Abstract
Aims
Traditional atrial fibrillation (AF) recurrence after catheter ablation is reported as a binary outcome. However, a paradigm shift towards a more granular definition, considering arrhythmic or symptomatic burden, is emerging. We hypothesize that ablation reduces AF burden independently of conventional recurrence status in patients with persistent AF, correlating with symptom burden reduction.
Methods and results
Ninety-eight patients with persistent AF from the DECAAF II trial with pre-ablation follow-up were included. Patients recorded daily single-lead electrocardiogram (ECG) strips, defining AF burden as the proportion of AF days among total submitted ECG days. The primary outcome was atrial arrhythmia recurrence. The AF severity scale was administered pre-ablation and at 12 months post-ablation. At follow-up, 69 patients had atrial arrhythmia recurrence and 29 remained in sinus rhythm. These patients were categorized into a recurrence (n = 69) and a no-recurrence group (n = 29). Both groups had similar baseline characteristics, but recurrence patients were older (P = 0.005), had a higher prevalence of hyperlipidaemia (P = 0.007), and had a larger left atrial (LA) volume (P = 0.01). There was a reduction in AF burden in the recurrence group when compared with their pre-ablation burden (65 vs. 15%, P < 0.0001). Utah Stage 4 fibrosis and diabetes predicted less improvement in AF burden. The symptom severity score at 12 months post-ablation was significantly reduced compared with the pre-ablation score in the recurrence group, and there was a significant correlation between the reduction in symptom severity score and the reduction in AF burden (R = 0.39, P = 0.001).
Conclusion
Catheter ablation reduces AF burden, irrespective of arrhythmia recurrence post-procedure. There is a strong correlation between AF burden reduction and symptom improvement post-ablation. Notably, elevated LA fibrosis impedes AF burden decrease following catheter ablation.
Keywords: Atrial fibrillation, AF burden, Smartphone ECG, Catheter ablation, Fibrosis, AF symptoms
Graphical Abstract
Graphical Abstract.
What’s new?
The study introduces a nuanced perspective on the outcomes of catheter ablation, emphasizing not just the binary metric of arrhythmia recurrence but also the more granular aspects such as arrhythmic burden and symptom severity, as well as an innovative and effective monitoring strategy, that is, smartphone-based single-lead electrocardiogram technology. This approach aligns with the evolving paradigm in electrophysiology that seeks to understand atrial fibrillation (AF) management outcomes beyond mere recurrence rates, highlighting a significant reduction in AF burden and symptom severity post-procedure. Clinicians can appreciate that lower pre-ablation left atrial fibrosis levels may predict better ablation outcomes.
Introduction
The assessment of catheter ablation outcome is traditionally based on a binary definition of atrial arrhythmia recurrence, which determines the presence or absence of atrial fibrillation (AF) episodes following a 90-day blanking period. However, recent studies have recommended a shift towards a more inclusive and meaningful outcome, known as AF burden.1 Various methods exist for determining AF burden, one of which involves calculating the proportion of time a patient experiences AF during a specified duration. This approach facilitates the generation of a continuous, or quasi-continuous, representation of the patient’s dynamic arrhythmic profile.
The evaluation of AF burden before and after catheter ablation has the potential to provide a more nuanced and detailed measurement of treatment efficacy than the binary recurrence definition. The latter only detects the presence or absence of AF episodes, while the former considers the amount of AF, offering a more objective measurement of improvement. Moreover, even in cases of AF recurrence, catheter ablation has been linked to an improvement in the quality of life and symptom burden.2
This study aims to propose a non-invasive method of evaluating AF burden using a smartphone single-lead electrocardiogram (ECG) device (SMURDEN). We hypothesize that catheter ablation decreases SMURDEN independently of conventional recurrence status in the persistent AF population, and a decrease in SMURDEN correlates with a reduction in symptom burden.
Methods
Study population
Details of the DECAAF II trial have been published.3 In short, the DECAAF II trial was a large investigator-initiated, industry-sponsored, prospective, multicentre (44 sites, 3 continents), randomized controlled clinical trial in which 843 patients with persistent AF were randomized into two treatment arms comparing magnetic resonance imaging (MRI)-guided fibrosis ablation + pulmonary vein isolation (PVI) vs. PVI alone. To be enrolled in the trial, patients had to have persistent AF (defined as 7 days or more of AF as evidenced by either a rhythm strip or documentation on a chart review) and must have been undergoing their first AF ablation. The main exclusion criteria were contraindication to gadolinium and/or MRI and previous AF ablation or valvular cardiac surgery. This study was approved by the Tulane University Biomedical IRB.
