Abstract
Background
In the United States, lung cancer death rates have been declining for decades, primarily as a result of pronounced decreases in cigarette smoking. It is unclear, however, whether there have been similar declines in mortality rates of lung cancer unrelated to smoking. We estimated trends in US lung cancer death rates attributable and not attributable to smoking from 1991 to 2018.
Methods
The study included 30- to 79-year-olds in the National Health Interview Survey who were linked to the National Death Index, 1991-2014. Adjusted hazard ratios for smoking status and lung cancer death were estimated, and age-specific population attributable fractions were calculated. Annual population attributable fractions were multiplied by annual US national lung cancer mortality, partitioning rates into smoking-attributable and smoking-unrelated lung cancer deaths. All statistical tests were 2-sided.
Results
During 1991-2018, the proportion of never smokers increased among both men (35.1%-54.6%) and women (54.0%-65.4%). Compared with those who had ever smoked, those who had never smoked had 86% lower risk (hazard ratio = 0.14; 95% confidence interval [CI] = 0.12 to 0.16) of lung cancer death. The fraction of lung cancer deaths attributable to smoking decreased from 81.4% (95% CI = 78.9 to 81.4) to 74.7% (95% CI = 78.1 to 71.4). Smoking-attributable lung cancer death rates declined 2.7% per year (95% CI = ‒2.9% to ‒2.5%) and smoking-unrelated lung cancer death rates declined 1.8% per year (95% CI = ‒2.0% to ‒1.5%); these declines have accelerated in recent years.
Conclusions
An increasing proportion of lung cancer deaths are unrelated to smoking based on declines in smoking prevalence. Smoking-unrelated lung cancer death rates have declined, however, perhaps because of decreases in secondhand smoke and air pollution exposure as well as treatment improvements.
Lung cancer is the leading cause of cancer death in the United States, with 127 000 deaths expected to occur in 2023, comprising 21% of all cancer deaths (1). Lung cancer death rates have declined substantially over the last 20 years, driven largely by declines in cigarette smoking. Declines have accelerated in recent years, attributed to improvements in lung cancer treatment (2,3).
Cigarette smoking has been estimated to cause 80% to 90% of lung cancer cases in the United States (4). Nevertheless, the number of lung cancer cases occurring among those who have never smoked (“never smokers”) is substantial, with 1 study estimating lung cancer among never smokers to be the seventh-largest contributor to cancer death in the United States in 2004 (5). Contemporary estimates of lung cancer mortality that reflect declining cigarette smoking prevalence (from 26% in 1991 to 13% in 2020) (6) and the increasing proportion of never smokers in the US population are needed. Several studies have reported an increasing fraction of lung cancer cases occurring among never smokers. Such changes could reflect rising rates of lung cancer from causes other than cigarettes. Such trends, however, could also simply reflect the increasing proportion of the US population that has never smoked (7,8). As smoking is not thoroughly collected either by US cancer registries or on death certificates, studies examining rates of smoking-unrelated lung cancer incidence and mortality have relied on large prospective cohort studies. Such studies generally do not show evidence for increasing rates of smoking-unrelated lung cancer over time (5,9,10). These cohorts are not representative of the US population, as they tend to include participants who are more affluent and healthier than the general population and thus may have different rates of lung cancer unrelated to smoking.
It is important to quantify trends in death rates due to lung cancers that are not attributable to smoking as increasing rates would imply growing exposure to other causes of lung cancer. In the current analysis, we used data from the National Health Interview Survey (NHIS) and national death certificate data to partition lung cancer death rates into those attributable and not attributable to cigarette smoking and examined trends from 1991 to 2018.
Methods
Study population and case definition
National Health Interview Survey
Population attributable fractions for smoking and lung cancer mortality were estimated with data from the NHIS, a nationally representative survey of the health of the US civilian noninstitutionalized population (11). Enrollees completed personal household surveys on which they reported demographics, health conditions, and health behaviors, including self-reported smoking status. Those with missing smoking status information (0.07%) were excluded. NHIS surveys from 1991 to 2015 were linked to the National Death Index to ascertain date and cause of death. Deaths due to neoplasms of the trachea, bronchus, and lung were defined by 113 Causes of Death Recode (International Statistical Classification of Diseases, Tenth Revision [ICD-10] codes C33-C34). Individuals were aged 30 years or older at baseline and were followed from the survey date to the first of death or age 80 years. NHIS-National Death Index linked data are available through application to the National Center for Health Statistics (NCHS). Institutional review board approval was not needed as the data were all deidentified and publicly available.
