Abstract
Behavioral and brain-related changes in word production have been claimed to predominantly occur after 70 years of age. Most studies investigating age-related changes in adulthood only compared young to older adults, failing to determine whether neural processes underlying word production change at an earlier age than observed in behavior. This study aims to fill this gap by investigating whether changes in neurophysiological processes underlying word production are aligned with behavioral changes. Behavior and the electrophysiological event-related potential patterns of word production were assessed during a picture naming task in 95 participants across five adult lifespan age groups (ranging from 16 to 80 years old). While behavioral performance decreased starting from 70 years of age, significant neurophysiological changes were present at the age of 40 years old, in a time window (between 150 and 220 ms) likely associated with lexical-semantic processes underlying referential word production. These results show that neurophysiological modifications precede the behavioral changes in language production; they can be interpreted in line with the suggestion that the lexical-semantic reorganization in mid-adulthood influences the maintenance of language skills longer than for other cognitive functions.
Keywords: aging, EEG, lexical-semantic processes, picture naming, topographic maps
Introduction
Life expectancy (considered as the average age at the time of death) in Europe has increased from approximately 44 years in 1900 to 74 years in 2008 (Mackenbach and Looman 2013), thus altering how adulthood is defined (Deschavanne and Tavoillot 2007). Nowadays, adults are surrounded by an extended period of youth, and an active third age still manifesting high cognitive performance. A typical example of preserved cognitive abilities with age is related to language production skills, which only significantly decline after the age of 70 (e.g. Salthouse 2010). This means that efficient utterance production is maintained for at least 50 years of adulthood. Along with behavioral changes it has been shown that older adults display differences in the neurofunctional substrates of word production compared to young adults (Baciu et al. 2021). Yet, few studies have investigated whether behavioral and/or neurophysiological differences are already present in intermediate adult groups. In this study, we examined when and how neurophysiological changes underlying word production occur over the course of adulthood and how they relate to behavioral changes.
Changes in language production during adulthood
Most cognitive functions start declining in early adulthood (e.g. Salthouse 2004, 2009; Spreng and Turner 2019): skills like episodic memory (e.g. Grady et al. 1999; Nyberg 2017) and working memory (e.g. Mitchell et al. 2000), attention and inhibitory control (e.g. Hasher et al. 1991), perceptual processing (e.g. Baltes and Lindenberger 1997; Ansado et al. 2012), and general processing speed (e.g. Salthouse 1996) show a progressive and linear decline starting from 20 to 25 years, which becomes more pronounced after 50 to 70 years (e.g. Anstey and Low 2004; Hedden and Gabrieli 2004; Salthouse 2004, 2009). In contrast, many language skills (e.g. vocabulary, word retrieval) are among the most stable cognitive domains throughout the lifespan, remaining better preserved with aging (e.g. Wierenga et al. 2008; Kahlaoui et al. 2012; Hartshorne and Germine 2015). While verbal fluency shows early age-related changes in performance, starting from the age of 50 (e.g. Kavé and Knafo-Noam 2015), performance in other language skills, notably word production, starts to decrease only from about 60 to 70 years of age (e.g. Albert et al. 1988; Hedden and Gabrieli 2004; Salthouse 2004; Verhaegen and Poncelet 2013; Diaz et al. 2021) with the steepest decline occurring only after 70 to 75 years (e.g. Feyereisen 1997; Zec et al. 2005; Kavé et al. 2010). Word-finding difficulties are reported with longer latencies, increased tip-of-the-tongue states (TOTs), and fewer accurate responses in picture naming tasks in older adulthood relative to young adults (e.g. Rabbitt et al. 1995; Mortensen et al. 2006; Neumann et al. 2009; Kavé et al. 2010; Saryazdi et al. 2019). In contrast to the decline observed in word retrieval, semantic knowledge, vocabulary and the size of the mental lexicon increase throughout adulthood, especially in older adults (e.g. Kemper and Sumner 2001; Park et al. 2002; Verhaeghen 2003; Salthouse 2004; Kavé and Halamish 2015; Keuleers et al. 2015; Brysbaert et al. 2016; Wulff et al. 2016; Mokhber et al. 2019). Such changes in the organization of the lexicon are at the core of recent interpretations of the preserved language skills in aging, as further detailed below. Note that in the studies taken into consideration, “older adulthood” is generally considered starting from around 70 years, while “young adults” generally involves participants aged around 20 to 30 years.
Different interpretations of the decline in word retrieval abilities in older adults have been proposed (Newman and German 2005; Burke and Shafto 2008; Lambon Ralph et al. 2017). A first group of explanatory hypotheses attribute the decrease in performance in word production to the same processes thought to underpin the global decline in cognitive performance, namely slowing down of processes by aging (i.e. the general slowing theories, e.g. Myerson et al. 1990; Salthouse 1996, 2000), a reduced capacity for processing information (the resource theory; e.g. Miller 1956), a decline in inhibitory processes that regulate attention and the contents of working memory (the inhibition deficit theory; Hasher and Zacks 1988), reduced working memory capacities (the working memory or resource theories; Kemper and Kemtes 1999), or a decline in sensory and perceptual processes (the degraded signal theory; e.g. Brown 2000; Schneider and Pichora-Fuller 2000). Other proposals, like the transmission deficit hypothesis (Burke et al. 2000), or the “semanticization of cognition”/DECHA (Default–Executive Coupling Hypothesis of Aging; Spreng and Turner 2019), are more specific to the language domain. The transmission deficit hypothesis (Burke et al. 2000) proposes a decrease in the strength of connections among representational units in aging, affecting the transmission of activation from the semantic to the phonological representations. Single connections from lexical to phonological representations make these connections more vulnerable to aging, compared to the connections within the semantic system, which are redundant. Semantic information is therefore thought to be more preserved with aging, whereas phonological retrieval failures would explain increased TOT states (Burke et al. 1991). Other authors related the longer maintenance of language production in older adults to enriched lexical-semantic networks over time. Indeed, Spreng and Turner (2019) hypothesized a shifting in the neurocognitive architecture during the adult lifespan (from 20 to 80 years). They proposed the concept of the “semanticization of cognition,” stating that, while cognitive control abilities decline with age, semantic abilities continue to grow and are well preserved during the adult’s lifetime. This means that in a word production task, where the lexical-semantic component is relevant, this neurocognitive reorganization may work in favor of maintaining good performance in older adults. However, word production tasks also rely on the processing speed and cognitive control abilities. As a consequence, when decline of these latter abilities becomes more pronounced in older adulthood, the advantage due to greater semantic abilities hypothesized by Spreng and Turner (2019) might not be enough to compensate and maintain high performance, leading to the behavioral changes observed in many studies on the older adult groups.
Changes in neuroanatomy and in neurophysiology of word production during adulthood
Research sought to relate these age-related behavioral changes to anatomical and functional neural changes. Age-related changes in both brain structure (Raz 2005; Raz et al. 2005, 2010; Peelle 2019) and neurophysiological activity have been reported when comparing word production in young and older adults (e.g. Wierenga et al. 2008; Valente and Laganaro 2015; Hoyau et al. 2017; Methqal et al. 2019; Mohan and Weber 2019). For example, the two studies by Wierenga et al. (2008) and Hoyau et al. (2017) showed larger activation of the frontal network in older adults (starting from around 70 years old) during word retrieval. These findings suggest that substrates involved in word retrieval decline with aging, and must be compensated with additional neural recruitment. In line with the integrity of semantic knowledge, these results may be interpreted within the framework of the “semanticization of cognition”.
Further evidence supporting this hypothesis is provided by Guichet et al. (2024), who analyzed white matter changes related to difficulties in lexical production during middle adulthood. Their results revealed a discontinuity in brain structure within distributed networks around the age of 50 years old, primarily in dorsal, ventral, and anterior cortico-subcortical pathways. The authors suggested that these findings support the idea of a decline in certain general cognitive processes with age, such as multitasking and fluid intelligence, which are associated with the onset of difficulties in lexical production, resulting in increased naming latencies. The authors also discuss how middle-aged adults may initially rely on semantic abilities to compensate for initial difficulties in lexical production; this strategy may be compromised in later adulthood due to the loss of the ability to exert cognitive control over semantic representations. However, what is the time-course of behavioral changes with respect to neurophysiological changes remains virtually unknown.
In addition, several fMRI studies reported significant differences between the brain activations of young and older adults in language production tasks, even when behavioral performance was equivalent across age groups (e.g. Wierenga et al. 2008; Meinzer et al. 2009; Diaz et al. 2014; Hoyau et al. 2017; Methqal et al. 2019). Generally, increased and/or more widespread brain activations in older adults (around 70 years old), compared to young adults (around 25 years old), are interpreted as compensatory activity when it is associated with better performance (Cabeza 2002; Reuter-Lorenz 2002) and simply as dedifferentiation when associated with worse performance (Bernard and Seidler 2012). According to the literature, the age-related neural dedifferentiation, characterized by less distinctive neural representations of perceptual and conceptual information, stems from reduced neural efficiency (e.g. Koen and Rugg 2019). However, word production is underlaid by several encoding processes that may be subject differently to compensatory and dedifferentiation. A better insight into the different word planning processes comes from studies with high-temporal-resolution approaches such as electroencephalography/event-related potentials (EEG/ERPs; Van Veen and Carter 2002). For instance, Valente and Laganaro (2015) reported ERP divergences between young (18 to 30 years) and older adults (60 to 80 years) in a picture naming task but not in a picture-word verification task. The authors observed divergences across age groups in the time window between 150 and 250 ms after the picture onset, a timeframe that has traditionally been associated with lexical-semantic processes (e.g. Dell’Acqua et al. 2010; Indefrey 2011). This suggests that age-related changes affecting the processing dynamics in the lexical-semantic system could determine word production performance, compatible with the aforementioned hypotheses of “semanticization of cognition” and related model of the “default-executive coupling hypothesis of aging” (DECHA; Spreng and Turner 2019; Turner and Spreng 2015).
