Abstract
The global ageing of populations calls for effective, ecologically valid methods to support brain health across adult life. Previous evidence suggests that music can promote white matter (WM) microstructure and grey matter (GM) volume while supporting auditory and cognitive functioning and emotional well‐being as well as counteracting age‐related cognitive decline. Adding a social component to music training, choir singing is a popular leisure activity among older adults, but a systematic account of its potential to support healthy brain structure, especially with regard to ageing, is currently missing. The present study used quantitative anisotropy (QA)‐based diffusion MRI connectometry and voxel‐based morphometry to explore the relationship of lifetime choir singing experience and brain structure at the whole‐brain level. Cross‐sectional multiple regression analyses were carried out in a large, balanced sample (N = 95; age range 21–88) of healthy adults with varying levels of choir singing experience across the whole age range and within subgroups defined by age (young, middle‐aged, and older adults). Independent of age, choir singing experience was associated with extensive increases in WM QA in commissural, association, and projection tracts across the brain. Corroborating previous work, these overlapped with language and limbic networks. Enhanced corpus callosum microstructure was associated with choir singing experience across all subgroups. In addition, choir singing experience was selectively associated with enhanced QA in the fornix in older participants. No associations between GM volume and choir singing were found. The present study offers the first systematic account of amateur‐level choir singing on brain structure. While no evidence for counteracting GM atrophy was found, the present evidence of enhanced structural connectivity coheres well with age‐typical structural changes. Corroborating previous behavioural studies, the present results suggest that regular choir singing holds great promise for supporting brain health across the adult life span.
Keywords: ageing, connectivity, grey matter, music, singing, white matter
Lifetime choir singing experience correlated positively with structural connectivity. Healthy adults, aged 21–88, showed extensive enhancements across the brain, including the arcuate fasciculus, inferior fronto‐occipital fasciculus, corticospinal tract, and corpus callosum. Older adults, aged 60–88, showed exclusive benefits in the fornix, a key pathway supporting memory function.
Practitioner Points.
Effects of regular amateur‐level choir singing on brain structure were explored in healthy adults (N = 95, age range 21–88 years) at the whole‐brain level.
Independent of age, regular choir singing is associated with extensive enhancements in structural connectivity along commissural, association, and projection pathways.
Older choir singers show selective enhancements of fornix microstructure bilaterally.
1. INTRODUCTION
Brain structure transforms across adulthood. After peaking in early adulthood, both white (WM) and grey matter (GM) undergo gradual reductions in structural integrity and volume (Bethlehem et al., 2022; Salat et al., 2004; Vinke et al., 2018). Consequent disruptions in WM (Charlton et al., 2006; Nicolas et al., 2020; Ritchie et al., 2015) and GM network integrity (Koini et al., 2018; Manard et al., 2016) are associated with declines in working and episodic memory, fluid intelligence, and executive function. Disrupted connectivity, among other factors, may contribute to impairments in neural plasticity (see Burke & Barnes, 2006). However, such changes are more regional and selectively constrained than historically assumed. Sufficient training may induce plastic changes that are both analogous to those observed in younger brains and unique to older brains (e.g., Boyke et al., 2008). Indeed, training‐induced plasticity can be utilised to support brain structure, such as WM microstructure (de Lange et al., 2018; Lövdén et al., 2010), brain volume, and cortical thickness (Colcombe et al., 2006; Kühn et al., 2017). Given the rapid pace of population ageing (United Nations Department of Economic and Social Affairs, 2022), however, there is an urgent need for the validation of widely applicable, ecologically valid, and cost‐effective training activities capable of supporting brain health at older age.
Previous evidence suggests that engagement in leisure activities, such as music, may contribute to the preservation of brain health and enhance specific cognitive functioning, including processing speed, episodic memory, and executive function in healthy ageing (Román‐Caballero et al., 2018; Rouse et al., 2022; Tremblay & Perron, 2023; for review, see also Sutcliffe et al., 2020) and in mild cognitive impairment (Doi et al., 2017; Dorris et al., 2021). Additionally, such activities have been shown to potentially reduce the risk of dementia (Arafa et al., 2022; Verghese et al., 2003). A recent randomised controlled trial (RCT) found that playing a musical instrument (the piano), can help preserve the integrity of the fornix in older adults, with a weak positive association with episodic memory (Jünemann et al., 2022; see also Burzynska et al., 2017). Some evidence also suggests that musical activities may support the preservation of brain volume in prefrontal and temporal regions (Chaddock‐Heyman et al., 2021; for review, see also Muiños & Ballesteros, 2021) and reduce overall signs of structural ageing at non‐professional level (Chaddock‐Heyman et al., 2021; Rogenmoser et al., 2018; however, see also Matziorinis et al., 2022). However, a systematic account on the influence of music on brain structure and its association with cognitive function in healthy ageing is currently missing (for review, see Sutcliffe et al., 2020). On a larger scale, the effects of musical activities on brain structure, particularly concerning its changing structural and dynamic functional properties across the adult lifespan, remain unknown.
In terms of ecological validity, singing is a particularly promising form of musical activity to support brain health as it requires neither access to instruments or other equipment nor the acquisition of a new motor skill. While both instrumental and vocal music training enhance frontotemporal and limbic connectivity bilaterally, singing has been associated with larger volumes of the left arcuate fasciculi (AF; Halwani et al., 2011; see also Perron et al., 2021) and larger‐scale enhancements of structural connectivity within vocal motor control, sensory feedback, and language processing networks (Cheng et al., 2023). Adding a social component to the positive effects of singing, group/choir singing has recently become of special interest for supporting healthy ageing. Recent findings suggest that group singing is effective for promoting long‐term quality of life, mood, cognitive function, and social engagement (Coulton et al., 2015; Johnson et al., 2020; Pentikäinen et al., 2021; Skingley et al., 2016; see also Cohen et al., 2006), thus providing a cost‐effective tool to support mental health in the ageing population (Coulton et al., 2015). Despite its great potential for promoting brain health across adult life, very few studies (e.g., Perron et al., 2021, 2022) have yet studied the effects of choir singing on the ageing brain. Direct evidence of whether choir singing might support the preservation of healthy brain structure at whole‐brain scale is currently missing.
The present study investigated the effects of the amateur‐level choir singing on age‐induced atrophy in WM and GM, as well as cognitive function, in a large, balanced sample (N = 95, age range 21–88 years). Specifically, the study employed a quantitative anisotropy (QA)‐based connectometry using multi‐shell diffusion MR imaging (dMRI) data for WM analysis and voxel‐based morphometry (VBM) for GM analysis. These measures were tested across the sample as well as separately within young (aged 20–39 years), middle‐aged (40–59 years), and older (60 years and above) participants. We hypothesised that a longer duration of amateur‐level choir singing would be associated with experience‐dependent specialisation in (i) WM QA, particularly in language‐related and vocal motor tracts, and (ii) GM volume, especially in the prefrontal and temporal regions, regardless of age.
2. METHODS
2.1. Participants
One hundred volunteers (55 females), aged 21–88 years (mean 49.2, SD 17.5), participated in this structural MRI study between November 2019 and December 2020. All participants were right‐handed native Finnish‐speakers (four bilingual) with no diagnosis of a hearing impairment, language or neurological disorder, cognitive decline, or dementia. Participants had no professional training or other background in music but were engaged in regular musical leisure activities at varying levels. Participants who reported having choir singing experience were required to have choir singing as their main musical hobby and to have been actively engaged in it, at minimum, for the past 1 year involving at least one hour of weekly choir singing. No upper limit on amateur‐level choir singing was set to allow for studying how the duration of lifetime choir singing experience might be associated with brain structure. For homogeneity, persons reporting prior choir singing experience without active participation during the past 1 year were excluded from the study.
The recruitment process controlled for the main demographic characteristics of the sample. Specifically, the recruitment process controlled for the distribution of (i) age to achieve a good representation of various ages across the desired age range (20–90), (ii) gender within the full sample and with respect to age to prevent overrepresentation of any gender‐associated characteristics in the structural data, and (iii) age‐corrected duration of choir singing experience to achieve a good representation of various degrees of experience but also of persons with no choir singing experience. All participants were pre‐screened for MRI contraindications upon recruitment and again immediately before scanning. Prior to participation, all participants provided written informed consent for participation and for the use of their data for the study's scientific purposes. The study was approved by the European Research Council Executive Agency and the University of Helsinki Ethical Review Board in the Humanities and Social and Behavioural Sciences.
2.2. Data acquisition
Data were acquired at the Advanced Magnetic Imaging (AMI) Centre of Aalto University, Espoo, Finland, with a MAGNETOM Skyra 3.0 T scanner (Siemens) equipped with a 32‐channel RF receiving head coil. Soft foam padding was used to stabilise participants' heads within the coil, minimizing head movement and providing hearing protection. Participants were instructed to immediately report any discomfort. A whole‐brain T1‐weighted anatomical volume was acquired using a 3D magnetisation‐prepared rapid gradient‐echo sequence with echo time (TE) = 3.3 ms, repetition time (TR) = 2530.0 ms, flip angle = 7°, voxel size = 1 × 1 × 1 mm3, field of view (FOV) = 256 mm, and number of slices = 176.
