 | – | – | – | 1400 | 1, 2 | |
| γ-Gurjunene | – | 0.1 | – | – | – | 1410 | 1668 | 1, 2 |
| Viridiflorene | – | – | – | 0.1 | – | 1414 | 1696 | 1, 2 |
| α-Amorphene | – | 1.9 | 0.1 | – | – | 1419 | 1693 | 1, 2 |
| trans-β-Guaiene | – | 0.8 | 0.2 | – | – | 1425 | 1651 | 1, 2 |
| Germacrene B | – | 1.1 | 0.7 | 0.3 | – | 1437 | 1795 | 1, 2 |
| Caryophyllene oxide | – | – | – | 1.0 | – | 1440 | 2000 | 1, 2 |
| Epiglobulol | – | 5.6 | 1.4 | – | – | 1444 | 2100 | 1, 2 |
| α-Cadinene | – | 1.6 | 0.2 | – | – | 1450 | 1769 | 1, 2 |
| Rosifoliol | – | 1.3 | 0.2 | – | – | 1461 | 1, 2 | |
| Khusimone | – | 0.4 | – | – | – | 1474 | 1, 2 | |
| γ-Eudesmol | – | 0.8 | – | – | – | 1477 | 2176 | 1, 2 |
| 5-epi-7-epi-α-Eudesmol | – | – | 0.2 | – | – | 1480 | 1, 2 | |
| Hinesol | – | 0.9 | – | – | – | 1482 | 2228 | 1, 2 |
| β-Eudesmol | – | 2.5 | 0.4 | – | – | 1485 | 2248 | 1, 2 |
| α-Eudesmol | – | 3.0 | 0.9 | – | – | 1489 | 2247 | 1, 2 |
| Total | 97.3 | 96.3 | 98.1 | 97.9 | 96.1 | |||
| Monoterpene hydrocarbons | 8.1 | 7.6 | 7.7 | 13.5 | 13.2 | |||
| Oxygenated monoterpenes | 73.2 | 39.4 | 85.1 | 82.2 | 76.7 | |||
| Sesquiterpene hydrocarbons | 16.0 | 20.1 | 1.7 | 0.4 | 6.2 | |||
| Oxygenated sesquiterpenes | – | 14.5 | 3.1 | 1.0 | – | |||
| Others | – | 14.7 | 0.5 | 0.8 | – |
a, b: Calculated Kovats retention indices on apolar HP-5 MS and polar HP Innowax capillary columns, respectively; c: identification method—1 = comparison of Kovats retention indices with published data, 2 = comparison of mass spectra with NIST 17 and Wiley 275 libraries and published data, 3 = coinjection with authentic compounds; - = absence.
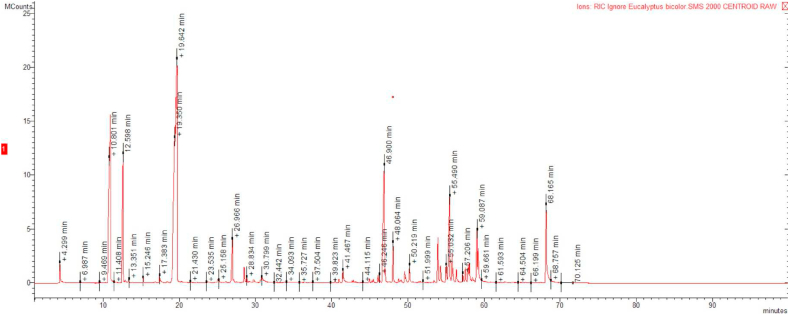
A representative GC profile of E. bicolor was present in Fig. 2. Chemical structure of identified compounds was reported in Fig. 3.
Fig. 2.
A representative gas chromatography (GC) profile of E. bicolor.
Fig. 3.
Chemical structure of identified compounds.
The analysis disclosed 21 components in E. amplifolia Naudin EO (97.3 % of the total), 39 in E. bicolor EO (96.3 %), 37 in E. campaspe EO (98,1 %), 36 in E. loxophleba EO (97,9 %) and 22 in E. salubris EO (96.1 %). Oxygenated monoterpenes predominated in all EOs: 73.2 % for E. amplifolia, 39.4 % for E. bicolor, 85.1 % for E. campaspe, 82.2 % for E. loxophleba and 76.7 % for E. salubris. The EO of E. amplifolia also contained hydrocarbon sesquiterpenes (16.0 %) and hydrocarbon monoterpenes (8.1 %). Eucalyptol (68.5 %) was the main compound while the other main components are allo-aromadendrene (7.7 %), α-pinene (4.3 %), 1,3,8-p-menthatriene, trans-β-guaiene (3.3 %), viridiflorene (2.6 %), borneol (2.2 %) and trans-pinocarveol (1.1 %). The EO of E. bicolor also contained sesquiterpene hydrocarbons (20.1 %), oxygenated sesquiterpenes (14.5 %), monoterpenes hydrocarbons (7.6 %) and other compounds (14.7 %). Eucalyptol (32.4 %) was the main compound while the other main components were 4-methyl-1,6-heptadien-4-ol (14.1 %), aromadendrene (10.6 %), α-pinene (6.4 %), epiglobulol (5.6 %), α-eudesmol (3.0 %), β-eudesmol (2.5 %), trans-pinocarveol (2.3 %), 9-epi-β-caryophyllene (2.0 %), α-amorphene (1.9 %), α-cadinene (1.6 %), rosifoliol (1.3 %), germacrene B (1.1 %), α-terpineol and α-terpinyl acetate (both 1.0 %). The EO of E. campaspe also contained monoterpene hydrocarbons (7.7 %), oxygenated sesquiterpenes (3.1 %), sesquiterpene hydrocarbons (1.7 %) and other compounds (0.5 %). Eucalyptol (72.1 %) was the main compound while the other main components were α-pinene (7.0 %), α-terpineol (3.3 %), trans-pinocarveol (2.9 %), borneol (2.1 %), epiglobulol (1.4 %) and exo-fenchol (1.0 %). The EO from E. loxophleba also contained monoterpene hydrocarbons (13.5 %), oxygenated sesquiterpenes (1.0 %), sesquiterpene hydrocarbons (0.4 %) and other compounds (0.8 %). The major component was eucalyptol (43.3 %) while the other main components were cryptone (7.2 %), trans-pinocarveol (5.4 %), p-cumic aldehyde (5.1 %), β-pinene (4.8 %), terpinen-4-ol (3.7 %), α-pinene (2.9 %), p-cymene (2.8 %), p-cymen-7-ol (2.4 %), α-terpineol (2.2 %), phellandral (2.1 %), pulegone (1.6 %), pinocarvone (1.3 %), safranal (1.2 %), myrtenol (1.1 %), sabina ketone (1.0 %) and caryophyllene oxide (1.0 %). The EO from E. salubris also contained monoterpene hydrocarbons (13.2 %) and sesquiterpene hydrocarbons (6.2 %). The major component wais eucalyptol (70.8 %) while the other main components were p-cymene (6.0 %), 1,3,8-p-menthatriene (4.8 %), cis-sabinenehydrate (2.5 %), trans-pinocarveol (2.3 %), α-pinene (1.8 %), aromadendrene (1.7 %), viridiflorene and δ-cadinene (1.2 %).
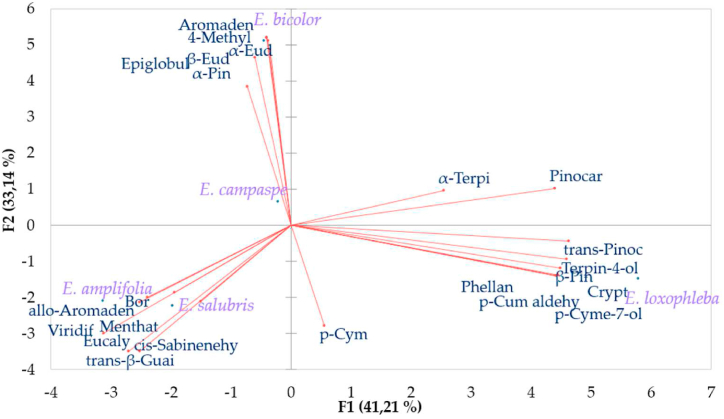
Principal Component Analysis (PCA) (Fig. 4) was applied on 23 major compounds of the chemical composition from tested species in order to process the structural and compositional descriptors of the samples.