NCHS death certificate data
Data on national trends in age-standardized death rates were estimated with NCHS death certificate data from 1991 to 2018 and analyzed in SEER*Stat software version 8.4.2 (12). Deaths from lung cancer were identified with International Classification of Diseases, Ninth Revision, code 162 and ICD-10 codes C33-C34. Death certificate data are publicly available through NCHS.
Statistical analysis
The association between smoking status (current, former, never smoker) and lung cancer mortality was estimated with hazard ratios (HRs) from Cox proportional hazards regression, with age as the time scale, adjusting for self-reported sex, self-reported race and ethnicity (Hispanic, non-Hispanic Black [ie, Black], non-Hispanic White [ie, White], non-Hispanic all other races combined, and missing), and education (high school or less, some college, ≥4 years of college, missing), using appropriate sample weights to account for the complex survey design. Other racial groups included individuals who identify as Asian, Pacific Islander, American Indian, or Alaska Native. Hazard ratios were estimated overall and stratified by survey year (1991-1993, 1994-1995, 1997-1999, 2000-2002, 2003-2005, 2006-2008, 2009-2011, and 2012-2014), sex, time, age group (30-49, 50-69, and 70-79 years), and race and ethnicity. In age-stratified analyses, participants were censored when they reached the upper limit of the age range. Prevalence of smoking was estimated from each annual NHIS, when this information was collected (1991-1995, 1997-2018). As smoking data were not available in 1996, the weighted number of never, former, and current smokers was estimated as the average of the 1995 and 1997 numbers.
Population attributable fractions for each calendar year were computed by combining the adjusted hazard ratios for all the covariates in the Cox regression model, with the covariate distributions from the NHIS time period (13). Population attributable fractions can be interpreted as the proportion of lung cancer deaths that could be avoided if everyone were a never smoker. As hazard ratios differed substantially by age group, population attributable fraction estimates were also estimated separately for those aged 30 to 49, 50 to 69, and 70 to 79 years. NHIS data used in this paper were analyzed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC) and SAS-callable SUDAAN statistical software, version 11.01 (RTI International, Research Triangle Park, NC), which accounted for the complex sample design of the NHIS for the estimation of smoking prevalence, hazard ratios, and population attributable fraction estimation.
Age-stratified population attributable fractions were then multiplied by annual lung cancer deaths among those aged 30 to 49, 50 to 69, and 70 to 79 years to partition the number of lung cancer deaths attributable to smoking (ie, smoking-attributable lung cancer deaths) and unrelated to smoking (ie, smoking-unrelated lung cancer deaths). We then estimated annual age-standardized lung cancer death rates and annual percent changes in smoking-attributable and smoking-unrelated lung cancer death rates with Joinpoint software (14), which identifies calendar years where there is a statistically significant change in the slope of mortality rate trends over time. Average annual percent changes also were estimated for the full 1991-2018 time period.
All statistical tests were 2-sided, and P are less than or equal to .05 was considered statistically significant.
Results
In total, 578 302 people aged 30 to 79 years participated in the annual NHIS from 1991 to 2014 (excluding 1996 because of missing smoking information) (Table 1). During follow-up through 2015, a total of 3221 study participants died from lung cancer (Supplementary Table 1, available online). Compared with the full population, people who went on to die from lung cancer were more likely to be men (58.0% vs 48.1%), 50 years of age or older at interview (88.8% vs 48.3%), White (82.5% vs 72.2%), and have no more than a high school education (66.3% vs 44.0%). More than half of the participants who died from lung cancer were current smokers at baseline (53.1%), 36.7% were former smokers, and 10.2% were never smokers.
Table 1.
