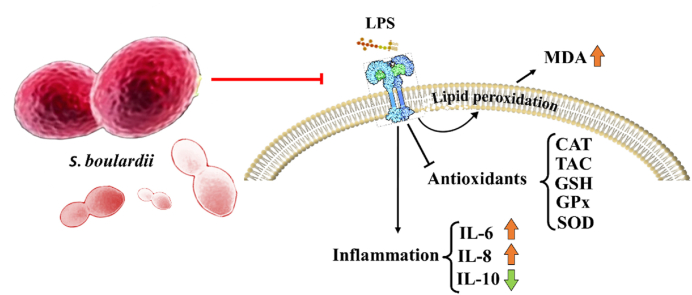
Abstract
The brain is sensitive to oxidative stress, which can trigger microglial activation and neuroinflammation. Antioxidant therapies may provide neuroprotection against oxidative stress. In recent years antioxidant effects of probiotics and their possible mechanisms in oxidative stress-related models have been determined. In the current study, for the first time, we assessed the effects of Saccharomyces boulardii on oxidative stress provoked by lipopolysaccharide (LPS) in the rat brain. Four groups of animals were used, including the control, LPS, S. boulardii + LPS, and S. boulardii groups. All animals received either saline or S. boulardii (1010 CFU) by gavage for four weeks. Between days 14 and 22, all animals received either LPS (250 μg/kg) or saline by intraperitoneal (i.p.) injection. S. boulardii was able to inhibit lipid peroxidation and prevent the reduction of antioxidant levels, including glutathione and catalase in the model of oxidative stress induced by LPS in the rat hippocampus and cortex. Also, it increased the lowered ratio of glutathione/oxidized glutathione in both tissues. Serum levels of anti-inflammatory interleukin 10 (IL-10) and proinflammatory cytokines IL-6 and IL-8 increased and decreased, respectively. S. boulardii has potential antioxidant activities in oxidative stress-related model, possibly modulating gut microbiota, immune defense, and antioxidant enzyme activities that can be considered in preventing oxidative stress-related central nervous system (CNS) diseases.
Keywords: Oxidative stress, Antioxidants, Saccharomyces boulardii, Lipopolysaccharide, Gut microbiota
Graphical abstract
1. Introduction
Oxidative stress is marked by an inequity between reactive oxygen species (ROS) and the antioxidant defense system, stemming from either elevated ROS levels or a diminished antioxidant response [1,2]. Biological systems generate ROS as aerobic metabolic by-products, including hydrogen peroxide (H2O2), superoxide (O2−), hydroxyl radicals (OH•), and singlet oxygen (1O2) [3,4]. There are two sources for the generation of cellular ROS: mitochondrial oxidative phosphorylation or cellular response to xenobiotics, cytokines, and bacterial invasion [2]. In this way, the released ROS cause lipid peroxidation under stress conditions resulting in the formation of products (e.g., malondialdehyde [MDA] and downregulation of antioxidant levels (e.g., glutathione [GSH]) [1,5]. The presence of ROS can lead to oxidative damage in cellular components [4].
The link between oxidative stress and several neurological diseases, including Parkinson's disease (PD), Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), depression, and memory loss, has been well established [6,7].
In this regard, lipopolysaccharide (LPS)-induced oxidative stress and mitochondrial dysfunction cause cell and tissue damage that can be considered the significant manifestations of acute and chronic inflammatory diseases [8] leading to ROS, nitric oxide (NO), cytokines, and chemokines generation [[8], [9], [10]]. LPS modulates inflammatory response through the secretion of the proinflammatory cytokines in the circulation. Then systemic inflammation can damage the blood-brain barrier (BBB) by several mechanisms including decreasing its integrity, increasing the permeability, and reducing the tight junction protein expression. Neuroinflammation and oxidative stress may be the next steps which are the most important molecular events in some neurodegenerative diseases, memory dysfunction, and mood disorders [11,12]. A single dose or chronic administration of LPS triggers the release of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukine-6 (IL-6), and IL-1β in the brain [[13], [14], [15]], and oxidative stress [16]. Therefore, the LPS animal model is used as a suitable model for investigating neuroinflammation and neurodegeneration. Our previous study also showed neuroinflammation, memory impairment, and amyloid-beta deposition in the hippocampus of a LPS rat model [17].
In recent years, the antioxidant properties of probiotics and their potential mechanisms in models related to oxidative stress have been a topic of discussion [[18], [19], [20], [21]]. The microorganism probiotics groups that are used include bacteria LAB (lactic acid bacteria), Lactobacillus, and Bifidobacterium which produce lactic acid, and the yeast Saccharomyces boulardii [22].
Numerous investigations have provided evidence of the protective properties of S. boulardii in diverse gastrointestinal disorders characterized by inflammation, such as inflammatory bowel diseases (IBD), Crohn's disease (CD), ulcerative colitis (UC), mucositis, intestinal infections, intestinal, lung, and hepatic injuries, and diabetic model [[23], [24], [25], [26], [27], [28], [29], [30]]. S. boulardii is used worldwide to prevent and treat infectious diarrhea of various etiologies. It appears that S. boulardii may suppress the generation of proinflammatory cytokines or enhance anti-inflammatory mediators [31]. The principal aim of this study was to investigate, for the first time, the influence of S. boulardii on oxidative stress induced by LPS in the rat brain. Furthermore, serum anti-inflammatory and proinflammatory cytokines levels were examined.
2. Materials and methods
2.1. Animals
Adult male Wistar rats (with weights ranging from 200 to 230 g) were procured from the Neuroscience Research Center and were randomly accommodated under standard conditions:
Rats were housed in standard polypropylene cages measuring 580 mm × 38 mm × 200 mm. Bedding consisting of wood shavings was provided, and the rats were given access to standard pellets and water ad libitum. The room was maintained at a constant temperature of 21 ± 2 °C, with a 12-h light/dark cycle. Environmental enrichment was provided in the form of nesting polyvinyl chloride pipes. All experiments were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Approval No. IR.SBMU.PHNS.REC.1398.129) and adhered to the guidelines set forth by the Animal Research: Reporting of In Vivo Experiments (ARRIVE).
2.2. Drugs
S. boulardii was obtained from Zist Takhmir Company (Iran). The DAILYEAST® capsule contains 1010 colony-forming units [CFU]). Ketamine and sedaxyl® were sourced from Bremer Pharma GMBH (Warburg, Germany) and KELA (Hoogstraten, Belgium), respectively. S. boulardii and LPS (Sigma, USA) were dissolved separately in saline and phosphate-buffered saline.
2.3. Experimental procedure
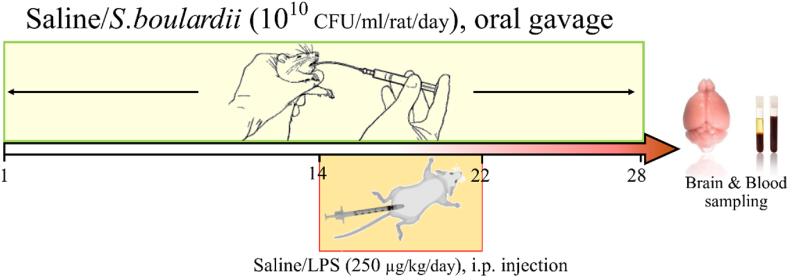
Animals were randomly allocated into four groups of eight animals. The control (Con) group was given saline (10 ml/kg) by gavage for four weeks and saline (1 ml/kg) by intraperitoneal (i.p.) injection for nine days (between days 14 and 22). The LPS group received saline (10 ml/kg) by gavage for four weeks and LPS (250 μg/kg/day) by i.p. injection for nine days. The S. boulardii (Sb) + LPS group was given Sb (1010 CFU/ml) by gavage for four weeks and LPS (250 μg/kg) by i.p. injection for nine days [17].