Multi‐shell dMRI was performed using two sequences with bidirectional phase encoding at anterior–posterior/posterior–anterior (AP/PA) directions. TR = 5000 ms; TE = 104.0 (AP)/101.0 (PA) ms; 13 volumes at b‐value of 0 s/mm2 (AP) interspersed between higher b‐values, 30 unique directions at b‐value of 1000 s/mm2 (AP), 100 unique directions at b‐value of 2500 s/mm2 (AP), and 7 volumes at b‐value of 0 s/mm2 (PA); number of slices = 72; voxel size = 2 × 2 × 2 mm3; and FOV = 240 mm.
Furthermore, all participants completed an extensive questionnaire battery that assessed demographic information, health and well‐being, social participation, leisure activities, and musical sophistication. Assessed musical activities encompassed singing, playing an instrument, dancing, and listening to music. Musical sophistication was assessed with the Goldsmith's Musical Sophistication Index questionnaire (Gold‐MSI; Müllensiefen et al., 2014).
2.3. Preprocessing
In the VBM analysis of structural MRI data, T1‐weighted images were segmented into GM, WM, and cerebrospinal fluid (CSF) using Unified Segmentation (Ashburner & Friston, 2005). These segmented images were then normalised into Montreal Neurological Institute (MNI) space using Geodesic Shooting (Ashburner & Friston, 2011). All processes were conducted within the Computational Anatomy Toolbox (CAT12; Jena University Hospital, Jena, Germany) running under the Statistical Parametric Mapping (SPM12; Wellcome Department of Cognitive Neurology, UCL) software package in Matlab R2019a (The MathWorks Inc., Natick, MA, USA). Following normalisation, the images underwent visual inspection and were smoothed with an 8‐mm full width at half maximum (FWHM) isotropic filter. Total intracranial volume (TIV) was computed from GM, WM, and CSF volumes for each participant.
For the dMRI data, initial processing steps included denoising for thermal noise (MP‐PCA method; Veraart et al., 2016) via the denoise tool in MRTrix 3 (Tournier et al., 2019). Additionally, the data were corrected for Gibbs ringing based on local sub‐voxel shifts (Kellner et al., 2016). We collected data with reversed phase‐encode blips, resulting in image pairs with opposite‐direction distortions. The susceptibility‐induced off‐resonance field was estimated from these image pairs using a method similar to that described by Andersson et al. (2003) as implemented in FSL (Smith et al., 2004). The images were then combined into a single corrected one. Finally, the data were corrected for motion and eddy currents using outlier detection and replacement (Andersson et al., 2016; Andersson & Sotiropoulos, 2016) and bias (Smith et al., 2004).
After these preprocessing steps, the diffusion data were reconstructed into MNI space in DSI Studio (version Chen, December 2022) available at http://dsi-studio.labsolver.org) using q‐space diffeomorphic reconstruction to obtain the spin distribution function (Yeh et al., 2010; Yeh & Tseng, 2011). The b‐table was checked by an automatic quality control routine to ensure its accuracy (Schilling et al., 2019). Normalisation was carried out using the anisotropy map of each participant, and a diffusion sampling length ratio of 1.25 was used. The output was resampled to 2‐mm isotropic resolution. Normalisation result was visually inspected using forceps major and forceps minor as anatomical benchmarks of quality (Hula et al., 2020; Sihvonen et al., 2021) for each participant, as well as using the R2 values denoting goodness‐of‐fit between the participant's anisotropy map and template. The restricted diffusion was quantified with restricted diffusion imaging (Yeh et al., 2017), and QA was extracted as the local connectome fingerprint (Yeh, Vettel, et al., 2016) for the following connectometry analysis. QA was selected as the marker for WM integrity as it has been shown to outperform traditional fractional anisotropy (FA) by being more specific to individual's connectivity patterns (Yeh, Vettel, et al., 2016) and less susceptible to the partial volume effect of crossing fibres and free water as well as to provide better resolution in tractography (Yeh et al., 2013; see also Sihvonen et al., 2023).
2.4. Analysis
For the analyses on both GM and WM, the main variable of interest was the experience of choir singing throughout one's lifetime, which was treated as a continuous variable to study how the extent of lifetime choir singing experience might be associated with brain structure. To mitigate the direct effects of age, the total years actively participating in choir singing was divided by each participant's age to obtain an age‐adjusted measure of choir singing experience. Similarly, the involvement in solo singing and instrument playing was age‐adjusted and included as covariates in the analyses to control for other types of musical activities reported by the participants. Four separate multiple regression models were executed for both GM and WM analyses: whole sample (N = 95), young adults (aged 20–39 years, N = 34), middle‐aged adults (40–59 years, N = 32), and older adults (60 years and above, N = 29). Each model controlled for the following factors: age, years of education, relative experience in solo singing and instrument playing, and TIV derived from T1 images. While age was considered a significant variable that could affect structural changes across the sample as well as in the older participants, gender had been controlled for a priori (see Section 3.1), thus showing a balanced distribution with respect to age, and was therefore not included as a covariate in the analyses. Also, the inclusion of TIV as a covariate also served to control for potential effects of gender, as TIV is closely associated with gender.
For the volumetric GM data, multiple regression analyses were performed in SPM12 and were thresholded across the whole brain at a familywise error rate (FWE) of p < .0125 (Bonferroni correction for multiple comparisons). In the case of dMRI connectometry (Yeh, Badre, & Verstynen, 2016), multiple regressions were carried out in DSI Studio. Seeding was based on the whole brain, and tracts were identified using a deterministic tracking algorithm (Yeh et al., 2013) exceeding a significance threshold of t = 3 (Hula et al., 2020) to obtain correlational tractography. The resulting tracts were filtered by topology‐informed pruning (Yeh et al., 2019) with 16 iterations and a length threshold of 30 voxels. To estimate the false discovery rate (FDR), 4000 randomised permutations were applied to the group label to obtain a null distribution for tract length. For each connectometric analysis, the whole‐brain FDR threshold was set to p < .0125 (Bonferroni correction for multiple comparisons). Tracts containing fewer than five surviving fibres were excluded to further minimise false positives.
3. RESULTS
3.1. Sample
The final sample size was N = 95, as dMRI data collection could not be completed with two participants and issues in data quality were found in three others. The demographic and musical background characteristics of the sample are summarised in Table 1. As intended, controlling for the main demographic characteristics during recruitment yielded a balanced gender distribution with respect to age. Consistent with the current demographic structure of the Finnish population (Statistics Finland, 2020, 2021), higher age was negatively associated with education [r s (93) = −.272, p = .008], with middle‐aged participants reporting the highest mean. Older participants also reported less lifetime experience (% of life active) in solo singing [r s (93) = −.251, p = .014] and playing an instrument [r s (93) = −.262, p = .010]. Besides age, education and other musical hobbies were thus accounted for in the structural regression models along with TIV.
TABLE 1.
Demographic information for the whole sample and age subgroups.
| Group | All (N = 95) | Young (N = 34) | Middle‐aged (N = 32) | Older (N = 29) |
|---|---|---|---|---|
| Demographic | ||||
| Age | 49.7 (17.6), 21–88 | 30.1 (5.5), 21–39 | 50.3 (6.4), 40–59 | 70.2 (7.5), 60–88 |
| Gender (female/male/other) | 50/45/0 | 18/16/0 | 16/16/0 | 16/13/0 |
| Education years | 16.6 (4.3), 2–34 | 17.4 (2.8), 12–24 | 18.1 (3.8), 12–34 | 13.9 (4.9), 2–20 |
| Choir singing | ||||
| Total years active | 10.3 (13.9), 0–59 | 6.5 (8.1), 0–29 | 9.4 (11.0), 0–36 | 15.8 (19.7), 0–59 |
| % of life active | 20.5 (24.1), 0–79 | 20.1 (24.8), 0–77 | 19.0 (21.9), 0–67 | 21.6 (26.3), 0–79 |
| Lifetime max. hours/week | 2.7 (2.7), 0–10 | 2.8 (2.6), 0–8 | 2.7 (2.6), 0–10 | 2.7 (2.9), 0–10 |
| Current hours/week | 1.8 (2.0), 0–8 | 1.2 (1.5), 0–5 | 1.9 (2.0), 0–8 | 2.3 (2.3), 0–8 |
| Solo singing | ||||
| Total years active | 3.9 (8.6), 0–50 | 3.6 (6.2), 0–26 | 6.8 (12.8), 0–50 | 1.1 (2.1), 0–8 |
| % of life active | 9.4 (19.7), 0–94 | 12.2 (20.2), 0–87 | 13.6 (25.4) 0–94 | 1.5 (2.8), 0–10 |
| Lifetime max. hours/week | 1.7 (3.2), 0–15 | 2.1 (3.7), 0–15 | 1.9 (3.5), 0–15 | 0.9 (2.1), 0–10 |
| Current hours/week | 0.5 (1.2), 0–8 | 0.8 (1.5), 0–8 | 0.5 (1.1), 0–5 | 0.2 (0.9), 0–5 |
| Playing an instrument | ||||
| Total years active | 9.9 (13.4), 0–60 | 9.8 (8.5), 0–30 | 10.0 (13.9), 0–48 | 10.0 (17.4), 0–60 |
| % of life active | 22.8 (27.3), 0–87 | 32.9 (28.1), 0–80 | 20.1 (26.8), 0–87 | 13.9 (23.6), 0–82 |
| Lifetime max. hours/week | 2.8 (4.0), 0–20 | 3.1 (4.0), 0–20 | 3.6 (5.0), 0–20 | 1.6 (2.3), 0–10 |
| Current hours/week | 0.6 (1.4), 0–10 | 0.5 (0.6), 0–2 | 0.9 (2.0), 0–10 | 0.5 (1.4), 0–7 |
| Gold‐MSI a | ||||
| Active engagement | 33.6 (9.2), 12–57 | 32.9 (9.7), 12–48 | 34.2 (10.3), 12–57 | 33.8 (6.7), 22–47 |
| Perceptual abilities | 48.3 (9.1), 24–62 | 48.3 (9.4), 30–62 | 50.0 (8.9), 24–61 | 45.9 (9.0), 28–62 |
| Musical training | 22.7 (10.5), 7–43 | 24.2 (12.4), 7–43 | 22.0 (8.9), 7–37 | 21.8 (10.0), 7–38 |
| Singing abilities | 30.3 (9.5), 7–45 | 31.2 (9.6), 11–45 | 30.2 (9.3), 7–44 | 29.2 (9.9), 11–42 |
| Emotions | 33.1 (5.5), 17–41 | 33.8 (5.0), 22–40 | 34.2 (5.6), 17–41 | 30.5 (5.5), 18–40 |
| General | 74.4 (22.2), 20–111 | 76.3 (25.1), 27–110 | 74.7 (21.0), 20–111 | 71.6 (20.4), 33–101 |
Note: All values reported as mean (SD), min–max, unless otherwise specified.
