Abstract
OBJECTIVE:
To investigate the effect of acupotomy, on mitophagy and the Pink1-Parkin pathway in chondrocytes from rabbits with knee osteoarthritis (KOA).
METHODS:
A KOA model was established via the modified Videman method. Rabbits were randomly divided into a control group (CON), KOA group and KOA + acupotomy group (Acu). Rabbits in the acupotomy group were subjected to acupotomy for 4 weeks after model establishment. The behavior of the rabbits before and after intervention was recorded. Cartilage degeneration was evaluated by optical microscopy and fluorescence microscopy. The level of mitophagy was evaluated by transmission electron microscopy, immunofluorescence and enzyme-linked immunosorbent assay (ELISA). The expression of phosphatase and tensin homolog (PTEN)-induced kinase 1 (Pink1)-Parkin mitophagy pathway components was evaluated by immunofluorescence, Western blotting and real-time polymerase chain reaction.
RESULTS:
In rabbits with KOA, joint pain, mobility disorders and cartilage degeneration were observed, the Mankin score was increased, collagen type Ⅱ (Col-Ⅱ) expression was significantly decreased, mitophagy was inhibited, mitochondrial function was impaired, and factors associated with the Pink1-Parkin pathway were inhibited. Acupotomy regulated the expression of Pink1-Parkin pathway-related proteins, the mitophagy-related protein microtubule-associated protein-1 light chain-3, the translocase of the outer membrane, and the inner mitochondrial membrane 23; increased the colocalization of mitochondria and autophagosomes; promoted the removal of damaged mitochondria; restored mitochondrial adenosine-triphosphate (ATP) production; and alleviated cartilage degeneration in rabbits with KOA.
CONCLUSIONS:
Acupotomy played a role in alleviating KOA in rabbits by activating mitophagy in chondrocytes via the regulation of proteins that are related to the Pink1-Parkin pathway.
Keywords: acupuncture therapy; osteoarthritis, knee; mitophagy; PTEN phosphohydrolase; cartilage
1. INTRODUCTION
Osteoarthritis (OA) is a chronic joint disease that is highly prevalent worldwide and can cause joint pain, impaired mobility and even disability. The 2019 Global Burden of Disease Study revealed that between 1990 and 2022, the number of OA patients increased from 248 million to 528 million, and the percentage of knee osteoarthritis (KOA) patients increased from 58.5% to 60.6%, resulting in significant economic burdens worldwide.1 However, no medication is available to cure KOA.
In recent years, mitochondrial dysfunction has been considered an important marker of the initiation and progression of KOA.2 Mitochondria are the primary sites of energy metabolism in chondrocytes, and mitochondrial dysfunction is closely related to extracellular matrix metabolism, chondrocyte apoptosis and chondrocyte senescence.3,4 Mitophagy is a type of selective autophagy that can specifically phagocytose damaged mitochondria to preserve mitochondrial stability and quality.5 During the initial stage of KOA, chondrocyte mitophagy is activated and functions as a compensatory mechanism that protects these cells from environmental changes (e.g., mechanical stress, hypoxia, and other stimuli). However, as KOA worsens, chondrocyte mitophagy is inhibited, the mitochondrial membrane potential is depolarized, adenosine-triphosphate (ATP) production decreases, and cartilage shows signs of degeneration.6 The inhibition of chondrocyte mitophagy causes damaged mitochondria to accumulate in these cells, increases the expression of Matrix metalloproteinases-1(MMP-1) and MMP-13, and accelerates cartilage degeneration.7
The Pink1-Parkin pathway is the most important pathway related to mitophagy, and it can initiate the specific clearance of damaged mitochondria by mediating a connection between damaged mitochondria and autophagosomes through the ubiquitination cascade.8 Wang et al 9 discovered that activating the Pink1-parkin pathway might decrease reactive oxygen species (ROS), MMP-3 and MMP-13 production and alleviate cartilage degradation. Numerous studies have shown that the activation of chondrocyte mitophagy in order to maintain cartilage tissue homeostasis is a potential target for the treatment of KOA.10,11
From the perspective of biomechanical mechanisms, Prof. DT Felson described that the vast majority of OA cases occur due to abnormal loading.12 Studies have shown that the function of mitochondria in chondrocytes is regulated by mechanical stimulation, and abnormal mechanical loading can directly impair chondrocyte mitophagy, resulting in decreased ATP production and accelerated cartilage degradation. Additionally, moderate mechanical loading can positively regulate the degree of mitophagy in chondrocytes, resulting in effective clearance of damaged mitochondria, increased chondrocyte survival and effective delays in cartilage degeneration.13,⇓-15
Acupotomy therapy, also known as miniscalpel needle therapy, is a biomechanical approach that is used to treat muscle fiber knots with a modified acupuncture tool. According to our clinical research, acupotomy can improve knee joint symptoms during weight-bearing activities better than electroacupuncture, and this effect is related to improvements in the mechanical environment of the knee joint16 Studies have also confirmed that acupotomy can improve the mechanical environment of the knee by releasing soft tissues around the knee, and this can promote chondrocyte proliferation and alleviate cartilage degeneration.17,18 Additionally, chondrocyte mitophagy is regulated by mechanical stimulation. Increases in mitochondrial autophagy in response to moderate mechanical force can increase the survival rate of OA chondrocytes, effectively protecting cartilage cells.19,20
In conclusion, whether the mechanical effect of acupotomy on KOA protects cartilage via the mitophagy pathway remains unknown. Additionally, whether this process is regulated by the Pink1-Parkin pathway deserves further exploration. To address these questions, we established a rabbit model of KOA with the modified Videman method and observed the effects of acupotomy on mitophagy, mitochondrial function and the Pink1-parkin pathway in rabbits with KOA.
