Abstract
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a central nervous system demyelinating disease. Current therapy methods, however, have limited effect on acute attacks except for intravenous methylprednisolone (IVMP). Efgartigimod is a first-in-class novel human immunoglobulin G1 (IgG1) Fc fragment approved for the treatment of generalized myasthenia gravis. Its capacity to rapidly decrease serum IgG levels, including pathogenic autoantibodies, positions it as a potentially effective option for managing the acute phase of NMOSD.
Case presentation
We report the case of a 59-year-old female patient with acute NMOSD, presenting with vision loss and numbness in all four limbs. Despite an initial inadequate response to intravenous methylprednisolone (IVMP), the addition of Efgartigimod to her treatment regimen led to rapid improvement, notably including a significant reduction in serum aquaporin-4 antibody titers, total IgG levels, and inflammation cytokine levels. Furthermore, no adverse events were reported during a four-month follow-up period.
Conclusion
As an adjunct to glucocorticoid therapy, Efgartigimod has proven effective and safe for this patient. However, to ascertain its potential as a novel therapeutic option for acute NMOSD, larger-scale prospective clinical trials are required.
Keywords: Neuromyelitis optica spectrum disorder (NMOSD), Efgartigimod, AQP4-IgG, FcRn. case report
1. Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune-mediated CNS demyelinating disease that has a high recurrence and impairment rate [1]. The primary treatment for the acute phase of NMOSD is intravenous methylprednisolone (IVMP), which is only successful in at most 80 % of patients [2]. Patients who do not respond well to IVMP can choose plasma exchange (PE) or intravenous immunoglobulin (IVIG) as second-line treatment. However, the efficacy of PE or IVIG is extremely limited most of the time [3,4]. Exploring innovative and effective therapies for acute NMOSD is an urgent dilemma for clinicians.
Efgartigimod represents a new class of neonatal Fc receptor (FcRn) antagonists, derived from the human immunoglobulin G1 (IgG1) Fc domain (residues D220-K447). It effectively reduces immunoglobulin G (IgG) levels in human serum [5]. Phase III clinical trials have demonstrated that Efgartigimod reduced IgG levels within one week, indicating its potential as a therapeutic for the acute phase of neurological autoimmune diseases. Furthermore, Efgartigimod was approved for the treatment in myasthenia gravis (MG) adults. This medication has the potential to treat patients suffering from acute NMOSD [6]. In this case, we report on the treatment of an acute NMOSD patient with Efgartigimod, aimed at exploring its efficacy during the acute phase of the disease.
2. Case Presentation
A 59-year-old woman with a history of congenital high myopia and a 3-year history of Sjogren's syndrome complained of the onset of vision loss in her left eye without any clear triggers. In less than a month, her left eye (left eye: no light perception, NLP; corrected vision of right eye: 20/32, Fig. 1) completely lost sight without ophthalmodynia or pain in eye movement. Regretfully, despite visiting several hospitals, her eyesight in her left eye remained unchanged. In the 6th month, she was diagnosed with ischemic optic neuropathy. Although she was given a compound anisodine hydrobromide injection (2 ml i.h., qd), her vision in the right eye similarly declined within a month (left eye: NLP, corrected vision of right eye: 20/500). In the 7th month, she went to the department of neurology at other hospitals, where positive aquaporin 4-IgG (AQP4-IgG) was found in the serum (titre: 1:320, cell-based assay, CBA) and cerebrospinal fluid (CSF, titre: 1:10, CBA); NMOSD was thereby diagnosed. Then she received IVMP 500mg × 5d (oral prednisone taper from 60mg/d) and IVIG (20g × 5d i.v.) sequentially. However, she felt that her right eye vision did not improve significantly (left eye: NLP, corrected vision of right eye: 20/100) and subsequently remained at that level. In the 10th month, she suffered numbness in four limbs and recurring visual loss, which had a significant impact on her daily activities. Therefore, she went to our department for further treatment.
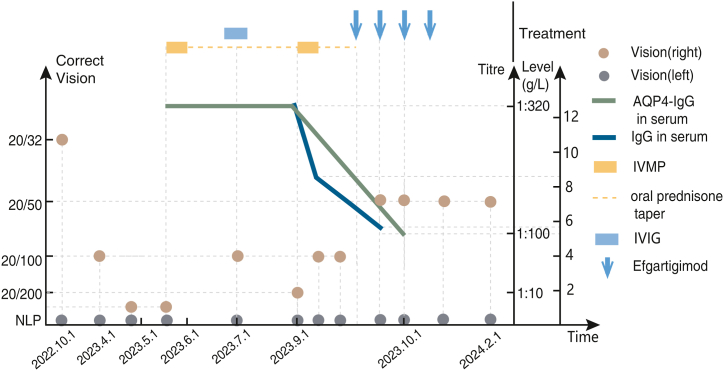
Fig. 1.