The remaining group received Sb (1010 CFU/ml) by gavage for four weeks and saline (1 ml/kg) by i.p. injection for nine days. On day 29, the animals underwent anesthesia through ketamine/xylazine administration (60/6 mg/kg, i.p.), and blood samples were collected to measure the levels of inflammatory cytokines. Subsequently, animals were euthanized through decapitation, and the hippocampus and cortex were carefully dissected and preserved at −80 °C for subsequent analysis. Fig. 1 is the timeline of the experimental procedure.
Fig. 1.
Timeline of the experimental procedure.
2.4. Sample preparation
The hippocampus and cortex samples were prepared according to the manufacturing protocols for each parameter. At first, to measure MDA levels, hippocampus and cortex tissues (approximately 100 mg) were homogenized in a cold 1.15 % KCl solution containing butylated hydroxytoluene (BHT) to prevent additional peroxidation. After centrifugation, the resulting supernatants were collected for further analysis as mentioned in the Biochemical Assays section (2.5.).
In addition, hippocampus and cortex tissues weighing 50–100 mg were homogenized in lysing buffer (500–1000 μl) and then centrifuged. The resulting supernatants were used for the assessment of various parameters including superoxide dismutase activity (SOD), total antioxidant capacity (TAC), catalase (CAT), glutathione (GSH), oxidized glutathione (GSSG), and glutathione peroxidase (GPx). The next steps completely were described in the Biochemical Assays section (2.5). To examine inflammatory cytokines concentration in serum, the blood samples were centrifuged at 3500 rpm for 15 min and serum samples were collected and stored at −80 °C.
2.5. Biochemical Assays
2.5.1. Measurement of lipid peroxidation
MDA, serving as an indicator of lipid peroxidation, was measured using the MDA assay kit according to the manufacturer's instructions (Teb Pazhouhan Razi Company, Tehran, Iran). MDA reacts with thiobarbituric acid (TBA) to produce an MDA-TBA adduct, resulting in the formation of a red-colored complex [32]. Previously collected brain supernatants (100 μl), as outlined in section 2.4, were combined with detergent (100 μl) and a chromogenic solution (comprising thiobarbituric acid, alkali, and acetic acid, 200 μl). The resulting mixture was heated in a boiling water bath for 1 h. Subsequently, the tubes were transferred to an ice bath for 10 min to stop the reactions, followed by centrifugation to remove any residual substances. The absorbance was determined at 530 nm and the quantification of lipid peroxides was based on the MDA standard curve, with results expressed as μmol/mg tissue protein. This kit has been utilized in multiple earlier studies to analyze the levels of MDA in the brain [33,34].
2.5.2. Superoxide dismutase activity
The measurement of SOD activity was conducted indirectly using a Nasdox™ assay kit (Navand Salamat Company, Urmia, Iran) based on its ability to inhibit pyrogallol autoxidation [35]. Fifty μl of brain sample supernatants, as outlined in section 2.4, were combined with 250 μl of the supplied reaction mixture from the kit and incubated at room temperature for 5 min. The absorbance was measured at 405 nm, and the SOD activity was calculated using the formula (OD of sample/OD of control) and reported as U/mg protein. This kit has been employed in various prior studies to evaluate SOD levels in in vivo experiments [36,37].
2.5.3. Total antioxidant capacity assay
The antioxidant potential of the samples, in terms of their ability to convert ferric iron (Fe3+) into ferrous iron (Fe2+), was determined using the ferric reduction antioxidant power (FRAP) method [38]. The Naxifer™ assay kit from Navand Salamat Company in Urmia, Iran, was utilized for the analysis. The brain sample supernatants (5 μl), as detailed in section 2.4, were combined with 250 μl of the kit's working solution. The change in color resulting from the reaction was measured at 590 nm after 5 min using a microplate reader. TAC was calculated by employing the standard curve and then represented as mmol Fe2+/mg tissue protein. This kit has been utilized in numerous prior studies to evaluate TAC levels in in vivo experiments [37,39].
2.5.4. Catalase activity
Catalase activity was determined by monitoring its interaction with hydrogen peroxide (H2O2) and methanol, resulting in the production of formaldehyde. The formaldehyde generated, in conjunction with a chromogen, was detected at 550 nm [40]. For the measurement of catalase activity, 20 μl of the supernatant, as described in section 2.4, was utilized following the instructions provided by the Nactaz™ assay kit from Navand Salamat Company in Urmia, Iran. Catalase activity was calculated using a standard curve and reported as nmol/min/mg tissue.
2.5.5. Glutathione, glutathione peroxidase, and oxidized glutathione assay
GPx initiates the oxidation of GSH to generate GSSG, subsequently employed in reducing cumene hydroperoxide within the glutathione peroxidase test method. Subsequently, GR transforms GSSG back to GSH through the reduction of NADPH [41]. Consequently, the GPx activity, measured at 340 nm, exhibits a decrease due to the utilization of NADPH [42]. The supernatants collected earlier (50 μl), as described in section 2.4, were utilized for the evaluation of GPx following the instructions provided by the Nagpix™ assay kit from Navand Salamat Company in Urmia, Iran. Absorbance was continuously recorded at 340 nm, and the obtained value was determined using the GPx standard curve. The result was expressed as mU/mg tissue protein. The assay had a sensitivity of detecting GPx activity as low as 0.5 mU/ml in the samples.
Glutathione levels were determined using the Nargul™ reduced glutathione (GSH) assay kit (Navand Salamat Company, Urmia, Iran) based on 2,2′-dinitro-5,5′-dithiodibenzoic acid (DTNB) measurement [43]. The brain supernatants collected earlier (20 μl), as described in section 2.4, were combined with GSH buffer (20 μl), and subsequently, an equal amount of DTNB (Ellman's reagent) and glutathione reductase (120 μl) were added. The mixture was incubated for 30 s. Afterward, a 60 μl cofactor solution was introduced, and the resultant absorbance was recorded at 412 nm. The measurement of GSH levels was conducted by utilizing a standard curve, and the outcomes were reported in μmol/mg tissue protein.
To assess GSSG, 25 μl of brain supernatants, as outlined in section 2.4, were combined with GSH buffer, enzyme, cofactor, and DTNB according to the instructions provided by the Naglu™ assay kit from Navand Salamat Company in Urmia, Iran. Subsequently, the absorbance at 412 nm was measured, and the obtained value was determined using the GSSG standard curve. The result was then reported as μm/mg tissue protein.
2.6. Serum cytokine levels
Following the collection of blood samples, the serum concentrations of IL-10 (DY522, Bio-Techne, USA), IL-6 (CSB-E04640r, Cusabio, USA), and IL-8 (abx576575, Abbexa, USA) were determined using ELISA (Enzyme-Linked Immunosorbent Assay) kits following the manufacturer's instructions. The serum cytokine concentrations were ascertained via a standard curve and presented in pg/ml.
2.7. Statistical analysis
The analysis was conducted using GraphPad Prism version 9.5.0. The normality of data variances was assessed using the Kolmogorov–Smirnov test. Variances among the experimental groups were analyzed through a one-way analysis of variance (ANOVA) followed by post hoc Tukey's and Bartlett's tests for multiple comparisons. Statistical significance was determined when P < 0.05. The data is presented as the mean ± SEM (standard error of the mean).