2. MATERIALS AND METHODS
2.1. Animal species and groupings
Six rabbits were selected from among 18 healthy male New Zealand rabbits (2.0-2.5 kg, Fulongtengfei Experimental Animal Research Institute, Beijing) and assigned to the control group (CON) according to the random number table method. After model establishment, the model rabbits were divided into 2 groups. The control group received no intervention except for normal grip and binding fixation for 4 weeks. The model group (KOA) was fed the same diet as the control group after 6 weeks of modeling. The model + acupotomy group (Acu) was subjected to acupotomy for 4 weeks after the 6-week modeling. The animals were housed in a single cage with a 12 h/12 h light/dark cycle and were given free access to standard food and water. The rabbits were allowed to acclimate for 1 week before the experiment was initiated. The experimental procedures followed the experimental design principles of minimizing the suffering and number of experimental animals used. The specific protocol was approved by the Laboratory Animal Committee of Beijing University of Chinese Medicine (approval number: BUCHM-4-2021-040105-2117).
2.2. Model establishment and model evaluation
After acclimation to feeding for one week, the modified Videman method was used to establish the KOA model.20 After the rabbits had fasted for 12 h, the ear edge vein was anesthetized using 3% pentobarbital sodium solution (30 mg/kg). Then, the rabbits were secured on an operating table, with a left hind limb preparation held in a straight position and wrapped in double-sided foam glue from the groin to below the ankle. The appropriate length of the polymer bandage was removed and quickly wrapped around the outside of the resin bandage for reinforcement. The toes of the rabbits were not wrapped so that the terminal blood supply to the limbs could be observed. The left knee of the rabbits was effectively immobilized for a total of 6 weeks.
After 6 weeks of modeling, the molds were disassembled. The rabbits were evaluated to assess model establishment according to the Lequesne MG scoring criteria (as shown in Section "2.4"): a score of 0 was considered normal, a score of 1-4 was considered mild KOA, a score of 5-8 was considered moderate KOA, and a score of 8 or higher was considered severe KOA. The data presented in this paper were collected after the experimental rabbits that died as well as the rabbits in which the model was not successfully established were excluded.
2.3. Acupotomy intervention
Acupotomy was performed after successful model establishment. A rabbit was fixed on an operating table, the hair was removed from the left knee joint. The tendon ending points of the vastus medialis, vastus lateralis, rectus femoris, and biceps femoris and 1-2 muscle knot nodes around the knee were selected as the treatment points. A disposable and flat head-shaped acupotomy (0.3 mm × 25 mm; Beijing Outstanding Huayou Medical Instrument Co., Ltd., Beijing, China) was used to perform the intervention according to the four-step protocol.21 The acupotomy was inserted vertically into the skin with the acupotomy parallel to the muscle fibers, after which the nodules were released. The intervention was administered weekly for 4 weeks.
2.4. Behavioral assessment
The passive range of motion (PROM) of the knee was detected using a digital angled ruler before and after 4 weeks of intervention. Moreover, the behavior of rabbits in each group was evaluated using the modified Lequesne MG KOA assessment scale to assess knee pain stimulus response, gait change, joint mobility and joint swelling.22 The knees of normal rabbits received scores of 0 points, whereas scores of 1-4 points indicated mild KOA, scores of 5-8 points indicated moderate KOA, and scores higher than 8 points indicated severe KOA. All the evaluations were observed and recorded by two researchers who were blinded to the experimental grouping.
2.5. Safranin O-fast green staining
Cartilage-subchondral bone units were dissected from the center of the medial tibial plateau and fixed in 4% paraformaldehyde for 24 h. The bone units were completely decalcified, embedded in paraffin, and cut into 4-μm-thick sections. The sections were stained with safranin O-fast green (Solarbio, Beijing, China) and observed with an optical microscope (Olympus, Nagano, Japan). Then, images of cartilage degeneration were captured. Photographs were scored using the Mankin scale by two investigators who were blinded to the group allocations.
2.6. Transmission electron microscopy
After the second behavioral assessment, the rabbits were sacrificed, and the cartilage (1 mm × 1 mm × 1 mm) was dissected with a blade. After fixation in glutaraldehyde for 2 h, the cartilage was cut into resin blocks, which were cut into 60-80-nm ultrathin sections. The sections were double stained with lead uranium. The morphology of the mitochondria and autophagic vesicles in chondrocytes was observed by investigators via transmission electron microscopy, and images were captured.
2.7. Enzyme-linked immunosorbent assay (ELISA)
After the second behavioral assessment ended, the rabbits were sacrificed, and the cartilage was dissected with a blade. Twenty milligrams of cartilage was weighed, and 0.9% normal saline was added at a weight (mg)/volume (μL) ratio of 1:9 for homogenization. The protein concentrations of the samples were determined with a bicinchoninic acid assay (BCA) kit. The ATP contents in the tissues were determined by an ATP kit.
2.8. Immunofluorescence
Paraffin sections of cartilage-subchondral bone units were dewaxed, dehydrated, subjected to antigen retrieval, cleared of spontaneous fluorescence, and blocked with serum. Diluted primary antibodies were added at a ratio of 1:100; the combination of primary antibodies included anti-collagen type Ⅱ (Col-Ⅱ) antibody alone, anti-Pink1 and anti-Parkin antibodies together for dual immunofluorescence staining, and anit-TOM20 and anti-LC3 antibodies together for dual immunofluorescence staining. The sections were incubated with Alexa Fluor-labeled secondary antibody at a ratio of 1:400 and finally sealed with Antifade Mounting Medium with C (DAPI) (ZSGB-Bio, Beijing, China). The sections were observed under a fluorescence microscope, and Image J (vers 1.53, National Institutes of Health, Bethesda, MD, USA) was used to analyze the Pearson coefficient of immunofluorescence colocalization and the fluorescence intensity of each protein.
