Abstract
We studied the carriage rate, distribution of serotype, and antimicrobial profile of Streptococcus pneumoniae (S. pneumoniae) among patients with acute respiratory tract infections (ARTI) in two primary health centres and a tertiary referral hospital from 2019 to 2020 in Manado, North Sulawesi, Indonesia before 13-valent pneumococcal conjugate vaccine (PCV13) introduction. A total of 106 nasopharyngeal swab samples were collected from children and adult patients. Serotyping of S. pneumoniae strain was performed by sequential multiplex PCR and Quellung reaction. Antimicrobial profile was performed by the disc diffusion method. We identified thirty-one patients carried S. pneumoniae strains (29 %). The S. pneumoniae carriage rate was found to be higher among children aged 2–5 years (13/32; 40.6 %) than in children under 1 year (8/27; 29.6 %), children and adolescents under 18 years of age (5/20; 25.0 %) and adult patients (5/27; 18.5 %). The distribution of serotypes varied, including 14, 18C, 19A, 23F, 19F and 35B (two strains each) and 1, 3, 6B, 6C, 31, 9V, 15C, 16F, 17F, 23A, 35F (one strain each) and non-typeable (9/31; 29 %). We found S. pneumoniae isolates were susceptible to vancomycin (30/31; 97 %), chloramphenicol (29/31; 94 %), clindamycin (29/31; 94 %), erythromycin (22/31; 71 %), azithromycin (22/31; 71 %), tetracycline (14/31; 45 %), penicillin (11/31; 35 %), and sulfamethoxazole/trimethoprim (10/31; 32 %). This study provides supporting baseline data on distribution of serotype and antimicrobial profile of S. pneumoniae among patients with ARTI before PCV13 introduction in Manado, North Sulawesi, Indonesia.
Keywords: acute respiratory infection, Indonesia, Streptococcus pneumoniae
Data Summary
All data on distribution of serotype and antimicrobial profiles of S. pneumoniae strains are available in Table S1, available in the online version of this article.
Introduction
Streptococcus pneumoniae (S. pneumoniae) or pneumococcus is a Gram-stain-positive bacterium able to asymptomatically colonize the nasopharynx of humans. The nasopharynx is the primary reservoir for the pneumococcus and a major source of transmission for the bacterium to spread into the community [1]. Prolonged pneumococcal nasopharyngeal carriage can in due course progress into invasive pneumococcal disease [1]. This opportunistic pathogen can cause severe disease when sterile sites such as the lungs, bloodstream, and meninges are encountered [2]. Viral upper respiratory tract infections often predispose to bacterial pneumonia as well, most likely by facilitating the invasion of bacteria such as the pneumococci colonizing the nasopharynx [3]. Pneumococcal disease has higher prevalence in individuals who have a weakened immune system such as young children, elderly adults, and the immunocompromised [4].
Several epidemiological data on S. pneumoniae carriage have been reported from the population in various regions of Indonesia [5,10]. Safari, D, et al. reported that S. pneumoniae strains were isolated from 37 of 159 adult and children outpatients with ARTI (18.5 %) at a district hospital in Tabanan, Bali, Indonesia, in 2017 with serotype 6A/6B being the most common serotype among cultured strains [11]. However, there are no other reports from other regions in Indonesia describing the pneumococcal serotype and antimicrobial profile isolated from nasopharynx of patients with ARTI. Meanwhile, in Indonesia, 13-valent pneumococcal conjugate vaccine (PCV13) vaccine, containing 13 invasive serotypes as follows: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, 23F, was introduced into routine infant immunization nationwide in 2022. Therefore, this study is important to support baseline community survey data in Indonesia before vaccine implementation. In this study, we have investigated the carriage rates, distribution of serotype, and antimicrobial profile of S. pneumoniae from children and adult patients in two primary health centres and a tertiary referral hospital in Manado, North Sulawesi, Indonesia between 2019 to 2020 before PCV13 vaccine introduction.
