Abstract
Background:
In Egypt, bladder cancer occupies the second rankamong reported cancers in men. Claudins are tight junctions that have a critical role in tumor pathogenesis, invasion, progression, and metastasis and currentlyare a focus of interest for targeting therapies.
Objectives:
We aimed to evaluatethe immunohistochemical expression of Claudin-1 and Claudin-4 in urinary bladder urothelial carcinoma and investigate the relationshipbetweenthe expressed Claudins with differentclinicopathological parameters.
Methods:
Claudin-1 and Claudin-4 immunohistochemical expression was studied in 62 cases of urinary bladder urothelial carcinomas. The cases were classified into two categories; low and high Claudin-1 and Claudin-4 expression.
Results:
High Claudin-1 expression was detected in67.7% of the studied urothelial carcinomas while 32.3% showed low expression. Claudin-1 expression was reduced significantly with high tumor grade, non-papillary tumors, muscle invasion, schistosomal infestation, and perineural invasion (p-value < 0.05). Claudin-4 high expression was detected in 82.3% of our cases while low expression was detected in 17.7%. Claudin-4 reduced expression was significantly associated with non-papillary tumors, muscle invasion, advanced T stages, and associated lympho-vascular emboli (P-value < 0.05).
Conclusion:
According to the results ofthe present study, the reduced expressions of Claudin-1 and Claudin-4 provide clues concerning the progression of urothelial carcinoma. Consequently, it is thought that Claudin-1 and Claudin-4 could help to differentiatelow-grade from high-grade and muscle-invasive from non-muscle-invasive urothelial carcinomas. In addition, it can be introduced as a possible therapeutic target.
Key Words: Urinary bladder, urothelial carcinoma, Claudin-1, Claudin-4, immunohistochemistry.
Introduction
Bladder cancer has become increasingly prevalent worldwide, constituting 3% of all cancer cases and occupying the 12th rank among diagnosed cancers globally [1]. Within the United States, bladder cancer ranks as the sixth most prevalent cancer overall and the third most frequently occurring cancer among men in 2022, following prostate and lung cancers. In Egypt, the urinary bladder ranks second following liver cancer in men [2]. Bladder cancer is three to four times more frequent in men than in women and typically presents in patientsaged 65 to 70 years [3]. It compromises 4.2% of all new cancer diagnoses, with 81,180 new cases diagnosed in 2022 [4].
The main factors that primarily influence the development of urothelial carcinoma are tobacco smoking and exposure to carcinogenic substances in some occupations, including polycyclic aromatic hydrocarbons, nitrosamines, aromatic amines, and arsenic. Additional risk factors include urinary schistosomal infestation, exposure to pelvic radiation therapy, the use of cyclophosphamide, and other factors related to one’s diet and lifestyle [5].
Urothelial carcinomas account for 90% of malignant tumors of the bladder. More than 70% of patients present in an early invasive or non-invasive stage and have a favorable prognosis. However, the high rate of recurrence for these tumors after transurethral resection has become a significant problem. On the other side, tumors with high histologic grade or muscle-invasive stages have significant mortality rates and are treated totally differently from non-invasive ones. Therefore, there is an emerging need for useful variables to help distinguish muscle-invasive from non-muscle-invasive carcinoma of the urinary bladder [6].
An overlap exists between the two oncogenic paths along which urothelial carcinoma develops: the luminal pathway, which results in low-grade papillary carcinoma (about 80% of bladder malignancies), and the basal pathway, which results in high-grade invasive non-papillary carcinoma (about 20% of bladder malignancies) [7].
Several studies have highlighted the important role of tight junctions, particularly claudins, in tumor pathogenesis [8]. In recent years, accumulating evidence has suggested that claudins may play a major role in many aspects of tumorigenesis, such as inflammatory response, tumor growth, progression, epithelial mesenchymal transition (EMT), tumor dissemination, resistance to therapy, and cancer stemness [9, 10] . However, the expression of claudins differs mainly depending on the affected organ and the histologic type of the tumor [11].
Loss of claudin expression induces an inflammatory response and an activation of carcinogenic pathways because the para-cellular barrier and signal transduction activities may be involved in the putative mechanisms by which claudins inhibit carcinogenesis [10].
Claudins can be targeted by multiple therapeutic agents, most notably clostridium perfringens endotoxins (CPE) and monoclonal antibodies (mAbs), due to their recognized role in cancer development and progression and their patterns of expression [12, 13, 10].
Although the histologic grade and stage of urothelial carcinoma are well-established parameters for predicting the tumor prognosis, new investigations to predict the clinical course of cancer have turned attention to non-surgical characteristics, particularly biomarkers [14].
Materials and Methods
Retrieval of Cases
This retrospective cros-sectional analytical study was conducted on 62 formalin-fixed, paraffin-embeddedtissue sections of bladder urothelial carcinoma collected from the archives of the Pathology Department, Faculty of Medicine,Cairo University. The authors obtained the approval of Kasr Alainy Research Ethics Committee (REC) in the Faculty of Medicine, Cairo University (MD-217-2021).
Twenty-six cases were radical cystectomy specimens and thirty-six cases were transurethral resection of tumour specimens (TUR-BT). Inclusion criteria was adequate viable tumor tissue and previous diagnosis of urothelial carcinoma. Exclusion criteria included tumour tissue with wide necrosis. TUR-BT specimens without muscularis propria,andcases with deficient data. The data collected from the pathology reports of these cases included age at the time of diagnosis, sex of patients, tumour site, maximum tumour diameter & multifocality.
Histopathological and ImmunohistochemicalStaining
Three serial sections of 4 microns thick were sliced from each block, one of them was prepared for mounting on a glass slide and was subjected tothe routine Hematoxylin and Eosin (H&E) stain for histological evaluation. The two other sections were mounted on charged slides for immunohistochemical staining. One was for immunohistochemical staining by Claudin-1 antibody. The other is for immunohistochemical staining by Claudin-4 antibody.