The present study
So far, studies have focused on language production changes in aging by comparing young adults (usually 20 to 30 years old) to older adults (mostly over the age of 70 years old), thus lacking a proper description of the reorganization that likely occurs during the years in the gap between these two extremes. Extending the investigation to other groups of the adult lifespan than the two adult lifespan extremes, may allow us to test whether the appearance of behavioral changes related to word production over the course of adult life is synchronous with the neurophysiological changes, and whether this is the case for all word encoding processes.
The present study thus aims to understand when and how word production changes over the adult lifespan, combining behavioral and neurophysiological investigations of picture naming. The study fills the gap in knowledge by enrolling adolescents as well as intermediate age groups between young adults and the elderly. We start the adult lifespan with 16- to 18-year-old adolescents, as it has been shown that the behavioral and neurophysiological correlates of picture naming are very similar in adolescents and young adults (e.g. Petanjek et al. 2008; Atanasova et al. 2020).
At the behavioral level, consistent with previous literature summarized above, we expect changes only in the older group (after the age of 70 years old), characterized by a decline in word production performance (e.g. Feyereisen 1997; Zec et al. 2005; Kavé et al. 2010). Concerning the neurophysiological data, our rationale is as follows: If the differences in neural patterns across age groups purely reflect the decline in word production abilities, they should be aligned with the behavioral results, with differences appearing only when a decrease in performance is observed; by contrast, if compensatory mechanisms are at play in age groups in which performance is still maintained, different neural patterns are expected in age groups before the behavioral decline is observed. In sum, this exploratory investigation examines when different ERP patterns emerge in adulthood, determining whether or not there is synchrony between behavioral changes and neural changes, and if they are observed in specific time windows underlying word production.
Beyond ERP waveform analyses, we compute topographic (microstate) analyses, i.e. an analysis of the quasi-stable periods of synchronized neural activity that evolve dynamically over time and that allow us to explore quantitative changes (same microstates but different time distributions across groups) and qualitative changes (different topographic patterns) reflecting the involvement of similar or different underlying neural networks across age groups (e.g. Michel et al. 2004; Laganaro 2014).
Performance in other cognitive tasks will also be assessed in all age groups with the aim of verifying that participants’ performance fell within the normal range for their age group.
Materials and methods
Participants
Ninety-five participants took part in the study. They were subdivided into five groups: “adolescents,” “young adults,” “adults,” “young-old adults” and “older adults” (all the details regarding the groups are summarized in Table 1).
Table 1.
Specifications of groups’ characteristics.
| Age group | N | Age ranges | meanage | SDage | Nb female participants | meanschooling | SDschooling |
|---|---|---|---|---|---|---|---|
| Adolescents | 19 | 16 to 18 | 17.1 | 0.875 | 11 | 11.5 | 1.264 |
| Young adults | 19 | 20 to 30 | 24.6 | 3.006 | 12 | 16.4 | 2.090 |
| Adults | 19 | 40 to 50 | 45.7 | 3.331 | 12 | 17.3 | 4.382 |
| Young-old adults | 19 | 59 to 69 | 64.1 | 3.195 | 13 | 14.1 | 2.460 |
| Older adults | 19 | 70 to 80 | 73.2 | 3.184 | 13 | 13.5 | 2.970 |
The choice to begin adulthood with a group of adolescents aged 16 to 18 years old stemmed from previous findings indicating that they exhibit behavioral performance and neurophysiological correlates very similar to those of young adults in picture naming tasks (Atanasova et al. 2020). The next four groups of adults, with a default age range of one decade, were composed of the two groups often compared in the literature (20 to 30 years and 70 to 80 years) and two intermediate groups. Sample size was based on previous studies using neurophysiological data, with between 15 and 20 participants per group (e.g. Maess et al. 2002; Wierenga et al. 2008; Strijkers et al. 2010; Baciu et al. 2016; Fargier and Laganaro 2016; Atanasova et al. 2020). Participants were all right-handed (according to the Edinburgh Handedness Scale; Oldfield 1971) and native French speakers. However, considering the socio-geographical context of the recruitment region (city of Geneva in Switzerland), the recruited population was likely to be bilingual. No subjects had diagnosed neurological diseases or speech disorders, and all had normal or corrected-to-normal vision. Participants were recruited through announcements posted at the University of Geneva and in surrounding gyms, as well as through word of mouth. All subjects volunteered and gave informed consent, in accordance with the Declaration of Helsinki. Study procedures were approved by the local faculty ethics committee of the University of Geneva (FPSE, University of Geneva, Geneva, Switzerland). Additional parental consent was collected for minors (under the age of 18 in Switzerland). All participants received monetary compensation for their participation.
Tasks and materials
Note that the data collected through the picture naming task have been made available and can be accessed via the link at the end of the text.
Picture naming
For the picture naming task, we selected 120 monosyllabic (n = 40), bisyllabic (n = 60), and trisyllabic (n = 20) concrete French words along with their corresponding pictures from two French datasets (Alario and Ferrand 1999; Bonin et al. 2003). The selected stimuli were all nouns and had a name agreement of over 75% (mean = 92.5%) to ensure consistent naming for the same picture (Alario et al. 2004). Their lexical frequency varied from 0.37 to 255 occurrences per million words (mean = 25.96) in the French database Lexique (New et al. 2004). The selected words had an age of acquisition range of 1.19 to 3.55 on a five-point scale (1: learned between 0 and 3 years; 5: learned after the age of 12; see also Laganaro et al. 2015), meaning that all words were acquired before 9 years of age. The images were black and white pictures with homogenized dimensions of 280 × 280 pixels.
Other cognitive assessments
The participants underwent six additional cognitive assessments in order to verify that all the groups followed the usual lifespan trend in performance.
The other cognitive tasks included: a simple reaction times test (which required pressing a specific key on the computer keyboard when a cross appeared on the screen) including 120 trials; a choice reaction times test (which required a choice in the response, pressing two different keys on the computer keyboard, depending on whether the longest of two bars appeared on the right or left side of the screen) including 120 trials; a working memory test (digit span test from the Wechsler Adult Intelligence Scale IV; Wechsler 1997); a vocabulary test (from the Wechsler Adult Intelligence Scale IV; Wechsler 1997); a Stroop Task (computerized serial four colors Stroop task requiring oral responses and including 180 trials divided among the congruent, incongruent and neutral conditions; for the complete procedure, see Ménétré and Laganaro 2019); and two verbal fluency tests (animal names and words beginning with the letter P given in 2 min each; Cardebat et al. 1990).
Procedures
The participants underwent the picture naming task under continuous EEG recording in a sound-proof booth, and the other cognitive assessment in a face-to-face setting. The order of presentation and administration of the different tasks, as well as the transition between the two settings, was balanced by dividing subjects into two groups (even and odd) to avoid an order effect on the results.
Picture naming
Each participant sat in front of a computer screen at an approximate distance of 60 cm (refreshment rate: 50 Hz), in a dimly lit, sound-attenuated room. The stimuli were presented using the E-Prime software (E-Studio). An experimental trial began with a fixation cross presented for 500 ms on a gray background screen, followed by the picture displayed for 2,000 ms. The participant was instructed to overtly produce, as quickly and accurately as possible, the word corresponding to the picture within 2,000 ms before responses were classified as “no response.” At the end of each trial, an inter-stimulus blank screen lasting 2000 ms was shown. Stimuli were presented in two different pseudo-randomized orders, with a self-managed break (after 60 items). Pseudo-randomization was preferred over complete randomization to avoid a succession of stimuli with phonological similarity or within the same semantic category. After receiving instructions, and before the task’s trials, each participant completed four training trials to familiarize themselves with the task. All responses were recorded with a microphone and digitized for off-line accuracy and latency check.
Other cognitive assessments
The tasks were conducted in a paper-and-pencil setting and audio-recorded, except for the two reaction time tests and the Stroop task, which were computer-based, and whose stimuli were presented using the E-Prime software (E-Studio).
EEG acquisition and preanalyses
EEG was recorded using the Active-Two Biosemi EEG system (Biosemi V.O.F., Amsterdam, Netherlands) with 128 channels at a 512 Hz sampling rate and filtered between 0.16 and 100 Hz. Data from all participants who completed all tasks were considered, without excluding any participant. The preprocessing was conducted with the Cartool 3.60 software (Brunet et al. 2011). Stimulus-aligned epochs (locked to the picture onset) of approximately 600 ms (including around 100 ms prestimulus signal) and response-aligned epochs (time-locked to around 100 ms before the acoustic onset of the vocal response, as described in the behavioral analyses below) of 500 ms were averaged across participants and age groups. Aligning epochs to 100 ms before the vocal onset of each single trial is performed to eliminate prearticulatory motor artifacts (see Fargier et al. 2018). Before the epoching, the data were high-pass-filtered at 0.2 Hz and low-pass-filtered at 30 Hz (using a second-order causal Butterworth filter with −12 dB/octave roll-off), and then averaged for each participant. In addition to an automated selection criterion the rejected epochs with amplitudes reaching ±100 μV, each trial was visually inspected. All epochs related to correct productions were recalculated against the average reference, they were manually inspected and only accepted if no artifacts were present, such as eye blinks, movement-related artifacts, or large amplitude variations. A minimum of 51 trials was averaged for each participant. Bad electrodes (up to 22% of the 128 electrodes) were interpolated using a 3D spline interpolation (Perrin et al. 1989).
