Abstract
Key Clinical Message
Mycoplasma myocarditis is a rare but potentially serious condition that can cause inflammation of the heart muscle, leading to arrhythmia and heart failure. It is important to consider this condition in the differential diagnosis of young patients presenting with unexplained signs of heart failure and SVT, even in the absence of signs of myocardiocytolysis and extra‐cardiac disease.
Abstract
Mycoplasma pneumoniae infections are often underdiagnosed as a great proportion of patients remain asymptomatic, pauci‐symptomatic, or exhibit varying presentations. M. Pneumoniae manifestations can affect different systems, including the heart, with the potential to lead to high degree of morbidity and debilitating sequelae. Here we present an atypical case of M. Pneumoniae associated myocarditis which presented with sustained refractory SVT, symptoms of heart failure, and with no signs of myocardiocytolysis, pulmonary involvement, or systemic infection. Given the lack of signs of myocardial inflammation, the patient was initially misdiagnosed with tachycardia induced cardiomyopathy (TIC), but later correctly diagnosed after showing signs of pneumonia during the hospitalization. The patient received the appropriate antibiotic treatment in addition to corticosteroids, was discharged on the 15th day of hospitalization, and completely recovered after 1 month with no arrhythmia recurrence and normalization of ventricular function.
Keywords: heart failure, mycoplasma pneumoniae myocarditis, myocarditis induced arrhythmia, supraventricular tachycardia, sustained refractory
1. INTRODUCTION
Mycoplasma pneumoniae is an atypical bacterium transmitted by respiratory droplets through close contact. 1 It typically triggers self‐limiting mild infections in both the upper and lower respiratory tracts, including conditions like pharyngitis, bronchitis, and pneumonia. 1 , 2 Its unique characteristic of lacking a cell wall renders it resistant to inhibitors targeting cell wall synthesis such as Beta‐Lactam antibiotics. 1 M. pneumoniae infections tend to occur as epidemics, with spread in institutional settings including schools, military bases, and camps, and they tend to cause more clinically significant illness in young children and adolescents. 1 , 2 , 3 Significant clinical features include the potential for extrapulmonary manifestations, best described in children and adolescents, 3 and often affecting the central nervous system, skin, heart, gastrointestinal tract, and joints. 1 , 3 Although the pathophysiology of these manifestations is not always clear, 3 it has been postulated that these might be caused by M. pneumoniae local effects via tissue invasion or indirect immune‐mediated injury. 1 , 3 Cardiac involvement is rare and occurs in the form of myopericarditis and, with a lower incidence, isolated myocarditis. Cardiac involvement tends to be found in older patients, in contrast to more typical Mycoplasma infections being found in young patients. 4 Mycoplasma infections are often underdiagnosed because of their asymptomatic or pauci‐symptomatic presentations, in fact only 3%–10% of patients develop pneumonia or severe symptoms. 5 , 6 , 7 Infections are mainly diagnosed with serology, 2 often associated with PCR testing in the early phases of the disease. 8 Myocarditis is also well known to be difficult to diagnose as many cases are pauci‐symptomatic. Cardiac magnetic resonance imaging or endomyocardial biopsy are needed for definitive diagnosis, which is often difficult to obtain. 9 Further, to establish a certain association between myocarditis and a particular pathogen, a tissue sample is often required, which is not routinely obtained in nonfatal cases of myocarditis given the poor diagnostic yield of endomyocardial biopsy. 10 , 11 , 12 Identification of myocarditis is essential as it is one of the main causes of dilated cardiomyopathy and one of the most common triggers of sudden cardiac death in young adults. 13 For these reasons Mycoplasma associated myocarditis represents a challenging pathological entity to diagnose, with a wide variety of presentations, often poorly described in literature. In this report, we present a unique case of acute mycoplasma myocarditis, presenting with sustained refractory SVT and acute heart failure. The patient did not show evidence of myocardiocytolysis and was initially misdiagnosed with tachycardia induced cardiomyopathy (TIC), leading to unsuccessful attempts to terminate the arrhythmia. Once the correct diagnosis was made, the patient was started on appropriate therapy and made a full recovery in 4 weeks.