3. Results
3.1. Effects of S. boulardii on oxidative stress parameters in the hippocampus
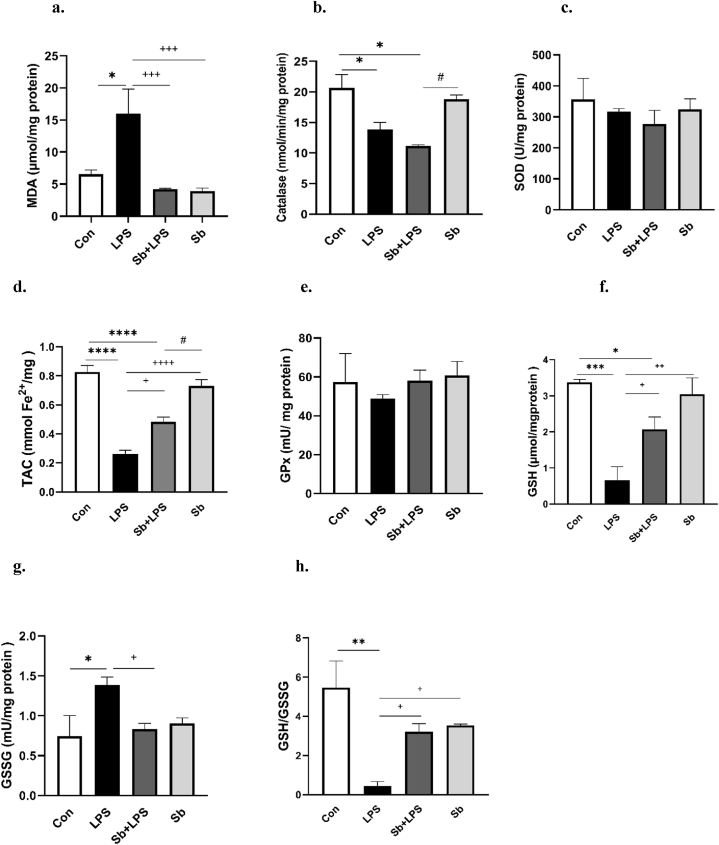
As presented in Fig. 2a, the MDA levels exhibited a significant increase in the hippocampal tissues of the LPS group compared to the Con group (P < 0.05). Conversely, the administration of S. boulardii with LPS effectively inhibited the LPS-induced elevation in MDA levels (P < 0.001, Fig. 2a). Both Sb + LPS and Sb-treated groups had no significant difference in MDA levels with control animals.
Fig. 2.
Panels a–h show the effects of S. boulardii on the MDA, CAT, SOD, TAC, GPx, GSH, GSSG and GSH/GSSG ratio respectively in the hippocampus. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, ****P < 0.0001, compared to control group. +P < 0.05, ++P < 0.01, +++P < 0.001 and ++++P < 0.0001 compared to LPS group, #P < 0.05 compared to Sb group, N = 8. Control (Con), lipopolysaccharide (LPS), S. boulardii + lipopolysaccharide (Sb + LPS), S. boulardii (Sb).
Catalase activity significantly decreased in the hippocampal tissues of the LPS-treated group in comparison with the Con group (P < 0.05). Similarly, as shown in Fig. 2b, the Sb + LPS-treated group significantly reduced catalase activity versus the Con group (P < 0.05). Consequently, catalase activity was not significant between the Con group and Sb-treated animals and also between Sb + LPS and LPS-treated (Fig. 2b). In other words, probiotic S. boulardii was unable to increase the catalase activity in the Sb + LPS group.
The groups did not exhibit any significant differences in SOD activity, as depicted in Fig. 2c. As shown in Fig. 2d, the TAC levels displayed a significant decrease in the hippocampal tissues of the LPS and Sb + LPS-treated groups versus the Con group (P < 0.0001). There was no difference in the TAC levels between the control animals and the Sb-treated group. However, the difference in TAC levels between Sb + LPS and LPS groups was significant (P < 0.05). As indicated in Fig. 2e, no significant variances were detected in the GPx activity within the hippocampus among the groups. As indicated by the findings presented in Fig. 2f, the GSH levels in the hippocampal tissues were notably reduced in both the LPS and Sb + LPS groups when compared to the Con group (P < 0.001 and P < 0.05, respectively). The Sb-treated animals had no difference in GSH levels from the control group. Remarkably, the Sb + LPS-treated group exhibited significantly higher GSH levels in comparison to the LPS-treated group (P < 0.05). As shown in Fig. 2g, in the LPS group, GSSG levels significantly increased in hippocampal tissues versus the Con group (P < 0.05). Both Sb + LPS and Sb-treated animals had no substantial difference in GSSG levels with the control group. However, pretreatment of S. boulardii with LPS significantly reduced the elevation of GSSG levels in comparison with the LPS-treated alone (P < 0.05). As presented in Fig. 2h, the LPS-treated group demonstrated a significantly lower GSH/GSSG ratio versus the Con group (P < 0.01). This ratio was not significant between the Con and Sb + LPS groups and also between control and Sb-treated animals. In contrast, the Sb + LPS-treated group exhibited a significantly higher GSH/GSSG ratio compared to the LPS alone (P < 0.05).
3.2. Effects of S. boulardii on oxidative stress parameters in the cortex
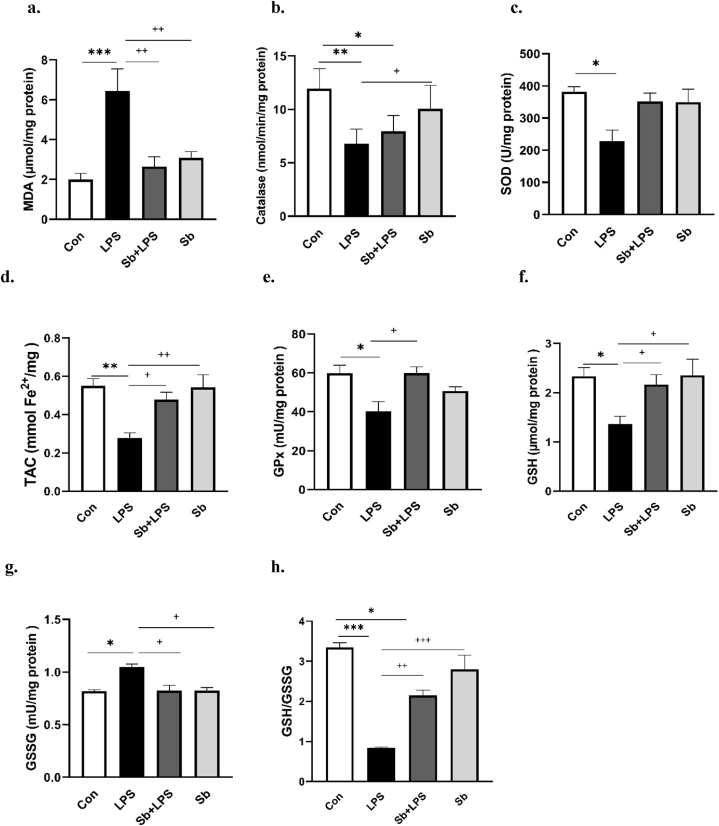
As presented in Fig. 3a, our findings revealed a significant increase in MDA levels in the cortical tissues of the LPS group versus the Con group (P < 0.001). MDA levels in the Sb + LPS and Sb groups were not different from those in the Con group. However, pretreatment of S. boulardii with LPS could significantly prevent the rise in MDA levels in comparison with the LPS alone (P < 0.01).
