Abstract
Methamphetamine is a potent and highly addictive neurotoxic psychostimulant that triggers a spectrum of adverse emotional responses during withdrawal. G-protein coupled receptor 55 (GPR55), a novel endocannabinoid receptor, is closely associated with mood regulation. Herein, we developed a murine model of methamphetamine-induced anxiety- and depressive-like behavior during abstinence which showed a decreased GPR55 expression in the hippocampus. Activation of GPR55 mitigated these behavioral symptoms, concomitantly ameliorating impairments in hippocampal neurogenesis and reducing neuroinflammation. These findings underscore the pivotal role of GPR55 in mediating the neuropsychological consequences of methamphetamine withdrawal, potentially via mechanisms involving the modulation of hippocampal neurogenesis and inflammation.
Keywords: Methamphetamine, GPR55, Anxiety, Depression
1. Introduction
Methamphetamine (METH) is an illegal psychostimulant abused globally [1]. Prolonged use and withdrawal from METH may lead to a range of neurobehavioral symptoms. A significant number of individuals undergoing substance use rehabilitation exhibit negative emotions, primarily manifested as anxiety and depression symptoms [2,3]. These can hinder recovery and increase relapse rates [[4], [5], [6]]. As part of the limbic system, the hippocampus is involved in regulating emotion-related processes and has a crucial role in negative emotions that occur with depression and anxiety [7,8]. Increasing hippocampal neurogenesis has been found to improve stress-induced anxiety- and depressive-like behaviors [9,10]. Additionally, activating microglia in the hippocampus promotes the occurrence of depressive-like behaviors [11], while inhibiting microglial activation and neuroinflammation improves depressive-like behaviors in mice [12].
G-protein coupled receptor 55 (GPR55) is a novel cannabinoid receptor highly expressed in brain regions like the hippocampus [13,14] and plays an essential role in maintaining brain function [15]. Activating GPR55 increased hippocampal neurogenesis [16], participated in microglial activation, and counteracted inflammatory damage [17]. GPR55 activation has also been found to improve anxiety- and depressive-like behaviors induced by acute stress and chronic social defeat stress [18,19]. However, it remains unknown whether activating GPR55 ameliorates METH-induced anxiety- and depressive-like withdrawal symptoms. Thus, this study explored the role of GPR55 in the regulation of anxiety- and depressive-like behaviors of mice in the context of METH abstinence.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice weighing 22–25 g were purchased from Hunan SJA Laboratory Animal Co. Mice were housed in a controlled environment at 23 ± 0.5 °C and 55 ± 5 % humidity. They had free access to food and water and were kept on a 12-h light/dark cycle. Before the start of the experiment, all animals were allowed to acclimate to the environment for 1 week. All experimental procedures were conducted following the ethical standards set by the Animal Ethics Committee of First Affiliated Hospital of Xinjiang Medical University (IACUC-20200331-53).
2.2. Reagents
METH was provided by the Changsha Public Security Bureau. O-1602 (HY-107541) was purchased from MedChemExpress (US). GPR55 antibody (AB203663) was purchased from Abcam (UK). NLRP3 antibody (R03102635), Caspase-1 antibody (R0742996), and ASC antibody (R06292462) were purchased from Shenyang Wanlei Biological Co., Ltd (CHN). β-Tubulin antibody (10068-1-AP) was purchased from Wuhan Sanying Company (CHN). The Corticosterone ELISA kit (MM-0061M1) was purchased from Jiangsu Enzyme-Free Company (CHN). TNF-α (AF2132-A), IL-6 (AF2163-A), and IL-1β (AF2040-A) ELISA kits were purchased from Aifang Biotechnology Company (CHN). DCX antibody (GB13434), Iba-1 antibody (GB113502), and secondary antibody (GB21303) were purchased from Wuhan Servicebio Company(CHN).
2.3. Animal model
To establish the model of METH-induced anxiety- and depression-like behaviors, mice were divided into two groups including a control group and a METH group. After a 1-week adaptation period, mice in the METH group were intraperitoneally injected with METH (10 mg/kg, once daily) for 14 consecutive days. Mice from the control group received an equivalent volume of saline. After a 2-day withdrawal period, behavioral experiments were conducted with no METH administration.
Next, we experimented to observe the effect of GPR55 activation by systematic administration of O-1602. O-1602 is a GPR55 agonist. The dosage and route of administration refer to previous literature [10,18]. Mice were randomly divided into the Vehicle group, METH group, and O-1602+METH group. After a 7-day adaptation period, mice from the METH group and O-1602+METH group were intraperitoneally injected with 10 mg/kg of METH once daily for 14 consecutive days, while mice from the control group received an equivalent volume of saline. During the 14 days of METH treatment, mice received an additional intraperitoneal injection of 10 mg/kg of O-1602 30 min before each METH injection in the O-1602+METH group, while in Vehicle and METH group mice received an equivalent volume of saline. Then a 2-day withdrawal was performed after METH treatment. On the next day, the behavioral tests were conducted for 4 consecutive days during the light cycle.
Mice were anesthetized 24 h after the behavioral tests. Heart perfusion with physiological saline was performed after blood collection. Tissues were quickly transferred to −80 °C for further use.
2.4. Behavioral tests
2.4.1. Open field test
We used a white opaque square arena (50 cm long × 50 cm wide × 40 cm high) as the experimental apparatus to assess anxiety-like behavior. At the beginning of each trial, mice were gently placed in the center of the square by facing the same direction each time. Then mice were allowed to freely explore the open field for 5 min. Time spent in the central area was recorded. After each trial, the apparatus was thoroughly cleaned with 75 % alcohol to eliminate any residual odor from the previous mouse.