Electrocardiogram acquisition
All patients received a handheld smartphone ECG device (ECG Check, Cardiac Designs4) and were required to record ECG strips daily and in case of any relevant symptoms during the study follow-up period. Ambulatory monitoring and 12-lead ECG data performed as part of clinical care were also reported as components of the primary outcome of the original trial, but are not part of this particular analysis. Electrocardiogram strips from the handheld device were transmitted automatically to the ECG core laboratory for analysis by trained experts masked from treatment assignment. A number of patients started submitting ECG strips for analysis from the initial screening visit. Electrocardiogram strips were intended for patient follow-up after the procedure, and pre-ablation rhythm monitoring was not required. For this analysis, which focuses on the difference between pre- and post-ablation arrhythmic burden, we included only those patients who recorded at least 10 ECG strips in the pre-ablation period.
Catheter ablation procedure
Fibrosis-guided ablation
For patients randomized to the fibrosis-guided ablation group, processed delayed-enhancement MRIs were merged with the 3D mapping system at each study site to be used during the procedure. All patients underwent PVI. After PV entrance block had been confirmed, fibrosis-guided ablation was pursued. The operator either encircled or covered with ablation lesions all fibrotic areas observed on delayed-enhancement MRI. Details regarding the ablation protocol for both treatment groups are included in the main manuscript.
Pulmonary vein isolation
All PVs were electrically isolated, as described by the Heart Rhythm Society Consensus Statement.5 If normal sinus rhythm could not be restored, despite cardioversion at the end of the PVI portion of the procedure in patients randomized to this group, the operator had the option to pursue other measures to eliminate recurrent arrhythmias if needed.
Imaging
Patients underwent a delayed-enhancement MRI within 30 days prior to the ablation procedure using the Merisight delayed-enhancement MRI protocol (MARREK Inc.). The purpose of the baseline MRI was to quantify left atrial (LA) fibrosis in all patients. Patients’ randomized treatment group was masked from reviewers who assessed MRI quality. MARREK Inc. assisted with image segmentation, processing, and quantification of LA fibrosis. Following ablation, delayed-enhancement MRIs were obtained at 90–180 days to quantify ablation-related scar formation.
Primary outcome
The primary endpoint of the study was the first confirmed recurrence of atrial arrhythmia (including AF, atrial flutter, or atrial tachycardia) lasting for at least 30 s after the 90-day blanking period, demonstrated by single-lead smartphone ECG device tracing, one positive reading on a clinical 12-lead ECG tracing, ambulatory monitor, or if the patient underwent repeat ablation, over a follow-up period of 12–18 months. The daily smartphone ECGs were intended as the primary method for assessing atrial arrhythmia recurrence, but clinical and ambulatory ECGs served as back-up methods for detecting recurrence in patients who failed to reliably transmit smartphone ECG readings. A core laboratory at the University of Washington adjudicated the ECG findings.
Smartphone atrial fibrillation burden (SMURDEN)
Atrial fibrillation burden was defined as the proportion of days on which the submitted ECG strips showed evidence of AF, out of the total number of days on which ECG strips were submitted for each patient during a specified period. The AF burden was calculated from the time of initial screening until the day of ablation, and from the end of the blanking period until the end of follow-up. If multiple ECG strips were provided on the same day, and all showed sinus rhythm, that day was considered a sinus rhythm day. However, if at least one ECG strip showed evidence of AF on that day, the day was classified as an AF day.
Atrial fibrillation severity scale
The AF severity scale (AFSS) is a self-administered questionnaire comprising 19 items, developed to measure both subjective and objective ratings of symptoms related to AF, healthcare utilization, and the overall burden of AF, including the frequency, duration, and severity of episodes. For the purposes of this analysis, we focused on Part C of the questionnaire, which specifically assesses the frequency of AF-related symptoms such as palpitation, shortness of breath at rest and during physical activity, exercise intolerance, fatigue at rest, lightheadedness/dizziness, and chest pain/pressure. The severity of each symptom is rated on a 6-point scale, ranging from 0 to 5 points. The resulting scores range from 0 to 35 points, with higher scores indicating greater AF symptom severity. The AFSS questionnaire was administered at the screening visit and 12 months’ post-ablation.
Statistics
The report of all continuous variables is given as the mean ± standard deviation (SD), with comparison achieved through Student’s t-tests and Wilcoxon tests, dependent on the outcome of the normality assumption verification via the Shapiro–Wilk test. For categorical variables, they are detailed as either percentages or frequencies, with comparisons made through χ2 tests.
