Abstract
Background
This study aimed to evaluate the efficacy and safety of intrathecal pemetrexed (IP) for treating patients with leptomeningeal metastases (LM) from non-small-cell lung cancer (NSCLC) who progressed from epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) treatment in an expanded, prospective, single-arm, phase II clinical study (ChiCTR1800016615).
Patients and methods
Patients with confirmed NSCLC-LM who progressed from TKI received IP (50 mg, day 1/day 5 for 1 week, then every 3 weeks for four cycles, and then once monthly) until disease progression or intolerance. Objectives were to assess overall survival (OS), response rate, and safety. Measurable lesions were assessed by investigator according to RECIST version 1.1. LM were assessed according to the Response Assessment in Neuro-Oncology (RANO) criteria.
Results
The study included 132 patients; 68% were female and median age was 52 years (31-74 years). The median OS was 12 months (95% confidence interval 10.4-13.6 months), RANO-assessed response rate was 80.3% (106/132), and the most common adverse event was myelosuppression (n = 42; 31.8%), which reversed after symptomatic treatment. The results of subgroup analysis showed that absence of brain parenchymal metastasis, good Eastern Cooperative Oncology Group score, good response to IP treatment, negative cytology after treatment, and patients without neck/back pain/difficult defecation had longer survival. Gender, age, previous intrathecal methotrexate/cytarabine, and whole-brain radiotherapy had no significant influence on OS.
Conclusions
This study further showed that IP is an effective and safe treatment method for the EGFR-TKI-failed NSCLC-LM, and should be recommended for these patients in clinical practice and guidelines.
Key words: non-small-cell lung cancer, leptomeningeal metastasis, epidermal growth factor receptor, intrathecal pemetrexed
Highlights
-
•
IP is an effective treatment method for the EGFR-TKI-failed NSCLC-LM with a median OS of 12 months.
-
•
Previous intrathecal methotrexate/cytarabine and whole-brain radiotherapy didn't have significant difference on IP treatment.
-
•
IP is a safe treatment method for the EGFR-TKI-failed NSCLC-LM with tolerated adverse events.
-
•
IP should be recommended for patients with EGFR-TKI-failed NSCLC-LM in clinical practice.
Introduction
Leptomeningeal metastases (LM) refer to the spread of malignant tumor cells to the leptomeninges and cerebral spinal fluid (CSF). The incidence rate of LM in non-small-cell lung cancer (NSCLC) is 3%-5%, and the prognosis is very poor.1,2 Recently, with the application of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), extended survival, and the improved diagnostic techniques, the incidence rate of LM is as high as 9.4% in EGFR-mutant NSCLC.3,4 Most patients develop LM during treatment with EGFR-TKI; therefore, effective treatment is urgently needed.5 Due to the presence of blood–brain barrier, intrathecal chemotherapy is an effective treatment option for LM. Previously, the commonly used drugs for intrathecal chemotherapy mainly included methotrexate (MTX), cytosine arabinoside, and thiotepa, but their efficacy is very low.1, 2, 3, 4 We have completed a phase I/II clinical trial, which determined that the recommended dose for intrathecal pemetrexed (IP) was 50 mg and confirmed that IP is a safe and effective treatment method for NSCLC-LM. The response rate was 84.6% (22/26), and the median overall survival (mOS) was 9 months [n = 30, 95% confidence interval (CI) 6.6-11.4 months].5 In addition, other researchers have also reported the efficacy and safety of IP treatment, but they are all clinical research studies with small sample sizes (n = 13-40).6, 7, 8, 9 This study is an expanded, prospective, phase II clinical trial (ChiCTR1800016615), and the purpose is to further evaluate the efficacy and safety of IP for the treatment of NSCLC-LM.
