Abstract
Objective:
HR-HPV types 16 and 18 are responsible for pre-invasive and invasive lesions of the cervix, accounting for 70-80% of the total subtypes. The aim of this study was to investigate the prevalence of high-risk HPV subtypes 16 and 18 in self-collected vaginal samples using real-time micro-PCR and to study the acceptability of self-sampling.
Methods:
Eligible women (30-65 years) were screened from a semi-urban area of Uttarakhand (India) using self-sampling. High-risk HPV genotypes (16/31 and 18/45) were tested using real-time micro-PCR technique with results available in one hour. The positive results were validated by standard RT-PCR for high-risk HPV 16, 18, separately and for 12 other high-risk genotypes, combined. Ease of the procedure, level of comfort, and recommendation to other women were studied and the acceptability of self-sampling was analyzed using the Likert scale.
Result:
Of 975 eligible women screened, 45 participants tested positive for HR-HPV (16/31,18/45) using real-time micro-PCR with a prevalence of 4.6%. Positive samples were further tested through routine RT-PCR and 60% were found to be HR-HPV 16 and 18 positive. For self-sampling, 96.72% (n=943) participants were ‘very satisfied’ and 94.15% (n=918) found self-sampling to be ‘very comfortable’ and 88.51% (n=863) stated that they will strongly recommend this test to other eligible women in the community.
Conclusion:
We conclude that HR-HPV testing with limited genotyping showed a prevalence of 4.6%, 60% of these were HPV 16/18 positive. Point of care testing was feasible in the community and self-sampling was acceptable.
Key Words: Cervical cancer screening, HPV, micro PCR, women’s health, community screening
Introduction
Cervical cancer is the fourth most common cancer worldwide with an estimated 604,000 new cases and 342,000 deaths. In India, it is the second most frequently diagnosed cancer with an estimated 79,103 new cases in 2022 and 85,241 cases projected for 2025 [1]. Preinvasive and invasive cervical cancer has well defined pathological criteria and is caused by persistent infection with carcinogenic types of Human Papillomavirus (HPV) [2]. High-risk HPV types (HPV 16 and 18) are mostly responsible for pre-invasive and invasive lesions of the cervix, accounting for 70-80% of the total subtypes [2, 3, 4]. Despite well-organized screening programs, cervical cancer is still missed because of high false negative rates of cytology (29%). The high number of false negative results could be credited to errors during sampling, preparation, and interpretation [4]. Hence, in recent years research efforts have focused on the detection of HPV DNA as an alternative method of screening for cervical cancer precursors. Several tests have been used for the detection of HR-HPV, namely hybrid capture 2 (Qiagen), Cobas 4800, mRNA (E6, E7) (Aptima), and real-time PCR for HR-HPV DNA [5]. These tests are expensive and need a laboratory infrastructure and have a high turnaround time. Thus, there is a need for affordable, easy-do, and ‘point-of-care’ tests where the results are available within hours. HR-HPV real-time micro-PCR system allows ‘point-of-care’ and rapid testing of HR-HPV types. Truenat HR-HPV has been validated using Hybrid Capture 2 (HC2) which is US Food and Drug Administration (FDA)-approved reference standard. The test uses an internal positive control to validate the run conditions. The sensitivity and specificity were found to be 97.7% and 98.9% respectively with a false negative rate of 6.2%. The sensitivity may further increase on adding 4 additional prevalent types (ie, 33, 35, 58, and 59) [3]. Self-sampling for HPV is physician-independent, and patient-centred. For HR-HPV DNA testing, self-collected vaginal samples are an acceptable strategy and have shown comparable sensitivity and specificity to clinician-collected samples [6].
We present the results of the study aimed to determine the prevalence of high-risk HPV (HPV 16 and 18) using real-time micro-PCR test in a self-collected vaginal sample and to study the acceptability of self-sampling in the community.
