Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-borne viral disease caused by the SFTS virus (Dabie bandavirus), which has become a substantial risk to public health. No specific treatment is available now, that calls for an effective vaccine. Given this, we aimed to develop a multi-epitope DNA vaccine through the help of bioinformatics. The final DNA vaccine was inserted into a special plasmid vector pVAX1, consisting of CD8+ T cell epitopes, CD4+ T cell epitopes and B cell epitopes (six epitopes each) screened from four genome-encoded proteins——nuclear protein (NP), glycoprotein (GP), RNA-dependent RNA polymerase (RdRp), as well as nonstructural protein (NSs). To ascertain if the predicted structure would be stable and successful in preventing infection, an immunological simulation was run on it. In conclusion, we designed a multi-epitope DNA vaccine that is expected to be effective against Dabie bandavirus, but in vivo trials are needed to verify this claim.
Keywords: Severe fever with thrombocytopenia syndrome, DNA vaccine, Bioinformatics, Cellular immune responses, pVAX1
Introduction
Emerging zoonoses are major and global challenges for public health [1]. Severe fever with thrombocytopenia syndrome (SFTS) is an emerging zoonosis caused by tick-borne SFTS virus (Dabie bandavirus), which was initially reported in China [2] and is prevalent at the moment in East Asia and has a high mortality rate of 12–50% [3, 4]. High fever and thrombocytopenia are the typical clinical signs of Dabie bandavirus infection. Additional symptoms include gastrointestinal problems, leukopenia, hemorrhagic tendencies, and others [5, 6]. The three stages of the SFTS clinical course include the high fever stage, multiple organ dysfunction (MOD) stage, and recovery stage [7]. Within five days of the disease’s onset, it will have advanced to the most serious MOD stage. Clinical signs of this stage include multi-organ failure, disseminated intravascular coagulation (DIC), persistently low platelet counts, neurological complaints, and bleeding manifestations [5]. Ribavirin with broad-spectrum antiviral activity, favipiravir targeting RNA-dependent RNA polymerase, and calcium channel inhibitors have been selected as treatments for SFTS in the expert consensus released in 2022 in China [8]. Among the three drugs, calcium channel inhibitors have the most potential for clinical application, since a Chinese clinical survey showed that nifedipine can reduce the mortality rate of Dabie bandavirus -infected patients by more than five times [9]. Still, there is no effective preventive method against this disease, this calls for a preventive vaccine that is reliable, long-lasting, low-cost, and secure, which is a permanent need in the battle against conventional, emerging and re-emerging infectious diseases [10]. Yu, et al. [11] reported and assessed two live attenuated viruses as vaccine candidates in ferret model. Dong, et al. [12] developed a live attenuated recombinant vesicular stomatitis virus-based vaccine candidate expressing the SFTSV Gn/Gc glycoproteins (rVSV-SFTSV/AH12-GP). Moreover, Kim, et al. [13] developed an mRNA-Gn vaccine administrated in lipid nanoparticle encapsule which successfully induced neutralizing antibodies and T-cell responses in mice. Notably, Kwak, et al. [14] have developed several DNA vaccines based on the genome of the virus.
Compared with traditional vaccines such as live attenuated vaccines, DNA vaccines have numerous potential advantages including inherent safety and a more rapid production time [15]. Plasmids with the desired antigen’s encoding genes are used to create DNA vaccines, which would be expressed in the host and induce the immune response [16, 17]. In the field of creating DNA vaccines, advances in immunoinformatics, bioinformatics and reverse vaccinology pipelines are increasingly widely applied [18]. These technologies were used to screen potential epitopes which include the conformational and linear epitopes that could be recognized by the immune system [19]. The genome of the Dabie bandavirus encodes four proteins, nuclear protein (NP), glycoprotein (GP), RNA-dependent RNA polymerase (RdRp), and a non-structural protein (NSs). GP is cleaved during synthesis into Gn and Gc [20]. Based on Gn/Gc, Kwak JE, et al. have developed a DNA vaccine [14]. The fact that numerous human monoclonal antibodies generated by SFTS patients recognized NP further indicated that the NP was important for humoral responses to Dabie bandavirus infection [21]. For NSs, it has been found that it could induce pro-viral autophagy by interacting with mTOR, where autophagy enhances Dabie bandavirus infection and propagation [22].
Based on the four proteins listed above, the goal of this study was to create a multi-epitope DNA vaccine against Dabie bandavirus. We assembled a vaccine containing high-potential CTL, HTL and B cells epitopes, and assessed the vaccine’s stability and effectiveness, providing a possible way to prevent Dabie bandavirus infection.
Methods
Sequence and structure retrieval
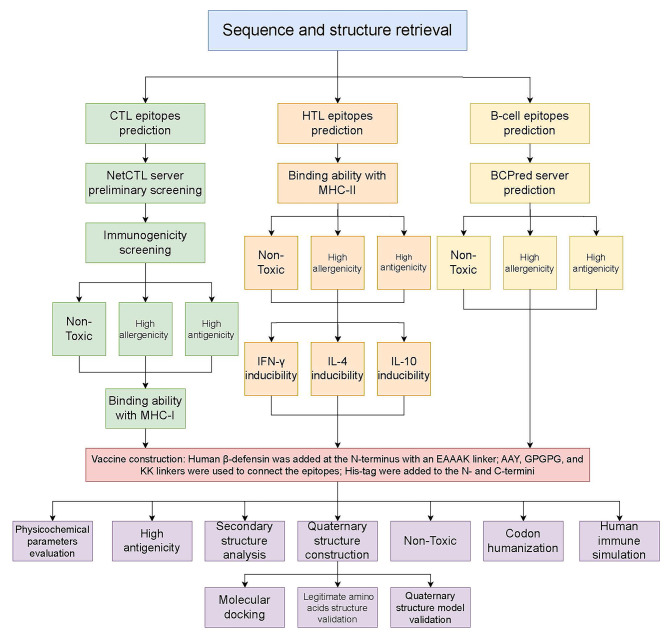
Details of the workflow are given in Fig. 1. We selected all four proteins encoded by the SFTS genome and the sequences were retrieved from GenBank (https://www.ncbi.nlm.nih.gov/genbank/), including glycoprotein (ID: AWW14922.1), RNA-dependent RNA polymerase (RdRp, ID: AWW14921.1), nucleoprotein (NP, ID: AVM39051.1) and nonstructural protein (NSs, ID: AVM39050.1).