Behavioral analyses
Picture naming
For the picture naming task, response latencies (RTs in milliseconds, i.e. the time separating the onset of the picture and the acoustic onset) and accuracy were systematically checked using speech analysis software (Check Vocal; Protopapas 2007), which allowed the visualization of both waveforms and spectrograms of each verbal response. Outlier RTs (shorter than 500 ms or longer than 2,000 ms, corresponding to 0.06% and 0.73% of the responses, respectively) and response errors (answers that did not match the expected answer, addition of articles before the word, or hesitation marks preceding the response, corresponding to 1.04% of the responses) were excluded from the RTs analyses. Mean RTs and accuracy data for each of the 120 items and each of the 95 participants were analyzed using the R software (R-project, R Development Core Team 2020) with the lme4 (Bates et al. 2007) and lmerTest packages (Kuznetsova et al. 2015). Linear mixed models were employed for RTs, and generalized mixed models were used assuming a binomial distribution for accuracy. Age group was considered as the independent variable, while participants and items were included as random-effect variables. The subdivision into age groups and thus the decision to consider age as a factorial, rather than a continuous predictor was primarily dictated by the choice to compare behavioral and ERP analyses, relying on a microstate analysis. Therefore, in order to align the two analyses, the same age groups were maintained. In addition, this approach allows for a more homogeneous representation of subjects in adulthood, making it possible to clearly observe differences between groups over such a wide age range in adulthood, while also avoiding overrepresentation of certain age groups.
Post-hoc tests were conducted using the emmeans package (Lenth et al. 2018), with Bonferroni correction for multiple comparisons (Bonferroni 1936). Visualization of the behavioral and neurophysiological data was performed using the ggplot2 (Wickham 2016) and NPL packages (Ménétré 2021).
Other cognitive assessments
For the other cognitive tasks, in order to evaluate whether participants’ performance fell within the normal range for their age group, comparisons were made to a normative corpus when possible (i.e. for the tasks from the Wechsler Adult Intelligence Scale IV). For the nonstandardized tasks, raw results were reported. Additionally, to analyze changes across the five age groups, separate mixed models were run for each task, considering age group as the independent variable. Raw scores were used for tasks belonging to standardized tests in these analyses. This approach allowed for the assessment of performance evolution on the same task across the lifespan. These results will only briefly be described below, as they are beyond the scope of this study, and their purpose was to verify that the participants’ performance in other cognitive tasks was consistent with what is typically observed in their age group.
ERP analyses
All analyses were run on average-referenced data. The ERPs were first subjected to a topographic analysis of variance (tANOVA) to determine the time periods that showed differences in the global distribution of the electric field between age groups. The tANOVA employs a nonparametric randomization test on global dissimilarity measures to identify periods of significant topographic modulation between experimental conditions or groups (Murray et al. 2008). Using this approach, it is possible to identify the time periods during which distinct topographies were observed between age groups, comparing time point by time point (with a time period criterion of 20 consecutive milliseconds).
Then, a spatio-temporal, or microstate segmentation, was applied on the group-averaged ERPs of each group to ascertain topographic differences across groups, both for stimulus- and response-locked ERPs. Topographic differences across groups would suggest different underlying brain processes (Lehmann and Skrandies 1984; Koukou and Lehmann 1987; Lehmann et al. 1998; Changeux and Michel 2004).
A temporal atomized and agglomerate hierarchical clustering algorithm was applied to the group-averaged data to identify the topographical maps (Murray et al. 2008). The Cartool meta-criterion for selecting the best topography (Tomescu et al. 2018) was initially followed, and then, a criterion of minimum duration (20 ms) was added. However, the segmentation based on the optimal number of microstates provided by the Cartool meta-criterion showed inconsistent results (e.g. segregation of the P1 component in two topographical maps), which is why a lower number of microstates (5 maps, with special attention to preserving entirely the P1 component, e.g. Laganaro 2017) was selected, which still explains 95% of the variance.
Based on the results of the microstate segmentation, we applied a “back-fitting procedure” that allows us to test how much topographic maps observed in the grand-averaged signals actually explain the ERP data of a single individual participant. Other quantifiers, such as measures of map presence/absence and duration in each individual ERP, were obtained and used to test statistical differences among groups using nonparametric statistical tests given the distribution of the data and the absence of variance for map presence in some of the groups (Kruskal–Wallis and Wilcoxon tests).
To better characterize the differences among the age groups, a second, more restricted back-fitting (shorter time window) was conducted on the time window exhibiting the biggest difference. In this additional analysis the same rank-based statistical analyses were applied to map presence and duration as well.
Brain electrical sources were also determined for this identified time period of significant ERP changes. Two separate source localization analyses were conducted based on the grand average ERPs of the “young adults” and “older adults” age groups (the groups with the largest difference). The procedure outlined by Michel and Brunet (2019) was followed using the Cartool 3.91 (Brunet et al. 2011) software. The head model for which the EEG forward solution is calculated was constructed from the MNI average brain (MNI 152, Montreal Neurological Institute, Montreal, Canada) with the cerebellum removed. The LSMAC (Locally Spherical Model with Anatomical Constraints) Lead Field was then calculated, adapting the absolute conductivity of the skull to the mean age of each group (0.0210 [S/m] for “young adults” and 0.0085 [S/m] for “older adults”), providing the matrix from which the inverse problem was solved using the algorithm LAURA (Local AUtoRegressive Average; De Peralta Menendez et al. 2004). After running these analyses, the P100 time window was checked to verify that this visual evoked potential (VEP) component was correctly localized in the posterior regions.
Results
Behavioral results
Picture naming task
Slower production latencies (RTs) were observed for the groups at the two extremities of the adult lifespan: “adolescents” versus “older adults” (see Fig. 1A). The mixed model indicated a significant effect of age group on RTs [F(4,89.9) = 2.78, P = 0.032], with only the “older adults” group being significantly slower than the “young adult” group by approximately 109 ms [t(90.0) = −3.147, P = 0.01]. Differences between the other groups did not reach significance. Regarding accuracy, although a lower score was observed in older adulthood (see Fig. 1B), the main effect of the age group was not significant on accuracy (χ2(4) = 7.36, P = 0.118). All details regarding these results are presented in Table IA and B in the Supplementary Materials.
Fig. 1.
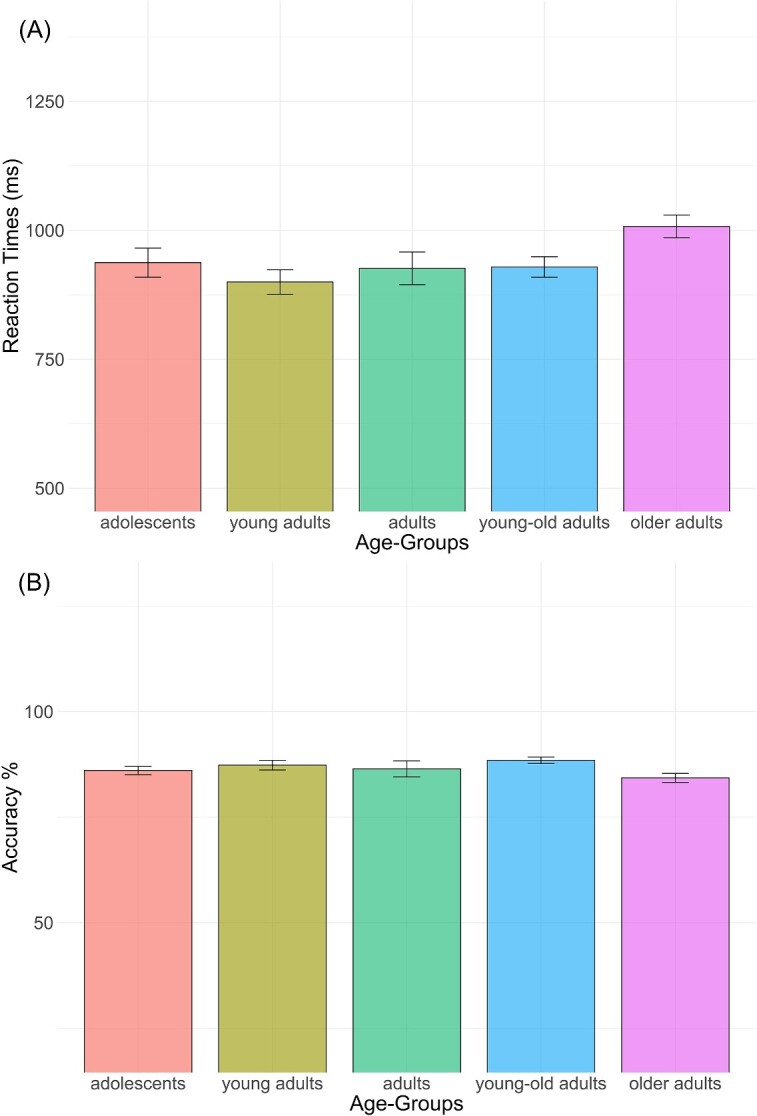
Mean RTs A) and mean accuracy B) for each age group in the picture naming task with error bars representing standard errors between subjects.
Other cognitive assessments
On the “Vocabulary” test and the “Working Memory” test, the performance of all participants was within the population norms for their age group. On the other cognitive assessments, none of the participants obtained outlying results compared to other participants in the same age group. As already mentioned, these results will not be further elaborated here, but additional details are presented in the Supplementary Materials (Fig. I and Tables II–VIII) and in the work of Fargier and Laganaro (2023).
ERP results
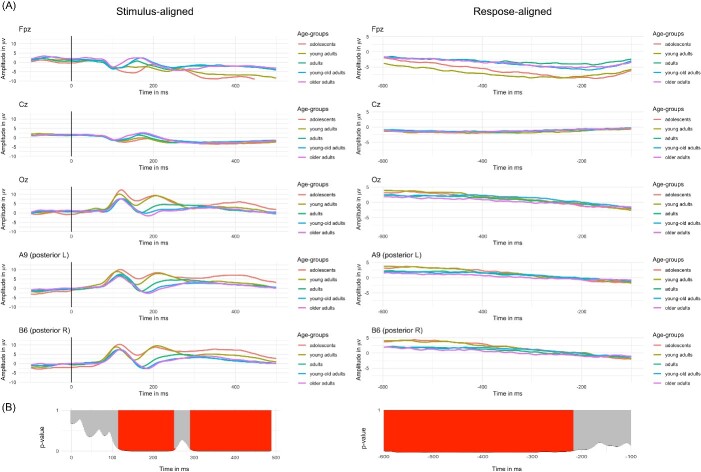
Representative waveforms are presented in Fig. 2A. The visual inspection revealed similar waveforms across age groups with differences in amplitudes, especially in the stimulus-aligned ERPs between 150 and 250 ms. The tANOVA results showed significant differences across groups from about 120 to 250 ms, and from 300 to 500 ms for the signal aligned to the stimulus, while for the signal aligned to the response, significant differences were present from about 600 to 210 ms before vocal onset (see results of tANOVA in Fig. 2B and contrasts between groups in Fig. II in Supplementary Materials). Significant differences were found for the “adolescents” and the “young adults” groups compared with the three older age groups from about 120 to 250 ms and from about 300 to 500 ms. Furthermore, the “adolescents” and the “young adults” groups were significantly different from each other between about 370 to 500 ms. Finally, the “adults” significantly differed from the two older age groups from about 160 to 260 ms. For the response-aligned signal significant differences were highlighted only among the two younger compared with the three older age groups in different fragmented time windows across the signal, with the “adolescents” and “young adults” groups differing from the “adults,” “young-old adults” and “older adults” groups from about −600 to −200 ms.