Fig. 4.
Principal Component Analysis (PCA) results carried out with chemical composition. Projection of 23 (>1 %) major compounds of eucalyptus species in the plane formed by the two main components. Abbreviations of compound β-Pinene (β-Pin), α-pinene (α-Pin), trans-Pinocarveol (trans-Pino), Terpinen-4-ol (Terpin-4-ol), p-Cumic aldehyde (p-Cum aldehy), Viridiflorene (Viridif), p-Cymen-7-ol (p-Cyme-7-ol), Cryptone (Crypt), eucalyptol (Eucaly), β-Eudesmol (β-Eud), α-Eudesmol (α-Eud), Aromadendrene (Aromade), Epiglobulol (Epiglobul), trans-β-Guaiene (trans-β-Guai), allo-Aromadendrene (allo-Aromaden), Phellandral (Phellan), 4-Methyl-1,6-heptadien-4-ol (4-Methyl), p-Cymene (p-Cym), 1,3,8-p-Menthatriene (Menthat), Borneol (Bor), cis-Sabinenehydrate (cis-Sabinenehy), α-Terpineol (α-Terpi).
The application of Principal Component Analysis (PCA) (Fig. 1) represent the horizontal component (F1) explained 41.21 % of the variance and the second (F2) 33.14 %, with β-Pinene (β-Pin), trans-Pinocarveol (trans-Pino), Terpinen-4-ol (Terpin-4-ol), p-Cumic aldehyde (p-Cum aldehy), Viridiflorene (Viridif), p-Cymen-7-ol (p-Cyme-7-ol), Cryptone (Crypt) being the variables with the greatest weight in horizontal component F1 and eucalyptol (Eucaly); α-pinene (α-Pin); β-Eudesmol(β-Eud); α-Eudesmol (α-Eud); Aromadendrene (Aromade); Epiglobulol (Epiglobul) being those in vertical component 2.
Fig. 1.
Schematic diagram of methodology used.
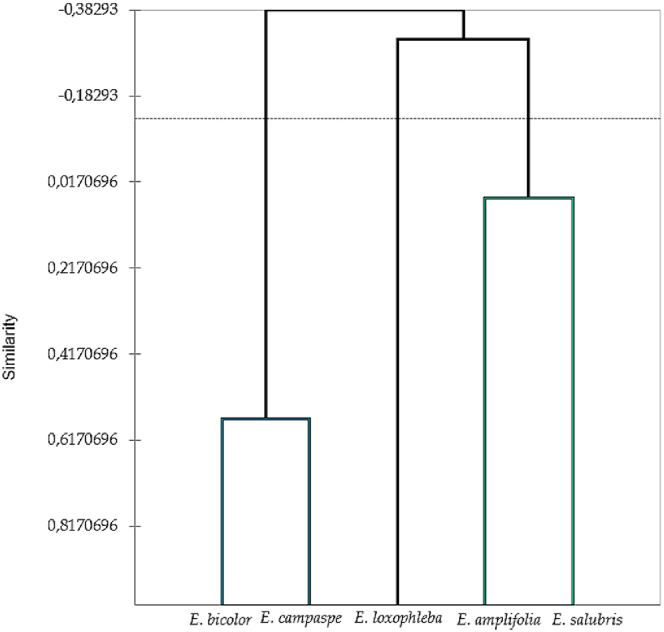
Concurrently, Hierarchical Cluster Analysis (HCA) (Fig. 5), utilizing Pearson correlation coefficients (r), delineated three distinct groups denoted as Group A, B, and C.
Fig. 5.
Hierarchical cluster analysis (HCA) of the EOs.
Group A (r = −0.332), predominantly comprising E. loxophleba, emerged prominently, establishing a distinctive dichotomy in the HCA. This group was characterized by elevated levels of cryptone and β-pinene, accompanied by a relatively low amount of eucalyptol. Simultaneously, this EO shares the presence of p-cymene with E. salubris EOs.
Group B (r = 0.567), consisting of E. salubris and E. amplifolia, was noteworthy for its highest concentration of eucalyptol.
Group C (r = 0.069) comprised E. bicolor and E. campaspae, distinguished by elevated levels of epiglobulol and aromadendrene. Intriguingly, E. campaspae exhibitrf a positive correlation with the second group, potentially attributed to the shared high level of eucalyptol.
An evident differentiation among the five eucalyptus species is discernible through the utilization of two statistical methodologies (PCA and HCA analysis). Consequently, it is deduced that these species exhibit distinctive chemical compositions, suggesting potential variations in their biological effects during subsequent testing procedures.
4.2. Phytotoxic activity
This research assessed the phytotoxic activities of the Eucalyptus EOs on the germination and radical growth of seeds from L. multiflorum, S. arvensis, R. sativus and C. sativus.
Table 3, Table 4 represented the radicle length in cm and the number of germinated seeds after treatment with the EOs.
Table 3.
Phytotoxic activity of the EOs against germination of L. multiflorum, S. arvensis, R. sativus, and C. sativus.
| Germination | ||||
|---|---|---|---|---|
| L. multiflorum | S. arvensis | R. sativus | C. sativus | |
| E. amplifolia | ||||
| CTR | 9.7 ± 0.6 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| CTR + C3H6O | 9.3 ± 0.6 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 1000 | 1.0 ± 0.0d | 0.0 ± 0.0d | 3.3 ± 0.3d | 5.3 ± 0.3d |
| 500 | 2.0 ± 0.0d | 0.0 ± 0.0d | 6.0 ± 0.0d | 7.0 ± 0.0d |
| 250 | 3.0 ± 0.0d | 2.0 ± 1.0d | 7.7 ± 0.3d | 7.7 ± 0.3d |
| 125 | 6.3 ± 0.6d | 5.7 ± 0.6d | 9.0 ± 0.0a | 9.0 ± 0.0a |
| E. bicolor | ||||
| CTR | 8.6 ± 0.6 | 9.7 ± 0.0 | 9.7 ± 0.3 | 10 ± 0.00 |
| CTR + C3H6O | 8.0 ± 0.0 | 9.6 ± 0.0 | 9 ± 0 | 10 ± 0.00 |
| 1000 | 1.3 ± 1.5b | 0.0 ± 0.0d | 2 ± 0d | 5.3 ± 0.3d |
| 500 | 5.3 ± 3.2 | 3.7 ± 0.9d | 2.0 ± 0.6d | 7.0 ± 0.0d |
| 250 | 6.0 ± 2.0 | 6.0 ± 1.0b | 3.3 ± 0.9d | 8.0 ± 0.0d |
| 125 | 9.0 ± 1.7 | 7.0 ± 0.0a | 4.7 ± 0.3d | 9.0 ± 0.0b |
| E. campaspe | ||||
| CTR | 8.7 ± 0.6 | 9.3 ± 1.5 | 8.3 ± 1.5 | 9.3 ± 0.6 |
| CTR + C3H6O | 7.3 ± 1.1 | 9.7 ± 0.6 | 5.7 ± 1.8 | 8.7 ± 0.6 |
| 1000 | 6.7 ± 0.6 | 0.7 ± 0.6d | 0.3 ± 0.6d | 5.0 ± 3.0b |
| 500 | 7.7 ± 1.5 | 1.7 ± 1.1d | 0.3 ± 0.6d | 9.0 ± 0.0 |
| 250 | 8.7 ± 0.6 | 5.7 ± 2.3a | 2.7 ± 0.6c | 9.3 ± 0.6 |
| 125 | 8.7 ± 1.1 | 9.7 ± 0.6 | 3.0 ± 1.0c | 10.0 ± 0.0 |
| E. loxophleba | ||||
| CTR | 7.0 ± 2.0 | 9.3 ± 1.1 | 7.3 ± 1.5 | 9.0 ± 1.0 |
| CTR + C3H6O | 7.3 ± 0.6 | 10.0 ± 0.0 | 5.3 ± 1.5 | 9.3 ± 0.6 |
| 1000 | 0.0 ± 0.0b | 0.0 ± 0.0d | 0.0 ± 0.0d | 8.7 ± 1.5 |
| 500 | 3.3 ± 2.5 | 0.0 ± 0.0d | 0.0 ± 0.0d | 8.5 ± 0.7 |
| 250 | 4.0 ± 1.7 | 0.0 ± 0.0d | 0.0 ± 0.0d | 9.3 ± 1.1 |
| 125 | 6.7 ± 2.5 | 1.3 ± 2.3d | 0.3 ± 0.6d | 9.0 ± 1.0 |
| E. salubris | ||||
| CTR | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| CTR + C3H6O | 10.0 ± 0.0 | 9.7 ± 0.3 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 1000 | 5.7 ± 0.3d | 0.0 ± 0.0d | 0.0 ± 0.0d | 6.0 ± 0.0d |
| 500 | 6.3 ± 0.7d | 0.0 ± 0.0d | 0.0 ± 0.0d | 6.7 ± 0.3d |
| 250 | 7.7 ± 0.3b | 0.0 ± 0.0d | 6.3 ± 0.3d | 8.0 ± 0.0d |
| 125 | 8.0 ± 0.00b | 8.3 ± 0.7b | 8.0 ± 0.0d | 8.3 ± 0.3c |
Results, mean ± SD of three experiments.
p < 0.05.
p < 0.01.
p < 0.001.
p < 0.0001 vs. control (ANOVA, Dunnett's test).CTR: deionized water; CTR + C3H6O: water–acetone mixture (99.5:0.5, v/v).