Methods
Nasopharyngeal swab specimen
We collected nasopharyngeal (NP) swab specimens from Puskesmas Tuminting and Puskesmas Bahu (primary health centres) and R. D. Kandou Hospital, a tertiary referral hospital in Manado, North Sulawesi, Indonesia between May 2019 to March 2020. The patients enrolled in the study included outpatients admitted to Tuminting and Bahu primary health centre sites and inpatients admitted to R. D. Kandou Hospital (Table S1). We enrolled the patients with fever, ≥37.5 °C, or history of fever with one or more of the respiratory symptoms: cough, nasal obstruction, rhinorrhea, and sore throat [12]. Data on demography, risk factors, and clinical symptoms were collected on day of admission by trained primary health centre or hospital staff.
NP swabs from patients were collected using a swab (flexible nasopharyngeal flocked swab; Catalogue no. 516CS01 for infant, Catalogue no. 503CS01 for subjects >1-year-old). Swab specimens were placed into STGG (1.0 ml of skim-milk, tryptone, glucose, and glycerol) transport medium and were shipped on ice pack box to the R. D. Kandou Hospital laboratory and were stored at −80 °C prior to testing.
Bacterial isolation and identification
Streptococcus pneumoniae strains were isolated and identified as described previously [6]. Briefly, NP-STGG sample (volume=200 µl) was inoculated to 5 ml Todd-Hewitt-0.5 % yeast extract broth supplemented with 1 ml of rabbit serum followed by vortexing. The mixture was then incubated for 5 h at 37 °C with 5 % CO2. Thereafter, 10 µl of each mixture was plated onto an 8 % sheep blood agar plate with TSA II [BD] as base agar followed by incubation at 37 °C with 5 % CO2 for 18–20 h. The alpha-hemolytic colonies were subcultured and tested for optochin susceptibility. Gram-staining, and bile solubility tests were conducted to confirm the identification of S. pneumoniae. Gram-stain-positive, and optochin susceptible isolates were harvested into STGG and stored at −80 °C for further identification. DNA extraction was performed by enzymatic fast DNA extraction as as described previously [13]. Serotype determination was performed by a sequential multiplex PCR and Quellung reaction [14]. The isolate with bile solubility positive results but negative antisera reaction was classified as a non-typeable isolate.
Antimicrobial profile testing
All S. pneumoniae isolates were tested for the antimicrobial profile using the disc diffusion method. The antimicrobial profile test was performed according to Clinical and Laboratory Standards Institute and on Mueller-Hinton agar with 5 % sheep blood using suspension prepared in Mueller-Hinton broth equal to 0.5 Mc Farland [6]. The antimicrobial test was included erythromycin [oxoid], chloramphenicol [oxoid], trimethoprim-sulfamethoxazole [oxoid], clindamycin [oxoid], azithromycin [oxoid], tetracycline [oxoid], and vancomycin [oxoid], Susceptibility to penicillin was tested with the oxacillin disc. S. pneumoniae ATCC 49619 was used as the control strain.
Data analysis
The chi-square test was performed to compare the differences in the proportion of positive S. pneumoniae cultures among age, sex, symptoms, onset of fever, family member, chest X-ray, and diagnosis groups. SPSS Statistics 25 (IBM Corp, NY, USA) and Prism v.9.0.2 (GraphPad Software, La Jolla, CA, USA) were used to performed statistical analysis.