Fig. 1.
The general workflow of the study
CTL epitopes prediction
NetCTL 1.2 (https://services.healthtech.dtu.dk/services/NetCTL-1.2/) was used to predict the CTL epitopes, using all the server-provided HLA supertypes, and the threshold of prediction score was 1.0. Then the immunogenicity of the epitopes was confirmed by the MHC-I Immunogenicity IEDB tool (http://tools.iedb.org/immunogenicity/). The MHC-I binding ability prediction was conducted by the IEDB MHC-I binding prediction tool (http://tools.iedb.org/mhci/), ensuring that the percentile rank < 2. Toxicity and allergenicity of the epitopes were checked by ToxinPred (https://webs.iiitd.edu.in/raghava/toxinpred/multi_submit.php) and AllerTOP 2.0 (https://www.ddg-pharmfac.net/AllerTOP/) respectively. The antigenicity of the epitopes was calculated using the VaxiJen server (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html).
HTL epitopes prediction
The IEDB MHC-II binding predictions tool (http://tools.iedb.org/mhcii/) was used to design HTL epitopes. A percentile rank < 0.25 was set as the threshold. The inducibility of interferon-γ (IFN-γ), interleukin-4 (IL-4), and interleukin-10 (IL-10) were conducted by IFNepitope (https://webs.iiitd.edu.in/raghava/ifnepitope/), IL10Pred (https://webs.iiitd.edu.in/raghava/il10pred/), and IL4Pred (https://webs.iiitd.edu.in/raghava/il4pred/index.php). We comprehensively considered the cytokine-inducing results in the final selection of epitopes. The toxicity, allergenicity and antigenicity were checked then.
B-cell epitopes prediction and selection
B-cell epitopes play an important role in B cell-mediated humoral immune response. Thus, B-cell epitopes up to standard should be selected as part of the multi-epitopes’ vaccine. Firstly, BCpred server was employed to predict the B-cell epitopes of the selected protein. Secondly, Vaxijen was used to perform antigenicity analysis on the B-cell epitopes predicted by BCpred server. As potential B-cell epitope candidates, six of the most antigenic epitopes were chosen. The toxicity and allergenicity of six candidate epitopes were further tested by ToxinPred and AllerCatPro 2.0.
Construction of the multi-epitope vaccine
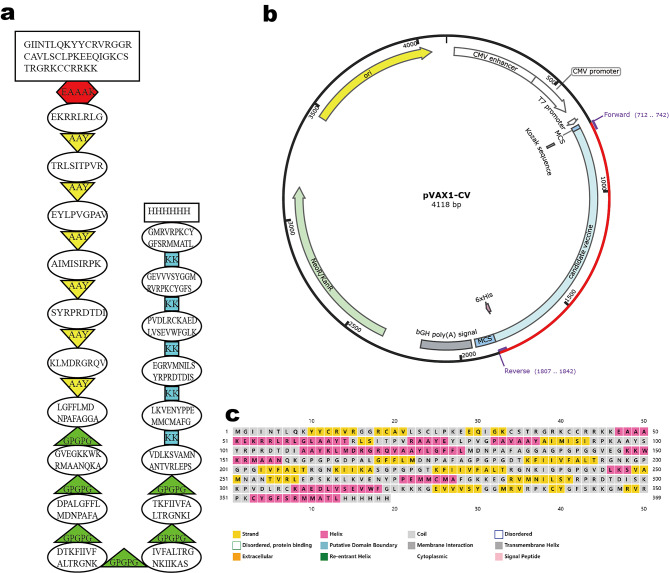
Different linkers, including AAY, GPGPG, and KK, were used to connect the epitopes. Human β-defensin-3 was added at the N-terminus with an EAAAK linker in order to enhance the immune response. At last, a methionine and a His-tag were added to the N- and C-termini, respectively (Fig. 2a). The cDNA of the vaccine was generated using the Java Codon Adaptation Tool (http://www.jcat.de/), then the Kozak sequence was added for initiation of replication. The cDNA was inserted between HindIII and EcoRI restriction sites of the pVAX1 vector, which was specially designed for DNA vaccine development (Fig. 2b).
Fig. 2.
(a) The final epitopes for DNA vaccine and the linkers. (b) The DNA sequence in pVAX1 vector. (c) The predicted secondary structure
Homology modeling and validation
We used I-TASSER server (https://zhanggroup.org/I-TASSER/) to predict the 3D structure of the vaccine. The obtained homology models were then refined by the GalaxyRefine tools (https://galaxy.seoklab.org/cgi-bin/submit.cgi?type=REFINE). The refined structures were analyzed by the SAVES 6 server (https://saves.mbi.ucla.edu/) and ProSA web (https://prosa.services.came.sbg.ac.at/prosa.php) and the structure with the best results synthetically was further analyzed.