Table 4.
Phytotoxic effects of the tested essential oils against radicle elongation of L. multiflorum, S. arvensis, R. sativum, and C. sativus.
| Radical elongation (cm) | L. multiflorum | S. arvensis | R. sativus | C. sativus |
|---|---|---|---|---|
| E. amplifolia | ||||
| CTR | 2.7 ± 0.3 | 2.1 ± 0.1 | 2.4 ± 0.3 | 2.6 ± 0.1 |
| CTR + C3H6O | 2.7 ± 0.2 | 2.0 ± 0.1 | 2.3 ± 0.2 | 2.5 ± 0.0 |
| 1000 | 0.1 ± 0.0d | 0.0 ± 0.0d | 0.5 ± 0.7d | 0.8 ± 0.0d |
| 500 | 0.3 ± 0.0d | 0.0 ± 0.0d | 1.3 ± 0.1b | 1.5 ± 0.1d |
| 250 | 0.6 ± 0.0d | 0.3 ± 0.0d | 1.6 ± 0.0b | 1.7 ± 0.1d |
| 125 | 1.8 ± 0.1a | 0.3 ± 0.1d | 2.8 ± 0.1 | 2.1 ± 0.1b |
| E. bicolor | ||||
| CTR | 4.5 ± 2.1 | 2.1 ± 0.1 | 2.3 ± 0.2 | 2.8 ± 0.1 |
| CTR + C3H6O | 4.0 ± 1.8 | 2.4 ± 0.2 | 2.1 ± 0.0 | 2.7 ± 0.1 |
| 1000 | 0.0 ± 0.0b | 0.1 ± 0.0d | 0.0 ± 0.0d | 0.5 ± 0.0d |
| 500 | 0.9 ± 0.3a | 0.2 ± 0.0d | 0.3 ± 0.1d | 1.8 ± 0.1d |
| 250 | 1.6 ± 0.7a | 0.3 ± 0.1d | 0.8 ± 0.2d | 1.6 ± 0.1d |
| 125 | 2.2 ± 0.8 | 0.8 ± 0.0d | 0.9 ± 0.1d | 2.1 ± 0.0d |
| E. campaspe | ||||
| CTR | 5.6 ± 1.1 | 2.0 ± 1.3 | 2.0 ± 0.7 | 3.1 ± 0.9 |
| CTR + C3H6O | 5.5 ± 1.1 | 0.8 ± 0.5 | 1.3 ± 0.3 | 2.9 ± 1.0 |
| 1000 | 1.0 ± 0.6b | 0.2 ± 0.0a | 0.0 ± 0.0c | 0.7 ± 0.5a |
| 500 | 1.9 ± 0.5b | 0.3 ± 0.1a | 0.0 ± 0.0c | 2.3 ± 0.9 |
| 250 | 2.6 ± 0.8a | 0.3 ± 0.1a | 0.8 ± 0.3a | 3.1 ± 0.5 |
| 125 | 2.5 ± 0.9a | 0.6 ± 0.3a | 1.2 ± 0.5 | 3.8 ± 0.9 |
| E. loxophleba | ||||
| CTR | 5.7 ± 1.4 | 2.5 ± 1.1 | 2.9 ± 0.8 | 3.6 ± 0.7 |
| CTR + C3H6O | 1.7 ± 0.7 | 1.0 ± 0.3 | 1.3 ± 0.7 | 1.5 ± 0.5 |
| 1000 | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.2 ± 0.1d |
| 500 | 0.3 ± 0.1d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.8 ± 0.4d |
| 250 | 0.3 ± 0.2d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.8 ± 0.3d |
| 125 | 1.4 ± 0.6d | 0.1 ± 0.0d | 0.4 ± 0.0d | 1.5 ± 0.3d |
| E. salubris | ||||
| CTR | 2.7 ± 0.1 | 2.2 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 |
| CTR + C3H6O | 2.6 ± 0.1 | 2.2 ± 0.1 | 2.6 ± 0.1 | 2.9 ± 0.2 |
| 1000 | 0.7 ± 0.1d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.9 ± 0.1d |
| 500 | 1.7 ± 0.3b | 0.0 ± 0.0d | 0.0 ± 0.0d | 1.6 ± 0.5b |
| 250 | 2.1 ± 0.2 | 0.0 ± 0.0d | 0.9 ± 0.1d | 1.5 ± 0.1b |
| 125 | 2.2 ± 0.1 | 1.4 ± 0.2b | 1.8 ± 0.1d | 1.4 ± 0.1b |
Results, mean ± SD of three experiments.
p < 0.05.
p < 0.01.
p < 0.001.
p < 0.0001 vs. control (ANOVA, Dunnett's test). CTR: deionized water; CTR + C3H6O: water–acetone mixture (99.5:0.5, v/v).
The finding indicated that the E. amplifolia EO effectively hindered the radical elongation of all tested seeds at concentration of 1000,500 and 250 μg/ml. Conversely, at the lowest concentration evaluated (125 μg/ml), this EO primarily impeded the growth of S. arvensis seeds, and was not active against radical growth of R. sativus seeds. Moreover, this EO was active in the same way against germination of all studied seeds at all concentrations used, except against the crops R. sativus and C. sativus: in fact, for these seeds fact data highlighted the lowest activity.
E. bicolor EO inhibited radical elongation and germination of S. arvensis, R. sativus and C. sativus seeds at all concentrations tested, instead the same EO was phytotoxic in different way for L. multiflorum. In fact, at concentrations of 1000, 500 and 250 μg/ml inhibited radical elongation, while only at the higher concentration (1000 μg/ml) inhibited seed germination.
The EO from E. campaspe exhibited activity in inhibiting the radical elongation of both L. multiflorum and S. arvensis at all concentrations tested. Moreover, this EO inhibited radical elongation of R. sativus seeds at concentrations of 1000,500 and 250 μg/ml, while was active against radical growth of C. sativus seeds only at the higher concentration tested. This EO hindered the germination of R. sativus seeds across all concentrations tested, while it did not exhibit activity against the germination of L. multiflorum.
E. loxophleba EO was the most phytotoxic essential oil, inhibiting radical elongation of all seeds studied at all concentrations tested. Moreover, this EO was active at lowest concentration against germination of S. arvensis and R. sativus.
E. salubris EO was active in similar way against radical elongation and germination of S. arvensis and R. sativus. Instead, for L. multiflorum and C. sativus seeds data showed a major activity against germination than radical elongation.
4.3. Anti-enzymatic activities
Table 5 showed the enzymatic inhibitory effects of the EOs. In particular, E. campaspae EO was the most active against AChE with an IC50 value of 51 μg/ml. The other EOs inhibition are as follow: E. salubris < E. amplifolia < E. loxophleba < E. bicolor. All EOs were less active against BChE than AChE. E. campaspae EO and E. loxophleba EO showed the higher activity against BChE with an IC50 value of 1.065 mg/ml followed by E. amplifolia and E. salubris EOs. Instead, E. bicolor EO was the least active with an IC50 value of 14.920 mg/ml.
Table 5.