2.9. Western blotting
After the second behavioral assessment ended, the rabbits were sacrificed, and the cartilage was dissected with a blade. The protein concentrations of the cartilage samples were quantified with a BCA kit, the proteins were denatured, and protein expression levels were ultimately analyzed via Western blotting. Primary antibodies against Pink1 (Proteintech, Wuhan, China), Parkin (Proteintech, Wuhan, China), LC3 (MBL, Beijing, China), TOM20 (Proteintech, Wuhan, China), TIM23 (Proteintech, Wuhan, China), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, UK) as well as horseradish peroxidase-labeled secondary antibodies (Zhongshanjinqiao, Beijing, China) were used for Western blotting. The gray value of each band was analyzed by ImageJ.
2.10. Polymerase chain reaction (PCR)
After the second behavioral assessment ended, the rabbits were sacrificed, and the cartilage was dissected with a blade. RNA extraction solution (Servicebio, Beijing, China) was used to homogenize the tissues and extract the total RNA. The RNA was reverse transcribed into cDNA according to the instructions of the RNA Reverse Transcription Kit. Real-time PCR was performed using qPCR SYBR Green Master Mix (Servicebio, Beijing, China) for cDNA amplification. Specific PCR primers for GAPDH, Pink1 and Parkin were generated based on published sequences. The relative expression levels of the target genes were calculated by the 2-△△Ct method and normalized to the mRNA expression level of GAPDH. PCR amplification was performed, and the specific primers that were used: for Pink1," forward primer (F.P): AGTA-CCTTCGCGTGAACACC and reverse Primer (R.P): TCAGGTCTCTGTGTGCGATG", for Parkin," F.P: ATTCTGACACCAGCATCTCCCA and R.P: AGTTCTGCACTGTTGACTCATCC", for GAPDH," F.P: TGAAGGTCGGAGTGAACGGAT and R.P: CGTTCTCAGCCTTGACCGTG"
2.11. Statistical analysis
SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA) was used for statistical analysis of the data, α = 0.05 was used as the test level, and P < 0.05 was considered to indicate statistical significance. Lequesne MG scores were analyzed by t test, the remaining data were analyzed by one-way analysis of variance (ANOVA), and all the data were expressed as the mean ± standard deviation ().
3. RESULTS
3.1. Acupotomy improved behavioral performance
As shown in Table 1, the rabbits in the model groups exhibited obvious pain responses after model establishment, and the affected limb was not used for walking. Compared with those in the CON group, the PROM of the model rabbits was significantly lower (P < 0.01), and the average MG score was 7.33. Moreover, there was no significant difference in the PROM or MG scores between the KOA group and the Acu group, suggesting a consistent baseline. After 4 weeks of acupotomy intervention, knee swelling and pain were reduced, and the PROM was significantly higher in the Acu group than in the KOA group (P < 0.01). Additionally, the MG score was significantly lower (P < 0.01), reaching 3.33 points.
Table 1.
Behavioral assessment of rabbits from the 3 groups (scores, )
| Item | Group | n | Before intervention | After intervention |
|---|---|---|---|---|
| PROM | CON | 6 | 141.2±2.1 | 140.7±3.5 |
| KOA | 6 | 31.2±6.5a | 53.2±10.0a | |
| Acu | 6 | 40.3±4.6a | 97.3±12.5ab | |
| Lequesne MG | CON | 6 | 0.0±0.0 | 0.0±0.0 |
| KOA | 6 | 7.3±0.5 | 6.5±1.0 | |
| Acu | 6 | 7.3±0.8 | 3.3±0.8b |
Notes: CON group: no modelling and no intervention; KOA group: 6 of week modeling followed by no intervention; Acu group: 6 of week modeling followed by acupotomy intervention for 4 weeks. PROM: passive range of motion; CON: control; KOA: knee osteoarthritis; Acu: acupotomy. The t-test was used for statistical analysis of both items. Compared with the control group, aP < 0.01; compared with the KOA group, bP < 0.01.
3.2. Acupotomy alleviated cartilage degeneration
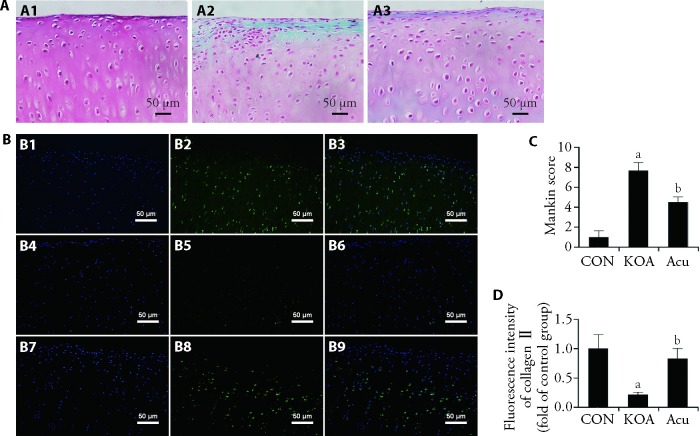
As shown in Figure 1A, cartilage from rabbits in each group was stained with Safranin O-fast green. The cartilage appeared red when stained with the basic dye saffranine, and the bone appeared green or blue when stained with the acid dye solid green. In the CON group, the cartilage structure was complete and smooth, the layers were clear and distinguishable, the chondrocytes were arranged in an orderly manner, the tidal line was clear and complete, and the staining was uniform. In the KOA group, the continuity of the cartilage surface was interrupted, and longitudinal cracks, cartilage thinning, disordered layers, absence of surface Safranin staining, chondrocyte aggregation and hypertrophy were observed; moreover, as shown in Figure 1C, the Mankin score was significantly higher in the KOA group than in the CON group (P < 0.01). Compared with that in the KOA group, the cartilage in the acupotomy group had a relatively intact structure, a smooth surface, and deeper saffranine staining, which indicated less proteoglycan degradation. The Mankin score in the Acu group was significantly lower than that in the KOA group (P < 0.01).