An exploration of univariate analyses was undertaken to identify potential predictors of the AF burden post-ablation from a group of variables of interest. The outcomes of these analyses are presented as regression coefficients (β). Inclusion in a multivariate model was considered for variables with a P-value of <0.3, using a stepwise selection process, where the threshold for inclusion was 0.05. For categorical variables with multiple levels, we combined the non-significant levels to obtain a parsimonious model.
The non-linear relationship between the change in AF burden and the change in AFSS score among the patients who underwent ablation was evaluated using the Spearman correlation coefficient. The entire statistical analyses was carried out using R 4.2.0 (http://www.R-project.org, The R Foundation), employing a two-sided significance level of 0.05 as the standard.
Results
Baseline characteristics
We included 98 patients who underwent pre-ablation ECG monitoring and submitted more than 10 single-lead ECG strips for analysis during that time period. The study population had a mean age of 61.6 years (SD = 9.3), with 72.4% being males, and a mean baseline LA fibrosis of 18.89% (SD = 7.08). At the end of the follow-up period, 69 patients had atrial arrhythmia recurrence, while 29 remained in sinus rhythm. We categorized these patients into a no-recurrence group (Group 1, n = 29) and a recurrence group (Group 2, n = 69). Overall, baseline characteristics, comorbidity profile, and medication history were similar between the two groups. Nevertheless, patients in Group 2 tended to be older (63.2 vs. 57.9 years, P = 0.005), had a higher prevalence of hyperlipidaemia (37.7 vs. 10.3%, P = 0.007), and a larger LA volume (111.4 vs. 92 mL, P = 0.01). The mean follow-up from screening to ablation in our population was 62 days. There was a significant difference in pre-ablation follow-up times between the two groups (55.9 vs. 75.6 days, P = 0.01). Additionally, the number of days with ECG strip submission in this period (pre-ablation) was significantly different between the two groups (51.7 vs. 37.5, P = 0.02). However, the proportion of days with ECG submissions from screening to ablation was similar between the two groups (68 vs. 67%, P = 0.74). There was no correlation between pre-ablation ECG submission rate and pre-ablation AFSS (r = 0.16, P = 0.12) or pre-ablation AF burden (r = 0.03, r = 0.78). Table 1 presents baseline characteristics, AFSS, and single-lead ECG data.
Table 1.
Baseline characteristics
| No-recurrence group (n = 29) | Recurrence group (n = 69) | Total (n = 98) | P-value | |
|---|---|---|---|---|
| Age (years) | 57.9 | 63.2 | 61.6 | 0.005 |
| Males (%) | 23 (79.3) | 48 (69.6) | 71 (72.4) | 0.32 |
| Baseline fibrosis (%) | 18.04 | 18.97 | 18.7 | 0.44 |
| Utah stage | 0.35 | |||
| I | 2 (6.9) | 9 (13) | 11 (11.2) | |
| II | 18 (62.1) | 30 (43.5) | 48 (49) | |
| III | 6 (20.7) | 23 (33.3) | 29 (29.6) | |
| IV | 3 (10.3) | 7 (10.1) | 10 (10.2) | |
| Treatment received | 0.06 | |||
| PVI | 19 | 31 | 50 | |
| PVI + fibrosis-guided ablation | 10 | 38 | 48 | |
| Hypertension | 17 (58.6) | 37 (53.6) | 54 (55.1) | 0.64 |
| Diabetes | 3 (10.3) | 8 (11.6) | 11 (11.2) | 0.85 |
| CHF | 7 (24.1) | 13 (18.8) | 20 (20.4) | 0.55 |
| Stroke | 2 (6.9) | 7 (10.1) | 9 (9.2) | 0.61 |
| Vascular disease | 2 (6.9) | 5 (7.2) | 7 (7.1) | 0.95 |
| Mitral regurgitation | 3 (10.3) | 4 (5.8) | 7 (7.1) | 0.42 |
| Tobacco | 15 (51.7) | 28 (40.6) | 43 (43.9) | 0.31 |
| CAD | 0 (0) | 8 (11.6) | 8 (8.2) | 0.06 |
| Hyperlipidaemia | 3 (10.3) | 26 (37.7) | 29 (29.6) | 0.0068 |
| BMI | 31.07 | 33.06 | 32.5 | 0.21 |
| AADs | 12 (41.4) | 30 (43.5) | 42 (42.9) | 0.84 |
| Statins | 3 (10.3) | 27 (39.1) | 30 (30.6) | 0.0048 |
| Failed AAD treatment | 16 (55.2) | 29 (42) | 45 (45.9) | 0.23 |
| LA volume (mL) | 92 | 111.4 | 105.6 | 0.01 |
| Pre-ablation AFSS | 16.52 | 13.94 | 14.7 | 0.11 |
| 1-Year post-ablation AFSS | 3.24 | 5.16 | 4.59 | 0.02 |
| AFSS decrease | 13.28 | 8.76 | 10.11 | 0.02 |
| Pre-ablation follow-up (days) | 75.6 | 55.9 | 61.7 | 0.01 |
| Pre-ablation number of days with ECG submission | 51.7 | 37.5 | 41.7 | 0.02 |
| Proportion of days with ECG submission over pre-ablation follow-up | 0.68 | 0.67 | 0.67 | 0.74 |
| Post-ablation number of days with ECG submission | 163.4 | 281.7 | 246.7 | 0.0002 |
AAD, antiarrhythmic drug; AFSS, atrial fibrillation severity scale; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; ECG, electrocardiogram; LA, left atrium.