2. CASE HISTORY
A previously healthy 29‐year‐old man presented to the Emergency Department (ED) complaining of persistent palpitations and abdominal fullness. The patient reported having symptoms for the previous 4 days and was prompted to come to the ED because of progressive shortness of breath. The patient denied having chest pain or gastrointestinal symptoms. His past medical history was remarkable for episodes of palpitations during adolescence, which were never investigated. On arrival, vitals showed blood pressure of 145/110 mmHg, heart rate 171 bpm, respiratory rate 23 breaths per minute, SpO2 89% on RA and 95% on 2 L O2 nasal canula, and temperature of 98 F. The EKG showed Supraventricular Tachycardia (SVT) at 160 bpm with no significant ischemic changes or other abnormalities (Figure 1). On physical examination, heart's apical impulse was noted to be displaced 3 cm to the left, a 3/6 diastolic murmur was auscultated on the cardiac apex with bilateral fine crackles at the lung bases. Blood test results showed white blood cells 14 × 10^3/uL, Hemoglobin 17 g/dL, Lymphocytes 0.63 × 10^3/mL (4.5%), Neutrophils 11.73 × 10^3/mL (83.7%), Eosinophils 0.15 × 10^3/mL (1.1%), electrolytes in range, Creatinine 1.43 mg/dL, Urea 20 mg/dL, BNP 9370 pg/mL, TSH 1.85 uIU/mL, C‐reactive protein high sensitivity >15 mg/L, erythrocyte sedimentation rate 15 mm/h, Troponins HS <0,012 ng/mL, CK‐MB 2 IU/L, Lactic acid <2 mmol/L, and LDH in range. Urine drug screen was negative and chest x‐ray showed cardiomegaly with pulmonary edema (Figure 2). The patient was not in apparent distress, and he was hemodynamically stable, therefore pharmacologic cardioversion was attempted. Adenosine 6 mg IVP was given, followed by another dose of Adenosine 12 mg IVP, with brief return to sinus rhythm and immediate arrhythmia recurrence. Electrical cardioversion was then attempted. The patient was sedated with Etomidate 20 mg IVP and then electrically cardioverted successfully with return to sinus rhythm. The EKG post cardioversion showed signs of diffuse ischemia with T waves inversion over the precordial leads (Figure 3).
FIGURE 1.
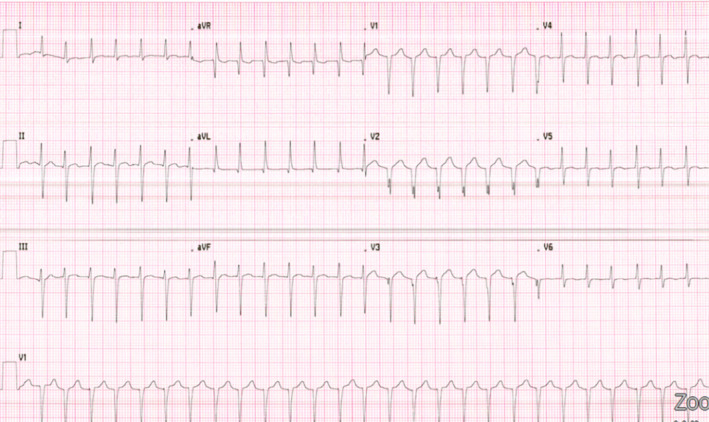
Electrocardiogram pre‐intervention upon ED evaluation on HD 0: SVT at 160 bpm. Abnormal left axis deviation. BPM, beats per minute; ED, emergency department; HD, hospital day.
FIGURE 2.
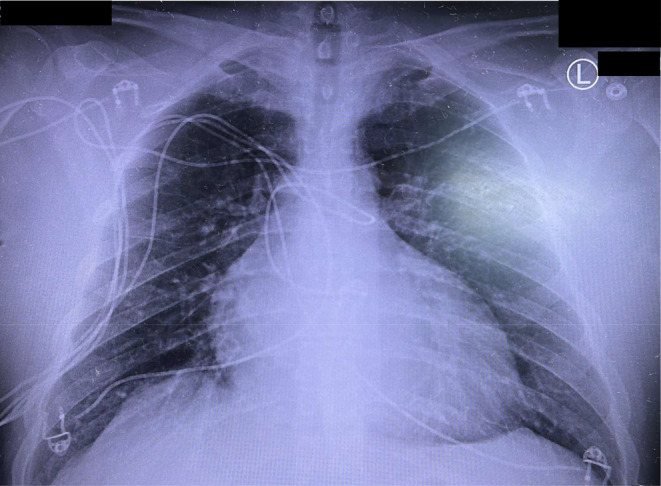
Chest radiographic findings upon Emergency Department evaluation (HD 0): cardiomegaly with pulmonary edema. No pleural effusion noted. HD, hospital day.
FIGURE 3.