Characteristics of the National Health Interview Survey participants included in the study and those who died from lung cancer during follow-up
| All participants |
Lung cancer deaths |
|||
|---|---|---|---|---|
| No. | Weighted % | No. | Weighted % | |
| All | 578 302 | 100.0 | 3221 | 100.0 |
| Sex | ||||
| Men | 256 273 | 48.1 | 1781 | 58.0 |
| Women | 322 029 | 51.9 | 1440 | 42.0 |
| Age at interview, y | ||||
| 30-39 | 149 251 | 25.9 | 57 | 1.8 |
| 40-49 | 136 986 | 25.7 | 279 | 9.5 |
| 50-59 | 119 486 | 21.7 | 715 | 23.4 |
| 60-69 | 100 577 | 16.2 | 1372 | 42.7 |
| 70-79 | 72 002 | 10.4 | 798 | 22.7 |
| Race and ethnicity | ||||
| Black | 79 656 | 11.1 | 495 | 10.9 |
| Hispanic | 81 898 | 11.5 | 157 | 3.5 |
| White | 388 069 | 72.2 | 2489 | 82.5 |
| Other racial groupsa | 28 114 | 5.2 | 75 | 3.0 |
| Missing | 565 | 0.1 | 5 | 0.1 |
| Education completed | ||||
| High school or less | 265 873 | 44.0 | 2173 | 66.3 |
| Some college (<4 y) | 154 528 | 26.6 | 663 | 20.8 |
| ≥4 y of college | 155 182 | 28.8 | 366 | 12.3 |
| Missing | 2719 | 0.5 | 19 | 0.6 |
| Smoking status | ||||
| Never | 306 210 | 53.4 | 319 | 10.2 |
| Former | 146 449 | 25.7 | 1157 | 36.7 |
| Current | 125 643 | 20.9 | 1745 | 53.1 |
“Other racial groups” include individuals who identify as Asian, Pacific Islander, American Indian, or Alaska Native.
The risk of lung cancer death was 86% less in never smokers than in ever smokers (ie, former or current smokers) (HR = 0.14, 95% confidence interval [CI] = 0.12 to 0.16), with a 78% lower risk than former smokers and a 92% lower risk than current smokers. The inverse association between never having smoked and lung cancer death was stronger in women than in men and among Black and White participants than among Hispanic participants and participants in other racial groups (Table 2). The association was also stronger among 70- to 79-year-olds (HR = 0.11, 95% CI = 0.09 to 0.14) and 50- to 69-year-olds (HR = 0.15, 95% CI = 0.12 to 0.18) than among 30- to 49-year-olds (HR = 0.31, 95% CI = 0.19 to 0.53). The association between smoking and lung cancer death did not differ by survey year (Supplementary Figure 1, available online).
Table 2.
Risk of lung cancer death, by baseline smoking statusa
| Never vs ever smokers | Never vs former smokers | Never vs current smokers | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Total | 0.14 (0.12 to 0.16) | 0.22 (0.19 to 0.26) | 0.08 (0.07 to 0.09) |
| Sex | |||
| Men | 0.19 (0.15 to 0.23) | 0.30 (0.24 to 0.38) | 0.10 (0.08 to 0.12) |
| Women | 0.11 (0.09 to 0.13) | 0.17 (0.14 to 0.21) | 0.07 (0.05 to 0.08) |
| Race and ethnicity | |||
| Black | 0.12 (0.09 to 0.17) | 0.19 (0.12 to 0.28) | 0.09 (0.06 to 0.12) |
| Hispanic | 0.48 (0.30 to 0.75) | 0.69 (0.38 to 1.22) | 0.30 (0.18 to 0.50) |
| White | 0.11 (0.09 to 0.13) | 0.19 (0.16 to 0.23) | 0.06 (0.05 to 0.07) |
| Other racial groupsb | 0.46 (0.24 to 0.87) | 0.71 (0.34 to 1.49) | 0.28 (0.13 to 0.61) |
| Age, y | |||
| 30-49 | 0.31 (0.19 to 0.53) | 0.63 (0.35 to 1.16) | 0.22 (0.12 to 0.39) |
| 50-69 | 0.15 (0.12 to 0.18) | 0.27 (0.21 to 0.34) | 0.08 (0.07 to 0.10) |
| 70-79 | 0.11 (0.09 to 0.14) | 0.16 (0.13 to 0.21) | 0.06 (0.05 to 0.07) |
Model used age as the time scale and adjusted for sex, race and ethnicity, and education level.
“Other” include individuals who identify as Asian, Pacific Islander, American Indian, or Alaska Native.