Delta atrial fibrillation burden
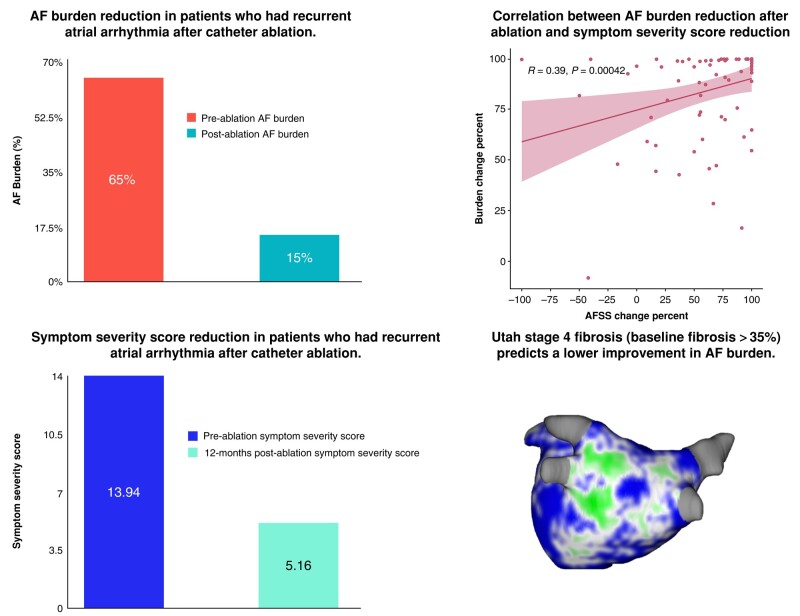
The pre-ablation AF burden was similar in both groups (59 vs. 65%, P = 0.58). At the conclusion of the observation period, the no-recurrence cohort demonstrated an anticipated post-ablation AF burden of 0%, while the recurrence cohort had a burden of 15%. Of note, there was a reduction in AF burden in the recurrence group when compared with their pre-ablation burden (65 vs. 15%, P < 0.0001), as illustrated in Figure 1. We performed additional analysis to calculate the AF burden after the first non-blanking period AF event, and compared it with the overall AF burden previously reported, and we found no significant difference (15 vs. 16.4%, P = 0.48).
Figure 1.
Atrial fibrillation burden reduction in patients who had recurrent atrial arrhythmia after catheter ablation. The grey lines represent individual patients.
Predictors of atrial fibrillation burden decrease
Univariate and multivariate predictors of AF burden decrease are presented in Table 2. Multivariate analysis showed that a higher pre-ablation AF burden is associated with a higher decrease in AF burden. Conversely, Utah Stage 4 fibrosis (baseline fibrosis > 35%) and diabetes predicted a lower improvement in AF burden.
Table 2.