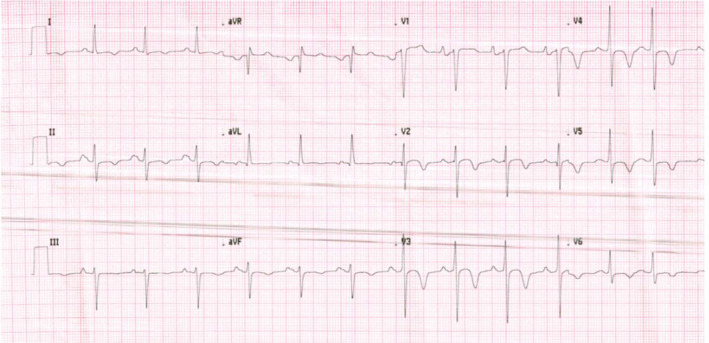
Electrocardiogram post‐electrical cardioversion in the ED on HD 0, after two unsuccessful attempts to terminate the arrhythmia with Adenosine: normal sinus rhythm at 80 bpm. Diffuse T wave inversion is noted, especially over the precordial leads, clinical correlation is advised. BPM, beats per minute; ED, emergency department; HD, hospital day.
2.1. Differential diagnosis, investigations, and treatment
Given the patient's past medical history of recurrent episodes of undiagnosed tachycardia, with the current episode lasting 5 days, his low risk for MI, the unexplained evidence of acute heart failure with apparently no extra‐cardiac disease or prodromal symptoms, and the absent laboratory signs of myocardial damage, the patient was admitted to the ICU with a working diagnosis of systolic heart failure secondary to TIC. Echocardiography showed a moderately dilated left ventricle with normal left ventricular wall thickness, moderate mitral and tricuspid regurgitation, and left ventricle ejection fraction of 30% with evidence of regional wall motion abnormalities in the basal infero‐lateral and antero‐septal walls. The patient was started on guideline‐directed medical therapy for heart failure as indicated by the American College of Cardiology (ACC)/American Heart Association (AHA), including Lisinopril 5 mg PO QD, Furosemide 40 mg IV QD, and DVT prophylaxis with Enoxaparin. On the initial day of hospitalization, the patient experienced two instances of sustained SVT, which were terminated on both occasions with 12 mg of Adenosine administered intravenously (IVP). To prevent recurrence of arrhythmia, a 150 mg bolus of Amiodarone was given, followed by a drip at a rate of 1 mg/min for 6 h, subsequently reduced to 0.5 mg/min for the next 18 h. The patient remained asymptomatic for chest pain, and oxygen therapy was discontinued given his SpO2 readings on room air and the absence of dyspnea or tachypnea. EP department was consulted for possible ablation. On day 2 the patient complained of a dry cough in the morning, and a chest CT was performed with findings suggestive of pneumonia with small parapneumonic effusion (Figure 4). The patient was started on empiric therapy for CAP with Ceftriaxone 1 g IV Q24 and Doxycycline 100 mg IV Q12H. Virus studies came back negative, Legionella and S. Pneumoniae urinary antigen tests (UAT) resulted negative, while M. pneumoniae IgM Ab titer was positive at 730.1 U/mL. These findings were furtherly validated with polymerase chain reaction (PCR) testing on sputum, which resulted positive for M. Pneumoniae. At this point a cardiac MRI was performed which revealed ventricular systolic dysfunction characterized by global hypokinesis with regional variations noted in the lateral wall. Additionally, there was severe dilation of the left ventricle, along with patchy late gadolinium enhancement observed along the inferolateral and anterolateral basal and anteroseptal wall (Figure 5). Based on the updated Lake Louise Criteria, 14 the observed pattern, along with the discussed serological findings, was deemed diagnostic of myocarditis, leading to a change in working diagnosis to systolic heart failure secondary to mycoplasma‐associated acute myocarditis. On the same day, the patient commenced Methylprednisolone intravenously at a dosage of 125 mg/day for 3 days, subsequently transitioning to oral Prednisone at 1 mg/kg for a duration of 2 weeks, followed by a tapering period of 4 weeks. The next day, the patient experienced another episode of SVT at 180 bpm, which was effectively treated with a single dose of 6 mg Adenosine. Following this, Metoprolol tartrate at a dosage of 100 mg orally twice daily was incorporated into the patient's medication regimen, Amiodarone was switched to 200 mg orally twice daily, and Ceftriaxone was discontinued.
FIGURE 4.