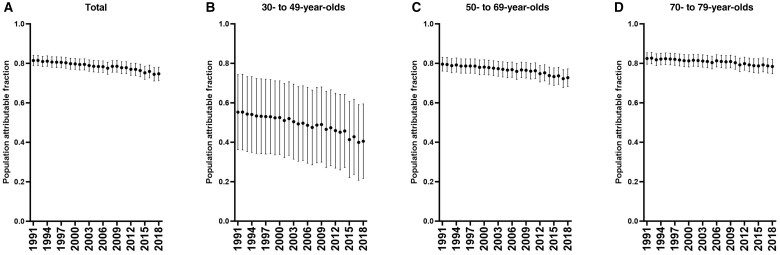
Population attributable fractions were estimated from age-specific hazard ratios (those aged 30-49, 50-69, and ≥70 years) and annual smoking prevalence estimates in 5-year age groups. During 1991-2018, the prevalence of current smoking declined from 29.6% to 16.9% among men and from 24.5% to 13.3% among women, while the prevalence of being a former smoker decreased among men (35.4% to 28.5%) and remained stable among women (21.5% to 21.3%). During 1991-2018, there were 3.36 million lung cancer deaths among people aged 30 to 79 years in the US population. The fraction of lung cancer deaths attributable to smoking decreased during 1991-2018 from 81.4% (95% CI = 78.9 to 84.0) to 74.7% (95% CI = 71.4 to 78.1). Declines in population attributable fractions occurred across age groups: 55.2% (95% CI = 36.3 to 74.2) to 40.5% (95% CI = 21.6 to 59.4) among 30- to 49-year-olds, 79.6% (95% CI = 76.1 to 83.1) to 72.7% (95% CI = 68.3 to 77.1) among 50- to 69-year-olds, and 82.5% (95% CI = 79.6 to 85.3) to 78.4% (95% CI = 74.8 to 82.0) among 70- to 79-year-olds (Figure 1).
Figure 1.
Population attributable fractions for ever smokers and lung cancer mortality, by calendar year, 1991-2018. Points represent population attributable fractions, and lines represent 95% confidence intervals. A) All ages; B) 30- to 49-year-olds; C) 50- to 69-year-olds; and D) 70- to 79-year-olds.
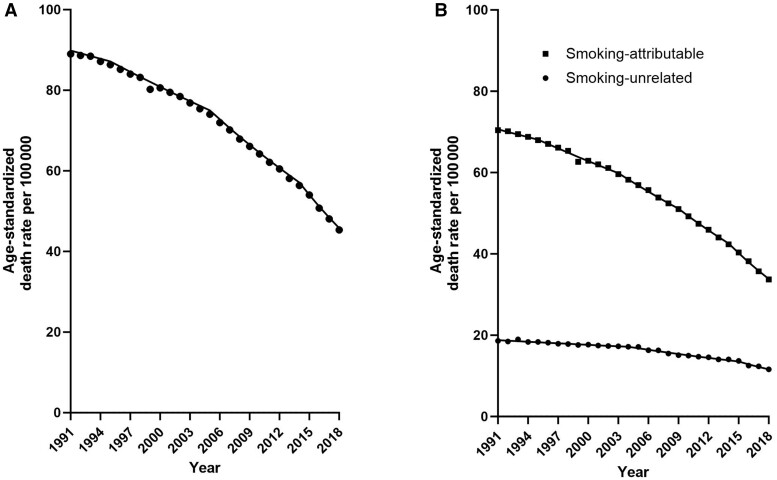
Age-standardized lung cancer death rates declined 49.0% from 89.0 per 100 000 (n = 122 154 deaths) to 45.3 per 100 000 (n = 105 248) during 1991-2018 (Figure 2). Rates of smoking-attributable lung cancer death declined from 70.4 to 33.7 per 100 000 (n = 96 897 in 1991 to n = 78 450 in 2018), and smoking-unrelated lung cancer death rates declined from 18.6 to 11.6 per 100 000 (n = 25 257 in 1991 to n = 26 798 in 2018) (Supplementary Table 2, available online). During 1991-2018, rates of smoking-attributable lung cancer declined an average of 2.7% per year (95% CI = ‒2.9 to ‒2.5), and smoking-unrelated lung cancer death rates declined an average of 1.8% per year (95% CI = ‒2.0 to ‒1.5) (Table 3). Declines in both smoking-attributable (2014-2018 annual percent change, ‒5.6%/year, 95% CI = ‒6.3% to ‒4.9%, vs 1991-1995 annual percent change, ‒0.9%/year, 95% CI = ‒1.4% to ‒0.4%) and smoking-unrelated lung cancer death rates (2015-2018 annual percent change, ‒5.0%/year, 95% CI = ‒6.8% to ‒3.0% vs 1991-2004 annual percent change, ‒0.7%/year, 95% CI = ‒0.9% to ‒0.5%) accelerated in recent years.