Figure 1. Cartilage degeneration under microscope.
A: safranin O-fast green staining of the knee cartilage (× 200); A1: CON group; A2: KOA group; A3: Acu group; B: Col-Ⅱ immunofluorescence staining in knee cartilage (× 200); B1-B3: CON group; B4-B6: KOA group; B7-B9: Acu group; C: analysis of the Mankin score; D: analysis of the Col-Ⅱ fluorescence intensity. CON group: no modelling or no intervention; KOA group: 6 weeks of modeling followed by no intervention; Acu group: 6 weeks of modeling followed by acupotomy intervention for 4 weeks. Col-Ⅱ: type Ⅱ collagen; CON: control; KOA: knee osteoarthritis; Acu: acupotomy. The data were presented as the mean ± standard deviation (n = 6). Compared with the CON group, aP < 0.01; compared with the KOA group, bP < 0.01.
Figure 1B shows the results of immunofluorescence staining for Col-Ⅱ in rabbit knee cartilage samples from each group. The nuclei were stained blue with DAPI and had an oval shape, and Col-Ⅱ was stained green and expressed in the cytoplasm and extracellular matrix. In the CON group, Col-Ⅱ was distributed in each layer of cartilage. In the KOA group, the expression of Col-Ⅱ was significantly reduced in all the cartilage layers, and only a small amount of Col-Ⅱ was observed in the deep cartilage layer. As shown in Figure 1D, compared with that in the CON group, the fluorescence intensity of Col-Ⅱ in the KOA group was significantly decreased (P < 0.01). A Col-Ⅱ distribution was observed in all the layers of cartilage in the Acu group, and the fluorescence intensity was significantly higher in the Acu group than in the KOA group (P < 0.01).
3.3. Acupotomy alleviated mitochondrial morphological damage in chondrocytes
As shown in Figure 2, transmission electron microscopy was used to observe the structure of the mitochondria and the morphology of autophagic vesicles in rabbit chondrocytes from each group. In the CON group, the bilateral mitochondrial membrane structure was intact, and the mitochondrial ridge was clear. In the KOA group, the mitochondria were swollen, the mitochondrial matrix was absent, the mitochondrial ridge disappeared, and the mitochondria were donut-shaped, indicating that the mitochondrial morphology was damaged and altered. In the Acu group, the mitochondrial morphology was relatively normal, mitochondrial cristae were observed, many mitochondrial autophagic vesicles were observed in the cells, and mitophagy might have been activated.
Figure 2. Ultramicrostructure of chondrocyte mitochondria.
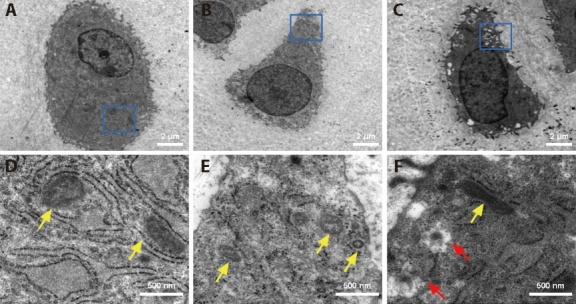
A, B, C: chondrocytes at low magnification (× 1200); D, E, F: chondrocytes at high magnification (× 8000); A, D: CON group; B, E: KOA group; C, F: Acu group; CON group: no modeling or no intervention; KOA group: 6 weeks of modeling followed by no intervention; Acu group: 6 weeks of modeling followed by acupotomy intervention for 4 weeks. The yellow arrow indicates mitochondria; the red arrow indicates mitochondrial autophagosomal vesicles.
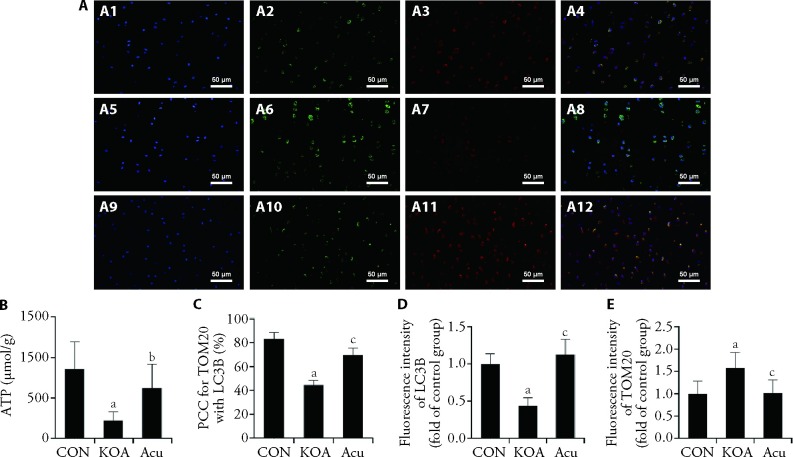
3.4. Acupotomy enhanced mitophagy and improved mitochondrial function
The function of mitochondria is related to normal morphology. Therefore, double immunofluorescence staining was used to further observe mitochondrial autophagy, and ATP levels were measured by ELISA to evaluate mitochondrial function. As shown in Figure 3-A, the colocalization coefficient of TOM20 and LC3B in the KOA group was significantly lower than that in the CON group (P < 0.01), the fluorescence intensity of LC3B and the level of ATP was significantly decreased (P < 0.01), and the fluorescence intensity of TOM20 was significantly increased (P < 0.01). Compared with those in the KOA group, the colocalization of TOM20 and LC3B was higher in the Acu intervention group (P < 0.01), the fluorescence intensity of LC3B and the level of ATP were higher (P < 0.01 or P < 0.05), and the fluorescence intensity of TOM20 was lower (P < 0.01).