Goldsmith's Musical Sophistication Index. Categorical scores available for N = 81 (young N = 29; middle‐aged N = 30; older N = 22) due to missing responses.
Factors measuring predisposition for music were not associated with age. Specifically, participants' lifetime experience in choir singing (% of age), which was used as the main study variable, was not associated with age. Also, the current level of choir singing activity (i.e., frequency; hours per week) and musical sophistication (Gold‐MSI) were not associated with age. It should be noted, however, that the sample size for Gold‐MSI subscale correlations was slightly lower (N = 81) due to missing responses.
With respect to choir singing experience, all the Gold‐MSI subscales except emotions were associated with choir singing (% of life active): active engagement r s (81) = .509, p < .001; perceptual abilities r s (81) = .555, p < .001; musical training r s (81) = .648, p < .001; singing abilities r s (81) = .572, p < .001; and general musical sophistication r s (81) = .627, p < .001. Other background factors (gender, education years) were not associated with choir singing experience (% of life active).
3.2. WM connectometry
The relationship between choir singing experience and whole‐brain QA was analysed using correlational tractography. Across the whole sample (N = 95), higher choir singing experience was positively associated with QA in associative [left AF, right extreme capsule, as well as bilateral cingulum, inferior longitudinal fasciculi (ILF), and inferior fronto‐occipital fasciculi (IFOF)], commissural [anterior commissure (AC) and corpus callosum (CC)], and projection pathways (bilateral corticopontine, corticospinal, corticostriatal, and dentatorubrothalamic tracts, medial lemnisci, and thalamic radiations). Separate analyses in the age groups showed that these results were largely driven by young adults, in whom higher choir singing experience was linked to higher QA in most of the WM tracts listed above. Across all age groups, a positive association was observed between choir singing experience and higher QA in the posterior part of the CC. Additionally, choir singing experience was linked to higher QA in the right corticospinal tract in middle‐aged adults and in the bilateral fornix in older adults. Negative associations between choir singing experience and whole‐brain QA were not found. The results are summarised in Table 2 and Figure 1.
TABLE 2.
Number of tracts showing increased QA as a function of higher lifetime choir singing experience.
| Tract | Hemisphere | All | Young | Middle‐aged | Older |
|---|---|---|---|---|---|
| 20–39 years | 40–59 years | 60+ years | |||
| Association | |||||
| AF | R | ‐ | 38 | ‐ | ‐ |
| L | 201 | 36 | ‐ | ‐ | |
| Cingulum | R | 15 | ‐ | ‐ | ‐ |
| L | 74 | 13 | ‐ | ‐ | |
| Extreme capsule | R | 6 | ‐ | ‐ | ‐ |
| L | ‐ | ‐ | ‐ | ‐ | |
| ILF | R | 8 | ‐ | ‐ | ‐ |
| L | 11 | ‐ | ‐ | ‐ | |
| IFOF | R | 568 | ‐ | ‐ | ‐ |
| L | 386 | 20 | ‐ | ‐ | |
| Uncinate fasciculus | R | ‐ | 5 | ‐ | ‐ |
| L | ‐ | 32 | ‐ | ‐ | |
| Commissural | |||||
| AC | R/L | 7 | ‐ | ‐ | ‐ |
| CC | R/L | 7481 | 3164 | 52 | 20 |
| Projection | |||||
| Corticopontine | R | 365 | 20 | ‐ | ‐ |
| L | 264 | 205 | ‐ | ‐ | |
| Corticospinal | R | 3275 | 1539 | 56 | ‐ |
| L | 445 | 630 | ‐ | ‐ | |
| Corticostriatal | R | 101 | 18 | ‐ | ‐ |
| L | 98 | 9 | ‐ | ‐ | |
| Dentatorubrothalamic | R | 446 | 25 | ‐ | ‐ |
| L | 5 | ‐ | ‐ | ‐ | |
| Medial lemniscus | R | 664 | 132 | ‐ | ‐ |
| L | 48 | 41 | ‐ | ‐ | |
| Fornix | R | ‐ | ‐ | ‐ | 5 |
| L | ‐ | ‐ | ‐ | 7 | |
| Thalamic radiation | R | 115 | ‐ | ‐ | ‐ |
| L | 14 | ‐ | ‐ | ‐ | |
| Cerebellar | |||||
| Cerebellum | R | ‐ | 59 | ‐ | ‐ |
| L | ‐ | ‐ | ‐ | ‐ | |
Note: Analyses corrected for multiple comparisons (Bonferroni) and surviving tracts with N < 5 rejected. No decreasing trends for QA were found.
Abbreviations: AC, anterior commissure; AF, arcuate fasciculus; CC, corpus callosum; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; MLF, middle longitudinal fasciculus.
FIGURE 1.
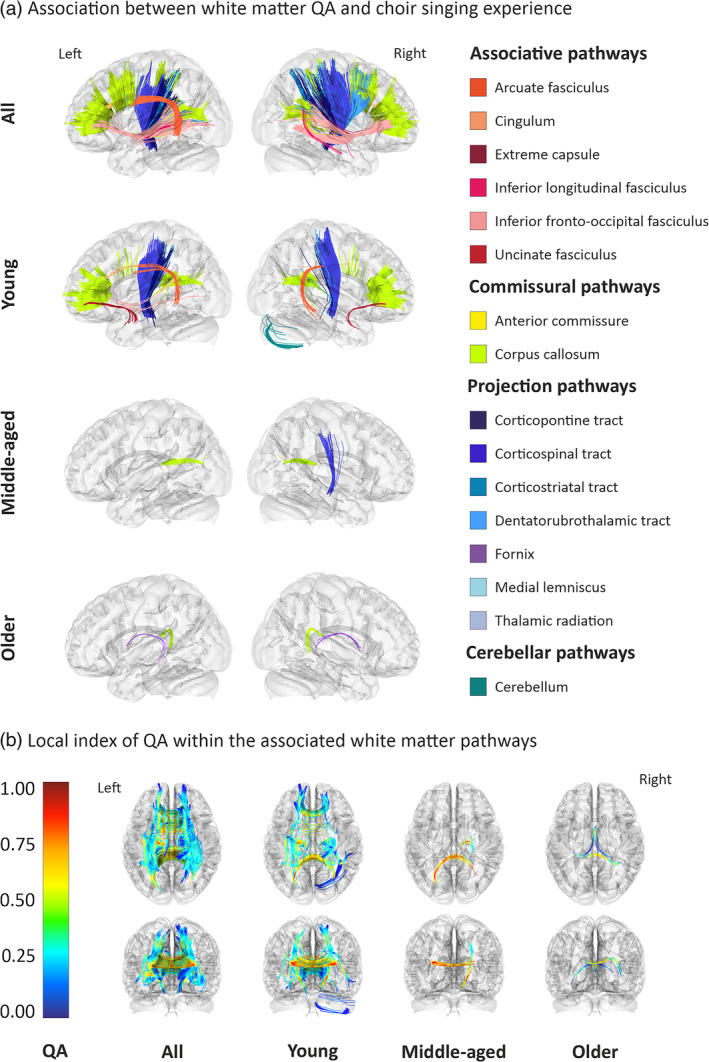
Positive associations between WM QA and lifetime choir singing experience. White matter (WM) pathways showing a positive association between quantitative anisotropy (QA) and choir singing experience regardless of age as well as within each age group (a) and the respective local indices for QA (b). Analyses were carried out using a significance threshold of t = 3.0 and filtered by topology‐informed pruning with 16 iterations and a length threshold of 30 voxels. All results corrected for multiple comparisons across the four models (FDR < 0.0125).
Furthermore, to learn more about the nature of these associations with respect to brain–behaviour correlations, supplementary follow‐up analyses between structural connectivity and choir singing frequency (hours/week lifetime maximum; hours/week current) were conducted in a similar manner in the full sample as well as in each age subgroup. Here, no significant associations with structural connectivity were found.
3.3. Voxel‐based morphometry
No significant whole‐brain effects on GM volume were observed in relation to choir singing experience, either in the overall sample or in any of age‐specific subgroups. To validate this null result, we conducted secondary analyses using more lenient thresholds (whole‐brain uncorrected p < .001 at the voxel‐level and FWE‐corrected p < .0125 at the cluster‐level). These analyses also failed to yield any significant results.