Patients and methods
Study patients
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University, and all patients provided written informed consent before IP treatment. The inclusion criteria were as follows: (i) patients with probable or confirmed EGFR-mutant LM from NSCLC, according to the European Society for Medical Oncology (ESMO)–European Association of Neuro-Oncology (EANO) guidelines; (ii) age 18-75 years; (iii) EGFR-TKI therapy failed, previously multiline therapy, including systemic chemotherapy, TKI treatment, intrathecal chemotherapy with MTX and cytarabine, or whole-brain radiation therapy (WBRT); (iv) adequate function of organs (absolute neutrophil count ≥1.5 × 109/l, platelet count ≥100 × 109/l, hemoglobin concentration ≥90 g/l, serum total bilirubin concentration ≤1.5 mg/dl, serum transaminase concentration ≤2.5 times the upper limit of normal, serum creatinine concentration ≤1.5 times the upper limit of normal, indoor air SpO2 ≥90%, and proteinuria ≤1+). Exclusion criteria were as follows: (i) human immunodeficiency virus-positive patients; (ii) history of severe pemetrexed allergies; (iii) severe infection or severe comorbidities, such as bleeding peptic ulcer, ileus, heart failure, renal failure, or poorly controlled diabetes; (iv) failure to observe test requirements; and (v) pregnancy.
Treatment methods and response evaluation
All eligible patients received IP twice a week for 1 week (day 1 and day 5) as induction treatment, followed by once every 3 weeks as consolidation therapy for four cycles, and then once monthly as maintenance therapy, until progressive disease was observed or intolerance or adverse events (AEs) developed. All patients received supplemental folic acid (400 μg, once daily, orally) and vitamin B12 (1 mg, intramuscular injection 1-2 weeks before the first dose of pemetrexed and repeated every 9 weeks) during the entire treatment period to avoid the side-effects of pemetrexed. Pemetrexed was administered by intrathecal injection by means of lumbar puncture or Ommaya reservoir. Pemetrexed lyophilized powder (200 mg) was dissolved in 0.9% sodium chloride solution (10 ml). Then, 2.5 ml was added to 0.9% sodium chloride solution to prepare 5 ml mixed solutions, and 5 ml was injected intrathecally each time. Dexamethasone (5 mg, 1 ml) was administered by intrathecal injection combined with pemetrexed during each IP to reduce the incidence of chemical myelitis. At the same time, systematic therapeutic strategy was continued (not changed, at least in the first 21 days of IP, mostly treated with third-generation EGFR-TKI). If grade 3 or above treatment-related AEs occurred, the dose of IP or systemic therapy was reduced as appropriate.
The main evaluating indices included OS, response rate, and AEs. OS was calculated from the time of start of IP therapy to the endpoint. LM response was evaluated using the Response Assessment in Neuro-Oncology (RANO) criteria: (i) standardized neurological examination, (ii) CSF cytology, and (iii) enhanced brain axonal magnetic resonance imaging (MRI) for radiological assessment.10 The assessment consists of three levels: response, stable, and progression. Clinical evaluation was carried out weekly from the start of treatment until 4 weeks after the full treatment cycle. Standardized neurological examination was carried out before each IP. Whole-brain spinal cord-enhanced MRI was carried out every 6 weeks (before IP). CSF was collected for cytology before each IP. AEs were assessed using the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). The basic clinical characteristics of NSCLC-LM patients were noted, including sex, age, time from NSCLC to LM diagnosis, gene test results, Eastern Cooperative Oncology Group (ECOG) score, clinical presentation, and previous treatment regimens.
Statistical analysis
SPSS 24.0 software (IBM Corporation, Armonk, NY) and R Studio 4.2.2 (Posit, Boston, MA) were used to analyze the data. To consider IP as a promising treatment strategy, we planned to detect an improvement in response rate from 60% (based on previous study11) to 80% with ∼90% power using the Pearson chi-square test at a one-sided significance level of 0.20. The planned sample size was 114 patients; assuming a 20% exclusion rate, 146 patients were screened. OS and survival curves were analyzed using the Kaplan–Meier method, and comparisons of survival were carried out using the log-rank test and CIs were calculated at the 95% confidence level. All statistical analyses were two-sided, with P < 0.05 indicating statistical significance.