Results
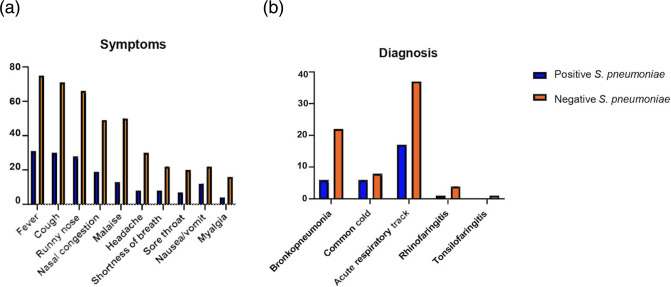
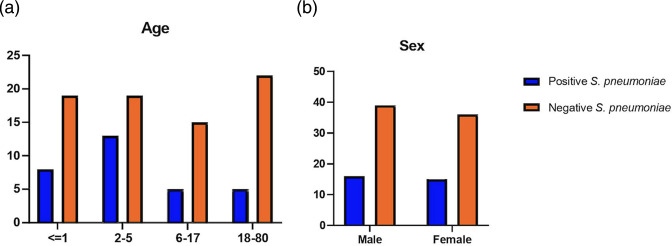
We collected NP Swab specimens from 106 of patients with ARTI in Manado, North Sulawesi (Data S1). The patient characteristics are presented in (Table 1). In this study, the majority of the subjects were children and adolescents under 18 years of age (79/106, 74.5 %). We found that the common clinical symptoms were fever, cough, and runny nose (Fig. 1a). Chest X-ray data were available from 21 out of 30 patients from hospital, which were reported as infiltrates. Majority of patients were diagnosed with acute respiratory tract infection (50.9 %) followed by bronchopneumonia (26.4 %) and common cold (13.2 %) (Fig. 1b). We found that 31 patients carried S. pneumoniae strains (29 %). The nasopharyngeal carriage of S. pneumoniae was found to be higher among children aged 2–5 years (13/32; 40.6 %) than in children under 1 year (8/27; 29.6 %), children and adolescents under 18 years of age (5/20; 25.0 %) and adult patients (5/27; 18.5 %) (Fig. 2a). This study shows that number of men and women infected with pneumococcus was almost equal (Fig. 2b).
Table 1. Characteritics of patients with acute respiratory tract infections in Manado, North Sulawesi, Indonesia.
| Variables | Total N (%) | Total positive pneumococcus N (%) | P value | |
| Age (years) | 0.2981 | |||
| 0–1 | 27 (25.5) | 8 (29.6) | ||
| 2–5 | 32 (30.2) | 13 (40.6) | ||
| 6–17 | 20 (18.9) | 5 (25.0) | ||
| 18–80 | 27 (25.5) | 5 (18.5) | ||
| Sex | ||||
| Male | 55 (51.9) | 16 (29.1) | 0.9711 | |
| Female | 51 (48.1) | 15 (29.4) | ||
| Type of facilities | ||||
| Primary Health Centre (PHC) | 74 (69.8) | 25 (32.9) | 0.1638 | |
| Hospital | 30 (28.3) | 6 (20.0) | ||
| Onset of fever (days) | 0.8797 | |||
| 1–5 | 99 (93.4) | 29 (29.3) | ||
| >5 | 3 (2.8) | 1 (33.3) | ||
| Antibiotic use | ||||
| Yes | 12 (11.3) | 2 (16.7) | N/A | |
| Fuel type | ||||
| Gas | 94 (88.7) | 29 (30.9) | 0.5396 | |
| Kerosene | 7 (6.6) | 1 (14.3) | ||
| Wood | 2 (1.9) | 1 (50.0) | ||
| Smoking | ||||
| Yes | 7 (6.6) | 2 (28.6) | N/A | |
| Exposure to cigarete | N/A | |||
| Yes | 68 (64.2) | 23 (33.8) | ||
| Number of family members | ||||
| 1–3 | 17 (16.0) | 4 (23.5) | 0.7313 | |
| 4–6 | 67 (63.2 | 22 (32.8) | ||
| >6 | 18 (17.0) | 5 (27.8) | ||
| Symptoms | ||||
| Fever | 106 (100.0) | 31 (29.2) | 0.8691 | |
| Cough | 101 (95.3) | 30 (28.3) | ||
| Runny nose | 94 (88.7) | 28 (26.4) | ||
| Nasal congestion | 68 (64.2) | 19 (17.9) | ||
| Malaise | 63 (59.4) | 13 (12.3) | ||
| Headache | 38 (35.8) | 8 (7.5) | ||
| Shortness of breath | 30 (28.3) | 8 (7.5) | ||
| Sore throat | 27 (25.5) | 7 (6.6) | ||
| Nausea/Vomit | 34 (32.1) | 12 (11.3) | ||
| Myalgia | 20 (18.9) | 4 (3.8) | ||
| Chest X-ray result | ||||
| Infiltrates | 21 (19.8) | 3 (2.8) | N/A | |
| Diagnosis | ||||
| Bronchopneumonia | 28 (26.4) | 6 (21.4) | 0.5884 | |
| Common cold | 14 (13.2) | 6 (42.9) | ||
| Acute respiratory infection | 54 (50.9) | 17 (31.5) | ||
| Rhinofaringitis | 5 (4.7) | 1 (20.0) | ||
| Tonsilofaringitis | 1 (0.9) | 0 (0.0) | ||
N = Total sample; p<0.05; N/A = Not Applicable.