Materials and Methods
Study site and population
This cross-sectional study was carried out in a semi-urban community of Uttarakhand (India). Sexually active or ever-married women between 30-65 years of age were included in the study. The exclusion criteria included pregnant women and women with a history of conization or hysterectomy. The study was approved by the institutional ethical committee (IEC number: EC/NEW/Inst/2020/1046) and informed consent was obtained from all subjects. The trial was registered with the Central Trials Registry of India (CTRI Reg No: CTRI/2021/06/033996).
A sample size of 975 women was calculated using the prevalence of HPV infection according to the largest Indian study with 95% confidence, 20% relative precision, and adjusting for a 10% non-responder rate [4]. A total of 975 eligible women were enrolled in the study.
Recruitment
Meetings were conducted with various stakeholders namely village sarpanch (head), community health workers, female representatives of the community, and medical officers in charge of cantonment health centers. They were briefed about the burden of cervical cancer, its severity, and that it can be prevented by HPV vaccination and screening. Informed written consent was obtained from eligible women. Initially, sample collection was done by house-to-house visits because of the COVID-19 pandemic. Later, every Friday eligible women who visited the health centre for other health issues and COVID vaccination were counselled and recruited for the self-sampling. Four awareness camps were also conducted in collaboration with local sarpanch and medical officers of the local cantonment.
Sample collection
The participants were provided with a sterile vaginal sampling stick with a brush and were asked to insert it through the vaginal introitus till resistance was felt and rotated 2-3 times in a clockwise direction to collect the vaginal sample. After withdrawal, the tip was transferred to VLM (Viral Lysis Media) vial.
Sample processing
The sample collected in Viral Lysis Media (VLM) was analyzed for HPV 16/31,18/45 using a micro-PCR analyzer. The collected sample was processed in the DNA extractor (20 minutes).
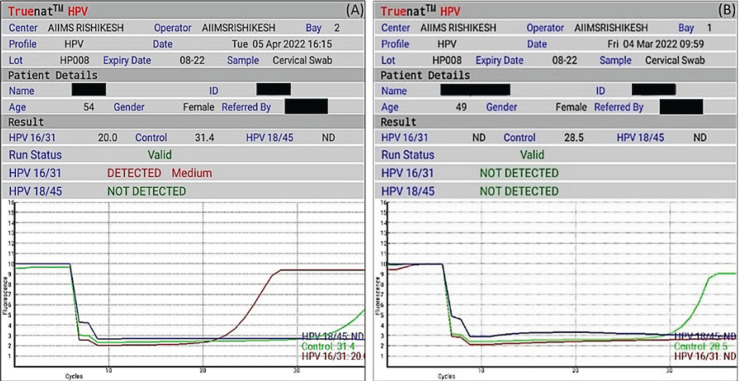
HPV-HR (micro-PCR) detected 4 HR subtypes of HPV (16/18/31/45). It differentiated the sample as HPV 16/31 and/or HPV 18/45 or negative for 16/31, 18/45. The test was initiated by selecting the profile name, entering sample details, and loading the master mix. Elute (6 µL) collected in the previous step was added to a microtube containing lyophilized PCR master mix; subsequently, the reconstituted solution was transferred to the chip well. During thermal cycling, fluorescent signals from three wavelengths were captured by the optoelectronic system, and data was visualized as a graph on the graph-user interface of the device. Results were auto-interpreted by the system and visualized as a simple readout form (Figure 1). A positive result was indicated by amplification in the either fluorescent channel for 16/31 or 18/45 or both (indicating mixed infection). Results were displayed within 40 minutes as “not detected” if only the internal positive control showed amplification and both the 16/31 and 18/45 channels did not show amplification. When there was no amplification in target channels and an absence of or shift of the internal positive control cycle threshold beyond a pre-set, the run was considered invalid.
Figure 1.
Results Generated at the End of the Test. (A) Sample positive for HPV 16/31. (B) HPV 16/31 & 18/45 not detected
All samples tested positive [HPV 16/31, 18/45] were further tested using the standard RT- PCR technique on Biorad CFX 96 platform using HPV-Q Real-time PCR kit (genes2me). Positivity of HPV 16, 18 separately, and other HR-HPV types (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) were reported by RT-PCR. It is a multiplex real-time reverse-transcription PCR system, containing specific primers and fluorescent probes targeting the sequence of HPV. Using probes linked to distinguishable dyes enables the parallel detection of HPV-specific DNA as well as the detection of the internal control in corresponding detector channels of the real-time PCR instrument.
