Abstract
Objectives
Determining the best available therapy for carbapenem-resistant Acinetobacter baumannii (CRAB) infections is a challenge. Cefiderocol is an attractive alternative drug effective against many resistance mechanisms in Gram-negative bacteria. However, its place in the treatment of Acinetobacter baumannii infections remains unclear and much debated, with contradictory results.
Methods
We describe here the case of a 37-year-old man with ventilator-associated bacteraemic CRAB pneumonia in an intensive care unit. He was initially treated with a combination of colistin and tigecycline, and was then switched onto colistin and cefiderocol. We then used a new accessible protocol to test 30 CRAB isolates (OXA-23/OXA-24/OXA-58/NDM-1) for adaptive resistance to cefiderocol (ARC) after exposure to this drug.
Results
After clinical failure with the initial combination, we noted a significant clinical improvement in the patient on the second combination, leading to clinical cure. No ARC was detected in the two OXA-23 case-CRAB isolates. All NDM-1 CRAB isolates were resistant to cefiderocol in standard tests; the OXA-23, OXA-24 and OXA-58 CRAB isolates presented 84.2 %, 50 % and 0 % ARC, respectively.
Conclusions
ARC is not routinely assessed for CRAB isolates despite frequently being reported in susceptible isolates (69.2 %). Subpopulations displaying ARC may account for treatment failure, but this hypothesis should be treated with caution in the absence of robust clinical data. The two main findings of this work are that (i) cefiderocol monotherapy should probably not be recommended for OXA-23/24 CRAB infections and (ii) the characterisation of carbapenemases in CRAB strains may be informative for clinical decision-making.
Keywords: Cefiderocol, Adaptive resistance, Acinetobacter baumannii, Therapeutic decision, NDM/OXA carbapenemases
Highlights
-
•
Test for adaptive resistance to cefiderocol in CRAB is a precious tool.
-
•
Cefiderocol-resistant subpopulations in CRAB may explain clinical failures.
-
•
Use of cefiderocol in combination therapy could be an option for treated CRAB infections.
-
•
Knowledge of the carbapenemase-type produced by CRAB is useful for managing this drug.
-
•
Collaboration between clinicians and microbiologists is essential.
1. Introduction
Multidrug- (MDR) and extensively drug-resistant (XDR) Gram-negative bacteria are increasingly emerging worldwide. The burden of these infections is considerable and was estimated at 192 000 years lost to disability and more than 1.27 million attributable deaths in the world in 2019 [1]. The development of new antibiotics is, thus, a global priority for the World Health Organisation (WHO) [2]. Cefiderocol, a siderophore cephalosporin, has emerged as a safe new alternative effective against bacteria displaying a large range of beta-lactam resistance mechanisms: β-lactamases (especially AmpC cephalosporinase; Klebsiella pneumoniae carbapenemase KPC, New Delhi Metallo-β-lactamase NDM and Oxacillinase OXA carbapenemases), porin mutations and efflux pumps [3]. However, its value for the clinical treatment of Acinetobacter baumannii infections remains unclear and a matter for debate. Cefiderocol has been shown to be effective for treating nosocomial pneumonia [4], but recent studies have reported higher mortality rates for patients on this drug, particularly in cases of bacteraemia and nosocomial pneumonia due to carbapenem-resistant A. baumannii (CRAB) [[5], [6], [7], [8]]. One possible reason for this discrepancy is the presence of bacterial subpopulations displaying hetero- or adaptive resistance, unmasked by the use of cefiderocol [9,10]. We report a case of nosocomial CRAB bacteraemia and pneumonia successfully treated with cefiderocol and colistin, which led us to use an accessible new protocol to explore adaptive resistance to cefiderocol (ARC) in our collection of CRAB isolates as a function of carbapenemase type (OXA/NDM).