The Variation of Immunotherapy with Corrected Vision, AQP4-IgG Level and IgG Level in the NMOSD patient
AQP4-IgG: aquaporin-4-IgG, IVIG: intravenous immunoglobulin, IVMP: intravenous methylprednisolone, NLP: no light perception.
Neurological examination: A fundus examination revealed retinal and choroid atrophy surrounding the optic disc. The bilateral pupils had a diameter of 4mm, with the left pupil fixed and the right pupillary light reflex sensitive. Vision in her left eye: NLP, corrected vision in the right eye: 20/200. There was diminished pinprick sensitivity below C5 on both sides. She had no other neurological abnormalities. Expanded disability status scale, EDSS = 4.5 (ambulation 1, visual functions 6, pyramidal functions 0, sensory functions 2). Average thickness of retinal nerve fibre layer (RNFL): 29μm (left eye), 37μm (right eye). MRI scan of the brain and spinal cord, after admission, revealed multifocal T2-hyperintensity in the cerebral white matter, brainstem and cervical cord without enhancement. Orbital MRI revealed that bilateral optic nerve sheath were enhanced, and suspicious enhancement was observed in the intracranial segments of both optic nerves (Fig. 2 a-f). Because of her symptoms of dry mouth and dry eyes, we conducted a re-examination related to Sjogren's syndrome for her. Schirmer I: 2mm in both eyes. Ocular staining score: 6 (left eye), 5 (right eye). Natural salivary flow rate: 0.4ml/min. Anti–SS–A antibody (+++), anti-Ro-52 antibody (+++), which confirmed the diagnosis of Sjogren's syndrome. The following are the results of her laboratory testing: IgG in serum: 12.60g/L, IgA in serum: 1.74g/L, IgM in serum: 0.49g/L, IgE in serum: 5 IU/mL; CSF white cell count: 2 × 106/L, CSF protein: 46 mg/dl, oligoclonal bands (−), AQP4-IgG in serum: 1:320 (CBA), AQP4-IgG in CSF: 1:10 (CBA). Her visual alterations are depicted in Fig. 1.
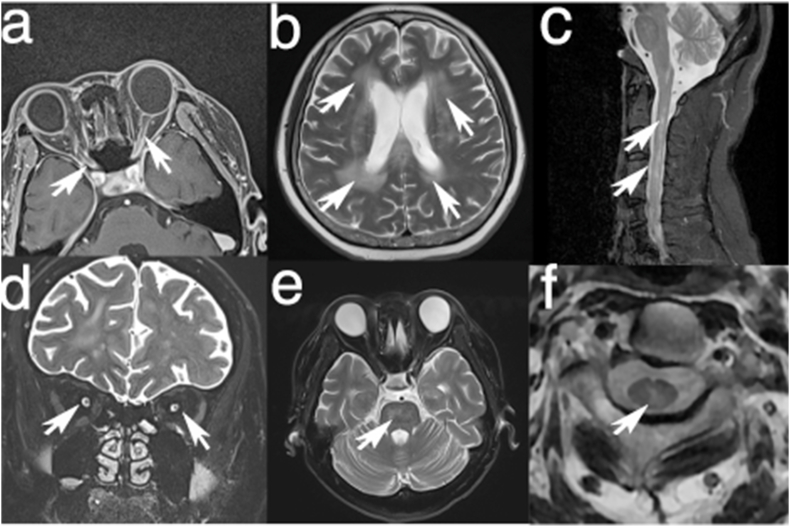
Fig. 2.
Patient's MRI features. The T1-weighted (T1WI) images with gadolinium enhancement by Gadobutrol reveal bilateral enhancement of the optic nerve sheaths and notable enhancement within the intracranial segments of the bilateral optic nerves (a); Coronal T2-weighted images (T2WI) display pronounced T2 hyperintensity in the orbital segments of both optic nerves (d); Multiple patchy T2-hyperintense lesions across the bilateral periventricular areas, basal ganglia, pons, and subcortical white matter (b, e); Longitudinally extensive myelitis lesions spanning the cervical spinal cord from C1 to C5 (c) with central located (f).