The result of the HPV test was generated within 1-2 hours and conveyed to the participant. Women with a negative report were asked to follow up after 5 years while those who tested positive were called to the tertiary care center for cervical cytology with Pap Test and colposcopy and were managed accordingly.
Post-sample collection, satisfaction, and comfort with the self-sampling method were recorded using a 10-point Likert scale. A score of 9-10 was categorized as very satisfied, 7-8 as moderately satisfied, 5-6 as neither satisfied nor dissatisfied, 3-4 as moderately dissatisfied, and 1-2 as very dissatisfied. Similarly, for the level of comfort a score of 9-10 was categorized as very comfortable, 7-8 as moderately comfortable 5-6 as neither comfortable nor uncomfortable, 3-4 as moderate discomfort, and 2-1 as very uncomfortable. Participants were asked whether they would recommend this test to others or not, and the answer was recorded as yes or no.
Statistical Analysis
Categorical variables were described as frequency and proportion. Continuous variables were described as mean ± standard deviation or median with interquartile range as applicable. Proportions were compared using the Chi-square test/ Fisher’s exact test. The means were compared using the students ‘t-test and Mann-Whitney U test as applicable. ANOVA was used to compare means in more than 2 groups. The univariate analysis was followed by multivariate analysis to document risk factors for HPV infection.
Results
A total of 975 eligible women between 30 to 65 years of age were recruited from the community for cervical cancer screening with HPV test using self-sampling. None of the eligible cases declined self-sampling for HPV. The prevalence of HR-HPV (16/31, 18/45) subtypes was found to be 4.6% (n=45) among which 60% were HPV 16 and 18 subtypes.
The mean age of the women in the present study was 39.89± 9.27 years. The majority (61.9%) of women belonged to the age group 30-40 years. The mean age of women at first sexual intercourse was higher in women who screened negative compared to the screen-positive women (20.25±3.49 versus 18.87±3.47). The mean age of women at first childbirth was significantly higher in women who screened negative (22.45±3.55 vs 22.28±3.73 years; P=0.020) years. However, other baseline parameters like parity, education, and socio-economic status were comparable between the women screened positive and negative (Table 1).
Table 1.
Baseline Characteristics of Study Population and Comparison between HPV Screen Positive and Negative Cases
| Demography variables | Total women screened (n=975) |
Screen positive (n=45) |
Screen negative (n=930) |
P-value |
| Age (mean±SD) | 39.89 ± 9.27 | 42.2 ± 9.03 | 39.75 ± 9.16 | 0.009 |
| Age at first sexual intercourse (mean±SD) | 20.11 ± 3.593 | 18.87 ± 3.47 | 20.25 ± 3.494 | 0.01 |
| Age at first childbirth (mean±SD) | 22.28 ± 3.728 | 21.34 ± 2.964 | 22.45 ± 3.551 | 0.02 |
| Parity (median) | 2 | 2.5 | 2 | - |
| Formal Education n(%) | 527 (54.05) | 27 (60) | 500 (53.76) | 0.23 |
| Primary school | 210 (21.54) | 06 (13.33) | 204 (21.94) | |
| High school | 151 (15.49) | 10 (22.22) | 141 (15.16) | |
| Graduate | 87 (8.92%) | 02 (4.44) | 85 (9.14) | |
| Post graduate | ||||
| Socio-economic status* n(%) | ||||
| Upper class | 12 (1.2) | 00 (0.0) | 12 (2.8) | |
| Upper middle class | 18 (1.8) | 00 (0.0) | 18 (49.7) | 0.5 |
| Middle class | 375 (38.5) | 03 (6.7) | 372 (40.0) | |
| Lower middle class | 428 (43.9) | 18 (40.0) | 410 (44.1) | |
| Lower class | 142 (14.6) | 24 (53.3) | 118 (12.7) |
*Modified BG Prasad socio-economic scale
Among 45 screen-positive samples by real-time micro-PCR, 64.4% (n=29) were positive for HPV 16/31, 31.1% (n=14) were positive for HPV 18/45 and 4.4%(n=02) were positive for both HPV 16/31 and HPV 18/45.