Figure 2.
US age-standardized lung cancer death rates, 1991-2018 A) overall and B) partitioned into death rates due to smoking-attributable (squares) and smoking-unrelated lung cancer (dots).
Table 3.
Annual percent change and average annual percent change in smoking-attributable and smoking-unrelated lung cancer death rates
| Smoking-attributable lung cancer deaths |
Smoking-unrelated lung cancer deaths |
|||
|---|---|---|---|---|
| Years | Estimate | Years | Estimate | |
| Age-standardized death rate per 100 000 | 1991 | 70.4 | 1991 | 18.6 |
| 2018 | 33.7 | 2018 | 11.6 | |
| Average annual percent change, %/y (95% confidence interval) | 1991-2018 | ‒2.7 (‒2.9 to ‒2.5) | 1991-2018 | ‒1.8 (‒2.0 to ‒1.5) |
| Annual percent change, %/y (95% confidence interval) | 1991-1995 | ‒0.9 (‒1.4 to ‒0.4) | 1991-2004 | ‒0.7 (‒0.9 to ‒0.5) |
| 1995-2003 | ‒1.6 (‒1.8 to ‒1.4) | 2004-2015 | ‒2.1 (‒2.4 to ‒1.8) | |
| 2003-2009 | ‒2.6 (‒3.0 to ‒2.2) | 2015-2018 | ‒5.0 (‒6.8 to ‒3.0) | |
| 2009-2014 | ‒3.6 (‒4.3 to ‒2.9) | — | — | |
| 2014-2018 | ‒5.6 (‒6.3 to ‒4.9) | — | — | |
Discussion
The fraction of lung cancer deaths attributable to smoking in the United States declined from 81% in 1991 to 75% in 2018, driven by consistent declines in smoking prevalence over time. We estimated that on average, death rates of smoking-attributable lung cancer declined 2.7% per year during 1991-2018, while smoking-unrelated lung cancer death rates declined 1.8% per year over the same time period. For both smoking-attributable lung cancer and smoking-unrelated lung cancer, the declines in death rates accelerated during the last several years of follow-up.
Several studies have reported that an increasing fraction of lung cancers are occurring among never smokers; however, the population of never smokers has grown over time (7,8). Adult smoking prevalence in the United States is at an all-time low, falling from 41.9% in 1965 to 12.7% in 2020, while youth smoking prevalence peaked in 1997 at 36.4% and declined to 5.4% in 2018 (6). These changes reflect both increases in smoking cessation and decreases in smoking initiation, and the proportion of never smokers in the US population continues to increase.
Overall declines in lung cancer mortality have been driven by both decreases in lung cancer incidence and improvements in survival after diagnosis (15). In our study, we separately estimated trends in smoking-attributable and smoking-unrelated lung cancer mortality. We found that rates of smoking-attributable lung cancer declined more rapidly than smoking-unrelated lung cancer mortality, likely driven by decreases in the prevalence of smoking and improvements in treatment (15). Survival has improved for non–small cell lung carcinomas, which make up about three-quarters of lung cancer cases in the United States. Recent improvements in lung cancer mortality have been attributed to immune-based therapies and therapies targeted at oncogenic driver mutations in the EGFR and ALK genes (3,15). Declines in smoking-unrelated lung cancer mortality may reflect improved treatments, given that lung cancer among nonsmokers is likely to be non–small cell adenocarcinoma, and alterations in EGFR and ALK have been found in lung cancers occurring in never smokers (16). Lung cancer screening with low-dose computed tomography has been recommended based on age and smoking history criteria by the US Preventive Services Task Force since 2013 (17,18). Low-dose computed tomography has been shown to reduce the risk of death and may have contributed to recent declines in lung cancer death rates (19), particularly for smoking-attributable lung cancers. The impact of low-dose computed tomography has been limited, however, given the low uptake of this screening modality (20-22).