Univariate and multivariate analysis of predictors of post-ablation AF burden
| Effect | SE | P-value | |
|---|---|---|---|
| Univariate analysis | |||
| Pre-ablation AF burden | 0.86 | 0.084 | <0.0001 |
| Treatment received (PVI only vs. PVI + MRI) | 0.074 | 0.079 | 0.35 |
| Age | 0.0020 | 0.0040 | 0.62 |
| Sex | −0.023 | 0.083 | 0.78 |
| Baseline Utah stage | 0.13 | ||
| I | Reference | Reference | Reference |
| II | 0.043 | 0.12 | |
| III | 0.15 | 0.13 | |
| IV | −0.13 | 0.16 | |
| Baseline terminal Utah Stage IV (compared with any other Utah stage) | −0.22 | 0.12 | 0.07 |
| AAD | −0.15 | 0.078 | 0.069 |
| ARB | −0.18 | 0.089 | 0.053 |
| Statins | 0.024 | 0.083 | 0.78 |
| CHF | −0.046 | 0.098 | 0.64 |
| HTN | 0.060 | 0.079 | 0.45 |
| DM | −0.19 | 0.11 | 0.11 |
| Stroke | −0.010 | 0.14 | 0.94 |
| Vascular disease | 0.16 | 0.17 | 0.36 |
| Tobacco | 0.028 | 0.082 | 0.73 |
| CAD | −0.20 | 0.11 | 0.082 |
| Hyperlipidaemia | −0.026 | 0.083 | 0.76 |
| Failed AAD | −0.16 | 0.077 | 0.039 |
| BMI | 0.0042 | 0.0059 | 0.48 |
| LA volume | 0.000011 | 0.0011 | 0.99 |
| Pre-ablation AFSS | 0.010 | 0.0053 | 0.059 |
| Mitral valve | 0.0087 | 0.15 | 0.95 |
| Multivariate analysis | |||
| Intercept | 0.018 | 0.063 | 0.77 |
| Pre-ablation AF burden | 0.84 | 0.073 | <0.0001 |
| Baseline Utah Stage IV | −0.18 | 0.063 | 0.0057 |
| DM | −0.19 | 0.059 | 0.0019 |
AAD, antiarrhythmic drug; AF, atrial fibrillation; AFSS, atrial fibrillation severity scale; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; HTN, hypertension; LA, left atrium; MRI, magnetic resonance imaging; PVI, pulmonary vein isolation; SE, standard error.
Atrial fibrillation severity scale
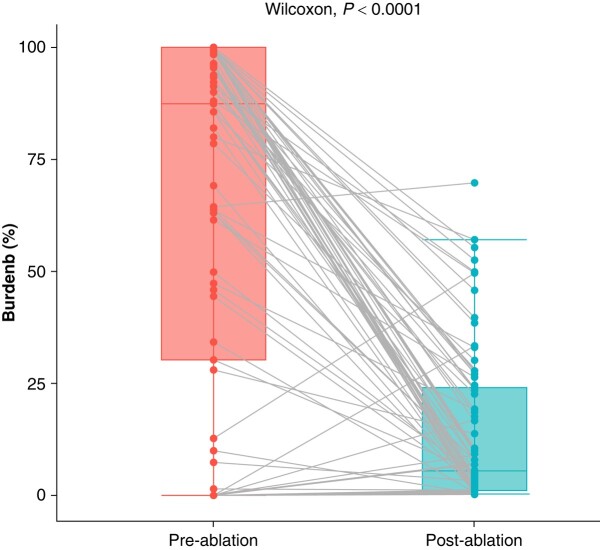
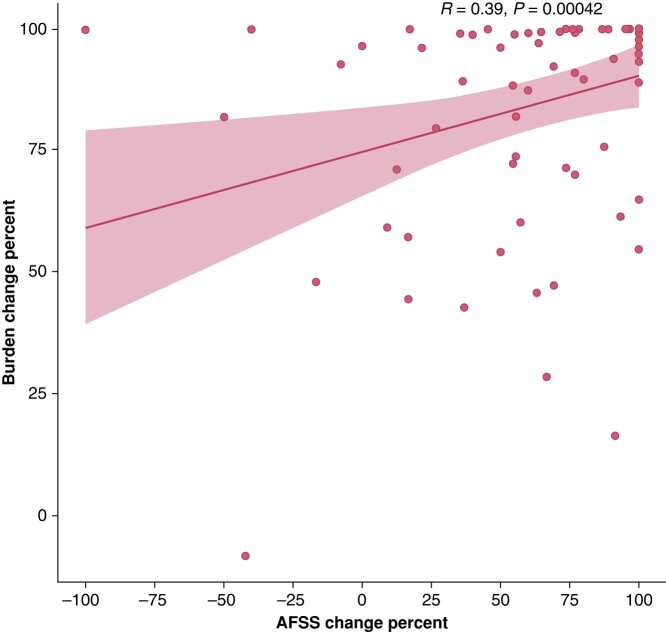
The pre-ablation symptom severity score was comparable between the two groups (16.5 vs. 13.9, P = 0.11). However, the 12-month post-ablation symptom severity score was significantly lower in the no-recurrence group when compared with the recurrence group (3.24 vs. 5.16, P = 0.02). Notably, the symptom severity score at 12 months post-ablation was significantly reduced compared with the pre-ablation score in the recurrence group (13.94 vs. 5.16, P = 0.01). Additionally, there was a significant correlation between the decrease in symptom severity score and the decrease in AF burden after the ablation (R = 0.39, P = 0.001), as illustrated in Figure 2.
