Summary
Background
Since June, 2019, more than 1000 new cases of e-cigarette, or vaping, product use associated lung injury (EVALI) have been reported in the USA. Patients presented with dyspnoea, cough, and were found to be hypoxaemic with bilateral airspace opacities on chest imaging. Most patients required management in the intensive care unit and steroid therapy. All patients recovered with cessation of vaping, supportive care, and steroid therapy and remained symptom free at follow up. E-cigarette use continues to rapidly escalate in the USA, particularly among youth.
Methods
Cases were defined as patients admitted to the University of Rochester Medical Center (Rochester, NY, USA) who had used e-cigarettes or another vaping device in the 30 days before presentation, and who had bilateral airspace opacification on chest imaging (CT or x-ray). Case details were obtained via medical record review and patient interviews over the past 3 months including symptomatology, physical exam data, imaging studies, laboratory data, vaping history, and subsequent outpatient follow-up data. In collaboration with the New York State Department of Health, our hospital developed a novel clinical practice algorithm based on statewide physician feedback along with input from experts in environmental health, medical toxicology, infectious disease, epidemiology, and chronic disease prevention.
Findings
We report 12 cases treated for suspected EVALI at our medical centre between June 6, 2019, and Sept 15, 2019. Ten (83%) patients had dyspnoea, fever, and emesis and nine (75%) had cough. 11 (92%) patients reported the use of e-cigarette cartridges containing tetrahydrocannabinol oil. Although eight (67%) patients required admission to the intensive care unit for hypoxaemic respiratory failure, no deaths occurred. The median hospitalisation duration was 7 days (IQR 7–8). All patients completing follow up (6 [50%]) had resolution of previous chest CT findings and normal spirometry. The clinical algorithm focuses on the key signs and symptoms of EVALI and the importance of ruling out infection and other cardiopulmonary conditions before making a presumptive diagnosis of EVALI.
Interpretation
Patients with suspected EVALI in our cohort had life-threatening hypoxaemia, with 67% requiring management in the intensive care unit. Despite the severity of presentation, similar to previous reports of patients with EVALI, most patients improved within 1–2 weeks of initial presentation after vaping cessation and administration of systemic corticosteroids when needed. Almost all (92%) patients with suspected EVALI reported vaping a THC product, making THC containing e-liquids or oils a key focus on the ongoing nationwide investigations into the cause of EVALI. Additional research is required to understand the potential toxins, underlying pathophysiological mechanisms, and identification of susceptible individuals at higher risk for hospitalisation due to EVALI. To our knowledge we present the first clinical practice algorithm for the evaluation and management of EVALI, which will be useful for both acute management and improved accurate reporting of this life-threatening respiratory illness.
Funding
None.
Introduction
The use of e-cigarettes has surged worldwide since 2000.1,2 E-cigarettes are devices that allow users to aerosolise (vape) liquid, which can contain nicotine or other substances, and are sometimes flavoured (e-liquid).3 Since their introduction in 2007, the USA has seen an increase in number of e-cigarette users and in vaping of non-nicotine liquids, such as tetrahydrocannibidiol (THC), cannabidiol (CBD), and other unknown substances.3,4
Many e-cigarette formulations come in attractive flavours to appeal to young adults and teenagers who tend to experiment and modify the product being vaped and the vaping method itself. New methods of inhalation, such as dabbing, have changed the content and properties of the compounds delivered to the pulmonary system. Dabbing is defined as the “consumption of cannabis whereby a cannabis concentrate is volatilised via application to a hot platform (holder) and the vapour is subsequently passed through a water pipe and inhaled by the end user”.5 The use of a metal platform during dabbing introduces the risk of inhaling solder, rust, and benzene, which are released at higher temperatures.3,6,7
One of the most popular methods of vaping involves a cartridge-based device normally filled with nicotine salts to deliver an aerosol to the user.8 Although cartridge-based systems are normally a closed non-modifiable system, users can modify the liquid composition within the cartridges. For the third-generation and fourth-generation refillable tank-based systems, users can enhance battery power and add any liquid that they choose. Any modification to either the e-liquid or the device itself has the potential to substantially change the chemical profile of the aerosol created.
Lung disease related to vaping has been previously documented mainly via isolated case reports with varied presentations, including mechanical injury (spontaneous pneumothorax), pneumonias (organising, eosinophilic, and lipoid), or hypersensitivity pneumonitis without any single uniting entity.9,10 Additionally, some additives have been shown to cause oxidative stress on lung epithelium.5
Since June, 2019, health-care professionals have documented 1888 cases of acute lung injury related to vaping in the USA.11 Presentations range from mild dyspnoea to acute hypoxaemic respiratory failure requiring mechanical ventilation, and have been associated with the use of THC-containing e-liquids among other types.11 Despite maximal medical therapy, 37 patients have died.12-15 The accumulation of cases of e-cigarette, or vaping, product use associated lung injury (EVALI) has attracted the attention of the general public, elected public officials, and law makers.16 Given the recency of these cases, there is no evidence-based approach to the diagnosis and management of patients who present with a history of vaping and dyspnoea. With the vast expansion of available devices and liquids (including THC oil), it is not surprising that the heterogeneity in presentation and case severity has in turn increased in the USA.17 With the strict regulations on e-liquids and e-cigarettes present in other countries, such as the UK, the risk of a similar outbreak of illness is likely to be lower; however, at least one case has been reported of lipoid pneumonia due to vaping.18
The presentation of a respiratory illness without any other known factors except vaping history leaves a broad differential diagnosis to consider. In this article, we summarise the clinical presentations of patients with probable and confirmed EVALI seen at a single academic medical centre, with a focus on diagnostic testing and clinical management. Additionally, we describe how coordination of efforts between multiple departments within the institution, the New York State Department of Health, and the New York City and Upstate New York Poison Control Centers has led to a consensus on testing and work up of patients in New York State.