Fig. 2.
A) Group-averaged stimulus- and response-aligned event-related potentials (ERPs) waveforms (128 electrodes) for five exemplar electrodes (Fpz, Cz, Oz, A9 and B6), plotted in microvolts in function of time and B) results of the tANOVA show in red time windows where topographies are significantly different between groups (P-value < 0.05).
Microstate analyses and fitting in the individual ERPs
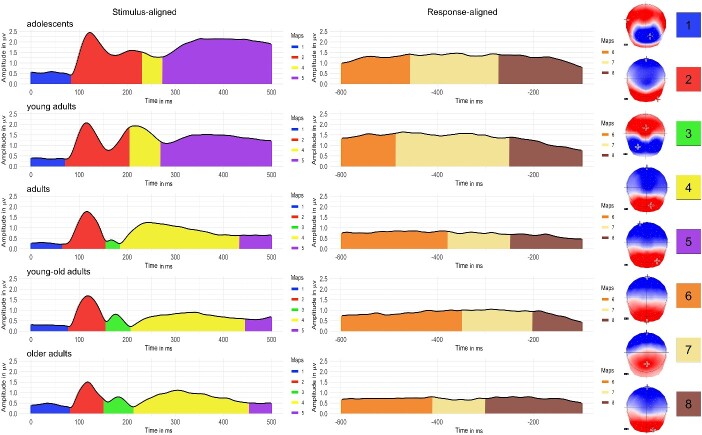
The spatio-temporal segmentation applied on the five grand-averages of groups revealed a best model explaining 95% of variance with 5 different topographic maps from the picture onset to the first 500 ms (stimulus-aligned epochs) and 3 different topographic maps on the time window starting 100 ms before vocal onset and covering the preceding 500 ms (response-aligned epochs; Fig. 3). A common pattern appears on the grand averaged ERPs between the two younger groups of “adolescents” and “young adults” and between the three older groups of “adults,” “young-old adults” and “older adults,” characterized by an additional microstate (Map 3 in Fig. 3) in the three older groups between 150 and 220 ms (Map3). Based on the distribution of the periods of topographic stability on the group averaged ERPs, one back-fitting period from 150 to 500 ms (Maps 2, 3, 4, 5) was applied to the individual stimulus-aligned signal in order to statistically assess the observed differences (the fitting in the individual ERPs starts at the end of P100, at 150 ms, to prevent the segmentation algorithm from confusing a relatively similar map with that of P1). One single back-fitting period on all the 500 ms (Maps 6 to 8) of the individual response-aligned signal was also applied.
Fig. 3.
Results of the spatio-temporal segmentation on the grand-averaged ERPs from each age group from the stimulus onset to 100 ms before vocal onset. The temporal distribution of the topographic maps is represented with color codes under the global field power. The 8 corresponding template maps are displayed with positive values in red and negative values in blue. Crosses on the maps represent the maximum and minimum amplitude of the electrical configuration.
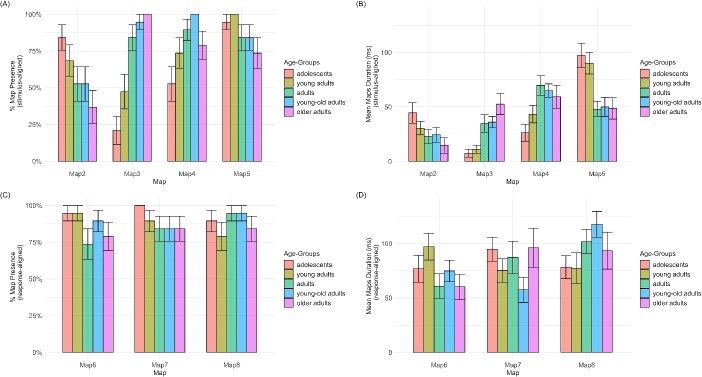
Figure 4 displays the mean presence and duration of each Map template in each age group. Presence of Map2 (and to a lesser extent Map5) decreases across ages, while Map3 (and to a lesser extent Map4) increases with ages. Duration of Map5 (and to a lesser extent Map2) progressively decreased over the lifespan, while Map3 and Map4 were longer in the three older age groups.
Fig. 4.
Bar plots representing the results of the fitting in the individual ERPs with standard error: percentage of maps presence per age group for A) stimulus-aligned and C) response-aligned signal; mean map duration per age group for B) stimulus-aligned and D) response-aligned signal.
Assessing the significance of differences in duration of the periods of stable electrophysiological signal at scalp (map’s number of time frames in the individual ERPs) across age groups was not possible using mixed models analyses since some groups had no or little variance (100% of presence within the group) (Analysis with mixed model shows a significant interaction effect between “age-groups” and “maps” on Maps Presence, with a χ2(12) = 32.05, P < 0.01. However, we will not take this data into account as this type of model is not suitable in a situation where one or more SD = 0.), (Analyses with mixed model show a significant interaction effect between “age-groups” and “maps” on Maps Duration, with an F(12, 260) = 2.698, P < 0.01. However, we will not take these data into account as this type of model is not suitable also in this situation.). However, given the high variability between the presence rate and duration among age groups, it seemed optimal to proceed with a detailed analysis comparing each group with the others for each map taken separately. This was achieved using Kruskal–Wallis tests to evaluate the main effect of groups and Wilcoxon tests to decompose the main effects (see Fig. 4). In the stimulus-aligned signal, the analyses confirmed that the microstate characterizing the P1 component (Map2) was present after 150 ms in particular in the first two groups (significant effect of Age-Groups for presence—χ2(4) = 10.071, P = 0.04—with only “adolescents” differing significantly from “older adults”—P = 0.034 on the Wilcoxon test). By contrast, Map3 characterized the ERP signal in the last three groups, with 100% of presence in “older adults” group (significant effect of Age-Groups for presence—χ2(4) = 40.959, P < 0.01). For this map “adolescents” and “young adults” significantly differed from “adults” (P < 0.01 and P = 0.032), “young-old adults” (both P < 0.01) and “older adults” (both P < 0.01). Presence of the microstate appearing in the following time window (Map4, starting around 210 ms) was higher in the three older groups, with 100% presence in the individual ERPs of “young-old adults” (significant effect of age groups—χ2(4) = 14.413, P < 0.01—but only the group of “adolescents” significantly differed from “young-old adults”—P < 0.01). Finally, even if the presence of Map5 (275 to 500 ms in the two younger age groups, 450 to 500 ms in the three older age groups) was higher in the first two groups, the effect of age groups was not significant.
On duration in the stimulus-aligned signal, a significant effect of age groups was observed only for Map5 (χ2(4) = 14.897, P < 0.01) which is progressively shorter across groups, with “adolescents” differing significantly from “adults” (P = 0.019) and “young-old adults” (P = 0.019). Moreover, only a tendency effect of age group was observed on duration of Map3 (χ2(4) = 9.016, P = 0.061, with “young adults” significantly differing from “young-old adults”—P = 0.039—and “older adults”—P = 0.039).
On the three maps characterizing the response-locked signal (Maps 6, 7, and 8) only minor differences appeared across groups in Fig. 4, and both analysis on Presence (Map6: χ2(4) = 3.459, P = 0.48; Map7: χ2(4) = 5.820, P = 0.21; Map8: χ2(4) = 3.459, P = 0.48) and Duration (Map6: χ2(4) = 5.741, P = 0.22; Map7: χ2(4) = 2.779, P = 0.60; Map8: χ2(4) = 6.329, P = 0.18) did not reveal any significant effect of age groups.
To analyze whether the duration of specific microstates was related to the performance of speakers, correlations between the duration of each map and the average RTs of individual subjects were calculated (on all participants, per group and on the 3 oldest age groups) (Correlations were not analyzed for Maps 5 and 6 (which are cut off by being at the borders of the analyzed stimulus-locked and response-locked time windows, respectively), neither for maps being present in less than 10 participants for a given age-group. Bonferroni corrections were applied for multiple comparisons (P-value was set at <0.013 when all participants, or the 3 oldest age-groups were considered, and at <0.003 for comparisons between each age-group).). The results are presented in Table IX in the Supplementary Materials. On all participants, significant positive correlations with RTs are observed for Map2 and Map8 (respectively r(54) = 0.41, P < 0.01; and r(82) = 0.38, P < 0.001) and only a marginal positive correlation was observed for Map3 (r(64) = 0.29, P = 0.016). When grouping the 3 older groups the only correlation that reaches significance is on Map8 (r(46) = 0.39, P < 0.001). Finally, on analyses carried out separately per age group only Map2 (r(14) = 0.77, P < 0.001) consistently correlates with RTs and only a marginal positive correlation was observed for Map8 (r(17) = 0.61, P < 0.01).
Further analyses on Map3—time period associated with Map3
A further analysis on the time window showing qualitative changes—Map3 progressively more present in the three older adults groups—was conducted in two steps: First, we proceeded to a second back-fitting analysis in the individual ERPs in the time period from 152 to 212 ms (including Map3 and the adjacent Maps 2 and 4) and second, we performed a source localization on that time period.