2.4.2. Elevated plus maze
The experimental apparatus consisted of two enclosed arms (5 cm wide × 35 cm long × 15 cm high) and two similar open arms, with the apparatus elevated approximately 55 cm above the ground. At the beginning of each trial, mice were placed in the central area of the apparatus by facing the same open arm. Then mice were allowed to freely explore for 5 min. Exploration of the open arms was recorded to assess anxiety-like behavior. After each trial, the apparatus was thoroughly cleaned with 75 % alcohol to eliminate any olfactory cues left by the previous mouse.
2.4.3. Forced swim test
The experimental apparatus was a cylindrical transparent tank (10 cm diameter × 25 cm high) with a water depth of approximately 16 cm and a water temperature of 24 ± 0.5 °C. At the beginning of each trial, mice were gently placed in the water from directly above and were allowed to move freely for 6 min. Immobility time during the last 4 min of the trial was recorded to assess depressive-like behavior. After each trial, mice were removed from the tank and dried, and the tank was emptied and cleaned. Fresh water was added to the tank for each trial.
2.4.4. Tail suspension test
The experimental apparatus was a blue opaque rectangular box (70 cm high × 30 cm long × 30 cm wide). At the beginning of each trial, mouse tails were taped and the mouse was then suspended from the top of the box, with its nose approximately 25 cm above the ground. The mouse was allowed to move freely for 6 min, and immobility time during the last 4 min of the trial was recorded to assess depressive-like behavior. After each trial, the apparatus was thoroughly cleaned with 75 % alcohol to eliminate any olfactory cues left by the previous mouse.
2.5. Enzyme linked immunosorbent assay (ELISA)
After anesthesia with isoflurane, the chest cavity of mice was opened to expose the heart. A sterile syringe was used to extract blood from the right atrium, which was then transferred to a 1.5 ml centrifuge tube. The tube was centrifuged at 4 °C and 1000g for 15 min. The supernatant was carefully aspirated into a new centrifuge tube and stored at −80 °C for further use. A commercial assay kit was used to measure the supernatant concentrations of corticosterone, TNF-α, IL-1β, and IL-6.
2.6. Immunofluorescence (IF)
Mice were perfused with saline until the organs, including the liver, turned from bright red to pale white. They were then perfused with 4 % paraformaldehyde until the body and all organs became stiff and rigid. After perfusion, the brain was immediately removed, fixed, dehydrated, and sliced. Brain slices were washed with phosphate-buffered saline (PBS) and blocked with bovine serum albumin (BSA) for 30 min. Then, DCX primary antibody (1:200) and Iba-1 primary antibody (1:500) were added and incubated overnight at 4 °C. After washing three times for 15 min with PBS, CY3-labeled goat anti-rabbit secondary antibody (1:300) was added and incubated at room temperature for 50 min in a dark room. Cell nuclei were stained with a DAPI staining solution and incubated in the dark for 10 min. Finally, the slides were sealed with an anti-fluorescence quenching mounting medium and observed under a microscope. ImageJ was used for statistical analyses.
2.7. Western blot
Mice were perfused with normal saline. When the liver and other organs changed from bright red to gray-white, the perfusion was stopped, and the mouse hippocampus tissue was taken immediately for Western blot analysis. Total hippocampal protein was extracted using RIPA lysis buffer (Beyotime, NO. P0013), and the protein concentration was measured using the bicinchoninic acid method (Beyotime, AWB0104c). Protein was separated by 10 % SDS-PAGE (Epizyme, PG112) and transferred onto a membrane (Millipore). The membrane was washed twice for 10 min with TBST, then blocked with 5 % BSA at 37 °C for 1 h. Primary antibodies, including GPR55 (1:1000), NLRP3 (1:1500), ASC (1:500), Caspase-1 (1:500), and β-Tubulin (1:5000) were added and incubated overnight at 4 °C. All primary antibodies were rabbit antibodies. After removing the primary antibodies, the membrane was washed three times for 15 min with TBST and incubated with the secondary antibody (1:5000) at 37 °C for 1 h. Enhanced chemiluminescence reagent was used for visualization, and the grayscale values were calculated using ImageJ.
2.8. Statistical analysis
All data were analyzed using GraphPad Prism 8.0.2. Quantitative data are presented as (mean ± standard error of the mean). One-way analysis of variance was used to compare multiple groups, and t-tests were used to compare two groups.p < 0.05 was considered statistically significant.