Inhibition of AChE, BChE, α-Amylase, and α-Glucosidase activities by tested EOs.
| Essential oils | IC50 (mg/ml) |
|||
|---|---|---|---|---|
| AChE | BChE | α-amylase | α-glucosidase | |
| E. amplifolia | 0.26 ± 0.02c | 2.78 ± 0.41a | 17.12 ± 1.25c | 26.32 ± 1.51b |
| E. bicolor | 0.371 ± 0.002d | 14.92 ± 3.787b | 8.097 ± 0.172b | >30 |
| E. campaspe | 0.051 ± 0.007a | 1.065 ± 0.098a | 20.125 ± 3.201c | 20.548 ± 1.236a |
| E. loxophleba | 0.341 ± 0.013d | 2.266 ± 0.024a | 0.909 ± 0.128a | 18.058 ± 2.451a |
| E. salubris | 0.22 ± 0.0b | 4.27 ± 0.26a | 0.57 ± 0.01a | 19.47 ± 1.25a |
| Galantamine | 0.009 ± 0.003 | 0.06 ± 0.02 | – | – |
| Acarbose | – | – | 0.003 ± 0.001 | 0.7 ± 0.2 |
Results (mean ± SD, n = 3) with different letters indicate significant differences at p < 0.05 (one-way ANOVA, Tukey's post-hoc test).
The EOs from E. salubris and E. loxophleba exhibited the highest activity against α-amylase with an IC50 value < 1 mg/ml followed by E. bicolor, E. amplifolia and E. campaspe EOs.
Instead, E. loxophleba, E. salubris and E. campaspe demonstrated the highest activity against α-glucosidase even if with high IC50 values. E. bicolor EO was inactive against α-glucosidase with an IC50 value > 30 mg/ml.
All samples showed IC50 values higher than those of standards used as positive control.
4.4. Antimicrobial activity
The in vitro antibacterial efficacy of the EOs was assessed through identification of the minimum inhibitory concentration (MIC) (Table 6). Additionally, the potential effects on biofilm formation and bacterial cell metabolism were elucidated and presented in Table 7, Table 8.
Table 6.
MIC value (mg/mL) of five eucalyptus EOs against assessed pathogens.
| Species | E. bicolor | E. loxophleba | E. campaspae | E. salubris | E. amplifolia |
|---|---|---|---|---|---|
| A. baumannii | >50a | 38 ± 4a | >50a | 16 ±3a | 14 ±1a |
| E. coli | >50a | 35 ± 2b | 35± 4b | 18 ±2b | 14 ±2a |
| L. monocytogenes | 30 ± 3b | 35 ± 3b | 35 ± 2b | 14 ±2c | 15 ±2 ab |
| P. aeruginosa | 38 ± 3c | 38 ± 2a | 38 ± 4c | 14 ±2c | 15 ±1 ab |
| S. aureus | 35 ± 2d | 30 ± 3c | 28 ± 2d | 14 ±1c | 16 ±2b |
The tests were conducted three times and the results are presented as the average ± SD) With different letters, denote significant differences at p < 0.05, following one-way ANOVA and SNK post hoc test.
Table 7.
Inhibitory activity of the EOs on the mature biofilm.
| E. amplifolia | E. bicolor | E. compaspae | E. loxophleba | E. salubris | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations (mg/mL) | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 |
| A. baumannii | 33.44 ± 4.32a |
50.79 ± 3,67a |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
21.82 ± 1.98a |
30.66 ± 2.09a | 3.41 ± 0.13a |
57.24 ± 4,44a |
| E. coli | 62.5 ± 3.96b |
78.27 ± 5,61b |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
42.65 ± 3.86a |
40.22 ± 3.67b |
45.85 ± 5.04b |
33.38 ± 2.41b |
35.35 ± 1,25b |
| L. monocytogenes | 63.86 ± 3.87b |
69.31 ± 5,01c |
0.00 ± 0.00 |
18.49 ± 1.04a |
12.32 ± 1.01a |
57.42 ± 5.04b |
23.92 ± 1.25a | 44.85 ± 4.54c |
68.54 ± 4.76c |
71.21 ± 3,23c |
| P. aeruginosa | 10.93 ± 0.87c |
61.45 ± 3,78c |
0.00 ± 0.00 |
34.14 ± 3.05b |
19.03 ± 2.01b |
23.35 ± 2.87c |
0.00 ± 0.00 |
24.39 ± 1.87d |
72.39 ± 2.51d |
79.45 ± 3,55c |
| S. aureus | 53.21 ± 3.53d |
57.86 ± 3.67a |
8.71 ± 0.92a | 45.53 ± 3.99c |
38.86 ± 2.44c |
76.61 ± 3.52d |
25.29 ± 1.55a |
70.94 ± 2.69e |
71.41 ± 3.01d |
88.24 ± 1.67d |
Results (mean ± SD, n = 3) with different letters denote significant differences at p < 0.05, following one-way ANOVA and SNK post hoc test.
Table 8.
Inhibation effects of EOs on bacterial sessile cell metabolism in mature biofilm.
| E. amplifolia | E. bicolor | E. compaspae | E. loxophleba | E. salubris | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations (mg/mL) | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 |
| A. baumannii | 60.55 ± 1.25a | 94.69 ± 2.08a | 30.3 ± 2.02a | 54.46 ± 4.87a |
85.96 ± 2.12a |
91.03 ± 3.11a |
93.47 ± 1.32a |
94.09 ± 2.86 |
53.04 ± 1.44a |
64.13 ± 1.17a |
| E. coli | 0.00 ± 0.00 | 78.81 ± 1.76b | 0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
1.88 ± 0.02b |
63.36 ± 2.61b |
77.65 ± 3.51 |
53.58 ± 2.21a |
81.72 ± 1.89b |
| L. monocytogenes | 0.00 ± 0.00 | 81.21 ± 4.02c | 14.2 ± 2.21b | 16.22 ± 1.55b |
0.00 ± 0.00 |
81.92 ± 2.21c |
74.04 ± 2.54c |
81.23 ± 1.88 |
28.89 ± 2.07b |
81.62 ± 3.92b |
| P. aeruginosa | 0.00 ± 0.00 | 80.88 ± 4.97c | 0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
71.82 ± 3.44 |
14.60 ± 1.01c |
57.81 ± 4.07c |
| S. aureus | 57.74 ± 2.01b | 93.40 ± 1.04a | 41.8 ± 3.13c | 44.48 ± 3.32c |
67.16 ± 1.67b |
81.78 ± 1.13c |
92.78 ± 2.09a |
94.35 ± 2.67 |
74.15 ± 2.25d |
92.62 ± 1.45d |
Results (mean ± SD, n = 3) with different letters denote significant differences at p < 0.05, following one-way ANOVA and SNK post hoc test.
The EOs manifested pronounced inhibitory effects, characterized by MIC values spanning from 14 mg/ml (E. salubris) to values surpassing 50 mg/ml (E. bicolor), as delineated in Fig. 3. The variability in MIC values was contingent upon the specific EOs and bacterial strains under scrutiny. Notably, each EO demonstrated unique inhibitory effects on biofilm formation, with efficacy varying according to bacterial species and concentrations.
The MIC reveal diverse sensitivities across the tested bacterial strains, with Gram-positive strains exhibiting greater sensitivity than Gram-negative ones which the MIC recorded exceeded 50 mg/mL. As for Gram-positive strains, the maximum values were observed as follows: P. aeruginosa (38 ± 4, E. compaspae), L. monocytogenes (35 ± 3, E. loxophleba), and S. aureus (35 ± 2, E. bicolor).
With the exception of the EOs of E. bicolor and E. campaspae, mature biofilms exhibited sensitivity to the effects of EOs, emphasizing their responsiveness. Notably, no inhibition was detected in the case of A. baumannii when exposed to E. bicolor and E. campaspae. However, a significant reduction in cellular metabolism was observed at the highest concentration, resulting in percentages of 54.46 ± 4.87 and 91.03 ± 3.11 %, respectively.
Similarly, E. coli demonstrated resistance when treated with E. campaspae EO; no inhibition was observed at 10 mg/ml for both mature biofilm and cellular metabolism. Yet, with an increased concentration, a 42.65 ± 3.86 % inhibition of mature biofilm and only 1.88 ± 0.02 % inhibition of cellular metabolism were observed. This highlights the distinctive effectiveness of these EOs in influencing microbial activity. After the application of E. amplifolia EO, similar percentage of inhibition was observed on mature biofilm (78.27 ± 5.61) and on cell metabolism (78.81 ± 1.76) at 10 mg/ml.