Methods
Case finding and investigations
We present a retrospective case series of patients presenting to a single academic medical centre (the University of Rochester Medical Center [URMC], Rochester, NY, USA]) over a period of 2 months with respiratory failure of unknown origin and history of e-cigarette or vape use. All patients had bilateral ground glass opacities on chest imaging (CT and x-ray) and unrevealing past medical histories (ie, nothing to indicate a potential source of lung injury). An extensive workup was performed with no clear cause identified. The histories of all patients did, however, involve vaping followed by gastrointestinal symptoms, and then respiratory symptoms within days before presentation. The pulmonologists and toxicologists created an internal vaping respiratory failure group and asked the emergency and internal medicine departments to monitor for patients presenting with unexplained respiratory failure and chest imaging abnormalities. Individual case details were obtained via medical record review and direct patient interviews by the primary, pulmonary, and toxicology teams focusing on vaping history and other risk factors for lung injury. EVALI was initially defined at our centre as respiratory symptoms, such as dyspnoea or cough, or gastrointestinal symptoms, such as emesis, with a history of vaping in the previous 30 days to hospital admission and with chest imaging showing bilateral airspace disease. An attempt was made to schedule a follow-up visit with each patient in the pulmonary clinic after discharge. This project was reviewed by the institution’s Research Subjects Review Board and it was determined to be exempt from obtaining informed consent because all data were de-identified and clinical information aggregated.
After aggregating suspected cases of EVALI, pulmonologists and clinical toxicologists reported case information to the regional poison control centre and the New York State Department of Health. As part of the active investigation of EVALI in New York State, the New York State Department of Health is coordinating the retrieval and shipping of product samples from the affected patients for chemical analysis (results pending), is conducting extensive patient interviews to better understand the histories of product use by patients, is developing a database inclusive of EVALI case reports from across the state to link case histories with product testing results, and is evaluating toxicological information relevant to various e-cigarette ingredients. The New York State Department of Health is coordinating their efforts with the outbreak investigation of the Centers for Disease Control and Prevention (CDC) by reporting case information and information sharing with other states and federal partners. The EVALI outbreak in New York has also led to the recognition of two serious public health crises (growth in e-cigerette use by young people and the EVALI outbreak) and has triggered new regulations, including a requirement for vape shops within the state of New York to post warning signs intended to alert users to the acute risks of vaping. Our efforts to determine the cause of the current EVALI crisis can help inform future statewide vaping policies.
Algorithm development
Medical staff within the University of Rochester saw a need for a diagnostic approach to these cases before the CDC guidance became available. Based on previous case reports of lung injury secondary to vaping, and a general approach to unexplained hypoxic respiratory failure, we developed an algorithm to allow for rapid identification of patients with suspected EVALI based on their history, clinical presentation, and chest imaging. This clinical algorithm was formulated in conjunction with the New York State Department of Health who provided review of the algorithm through physician experts within the Department, including experts in environmental health, infectious disease, epidemiology, and chronic disease prevention. This algorithm was created based upon the case presentations at our medical centre and refined by our statewide experience in this crisis (currently 165 reported cases). Additionally, medical toxicologists provided input through local poison control centres.
Role of the funding source
There was no funding source for this study.
Results
Between June 1, 2019, and Sept 15, 2019, a total of 12 patients were admitted to URMC with suspected EVALI. The median age of these cases was 27 years (IQR 21–35) years and seven (58%) patients were men. All 12 patients were reportedly healthy with no functional deficits before presentation. Only three (25%) had a documented history of pre-existing pulmonary disease (asthma).
Patients typically had symptoms for around 1 week before presentation (median of 7 days [IQR 6·5–10]). The presenting symptoms were varied with cases involving pulmonary or gastrointestinal complaints (table 1). The most common presenting symptoms were dyspnoea in ten (83%) patients, subjective (ie, patient-reported) fevers in ten (83%) patients, emesis in ten (83%) patients, and cough in nine (75%) patients. The cough was nonproductive in five (56%) of nine patients with cough and six (50%) had pleuritic chest pain. One (8%) patient presented with haemoptysis that he attributed to an episode of dabbing, in addition to his regular use of THC-based e-liquid in a cartridge. None of the patients reported being in contact with anyone with an infection or compounding environmental exposures. Two (17%) presented after a failed course of antibiotics. 11 (92%) patients reported using THC oil or cartridges in their e-cigarettes; one (8%) patient used nicotine only. Six (55%) of 11 cases using THC oil also reported using nicotine-containing cartridges. Only five (42%) of 12 reported using cannabis via methods in addition to vaping (ie, joint or pipes) and only one (8%) person reported actively smoking combustible tobacco.