Then she was given IVMP 1000mg × 5d. However, her right eye vision did not improve a lot (left eye: NLP, corrected vision of right eye: 20/100), which is a common treatment dilemma in the NMOSD acute phase when there is poor response to conventional treatments. The patient sincerely desired to improve her vision. As an invasive therapy approach, PE has a limited therapeutic effect. Efgartigimod was accessible in some countries at that moment and licensed for generalized myasthenia gravis. As a result, we chose to prioritize Efgartigimod as an add-on therapy according to the drug instructions (10 mg/kg IV, weekly × 4) after she signed the informed consent form. After 3 days of the first dose of Efgartigimod treatment (Day 4), the IgG level in her serum decreased by around 34.5 % (IgG before Efgartigimod treatment (Day 0):12.60g/L; Day 4: 8.26g/L; Day 30: 5.81g/L, Fig. 1). On Day 8, her corrected vision of the right eye improved to 20/50 (EDSS = 3, ambulation 1, visual functions 5, pyramidal functions 0, sensory functions 0) and her numbness entirely disappeared, then she received her second dose of Efgartigimod (10 mg/kg IV). The serum AQP4-IgG titer decreased to 1:100 (serum, CBA) on Day 30 (Fig. 1). Cytokine levels in her peripheral blood also changed (Before Efgartigimod therapy: interleukin-6, IL-6 4.53pg/ml; interleukin-8, IL-8 6.92pg/ml; interleukin-17, IL-17 5.52pg/ml; interferon-γ, IFN-γ 5.52pg/ml; interleukin-10, IL-10 11.71pg/ml. Day 30: IL-6 3.03pg/ml, IL-8 4.67pg/ml, IL-17 5.03pg/ml, IFN-γ 2.26pg/ml, IL-10 23.36pg/ml). During the subsequent 4-month follow-up, the corrected vision in the right eye in this patient stayed at 20/50.There were no obvious adverse effects during the administration of Efgartigimod and subsequent 4-month follow-up. However, long-term monitoring is still required for potential side effects such as hypogammaglobulinemia and concerns about opportunistic infections related to Efgartigimod.
3. Discussions
We describe the efficacy and safety of Efgartigimod in a 59-year-old female with an acute NMOSD attack. For the first time, Efgartigimod was prescribed as a rescue treatment after IVMP in the acute phase of NMOSD which relieved the serious vision loss in this patient.
FcRn has the potential to be a novel target for the treatment of acute phase of NMOSD, inspired by the mechanism of IVIG. In general, the Fc domain of absorbed IgG binds to FcRn with great affinity, causing IgG to be transferred to the cell surface and released. Lysosomes will destroy IgG that is not coupled to FcRn. This procedure increases IgG (including pathogenic IgG) half-life [7]. In high quantities (1–2 g/kg), infused IgG saturate FcRn and cause autoantibody catabolism, resulting in a shorter period of autoimmune IgG in the body [8]. Similarly, as FcRn antagonists, Efgartigimod injection eliminated blood IgG in healthy volunteers and MG patients rapidly and specifically: a single injection lowered by 50 %, while successive dosages reduced by almost 75 % [5,9]. Efgartigimod, compared to ineblizumab (a CD19 monoclonal antibody) [10] and satralizumab (an IL-6 monoclonal antibody) [11], demonstrates a more rapid onset of action. However, the onset time of efgartigimod may be close to that of eculizumab (an anti-C5 complement inhibitor) [12,13]. Therefore, we speculate that this rapid efficacy is likely to significantly enhance symptom management in patients experiencing acute NMOSD episodes. Given the acute nature of this patient's condition, efgartigimod was selected for treatment.
In addition to NMOSD, the patient was diagnosed with Sjögren's Syndrome, a condition that may further contribute to the observed visual impairment and hindered recovery. Prior research has indicated that the Fc receptor plays a significant role in the pathogenesis of Sjögren's Syndrome [14]. Consequently, antagonists of the neonatal Fc receptor could potentially offer a therapeutic strategy for treating Sjögren's Syndrome.