Figure 3. Immunofluorescence staining of mitophagy and mitochondrial function.
A: double immunofluorescence staining for TOM20 and LC3B (× 400); A1, A2, A3, A4: CON group; A5, A6, A7, A8: KOA group; A9, A10, A11, A12: Acu group; A1, A5, A9: DAPI staining; A2, A6, A10: TOM20 staining; A3, A7, A11: LC3B staining; A4, A8, A12: merge of TOM20 and LC3B staining; B: level of ATP; C: colocalization coefficient; D, E: analysis of TOM20 and LC3B fluorescence intensity. CON group: no modeling or no intervention; KOA group: 6 weeks of modeling followed by no intervention; Acu group: 6 weeks of modeling followed by acupotomy intervention for 4 weeks. ATP: adenosine triphosphate; PCC: Pearson's correlation coefficient; TOM20: translocase of the outer membrane 20; LC3B: microtubule-associated protein-1 light chain-3B; CON: control; KOA: knee osteoarthritis; Acu: acupotomy; DAPI: 4',6-diamidino-2-phenylindole. The data were presented as the mean ± standard deviation (n = 6). aP < 0.01 compared with the control group; bP < 0.05, cP < 0.01, compared with the KOA group.
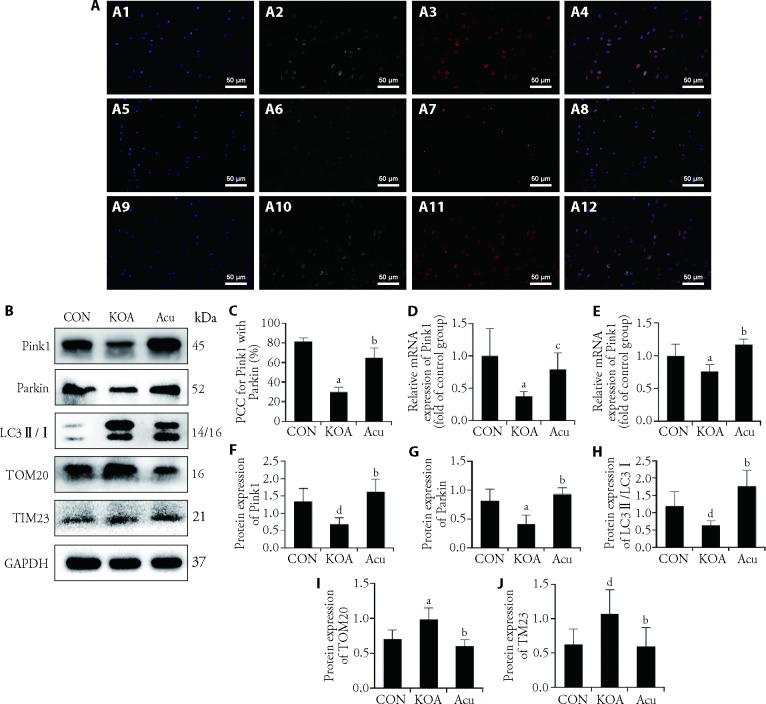
3.5. Acupotomy regulated factors associated with the Pink1-Parkin pathway
As shown in Figure 4A and Figure 4C, compared with that in the CON group, the colocalization of Pink1 and Parkin in the KOA group was significantly decreased (P < 0.01). Compared with that in the KOA group, the colocalization of PCC in the Acu group was significantly higher (P < 0.01). As shown in Figure 4D and 4E, the transcription levels of Pink and Parkin in the KOA group were significantly lower than those in the CON group (P < 0.01), and acupotomy intervention inhibited these KOA-induced changes. As shown in Figure 4B and Figure 4F to Figure 4K, compared with those in the CON group, the protein expression levels of Pink1, Parkin and LC3Ⅱ/Ⅰ in the KOA group were significantly inhibited (P < 0.01 or P < 0.05), and the protein expression levels of the mitochondrial markers TOM20 and TIM23 were significantly increased (P < 0.01 or P < 0.05). Compared with those in the KOA group, acupotomy increased the protein expression of Pink1, Parkin and LC3Ⅱ/Ⅰ (P < 0.01) and decreased the accumulation of TOM20 and TIM23 (P < 0.01).
Figure 4. Expression of factors associated with the Pink1-Parkin pathway.
A: double immunofluorescence staining for Pink1 and Parkin (× 400); A-1A4: CON group; A5-A8: KOA group; A9-A12: Acu group; A1, A5, A9: DAPI staining; A2, A6, A10: Pink1 staining; A3, A7, A11: Parkin staining; A4, A8, A12: merge of Pink1 and Parkin staining; B: Pink1, Parkin, LC3Ⅱ/Ⅰ, TOM20 and TIM23 expression was detected by Western blot analyses. C: colocalization coefficient; D: mRNA expression of Pink1; E: mRNA expression of Parkin; F: Western blotting analysis of Pink1 expression; G: Western blotting analysis of Parkin expression; H: Western blotting analysis of LC3Ⅱ/Ⅰ expression; I: Western blotting analysis of TOM20 expression; J: Western blotting analysis of TIM23 expression. CON group: no modeling or no intervention; KOA group: 6 weeks of modeling followed by no intervention; Acu group: 6 weeks of modeling followed by acupotomy intervention for 4 weeks. PCC: Pearson's correlation coefficient; Pink1: PTEN-induced putative kinase 1; LC3Ⅱ/Ⅰ: microtubule-associated protein-1 light chain-3Ⅱ/Ⅰ; TOM20: translocase of the outer membrane 20; TIM23: translocase of the inner membrane 23; CON: control; KOA: knee osteoarthritis; Acu: acupotomy; DAPI: 4', 6-diamidino-2-phenylindole; PTEN: phosphatase and Tensin Homolog. The data were presented as the mean ± standard deviation (n = 6). aP < 0.01 and dP < 0.01 compared with the control group; bP < 0.01 and cP < 0.05, compared with the KOA group.