3. Results
3.1. 3.1METH resulted in anxiety- and depressive-like behaviors and decreased hippocampal GPR55 protein expression
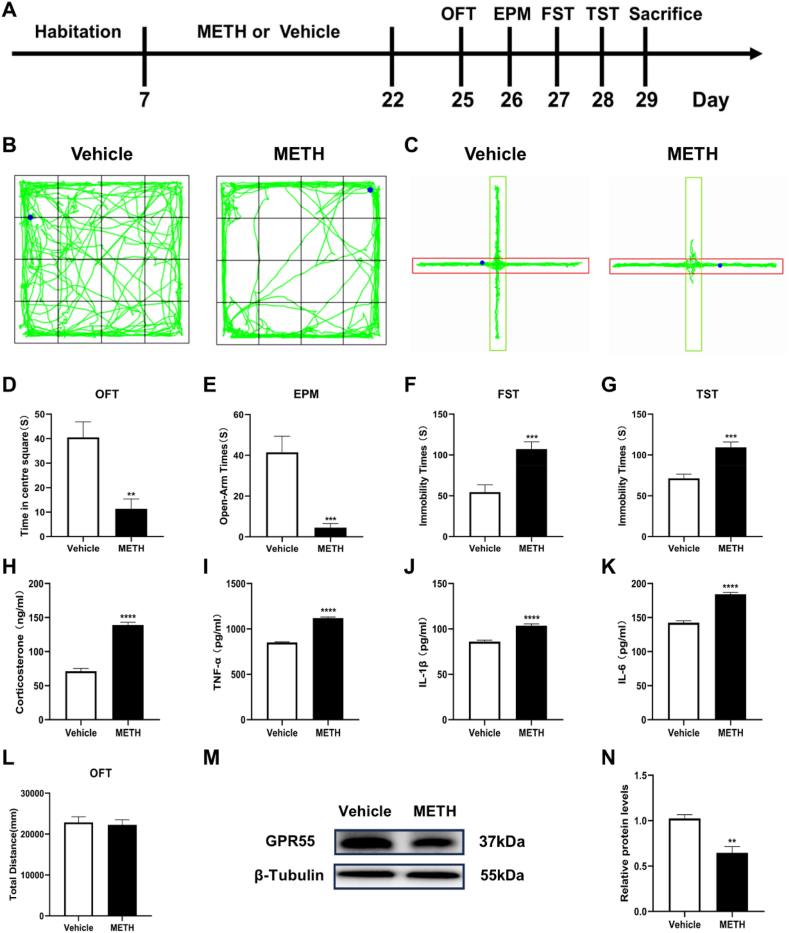
After METH administration, behavioral tests were conducted to detect whether mice exhibited anxiety- and depressive-like behaviors (Fig. 1A). Open-field test results showed that METH-treated mice spent significantly less time in the center area compared with that in the Vehicle group (t = 3.855, p < 0.01) (Fig. 1B–D). However, there was no difference in total distance between the two groups (Fig. 1L). In the elevated plus maze, METH-treated mice spent less time exploring the open arms (t = 4.522, p < 0.001) (Fig. 1C–E). In the forced swim and tail suspension tests, METH-treated mice exhibited significantly longer immobility time compared with the Vehicle group (t = 4.145, t = 4.587, p < 0.001) (Fig. 1F and G), ELISA assays revealed significantly increased levels of corticosterone, TNF-α, IL-1β, and IL-6 in the serum of METH-treated mice (t = 11.82, t = 19.49, t = 6.740, t = 10.84, p < 0.0001) (Fig. 1H–K). Subsequent analyses of hippocampal tissue revealed a decrease in GPR55 protein expression in METH-treated mice (t = 4.998, p < 0.01) (Fig. 1M and N). These results indicate the presence of anxiety- and depressive-like behaviors and the decrease of hippocampal GPR55 protein during METH withdrawal.
Fig. 1.
METH-induced anxiety- and depressive-like behaviors, accompanied by elevated serum corticosterone and pro-inflammatory cytokine levels, and decreased hippocampal GPR55 protein expression. (A) Experimental procedure. (B) Representative activity traces in the open field test. (C) Representative activity traces in the elevated plus maze test. (D) Time spent in the center area in the open field test. (E) Time spent exploring the open arms in the elevated plus maze. (F) Immobility time in the forced swim test. (G) Immobility time in the tail suspension test. (H) Serum corticosterone levels. (I–K) Serum TNF-α, IL-1β, and IL-6 levels. (L) Total distance in the open field test. (M) Representative bands in Western blot analysis. (The original image is provided in the Supplementary file). (N) Relative expression levels of GPR55 protein in each group. Each group n = 8, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.2. GPR55 agonist O-1602 inhibited METH-induced anxiety- and depressive-like behaviors
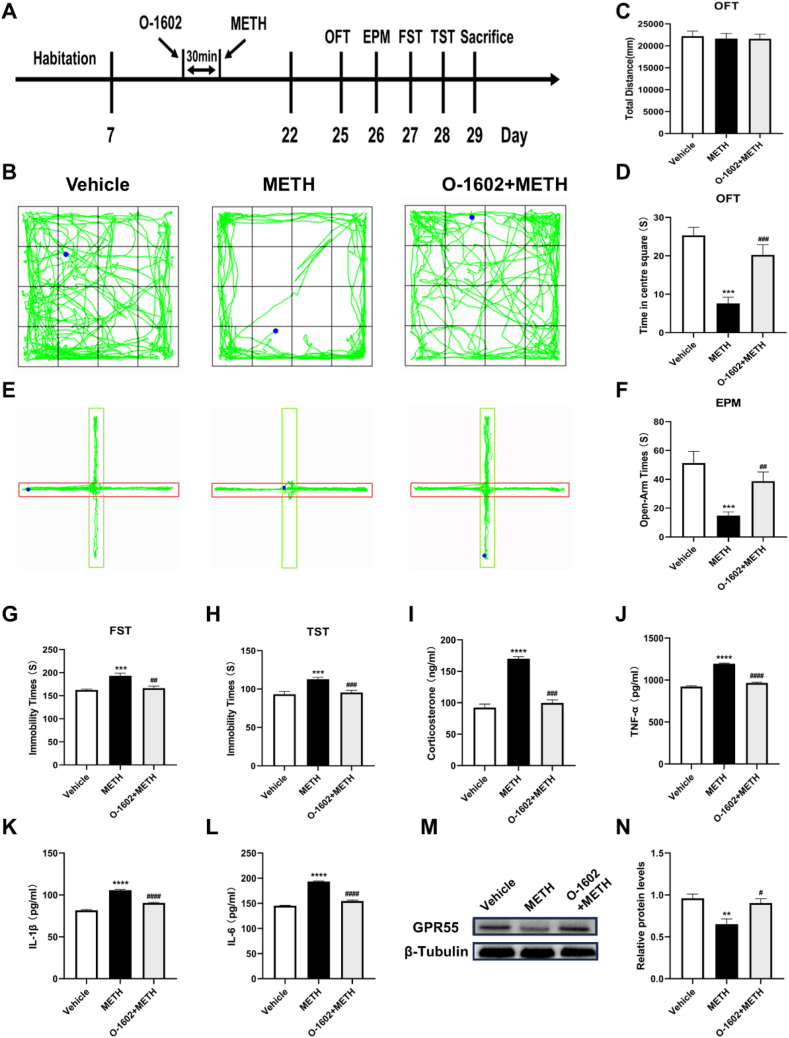
We administered the GPR55 agonist O-1602 30 min before METH treatment and observed behavior changes of mice (Fig. 2A). O-1602 pre-treatment significantly increased both time spent in the center area of the open field test (t = 4.100, p < 0.001) (Fig. 2B–D) and time exploring the open arms in the elevated plus maze test (t = 3.487, p < 0.01) (Fig. 2E and F), while there was no difference in the total distance traveled in the open field test (Fig. 2C), indicating a relief of anxiety-like behavior. In the forced swim and tail suspension tests, mice pre-treated with O-1602 exhibited significantly reduced immobility time (t = 3.754, t = 4.510, p < 0.01) (Fig. 2G and H). Furthermore, O-1602 pre-treatment decreased levels of serum corticosterone, TNF-α, IL-1β, and IL-6 in the serum (Fig. 2I–L) and increased hippocampal GPR55 protein expression (t = 3.187, p < 0.05) (Fig. 2M and N).