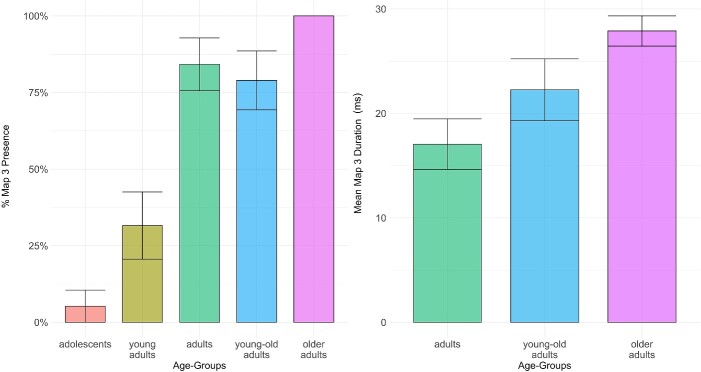
The presence of Map3 increased progressively over the lifespan, until reaching 100% of presence in “older adults” (see Fig. 5). A significant difference in the presence of this topographic pattern was confirmed by a significant main effect of age groups [χ2(4) = 49.734, P < 0.01]. The Wilcoxon test showed that the groups of “adolescents” and “young adults” (who did not significantly differ from each other) significantly differed from “adults” (both P < 0.01), “young-old adults” (both P < 0.01) and “older adults” (both P < 0.01). The latter two groups did not significantly differ from each other. Analysis of duration was only applied to the 3 groups showing at least 50% of presence (“adults,” “young-old adults” and “older adults”). We found a significant effect of age groups (χ2(2) = 11.693, P < 0.01) with longer duration in both groups of “young-old adults” and “older adults” (who did not significantly differ from each other) relative to “adults” (both P < 0.01; Fig. 5).
Fig. 5.
Bar plots representing on the left the percentage of presence and on the right the mean duration for Map3 (152 to 212 ms) in the individual ERPs for each age group, with error bars representing standard errors.
A source localization analysis was performed on this same time period (from 152 to 212 ms), comparing the age group that showed the maximum presence of Map3 (“older adults”) and the most efficient group in terms of RTs (“young adults,” in which Map3 was very slightly represented). For “young adults,” results show an activity mainly located in the occipital bilateral and left posterior frontal regions. “Older adults” showed more extended activation in the left hemisphere, especially in the left temporo-parietal and frontal superior areas (see Fig. 6).
Fig. 6.
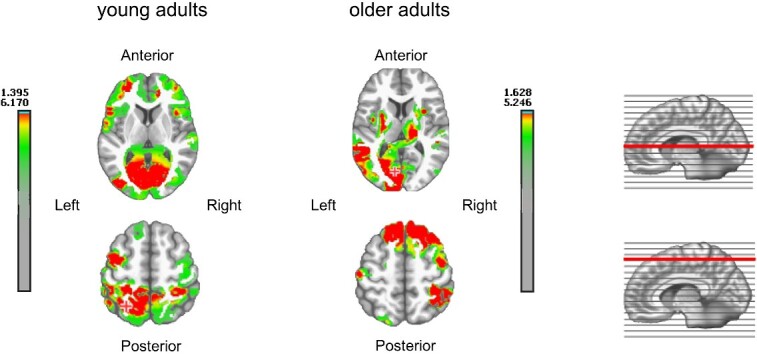
Source localization of activation in the 152 to 212 ms time window in the group of “young adults” on the left and the group of “older adults” on the right displayed on two transversal sections.
Exploratory analysis on Map3
Although the results of other cognitive tasks were anticipated as a control to ensure that the performance of all participants was in accordance with the expected outcomes for their age group, we decided to exploit them for an exploratory analysis to provide additional insights aimed to test, whether the duration of the additional topographic pattern observed starting from the age of 40 to 50 years old (Map3) was modulated by the performance on the other cognitive assessments. Results to the “Vocabulary,” “Verbal Fluency – Animals,” “Verbal Fluency – P letter,” “Simple Reaction Times,” “Working Memory,” and “Stroop” tasks were considered as independent variables in a linear model in order to explore their effect on the duration of Map3. Results showed that only performance on the “Verbal Fluency – Animals” task predicted the duration of Map3 [F(1,82) = 5.055, P = 0.027]. The effect indicated a longer duration of Map3 when performance on the “Verbal Fluency – Animals” task was lower (β = −0.907).
Discussion
In this study, we aimed to investigate whether changes in the neurophysiological correlates of word production are aligned with behavioral changes occurring across the adult lifespan. A decrease in behavioral performance was observed only in the group aged over 70 years old (“older adults”). However, the neurophysiological results were not aligned with the behavioral results, as inter-group differences in ERPs began to be present at the age of 40 (“adults group”) and continued through the two older age groups. The main differences in the ERP signal were consistent and limited to a specific time window, between 150 and 220 ms after the onset of the stimulus (Map3 in Fig. 3), and were characterized by an additional increasingly longer period of stable electrophysiological activity on the scalp. These qualitative changes were first observed at the age of 40 when behavioral performance was still maintained.
We will briefly discuss the behavioral results before presenting an in-depth interpretation of these ERP results and the misalignment between behavioral and ERP differences across age groups.
Behavioral differences across age groups
Behavioral results in our target picture naming task are consistent with the literature, as only the group of “older adults” differed from the group of “young adults” in terms of RTs, while on the other nonverbal cognitive assessments, the expected progressive decrease in performance is also observed. In addition, the results across all other cognitive tasks confirmed that the performance of all subjects was consistent with what is expected for their age group.
No significant differences across age groups were observed for accuracy, which also aligns with several previous studies (e.g. Wierenga et al. 2008; Kavé et al. 2010; Kahlaoui et al. 2012). Note that changes in performance in terms of production latencies versus accuracy are debated in the literature (e.g. Goulet et al. 1994; Verhaegen and Poncelet 2013; Valente and Laganaro 2015). For example, Kavé et al. (2010) found a “bow-shaped” pattern for accuracy in response to a picture naming task, but only the groups of children up to 8 years old significantly differed from the other age groups. This was interpreted as a combined effect of increased vocabulary skills and changes in retrieval processes. Wierenga et al. (2008) also argued that the differences observed in both RTs and accuracy suggest that decreased performance in word retrieval is likely due to difficulty accessing lexical-semantic information rather than progressive decline in knowledge or in the neural substrates underlying semantic knowledge. Additional analyses performed on our data to explore this hypothesis confirmed that performance on the “Simple Reaction Times” task predicted picture naming RTs, while performance on the “Vocabulary” task predicted accuracy. In contrast, no significant effect of “Vocabulary” was found on RTs, and no significant effect of “Simple Reaction Times” was found on accuracy (Results to the “Simple Reaction Times” task and to the “Vocabulary” task were considered as independent variables in linear models in order to verify the hypothesis that RTs are more related to general processing speed while accuracy mainly reflects word retrieval capabilities. Results showed that performance on the “Simple Reaction Times” task predicted picture naming RTs [F(1) = 4.041, P = 0.048], while performance on the “Vocabulary” task predicted accuracy [F(1) = 4.918, P = 0.029]. The effects indicated faster production speed when “Simple Reaction Times” were shorter (β = 3.912e−4) and more accurate responses when results on “Vocabulary” task were higher (β = 1.929e−3). No significant effect of age-group was found.). Note that the absence of significant differences in accuracy across age groups may also be related to high accuracy due to material selection with relatively high-frequency nouns (e.g. “train”, “dog”, “sun”) compared to other studies (Gertel et al. 2020).
Crucially for our purpose here, these behavioral changes were not aligned with the neurophysiological changes across adulthood.
ERP changes are not aligned to behavioral changes
Our results showed similar ERP patterns associated with picture naming for the two youngest groups and ERP differences across groups starting from the age of 40 to 50 years old, in the absence of any behavioral changes until the oldest age group. The main inter-group difference was reflected by the increasingly longer period of stable electrophysiological activity on the scalp (Map3 in Fig. 3) in the three older age groups. The presence and duration of this topographic pattern significantly increased for the latter groups and was virtually absent in the signal of the two youngest groups. The progressive increase in the duration of this microstate in the neural signal thus suggests a gradual change that is related to detrimental performance only after 70 years old. Note that qualitative differences in topographic patterns (microstates) are assumed to reflect the involvement of different underlying neural networks (Michel et al. 2004; Michel and Murray 2012). As support of this idea, source localization applied in this time window of interest revealed different activations in young and older adults, with mainly occipital activation in “young adults” and more extended activations in left temporal and bilateral frontal areas in older adults. In the following, we interpret the loci and nature of these neural changes and tackle the issue of the discrepancy between neurophysiological and behavioral results.
Qualitative age-related changes and functional reorganization
The major ERP differences consisted of qualitative changes between 150 and 220 ms: distinct topographic patterns were observed for younger and older individuals, as evidenced by the presence of Map3 only for adults above 40 years old. This time window has been associated to lexical-semantic processes, i.e. the activation of semantic features guiding lexical selection (Indefrey and Levelt 2004; Indefrey 2011 see also Python et al. 2018; Schendan and Kutas 2003; Simon et al. 2004). This interpretation is also supported by the exploratory analyses conducted on the other cognitive tasks, which highlighted that only performance on the “Verbal Fluency – Animals” task predicted the duration of Map3. In fact, the semantic (here animal) fluency task is the only one among those tested that could be more representative of lexical-semantic selection effort.