On further evaluation of positive samples with RT-PCR, 27 out of 45 (60%) were either HPV 16 (n=20), 18 (n=06), or both (n=1) positive, while other high-risk HPV types (n=16) comprised 35.6% of HR-HPV positives. Two were found to be negative out of 45 tested.
Most of the participants were very satisfied with the self-sampling method based on the 10-point Likert scale. The mean satisfaction score in the present study was 9.84±0.63. 96.7% (n=943) were ‘very satisfied’ with the self-sampling method. The mean score for comfort (10-point Likert scale) was 9.79±0.73 and 94.2% of participants (n=918) found self-sampling to be very comfortable. Most participants (88.5%, n=863) responded that they will strongly recommend it to other eligible women. Overall acceptability of the self-sampling method was found to be 93.13% in terms of satisfaction, comfort, and recommendation to others (Table 2).
Table 2.
Level of Satisfaction, Comfort, and Recommendation for Self-Sampling
| Satisfaction level with self-sampling |
n(%) | Comfort level with self-sampling |
n(%) | Recommendation of self-sampling method to others | n(%) |
|---|---|---|---|---|---|
| Very satisfied | 943 (96.7) | Very comfortable | 918 (94.2) | Yes | 863 (88.5) |
| Moderately satisfied | 25 (2.6) | Moderately comfortable | 41 (4.2) | No | 112 (11.5) |
| Neither satisfied nor dissatisfied |
04 (0.4) | Neither comfortable nor discomfort | 11 (1.1) | ||
| Moderately dissatisfied | 01 (0.1) | Moderate discomfort | 03 (0.3) | ||
| Very dissatisfied | 2 (0.2) | Very uncomfortable | 2 (0.2) |
Follow up
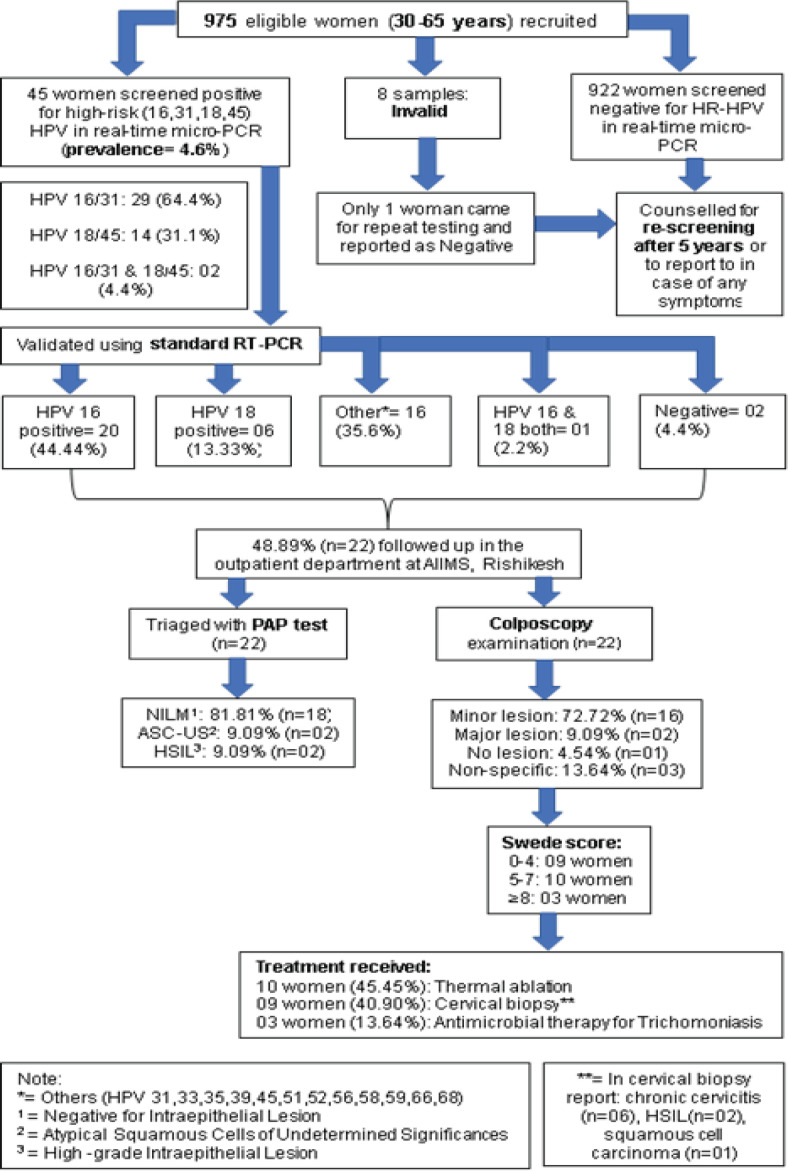
Among 45 women who were screened positive for HR-HPV, 48.9% (n=22) reported for Papanicolaou (PAP) test and colposcopy. PAP test was reported as NILM/ inflammatory in 18 women i.e., 81.8%, ASCUS in two women (9.1%), and HSIL in two women (9.1%). Further, a colposcopy examination was done. Of these 22 women, two women had major lesions (dense aceto-whitening with cuffed glands openings), 16 women had minor lesions (thin aceto-whitening, irregular geographic border), one woman had no lesion on colposcopy and three women had non-specific lesions. 45.5% (n=10) women had a Swede score between 0-4, 40.9% (n=09) women had a score between 5-7, while 13.64% (n=03) women had a score of 8. With these findings, 10 women were treated using thermal ablation, nine women underwent cervical biopsy, and three women received antimicrobial therapy for trichomonas infection. One woman (HPV 16/31 on micro-PCR) was diagnosed with non-keratinizing squamous cell carcinoma on cervical biopsy and received chemoradiation, two women had a high-grade intraepithelial lesion (HPV 16/31 on micro-PCR) and were managed with LEEP, six women had chronic cervicitis with no evidence of malignancy (Figure 2).