It is also possible that the decreasing prevalence of other exposures has contributed to the declines in smoking-unrelated lung cancer deaths. For example, secondhand smoke exposure among never smokers decreased substantially, from 83% in 1988-1994 to 26% in 2017-18 (6), and secondhand smoke exposure increases lung cancer risk 20% to 30% (23). Radon exposure also increases lung cancer risk among both smokers and never smokers (24,25). The number of homes that have operating radon mitigation systems has increased over time (26), which could also have influenced lung cancer mortality rates. Air pollution is another cause of lung cancer (27), and exposure to particulate matter 2.5 µm or smaller in diameter has declined over the past 20 years (28).
In this study, 15.3% of lung cancer deaths were not attributable to smoking in 2018, which would translate to about 19 400 deaths in 2023 if this fraction remained stable. Ideally, we would have directly estimated trends in lung cancer mortality rates among people who have never smoked; however, this was not possible, given the limited number of lung cancer deaths in this group in the linked NHIS-mortality data. Instead, we estimated trends in smoking-unrelated lung cancer deaths using population attributable fractions, with the entire population as the rate denominator. Although a fraction of these deaths occurred in smokers, most are likely to have occurred among never smokers due to the strong association between smoking and lung cancer. Given that the population of never smokers has grown over time in the United States, our results strongly suggest that lung cancer mortality rates in never smokers have declined over the time period.
The main strength of this analysis is the use of nationally representative survey data from a large sample of the US population over nearly 2 decades combined with lung cancer death rates from national death certificate data. Even with such a large study, the limited number of lung cancer deaths that occurred among never smokers precluded us from cross-stratifying hazard ratios by age, sex, and racial and ethnic groups. As the largest differences in hazard ratios were seen across age groups, we stratified population attributable fractions in 3 age groups and assumed that the hazard ratios were constant within each age group across sex and race and ethnicity. Given prior evidence that the fraction of lung cancers among never smokers varies substantially across joint categories of sex and race and ethnicity, with a notably higher fraction of lung cancers among Asian women who never smoked (29), this is an important limitation. The weaker association between smoking and lung cancer death among Hispanic individuals may be due to fewer cigarettes smoked per day in this group (22). Additional years of follow-up in the NHIS-mortality linkage may yield sufficient power to further stratify these calculations in the future. In addition, though lung cancer mortality largely reflects lung cancer incidence due to the overall poor prognosis of lung cancer, it would be of interest to similarly estimate trends for lung cancer incidence overall and by histology, given the strength of the association between smoking and lung cancer, and survival differs by histologic subtype (3,4). Finally, smoking status was self-reported in the NHIS. Misreporting of smoking status could have affected our hazard ratios and smoking prevalence estimates.
We showed that in the United States, rates of both smoking-attributable and smoking-unrelated lung cancer mortality declined significantly during 1999-2018, with accelerated declines observed in the most recent years. Declines in rates of smoking-unrelated lung cancer deaths are likely driven by improved treatments, decreased exposure to secondhand smoke, and possibly by decreased exposure to nontobacco risk factors. As the prevalence of never smokers continues to increase, the fraction of lung cancer caused by smoking will continue to decline over time. Cigarette smoking still causes 3 of 4 lung cancer deaths, however, as well as other cancers and chronic diseases. Sustained public health efforts are urgently needed to further reduce smoking prevalence and disparities, particularly in communities where smoking prevalence remains high.
Supplementary Material
Acknowledgements
The funder reviewed a final version of this paper but was not involved in the study inception, analysis, or interpretation of findings. Results were previously presented in an oral presentation at the June 2022 Annual Meeting of the Society of Epidemiologic Research in Chicago, Illinois.
Contributor Information
Meredith S Shiels, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Barry I Graubard, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Timothy S McNeel, Information Management Services, Calverton, MD, USA.
Lisa Kahle, Information Management Services, Calverton, MD, USA.
Neal D Freedman, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Data availability
NHIS-National Death Index linked data are available through application to the NCHS: https://www.cdc.gov/nchs/data-linkage/mortality-restricted.htm. NHIS prevalence data and NCHS death certificate data are publicly available.
Author contributions
Meredith Shiels, PhD (Conceptualization; Formal analysis; Writing—original draft), Barry Graubard, PhD (Formal analysis; Methodology; Writing—review & editing), Tim McNeel, BA (Data curation; Formal analysis; Writing—review & editing), Lisa Kahle, BA (Data curation; Formal analysis; Writing—review & editing), Neal Freedman, PhD (Conceptualization; Writing—review & editing).
Funding
This work was funded by the Intramural Research Program of the National Cancer Institute.
Conflict of interest
The authors have no disclosures or conflicts of interest to declare.