Fig. 1. Graph (a) symptoms and (b) diagnosis of of patients with acute respiratory tract infections (number) in Manado, North Sulawesi, Indonesia.
Fig. 2. Graph (a) age and (b) sex of of patients with acute respiratory tract infections (number) in Manado, North Sulawesi, Indonesia.
In this study, we identified 31 S. pneumoniae strains. The distribution of serotypes varied, including 14, 18C, 19A, 23F, and 35B (two strains each) and 1, 3, 6B, 6C, 31, 9V, 15C, 16F, 17F, 23A, 19F and 35F (one strain each). Whereas, 29 % (9/31) of the isolates were non-typeable (Table 2). We found that vaccine type (13-valent pneumococcal conjugate vaccine, PCV13) strains were found higher in children under 5 years old (9/21; 43 %) than in children above 5 years old (4/13; 40 %) and adults (1/5; 20 %).
Table 2. Serotype distribution and antimicrobial profile of Streptococcus pneumoniae strains isolated from patients with acute respiratory tract infections in Manado, North Sulawesi, Indonesia.
| Isolate ID | Serotype | CHL | CLI | PEN* | AZM | SXT | ERY | VAN | TET |
| RMD.024 | 14 | S | S | R | S | R | S | S | R |
| RMD.015 | 14 | S | S | R | S | R | S | S | R |
| RMD.001 | 18C | S | S | R | R | R | R | S | S |
| RMD.028 | 18C | S | S | S | S | R | S | S | R |
| RMD.026 | 19A | S | S | S | S | S | S | S | S |
| RMD.208 | 19A | S | S | R | S | R | S | S | R |
| RMD.031 | 23F | S | S | R | S | S | R | S | R |
| RMD.221 | 23F | S | S | R | R | R | R | R | R |
| RMD.131 | 1 | R | S | S | S | R | S | S | R |
| RMD.139 | 3 | S | S | S | S | S | S | S | S |
| RMD.013 | 6B | S | S | R | S | R | S | S | S |
| RMD.222 | 19F | S | S | R | R | R | R | S | R |
| RMD.122 | 9V | S | S | R | R | R | R | S | R |
| RMD.027 | 35B | S | S | R | R | R | R | S | R |
| RMD.225 | 35B | S | R | R | R | R | R | S | R |
| RMD.020 | 6C | S | S | S | S | S | S | S | S |
| RMD.006 | 15C | S | S | R | S | S | S | S | S |
| RMD.009 | 16F | S | S | S | S | S | S | S | S |
| RMD.130 | 17F | S | R | S | R | R | S | S | R |
| RMD.035 | 23A | S | S | S | S | S | S | S | S |
| RMD.002 | 31 | R | S | S | S | S | S | S | R |
| RMD.132 | 35F | S | S | S | S | S | S | S | S |
| RMD.003 | Untypeable | S | S | R | S | R | S | S | R |
| RMD.014 | Untypeable | S | S | R | S | R | S | S | S |
| RMD.107 | Untypeable | S | S | R | R | R | R | S | R |
| RMD.112 | Untypeable | S | S | R | R | R | R | S | R |
| RMD.005 | Untypeable | S | S | R | S | R | S | S | S |
| RMD.033 | Untypeable | S | S | S | S | S | S | S | S |
| RMD.037 | Untypeable | S | S | R | S | R | S | S | R |
| RMD.216 | Untypeable | S | S | R | S | R | S | S | S |
| RMD.232 | Untypeable | S | S | R | S | R | S | S | S |
Grey colour indicates: Vaccine serotypes (PCV13=1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, 23F).
*Susceptibility to penicillin was tested with the oxacillin disk.