The histologic classification and grading of urothelial carcinoma were based on the most recent recommendations of the World Health Organization (WHO classification of urinary tract tumors, fifth edition, 2022) [15].The cases were graded into low and high histologic grades [15, 16]. The extent of tumour invasion (T stage) and lymph nodal involvement (LN stage) were staged according to the American Joint, AJCC 2019 staging of urinary bladder cancer, 8th edition [17, 18]. The presence of lympho-vascular emboli (LVE) and perineural invasion was assessed. Associated schistosomal infestation based on detection of schistosoma ova in tumor tissues was also evaluated.
Immunohistochemical staining using BenchMark XT (Ventana) autostainer for Claudin1 and claudin-4 was done. The slides, were immunostained for Claudin-1 polyclonal antibody Cat.#RB-9209-R7 and Claudin-4 polyclonal antibody Cat.#RB-9043-R7,bothdiluted at 1:100, at room temperature for 30 minutes. Immunodetection was performed, using a labeled streptavidin-biotin (LSAB) system. A skin section with a histologically normal epidermis was used as a positive control for Claudin-1 and a section from colonic mucosa normal epithelium was used as a positive control for Claudin-4 [6, 19]. For negative control of both Claudin-1 and Claudin-4, omitting the primary antibodies was done.
Claudin1 & Claudin4 expression were detected as cytoplasmic and/or membranous brown staining. Immunoreactivity was assessed, based on a combined multiplied score of the percentage of immunostained cellsand the intensity of staining. The staining intensity was evaluated as follows: 0 indicates absent reaction; 1 denotesweak intensity; 2 indicates moderate intensity; 3 signifies strong intensity; and the percentage of positive tumour cells was evaluated as: 0 indicates 0%; 1 meansless than 25 %; 2 denotes 25 to 50%; 3 means more than or equal 51%. The final comprehensive score was calculated by multiplying the intensity and percentage of positive staining scores. Accordingly, it is evaluated as follows: 0 indicated negative results, 1–2 represented weak expression, 3–6 denoted moderate expression, and 9 meant strong expression. Additionally and weak expressions were classified as low, while moderate and strong were considered as high expressions [6, 19].
Statistical Analysis
The entry of data was performed using Microsoft Excel 2013, while data analysis was carried out using the Statistical Package for Social Sciences (SPSS) version 27. Simple descriptive tests, such as arithmetic mean and standard deviation, were performed for age, but frequencies were used for qualitative data. Bivariate correlations were presented in cross-tabulations, and the comparison of proportions was applied using chi-square tests. Statistical significance was assessed by calculating the p-value, and a p-value<0.05 was regarded as statistically significant.
Results
This study included 62 cases of bladder urothelial carcinoma, twenty-six (26) cases were radical cystectomy specimens and thirty-six (36) cases were transurethral resection of tumour specimens (TURT). The age of the patients ranged from 17 to 85 years, with a mean age of 58.27 years ± 10.59 years old. Out of the 62 enrolled patients, 56 were maleswhile 6 were females, with a 9.3:1 male-to-female ratio. The tumorsite was documented in 38 cases only. The commonesttumor site was the lateral wall representing (42.1%) 16/38 cases. The size of the tumor was documented in 26 cases and ranged from 1.2 cm to 9.5 cm; with a mean diameterof 4.48 cm ± 2.18 cm SD. According to the 2022 WHO grading system, 61.3 % were high-grade tumors and 38.7 % were low-grade. Papillary tumors (non invasive and invasive) represented 54.8 % of the cases and non-papillary invasive tumors represented 45.2 %.
Muscle invasive tumors represented 41.9% of the enrolled cases while58.1% were non-muscle invasive. The studied cases were classified as Ta (24.2%), T1 (33.9%), T2 (6.5%), T3 (30.6%) and T4 (4.8%). Lymph node metastases were found in 8 cases (30.8%) .Schistossomal infestation, LVE, and perineural invasion were present in 19.4 %, 30.6%, and 11.3% of the cases respectively. The clinicopathologic characteristics of the studied cases are summarized in Table 1.
Table 1.
The Clinicopathologiccharacteristics of the Enrolled Urothelial Carcinoma Cases
| ClinicopathologicalCharacteristics | NO. (%) |
|---|---|
| Age category | |
| <60 years | 33 (53.2%) |
| ≥60 years | 29 (46.8%) |
| Sex | |
| Male | 56 (90.3%) |
| Female | 6 (9.7%) |
| Tumor location | |
| Bladder dome | 3 (7.9%) |
| Lateral wall | 16 (42.1%) |
| Anterior wall | 4 (10.5%) |
| Posterior wall | 5 (13.2%) |
| Bladder neck | 1 (2.6%) |
| Multifocal | 9 (23.7%) |
| Tumor size | |
| < 4.48 cm | 15 (57.7%) |
| ≥4.48 cm | 11 (42.3%) |
| Tumor grade | |
| High grade | 38 (61.3%) |
| Low grade | 24 (38.7%) |
| Tumor histological type | |
| Papillary | 34 (54.8%) |
| Non-papillary | 28 (45.2%) |
| Muscle invasion | |
| Muscle invasive | 26 (41.9%) |
| Non-muscle invasive | 36 (58.1%) |
| Schistosomal infestation | |
| Present | 12 (19.4%) |
| Absent | 50 (80.6%) |
| Lympho-vascular emboli | |
| Present | 19 (30.6%) |
| Absent | 43 (69.4%) |
| Perineural invasion | |
| Present | 7 (11.3%) |
| Absent | 55 (88.7%) |
| T-stage | |
| Ta | 15 (24.2%) |
| T1 | 21 (33.9%) |
| T2 | 4 (6.5%) |
| T3 | 19 (30.6%) |
| T4 | 3 (4.8%) |
| Lymph node stage | |
| N0 | 18 (69.2%) |
| N1 | 6 (23.1%) |
| N2 | 2 (7.7%) |
According to Claudin-1 expression, forty-two cases (67.7%) showed high Claudin-1 expression, and the remaining 20 cases (32.3%) showed low expression. Most of low-grade cases were high Claudin-1 expression with a statistically significant difference (P-value= 0.008). A significant relationship between histological type and Claudin-1 expression (P-value =0.007) was observed, where most of the studied papillary carcinoma cases tend to show high Claudin-1 expression. Higher Claudin-1 expression was noticed with non-muscle invasive cases with a statistically significant correlation (P-value= 0.047). Claudin-1 expression was reduced significantly with associated schistosomal infestation and associated perineural invasion (P value= 0.031) and (P value=0.019) respectively. Although low Claudin-1 expression was more frequently observed in higher T stages than in lower T stages, the relation between Claudin-1 expression and T stage was statistically insignificant. The correlation of Claudin-1 expression with various clinicopathologic parameters of the enrolled cases is summarized in Table 2.