Table 1:
Patient characteristics and presentations
| Patients (n=12) | |
|---|---|
| Median age (years) | 27 (21–35) |
| Sex | |
| Male | 7 (58%) |
| Female | 5 (42%) |
| Past medical history | |
| Anxiety | 4 (33%) |
| Asthma | 3 (25%) |
| Reflux | 1 (8%) |
| Epilepsy | 1 (8%) |
| Congenital Heart Disease | 1 (8%) |
| Median length of hospital stay (days) | 7 (7–8) |
| Median length of intensive care unit stay (days) | 3·5 (0–5) |
| Median symptom duration before admission (days) | 7 (6·5–10) |
| Respiratory symptoms | 11 (92%) |
| Dyspnoea | 10/11 (91%) |
| Cough | 9/11 (82%) |
| Pleuritic pain | 6/11 (55%) |
| Sputum | 4/11 (36%) |
| Haemoptysis | 1/11 (9%) |
| Systemic symptoms | 12 (100%) |
| Fever (subjunctive) | 10 (83%) |
| Malaise | 9 (75%) |
| Sweats | 5 (42%) |
| Chills | 3 (25%) |
| Myalgias | 2 (17%) |
| Gastrointestinal symptoms | 11 (92%) |
| Emesis | 10/11 (91%) |
| Nausea | 7/11 (64%) |
| Abdominal pain | 3/11 (27%) |
| Diarrhoea | 3/11 (27%) |
| Miscellaneous | |
| Previous health-care visit for symptoms | 7 (58%) |
| Sore throat | 3 (25%) |
| Nasal congestion | 3 (25%) |
| Headache | 3 (25%) |
| Previous antibiotic therapy | 2 (17%) |
| Epistaxis | 1 (8%) |
| Odynophagia | 1 (8%) |
| Leg pain | 1 (8%) |
| Back pain | 1 (8%) |
| Patients (n=12) | |
| Substance use | |
| Tetrahydrocannibidiol vaping | 11 (92%) |
| Nicotine vaping | 7 (58%) |
| Cannabis use (non-vape) | 5 (42%) |
| Cannibidiol vaping | 1 (8%) |
| Nicotine vaping only | 1 (8%) |
| Tobacco cigarettes | 1 (8%) |
| Vital signs | |
| Temperature >100·3 degrees Fahrenheit within 48 h of admission | 9 (75%); 100·6 (100·4–102·7) |
| Heart rate >100 beats per minute on triage | 8 (67%) |
| Respiratory rate >20 breaths per minute on triage | 3 (25%) |
| Systolic blood pressure <90 mm Hg on triage | 1 (8%) |
| Oxygen saturation <94% on triage | 9 (75%) |
| Median lowest recorded oxygen saturation throughout stay (%) | 82·5 (78–85) |
| Respiratory interventions | |
| High flow nasal cannula | 6 (50%) |
| Mechanical ventilation | 1 (8%) |
| BiPAP | 1 (8%) |
| Nasal cannula | 4 (33%) |
| Bronchoscopy performed | 4 (33%) |
| Intensive care unit needed | 8 (67%) |
Data are n (%) or mean (IQR). BiPAP=bilevel positive airway pressure.
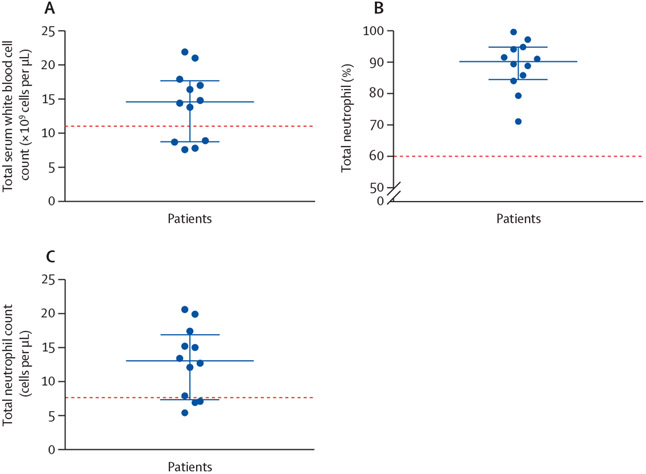
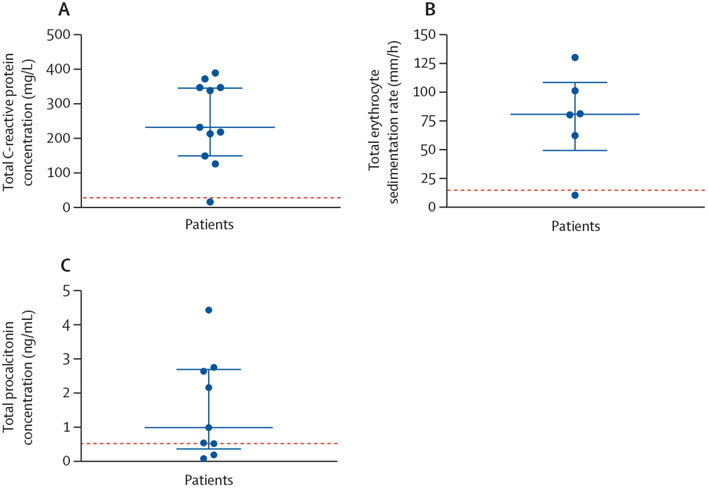
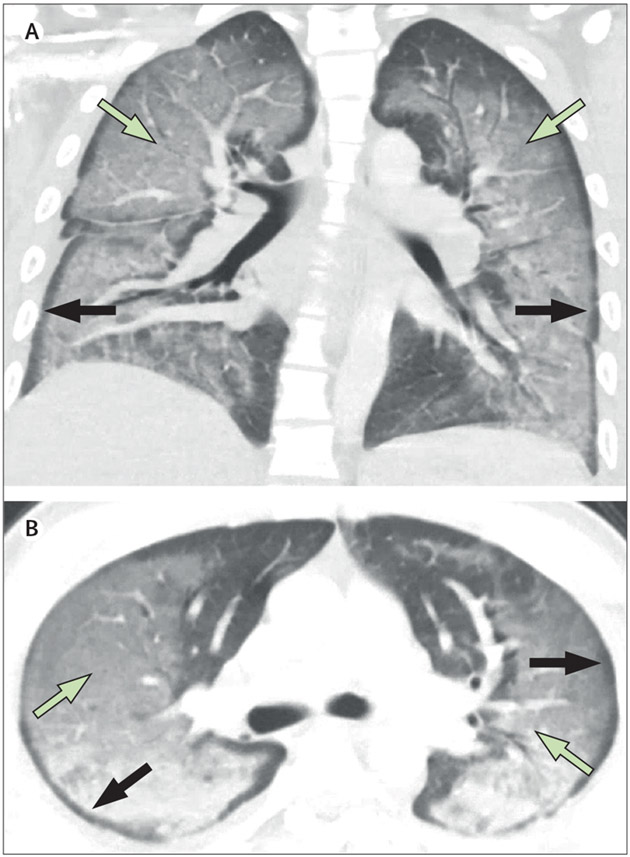
On arrival to the emergency department, nine (75%) of 12 patients were hypoxaemic, eight (67%) were tachycardic, and 11 (92%) were normotensive. Nine (75%) had a fever within 48 h of admission to hospital. The median admission white blood cell count was 14 600 per μL (IQR 8800–17 700; normal range 4200–9100) and all patients generally had a neutrophil predominance (median neutrophil count 13 000 per μL [7300–16 850]; figure 1). No eosinophilia was observed. Inflammatory markers were severely elevated in ten (83%) patients (figure 2). Patients had a median C-reactive protein (CRP) concentration of 232 mg/L (129–347; normal range 0–10). Erythrocyte sedimentation rate (ESR) was elevated in five (83%) of six patients tested with a median value of 80·5 mm/h (49·0–108·3; normal range 0–15). Procalcitonin concentration was elevated in eight (89%) of nine patients tested with a median value of 0·99 ng/mL (0·36–2·70; normal range 0·00–0·09). All patients had infectious disease testing with blood cultures, respiratory viral panels, and urine antigens for streptococcus and legionella (table 2). However, no patients had a positive culture or laboratory test for infection. HIV screening test was negative in 11 of 11 patients who were tested. Antinuclear antibodies and antineutrophil cytoplasmic antibody testing was negative for six patients for whom testing was available. 11 (92%) patients had cross-sectional CT imaging of the chest with the predominant finding being bilateral ground glass opacification (seen in all patients), with seven (64%) patients also exhibiting subpleural sparing (figure 3). Four (33%) patients had bronchoscopy, with all four bronchoalveolar lavage samples revealing macrophage predominance (one haemosiderin-laden and lipid-laden, one pigment-laden, two alveolar macrophages) and negative infectious analyses (including routine bacterial, fungal, acid-fast bacilli cultures, pneumocystis PCR, aspergillus antigens, and routine viral PCR studies).