Figure 2.
Study Flow Diagram
Discussion
Of the 975 women recruited in the study, 45 (4.6%) tested positive for the presence of high-risk HPV DNA in the self-collected vaginal samples. The overall global burden of HPV infection assessed using data from 194 studies, including testing of one million women using PCR or HC2 tests for HPV infection, indicated the global prevalence of HPV infection to be 11-12%. The highest prevalence was seen in sub-Saharan Africa (24%), Eastern Europe (21%), and Latin America (16%), while rates in North Africa and Western Asia were found to be 9% and 2%, respectively [7].
In a cohort study conducted in the United States, it was found that women who tested positive for HPV16 were at the highest risk for developing Cervical Intraepithelial Neoplasia grade 3 or worse (CIN3+), followed by those who tested positive for HPV18. This finding was echoed in a longitudinal study by Kitamura (2021) [8] in Japan and a large clinical trial known as ATHENA (Addressing the Need for Advanced HPV Diagnosis). A study from Norway also found that the risks of CIN3+ were higher for HPV16/18 compared to other high-risk HPV genotypes. Among women with any cytologic abnormality [atypical squamous cells of undetermined significance or worse], the immediate risks were 57.8% (95%CI = 53.0–62.6%) for HPV16, 40.2% (95%CI = 32.3–49.2%) for HPV18, and 31.4% (95%CI = 28.7–34.3%) for other high-risk HPV [9].
Various Indian studies have shown a prevalence between 12.7 and 3%. In a cross-sectional study, Hariprasad et al. [3] showed the prevalence of HR-HPV to be 12.7% by HC2 and 8% by micro-PCR Truenat testing and reported the sensitivity and specificity of Truenat HPV-HR to be 97.7% and 98.9%, respectively [3]. Similarly, in another study by Mittal (2016) a prevalence of 4.7% was noted in demonstration projects in primary care settings using the HC2 test. Another community-based study using self-sampling by Peedicayil (2016) [10] found the prevalence to be 5.9% in which HPV testing by PCR and genotyping of 15 HR-HPV subtypes using the line blot assay was done. In the study done by Labani (2014) [11] using the careHPV test, the prevalence was lower at 3% in a rural population in north India. The prevalence rate in our study was comparable to various Indian studies and was an effort to study the prevalence rate from a semi-urban population in the state of Uttarakhand which was not studied earlier. An attempt was made to study a point-of-care test and implement the method of self-sampling for cervical cancer screening during the pandemic when screening at health facilities came to a standstill.