- AZM
- Azythromycin
- CHL
- Chloramphenicol
- CLI
- Clindamycin
- ERY
- Erythromycin
- PEN
- Oxacillin
- SXT
- Sulphametoxazole/trimetrophim
- TET
- Tetracycline
- VAN
- Vancomycin
We found that the majority of the strains were susceptible to vancomycin (30/31; 97 %), chloramphenicol (29/31; 94 %), clindamycin (29/31; 94 %), erythromycin (22/31; 71 %), and azithromycin (22/31; 71 %). Meanwhile, the strains were less susceptible to tetracycline (14/31; 45 %), penicillin (11/31; 35 %), and sulfamethoxazole/trimethoprim (10/31; 32 %) (Table 2). Sixteen of the 31 isolates (52 %) expressed less susceptibility to three or more antimicrobial agents of different classes.
Discussion
We identified that the S. pneumoniae carriage rate was 40.6 % among children under 5 years-old diagnosed with ARTI symptoms. The prevalence of S. pneumoniae carriage rate in Manado was less than previously reported in children in Lombok (48 %) [15], in HIV-infected children in Jakarta (46 %) [16], and children in Semarang, Central Java (43 %) [17]. However, the carriage rate of S. pneumoniae among adult patients in Manado (18.5 %) was higher than previously reported for adults 45–75 years of age in Semarang (11 %), adults in Jakarta (3 %) and HIV-infected adults in Jakarta (10 %) [5,17,19]. Furthermore, we observed that serotypes included in the PCV13 were the commonest serotypes found in this study (42 %) before PCV13 vaccine introduction in Indonesia, which was also in agreement with a previous study [5].
Among 42 % of vaccine serotypes found in this study, we identified serotype three as one of prevalent serotypes which was previously reported as a common serotype found among adult patients with community-acquired pneumonia in Jakarta, Indonesia [20]. Vaccine serotypes including serotype 3, 7F, 19A, 1, and 14 were reported as the most frequent serotypes found in adult patients with invasive pneumococcal disease in Portugal before PCV13 [21]. Serotype 14 of S. pneumoniae was the most prevalent aetiology of pneumococcal community-acquired pneumonia in children [22]. We detected that 42 % of serotypes in this study were covered by the PCV13. Serotype analysis of pneumococcal nasopharyngeal carriage isolates in Indonesia revealed that 38–60 % of isolates are covered by the PCV13 [5].
There was an increasing number of non-susceptible S. pneumoniae strains to sulfamethoxazole/trimethoprim from 38 % in 2010 [15] to 68 % indicated in this study. Sulfamethoxazole/trimethoprim is the second most common antimicrobial prescribed for children and is included in the antibacterials that have to be reconsidered by physicians for use in public healthcare facilities in Indonesia [23]. Penicillin and sulfonamides are the most common classes of antibiotics consumed by infants from birth to 18 months of age (38.81 and 24.48 %, respectively) [24], while penicillin and tetracyclines accounted for 80 % of the prescribed antibiotics in public healthcare facilities [23].
In addition, a variety of factors associated with pneumococcal carriage were analysed in this study for their carriage rates among patients. Factors such as younger age, greater family size, and exposure to cigarette smoke have been reported positively associated with pneumococcal carriage while factors such as antibiotics usage have otherwise been negatively associated [25].
Carriage prevalence of S. pneumoniae varies among age groups. Children under the age of five had a greater pneumococcal nasopharyngeal carriage rate compared to other age groups classified in this study. In this study, the carriage rate for children under the age of five was 37 %, followed by 24 % for children in the age range of 12–17 and 18 % for adults 18 years old and above. Children are repeatedly reported as having higher carriage rates compared to adults, and the highest rates reported are often among children of 2 years of age [4,25]. A carriage study previously conducted in Kotabaru, South Kalimantan, Indonesia had relatively high carriage rates among children under 5 years old, ranging from 42.7–51.4 % [6]. Another survey conducted in Nigeria also reported their highest carriage rates among children ages 0–4, 33.9 % in Kumbotso and 36.5 % in Pakoto [26].