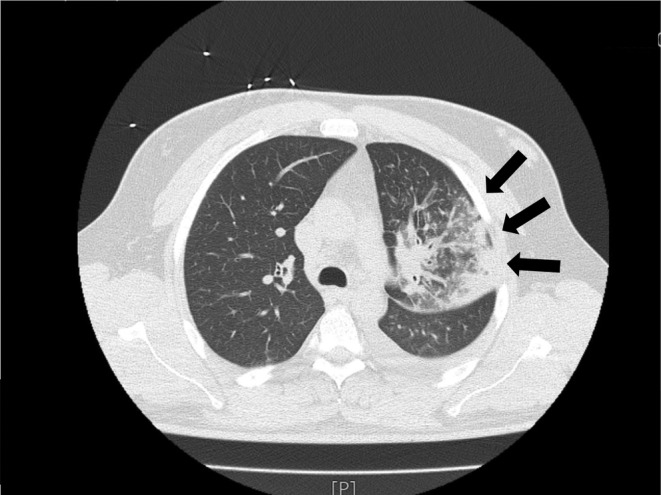
Chest CT findings on HD 2, lobar pneumonia (black arrows). HD, hospital day.
FIGURE 5.
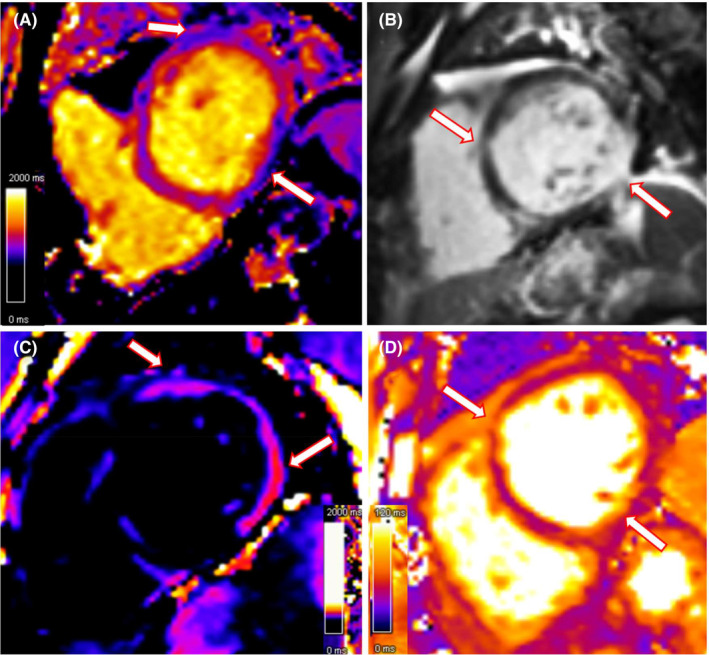
Cardiac MRI findings on HD 3, (A) Native T1 map showing regional prolonged T1 relaxation time, indicative of edema/hyperemia; (B) T1 delayed post contrast sequence demonstrate diffuse patchy areas of intramyocardial late gadolinium enhancement (LGE), mainly involving the inferolateral basal, anterolateral basal and anteroseptal wall (white arrows); (C) Corresponding prolonged T1 relaxation time in the LGE map confirms edema; (D) T2 map shows diffuse prolongation of relaxation time as a consequence of increased tissue water content (edema), which is a hallmark of myocardial inflammation.
2.2. Outcomes and follow‐up
The patient's condition gradually improved throughout the hospital staying, markers of myocardial damage remained negative, HS‐CRP and BNP trended down (Table 1, Figure 6), Amiodarone was discontinued, Metoprolol was gradually tapered and switched to succinate 50 mg once a day, while Doxycycline was discontinued at day 12. The patient was finally discharged on day 15 without any recurrence of the arrhythmia. The patient was advised to abstain from exercise until normalization of the ejection fraction. A follow‐up echocardiogram was performed 4 weeks after discharge, showing improvement of the left ventricular dilatation, valve regurgitations, and normalization of the ejection fraction. The patient was re‐evaluated regularly every 8 weeks for the first 6 months, showing no sequelae. During the follow‐up the patient did not report any episode of palpitations, and this was confirmed by Holter monitor tests.
TABLE 1.