Table 2.
The Correlation of Claudin-1 Expression with Various Clinicopathologic Parameters of the Enrolled Cases
| Clinico-pathological parameter | Claudin-1 expression | P-Value | ||
|---|---|---|---|---|
| Criteria | NO. (%) | Low expression (%) | High expression (%) | |
| Age category | ||||
| <60 | 33 (53.2%) | 10 (30.3%) | 23 (69.7%) | 0.725 |
| ≥60 | 29 (46.8%) | 10 (34.5%) | 19 (65.5%) | |
| Sex | ||||
| Male | 56 (90.3%) | 20 (35.7%) | 36 (64.3%) | 0.075 |
| Female | 6 (9.7%) | 0 (0%) | 6 (100%) | |
| Tumor location | ||||
| Bladder dome | 3 (7.9%) | 2 (66.7%) | 1 (33.3%) | 0.332 |
| Lateral wall | 16 (42.1%) | 6 (37.5%) | 10 (62.5%) | |
| Anterior wall | 4 (10.5%) | 2 (50 %) | 2 (50%) | |
| Posterior wall | 5 (13.2%) | 2 (40%) | 3 (60%) | |
| Bladder neck | 1 (2.6%) | 1 (100%) | 0 (0%) | |
| Multifocal | 9 (23.7%) | 1 (11.1%) | 8 (88.9%) | |
| Tumor size | ||||
| < 4.48 | 15 (57.7%) | 8 (53.3%) | 7 (46.7%) | 0.391 |
| >4.48 | 11 (42.3%) | 4 (36.4%) | 7 (63.6%) | |
| Tumor grade | ||||
| High grade | 38 (61.3%) | 17 (44.7%) | 21 (55.3%) | 0.008* |
| Low grade | 24 (38.7%) | 3 (12.5%) | 21 (87.5%) | |
| Tumor histological type | ||||
| Papillary | 34 (54.8%) | 6 (17.6%) | 28 (82.4%) | 0.007* |
| Non-papillary | 28 (45.2%) | 14 (50%) | 14 (50%) | |
| Muscle invasion | ||||
| Muscle invasive | 26 (41.9%) | 12 (46.2%) | 14 (53.8%) | 0.047* |
| Non-muscle invasive | 36 (58.1%) | 8 (22.2%) | 28 (77.8%) | |
| Schistosomal infestation | ||||
| Present | 12 (19.4%) | 7 (58.3%) | 5 (41.7%) | 0.031* |
| Absent | 50 (80.6%) | 13 (26%) | 37 (74%) | |
| Lympho-vascular emboli | ||||
| Present | 19 (30.6%) | 9 (47.4%) | 10 (52.6%) | 0.091 |
| Absent | 43 (69.4%) | 11 (25.6%) | 32 (74.4%) | |
| Perineural invasion | ||||
| Present | 7 (11.3%) | 5 (71.4%) | 2 (28.6%) | 0.019* |
| Absent | 55 (88.7%) | 15 (27.3%) | 40 (72.7%) | |
| pT-stage | ||||
| Ta | 15 (24.2%) | 3 (20%) | 12 (80%) | 0.119 |
| T1 | 21 (33.9%) | 5 (23.8%) | 16 (76.2%) | |
| T2 | 4 (6.5%) | 2 (50%) | 2 (50%) | |
| T3 | 19 (30.6%) | 10 (52.6%) | 9 (47.4%) | |
| T4 | 3 (4.8%) | 0 (0%) | 3 (100%) | |
| Lymph node stage | ||||
| N0 | 18 (69.2%) | 8 (44.4%) | 10 (55.6%) | 0.966 |
| N1 | 6 (23.1%) | 3 (50%) | 3 (50%) | |
| N2 | 2 (7.7%) | 1 (50%) | 1 (50%) | |
*Statistically Significant
Regarding Claudin-4 expression, high Claudin-4 expression was noticed in 82.3% of the studied cases. Reduced Claudin-4 expression was found more frequently in high-grade tumors, however, no statistically significant difference was found between Claudin-4 expression and tumor grade. A significant relationship between histological type and claudin-4 expression (P-value =0.007) was observed, where most of the studied papillary carcinoma cases had high Claudin-4 expression. Most of non-muscle-invasive tumors (94.4%) showed high Claudin-4 expression with a statistically significant correlation (P-value= 0.003). Claudin-4 expression was reduced significantly with higher stages and associated LVE (P value= 0.024) and (P value=0.001) respectively. The correlation of Claudin-4 expression with various clinicopathologic parameters of the enrolled cases is summarized in Table 3.
Table 3.