The changes across age groups observed with this additional microstate in the 150 to 220 ms time window may therefore be related to previous observations suggesting lifespan changes in the lexical-semantic network (see Wulff et al. 2019, 2022; Krethlow et al. 2020; Cosgrove et al. 2023; Guichet et al. 2024). Indeed, age-specific characteristics of the lexical-semantic network have been demonstrated to influence performance in word production (Krethlow et al. 2020), resulting in faster word production speed when the semantic network was richer and more prototypical. Further evidence supporting this interpretation is derived from a deeper look at the neural changes. In “older adults” where Map3 was highly present, we found bilateral frontal activation, which is coherent with a functional reorganization of the underlying brain networks with aging (Cabeza 2002; Ansado et al. 2013; Shafto and Tyler 2014). The activation of a larger network, including the left temporo-parietal lobe in the older age group, is compatible with the “semanticization of cognition”/DECHA hypothesis. Within this framework, there are two commonly cited patterns of change in brain function (Turner and Spreng 2015; Spreng and Turner 2019). The first one involves an increased recruitment of lateral prefrontal brain regions underlying cognitive control, with a reduced suppression of the default network. The second one involves the recruitment of functionally connected brain regions associated with the storage and retrieval of prior-knowledge representations. Based on these functional brain changes, the DECHA model (Turner and Spreng 2015; Spreng and Turner 2019) proposes that as efficiency in executive skills declines, the engaged default network becomes increasingly dependent on the lateral prefrontal cortex. Considering the patterns observed in young and old adults, our results are also compatible with the posterior-anterior shift in aging (PASA) phenomenon of intra-hemispheric reorganization of activation patterns, from the occipitotemporal to frontal cortex (Davis et al. 2008; Dennis and Cabeza 2008). These assumptions of functional reorganization are thus coherent with the timing of the observed changes (150 to 220 ms), tapping into processes that deal with the temporal coactivation of multiple lexical representations driving the selection of the correct word to be produced (Nozari and Pinet 2020).
Dedifferentiation in the context of maintained or reduced performance
Generally, aging is studied through the prism of cognitive aging and decline, with many studies using fMRI approaches. When more widespread and frequently bilateral brain activations are observed in older adults, and when performance is maintained or even improved relative to young adults, this pattern is seen as compensatory (Cabeza 2002; Reuter-Lorenz 2002; Reuter-Lorenz and Lustig 2005; Cabeza et al. 2018). When different neural patterns are associated with worse performance or are unrelated to the task, this is interpreted as dedifferentiation of brain networks (Riecker et al. 2006; Heuninckx et al. 2008; Bernard and Seidler 2012; Koen and Rugg 2019; Cassady et al. 2020). The crucial aspect of the present work is that the same pattern could be seen as compensatory—in adults and young-old adults—or as dedifferentiation in older adults, if interpreted in isolation. So far, whether dedifferentiation is compensatory is not clearly understood, probably because studies have been confined to comparisons of the two extremes of adulthood. Our results are thus in line with accumulating evidence that neural dedifferentiation does not exclusively reflect detrimental aging (Koen and Rugg 2019), but it is likely a stage of progressive qualitative change undergone by the brain during healthy aging, which cannot indefinitely support compensation. Actually, the misalignment between neural changes and behavioral changes is not a new phenomenon. For example, McLaughlin et al. (2004) also found discrepancies between the results of the two approaches in a longitudinal study of word learning in young adults. This allowed the authors to conclude that these different methodologies highlight slightly different processes, both contributing to a more complete understanding of the cognitive processes underlying the examined language skills. Focusing on aging, previous fMRI studies have found differences in neural activation between young and older adults, in the absence of differences at the behavioral level (Grady et al. 2003; Aine et al. 2006; Ansado et al. 2013), but to our knowledge, none have investigated intermediate age groups. The discrepancy between behavioral performance and neurophysiological correlates on verbal tasks may be due to a compensatory mechanism related either to an increase in lexical-semantic abilities throughout adulthood or/and to the reorganization of brain activation underlying the preserved function (Wierenga et al. 2008; Baciu et al. 2021), or a combination of both. More work is needed to clarify this specific issue.
Other age-related changes in the ERP signal
Differences across age groups were also present in other time windows beyond the time period between 150 and 220 ms, but they were related to a different distribution of the same microstates rather than different microstates. For instance, Map2 was more prominent in “adolescents” and “young adults” due to the analyzed time window (Map3 time window corresponds to the end of Map2 for the two youngest groups). This map corresponds to the P100 component related to visual processing and object recognition, and it had an extended duration in the two youngest groups. After 300 ms, the duration of patterns was distributed differently between the youngest and the older groups (Map4 and Map5). Map4 was observed in all groups in the P2 time window. This component has been associated with lexical selection and phonological code retrieval (Indefrey and Levelt 2004; Costa et al. 2009; Indefrey 2011; Cai et al. 2020). Here, the same brain process seems to underpin lexical selection and phonological code retrieval in all groups, possibly with shorter duration in the two youngest groups. Part of these differences is possibly due to the additional Map3 in the older groups, as discussed above. Considering the absence of Map3 and the shorter duration of Map4 in the youngest group despite similar production latencies with the next two adult groups, there might be faster processing in early time windows in the younger adults, which would then be slowed down in the later time window (after 300 ms). Hence, along with the different brain processes discussed above for Map3, the results of the following microstates suggest different time distributions of similar processes, which even out in terms of the behavioral final outcome up to the oldest group. The lengthening of Map4 after 300 ms for the three older age groups in a time window likely associated with lexical selection and phonological code retrieval (Indefrey 2011) is compatible with the “transmission deficit hypothesis” (Burke et al. 1991, 2000), where the priming from the semantic to the phonological representations is thought to decrease with aging. Note that in the response-aligned signal, significant differences were only observed in a time window relatively far from the response, which may overlap with the results observed on the stimulus-aligned data.
Conclusion
The present study clearly shows a misalignment of behavioral and neurophysiological changes in word production, revealing significant differences at the neurophysiological level that appear as early as the age of 40, while a decrease in performance in word production was only observed from the age of 70. The main ERP changes were qualitative differences characterized by an additional activated brain network, which progressively increased in presence and duration in the three older age groups. Converging evidence from the corresponding source localization and the time window of effects suggests dedifferentiation of the neural networks supporting lexical-semantic processes. The results indicate compensatory mechanisms that allow the maintenance of language skills longer than other cognitive functions up to 70 years, which, however, are no longer sufficient to compensate for the decrease in performance afterwards. The lifespan approach adopted here, especially the inclusion of middle-aged adults, allowed us to demonstrate that the same qualitative neural change—likely restricted to lexical-semantic processes—can be seen in two ways: as compensation or dedifferentiation. The present study sheds new light on whether dedifferentiation is compensatory (Koen and Rugg 2019), illustrating that it is actually a progressive phenomenon, and contributes to the emerging trend of refining brain–behavior relationships through neural degeneracy (Westlin et al. 2023).
Supplementary Material
Contributor Information
Giulia Krethlow, Faculty of Psychology and Educational Sciences, University of Geneva, Bd du Pont d’Arve 40, 1205, Geneva, Switzerland.
Raphaël Fargier, Université Côte d’Azur, CNRS, BCL, France.
Tanja Atanasova, Faculty of Psychology and Educational Sciences, University of Geneva, Bd du Pont d’Arve 40, 1205, Geneva, Switzerland.
Eric Ménétré, Faculty of Psychology and Educational Sciences, University of Geneva, Bd du Pont d’Arve 40, 1205, Geneva, Switzerland.
Marina Laganaro, Faculty of Psychology and Educational Sciences, University of Geneva, Bd du Pont d’Arve 40, 1205, Geneva, Switzerland.
Author contributions
Giulia Krethlow (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—original draft), Raphaël Fargier (Data curation, Writing—review & editing), Tanja Atanasova (Data curation, Writing—review & editing), Eric Ménétré (Data curation, Formal analysis, Writing—review & editing), Marina Laganaro (Conceptualization, Formal analysis, Funding acquisition, Supervision, Validation, Writing—review & editing).
Funding
This work was supported by the Swiss National Science Foundation (SNSF) under Grant no. 100014_165647.
Conflict of interest statement: None declared.
Data availability
Behavioral and ERP data are available at the following link: https://doi.org/10.26037/yareta:ves7bzbscjebhaurlfs7rniscy.