4. DISCUSSION
According to this study, acupotomy improved behavioral performance and reduce cartilage degradation in rabbits with KOA. After 6 weeks of modeling by the modified Videman method, factors associated with the Pink1-Parkin pathway as well as mitophagy in chondrocytes were inhibited, the morphology and function of mitochondria were damaged, damaged mitochondria accumulated, and the distribution of collagen and proteoglycans in cartilage was reduced. Acupotomy increased the level of mitophagy in chondrocytes, improved mitochondrial morphology and function, and enhanced the distribution of Col-Ⅱ and proteoglycans in cartilage, and these effects were mediated through the activation of the Pink1-Parkin pathway.
In our previous studies, we discovered that the modified Videman method effectively establishes a KOA model that affects the complete knee capsule and includes noticeable cartilage degeneration and periarticular muscle atrophy.23,24 In this study, after 6 weeks of modeling, the knees of the rabbits were slightly swollen, the joints were stiff, pain responses were obvious during passive activity, and arthritis scores were significantly increased, reaching approximately 7 points. According to the scoring standard of Lequesne MG, 0 is classified as normal, 1-4 is classified as mild KOA, 5-8 is classified as moderate KOA, and more than 8 is classified as severe KOA.25 After modeling in this study, the scores of the rabbits in each group indicated that they had moderate KOA, confirming successful establishment of the model. As a result of acupotomy, joint swelling and pain were significantly reduced, the passive range of motion was improved, and arthritis scores were reduced in the rabbits. Hence, it can be concluded that acupotomy has the potential to improve the behavioral performance of rabbits with KOA.
Cartilage consists of chondrocytes and extracellular matrix, which includes 75% water, 15% collagen (mainly col-Ⅱ), and 10% proteoglycan. Col-Ⅱ interacts with proteoglycans in the extracellular matrix to form a network structure that provides the compressive and shock absorption properties of cartilage.26 Cartilage appears red when stained with safranin, and safranin staining indirectly reflects the distribution and content of proteoglycans in cartilage.27 Loss of Col-Ⅱand proteoglycan during KOA is a typical manifestation of cartilage degeneration, and it causes cartilage to lose potential elastic energy and be more susceptible to injury due to joint load.28 This study revealed that acupotomy reduced the degradation of proteoglycans and Col-Ⅱ in rabbits with KOA, thereby delaying articular cartilage degeneration.
Researchers have believed that due to the physiological hypoxic environment of cartilage, mitochondrial function is not important.29 However, in recent years, an increasing number of studies have shown that mitochondria perform important functions in chondrocytes and are closely related to the occurrence and development of KOA.30 In KOA cartilage, chondrocyte mitochondria undergo swelling and transition from an elongated to a circular shape, resulting in a loss of mitochondrial membrane potential, increased production of reactive oxygen species, and significant decreases in ATP synthesis.31,32 ATP can participate in the synthesis of collagen and proteoglycans in the extracellular matrix of cartilage, and it plays an important role in the physiological function of cartilage cells and cartilage repair.33 In this study, the mitochondria were swollen, ATP production was decreased, and proteoglycans and Col-Ⅱ were degraded in the KOA group, which was consistent with the findings of other studies.
Mitophagy is a kind of selective autophagy in which damaged and dysfunctional mitochondria are degraded through the autophagic lysosomal system to achieve mitochondrial self-renewal and maintain mitochondrial quality, and mitophagy is an important regulatory mechanism for maintaining homeostasis of the intracellular environment and cellular activity.34 In the early stage of KOA, excessive ROS in chondrocytes can destroy the mitochondrial membrane potential and mitochondrial permeability and induce mitochondrial autophagy.35 However, with the progression of KOA, chondrocyte mitophagy is inhibited, and damaged mitochondria accumulate.36 Dysfunction of mitophagy can lead to impaired mitochondrial function in KOA chondrocytes and exacerbate cartilage degeneration.37 Activation of mitophagy in KOA chondrocytes can enhance the mitochondrial membrane potential and ATP production as well as alleviate oxidative damage and chondrocyte apoptosis.38 In this study, chondrocyte mitophagy was inhibited in the KOA group, and mitochondrial function was significantly impaired. After acupotomy, the colocalization of the mitochondrial marker TOM20 and the autophagosome marker LC3B in chondrocytes was significantly increased, mitophagy was activated, and ATP production was increased. The recovery of ATP production in the Acu group promoted cartilage repair and increased the distribution of Col-Ⅱand proteoglycans.
The Pink1-Parkin pathway is one of the most important pathways that regulates mitophagy.39 When mitochondria are damaged, their membrane potential decreases, and Pink1 cannot easily cross the double membrane of the mitochondria; rather, Pink1 binds to the TOM complex and is anchored to the outer membrane of the mitochondria.40 Subsequently, Pink1 recruits and binds to Parkin in the cytoplasm to label damaged mitochondria that need to be degraded, causing the ubiquitination of mitochondrial outer membrane proteins and initiating the mitophagy degradation process.41 Finally, TOM20 binds to the autophagy-related factor LC3 to complete the docking of mitochondria and autophagosomes, promoting the degradation of damaged mitochondria.42 Timely and moderate activation of the Pink1-Parkin pathway can ensure that mitophagy effectively eliminates damaged mitochondria when cells become stressed, prevent the accumulation of harmful substances, and increase the survival rate of cells.43 Activating mitophagy reduces reactive oxygen species and matrix metalloproteinase production while increasing Col-Ⅱ production by chondrocytes.9 In addition, studies have shown that mitochondria are connected to the extracellular matrix via the chondrocyte cytoskeleton and are sensitive to mechanical load.44 Mechanical load can directly regulate the level of mitochondrial autophagy in chondrocytes, and a mechanical load that is too high or too low leads to inhibition of the Pink1-Parkin pathway and mitophagy in chondrocytes.15,45 Due to differences in the mode, intensity and duration of mechanical force stimulation, dual effects of mechanical load regulation on mitophagy in chondrocytes were observed, and chondrocytes could be effectively protected only when exposed to moderate mechanical load.46 One study showed that the modeling method that involves knee joint immobilization results in joint load unloading.47 In this study, a KOA rabbit model was established by immobilizing the left hind limb for 6 weeks (the Videman method), after which the Pink1-Parkin pathway was inhibited in the chondrocytes of rabbits with KOA, and mitophagy was reduced. Early studies confirmed that acupotomy could restore the mechanical environment of the knee and improve the mechanical load on the cartilage by releasing the adhesion muscle and tendon around the knee.48 In this study, acupotomy activated the Pink1-Parkin pathway and downstream factors in chondrocytes of rabbits with KOA, improved mitophagy and protected cartilage, possibly through the mechanical effect of acupotomy.