The Correlation of Claudin-4 Expression with Various Clinicopathologic Parameters of the Enrolled Cases
| Clinico-pathological parameter | Claudin-4 expression | P-Value | ||
|---|---|---|---|---|
| Criteria | NO. (%) | Low expression (%) | High expression (%) | |
| Age category | ||||
| <60 | 33 (53.2%) | 7 (21.2%) | 26 (78.8%) | 0.445 |
| ≥60 | 29 (46.8%) | 4 (13.8%) | 25 (86.2%) | |
| Sex | ||||
| Male | 56 (90.3%) | 10 (17.9%) | 46 (82.1%) | 0.942 |
| Female | 6 (9.7%) | 1 (16.7%) | 5 (83.3%) | |
| Tumor location | ||||
| Bladder dome | 3 (7.9%) | 1 (33.3%) | 2 (66.7%) | 0.758 |
| Lateral wall | 16 (42.1%) | 3 (18.8%) | 13 (81.2%) | |
| Anterior wall | 4 (10.5%) | 2 (50 %) | 2 (50%) | |
| Posterior wall | 5 (13.2%) | 2 (40%) | 3 (60%) | |
| Bladder neck | 1 (2.6%) | 0 (0%) | 1 (100%) | |
| Multifocal | 9 (23.7%) | 2 (22.2%) | 7 (77.8%) | |
| Tumor size | ||||
| < 4.48 | 15 (57.7%) | 5 (33.3%) | 10 (66.7%) | 0.873 |
| >4.48 | 11 (42.3%) | 4 (36.4%) | 7 (63.6%) | |
| Tumor grade | ||||
| High grade | 38 (61.3%) | 9 (23.7%) | 29 (76.3%) | 0.123 |
| Low grade | 24 (38.7%) | 2 (8.3%) | 22 (91.7%) | |
| Tumor histological type | ||||
| Papillary | 34 (54.8%) | 2 (5.9%) | 32 (94.1%) | 0.007* |
| Non-papillary | 28 (45.2%) | 9 (32.1%) | 19 (67.9%) | |
| Muscle invasion | ||||
| Muscle invasive | 26 (41.9%) | 9 (34.6%) | 17 (65.4%) | 0.003* |
| Non-muscle invasive | 36 (58.1%) | 2 (5.6%) | 34 (94.4%) | |
| Schistosomal infestation | ||||
| Present | 12 (19.4%) | 2 (16.7%) | 10 (83.3%) | |
| Absent | 50 (80.6%) | 9 (18%) | 41 (82%) | 0.914 |
| Lympho-vascular emboli | ||||
| Present | 19 (30.6%) | 8 (42.1%) | 11 (57.9%) | 0.001* |
| Absent | 43 (69.4%) | 3 (7%) | 40 (93%) | |
| Perineural invasion | ||||
| Present | 7 (11.3%) | 3 (42.9%) | 4 (57.1%) | 0.065 |
| Absent | 55 (88.7%) | 8 (14.5%) | 47 (85.5%) | |
| pT-stage | ||||
| Ta | 15 (24.2%) | 1 (6.7%) | 14 (93.3%) | 0.024* |
| T1 | 21 (33.9%) | 1 (4.8%) | 20 (95.2%) | |
| T2 | 4 (6.5%) | 1 (25%) | 3 (75%) | |
| T3 | 19 (30.6%) | 6 (31.6%) | 13 (68.4%) | |
| T4 | 3 (4.8%) | 2 (66.7%) | 1 (33.3%) | |
| Lymph node stage | ||||
| N0 | 18 (69.2%) | 4 (22.2%) | 14 (77.8%) | 0.06 |
| N1 | 6 (23.1%) | 3 (50%) | 3 (50%) | |
| N2 | 2 (7.7%) | 2 (100%) | 0 (0%) | |
*Statistically Significant
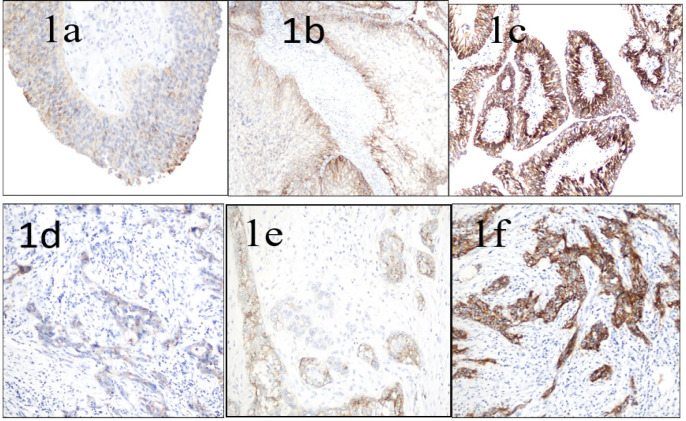
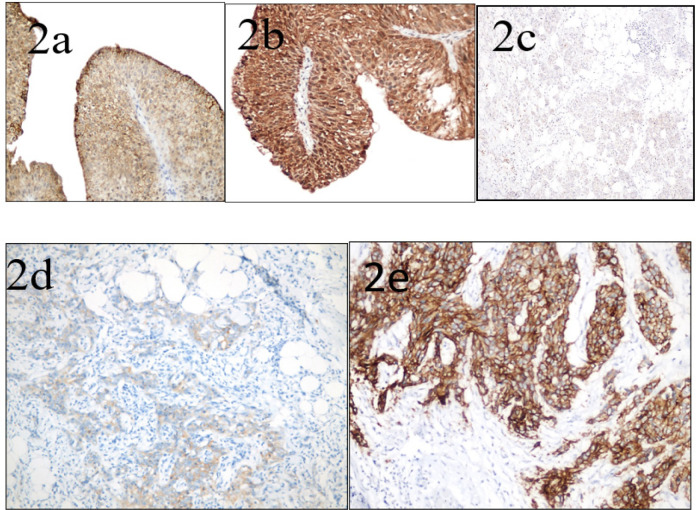
Both Claudin1 and Claudin4 expression were displayed as cytoplasmic and, or membranous brown immunostaining with either variable degrees of intensity as displayed in Figure 1 or Figure 2 respectively.
Figure 1.