Fig. 2.
Anxiety- and depressive-like behaviors, serum corticosterone and pro-inflammatory cytokine levels, and GPR55 expression levels after GPR55 activation. (A) Experimental protocol. (B) Representative activity tracks in the open field test for each group. (C) Total distance in the open field test. (D) Time spent in the center area of the open field test. (E) Representative activity tracks in the elevated plus maze test. (F) Time exploring the open arms in the elevated plus maze. (G) Immobility time in the forced swim test. (H) Immobility time in the tail suspension test. (I) Serum corticosterone levels. (J–L) Serum TNF-α, IL-1β, and IL-6 levels. (M) Representative bands in Western blot analysis. (The original image is provided in the Supplementary file). (N) Relative expression levels of GPR55 protein in each group. Each group n = 9. Compared with the Vehicle group, **P < 0.01, ***P < 0.001, ****P < 0.0001; Compared with the METH group, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
3.3. GPR55 activation prevented hippocampal neurogenesis impairment
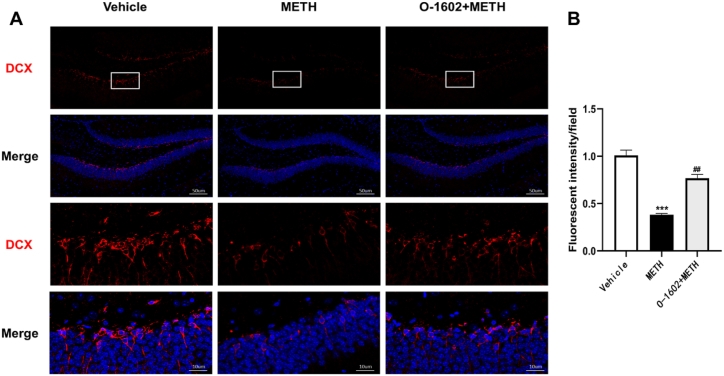
The hippocampal dentate gyrus (DG) is a major brain region in which neurogenesis occurs. IF detection of expression of the neurogenesis marker DCX in the DG was performed in the Vehicle, METH, and O-1602+METH groups. The extent of neurogenesis was reflected by analyzing the area of fluorescence. Compared with the Vehicle group, DCX expression in the DG was significantly reduced in the METH group (t = 11.16, p < 0.001). In the O-1602+METH group, DCX expression in the DG was significantly increased compared with the METH group (t = 9.097, p < 0.01) (Fig. 3A and B). These results indicate that METH inhibits hippocampal neurogenesis, while GPR55 activation may restore the neurogenesis suppressed by METH.
Fig. 3.
GPR55 activation improves METH-induced inhibition of neurogenesis. (A) Representative images of DCX immunofluorescence staining, with DCX stained in red fluorescence and DAPI staining cell nuclei in blue fluorescence. (B) Analysis of DCX immunofluorescence. Each group n = 3. Scale bar = 50μm and 10um. ***P < 0.001 compared with the Vehicle group; ##P < 0.01 compared with the METH group.
3.4. GPR55 activation prevented microglial activation and reduce neuroinflammation
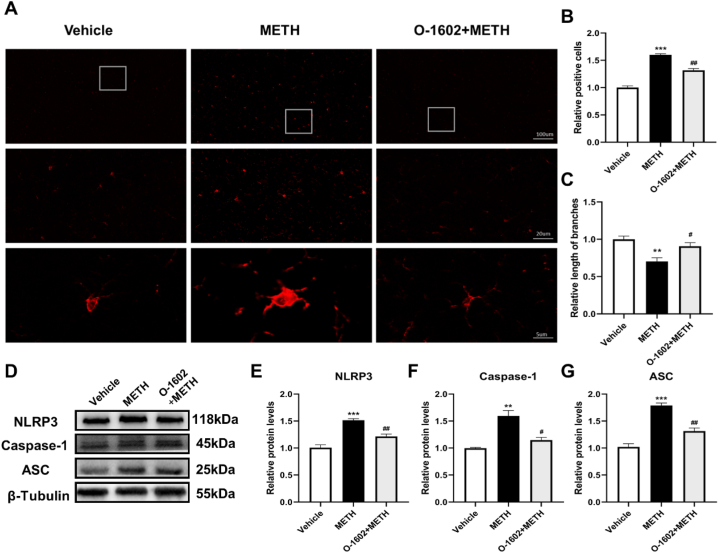
We further investigated the effects of GPR55 activation on microglial activation and neuroinflammation. The activation and quantity of microglia can reflect the extent of the inflammatory response. We performed IF staining using the microglial marker Iba-1. Compared with the Vehicle group, mice in the METH group had significantly increased numbers of microglia in the hippocampus (t = 14.92, p < 0.001). The microglial processes were retracted and less complex (t = 4.586, p < 0.01). However, the O-1602+METH group mice had a significant decrease in the number of microglia in the hippocampus compared with the METH group (t = 7.140, p < 0.01). The length and quantity of microglial processes showed some recovery (t = 3.004, p < 0.05) (Fig. 4A, B, C).