Based on the findings of previous studies, this study confirmed the regulatory effects of acupotomy on autophagy activation and energy metabolism in the cartilage of rabbits with KOA, and this study confirmed that these phenomena occurred due to the mechanical effect of acupotomy on activating mitophagy via the Pink1-Parkin pathway.18,19 This study has several limitations. Probes to measure mitophagy can be used only in live cells and cannot be used on tissue samples that have been fixed with paraformaldehyde. Therefore, the degree of mitochondrial damage and the level of mitophagy in cartilage could not be dynamically and accurately evaluated.
In conclusion, acupotomy could play a therapeutic role in KOA by regulating the Pink1-Parkin pathway in rabbit chondrocytes, activating mitophagy, effectively clearing damaged mitochondria, restoring mitochondrial function, and reducing the degradation of the cartilage matrix.
REFERENCES
- 1. Long H, Liu Q, Yin H, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol 2022; 74: 1172-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He Y, Wu Z, Xu L, et al. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell Mol Life Sci 2020; 77: 3729-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu HY, Chang CF, Lu CC, et al. The role of mitochondrial metabolism, AMPK-SIRT mediated pathway, LncRNA and MicroRNA in osteoarthritis. Biomedicines 2022; 10: 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolduc JA, Collins JA, Loeser RF. . Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med 2019; 132: 73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lemasters JJ. . Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 2005; 8: 3-5. [DOI] [PubMed] [Google Scholar]
- 6. Zeng Z, Zhou X, Wang Y, et al. Mitophagy-a new target of bone disease. Biomolecules 2022; 12: 1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Z, Chang J, Huang W, et al. Inhibition of chondrocyte mitochondrial autophagy increased the expression of MMP-1 and MMP-13. Anhui Yi Ke Da Xue Xue Bao 2018; 53: 600-4. [Google Scholar]
- 8. Sun K, Jing X, Guo J, Yao X, Guo F. . Mitophagy in degenerative joint diseases. Autophagy 2021; 17: 2082-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang C, Yang Y, Zhang Y, Liu J, Yao Z, Zhang C. . Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci Trends 2019; 12: 605-12. [DOI] [PubMed] [Google Scholar]
- 10. Ansari MY, Khan NM, Ahmad I, Haqqi TM. . Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage 2018; 26: 1087-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang LW, Huang TC, Hu YC, et al. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem Biophys Res Commun 2020; 521: 50-6. [DOI] [PubMed] [Google Scholar]
- 12. Felson DT. . Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 2013; 21: 10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kong DC, Zheng TS, Zhang M, et al. Static mechanical stress induces apoptosis in rat endplate chondrocytes through MAPK and mitochondria-dependent caspase activation signaling pathways. PLoS One 2017; 8: e69403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coleman MC, Ramakrishnan PS, Brouillette MJ, Martin JA. . Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheumatol 2016; 68: 662-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He YC, Yocum L, Alexander PG, Jurczak MJ, Lin H. . Urolithin a protects chondrocytes from mechanical overloading-induced injuries. Front Pharmacol 2021; 12: 703847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo CQ, Si T, Wen JM, et al. A randomized controlled clinical study on the improvement of pain symptoms of knee osteoarthritis with acupotomy. Tianjin Zhong Yi Yao Za Zhi 2012; 29: 35-8. [Google Scholar]
- 17. An XY, Wang T, Zhang W et al. Chondroprotective effects of combination therapy of acupotomy and human adipose mesenchymal stem cells in knee osteoarthritis rabbits via the GSK3β-Cyclin D1-CDK4/CDK 6 signaling pathway. Aging Dis 2020; 11: 1116-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma SN, Xie ZG, Guo Y, et al. Effect of acupotomy on FAK-PI3K aignaling pathways in KOA rabbit articular cartilages. Evid Based Complement Alternat Med 2017; 2017: 4535326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He YC, Yocum L, Alexander PG, Jurczak MJ, Lin H. . Urolithin a protects chondrocytes from mechanical overloading-induced injuries. Front Pharmacol 2021, 12: 703847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang JM, Hao XX, Chi RM, Qi J, Xu T. . Moderate mechanical stress suppresses the IL-1beta-induced chondrocyte apoptosis by regulating mitochondrial dynamics. J Cell Physiol 2021, 236: 7504-15. [DOI] [PubMed] [Google Scholar]
- 21. Videman T. . Experimental osteoarthritis in the rabbit: comparison of different periods of repeated immobilization. Acta orthopaedica Scandinavica 1982; 53: 339-47. [DOI] [PubMed] [Google Scholar]
- 22. Guo C. . Acupotomology. 3rd ed. Beijing: China Press of Traditional Chinese Medicine, 2017: 199- 204. [Google Scholar]
- 23. Lequesne MG, Mery C, Samson M, Gerard P. . Indexes of severity for osteoarthritis of the hip and knee. Validation--value in comparison with other assessment tests. Scand J Rheumatol Suppl 1987; 65: 85-9. [DOI] [PubMed] [Google Scholar]