Physiochemical properties and immune properties prediction
A flexible and powerful server, ProtParam server could help us predict the Physiochemical properties of the vaccine more easily. In addition, to confirm that the constructed vaccine can indeed produce immunological effects, we analyzed the antigenicity of the vaccine by Vaxijen and AntigenPro, and predicted the allergenicity of the vaccine by AllerTOP2.0 network tool. Finally, the designed vaccine was compared to human proteins using NCBI’s BLASTp tool to ensure that the immune system could be properly activated.
Molecular interaction analysis of the vaccine with TLR-3
HDOCK server (http://hdock.phys.hust.edu.cn/) carried out the molecular docking experiments of the vaccine with Toll-like receptor 3 (TLR-3). The PyMol was used to generate the final images. In-depth knowledge regarding the relationships between vaccine-receptor binding was obtained using the PDBsum server. In order to gain a better understanding of the vaccine-TLR3 complex’s dynamic stability, the final stage required putting it to molecular modeling. For the complex, we ran 10ns of simulation using the AMBER20 tool. We applied the MM/GBSA method to evaluate the binding free energies inside the complex. This platform predicted and analyzed the protein-protein complex using MM/GBSA and computational docking.
Human immune simulation
To mimic the immunological response following the administration of the vaccine, C-ImmSim sever (https://kraken.iac.rm.cnr.it/C-IMMSIM/) was utilized [23–25]. All of the simulation’s default settings were applied.
Results
CTL epitopes prediction
CTL epitopes were screened by using the NetCTL 1.2 server. These epitopes were shortlisted based on the MHC-I binding affinity, C-terminal cleavage affinity and TAP transport efficiency. Epitopes were further analyzed, including their immunogenicity, toxicity, antigenicity, etc. Taking all the analysis results into consideration, 6 epitopes with the highest percentile ranks from the four proteins (GP, RdRp, NP, NSs) were finally selected (Table 1).
Table 1.
List of final CTL epitopes and detailed information
| Protein | Peptide sequence | MHC binding affinity | C-terminal cleavage affinity | TAP transport efficiency | Prediction score | Immunogenicity | Toxicity | Allergenicity | Antigenicity score (> 0.5) | MHC-I binding prediction |
|---|---|---|---|---|---|---|---|---|---|---|
| NSs | ||||||||||
| EKRRLRLGL | 0.3325 | 0.1229 | 0.858 | 1.1969 | 0.11724 | None | None | 1.5340 | < 2 | |
| NP | ||||||||||
| TRLSITPVR | 0.3769 | 0.7224 | 1.521 | 1.1863 | 0.01083 | None | None | 2.1836 | < 2 | |
| EYLPVGPAV | 0.5025 | 0.8077 | 0.494 | 1.2159 | 0.07084 | None | None | 1.4436 | < 2 | |
| RdRp | ||||||||||
| AIMISIRPK | 0.528 | 0.1913 | 0.856 | 1.0653 | 0.0783 | None | None | 2.6252 | < 2 | |
| SYRPRDTDI | 0.3845 | 0.9207 | 0.949 | 1.0043 | 0.12264 | None | None | 2.4142 | < 2 | |
| GP | ||||||||||
| KLMDRGRQV | 0.6154 | 0.7269 | 0.476 | 1.0502 | 0.02362 | None | None | 1.3673 | < 2 |
HTL epitopes prediction
The IEDB MHC-II binding predictions tool’s percentile rank (< 0.25) and its capacity to induce cytokines, particularly IFN-γ, were used to identify the HTL epitopes. Six epitopes from RdRp and NP met the rules (Table 2). The antigenicity, toxicity and allergenicity were also noted in Table 2.
Table 2.
List of final HTL epitopes and detailed information
| Protein | Peptide sequence | Method | Percentile rank(<0.25) | Antigenicity score (> 0.5) | Toxicity | Allergenicity | IFN-γ | IL-4 | IL-10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| RdRp | ||||||||||
| LGFFLMDNPAFAGGA | NetMHCIIpan | 0.11 | 0.5485 | None | None | Positive | - | Inducer | ||
| GVEGKKWKRMAANQK | Consensus (smm/nn/sturniolo) | 0.21 | 0.6958 | None | None | Positive | Inducer | Inducer | ||
| DPALGFFLMDNPAFA | Consensus (comb.lib./smm/nn) | 0.15 | 0.5923 | None | None | Positive | Inducer | - | ||
| NP | ||||||||||
| DTKFIIVFALTRGNK | Consensus (smm/nn/sturniolo) | 0.04 | 0.7901 | None | None | Negative | Inducer | Inducer | ||
| IVFALTRGNKIIKAS | Consensus (comb.lib./smm/nn) | 0.01 | 0.715 | None | None | Negative | Inducer | Inducer | ||
| TKFIIVFALTRGNKI | Consensus (comb.lib./smm/nn) | 0.01 | 0.5124 | None | None | Negative | Inducer | Inducer |
Linear B-cell epitopes prediction and selection
High antigenicity is a crucial factor to consider when choosing B-cell epitopes to create an effective multi-epitope vaccine. Therefore, B-cell epitopes with 16,18,20-mer lengths, predicted by the BCPred server (Threshold > 0.8), were ordered from highest to lowest antigenicity. Six of the most antigenic epitopes were selected for the final vaccine. Additionally, these six B-cell epitopes were confirmed to be non-toxic and non-allergic using ToxinPred and AllerCatPro 2.0, respectively. The six B-cell epitopes chosen for further analysis was shown in Table 3.
Table 3.