Figure 1: Serum complete white blood cell count (A), percentage neutrophil (B), and neutrophil cell count differential (C).
The dotted red line represents the upper limit of normal for each test. The blue bars represent the median value, with the minimum and maximum range.
Figure 2: Serum inflammatory markers: C-reactive protein concentration (A), erythrocyte sedimentation rate (B), and procalcitonin concentration (C).
The dotted red line represents the upper limit of normal for each test. The blue bars represent the median value, with the IQR.
Table 2:
Patient laboratory and CT imaging results
| Patients (n=12) | |
|---|---|
| Tetrahydrocannibidiol on urine toxicology screen | 8/9 (89%) |
| Median blood urea nitrogen concentration (mg/dL) | 11 (9·5–15) |
| Median creatinine concentration (mg/dL) | 0·77 (0·69–0·87) |
| Median white blood cell count on day 1 of hospital admission (thousands per μL) | 14·6 (8·8–17·7) |
| White blood cell count >10 (thousands per μL) | 8/12 (67%) |
| Median serum eosinophil count (thousands per μL) | 0·03 (0–0·1) |
| Median serum neutrophil count (thousands per μL) | 13·1 (7·3–16·9) |
| Median serum lymphocyte count (thousands per μL) | 0·91 (0·5–1·5) |
| Median platelet count (thousands per μL) | 308 (256–385·5) |
| Median CRP concentration (mg/dL) | 232 (149–347) |
| CRP concentration >10 (mg/dL) | 10/10 (100%) |
| Median erythrocyte sedimentation rate (mm/h) | 80·5 (1–130) |
| Erythrocyte sedimentation rate >15 (mm/h) | 5/6 (83%) |
| Median procalcitonin concentration (ng/ml) | 1·59 (0·08–4·43) |
| Procalcitonin concentration >0·09 (ng/mL) | 8/9 (89%) |
| Lactate dehydrogenase concentration (U/L) | 484 (248–601) |
| Antinuclear antibodies screen (negative) | 6/6 (100%) |
| Antineutrophil cytoplasmic antibody screen (negative) | 6/6 (100%) |
| Rheumatoid factor (negative) | 3/3 (100%) |
| Double-stranded DNA antibodies (negative) | 3/3 (100%) |
| Anti-smith DNA antibodies (negative) | 2/2 (100%) |
| Anti-Ro/anti-La antibodies (negative) | 3/3 (100%) |
| Anti-citrullinated protein antibodies (negative) | 3/3 (100%) |
| Median IgE concentration (mg/dL) | 81·67 (9–143) |
| Infection analyses | |
| Blood cultures (no growth) | 12/12 (100%) |
| Sputum cultures (no growth) | 3/3 (100%) |
| Urine cultures (no growth) | 2/3 (67%) |
| Legionella cultures (no growth) | 8/8 (100%) |
| Rhinovirus PCR (negative) | 9/9 (100%) |
| Adenovirus PCR (negative) | 9/9 (100%) |
| Metapneumovirus DNA PCR (negative) | 9/9 (100%) |
| Parainfluenza 1–4 PCR (negative) | 9/9 (100%) |
| Influenza A (negative) | 10/10 (100%) |
| Influenza B (negative) | 10/10 (100%) |
| Respiratory syncytial virus (negative) | 9/9 (100%) |
| HIV 1/2 Ag/AB (non-reactive) | 11/11 (100%) |
| Mycoplasma pneumoniae nucleic acid amplification test (negative) | 7/7 (100%) |
| Mycoplasma pneumoniae IgM (negative) | 0/1 (0%) |
| Strep pneumoniae urinary Ag (negative) | 12/12 (100%) |
| Legionella urinary Ag (negative) | 12 (100%) |
| Cryptococcal antigen (negative) | 2/2 (100%) |
| Histoplasma antigen (negative) | 4/4 (100%) |
| Aspergillus antigen (negative) | 3/3 (100%) |
| Pneumocystis DNA PCR (negative) | 4/4 (100%) |
| Stool studies (negative) | 3/3 (100%) |
| CT findings | 11/11 |
| Subpleural sparing | 7 (64%) |
| Bilateral ground glass opacification | 11 (100%) |
| Nodules | 0 |
| Pleural effusions | 1 (9%) |
| Fibrotic features (reticulation, bronchiectasis, honeycombing) | 2 (18%) |
| Ground glass opacification pattern | |
| Patchy | 7 (64%) |
| Confluent | 3 (27%) |
| Both | 1 (9%) |
| Mediastinal lymphadenopathy | 3 (27%) |
Median (IQR), n/N (%), or n (%). One patient did not have CT and had a chest x-ray instead.