2. Case description
A 37-year-old man was admitted to the intensive care unit (ICU) in April 2022 for acute respiratory failure and shock. He had a history of asthma since childhood, Biermer's disease, diagnosed in 2021, and chronic alcoholism. His treatments included monthly vitamin B12 supplements and the use of β-2 mimetics as required. He was initially admitted to the hepatogastroenterology unit on April 9th, 2022, for acute necrotic-haemorrhagic pancreatitis. The next day, he was transferred to the ICU for respiratory failure requiring oxygen supplementation at a rate of 15 L/min and low blood pressure (76/33 mmHg). Biological tests revealed an inflammatory syndrome with 20.6 x 109 leukocytes/L, a C-reactive protein concentration of 252 mg/L and acute renal failure, with a creatinaemia of 113 μmol/L and a lactate concentration of 2.5 mmol/L. Chest X ray showed a bilateral pulmonary infiltrate. Trans-thoracic ultrasound revealed no signs of heart failure. The patient was treated with oxygen therapy and intravenous fluid therapy. The patient's respiratory condition declined, rendering oro-tracheal intubation with one ventral decubitus session necessary on April 12th. A computed tomography scanner showed bibasal pleuropneumonia, probably related to inhalation. All respiratory samples were sterile. Empiric treatment with 2 g cefotaxime and 500 mg metronidazole every 8 h for seven days was initiated. On April 18th, the patient's respiratory condition declined again and ventilator-acquired CRAB and Extended-Spectrum-β-Lactamase (ESBL)-producing Enterobacter cloacae pneumonia was documented (Fig. 1A and Table S1). Antimicrobial susceptibility testing for A. baumannii revealed resistance to all antibiotics tested (cefiderocol was not initially tested) except colistin and tygecycline. Treatment was resumed with 9 MIU colistin, followed by 4.2 MIU every 12 h, and 200 mg tygecycline followed by 100 mg every 12 h for nine days. On May 6th, the patient's condition worsened, with the development of bacteraemic CRAB pleuropneumonia (Fig. 1B and Table S1). The patient was then switched onto colistin plus 2 g cefiderocol every 6 h until May 24, after standard determination of the susceptibility of the CRAB isolate to cefiderocol (disk diffusion method, European Committee on Antimicrobial Susceptibility Testing, EUCAST 2021 note, MIC assessment not available, The European Committee on Antimicrobial Susceptibility Testing. Breakpoint for cefiderocol from EUCAST. Addendum (May 2020) to EUCAST breakpoint v. 10.0. Breakpoints to be included EUCAST breakpoint tables v 11.0, January 2021Version 10.0, 2024. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf). A significant improvement in the patient's respiratory condition was noted after 10 days of bitherapy, and the patient was weaned off of the ventilator on May 22nd. The patient was transferred to the medical unit on May 23rd, and was discharged home on June 8th, with a favourable clinical outcome (Fig. 1C).
Fig. 1.
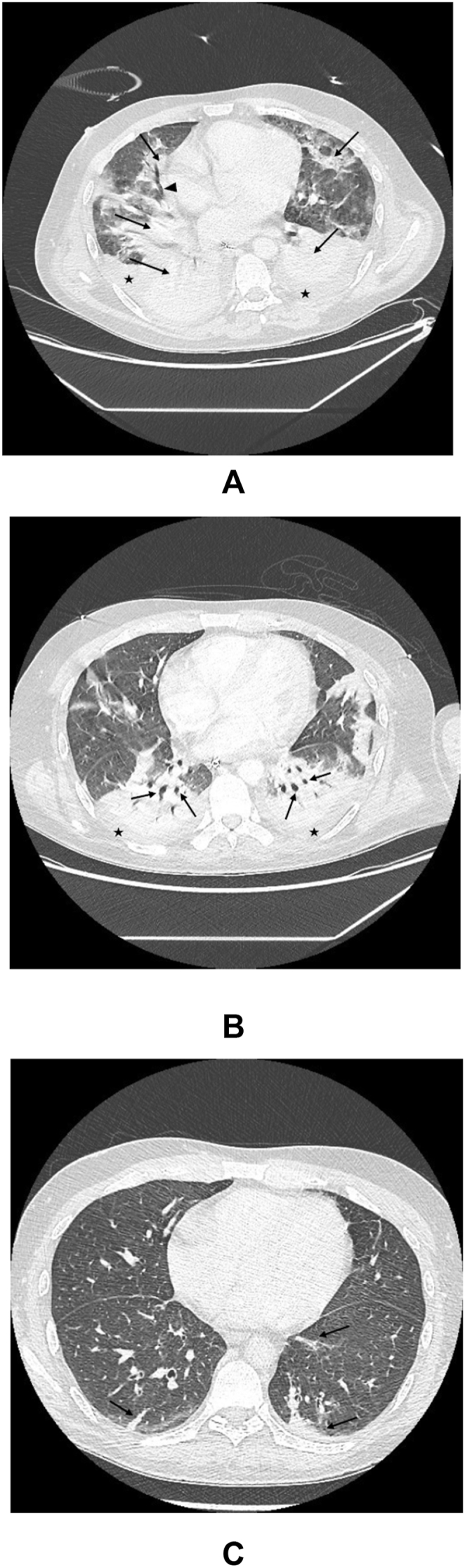
Progression of lung involvement on CT scans
A. Contrast-enhanced computed tomography (CECT) of the chest, showing bilateral multifocal areas of consolidation (arrows) with a rare air bronchogram in the right middle lobe (arrowhead) and no sign of necrosis or cavitation. Bilateral pleural effusion (★).