Results
Patient characteristics
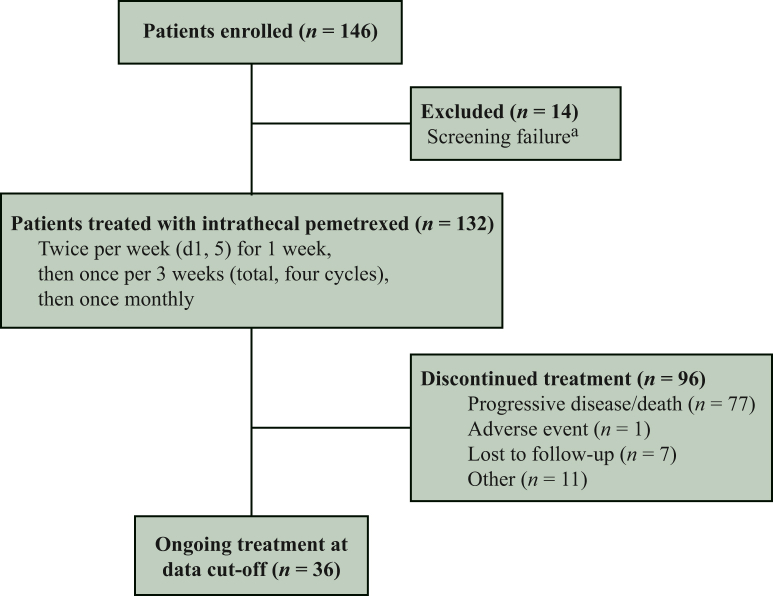
Between 3 December 2019 and 16 May 2022, a total of 132 patients were included, as detailed in Figure 1 and Table 1. There were 68% female patients and the median age was 52 years (31-74 years). The median time from diagnosis of NSCLC to development of LM was 26 months (0-156 months). ECOG scores of 3-4 accounted for 64%. There were 73% of patients with brain parenchymal metastases. All patients had confirmed EGFR mutations and had received TKI treatment, and most had common mutations. There were 92% of patients with symptoms of intracranial hypertension, such as headache, nausea, and vomiting, 30% of patients with consciousness disturbances or epilepsy, 51% of patients with walking difficulty, 64% of patients with optic, auditory, or facial nerve involvement, and 11% of patients with neck pain, back pain, or defecation difficulty. There were 56% of patients with LM as assessed by enhanced MRI, 80% of patients with positive CSF cytology for the first time, and 10% of patients with positive CSF cytology for the second time. All patients had failed TKI treatment, of which 73% of patients had received a first-generation TKI, 22% of patients had received a second-generation TKI, and 84% had received a third-generation TKI. Seventy-two percent of patients had received systemic chemotherapy, 11% had received intrathecal MTX and cytarabine, 39% had undergone brain radiotherapy, and 14% had received WBRT. A total of 51 (39%) patients had concurrent gene testing of CSF and plasma, and EGFR mutation was highly concordant between NSCLC tissue and CSF. A much higher positive detection rate of mutant gene was found in the CSF than in the plasma (90% versus 48%). The T790M mutation of EGFR was detected in the plasma of five patients, but not in the CSF, and this mutation was detected in the CSF of another three patients, but not in these patients’ plasma.
Figure 1.
Flow diagram.
aReasons for screening failure: epidermal growth factor receptor (EGFR) status negative or not assessed (n = 1), screening failure as results of other inclusion/exclusion criteria (n = 13). d, day.
Table 1.
Clinical characteristics
| Characteristic | n (%) |
|---|---|
| Sex, male | 42 (32) |
| Sex, female | 90 (68) |
| Age at diagnosis, years, mean (range) | 52 (31-74) |
| Time from NSCLC diagnosis to LM, months (range) | 26 (0-156) |
| ECOG score at admission | |
| 0-2 | 47 (36) |
| 3-4 | 85 (64) |
| Brain metastasis | |
| With | 96 (73) |
| Without | 36 (27) |
| Brain radiotherapy before LM | |
| Local | 35 (27) |
| Whole | 19 (14) |
| Without | 80 (61) |
| Cytotoxic chemotherapy before LM | |
| With | 95 (72) |
| Without | 37 (28) |
| EGFR-TKI therapy before IP | |
| First generation | 96 (73) |
| Second generation | 29 (22) |
| Third generation | 111 (84) |
| Clinical manifestation | |
| Headache, nausea, or vomiting | 122 (92) |
| Disorders of consciousness or epilepsy | 39 (30) |
| Walking instability | 67 (51) |
| Optic, auditory, or facial nerve involvement | 85 (64) |
| Neck pain, back pain, or defecation difficulty | 14 (11) |
| Gene mutation detection | |
| EGFR 19 mutation | 46 (35) |
| EGFR 21 mutation | 64 (48) |
| EGFR 18 mutation | 9 (7) |
| EGFR 20 mutation | 6 (5) |
| Others | 7 (5) |
| The modality of LM diagnosis | |
| MRI+/cytology+ | 61 (46) |
| MRI−/cytology+ | 58 (44) |
| MRI+/cytology− | 13 (10) |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IP, intrathecal pemetrexed; LM, leptomeningeal metastases; MRI, magnetic resonance imaging; NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor.