Fig. 3.
Panels a–h show the effects of S. boulardii on the MDA, CAT, SOD, TAC, GPx, GSH, GSSG and GSH/GSSG ratio respectively in the cortex. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, compared to control group. +P < 0.05, ++P < 0.01 and +++P < 0.001, compared to LPS group, N = 8. Control (Con), lipopolysaccharide (LPS), S. boulardii + lipopolysaccharide (Sb + LPS), S. boulardii (Sb).
As shown in Fig. 3b–a significant decrease was found in the catalase activity of cortical tissues of the LPS-treated group versus the Con (P < 0.01). Furthermore, pretreatment of S. boulardii with LPS significantly decreased catalase activity versus the Con group (P < 0.05). However, the difference in catalase activity between the Sb + LPS and LPS groups and between control and Sb-treated animals was not significant (Fig. 3b).
As shown in Fig. 3c, the SOD activity in the cortical tissues of the LPS group exhibited a significant decrease versus the Con group (P < 0.05). S. boulardii was effective in preventing the decline in SOD activity in the Sb + LPS group, which led to the absence of a significant difference between the Sb + LPS group and the Con group. There was no difference in the SOD activity between the control animals and the Sb-treated group. As shown in Fig. 3d, the TAC levels in the cortical tissues of the LPS group significantly decreased versus the Con group (P < 0.01). Both the Sb + LPS and Sb-treated animals had no substantial difference in TAC levels with the control group. Pretreatment of S. boulardii with LPS could significantly increase the TAC levels versus the LPS alone (P < 0.05). Notably, the TAC levels of the Sb + LPS-treated group were not different from the Con group.
As shown in Fig. 3e and f, the LPS-treated group exhibited significant decreases in GPx activity and GSH levels versus the Con group (P < 0.05). However, pretreatment of S. boulardii in the Sb + LPS animals could significantly increase GPx activity and GSH levels of cortical tissues versus LPS-treated alone (P < 0.05). Furthermore, the difference in GPx activity and GSH levels between the Sb + LPS and Con groups and also between control and Sb-treated animals were not significant.
As shown in Fig. 3g, the LPS-treated group exhibited a significant rise in the GSSG levels of cortical tissues compared to the Con group (P < 0.05). However, when S. boulardii was administered with LPS, it prevented the increase in GSSG levels versus the LPS alone (P < 0.05).
The distinction between control animals and the Sb + LPS and Sb groups did not reach statistical significance in Fig. 3g. As presented in Fig. 3h, the GSH/GSSG ratio in the cortex exhibited a notable reduction in the LPS-treated group compared to the Con group (P < 0.001). Within the Sb + LPS-treated group, S. boulardii significantly elevated the diminished GSH/GSSG ratio in comparison to the LPS-treated group (P < 0.01). The difference in GSH/GSSG ratio was significant between the Sb + LPS and Con groups (P < 0.05) but was not significant between the control and Sb-treated animals.
3.3. Effects of S. boulardii on the serum cytokine levels
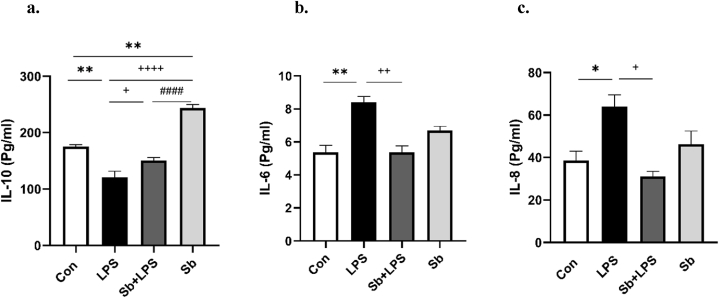
As presented in Fig. 4a, the LPS-treated group significantly decreased serum levels of IL-10 versus the Con group (P < 0.01). Pretreatment of S. boulardii with LPS significantly elevated IL-10 levels compared to LPS treatment alone (P < 0.05). The IL-10 levels were not different between both groups of the Sb + LPS and Con (Fig. 4a). Besides, the Sb-treated animals showed a significant increase in IL-10 levels compared to controls.
Fig. 4.
Panels a–c show the effects of S. boulardii on the serum of IL-10, IL-6, and IL-8 respectively. Data are presented as mean ± SEM. *P < 0.05, and **P < 0.01, compared to control group. +P < 0.05, ++P < 0.01, and ++++P < 0.0001 compared to LPS group, ####P < 0.0001, compared to Sb group. N = 8. Control (Con), lipopolysaccharide (LPS), S. boulardii + lipopolysaccharide (Sb + LPS), S. boulardii (Sb).
As shown in Fig. 4b and c, the LPS-treated group showed significant increases in the IL-6 and IL-8 serum levels versus the Con group (P < 0.01 and P < 0.05, respectively). On the contrary, the Sb + LPS-treated group exhibited a notable reduction in the serum concentrations of IL-6 and IL-8 in comparison to the LPS-treated group (P < 0.01 and P < 0.05, respectively). Both the Sb + LPS and Sb-treated animals had no substantial difference in IL-6 and IL-8 levels with the control group.
4. Discussion
Our findings present novel evidence indicating that the utilization of S. boulardii has the potential to mitigate the rise in lipid peroxidation in the hippocampus and cortex of rats within an oxidative stress model induced by LPS. In this regard, it prevented the decrement of the levels of antioxidants, including GSH and catalase especially in the cortex, and the increment of GSSG levels against oxidative stress induced by LPS in both the hippocampus and cortex. Therefore, the ratio of GSH/GSSG was preserved after the administration of S. boulardii with LPS in both tissues. Also, S. boulardii could prevent a decrease in TAC induced by LPS. We observed that SOD and GPx activities did not change in the LPS model in the hippocampus but were reduced in the rat cortex. The concurrent administration of S. boulardii with LPS effectively mitigated the decline in SOD activity observed in the cortex. Additionally, an interesting point is that the lack of a difference between Sb and LPS + Sb groups in all parameters except for catalase activity in the hippocampus tissue indicates that probiotic S. boulardii could successfully modulate oxidant and antioxidant parameters in the context of oxidative stress and inflammation that induced by LPS.
The progression of neurodegenerative diseases and conditions involves crucial steps, including oxidative stress, lipid peroxidation, and subsequent modifications to proteins and lipids through oxidation and nitration. Microglial activation and neuroinflammation are triggered by oxidative stress. Consequently, antioxidant therapies hold promise for providing neuroprotection in the presence of oxidative/nitrative stress [45]. S. boulardii significantly prevented lipid peroxidation due to LPS-induced oxidative stress in rat hippocampus and cortex that could inhibit the progression of neuroinflammation. In parallel with our study, S. boulardii pretreatment alleviated ischemia-reperfusion-induced lung injury by significantly suppressing increases in proinflammatory cytokine of serum and lung tissue MDA and TNF-α levels in rats [30].