B. CECT of the chest, showing a partial regression of consolidation and additional air bronchograms in the upper segments of the lower lobes (arrows). Partial regression of bilateral pleural effusion (★).
C. Non-enhanced computed tomography (NECT) of the chest, showing small residual consolidations in the upper segments of the lower lobes (arrows). No pleural effusion.
3. Methods
3.1. Microbiological analyses
Thirty carbapenem-resistant Acinetobacter sp. isolates from the biobank of Reunion Island University Hospital (UHRI) were used and specifically tested for ARC, according to carbapenemase status (NDM/OXA). We also included the two isolates from the patient studied here (A3a and A3b). For each isolate, susceptibility to cefiderocol was determined by the disk diffusion and microdilution methods (EUCAST recommendations). We used a new method, as described below, to investigate ARC in non-NDM-1 isolates.
3.2. Molecular characterisation of resistance
The two CRAB isolates from the patient with ventilator-associated pneumonia and bacteraemia were screened for the presence of OXA-23-like, OXA-24/58-like and NDM carbapenemases with a lateral flow assay (Coris RESIST Acineto, Coris bioConcept, Gembloux, Belgium). The two isolates were then sent to the French National Centre for Antimicrobial Resistance (FNCAR) for confirmation of their molecular resistance profiles by PCR or whole-genome sequencing.
3.3. Standard antimicrobial susceptibility testing (AST)
We assessed the susceptibility of each isolate to cefiderocol by the disk diffusion and broth microdilution methods, in accordance with the EUCAST 2020 recommendations. Breakpoint for cefiderocol from EUCAST. Addendum (May 2020) to EUCAST breakpoint v. 10.0. Breakpoints to be included EUCAST breakpoint tables v 11.0, January 2021Version 10.0, 2024. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf). We used a disk containing 30 μg cefiderocol (Liofilchem Diagnostics, Roseto degli Abruzzi, Italia) on Mueller Hinton agar plates (MHE, bioMérieux, Marcy l’Étoile, France), with a cut-off of 17 mm. Note that the 2022 EUCAST warning does not apply to this combination of reagents. The minimum inhibitory concentration (MIC) of cefiderocol was determined with UMIC®Cefiderocol (Bruker daltonics, Bremen, Germany) on ID-CA-MHB (iron-depleted cation-adjusted Muller Hinton broth) according to EUCAST recommendations. An isolate was considered susceptible to cefiderocol if its MIC was ≤2 mg/L. The diameter of inhibition was measured and photographed, and the results were read twice, independently, by two different operators.