References
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualis C, Bockholt J, Kovacevic S, Cobb W, Padilla D, et al. Aging: compensation or maturation? NeuroImage. 2006:32(4):1891–1904. 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alario FX, Ferrand L. A set of 400 pictures standardized for French: norms for name agreement, image agreement, familiarity, visual complexity, image variability, and age of acquisition. Behav Res Methods Instrum Comput. 1999:31(3):531–552. 10.3758/BF03200732. [DOI] [PubMed] [Google Scholar]
- Alario FX, Ferrand L, Laganaro M, New B, Frauenfelder UH, Segui J. Predictors of picture naming speed. Behav Res Methods Instrum Comput. 2004:36(1):140–155. 10.3758/BF03195559. [DOI] [PubMed] [Google Scholar]
- Albert MS, Heller HS, Milberg W. Changes in naming ability with age. Psychol Aging. 1988:3(2):173–178. 10.1037/0882-7974.3.2.173. [DOI] [PubMed] [Google Scholar]
- Ansado J, Monchi O, Ennabil N, Faure S, Joanette Y. Load-dependent posterior–anterior shift in aging in complex visual selective attention situations. Brain Res. 2012:1454:14–22. 10.1016/j.brainres.2012.02.061. [DOI] [PubMed] [Google Scholar]
- Ansado J, Marsolais Y, Methqal I, Alary F, Joanette Y. The adaptive aging brain: evidence from the preservation of communication abilities with age. Eur J Neurosci. 2013:37(12):1887–1895. 10.1111/ejn.12252. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician. 2004:33(10):783–787. [PubMed] [Google Scholar]
- Atanasova T, Fargier R, Zesiger P, Laganaro M. Dynamics of word production in the transition from adolescence to adulthood. Neurobiology of Language. 2020:2(1):1–21. 10.1162/nol_a_00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M, Boudiaf N, Cousin E, Perrone-Bertolotti M, Pichat C, Fournet N, Chainay H, Lamalle L, Krainik A. Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age. 2016:38(1):1–22. 10.1007/s11357-015-9857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M, Banjac S, Roger E, Haldin C, Perrone-Bertolotti M, Lœvenbruck H, Démonet JF. Strategies and cognitive reserve to preserve lexical production in aging. GeroScience. 2021:43(4):1725–1765. 10.1007/s11357-021-00367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997:12(1):12–21. 10.1037/0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bates D, Sarkar D, Bates MD, Matrix L. The lme4 package. R package version. 2007:2(1):74. [Google Scholar]
- Bernard JA, Seidler RD. Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging. 2012:33(9):1890–1899. 10.1016/j.neurobiolaging.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonferroni C. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commericiali di Firenze. 1936:8:3–62. [Google Scholar]
- Bonin P, Peereman R, Malardier N, Méot A, Chalard M. A new set of 299 pictures for psycholinguistic studies: French norms for name agreement, image agreement, conceptual familiarity, visual complexity, image variability, age of acquisition, and naming latencies. Behav Res Methods Instrum Comput. 2003:35(1):158–167. 10.3758/BF03195507. [DOI] [PubMed] [Google Scholar]
- Brown S. Temporal jitter mimics effects of aging on word identification and word recall in noise, Doctoral dissertation. Vancouver: University of British Columbia; 2000. [Google Scholar]
- Brunet D, Murray MM, Michel CM. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput Intell Neurosci. 2011:2011:1–15. 10.1155/2011/813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, Stevens M, Mandera P, Keuleers E. How many words do we know? Practical estimates of vocabulary size dependent on word definition, the degree of language input and the participant’s age. Front Psychol. 2016:7:1116. 10.3389/fpsyg.2016.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York, NY: Psychology Press; 2008, pp. 373–443. [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, Wade E. On the tip of the tongue: what causes word finding failures in young and older adults? J Mem Lang. 1991:30(5):542–579. 10.1016/0749-596X(91)90026-G. [DOI] [Google Scholar]
- Burke DM, MacKay DG, James LE. Theoretical approaches to language and aging. In: Perfect T, Maylor E, editors. Models of cognitive aging. Oxford: Oxford University Press; 2000, pp. 204–237. 10.1093/oso/9780198524380.003.0008. [DOI] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002:17(1):85–100. 10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FI, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park CD, Reuter-Lorenz PA, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018:19(11):701–710. 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Ouyang M, Yin Y, Zhang Q. The effect of time pressure and semantic relatedness in spoken word production: a topographic ERP study. Behav Brain Res. 2020:387:112587. 10.1016/j.bbr.2020.112587. [DOI] [PubMed] [Google Scholar]
- Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990:90(4):207–217. [PubMed] [Google Scholar]
- Cassady K, Ruitenberg MF, Reuter-Lorenz PA, Tommerdahl M, Seidler RD. Neural dedifferentiation across the lifespan in the motor and somatosensory systems. Cereb Cortex. 2020:30(6):3704–3716. 10.1093/cercor/bhz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Michel CM. Mechanisms of neural integration at the brain scale level: The neuronal workspace and microstate models. In: Grillner S, Grabyel AM, editors. Microcircuits: the interface between neurons and global brain function. Cambridge, MA: MIT Press; 2004, pp. 347–370. [Google Scholar]
- Cosgrove AL, Beaty RE, Diaz MT, Kenett YN. Age differences in semantic network structure: acquiring knowledge shapes semantic memory. Psychol Aging. 2023:38(2):87–102. 10.1037/pag0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Strijkers K, Martin C, Thierry G. The time course of word retrieval revealed by event-related brain potentials during overt speech. Proc Natl Acad Sci. 2009:106(50):21442–21446. 10.1073/pnas.0908921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior–anterior shift in aging. Cereb Cortex. 2008:18(5):1201–1209. 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Peralta Menendez RG, Murray MM, Michel CM, Martuzzi R, Andino SLG. Electrical neuroimaging based on biophysical constraints. NeuroImage. 2004:21(2):527–539. 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua R, Sessa P, Peressotti F, Mulatti C, Navarrete E, Grainger J. ERP evidence for ultra-fast semantic processing in the picture–word interference paradigm. Front Psychol. 2010:1:177. 10.3389/fpsyg.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FI, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. New York: Psychology Press; 2008, pp. 1–54. [Google Scholar]
- Deschavanne E, Tavoillot PH. Philosophie des âges de la vie. Paris: Grasset; 2007. [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Madden DJ. Age-related differences in the neural bases of phonological and semantic processes. J Cogn Neurosci. 2014:26(12):2798–2811. 10.1162/jocn_a_00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Karimi H, Troutman SB, Gertel VH, Cosgrove AL, Zhang H. Neural sensitivity to phonological characteristics is stable across the lifespan. NeuroImage. 2021:225:117511. 10.1016/j.neuroimage.2020.117511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargier R, Laganaro M. Neurophysiological modulations of non-verbal and verbal dual-tasks interference during word planning. PLoS One. 2016:11(12):e0168358. 10.1371/journal.pone.0168358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargier R, Laganaro M. Referential and inferential production across the lifespan: different patterns and different predictive cognitive factors. Front Psychol. 2023:14. 10.3389/fpsyg.2023.1237523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargier R, Buerki A, Pinet S, Alario FX, Laganaro M. Word onset phonetic properties and motor artifacts in speech production EEG recordings. Psychophysiology. 2018:55(2):e12982. 10.1111/psyp.12982. [DOI] [PubMed] [Google Scholar]
- Feyereisen P. A meta-analytic procedure shows an age-related decline in picture naming: comments on Goulet, Ska, and Kahn (1994). J Speech Lang Hear Res. 1997:40(6):1328–1333. 10.1044/jslhr.4006.1328. [DOI] [PubMed] [Google Scholar]
- Gertel VH, Karimi H, Dennis NA, Neely KA, Diaz MT. Lexical frequency affects functional activation and accuracy in picture naming among older and younger adults. Psychol Aging. 2020:35(4):536–552. 10.1037/pag0000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet P, Ska B, Kahn HJ. Is there a decline in picture naming with advancing age? J Speech Lang Hear Res. 1994:37(3):629–644. 10.1044/jshr.3703.629. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999:9(8):805–814. 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci. 2003:23(3):986–993. 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichet C, Roger E, Attye A, Achard S, Mermillod M, Baciu M. Dynamics of white matter architecture in lexical production among middle-aged adults. bioRxiv. 2024:2024:02. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK, Germine LT. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015:26(4):433–443. 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. Psychol Learn Motiv. 1988:22:193–225. [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn Mem Cogn. 1991:17(1):163–169. 10.1037/0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004:5(2):87–96. 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008:28(1):91–99. 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyau E, Boudiaf N, Cousin E, Pichat C, Fournet N, Krainik A, Jaillard A, Baciu M. Aging modulates the hemispheric specialization during word production. Front Aging Neurosci. 2017:9:125. 10.3389/fnagi.2017.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol. 2011:2:255. 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004:92(1–2):101–144. 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kahlaoui K, Di Sante G, Barbeau J, Maheux M, Lesage F, Ska B, Joanette Y. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang. 2012:121(2):164–173. 10.1016/j.bandl.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Kavé G, Halamish V. Doubly blessed: older adults know more vocabulary and know better what they know. Psychol Aging. 2015:30(1):68–73. 10.1037/a0038669. [DOI] [PubMed] [Google Scholar]
- Kavé G, Knafo-Noam A. Lifespan development of phonemic and semantic fluency: universal increase, differential decrease. J Clin Exp Neuropsychol. 2015:37(7):751–763. 10.1080/13803395.2015.1065958. [DOI] [PubMed] [Google Scholar]
- Kavé G, Knafo A, Gilboa A. The rise and fall of word retrieval across the lifespan. Psychol Aging. 2010:25(3):719–724. 10.1037/a0018927. [DOI] [PubMed] [Google Scholar]
- Kemper S, Kemtes KA. The age-invariance of working memory measures and non-invariance of producing complex syntax. Behav Brain Sci. 1999:22(1):102–103. 10.1017/S0140525X99301783. [DOI] [Google Scholar]
- Kemper S, Sumner A. The structure of verbal abilities in young and older adults. Psychol Aging. 2001:16(2):312–322. 10.1037/0882-7974.16.2.312. [DOI] [PubMed] [Google Scholar]
- Keuleers E, Stevens M, Mandera P, Brysbaert M. Word knowledge in the crowd: measuring vocabulary size and word prevalence in a massive online experiment. Q J Exp Psychol. 2015:68(8):1665–1692. 10.1080/17470218.2015.1022560. [DOI] [PubMed] [Google Scholar]
- Koen JD, Rugg MD. Neural dedifferentiation in the aging brain. Trends Cogn Sci. 2019:23(7):547–559. 10.1016/j.tics.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukou M, Lehmann D. An information processing perspective of psychophysiological measurements. J Psychophysiol. 1987:1:109–112. [Google Scholar]
- Krethlow G, Fargier R, Laganaro M. Age-specific effects of lexical–semantic networks on word production. Cogn Sci. 2020:44(11):e12915. 10.1111/cogs.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. Package “lmertest”. R package version. 2015:2(0):734. [Google Scholar]
- Laganaro M. ERP topographic analyses from concept to articulation in word production studies. Front Psychol. 2014:5:493. 10.3389/fpsyg.2014.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganaro M. Inter-study and inter-individual consistency and variability of EEG/ERP microstate sequences in referential word production. Brain Topogr. 2017:30(6):785–796. 10.1007/s10548-017-0580-0. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Tzieropoulos H, Frauenfelder UH, Zesiger P. Functional and time-course changes in single word production from childhood to adulthood. NeuroImage. 2015:111:204–214. 10.1016/j.neuroimage.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017:18(1):42–55. 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Spatial analysis of evoked potentials in man—a review. Prog Neurobiol. 1984:23(3):227–250. 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik WK, Henggeler B, König T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int J Psychophysiol. 1998:29(1):1–11. 10.1016/S0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M. Package “Emmeans”. R package version 4.0-3. 2018. https://cran.r-project.org/web/packages/emmeans/index.html.