GSH is an endogenous non-protein thiol-containing tripeptide with antioxidant properties [5,45]. GSH is synthesized in the cytoplasm and exists in higher concentrations in the mitochondrial matrix which can oxidize to GSSG. Total GSH levels and GSH/GSSG ratio are two important indices of the protective ability of cells under oxidative stress [1,5]. Consequently, a reduction in both of these indicators elevates vulnerability to oxidative stress, leading to potential harm that could be significant in the advancement of various conditions including cancer, cardiovascular ailments, respiratory disorders, inflammatory diseases, diabetes mellitus, and neurodegenerative conditions like PD and AD [46]. In this study, the prophylactic administration of S. boulardii effectively maintained the GSH/GSSG ratio in both tissues, counteracting the decremental effect induced by LPS. These findings offer promising prospects for protecting against neuroinflammation caused by oxidative stress. This finding agrees with a previous study that has prevented chloride secretion due to rotavirus-non-structural protein 4 (NSP4) by inhibiting the imbalance between ROS and GSH/GSSH ratio [47]. Other endogenous antioxidative defense system includes SOD, CAT, and GPx, which act as scavengers, protect cells from active oxygen, and produce O2 and H2O [3,20].
Our results indicated that S. boulardii could preserve the SOD, CAT, and GPx activities in the cortex against oxidative stress induced by LPS, which can protect the antioxidative defense system. Similar to our finding, S. boulardii exhibited neuroprotective effects and improved memory impairment by preventing antibiotic-induced gut dysbiosis in mice and reducing inflammation and oxidative stress in the intestines and brain [11]. Also, S. boulardii mitigated oxidative stress by increasing colonic SOD and CAT activities and GSH levels and decreasing colonic MDA levels in UC induced by dextran sulfate sodium (DSS) in mice. Furthermore, it inhibited colonic inflammation by reducing proinflammatory cytokine levels such as IL-1, IL-6, and TNF-α possibly by inhibiting NF-κB and promoting Nrf2 pathways, essential targets in the treatment of UC [48]. In this study, SOD activity in the hippocampus did not significantly affect by LPS which may be related to the different responses of SOD to the oxidative stress model [49].
The study findings demonstrated that administering S. boulardii significantly elevated IL-10, an anti-inflammatory cytokine, in serum levels, and simultaneously reduced the proinflammatory cytokines IL-6 and IL-8 in the LPS model of oxidative stress. Likewise, the application of S. boulardii culture supernatant showed a reduction in the inflammatory response triggered by LPS in human dendritic cells. This resulted in a reduction in IL-6 and TNF-α levels, coupled with an elevation in IL-10 production [50]. S. boulardii exhibited anti-inflammatory ability against Candida albicans by diminishing the production of proinflammatory cytokines, through the regulation of interferon-gamma (IFN-γ) and IL-1β. Simultaneously, it promoted the secretion of anti-inflammatory cytokines like IL-4 and IL-10 [51]. Additionally, it reduced toll-like receptor 2 (TLR2) stimulation [52].
Moreover, S. boulardii has demonstrated its anti-inflammatory effects against Clostridium difficile by inhibiting the activation of MAP kinases ERK1/2 and JNK/SAPK, resulting in a reduction in IL-8 production [53]. Similarly, S. boulardii exhibited this effect against Salmonella by inhibiting MAP kinases ERK1/2, p38, and JNK, as well as nuclear factor kappa B (NF-κB) activation, resulting in decreased levels of IL-8 [54], IL-6, and TNF-α [29]. Furthermore, it inhibited the mRNA expression associated with TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), CXC motif chemokine ligand 8 (CXCL8), and chemokine ligand 2 (CCL2) genes [31]. Moreover, S. boulardii exhibited the ability to up-regulate peroxisome proliferator-activated receptor gamma and down-regulate IL-8 expression in human HT-29 colonocytes and rat model colitis, respectively [55]. Also, S. boulardii inhibited the expression of proinflammatory cytokines and inducible nitric oxide synthase (iNOS) genes in the colonic mucosa of infected rats with Blastocystis subtype-3 cysts [56].
The antioxidant effects of various probiotics against oxidative stress have been documented in several studies [57,58]. A meta-analysis of clinical trials on probiotics showed that their consumption could decrease high-sensitive C-reactive protein (hs-CRP) and MDA and increase GSH and TAC. As a result, they are effective against inflammation and oxidative stress biomarkers. Furthermore, their overall effects on serum TAC levels may be more evident on probiotic dose> 5 billion CFU/day [59]. On the other hand, several possible mechanisms for neuroinflammation induced by LPS have been discussed in a prior study: 1) cellular damage caused by ROS generation and cytokines activation, 2) astrocytes and microglia activation in the CNS that can potentiate the expression of IL-6 that is considered as a biomarker of neuroinflammation, 3) neurodegeneration and memory impairment [60].
We found that S. boulardii could preserve the antioxidant enzymes' activities against LPS-induced oxidative stress. The levels of serum proinflammatory cytokines IL6 and Il-8 and the anti-inflammatory IL-10 decreased and increased, respectively. Furthermore, we have shown that S. boulardii ameliorates neuroinflammation induced by LPS, amyloid-β deposition, and subsequent memory impairment or anxiety in animals, possibly by modulating the gut microbiota [17,61]. All of these observations confirm that S. boulardii exhibits protective effects against LPS-induced neuroinflammation.
S. boulardii possibly exerts part of its vital activity by modulating gut microbiota. The production of microbial metabolite short-chain fatty acids (SCFAs), including isobutyrate and valerate, has been reported to increase with S. boulardii supplementation in various studies which may be necessary for preventing and treating diseases [[62], [63], [64]]. S. boulardii showed anti-inflammatory and protective effects in the DSS model of UC in mice by decreasing the inflammatory cytokine levels (TNF-α and IL-8), protecting intestinal histological structures and tight junctions, which upregulation the percentage of S24-7 in the intestinal flora may be considered a fundamental mechanism of its activities [65].
In the current study, we examined the effect of probiotic Saccharomyces boulardii on brain oxidative stress in a LPS rat model which is known and used as a model for investigating neuroinflammation, neuronal loss, and neurodegeneration. According to previous studies, we assumed that the gut epithelium barrier and blood-brain barrier were disrupted following LPS-induced inflammation and then restored after probiotic treatment. Peng et al. (2021) discussed completely the mechanisms of BBB permeability and disruption by LPS [66]. Another study that showed BBB dysfunction and permeability in the LPS animal model was performed by Li et al. (2020). They found that LPS markedly disrupted the BBB and reduced Occldin and ZO-1 in the microvessels samples isolated from the brains of LPS-induced mice [67]. In this regard, recent studies revealed that probiotics can modulate BBB integrity. The BBB permeability and the expression of tight junction proteins were normalized in germ-free mice colonized with a commensal microbiota [68]. The probiotic Lactobacillus reuteri improved BBB dysfunction and cognitive deficit in offspring of LPS-induced dams [69].
There was no study addressing the effect of S. boulardii on BBB integrity and function. But, according to our results, the anti-inflammatory effects of S. boulardii in the circulation especially through its metabolites like SCFAs may affect some properties in BBB including its integrity and permeability to reverse the harmful effects of LPS which can result in decreasing oxidative stress in the brain samples.
Moreover, the intrinsic antioxidant activity of S. boulardii is evident. Previous research has shown that cysteine ethyl ester, a GSH inducer, effectively enhances intracellular GSH levels in various Saccharomyces strains, including S. cerevisiae, S. bayanus, and S. boulardii. This discovery suggests the possibility of merging the antioxidant properties of GSH to counteract oxidative stress with the probiotic characteristics of S. boulardii in the treatment of gastrointestinal disorders [70]. Furthermore, the synthesis of GSH by S. boulardii (CNCM I-745) is consistent with the results obtained in a prior study [45]. S. boulardii has intracellular ROS scavenging activity, and this antioxidant activity may be related to the presence of vanillin/vanillic acid as an active constituent in its fermentation broth [71].