Claudin-1 Expression in Urothelial Carcinoma of the Urinary Bladder (a) Low-grade papillary urothelial carcinoma, showing weak membranous and cytoplasmic Claudin-1 immunostaining of 25- 50% of tumor cells, considered as low Claudin-1 expression (score 2) (x400 original magnification). (b) Papillary urothelial carcinoma, low-grade, displaying moderate cytoplasmic and membranous Claudin-1 immunostaining (especially at basal layers) of more than 50% of tumor cells, considered as high Claudin-1 expression (score 6) (x200 original magnification). (c)High-grade papillary urothelial carcinoma, showing strong membranous Claudin-1 immunostaining (especially at basal layers) of more than 50% of tumor cells, considered as high Claudin-1 expression (score 9) (x400 original magnification). (d) High-grade-invasive urothelial carcinoma, showing weak membranous and cytoplasmic Claudin-1 immunostaining of 25-50% of tumor cells, considered as low Claudin-1 expression (score2) (x 400 original magnifications). (e) High-grade invasive urothelial carcinoma, displaying moderate cytoplasmic and membranous Claudin-1 immunostaining of more than 50% of tumor cells, considered as high Claudin-1 expression (score 6) (x400 original magnification). (f) High-grade invasive urothelial carcinoma, showing strong membranous and cytoplasmic Claudin-1 immunostaining of more than 50% of tumor cells, is considered as high Claudin-1 expression (score 9) ( x400 original magnification)
Figure 2.
Claudin-4 Expression of Urothelial Carcinoma of the Urinary Bladder among Studied Cases (a) Low-grade papillary urothelial carcinoma, showing moderate cytoplasmic and membranous Claudin-4 immunostaining of more than 50% of tumor cells, considered as high Claudin-4 expression (score 6) (x400 original magnification). (b) Low-grade papillary urothelial carcinoma, showing strong cytoplasmic and membranous Claudin-4 immunostaining of more than 50% of tumor cells, considered as high Claudin-4 expression (score 9) (x400 original magnification). (c) High-grade invasive urothelial carcinoma infiltrating fat, showing weak cytoplasmic and membranous Claudin-4 immunostaining of 25-50% of tumor cells, considered as low Claudin-4 expression (score 2) (x200 original magnification). (d) High-grade invasive urothelial carcinoma infiltrating fat, showing weak cytoplasmic and membranous Claudin-4 immunostaining of more than 50% of tumor cells, considered as high Claudin-4 expression (score 3 ) (x400 original magnification). (e) High-grade invasive urothelial carcinoma, showing strong cytoplasmic and membranous Claudin-4 immunostaining of more than 50% of tumor cells, considered as high Claudin-4 expression (score 9) (x400 original magnification)
Discussion
Bladder cancer ranks as the 13th most fatal malignancy worldwide, resulting in a loss of about 200,000 individuals in 2018. Regions such as North and East Africa, as well as the Middle East, exhibit the highest mortality rates where there is a high incidence due to schistosomiasis infestation. Notably, Egypt experiences the highest mortality rate, reaching 6.6 deaths per 100,000 individuals [20].
Tight junctions, which are dynamic structures, play a major role in regulating intercellular diffusion and maintaining cellular polarity. These tight junctions are primarily composed of transmembrane components known as Claudins [21]. Additionally, Claudins may have an impact on signaling pathways [22, 21]. The expression patterns of various types of Claudins, both qualitatively and quantitatively, can enhance the distinction between normal and cancerous epithelium. However, it is difficult to predict the specific alterations in Claudin expression in different organs based on the assumption that malignant tumors generally experience a loss of intercellular connections during progression [23]. Some Claudins exhibit decreased expression in tumors [24], while others are overexpressed [25]. Moreover, different expression patterns were stated; as Claudin-1 expression was documented on the basal layer of papillary tumors as well as plasma membrane of tumor cells while Claudin-4 expression was restricted to the tumor cells plasma membrane in papillary and other nodular tumors [26].
In the current study, we detected high Claudin-1 expression in forty-two cases (67.7%), and low expression was noticed in the remaining twenty cases (32.3%). That is near to the percentages in the study done by Saad et al. [19] whichreported high Claudin-1 expression in 60% and low Claudin-1 expression in 40% of their studied cases. Moreover, Abd El-Fattah et al. [14] reported high Claudin-1 expression in 50% of carcinoma cases included in their study.
As regards Claudin-4 expression, fifty-one cases (82.3%) demonstrated high Claudin-4 expression, and eleven cases (17.7%) demonstrated low Claudin-4 expression. In contrary Saad et al. [19] and Abd El-Fattah et al. [14] reported high Claudin-4 expression in 44% and 46.7% and low Claudin-4 in 56% and 53.3% of carcinoma cases included in their studies respectively. The difference observed in these results may be attributed to the difference in the number of studied cases conducted in their studies.
Regarding the tumour histologic grade, a significant inverse correlation was detected between Claudin-1 expression and histological grade (P-value= 0.008), where most of the low-grade cases (87.5%) showed high Claudin-1 expression. Similarly, Törzsök et al. [27] reported significantly lower expression of Claudin-1 in high-grade tumours in comparison to low grade ones (P- value <0.035). In contrast, Saad et al. [19] and Abd El-Fattah et al. [14] found that high Claudin-1 expression was significantly associated with high grade urothelial carcinomas (P-value = 0.009) and (P-value= 0.012) respectively. Also, a study done by Kokenek-Unal, et al. [6] showed that Claudin-1 had significantly lower expression in low grade non-invasive papillary urothelial carcinoma (NPUCs) compared to high grade and invasive papillary urothelial carcinoma (PUCs). In addition, they noted that Claudin-1 expression in non-invasive papillary urothelial carcinomas (NPUCs) was lower than in papillary urothelial neoplasm of low malignant potential (PUNLMPs) (P-value= 0.025).