Figure 3: High-resolution CT imaging.
This patient has e-cigarette, or vaping, product use associated lung injury, with diffuse bilateral ground glass opacities (green arrows) in a peribronchial distribution, with subpleural sparing (black arrows).
Eight (67%) of 12 patients were admitted to the intensive care unit (ICU) for advanced respiratory support with a median ICU length of stay of 3·5 days (IQR 0–5·3). Half (50%) of the patients required high flow nasal cannula, one (8%) required bilevel positive pressure ventilation (BiPAP) and one (8%) required intubation with mechanical ventilation. Eight (67%) patients were given corticosteroids, the most typical dose and route being 40 mg intravenous methylprednisolone every 6–12 h. 11 (92%) patients were initially given pre-emptive antibiotics to treat community-acquired pneumonia pathogens for a median duration of 5·5 days (3·8–6·3). All 12 patients recovered, with a median hospital stay of 7·0 days (6·8–8·0). Those who received corticosteroids were discharged on a prednisone taper with a median of 25·0 days duration (19·3–29·8). Notably, the four patients who did not receive corticosteroids were admitted for a decreased length of time (median 5·5 days [4·5–7·0]) but had similar laboratory findings and vital sign characteristics, without apparent effect on outcome. Of the six (50%) patients who had follow up (table 3), all had complete resolution of ground glass opacities on chest imaging, five of five patients had complete resolution of respiratory symptoms, and five of five had normal FEV1, forced vital capacity (FVC), and FEV1/FVC ratio on spirometry, which included one patient who did not receive corticosteroids. The median time to follow up was 13·5 days (6·8–16·5).
Table 3:
Duration of antibiotic treatment
| Patients (n=12) | |
|---|---|
| Median antibiotic duration (days) | 5·5 (3·8–6·3) |
| Steroids used | 8 (67%) |
| Median steroid duration (days) | 24·5 (19·3–29·8) |
| Follow up | |
| Median time to follow up (days) | 13·5 (6·8–16·5) |
| Resolution on follow up imaging | 6/6 (100%) |
| Normal spirometry | 5/6 (83%) |
| Resolution of symptoms | 6/6 (100%) |
Data are median (IQR) or n (%).
Diagnostic approach
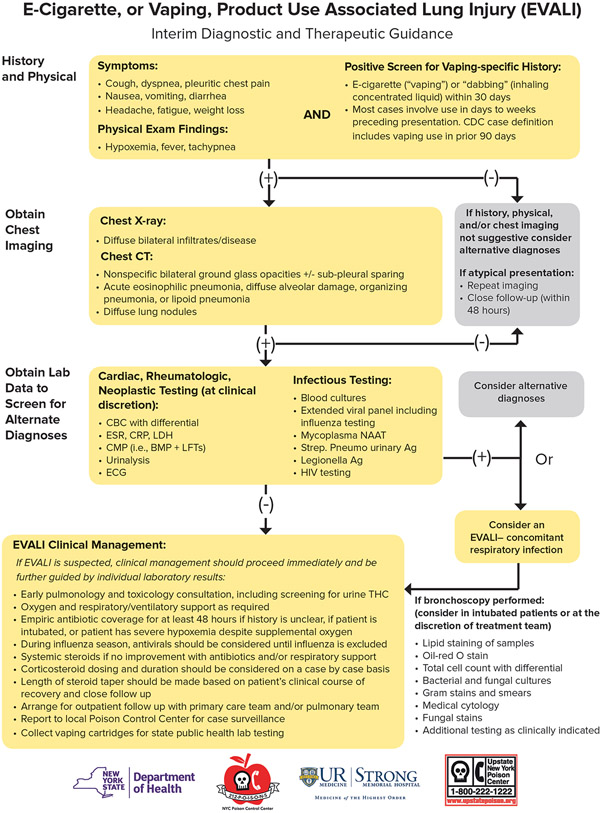
Given the challenges in the diagnosis and treatment of EVALI, we propose the following diagnostic pathway to evaluate patients with suspected EVALI (figure 4). Our approach hinges on the lack of a single, obvious pathophysiological mechanism for this clinical syndrome. The CDC diagnostic and management approach19 now available is generally consistent with our algorithm, aside from our use of a 30-day period for vaping exposure before presentation rather than a 90-day window in the CDC version, so that we could minimise exposure misclassification. The maximum time after exposure to vaping that symptoms can manifest is unknown; however, it seems unlikely that exposure more than 30 days before presentation would result in EVALI. In fact the largest current case series of EVALI12 observed 32 of 34 cases vaped within 1 week of symptom onset, making remote exposure (over 1 month) less likely to contribute. Our algorithm also details e-cigarette history and coordination with the New York State Department of Health for collecting and reporting case information to the CDC so that local information can be included in the data describing the national outbreak. Most importantly, the flow diagram presentation of the algorithm is easier to use than plain text and it provides greater specificity of diagnostic findings and medical management.