Among Gram-positive strains, S. aureus demonstrated the highest sensitivity. The observed inhibition of mature biofilm varied from 8.71 ± 0.92 % (E. bicolor) to 88.24 ± 1.67 % (E. salubris). In the case of cell metabolism, the inhibition ranged from 41.83 ± 3.13 (E. bicolor) to 94.35 ± 2.67 % (E. loxophleba).
The effectiveness of EOs from E. salubris, E. amplifolia, and E. loxophleba was evident in reducing biofilm formation across various bacterial strains. Additionally, these EOs exhibited a hindering effect on the cellular metabolism of these pathogens. Interestingly, the inhibition caused by the EOs did not consistently correlate with a comparable influence on the metabolic activity of bacterial cells within the biofilm. For example, E. loxophleba showed a notably higher impact on P. aeruginosa cellular metabolism, reaching 71.82 ± 3.14 %, compared to its effect on mature biofilm at 24.29 ± 1.87 %, at the identical concentration of 20 mg/ml. Similarly, E. compaspae EO demonstrated a substantial 81.92 ± 2.21 % inhibition of L. monocytogenes cellular metabolism. However, this value diminished to 57.42 ± 5.04 % when the same EO was applied to mature biofilm, at of 20 mg/ml.
5. Discussion
In the majority of cases, the quantities of EO obtained closely match the data reported in the literature. For instance, the EO from E. bicolor demonstrated similarity to EO yields from Iraq (1.45 %) as reported by Dehghani et al. (2023) [28]. A separate study conducted in Tunisia indicated 1.50 ± 0.3 % EO yield [29], while another study from the Province of Isfahan, Iran reported 1.85 % EO yield [30]. However, for species such as E. amplifolia, E. salubris, and E. campaspe, the yields observed in this study surpass previously documented values. E. amplifolia EO was reported to yield 0.9–1.2 % from trees growing in South Wales, Australia [31], and 0.36 % from trees growing in Uruguay [32].
E. salubris EO was reported to yield 1.5–1.65 % from trees growing in Morocco [33] and 4–3.8 % from trees growing in Tunisia [34]. E. campaspe EO was reported to yield 1.90 % from trees growing in Arboretum de Waite, South Australia l [35]. Conversely, for E. loxophleba EO, the yields are lower than previous references. A yield of 7.1 % was reported from trees growing in southwest Iran [36], and a similar yield to our result, 3.8 %, was reported from trees growing in Narrogin, southwest Western Australia [37].
The disparities in yield compared to existing data may be ascribed to various factors, encompassing climate, soil characteristics [38] timing of collection [38] and geoclimatic conditions [39].
By exploring the biological potential of Eucalyptus EOs, this study contributed valuable insights into their diverse applications and effects.
The analysis of their chemical composition highlighted interspecific diversity which is confirmed by PCA and HCA analyses.
Notably, the chemical composition of E. amplifolia EO has been previously documented by Hellyer and McKern [31], reporting a composition rich in isovaleric aldehyde, α-pinene, eucalyptol, limonene, α-terpineol, aromadendrene, α- and β-eudesmol. However, the quantity of the most present components was not extensively detailed in the cited work, except for eucalyptol, which was present at 19.5 %. A comparison with subsequent works revealed variations in the concentrations of shared compounds, such as α-pinene, eucalyptol, and aromadendrene. In 1990, Dellacassa and collaborators [32] presented a different composition of E. amplifolia essential oil, with eucalyptol as the main component (40.8 %), accompanied by limonene, α-pinene, globulol, and aromadendrene. Comparisons with earlier works highlighted differences in the concentration of eucalyptol and other shared compounds, underscoring the dynamic nature of the chemical composition of E. amplifolia EO. A similar trend was observed in study by Bignell and coworkers [35], who reported an oil composition similar to the one examined in this study. Although both EOs shared eucalyptol as the main component, significant differences in concentration were noted for eucalyptol and allo-aromadendrene, reinforcing the importance of considering variations in chemical profiles.
The examination of E. bicolor EO by Elaissi and coworkers [29] highlighted similarities in composition with our study, presenting shared compounds such as eucalyptol, aromadendrene, α-pinene, and trans-pinocarveol. While the quantities differed, the commonality in major components highlights consistency in the chemical composition. Similarly, Al-Snafi [40], emphasized the prevalence of eucalyptol in, aligning with the findings of our study. Although variations in concentrations were noted, the shared presence of eucalyptol signifies its significance in defining the chemical profile.
Examining E. campaspe EO, Dhaouadi et al. [41] identified eucalyptol as the main component.
The investigation into E. loxophleba EOs by Aldoghaim [42], revealed differences in the concentration of eucalyptol when compared to a 2011 study by Rahimi-Nasrabadi and Batooli [36]. While eucalyptol was the only shared main component, the divergent quantities highlight the inherent variability in E. loxophleba EO. Similarly, the study of aTunisian E. loxophleba EO by Elaissi in 2010 [43] exhibited variations in the concentration of eucalyptol compared to other works. Despite differences, the common presence of eucalyptol reinforces its significance in defining the chemical composition.
Exploring E. salubris EOs, Elaissi and collaborators [34] demonstrated common compounds like limonene, eucalyptol, cryptone, and α-pinene, albeit with different concentrations. While variations exist, the presence of eucalyptol as a shared major component underscores its significance in defining the chemical profile. Zhang and collaborators [44] reported an EO of E. salubris whose main components were eucalyptol, α-pinene, p-cymene and trans-pinocarveol. The paper does not report the quantity of these components but all four are present as majority compounds in the EO reported in this work. In 2020 Abd-ElGawad and collaborators [45] report an EO of E. salubris which had eucalyptol, α-pinene and p-cymene as main components. This work does not report the quantity of these components either but all three are present as majority compounds in the OE reported in this work.
Evidently, the chemical analyses underscore the necessity to discern the potential influence of environmental factors on their compositional dynamics. This assertion underscores the intricacies involved in elucidating the complex chemical constitution of Eucalyptus EOs and the concomitant environmental variables that may exert discernible effects on their overall composition [[46], [47]].
In existing literature, there is an absence of prior research elucidating the phytotoxic effects on the seeds of L. multiflorum, S. arvensis, R. sativus, and C. sativus induced by various Eucalyptus species.
Numerous studies have underscored the capacity of Eucalyptus EOs to impede or delay the germination processes of seeds such as Triticum aestivum L., Zea mays L. Cassia occidentalis L., Amaranthus viridis L., Echinochloa crus-galli (L.) P. Beauv., Parthenium hysterophorus L., and Bidens pilosa L [[48], [49], [50]].
Our preceding investigations have also identified the phytotoxicity of several Eucalyptus EOs against R. sativus, L. multiflorum, and S. arvensis [9,51]
The phytotoxicity associated with Eucalyptus EOs is likely attributed to the elevated presence of the monoterpene eucalyptol (1,8 cineole), known for inhibiting mitochondrial respiration, mitosis, and DNA synthesis, thereby influencing the germination process [52]. This inhibitory activity is closely tied to the chemical structure of the compound, as exemplified by the structural similarity between the first commercialized allelopathic herbicide, cinmethylin and natural 1,8-cineole [53].
Conversely, the variation in phytotoxic activities observed among different EOs may stem from potential synergistic interactions between their constituents, influencing enzymes crucial to the germination process [52].
Key enzymes in the germination process include α-amylase and α-glucosidase, responsible for hydrolyzing starch reserves into maltose and further converting maltose into glucose, respectively [54,55].
Consequently, following the determination of phytotoxic activities from EOs, an assessment of their potential impact on the regulation of these enzymes was conducted. The results align with the phytotoxic assays, indicating that E. loxophleba and E. salubris EOs, exhibiting the highest phytotoxicity at the lowest concentrations tested (125 μg/ml), also demonstrated the lowest IC50 values for both α-amylase and α-glucosidase. Furthermore, although E. bicolor EO did not display activity against α-glucosidase (IC50 > 30 mg/ml), it exhibited an IC50 < 10 mg/ml against α-amylase and proved more phytotoxic at the lowest concentration (125 μg/ml) compared to E. amplifolia and E. campaspe EOs, which showed IC50 values > 20 mg/ml for one or both enzymes.