3.4. Adaptive resistance to cefiderocol
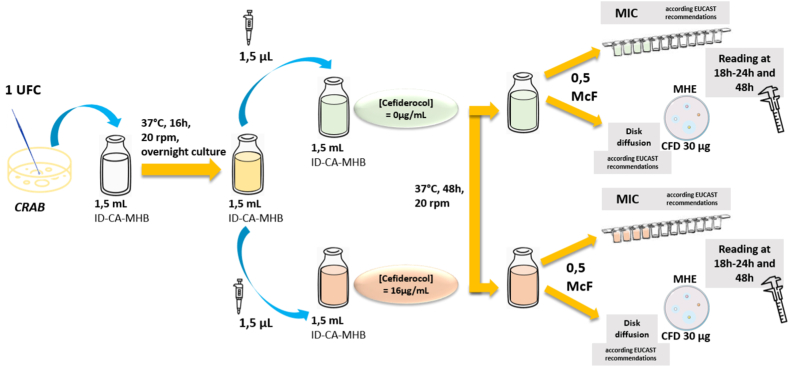
We investigated the ARC of the isolates with a new protocol adapted from that described by Choby et al. [10]: a single colony of carbapenem-resistant A. baumannii from a frozen streak was used to inoculate 1.5 mL ID-CA-MHB (Bruker daltonics). The overnight culture was incubated at 37 °C for 16 h, with shaking at 20 rpm. We then added 1.5 μL of this overnight culture of Acinetobacter sp. to 1.5 ml ID-CA-MHB containing 0 or 16 μg/ml cefiderocol (Fetcroja, Shionogi & Co.) and incubated the resulting suspension for 48 h at 37 °C with shaking at 20 rpm. The disk diffusion and microdilution assays were performed as described above, with a reading time of 18–24 h and 48 h for inhibition diameters and 18–24 h for MIC (Fig. 2). For the calculation of resistance percentages, the two isolates obtained from the case described here were considered as a single isolate (A3) among a total of 30 isolates tested (see Table 1).
Fig. 2.
Procedure used to measure adaptive resistance to cefiderocol by the disk diffusion and minimum inhibitory concentration methods, for each sample (blood culture and lower respiratory tract).
Overnight cultures of carbapenem-resistant A. baumannii isolates were prepared by using a single colony from a frozen streak to inoculate 1.5 mL iron-depleted cation-adjusted Mueller Hinton Broth (ID-CA-MHB, Bruker Daltonics). The cultures were incubated at 37 °C for 16 h with shaking at 20 rpm. We then added 1.5 μL of the overnight culture of A. baumannii to 1.5 mL ID-CA-MHB supplemented with 0 or 16 μg/mL cefiderocol (Fetcroja, Shionogi & Co.) and incubated the culture at 37 °C for 48 h, with shaking at 20 rpm. Readings were taken after 18–24 h and 48 h of growth for inhibition diameters and after 18–24 h for MIC determination.
CFD: cefiderocol; ID-CA-MHB: iron-depleted cation-adjusted Mueller-Hinton broth; McF: MacFarland; MHE: Mueller-Hinton E agar; MIC: minimum inhibitory concentration; RPM: rotations per minute; CFU: colony-forming units.
Table 1.
Tests for adaptive resistance to cefiderocol performed on 30 carbapenem-resistant Acinetobacter spp. isolates with the new protocol adapted from that described by Choby et al. [10].
| CFD 0 μg/mL |
CFD 16 μg/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | Origin | Species | Carbapenemase | Resistance genes | ID (mm) |
MIC (mg/L) |
ID (mm) |
MIC (mg/L) |
||
| 18–24 h | 48 h | 18–24 h | 18–24 h | 48 h | 18–24 h | |||||
| A1 | Madagascar | A. baumannii | OXA-24 | AmpC, TEM | S (22) | S (21) | S (0,5) | R (15) | R (13) | R (32) |
| A2 | Mainland France | A. baumannii | OXA-24 | AmpC | S (24) | S (24) | S (0.25) | R (16) | R (11) | R (16) |
| A3a | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (22) | S (22) | S (2) | S (18) | S (18) | S (2) |
| A3b | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (21) | S (20) | S (2) | S (21) | S (19) | S (2) |
| A4 | Mayotte | A. baumannii | OXA-23 | – | S (23) | S (23) | S (0.25) | R (16) | R (16) | R (8) |
| A5 | Comoros | A. baumannii | OXA-58 | – | S (27) | S (27) | S (0.06) | S (32) | S (32) | S (0.06) |
| A6 | Ivory Coast | A. baumannii | OXA-58 | – | S (22) | S (22) | S (0.25) | S (17) | S (17) | S (0,03) |
| A7 | Mainland France | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (19) | S (18) | S (1) | R (11) | R (6) | R (16) |