Figure 2.
Correlation between atrial fibrillation burden variation and symptom severity variation.
We developed two multivariable models to predict post-ablation AFSS. Model 1 uses AF recurrence status, time to recurrence, and post-ablation AF burden. Model 2 used the same variables except for post-ablation AF burden. By conducting a likelihood ratio test to compare these two models, we find that adding post burden in addition to recurrence and time to recurrence improved the model significantly (Log likelihood −284.38 vs. −289.49, P-value = 0.0014; Table 3).
Table 3.
Multivariable models predicting post-ablation AFSS score
| Estimate | SE | P-value | |
|---|---|---|---|
| Intercept | 3.236 | 1.843 | 0.082 |
| AF recurrence | −0.146 | 1.300 | 0.911 |
| Time to AF recurrence | 0.0001 | 0.004 | 0.975 |
| Smartphone AF burden post-ablation | 14.168 | 4.409 | 0.002 |
| AIC = 578.77 |
| Estimate | SE | P-value | |
|---|---|---|---|
| Intercept | 6.332 | 1.649 | 0.000 |
| AF recurrence | 0.501 | 1.349 | 0.711 |
| Time to AF recurrence | −0.008 | 0.003 | 0.022 |
| AIC = 586.97 |
AF, atrial fibrillation; AFSS, atrial fibrillation severity scale; AIC, akaike information criterion; SE, standard error.
Antiarrhythmic drugs post-ablation
The most common drugs used in our patient population were flecainide (12.2%), digoxin (11.2%), and amiodarone (10.2%; Table 4). In the DECAAF II trial protocol, the use of antiarrhythmic drugs (AADs) post-ablation was left to the discretion of the treating physician, including the decision to start the drug, type of drug, and timing of drug initiation post-ablation. Importantly, we found that the use of AADs was not different between the two groups (Table 4). In addition, the multivariable model did not show any significant impact of AAD use on the drop in AF burden.
Table 4.
Antiarrhythmic drug use post-ablation in both the recurrence and non-recurrence groups
| No recurrence group (n = 29) n (%) |
Recurrence group (n = 69) n (%) |
Total (n = 98) n (%) |
P-value | |
|---|---|---|---|---|
| Any antiarrhythmic drug | 12 (41.4) | 30 (43.5) | 42 (42.9) | 0.85 |
| Amiodarone | 3 (10.3) | 7 (10.1) | 10 (10.2) | 0.98 |
| Digoxin | 5 (17.2) | 6 (8.7) | 11 (11.2) | 0.22 |
| Dofetilide | 0 (0.0) | 4 (5.8) | 4 (4.1) | 0.19 |
| Dronedarone | 0 (0.0) | 1 (1.4) | 1 (1.0) | 0.51 |
| Flecainide | 3 (10.3) | 9 (13.0) | 12 (12.2) | 0.71 |
| Propafenone | 0 (0.0) | 1 (1.4) | 1 (1.0) | 0.51 |
| Sotalol | 3 (10.3) | 6 (8.7) | 9 (9.2) | 0.80 |
Atrial fibrillation burden post-ablation and time to recurrence
We performed Cox analysis, which showed that higher AF burden post-ablation is strongly associated with earlier AF recurrence (β coefficient = 6.099, P < 0.001).
Discussion
Our analysis has revealed several significant findings. First, our study shows that catheter ablation for AF reduces AF burden, irrespective of conventional binary recurrence status. Secondly, we found that a higher degree of atrial fibrosis is associated with a lower degree of improvement in AF burden following ablation. Thirdly, we observed a reduction in symptom severity following ablation. Lastly, there is a correlation between the decrease in symptom severity and the decrease in AF burden following the ablation procedure.