Existing literature only encompasses two prior studies that hint at a potential correlation between the phytotoxic and anti-α-amylase activities of Eucalyptus pulverulenta Sims, E. gunnii Hook. f., and O. heracleoticum L. EOs [8,56].
Remarkably, no previous studies have reported a plausible correlation between phytotoxicity and the inhibition of α-glucosidase activity.
Building upon the recognized advantages of natural pesticides over chemical counterparts, biopesticides, such as those derived from Eucalyptus EOs, present a promising alternative to conventional pest control methods [57]. The diverse and desirable properties of Eucalyptus EOs for pest management have been extensively explored in the literature, encompassing various species like E. camaldulensis Dehnh., E. citriodora Hook., E. globulus, E. grandis W. Hill., E. robusta Sm., and E. saligna Sm [[58], [59], [60], [61], [62], [63], [64]].
Despite the well-established pesticidal potential of Eucalyptus EOs, the exploration of their impact on cholinesterases, crucial enzymes targeted by pesticides, has been limited in previous studies [65,[66], [67]]. Addressing this research gap, our study delves into the pesticidal capabilities of five Eucalyptus EOs. The results unequivocally demonstrate the inhibitory effects on acetylcholinesterase (AChE) and activity against butyrylcholinesterase (BChE) for all EOs, except for E. bicolor, which exhibited relatively lower activity against BChE. These findings not only underscore the multifaceted utility of Eucalyptus EOs in pest management but also emphasize their potential as biopesticides with dual efficacy against key enzymatic targets.
In examining the antimicrobial potential of Eucalyptus EOs, our investigation extends to diverse geographical sources, revealing unique properties of each species. The E. loxophleba EO sourced from western Australia exhibited moderate antimicrobial potential, with zone of inhibition values ranging from 14.7 ± 0.6 to 16.7 ± 0.6 against E. coli ATCC 25922 and 15.3 ± 0.6 to 16.7 ± 0.6 against S. aureus ATCC 29213 when exposed to 25 and 50 μL of EOs. Additionally, the MIC was determined to be 8 % v/v for E. coli and 4 % v/v for S. aureus [42].
The examination of Tunisian E. salubris EOs revealed noteworthy effectiveness against various microorganisms, except for S. aureus and E. coli. Significant activity was observed against L. monocytogenes (18 mm) and K. pneumoniae (12 mm) [68].
In the case of E. salubris EOs from Jordanian trees, significant antibacterial effectiveness was observed against both Gram-negative and Gram-positive bacteria, with MIC values ranging from 98 μg/ml for S. aureus to 140 μg/ml for P. aeruginosa, and 119 μg/ml against E. coli [[69], [70]].
An E. bicolor EO from Iran demonstrated moderate to high antimicrobial impact against various bacteria, fungi, and yeast, with no effect against P. aeruginosa or E. coli [30].
Subsequent studies confirmed the absence of antibacterial effects on P. aeruginosa, while revealing a significant impact on E., and an MIC range of 62.4–61.4 μg/ml against S. aureus [[71], [72]].
Notably, while previous reports have documented the antibacterial activities of E. bicolor, E. salubris, and E. loxophleba, a comprehensive literature search revealed a dearth of information regarding the antibacterial activities of the EOs from the two additional species under examination, namely E. campaespe and E. amplifolia. This underscores the need for further exploration and understanding of the antimicrobial potential of these Eucalyptus species.
In conclusion, this study not only contributes valuable insights into the diverse applications of Eucalyptus EOs but also emphasizes the need for continued exploration and understanding of their complex biological interactions. The multifaceted nature of Eucalyptus EOs, as revealed through this research, underscores their potential for various applications in agriculture, pest management, and healthcare, paving the way for future studies and practical applications in these domains.
Funding
This research received no external funding.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Sana Khedhri: Writing – original draft, Writing – review & editing, Formal analysis, Data curation. Flavio Polito: Writing – review & editing, Investigation, Data curation. Lucia Caputo: Writing – review & editing, Software, Methodology, Data curation. Marwa Khammassi: Investigation. Ferjani Dhaouadi: Investigation. Ismail Amri: Project administration. Lamia Hamrouni: Resources, Conceptualization. Yassine Mabrouk: Investigation, Data curation. Florinda Fratianni: Data curation. Filomena Nazzaro: Validation, Methodology, Formal analysis, Data curation. Vincenzo De Feo: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Sana Khedhri, Email: sanakhedhrii@gmail.com.
Flavio Polito, Email: fpolito@unisa.it.
Lucia Caputo, Email: lcaputo@unisa.it.
Marwa Khammassi, Email: khammassi_marwa@yahoo.fr.
Ferjani Dhaouadi, Email: abdotasnime2014@gmail.com.
Ismail Amri, Email: amri_amri@lice.fr.
Lamia Hamrouni, Email: hamrounilam@yahoo.fr.
Yassine Mabrouk, Email: yassinemabrouk@g.mail.com.
Florinda Fratianni, Email: florinda.fratianni@isa.cn.it.
Filomena Nazzaro, Email: filomena.nazzaro@isa.cnr.it.
References
- 1.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Hanif M.A., Nisar S., Khan G.S., Mushtaq Z., Zubair M. In: Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production. Malik S., editor. Springer International Publishing; Berlin, Germany: 2019. Essential oils; pp. 3–17. [DOI] [Google Scholar]
- 3.Elshafie H.S., Camele I. An Overview of the biological effects of some Mediterranean essential oils on human Health. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fometu S., Shittu S., Herman R., Ayepa E. Essential Oils and their applications-A mini review. Adv. Nutr. Food Sci. 2019;4:1–13. [Google Scholar]
- 5.Sharma A.D., Farmaha M., Kaur I., Singh N. Phytochemical analysis using GC-FID, FPLC fingerprinting, antioxidant, antimicrobial, anti- inflammatory activities analysis of traditionally used Eucalyptus globulus essential oil. Drug Anal. Res. 2021;5:26–38. doi: 10.22456/2527-2616.110344. [DOI] [Google Scholar]
- 6.Polito F., Fratianni F., Nazzaro F., Amri I., Kouchi H., Khammassi M., Hamrouni L., Malaspina P., Cornara L., Khedri S., Romano B., Maresca D.C., Ianaro A., Ercolano G., De Feo V. Essential oil composition, antioxidant activity and leaf Micromorphology of five Tunisian Eucalyptus species. Antioxidants. 2023;12:867. doi: 10.3390/antiox12040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashour H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008;7:399–403. doi: 10.4161/cbt.7.3.5367. [DOI] [PubMed] [Google Scholar]
- 8.Danna C., Cornara L., Smeriglio A., Trombetta D., Amato G., Aicardi P., De Martino L., De Feo V., Caputo L. Eucalyptus gunnii and Eucalyptus pulverulenta ‘Baby Blue’ essential oils as potential natural herbicides. Molecules. 2021;26:21. doi: 10.3390/molecules26216749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khedhri S., Polito F., Caputo L., Manna F., Khammassi M., Hamrouni M., Amri I., Nazzaro F., De Feo V., Fratianni F. Chemical composition, phytotoxic and Antibiofilm activity of seven Eucalyptus species from Tunisia. Molecules. 2022;27:23. doi: 10.3390/molecules27238227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores-Macías A., Reyes-Zarate G.G., da Camara C.A.G., López-Ordaz R., Guillén J.C., Ramos-López M.Á. Chemical composition and phytotoxic potential of Eucalyptus globulus essential oil against Lactuca sativa and two herbicide-resistant weeds: Avena fatua and Amaranthus hybridus. Tip rev. espec. cienc. 2022;24(1) [Google Scholar]