| A8 | Mayotte | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (24) | S (21) | S (1) | S (20) | R (15) | R (16) |
| A9 | Reunion Island | A. baumannii | OXA-72 (OXA-24 type) | – | S (25) | S (24) | S (0.125) | S (25) | S (20) | S (0.5) |
| A10 | Madagascar | A. baumannii | OXA-23 | AmpC | S (20) | S (19) | S (0.25) | R (12) | R (7) | R (16) |
| A11 | Madagascar | A. baumannii | OXA-23 | AmpC | S (20) | S (19) | S (1) | R (13) | R (8) | R (8) |
| A12 | Madagascar | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (22) | S (21) | S (2) | R (6) | R (6) | R (16) |
| A13 | Mauritius | A. baumannii | OXA-23 | AmpC | S (20) | S (19) | S (2) | S (18) | R (14) | R (16) |
| A14 | Reunion Island | A. baumannii | OXA-23 | AmpC | S (21) | S (18) | S (0.5) | S (14) | S (21) | S (0.25) |
| A15 | Reunion Island | A. baumannii | OXA-23 | AmpC | S (23) | S (23) | S (0.5) | S (30) | S (26) | S (0.125) |
| A16 | Reunion Island | A. baumannii | OXA-24 | AmpC, TEM | S (22) | S (20) | S (1) | S (23) | S (21) | S (0.5) |
| A17 | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (20) | S (19) | S (2) | R (16) | R (14) | R (16) |
| A18 | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (26) | S (20) | S (0.25) | R (10) | R (10) | R (16) |
| A19 | Madagascar | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (28) | S (25) | S (1) | R (8) | R (8) | R (8) |
| A20 | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM, ArmA | S (25) | S (24) | S (0.5) | R (10) | R (12) | R (8) |
| A21 | Mauritius | A. baumannii | OXA-23 | ArmA | S (25) | S (24) | S (0.25) | R (14) | R (14) | R (8) |
| A22 | Madagascar | A. baumannii | OXA-23 | PER-7, ArmA | R (12) | R (12) | R (8) | R (8) | R (8) | R (16) |
| A23 | Mainland France | A. baumannii | OXA-23 | ArmA | S (25) | S (25) | S (0.25) | R (16) | R (14) | R (4) |
| A24 | Mayotte | A. nosocomialis | OXA-420 (OXA-58 type) | – | S (21) | S (21) | S (0.5) | S (23) | S (23) | S (1) |
| A25 | Reunion Island | A. baumannii | OXA-23 | AmpC, ArmA | S (21) | S (19) | S (0.5) | R (14) | R (14) | R (8) |
| A26 | Reunion Island | A. baumannii | OXA-23 | AmpC, TEM | S (21) | S (19) | S (0.5) | R (16) | R (16) | R (8) |
| A27 | Madagascar | A. baumannii | OXA-23 | AmpC, TEM | S (22) | S (19) | S (0.5) | R (14) | R (14) | R (32) |
| A28 | Mayotte | A. ursingii | NDM-1 | – | R (14) | – | R (4) | – | – | – |
| A29 | Reunion Island | A. baumannii | NDM-1 | – | R (11) | – | R (16) | – | – | – |
| A30 | Mayotte | A. baumannii | NDM-1 + OXA-23 | - | R (8) | – | R (32) | – | – | – |
Resistance to cefiderocol was detected in all NDM-1 (3/3) and OXA-23+PER-7 isolates in standard AST. Adaptive resistance to cefiderocol was detected in 84.2 % (16/19) and 50 % (2/4) of OXA-23 and OXA-24-type isolates, respectively.
MIC: minimum inhibitory concentration; R: resistant; S: susceptible; ID: inhibition diameter.
4. Results
4.1. Two case isolates
Molecular investigation by sequencing revealed an overproduction of AmpC, the production of OXA-23 and TEMoneira (TEM) leading to β-lactam resistance, and an ArmA-type 16sRNA methylase responsible for resistance to aminoglycosides.
In standard AST, the two isolates from the studied case, obtained from lung and blood cultures, were classified as susceptible to cefiderocol in disk diffusion (≥21 mm) and broth microdilution (MIC = 2 mg/L) assays. No ARC was detected in the two isolates from the case, with an MIC = 2 mg/L before and after 48 h of exposure to cefiderocol (see Table 1).