Longitudinal variations of main hematological, inflammatory, and biochemical parameters.
| HD 0 | HD 2 | HD 7 | HD 15 | |
|---|---|---|---|---|
| WBC | 14 10^3/uL | 12.7 10^3/uL | 17.5 10^3/uL | 14.8 10^3/uL |
| Troponin | <0,012 ng/mL | <0,012 ng/mL | – | – |
| CK–MB | 2 IU/L | – | – | – |
| BNP | 9370 pg/mL | 7945 pg/mL | – | 2357 pg/mL |
| HS–CRP | >15 mg/L | >15 mg/L | 4.6 mg/L | 2.1 mg/L |
| Lactic acid | <2 mmol/L | <2 mmol/L | – | – |
| Resp virus panel | Negative | – | – | – |
| Mycoplasma pneumoniae IgM | – | 730.1 U/mL | – | – |
| Mycoplasma pneumoniae PCR | – | Positive | – | – |
Note: Resp Virus Panel, PCR detecting influenza (A, B), respiratory syncytial virus (RSV), parainfluenza (1, 2, 3, 4), human metapneumovirus (hMPV), human rhinovirus, and adenovirus.
Abbreviations: BNP, brain natriuretic peptide; CK‐MB, creatine phosphokinase‐MB; HD, hospital day; HS‐CRP, high sensitivity C‐reactive protein; PCR, protein chain reaction; WBC, white blood count.
FIGURE 6.
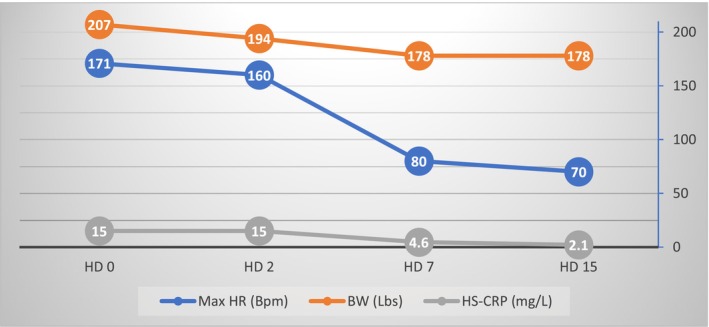
Longitudinal representation of significant clinical and biochemical parameters. Bpm, beats per minute; BW, body weight; HS‐CRP, high‐sensitivity C‐reactive Protein; HD, hospital day; Max HR, maximum heart rate.
3. DISCUSSION
This case report underscores the numerous hurdles encountered in diagnosing and managing a rare instance of M. pneumoniae myocarditis. It emphasizes the diverse ways the disease can manifest, the uncommon absence of typical indicators of myocardial damage and extra‐cardiac disease, and the inherent arrhythmogenic risk posed by this bacterium.
M. pneumoniae infections are often underdiagnosed as a great proportion of patients remain asymptomatic or pauci‐symptomatic, with approximately 50% of patients exhibiting varied and nonspecific mild upper respiratory tract symptoms, accompanied by malaise and low‐grade fever. This condition affects mainly children and young adults, the majority of which will develop mild and benign upper respiratory infections, 3%–10% will present with Pneumonia, and 5%–10% will exhibit extrapulmonary involvement, often of greater clinical severity than the primary focus of infection. 5 , 6 The diagnosis of M. Pneumoniae is challenging due to its myriad presentations, but at the same time, its diagnosis is of vital importance given the potential to cause serious infections with a high degree of morbidity and debilitating sequelae. 7
Cardiac involvement is rare, but often severe. It is more prevalent in the adult population 15 , 16 , 17 and presents mainly in the form of pericarditis, endocarditis, and myocarditis. Although the pathophysiology is not entirely known, it is postulated that the cardiac damage in pericarditis and endocarditis is caused by the development of mycoplasma bacteremia, local invasion, and cytokines induction. 18 Mycoplasma associated myocarditis, on the other hand, appears to be the byproduct of immune modulations in the form of autoimmunity, allergy, or immune complex formation. 19 , 20
Myocarditis itself is often an underdiagnosed cardiac disease caused by a wide range of infectious disease, immunologic, and toxic processes. Possible outcomes include recovery, DCM development, abrupt deterioration, heart failure, and death. 21 Among the spectrum of myocarditis, the most prevalent forms are of infectious etiology, with viruses being the most common agents, especially coxsackievirus, CMV, adenovirus, parvovirus B19, hepatitis C, HIV, Varicella, and EBV. 13 M. pneumoniae is a rare cause of myocarditis, which is more frequently associated with other bacteria, such as: Corynebacterium diphtheriae and Staphylococcus aureus. Nonetheless, Mycoplasma associated myocarditis should be considered among myocarditis differentials because it requires prompt diagnosis and treatment to reduce its morbidity and debilitating sequelae. 22 This is evident when comparing the long‐term sequelae rate in the first review on M. Pneumonia associated carditis by Pönkä et al with the more recent by Paz et al. In the earlier review from 1979, because of the less frequent use of echocardiography and the scarcity of diagnostic tools, a 44% rate of long‐term sequelae was reported against the 25% rate reported in 2002. 15 , 16 In addition to this, Paz et al found evidence that patients with a more severe respiratory involvement had a higher chance to develop carditis; in details 43% of patients diagnosed with carditis had pneumonia and 19% had pleural effusion. The symptoms at presentation varied, with 38% of cases presenting with chest pain and 43% with palpitations. Of note, EKG abnormalities were present in 100% of the patients. 15