Patients having more family members also had a higher pneumococcal nasopharyngeal carriage rate compared to patients having fewer. In this study, patients having family members of 4–6 had a carriage rate of 32 %. The same carriage rate was also observed in patients having seven family members and above. However, patients having 1–3 family members had a lower carriage rate of 24 %. Increase in pneumococcal colonization in patients having larger family size have previously been observed as well in children who have siblings [27,28].
Furthermore, patients exposed to cigarette smoke had a higher risk of pneumococcal colonization in their nasopharynx. Cigarette smoke has previously been reported to increase susceptibility to colonization by bacterial pathogens such as S. pneumoniae and H. influenzae in the upper airways [29]. In this study, the carriage rate of paediatric patients exposed to cigarette smoke was 35 % while those not exposed to cigarette smoke had a carriage rate of 31 %.
Meanwhile, antibiotics usage has been observed to reduce the odds of pneumococcal colonization. Antibiotics use have been reported in a review as being protective against pneumococcal carriage [25]. In this study, patients that were on antibiotics during the period of study, had a carriage rate of 17 % while patients that were not using antibiotics had a carriage rate of 31 %. Another pneumococcal carriage study conducted in Kilifi district, Kenya have also observed lower odds of carriage in children who reported taking antibiotics in the previous 2 weeks of study [30].
This study described serotype distribution and antimicrobial profile among adult patients with ARTI only with nasopharyngeal specimens. The combination of other invasive clinical specimens representing the ARTI cases would elaborate better understanding on correlation of colonization and ARTI. We declared this as limitation of the study.
This study provides supporting baseline data on distribution of serotype and antimicrobial profile of S. pneumoniae among acute respiratory infections patients before PCV13 vaccine introduction in Manado, North Sulawesi, Indonesia.
supplementary material
Acknowledgements
The authors thank all study participants and their families, the doctors and laboratory staff of Puskesmas Tuminting and Puskesmas Bahu, and the doctors and nurses of Department of Paediatric of R. D. Kandou General Hospital, Manado, for sample collections. We acknowledge Vecky Wowiling and Geralfy Rotty from Laboratorium Klinik R. D Kandou General Hospital Manado. We also thank Wisiva Tofriska, Hanifah Fajri, and I Gusti Ayu Inten Wulandari for technical assistance and manuscript editing.
Abbreviations
- ARTI
acute respiratory tract infections
- NP
nasopharyngeal
- PCV13
13-valent pneumococcal conjugate vaccine
- STGG
skim-milk, tryptone, glucose, and glycerol
Footnotes
Funding: This study was financially supported by the Directorate of Research and Community Service, Ministry of Research, Technology and Higher Education, Republic of Indonesia through applied research schemes fiscal year 2019–2020. This study was also supported by a grant from the Joint Funding Scheme SOUTHEAST Asia-EUROPE for Research and Innovation (FZK 01DP19007 to SH and 206/SP2H/AMD/LT/DPRM/2020 to SP).
Ethical statement: This study was approved by the Ethics Committee of R. D. Kandou General Hospital, Manado (Ethical approval number: 042/EC-KEPK/III/2019). Written informed consent was obtained from all patients and specifically for children under 18 years old, written consent was provided by their parents or legal guardians.
Author contributions: D.S.P.: chief investigator, study design, data collection, preparation of manuscript. M.M.K.: coinvestigator, study design, data collection, data analysis, review draft manuscript. W.T.: coinvestigator, data collection, data analysis, review draft manuscript. S.H.M.K.: data collection, review draft manuscript. R.W.: data collection, review draft manuscript. B.J.K.: data collection, review draft manuscript. H.P.T.R.: data collection, review draft manuscript. L.G.: data collection, review draft manuscript. S.V.: co-investigator, study design, review draft manuscript. W.F.E.: data analysis, review draft manuscript. D.S.: co-investigator, study design, data analysis, preparation of manuscript.
Contributor Information
Diana Shintawati Purwanto, Email: shintapurwanto@yahoo.co.id.
Miftahuddin Majid Khoeri, Email: majid.khoeri@gmail.com.
Wisnu Tafroji, Email: wisnutafroji@gmail.com.
Stefana Helena Margaretha Kaligis, Email: stefana.kaligis@unsrat.ac.id.
Rocky Wilar, Email: wilarrocky@gmail.com.