Figure 4: Clinical algorithm for the workup of e-cigarette, or vaping, product use associated lung injury (EVALI).
Reproduced with permission from New York State Department of Health and the University of Rochester Medical Center © 2019.
If the history and imaging are suspicious for EVALI, we recommend a thorough infectious and potentially autoimmune workup as outlined in figure 4. Although respiratory infection is not part of the case definition of EVALI, one key determination is the presence or absence of infection. Truly ruling out infection is difficult due to the limitations of modern microbiological diagnostic methods.20,21 If clinically appropriate, we recommend a bronchoscopy to bolster the microbiological data and to avoid missing a serious microbiological infection,22 as e-cigarette use is associated with an increased risk of pulmonary infections due to epithelial cell damage.23,24 During the initial diagnostic testing it is imperative to maintain a broad differential diagnoses, and we advocate for the administration of empiric antibiotics, particularly if respiratory support is required. Given the apparent inflammatory nature of EVALI (ie, high inflammatory markers and fever) and the rapid improvement in oxygenation observed in patients receiving corticosteroids, we advocate for administration of systemic corticosteroids in patients with suspected EVALI. As no data exists to guide dosing, we recommend starting with 40 mg methylprednisolone every 8 h and, if the patient shows improvement, to then transition to oral prednisone with a tapering dose for a total duration of 2 weeks. For the cases given corticosteroid treatment in this case series, the total length of treatment was approximately 3 weeks. Empiric treatment with corticosteroids for longer than 2 weeks was unlikely to be necessary, based on the observation that several patients who received corticosteroids received courses as short as 5 days with symptom resolution at follow-up.
Discussion
This is a case series of 12 patients presenting with respiratory failure, gastrointestinal symptoms, and evidence of systemic inflammation in the context of vaping, ultimately attributed to EVALI. Patients in this case series had increased oxygen requirements and none had positive results for infectious disease. All patients had bilateral airspace opacification on chest imaging. All patients recovered and were discharged on room air, with a median length of stay in the hospital of 7 days. Of the six who had outpatient follow-up, all had complete resolution of imaging findings and symptoms.
Clinically, patients in our case series generally presented with similar respiratory and gastrointestinal symptoms as patients reported in other states nationwide;12,25,26 such as in Illinois and Wisconsin earlier this year,12 indicating a common clinical syndrome marked by recent history of vaping THC- and nicotine-based products. EVALI appears to be a syndrome characterised by respiratory failure with an intense inflammatory response. Patients showed fever, leucocytosis, elevated CRP concentration and ESR with no evidence of viral or bacterial infection in urine, blood, sputum, or serum laboratory samples. Of the 11 patients who had inflammatory marker testing, 10 (91%) showed what we defined as an inflammatory phenotype, with elevated concentrations of at least two of four markers (CRP, ESR, white blood cells, procalcitonin). Additionally, basic autoimmune workups were negative in all of the patients tested. Although the chest imaging findings are heterogeneous among our patients, bilateral ground glass opacities were the common finding in all 12, which supports the suspicion that diffuse pulmonary inflammation is a key step in the pathophysiological pathway. Based on our data, all 12 cases would meet the CDC definition of probable or confirmed EVALI.11
At the time of this publication, a specific toxin or a clear pathological mechanism explaining the disease process that is being observed has yet to be identified. While the majority of cases report combined use of nicotine and cannabinoid-containing products, there are some cases on both state and national levels that report exclusive use of nicotine-containing products. Therefore, nicotine-containing vaping products have not been excluded from the current investigation into the causal pathway of EVALI. Future investigations require continued collection of vape devices and cartridges for analysis in research laboratories and thorough patient follow-up to establish vaping and other substance use history, with careful documentation of clinical information, with the aim being that the vaping products can be tested and association studies can be carried out to try and identify the problematic substances. Notably, eight (89%) of nine patients in our cohort who had a urine toxicology screen were positive for THC. The patient with a negative urine toxicology screen denied using THC and only used nicotine cartridges for vaping. Interestingly, several patients noted that the THC cartridges they used were loaded with a less viscous material than usual and did not produce the same high as previous THC-containing cartridges. Counterfeit e-liquids with mislabelled contents surfaced within the vaping market several years ago.27 One hypothesis is that counterfeit, low cost, THC-containing cartridges, with poorly tested diluents, might be contributing to the epidemic of EVALI. For example, it has been suggested that unregulated manufacturers of THC cartridges are introducing novel compounds, such as vitamin E acetate as diluents.28 It is possible these modifications are being made to modify the viscosity and mimic the more expensive and better regulated THC cartridges.29 When heated and aerosolised, diluting agents, vehicles, and their breakdown products might result in an inflammatory cascade that results in EVALI.