Due to the physical and psychological discomfort associated with pelvic examination associated with physician-collected samples, self-sampling may be an acceptable alternative, which is also supported by WHO recommendations on self-care interventions. Furthermore, Indian women continue to be hesitant and shy about undergoing a pelvic exam culturally [12]. Self-sampling has provided a good opportunity to screen a large population of women in India. Yeh (2019) [13] in a systematic review and meta-analysis reported that HPV self-sampling could increase cervical cancer screening uptake compared with standard of care, with a marginal effect on linkage to clinical assessment/treatment. The present study reported a high acceptability of self-sampling and there was no participant refusal for self-sampling which was probably due to effective counselling and the semi-urban study population was a part of institute’s area of care. Poli (2020) [14] tested 4,643 self-collected vaginal samples with the careHPV test (Qiagen) of which 6.4% (n=297) were positive for HR-HPV infection. Screen positivity among women aged 30-59 years ranged between 5.6% and 6.8%. We found that a simple point-of-care HPV test may be used for community cervical cancer screening with high acceptability similar to that reported by Poli (2020) [14].
Compliance with follow-up in our study was approximately 48.9%, which was lower compared to a study by Mittal (2016) [15] who reported a follow-up rate of 80.1% when screening was implemented in primary healthcare centers. This situation can be avoided by implementing a screen-and-treat policy in future implementation programs [16-18].
To our knowledge, it was one of the few studies on cervical cancer screening utilizing a self-sampling and point-of -care approach for HPV testing in a semi-urban area of Uttarakhand. The study had an adequate sample size. One of the major strengths of the study was that the micro-PCR-positive samples were validated with standard RT-PCR. A few limitations of the study were that only four strains of HR-HPV were tested and all 975 samples were not validated with standard PCR tests due to which sensitivity, specificity and false negative rate of the micro-PCR test could not be studied. Point-of-care testing was feasible in the community and self-sampling was acceptable. In conclusion, this study adds to the existing literature on point-of-care testing and self-sampling from a low-resource setting.
Author Contribution Statement
Shilpa Panta and Shalini Rajaram planned the study design. Shilpa Panta collected clinical and follow up data. Ayush Heda and Shilpa Panta wrote the initial draft of the manuscript with input of all authors. Ajeet Singh Bhadoria helped in data analysis. Deepjyoti Kalita was involved with the technical aspects of methodology and HPV DNA testing. Latika Chawla and Jaya Chaturvedi provided feedback and helped shape the research. All authors were actively involved at all stages of the study.
Acknowledgements
General
We thank Prof. Vartika Saxena, Head of the Department of Community and Family Medicine for her valuable support and advise through the study. We extend our heartfelt appreciation to Dr Himani, Dr Varsha Kamath, Dr Nivetha R and Dr Ravi Kajal for their invaluable efforts in case recruitment and meticulous data collection.
Funding statement
Micro-PCR and RT-PCR kits were provided by Molbio Diagnostics Pvt. Ltd.