References
- 1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta, GA: American Cancer Society; 2023.
- 2. Islami F, Ward EM, Sung H, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst. 2021;113(12):1648-1669. doi: 10.1093/jnci/djab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freedman ND, Abnet CC, Caporaso NE, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol. 2016;45(3):846-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Cancer Institute. Online Summary of Trends in US Cancer Control Measures: Prevention. https://progressreport.cancer.gov/prevention. Accessed September 6, 2023.
- 7. Pelosof L, Ahn C, Gao A, et al. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J Natl Cancer Inst. 2017;109(7):djw295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samet JM. Is the incidence of adenocarcinoma of the lung rising in never smokers? J Natl Cancer Inst. 2017;109(7):djw235. [DOI] [PubMed] [Google Scholar]
- 9. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakoda LC, Alabaster A, Sumner ET, et al. Trends in smoking-specific lung cancer incidence rates within a US integrated health system, 2007-2018. Chest. 2023;164(3):785-795. 10.1016/j.chest.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 11. National Center for Health Statistics. National Health Interview Survey. https://www.cdc.gov/nchs/nhis/index.htm. Accessed September 6, 2023.
- 12.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. SEERStat Database: Mortality—All COD, Total U.S. (1990-2019) <Katrina/Rita Population Adjustment> - Linked To County Attributes—Total U.S., 1969-2019 Counties. DCCPS, Surveillance Research Program. 2021. https://www.seer.cancer.gov. Accessed September 6, 2023.
- 13. Graubard BI, Flegal KM, Williamson DF, Gail MH.. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Stat Med. 2007;26(13):2639-2649. [DOI] [PubMed] [Google Scholar]
- 14. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Joinpoint Regression Program, Version 5.0.2.2023. https://surveillance.cancer.gov/joinpoint/. Accessed September 6, 2023.
- 15. Shiels MS, Lipkowitz S, Campos NG, et al. Opportunities for achieving the cancer moonshot goal of a 50% reduction in cancer mortality by 2047. Cancer Discov. 2023;13(5):1084-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang T, Joubert P, Ansari-Pour N, et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat Genet. 2021;53(9):1348-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. [DOI] [PubMed] [Google Scholar]
- 18. Krist AH, Davidson KW, Mangione CM, et al. ; USPST Force. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962-970. [DOI] [PubMed] [Google Scholar]
- 19. Church TR, Black WC, Aberle DR, et al. National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams RM, Li T, Luta G, et al. Lung cancer screening use and implications of varying eligibility criteria by race and ethnicity: 2019 Behavioral Risk Factor Surveillance System data. Cancer. 2022;128(9):1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rustagi AS, Byers AL, Keyhani S.. Likelihood of lung cancer screening by poor health status and race and ethnicity in US adults, 2017 to 2020. JAMA Netw Open. 2022;5(3):e225318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen-Grozavu FT, Pierce JP, Sakuma KK, et al. Widening disparities in cigarette smoking by race/ethnicity across education level in the United States. Prev Med. 2020;139:106220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA; 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed September 6, 2023.
- 24. Field RW, Steck DJ, Smith BJ, et al. Residential radon gas exposure and lung cancer: The Iowa Radon Lung Cancer Study. Am J Epidemiol. 2000;151(11):1091-1102. [DOI] [PubMed] [Google Scholar]
- 25. Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330(7485):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. National Cancer Institute Cancer Trends Progress Report. https://progressreport.cancer.gov/prevention/radon. Accessed November 10, 2023.
- 27. Wang N, Mengersen K, Kimlin M, et al. Lung cancer and particulate pollution: a critical review of spatial and temporal analysis evidence. Environ Res. 2018;164:585-596. [DOI] [PubMed] [Google Scholar]
- 28.United States Environmental Protection Agency. Particulate Matter (PM2.5) Trends. https://www.epa.gov/air-trends/particulate-matter-pm25-trends. Accessed September 6, 2023.
- 29. Pinheiro PS, Callahan KE, Medina HN, et al. Lung cancer in never smokers: distinct population-based patterns by age, sex, and race/ethnicity. Lung Cancer. 2022;174:50-56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NHIS-National Death Index linked data are available through application to the NCHS: https://www.cdc.gov/nchs/data-linkage/mortality-restricted.htm. NHIS prevalence data and NCHS death certificate data are publicly available.