Fig. 4.
O-1602 can improve METH-induced microglial activation and neuroinflammation. (A) Representative images of Iba-1 immunofluorescence. (B) Analysis of Iba-1 immunofluorescence. (C) Length analysis of Iba-1 immunofluorescent. (D) Representative bands of Western blot. (The original image is provided in the Supplementary file). (F–G) Expression levels of NLRP3, Caspase-1, and ASC proteins. Scale bars = 100 μm, 20 μm, and 5 μm. Compared with the Vehicle group, **P < 0.01, ***P < 0.001; compared with the METH group, #P < 0.05, ##P < 0.01.
Expression levels of NLRP3 inflammasome proteins were also detected. Compared with the Vehicle group, mice in the METH group had increased expression levels of NLRP3, ASC, and Caspase-1 in the hippocampus (t = 8.502, t = 10.36, t = 5.929, p < 0.01), which were decreased upon GPR55 activation (t = 5.794, t = 6.405, t = 4.016, p < 0.05) (Fig. 4D–G). These findings indicate that METH may activate hippocampal microglia and induce neuroinflammation, while GPR55 activation results in inhibition of these cascades.
4. Discussion
METH use may damage brain structure and function, leading to negative emotions including anxiety and depression [[20], [21], [22]]. Even several years after treatment, those in recovery from METH addiction may continue to experience varying degrees of depression [23]. Reasons for variance in severity may include METH use duration and dosage, number of relapses, and gender difference. Numerous studies have explored the molecular mechanisms underlying METH-induced neurotoxicity such as brain inflammation, oxidative damage, synaptic plasticity changes, and abnormal hippocampal neurogenesis [[24], [25], [26]]. However, the mechanisms underlying METH-induced withdrawal symptoms have not been fully elucidated. In our study, anxiety and depression-like behaviors were elicited in a METH abstinence model. The negative behavior effects were improved by administration of GPR55 agonist O-1602, as well as alleviation of the hippocampal neurogenic damage and neuroinflammation caused by METH.
Different dose regimens including a single high-dose (30 mg/kg) [27], 5 mg/kg for five days [28], and 8 weeks of METH administration [29], have been employed; all of these result in the manifestation of anxiety- or depression-like behavior. Such effects were also observed following intraperitoneal injections of 5 mg/kg METH for 21 consecutive days, as well as with escalating doses for 14 consecutive days [20,21]. The dosage of 10 mg/kg for 14 days applied in our study was informed by these previous studies and we successfully identified these psychological conditions. Corticosterone secretion is a major neuroendocrine response to anxiety and depression [30,31]. Increased serum corticosterone levels were found during early withdrawal, accompanied by mental symptoms such as anxiety and depression in patients with substance use disorder and rodents [29,32,33]. Further, patients with anxiety and depression often exhibit increased serum levels of TNF-α, IL-1β, and IL-6 [34]. Similarly, elevated levels of these factors were detected in mice during METH withdrawal, implying the successful model construction in our study.
The endocannabinoid system has been extensively investigated in substance use disorder. For example, intervening in the endocannabinoid system affected METH self-administration withdrawal [35] and conditioned place preference [36]. The endocannabinoid system may be involved in the development of depression by regulating hippocampal neurogenesis and neuroinflammation [37]. GPR55, an atypical cannabinoid receptor, has been reported to be expressed at various levels in different brain regions during emotional regulation. For example, intracerebroventricular administration of the GPR55 agonist O-1602 produced anxiolytic effects [38]. Intraperitoneal O-1602 administration prevented anxiety and depression caused by chronic social defeat [10]. In line with previous research, GPR55 was inhibited in our study, and activating GPR55 by O-1602 alleviated METH-induced anxiety- and depression-like behavior; these findings demonstrate the involvement of the endocannabinoid system in METH-induced negative emotion. It is worth mentioning that inhibiting GPR55 expression hinders the anxiolytic and antidepressant effects of electroacupuncture [10]. At this point, the impact of low GPR55 expression on METH-induced withdrawal symptoms still needs further exploration.
Research has established a causal relationship between the use of psychostimulants and opioids and the onset of anxiety- and depression-like behaviors [[39], [40], [41]]. Clinical studies report that these negative performances of METH are the most severe [42]. Long-term use of METH impairs hippocampal neurogenesis which may contribute to the development of psychiatric disorders [43]. We found a significant decrease upon METH administration and a significant increase after O-1602 intervention in hippocampal neurogenesis, indicating that GPR55 is likely involved in METH withdrawal via mediating hippocampal neurogenesis. These results are in accordance with the observation of decreased hippocampal neurogenesis after drug use [44]. Studies indicate that reduced neurogenesis in the hippocampus is associated with the pathogenesis of stress-related anxiety and depression disorders [45]. However, limited research has addressed the correlation of neurogenesis with drug induced anxiety- and depression-like behaviors. Detailed mechanisms should be investigated regarding this issue.