Efficacy evaluation
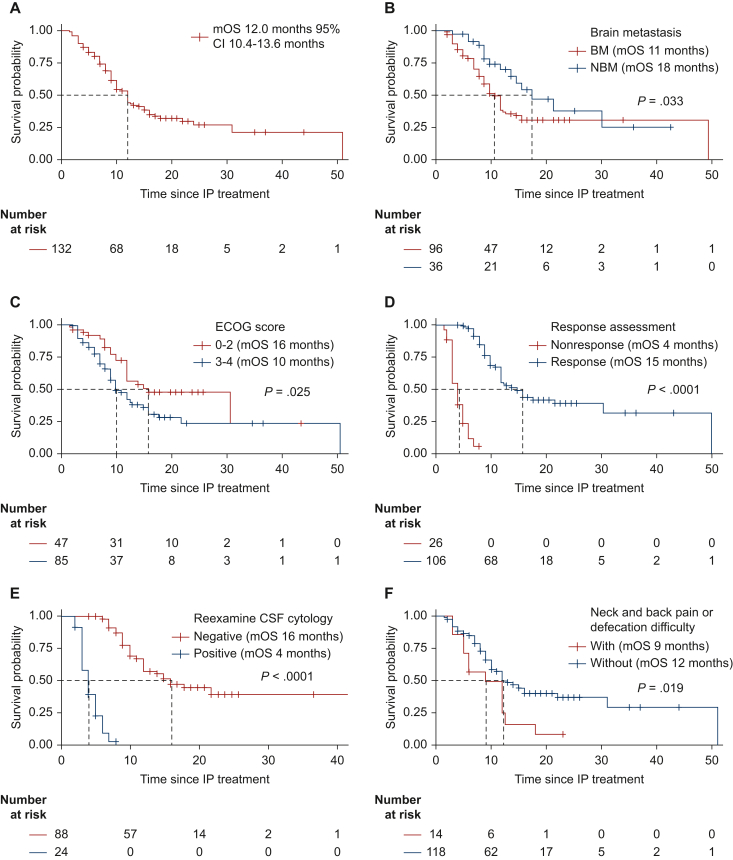
The median follow-up time was 18 months and the inverse Kaplan–Meier method was used for patients who were evaluated for efficacy until 30 September 2022. Patients received IP for an average of six times. At the end of the study, 73 (55.3%) patients died, 52 (39.4%) patients survived, and 7 (5.3%) patients were lost to follow-up. Overall, patients had an mOS of 12.0 months (95% CI 10.4-13.6 months) (Figure 2A). In the subgroup survival analysis, mOS was 11 months in patients with brain parenchymal metastases compared with 18 months in patients without brain parenchymal metastases (P = 0.033) (Figure 2B). Patients with good ECOG scores had better mOS than those with poor ECOG scores (0-2 versus 3-4: 16.0 versus 10.0 months, P = 0.025) (Figure 2C). The mOS was 15 months in patients with response for IP treatment compared with 4 months in patients without IP response (P = 0.033) (Figure 2D). CSF cytological transformation to negative was associated with longer survival than no CSF cytological transformed negativity (mOS 16.0 versus 4.0 months, P < 0.001) (Figure 2E). The survival time of patients with and without neck pain, back pain, or defecation difficulty was different and the difference was statistically significant (mOS 9 versus 12 months, P = 0.0187) (Figure 2F). There were no significant differences in OS with respect to different EGFR mutations (exon 19 versus 21), intrathecal MTX/cytarabine treatment history (yes versus no), WBRT (yes versus no), contrast-enhanced MRI (positive versus negative), and intrathecal methods (Ommaya versus lumbar puncture).