Given the nonspecific signs and symptoms associated with Mycoplasma associated myocarditis, it is of paramount importance to contribute to the current body of literature with cases of unique presentation. In this report, we describe a case of a young adult who presented exclusively with signs of heart failure, and sustained refractory SVT. SVT in association with M. Pneumonia carditis has never been reported in the literature, with sinus tachycardia, ventricular tachycardia, and AV block being the most often described dysrhythmias. 15 , 23 , 24 , 25 The diagnostic process was additionally complicated by the absence of myocardiocytolysis markers, a phenomenon that, while uncommon, has been previously attributed to the observation that severe myocarditis can sometimes present without necrosis. 26 , 27 , 28 The lack of signs of an extra‐cardiac disease, fever, chest pain, systemic symptoms, and cough delayed the diagnostic process as well. This is primarily due to the varied presentation of M. pneumoniae infections as discussed above Moreover, the patient had a history of unspecified recurrent episodes of sustained palpitations during adolescence, prompting the initial misdiagnosis of TIC. This led to several unsuccessful attempts to terminate the arrhythmia. Once the diagnosis of M. Pneumonia‐associated myocarditis was established and the appropriate therapy was initiated, the patient eventually recovered completely with no cardiac sequelae at 6 months and no other episodes of tachyarrhythmia.
The mechanism of arrhythmia in association with myocarditis is poorly understood. Dysrhythmias are commonly seen in Diphtheria‐associated myocarditis as a result of the Diphtheria Exotoxin's activity on the heart conduction system. 23 , 29 M. pneumoniae has been shown to produce an exotoxin as well. 30 , 31 This exotoxin is very similar in structure to the Pertussis exotoxin, which has been associated with a weakening of the negative chronotropic and inotropic action mediated by muscarinic receptors, 32 , 33 leading to tachyarrhythmias. Another possible explanation has been postulated, which sees the myocarditis‐induced interstitial edema as the culprit of conduction disturbances. 34
4. CONCLUSION
We report an unusual case of a young adult exhibiting an atypical presentation of M. Pneumoniae associated myocarditis, with no signs of myocardiocytolysis, pulmonary involvement, or systemic infection. The late appearance of respiratory symptoms, and the refractory, sustained tachyarrhythmia prompted the investigation of possible infectious causes of myocardial disfunction. M. Pneumonia carditis with pneumonia was clinically diagnosed, and corroborated by cardiac MRI findings, IgM antibody titer and PCR test on sputum, and the patient was treated effectively with corticosteroids and Doxycycline, given the higher rates of macrolide‐resistant M.Pneumoniae on the East Coast of the United States. 35 The patient's arrhythmia was terminated 48 h after therapy initiation, and he recovered completely in 4 weeks.
AUTHOR CONTRIBUTIONS
Marco Radaelli: Conceptualization; data curation; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. C. P. T. Leah Keller: Writing – review and editing. Hudson Franca: Data curation; investigation; writing – review and editing. Kshitij Mehrotra: Writing – review and editing.
FUNDING INFORMATION
This research received no specific funding in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
CONSENT
The authors collected patient's permission to anonymously publish his clinical course. The patient accepted and verbalized understanding of the possible implications, he also signed an informed consent which was also signed by all the authors.
Radaelli M, Keller CPTL, Franca H, Mehrotra K. Mycoplasma myocarditis presenting with sustained SVT and acute heart failure without signs of myocardiocytolysis and extra‐cardiac disease. Clin Case Rep. 2024;0:e8851. doi: 10.1002/ccr3.8851
DATA AVAILABILITY STATEMENT
All data underlying the results are available as part of the article and no additional source data are required.