S. boulardii has antimicrobial, metabolic, and enzymatic activities, increasing immune defense in the gut and decreasing the synthesis of inflammatory cytokines, antitoxin, and modulatory effects on intestinal flora [72]. Our results determined that S. boulardii has the potential to be neuroprotective against oxidative stress induced by LPS. On the other hand, S. boulardii indicates some advantages including growing at a temperature of 37 °C despite different strains of S. cerevisiae. Additionally, it displays resistance to low pH levels and demonstrates tolerance to bile acids [[72], [73], [74]]. It is naturally resistant to antibiotics [44]. It does not colonize the host gastrointestinal tract [75]. It does not engage in the DNA exchange of resistance genes with bacteria during antibiotic treatment [76,77].
5. Conclusions
The findings demonstrated for the first time that S. boulardii has potential antioxidant activities in oxidative stress-related model, possibly modulating gut microbiota, immune defense, and antioxidant enzyme activity that can be considered in preventing oxidative stress-related CNS diseases.
In this regard, the future directions for research in the field of the gut-brain axis should explore a new approach that considers it as a potential factor influencing brain health. Furthermore, the use of probiotics such as S. boulardii, with their neuroprotective effects, may be considered as a therapeutic intervention in CNS disorders related to neuroinflammation. However, the mechanisms behind the associations between the actions of S. boulardii and neurological functions need to be further investigated in future studies. This study also emphasizes the importance of fully understanding specific aspects of S. bouvardia's mechanisms such as analyzing the gut composition when manipulated with this probiotic and evaluating the immunological, endocrine, and nervous pathways that S. boulardii can impact the BBB and the brain.
6. Limitations
The current study investigated the antioxidant impact of probiotic S. boulardii in the LPS-induced model for the first time. However, we had some limitations such as a confounding factor related to the complete dissimilarity of gut microbiota composition between animals and humans in this research context, which could complicate translating the results to human-based conditions. However, due to the limitations in human brain tissue collection, extensive study of animal samples is common. Moreover, in animal ethics, we had to limit the gavage time in our study to avoid damaging the animals for a long time. The limitation in the brain sample size was another challenge in evaluating all the oxidative stress markers in our research.
Foundation
This study was funded by the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No 98.20971).
Data availability
Data has been included in the article.
Ethics statement
All experiments were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1398.129) and performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
CRediT authorship contribution statement
Fatemeh Babaei: Writing – original draft, Investigation, Formal analysis, Data curation. Ava Navidi-Moghaddam: Writing – original draft, Investigation, Formal analysis, Data curation. Ariyan Naderi: Investigation. Shiva Ghafghazi: Investigation. Mohammadreza Mirzababaei: Visualization, Investigation. Leila Dargahi: Writing – review & editing, Methodology. Ghazaleh Mohammadi: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Marjan Nassiri-Asl: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Neurobiology and Neuroscience Research Centers, Shahid Beheshti University of Medical Sciences, for their scientific and technical support.
Contributor Information
Ghazaleh Mohammadi, Email: qmohammadi@qums.ac.ir.
Marjan Nassiri-Asl, Email: marjannassiriasl@sbmu.ac.ir.
References
- 1.Ansari M.A., Scheff S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez Macarro M., Avila-Gandia V., Perez-Pinero S., Canovas F., Garcia-Munoz A.M., Abellan-Ruiz M.S., Victoria-Montesinos D., Luque-Rubia A.J., Climent E., Genoves S., Ramon D., Chenoll E., Lopez-Roman F.J. Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants. 2021;10(2):323. doi: 10.3390/antiox10020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Jh L.Y., Im J.H., Ham Y.W., Lee H.P., Han S.B., Hong J.T. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar. Drugs. 2019;17(2):123. doi: 10.3390/md17020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobielska M., Bartosik N.K., Zyzik K.A., Kowalczyk E., Karbownik M.S. Mechanisms of cognitive impairment in depression. May probiotics help? Front. Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.904426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevit. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fock E.M., Parnova R.G. Protective effect of mitochondria-targeted antioxidants against inflammatory response to lipopolysaccharide challenge: a review. Pharmaceutics. 2021;13(2):144. doi: 10.3390/pharmaceutics13020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Z., Yuan Y. Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: iotanhibition by ST1926. Int. J. Mol. Med. 2018;41(6):3405–3421. doi: 10.3892/ijmm.2018.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Q., Li Y., Chen J., Wang N. Azilsartan suppressed LPS-induced inflammation in U937 macrophages through suppressing oxidative stress and inhibiting the TLR2/MyD88 signal pathway. ACS Omega. 2020;6(1):113–114. doi: 10.1021/acsomega.0c03655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy Sarkar S., Mitra Mazumder P., Chatterjee K., Sarkar A., Adhikary M., Mukhopadhyay K., Banerjee S. Saccharomyces boulardii ameliorates gut dysbiosis associated cognitive decline. Physiol. Behav. 2021;236 doi: 10.1016/j.physbeh.2021.113411. [DOI] [PubMed] [Google Scholar]
- 12.Collins S.M., Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136(6):2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi G., Dargahi L., Peymani A., Mirzanejad Y., Alizadeh S.A., Naserpour T., Nassiri-Asl M. The effects of probiotic formulation pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a lipopolysaccharide rat model. J. Am. Coll. Nutr. 2019;38(3):209–217. doi: 10.1080/07315724.2018.1487346. [DOI] [PubMed] [Google Scholar]
- 14.Sulakhiya K., Kumar P., Jangra A., Dwivedi S., Hazarika N.K., Baruah C.C., Lahkar M. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur. J. Pharmacol. 2014;744:124–131. doi: 10.1016/j.ejphar.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Al-Amin M.M., Choudhury M.F.R., Chowdhury A.S., Chowdhury T.R., Jain P., Kazi M., Alkholief M., Alshehri S.M., Reza H.M. Pretreatment with risperidone ameliorates systemic LPS-induced oxidative stress in the cortex and Hippocampus. Front. Neurosci. 2018;12:384. doi: 10.3389/fnins.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullah R., Ali G., Baseer A., Irum Khan S., Akram M., Khan S., Ahmad N., Farooq U., Kanwal Nawaz N., Shaheen S., Kumari G., Ullah I. Tannic acid inhibits lipopolysaccharide-induced cognitive impairment in adult mice by targeting multiple pathological features. Int. Immunopharm. 2022;110 doi: 10.1016/j.intimp.2022.108970. [DOI] [PubMed] [Google Scholar]
- 17.Babaei F., Mirzababaei M., Dargahi L., Shahsavari Z., Nassiri-Asl M., Karima S. Preventive effect of Saccharomyces boulardii on memory impairment induced by lipopolysaccharide in rats. ACS Chem. Neurosci. 2022;13(22):3180–3187. doi: 10.1021/acschemneuro.2c00500. [DOI] [PubMed] [Google Scholar]