Figure 2.
OS of all patients with NSCLC-LM and subgroup analysis. (A) The OS of all the patients. (B) Subgroup analysis for patients with and without brain parenchymal metastases. (C) Subgroup analysis for patients with good and bad ECOG scores. (D) Subgroup analysis for patients with and without clinical response for IP treatment. (E) With and without CSF cytological transformation to negative subgroup. (F) With and without neck pain, back pain, or defecation difficulty subgroup.
BM, brain parenchymal metastasis; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; NBM, no brain parenchymal metastasis; OS, overall survival.
According to RANO criteria, 80.3% (106/132) of patients were assessed as response (OS 4.0-51.0 months, median 15.0 months), 14.4% (19/132) of patients were stable (OS 2.0-8.0 months, median 4.0 months), and progression was seen in 5.3% (7/132) of patients (OS 1.5-3.0 months, median 3.0 months) (Table 2).
Table 2.
Clinical response rate and the patients’ survival
| Response | n (%) | OS (months) | Median OS (months) |
|---|---|---|---|
| Response | 106 (80.3) | 4.0-51.0 | 15.0 |
| Stable | 19 (14.4) | 2.0-8.0 | 4.0 |
| Progression | 7 (5.3) | 1.5-3.0 | 3.0 |
OS, overall survival.
Safety and toxicity
In this study, AEs included elevated transaminases, nausea, vomiting, myelosuppression, neurotoxicity, and leukoencephalopathy (Table 3). Most AEs were mild (grade 1-2). Two patients had grade 3 limb pain and one patient had grade 3 headache. Two patients developed mucositis. Nausea and vomiting were observed in 7 and 11 patients, respectively. The most common AE was myelosuppression (n = 42; 31.8%); grade 3 or greater myelosuppression was observed in 12 (9%) patients, which reversed after administration of supportive care.
Table 3.
Adverse events
| Toxicity, n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Myelosuppression | 17 (12.9) | 13 (9.8) | 6 (4.5) | 6 (4.5) |
| Nausea | 6 (4.5) | 1 (0.8) | 0 | 0 |
| Vomiting | 9 (6.8) | 2 (1.5) | 0 | 0 |
| ALT/AST | 0 | 2 (1.5) | 1 (0.8) | 0 |
| Neurotoxicity | ||||
| Limb | 3 (2.3) | 2 (1.5) | 2 (1.5) | 0 |
| Back pain | 4 (3.0) | 1 (0.8) | 0 | 0 |
| Paralysis | 2 (1.5) | 2 (1.5) | 0 | 0 |
| Headache | 0 | 5 (3.8) | 1 (0.8) | 0 |
| Mucositis | 0 | 1 (0.8) | 1 (0.8) | 0 |
| Leukoencephalopathy | 10 (7.6) | 0 | 0 | 0 |
| Acute cerebral meningitis | 0 | 0 | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
This study shows that IP is an effective and safe treatment method for EGFR-mutant NSCLC-LM with a median survival of 12 months and a response rate of 80.3% (106/132), as assessed by RANO criteria. To our knowledge, this is the largest prospective study of IP therapy for NSCLC-LM to date. As the majority of NSCLC-LM patients had poor performance status, this trial did not preclude patients with ECOG scores of 3-4, which accounted for 64% (85/132). There were more female patients (68%) in our study and this is probably due to more female NSCLC patients with EGFR mutations. Most patients (84%) developed LM during third-generation EGFR-TKI treatment, and neurological symptoms and signs subsided after IP treatment. Therefore, our study shows that IP has good efficacy in NSCLC-LM patients who have failed third-generation TKI therapy. The BLOOM study showed an objective response rate of 41% and an mOS of 11.0 months in patients with EGFR-mutant NSCLC-LM treated with a double dose of osimertinib (160 mg).11 Unlike the BLOOM study, which included patients with an ECOG score of 0-2, 64% of patients in this study had an ECOG score of 3-4 and were allowed to receive multiple lines of therapy. Geng et al.’s retrospective study, which included 34 patients, showed a 76.5% response rate with IP for NSCLC-LM and an mOS of 20 months.6 However, Geng et al.’s study calculated OS from the time of LM diagnosis, and the OS calculated from IP therapy was 3.5 months (mentioned in the discussion). In addition to EGFR mutations, anaplastic lymphoma kinase (ALK) mutations were also present in Geng et al.’s study, and treatment history of patients was different from our study. Therefore, the comparisons between different studies should be done paying careful attention to these differences. Whether double-dose osimertinib combined with IP for the treatment of NSCLC-LM has good efficacy and tolerability of side-effects is worthy of further study.