4. DISCUSSION
The present cross‐sectional study set out to examine the training‐induced association between amateur‐level choir singing on whole‐brain WM integrity (QA) and GM volume from early adulthood to older age. Independent of age, the multiple regression models showed positive effects of lifetime choir singing experience on the integrity of commissural (AC and CC) pathways and of associative (left AF and bilateral cingulum, ILF, and IFOF) and motor pathways (bilateral medial lemnisci, thalamic radiations, and corticostriatal, corticospinal, corticopontine, and dentatrorubrothalamic tracts). The whole‐sample results were largely driven by effects in young adults, possibly relating to higher intensity of musical activities following earlier starting age (see Gaser & Schlaug, 2003; Hutchinson, 2003). The integrity of the posterior CC showed experience‐dependent increase in all age groups (young, middle‐aged, and older adults). In addition, older adults showed positive effects of choir singing experience in the bilateral fornix, which was not observed in the other age groups. Neither negative effects on WM integrity nor associations with GM volume were found in relation to lifetime choir singing experience. Also supplementary follow‐up analyses on the frequency of choir singing experience (lifetime maximum; current) showed no significant associations with WM integrity.
4.1. Language network and integrative processing
Previous studies have reported experience‐dependent specialisation related to vocal training and amateur‐level singing in AF microstructure (Halwani et al., 2011; Perron et al., 2021) and network density in the left hemisphere (Cheng et al., 2023). In line with these findings, we observed that choir singing experience was associated with increased QA values in both the left AF and bilateral IFOF across the entire sample. A beneficial transfer effect from regular musical activities to linguistic functions (Patel, 2011; Román‐Caballero et al., 2018; see also Vuust et al., 2022), often attributed to functional overlap (see Musso et al., 2015; Patel, 2011), is a classical finding in music neuroscience. As AF and IFOF are thought to convey much of the information along the dorsal and ventral streams of the language network (Friederici & Gierhan, 2013; see also Conner et al., 2018), respectively, the present results corroborate behavioural findings and tentatively suggest enhanced structural network connectivity as one potential cause for such benefits (see also Halwani et al., 2011; Perron et al., 2021).
Interestingly, the present results also showed increased IFOF QA in the non‐language‐dominant right hemisphere. While its precise function remains unclear, previous evidence suggests that IFOF may also act as a connector of several networks across the brain (see, e.g., Sarubbo et al., 2013), contributing to emotional processing, nonlinguistic semantics, and mentalisation (e.g., H. Li et al., 2020; Matyi & Spielberg, 2023; Roux et al., 2021), for instance. A recent study on prosodic deficits and amusia in stroke patients (Sihvonen et al., 2022) also found right IFOF damage to predict the incidence of both, hypothesising that disrupted ventral stream processing might lead to inefficient rhythmic‐melodic integration along the far‐branching tract. Building on this idea, the observed bilateral increases in IFOF QA in the present study might further explain the enhanced auditory skills, such as pitch, timing, and timbre processing, commonly seen in musicians (for review, see Kraus & Chandrasekaran, 2010). Future investigations on this topic could benefit from a combination of structural and functional network measures.
Music is known to trigger strong emotional responses, engaging brain areas such as the anterior cingulate and insular regions for emotional expression during musical production (Koelsch, 2014; Pando‐Naude et al., 2021). This also engages the dopaminergic mesolimbic reward system (Koelsch, 2014; see also Menon & Levitin, 2005), which could potentially enhance the cingulum pathways as observed in the present data. Some previous work also links musical emotions to mnemonics (e.g., Ratovohery et al., 2018, 2019), suggesting that musical emotions may influence the mnemonic effects in music, potentially engaging overlapping subcortical structures (Groussard et al., 2010; see also Koelsch, 2014). As cingulum is also centrally involved in goal‐directed behaviour and motor control (Bubb et al., 2018), it would be highly interesting to learn how its structural specialisation maps to the multifaceted sensory‐emotional‐motor integration involved in regular musical activity through time.
Finally, choir singers also showed extensive QA increases along the somatomotor control circuitry, including the corticospinal tracts. Similar neuroplastic effects on descending motor tracts have also been documented for instrumental musical practice in previous dMRI studies (Engel et al., 2014; Imfeld et al., 2009; Rüber et al., 2015). These pathways convey motor signals to the musculature of the body and seem to be primed by emotional stimuli, such as facial expressions and music (Baumgartner et al., 2007; Borgomaneri et al., 2021; Haber, 2016; see also Pisner et al., 2017). Considering the brains' strong sensory‐motor (for review, see Vuust et al., 2022) and emotional‐motor response integration in music (Putkinen et al., 2021; see also Matthews et al., 2020), it seems plausible that choir singing, although not necessarily involving much active bodily movement aside from balance and high postural control, could enhance the microstructure of corticospinal tracts through coupled network processing of the music.
Interestingly, many of these general effects were largely driven by the young group. This observation suggests that singing‐associated structural specialisation was most pronounced in participants under 40 years of age. Given that the relative lifetime experience of choir singing was consistent across all age groups, this might be related to an earlier onset of training (Gaser & Schlaug, 2003). In the case of highly trained young participants, this early training would coincide with the highly plastic periods extending from childhood to early adulthood (see Altenmüller & Furuya, 2016). Furthermore, while the present analyses considered the influence of other musical hobbies (i.e., solo singing and instrument playing), interaction effects that cannot be attributed to a single origin, such as choir singing, should not be discounted. One potential factor is plain exposure to music; the neural networks supporting the perception of different types of music are likely to overlap, thereby potentially amplifying the plastic effects through increased overall frequency during these developmental periods (Altenmüller & Furuya, 2016; see also Hanna‐Pladdy & Gajewski, 2012; Hanna‐Pladdy & MacKay, 2011; Mansens et al., 2018). Since a direct comparison between the age groups was beyond the scope of this study, the contribution of the young choir singers to the full‐sample effects should be interpreted cautiously.
4.2. Corpus callosum, fornix, and memory at older age
The main finding of structural specialisation was found across the CC, with a more focal effect in the posterior parts in middle‐aged and older adults. Although the present study controlled for age also within the subgroups, the typically faster degradation of the anterior parts of CC (e.g., Fan et al., 2019) might drive this trend. Forming the largest commissural pathways in the human brain, CC contributes to a plethora of processes involving interhemispheric coordination (for review, see Innocenti et al., 2022), showing a positive relationship between its microstructural integrity and general cognition (Coelho et al., 2021; Raghavan et al., 2020; see also Shafer et al., 2022). Specifically, accelerated degradation in its posterior parts (posterior body, splenium, forceps major) have been found in older populations with mild cognitive impairment and Alzheimer's disease (Qiu et al., 2016; Xiao et al., 2022; for review, see also Teipel et al., 2016), potentially mediating episodic memory performance in these groups via disrupted functional connectivity (Qiu et al., 2016).
Like the posterior regions of the CC, structural disintegration of the adjacent fornices—main pathways connecting to the hippocampi—have been linked to memory decline (for reviews, see Li et al., 2022; Senova et al., 2020). The fornices typically mature and decline among the first WM tracts in the brain (Jang et al., 2011; Korbmacher et al., 2023; Lebel et al., 2012; Yap et al., 2013), which may explain why the observed singing‐related QA enhancements were seen exclusively in older adults. Disruptions in fornix microstructure also typically precede those seen in posterior CC in AD and may already appear at the pre‐clinical stage (see Teipel et al., 2016). Therefore, relatively longer duration of singing experience might offer greater potential in preserving memory‐related structures throughout adulthood.
Previous studies on the effects of musical activities on CC and fornix microstructure in older adults remain sparse, yet two RCTs have found that already six months of dancing (Burzynska et al., 2017) and instrumental training (Jünemann et al., 2022) can improve the structural integrity of the fornix, with a positive association to episodic memory in healthy, musically naïve older adults (Jünemann et al., 2022). Considering that both dancing and instrument playing have also been associated with a reduced risk of dementia (Verghese et al., 2003) and improved cognitive and memory functioning in older persons with mild cognitive impairment (Doi et al., 2017), choir singing would seem another promising leisure activity to protect structures supporting memory function.
Paradoxically, previous evidence on the effects from singing on cognitive function in older adults is somewhat mixed, with some studies reporting singing‐related benefits across a range of functions, episodic memory included (Rouse et al., 2022), and some no cognitive effects (Feng et al., 2020; Johnson et al., 2020). Methodological variability, the abovementioned duration as well as the frequency of musical activity might cause this discrepancy (see Hanna‐Pladdy & Gajewski, 2012; Hanna‐Pladdy & MacKay, 2011; Mansens et al., 2018). While the effects of choir singing on cognitive function were outside the scope of this structural mapping study, it is recommended that future work aiming to establish such a connection would consider methodological choices of previous studies, including the duration and frequency of singing, typical ageing patterns of the structures of interest (e.g., Korbmacher et al., 2023), as well as individual variability, including the contribution of unique lifetime factors (e.g., Chan et al., 2018).
4.3. Choir singing and GM volume
In contrast to large‐scale WM changes, we found no association between choir singing and whole‐brain GM. While GM and WM follow different ageing trajectories (Bethlehem et al., 2022) and potential effects may not be focal enough to reach statistical significance, volumetric methods may also be less sensitive to subtle variations in ageing effects than dMRI (e.g., Giorgio et al., 2010). This may explain the asymmetry of the effects within the present sample. However, it should also be noted that our null result contradicts previous findings involving a range of musical activities from amateur‐level musicianship (Chaddock‐Heyman et al., 2021) to weekly exercise with music (Tabei et al., 2017). A methodological reason for the discrepant findings could be that only the present study used a free whole‐brain analysis in a relatively large sample, while differences in controlling for multiple comparisons and for the effects from demographic factors, for instance, might further differentiate the outcomes.