4.2. ARC in the UHRI collection
In the second phase of our study, we tested a collection of 30 carbapenem-resistant Acinetobacter spp. isolates (OXA-23 without NDM-1, n = 20; OXA-24 type, n = 4; OXA-58 type, n = 3; NDM-1, n = 3). All NDM-1 isolates (n = 3) were categorised resistant to cefiderocol in standard AST (MIC >2 mg/L). One (OXA-23+PER-7) of the 27 isolates with OXA-type carbapenemases was resistant, whereas the other 26 were categorised susceptible in standard AST (MIC ≤2 mg/L). None of the OXA-58 type isolates had adaptive resistance, with no difference observed in the inhibition diameter (≥17 mm with no colonies appearing close to the disk) or MIC (≤2 mg/mL) determinations with and without cefiderocol exposure. We found that 84.2 % (16/19) of OXA-23 isolates and 50 % (2/4) of OXA-24 type isolates (excluding OXA-23+PER-7) displayed a significant decrease in inhibition diameter (<17 mm, 6–16 mm) and an increase in MIC (>2 mg/L, 4–32 mg/L) after 48 h of exposure to cefiderocol; these isolates were considered to display adaptive resistance to cefiderocol (see Table 1 and Fig. S1). For two isolates, only a reading of inhibition diameter after 48 h was able to distinguish a subpopulation with a reduced inhibition diameter (<17 mm; see supplementary data, Fig. S1). Finally, 69.2 % of the isolates initially categorised as susceptible to cefiderocol in standard AST (n = 26) were found to display ARC. All these isolates harboured OXA-23 or OXA-24 class D carbapenemases.
5. Discussion
A. baumannii infections are associated with significant mortality, particularly in vulnerable ICU patients. Discordant results between the APEKS-NP and CREDIBLE-CR [5] studies and other case series [4,6] have led to a reluctance to use cefiderocol to treat A. baumannii complex infections. In two correspondences, Choby et al., 2021 [9,10] suggested that these discrepancies might be due to cefiderocol-heteroresistant subpopulations not detected by standard AST. This hypothesis seems to be invalidated by the latest results from the CREDIBLE-CR study presented by Longshaw et al. [11]. However, the apparently high rate of clinical or microbiological failure in some case series cannot be ignored, although other factors, such as clinical severity (septic shock) or strong immunosuppression, may influence these poor patient outcomes [6,12]. Recently, the rapid development of adaptive resistance to this molecule in vitro was well documented by Stracquadanio et al. [13]. As mentioned in two recent reviews [14,15], the clinical relevance of heteroresistance in the context of treatment failure has not been demonstrated with a sufficient level of evidence (retrospective studies, case reports) for any species other than Staphylococcus aureus (meta-analyses). We prefer the term "adaptive resistance" over heteroresistance, as it is deliberately less precise (heteroresistance being defined as a subpopulation with a frequency ≤10−6 [15]). Indeed, we wished to highlight the intrinsic capacity of this bacterial genus to adapt rapidly to this new drug. Moreover, we used a technique simpler using commercial reagents and more accessible than the current reference methods (Population analysis profile, PAPs, [10]), which cannot quantify the fraction of the subpopulation displaying ARC. We focused on the presence/absence of ARC in the isolates tested, to simulate as closely as possible the situation encountered in vivo and to orient potential use by clinicians.
We observed a clear clinical improvement in the patient studied here following combination therapy with cefiderocol and colistin. It should be noted that, despite the susceptibility of the isolate to cefiderocol in the standard AST performed at the time of diagnosis (four days after diagnosis), the ICU clinician did not select this treatment option, even though the microbiologist advised the use of a ꞵ-lactam, because of the results for the Acinetobacter subgroup obtained in the CREDIBLE-CR study. The two key points to note in this case are that (i) the isolate responsible for the infection did not develop ARC in vitro and (ii) the patient was treated with combination therapy, in this case with colistin, rather than monotherapy.