One proposed mechanism is that oil heating, aerosolisation, and deposition into the lower airways results in lipoid pneumonia, which has been suggested in several cases of the outbreak of suspected EVALI cases in the USA30 and in many previous cases associated with e-cigarette use.9 However, the well described presentations31 of typical exogenous lipoid pneumonia are more indolent in nature with chronic cough relating to recurrent aspiration of oils, which does not appear to fit the current presentations. These cases might illustrate a different phenomenon, such as endogenous lipoid pneumonia representing macrophage accumulation of lipids released after injury to and breakdown of alveolar epithelial cells. Previous publications have outlined other very reasonable differential diagnoses that broadly include inflammatory or immune processes, such as lipoid pneumonia, organising pneumonia, diffuse alveolar haemorrhage, or hypersensitivity pneumonitis.12,32 Radiologically, several of these cases have a similar appearance to conditions on this previously described differential, including bilateral ground glass opacities with subpleural sparing consistent with non-specific interstitial pneumonia (figure 3). Various lines of evidence in mice and human cells indicate damage, inflammation, and compromised lung function in response to vaping ingredients, such as aerosolised propylene glycol and vegetable glycerin, which are components of e-liquid.33,34 These studies raise concern for a decreased innate immune response and increased infection risk related to e-cigarette use. The diversity in presentation and findings of cases seen both at our institution and in other published studies12,26,29,30 suggests that there are variations within the possible common pathway of inflammatory injury to the lung epithelium and lung parenchyma, manifesting as an acute pneumonitis. The risk to the e-cigarette user is likely to vary depending on the device, intensity of use, type (ie, oil vs nicotine salt), and quality (ie, counterfeit vs retail) of e-liquid and underlying lung health. This broad collection of variables could explain the wide range of respiratory illness from mild dyspnoea to acute respiratory distress syndrome and death. It is also possible that an interaction occurs between inhalation of aerosolised THC-based products with the vaping of nicotine or flavourings, such that the inflammation of the lining of the airways caused by both might be cumulative or synergistic. This alternate hypothesis is supported by the substantial evidence of inflammation and oxidative stress in a number of in-vivo mouse models and in-vitro human cell models caused by exposure to e-liquids and their aerosols.35
As we work to determine the underlying mechanism of EVALI, providers can benefit from a structured diagnostic approach based on the best available evidence. To our knowledge, this is the first study to present a clinical practice algorithm meant for real time use as a decision matrix to support the evaluation and management of EVALI. Our clinical algorithm was informed by our clinical experience and guidance from local and state experts. When counselling patients, families, and the public, in general the appropriate guidance is that the use of any vaping product should be stopped. Importantly, anyone vaping to help quit combustible cigarette use should wean off of vaping as soon as possible and avoid converting back to combustible cigarette use. Nicotine-based e-cigarettes are not an approved US Food and Drug Administration (FDA) cessation approach, while other approaches (eg transdermal nicotine patch) are approved and have proven benefits.36 Providers are encouraged to counsel patients with EVALI and all others in the community to only use approved cessation approaches and thus avoid the risks associated with vaping products. Additional cessation assistance can be found online.
This reported case series highlights the clinical features and laboratory findings associated with patients presenting to a single academic centre over the course of 2 months. A cooperative effort between departments within the institution, the regional poison centre, and the New York State Department of Health triggered a prompt public health response with a broad positive effect within our state.
Research in context.
Evidence before this study
We first searched PubMed on Sept 10, 2019, with the search terms “e-cigarette”OR “e-cigarettes”OR “vaping” AND “pulmonary disease”. We have been repeating this search to ensure we have included the most relevant and up-to-date information. This search revealed several case reports or case series detailing other cases of e-cigarette, or vaping, product use associated lung injury (EVALI). The US Centers for Disease Control and Prevention (CDC) has been tracking cases nationally and provided clinical guidance via its website and series of publications in the Morbidity and Mortality Weekly Report (MMWR). This guidance gave helpful information regarding the presenting symptoms of cases and the types of exposures patients encountered. Current data on EVALI are limited by challenges in accurate patient and provider reporting in part due to a lack of a standardised algorithm for workup and management of this condition. We independently developed a clinical algorithm for the diagnosis, medical management, and reporting of EVALI based upon our case series of 12 patients reported presently, as well as the emerging information on this outbreak available statewide and nationally.
Added value of this study
To our knowledge, this is the first study presenting a clinical practice algorithm meant for direct patient care of EVALI. The current case definition and clinical guidance available from CDC provides useful general principles, which has been enhanced presently through the experience we have gained in the 12 patient case series. We present a practical algorithm for efficient screening, diagnosis, and medical management of EVALI. This case series also highlights the important challenge of post hospitalisation follow up on patients and the degree to which the severe changes in imaging and symptoms can improve with the cessation of vaping and systemic corticosteroids (in severe cases).
Implications of all the available evidence
A significant proportion of the EVALI cases reported here had exposure to aerosolised tetrahydrocannabinol oil, also consistent with national reports by the CDC. Most patients improved within 1–2 weeks of initial presentation after vaping cessation and administration of systemic corticosteroids when needed. Our clinical practice algorithm is similar in content to the recommendations provided by the CDC, though our algorithm focuses on vaping activity within the past 30 days and is arranged to allow for efficient exclusion of cases that bear no similarity to our current understanding of EVALI. Further research is needed to identify the inciting toxins of EVALI, underlying pathophysiology behind acute hypoxaemic respiratory failure, and the susceptibility of hospitalised individuals.
Acknowledgments
We thank Phillip Disalvo, Mark Su, and the staff of the New York City Poison Control Centre, as well as the staff of the Upstate New York Poison Control Centre for their substantial contributions to the diagnostic algorithm. We also thank Lisa Ryder, Justin Weisenberger, Heidi Connelly, Justin Weis, Serban Staicu, Michael Nead, Sandhya Khurana, Matt Kottmann, Mary Anne Morgan, and all other faculty members at the University of Rochester Medical Center who managed these cases and informed the author team. Finally we would like to thank Mark Utell for his mentorship and review of this manuscript.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Aleksandr Kalininskiy, Department of Medicine, Pulmonary Diseases and Critical Care, University of Rochester, Rochester NY, USA.
Christina T Bach, Department of Medicine, Pulmonary Diseases and Critical Care, University of Rochester, Rochester NY, USA; Strong Memorial Hospital, Rochester, NY, USA.
Nicholas E Nacca, Department of Emergency Medicine, University of Rochester, Rochester NY, USA; Strong Memorial Hospital, Rochester, NY, USA; Upstate New York Poison Center, Syracuse, NY, USA.
Gary Ginsberg, New York State Department of Health, Albany, NY, USA; Center for Environmental Health, Albany, NY, USA.
Jeanna Marraffa, Upstate New York Poison Center, Syracuse, NY, USA; Department of Emergency Medicine, Upstate Medical University, Syracuse, NY, USA.