4.4. Conclusion
The present study systematically mapped the effects of lifetime amateur‐level choir singing experience on GM volume and structural connectivity, showing extensive enhancements in commissural as well as bilateral association and projection tracts associated with lifetime experience in choir singing across the sample. By contrast, structural connectivity was not associated with the frequency of weekly choir singing. Considering that the participants reported a lifetime maximum of amateur‐level choir singing activities up to 10 h per week and the current level up to 8 h per week (Table 1), this asymmetry between duration and frequency of choir singing with respect to structural connectivity seems to suggest a more determinant role for the regularity rather than volume of activity at the amateur level (see also Hanna‐Pladdy & MacKay, 2011; Mansens et al., 2018). Specifically, the present inclusion criteria required a minimum of one weekly hour of choir singing for the past year for participants who were engaged in choir (for more detail, see Section 2.1).
However, marked limitations apply. First, although the chosen study variable represented lifetime experience with choir singing, thus offering a longitudinal perspective on the significant effects, a cross‐sectional design does not allow establishing a causal relationship between choir singing and brain structure. Long‐term RCTs involving structural methods as well as recorded history of long‐term engagement in leisure activities (Chan et al., 2018) are required. Second, despite careful demographic matching within the sample and controlling for differences in key factors, a potential bias, as seen in the above sections, could be caused by the complex relationships of different factors related to musical (and other) activities, for instance. These could not be thoroughly controlled for with the present sample size (see, e.g., Harrell, 2015). Each of these issues could also be overcome by focusing future studies on RCTs involving musically naïve adults and a comprehensive account of musical aptitudes as well as records of leisure activities and other unique lifetime factors in addition to standard demographic information.
Third, self‐selection of musical activities, such as singing, may lead to population bias through natural disposition for music; a limitation which has been recognised in the field for some time (e.g., Schlaug, 2005, pp. 367–377). Specifically, while lifetime‐range correlational studies, such as the present one, can help map effects associated with various degrees of experience, controlling for musical aptitude and sophistication as well as their structural and other biological underpinnings (e.g., Järvelä, 2018) in a naturalistic sample is highly challenging. Indeed, the present demographic results for musical sophistication showed positive associations between choir singing experience and the Gold‐MSI scales except for the Emotion subscale. This suggests that, although the ability to enjoy music emotionally was not restricted to musically active participants, a general selection bias towards musical activities may apply. Besides continuing the work with an RCT, higher reliability could be achieved through careful control over these factors using pooled samples, for instance.
The stated limitations considered, the present study provides the first evidence of an association between choir singing and enhanced WM microstructure at the whole‐brain level across a large age range, and corroborates previous findings from cross‐sectional and longitudinal studies on the benefits of musical activities. Importantly, large‐scale specialisation effects were seen across the age range, with beneficial effects on associative language and limbic tracts supporting previous findings of enhanced processing in related functional domains. With respect to maintaining brain health at older age, a key finding in the present study was the enhancement of posterior CC and fornix integrity in older choir singers, which may serve as interesting neuroimaging biomarkers for future singing intervention studies in ageing. The present results encourage further investigations on the effects of choir singing as well as other musical leisure activities. Considering the high ecological validity and cost‐efficiency of such activities, the present results suggest great potential for using regular, long‐term singing as method to maintain healthy brain structure across the adult life span.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ACKNOWLEDGEMENT
The authors would like to thank all the participants as well as the personnel of the AMI Centre for their invaluable contribution to this study.
Moisseinen, N. , Ahveninen, L. , Martínez‐Molina, N. , Sairanen, V. , Melkas, S. , Kleber, B. , Sihvonen, A. J. , & Särkämö, T. (2024). Choir singing is associated with enhanced structural connectivity across the adult lifespan. Human Brain Mapping, 45(7), e26705. 10.1002/hbm.26705
Aleksi J. Sihvonen and Teppo Särkämö contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Altenmüller, E. , & Furuya, S. (2016). Brain plasticity and the concept of metaplasticity in skilled musicians. In Laczko J. & Latash M. (Eds.), Advances in experimental medicine and biology (Vol. 957, pp. 197–208). Springer. 10.1007/978-3-319-47313-0_11 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , Graham, M. S. , Zsoldos, E. , & Sotiropoulos, S. N. (2016). Incorporating outlier detection and replacement into a non‐parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141, 556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa, A. , Teramoto, M. , Maeda, S. , Sakai, Y. , Nosaka, S. , Gao, Q. , Kawachi, H. , Kashima, R. , Matsumoto, C. , & Kokubo, Y. (2022). Playing a musical instrument and the risk of dementia among older adults: A systematic review and meta‐analysis of prospective cohort studies. BMC Neurology, 22(1), 395. 10.1186/s12883-022-02902-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2011). Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation. NeuroImage, 55(3), 954–967. 10.1016/j.neuroimage.2010.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner, T. , Willi, M. , & Jäncke, L. (2007). Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: A transcranial magnetic stimulation study. Neuroreport, 18(3), 261–265. 10.1097/WNR.0b013e328012272e [DOI] [PubMed] [Google Scholar]
- Bethlehem, R. A. I. , Seidlitz, J. , White, S. R. , Vogel, J. W. , Anderson, K. M. , Adamson, C. , Adler, S. , Alexopoulos, G. S. , Anagnostou, E. , Areces‐Gonzalez, A. , Astle, D. E. , Auyeung, B. , Ayub, M. , Bae, J. , Ball, G. , Baron‐Cohen, S. , Beare, R. , Bedford, S. A. , Benegal, V. , … Alexander‐Bloch, A. F. (2022). Brain charts for the human lifespan. Nature, 604(7906), 525–533. 10.1038/s41586-022-04554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgomaneri, S. , Vitale, F. , Battaglia, S. , & Avenanti, A. (2021). Early right motor cortex response to happy and fearful facial expressions: A TMS motor‐evoked potential study. Brain Sciences, 11(9), 1203. 10.3390/brainsci11091203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke, J. , Driemeyer, J. , Gaser, C. , Büchel, C. , & May, A. (2008). Training‐induced brain structure changes in the elderly. The Journal of Neuroscience, 28(28), 7031–7035. 10.1523/JNEUROSCI.0742-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb, E. J. , Metzler‐Baddeley, C. , & Aggleton, J. P. (2018). The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews, 92, 104–127. 10.1016/j.neubiorev.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, S. N. , & Barnes, C. A. (2006). Neural plasticity in the ageing brain. Nature Reviews Neuroscience, 7(1), 30–40. 10.1038/nrn1809 [DOI] [PubMed] [Google Scholar]
- Burzynska, A. Z. , Jiao, Y. , Knecht, A. M. , Fanning, J. , Awick, E. A. , Chen, T. , Gothe, N. , Voss, M. W. , McAuley, E. , & Kramer, A. F. (2017). White matter integrity declined over 6‐months, but dance intervention improved integrity of the fornix of older adults. Frontiers in Aging Neuroscience, 9, 59. 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock‐Heyman, L. , Loui, P. , Weng, T. B. , Weisshappel, R. , Mcauley, E. , & Kramer, A. F. (2021). Musical training and brain volume in older adults. Brain Sciences, 11, 50. 10.3390/brainsci11010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, D. , Shafto, M. , Kievit, R. , Matthews, F. , Spink, M. , Valenzuela, M. , & Henson, R. N. (2018). Lifestyle activities in mid‐life contribute to cognitive reserve in late‐life, independent of education, occupation, and late‐life activities. Neurobiology of Aging, 70, 180–183. 10.1016/j.neurobiolaging.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton, R. A. , Barrick, T. R. , McIntyre, D. J. , Shen, Y. , O'Sullivan, M. , Howe, F. A. , Clark, C. A. , Morris, R. G. , & Markus, H. S. (2006). White matter damage on diffusion tensor imaging correlates with age‐related cognitive decline. Neurology, 66(2), 217–222. 10.1212/01.wnl.0000194256.15247.83 [DOI] [PubMed] [Google Scholar]