In our subgroup analysis, OS benefit was significant in patients with no brain parenchymal metastasis, good physical status (ECOG 0-2 score), effective IP therapy, and negative CSF cytology after treatment (P < 0.05); this was consistent with our previous study.5 In addition, subgroup analysis showed that patients with symptoms of neck pain, back pain, or difficulty in defecation had shorter OS than those without these symptoms; this may be due to the presence of spinal metastases and poorer physical performance (ECOG scores of 3-4 in 13 of 14 patients). There was no significant difference in OS with or without prior intrathecal MTX/cytosine arabinoside therapy and no significant increase in AEs after IP therapy (P = 0.205). This suggests that IP has a good antitumor effect even after MTX/cytosine arabinoside resistance. There was no significant difference in OS with or without WBRT treatment, which was related to the insufficient irradiation range, low dose, and extensive adverse reaction. In this study, nine patients were treated with IP by Ommaya reservoir. There was no significant difference in survival time between the two groups (P = 0.066). This is at odds with Montes de Oca Delgado et al.’s report, which included 10 patients with Ommaya reservoir and 30 patients with lumbar puncture treated with intrathecal chemotherapy, and the results showed that intrathecal chemotherapy by Ommaya conferred greater survival benefit.12 The reasons for this inconsistency may be related to the differences in basic characteristics of enrolled patients, differences in treatment methods, and the limited number of patients. Therefore, we believe that the benefits of IP via Ommaya reservoir need to be confirmed by further studies. Twenty patients received PCR and 112 patients received next-generation sequencing (NGS) on the initial testing of EGFR mutations. Only NGS was carried out for patients at the time of LM with CSF. Most common co-mutations include TP53 mutation (31/51) and EGFR copy number variation (CNV) (17/51) in patients who received NGS test with CSF. There were no significant differences in OS with respect to TP53 mutations or not [mOS for TP53+ versus TP53− 12 months (95% CI 4.8-19.2 months) versus 15 months (95% CI 10.0-20.0 months), P = 0.468]. The mOS was 10 months (95% CI 7.8-12.1 months) in patients with EGFR CNV compared with 31 months (95% CI 9.6-52.3 months) in patients without (P = 0.041, Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102384).
According to ESMO–EANO guidelines, LM can be confirmed by finding tumor cells or suspicious tumor cells in CSF, whereas patients with overt neuroimaging and typical clinical symptoms are diagnosed with probable LM.13 The guidelines recommend that patients with confirmed and probable LM should participate in clinical trials or receive tumor-specific therapy. In the past, a positive CSF cytopathology rate of 50% was detected using the test tube in the lumbar puncture package in our hospital; in our study, CSF (10 ml) was isolated and immediately stored in a methanol-containing cell-fixing solution (TIB, Tepper Bioscience, Beijing, China); a positive CSF cytopathology rate of 80% was detected.14 A previous study has reported that CSF cell-free DNA (cfDNA) detection was helpful in the diagnosis of LM.15 MRI scan was positive in 56% (74/132) of all patients in this study, including 13 patients with enhanced MRI suggestive of LM and with LM-associated neurological symptoms but negative CSF cytology. Ten of these patients had CSF genetic testing, nine (90%) had EGFR mutations in their CSF consistent with the primary tumor, and one patient had no EGFR mutations in his CSF, who had remission of symptoms after IP therapy. A total of 51 patients (48 had a history of receiving third-generation EGFR-TKI) underwent plasma and CSF genetic testing in this study. The T790M mutation was found in the plasma of five patients, but not in the corresponding CSF samples. T790M mutation was detected in the CSF of three patients, but not in the corresponding plasma. These results are consistent with previous reports that T790M is relatively rare in central nervous system lesions compared to extracranial sites, possibly because the presence of the blood–brain barrier results in lower levels of first-generation EGFR-TKIs in the CSF.16 Intracranial tumor cells are less affected by TKI and do not readily acquire secondary T790M mutations; hence, LM may occur before TKI treatment failure rather than progress after TKI treatment failure. The findings in this study also support the use of CSF cfDNA as an adjunct method for the diagnosis of LM, an aspect which needs further study.