List of final B-cell epitopes
| Protein | Peptide sequence | Prediction Score (> 0.8) | Antigenicity score (> 0.5) |
|---|---|---|---|
| NSs | |||
| VDLKSVAMNANTVRLEPS | 0.82 | 1.0716 | |
| NP | |||
| LKVENYPPEMMCMAFG | 0.87 | 1.6071 | |
| RdRp | |||
| EGRVMNILSYRPRDTDIS | 0.82 | 1.4985 | |
| PVDLRCKAEDLVSEVWFGLK | 0.81 | 1.4377 | |
| Gly | |||
| GEVVVSYGGMRVRPKCYGFS | 0.87 | 1.8168 | |
| GMRVRPKCYGFSRMMATL | 0.83 | 1.7512 |
Physicochemical parameters evaluation
The physiochemical properties of the final vaccine were computed using the ProtParam service, which is often regarded as the initial phase of built amino sequence assessment. The results showed that the designed vaccine’s molecular weight was 40434.68 g/mol with the theoretical Isoelectric point (pI) of 10.19. It has 25 negatively and 67 positively charged residues in the vaccine. This candidate vaccine’s Instability index was computed to be 24.91, indicating that the designed vaccine had good stability in different temperatures (instability index < 40). At the same time, the vaccine’s estimated half-life differed in mammalian reticulocytes (in vitro), yeast (in vivo) and Escherichia coli (in vivo), where it took 30 h, more than 20 h and more than 10 h to reach its certain concentration. In addition, the aliphatic index is a critical parameter to be evaluated because high aliphatic index is associated with thermal stability in usual. The final vaccine construct could be regarded as a thermostable protein for its aliphatic index 71.95. Last but not least, the grand average of hydropathicity (GRAVY) of this vaccine construct was − 0.314, classifying the protein as hydrophilic. The specific value was illustrated in Table 4.
Table 4.
The physicochemical properties of the Dabie bandavirus candidate vaccine evaluated by ProtScale server
| Number of amino acids | Molecular weight | Theoretical pI | Instability index(< 40) | Aliphatic index | GRAVY | |
|---|---|---|---|---|---|---|
| Recombinant Vaccine | 369 | 40434.68 | 10.19 | 24.91 | 71.95 | -0.314 |
Antigenicity and allergenicity prediction
Vaccine antigenicity was estimated using the Vaxijen server to ensure it could stimulate protective humoral immune responses. The result indicated that the vaccine was a potential antigen with a high antigenicity score of 0.9018, setting 0.4 as the threshold. Besides, the allergenicity of the vaccine needs to be predicted to avoid unnecessary hypersensitivity. The AllerTOP v.2.0 server’s anticipated outcome showed that the candidate vaccination was classified as non-allergen (not shown in the figures).
Homology modeling and validation
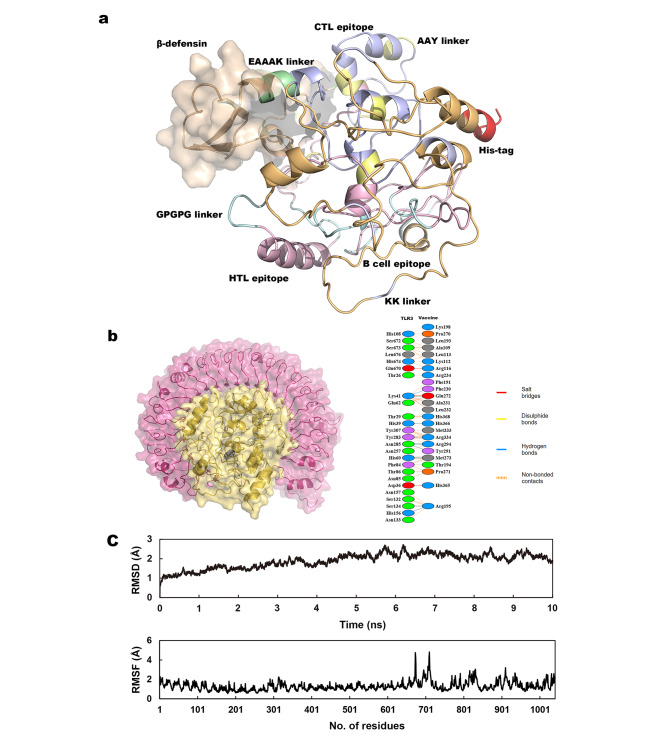
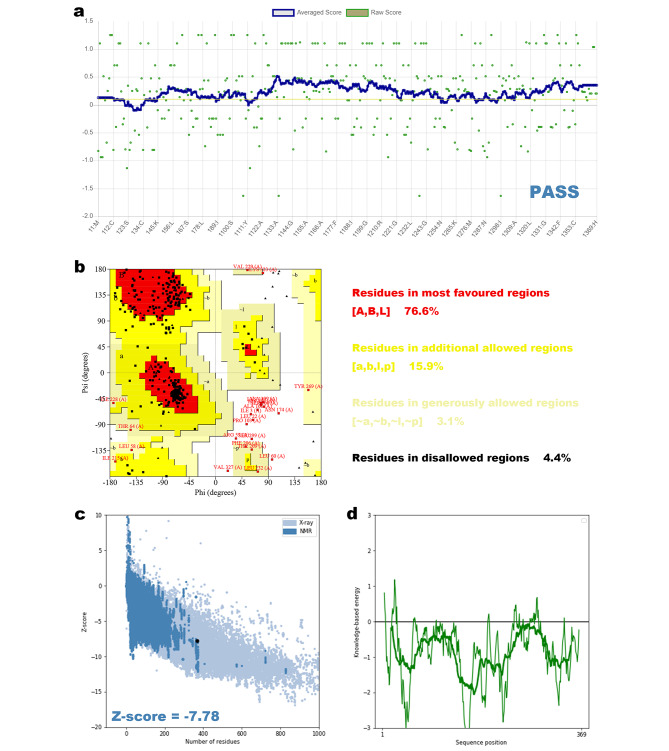
The I-TASSER server generated five models of the 3D structures, after further analyses, the refined model 3 (Fig. 3a) was selected for further study. The Ramachandran plot was performed by the SAVES 6 server (Fig. 4b), the result shows that 76.6% of the residues were in the most favored regions, 15.9% were in additional allowed regions, 3.1% were in generously allowed regions, with only 4.4% residues in disallowed regions. More than 80% of the amino acids scored higher than 0.2 in the 3D/1D profile utilizing, according to Verify3D, validating a legitimate structure (Fig. 4a). To identify any potential model flaws, ProSA-web additionally assessed the refined structure. The Z-score was − 7.78 (Fig. 4c and d).