- Cheng, L. , Chiu, Y. , Lin, Y. , Li, W. , Hong, T. , Yang, C. , Shih, C. , Yeh, T. , Tseng, W. I. , Yu, H. , Hsieh, J. , & Chen, L. (2023). Long‐term musical training induces white matter plasticity in emotion and language networks. Human Brain Mapping, 44(1), 5–17. 10.1002/hbm.26054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, A. , Fernandes, H. M. , Magalhães, R. , Moreira, P. S. , Marques, P. , Soares, J. M. , Amorim, L. , Portugal‐Nunes, C. , Castanho, T. , Santos, N. C. , & Sousa, N. (2021). Signatures of white‐matter microstructure degradation during aging and its association with cognitive status. Scientific Reports, 11(1), 4517. 10.1038/s41598-021-83983-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, G. D. , Perlstein, S. , Chapline, J. , Kelly, J. , Firth, K. M. , & Simmens, S. (2006). The impact of professionally conducted cultural programs on the physical health, mental health, and social functioning of older adults. Gerontologist, 46(6), 726–734. 10.1093/geront/46.6.726 [DOI] [PubMed] [Google Scholar]
- Colcombe, S. J. , Erickson, K. I. , Scalf, P. E. , Kim, J. S. , Prakash, R. , McAuley, E. , Elavsky, S. , Marquez, D. X. , Hu, L. , & Kramer, A. F. (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(11), 1166–1170. 10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- Conner, A. K. , Briggs, R. G. , Sali, G. , Rahimi, M. , Baker, C. M. , Burks, J. D. , Glenn, C. A. , Battiste, J. D. , & Sughrue, M. E. (2018). A connectomic atlas of the human cerebrum—Chapter 13: Tractographic description of the inferior fronto‐occipital fasciculus. Operative Neurosurgery, 15(suppl_1), S436–S443. 10.1093/ons/opy267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton, S. , Clift, S. , Skingley, A. , & Rodriguez, J. (2015). Effectiveness and cost‐effectiveness of community singing on mental health‐related quality of life of older people: Randomised controlled trial. British Journal of Psychiatry, 207(3), 250–255. 10.1192/bjp.bp.113.129908 [DOI] [PubMed] [Google Scholar]
- de Lange, A.‐M. G. , Bråthen, A. C. S. , Rohani, D. A. , Fjell, A. M. , & Walhovd, K. B. (2018). The temporal dynamics of brain plasticity in aging. Cerebral Cortex, 28(5), 1857–1865. 10.1093/cercor/bhy003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, T. , Verghese, J. , Makizako, H. , Tsutsumimoto, K. , Hotta, R. , Nakakubo, S. , Suzuki, T. , & Shimada, H. (2017). Effects of cognitive leisure activity on cognition in mild cognitive impairment: Results of a randomized controlled trial. Journal of the American Medical Directors Association, 18(8), 686–691. 10.1016/j.jamda.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Dorris, J. L. , Neely, S. , Terhorst, L. , VonVille, H. M. , & Rodakowski, J. (2021). Effects of music participation for mild cognitive impairment and dementia: A systematic review and meta‐analysis. Journal of the American Geriatrics Society, 69(9), 2659–2667. 10.1111/jgs.17208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, A. , Hijmans, B. S. , Cerliani, L. , Bangert, M. , Nanetti, L. , Keller, P. E. , & Keysers, C. (2014). Inter‐individual differences in audio‐motor learning of piano melodies and white matter fiber tract architecture. Human Brain Mapping, 35(5), 2483–2497. 10.1002/hbm.22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Q. , Tian, Q. , Ohringer, N. A. , Nummenmaa, A. , Witzel, T. , Tobyne, S. M. , Klawiter, E. C. , Mekkaoui, C. , Rosen, B. R. , Wald, L. L. , Salat, D. H. , & Huang, S. Y. (2019). Age‐related alterations in axonal microstructure in the corpus callosum measured by high‐gradient diffusion MRI. NeuroImage, 191, 325–336. 10.1016/j.neuroimage.2019.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L. , Romero‐Garcia, R. , Suckling, J. , Tan, J. , Larbi, A. , Cheah, I. , Wong, G. , Tsakok, M. , Lanskey, B. , Lim, D. , Li, J. , Yang, J. , Goh, B. , Teck, T. G. C. , Ho, A. , Wang, X. , Yu, J.‐T. , Zhang, C. , Tan, C. , … Kua, E.‐H. (2020). Effects of choral singing versus health education on cognitive decline and aging: A randomized controlled trial. Aging, 12(24), 24798–24816. 10.18632/aging.202374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici, A. D. , & Gierhan, S. M. (2013). The language network. Current Opinion in Neurobiology, 23(2), 250–254. 10.1016/j.conb.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Gaser, C. , & Schlaug, G. (2003). Brain structures differ between musicians and non‐musicians. The Journal of Neuroscience, 23(27), 9240–9245. 10.1523/JNEUROSCI.23-27-09240.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio, A. , Santelli, L. , Tomassini, V. , Bosnell, R. , Smith, S. , De Stefano, N. , & Johansen‐Berg, H. (2010). Age‐related changes in grey and white matter structure throughout adulthood. NeuroImage, 51(3), 943–951. 10.1016/j.neuroimage.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussard, M. , Rauchs, G. , Landeau, B. , Viader, F. , Desgranges, B. , Eustache, F. , & Platel, H. (2010). The neural substrates of musical memory revealed by fMRI and two semantic tasks. NeuroImage, 53(4), 1301–1309. 10.1016/j.neuroimage.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. 10.31887/DCNS.2016.18.1/shaber [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani, G. , Loui, P. , Rueber, T. , & Schlaug, G. (2011). Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non‐musicians. Frontiers in Psychology, 2, 156. 10.3389/fpsyg.2011.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna‐Pladdy, B. , & Gajewski, B. (2012). Recent and past musical activity predicts cognitive aging variability: Direct comparison with general lifestyle activities. Frontiers in Human Neuroscience, 6, 198. 10.3389/fnhum.2012.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna‐Pladdy, B. , & MacKay, A. (2011). The relation between instrumental musical activity and cognitive aging. Neuropsychology, 25(3), 378–386. 10.1037/a0021895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, F. E. (2015). Multivariable modeling strategies (pp. 63–102). Springer. 10.1007/978-3-319-19425-7_4 [DOI] [Google Scholar]
- Hula, W. D. , Panesar, S. , Gravier, M. L. , Yeh, F.‐C. , Dresang, H. C. , Dickey, M. W. , & Fernandez‐Miranda, J. C. (2020). Structural white matter connectometry of word production in aphasia: An observational study. Brain, 143(8), 2532–2544. 10.1093/brain/awaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, S. (2003). Cerebellar volume of musicians. Cerebral Cortex, 13(9), 943–949. 10.1093/cercor/13.9.943 [DOI] [PubMed] [Google Scholar]
- Imfeld, A. , Oechslin, M. S. , Meyer, M. , Loenneker, T. , & Jancke, L. (2009). White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage, 46(3), 600–607. 10.1016/j.neuroimage.2009.02.025 [DOI] [PubMed] [Google Scholar]
- Innocenti, G. M. , Schmidt, K. , Milleret, C. , Fabri, M. , Knyazeva, M. G. , Battaglia‐Mayer, A. , Aboitiz, F. , Ptito, M. , Caleo, M. , Marzi, C. A. , Barakovic, M. , Lepore, F. , & Caminiti, R. (2022). The functional characterization of callosal connections. Progress in Neurobiology, 208, 102186. 10.1016/j.pneurobio.2021.102186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, S. H. , Cho, S.‐H. , & Chang, M. C. (2011). Age‐related degeneration of the fornix in the human brain: A diffusion tensor imaging study. International Journal of Neuroscience, 121(2), 94–100. 10.3109/00207454.2010.531894 [DOI] [PubMed] [Google Scholar]
- Johnson, J. K. , Stewart, A. L. , Acree, M. , Nápoles, A. M. , Flatt, J. D. , Max, W. B. , & Gregorich, S. E. (2020). A community choir intervention to promote well‐being among diverse older adults: Results from the community of voices trial. The Journals of Gerontology: Series B, 75(3), 549–559. 10.1093/geronb/gby132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvelä, I. (2018). Genomics studies on musical aptitude, music perception, and practice. Annals of the New York Academy of Sciences, 1423, 82–91. 10.1111/nyas.13620 [DOI] [PubMed] [Google Scholar]
- Jünemann, K. , Marie, D. , Worschech, F. , Scholz, D. S. , Grouiller, F. , Kliegel, M. , Van De Ville, D. , James, C. E. , Krüger, T. H. C. , Altenmüller, E. , & Sinke, C. (2022). Six months of piano training in healthy elderly stabilizes white matter microstructure in the fornix, compared to an active control group. Frontiers in Aging Neuroscience, 14, 817889. 10.3389/fnagi.2022.817889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, E. , Dhital, B. , Kiselev, V. G. , & Reisert, M. (2016). Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic Resonance in Medicine, 76(5), 1574–1581. 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- Koelsch, S. (2014). Brain correlates of music‐evoked emotions. Nature Reviews Neuroscience, 15(3), 170–180. 10.1038/nrn3666 [DOI] [PubMed] [Google Scholar]