Microglia, the immune cells in the brain, are the primary targets for the release of inflammatory factors. In this study, compared to the control group, the number and activation level of microglia in the hippocampus of mice treated with METH increased. Upon activation of GPR55, both the number and activation degree of microglia significantly decreased. Previous studies have shown significant activation of the NLRP3 inflammasome and that the NLRP3 inhibitor MCC950 can alleviate METH-induced cerebellar motor disorders [46]. Inhibiting activation of the NLRP3 inflammasome improved depression-like behavior [47]. Our results show that expressions of hippocampal NLRP3, ASC, and Caspase-1 significantly increased in the METH group; their expressions all decreased after GPR55 activation, indicating that GPR55 may regulate anxiety- and depression-like behaviors possibly by mediating microglial activation and NLRP3-involved neuroinflammation.
Several limitations should be addressed. Firstly, there are sex differences in substance use disorders [48]. Women are more susceptible to developing METH dependence [49], and female animals are more prone to self-administer METH and develop conditioned place preference [50,51]. Female METH users experience more severe psychiatric symptoms, including depression [52,53], and estradiol is considered a protective factor for females, helping them combat mental disorders [54,55]. Male mice were used exclusively herein, so it is necessary to further clarify the roles of GPR55 in females. Secondly, in prior literature, administering O-1602 at the same dosage used in our study did not induce anxiety or depression-like behaviors, nor did it promote hippocampal neurogenesis in male mice [10]. However, differences may exist between studies due to the distinct species and dose regimens. Future research is necessary to determine whether O-1602 itself triggers mood disorders related to withdrawal and enhances hippocampal neurogenesis. The dose-dependent effects of O-1602 should also be studied to confirm the role of GPR55 and potential translation.
Furthermore, the specificity of systemic O-1602 injection is inadequate. Directly injecting a GPR55 agonist into the hippocampus or employing viral tools would offer more conclusive evidence. Previous reports have shown that GPR55 colocalizes with the microglial marker Iba-1 but not with astrocyte marker GFAP, mature neuron marker MAP2, or neural stem cell marker Nestin, indicating that the localization of GPR55 is mainly on microglia [10,17]. The cell type of GPR55 localization in our study is more likely referred to as microglia in the hippocampus. However, future studies employing cell type-specific manipulations of GPR55 will be invaluable in dissecting the cellular pathways through which GPR55 modulates METH withdrawal symptoms.
In conclusion, our findings underscore the role of GPR55 in mitigating anxiety- and depression-like behaviors related to METH withdrawal, suggesting that targeting GPR55 could represent a promising therapeutic approach for the medication of METH use disorder.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Shuliang Niu], upon reasonable request.
Ethics declarations
This study was reviewed and approved by the Animal Ethics Committee of First Affiliated Hospital of Xinjiang Medical University, with the approval number: IACUC-20200331-53.
CRediT authorship contribution statement
Jinlong Zhang: Writing – review & editing, Writing – original draft, Data curation. Jie Yan: Conceptualization. Shuyue Li: Data curation. Qianqian Chen: Data curation. Jiang Lin: Data curation. Yilin Peng: Data curation. Yuhang Liu: Data curation. Binbin Wang: Data curation. Xinrong Wei: Data curation. Chen Sun: Data curation. Shuliang Niu: Writing – review & editing, Conceptualization.
Declaration of competing interest
No conflict of interest was declared.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (82060339) and the Xinjiang Uygur Autonomous Region Graduate Innovation Project (XJ2023G191).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30462.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Huang J., Ding J., Wang X., et al. Transfer of neuron-derived α-synuclein to astrocytes induces neuroinflammation and blood–brain barrier damage after methamphetamine exposure: involving the regulation of nuclear receptor-associated protein 1. Brain Behav. Immun. 2022;106:247–261. doi: 10.1016/j.bbi.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Su M.F., Liu M.X., Li J.Q., et al. Epidemiological characteristics and risk factors of methamphetamine-associated psychotic symptoms. Front. Psychiatr. 2018;9:489. doi: 10.3389/fpsyt.2018.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J., Sun X.J., Wang R.J., et al. Profile of psychiatric symptoms in methamphetamine users in China: greater risk of psychiatric symptoms with a longer duration of use. Psychiatr. Res. 2018;262:184–192. doi: 10.1016/j.psychres.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Monroe S.C., Radke A.K. Opioid withdrawal: role in addiction and neural mechanisms. Psychopharmacology (Berl) 2023;240(7):1417–1433. doi: 10.1007/s00213-023-06370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen W., Liu Y., Li L., et al. Negative moods correlate with craving in female methamphetamine users enrolled in compulsory detoxification. Subst. Abuse Treat. Prev. Pol. 2012;7:44. doi: 10.1186/1747-597x-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F., Tian Q., Tang H.L., et al. Hydrogen sulfide attenuates depression-like behaviours in Parkinson's disease model rats by improving synaptic plasticity in a hippocampal warburg effect-dependent manner. Pharmacol. Biochem. Behav. 2023;234 doi: 10.1016/j.pbb.2023.173677. [DOI] [PubMed] [Google Scholar]