- 18.Shabani M., Hasanpour E., Mohammadifar M., Bahmani F., Talaei S.A., Aghighi F. Evaluating the effects of probiotic supplementation on neuropathic pain and oxidative stress factors in an animal model of chronic constriction injury of the sciatic nerve. Basic and Clinical Neuroscience Journal. 2021:1–19. doi: 10.32598/bcn.2022.3772.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi A.A., Jazayeri S., Khosravi-Darani K., Solati Z., Mohammadpour N., Asemi Z., Adab Z., Djalali M., Tehrani-Doost M., Hosseini M., Eghtesadi S. Effects of probiotics on biomarkers of oxidative stress and inflammatory factors in petrochemical workers: a randomized, double-blind, placebo-controlled trial. Int. J. Prev. Med. 2015;6:82. doi: 10.4103/2008-7802.164146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Jy K.C. Probiotics alleviate oxidative stress in H2O2-exposed hepatocytes and t-BHP-induced C57bl/6 mice. Microorganisms. 2022;10(2):234. doi: 10.3390/microorganisms10020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkushi A.G., Abdelfattah-Hassan A., Eldoumani H., Elazab S.T., Mohamed S.A.M., Metwally A.S., E S.E.-S., Saleh A.A., ElSawy N.A., Ibrahim D. Probiotics-loaded nanoparticles attenuated colon inflammation, oxidative stress, and apoptosis in colitis. Sci. Rep. 2022;12(1):5116. doi: 10.1038/s41598-022-08915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazmierczyk-Winciorek M., Nedzi-Gora M., Slotwinska S.M. The immunomodulating role of probiotics in the prevention and treatment of oral diseases. Cent. Eur. J. Immunol. 2021;46(1):99–104. doi: 10.5114/ceji.2021.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2009;30(8):826–833. doi: 10.1111/j.1365-2036.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas S., Metzke D., Schmitz J., Dorffel Y., Baumgart D.C. Anti-inflammatory effects of Saccharomyces boulardii mediated by myeloid dendritic cells from patients with Crohn's disease and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301(6):G1083–G1092. doi: 10.1152/ajpgi.00217.2011. [DOI] [PubMed] [Google Scholar]
- 25.Justino P.F., Melo L.F., Nogueira A.F., Costa J.V., Silva L.M., Santos C.M., Mendes W.O., Costa M.R., Franco A.X., Lima A.A., Ribeiro R.A., Souza M.H., Soares P.M. Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice. Br. J. Nutr. 2014;111(9):1611–1621. doi: 10.1017/S0007114513004248. [DOI] [PubMed] [Google Scholar]
- 26.Duman D.G., Kumral Z.N., Ercan F., Deniz M., Can G., Caglayan Yegen B. Saccharomyces boulardii ameliorates clarithromycin- and methotrexate-induced intestinal and hepatic injury in rats. Br. J. Nutr. 2013;110(3):493–499. doi: 10.1017/S000711451200517X. [DOI] [PubMed] [Google Scholar]
- 27.Abreu I., Albuquerque R., Brandao A.B.P., Barssotti L., de Souza L.B., Ferreira F.G., Oliveira L.C.G., Yokota R., Sparvoli L.G., Dias D.D.S., Salgado M.A.C., Taddei C., De Angelis K., Casarini D.E., Cunha T.S. Saccharomyces boulardii exerts renoprotection by modulating oxidative stress, renin angiotensin system and uropathogenic microbiota in a murine model of diabetes. Life Sci. 2022;301 doi: 10.1016/j.lfs.2022.120616. [DOI] [PubMed] [Google Scholar]
- 28.Barssotti L., Abreu I., Brandao A.B.P., Albuquerque R., Ferreira F.G., Salgado M.A.C., Dias D.D.S., De Angelis K., Yokota R., Casarini D.E., Souza L.B., Taddei C.R., Cunha T.S. Saccharomyces boulardii modulates oxidative stress and renin angiotensin system attenuating diabetes-induced liver injury in mice. Sci. Rep. 2021;11(1):9189. doi: 10.1038/s41598-021-88497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins Fs V.A., Elian S.D., Arantes R.M., Tiago F.C., Sousa L.P. Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microb. Infect. 2013;15(4):270–279. doi: 10.1016/j.micinf.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Durmaz S., Kurtoglu T., Barbarus E., Cetin N.K., Yilmaz M., Rahman O.F., Abacigil F. Probiotic Saccharomyces boulardii alleviates lung injury by reduction of oxidative stress and cytokine response induced by supraceliac aortic ischemia-reperfusion injury in rats. Braz. J. Cardiovasc. Surg. 2021;36(4):515–521. doi: 10.21470/1678-9741-2020-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stier H., Bischoff S.C. Influence of Saccharomyces boulardii CNCM I-745on the gut-associated immune system. Clin. Exp. Gastroenterol. 2016;9:269–279. doi: 10.2147/CEG.S111003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguilar Diaz De Leon J., Borges C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020;12(159) doi: 10.3791/61122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarbali S., Zahmatkesh M.M., Torkaman-Boutorabi A., Khodagholi F. Assessment of lipophilic fluorescence products in β-amyloid-induced cognitive decline: a parallel track in hippocampus, CSF, plasma and erythrocytes. Exp. Gerontol. 2022;157 doi: 10.1016/j.exger.2021.111645. [DOI] [PubMed] [Google Scholar]
- 34.Goudarzi M., Mombeini M.A., Fatemi I., Aminzadeh A., Kalantari H., Najafzadehvarzi A. Nesari H., Mehrzadi S S. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol. Res. 2019;41(5):419–428. doi: 10.1080/01616412.2019.1576319. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed H., Schott E.J., Gauthier J.D., Vasta G.R. Superoxide dismutases from the oyster parasite Perkinsus marinus: purification, biochemical characterization, and development of a plate microassay for activity. Anal. Biochem. 2003;318(1):132–141. doi: 10.1016/s0003-2697(03)00192-1. [DOI] [PubMed] [Google Scholar]
- 36.Abootorabi S., Akbari J., Saeedi M., Seyedabadi M., Ranaee M., Asare-Addo K., Nokhodchi A. Atorvastatin entrapped noisome (atrosome): green preparation approach for wound healing. AAPS PharmSciTech. 2022;23(3):81. doi: 10.1208/s12249-022-02231-x. [DOI] [PubMed] [Google Scholar]
- 37.Tajaldini M., Samadi F F., Khosravi A., Ghasemnejad A., Asadi J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109594. [DOI] [PubMed] [Google Scholar]
- 38.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 39.Babaei A., Asadpour R., Mansouri K., Sabrivand A., Kazemi-Darabadi S. Lycopene protects sperm from oxidative stress in the experimental varicocele model. Food Sci. Nutr. 2021;9(12):6806–6817. doi: 10.1002/fsn3.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katerji M., Filippova M., Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei J., Pan X., Wei G., Hua Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1147414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biasibetti H., Pierozan P., Rodrigues A.F., Manfredini V., Wyse A.T.S. Hypoxanthine intrastriatal administration alters neuroinflammatory profile and redox status in striatum of infant and young adult rats. Mol. Neurobiol. 2017;54(4):2790–2800. doi: 10.1007/s12035-016-9866-6. [DOI] [PubMed] [Google Scholar]
- 43.Sadeghi M.A., Hemmati S., Mohammadi S., Yousefi-Manesh H., Vafaei A., Zare M., Dehpour A.R. Chronically altered NMDAR signaling in epilepsy mediates comorbid depression. Acta Neuropathol Commun. 2021;9(1):53. doi: 10.1186/s40478-021-01153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cobb C.A., Cole M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015;84:4–21. doi: 10.1016/j.nbd.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badr H., El-Baz A., Mohamed I., Shetaia Y., El-Sayed A.S.A., Sorour N. Bioprocess optimization of glutathione production by Saccharomyces boulardii: biochemical characterization of glutathione peroxidase. Arch. Microbiol. 2021;203(10):6183–6196. doi: 10.1007/s00203-021-02584-0. [DOI] [PubMed] [Google Scholar]