RANO criteria were used in this analysis to evaluate efficacy.10 The response rate in this trial was 80.3% (106/132), which was higher than that in patients who received intrathecal MTX, cytarabine, and thiotepa.17 Compared with the results of our previous phase I/II clinical trial,5 the response rate was almost the same (80.3% versus 84.6%), but the median survival was higher in this study (12 versus 9 months). This may be due to differences in ECOG scores, percentage of brain parenchymal metastases, and assessment criteria used in the two studies.
Myelosuppression, elevation of transaminases, nausea, vomiting, and neurotoxicity were common AEs in IP-treated patients. Consistent with our previous study,5 the most common AE was myelosuppression rather than neurotoxicity; the incidence of myelosuppression was largely consistent with previous studies and was mostly grade 1-2. Neurotoxicity mainly included headache, back pain, limb pain, and transient limb weakness, but these improved after symptomatic treatment. Leukoencephalopathy in the brain was observed in 10 patients in this study, which was not observed in previous studies, possibly because of relatively few patients in those studies.5,6,8 We could not confirm that leukoencephalopathy is caused by intrathecal chemotherapy, because leukoencephalopathy can also be caused by LM, parenchymal metastases, and WBRT. Liver damage was observed in three patients in this study, which was not observed in our previous study5; this may be related to the small sample size in previous study and the different combination of systemic therapies administered to the patients in our study.
This study, however, has some limitations. Firstly, this study included NSCLC-LM patients with EGFR mutations, which were currently the most common in our clinical work. However, we also detected ALK-positive, wild-type NSCLC-LM and small-cell lung cancer-LM, and the effect of IP therapy in these patients needs further study. Secondly, this study included patients with diverse pre-treatment protocols and combined systemic treatment with IP, which might have influenced the evaluation of the effect of IP treatment. However, these were also some characteristics of NSCLC-LM patients, which mostly occurred after multiline treatment. The number of patients included in the trial would have been significantly affected by stringent restrictions on prior and concurrent treatment. Thirdly, neuroimaging studies of LM are generally not good at assessing LM in the absence of massive, nodular disease, and it is difficult to define a measurable target lesion even under ideal conditions. The false negativity rate of CSF cytology also influences the evaluation of treatment effect; this may have led to some errors in the evaluation of IP treatment effect on LM. However, the RANO criteria used in this study are currently a better method for evaluating LM. In addition, the frequency and interval of IP treatment for LM need to be further refined. Despite these potential limitations, our results provide strong evidence for this promising treatment strategy and can potentially change the standard of care for a difficult-to-treat patient population.
Conclusions
The median survival of patients with NSCLC-LM who had failed EGFR-TKI and were treated with IP in this study was 12 months, with a response rate of 80.3% and manageable adverse effects. Our study further confirmed that IP is an effective and safe treatment for EGFR-TKI-failed NSCLC-LM patients, and has potential application in clinical practice, and should be recommended for these patients in clinical practice and guidelines.
Acknowledgements
We would like to thank Yashuang Zhao (Department of Epidemiology, School of Public Health, Harbin Medical University, Harbin, China) for help in statistical analysis.
Funding
This study was supported by Jiangsu Hansoh Pharmaceutical Group Co., Ltd [grant number HL-HS2020-76] and Health Commission of Heilongjiang Province [grant number 20220303101064].