- Mackenbach JP, Looman CW. Life expectancy and national income in Europe, 1900-2008: an update of Preston’s analysis. Int J Epidemiol. 2013:42(4):1100–1110. 10.1093/ije/dyt122. [DOI] [PubMed] [Google Scholar]
- Maess B, Friederici AD, Damian M, Meyer AS, Levelt WJ. Semantic category interference in overt picture naming: sharpening current density localization by PCA. J Cogn Neurosci. 2002:14(3):455–462. 10.1162/089892902317361967. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Osterhout L, Kim A. Neural correlates of second-language word learning: minimal instruction produces rapid change. Nat Neurosci. 2004:7(7):703–704. 10.1038/nn1264. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi L, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. J Cogn Neurosci. 2009:21(10):2007–2018. 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménétré E. NPL: an incomplete toolkit for the researcher in NeuroPsycholinguistic. Geneva: https://github.com/EricMenetre/NPL: Github; Retrieved from; 2021. [Google Scholar]
- Ménétré E, Laganaro M. Attentional reorientation and inhibition adjustment in a verbal Stroop task: a lifespan approach to interference and sequential congruency effect. Front Psychol. 2019:10:2028. 10.3389/fpsyg.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methqal I, Marsolais Y, Wilson MA, Monchi O, Joanette Y. More expertise for a better perspective: task and strategy-driven adaptive neurofunctional reorganization for word production in high-performing older adults. Aging Neuropsychol Cognit. 2019:26(2):190–221. 10.1080/13825585.2017.1423021. [DOI] [PubMed] [Google Scholar]
- Michel CM, Brunet D. EEG source imaging: a practical review of the analysis steps. Front Neurol. 2019:10:325. 10.3389/fneur.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM. Towards the utilization of EEG as a brain imaging tool. NeuroImage. 2012:61(2):371–385. 10.1016/j.neuroimage.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, De Peralta RG. EEG source imaging. Clin Neurophysiol. 2004:115(10):2195–2222. 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956:63(2):81–97. 10.1037/h0043158. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D’Esposito M. Aging and reflective processes of working memory: binding and test load deficits. Psychol Aging. 2000:15(3):527–541. 10.1037/0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Mohan R, Weber C. Neural activity reveals effects of aging on inhibitory processes during word retrieval. Aging Neuropsychol Cognit. 2019:26(5):660–687. 10.1080/13825585.2018.1519105. [DOI] [PubMed] [Google Scholar]
- Mokhber N, Avan A, Delbari A, Shojaeianbabaei G, Azarpazhooh M, Chaimowitz G. Ageing and cognitive function: a mini-review. EC Neurology. 2019:11:475–480. [Google Scholar]
- Mortensen L, Meyer AS, Humphreys GW. Agerelated effects on speech production: a review. Lang Cogn Process. 2006:21(1–3):238–290. 10.1080/01690960444000278. [DOI] [Google Scholar]
- Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008:20(4):249–264. 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- Myerson J, Hale S, Wagstaff D, Poon LW, Smith GA. The information-loss model: a mathematical theory of age-related cognitive slowing. Psychol Rev. 1990:97(4):475–487. 10.1037/0033-295X.97.4.475. [DOI] [PubMed] [Google Scholar]
- Neumann Y, Obler LK, Gomes H, Shafer V. Phonological vs. sensory contributions to age effects in naming: an electrophysiological study. Aphasiology. 2009:23(7–8):1028–1039. 10.1080/02687030802661630. [DOI] [Google Scholar]
- New B, Pallier C, Brysbaert M, Ferrand L. Lexique 2: a new French lexical database. Behav Res Methods Instrum Comput. 2004:36(3):516–524. 10.3758/BF03195598. [DOI] [PubMed] [Google Scholar]
- Newman RS, German DJ. Life span effects of lexical factors on oral naming. Lang Speech. 2005:48(2):123–156. 10.1177/00238309050480020101. [DOI] [PubMed] [Google Scholar]
- Nozari N, Pinet S. A critical review of the behavioral, neuroimaging, and electrophysiological studies of co-activation of representations during word production. J Neurolinguistics. 2020:53:100875. 10.1016/j.jneuroling.2019.100875. [DOI] [Google Scholar]
- Nyberg L. Functional brain imaging of episodic memory decline in ageing. J Intern Med. 2017:281(1):65–74. 10.1111/joim.12533. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:9(1):97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002:17(2):299–320. 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Peelle JE. Language and aging. The Oxford handbook of neurolinguistics. 2019:10:1–21. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989:72(2):184–187. 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Kostović I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008:18(4):915–929. 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Protopapas A. Check vocal: a program to facilitate checking the accuracy and response time of vocal responses from DMDX. Behav Res Methods. 2007:39(4):859–862. 10.3758/BF03192979. [DOI] [PubMed] [Google Scholar]
- Python G, Fargier R, Laganaro M. ERP evidence of distinct processes underlying semantic facilitation and interference in word production. Cortex. 2018:99:1–12. 10.1016/j.cortex.2017.09.008. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria; 2020. [accessed 2020 March 30]. https://www.r-project.org/. [Google Scholar]
- Rabbitt P, Maylor E, McInnes L, Bent N, Moore B. What goods can self-assessment questionnaires deliver for cognitive gerontology? Appl Cogn Psychol. 1995:9(7):S127–S152. 10.1002/acp.2350090709. [DOI] [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Craik IM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. New York: Oxford University Press; 2005, pp. 246–271. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005:15(11):1676–1689. 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage. 2010:51(2):501–511. 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. Trends Cogn Sci. 2002:6(9):394–400. 10.1016/S1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005:15(2):245–251. 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. NeuroImage. 2006:32(3):1345–1354. 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996:103(3):403–428. 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Steps toward the explanation of adult age differences in cognition. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford: Oxford University Press; 2000, pp. 19–49. 10.1093/oso/9780198524380.003.0002. [DOI] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Curr Dir Psychol Sci. 2004:13(4):140–144. 10.1111/j.0963-7214.2004.00293.x. [DOI] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009:30(4):507–514. 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society: JINS. 2010:16(5):754–760. 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saryazdi R, Bannon J, Chambers CG. Age-related differences in referential production: a multiple-measures study. Psychol Aging. 2019:34(6):791–804. 10.1037/pag0000372. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Kutas M. Time course of processes and representations supporting visual object identification and memory. J Cogn Neurosci. 2003:15(1):111–135. 10.1162/089892903321107864. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2000, pp. 155–219. [Google Scholar]
- Shafto MA, Tyler LK. Language in the aging brain: the network dynamics of cognitive decline and preservation. Science. 2014:346(6209):583–587. 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- Simon G, Bernard C, Largy P, Lalonde R, Rebai M. Chronometry of visual word recognition during passive and lexical decision tasks: an ERP investigation. Int J Neurosci. 2004:114(11):1401–1432. 10.1080/00207450490476057. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Turner GR. The shifting architecture of cognition and brain function in older adulthood. Perspect Psychol Sci. 2019:14(4):523–542. 10.1177/1745691619827511. [DOI] [PubMed] [Google Scholar]
- Strijkers K, Costa A, Thierry G. Tracking lexical access in speech production: electrophysiological correlates of word frequency and cognate effects. Cereb Cortex. 2010:20(4):912–928. 10.1093/cercor/bhp153. [DOI] [PubMed] [Google Scholar]
- Tomescu MI, Rihs TA, Rochas V, Hardmeier M, Britz J, Allali G, Fuhr P, Eliez S, Michel CM. From swing to cane: sex differences of EEG resting-state temporal patterns during maturation and aging. Dev Cogn Neurosci. 2018:31:58–66. 10.1016/j.dcn.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Prefrontal engagement and reduced default network suppression cooccur and are dynamically coupled in older adults: the default-executive coupling hypothesis of aging. J Cogn Neurosci. 2015:27(12):2462–2476. 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- Valente A, Laganaro M. Ageing effects on word production processes: an ERP topographic analysis. Language, Cognition and Neuroscience. 2015:30(10):1259–1272. 10.1080/23273798.2015.1059950. [DOI] [Google Scholar]
- Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002:77(4–5):477–482. 10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Verhaegen C, Poncelet M. Changes in naming and semantic abilities with aging from 50 to 90 years. J Int Neuropsychol Soc. 2013:19(2):119–126. 10.1017/S1355617712001178. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and vocabulary score: a meta-analysis. Psychol Aging. 2003:18(2):332–339. 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-III: Wechsler adult intelligence scale. 3rd ed. San Antonio (TX): Psychological Corporation; 1997. [Google Scholar]
- Westlin C, Theriault JE, Katsumi Y, Nieto-Castanon A, Kucyi A, Ruf SF, Brown SM, Pavel M, Erdogmus D, Brooks DH, et al. Improving the study of brain-behavior relationships by revisiting basic assumptions. Trends Cogn Sci. 2023:27(3):246–257. 10.1016/j.tics.2022.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Programming with ggplot2. In: ggplot2: elegant graphics for data analysis. Cham: Springer Nature; 2016, pp. 241–253. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008:29(3):436–451. 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wulff DU, Hills TT, Lachman M, Mata R. The aging lexicon: Differences in the semantic networks of younger and older adults. In: Papafragou A, Grodner D, Mirman D, Trueswell JC, editors. Proceedings of the 38th annual meeting of the cognitive science society. Austin: Cognitive Science Society; 2016, pp. 907–912. [Google Scholar]
- Wulff DU, De Deyne S, Jones MN, Mata R. New perspectives on the aging lexicon. Trends Cogn Sci. 2019:23(8):686–698. 10.1016/j.tics.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Wulff DU, Hills TT, Mata R. Structural differences in the semantic networks of younger and older adults. Sci Rep. 2022:12(1):21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec RF, Markwell SJ, Burkett NR, Larsen DL. A longitudinal study of confrontation naming in the “normal” elderly. J Int Neuropsychol Soc. 2005:11(6):716–726. 10.1017/S1355617705050897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral and ERP data are available at the following link: https://doi.org/10.26037/yareta:ves7bzbscjebhaurlfs7rniscy.