- 8.Loef D., Tendolkar I., van Eijndhoven P.F.P., et al. Electroconvulsive therapy is associated with increased immunoreactivity of neuroplasticity markers in the hippocampus of depressed patients. Transl. Psychiatry. 2023;13(1):355. doi: 10.1038/s41398-023-02658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao C., Zhang Y., Sun X., et al. Areca catechu l. Ameliorates chronic unpredictable mild stress-induced depression behavior in rats by the promotion of the bdnf signaling pathway. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114459. [DOI] [PubMed] [Google Scholar]
- 10.Shen S.Y., Yu R., Li W., et al. The neuroprotective effects of gpr55 against hippocampal neuroinflammation and impaired adult neurogenesis in csds mice. Neurobiol. Dis. 2022;169 doi: 10.1016/j.nbd.2022.105743. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.L., Han Q.Q., Gong W.Q., et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018;15(1):21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun C., Shen Y., Liu P., et al. Nlrc5 deficiency reduces lps-induced microglial activation via inhibition of nf-κb signaling and ameliorates mice's depressive-like behavior. Int. J. Mol. Sci. 2023;24(17) doi: 10.3390/ijms241713265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewer R.C., Christie L.A., Doyle K.J., et al. Discovery and characterization of novel cns-penetrant gpr55 agonists. J. Med. Chem. 2023;66(18):12858–12876. doi: 10.1021/acs.jmedchem.3c00784. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Zavaleta R., Segovia J., Ruiz-Contreras A.E., et al. Gpr55 activation prevents amphetamine-induced conditioned place preference and decrease the amphetamine-stimulated inflammatory response in the ventral hippocampus in male rats. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2023;120 doi: 10.1016/j.pnpbp.2022.110636. [DOI] [PubMed] [Google Scholar]
- 15.Marichal-Cancino B.A., Fajardo-Valdez A., Ruiz-Contreras A.E., et al. Advances in the physiology of gpr55 in the central nervous system. Curr. Neuropharmacol. 2017;15(5):771–778. doi: 10.2174/1570159X14666160729155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill J.D., Zuluaga-Ramirez V., Gajghate S., et al. Activation of gpr55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation. Brain Behav. Immun. 2019;76:165–181. doi: 10.1016/j.bbi.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietr M., Kozela E., Levy R., et al. Differential changes in gpr55 during microglial cell activation. FEBS Lett. 2009;583(12):2071–2076. doi: 10.1016/j.febslet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q.X., Yang L.K., Shi W.L., et al. The novel cannabinoid receptor gpr55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain. 2017;10(1):38. doi: 10.1186/s13041-017-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen S.-Y., Yu R., Li W., et al. The neuroprotective effects of gpr55 against hippocampal neuroinflammation and impaired adult neurogenesis in csds mice. Neurobiol. Dis. 2022;169 doi: 10.1016/j.nbd.2022.105743. [DOI] [PubMed] [Google Scholar]
- 20.Yang J., Zhang Z., Xie Z., et al. Metformin modulates microbiota-derived inosine and ameliorates methamphetamine-induced anxiety and depression-like withdrawal symptoms in mice. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112837. [DOI] [PubMed] [Google Scholar]
- 21.Re G.F., Li H., Yang J.Q., et al. Exercise modulates central and peripheral inflammatory responses and ameliorates methamphetamine-induced anxiety-like symptoms in mice. Front. Mol. Neurosci. 2022;15 doi: 10.3389/fnmol.2022.955799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMiceli L.E., Sherman S.G., Aramrattana A., et al. Methamphetamine use is associated with high levels of depressive symptoms in adolescents and young adults in rural chiang mai province, Thailand. BMC Publ. Health. 2016;16 doi: 10.1186/s12889-016-2851-1. 16810.1186/s12889-016-2851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasner-Edwards S., Marinelli-Casey P., Hillhouse M., et al. Depression among methamphetamine users: association with outcomes from the methamphetamine treatment project at 3-year follow-up. J. Nerv. Ment. Dis. 2009;197(4):225–231. doi: 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Wang Y., Li Q., et al. The main molecular mechanisms underlying methamphetamine- induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci. 2018;11:186. doi: 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong K., Liao H., Long L., et al. Necroptosis contributes to methamphetamine-induced cytotoxicity in rat cortical neurons. Toxicol. Vitro. 2016;35:163–168. doi: 10.1016/j.tiv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Lu S., Liao L., Zhang B., et al. Antioxidant cascades confer neuroprotection in ethanol, morphine, and methamphetamine preconditioning. Neurochem. Int. 2019;131 doi: 10.1016/j.neuint.2019.104540. [DOI] [PubMed] [Google Scholar]
- 27.Silva C.D., Neves A.F., Dias A.I., et al. A single neurotoxic dose of methamphetamine induces a long-lasting depressive-like behaviour in mice. Neurotox. Res. 2014;25(3):295–304. doi: 10.1007/s12640-013-9423-2. [DOI] [PubMed] [Google Scholar]
- 28.Iijima M., Koike H., Chaki S. Effect of an mglu2/3 receptor antagonist on depressive behavior induced by withdrawal from chronic treatment with methamphetamine. Behav. Brain Res. 2013;246:24–28. doi: 10.1016/j.bbr.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Ru Q., Xiong Q., Zhou M., et al. Withdrawal from chronic treatment with methamphetamine induces anxiety and depression-like behavior in mice. Psychiatr. Res. 2019;271:476–483. doi: 10.1016/j.psychres.2018.11.072. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R., Asai M., Mahoney C.E., et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatr. 2016;22(5):733–744. doi: 10.1038/mp.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang B., Xiong Z., Yang J., et al. Antidepressant‐like effects of ginsenoside rg1 are due to activation of the bdnf signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 2012;166(6):1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichniak A., Brunner H., Ising M., et al. Impaired hypothalamic-pituitary-adrenocortical (hpa) system is related to severity of benzodiazepine withdrawal in patients with depression. Psychoneuroendocrinology. 2004;29(9):1101–1108. doi: 10.1016/j.psyneuen.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Motaghinejad M., Bangash M.Y., Motaghinejad O. Attenuation of alcohol withdrawal syndrome and blood cortisol level with forced exercise in comparison with diazepam. Acta Med. Iran. 2015;53(5):311–316. [PubMed] [Google Scholar]