Fig. 3.
(a) The fined 3D structure of the final product. (b) The complex of the vaccine with TLR-3. (c) The molecular dynamics simulations of the vaccine with TLR-3
Fig. 4.
(a) Verify3D confirmed a valid structure of the final product. (b) Validation of the final structure by a Ramachandran plot. (c and d) ProSA-web Z-score plot and local model quality for the 3D structure
Molecular interaction analysis of the vaccine with TLR-3
HDOCK predicted 10 complexes for the complexes of the vaccine with TLR-3 and ranked them beyond the docking energy score (the lower the energy score, the higher the rank). We selected the structure with the highest rank and used Pymol to generate the 3D structures (Fig. 3b and c). The PDBsum server was used to further show the protein-protein interactions. The results revealed the hydrogen bonds and non-bond interactions between the vaccine and TLR-3. 11 H-bonds and 164 non-bond interactions were predicted in the complex with TLR-3 (Fig. 3b). Molecular simulation of the vaccines-TLR3 complexes demonstrated a stable dynamic behavior during the simulation (Fig. 3c), the RMSD of each complex stabilized at 1.9 Å following a uniform pattern then until 10ns with no significant deviation revealing that the vaccine binds stably to the TLR-3. On the other hand, the RMSF (1.3 Å on average) of the complex as shown in Fig. 3c is within acceptable range. The binding free energies of the vaccine with TLR-3 was shown in Table 5.
Table 5.
Binding free energy of the vaccine-TLR-3 complex
| VDW | ELE | GB | SA | TOTAL | |
|---|---|---|---|---|---|
| Complex | -94.96 | -2775.07 | 2828.91 | -12.54 | -53.66 |
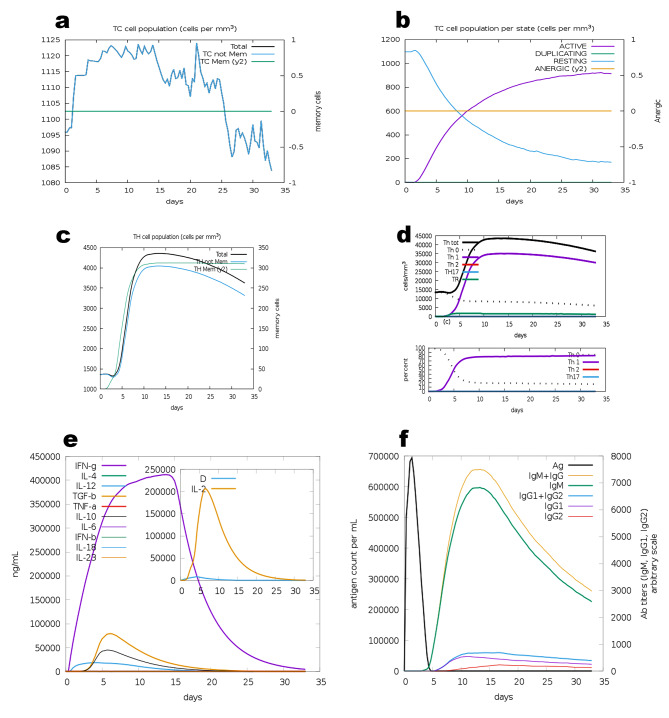
Human immune simulation
The C-ImmSim server modeled the immunological response to the final vaccine design. A sharp increase in Tc (cytotoxic) cell population was observed after the injection (Fig. 5a), with the number of active Tc cells keeping rising (Fig. 5b). The Th (helper) cell was also responsive to the vaccine (Fig. 5c). In addition, Th1 cells were activated after the injection (Fig. 5d), under this, a significant increase in IFN-γ could be observed (Fig. 5e). A significant antibody response was also observed (Fig. 5f).
Fig. 5.
Immune simulation of the vaccine. (a and b) The change in total TC cell population and population per state. (c and d) The change in total TH cell population and population per state. (e) The changes in the secretion of cytokines. (f) The changes in the secretion of antibodies
Discussion
Since its initial identification in China in 2009, SFTS has become an emerging infectious disease and a substantial risk to public health, with a steadily expanding geographic dispersion, especially in China, Japan and Korea [2, 26, 27]. Up until December 2020, epidemiological data showed that there were 13,305 confirmed cases of SFTS in 24 Chinese provinces, and that the incidence was rising annually [28, 29]. In addition, the Dabie bandavirus is able to rapidly evolve and enhance genetic diversity, which has garnered significant attention already [30]. Worse still, apart from preventing tick bites, there is no specific treatment for SFTS available [31]. The creation of protective vaccinations is therefore becoming a major problem and preparedness strategies.