- 11.Amri I., Khammassi M., Ben Ayed R., Khedhri S., Mansour M.B., Kochti O., Hamrouni L. Essential oils and biological activities of Eucalyptus falcata, E. Sideroxylon and E. Citriodora growing in Tunisia. Plants. 2023;12(4):816. doi: 10.3390/plants12040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khedhri S., Polito F., Caputo L., De Feo V., Khammassi M., Kochti O., Hamrouni L., Mabrouk Y., Nazzaro F., Fratianni F., Amri I. Chemical composition, antibacterial properties, and anti-enzymatic effects of Eucalyptus essential oils sourced from Tunisia. Molecules. 2023;28:7211. doi: 10.3390/molecules28207211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usman L.A., Oguntoye S.O., Ismaeel R.O. Effect of collection time on the chemical composition, antioxidant and antidiabetic potentials of leaf essential oil of Eucalyptus globulus L. growing in North-Central, Nigeria. Chemistry Africa. 2022;5(2):257–267. [Google Scholar]
- 14.Jaradat N., Al-Maharik N., Hawash M., Qadi M., Issa L., Anaya R., Aboturabi R.A. Eucalyptus camaldulensis Dehnh leaf essential oil from Palestine exhibits antimicrobial and antioxidant activity but No effect on Porcine Pancreatic Lipase and α-amylase. Plants. 2023;12(22):3805. doi: 10.3390/plants12223805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khammassi M., Polito F., Amri I., Khedri S., Hamrouni L., Nazzaro F., Fratianni F., De Feo V. Chemical composition and phytotoxic, antibacterial and Antibiofilm activity of the essential oils of Eucalyptus occidentalis, E. Striaticalyx and E. Stricklandii. Molecules. 2022;27:5820. doi: 10.3390/molecules27185820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Directorate for the Quality of Medicines & HealthCare (EDQM) 2022. European Pharmacopoeia (Ph. Eur.) Edition 11.0; p. 42. [Google Scholar]
- 17.Adams R.P. Allured Publishing Co.; Carol Stream, IL, USA: 2007. Identification of Essential Oil Components by Gas Cromatography/Mass Spectrometry. [Google Scholar]
- 18.Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A. 1990;503:1–24. [Google Scholar]
- 19.Jenings W., Shibamoto T. Academic Press; New York, NY, USA: 1980. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography. [Google Scholar]
- 20.NIST . 2017. Wiley Registry 11th Edition/NIST 2017 Mass Spectral Library DVD-ROM. ISBN: 978-1-119-41223- 6. [Google Scholar]
- 21.Bewley J.D. Seed germination and Dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellman G.L., Courtney K.D., Andres V., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Bernfeld P. In: Methods in Enzymology. Colowick S.P., Kaplan N.O., editors. Academic Press Inc.; New York, NY, USA: 1955. Amylase α and β; pp. 149–158. [Google Scholar]
- 24.Si M., Lou J., Zhou C.X., Shen J.-N., Wu H.-H., Yang B., He Q.-J., Wu H.-S. Insulin releasing and alpha-glucosidase inhibitory activity of ethyl acetate fraction of Acorus calamus in vitro and in vivo. J. Ethnopharmacol. 2010;128:154–159. doi: 10.1016/j.jep.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 25.Sarker S., Nahar D.L. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalitha M. vol. 56238. CLSI; Wayne, PA, USA: 2004. Manual on antimicrobial susceptibility testing; pp. 454–456. (Performance Standards for Antimicrobial Testing: Twelfth Informational Supplement). [Google Scholar]
- 27.Nazzaro F., Polito F., Amato G., Caputo G., Francolino R., d'Acierno A., Fratianni F., Candido V., Coppola R., De Feo V. Chemical composition of essential oils of Bulbs and Aerial Parts of two Cultivars of Allium sativum and their Antibiofilm activity against food and Nosocomial pathogens. Antibiotics. 2022;11:724. doi: 10.3390/antibiotics11060724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehghani Bidgoli R., O Abdullah F., Budriesi R., Beatrice Mattioli L., Spadoni G., Mari M., Micucci M. Essential oil chemical composition, antioxidant and antibacterial activities of Eucalyptus largiflorens F. Muell. Pol. J. Environ. Stud. 2023;32:3043–3052. [Google Scholar]
- 29.Elaissi A., Moumni S., Roeleveld K., Larbi Khouja M. Chemical Characterization of five Tunisian Eucalyptus essential oils species. Chem. Biodivers. 2020;17 doi: 10.1002/cbdv.201900378. [DOI] [PubMed] [Google Scholar]
- 30.Safaei-Ghomi J., Abbasi Ahd A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. 2010;6:172–175. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellyer R.O., McKern H.H.G. The volatile oils of the genus Eucalyptus (family Myrtaceae). IV. The leaf oils of E. amplifolia Naudin and E. kitsoniana Maiden. Aust. J. Chem. 1966;19:1541–1543. doi: 10.1071/ch9661541. [DOI] [Google Scholar]
- 32.Dellacassa E., Menéndez P., Moyna P., Soler E. Chemical composition of Eucalyptus essential oils grown in Uruguay. Flavour Fragrance J. 1990;5:91–95. doi: 10.1002/ffj.2730050207. [DOI] [Google Scholar]
- 33.Zrira S., El Khirani F., Benjilali B. Huiles essentielles de six espèces xérophyles d'Eucalyptus: effet du milieu sur les rendements et la composition chimique. Revue Marocaine des Sciences Agronomiques et Vétérinaires. 1994;14(1):5–9. [Google Scholar]
- 34.Elaissi A., Chraif I., Bannour F., Farhat F., Ben Salah M., Chemli R., Khouja M.L. Contribution to the Qualitative and Quantitative study of seven Eucalyptus species essential oil harvested of Hajeb Layoun Arboreta (Tunisia) J. Essen. Oil-Bear. Plants. 2007;10:15–25. doi: 10.1080/0972060X.2007.10643513. [DOI] [Google Scholar]
- 35.Bignell C.M., Dunlop P.J., Brophy J.J., Jackson J.F. Volatile leaf oils of some South-western and southern Australian species of the genus Eucalyptus. Part VIII. Subgenus Symphyomyrtus, (a) section Bisectaria, Series Cornutae and Series Bakeranae, and (b) section Dumaria, Unpublished Series Furfuraceae group. Flavour Fragrance J. 1996;11:43–47. doi: 10.1002/(SICI)1099-1026(199601)11:1<43::AID-FFJ543>3.0.CO;2-E. [DOI] [Google Scholar]
- 36.Rahimi-Nasrabadi M., Ahmadi F., Batooli H. Chemical composition of essential oil and in vitro antioxidant activities of the essential oil and methanol extracts of Eucalyptus loxophleba. Nat. Prod. Res. 2012;26:669–674. doi: 10.1080/14786419.2011.593516. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S., Zhang D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014;133:443–451. doi: 10.1016/j.seppur.2014.07.018. [DOI] [Google Scholar]
- 38.Kahkashan P., Najat B., Iram S., Iffat S. Influence of soil Type on the growth Parameters, essential oil yield and Biochemical Contents of Mentha arvensis L. J. Essent. Oil Bear Plants. 2016;19:76–81. doi: 10.1080/0972060X.2015.1086285. [DOI] [Google Scholar]
- 39.Laftouhi A., Eloutassi N., Drioua S., Ech-Chihbi E., Rais Z., Abdellaoui A., Taleb M. Impact of water stress and temperature on Metabolites and essential oil of Rosmarinus officinalis (Phytochemical screening, extraction, and gas chromatography) J. Ecol. Eng. 2023;24(5):237–248. doi: 10.12911/22998993/161760. [DOI] [Google Scholar]
- 40.Al-Snafi A. The pharmacological and therapeutic importance of Eucalyptus species grown in Iraq. IOSR J. Pharm. 2017;7:72–91. doi: 10.9790/3013-0703017291. [DOI] [Google Scholar]
- 41.Dhaouadi F., Bargougui A., Maamer S., Amri I., Guerfali M.M., Hamrouni L., Flamini G., Mejri N. Chemical composition and insecticidal activity of two Eucalyptus essential oils against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) J. Plant Dis. Prot. 2023;3:483–493. doi: 10.1007/s41348-022-00702-8. [DOI] [Google Scholar]