Improvement in the vision of this patient's right eye was observed after intravenous infusion of Efgartigimod. Subsequent to receiving four doses over the course of one month, there was a significant reduction in her total IgG, AQP4-IgG, and several inflammation-related cytokines in the peripheral blood. This suggests that the integration of Efgartigimod with IVMP may offer promise for the future management of acute NMOSD. Notably, visual improvement was observed after just one week of Efgartigimod treatment, leaving open the possibility that this patient's improvement might partially be attributed to lingering glucocorticoids. The patient experienced no adverse events during or subsequent to treatment. Earlier studies have demonstrated that the addition of the FcRn-monoclonal antibody, Batoclimab, to IVMP therapy enhances the EDSS scores in patients with acute NMOSD within 3–6 months [15]. Thus, FcRn antagonists are probably beneficial not only during the acute phase of NMOSD but may also contribute to sustained neurological function improvement. Extended observation and a larger patient cohort are required to validate the long-term efficacy and safety of Efgartigimod.
4. Conclusions
Under the results of the case of efgartigimod treatment in NMOSD, we speculate that efgartigimod has the potential to be a rescue treatment target for the acute phase of NMOSD. Additional randomized controlled trials or cases will be necessary to further confirm the well-documented efficacy and safety.
Ethics statement
This study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, with the approval number: KY2023-488. All patients provided informed consent to participate in the study. All patients provided informed consent for the publication of their anonymised case details and images.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Zhizhong Li: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Qiao Xu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jialu Huang: Writing – review & editing, Resources, Investigation. Qiyuan Zhu: Resources, Methodology, Investigation. Xiaolin Yang: Writing – review & editing, Investigation. Mengjie Zhang: Methodology, Investigation. Shaoru Zhang: Investigation. Siyuan Huang: Investigation. Gang Yu: Resources. Peng Zheng: Resources. Xinyue Qin: Writing – review & editing, Supervision. Jinzhou Feng: Writing – review & editing, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xinyue Qin, Email: qinxinyuecqchina@hotmail.com.
Jinzhou Feng, Email: 203756@cqmu.edu.cn.
References
- 1.Wingerchuk D.M., et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sellner J., et al. EFNS guidelines on diagnosis and management of neuromyelitis optica: diagnosis and management of neuromyelitis optica. Eur. J. Neurol. 2010;17:1019–1032. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 3.Li X., et al. Intravenous immunoglobulin for acute attacks in neuromyelitis optica spectrum disorders (NMOSD) Multiple Sclerosis and Related Disorders. 2020;44 doi: 10.1016/j.msard.2020.102325. [DOI] [PubMed] [Google Scholar]
- 4.Siritho S., Nopsopon T., Pongpirul K. Therapeutic plasma exchange vs conventional treatment with intravenous high dose steroid for neuromyelitis optica spectrum disorders (NMOSD): a systematic review and meta-analysis. J. Neurol. 2021;268:4549–4562. doi: 10.1007/s00415-020-10257-z. [DOI] [PubMed] [Google Scholar]
- 5.Ulrichts P., et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J. Clin. Invest. 2018;128:4372–4386. doi: 10.1172/JCI97911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yunce M., et al. Neonatal Fc receptor blockade as emerging therapy in diseases with plasma exchange indications. J. Clin. Apher. 2023;38:632–640. doi: 10.1002/jca.22055. [DOI] [PubMed] [Google Scholar]
- 7.Bayry J., Kaveri S.V. Kill ‘em all: efgartigimod Immunotherapy for autoimmune diseases. Trends Pharmacol. Sci. 2018;39:919–922. doi: 10.1016/j.tips.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Akilesh S., et al. The MHC class I–like Fc receptor promotes humorally mediated autoimmune disease. J. Clin. Invest. 2004;113:1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard J.F., et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20:526–536. doi: 10.1016/S1474-4422(21)00159-9. [DOI] [PubMed] [Google Scholar]
- 10.Cree B.A.C., et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 11.Traboulsee A., et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19:402–412. doi: 10.1016/S1474-4422(20)30078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingerchuk D.M., et al. PREVENT study group, long-term safety and efficacy of eculizumab in aquaporin-4 IgG-positive NMOSD. Ann. Neurol. 2021;89:1088–1098. doi: 10.1002/ana.26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijnsma K.L., et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin. Pharmacokinet. 2019;58:859–874. doi: 10.1007/s40262-019-00742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalayer E., et al. Fc receptors gone wrong: a comprehensive review of their roles in autoimmune and inflammatory diseases. Autoimmun. Rev. 2022;21 doi: 10.1016/j.autrev.2021.103016. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., et al. Batoclimab NMOSD Study Group, Batoclimab as an add‐on therapy in neuromyelitis optica spectrum disorder patients with acute attacks. Eur. J. Neurol. 2023;30:195–203. doi: 10.1111/ene.15561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.