With the availability of large-scale sequencing data in well-organized databases and the advancement in sequencing techniques, reverse vaccinology places a strong emphasis on developing vaccines through a genome-based approach [32]. Bioinformatics procedures have played essential roles in designing more robust vaccines, which have saved a great deal of time and without the necessity to manipulate a pathogenic microorganism in vitro [33]. Furthermore, in comparison to mRNA vaccines and other conventional vaccine strategies, DNA vaccines may have excellent prophylactic potential since they can rapidly modify sequences to adapt to the emergence of new diseases [34]. Besides this, the strength of DNA vaccines such as the simplicity of preparation and storage are becoming more obvious and popular [35]. In the past few years, the multiple epitope-based vaccine design approach has become feasible and efficient, which can enhance the safety and effectiveness of vaccines [36, 37]. Therefore, the accurate epitope prediction for both T cells and B cells of a special genome-encoded protein plays a crucial role in the properties of multi-epitope DNA vaccines [38]. Up to now, the efficacy and safety of multi-epitope DNA vaccines have been verified through human clinical trials [39]. For instance, the first genetic vaccination to combat the COVID-19 pandemic was licensed [40].
In this study, we used bioinformatics methods to predict the CTL, HTL and B-cell epitopes of Dabie bandavirus’s genome-encode proteins and constructed a multi-epitope DNA vaccine via in silico genomic databases. Additionally, high antigenicity and immunogenicity of the vaccine have been confirmed using bioinformatics resources available online, including VaxiJen and IEDB. Instead of using the full-length proteins, we selected several high-potential epitopes from each protein to obtain a reduced size of the final products, which means the prevention of the possibility of insolubleness due to the large molecular weight [41]. The screening of dominant epitopes made the most use of the immunogenicity of the antigen and evaluated the toxicity and allergenicity, and both the CTL, HTL and B-cell epitopes were screened separately and combined in the multi-epitope construction to expand the width of immune response and ensure safety. For CTL and HTL epitopes, they all have a good binding ability with MHC-I/MHC-II, which is predicted by online services. Meanwhile, for HTL, the inducibility of IFN-γ [42], IL-4 [43] and IL-10 [44] was taken in consideration [45]. As a result, this multi-epitope DNA vaccine had a substantial advantage over currently employed vaccinations in that its capability to stimulate both humoral and cellular immune responses [46].
The four proteins (GP, NP, RdRp, NSs) encoded by the genome of the Dabie bandavirus play crucial roles in the infection and propagation process of the virus [47]. Therefore, in this study, candidate epitopes were screened from all four proteins, which was also strategically different from other vaccine designs such as the one Suleman M, et al. developed [48]. Suleman M, et al. selected dominant epitopes only from RdRp and GP beyond the antigenic score, while we not only focused on the antigenicity but also attempted to stimulate the immune system against Dabie bandavirus in the whole process of Dabie bandavirus infection. After the preliminary designing, we conducted a homology modeling and validated that the target post-transcriptional product was structurally stable. Also, we conducted the molecular dynamics simulations of the vaccine with TLR-3, because of the incorporation of the Human β-defensin 3 (hBD3) sequence at the N-terminus. This decision was informed by compelling research demonstrating the ability of hBD3 to augment the production of type I Interferon-β in response to the viral ligand mimic polyinosinic: polycytidylic acid (polyI: C) in both human and mouse primary cells [49]. Notably, this augmentation is intricately linked to TLR-3, a pivotal factor in antiviral innate immune responses. By targeting TLR-3 in our molecular docking, we evaluated our vaccine’s potential effectiveness, leveraging the known mechanism of action associated with hBD3 and its correlation to TLR-3-mediated immune responses. Last but not least, to determine the potency of the multi-epitope DNA vaccines as a final prevention option, more research utilizing in silico and in vivo patterns must be performed in future studies.
Conclusions
In this study, a novel multi-epitope DNA vaccine against Dabie bandavirus was created using bioinformatics methods. More in vivo research is necessary, as well as testing on mouse models.
Author contributions
ZPX conceived and designed the study. YRT, YZ and YML performed the formal analysis. YRT and YML acquired and analyzed the data. YRT, YZ, TML, QL, JZ, MJJ, GQF and ZPX wrote the first draft, YRT, YZ and ZPX subsequent revised the manuscript. All authors edited the manuscript and commented on the text. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20211586), Medical scientific research project of Jiangsu Provincial Health Commission (Grant No. Z2021004), Qing Lan Project of Jiangsu Province of China (Grant No. KY101R202127), Research Project of Educational Science in Nanjing Medical University (Grant No. 2023YJS-ZD003).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong X, Soong L. Emerging and re-emerging zoonoses are major and global challenges for public health. Zoonoses. 2021;1(1):e999.
- 2.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523-1532. [DOI] [PMC free article] [PubMed]
- 3.Shimojima M, Sugimoto S, Umekita K, Onodera T, Sano K, Tani H, Takamatsu Y, Yoshikawa T, Kurosu T, Suzuki T et al. Neutralizing mAbs against SFTS Virus Gn Protein Show strong therapeutic effects in an SFTS Animal Model. Viruses. 2022;14(8):1665. [DOI] [PMC free article] [PubMed]
- 4.Li S, Li H, Zhang YL, Xin QL, Guan ZQ, Chen X, Zhang XA, Li XK, Xiao GF, Lozach PY et al. SFTSV infection induces BAK/BAX-Dependent mitochondrial DNA release to trigger NLRP3 inflammasome activation. Cell Rep. 2020;30(13):4370-4385 e4377. [DOI] [PubMed]
- 5.Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, Li C, Li XY, Zhang QF, Bian PF et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis. 2012;206(7):1095-1102. [DOI] [PubMed]
- 6.Li DX. Severe fever with thrombocytopenia syndrome: a newly discovered emerging infectious disease. Clin Microbiol Infect. 2015;21(7):614-620. [DOI] [PubMed]
- 7.Park ES, Shimojima M, Nagata N, Ami Y, Yoshikawa T, Iwata-Yoshikawa N, Fukushi S, Watanabe S, Kurosu T, Kataoka M et al. Severe fever with Thrombocytopenia Syndrome Phlebovirus causes lethal viral hemorrhagic fever in cats. Sci Rep. 2019;9(1):11990. [DOI] [PMC free article] [PubMed]
- 8.Chen G, Chen T, Shu SN, Ma K, Wang XJ, Wu D, Wang HW, Han MF, Jia XJ, Liu MY et al. Expert consensus on diagnosis and treatment of severe fever with thrombocytopenia syndrome. Infectious Disease Information. 2022;35(05):385-393.