- Koini, M. , Duering, M. , Gesierich, B. G. , Rombouts, S. A. R. B. , Ropele, S. , Wagner, F. , Enzinger, C. , & Schmidt, R. (2018). Grey‐matter network disintegration as predictor of cognitive and motor function with aging. Brain Structure and Function, 223(5), 2475–2487. 10.1007/s00429-018-1642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbmacher, M. , de Lange, A. M. , van der Meer, D. , Beck, D. , Eikefjord, E. , Lundervold, A. , Andreassen, O. A. , Westlye, L. T. , & Maximov, I. I. (2023). Brain‐wide associations between white matter and age highlight the role of fornix microstructure in brain ageing. Human Brain Mapping, 44(10), 4101–4119. 10.1002/hbm.26333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, N. , & Chandrasekaran, B. (2010). Music training for the development of auditory skills. Nature Reviews Neuroscience, 11(8), 599–605. 10.1038/nrn2882 [DOI] [PubMed] [Google Scholar]
- Kühn, S. , Lorenz, R. C. , Weichenberger, M. , Becker, M. , Haesner, M. , O'Sullivan, J. , Steinert, A. , Steinhagen‐Thiessen, E. , Brandhorst, S. , Bremer, T. , & Gallinat, J. (2017). Taking control! Structural and behavioural plasticity in response to game‐based inhibition training in older adults. NeuroImage, 156, 199–206. 10.1016/j.neuroimage.2017.05.026 [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Li, H. , Lin, X. , Liu, L. , Su, S. , Zhu, X. , Zheng, Y. , Huang, W. , Que, J. , Shi, L. , Bao, Y. , Lu, L. , Deng, J. , & Sun, X. (2020). Disruption of the structural and functional connectivity of the frontoparietal network underlies symptomatic anxiety in late‐life depression. NeuroImage: Clinical, 28, 102398. 10.1016/j.nicl.2020.102398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Zhang, C. , Rao, Y. , & Yuan, T.‐F. (2022). Deep brain stimulation of fornix for memory improvement in Alzheimer's disease: A critical review. Ageing Research Reviews, 79, 101668. 10.1016/j.arr.2022.101668 [DOI] [PubMed] [Google Scholar]
- Lövdén, M. , Bodammer, N. C. , Kühn, S. , Kaufmann, J. , Schütze, H. , Tempelmann, C. , Heinze, H.‐J. , Düzel, E. , Schmiedek, F. , & Lindenberger, U. (2010). Experience‐dependent plasticity of white‐matter microstructure extends into old age. Neuropsychologia, 48(13), 3878–3883. 10.1016/j.neuropsychologia.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Manard, M. , Bahri, M. A. , Salmon, E. , & Collette, F. (2016). Relationship between grey matter integrity and executive abilities in aging. Brain Research, 1642, 562–580. 10.1016/j.brainres.2016.04.045 [DOI] [PubMed] [Google Scholar]
- Mansens, D. , Deeg, D. J. H. , & Comijs, H. C. (2018). The association between singing and/or playing a musical instrument and cognitive functions in older adults. Aging and Mental Health, 22(8), 964–971. 10.1080/13607863.2017.1328481 [DOI] [PubMed] [Google Scholar]
- Matthews, T. E. , Witek, M. A. G. , Lund, T. , Vuust, P. , & Penhune, V. B. (2020). The sensation of groove engages motor and reward networks. NeuroImage, 214, 116768. 10.1016/j.neuroimage.2020.116768 [DOI] [PubMed] [Google Scholar]
- Matyi, M. A. , & Spielberg, J. M. (2023). Negative emotion differentiation and white matter microstructure. Journal of Affective Disorders, 332, 238–246. 10.1016/j.jad.2023.04.010 [DOI] [PubMed] [Google Scholar]
- Matziorinis, A. M. , Gaser, C. , & Koelsch, S. (2022). Is musical engagement enough to keep the brain young? Brain Structure and Function, 228(2), 577–588. 10.1007/s00429-022-02602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Levitin, D. J. (2005). The rewards of music listening: Response and physiological connectivity of the mesolimbic system. NeuroImage, 28(1), 175–184. 10.1016/j.neuroimage.2005.05.053 [DOI] [PubMed] [Google Scholar]
- Muiños, M. , & Ballesteros, S. (2021). Does dance counteract age‐related cognitive and brain declines in middle‐aged and older adults? A systematic review. Neuroscience & Biobehavioral Reviews, 121, 259–276. 10.1016/j.neubiorev.2020.11.028 [DOI] [PubMed] [Google Scholar]
- Musso, M. , Weiller, C. , Horn, A. , Glauche, V. , Umarova, R. , Hennig, J. , Schneider, A. , & Rijntjes, M. (2015). A single dual‐stream framework for syntactic computations in music and language. NeuroImage, 117, 267–283. 10.1016/j.neuroimage.2015.05.020 [DOI] [PubMed] [Google Scholar]
- Müllensiefen, D. , Gingras, B. , Musil, J. , & Stewart, L. (2014). The musicality of non‐musicians: An index for assessing musical sophistication in the general population. PLoS One, 9(2), e89642. 10.1371/journal.pone.0089642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas, R. , Hiba, B. , Dilharreguy, B. , Barse, E. , Baillet, M. , Edde, M. , Pelletier, A. , Periot, O. , Helmer, C. , Allard, M. , Dartigues, J.‐F. , Amieva, H. , Pérès, K. , Fernandez, P. , & Catheline, G. (2020). Changes over time of diffusion MRI in the white matter of aging brain, a good predictor of verbal recall. Frontiers in Aging Neuroscience, 12, 218. 10.3389/fnagi.2020.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pando‐Naude, V. , Patyczek, A. , Bonetti, L. , & Vuust, P. (2021). An ALE meta‐analytic review of top‐down and bottom‐up processing of music in the brain. Scientific Reports, 11(1), 20813. 10.1038/s41598-021-00139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. D. (2011). Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Frontiers in Psychology, 2, 142. 10.3389/fpsyg.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentikäinen, E. , Pitkäniemi, A. , Siponkoski, S.‐T. , Jansson, M. , Louhivuori, J. , Johnson, J. K. , Paajanen, T. , & Särkämö, T. (2021). Beneficial effects of choir singing on cognition and well‐being of older adults: Evidence from a cross‐sectional study. PLoS One, 16(2), e0245666. 10.1371/journal.pone.0245666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron, M. , Theaud, G. , Descoteaux, M. , & Tremblay, P. (2021). The frontotemporal organization of the arcuate fasciculus and its relationship with speech perception in young and older amateur singers and non‐singers. Human Brain Mapping, 42(10), 3058–3076. 10.1002/hbm.25416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron, M. , Vaillancourt, J. , & Tremblay, P. (2022). Amateur singing benefits speech perception in aging under certain conditions of practice: behavioural and neurobiological mechanisms. Brain Structure and Function, 227(3), 943–962. 10.1007/s00429-021-02433-2 [DOI] [PubMed] [Google Scholar]
- Pisner, D. A. , Smith, R. , Alkozei, A. , Klimova, A. , & Killgore, W. D. S. (2017). Highways of the emotional intellect: White matter microstructural correlates of an ability‐based measure of emotional intelligence. Social Neuroscience, 12(3), 253–267. 10.1080/17470919.2016.1176600 [DOI] [PubMed] [Google Scholar]
- Putkinen, V. , Nazari‐Farsani, S. , Seppälä, K. , Karjalainen, T. , Sun, L. , Karlsson, H. K. , Hudson, M. , Heikkilä, T. T. , Hirvonen, J. , & Nummenmaa, L. (2021). Decoding music‐evoked emotions in the auditory and motor cortex. Cerebral Cortex, 31(5), 2549–2560. 10.1093/cercor/bhaa373 [DOI] [PubMed] [Google Scholar]
- Qiu, Y. , Liu, S. , Hilal, S. , Loke, Y. M. , Ikram, M. K. , Xu, X. , Yeow Tan, B. , Venketasubramanian, N. , Chen, C. L.‐H. , & Zhou, J. (2016). Inter‐hemispheric functional dysconnectivity mediates the association of corpus callosum degeneration with memory impairment in AD and amnestic MCI. Scientific Reports, 6(1), 32573. 10.1038/srep32573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan, S. , Przybelski, S. A. , Reid, R. I. , Graff‐Radford, J. , Lesnick, T. G. , Zuk, S. M. , Knopman, D. S. , Machulda, M. M. , Mielke, M. M. , Petersen, R. C. , Jack, C. R. , & Vemuri, P. (2020). Reduced fractional anisotropy of the genu of the corpus callosum as a cerebrovascular disease marker and predictor of longitudinal cognition in MCI. Neurobiology of Aging, 96, 176–183. 10.1016/j.neurobiolaging.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovohery, S. , Baudouin, A. , Gachet, A. , Palisson, J. , & Narme, P. (2018). Is music a memory booster in normal aging? The influence of emotion. Memory, 26(10), 1344–1354. 10.1080/09658211.2018.1475571 [DOI] [PubMed] [Google Scholar]
- Ratovohery, S. , Baudouin, A. , Palisson, J. , Maillet, D. , Bailon, O. , Belin, C. , & Narme, P. (2019). Music as a mnemonic strategy to mitigate verbal episodic memory in Alzheimer's disease: Does musical valence matter? Journal of Clinical and Experimental Neuropsychology, 41(10), 1060–1073. 10.1080/13803395.2019.1650897 [DOI] [PubMed] [Google Scholar]
- Ritchie, S. J. , Bastin, M. E. , Tucker‐Drob, E. M. , Maniega, S. M. , Engelhardt, L. E. , Cox, S. R. , Royle, N. A. , Gow, A. J. , Corley, J. , Pattie, A. , Taylor, A. M. , Valdés Hernández, M. D. C. , Starr, J. M. , Wardlaw, J. M. , & Deary, I. J. (2015). Coupled changes in brain white matter microstructure and fluid intelligence in later life. The Journal of Neuroscience, 35(22), 8672–8682. 10.1523/JNEUROSCI.0862-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogenmoser, L. , Kernbach, J. , Schlaug, G. , & Gaser, C. (2018). Keeping brains young with making music. Brain Structure and Function, 223(1), 297–305. 10.1007/s00429-017-1491-2 [DOI] [PubMed] [Google Scholar]