- 34.Zunszain P.A., Hepgul N., Pariante C.M. Behavioral Neurobiology of Depression and its Treatment. 2012. Inflammation and depression; pp. 135–151. [Google Scholar]
- 35.Jayanthi S., Peesapati R., McCoy M.T., et al. Footshock-induced abstinence from compulsive methamphetamine self-administration in rat model is accompanied by increased hippocampal expression of cannabinoid receptors (cb1 and cb2) Mol. Neurobiol. 2022;59(2):1238–1248. doi: 10.1007/s12035-021-02656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassanlou A.A., Jamali S., RayatSanati K., et al. Cannabidiol modulates the meth-induced conditioned place preference through d2-like dopamine receptors in the hippocampal ca1 region. Brain Res. Bull. 2021;172:43–51. doi: 10.1016/j.brainresbull.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Giacobbe J., Marrocu A., Di Benedetto M.G., et al. A systematic, integrative review of the effects of the endocannabinoid system on inflammation and neurogenesis in animal models of affective disorders. Brain Behav. Immun. 2021;93:353–367. doi: 10.1016/j.bbi.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Rahimi A., Hajizadeh Moghaddam A., Roohbakhsh A. Central administration of gpr55 receptor agonist and antagonist modulates anxiety-related behaviors in rats. Fundam. Clin. Pharmacol. 2015;29(2):185–190. doi: 10.1111/fcp.12099. [DOI] [PubMed] [Google Scholar]
- 39.Butler A.J., Rehm J., Fischer B. Health outcomes associated with crack-cocaine use: systematic review and meta-analyses. Drug Alcohol Depend. 2017;180:401–416. doi: 10.1016/j.drugalcdep.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 40.Farrell M., Martin N.K., Stockings E., et al. Responding to global stimulant use: challenges and opportunities. Lancet. 2019;394(10209):1652–1667. doi: 10.1016/s0140-6736(19)32230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Lu Y., Jia M., et al. Kappa opioid receptor in nucleus accumbens regulates depressive-like behaviors following prolonged morphine withdrawal in mice. iScience. 2023;26(9) doi: 10.1016/j.isci.2023.107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo D., Tan L., Shen D., et al. Characteristics of depression, anxiety, impulsivity, and aggression among various types of drug users and factors for developing severe depression: a cross-sectional study. BMC Psychiatr. 2022;22(1):274. doi: 10.1186/s12888-022-03933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singhakumar R., Boontem P., Ekthuwapranee K., et al. Melatonin attenuates methamphetamine-induced inhibition of neurogenesis in the adult mouse hippocampus: an in vivo study. Neurosci. Lett. 2015;606:209–214. doi: 10.1016/j.neulet.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Xu C., Loh H.H., Law P.Y. Effects of addictive drugs on adult neural stem/progenitor cells. Cell. Mol. Life Sci. 2016;73(2):327–348. doi: 10.1007/s00018-015-2067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder J.S., Soumier A., Brewer M., et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding J., Shen L., Ye Y., et al. Inflammasome inhibition prevents motor deficit and cerebellar degeneration induced by chronic methamphetamine administration. Front. Mol. Neurosci. 2022;15 doi: 10.3389/fnmol.2022.861340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He H., Xie X., Kang X., et al. Ginsenoside rg1 ameliorates depressive-like behavior by inhibiting nlrp3 inflammasome activation in mice exposed to chronic stress. Eur. J. Pharmacol. 2023;960 doi: 10.1016/j.ejphar.2023.176120. [DOI] [PubMed] [Google Scholar]
- 48.Simpson J.L., Grant K.M., Daly P.M., et al. Psychological burden and gender differences in methamphetamine-dependent individuals in treatment. J. Psychoact. Drugs. 2016;48(4):261–269. doi: 10.1080/02791072.2016.1213470. [DOI] [PubMed] [Google Scholar]
- 49.Brecht M.L., O'Brien A., von Mayrhauser C., et al. Methamphetamine use behaviors and gender differences. Addict. Behav. 2004;29(1):89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 50.Roth M.E., Carroll M.E. Sex differences in the acquisition of iv methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- 51.Chen H.H., Yang Y.K., Yeh T.L., et al. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin. J. Physiol. 2003;46(4):169–174. [PubMed] [Google Scholar]
- 52.Semple S.J., Zians J., Strathdee S.A., et al. Psychosocial and behavioral correlates of depressed mood among female methamphetamine users. J. Psychoact. Drugs. 2007;(Suppl 4):353–366. doi: 10.1080/02791072.2007.10399897. [DOI] [PubMed] [Google Scholar]
- 53.King G., Alicata D., Cloak C., et al. Psychiatric symptoms and hpa axis function in adolescent methamphetamine users. J. Neuroimmune Pharmacol. 2010;5(4):582–591. doi: 10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulkarni J. Estrogen - a key neurosteroid in the understanding and treatment of mental illness in women. Psychiatr. Res. 2023;319 doi: 10.1016/j.psychres.2022.114991. [DOI] [PubMed] [Google Scholar]
- 55.Newhouse P., Albert K. Estrogen, stress, and depression: a neurocognitive model. JAMA Psychiatr. 2015;72(7):727–729. doi: 10.1001/jamapsychiatry.2015.0487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Shuliang Niu], upon reasonable request.