Săndulescu et al. [28] also documented that the high FSS (final staining score) values of Claudin-1 were more frequent in high-grade urothelial carcinomas, but they didn’t find a statistical difference between them ( P-value= 0.0394). Our study didn’t demonstrate a significant difference between Claudin-4 expression and histological grade (P-value= 0.123). However, most of the low-grade tumors were high Claudin-4 expression. Our findings were consistent with those reported by Boireau et al. [29], Kokenek-Unal et al. [6], Saad et al. [19] and Abd El-Fattah et al. [14] as they observed that Claudin-4 expression decreases with increasing histological grade, with a positive significant correlation (P-value= 0.029), (P-value = 0.003), (P-value <0.001) and (P-value 0.006) respectively.
In contrast, Törzsök et al. [27] showed significantly elevated Claudin-4 expression in high-grade urothelial carcinomas in comparison to low-grad ones (P-value= 0.037). In addition, Székely et al. [30] found overexpressed CLDN4 in PUNLMP and low-grade urothelial carcinoma than in hyperplastic tissue. Also, SĂndulescu et al. [31] suggested that Claudin-4 overexpression was associated with high-grade urothelial carcinomas. The difference observed in these results may be attributed to the different scoring systems.
Maesaka et al. [32] found a correlation between Claudin-4 promoter DNA hypomethylation with subsequent Claudin-4 overexpression and advanced bladder urothelial grade. This result might be different from ours due to different techniques used.
Claudins may play a role in suppressing carcinogenesis through the para-cellular barrier and signal transduction functions, so loss of their expressions and/or alteration in their number or appearance leads to fluid leakage, flux of growth factors, inflammation, and activation of oncogenic pathways [10]. Hence more advanced grade of tumor was supposed to be associated with more destruction and subsequently loss of Claudins.
Regarding tumor histologic type, our study showed a significant relationship between histological type and Claudin-1 expression (P-value =0.007), where most of the studied papillary carcinoma cases tend to show high Claudin-1 expression. In contrast, Saad et al. [19] found most of the higher Claudin-1 expression (82%) were non-papillary tumors, with a positive significant correlation (P-value= 0.009). When evaluating the relationship between Claudin-4 expression and tumor histologic type, there was a significant positive association (P-value = 0.007), where most of the papillary carcinoma cases where high Claudin-4 exprssors (94.1%). The same significant correlation between Claudin-4 immunoreaction and the tumor histologic type was also mentioned by Saad et al. [19] as they noticed high Claudin-4 expression in papillary lesions (P-value= 0.003). As the cellular organization is lost in cancer, not uncommonly a reduction in tight junction function is detectable, consistent with changes in cellular polarity as stated Landers et al. [33] that can explain our finding.
Evaluation of Claudin-1 expression in relation to the state of muscle infiltration in our study showed a significant negative correlation (P-value = 0.047), where higher Claudin-1 expression was noticed with non-muscle invasive cases. On the other hand, Kokenek-Unal et al. [6] and Abd El-Fattah et al. [14] noticed that Claudin-1 significantly had the highest expression in cases that exhibited muscle invasion (P-value= 0.001) and (P-value= 0.000) respectively. Also, Săndulescu et al. [28] reported that high FSS of Claudin-1 was associated with invasive tumors, but no significant difference was found between them (P-value= 0.369).
Comparison of Claudin-4 expression with the state of muscle invasion showed high claudin-4 expression in 94.4% of non-muscle invasive tumors with a statistically significant correlation (P-value =0.003). Our result was consistent with those obtained by Boireau et al. [29], Kokenek-Unal et al. [6] and Abd El-Fattah et al. [14] whose data found an association between high claudin-4 expression and superficial urothelial carcinomas without muscle invasion (P-value=0.000) in the first and third study and (P-value <0.001) in the second study.
However, SĂndulescu et al. [31] reported that Claudin-4 overexpression was associated with muscle invasion, and negative reactions were associated with deep-invasive carcinomas throughout the bladder wall. Their results support the involvement of Claudin-4 in the progression of urothelial carcinomas, both in the non-invasive and tumor invasion phases. Also Maesaka et al. [32] found a correlation between Claudin-4 promoter DNA hypomethylation with subsequent Claudin-4overexpression and invasion of bladder urothelial carcinoma. They found that its overexpression is due to increased levels of non-tight junction CDLN4, which promotes stemness through the activation of integrin β1. These differences can be attributed to the different scoring systems in the first study and the different techniques used in the second one.
Epithelial-mesenchymal transition (EMT) is a recently recognized phenomenon associated with epithelial carcinogenesis. Nowadays it has been proved by molecular studies that two major subtypes of bladder urothelial carcinoma (superficial /papillary and invasive/non-papillary) are two different molecular entities [34]. It was also believed that muscle-invasive tumors develop through the “epithelial-mesenchymal transition” process [35]. Some EMT-inducing transcription factors Snail and Slug were implicated as potential repressors of Claudin-1 expression [36].
In our study, we found that Claudin-1 and Claudin-4 expression was decreased markedly in non-papillary muscle-invasive tumors. We believe that this result can be explained by damage of the structural integrity of claudins during epithelial-mesenchymal transition and their suppression by EMT transcription factors, causing its low expression.
Although high Claudin-1 expression was observed more frequently in early stages (Ta and T1) than in other advanced stages, the relation between Claudin-1expression and tumor invasiveness (T stage) was statistically insignificant (P value= 0.119). However, other studies done by Saad et al. [19]and Abd El-Fattah et al. [14] showed that high Claudin-1 expression was significantly correlated with higher stages ( P-value= 0.03) and (P-value=0.000) respectively.
In our study, Claudin-4 has an inverse statistically significant relationship with T stage of tumor in studied cases (P-value= 0.024), as high Claudin-4expression was detected in 93.3% and 95.2% of Ta and T1 stages respectively. This was found to be parallel with the previous studies done by Boireau et al. [29], Saad et al. [19] and Abd El-Fattah et al. [14] as there was an inverse significant correlation between Claudin-4 expression and the T stage in their studies (P-value< 0.001), (P-value=0.022) and (P-value = 0.000) respectively.