- Román‐Caballero, R. , Arnedo, M. , Triviño, M. , & Lupiáñez, J. (2018). Musical practice as an enhancer of cognitive function in healthy aging—A systematic review and meta‐analysis. PLoS One, 13(11), e0207957. 10.1371/journal.pone.0207957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse, H. J. , Jin, Y. , Hueluer, G. , Huo, M. , Bugos, J. A. , Veal, B. , Torres, M. , Peterson, L. , Dobbs, D. , & Meng, H. (2022). Association between music engagement and episodic memory among middle‐aged and older adults: A national cross‐sectional analysis. The Journals of Gerontology: Series B, 77(3), 558–566. 10.1093/geronb/gbab044 [DOI] [PubMed] [Google Scholar]
- Roux, A. , Lemaitre, A.‐L. , Deverdun, J. , Ng, S. , Duffau, H. , & Herbet, G. (2021). Combining electrostimulation with fiber tracking to stratify the inferior fronto‐occipital fasciculus. Frontiers in Neuroscience, 15, 683348. 10.3389/fnins.2021.683348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüber, T. , Lindenberg, R. , & Schlaug, G. (2015). Differential adaptation of descending motor tracts in musicians. Cerebral Cortex, 25(6), 1490–1498. 10.1093/cercor/bht331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner, R. L. , Snyder, A. Z. , Greve, D. N. , Desikan, R. S. R. , Busa, E. , Morris, J. C. , Dale, A. M. , & Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- Sarubbo, S. , De Benedictis, A. , Maldonado, I. L. , Basso, G. , & Duffau, H. (2013). Frontal terminations for the inferior fronto‐occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi‐component bundle. Brain Structure and Function, 218(1), 21–37. 10.1007/s00429-011-0372-3 [DOI] [PubMed] [Google Scholar]
- Schilling, K. G. , Yeh, F.‐C. , Nath, V. , Hansen, C. , Williams, O. , Resnick, S. , Anderson, A. W. , & Landman, B. A. (2019). A fiber coherence index for quality control of B‐table orientation in diffusion MRI scans. Magnetic Resonance Imaging, 58, 82–89. 10.1016/j.mri.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug, G. (2005). The brain of musicians. In Peretz I. & Zatorre R. (Eds.), The cognitive neuroscience of music (pp. 366–381). Oxford University Press. [Google Scholar]
- Senova, S. , Fomenko, A. , Gondard, E. , & Lozano, A. M. (2020). Anatomy and function of the fornix in the context of its potential as a therapeutic target. Journal of Neurology, Neurosurgery & Psychiatry, 91(5), 547–559. 10.1136/jnnp-2019-322375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer, A. T. , Williams, O. A. , Perez, E. , An, Y. , Landman, B. A. , Ferrucci, L. , & Resnick, S. M. (2022). Accelerated decline in white matter microstructure in subsequently impaired older adults and its relationship with cognitive decline. Brain Communications, 4(2), fcac051. 10.1093/braincomms/fcac051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen, A. J. , Sammler, D. , Ripollés, P. , Leo, V. , Rodríguez‐Fornells, A. , Soinila, S. , & Särkämö, T. (2022). Right ventral stream damage underlies both poststroke aprosodia and amusia. European Journal of Neurology, 29(3), 873–882. 10.1111/ene.15148 [DOI] [PubMed] [Google Scholar]
- Sihvonen, A. J. , Vadinova, V. , Garden, K. L. , Meinzer, M. , Roxbury, T. , O'Brien, K. , Copland, D. , McMahon, K. L. , & Brownsett, S. L. E. (2023). Right hemispheric structural connectivity and poststroke language recovery. Human Brain Mapping, 44(7), 2897–2904. 10.1002/hbm.26252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen, A. J. , Virtala, P. , Thiede, A. , Laasonen, M. , & Kujala, T. (2021). Structural white matter connectometry of reading and dyslexia. NeuroImage, 241, 118411. 10.1016/j.neuroimage.2021.118411 [DOI] [PubMed] [Google Scholar]
- Skingley, A. , Martin, A. , & Clift, S. (2016). The contribution of community singing groups to the well‐being of older people. Journal of Applied Gerontology, 35(12), 1302–1324. 10.1177/0733464815577141 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , Bannister, P. R. , De Luca, M. , Drobnjak, I. , Flitney, D. E. , Niazy, R. K. , Saunders, J. , Vickers, J. , Zhang, Y. , De Stefano, N. , Brady, J. M. , & Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Statistics Finland . (2020). Educational structure of population (e‐publication). In Official Statistics of Finland (OSF). Helsinki: Statistics Finland.
- Statistics Finland . (2021). Share of population with educational qualifications has multiplied in 50 years. Official Statistics of Finland (OSF).
- Sutcliffe, R. , Du, K. , & Ruffman, T. (2020). Music making and neuropsychological aging: A review. Neuroscience & Biobehavioral Reviews, 113, 479–491. 10.1016/j.neubiorev.2020.03.026 [DOI] [PubMed] [Google Scholar]
- Tabei, K. , Satoh, M. , Ogawa, J. , Tokita, T. , Nakaguchi, N. , Nakao, K. , Kida, H. , & Tomimoto, H. (2017). Physical exercise with music reduces gray and white matter loss in the frontal cortex of elderly people: The Mihama‐Kiho scan project. Frontiers in Aging Neuroscience, 9, 174. 10.3389/fnagi.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel, S. , Grothe, M. J. , Zhou, J. , Sepulcre, J. , Dyrba, M. , Sorg, C. , & Babiloni, C. (2016). Measuring cortical connectivity in Alzheimer's disease as a brain neural network pathology: Toward clinical applications. Journal of the International Neuropsychological Society, 22(2), 138–163. 10.1017/S1355617715000995 [DOI] [PubMed] [Google Scholar]
- Tournier, J.‐D. , Smith, R. , Raffelt, D. , Tabbara, R. , Dhollander, T. , Pietsch, M. , Christiaens, D. , Jeurissen, B. , Yeh, C.‐H. , & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tremblay, P. , & Perron, M. (2023). Auditory cognitive aging in amateur singers and non‐singers. Cognition, 230, 105311. 10.1016/j.cognition.2022.105311 [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs . (2022). World Population Prospects 2022: Summary of results. In UN DESA/POP/2022/TR/NO. 3.
- Veraart, J. , Novikov, D. S. , Christiaens, D. , Ades‐aron, B. , Sijbers, J. , & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese, J. , Lipton, R. B. , Katz, M. J. , Hall, C. B. , Derby, C. A. , Kuslansky, G. , Ambrose, A. F. , Sliwinski, M. , & Buschke, H. (2003). Leisure activities and the risk of dementia in the elderly from the Einstein aging study. The New England Journal of Medicine, 25). Retrieved from www.nejm.org, 2508–2516. [DOI] [PubMed] [Google Scholar]
- Vinke, E. J. , de Groot, M. , Venkatraghavan, V. , Klein, S. , Niessen, W. J. , Ikram, M. A. , & Vernooij, M. W. (2018). Trajectories of imaging markers in brain aging: the Rotterdam study. Neurobiology of Aging, 71, 32–40. 10.1016/j.neurobiolaging.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Vuust, P. , Heggli, O. A. , Friston, K. J. , & Kringelbach, M. L. (2022). Music in the brain. Nature Reviews Neuroscience, 23(5), 287–305. 10.1038/s41583-022-00578-5 [DOI] [PubMed] [Google Scholar]
- Xiao, D. , Wang, K. , Theriault, L. , & Charbel, E. (2022). White matter integrity and key structures affected in Alzheimer's disease characterized by diffusion tensor imaging. European Journal of Neuroscience, 56(8), 5319–5331. 10.1111/ejn.15815 [DOI] [PubMed] [Google Scholar]
- Yap, Q. J. , Teh, I. , Fusar‐Poli, P. , Sum, M. Y. , Kuswanto, C. , & Sim, K. (2013). Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. Journal of Neural Transmission, 120(9), 1369–1395. 10.1007/s00702-013-0971-7 [DOI] [PubMed] [Google Scholar]
- Yeh, F. , Liu, L. , Hitchens, T. K. , & Wu, Y. L. (2017). Mapping immune cell infiltration using restricted diffusion <scp>MRI</scp>. Magnetic Resonance in Medicine, 77(2), 603–612. 10.1002/mrm.26143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Badre, D. , & Verstynen, T. (2016). Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage, 125, 162–171. 10.1016/j.neuroimage.2015.10.053 [DOI] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Panesar, S. , Barrios, J. , Fernandes, D. , Abhinav, K. , Meola, A. , & Fernandez‐Miranda, J. C. (2019). Automatic removal of false connections in diffusion MRI tractography using topology‐informed pruning (TIP). Neurotherapeutics, 16(1), 52–58. 10.1007/s13311-018-0663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , & Tseng, W.‐Y. I. (2011). NTU‐90: A high angular resolution brain atlas constructed by q‐space diffeomorphic reconstruction. NeuroImage, 58(1), 91–99. 10.1016/j.neuroimage.2011.06.021 [DOI] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Verstynen, T. D. , Wang, Y. , Fernández‐Miranda, J. C. , & Tseng, W.‐Y. I. (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One, 8(11), e80713. 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Vettel, J. M. , Singh, A. , Poczos, B. , Grafton, S. T. , Erickson, K. I. , Tseng, W.‐Y. I. , & Verstynen, T. D. (2016). Quantifying differences and similarities in whole‐brain white matter architecture using local connectome fingerprints. PLoS Computational Biology, 12(11), e1005203. 10.1371/journal.pcbi.1005203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Wedeen, V. J. , & Tseng, W.‐Y. I. (2010). Generalized q‐sampling imaging. IEEE Transactions on Medical Imaging, 29(9), 1626–1635. 10.1109/TMI.2010.2045126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