In electrophysiology trials, the occurrence of AF after an ablation procedure is typically categorized as a binary outcome. This involves patients either experiencing AF recurrence or not, with the method used to detect recurrence determined by techniques, such as 24 h Holter monitoring, symptom recording, or transtelephonic transmission of ECG recordings. Despite their established effectiveness, these methods only offer a static view of the constantly changing nature of cardiac arrhythmias. Recently, there has been a paradigm shift in defining AF as a disease reflected by its burden, such as the percentage of time spent in AF6–10 or symptom burden.11 Various tools can be used to assess AF burden, including continuous monitoring by subcutaneous insertable cardiac monitors, which provide the most comprehensive picture of AF but require invasive implantation and carry a small risk of infection.12 Our study offers a non-invasive, widely available, and cost-effective alternative using single-lead ECG technology, which has high sensitivity and specificity in detecting AF. Intermittent 19 min recordings using single-lead ECG showed comparable detection capacity to 24 h Holter monitoring.13 Additionally, smartphone ECG has demonstrated significant benefits in detecting AF compared with usual monitoring methods, doubling treatment-relevant AF detection.14 It is widely accepted across multiple studies that a higher AF burden is an independent predictor of ischaemic stroke and cardiovascular mortality,15 highlighting the clinical significance of assessing AF burden. Furthermore, the temporal and diurnal variations in AF patterns have shown significant clinical correlates. Results from the RACE V trial demonstrated that patients with a predominant pattern of AF occurrence (daytime or nocturnal) have a better comorbidity profile compared with those with a mixed pattern of AF.16
Catheter ablation has been demonstrated to be an effective therapeutic intervention for AF. It has been demonstrated to restore sinus rhythm, reduce symptom burden, and enhance quality of life.2 Andrade et al.17 found that early ablation reduced the progression from paroxysmal AF to persistent AF, characterizing it as a disease-modifying procedure. Our results suggest that catheter ablation is indeed a disease-modifying procedure since it substantially decreases AF burden after the procedure, despite the recurrence of arrhythmia. This could be explained by the fact that ablation targets and eliminates AF triggers during PVI and modifies the substrate surrounding the pulmonary veins.18 Additionally, it could be attributed to the fact that ablation creates a modulation of the autonomic nervous system through vagal denervation.19 Multiple studies have reported the positive impact of ablation on structural and functional remodelling.20 We also report that ablation decreases symptom burden in patients with persistent AF, which is consistent with prior research.11 Furthermore, we demonstrate that symptom improvement after the procedure is highly correlated with the reduction of AF burden.
The predictors of successful ablation have been extensively investigated in the existing literature. Voskoboinik et al.21 conducted a meta-analysis and identified age and arrhythmia recurrence during the blanking period as significant predictors of arrhythmia recurrence. Gender has also been shown to be a significant predictor of ablation success.22 However, we did not observe this trend in our study since our sample was predominantly male (77.3%). Furthermore, our study population was relatively young, with a mean age of 62 years. Creta et al.23 found that diabetes is a significant predictor of ablation outcomes, with diabetic patients being more likely to experience procedure failure. Additionally, LA fibrosis, as detected by late gadolinium enhancement MRI, has been found to be a strong predictor of ablation failure in the DECAAF I trial.24 Using a multivariable model, we demonstrate that Utah Stage 4 (fibrosis > 35%) is an independent predictor of a minimal decrease in AF burden following ablation, which is in line with prior research. This finding raises the question of whether patients with a high degree of fibrosis should undergo ablation.
Conclusions
To summarize, our study’s findings suggest that catheter ablation is effective in reducing AF burden despite arrhythmia recurrence after the procedure. We also observed a strong correlation between the reduction in AF burden and the improvement in symptom burden following the procedure. Additionally, our study highlights that elevated LA fibrosis significantly impedes the decrease in AF burden following catheter ablation.
Limitation
This study is a retrospective post hoc analysis of the DECAAF II trial, which introduces inherent bias by design. Additionally, the design of the DECAAF II trial did not include a control group that abstained from ablation, limiting our ability to compare results. While the ECG monitoring was comprehensive, with patients submitting daily ECG strips through a handheld device, this monitoring method is less rigorous when compared with implantable loop recorders. In addition, we were unable to perform a one-to-one comparison with an implanted loop recorder (ILR) or Holter monitor. Prior research evaluating AF recurrence rates following ablation with implantable loop recorders has demonstrated higher recurrence rates than previously reported with intermittent monitoring. Furthermore, ECG reporting compliance decreased over the trial duration, implying that the actual recurrence rate of AF and AF burden could have been underestimated in the current study. Additionally, the study population was predominantly male (72.6%) and relatively young, which may have influenced the results when compared with previous studies. Moreover, patients in the no-recurrence group submitted significantly fewer ECG strips for analysis than those in the recurrence group, which may have biased the primary outcome of the study, given that a more complete monitoring could detect more arrhythmia. This could be attributed to the possibility that some patients in the recurrence group experienced symptomatic arrhythmia, which may have resulted in a higher ECG submission rate.