Hessyani Patricia Theodora Raranta, Email: hessypatra@gmail.com.
Lidia Gaghiwu, Email: gaghiwulidia@gmail.com.
Sven Hammerschmidt, Email: sven.hammerschmidt@uni-greifswald.de.
Waode Fifin Ervina, Email: fifinervina92@gmail.com.
Dodi Safari, Email: dodi004@brin.go.id.
References
- 1.Henriques-Normark B, Tuomanen EI. The Pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3:a010215. doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morpeth SC, Munywoki P, Hammitt LL, Bett A, Bottomley C, et al. Impact of viral upper respiratory tract infection on the concentration of nasopharyngeal pneumococcal carriage among Kenyan children. Sci Rep. 2018;8:11030. doi: 10.1038/s41598-018-29119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks LRK, Mias GI. Streptococcus pneumoniae’s virulence and host immunity: aging, diagnostics, and revention. Front Immunol. 2018;9:1366. doi: 10.3389/fimmu.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kartasasmita CB, Rezeki Hadinegoro S, Kurniati N, Triasih R, Halim C, et al. Epidemiology, nasopharyngeal carriage, serotype prevalence, and antibiotic resistance of Streptococcus pneumoniae in Indonesia. Infect Dis Ther. 2020;9:723–736. doi: 10.1007/s40121-020-00330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salsabila K, Paramaiswari WT, Amalia H, Ruyani A, Tafroji W, et al. Nasopharyngeal carriage rate, Serotype distribution, and antimicrobial susceptibility profile of Streptococcus Pneumoniae isolated from children under five years old in Kotabaru, South Kalimantan, Indonesia. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2021;S1684-1182:00138–00139. doi: 10.1016/j.jmii.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Harimurti K, Saldi SRF, Dewiasty E, Alfarizi T, Dharmayuli M, et al. Streptococcus pneumoniae carriage and antibiotic susceptibility among Indonesian pilgrims during the Hajj pilgrimage in 2015. PLoS One. 2021;16:e0246122. doi: 10.1371/journal.pone.0246122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prayitno A, Supriyatno B, Munasir Z, Karuniawati A, Hadinegoro SRS, et al. Pneumococcal nasopharyngeal carriage in Indonesia infants and toddlers post-PCV13 vaccination in A 2+1 schedule: a prospective cohort study. PLoS One. 2021;16:e0245789. doi: 10.1371/journal.pone.0245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muktiarti D, Khoeri MM, Tafroji W, Waslia L, Safari D. Serotypes and antibiotic susceptibility profile of Streptococcus pneumoniae isolated from nasopharynges of children infected with HIV in Jakarta, Indonesia, pre- and post-pneumococcal vaccination. Access Microbiol. 2021;3:000215. doi: 10.1099/acmi.0.000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne EM, Murad C, Sudigdoadi S, Fadlyana E, Tarigan R, et al. Carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in Indonesian children: a cross-sectional study. PLoS One. 2018;13:e0195098. doi: 10.1371/journal.pone.0195098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safari D, Widhidewi NW, Paramaiswari WT, Lila Paramasatiari AAA, Maharani Putri HF, et al. Prevalence, serotype distribution, and antimicrobial susceptibility profile of Streptococcus pneumoniae among patients with acute respiratory tract infection. WHO South East Asia J Public Health. 2021;10:66–69. doi: 10.4103/WHO-SEAJPH.WHO-SEAJPH_58_21. [DOI] [PubMed] [Google Scholar]
- 12.Widhidewi NW, Wiyatno A, Dewantari AK, Paramasatiari L, Aryastuti SA, et al. Identification of viral etiology of acute respiratory tract infections in children and adults in Tabanan, Bali, Indonesia. Access Microbiol. 2020;2:acmi000120. doi: 10.1099/acmi.0.000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yani FF, Julianty RJ, Tafroji W, Linosefa L, Ihsan I, et al. Nasopharyngeal carriage and antimicrobial susceptibility profiles of Streptococcus pneumoniae among children with pneumonia and healthy children in Padang, Indonesia. Access Microbiol. 2023;5:acmi000584.v3. doi: 10.1099/acmi.0.000584.v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Gloria Carvalho M, Pimenta FC, Jackson D, Roundtree A, Ahmad Y, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. 2010;48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadinegoro SR, Prayitno A, Khoeri MM, Djelantik IGG, Dewi NE, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children under five years old in central lombok regency, Indonesia. Southeast Asian J Trop Med Public Health. 2016;47:485–493. [PubMed] [Google Scholar]