REFERENCES
- 1. Meyer Sauteur PM, Unger WWJ, Van Rossum AMC, Berger C. The art and science of diagnosing mycoplasma pneumoniae infection. Pediatr Infect Dis J. 2018;37:1192‐1195. doi: 10.1097/INF.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 2. Hammerschlag MR. Mycoplasma pneumoniae infections. Curr Opin Infect Dis. 2001;14:181‐186. doi: 10.1097/00001432-200104000-00012 [DOI] [PubMed] [Google Scholar]
- 3. Poddighe D. Extra‐pulmonary diseases related to mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. 2018;30:380‐387. doi: 10.1097/BOR.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 4. Vijay A, Stendahl JC, Rosenfeld LE. Mycoplasma pneumoniae pericarditis. Am J Cardiol. 2019;123:1383‐1384. doi: 10.1016/j.amjcard.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 5. Abdulhadi B, Kiel J. Mycoplasma Pneumonia. PCR Clin Microbiol an Aust Int Perspect. 2022;179‐182. doi: 10.1007/978-90-481-9039-3_21 [DOI] [Google Scholar]
- 6. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697‐728. 10.1128/CMR.17.4.697-728.2004/ASSET/B3CEAEA9-E24C-4462-A3D9-5348EF127B0D/ASSETS/GRAPHIC/ZCM0030420960006.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bajantri B, Venkatram S, Diaz‐Fuentes G. Mycoplasma pneumoniae : a potentially severe infection. J Clin Med Res. 2018;10:535‐544. 10.14740/JOCMR3421W [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manoharan A, Ramya MS, Chandy S, et al. Evaluation of real‐time PCR with serology for diagnosis of community acquired pneumonia caused by mycoplasma pneumoniae . Clin Epidemiol Glob Heal. 2022;16:101084. doi: 10.1016/j.cegh.2022.101084 [DOI] [Google Scholar]
- 9. Ammirati E, Moslehi JJ. Diagnosis and treatment of acute myocarditis: a review. JAMA. 2023;329:1098‐1113. doi: 10.1001/JAMA.2023.3371 [DOI] [PubMed] [Google Scholar]
- 10. Ammirati E, Buono A, Moroni F, et al. State‐of‐the‐art of endomyocardial biopsy on acute myocarditis and chronic inflammatory cardiomyopathy. Curr Cardiol Rep. 2022;24:597‐609. doi: 10.1007/s11886-022-01680-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sands MJ, Satz JE, Turner WE, Soloff LA. Pericarditis and perimyocarditis associated with active mycoplasma pneumoniae infection. Ann Intern Med. 1977;86:544‐548. doi: 10.7326/0003-4819-86-5-544 [DOI] [PubMed] [Google Scholar]
- 12. Marquet Y, Hékimian G, Lebreton G, et al. Diagnostic Yield, Safety and Therapeutic Consequences of Myocardial Biopsy in Clinically Suspected Fulminant Myocarditis Unweanable from Mechanical Circulatory Support. Ann Intensive Care. 2023;13(1):78. doi: 10.1186/s13613-023-01169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol. 2008;127:17‐26. doi: 10.1016/J.IJCARD.2007.10.053 [DOI] [PubMed] [Google Scholar]
- 14. Ferreira VM, Schulz‐Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158‐3176. doi: 10.1016/J.JACC.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 15. Paz A, Potasman I. Mycoplasma‐associated carditis: case reports and review. Cardiology. 2002;97:83‐88. doi: 10.1159/000057677 [DOI] [PubMed] [Google Scholar]
- 16. Pönkä A. Carditis associated with mycoplasma pneumoniae infection. Acta Med Scand. 1979;206:77‐86. doi: 10.1111/J.0954-6820.1979.TB13473.X [DOI] [PubMed] [Google Scholar]
- 17. Defilippi A, Silvestri M, Tacchella A, et al. Epidemiology and clinical features of mycoplasma pneumoniae infection in children. Respir Med. 2008;102:1762‐1768. doi: 10.1016/J.RMED.2008.06.022 [DOI] [PubMed] [Google Scholar]
- 18. Narita M. Pathogenesis of extrapulmonary manifestations of mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16:162‐169. doi: 10.1007/S10156-010-0044-X [DOI] [PubMed] [Google Scholar]
- 19. Fan Q, Meng J, Li P, Liu Z, Sun Y, Yan P. Pathogenesis and association of mycoplasma pneumoniae infection with cardiac and hepatic damage. Microbiol Immunol. 2015;59:375‐380. doi: 10.1111/1348-0421.12267 [DOI] [PubMed] [Google Scholar]