- 46.Ballatori N., Krance S.M., Notenboom S., Shi S., Tieu K., Hammond C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buccigrossi V L.G., Russo C., Miele E., Sofia M., Monini M., Ruggeri F.M., Guarino A. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao H., Li Y., Sun J., Xu H., Wang M., Zuo X., Fu Q., Guo Y., Chen Z., Zhang P., Li X., Wang N., Ye T., Yao Y. Saccharomyces boulardii ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating NF-kappaB and Nrf2 signaling pathways. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/1622375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kheir-Eldin A.A., Motawi T.K., Gad M.Z., Abd-ElGawad H.M. Protective effect of vitamin E, beta-carotene and N-acetylcysteine from the brain oxidative stress induced in rats by lipopolysaccharide. Int. J. Biochem. Cell Biol. 2001;33(5):475–482. doi: 10.1016/s1357-2725(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 50.Thomas S., Przesdzing I., Metzke D., Schmitz J., Radbruch A., Baumgart D.C. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin. Exp. Immunol. 2009;156(1):78–87. doi: 10.1111/j.1365-2249.2009.03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fidan I., Kalkanci A., Yesilyurt E., Yalcin B., Erdal B., Kustimur S., Imir T. Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans. Mycoses. 2009;52(1):29–34. doi: 10.1111/j.1439-0507.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 52.Jawhara S., Poulain D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007;45(8):691–700. doi: 10.1080/13693780701523013. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Kokkotou E.G., Mustafa N., Bhaskar K.R., Sougioultzis S., O'Brien M., Pothoulakis C., Kelly C.P. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J. Biol. Chem. 2006;281(34):24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 54.Martins F.S., Dalmasso G., Arantes R.M., Doye A., Lemichez E., Lagadec P., Imbert V., Peyron J.F., Rampal P., Nicoli J.R., Czerucka D. Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S.K., Kim Y.W., Chi S.G., Joo Y.S., Kim H.J. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig. Dis. Sci. 2009;54(2):255–263. doi: 10.1007/s10620-008-0357-0. [DOI] [PubMed] [Google Scholar]
- 56.Meabed E.M.H., Abdelhafez D.N., Abdelaliem Y.F. Saccharomyces boulardii inhibits the expression of pro-inflammatory cytokines and inducible nitric oxide synthase genes in the colonic mucosa of rats experimentally-infected with Blastocystis subtype-3 cysts. Parasitology. 2019;146(12):1532–1540. doi: 10.1017/S0031182019000696. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y., Wang B., Xu H., Tang L., Li Y., Gong L., Wang Y., Li W. Probiotic Bacillus attenuates oxidative stress- induced intestinal injury via p38-mediated autophagy. Front. Microbiol. 2019;10:2185. doi: 10.3389/fmicb.2019.02185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Wu Y., Wang Y., Fu A., Gong L., Li W., Li Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017;101(7):3015–3026. doi: 10.1007/s00253-016-8032-4. [DOI] [PubMed] [Google Scholar]
- 59.Bohlouli J., Namjoo I., Borzoo-Isfahani M., Hojjati Kermani M.A., Balouch Zehi Z., Moravejolahkami A.R. Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: a systematic review and meta-analysis of clinical trials. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2021.e05925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alam Q., Krishnamurthy S. Dihydroquercetin ameliorates LPS-induced neuroinflammation and memory deficit. Curr Res Pharmacol Drug Discov. 2022;3 doi: 10.1016/j.crphar.2022.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babaei F., Mirzababaei M., Mohammadi G., Dargahi L., Nassiri-Asl M. Saccharomyces boulardii attenuates lipopolysaccharide-induced anxiety-like behaviors in rats. Neurosci. Lett. 2022;778 doi: 10.1016/j.neulet.2022.136600. [DOI] [PubMed] [Google Scholar]
- 62.Li B., Zhang H., Shi L., Li R., Luo Y., Deng Y., Li S., Li R., Liu Z. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 2022;13(1):102–112. doi: 10.1039/d1fo02752b. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W B.C., Wang J., Zang J., Cao Y. Administration of Saccharomyces boulardii mafic-1701 improves feed conversion ratio, promotes antioxidant capacity, alleviates intestinal inflammation and modulates gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 2020;11(1):112. doi: 10.1186/s40104-020-00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider S.M., Girard-Pipau F., Filippi J., Hebuterne X., Moyse D., Hinojosa G.C., Pompei A., Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J. Gastroenterol. 2005;11(39):6165–6169. doi: 10.3748/wjg.v11.i39.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong J.P., Zheng Y., Wu T., He Q., Teng G.G., Wang H.H. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin. Med. J. 2019;132(16):1951–1958. doi: 10.1097/CM9.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng X., Luo Z., He S., Zhang L., Li Y. Blood-brain barrier disruption by lipopolysaccharide and sepsis-associated encephalopathy. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.768108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T., Zheng L.N., Han X.H. Fenretinide attenuates lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2019.109680. [DOI] [PubMed] [Google Scholar]
- 68.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyás B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263) doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu J., Fan X., Lu L., Yu Y., Markiewicz E., Little J.C., Sidebottom A.M., Claud E.C. Limosilactobacillus reuteri normalizes blood-brain barrier dysfunction and neurodevelopment deficits associated with prenatal exposure to lipopolysaccharide. Gut Microb. 2023;15(1) doi: 10.1080/19490976.2023.2178800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorenz E S.M., Senz M. Evaluation of cysteine ethyl ester as efficient inducer for glutathione overproduction in Saccharomyces spp. Enzym. Microb. Technol. 2016;94:121–131. doi: 10.1016/j.enzmictec.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Suryavanshi A A.A., Kaler A., Bihade U., Kaur J., Tikoo K.B., Banerjee U.C. Comparative studies on the antioxidant potential of vanillin-producing Saccharomyces boulardii extracts. Oxidants and Antioxidants in Medical Science. 2013;2:1. [Google Scholar]
- 72.McFarland L.V. Academic press; 2017. Saccharomyces Boulardii. [Google Scholar]
- 73.Edwards-Ingram L., Gitsham P., Burton N., Warhurst G., Clarke I., Hoyle D., Oliver S.G., Stateva L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007;73(8):2458–2467. doi: 10.1128/AEM.02201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fietto Jl A.R., Valadão F.N., Fietto L.G., Brandão R.L., Neves M.J., Gomes F.C., Nicoli J.R., Castro I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004;50(8):615–621. doi: 10.1139/w04-050. [DOI] [PubMed] [Google Scholar]
- 75.Girard P., Pansart Y., Lorette I., Gillardin J.M. Dose-response relationship and mechanism of action of Saccharomyces boulardii in castor oil-induced diarrhea in rats. Dig. Dis. Sci. 2003;48(4):770–774. doi: 10.1023/a:1022801228938. [DOI] [PubMed] [Google Scholar]
- 76.Mu Z., Yang Y., Xia Y., Wang F., Sun Y., Yang Y., Ai L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food Funct. 2021;12(18):8386–8398. doi: 10.1039/d1fo01314a. [DOI] [PubMed] [Google Scholar]
- 77.Czerucka D P.T., Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26(6):767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data has been included in the article.