- 16.Safari D, Kurniati N, Waslia L, Khoeri MM, Putri T, et al. Serotype distribution and antibiotic susceptibility of Streptococcus pneumoniae strains carried by children infected with human immunodeficiency virus. PLoS One. 2014;9:e110526. doi: 10.1371/journal.pone.0110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farida H, Severin JA, Gasem MH, Keuter M, Wahyono H, et al. Nasopharyngeal carriage of Streptococcus pneumonia in pneumonia-prone age groups in Semarang, Java Island, Indonesia. PLoS One. 2014;9:e87431. doi: 10.1371/journal.pone.0087431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harimurti K, Saldi SRF, Dewiasty E, Khoeri MM, Yunihastuti E, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in adults infected with human immunodeficiency virus in Jakarta, Indonesia. J Infect Public Health. 2016;9:633–638. doi: 10.1016/j.jiph.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Safari D, Harimurti K, Khoeri MM, Waslia L, Mudaliana S, et al. Staphylococcus aureus and Streptococcus pneumoniae prevalence among elderly adults in Jakarta, Indonesia. Southeast Asian J Trop Med Public Health. 2015;46:465–471. [PubMed] [Google Scholar]
- 20.Amanda G, Tafroji W, Sutoyo DK, Burhan E, Haryanto B, et al. Serotype distribution and antimicrobial profile of Streptococcus pneumoniae isolated from adult patients with community-acquired pneumonia in Jakarta, Indonesia. J Microbiol Immunol Infect. 2021;54:1175–1178. doi: 10.1016/j.jmii.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Horácio AN, Silva-Costa C, Diamantino-Miranda J, Lopes JP, Ramirez M, et al. Population structure of Streptococcus pneumoniae causing invasive disease in adults in Portugal before PCV13 availability for adults: 2008-2011. PLoS One. 2016;11:e0153602. doi: 10.1371/journal.pone.0153602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentile A, Bardach A, Ciapponi A, Garcia-Marti S, Aruj P, et al. Epidemiology of community-acquired pneumonia in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Infect Dis. 2012;16:e5–15. doi: 10.1016/j.ijid.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Werter S, et al. Survey of antibiotic use of individuals visiting public healthcare facilities in Indonesia. Int J Infect Dis. 2008;12:622–629. doi: 10.1016/j.ijid.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.At Thobari J, Satria CD, Ridora Y, Watts E, Handley A, et al. Antimicrobial use in an Indonesian community cohort 0-18 months of age. PLoS One. 2019;14:e0219097. doi: 10.1371/journal.pone.0219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal EFG, Chan J, Nguyen CD, Russell FM. Factors associated with pneumococcal nasopharyngeal carriage: a systematic review. PLoS Glob Public Health. 2022;2:e0000327. doi: 10.1371/journal.pgph.0000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adetifa IMO, Adamu AL, Karani A, Waithaka M, Odeyemi KA, et al. Nasopharyngeal pneumococcal carriage in Nigeria: a two-site, population-based survey. Sci Rep. 2018;8:3509. doi: 10.1038/s41598-018-21837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, et al. Trends in asymptomatic nasopharyngeal colonization with Streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J. 2014;33:724–730. doi: 10.1097/INF.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 28.Hadjipanayis A, Efstathiou E, Alexandrou M, Panayiotou L, Zachariadou C, et al. Nasopharyngeal pneumococcal carriage among healthy children in Cyprus post widespread simultaneous implementation of PCV10 and PCV13 vaccines. PLoS One. 2016;11:e0163269. doi: 10.1371/journal.pone.0163269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss M, Wonnenberg B, Honecker A, Kamyschnikow A, Herr C, et al. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir Res. 2015;16:41. doi: 10.1186/s12931-015-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2012;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.