- 20. Narita M. Classification of extrapulmonary manifestations due to mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. 2016;7:23. doi: 10.3389/FMICB.2016.00023/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rroku A, Kottwitz J, Heidecker B. Update on myocarditis—what we know so far and where we may be heading. Eur Hear Journal Acute Cardiovasc Care. 2021;10:455‐467. doi: 10.1177/2048872620910109 [DOI] [PubMed] [Google Scholar]
- 22. Park IH, Choi DY, Oh YK, Kim JD, Yu ST. A case of acute Myopericarditis associated with mycoplasma pneumoniae infection in a child. Korean Circ J. 2012;42:709‐713. doi: 10.4070/KCJ.2012.42.10.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umemoto M, Fujii I, Take H. Advanced atrioventricular block associated with atrial tachycardia caused by mycoplasma pneumoniae infection. Pediatr Int. 1995;37:518‐520. doi: 10.1111/J.1442-200X.1995.TB03367.X [DOI] [PubMed] [Google Scholar]
- 24. Agarwala BN, Ruschhaupt DG. Complete heart block from mycoplasma pneumoniae infection. Pediatr Cardiol. 1991;12:233‐236. doi: 10.1007/BF02310573 [DOI] [PubMed] [Google Scholar]
- 25. Chergui K, Fourme T, Veillard‐Baron A, Loubieres Y, Voyer C, Jardin F. Mycoplasma pneumoniae and second‐degree heart block. Clin Infect Dis. 1998;27:1534‐1535. doi: 10.1086/517739/2/27-6-1534.PDF.GIF [DOI] [PubMed] [Google Scholar]
- 26. Berg J, Kottwitz J, Baltensperger N, et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3‐month follow‐up. Circ Heart Fail. 2017;10:10. doi: 10.1161/CIRCHEARTFAILURE.117.004262 [DOI] [PubMed] [Google Scholar]
- 27. Gilotra NA, Minkove N, Bennett MK, et al. Lack of relationship between serum cardiac troponin I level and Giant cell myocarditis diagnosis and outcomes. J Card Fail. 2016;22:583‐585. doi: 10.1016/J.CARDFAIL.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 28. Fairweather DL, Beetler DJ, Di Florio DN, Musigk N, Heidecker B, Cooper LT. COVID‐19, myocarditis and pericarditis. Circ Res. 2023;132:1302‐1319. doi: 10.1161/CIRCRESAHA.123.321878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh S, Gupta N, Saple P. Diphtheritic myocarditis: a case series and review of literature. J Family Med Prim Care. 2020;9:5769‐5771. doi: 10.4103/JFMPC.JFMPC_1396_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Techasaensiri C, Tagliabue C, Cagle M, et al. Variation in colonization, ADP‐ribosylating and vacuolating cytotoxin, and pulmonary disease severity among mycoplasma pneumoniae strains. Am J Respir Crit Care Med. 2010;182:797‐804. doi: 10.1164/RCCM.201001-0080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kannan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein a binding protein of mycoplasma pneumoniae . Infect Immun. 2005;73:2828‐2834. doi: 10.1128/IAI.73.5.2828-2834.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Böhm M, Schmitz W, Scholz H, Wilken A. Pertussis toxin prevents adenosine receptor‐ and m‐cholinoceptor‐mediated sinus rate slowing and AV conduction block in the Guinea‐pig heart. Naunyn Schmiedeberg's Arch Pharmacol. 1989;339:152‐158. doi: 10.1007/BF00165137 [DOI] [PubMed] [Google Scholar]
- 33. Tuček S, Doležal V, Folbergrová J, Hynie S, Kolář F, Oštádal B. Pertussis toxin inhibits negative inotropic and negative chronotropic muscarinic cholinergic effects on the heart. Pflugers Arch. 1987;408:167‐172. doi: 10.1007/BF00581347 [DOI] [PubMed] [Google Scholar]
- 34. Morimoto SI, Kato S, Hiramitsu S, et al. Role of myocardial interstitial edema in conduction disturbances in acute myocarditis. Heart Vessel. 2006;21:356‐360. doi: 10.1007/S00380-006-0922-4 [DOI] [PubMed] [Google Scholar]
- 35. Waites KB, Ratliff A, Crabb DM, et al. Macrolide‐resistant mycoplasma pneumoniae in the United States as determined from a national surveillance program. J Clin Microbiol. 2019;57(11):e00968‐19. doi: 10.1128/JCM.00968-19/SUPPL_FILE/JCM.00968-19-S0001.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.