- 9.Li H, Zhang LK, Li SF, Zhang SF, Wan WW, Zhang YL, Xin QL, Dai K, Hu YY, Wang ZB et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019;29(9):739-753. [DOI] [PMC free article] [PubMed]
- 10.Cao J, Jiang L, Miller LH. Decoding infection and transmission: deciphering the mystery of infectious diseases from data-based research. Decoding Infection Transmission. 2023;1:100001.
- 11.Yu KM, Park SJ, Yu MA, Kim YI, Choi Y, Jung JU, Brennan B, Choi YK. Cross-genotype protection of live-attenuated vaccine candidate for severe fever with thrombocytopenia syndrome virus in a ferret model. Proc Natl Acad Sci U S A 2019, 116(52). [DOI] [PMC free article] [PubMed]
- 12.Dong F, Li D, Wen D, Li S, Zhao C, Qi Y, Jangra RK, Wu C, Xia D, Zhang X et al. Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. NPJ Vaccines. 2019;4:5. [DOI] [PMC free article] [PubMed]
- 13.Kim JY, Jeon K, Park SI, Bang YJ, Park HJ, Kwak HW, Kim DH, Lee SY, Choi EJ, Cho NH et al. mRNA vaccine encoding Gn provides protection against severe fever with thrombocytopenia syndrome virus in mice. NPJ Vaccines. 2023;8(1):167. [DOI] [PMC free article] [PubMed]
- 14.Kwak J-E, Kim Y-I, Park S-J, Yu M-A, Kwon H-I, Eo S, Kim T-S, Seok J, Choi W-S, Jeong JH. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nature communications. 2019;10(1):3836. [DOI] [PMC free article] [PubMed]
- 15.Moss RB. Prospects for control of emerging infectious diseases with plasmid DNA vaccines. J Immune Based Ther Vaccines. 2009;7:3. [DOI] [PMC free article] [PubMed]
- 16.Hasson SSAA, Al-Busaidi JKZ, Sallam TA. The past, current and future trends in DNA vaccine immunisations. Asian Pacific Journal of Tropical Biomedicine. 2015;5(5):344-353.
- 17.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62-84. [DOI] [PubMed]
- 18.Yero D, Conchillo-Sole O, Daura X. Antigen Discovery in Bacterial panproteomes. Methods Mol Biol. 2021;2183:43-62. [DOI] [PubMed]
- 19.Ansari HR, Raghava GP. Identification of conformational B-cell epitopes in an antigen from its primary sequence. Immunome Res. 2010;6(1):6. [DOI] [PMC free article] [PubMed]
- 20.Shi J, Hu Z, Deng F, Shen S. Tick-Borne viruses. Virol Sin. 2018;33(1):21-43. [DOI] [PMC free article] [PubMed]
- 21.Yu L, Zhang L, Sun L, Lu J, Wu W, Li C, Zhang Q, Zhang F, Jin C, Wang X et al. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One. 2012;7(6):e38291. [DOI] [PMC free article] [PubMed]
- 22.Feng K, Zhang H, Jiang Z, Zhou M, Min YQ, Deng F, Li P, Wang H, Ning YJ. SFTS Bunyavirus NSs protein sequestrates mTOR into inclusion bodies and deregulates mTOR-ULK1 signaling, provoking pro‐viral autophagy. Journal of Medical Virology. 2023;95(1). [DOI] [PubMed]
- 23.Ragone C, Manolio C, Cavalluzzo B, Mauriello A, Tornesello ML, Buonaguro FM, Castiglione F, Vitagliano L, Iaccarino E, Ruvo M et al. Identification and validation of viral antigens sharing sequence and structural homology with tumor-associated antigens (TAAs). J Immunother Cancer. 2021;9(5). [DOI] [PMC free article] [PubMed]
- 24.Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS One. 2010;5(4):e9862. [DOI] [PMC free article] [PubMed]
- 25.Stolfi P, Castiglione F, Mastrostefano E, Di Biase I, Di Biase S, Palmieri G, Prisco A. In-silico evaluation of adenoviral COVID-19 vaccination protocols: Assessment of immunological memory up to 6 months after the third dose. Front Immunol. 2022;13:998262. [DOI] [PMC free article] [PubMed]
- 26.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19(11):1892-1894. [DOI] [PMC free article] [PubMed]
- 27.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T et al. The first identification and retrospective study of severe fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209(6):816-827. [DOI] [PMC free article] [PubMed]
- 28.Che TL, Li XL, Tian JB, Wang G, Peng XF, Zhang HY, Chen JH, Zhu Y, Zhang WH, Wang T et al. The role of selenium in severe fever with thrombocytopenia syndrome: an integrative analysis of surveillance data and clinical data. Int J Infect Dis. 2022;122:38-45. [DOI] [PubMed]
- 29.Chen QL, Zhu MT, Chen N, Yang D, Yin WW, Mu D, Li Y, Zhang YP, Zainawudong Y. [Epidemiological characteristics of severe fever with Thtrombocytopenia syndrome in China, 2011–2021]. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(6):852-859. [DOI] [PubMed]