Kristen A Navarette, New York State Department of Health, Albany, NY, USA; Center for Environmental Health, Albany, NY, USA; Department of Pediatrics, Albany Medical Center, Albany, NY, USA.
Matthew D McGraw, Department of Pediatrics, Pulmonology, University of Rochester, Rochester NY, USA; Strong Memorial Hospital, Rochester, NY, USA.
Daniel P Croft, Department of Medicine, Pulmonary Diseases and Critical Care, University of Rochester, Rochester NY, USA; Strong Memorial Hospital, Rochester, NY, USA.
References
- 1.WHO. Global report on trends in prevalence of tobacco smoking 2000–25. Geneva: World Health Organization, 2018. [Google Scholar]
- 2.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67: 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitbarth AK, Morgan J, Jones AL. E-cigarettes—an unintended illicit drug delivery system. Drug Alcohol Depend 2018; 192: 98–111. [DOI] [PubMed] [Google Scholar]
- 4.Trivers KF, Phillips E, Gentzke AS, Tynan MA, Neff LJ. Prevalence of cannabis use in electronic cigarettes among US youth. JAMA Pediatr 2018; 172: 1097–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman I, Lamb T, Muthumalage T. JUUL e-cigarette and vape pen flavors impose oxidative stress and inflammatory responses in lung epithelial cells and macrophages. American Thoracic Society 2019; May 20,2019. (abstr A4186-A). [Google Scholar]
- 6.Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci 2015; 40: 797–803. [DOI] [PubMed] [Google Scholar]
- 7.Wester RC, Maibach HI, Gruenke LD, Craig JC. Benzene levels in ambient air and breath of smokers and non-smokers in urban and pristine environments. J Toxicol Environ Health 1986; 18: 567–73. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28: 146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clinical Respir J 2018; 12: 1295–99. [DOI] [PubMed] [Google Scholar]
- 10.Bonilla A, Blair AJ, Alamro SM, et al. Recurrent spontaneous pneumothoraces and vaping in an 18-year-old man: a case report and review of the literature. J Med Case Rep 2019; 13: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jatlaoui TC. Outbreak of lung injury associated with e-cigarette product use or vaping: information for clinicians. Centers for Disease Control and Prevention. Oct 24, 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#what-we-know (accessed Oct 26, 2019). [Google Scholar]
- 12.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—preliminary report. N Engl J Med 2019; published online Sept 6. DOI: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 13.Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep 2017; 5: e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RP, Zechar K. Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep 2019; 26: 171–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol 2018; 2018: 9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Lung illnesses associated with use of vaping products. 2019. https://www.fda.gov/news-events/public-health-focus/lung-illnesses-associated-use-vaping-products (accessed Oct 25, 2019).
- 17.McGraw MD, Houser GH, Galambos C, Wartchow EP, Stillwell PC, Weinman JP. Marijuana medusa: the many pulmonary faces of marijuana inhalation in adolescent males. Pediatr Pulmonol 2018; 53: 1619–26. [DOI] [PubMed] [Google Scholar]
- 18.Viswam D, Trotter S, Burge PS, Walters GI. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep 2018; 2018: bcr-2018–224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel DA. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—United States, October 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57(suppl 3): S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zumla A, Al-Tawfiq JA, Enne VI, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis 2014; 14: 1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Örtqvist Å, Kalin M, Lejdeborn L, Lundberg B. Diagnostic fiberoptic bronchoscopy and protected brush culture in patients with community-acquired pneumonia. Chest 1990; 97: 576–82. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 2014; 9: e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyashita L, Suri R, Dearing E, et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J 2018; 51: 1701592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azad A. 14 young people in two states hospitalized after vaping, health officials say. 2019. https://www.cnn.com/2019/08/03/health/vaping-hospitalizations-wisconsin-illinois/index.html?nost=1568563695 (accessed Oct 25, 2019). [Google Scholar]
- 26.Triantafyllou GA, Tiberio PJ, Zou RH, et al. Vaping-associated acute lung injury: a case series. Am J Respir Crit Care Med 2019; published online Oct 1. DOI: 10.1164/rccm.201909-1809LE. [DOI] [PubMed] [Google Scholar]
- 27.Omaiye EE, Cordova I, Davis B, Talbot P. Counterfeit electronic cigarette products with mislabeled nicotine concentrations. Tob Regul Sci 2017; 3: 347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New York State Department of Health. New York State Department of Health announces update on investigation into vaping-associated pulmonary illnesses. 2019. https://www.health.ny.gov/press/releases/2019/2019-09-05_vaping.htm (accessed Oct 26, 2019).
- 29.Davidson K BA, Heetderks P, et al. Outbreak of electronic-cigarette–associated acute lipoid pneumonia—North Carolina, July–August, 2019. 2019. https://www.cdc.gov/mmwr/volumes/68/wr/mm6836e1.htm (accessed Oct 25, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med 2019; 381: 1488–89. [DOI] [PubMed] [Google Scholar]
- 31.Betancourt SL, Martinez-Jimenez S, Rossi SE, Truong MT, Carrillo J, Erasmus JJ. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. Am J Roentgenol 2010; 194: 103–09. [DOI] [PubMed] [Google Scholar]
- 32.Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; published online Sept 6. DOI: 10.1056/NEJMe1912032. [DOI] [Google Scholar]
- 33.Madison MC, Landers CT, Gu B-H, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 2019; 129: 4290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reidel B, Radicioni G, Clapp PW, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med 2018; 197: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The National Academies of Sciences, Engineering, Medicine. Public health consequences of e-cigarettes. Jan 23, 2018. http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx (accessed Oct 25, 2019).
- 36.Wadgave U, Nagesh L. Nicotine replacement therapy: an overview. Int J Health Sci 2016; 10: 425–35. [PMC free article] [PubMed] [Google Scholar]