Our microbiological analyses with this new protocol (adapted from that described by Weiss et al.) yielded very similar results to recent studies based on the reference technique (PAPs): 84.2 % of the OXA-23 carbapenemase-producing CRAB isolates initially categorised as susceptible, 80 % if we include the OXA-23+PER-7 isolate initially categorised as resistant, developed ARC (80 % in the study by Stracqadanio et al.) [13]. For OXA-24-producing CRAB, it is difficult to draw any firm conclusions due to the very small number of isolates. ARC developed in 69.2 % of the isolates initially classified as susceptible in standard AST, considered together. Isolates producing another class D carbapenemase, OXA-58, did not develop ARC. All the isolates producing the NDM-1 metallo-β-lactamase or PER extended-spectrum β-lactamase were initially categorised as resistant to cefiderocol in standard AST, as previously described [16]. Thus, the type of carbapenemase type can potentially guide the choice of treatment, with a presumptive prediction of microbiological category (NDM-1: R, OXA-23/24: HR, OXA-58: S). Greater understanding of the genetic determinants (single-nucleotide polymorphisms) involved in this adaptive resistance needs to be explored. New-generation sequencing (NGS) of iron transport genes seems to be a promising way, as highlighted by of Stracquadanio S et al., [13]. The presence/absence of nonsynonymous mutations at variable frequencies in OXA-23, OXA-24 or OXA-58 genes, could be a potential pathway for the discrepancies observed. These preliminary results, of course, require confirmation in studies on larger numbers of isolates, but to our knowledge, this is the first study to establish a link between adaptive resistance and OXA-type carbapenemases. Nevertheless, this finding remains highly relevant in the context of the development of new rapid and accessible lateral flow assays (Coris bioConcept, NG biotech) allowing the rapid characterisation of the carbapenemase type in an Acinetobacter spp. isolate. With effective clinical-biological dialogue, these rapid tests can provide clinicians indications within a few minutes.
Heteroresistance is not a new phenomenon in microbiology, but its clinical implications remain unclear (except for S. aureus). It has been described for many antibiotics and combination treatments are generally used to prevent it; this would be an interesting to confirm in the context of CRAB infections [17]. Moreover, the toned to simulate iron depletion in vitro, as such depletion is thought to occur in vivo, should lead to caution in the interpretation of these microbiological results and the in vitro/in vivo correlation. Our findings suggest that cefiderocol should be considered as an alternative treatment for CRAB infections if the isolate is classified as susceptible to this antibiotic in standard AST and no other ꞵ-lactam is available. However, it should be used in combinations (colistin, tigecycline or rifampicin) to prevent the development of adaptive resistance phenomena [12,13]. Finally, it may be useful to advise microbiologists to repeat standard AST (MIC) on Acinetobacter spp. isolates after 48 or 72 h of exposure in vivo to cefiderocol in cases of infection, to check that the strain remains susceptible.
The chief limitations of this study are that we were unable to test other strains exposed to cefiderocol in vivo and we did not compare our technique to the reference technique (PAPs).
However, the protocol proposed here offers the benefit of accessibility, although it remains time-consuming (five days). To date, the clinical impact of heteroresistance remains uncertain and has been the subject of scientific world debate since 2021 [[4], [5], [6],9,10,12,13,18]. Understanding the genetic determinants in the emergence of resistance to cefiderocol, whether adaptive or not, will both require the use of standardised methods and large-scale, ideally prospective, studies [14]. Our study adds to knowledge of this phenomenon by linking the clinical course of the infection and microbiological investigations. We have three main conclusions: (i) for cefiderocol, EUCAST susceptibility testing is unable to detect adaptive resistance in Acinetobacter spp., (ii) knowledge of the carbapenemase produced by the CRAB isolate is useful, to guide the use of this drug, and (iii) the use of cefiderocol in monotherapy for OXA-23/24-producing CRAB should probably not be recommended, or should be considered only after an assessment of ARC. These last two conclusions could gain further value if more data can be generated from both work on a wider collection of strains to see if difference between OXA-58 and others may be confirmed understanding their genetic support and on more clinical isolates after cefiderocol treatment. According to the WHO, research in this field is a matter of priority, to provide clinicians with guidance in the management of CRAB infections.
Funding
None.
Ethics approval and consent to participate
Informed consent was obtained from the patient for the publication of this case report and the corresponding images.
Consent for publication
All authors have seen and approved the final manuscript. All authors consent to publication.
Data availability statement
All the data are available from the manuscript text, tables, the figures and the supplementary materials.