- 30.Wang Y, Pang B, Wang Z, Tian X, Xu X, Chong X, Liang H, Ma W, Kou Z, Wen H. Genomic diversity and evolution analysis of severe fever with thrombocytopenia syndrome in East Asia from 2010 to 2022. Front Microbiol. 2023;14:1233693. [DOI] [PMC free article] [PubMed]
- 31.Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763-772. [DOI] [PubMed]
- 32.Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001;19:17–9. doi: 10.1016/S0264-410X(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 33.Taheri-Anganeh M, Savardashtaki A, Vafadar A, Movahedpour A, Shabaninejad Z, Maleksabet A, Amiri A, Ghasemi Y, Irajie C. In Silico Design and evaluation of PRAME + FliC∆D2D3 as a new breast Cancer vaccine candidate. Iran J Med Sci. 2021;46(1):52-60. [DOI] [PMC free article] [PubMed]
- 34.Lim M, Badruddoza AZM, Firdous J, Azad M, Mannan A, Al-Hilal TA, Cho CS, Islam MA. Engineered Nanodelivery systems to improve DNA Vaccine technologies. Pharmaceutics. 2020;12(1). [DOI] [PMC free article] [PubMed]
- 35.Escalona E, Sáez D, Oñate A. Immunogenicity of a multi-epitope DNA vaccine encoding epitopes from Cu-Zn Superoxide dismutase and open reading frames of Brucella abortus in mice. Front Immunol. 2017;8:125. [DOI] [PMC free article] [PubMed]
- 36.Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol. 2003;15(4):461-470. [DOI] [PubMed]
- 37.Mears MC, Bente DA. In silico design of a Crimean-Congo Hemorrhagic Fever Virus Glycoprotein Multi-epitope Antigen for Vaccine Development. Zoonoses (Burlingt). 2022;2(1). [DOI] [PMC free article] [PubMed]
- 38.Song P, He S, Zhou A, Lv G, Guo J, Zhou J, Han Y, Zhou H, Hao Z, Cong H. Vaccination with toxofilin DNA in combination with an alum-monophosphoryl lipid a mixed adjuvant induces significant protective immunity against Toxoplasma Gondii. BMC Infect Dis. 2017;17(1):19. [DOI] [PMC free article] [PubMed]
- 39.Chang GJ, Kuno G, Purdy DE, Davis BS. Recent advancement in flavivirus vaccine development. Expert Rev Vaccines. 2004;3(2):199-220. [DOI] [PubMed]
- 40.Franck CO, Fanslau L, Bistrovic Popov A, Tyagi P, Fruk L. Biopolymer-based carriers for DNA Vaccine Design. Angew Chem Int Ed Engl. 2021;60(24):13225-13243. [DOI] [PMC free article] [PubMed]
- 41.Rashidi S, Faraji SN, Mamaghani AJ, Hatam S, Kazemi B, Bemani P, Tabaei SJS, Hatam G. Bioinformatics analysis for the purpose of designing a novel multi-epitope DNA vaccine against Leishmania major. Sci Rep. 2022;12(1):18119. [DOI] [PMC free article] [PubMed]
- 42.Dhanda SK, Vir P, Raghava GP. Designing of interferon-gamma inducing MHC class-II binders. Biol Direct. 2013;8(1):30. [DOI] [PMC free article] [PubMed]
- 43.Dhanda SK, Gupta S, Vir P, Raghava GP. Prediction of IL4 inducing peptides. Clin Dev Immunol. 2013;2013:263952. [DOI] [PMC free article] [PubMed]
- 44.Nagpal G, Usmani SS, Dhanda SK, Kaur H, Singh S, Sharma M, Raghava GP. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci Rep. 2017;7:42851. [DOI] [PMC free article] [PubMed]
- 45.Ahammad I, Lira SS. Designing a novel mRNA vaccine against SARS-CoV-2: an immunoinformatics approach. Int J Biol Macromol. 2020;162:820-837. [DOI] [PMC free article] [PubMed]
- 46.San Zhao L, Qin S, Zhou TY, Tang H, Liu L, Lei BJ. DNA-based vaccination induces humoral and cellular immune responses against hepatitis B virus surface antigen in mice without activation of C-myc. World journal of gastroenterology. 2000;6(2):239. [DOI] [PMC free article] [PubMed]
- 47.Mendoza CA, Ebihara H, Yamaoka S. Immune Modulation and Immune-mediated pathogenesis of emerging Tickborne Banyangviruses. Vaccines (Basel). 2019;7(4). [DOI] [PMC free article] [PubMed]
- 48.Suleman M, Asad U, Arshad S, Rahman AU, Akbar F, Khan H, Hussain Z, Ali SS, Mohammad A, Khan A et al. Screening of immune epitope in the proteome of the Dabie bandavirus, SFTS, to design a protein-specific and proteome-wide vaccine for immune response instigation using an immunoinformatics approaches. Comput Biol Med. 2022;148:105893. [DOI] [PubMed]
- 49.Semple F, MacPherson H, Webb S, Kilanowski F, Lettice L, McGlasson SL, Wheeler AP, Chen V, Millhauser GL, Melrose L. Human β-D-3 exacerbates MDA5 but suppresses TLR3 responses to the viral molecular pattern mimic polyinosinic: polycytidylic acid. PLoS genetics. 2015;11(12):e1005673. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.