- 42.Aldoghaim F.S., Flematti G.R., Hammer K.A. Antimicrobial activity of several cineole-rich western Australian Eucalyptus essential oils. Microorganisms. 2018;6:122. doi: 10.3390/microorganisms6040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elaissi A., Medini H., Larbi Khouja M., Simmonds M., Lynene F., Farhat F., Chemli R., Harzallah Skhiri F. Variation in volatile leaf oils of eleven Eucalyptus species harvested from Korbous Arboreta (Tunisia) Chem. Biodivers. 2010;7:1841–1854. doi: 10.1002/cbdv.200900381. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J., An M., Wu H., Stanton R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012;68:231–237. doi: 10.1007/s10725-012-9711-5. [DOI] [Google Scholar]
- 45.Abd-ElGawad A.M., El Gendy A.E.-N.G., Assaeed A.M., Al-Rowaily S.L., Alharthi A.S., Mohamed T.A., Nassar M.I., Dewir Y.H., Elshamy A.I. Phytotoxic effects of plant essential oils: a Systematic review and structure-activity relationship based on Chemometric analyses. Plants. 2020;10:36. doi: 10.3390/plants10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barra A. Factors affecting chemical variability of essential oils: a review of recent Developments. Nat. Prod. Res. 2009;4:1147–1154. doi: 10.1177/1934578X0900400827. [DOI] [PubMed] [Google Scholar]
- 47.Figueiredo A.C., Barroso J.G., Pedro L.G., Scheffer J.J.C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragrance J. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- 48.Batish D., Setia N., Singh D.H., Kohli R. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Protect. 2004;23:1209–1214. doi: 10.1016/j.cropro.2004.05.009. [DOI] [Google Scholar]
- 49.Singh H.P., Batish D.R., Setia N., Kohli R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005;146:89–94. doi: 10.1111/j.1744-7348.2005.04018.x. [DOI] [Google Scholar]
- 50.Setia N., Batish D.R., Singh H.P., Kohli R.K. Phytotoxicity of volatile oil from Eucalyptus citriodora against some weedy species. J. Environ. Biol. 2007;28:63–66. [PubMed] [Google Scholar]
- 51.Polito F., Kouki H., Khedhri S., Hamrouni L., Mabrouk Y., Amri I., Nazzaro F., Fratianni F., De Feo V. Chemical composition and phytotoxic and Antibiofilm activity of the essential oils of Eucalyptus bicostata, E. Gigantea, E. Intertexta, E. Obliqua, E. Pauciflora and E. Tereticornis. Plants. 2022;11:22. doi: 10.3390/plants11223017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macías F.A., Molinillo J.M.G., Varela R.M., Galindo J.C.G. Allelopathy--a natural alternative for weed control. Pest Manag. Sci. 2007;63:327–348. doi: 10.1002/ps.1342. [DOI] [PubMed] [Google Scholar]
- 53.Macías F.A., Molinillo J.M.G., Galindo J.C.G., Varela R.M., Simonet A.M., Castellano D. The Use of allelopathic studies in the search for natural herbicides. J. Crop Prod. 2001;4:237–255. doi: 10.1300/J144v04n02_08. [DOI] [Google Scholar]
- 54.Murtaza G., Asghar R. α-Amylase activities during seed development and germination in pea (Pisum sativum L.) treated with salicylic acid. Pakistan J. Bot. 2012;44:1823–1829. [Google Scholar]
- 55.Stanley D., Rejzek M., Naested H., Smedley M., Otero S., Fahy B., Thorpe F., Nsh R.J., Harwood W., Svensson B., Denyer K., Field R.A., Smith A.M. The role of α-glucosidase in germinating Barley Grains. Plant Physiol. 2011;155:932–943. doi: 10.1104/pp.110.168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amato G., Caputo L., Francolino R., Martino M., De Feo V., De Martino L. Origanum heracleoticum essential oils: chemical composition, phytotoxic and alpha-amylase inhibitory activities. Plants. 2023;12:4. doi: 10.3390/plants12040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimetry N.Z. In: Advances in Plant Biopesticides. Singh D., editor. Springer India; New Dehli: 2014. Different plant Families as Bioresources for pesticides. [DOI] [Google Scholar]
- 58.Watanabe K., Shomo Y., Kakimizu A., Okada A., Matsuo N., Satoh A., Nishimura H. New mosquito repellent from Eucalyptus camaldulensis. J. Agric. Food Chem. 1993;41:2164–2166. doi: 10.1021/jf00035a065. [DOI] [Google Scholar]
- 59.Ramezani H., Singh H.P., Batish D.R., Kohli R.K. Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia. 2002;73:261–262. doi: 10.1016/s0367-326x(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 60.Lucia A., Gonzalez Audino P., Seccacini E., Licastro S., Zerba E., Masuh H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control Assoc. 2007;23:299–303. doi: 10.2987/8756-971X(2007)23[299:LEOEGE]2.0.CO,2. [DOI] [PubMed] [Google Scholar]
- 61.Sartorelli P., Marquioreto A.D., Amaral-Baroli A., Lima M.E.L., Moreno P.R.H. Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother Res. 2007;21:231–233. doi: 10.1002/ptr.2051. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y.C., Choi H.Y., Choi W.S., Clark J.M., Ahn Y.J. Ovicidal and Adulticidal activity of Eucalyptus globulus leaf oil Terpenoids against Pediculus humanus capitis (Anoplura: Pediculidae) J. Agric. Food Chem. 2004;52:2507–2511. doi: 10.1021/jf0354803. [DOI] [PubMed] [Google Scholar]
- 63.Boland D.J., Brophy J.J., House A.P.N., editors. Eucalyptus Leaf Oils. Use, Chemistry, Distillation and Marketing. Inkata Press; Melbourne/Sydney: 1991. [Google Scholar]
- 64.Danna C., Malaspina P., Cornara L., Smeriglio A., Trombetta D., De Feo V., Vanin S. Eucalyptus essential oils in pest control: a review of chemical composition and applications against insects and mites. Crop Protect. 2024;176 doi: 10.1016/j.cropro.2023.106319. [DOI] [Google Scholar]
- 65.Kobenan K.C., Ochou G.E.C., Sinan Kouadio I., Kouakou M., Bini K.K.N., Ceylan R., Zengin G., Kosso Boka N.R., Ochou O.G. Chemical composition, antioxidant activity, cholinesterase inhibitor and in vitro insecticidal Potentiality of essential oils of Lippia multiflora Moldenke and Eucalyptus globulus Labill. On the main Carpophagous pests of Cotton plant in Ivory Coast. Chem. Biodivers. 2022;19 doi: 10.1002/cbdv.202100993. [DOI] [PubMed] [Google Scholar]
- 66.Arafa W.M., Aboelhadid S.M., Moawad A., Shokeir K.M., Ahmed O. Toxicity, repellency and anti-cholinesterase activities of thymol-eucalyptus combinations against phenotypically resistant Rhipicephalus annulatus ticks. Exp. Appl. Acarol. 2020;81:265–277. doi: 10.1007/S10493-020-00506-1. [DOI] [PubMed] [Google Scholar]
- 67.Santos E.G.G.D., Bezerra W.A.D.S., Temeyer K.B., de León A.A.P., Costa-Junior L.M., Soares A.M.D.S. Effects of essential oils on native and recombinant acetylcholinesterases of Rhipicephalus microplus. Rev. Bras. Parasitol. Vet. 2021;30 doi: 10.1590/S1984-29612021024. [DOI] [PubMed] [Google Scholar]
- 68.Ben Marzoug H.N., Nouajila J., Ennajr M., Lebrihi A., Mathieu F., Couderc G., Abderraba M., Romdhane M. Eucalyptus (gracilis, oleosa, salubris, and salmonophloia) essential oils: their chemical composition and antioxidant and antimicrobial activities. J. Med. Food. 2010;13:1005–1012. doi: 10.1089/jmf.2009.0153. [DOI] [PubMed] [Google Scholar]
- 69.Bardaweel S., Hudaib M., Tawaha K. Evaluation of antibacterial, antifungal, and anticancer activities of essential oils from six species of Eucalyptus. J. Essent. Oil-Bear. Plants. 2014;17:1165–1174. doi: 10.1080/0972060X.2014.963169. [DOI] [Google Scholar]
- 70.Bardaweel S., Hudaib M., Tawaha K., Bashatwah R. Antimicrobial, antioxidant and free radical Scavenging activities of essential oils extracted from six Eucalyptus species. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015;9:66. [Google Scholar]
- 71.Rahimi-Nasrabadi M., Nazarian S., Koohbijari G., Ahmadi F., Batooli H. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell. Int. J. Food Prop. 2011;16:369–381. doi: 10.1080/10942912.2010.551310. [DOI] [Google Scholar]
- 72.Bidgoli R.D., Abdullah F.O., Budriesi R., Mattioli L.B., Spadoni G., Mari M., Micucci M. Essential oil chemical composition, antioxidant and antibacterial activities of Eucalyptus largiflorens F. Muell. Pol. J. Environ. Stud. 2023;32:3043–3052. doi: 10.15244/pjoes/161886. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.