Contributor Information
Charbel Noujaim, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Ala Assaf, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Chanho Lim, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Han Feng, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Hadi Younes, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Mario Mekhael, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Nour Chouman, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Ghaith Shamaileh, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Abdel Hadi El Hajjar, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Tarek Ayoub, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Nino Isakadze, Department of Cardiovascular Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Mihail G Chelu, Department of Internal Medicine, Baylor College of Medicine, Houston, TX, USA; Division of Cardiology, Baylor College of Medicine, Houston, TX, USA; Baylor St Luke’s Medical Center, Houston, TX, USA; Texas Heart Institute, Houston, TX, USA.
Nassir Marrouche, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Eoin Donnellan, Tulane Research Innovation for Arrhythmia Discovery, 1430 Tulane Avenue, New Orleans, LA, USA.
Funding
No outside funding was received for the realization of this manuscript.
Data availability
The data will be made available upon reasonable request.
References
- 1. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 2018;137:e623–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aljuaid M, Marashly Q, AlDanaf J, Tawhari I, Barakat M, Barakat R et al. Smartphone ECG monitoring system helps lower emergency room and clinic visits in post-atrial fibrillation ablation patients. Clin Med Insights Cardiol 2020;14:1179546820901508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]
- 7. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 8. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–8. [DOI] [PubMed] [Google Scholar]
- 9. Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913–20. [DOI] [PubMed] [Google Scholar]
- 10. Noujaim C, Lim C, Mekhael M, Feng H, Chouman N, Younes H et al. Identifying the prognostic significance of early arrhythmia recurrence during the blanking period and the optimal blanking period duration: insights from the DECAAF II study. Europace 2023;25:euad173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schnabel RB, Pecen L, Rzayeva N, Lucerna M, Purmah Y, Ojeda FM et al. Symptom burden of atrial fibrillation and its relation to interventions and outcome in Europe. J Am Heart Assoc 2018;7:e007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hercé B, Nazeyrollas P, Lesaffre F, Sandras R, Chabert J-P, Martin A et al. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace 2013;15:66–70. [DOI] [PubMed] [Google Scholar]
- 13. Duarte R, Stainthorpe A, Mahon J, Greenhalgh J, Richardson M, Nevitt S. Lead-I ECG for detecting atrial fibrillation in patients attending primary care with an irregular pulse using single-time point testing: a systematic review and economic evaluation. PLoS One 2019;14:e0226671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizas KD, Freyer L, Sappler N, von Stülpnagel L, Spielbichler P, Krasniqi A et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–30. [DOI] [PubMed] [Google Scholar]
- 15. Botto GL, Tortora G, Casale MC, Canevese FL, Brasca FAM. Impact of the pattern of atrial fibrillation on stroke risk and mortality. Arrhythm Electrophysiol Rev 2021;10:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van de Lande ME, Rama RS, Koldenhof T, Arita VA, Nguyen B-O, van Deutekom C et al. Time of onset of atrial fibrillation and atrial fibrillation progression data from the RACE V study. Europace 2023;25:euad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023;388:105–16. [DOI] [PubMed] [Google Scholar]
- 18. Link MS, Haïssaguerre M, Natale A. Ablation of atrial fibrillation. Circulation 2016;134:339–52. [DOI] [PubMed] [Google Scholar]
- 19. Qin M, Liu X, Jiang WF, Wu SH, Zhang XD, Po S. Vagal response during pulmonary vein isolation: re-recognized its characteristics and implications in lone paroxysmal atrial fibrillation. Int J Cardiol 2016;211:7–13. [DOI] [PubMed] [Google Scholar]
- 20. Walters TE, Nisbet A, Morris GM, Tan G, Mearns M, Teo E et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: implications for early ablative intervention. Heart Rhythm 2016;13:331–9. [DOI] [PubMed] [Google Scholar]
- 21. Voskoboinik A, Moskovitch JT, Harel N, Sanders P, Kistler PM, Kalman JM. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm 2017;14:661–7. [DOI] [PubMed] [Google Scholar]
- 22. Santangeli P, Di Biase L, Basile E, Al-Ahmad A, Natale A. Outcomes in women undergoing electrophysiological procedures. Arrhythm Electrophysiol Rev 2013;2:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Creta A, Providência R, Adragão P, de Asmundis C, Chun J, Chierchia G et al. Impact of type-2 diabetes mellitus on the outcomes of catheter ablation of atrial fibrillation (European Observational Multicentre Study). Am J Cardiol 2020;125:901–6. [DOI] [PubMed] [Google Scholar]
- 24. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available upon reasonable request.