Approval
The study was approved by the institutional ethical committee of All India Institute of Medical Sciences, Rishikesh (IEC number: EC/NEW/Inst/2020/1046) as student thesis
Conflict of Interest
None
Data Availability
Data generated by the authors available on request
Study Registration
Central Trials Registry of India (CTRI Reg No: CTRI/2021/06/033996)
References
- 1.Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P. Cancer incidence estimates for 2022 & projection for 2025: Result from national cancer registry programme, india. Indian J Med Res. 2022;156(4-5):598–607. doi: 10.4103/ijmr.ijmr_1821_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124(3):516–20. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariprasad R, Tulsyan S, Babu R, Dhanasekaran K, Thakur N, Hussain S, et al. Evaluation of a chip-based, point-of-care, portable, real-time micro pcr analyzer for the detection of high-risk human papillomavirus in uterine cervix in india. JCO Glob Oncol. 2020;6:1147–54. doi: 10.1200/GO.20.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. Hpv screening for cervical cancer in rural india. N Engl J Med. 2009;360(14):1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Simon M, Peeters E, Xu L, Meijer C, Berkhof J, et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infect. 2021;27(8):1083–95. doi: 10.1016/j.cmi.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Adsul P, Srinivas V, Gowda S, Nayaka S, Pramathesh R, Chandrappa K, et al. A community-based, cross-sectional study of hrhpv DNA self-sampling-based cervical cancer screening in rural karnataka, india. Int J Gynaecol Obstet. 2019;146(2):170–6. doi: 10.1002/ijgo.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30 Suppl 5:F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura T, Suzuki M, Shigehara K, Fukuda K. Prevalence and risk factors of human papillomavirus infection among japanese female people: A nationwide epidemiological survey by self-sampling. Asian Pac J Cancer Prev. 2021;22(6):1843–9. doi: 10.31557/APJCP.2021.22.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashim D, Engesæter B, Baadstrand Skare G, Castle PE, Bjørge T, Tropé A, et al. Real-world data on cervical cancer risk stratification by cytology and hpv genotype to inform the management of hpv-positive women in routine cervical screening. Br J Cancer. 2020;122(11):1715–23. doi: 10.1038/s41416-020-0790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peedicayil A, Abraham P, Prasad J, Jeyaseelan L, Abraham S, Kurian S, et al. Community prevalence of human papillomavirus by self-collected samples in south india. Indian J Gynecol Oncol. 2016;14(1):16 . [Google Scholar]
- 11.Labani S, Asthana S, Sodhani P, Gupta S, Bhambhani S, Pooja B, et al. Carehpv cervical cancer screening demonstration in a rural population of north india. Eur J Obstet Gynecol Reprod Biol. 2014;176:75–9. doi: 10.1016/j.ejogrb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Madhivanan P, Nishimura H, Ravi K, Pope B, Coudray M, Arun A, et al. Acceptability and concordance of self- versus clinician- sampling for hpv testing among rural south indian women. Asian Pac J Cancer Prev. 2021;22(3):971–6. doi: 10.31557/APJCP.2021.22.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (hpv) testing: A systematic review and meta-analysis. BMJ Glob Health. 2019;4(3):e001351. doi: 10.1136/bmjgh-2018-001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli UR, Muwonge R, Bhoopal T, Lucas E, Basu P. Feasibility, acceptability, and efficacy of a community health worker-driven approach to screen hard-to-reach periurban women using self-sampled hpv detection test in india. JCO Glob Oncol. 2020;6:658–66. doi: 10.1200/GO.20.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal S, Mandal R, Banerjee D, Das P, Ghosh I, Panda C, et al. Hpv detection-based cervical cancer screening program in low-resource setting: Lessons learnt from a community-based demonstration project in india. Cancer Causes Control. 2016;27(3):351–8. doi: 10.1007/s10552-015-0708-z. [DOI] [PubMed] [Google Scholar]
- 16.Joshi S, Kulkarni V, Darak T, Mahajan U, Srivastava Y, Gupta S, et al. Cervical cancer screening and treatment of cervical intraepithelial neoplasia in female sex workers using “screen and treat” approach. Int J Womens Health. 2015;7:477–83. doi: 10.2147/IJWH.S80624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahnaz S, Hira HM, Begum KN, Akhter R, Sharmin S. “Screen and treat” approach among via positive women during cervical cancer screening program: Experience at low resource setting. Mymensingh Med J. 2021;30(4):1100–6. [PubMed] [Google Scholar]
- 18.Toliman PJ, Kaldor JM, Tabrizi SN, Vallely AJ. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric. 2018;21(3):235–8. doi: 10.1080/13697137.2018.1439917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated by the authors available on request