CRediT authorship contribution statement
Anissa Desmoulin: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Loïk Sababadichetty: Investigation. Laure Kamus: Writing – review & editing, Writing – original draft. Marion Daniel: Investigation, Formal analysis. Lucie Feletti: Writing – review & editing, Investigation, Formal analysis. Nicolas Allou: Writing – review & editing, Supervision, Investigation, Formal analysis, Conceptualization. Anaïs Potron: Writing – review & editing, Formal analysis. Anne-Gaëlle Leroy: Writing – review & editing, Formal analysis. Marie-Christine Jaffar-Bandjee: Writing – review & editing, Supervision. Olivier Belmonte: Writing – review & editing, Supervision. Thomas Garrigos: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Guillaume Miltgen: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the patient for providing informed consent for the publication of his case. We would like to thank Elodie Bonhomme and Bruker for providing us with the UMIC®Cefiderocol (Bruker daltonics, Bremen, Germany) and ID-CA-MHB (iron-depleted cation-adjusted Muller Hinton broth) samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30365.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Global priority list of antibiotics-resistant bacteria to guide research, discovery, and developpement of new antibiotics. 2017 https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed [Google Scholar]
- 3.Mushtaq S., Sadouki Z., Vickers A., Livermore D.M., Woodford N. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant gram-negative bacteria. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01582-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larcher R., Laffont-Lozes P., Loubet P., Laureillard D., Naciri T., Sotto A. Re: “Real world clinical outcome of cefiderocol for treatment of multidrug-resistant nonfermenting gram-negative bacilli infections” by Hoellinger et al. Clin. Microbiol. Infect. 2023 doi: 10.1016/j.cmi.2023.01.022. S1198-743X(23)00047-2. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 6.Hoellinger B., Simand C., Jeannot K., Garijo C., Cristinar M., Reisz F., et al. Real-world clinical outcome of cefiderocol for treatment of multidrug-resistant non-fermenting, gram negative bacilli infections: a case series. Clin. Microbiol. Infect. 2023;29:393–395. doi: 10.1016/j.cmi.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Kollef M., Dupont H., Greenberg D.E., Viale P., Echols R., Yamano Y., et al. Prospective role of cefiderocol in the management of carbapenem-resistant Acinetobacter baumannii infections: review of the evidence. Int. J. Antimicrob. Agents. 2023;62 doi: 10.1016/j.ijantimicag.2023.106882. [DOI] [PubMed] [Google Scholar]
- 8.McCreary E.K., Heil E.L., Tamma P.D. New perspectives on antimicrobial agents: cefiderocol. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/aac.02171-20. 10.1128/aac.02171-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choby J.E., Ozturk T., Satola S.W., Jacob J.T., Weiss D.S. Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results? Lancet Microbe. 2021;2:e648–e649. doi: 10.1016/S2666-5247(21)00271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choby J.E., Ozturk T., Satola S.W., Jacob J.T., Weiss D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021;21:597–598. doi: 10.1016/S1473-3099(21)00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longshaw C., Santerre Henriksen A., Dressel D., Malysa M., Silvestri C., Takemura M., et al. Heteroresistance to cefiderocol in carbapenem-resistant Acinetobacter baumannii in the CREDIBLE-CR study was not linked to clinical outcomes: a post hoc analysis. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.02371-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcone M., Tiseo G., Leonildi A., Della Sala L., Vecchione A., Barnini S., et al. Cefiderocol- compared to colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant acinetobacter baumannii. Antimicrob. Agents Chemother. 2022;66 doi: 10.1128/aac.02142-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stracquadanio S., Bonomo C., Marino A., Bongiorno D., Privitera G.F., Bivona D.A., et al. Acinetobacter baumannii and cefiderocol, between cidality and adaptability. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02347-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roch M., Sierra R., Andrey D.O. Antibiotic heteroresistance in ESKAPE pathogens, from bench to bedside. Clin. Microbiol. Infect. 2022 doi: 10.1016/j.cmi.2022.10.018. S1198743X22005328. [DOI] [PubMed] [Google Scholar]
- 15.Karakonstantis S., Rousaki M., Kritsotakis E.I. Cefiderocol: systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics (Basel) 2022;11:723. doi: 10.3390/antibiotics11060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirel L., Sadek M., Nordmann P. Contribution of PER-type and NDM-type β-lactamases to cefiderocol resistance in acinetobacter baumannii. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.00877-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Band V.I., Hufnagel D.A., Jaggavarapu S., Sherman E.X., Wozniak J.E., Satola S.W., et al. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat Microbiol. 2019;4:1627–1635. doi: 10.1038/s41564-019-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiseo G., Galfo V., Falcone M. What is the clinical significance of “heteroresistance” in non-fermenting Gram-negative strains? Curr. Opin. Infect. Dis. 2023 doi: 10.1097/QCO.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are available from the manuscript text, tables, the figures and the supplementary materials.