- 24. Shi XW, Yu WJ, Wang T, et al. Electroacupuncture alleviates cartilage degradation: improvement in cartilage biomechanics via pain relief and potentiation of muscle function in a rabbit model of knee osteoarthritis. Biomed Pharmacother 2020; 123: 109724. [DOI] [PubMed] [Google Scholar]
- 25. An XY, Wang T, Zhang W, et al. Chondroprotective effects of combination therapy of acupotomy and human adipose mesenchymal stem cells in knee osteoarthritis rabbits via the GSK3beta-Cyclin D1-CDK4/CDK6 signaling pathway. Aging Dis 2020; 11: 1116-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin X, Yang Y, Zhao J, Cao Y, Li J, Hao C. . Rational analysis of using braking method to build rabbit KOA stage model. Zhong Guo Bi Jiao Yi Xue Za Zhi 2021; 31: 78-82. [Google Scholar]
- 27. Adarmes H, Donders L, Dörner C, et al. Glycosaminoglycans (GAGs) determination in healthy and damaged equine articular cartilage. Austral J Vet Sci 2017; 49: 129-133. [Google Scholar]
- 28. Xu WC, Zhao X, Sun PP, Zhang C, Fu ZJ, Zhou DS. . The effect of medical ozone treatment on cartilage chondrocyte autophagy in a rat model of osteoarthritis. Am J Transl Res 2020; 12: 5967-76. [PMC free article] [PubMed] [Google Scholar]
- 29. Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010; 69: 761-5. [DOI] [PubMed] [Google Scholar]
- 30. Otte P. . Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol 1991; 50: 304-12. [PubMed] [Google Scholar]
- 31. Chen Y, Wu YY, Si HB, Lu YR, Shen B. . Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol Res 2021; 166: 105497. [DOI] [PubMed] [Google Scholar]
- 32. Eitner A, Sparing S, Kohler FC, et al. Osteoarthritis-induced metabolic alterations of human hip chondrocytes. Biomedicines 2022; 10: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu H, Li ZY, Cao YP, et al. Effect of chondrocyte mitochondrial dysfunction on cartilage degeneration: a possible pathway for osteoarthritis pathology at the subcellular level. Mol Med Rep 2019; 20: 3308-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Croucher LJ, Crawford A, Hatton PV, Russell RG, Buttle DJ. . Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochim Biophys Acta 2000; 1502: 297-306. [DOI] [PubMed] [Google Scholar]
- 35. Liu D, Cai Z, Yang Y, et al. Mitochondrial quality control in cartilage damage and osteoarthritis: new insights and potential therapeutic targets. Osteoarthr Cartilage 2021; 30: 395-405. [DOI] [PubMed] [Google Scholar]
- 36. Insil K, Sara R, John JL. . Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 2007; 462: 245-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Staines KA, Wood L, Mainenti M, et al. Mitophagy dysfunction localises to regions of natural osteoarthritis in str/ort mice. Osteoarthr Cartilage 2014; 22: 340-1. [Google Scholar]
- 38. Kuwahara M, Akasaki Y, Kurakazu I, et al. C10orf10/DEPP activates mitochondrial autophagy and maintains chondrocyte viability in the pathogenesis of osteoarthritis. FASEB J 2022; 36: e22145. [DOI] [PubMed] [Google Scholar]
- 39. Tang Q, Zheng G, Feng Z, et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis 2017; 8: e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun K, Jing X, Guo J, Yao X, Guo F. . Mitophagy in degenerative joint diseases. Autophagy 2020; 17: 2082-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Christian S, Alexander S, Peter R. . Unlocking the presequence import pathway. Trends Cell Biol 2015; 25: 265-75. [DOI] [PubMed] [Google Scholar]
- 42. Gong G, Song M, Gyorgy C, Daniel PK, Scot JM, Gerald WD. . Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 2015; 350: aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kathryn M, Michael AH, Ryan L, Reed F. . A systematic review and Meta-analysis of lower limb neuromuscular alterations associated with knee osteoarthritis during level walking. Clin Biomech (Bristol, Avon) 2013; 28: 713-24. [DOI] [PubMed] [Google Scholar]
- 44. Jin Z, Xu S, Yang Y, Yue Y, Bai L. . Research progress of PINK1/ Parkin-mediated mitochondrial autophagy in osteoarthritis. Zhong Guo Lin Chuang Yan Jiu 2021; 34: 258-61. [Google Scholar]
- 45. Steele HE, Guo Y, Li B, Na S. . Mechanotransduction of mitochondrial AMPK and its distinct role in flow-induced breast cancer cell migration. Biochem Biophys Res Commun 2019; 514: 524-29. [DOI] [PubMed] [Google Scholar]
- 46. Zhang J, Hao X, Chi R, Qi J, Xu T. . Moderate mechanical stress suppresses the IL-1beta-induced chondrocyte apoptosis by regulating mitochondrial dynamics. J Cell Physiol 2021; 236: 7504-15. [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Wang Q, Wang M. . The role of mitophagy in cartilage degeneration in osteoarthritis. Sheng Li Ke Xue Jin Zhan 2022: 1-11. [Google Scholar]
- 48. Nomura M, Sakitani N, Iwasawa H, et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr Cartilage 2016; 25: 727-36. [DOI] [PubMed] [Google Scholar]
- 49. Ma SN, Xie ZG, Guo Y, et al. Effect of acupotomy on FAK-PI3K signaling pathways in KOA rabbit articular cartilages. Evid Based Complement Alternat Med 2017; 2017: 4535326. [DOI] [PMC free article] [PubMed] [Google Scholar]