Our findings are consistent with current concepts of the carcinogenesis/invasion process and agree with the role of Claudin-1 and Claudin-4 in enhancing the barrier function of tight junctions and inhibiting cancer cell migration, and invasion. Therefore, their loss aid in the development of metastatic phenotype and hence, progression to more advanced stages.
A study done by Nakanishi et al. [26] on urothelial carcinoma of the upper urinary tract, demonstrated a significantly higher expression of Claudin-1 and claudin-4 in high grade and advanced stages and had a significant impact on the reduction of survival rate, which disagreed our results on both markers. This difference may be attributed to diverse features of the urothelium of the lower and upper urinary tract.
No relationship was found between immunolabeling of cancer cells for (Claudin-1 and Caludin-4) and the presence of lymph node metastasis (P-value= 0.966) and (P-value= 0.060) respectively. Other comparative studies didn’t mention the relation of the N stage to either Claudin-1 or claudin-4 expression in their results.
Claudin-1 expression was negatively correlated with associated schistosomal infestation and presence of perineural invasion in the present study, (P-value= 0.031) and (P-value= 0.019) respectively, where its low expression was associated with the presence of these features. However, these parameters weren’t mentioned in previous comparative studies. Our finding can be clarified as we found most of the studied cases with associated schistosomal infestation and all studied cases showed perineural invasion are high grade tumors and exhibited more advanced stages, so they were subsequently accompanied by reduced Claudin 1-expression.
Although we noticed higher Claudin-1 expression in cases with absent LVE, this relationship was statistically insignificant (P-value= 0.091), in contrast Saad et al. [19]found a positive correlation between high Claudin-1 expression and associated LVE.
Claudin-4 expression was negatively correlated with LVE (P-value = 0.001), this matched with data obtained by Saad et al. [19] who also found a negative significant correlation between Claudin-4 expression and the presence of LVE (P-value < 0.05).
Our results can be explained by, that besides the role of Claudins as tight junctions in urothelial cells, they were also noted to have a role in endothelial cell homeostasis, as it act as a barrier, regulating the passage of molecules, water, and cells. Loss of these junctions will subsequently increase vascular permeability, thus allowing tumour cells to penetrate vessels and metastasize. This finding was first described by Liebner et al. [37]. In tumor microvessels in glioblastoma (GB) and subsequently described in metastatic melanoma cases in a study done by Cohn et al. [38]. So we believe that reduced Claudin-1 and Claudin-4 expression in both malignant urothelial cells and endothelial cells of tumor-associated vessels were accompanied by metastasis of tumor cells and aiding in vessel penetration.
Several explanations can be also suggested for the conflicting results reported in different studies regarding Claudins expression in bladder urothelial carcinoma. The number of analyzed cases, differences in surgical approach (TUR-BT Vs. radical cystectomy), the application of different immune-staining methods, fixation time, antigen preservation, and the variations in scoring systems, either individually or in combination may potentially account for the diverse results.
In conclusion, to sum up, the present study provides clues that reduced expression of Claudin-1 and Claudin-4 are indicative of the progression of urothelial carcinoma. Consequently, it is thought that Claudin-1 and Claudin-4 could help in differentiating low-grade from high-grade and invasive from non-invasive urothelial carcinomas However, because of the diverse results of Claudin-1 and Claudin-4 staining results in several studies regarding urothelial carcinoma, it should be investigated in a larger series.
Author Contribution Statement
All authors contributed efficiently to the study. Aya Magdy Elyamany shared in the study design, data analysis and interpretation of results. Eman Ibrahim Mahmoud shared in data collection, data analysis, interpretation of results and, writing the manuscript. Mostafa Mohamed Salem, shared in the research idea, and revising the manuscript. Rasha Ahmed Khairy shared in the data analysis, interpretation of results and writing the manuscript.
Acknowledgements
Scientific approval
This study was approved by the scientific committee of the pathology department, faculty of medicine, Cairo University.
Conflict of interest
The authors declare there is no conflict of interest
Approval of ethical committee
This study was approved by the Kasr Alainy Research Ethics Committee (REC) with an approval number code: MD-217-2021.
Availability of data
The data is available upon request according to the institution’s guidelines and approval.
References
- 1.Chhikara BS, Parang K. Global cancer statistics 2022: The trends projection analysis. Chem Biol Lett. 2023;10:451. [Google Scholar]
- 2.Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in egypt: Results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu C, Porten S. Epidemiology of Bladder Cancer: Trends and Disparities. In: Bjurlin MA, Matulewicz RS, editors. Comprehensive Diagnostic Approach to Bladder Cancer. Springer Cham; 2021. pp. 1–12. [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Guillaume L, Guy L. Epidemiology of and risk factors for bladder cancer and for urothelial tumor. Rev Prat. 2014;64(10):1372–4, 8-80. [PubMed] [Google Scholar]
- 6.Kokenek-Unal TD CI, Oguz-Erdogan AS, et al. Differential expression of claudin-1, claudin-3, and claudin-4 in bladder lesions. J Cancer Tumor Int. 2015;2:117–27. [Google Scholar]
- 7.Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefker-Radtke A, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine. 2016;12:105–17. doi: 10.1016/j.ebiom.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: An overview. J Oncol. 2010;2010:541957. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowrikumar S, Singh AB, Dhawan P. Role of claudin proteins in regulating cancer stem cells and chemoresistance-potential implication in disease prognosis and therapy. Int J Mol Sci. 2019;21(1) doi: 10.3390/ijms21010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J. Targeting claudins in cancer: Diagnosis, prognosis and therapy. Am J Cancer Res. 2021;11(7):3406–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Fatima Z, Riaz SK, Khan JS, Haq F, Malik MFA. Dysregulated claudin expression significantly effect breast cancer disease progression. J Cancer Res Ther. 2022;18(6):1771–5. doi: 10.4103/jcrt.JCRT_427_20. [DOI] [PubMed] [Google Scholar]