Disclosure
TX reports research support from Hansoh; speaker fees from AstraZeneca, Roche, Hansoh, and Hengrui Therapeutics. All other authors have declared no conflicts of interest.
Data sharing
Individual participant data underlying the results reported in this study can be made available to other qualified medical investigators after de-identification, via a request to the corresponding author. Requestors will need to sign a data access agreement. Data will be available beginning at 12 months and ending at 36 months after article publication.
Supplementary data
References
- 1.Thakkar J., Kumthekar P., Dixit K., et al. Leptomeningeal metastasis from solid tumors. J Neurol Sci. 2020;411 doi: 10.1016/j.jns.2020.116706. [DOI] [PubMed] [Google Scholar]
- 2.Lukas R.V., Thakkar J.P., Cristofanilli M., et al. Leptomeningeal metastases: the future is now. J Neurooncol. 2022;156:443–452. doi: 10.1007/s11060-021-03924-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Yang X., Li N.J., Xue J.X. Leptomeningeal metastases in non-small cell lung cancer: diagnosis and treatment. Lung Cancer. 2022;174:1–13. doi: 10.1016/j.lungcan.2022.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Ozcan G., Singh M., Vredenburgh J. Leptomeningeal metastasis from non-small cell lung cancer and current landscape of treatments. Clin Cancer Res. 2023;29:11–29. doi: 10.1158/1078-0432.CCR-22-1585. [DOI] [PubMed] [Google Scholar]
- 5.Fan C., Zhao Q., Li L., et al. Efficacy and safety of intrathecal pemetrexed combined with dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (unique identifier: ChiCTR1800016615) J Thorac Oncol. 2021;16:1359–1368. doi: 10.1016/j.jtho.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Geng D., Guo Q., Huang S., et al. A retrospective study of intrathecal pemetrexed combined with systemic therapy for leptomeningeal metastasis of lung cancer. Technol Cancer Res Treat. 2022;21 doi: 10.1177/15330338221078429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Z., Yang G., He H., et al. Intrathecal pemetrexed combined with involved-field radiotherapy as a first-line intra-CSF therapy for leptomeningeal metastases from solid tumors: a phase I/II study. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920937953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao Q., Zheng X., Zhang L., et al. Multiple combination therapy based on intrathecal pemetrexed in non-small cell lung cancer patients with refractory leptomeningeal metastasis. Ann Palliat Med. 2020;9:4233–4245. doi: 10.21037/apm-20-2086. [DOI] [PubMed] [Google Scholar]
- 9.Pan Z., Yang G., Cui J., et al. A pilot phase 1 study of intrathecal pemetrexed for refractory leptomeningeal metastases from non-small-cell lung cancer. Front Oncol. 2019;9:838. doi: 10.3389/fonc.2019.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain M., Junck L., Brandsma D., et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19:484–492. doi: 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J.C.H., Kim S.W., Kim D.W., et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montes de Oca Delgado M., Cacho Díaz B., Santos Zambrano J., et al. The comparative treatment of intraventricular chemotherapy by ommaya reservoir vs. lumbar puncture in patients with leptomeningeal carcinomatosis. Front Oncol. 2018;8:509. doi: 10.3389/fonc.2018.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Rhun E., Weller M., Brandsma D., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 14.Gao N., Teng C., Fan C., Xin T. [Investigation of methods and influencing factors to increase the positive rate of cytological pathology of cerebrospinal fluid from lung cancer leptomeningeal metastases] Zhongguo Fei Ai Za Zhi. 2022;25:789–796. doi: 10.3779/j.issn.1009-3419.2022.102.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White M.D., Klein R.H., Shaw B., et al. Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Ye X., Xu Y., et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol. 2016;78:1305–1310. doi: 10.1007/s00280-016-3155-y. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.L., Zhou L., Lu Y. Intrathecal chemotherapy as a treatment for leptomeningeal metastasis of non-small cell lung cancer: a pooled analysis. Oncol Lett. 2016;12:1301–1314. doi: 10.3892/ol.2016.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.