- 12.Cherradi S, Ayrolles-Torro A, Vezzo-Vié N, Gueguinou N, Denis V, Combes E, et al. Antibody targeting of Claudin-1 as a potential colorectal cancer therapy. J Exp Clin Cancer Res. 2017;36(1):89. doi: 10.1186/s13046-017-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichner M, Augustin C, Fromm A, Piontek A, Walther W, Bücker R, et al. In colon epithelia, clostridium perfringens enterotoxin causes focal leaks by targeting claudins which are apically accessible due to tight junction derangement. J Infect Dis. 2017;217(1):147–57. doi: 10.1093/infdis/jix485. [DOI] [PubMed] [Google Scholar]
- 14.El-Fattah GAA, Said EM, Roshdy RG. Evaluation of the role of tight junction molecules: Claudin-1 and claudin-4 in urothelial neoplasms. Egypt J Pathol. 2021;41(1):34–40. [Google Scholar]
- 15.Moch H, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 world health organization classification of tumours of the urinary system and male genital organs-part a: Renal, penile, and testicular tumours. Eur Urol. 2022;82(5):458–68. doi: 10.1016/j.eururo.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty SK, Lobo A, Cheng L. The 2022 revision of the world health organization classification of tumors of the urinary system and male genital organs: Advances and challenges. Hum Pathol. 2023;136:123–43. doi: 10.1016/j.humpath.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Magers MJ, Lopez-Beltran A, Montironi R, Williamson SR, Kaimakliotis HZ, Cheng L. Staging of bladder cancer. Histopathology. 2019;74(1):112–34. doi: 10.1111/his.13734. [DOI] [PubMed] [Google Scholar]
- 18.American Cancer Society. American Cancer Society Facts & Figures. Atlanta: 2019. [Google Scholar]
- 19.Saad E, Ibrahim K, Emaraa N, El-Sebaaie A, omar E, youssef S. Role of her2 and claudins in subtypes of urothelial carcinoma identified bygata3 and cytokeratin5\6 immunohistochemical study. Benha Med J. 2021;38(Academic issue):128–46. [Google Scholar]
- 20.Fong MHY, Feng M, McConkey DJ, Choi W. Update on bladder cancer molecular subtypes. Transl Androl Urol. 2020;9(6):2881–9. doi: 10.21037/tau-2019-mibc-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10(8):235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788(4):761–7. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788(4):872–91. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, et al. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179(2):123–33. doi: 10.1164/rccm.200803-456OC. [DOI] [PubMed] [Google Scholar]
- 25.Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, McBryan J, et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 2009;124(9):2088–97. doi: 10.1002/ijc.24159. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K, Ogata S, Hiroi S, Tominaga S, Aida S, Kawai T. Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am J Clin Pathol. 2008;130(1):43–9. doi: 10.1309/U77A6BTEXVCA5D0E. [DOI] [PubMed] [Google Scholar]
- 27.Törzsök P, Riesz P, Kenessey I, Székely E, Somorácz A, Nyirády P, et al. Claudins and ki-67: Potential markers to differentiate low- and high-grade transitional cell carcinomas of the urinary bladder. J Histochem Cytochem. 2011;59(11):1022–30. doi: 10.1369/0022155411424606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Săndulescu A, Stepan AE, Mărgăritescu C, Enăchescu V, Mitroi G, Simionescu CE. The role of cell adhesion molecules in the progression of bladder urothelial carcinomas. Rom J Morphol Embryol. 2022;63(1):145–51. doi: 10.47162/RJME.63.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boireau S, Buchert M, Samuel MS, Pannequin J, Ryan JL, Choquet A, et al. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis. 2007;28(2):246–58. doi: 10.1093/carcin/bgl120. [DOI] [PubMed] [Google Scholar]
- 30.Székely E, Törzsök P, Riesz P, Korompay A, Fintha A, Székely T, et al. Expression of claudins and their prognostic significance in noninvasive urothelial neoplasms of the human urinary bladder. J Histochem Cytochem. 2011;59(10):932–41. doi: 10.1369/0022155411418829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SĂndulescu A, Stepan AE, MĂrgĂritescu C, Badiu AM, Matei M, Simionescu CE. Claudin-4 immunoexpression in urothelial carcinomas. Curr Health Sci J. 2020;46(4):379–82. doi: 10.12865/CHSJ.46.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maesaka F, Kuwada M, Horii S, Kishi S, Fujiwara-Tani R, Mori S, et al. Hypomethylation of cldn4 gene promoter is associated with malignant phenotype in urinary bladder cancer. Int J Mol Sci. 2022;23(12) doi: 10.3390/ijms23126516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landers KA, Samaratunga H, Teng L, Buck M, Burger MJ, Scells B, et al. Identification of claudin-4 as a marker highly overexpressed in both primary and metastatic prostate cancer. Br J Cancer. 2008;99(3):491–501. doi: 10.1038/sj.bjc.6604486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baydar DE. Molecular Pathology. In: ZhouH D, Guo CC, Ro JY, editors. Urinary Bladder Pathology. Switzerland: Springer Nature; 2021. p. 175. [Google Scholar]
- 35.McConkey DJ, Lee S, Choi W, Tran M, Majewski T, Lee S, et al. Molecular genetics of bladder cancer: Emerging mechanisms of tumor initiation and progression. Urol Oncol. 2010;28(4):429–40. doi: 10.1016/j.urolonc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez-Estrada OM, Cullerés A, Soriano FX, Peinado H, Bolós V, Martínez FO, et al. The transcription factors slug and snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394(Pt 2):449–57. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 38.Cohn ML, Goncharuk VN, Diwan AH, Zhang PS, Shen SS, Prieto VG. Loss of claudin-1 expression in tumor-associated vessels correlates with acquisition of metastatic phenotype in melanocytic neoplasms. J Cutan Pathol. 2005;32(8):533–6. doi: 10.1111/j.0303-6987.2005.00324.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available upon request according to the institution’s guidelines and approval.